Abstract
Background
Patients with hematologic diseases are at higher risk of the SARS-CoV-2 infection and more severe clinical outcomes of the coronavirus disease. CHRONOS19 is an observational prospective cohort study with the aim to determine the short and longer-term clinical outcomes, risk factors for disease severity and mortality, and rates of postinfectious immunity in patients with malignant and nonmalignant hematologic diseases and COVID-19.
Patients and Methods
: Overall, 666 patients were enrolled in the study, of which 626 were included in the final data analysis. The primary endpoint was 30-days all-cause mortality. Secondary endpoints included COVID-19 complications, rates of ICU admission and mechanical ventilation, outcomes of a hematologic disease in SARS-CoV-2 infected patients, overall survival, and risk factors for disease severity and mortality. Data from 15 centers were collected at 30, 90, and 180 days after COVID-19 was diagnosed and were managed using a web-based e-data capture platform. All evaluations were performed in the pre-omicron period of COVID-19 pandemic.
Results
Thirty-days all-cause mortality was 18.9%. The predominant cause of death (in 80% of cases) were COVID-19 complications. At 180 days, the majority (70%) of additional deaths were due to hematologic disease progression. At a median follow-up of 5.7 [0.03-19.04] months, 6-months overall survival was 72% [95% CI: 0.69-0.76]. One-third of patients had severe SARS-CoV-2 disease. The rate of ICU admission was 22% with 77% of these patients requiring mechanical ventilation, with poor survival rate. A univariate analysis revealed that older age (≥ 60 years), male sex, malignant hematologic disease, myelotoxic agranulocytosis, transfusion dependence, refractory disease or relapse, diabetes among comorbidities, any complications, especially ARDS alone or in combination with CRS, admission to an ICU, and mechanical ventilation were associated with higher risks of mortality. Treatment of the hematologic disease was changed, postponed, or canceled in 63% of patients. At a longer follow-up (90 and 180 days), the status of the hematologic disease changed in 7.5% of patients.
Conclusion
Patients with hematologic disease and COVID-19 have high mortality rates, predominantly due to COVID-19 complications. At a longer-term follow-up, no significant impact of COVID-19 on the course of a hematologic disease was revealed.
Keywords: Mortality, Lymphomas, Leukemia, Risk factors, SARS-CoV-2
Micro-Abstract
Patients with hematologic conditions are at a higher risk of mortality due to COVID-19 compared to the general population. Further research and implementation of appropriate interventions in order to prevent the spread of the SARS-CoV-2 infection in this vulnerable patient population are warranted.
Introduction
Since the beginning of the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection outbreak, more vulnerable patient populations with various concomitant acute and chronic diseases have drawn particular attention from the medical and scientific community due to the numerous concerns regarding the complications, consequences, and outcomes of both the coronavirus infectious disease (COVID-19) and the underlying condition. Early reports demonstrated that patients with cancer had significantly higher risks of poor outcomes of COVID-19, including higher mortality rates, compared to the general population infected with SARS-CoV-2, whereas hematologic malignancies and lung cancer were generally associated with the highest incidence of severe events.1, 2, 3, 4, 5 This led to the initiation of several registries in various countries, including those organized by research institutions and professional associations such as the American Society of Clinical Oncology (ASCO), American Society of Hematology (ASH), European Society for Medical Oncology (ESMO), European Hematology Association (EHA), National Cancer Institute (NCI), and others. One of the largest and the earliest to report the preliminary results was a collaborative research project between the USA and Canada sites–the COVID-19 and Cancer Consortium (CCC19), with about 5000 patients included in the registry database throughout the pandemic.4 , 6
Based on the acquired knowledge, patients with hematologic diseases are at increased risk of SARS-CoV-2 infection due to the nature of the underlying hematologic disease, comorbidities, and immunosuppressive treatments.7, 8, 9, 10, 11, 12, 13 ASH, EHA registries and several reports from single- or multi-center observations demonstrated clinical outcomes, risk factors, and immunity in hematologic patients with COVID-19.14, 15, 16, 17, 18, 19, 20, 21, 22 However, to the best of our knowledge, this observational study is the first to explore COVID-19 in patients with various hematologic diseases that included short-term and longer-term follow-ups with the objective to assess both COVID-19 immediate outcomes as well as any potential impact on hematologic disease caused by either the infection or any change in the treatment schedule.
Here, we report the final results of the Covid-19 & Hematologic diseases Registry: cOhort NOninterventional Study (CHRONOS19).
Methods
Study Design
CHRONOS19 is a multi-center observational prospective cohort study with the aim to determine the clinical outcomes in SARS-CoV-2 infected patients with hematologic diseases. The study was conducted among adult patients (≥ 18 years) with malignant or nonmalignant hematologic diseases and laboratory-confirmed or suspected (based on clinical symptoms and/or CT) COVID-19. Between June 2020 and January 2022, data from 15 centers in Russia were collected at 30, 90, and 180 days after COVID-19 was diagnosed and were managed with the assistance of Enrollme.ru, a web-based EDC/CTMS Research Network, in a de-identified manner. This e-platform provided data capture, storage, and management in compliance with ISPE GAMP5 and 21CFR Part 11 requirements.
After the registration, investigators filled out the online case report forms including the initial complete form on Day 30 and 2 abbreviated follow-up forms on Day 90 and Day 180. Retrospective data input was allowed for the first follow-up (at 30 days). The case report form consisted of 6 sections with a total of 48 single- or multiple-choice questions including the general clinical history of a hematologic disease; concomitant conditions; manifestation, treatment, and the clinical course of COVID-19; complications, clinical outcomes, and antibody immunity; impact on a hematologic disease and its specific treatment; and a field for additional comments. Both abbreviated forms contained only a section on the outcomes of a hematologic disease and a field for additional comment.
The primary endpoint was 30-days all-cause mortality. Secondary endpoints included COVID-19 complications, rates of ICU admission and mechanical ventilation, outcomes of a hematologic disease in SARS-CoV-2 infected patients, overall survival, and risk factors for disease severity and mortality. Only patients with a known outcome of COVID-19 were included in the final analysis.
Severity of the coronavirus disease was determined in accordance with the local recommendations and divided into mild, moderate, moderately severe, and severe.
This study was approved by the Institutional Review Board and the Ethical Committee of the National Research Center for Hematology (Moscow, Russia).
This study was registered at clinicaltrials.gov. NCT04422470.
Statistical Considerations
Continuous variables were described with median and max/min interval. Categorical variables were expressed as frequencies. The survival analysis with Kaplan-Maier estimates and log-rank test were used for the comparison of overall survival (OS) in different groups of patients. Cox proportional-hazards models were applied to determine hazard ratios (HRs) and 95% confidence intervals (CIs), used for both univariate and multivariate analyses. The initial set of cofounders for multivariate analysis was selected among the variables that demonstrated statistical significance level less than 0.05 by the univariate analysis. For multivariate analysis, we used forward selection with the entry significance level of 0.5 and the significance level of 0.05 for retaining. A two-sided P < .05 was considered statistically significant. Landmark approach was utilized for time-dependent factors. All statistical analyses were performed using SAS (version 9.4) and SPSS (version 22.0).
Results
Patient Disposition
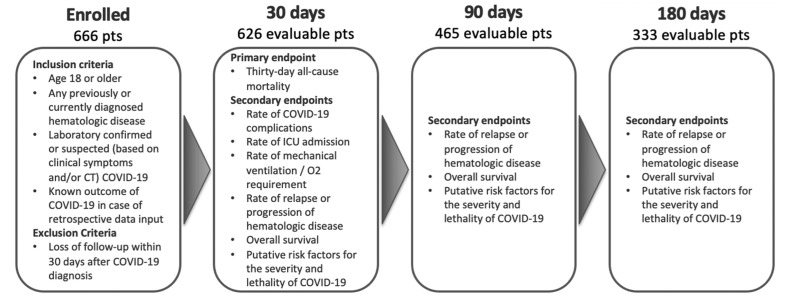
Between June 22, 2020 and August 22, 2021, overall, 666 patients were enrolled in the registry with 626 being included in the final data analysis as eligible for the primary endpoint assessment at Day 30. Follow-up data were available for 465 and 333 evaluable patients at Day 90 and Day 180, respectively (Figure 1 ). Patient demographics and clinical characteristics are described in Table 1 .
Figure 1.
CHRONOS19 study design and patient disposition
Table 1.
Patient Demographics (n = 626)
| Age, Median [Range] | 56 (18-90) Years |
|---|---|
| Males/Females, n (%) | 329 (52%) / 297 (48%) |
| Disease type, n (%): | |
| Malignant / Nonmalignant | 581 (93%) / 45 (7%) |
| Disease status, n (%)a: | |
| Induction | 218 (37.5%) |
| Remission | 221 (38%) |
| Relapse/Refractory | 142 (24.5%) |
| adata available for 581 (93%) of patients | |
| HSCT, n: allogeneic / autologous | 19 / 25 |
| Myelotoxic agranulocytosis, n (%)a: yes / no | 119 (19%) / 496 (82%) |
| adata available for 615 (98%) of patients | |
| Transfusion dependency, n (%)a: yes / no | 208 (34%) / 401 (66%) |
| adata available for 609 (97%) of patients | |
| Comorbidities, n (%)a: yes / no | 358 (58%) / 262 (42%) |
| Cardiovascular | 234 (65%) |
| Diabetes | 68 (19%) |
| Obesity | 52 (14.5%) |
| Pulmonary | 38 (11%) |
| Renal | 42 (12%) |
| Hepatic | 30 (8%) |
| Other | 85 (24%) |
| adata available for 620 (99%) of patients | |
| ECOG at COVID-19 diagnosis, n (%)a: | |
| 1 | 190 (33%) |
| 2 | 229 (39%) |
| 3 | 153 (26%) |
| 4 | 9 (2%) |
data available for 581 (93%) of patients
The majority of patients (93%) had malignant disease (for hematologic disease distribution, Table 2 ). Concomitant conditions were reported in 358 (58%) patients.
Table 2.
Hematologic Disease Distribution (N = 626; The Proportions of Individual Diagnoses Were Calculated From the Total Number of Malignant or Nonmalignant Diseases)
| Disease | n (%) |
|---|---|
| Malignant | 581 (93%) |
| Acute myeloid leukemia (AML) | 108 (19%) |
| Multiple myeloma (MM) | 107 (18%) |
| Non-Hodgkin lymphoma (NHL) | 102 (18%) |
| Chronic myeloid leukemia (CML) | 59 (10%) |
| Acute lymphoblastic leukemia (ALL) | 47 (8%) |
| Chronic lymphocytic leukemia (CLL) | 45 (8%) |
| Chronic myeloproliferative neoplasms (CMPN) | 30 (5%) |
| Myelodysplastic syndrome (MDS) | 23 (4%) |
| Other | 60 (10%) |
| Hairy cell leukemia (HCL) | 22 |
| Hodgkin's lymphoma (HL) | 20 |
| Acute promyelocytic leukemia (APL) | 11 |
| Waldenstrom macroglobulinemia (WM) | 7 |
| Non-malignant | 45 (7%) |
| Aplastic anemia | 15 (33%) |
| Autoimmune hemolytic anemia | 8 (18%) |
| Idiopathic thrombocytopenic purpura | 6 (13%) |
| Paroxysmal nocturnal hemoglobinuria | 4 (9%) |
| Hemophilia | 3 (7%) |
| Gaucher disease | 3 (7%) |
| Other | 6 (13%) |
All-Cause Mortality
Thirty-days all-cause mortality was 18.9% (118 patients died). Death due to COVID-19 complications occurred in 95 (80%) patients, 16 (14%) patients died due to the progression of a hematologic disease (15 of them were malignant, in 1 case the hemolytic crisis occurred in a patient with autoimmune hemolytic anemia (AIHA)), in 7 remaining cases the cause of death was either not available (n = 5) or unknown (n = 2).
The highest 30-days mortality rate was detected among patients with CLL (14 of 45 patients 31%), acute leukemia (48 of 166%-29%: AML, 39 of 108%-36%, ALL, 8 of 47%-17%), MDS (6 of 23%-26%), and CMPN (7 of 30%-23%). The lowest 30-days mortality was seen in patients with CML (1 of 59%-2%), Hodgkin's lymphoma (1 of 20%-5%), and nonmalignant hematologic diseases (3 of 45%-7%) (Figure 2 ).
Figure 2.
Overall survival in patients with various types of hematologic malignancies and COVID-19 who were eligible for the primary endpoint assessment in CHRONOS19. Survival analysis was performed with Kaplan-Maier estimates. Abbreviations: AL, acute leukemia; CLL, chronic lymphocytic leukemia; CML, chronic myeloid leukemia; CMPN, chronic myeloproliferative neoplasms; HL, Hodgkin's lymphoma; MDS, myelodysplastic syndrome; MM, multiple myeloma; NHL, non-Hodgkin lymphoma
At 90 days, 31 more deaths occurred with 19 cases (61%) being still due to COVID-19 and its complications. However, at 180 days, among 23 additional deaths, the majority (70%) were due to the progression of the hematologic disease. Still, in 7 cases, the second or the third episode of COVID-19 proved to be lethal.
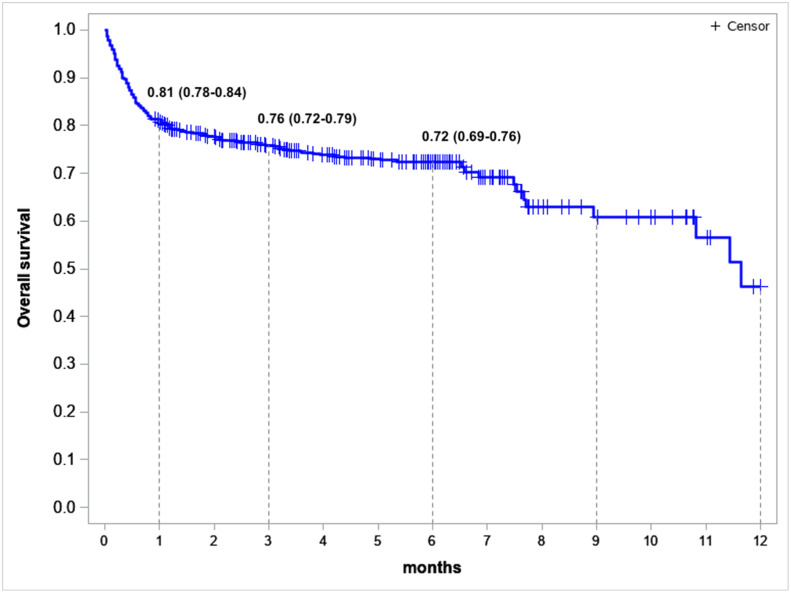
At a median follow-up of 5.7 [0.03-19.04] months, 6-months OS was 72% (95% CI: 0.69-0.76) (Figure 3 ).
Figure 3.
Overall survival in the evaluable population of patients who were eligible for the primary endpoint assessment in CHRONOS19, n = 626: survival estimates (95% CI) at 1, 3, and 6 months after the COVID-19 diagnosis. Survival analysis was performed with Kaplan-Maier estimates.
COVID-19: Course of Disease and Treatment
PCR detection of SARS-CoV-2 was reported in 85% of cases, predominantly in nasopharyngeal swabs (96%). In 3 cases, bronchoalveolar lavage (BAL) fluid was used to detect coronavirus. The leading COVID-19 symptoms included fever (77%), fatigue (62%), and cough (58%). In 12% of patients, the disease was asymptomatic. Median duration of COVID-19 was 15.51-125 days.
Overall, 207 (33%) patients had severe disease (including moderate severe and severe cases, for definitions, please see Table 3 ). The worst ECOG 3 to 4 was detected in 53% of patients, 49% of these cases had ECOG 0 to 2 at diagnosis. The most common disease manifestations and complications included pneumonia in 398 (65%) patients, respiratory failure in 92 (15%) patients, multiple organ failure in 56 (9%) patients, cytokine release syndrome in 48 (8%) patients, ARDS in 47 (8%) patients, and sepsis in 44 (7%) patients. In patients with pneumonia, the worst computer tomography (CT) results detected during the disease were CT I (< 25% of lung tissue damage)–in 19%; CT II (25%-50%)–in 41%; CT III (50%-75%)–in 26%; and CT IV (> 75%)–in 14% of cases.
Table 3.
Definitions of the COVID-19 Severity According to Local Regulations31
| Illness Severity (Stage) | Symptoms |
|---|---|
| Mild (Stage I) | Individuals who have any of the various signs and symptoms of COVID-19 (eg, fever, cough, sore throat, malaise, headache, muscle pain, nausea, vomiting, diarrhea, loss of taste and smell) but who do not have shortness of breath, dyspnea, or abnormal chest imaging, and body temperature T< 38°C. |
| Moderate (Stage II) | Individuals who show evidence of lower respiratory disease during clinical assessment or imaging and who have saturation of oxygen 95>(SpO2) ≥ 94% on room air at sea level, respiratory frequency > 22 breaths per minute, with T> 38°C, abnormal chest imaging (typical of a viral infection – CT 1-2), C-reactive protein in blood serum>10 mg/l. |
| Moderate severe (Stage III) | Individuals who have SpO2 ≤ 93% on room air at sea level, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) ≤ 300 mm Hg, respiratory frequency >30 breaths per minute, or lung infiltrates >50% abnormal chest imaging (typical of a viral infection – CT 3-4), lactate in arterial blood>2 mmol/l, qSOFA>2. |
| Severe (Stage IV) | Individuals who have respiratory failure, septic shock, and/or multiple organ dysfunction, and/or abnormal chest imaging with critical changes of a viral infection (CT 4), or acute respiratory distress syndrome. |
Specific or symptomatic treatment was reported in 459 patients. Among them, antiviral (including favipiravir, umifenovir, lopinavir/ritonavir, etc.) and/or antibacterial therapeutics were given to 342 (75%) patients, anti-IL-6 monoclonal antibodies were administered in 81 (18%) patients, and 18 (4%) patients received JAK inhibitors. Prevention of thrombotic complications was used in 334 (60%) patients. Prior to SARS-CoV-2 infection, 221 (37%) patients received systemic corticosteroids which didn't significantly affect the clinical outcomes. Median overall survival in patients who received systemic corticosteroids was 11.6 (7.5-NE) months vs. 12.3 (10.8-NE) months in those with no corticosteroid treatment (HR, 1.29 [95% CI, 0.96-1.76], P = .1040).
The hospitalization rate due to COVID-19 was 30.33%, another 224 patients (33.63%) had already been in the hospital at the time of COVID-19 diagnosis. The rate of admission to an intensive care unit (ICU) was 22% (140 patients) with a high 30-days mortality rate in this group of patients (79%); 108 (77%) patients in ICU required mechanical ventilation, among them, only 3 (3%) patients survived.
It was observed that patients with CLL were significantly more likely to require admission to ICU than patients with other oncohematologic diseases (41% vs. 22%, P < .003). Also, they required mechanical ventilation more frequently than other patients (33% vs. 17%, P = .019), had higher incidence rates of pneumonia (98% vs. 65%, P < .001), and more frequently developed moderate severe (34% vs. 20%, P = .019) and severe (27% vs. 17%, P =.03) infection. Thus, among all patients, those with CLL were significantly more likely to have severe COVID-19 and to develop life-threatening complications.
Risk Factors
A univariate analysis revealed that older age ≥ 60 years (30-days OS: 75% vs. 85%; HR, 1.52 [95% CI, 1.13-2.04]), male sex (80% vs. 82%; HR, 1.37 [95% CI, 1.01-1.85]), malignant disease vs. nonmalignant (80% vs. 93%; HR, 2.34 [95% CI, 1.04-5.28]), ECOG 3-4 vs. 0-2 at the onset of COVID-19 (63% vs. 86%; HR, 2.77 [95% CI2.06-3.74]) and the worst ECOG 3-4 vs. 0-2 during the course of SARS-CoV-2 disease (73% vs. 98%; HR, 4.50 [95% CI, 2.95-6.86]), myelotoxic agranulocytosis (78% vs. 89%; HR, 2.27 [95% CI, 1.55-3.34]), transfusion dependence (75% vs. 92%; HR, 3.33 ([95% CI, 2.31-4.79]), refractory disease or relapse vs. remission of a hematologic disease (66% vs. 87%; HR, 2.31 [95% CI, 1.68-3.18]), diabetes among comorbidities (72% vs. 86%; HR, 1.97 ([95% CI, 1.27-3.05]), ARDS alone or with CRS vs. no complications (45% or 53% vs. 100%; HR, 15.12 [95% CI, 7.19-31.78] or 16.67 [95% CI, 7.64-36.37]) as well as other complications (84% vs. 100%; HR, 3.58 [95% CI, 2.08-6.15]), admission to an ICU (31% vs. 98%; HR, 20.13 [95% CI, 13.68-29.63]), and mechanical ventilation (13% vs. 97%, HR, 66.38 [95% CI, 40.77-108.08]) were associated with higher risks of mortality (Figure 4 ). Interestingly, ICU without mechanical ventilation was associated with higher OS (88% at 30 days; 95% CI, 4.23 [1.98-9.04]), however, 30-day OS in patients who had been admitted to ICU and required mechanical ventilation was only 10% (P < .0001). In a multivariate analysis, transfusion dependence (HR, 2.6 [1.91-3.73], P < .0001) and ECOG 3-4 vs. 0-2 at the onset of COVID-19 (HR, 2.43 [2.43-3.39], P < .0001) were associated with higher mortality.
Figure 4.
Forrest plot representing risk factors for COVID-19 mortality (univariate analysis). Abbreviations: ARDS, acute respiratory distress syndrome; CRS, cytokine release syndrome; ECOG, Eastern Cooperative Oncology Group; ICU, intensive care unit; MTA, myelotoxic agranulocytosis.
The results of a univariate analysis of various factors at the onset of COVID-19 showed that a malignant hematologic disease, blood transfusion dependence, myelotoxic agranulocytosis, a relapse or a refractory hematologic disease were significantly associated with the severity of COVID-19 (Table 4 ).
Table 4.
Potential Risk Factors of Severe COVID-19 at the Onset of the Disease (Univariate Analysis)
| Factor | HR [95% CI] | Р |
|---|---|---|
| Blood transfusion dependence | 2.08 [1.46-2.96] | <.0001 |
| Myelotoxic agranulocytosis | 2.12 [1.41-3.20] | .0003 |
| Relapse or refractory disease | 1.73 [1.17-2.56] | .0061 |
| Malignant hematologic disease | 4.24 [1.64-10.94] | .0012 |
Hematologic Outcomes
Treatment of a hematologic disease was changed, postponed, or canceled in 385 (63%) patients with a median treatment delay of 41, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24 weeks. At 30 days, the rate of relapse / progression of a hematologic disease was 4%/11% (22 / 57 of 503 evaluable patients); refractory disease was reported in 64 (13%) patients.
At a longer follow-up (90 and 180 days), the status of a hematologic disease changed in 24 of 319 evaluable patients (7.5%). At a 90-days follow-up, 15 cases of relapsed or refractory disease were detected in patients with acute leukemia (1 AML and 3 ALL cases), CML (6), Hodgkin lymphoma (2), MDS (1), follicular lymphoma (1), and aplastic anemia (1)–3 patients with ALL (2) and MDS (1) died. Among these patients, the hematologic disease reversed back to remission at 180 days after the COVID-19 diagnosis in 4 patients (2 CML, 1 AML, and 1 HL) – all these patients were alive during the study follow-up. At a 180-days follow-up, relapsed or refractory disease was reported in 3 more patients with AML, one of them died. Progressive disease was detected in 2 patients at 90 days and 4 patients at 180 days. These patients had been diagnosed with CML (1), multiple myeloma (1), T cell lymphoma (1), and aggressive non-Hodgkin B cell lymphomas (2 DLBCL cases and 1 case of Burkitt lymphoma). One patient with the progression of MM and 1 patient with progressive T cell lymphoma died.
Treatment change or rescheduling did not have a significant impact on OS, with HR, 0.98 [95% CI, 0.66-1.46]. However, cancelation or noninitiation of treatment of a hematologic disease was associated with a significantly lower OS, with HR, 4.35 [95% CI, 2.98-6.35] compared to no impact on the treatment (Figure 5 ). Six-months OS rates in patients who experienced treatment cancelation were 42% vs. 81% in both those with treatment schedule change and no impact on treatment. There were no statistically significant differences in the causes of death in these 3 groups.
Figure 5.
Impact of a hematologic disease treatment and its schedule change on overall survival. Survival analysis was performed with Kaplan-Maier estimates.
Discussion
In the face of the new coronavirus pandemic, real-world data became the cornerstone of clinical decisions for the prevention and treatment of COVID-19 in the early days of pandemic before the data from randomized trials became available, allowing for the improvement of clinical outcomes for patients both infected and at a higher risk of infection or severe disease. Therefore, the development of registries, such as CHRONOS19, was crucial for further providing insights and developing clinical guidance in special patient populations. In a recent review, summaries of 35 studies of COVID-19 in > 50 patients with hematologic malignancy (HM) from all over the world were presented comprising a total population of over 17,500 hematologic patients.23 The largest studies included a retrospective NCATS' National COVID Cohort Collaborative (63 413 cancer patients with COVID-19, 10.5% among them with HM) and a survey EPICOVIDEHA (3 801 patients with HM with COVID-19). It should be noted that the majority of the reported studies were retrospective. Only a few prospective studies enrolled between approximately 150 and 400 patients each.
Our data suggest that mortality in patients with hematologic disease exceeds COVID-19-related death rates in the general population. Thus, 1.8% of patients infected with SARS-CoV-2 died overall in Russia compared to a 30-days all-cause mortality of 18.9% in CHRONOS19.24 , 25 High mortality rates in hematologic subpopulation were consistent among different studies across the countries all over the world and varied between 12% and 37%.23 According to our data, the predominant cause of death, in short term, was COVID-19 complications (80%) which once again speaks for a vast significance of preventive measures that should be taken in this vulnerable patient population, including isolation, appropriate vaccination, special precautions when organizing patient flow in the hospital setting, etc.
There is no doubt that vaccination along with additional prophylactic measures changed the entire landscape of the pandemic, and patients with cancer are no exception. For instance, the EPICOVIDEHA registry included over 1500 cases of breakthrough COVID-19 in patients with HM after anti-SARS-CoV-2 vaccination, with a vast majority of them having lymphoid malignancies. In this analysis, the mortality rate after a 30-days follow-up from the COVID-19 diagnosis was significantly lower than in the pre-vaccine era (9% vs. 31%).26 Nevertheless, breakthrough COVID-19 in patients with HM is still associated with considerable mortality, even in patients with Omicron variant (7.9%), while the reported overall death rate remains of over 10% in cancer patients during the Omicron wave.27 In a UK national COVID-19 cancer cross-sectional study involving 4249 antibody test results from patients with cancer, those with HM had the lowest antibody titers. The findings from this study suggested that patients with an undetectable antibody vaccine response were at much greater risk for SARS-CoV-2 breakthrough infection.28 Therefore, similar precaution recommendations should still be followed to lower the risk of infection or hospitalization among patients with HM.
For proper clinical assessments and prognosis, it is important to identify the risk factors associated with disease severity and mortality. Our data confirmed the most common risk factors reported earlier.29 , 30 Admission to ICU and mechanical ventilation reflect the severity of disease with the highest death rate in the latter group. Conversely, nonmalignant hematologic disease and remission of any hematologic disease, as well as absence of any concomitant conditions were associated with more favorable prognosis of COVID-19 in this patient population.
At a longer-term follow-up, no significant impact of COVID-19 on the course of a hematologic disease was shown. There were only a few cases of hematologic disease relapse or progression reported. However, these data are limited by a scarce number of cases reported at a longer term and only 180 days of follow-up. There was no data collection beyond this time point, therefore, additional events could have taken place after Day 180 with potential impact on the outcomes of the hematologic disease. Moreover, the study was not designed to include a patient cohort who were not infected with SARS-CoV-2, therefore, no robust conclusions regarding relapse or progression rates can be derived. Furthermore, the lack of matched control limits the strengths of the observed statements.
Other limitations of the presented study include observational design with an opportunity to insert data retrospectively for the first data collection point (30 days), hence possible selection bias, and a small number of patients for subgroup analyses.
Conclusion
The COVID-19 pandemic continues, and our data confirm that hematologic patients are at a greater risk for severe disease and death. Therefore, prevention of SARS-CoV-2 infection, including vaccination, pre- and postexposure prophylaxis, and other measures, should remain the priority with the aim to diminish its impact on clinical outcomes and provide superior care for patients with hematologic diseases during the ongoing pandemic.
Ethics Approval Statement
The authors confirm this analysis and manuscript were created in accordance with the Declaration of Helsinki and have been approved by the IRB at National Research Center for Hematology (Moscow, Russia).
Author Contributions
K.Z and O.A.A designed study concept. A.N.V, V.S.D, L.S.B., L.V.G., A.A.S, A.A.P, M.A.G, A.F.R collected data. O.A.A, K.Z, and A.N.V organized data. Y.A.C and S.M.K cleaned data and performed statistical analysis. K.Z and O.A.A wrote and edited the manuscript. All listed authors have contributed to the manuscript substantially and have agreed to the final submitted version.
Disclosure
The authors declare no competing financial interests. There was no funding used for this study or preparation of this manuscript. This article has not been published elsewhere, and is not currently under consideration for publication elsewhere.
Acknowledgments
The authors would like to thank Mikhail Getman (Enrollme.ru) for his support in data capture and study management, Dr. Ilya Tsimafeyeu for his help in developing eCRF, Foundation for Cancer Research Support (RakFond) for setting up the study, all participating investigators, patients, their families, and caregivers.
Footnotes
O.A.A and K.Z. contributed equally to this work and Co-first authors.
References
- 1.Dai M, Liu D, Liu M, et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10(6):783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jee Justin, Foote Michael B., Lumish Melissa, et al. Chemotherapy and COVID-19 outcomes in patients with cancer. J Clin Oncol. 2020;38(30):3538–3546. doi: 10.1200/JCO.20.01307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brar G, Pinheiro LC, Shusterman M, et al. COVID-19 severity and outcomes in patients with cancer: a matched cohort study. J Clin Oncol. 2020;38(33):3914–3924. doi: 10.1200/JCO.20.01580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grivas P, Khaki AR, Wise-Draper TM, et al. Association of clinical factors and recent anticancer therapy with COVID-19 severity among patients with cancer: a report from the COVID-19 and Cancer Consortium. Ann Oncol. 2021;32(6):787–800. doi: 10.1016/j.annonc.2021.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu J, Ouyang W, Chua MLK, et al. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020;6(7):1108–1110. doi: 10.1001/jamaoncol.2020.0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vijenthira A, Gong IY, Fox TA, et al. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood. 2020;136:2881–2892. doi: 10.1182/blood.2020008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cattaneo C, Daffini R, Pagani C, et al. Clinical characteristics and risk factors for mortality in hematologic patients affected by COVID-19. Cancer. 2020;126:5069–5076. doi: 10.1002/cncr.33160. [DOI] [PubMed] [Google Scholar]
- 10.Borah P, Mirgh S, Sharma SK, et al. Effect of age, comorbidity and remission status on outcome of COVID-19 in patients with hematological malignancies. Blood Cells Mol Dis. 2021;87 doi: 10.1016/j.bcmd.2020.102525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wood WA, Neuberg DS, Thompson JC, et al. Outcomes of patients with hematologic malignancies and COVID-19: a report from the ASH Research Collaborative Data Hub. Blood Adv. 2020;4:5966–5975. doi: 10.1182/bloodadvances.2020003170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee LYW, Cazier JB, Starkey T, et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol. 2020;21:1309–1316. doi: 10.1016/S1470-2045(20)30442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glenthoj A, Jakobsen LH, Sengelov H, et al. SARS-CoV-2 infection among patients with hematological disorders: severity and one-month outcome in 66 Danish patients in a nationwide cohort study. Eur J Haematol. 2021;106:72–81. doi: 10.1111/ejh.13519. [DOI] [PubMed] [Google Scholar]
- 14.Malard F, Genthon A, Brissot E, et al. COVID-19 outcomes in patients with haematology disease. Bone Marrow Transplant. 2020;55(11):2180–2184. doi: 10.1038/s41409-020-0931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Available at: https://www.ashresearchcollaborative.org/s/covid-19-registry/data-summaries (Accessed: July 28, 2022)
- 16.Pagano L, Salmanton-García J, Marchesi F, et al. COVID-19 infection in adult patients with hematological malignancies: a European Hematology Association Survey (EPICOVIDEHA) J Hematol Oncol. 2021;14(1):168. doi: 10.1186/s13045-021-01177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma A, Bhatt NS, St Martin A, et al. Clinical characteristics and outcomes of COVID-19 in hematopoietic stem-cell transplantation recipients: an observational cohort study. Lancet Haematol. 2021;8:e185–e193. doi: 10.1016/S2352-3026(20)30429-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ljungman P, de la Camara R, Mikulska M, et al. COVID-19 and stem cell transplantation; results from an EBMT and GETH multicentre prospective study. Leukemia. 2023;5(10):2885–2894. doi: 10.1038/s41375-021-01302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinana JL, Martino R, Garcia-Garcia I, et al. Risk factors and outcome of COVID-19 in patients with hematological malignancies. Exp Hematol Oncol. 2020;9:21. doi: 10.1186/s40164-020-00177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Passamonti F, Cattaneo C, Arcaini L, et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with hematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. 2020;7:e737–e745. doi: 10.1016/S2352-3026(20)30251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 22.García-Suárez J, de la Cruz J, Cedillo Á, et al. Impact of hematologic malignancy and type of cancer therapy on COVID-19 severity and mortality: lessons from a large population-based registry study. J Hematol Oncol. 2020;13(1):133. doi: 10.1186/s13045-020-00970-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langerbeins P, Hallek M. COVID-19 in patients with hematologic malignancy. Blood. 2022;140(3):236–252. doi: 10.1182/blood.2021012251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Available at: https://coronavirus.jhu.edu/map.html (Accessed: January 06, 2023)
- 25.Available at: https://xn–80aesfpebagmfblc0a.xn–p1ai/information/ (Accessed: January 06, 2023)
- 26.Pagano L., Salmanton-García J., Marchesi F., et al. Breakthrough COVID-19 in vaccinated patients with hematologic malignancies: results from the EPICOVIDEHA survey. Blood. 2022;140(26):2773–2787. doi: 10.1182/blood.2022017257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kareff SA, Khan A, Barreto-Coelho P, et al. Prevalence and outcomes of COVID-19 among hematology/oncology patients and providers of a community-facing health system during the B1.1.529 ("Omicron") SARS-CoV-2 variant wave. Cancers (Basel) 2022;14(19):4629. doi: 10.3390/cancers14194629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee LYW, Tilby M, Starkey T, et al. Association of SARS-CoV-2 spike protein antibody vaccine response with infection severity in patients with cancer: a national COVID cancer cross-sectional evaluation. JAMA Oncol. 2023;9(2):188–196. doi: 10.1001/jamaoncol.2022.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piñana JL, Martino R, García-García I, et al. Infectious complications subcommittee of the Spanish hematopoietic stem cell transplantation and cell therapy group (GETH). Risk factors and outcome of COVID-19 in patients with hematological malignancies. Exp Hematol Oncol. 2020;9:21. doi: 10.1186/s40164-020-00177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eryilmaz-Eren E, Ture Z, Kilinç-Toker A, et al. The course of COVID-19 in patients with hematological malignancies and risk factors affecting mortality: A cross-sectional study. Hematol Transfus Cell Ther. 2022 doi: 10.1016/j.htct.2022.10.001. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Temporary Recommendations. Prevention, Diagnosis and Treatment of COVID-19. Ministry of Health of the Russian Federation; 2020. Version 7. Available at: https://static-0.minzdrav.gov.ru/system/attachments/attaches/000/050/584/original/03062020_%D0%9CR_COVID-19_v7.pdf. (Accessed: June 03, 2020).