Highlights
-
•
Analyses of test–retest functional connectomes in first episode of psychosis.
-
•
Connectomes from patients show higher inter subject variability.
-
•
Variability of functional connectomes in psychosis relates to symptom’s severity.
-
•
Changes in symptoms severity correlates with deviation from healthy connectomes.
Keywords: Functional connectome, Variability, Fingerprint, First-episode of psychosis
Abstract
Patients with Schizophrenia may show different clinical presentations, not only regarding inter-individual comparisons but also in one specific subject over time. In fMRI studies, functional connectomes have been shown to carry valuable individual level information, which can be associated with cognitive and behavioral variables. Moreover, functional connectomes have been used to identify subjects within a group, as if they were fingerprints. For the particular case of Schizophrenia, it has been shown that there is reduced connectome stability as well as higher inter-individual variability. Here, we studied inter and intra-individual heterogeneity by exploring functional connectomes’ variability and related it with clinical variables (PANSS Total scores and antipsychotic’s doses). Our sample consisted of 30 patients with First Episode of Psychosis and 32 Healthy Controls, with a test–retest approach of two resting-state fMRI scanning sessions. In our patients’ group, we found increased deviation from healthy functional connectomes and increased intragroup inter-subject variability, which was positively correlated to symptoms’ levels in six subnetworks (visual, somatomotor, dorsal attention, ventral attention, frontoparietal and DMN). Moreover, changes in symptom severity were positively related to changes in deviation from healthy functional connectomes. Regarding intra-subject variability, we were unable to replicate previous findings of reduced connectome stability (i.e., increased intra-subject variability), but we found a trend suggesting that result. Our findings highlight the relevance of variability characterization in Schizophrenia, and they can be related to evidence of Schizophrenia patients having a noisy functional connectome.
1. Introduction
Schizophrenia is a heterogenous mental disorder with high variability in clinical profiles displayed by patients. Moreover, there is both inter-individual and intra-individual variability in Schizophrenia. In other words, not only two people diagnosed with this disorder may show different clinical presentations, but also a single patient may show different symptomatic patterns along their medical history (Kirkpatrick et al., 2001, Wong and van Tol, 2003).
Even in healthy populations, despite having gross similarities, brains of different individuals are unique (Barch et al., 2013, Gordon et al., 2017). Functional connectomes derived from fMRI studies capture such individual variability, and they have been shown to be stable in time, allowing for the identification of a particular subject among others, as if they were fingerprints (Finn et al., 2015, Amico and Goñi, 2018, Liu et al., 2018). However, traditional neuroimaging studies are advocated to the estimation of mean group-level characteristics. As such, they fall short when assessing individual-level characteristics, which can be so relevant for a heterogenic and complex disorder such as Schizophrenia. For fMRI measures to provide insights into the clinical manifestations of Schizophrenia, they should correlate with variations in symptoms expression and, ideally, predict outcome, treatment response or risk of disease onset (van den Heuvel and Fornito, 2014).
There is evidence that functional connectomes’ variability is associated with cognitive and behavioral variables (Barch et al., 2013). Several studies have used this information to predict individuals’ performance variability in cognitive domains or symptom severity in neuropsychiatric disorders (Finn et al., 2015, Shen et al., 2017, Svaldi et al., 2021). Relatedly, the stability of a subject’s functional connectome has been shown to be age-related: it increases during development, peaks in early adulthood, and decreases thereafter (Kaufmann et al., 2017, Ousdal et al., 2020). A delay in the stabilization of functional connectomes has been related to mental health in adolescents (Kaufmann et al., 2017).
Particularly, previous research suggests there is reduced connectome stability in patients with Schizophrenia, as the connectomic fingerprint of patients has been found to be less stable than that of healthy-control subjects (Kaufmann et al., 2018) This reduced stability of the connectome in Schizophrenia recalls the intra-individual clinical variability usually seen in patients. Moreover, it could be related to changes in symptom expression, although it has not been directly explored. In addition, inter-individual variability in Schizophrenia was also found to be higher in patients for whole brain analysis, frontoparietal, DMN, and ventral attention networks (Santo-Angles et al., 2021). Furthermore, this variability was negatively associated with PANSS scores (Santo-Angles et al., 2021), with more symptomatic patients being more similar to a patient’s mean-connectome.
Regardless of their individually less stable connectomes, and in support of group-average traditional approaches, group-level mean functional connectomes have been shown to be useful for the classification of subjects with Schizophrenia (Ji et al., 2019, Talpalaru et al., 2019). Then, functional connectomes may also capture some common patterns of Schizophrenia, which make patients differ from healthy subjects. Functional connectomes can then capture subject-specific and group-average patterns, both of which are relevant for the future description of a Schizophrenia biomarker.
Here, we explored inter and intra-individual variabilities in a longitudinal sample (two resting state fMRI sessions) of patients with First Episode of Psychosis (FEP) and Healthy Controls (HC). In the patients’ group, we looked for associations with clinical variables --particularly antipsychotic medication doses and symptoms severity (measured by Positive and Negative Syndrome Scale, PANSS). The early period of psychosis has been proposed as the most dynamic period of the illness (Birchwood et al., 1998), so studying variabilities and clinical changes in FEP patients, instead of looking for common group-patterns, can be a way to individualize subjects in their evolution throughout the disease. Based on previous literature, we expected to find reduced connectome stability (i.e., greater intra-individual variability and greater inter-individual variability) in psychotic patients.
2. Material and methods
2.1. Sample
Our data sample consisted of 30 patients with a First Episode of Psychosis (FEP), recruited at the Psychiatric Institute “Dr. José Horwitz Barak”, in Santiago, Chile, and 32 Healthy Controls (HC). Patients fulfilled criteria for a psychotic episode according to the MINI neuropsychiatric interview (Lecrubier et al., 1997, Sheehan et al., 1998). Patients with a psychotic episode secondary to drugs abuse or a neurological or medical condition were excluded.
Each subject was scanned twice (two resting state fMRI sessions). In the patients’ group, the first scan was taken during their first psychotic episode (with a mean duration of treatment with antipsychotic medication of 23.83 ± 10.69 days) and the second scan, after 122.6 ± 44.22 days (or 17.51 ± 6.31 weeks) of treatment (Undurraga et al., 2022). In the case of healthy controls, the interval between scans was similar to that of patients (p = 0.605), with 128.87 ± 47. 59 days (or 18.3 ± 6.83 weeks).
Treatment was provided according to local guidelines and the clinical team’s criteria, with no intervention from the research team. At both sessions, a psychiatrist assessed patients' symptoms with the PANSS scale (Stanley R Kay et al., 1987).
The study was approved by the Ethics and Research Committee of the Northern Metropolitan Health Service (SSMN) and the Ethics Committee of the Pontificia Universidad Católica de Chile. All participants signed informed consent.
2.2. Clinical variables
To compare doses of antipsychotic medication across patients taking different antipsychotic drugs, we used Chlorpromazine (CPZ) dose equivalents calculated according to Defined Daily Doses method (DDDs) (Leucht et al., 2016).
To assess symptoms severity, we used Positive and Negative Syndrome Scale (PANSS) (Stanley R Kay et al., 1987). This instrument is constituted by three subscales measuring positive and negative syndromes, and general psychopathology. The subscales are:
-
-
Positive Scale (range 7 to 49): measures so-called positive symptoms, including delusions and hallucinations, which originally were conceptualized as productive features superadded to the mental status of patients.
-
-
Negative Scale (range 7 to 49): measures negative symptoms such as a lack of motivation, conceptualized originally as deficit features characterized by loss of functioning.
-
-
General Psychopathology (range 16 to 112): an important adjunct to the positive–negative assessment, to provide a parallel but separate measure of illness severity.
PANSS Total score (range 30 to 210) is obtained by summation across Positive, Negative and General Psychopathology subscales.
2.3. MRI protocol and preprocessing
Resting-state functional MRIs were acquired with a Philips Ingenia 3 T MRI scanner with a 16-channel brain coil. The scanning parameters were the following: total scan time 8.33 min, single-shot EPI, TR 2.5 s, TE 35 ms, flip angle of 82°, FOV of 220x220x110mm, and an isotropic spatial resolution of 2.75 mm. Subjects were asked to remain still and with their eyes opened. A structural T1-weighted image was also acquired with a voxel size of 1.0 mm3 isotropic, a TI delay of 965.2 ms, TE 3.6 ms, TR 7.7 ms, and flip angle of 8°.
Preprocessing of the functional images followed the same pipeline described elsewhere (Tepper et al., 2022), which includes usual steps of realignment and slice-time correction with SPM12 (Ashburner et al., 2021), co-registration and nonlinear transformation to MNI space with ANTs, (Avants et al., 2014), linear detrending, intensity normalization, spatial smoothing and bandpass-filtering (MATLAB, 2021). ICA-AROMA (Pruim et al., 2015) was used to remove residual movement artifacts, as well as the regression of mean white matter and cerebrospinal fluid. Also, one iteration of DiCER (Diffuse Cluster Estimation and Regression) (Aquino et al., 2020) with a full gray matter mask was implemented in order to correct biphasic global signal artifacts. This algorithm performs a clustering analysis of the voxels’ time series to find wide-spread signal deflections (WSD); then it computes an adjusted-mean of such identified voxels, in which any anticorrelated voxel is flip around in sign. Finally, this adjusted mean of a detected WSD is regress-out to clean the BOLD signal. By using a full gray matter mask and one iteration in our implementation, we performed an adjusted version of Global Signal Regression (GSR), in which the flipping of anticorrelated voxels allows for correction of biphasic signal artifacts that are not cleaned-out by GSR, because its global mean value would turn out to be close to zero.
2.4. Analyses of functional connectomes
To define the ROIs in our functional connectomes, we used a 400 parcellations atlas (Schaefer et al., 2018) in which each parcel is matched to a corresponding network in the 7 Yeo networks (Yeo et al., 2014). We also incorporated a subcortical parcellation with 32 ROIs (Tian et al., 2020). For our analyses including subnetworks computations, 7 Yeo networks plus 1 subcortical ‘network’ were considered.
We computed functional connectomes (FC) by performing pairwise Pearson’s correlations between time-series of the previously mentioned 432 ROIs. Fisher’s z transform was applied to all subjects’ FCs to get normal distributions of correlation values (Fisher, 1992) before computing our three variability measures (Deviation from Healthy FC, Iself and Iothers).
2.4.1. Intra-individual variability: Iself
We analyzed connectome stability to study whether patients’ FCs change more than those from healthy controls from one session to the other (i.e., intra-subject variability).
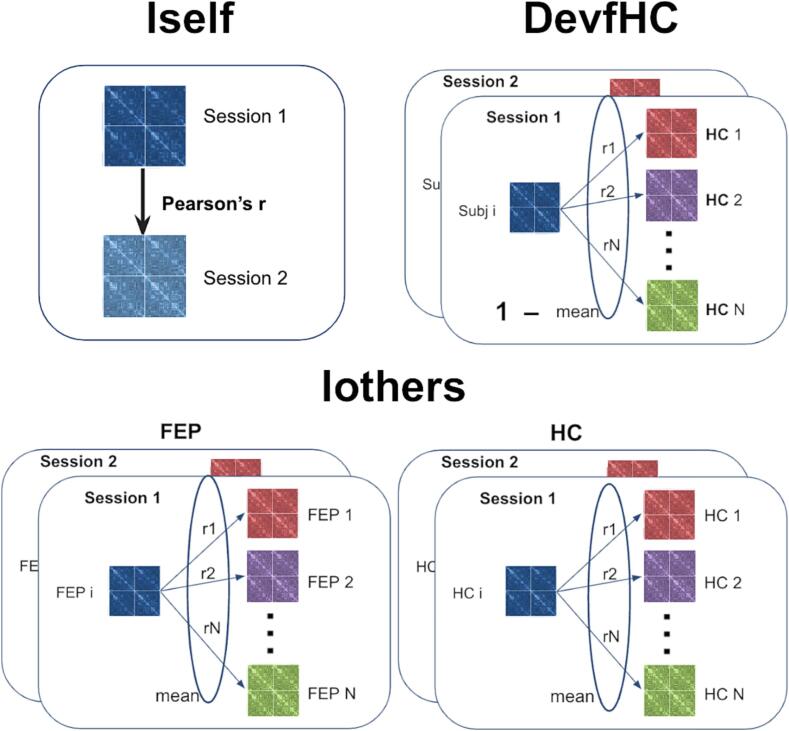
Connectome stability was computed as the within-subject Pearson’s correlation coefficient (i.e., Pearson’s correlation between the two connectivity matrices from the same subject, taken in the two fMRI scan sessions) (see Fig. 1, top-left panel). This measure can also be referred to as the individual’s fingerprint (Finn et al., 2015) or as Iself (Amico and Goñi, 2018), which will be used from now on. We computed Iself both from whole brain functional connectomes and separately from the 7 Yeo canonical functional networks (Yeo et al., 2014, Schaefer et al., 2018) plus one additional ‘network’ of subcortical regions (Tian et al., 2020).
Fig. 1.
Methods to compute Iself, DevfHC and Iothers measures (schematic representation). Different color functional connectomes (FCs) represent different subjects. For Iself (top-left panel) blue and light blue represent two FCs from the same subject, taken in two different sessions. Top-left panel: Iself was computed as the within-subject Pearson’s correlation coefficient (i.e., Pearson’s correlation between two FCs from the same subject). Top-right panel: for each subject and each session, DevfHC was computed as 1 minus the mean of pairwise Pearson’s correlations between its FC and each healthy subjects’ FC, for the same session. Bottom panel: Iothers was computed as the mean of Pearson’s correlations between pairs of FCs from different subjects from the same group, and the same session. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Then, we compared mean Iself values of FEP patients and HC on a group-level analysis. For these analyses, we modeled Iself with a GLM model including age, sex, days between sessions (SessInterval) and maximum framewise displacement (maximum value across the two mean values, each from one scan session) as covariates of no interest, and a categorical variable ‘Group’ with values ‘FEP’ or ‘HC’. All numerical variables were standardized (centered and scaled) to aid in the interpretation of results.
| Iself_network ∼ Intercept + Age + Sex + maxFD + SessInterval + Group |
Where Iself_network refers to either Iself computed from whole brain FC or subnetworks’ FCs.
We used a second model, only with data from our patient’s sample, to find out whether differences in connectome stability were related to clinical measures. Our predictors in this model were: deltaPANSS, which measured absolute changes in symptoms between the two sessions (measured as the absolute value of the inter-sessions difference of PANSS Total scores), and deltaAP, which measured absolute changes in treatment between the two sessions (measured as the absolute value of the inter-sessions difference of antipsychotic doses, APdose). Antipsychotic doses were expressed in mg of chlorpromazine (CPZ) equivalents using the DDD method (Leucht et al., 2016). As in the previous model, we included age, sex, days between sessions, and maximum framewise displacement as covariates of no interest, and all numerical variables were standardized to aid in the interpretation of results.
| Iself_patient ∼ Intercept + Age + Sex + maxFD + SessInterval + deltaPANSS + deltaAP |
| With: deltaPANSS = abs(PANSSTot_sess2 – PANSSTot_sess1) |
| and deltaAP = abs(APdose_sess2 – APdose_sess1) |
2.4.2. Deviation from healthy FC
We then studied whether patients’ FCs were more different to healthy FCs than HC intra-group differences. For this, we computed the “Deviation from Healthy FCs” (DevfHC) measure for each subject and each session as 1 minus the mean of pairwise Pearson’s correlation between their connectome and each healthy subjects’ FC, for the same session (see Fig. 1, top-right panel). As each subject has two FCs (one per session), we computed two DevfHC for each subject. Accordingly, we used the following mixed linear model for repeated measures to explore group differences in DevfHC:
| DevfHC ∼ Intercept + Age + Sex + FD + Group + (1|subject) |
Where age and sex were time-invariant covariates (just one value was used for the repeated observations from a given subject), while FD was a time-varying covariate, taking on a different value (mean FD from session 1 or from session 2) for each of the repeated observations. We did not control for SessInterval in this model because DevfHC is computed independently for each session of each subject. All numerical variables were standardized to aid in the later interpretation of results.
Then, we tested whether different values of DevfHC in the FEP group were associated with clinical variables, specifically with symptoms levels (PANSS Total scores) and doses of antipsychotic medication (AP dose), with the following model:
| DevfHC ∼ Intercept + Age + Sex + FD + Total PANSS + AP dose + (1|subject) |
In this model, both Total PANSS and AP dose were modeled as time-varying factors (with the corresponding values measured in each of the two sessions of each subject), to account for changes in between sessions. Antipsychotic doses were expressed in mg of chlorpromazine (CPZ) equivalents using the DDD method (Leucht et al., 2016), and all numerical variables were standardized before fitting the model.
In order to study how changes in clinical variables might relate to changes in DevfHC, we used the following model:
| deltaDevfHC ∼ Intercept + Age + Sex + FDmax + deltaPANSS_signed + deltaAPdose_signed |
in which all ‘delta’ variables were computed as the difference between their value as measured in session 1 minus their value in session 2 (different from what we used in models for Iself values, in which we were not interested in the direction of the change, but only on its absolute value).
| Here, then: deltaDevfHC = DevfHC_sess1 – DevfHC_sess2 |
| deltaPANSS_signed = PANSSTotal_sess1 – PANSSTotal_sess2 |
| deltaAP_signed = APdose_sess1 – APdose_sess2 |
Also, in this model we do not control for SessInterval because each value of DevfHC is computed by comparisons with healthy subjects on that same session. So, any physiological changes due to time will be present not only in the FC of each individual but also in those FCs with which we are comparing them.
2.4.3. Inter-individual variability: Iothers
To evaluate inter-individual differences between patients and controls, we used the fingerprint-like measure “Iothers” (as defined by Amico and Goñi, 2018), which can be calculated as the average of Pearson’s correlations between pairs of connectivity matrices from different subjects from the same group. In other words, this is a measure of how much alike different subjects are in an intra-group fashion.
With our dataset, with two sessions per subject, we computed two measures of Iothers per subject as the average of Pearson’s correlations between their own FC and those of the other subjects of the same group (see Fig. 1, bottom panel). For the case of HC group, Iothers is the inverse of DevfHC; but for FEP patients, Iothers is an intragroup inter-individual variability measure, whereas DevfHC is an intergroups inter-individual comparative measure. This is, DevfHC measured differences between patients and HC, and Iothers measured how different patients were among themselves.
We tested whether FEP patients had different Iothers than HC by means of the following mixed linear model (with all numerical variables standardized, and with FD as the only time-varying covariate). We did not control for SessInterval because Iothers is computed independently for each session of each subject.
| Iothers ∼ Intercept + Age + Sex + FD + Group + (1|subject) |
Then, we evaluated whether different values of Iothers in FEP were related to clinical variables (both PANSS Total scores and doses of antipsychotic medication as time-varying factors) with the following model:
| Iothers_patients ∼ Intercept + Age + Sex + FD + Total PANSS + AP dose + (1|subject) |
We also repeated these analyses computing Iothers from the 7 Yeo canonical functional networks (Yeo et al., 2014, Schaefer et al., 2018) plus the additional subcortical ROIs (Tian et al., 2020).
3. Results
Demographic and clinical information can be found in Table 1. Patients and healthy controls differed significantly in age (p = 2.12e-05). According to PANSS Total scores, and its correspondence to Clinical Global Impressions ratings (Leucht et al., 2005), our patients were “moderately ill” in session 1 and improved to “mildly ill” in session 2, with 122.6 ± 44.22 days of treatment in between sessions. Statistically significant differences between symptoms in sessions 1 and 2 were found for PANSS Total scores (p = 5.62e-08) and also for Positive, Negative and General subscales (p = 6.93e-05, p = 8.68e-04 and p = 1.18e-07, respectively). We modeled clinical symptoms severity by including PANSS Total scores in our models (results from models including PANSS subscales as separate factors can be found in Supplementary Information).
Table 1.
Demographic and clinical information of the sample. Groups differed significantly in age, but not in sex, days between sessions nor framewise displacement. In the First Episode of Psychosis group (FEP), patients showed statistically significant differences in symptoms severity between sessions 1 and 2 (for PANSS Total scores and independently for each subscale). Not statistically significant changes were found in doses of antipsychotic medication between sessions.
| FEP (N = 30) |
HC (N = 32) |
Statistics | |||
|---|---|---|---|---|---|
| Sess1 | Sess2 | Sess1 | Sess2 | ||
| Sex (F/M) | 8/22 | 13/19 | Chi-square = 1.34 p = 0.246 |
||
| Age (mean ± sd) | 20.4 ± 2.57 | 24.06 ± 3.56 | t = − 4.62 p = 2.12e-05 |
||
|
Days of treatment before first session (mean ± sd) |
23.83 ± 10.69 | NA | NA | ||
|
Days between sessions 1 and 2 (mean ± sd) |
122.6 ± 44.22 (17.51 ± 6.31 weeks) |
128.87 ± 47.59 (18.3 ± 6.83 weeks) |
t = 0.52 p = 0.605 |
||
| PANSS Total score (mean ± sd) | 68.83 ± 13.20 | 45.86 ± 15.27 | NA | NA | t = 6.23 p = 5.62e-08 |
|
PANSS Positive scale (mean ± sd) |
16.33 ± 6.18 | 10.10 ± 5.03 | NA | NA |
t = 4.29 p = 6.93e-05 |
|
PANSS Negative scale (mean ± sd) |
19.93 ± 7.43 | 13.33 ± 7.13 | NA | NA | t = 3.51 p = 8.68e-04 |
|
PANSS General scale (mean ± sd) |
32.57 ± 6.69 | 22.43 ± 6.31 | NA | NA | t = 6.04 p = 1.18e-07 |
| Antipsychotic (AP) medication doses (CPZ equivalents in mg) | 510.0 ± 303.81 | 450.36 ± 268.49 | NA | NA | t = 0.79 p = 0.433 |
| Mean Framewise Displacement (mean ± sd) | 0.325 ± 0.129 | 0.318 ± 0.118 | 0.282 ± 0.071 | 0.322 ± 0.117 | t (FEPsess1, HCsess1) = 1.64 p = 0.106 t (FEPsess2, HCsess2) = -0.14 p = 0.885 |
NA = Not Applicable. FEP = First Episode of Psychosis. HC = Healthy Controls.
Where group comparisons were made between FEP and HC (models without inclusion of clinical variables), 1 HC subject was excluded due to missing values for days between sessions (N = 61, where NFEP = 30 and NHC = 31). In all FEP analyses including AP doses, 2 FEP subjects were excluded because of missing values (remaining N = 28).
3.1. General results
Summary of the computed measures and uncorrected group comparisons (2 sample t-test) can be found in Table 2. We found no differences in Iself values (p = 0.120), but patients differed significantly from healthy subjects in DevfHC and in Iothers in their first fMRI scanning session (p = 0.021 and p = 0.0001, respectively), but not in the second session (p = 0.230 and p = 0.163). Results from more refined analyses including confounds are described in the following sections.
Table 2.
Summary of computed measures (whole brain FCs) and uncorrected group comparisons. We found no significant difference for Iself values between groups. Groups differed significantly in DevfHC and Iothers in session 1, but not in session 2.
| FEP (N = 30) |
HC (N = 32) |
Statistics | |||
|---|---|---|---|---|---|
| Sess1 | Sess2 | Sess1 | Sess2 | ||
|
Iself (mean ± sd) |
0.476 ± 0.087 | 0.508 ± 0.074 |
t = -1.58 p = 0.120 |
||
|
DevfHC (mean ± sd) |
0.629 ± 0.035 | 0.630 ± 0.026 | 0.609 ± 0.033 | 0.621 ± 0.033 | t (FEPsess1, HCsess1) = 2.36 p = 0.021 t (FEPsess2, HCsess2) = 1.21 p = 0.230 |
|
Iothers (mean ± sd) |
0.356 ± 0.033 | 0.368 ± 0.024 | 0.391 ± 0.033 | 0.379 ± 0.033 | t (FEPsess1, HCsess1) = -4.13 p = 0.0001 t (FEPsess2, HCsess2) = -1.41 p = 0.163 |
3.2. Intra-individual variability: Iself
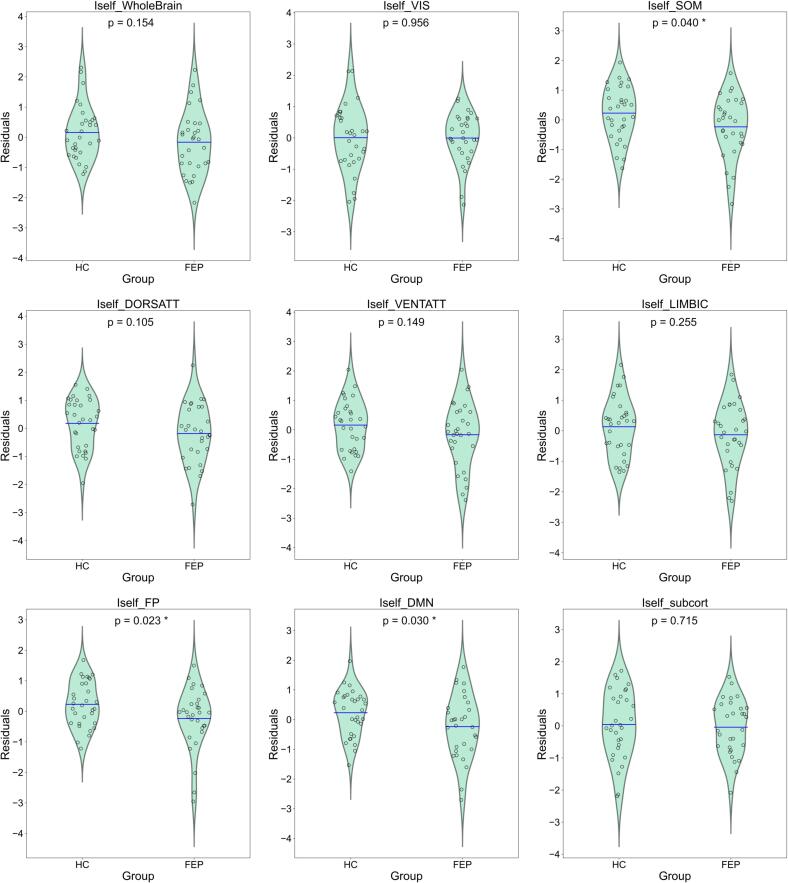
As it can be seen in Table 3, Table 4, we found no group differences for whole brain functional connectomes (Group p-val = 0.154). Uncorrected p values point to a significant effect of Group for Iself values from somatomotor, frontoparietal and DMN network, but none of them survives multiple comparisons correction (FDR Benjamini & Hochberg). The direction was similar for lower Iself in FEP patients for all subnetworks (see Table 4 and Fig. 2).
Table 3.
Models for Iself computed from whole brain FCs. Top table shows results for group comparisons (no statistically significant differences were found when correcting for age, sex, framewise displacement (FDmax) and days between sessions (SessInterval)). Bottom table shows model looking for associations with changes in clinical variables in the FEP group. No statistically significant results were found. All coefficients showed are standardized.
| Iself ∼ Age + Sex + FDmax + SessInterval + Group N = 61 | |||||
|---|---|---|---|---|---|
| Coef. | Std.Err | t | P > |t| | [0.025–0.975] | |
| Intercept | 0.2994 | 0.265 | 1.132 | 0.263 | −0.231 – 0.829 |
| Age | −0.1594 | 0.157 | −1.014 | 0.315 | −0.474 – 0.156 |
| Sex[T.M] | −0.1328 | 0.286 | −0.464 | 0.644 | −0.706 – 0.440 |
| FDmax | −0.2345 | 0.137 | −1.709 | 0.093 | −0.509 – 0.040 |
| SessInterval | 0.0304 | 0.129 | 0.235 | 0.815 | −0.229 – 0.289 |
| Group[T.FEP] | −0.4317 | 0.298 | −1.447 | 0.154 | −1.029–0.166 |
| Iself_patients ∼ Age + Sex + FDmax + SessInterval + deltaPANSS + deltaAPdose N = 28 | |||||
| Coef. (std) | Std.Err | t | P > |t| | [0.025–0.975] | |
| Intercept | −0.0478 | 0.418 | −0.114 | 0.910 | −0.916 – 0.821 |
| Age | −0.2098 | 0.227 | −0.925 | 0.365 | −0.681 – 0.262 |
| Sex[T.M] | 0.0669 | 0.520 | 0.129 | 0.899 | −1.014 –1.148 |
| FDmax | −0.1155 | 0.217 | −0.532 | 0.600 | −0.567 – 0.336 |
| SessInterval | 0.1852 | 0.227 | 0.814 | 0.425 | −0.288 – 0.658 |
| deltaPANSS | −0.1886 | 0.214 | −0.883 | 0.387 | −0.633 – 0.225 |
| deltaAPdose | 0.3332 | 0.205 | 1.629 | 0.118 | −0.092 – 0.759 |
Table 4.
Iself subnetworks: groups comparison. Summary of results for categorical variable ‘Group’, across models for Iself values computed from subnetworks. Uncorrected p values point to significant group differences in somatomotor, frontoparietal and DMN networks, with reduced Iself values in patients. No results remain significant after correction for multiple comparisons with FDR Benjamini & Hochberg. All coefficients showed are standardized.
| Iself_network | Group (T.FEP) | ||
|---|---|---|---|
| Coefficient | Uncorrected p | Corrected p (fdr) | |
| Whole brain | −0.4317 | 0.154 | ---- |
| Visual | −0.0157 | 0.956 | 0.956 |
| Somatomotor | −0.6193 | 0.040 | 0.106 |
| Dorsal Attention | −0.4891 | 0.105 | 0.210 |
| Ventral Attention | −0.4374 | 0.149 | 0.239 |
| Limbic | −0.3526 | 0.255 | 0.340 |
| Frontoparietal | −0.6249 | 0.023 | 0.106 |
| DMN | −0.6301 | 0.030 | 0.106 |
| Subcortical | −0.1092 | 0.715 | 0.817 |
Fig. 2.
Residualized Iself group differences. Residuals from model Iself ∼ Intercept + Age + Sex + FD + SessInterval (all numerical variables standardized) plotted according to Group (FEP or HC). Blue line represents the mean value of the distribution. Shown p-values correspond to uncorrected p-values for ‘Group’ beta coefficient from linear models with Iself as dependent variable (* highlights p < 0.05) (See Table 4). Uncorrected p values point to significant group differences in somatomotor, frontoparietal and DMN networks, with reduced Iself in patients. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Results from modelling Iself values with clinical variables can be seen in Table 3 (whole brain) and Table 5 (all networks). For these models, no results were statistically significant after correcting for multiple comparisons with FDR Benjamini & Hochberg method. When substituting in the model the predictor deltaPANSS by its three subscales (deltaPositive, deltaNegative and deltaGeneral), results suggest a significant association between changes in General PANSS subscale and Iself values in Ventral Attention networks. For more details on those results see Supplementary information (Table S1 and S2).
Table 5.
Iself subnetworks: models with clinical variables. Summary of results for variables ‘deltaPANSS’ and ‘deltaAP’ (see Methods) across models for Iself values computed from subnetworks, and only for FEP subjects. Uncorrected p values point to significant associations between deltaPANSS and Iself from Ventral Attention network, and between deltaAPdose and Visual, Ventral Attention and Frontoparietal networks. No results remain significant after correction for multiple comparisons with FDR Benjamini & Hochberg. All coefficients showed are standardized.
| Iself_patients | deltaPANSS | deltaAPdose | ||||
|---|---|---|---|---|---|---|
| Coefficient | Uncorrected p | Corrected p (fdr) | Coefficient | Uncorrected p | Corrected p (fdr) | |
| Whole brain | −0.1886 | 0.387 | ---- | 0.3332 | 0.118 | ---- |
| Visual | −0.1251 | 0.534 | 0.611 | 0.4743 | 0.021 | 0.092 |
| Somatomotor | −0.1070 | 0.642 | 0.642 | 0.3210 | 0.154 | 0.206 |
| Dorsal Attention | −0.1509 | 0.456 | 0.611 | 0.1747 | 0.369 | 0.422 |
| Ventral Attention | −0.4886 | 0.018 | 0.146 | 0.4142 | 0.034 | 0.092 |
| Limbic | 0.1876 | 0.394 | 0.611 | 0.3278 | 0.127 | 0.203 |
| Frontoparietal | 0.1246 | 0.513 | 0.611 | 0.4054 | 0.032 | 0.092 |
| DMN | −0.1610 | 0.445 | 0.611 | 0.3214 | 0.120 | 0.203 |
| Subcortical | 0.1446 | 0.500 | 0.611 | −0.0057 | 0.978 | 0.978 |
3.3. Deviation from healthy FC
We found a significant association between Deviation from healthy connectomes (DevfHC) and being in the FEP group (standardized beta of 0.491 ± 0.237, p = 0.038; Table 6 top panel), controlling for differences in sex, age and framewise displacement. We also looked at whether this association was related to clinical variables. As shown in Table 6 (bottom panel), we did not find significant associations (p values for variables Total PANSS and APdose were 0.136 and 0.060 respectively). Results looking at PANSS subscales were similar and can be found in Supplementary information (Table S3).
Table 6.
Results for DevfHC mixed models. Top table shows results for group comparisons: FEP subjects showed greater DevfHC when correcting for age, sex and framewise displacement (FD) (p = 0.038). Bottom table shows model looking for associations with clinical variables in the FEP group. No statistically significant results were found. All coefficients showed are standardized.
| DevfHC ∼ Age + Sex + FD + Group + (1|subject) N = 61 | |||||
|---|---|---|---|---|---|
| Coef. | Std.Err | z | P > |z| | [0.025 – 0.975] | |
| Intercept | −0.152 | 0.209 | −0.727 | 0.467 | −0.563 – 0.258 |
| Age | 0.033 | 0.122 | 0.275 | 0.783 | −0.205 – 0.272 |
| Sex[T.M] | −0.136 | 0.224 | −0.610 | 0.542 | −0.575 – 0.302 |
| FD | 0.027 | 0.088 | 0.307 | 0.759 | −0.145 – 0.199 |
| Group[T.FEP] | 0.491 | 0.237 | 2.071 | 0.038 | 0.026 – 0.957 |
| Group Var (random factor) | 0.288 | 0.199 | |||
| DevfHC ∼ Age + Sex + FD + TotalPANSS + APdose + (1|subject) N = 28 | |||||
| Coef. | Std.Err | z | P > |z| | [0.025 – 0.975] | |
| Intercept | 0.288 | 0.256 | 1.124 | 0.261 | −0.214 – 0.790 |
| Age | 0.096 | 0.135 | 0.709 | 0.478 | −0.169 – 0.360 |
| Sex[T.M] | −0.403 | 0.310 | −1.301 | 0.193 | −1.011 – 0.204 |
| FD | −0.169 | 0.134 | −1.264 | 0.206 | −0.432 – 0.093 |
| Total PANSS | 0.197 | 0.132 | 1.489 | 0.136 | −0.062 – 0.456 |
| APdose | 0.264 | 0.141 | 1.878 | 0.060 | −0.011 – 0.539 |
| Group Var (random factor) | 0.000 | 0.201 | |||
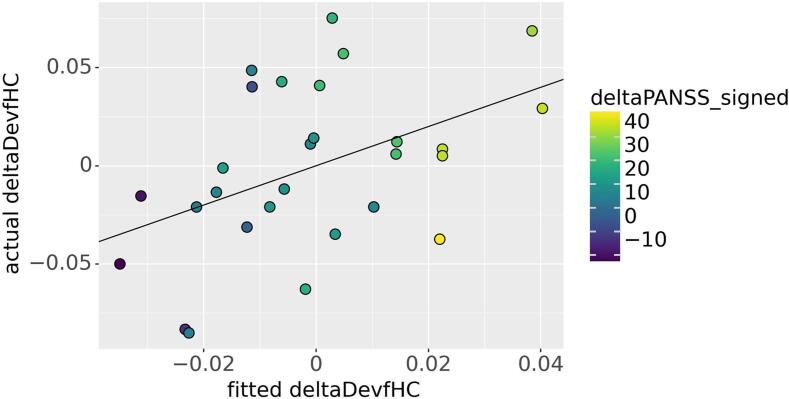
When assessing changes in DevfHC (deltaDevfHC), we found no group differences (see Table 7). However, in the group of patients, we found a positive association between deltaPANSS_signed and deltaDevfHC (p = 0.035) (see Table 7 and Fig. 3). Results looking at PANSS subscales suggest this association to be mainly attributable to changes in Negative symptoms (see Table S4).
Table 7.
Results for deltaDevfHC models. Top table shows results for group comparisons: no statiscally significant difference were found when accounting for age, sex and framewise displacement (FDmax). Bottom table shows model looking for associations with changes in clinical variables in the FEP group: deltaPANSS_signed was positively associated with deltaDevfHC (p = 0.035). All coefficients showed are standardized.
| deltaDevfHC ∼ Age + Sex + FDmax + Group N = 61 | |||||
|---|---|---|---|---|---|
| Coef. | Std.Err | t | P > |t| | [0.025 – 0.975] | |
| Intercept | −0.3122 | 0.269 | −1.161 | 0.250 | −0.851 – 0.226 |
| Age | 0.1030 | 0.160 | 0.646 | 0.521 | −0.217 – 0.423 |
| Sex[T.M] | 0.2318 | 0.290 | 0.799 | 0.428 | −0.349 – 0.813 |
| FDmax | 0.1127 | 0.139 | 0.809 | 0.422 | −0.167 – 0.392 |
| Group[T.FEP] | 0.3256 | 0.303 | 1.075 | 0.287 | −0.281 – 0.932 |
| deltaDevfHC ∼ Age + Sex + FDmax + deltaPANSS_signed + deltaAPdose_signed N = 28 | |||||
| Coef. | Std.Err | t | P > |t| | [0.025 – 0.975] | |
| Intercept | 0.0063 | 0.388 | 0.016 | 0.987 | −0.799 – 0.811 |
| Age | −0.2057 | 0.220 | −0.935 | 0.360 | −0.662 – 0.250 |
| Sex[T.M] | −0.0089 | 0.474 | −0.019 | 0.985 | −0.992 – 0.974 |
| FDmax | −0.0222 | 0.209 | −0.106 | 0.916 | −0.455 – 0.411 |
| deltaPANSS_signed | 0.4906 | 0.218 | 2.254 | 0.035 | 0.039 – 0.942 |
| deltaAPdose_signed | 0.1660 | 0.206 | 0.806 | 0.429 | −0.261 – 0.593 |
Fig. 3.
Model fit for deltaDevfHC and association with deltaPANSS_signed. The positive association between deltaDevfHC and deltaPANSS_signed is colour coded. deltaDevfHC was positively associated with deltaPANSS_signed when accounting for age, sex, FDmax and deltaAPdose_signed (p = 0.035). See Table 7 for more information on this model.
3.4. Inter-individual variability: Iothers
Regarding Iothers (i.e., how similar a subject is to their group), we found a statistically significant difference between groups (p = 0.001), after controlling for sex, age and movement within the scanner. FEP patients showed reduced Iothers values (more inter-subject variability) with standardized beta coefficient of −0.716.
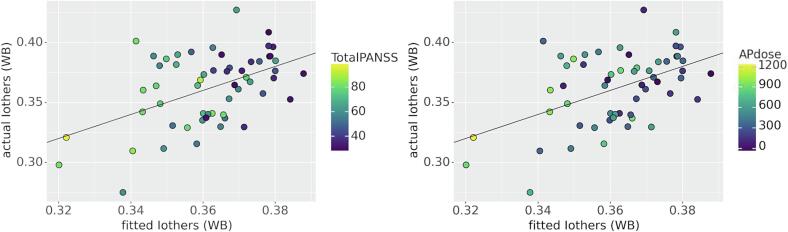
Then, we tested whether these differences in whole brain Iothers of FEP patients were attributable to clinical differences (PANSS Total score and doses of antipsychotic medication). When correcting for age, sex and framewise displacement, we found a statistically significant association with PANSS Total scores (p = 0.027) and a trending association with AP doses (p = 0.057) (see Fig. 4). We also found an association between Iothers and sex, with male subjects being more similar to other patients (p = 0.049). More information about these analyses can be found in Table 8.
Fig. 4.
Model fit for Iothers (whole brain) and association with clinical variables. Associations between Iothers WB (whole brain) and clinical variables are colour coded. Left panel: Iothers and PANSS Total scores were negatively associated (p = 0.027). Right panel: Iothers and AP doses showed a trend to a negative association (p = 0.057). For more information on this model see Table 8.
Table 8.
Models for Iothers computed from whole brain FCs. Top table shows results for group comparisons: FEP subjects showed reduced Iothers when accounting for age, sex and framewise displacement (FD) (p = 0.001). Bottom table shows model looking for associations with clinical variables in the FEP group: PANSS Total score was negatively associated with Iothers (p = 0.027), and male subjects had higher Iothers values (p = 0.049). All coefficients showed are standardized.
| Iothers ∼ Age + Sex + FD + Group + (1|subject) N = 61 | |||||
|---|---|---|---|---|---|
| Coef. | Std.Err | z | P > |z| | [0.025 – 0.975] | |
| Intercept | 0.216 | 0.198 | 1.091 | 0.275 | −0.172 – 0.604 |
| Age | −0.021 | 0.115 | −0.187 | 0.852 | −0.247 – 0.204 |
| Sex[T.M] | 0.208 | 0.211 | 0.982 | 0.326 | −0.207 – 0.622 |
| FD | −0.029 | 0.086 | −0.337 | 0.736 | −0.197 – 0.139 |
| Group[T.FEP] | −0.716 | 0.224 | −3.193 | 0.001 | −1.156 – −0.276 |
| Group Var (random factor) | 0.224 | 0.179 | |||
| Iothers_patients ∼ Age + Sex + FD + TotalPANSS + APdose + (1|subject) N = 28 | |||||
| Coef. | Std.Err | z | P > |z| | [0.025 – 0.975] | |
| Intercept | −0.419 | 0.246 | −1.703 | 0.089 | −0.900 – 0.063 |
| Age | −0.076 | 0.129 | −0.587 | 0.557 | −0.329 – 0.177 |
| Sex[T.M] | 0.586 | 0.297 | 1.970 | 0.049 | 0.003 – 1.169 |
| FD | 0.141 | 0.128 | 1.099 | 0.272 | −0.111 – 0.393 |
| Total PANSS | −0.286 | 0.129 | −2.217 | 0.027 | −0.539 – −0.033 |
| APdose | −0.259 | 0.136 | −1.905 | 0.057 | −0.526 – 0.007 |
| Group Var (random factor) | 0.000 | 0.205 | |||
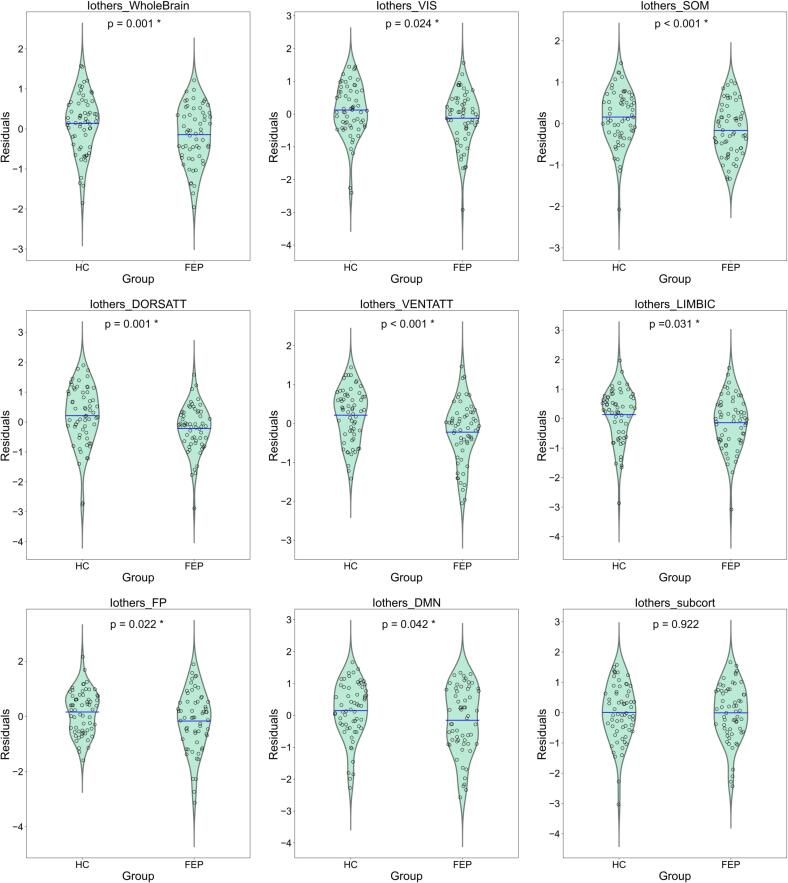
We then analyzed Iothers computed from subnetworks. After controlling for sex, age, and movement within the scanner, and correcting for multiple comparisons (FDR Benjamini & Hochberg), we found a significant group difference in all 7 Yeo networks (but not for subcortical regions) (see Table 9). As it can be seen in Table 8, for all significant subnetworks, FEP patients showed reduced Iothers values (more inter-subject variability). Residualized Iothers group differences (correcting for sex, age and FD) can be seen in Fig. 5.
Table 9.
Iothers subnetworks: groups comparison. Summary of results for variable ‘Group’ across models for Iothers computed from subnetworks. Before and after correction for multiple comparisons with FDR Benjamini & Hochberg, FEP subjects showed reduced Iothers when computed from whole brain functional connectomes and also from all 7 Yeo networks, but not for subcortical regions. All coefficients showed are standardized.
| Iothers_network | Group (T.FEP) | ||
|---|---|---|---|
|
Coefficient (beta std) |
Uncorrected p | Corrected p (fdr) | |
| Whole brain | −0.716 | 0.001 | ---- |
| Visual | −0.505 | 0.024 | 0.038 |
| Somatomotor | −0.966 | 0.000 | 0.000 |
| Dorsal Attention | −0.691 | 0.001 | 0.001 |
| Ventral Attention | −1.024 | 0.000 | 0.000 |
| Limbic | −0.473 | 0.031 | 0.041 |
| Frontoparietal | −0.462 | 0.022 | 0.038 |
| DMN | −0.423 | 0.042 | 0.048 |
| Subcortical | −0.022 | 0.922 | 0.922 |
Fig. 5.
Residualized Iothers group differences. Residuals from model Iothers ∼ Age + Sex + FD + (1|subject) (all numerical variables standardized) plotted according to Group (FEP or HC). Blue line represents the mean value of the distribution. Shown p-values correspond to uncorrected p-values for ‘Group’ beta coefficient from mixed models with Iothers as dependent variable (* highlights p < 0.05) (See Table 9). FEP subjects showed reduced Iothers when computed from whole brain functional connectomes and also from all 7 Yeo networks, but not for subcortical regions. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
In 6 out of these 7 networks (visual, somatomotor, dorsal attention, ventral attention, frontoparietal and DMN), Iothers was negatively correlated with PANSS Total scores (more symptomatic patients would be more different from other patients) (see Table 10). Associations with AP doses did not reach statistical significance. Results looking at PANSS subscales suggest associations between changes in Negative symptoms and Iothers computed from whole brain, as well as somatomotor and ventral attention networks (see Table S5 and S6).
Table 10.
Iothers subnetworks: models with clinical variables. Summary of results for variables ‘TotalPANSS’ and ‘APdose’ across models for Iothers computed from subnetworks, and only for FEP subjects. After correction for multiple comparisons with FDR Benjamini & Hochberg, Iothers was negatively associated to TotalPANSS when computed from whole brain as well as networks Visual, Somatomotor, Dorsal Attention, Ventral Attention, Frontoparietal and DMN. No significant associations between Iothers and APdose were found. All coefficients showed are standardized.
| Iothers_network | TotalPANSS | APdose | ||||
|---|---|---|---|---|---|---|
| Coefficient | Uncorrected p | Corrected p (fdr) | Coefficient | Uncorrected p | Corrected p (fdr) | |
| Whole brain | −0.286 | 0.027 | ---- | −0.259 | 0.057 | ---- |
| Visual | −0.304 | 0.026 | 0.035 | −0.131 | 0.348 | 0.901 |
| Somatomotor | −0.303 | 0.016 | 0.026 | −0.015 | 0.917 | 0.917 |
| Dorsal Attention | −0.355 | 0.005 | 0.013 | 0.066 | 0.612 | 0.901 |
| Ventral Attention | −0.417 | 0.001 | 0.005 | 0.040 | 0.762 | 0.901 |
| Limbic | −0.096 | 0.557 | 0.557 | −0.125 | 0.445 | 0.901 |
| Frontoparietal | −0.534 | 0.000 | 0.001 | −0.083 | 0.520 | 0.901 |
| DMN | −0.359 | 0.009 | 0.018 | −0.036 | 0.788 | 0.901 |
| Subcortical | −0.205 | 0.135 | 0.154 | −0.169 | 0.215 | 0.901 |
4. Discussion
In this study, we explored inter and intra-individual variabilities in a group of subjects with First Episode of Psychosis (FEP) using resting state fMRI data and fingerprint-like measures derived from functional connectomes. Particularly, we explored three measures: Iself, Deviation from Healthy FC, and Iothers.
As mentioned in the introduction, it has been previously reported that adults with Schizophrenia spectrum disorders have decreased connectome stability (Kaufmann et al., 2018). We expected our results to replicate such increased intra-subject variability, but no statistically significant values emerged from our analysis of Iself measures (whole brain p-value = 0.154). However, the tendency of all of our comparisons pointed in the same direction as previously reported findings, showing reduced Iself for FEP patients. Moreover, uncorrected p-values (without correcting for multiple comparisons) showed three Schizophrenia-relevant networks as focuses of reduced connectome stability (i.e., somatomotor, frontoparietal and DMN networks).
Even if our results did not back up inter-group differences in connectome stability, the idea of greater intra-individual variability inevitably recalls the clinical variability seen in patients with Schizophrenia. As such, we decided to explore whether intra-group differences in Iself were associated to changes in symptoms (as measured by PANSS Total scores) and/or changes in antipsychotic doses from one session to the other (with a few months of treatment in between) in our FEP sample. If changes of symptomatology scores (or medication doses) were related to Iself in a negative direction, this could imply that patients who remain more stable in a certain clinical status have greater congruence with their own FC from a different session. However, our results were not statistically significant and therefore the data did not support this interpretation.
Regarding inter-individual variance, first, with our measure of Deviation from Healthy FC, we studied individual mean distance to healthy functional connectomes. As expected, we found greater distances in the patients’ group (p = 0.038). This result means that patients’ FCs are generally more different from healthy FC than those of other healthy subjects. However, when analyzing our patients’ group, our results were not conclusive regarding whether this distance to healthy FCs is associated to symptoms severity or medication doses, although patients who were more medicated seem to differ more (with trend p value 0.060 for antipsychotic doses).
Relatedly, we also evaluated deltaDevfHC to analyze not only the relationship between illness severity and the distance to healthy FC, but how changes in clinical variables affect such distance. In this case, we found no group differences (p = 0.287), meaning patients and controls distances to healthy FCs changed approximately the same from session 1 to session 2 (there might be healthy physiological changes in between sessions). However, we did find a positive association in the patients’ group between deltaPANSS_signed and deltaDevfHC (p = 0.035). Patients whose symptoms were reduced from session 1 to session 2 (deltaPANSS_signed > 0), got closer to healthy FCs (deltaDevfHC > 0) (recall from Methods that delta is computed as DevfHC_Sess1 – DevfHC_Sess2, so a positive value means that distance was larger in session 1). According to analyses with PANSS subscales as covariables, this associations might be mainly driven by changes in Negative symptoms (see Table S4).
The idea of Deviation from Healthy FC can be related to previous studies in which group-level mean functional connectomes help classify subjects with Schizophrenia (Ji et al., 2019). In this matter, machine learning techniques have been used to train relatively successful classifiers (with an average of 75% accuracy) to distinguish between patients and healthy controls based on their functional connectomes (Ji et al., 2019, Talpalaru et al., 2019). Results of such classification studies are usually interpreted as the same phenotypic group having some similar functional connectome features (as connectivity signatures), which are used by the trained algorithms to discriminate among subjects of different groups. Then, functional connectomes of Schizophrenia patients may have some distinct patterns which make them different from healthy subjects, and our results point to clinical variables (particularly symptoms severity) being related to changes in these abnormal patterns.
Regarding inter-individual variance, we also explored whether patients are more (or less) similar than healthy controls in an intra-group comparison. By evaluating group differences of Iothers (i.e., how much similar a subject is to their group) computed from whole brain FCs, we found a statistically significant difference (p = 0.001), with FEP patients showing reduced Iothers values (more inter-subject variability). We also found a statistically significant association with PANSS score (p = 0.027), and a trending association with AP doses (p = 0.057), which indicates that patients with higher PANSS scores (and higher AP doses) have lower values of Iothers (they are less similar to other patients). We also found an association with sex (p = 0.049), indicating male patients to be more similar to other patients; which might be related to the fact that 73% of our patients are men (Ritchie et al., 2018).
When analyzing Iothers from subnetworks, we found a group difference for Iothers in all 7 Yeo networks (but not for subcortical regions). In all subnetworks, FEP patients showed higher inter-subject variability (reduced Iothers), which in 6 networks (visual, somatomotor, dorsal attention, ventral attention, frontoparietal and DMN) was correlated with PANSS Total scores (the more symptomatic, the more different to other patients). According to analyses with PANSS subscales as covariables, these associations might be mainly driven by Negative symptoms (see supplementary information). Associations with AP doses did not reach statistical significance.
A recent study exploring inter-individual variability in Schizophrenia found a similar result of higher variability in patients in whole brain and in frontoparietal, DMN, and ventral attention networks (Santo-Angles et al., 2021). However, in that study they found an opposite association with symptomatology: more symptoms were associated with more similar connectomes (Santo-Angles et al., 2021). The authors argued that more symptomatic patients may be overrepresented in their mean connectome, which they used for assessing inter-individual similarity. When exploring inter-individual variability in an intragroup fashion, we should keep in consideration that outcomes will depend on the nature of our samples (this is, who we are comparing each subject against). Particularly, when regarding associations with clinical variables, the degree and heterogeneity in illness severity of the sample under study might have an impact on the direction of results. Here we computed Iothers by pairwise comparisons between subjects (and later we took the average of those values), and our results point to more symptomatic patients having a greater difference to others in their group.
Our findings of higher variability in FEP patients are consistent with the heterogeneity of the disorder and could be related to studies showing that Schizophrenia subjects have noisier (i.e., more variable) brains. There have been studies using entropy measures, which related Schizophrenia with more complex signal patterns than healthy subjects and dysregulation of brain dynamics (Sokunbi et al., 2014). Also, biologically realistic computational models have shown how Schizophrenia is characterized by diminished signal-to-noise ratios (Rolls et al., 2008). These models have shown that neurons firing irregularly and less synchronized, would be reflected in a more variable (that is, noisier) BOLD signal (Rolls et al., 2008). Both findings of higher intra and inter-individual variabilities in Schizophrenia would be consistent with such an assumption.
These non-uniformly distributed variabilities of functional features in Schizophrenia can lead to biased results in group-means comparisons. In healthy subjects, intra-subject variability is concentrated in visual and somatomotor regions (Laumann et al., 2015, Poldrack et al., 2015), while inter-subject variability has been shown to be significantly higher in multimodal association areas and lower in unimodal sensory and primary motor areas (Mueller et al., 2013). Our findings of reduced connectome stability (with uncorrected p-values) showed that patients would have higher intra-subject variability in frontoparietal, DMN, and somatomotor networks. On top of that, our results showed higher inter-subject variability for patients across all cortical networks. In particular, inter-subject variability can be a crucial factor for group-means comparisons, where brain regions with low inter-subject variability reach with higher probability the cut-off for significance than regions with higher inter-subject variability (Zilles and Amunts, 2013).
Our results here show that there is increased inter-individual variability in FEP patients, both intragroup and when comparing them to healthy subjects. Moreover, inter-individual variability between patients seems to be associated with illness severity, with connectomes from more symptomatic patients differing more. This resonates with results from studies looking at more chronic patients (Kaufmann et al., 2018, Santo-Angles et al., 2021), and extends them to the first period of the illness, where changes are more dynamic (Birchwood et al., 1998). Our findings could support the theory of a noisy connectome. If neurotransmitter dysregulations result in reduced signal-to-noise ratios, then the information captured by functional connectomes would also be noisy and any comparisons like the ones made here would result in greater variabilities.
Limitations
This study has some limitations worth noting. First, our analyses (particularly for Iself) could have been underpowered (see Supplementary Information for power analysis and sample size estimation). Iself is the only measure in which no averaging (which increases signal-to-noise ratios) is done at any point of the computation. Moreover, statistical models for Iself values do not have repeated measures, since only one measure of Iself is obtained for each subject from the two repeated scans. These methodological characteristics would require analyses to have larger sample sizes to reach same levels of statistical power. Kaufmann et al., 2018 found reduced connectome stability in Schizophrenia patients using a sample>5 times larger than the one used here (Number of patients = 167, Number of controls = 202). Similarly, Santo-Angles et al., 2021 assessed inter individual variability in 110 patients and 110 control subjects.
On top of that, in this study we computed Iself and Iothers using Pearson’s correlation of full connectomes (or complete subnetworks). Some other more spatially localized measures (such as Regional Homogeneity - ReHo) have been shown to more strongly identify subjects (even from Schizophrenia samples) (Larabi et al., 2022). Future studies could examine more localized metrics from a fingerprint perspective.
5. Conclusions
We showed a trend towards reduced connectome stability (i.e., increased intra-subject variability). We found increased deviation from healthy FCs in patients with first episode of psychosis, whose changes were related to changes in symptoms severity; and we found increased intragroup inter-subject variability which was related to symptoms’ levels in 6 Yeo networks (visual, somatomotor, dorsal attention, ventral attention, frontoparietal and DMN).
In the search for a Schizophrenia biomarker, and given the heterogeneity of this disorder, it is highly relevant to find individual-level measures which relate to clinical aspects. We here showed that the functional connectome can capture individual variability related to the severity of the disorder. However, it is possible that some of our analyses were underpower. Further research is needed to shed light on the real prognostic value of functional connectome’s variability.
Data and code availability
Functional connectomes and behavioral data used in this work is available in Zenodo https://doi.org/10.5281/zenodo.7569166
Code used for analyses is available in this GitHub repository: https://github.com/angietep/Inter-and-Intra-Indiv-Variability
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
This work was supported by the Agencia Nacional de Investigación y Desarrollo from Chile (ANID), through its grants ANILLO PIA ACT192064 and ACT1414, FONDECYT regular 1200601, doctoral fellowship 21190222, and the Millenium Science Initiative Program – ICN2021_004.
Role of the Funding Source
The funding sources did not have any role in the study design, collection of the data, analysis, interpretation of the data, writing the report, or decision to submit the paper for publication.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2023.103391.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data is available from Zenodo and scripts from Github,as described in the paper (see Conclusions).
References
- Amico E., Goñi J. The quest for identifiability in human functional connectomes. Sci Rep. 2018;8:1–14. doi: 10.1038/s41598-018-25089-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquino K.M., Fulcher B.D., Parkes L., Sabaroedin K., Fornito A. Identifying and removing widespread signal deflections from fMRI data: Rethinking the global signal regression problem. Neuroimage. 2020;212 doi: 10.1016/J.NEUROIMAGE.2020.116614. [DOI] [PubMed] [Google Scholar]
- Ashburner, J., Barnes, G., Chen, C.-C., Daunizeau, J., Flandin, G., Friston, K., Gitelman, D., Glauche, V., Henson, R., Hutton, C., Jafarian, A., Kiebel, S., Kilner, J., Litvak, V., Mattout, J., Moran, R., Penny, W., Phillips, C., Razi, A., Stephan, K., Tak, S., Tyrer, A., Zeidman, P., 2021. SPM12 manual. researchgate.net.
- Avants, B.B., Tustison, N., Johnson, H., 2014. Advanced Normalization Tools (ANTS) Release 2.x.
- Barch D.M., Burgess G.C., Harms M.P., Petersen S.E., Schlaggar B.L., Corbetta M., Glasser M.F., Curtiss S., Dixit S., Feldt C., Nolan D., Bryant E., Hartley T., Footer O., Bjork J.M., Poldrack R., Smith S., Johansen-Berg H., Snyder A.Z., van Essen D.C. Function in the human connectome: Task-fMRI and individual differences in behavior. Neuroimage, Mapping the Connectome. 2013;80:169–189. doi: 10.1016/j.neuroimage.2013.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchwood M., Todd P., Jackson C. Early intervention in psychosis: The critical period hypothesis. The British Journal of Psychiatry. 1998;172:53–59. doi: 10.1192/S0007125000297663. [DOI] [PubMed] [Google Scholar]
- Finn E.S., Shen X., Scheinost D., Rosenberg M.D., Huang J., Chun M.M., Papademetris X., Constable R.T. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat Neurosci. 2015;18:1664–1671. doi: 10.1038/nn.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, R.A., 1992. Statistical Methods for Research Workers 66–70. 10.1007/978-1-4612-4380-9_6.
- Gordon E.M., Laumann T.O., Adeyemo B., Petersen S.E. Individual Variability of the System-Level Organization of the Human Brain. Cereb Cortex. 2017;27:386–399. doi: 10.1093/cercor/bhv239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G.J., Chen X., Bai T., Wang L., Wei Q., Gao Y., Tao L., He K., Li D., Dong Y., Hu P., Yu F., Zhu C., Tian Y., Yu Y., Wang K. Classification of schizophrenia by intersubject correlation in functional connectome. Hum Brain Mapp. 2019;40:2347–2357. doi: 10.1002/hbm.24527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann T., Alnæs D., Doan N.T., Brandt C.L., Andreassen O.A., Westlye L.T. Delayed stabilization and individualization in connectome development are related to psychiatric disorders. Nat Neurosci. 2017;20:513–515. doi: 10.1038/nn.4511. [DOI] [PubMed] [Google Scholar]
- Kaufmann T., Alnæs D., Brandt C.L., Bettella F., Djurovic S., Andreassen O.A., Westlye L.T. Stability of the Brain Functional Connectome Fingerprint in Individuals With Schizophrenia. JAMA Psychiatry. 2018;75:749–751. doi: 10.1001/JAMAPSYCHIATRY.2018.0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B., Buchanan R.W., Ross D.E., Carpenter J. A Separate Disease Within the Syndrome of Schizophrenia. Arch Gen Psychiatry. 2001;58:165–171. doi: 10.1001/ARCHPSYC.58.2.165. [DOI] [PubMed] [Google Scholar]
- Larabi, D.I., Gell, M., Amico, E., Eickhoff, S.B., Patil, K.R., 2022. Spatially localized fMRI metrics as predictive and highly distinct state-independent fingerprints. bioRxiv 2021.08.03.454862. 10.1101/2021.08.03.454862.
- Laumann T.O., Gordon E.M., Adeyemo B., Snyder A.Z., Joo S.J., Chen M.-Y., Gilmore A.W., McDermott K.B., Nelson S.M., Dosenbach N.U.F., et al. Functional system and areal organization of a highly sampled individual human brain. Neuron. 2015;87:657–670. doi: 10.1016/j.neuron.2015.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecrubier, Y., Sheehan, D. v., Weiller, E., Amorim, P., Bonora, I., Sheehan, K.H., Janavs, J., Dunbar, G.C., 1997. The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: Reliability and validity according to the CIDI. European Psychiatry 12, 224–231. 10.1016/S0924-9338(97)83296-8.
- Leucht S., Kane J.M., Kissling W., Hamann J., Etschel E., Engel R.R. What does the PANSS mean? Schizophr Res. 2005;79:231–238. doi: 10.1016/j.schres.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Leucht S., Samara M., Heres S., Davis J.M. Dose Equivalents for Antipsychotic Drugs: The DDD Method. Schizophr Bull. 2016;42:S90. doi: 10.1093/SCHBUL/SBV167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Liao X., Xia M., He Y. Chronnectome fingerprinting: identifying individuals and predicting higher cognitive functions using dynamic brain connectivity patterns. Hum Brain Mapp. 2018;39:902–915. doi: 10.1002/hbm.23890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlab . The MathWorks Inc.; Natick, Massachusetts: 2021. Version: 9.11.0.1769968 (R2021b) [Google Scholar]
- Mueller S., Wang D., Fox M.D., Yeo B.T.T., Sepulcre J., Sabuncu M.R., Shafee R., Lu J., Liu H. Individual variability in functional connectivity architecture of the human brain. Neuron. 2013;77:586–595. doi: 10.1016/J.NEURON.2012.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousdal O.T., Kaufmann T., Kolskår K., Vik A., Wehling E., Lundervold A.J., Lundervold A., Westlye L.T. Longitudinal stability of the brain functional connectome is associated with episodic memory performance in aging. Hum Brain Mapp. 2020;41:697–709. doi: 10.1002/hbm.24833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack, R.A., Laumann, T.O., Koyejo, O., Gregory, B., Hover, A., Chen, M.Y., Gorgolewski, K.J., Luci, J., Joo, S.J., Boyd, R.L., Hunicke-Smith, S., Simpson, Z.B., Caven, T., Sochat, V., Shine, J.M., Gordon, E., Snyder, A.Z., Adeyemo, B., Petersen, S.E., Glahn, D.C., Mckay, D.R., Curran, J.E., Göring, H.H.H., Carless, M.A., Blangero, J., Dougherty, R., Leemans, A., Handwerker, D.A., Frick, L., Marcotte, E.M., Mumford, J.A., 2015. Long-term neural and physiological phenotyping of a single human. Nature Communications 2015 6:1 6, 1–15. 10.1038/ncomms9885. [DOI] [PMC free article] [PubMed]
- Pruim R.H.R., Mennes M., van Rooij D., Llera A., Buitelaar J.K., Beckmann C.F. ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage. 2015;112:267–277. doi: 10.1016/j.neuroimage.2015.02.064. [DOI] [PubMed] [Google Scholar]
- Ritchie, S.J., Cox, S.R., Shen, X., Lombardo, M. v., Reus, L.M., Alloza, C., Harris, M.A., Alderson, H.L., Hunter, S., Neilson, E., Liewald, D.C.M., Auyeung, B., Whalley, H.C., Lawrie, S.M., Gale, C.R., Bastin, M.E., McIntosh, A.M., Deary, I.J., 2018. Sex Differences in the Adult Human Brain: Evidence from 5216 UK Biobank Participants. Cerebral Cortex 28, 2959–2975. 10.1093/CERCOR/BHY109. [DOI] [PMC free article] [PubMed]
- Rolls, E.T., Loh, M., Deco, G., Winterer, G., 2008. Computational models of schizophrenia and dopamine modulation in the prefrontal cortex. Nature Reviews Neuroscience 2008 9:9 9, 696–709. 10.1038/nrn2462. [DOI] [PubMed]
- Santo-Angles A., Salvador R., Gomar J.J., Guerrero-Pedraza A., Ramiro N., Tristany J., Teixidó C., Ortiz-Gil J., Aguirre C., Bosque C., López-Araquistain L., Maristany T., Salgado-Pineda P., Sarró S., McKenna P.J., Bernardo M., Pomarol-Clotet E., Schwarzbach J. Interindividual variability of functional connectome in schizophrenia. Schizophr Res. 2021;235:65–73. doi: 10.1016/J.SCHRES.2021.07.010. [DOI] [PubMed] [Google Scholar]
- Schaefer A., Kong R., Gordon E.M., Laumann T.O., Zuo X.-N., Holmes A.J., Eickhoff S.B., Yeo B.T.T. Local-Global Parcellation of the Human Cerebral Cortex from Intrinsic Functional Connectivity MRI. Cerebral Cortex. 2018;28:3095–3114. doi: 10.1093/CERCOR/BHX179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan, D. v., Lecrubier, Y., Sheehan, K.H., Amorim, P., Janavs, J., Weiller, E., Hergueta, T., Baker, R., Dunbar, G.C., 1998. The Mini-International Neuropsychiatric Interview (M.I.N.I): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59, 20–33. [PubMed]
- Shen, X., Finn, E.S., Scheinost, D., Rosenberg, M.D., Chun, M.M., Papademetris, X., Constable, R.T., 2017. Using connectome-based predictive modeling to predict individual behavior from brain connectivity. Nature Protocols 2017 12:3 12, 506–518. 10.1038/nprot.2016.178. [DOI] [PMC free article] [PubMed]
- Sokunbi M.O., Gradin V.B., Waiter G.D., Cameron G.G., Ahearn T.S., Murray A.D., Steele D.J., Staff R.T. Nonlinear Complexity Analysis of Brain fMRI Signals in Schizophrenia. PLoS One. 2014;9:e95146. doi: 10.1371/journal.pone.0095146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svaldi D.O., Goñi J., Abbas K., Amico E., Clark D.G., Muralidharan C., Dzemidzic M., West J.D., Risacher S.L., Saykin A.J., Apostolova L.G. Optimizing differential identifiability improves connectome predictive modeling of cognitive deficits from functional connectivity in Alzheimer’s disease. Hum Brain Mapp. 2021;42:3500–3516. doi: 10.1002/HBM.25448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talpalaru A., Bhagwat N., Devenyi G.A., Lepage M., Chakravarty M.M. Identifying schizophrenia subgroups using clustering and supervised learning. Schizophr Res. 2019;214:51–59. doi: 10.1016/J.SCHRES.2019.05.044. [DOI] [PubMed] [Google Scholar]
- Tepper Á., Cuiza A., Alliende L.M., Mena C., Ramirez-Mahaluf J.P., Iruretagoyena B., Ornstein C., Fritsch R., Nachar R., González-Valderrama A., Undurraga J., Cruz J.P., Tejos C., Fornito A., Repetto G., Crossley N. Functional Dysconnectivity in Ventral Striatocortical Systems in 22q11.2 Deletion Syndrome. Schizophr Bull. 2022;48:485–494. doi: 10.1093/SCHBUL/SBAB139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, Y., Margulies, D.S., Breakspear, M., Zalesky, A., 2020. Topographic organization of the human subcortex unveiled with functional connectivity gradients. Nature Neuroscience 2020 23:11 23, 1421–1432. 10.1038/s41593-020-00711-6. [DOI] [PubMed]
- Undurraga, J., Negussie, H., Wendler, D., 2022. Consent, decisional capacity and guardianship in mental health research. Wellcome Open Research 2022 7:183 7, 183. 10.12688/wellcomeopenres.18003.1. [DOI] [PMC free article] [PubMed]
- van den Heuvel M.P., Fornito A. Brain networks in schizophrenia. Neuropsychol Rev. 2014;24:32–48. doi: 10.1007/s11065-014-9248-7. [DOI] [PubMed] [Google Scholar]
- Wong A.H.C., van Tol H.H.M. Schizophrenia: from phenomenology to neurobiology. Neurosci Biobehav Rev. 2003;27:269–306. doi: 10.1016/S0149-7634(03)00035-6. [DOI] [PubMed] [Google Scholar]
- Yeo B.T.T., Krienen F.M., Chee M.W.L., Buckner R.L. Estimates of segregation and overlap of functional connectivity networks in the human cerebral cortex. Neuroimage. 2014;88:212–227. doi: 10.1016/J.NEUROIMAGE.2013.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K., Amunts K. Individual variability is not noise. Trends Cogn Sci. 2013;17:153–155. doi: 10.1016/J.TICS.2013.02.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available from Zenodo and scripts from Github,as described in the paper (see Conclusions).