Abstract
Objectives
Accurate determination of the immediate causes of death in patients with COVID-19 is important for optimal care and mitigation strategies.
Methods
All deaths in Qatar between March 01, 2020, and August 31, 2022, flagged for likely relationship to COVID-19 were reviewed by two independent, trained reviewers using a standardized methodology to determine the immediate and contributory causes of death.
Results
Among 749 flagged deaths, the most common admitting diagnoses were respiratory tract infection (91%) and major adverse cardiac event (MACE, 2.3%). The most common immediate causes of death were COVID-19 pneumonia (66.2%), MACE (7.1%), hospital-associated pneumonia (HAP, 6.8%), bacteremia (6.3%), disseminated fungal infection (DFI, 5.2%), and thromboembolism (4.5%). After COVID-19 pneumonia, MACE was the predominant cause of death in the first 2 weeks but declined thereafter. No death occurred due to bacteremia, HAP, or DFI in the first week after hospitalization, but became increasingly common with increased length of stay in the hospital accounting for 9%, 12%, and 10% of all deaths after 4 weeks in the hospital, respectively.
Conclusion
Nearly one-third of patients with COVID-19 infection die of non-COVID-19 causes, some of which are preventable. Mitigation strategies should be instituted to reduce the risk of such deaths.
Keywords: SARS-CoV-2, COVID-19, Mortality, Cause of death
Introduction
As of November 15, 2022, more than 640 million cases of COVID-19 with over 6.6 million deaths had been reported globally, which translates to a crude case fatality of rate of just over 1% [1]. These may represent under- or over-estimation of the numbers and rates, as the mild or asymptomatic cases may not be tested or reported, and cause of death may not be accurately identified. While incidence of COVID-19 infection is still high in many countries, disease severity and case fatality have declined substantially over time [1], [2], [3], [4], [5]. A large proportion of deaths in patients with COVID-19 occur among those with other significant comorbidities [6,7]. In such cases, it is difficult to ascertain whether COVID-19 or other comorbidities were the immediate cause of death. To complicate matters further, existing comorbidities can be exacerbated by COVID-19 infection and vice versa. It is important to know the number of patients who “die of COVID-19” as opposed to those who “die with COVID-19”, for public health, public policy, and clinical care decision-making purposes. In other words, quantifying the immediate cause(s) of death in patients with COVID-19 may help devise strategies to improve outcomes in these patients. We conducted this study to determine the immediate and contributory cause(s) that caused the terminal event in patients with COVID-19.
Methods
Study setting and population
The study was conducted in the State of Qatar between March 01, 2020, and August 31, 2022. All deaths in Qatar are evaluated and processed through a single public sector healthcare system with a unified electronic health records platform. Trained medical examiners review each death and assign a primary cause of death and contributory causes according to the World Health Organization (WHO) criteria for reporting causes of death based on individual chart review [8]. Since reporting of all deaths is mandatory and death certificates are required for burial and any post-mortem benefits to the descendants, capture of death data is complete in Qatar. All deaths that occurred in Qatar during the study period which were flagged by the mortality review team for likely relationship to COVID-19 were evaluated. All deaths in Qatar in patients with a recent positive COVID-19 test were reviewed by a dedicated team of physicians who made a determination of its possible association with COVID-19. Any death deemed to be linked to the COVID-19 diagnosis was labeled as such based on the WHO reporting criteria for COVID-19-related deaths.
Vaccination and COVID-19 testing data
COVID-19 laboratory testing, vaccination, and clinical infection data were extracted from the integrated, nationwide, digital-health information platform that hosts the national, federated SARS-CoV-2 databases. These databases are complete with no missing information for polymerase chain reaction testing, COVID-19 vaccinations, COVID-19 hospitalizations, and basic demographic details, and have captured all SARS-CoV-2-related data since epidemic onset. Nearly all individuals who were vaccinated received their vaccines in Qatar, through the public healthcare system for all nationals and residents of Qatar [2,3,[9], [10], [11], [12].
Ascertainment of cause(s) of death
For the current study, each death flagged for likely being related to COVID-19 in Qatar was evaluated by two independent physician reviewers. The reviewers were trained to determine and assign the most likely immediate cause of death. Each decedent's electronic medical records were comprehensively reviewed, and the cause of death was assigned based on the most plausible immediate event that triggered the event(s) that led to death. Such ascertainment was based on physician notes, supporting notes from other care providers, and a review of laboratory, microbiology, pathology, and radiology data, wherever applicable. The reviewers were specifically trained to separate the mode of death (generic terms like cardiac arrest, respiratory failure, etc.) from the immediate cause(s) of death that led to those terminal events (e.g., acute myocardial infarction, hospital-associated pneumonia [HAP], etc.). Reviewers were initially trained on a smaller dataset of non-COVID-19 related deaths and the methodology was refined through team feedback and consensus. Discordant determinations were adjudicated by a third reviewer, also trained in a similar fashion. In addition to the primary immediate cause of death, reviewers also determined additional major and direct contributory factors that led to death.
Categorizing cause(s) of death
After the primary reviewers had assigned the immediate and contributory causes of death, they were categorized into major diagnostic groups by organ system, syndrome, or disease classification. A uniform categorization algorithm was used for all cases. Details of mapping individual causes to categories are provided in Supplementary Table 1. After categorization, any discordant causes were adjudicated by a third reviewer.
Ethical considerations
The study was approved by the Institutional Review Board at Hamad Medical Corporation. Since the study involved only decedents, the requirement for informed consent was not applicable. There was no funding source, hence no such body had any influence over any aspects of this study.
Results
A total of 749 deaths were flagged for likely association with COVID-19 during the study period. The median age of the decedents was 56 years (interquartile range [IQR] 47,83), 76.4% were male, and 19.5% were Qatari nationals. Most (79.6%) were unvaccinated at the time of diagnosis. All were admitted to the hospital with the most common admitting diagnoses being respiratory tract infection (91%) and major adverse cardiac event (MACE, 2.3%) (Table 1 ).
Table 1.
Baseline characteristics of the decedents included in our study.
| n N = 749 |
% | |
|---|---|---|
| Median age (IQR), years | 56 (47,83) | |
| Sex | ||
| Male | 572 | 76.4% |
| Female | 176 | 23.5% |
| Missing | 1 | 0.1% |
| Nationalitya | ||
| Qatari | 146 | 19.5% |
| Southeast Asian | 337 | 45.0% |
| Middle East and North Africa (MENA) | 145 | 19.4% |
| Far Eastern | 67 | 8.9% |
| African | 44 | 5.9% |
| All others | 9 | 1.2% |
| Missing | 1 | 0.1% |
| Admitting diagnoses | ||
| Respiratory tract infection | 682 | 91.1% |
| Major adverse cardiac event | 17 | 2.3% |
| Malignancy | 6 | 0.8% |
| Infection, other acute bacterial or viral | 4 | 0.5% |
| Acute kidney injury | 3 | 0.4% |
| Trauma, fracture, or burns | 3 | 0.4% |
| Metabolic or electrolyte abnormalities | 2 | 0.3% |
| Thromboembolism | 2 | 0.3% |
| Liver failure, acute or acute on chronic | 1 | 0.1% |
| Missing | 22 | 2.9% |
| Other | 7 | 0.9% |
| Vaccination status | ||
| No dose received | 596 | 79.6% |
| 1 dose only | 97 | 13.0% |
| 2 doses | 48 | 6.4% |
| 3 doses | 8 | 1.1% |
| Median (IQR) hospital length of stay, days (10 missing values were excluded) | 23 (14,38) |
Nationalities are grouped as follows:
Southeast Asian: Indian, Bangladeshi, Pakistani, Nepalese, Sri Lankan
Far Eastern: Filipino, Indonesian, Malaysian
MENA: Algerian, Bahraini, Egyptian, Emirati, Iranian, Iraqi, Jordanian,
Lebanese, Omani, Palestinian, Saudi, Syrian, Tunisian, Yemeni, Emirati, Iraqi
African include: Sudanese, Somali, Nigerian, Ghanaian, Eritrean, Mauritanian
Others include: United Kingdom, the Netherlands, American, Canadian, and Afghan
IQR, interquartile range.
The most common immediate cause of death was COVID-19 pneumonia (66.2%), followed by MACE (7.1%), HAP (6.8%), bacteremia (6.3%), disseminated fungal infection (DFI, 5.2%), and thromboembolism (4.5%). (Table 2 ) The median length of hospital stay was 23 days (IQR 14,38).
Table 2.
Immediate cause of death in patients diagnosed with COVID-19.
| Immediate cause of death N = 749 |
||
|---|---|---|
| n | % | |
| COVID-19 pneumonia | 496 | 66.2% |
| Major adverse cardiovascular event | 53 | 7.1% |
| Hospital-associated pneumonia | 51 | 6.8% |
| Bacteremia | 47 | 6.3% |
| Disseminated fungal infection | 39 | 5.2% |
| Thromboembolism | 34 | 4.5% |
| Acute ischemia or bleeding | 11 | 1.5% |
| Malignancy | 8 | 1.1% |
| No consensus | 8 | 1.1% |
| Trauma | 2 | 0.3% |
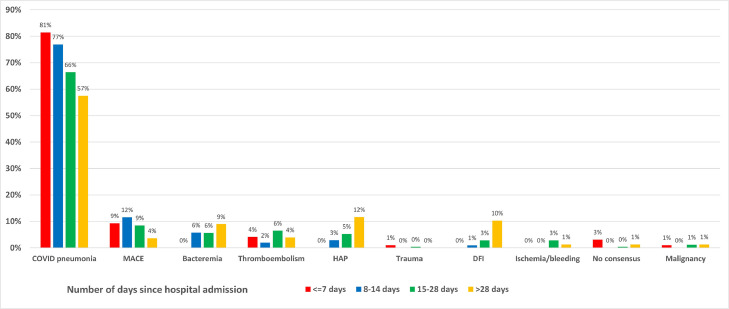
When broken down by the time of death after hospitalization, COVID-19 pneumonia remained the predominant cause irrespective of the time from admission, though the proportion dropped with the increasing length of stay in the hospital. Other than COVID-19 pneumonia, MACE was the predominant cause of death in the first 2 weeks but declined thereafter. No death occurred due to bacteremia, HAP, or DFI in the first week after hospitalization, but they accounted for 9%, 12%, and 10% of all deaths after 4 weeks in the hospital, respectively (Figure 1 and Supplementary Table 2).
Figure 1.
Immediate cause of death by time of death after hospital admission. Main graph shows major causes of death other than COVID-19 pneumonia, which is shown in the inset.
The majority of deaths (86%) occurred in the intensive care unit setting. A breakdown of causes of death in the intensive care vs non-intensive care unit setting is provided in Table 3 . COVID-19 pneumonia remained the major cause of death in both setting accounting for approximately two-thirds of deaths in each setting. MACE and HAP were approximately equally represented in both settings while bacteremia and DFI were more common in the intensive care unit setting.
Table 3.
Cause of death, by location.
| ICU deaths n = 648 |
Non-ICU deaths n = 101 |
|||
|---|---|---|---|---|
| N | % | N | % | |
| COVID-19 pneumonia | 426 | 65.7% | 70 | 69.3% |
| Major adverse cardiac event | 45 | 6.9% | 8 | 7.9% |
| Hospital-associated pneumonia | 44 | 6.8% | 7 | 6.9% |
| Bacteremia | 42 | 6.5% | 5 | 5.0% |
| Disseminated fungal infection | 37 | 5.7% | 2 | 2.0% |
| Thromboembolism | 31 | 4.8% | 3 | 3.0% |
| Acute ischemia or bleeding | 11 | 1.7% | 0 | 0.0% |
| Metastatic cancer | 6 | 0.9% | 2 | 2.0% |
| No consensus | 4 | 0.6% | 5 | 5.0% |
| Trauma | 2 | 0.3% | 0 | 0.0% |
ICU, intensive care unit.
ICU deaths, deaths occurring in an intensive care unit setting; non-ICU deaths, death occurring in a non-intensive care unit setting including deaths occurring in step-down and other high-dependency units.
Additional analyses
We also ascertained the additional contributing cause(s) of death among all decedents in the study. In about one-third of the cases, the reviewers did not reach a consensus regarding the most significant contributing causes. In about one-quarter of the cases, no additional cause was listed. A list of all causes regardless of the immediate cause, as well as a list of causes excluding the same immediate cause is provided in Supplementary Table 3. Among the 224 cases with no consensus, we listed the causes as determined by the two primary reviewers (Supplementary Table 4).
Discussion
Understanding the immediate cause of death is critical in addressing any potential gaps in care and improving outcomes in patients with COVID-19. We provide the first large national analysis of the immediate cause(s) of death in hospitalized patients with COVID-19 using a robust methodology involving individual medical records review by trained clinicians.
The median age of the decedents in our study was 56 years, which is considerable younger than the reported life expectancy in Qatar at birth of 79 years [13]. This indicates a substantial impact of COVID-19 on life expectancy. While we did not calculate the quantitative decline in life expectancy in Qatar, recently published data demonstrate a decline of 2-3 years in several European countries and the United States [14]. In countries in the lower socioeconomic strata, the effect has been even more profound, with Latin American countries experiencing up to a 10-year decline in life expectancy at birth due to COVID-19 [15]. Approximately 80% of the decedents in our study were not vaccinated, which reinforces the role of vaccination in preventing severe disease and death among those infected with COVID-19. A negative correlation between the decline in life expectancy and national vaccination rates has been reported across several countries [14]. The median length of hospital stay was 23 days, which indicates the high burden of healthcare utilization and financial implications of the disease.
As expected, the most common immediate cause of death was COVID-19 pneumonia. COVID-19 primarily affects the lungs, though many other organ systems may be involved early or late in the disease [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27]. The most common admitting diagnosis was also respiratory tract infection, which is compatible with the most common disease presentation for COVID-19 as well as subsequent complications [28,29]. However, while 91% of the patients presented with a respiratory tract infection diagnosis, only two-thirds died of COVID-19 pneumonia. This underscores the importance of other conditions that may lead to death in these patients.
After COVID-19, the most frequent cause of death was MACE, which includes acute myocardial infarction, stroke, heart failure, and cardiac rhythm abnormalities. Cardiovascular events have clearly been associated with COVID-19 infection, though their exact nature, temporal relationship to infection, and their relative contribution toward acute mortality are far from clear [20], [21], [22]. In our study, these events were frequently seen early after admission, with 21% of those events occurring within 14 days of hospitalization and declining thereafter. This supports, though does not prove, a causal relationship between COVID-19 and MACE. Another possible explanation is that the acute stress of infection may have precipitated previously known or subclinical cardiovascular disease leading to an acute event resulting in death [30].
A striking finding from our study is the high rate of bacteremia and DFI. Most of the latter (18/39, 46.2%) were candidemia infections. Candida bloodstream infections are among the leading cause of bloodstream infections in hospitalized patients. Increased total hospital length of stay, intensive care unit admission and length of stay, central venous catheters, total parenteral nutrition, and use of broad-spectrum antibiotics are among the risk factors for candida bloodstream infections. A large proportion of our patient population was admitted to an intensive care setting (86%) and had a hospital length of stay far above most national averages. These could certainly have contributed to the high risk of DFIs. Risk factors for bacteremia in hospitalized patients are somewhat similar and could explain the high incidence of bacteremia as the cause of death in our patient population [31], [32], [33]. Both bacteremia and DFIs were seen later in the hospital stay, strongly suggesting their acquisition in the healthcare setting. None of these infections contributed to death in the first week of hospital stay in our patient population. These results underscore the need for vigilance and steps to mitigate the risk of these infections in COVID-19 patients who stay in the hospital beyond 1 week.
Hospital-associated pneumonia also showed similar trends to bloodstream infections. As the definitions would suggest, there were no cases early on during hospitalization, but the number increased significantly from the second week of hospitalizations onwards. Nearly half the cases (24/51, 47%) were classified as ventilator-associated pneumonia. These patients had either admitted with a diagnosis other than respiratory tract infection or had recovered from that and then developed a new pneumonia while in hospital. Again, the overall length of stay in the hospital and being in the intensive care unit setting on a ventilatory may have contributed significantly to this outcome [34].
The fact that a large proportion of deaths other than COVID-19 pneumonia itself were due to causes associated with a prolonged hospital stay, and may in many cases be preventable, is a reminder to institute mitigation strategies to prevent these outcomes. Such strategies could include minimizing hospital stay wherever possible, enhanced surveillance for bloodstream infections, strict aseptic techniques for all procedural interventions, eliminating inappropriate use of antibiotics, removal of unnecessary central venous or arterial catheters, and aggressive ventilator management. Clinicians should also be vigilant in monitoring, assessing, and intervening for less anticipated events like MACE.
Strengths of our study include a robust methodology to ascertain the immediate and additional contributory causes of death using trained reviewers minimizing reporting or ascertainment bias, and a national study population under care at a single healthcare system eliminating bias due to regional variations in practices. Limitations include the lack of autopsy data to confirm the adjudicated causes. Our aim was not to determine the risk factors for the identified causes; hence we did not conduct additional analyses to determine those risk factors and their association with the causes of death. We did not determine the impact of vaccination or viral variants on the causes of death. Our study population was derived from those who were flagged by the Qatar national mortality review team for possible association with COVID-19. While unlikely, there is a possibility that some deaths may have been missed due to non-inclusion in the flagged population.
In summary, a large proportion of patients with COVID-19 infection die of non-COVID-19 causes, some of which are preventable. Mitigation strategies should be instituted to reduce the risk of such deaths.
Declaration of competing interest
Dr. Butt has received investigator-initiated grant funding from Gilead Sciences and Merck and Company (to the institution, Veterans Health Foundation of Pittsburgh) which is unrelated to the work presented here. Other authors declare no financial conflict of interest regarding the content of this article.
Acknowledgments
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Support for open access publication was provided by the Medical Research Center, Hamad Medical Corporation, Doha, Qatar.
Ethical considerations
The study was approved by the Institutional Review Board at Hamad Medical Corporation. A waiver of informed consent was granted for the study. There was no external funding source for the study.
Acknowledgments and disclaimer
The authors are grateful for the leadership and assistance provided by the Business Intelligence Unit at Hamad Medical Corporation, the Ministry of Public Health in Qatar, partner institutions within the Academic Health System in Qatar, and all the dedicated frontline healthcare workers who have selflessly served and provided care and comfort to all patients in Qatar. The views expressed in this article are those of the authors and do not necessarily represent official government views or policy of the State of Qatar or Hamad Medical Corporation.
Author contributions
Concept and study design: AAB. Drafting of the manuscript: AAB. Data acquisition: MDG, HA, AHSA, TO, AASSA, MFSM, AGT, SS, SS, FH, AHK, ANL, and RMS. Data analysis and interpretation: AAB and ABAS. Critical appraisal and review: All authors. Final approval of the article: All authors.
Data access
Drs. Butt and Abou-Samra had complete access to the data at all times and accept responsibility for the integrity of this article.
Role of the funding source
Support for open access publication was provided by the Medical Research Center, Hamad Medical Corporation, Doha, Qatar, which had no role in conduct of the study or the decision to publish.
Role of sponsors and affiliate institutions
Design and conduct of the study: None. Collection, management, analysis, and interpretation of the data: Data used in this study were collected as a part of the National COVID-19 Response, and were overseen by the Ministry of Public Health, Qatar and Hamad Medical Corporation, Qatar. Neither had a role in the analysis or interpretation of the data. Preparation, review, or approval of the manuscript: None. Decision to submit the manuscript for publication: None.
Data sharing
The national dataset used for this study is the property of the Qatar Ministry of Public Health that was provided to the researchers through a restricted-access agreement that prevents sharing the dataset with a third party or publicly. Future access to this dataset can be considered through a direct application for data access to the Ministry of Public Health Qatar (https://www.moph.gov.qa/english/Pages/default.aspx).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2023.04.385.
Appendix. Supplementary materials
References
- 1.Worldometer. Coronavirus updates, https://www.worldometers.info/coronavirus/?utm_campaign=homeAdTOA; 2022 [accessed 13 November 2022].
- 2.Butt AA, Dargham SR, Chemaitelly H, Al Khal A, Tang P, Hasan MR, et al. Severity of illness in persons infected with the SARS-CoV-2 Delta variant vs beta variant in Qatar. JAMA Intern Med. 2022;182:197–205. doi: 10.1001/jamainternmed.2021.7949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butt AA, Dargham SR, Coyle P, Yassine HM, Al-Khal A, Abou-Samra AB, et al. COVID-19 disease severity in persons infected with omicron BA.1 and BA.2 sublineages and association with vaccination status. JAMA Intern Med. 2022;182:1097–1099. doi: 10.1001/jamainternmed.2022.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butt AA, Dargham SR, Tang P, Chemaitelly H, Hasan MR, Coyle PV, et al. COVID-19 disease severity in persons infected with the Omicron variant compared with the Delta variant in Qatar. J Glob Health. 2022;12:05032. doi: 10.7189/jogh.12.05032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayr FB, Talisa VB, Castro AD, Shaikh OS, Omer SB, Butt AA. COVID-19 disease severity in US Veterans infected during Omicron and Delta variant predominant periods. Nat Commun. 2022;13:3647. doi: 10.1038/s41467-022-31402-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrison SL, Fazio-Eynullayeva E, Lane DA, Underhill P, Lip GYH. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: a federated electronic medical record analysis. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization . 2010. Cause of Death on the Death Certificate in line with ICD-10 - Quick Reference Guide.https://apps.who.int/classifications/apps/icd/icd10training/ICD-10%20Death%20Certificate/html/ICD-10_Resources/causeofdeathflyer.pdf [accessed 05 March 2023] [Google Scholar]
- 9.Abu-Raddad LJ, Chemaitelly H, Ayoub HH, AlMukdad S, Yassine HM, Al-Khatib HA, et al. Effect of mRNA vaccine boosters against SARS-CoV-2 omicron infection in Qatar. N Engl J Med. 2022;386:1804–1816. doi: 10.1056/NEJMoa2200797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abu-Raddad LJ, Chemaitelly H, Bertollini R. National Study Group for COVID-19 Vaccination. Effectiveness of mRNA-1273 and BNT162b2 vaccines in Qatar. N Engl J Med. 2022;386:799–800. doi: 10.1056/NEJMc2117933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chemaitelly H, Yassine HM, Benslimane FM, Al Khatib HA, Tang P, Hasan MR, et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med. 2021;27:1614–1621. doi: 10.1038/s41591-021-01446-y. [DOI] [PubMed] [Google Scholar]
- 12.Tang P, Hasan MR, Chemaitelly H, Yassine HM, Benslimane FM, Al Khatib HA, et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 Delta variant in Qatar. Nat Med. 2021;27:2136–2143. doi: 10.1038/s41591-021-01583-4. [DOI] [PubMed] [Google Scholar]
- 13.WorldBank. Life expectancy at birth, total (years). Qatar, https://data.worldbank.org/indicator/SP.DYN.LE00.IN?locations=QA; 2022 [accessed 05 March 2023].
- 14.Schöley J, Aburto JM, Kashnitsky I, Kniffka MS, Zhang L, Jaadla H, et al. Life expectancy changes since COVID-19. Nat Hum Behav. 2022;6:1649–1659. doi: 10.1038/s41562-022-01450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lima EEC, Vilela EA, Peralta A, Rocha M, Queiroz BL, Gonzaga MR, et al. Investigating regional excess mortality during 2020 COVID-19 pandemic in selected Latin American countries. Genus. 2021;77:30. doi: 10.1186/s41118-021-00139-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X, Jin Y, Li R, Zhang Z, Sun R, Chen D. Prevalence and impact of acute renal impairment on COVID-19: a systematic review and meta-analysis. Crit Care. 2020;24:356. doi: 10.1186/s13054-020-03065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunutsor SK, Laukkanen JA. Renal complications in COVID-19: a systematic review and meta-analysis. Ann Med. 2020;52:345–353. doi: 10.1080/07853890.2020.1790643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zarifian A, Zamiri Bidary M, Arekhi S, Rafiee M, Gholamalizadeh H, Amiriani A, et al. Gastrointestinal and hepatic abnormalities in patients with confirmed COVID-19: a systematic review and meta-analysis. J Med Virol. 2021;93:336–350. doi: 10.1002/jmv.26314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rokkas T. Gastrointestinal involvement in COVID-19: a systematic review and meta-analysis. Ann Gastroenterol. 2020;33:355–365. doi: 10.20524/aog.2020.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;76:533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang L, Zhao P, Tang D, Zhu T, Han R, Zhan C, et al. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. JACC Cardiovasc Imaging. 2020;13:2330–2339. doi: 10.1016/j.jcmg.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marchand L, Pecquet M, Luyton C. Type 1 diabetes onset triggered by COVID-19. Acta Diabetol. 2020;57:1265–1266. doi: 10.1007/s00592-020-01570-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vogrig A, Bagatto D, Gigli GL, Cobelli M, D'Agostini S, Bnà C, et al. Causality in COVID-19-associated stroke: a uniform case definition for use in clinical research. J Neurol. 2021;268:758–761. doi: 10.1007/s00415-020-10103-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Sanctis P, Doneddu PE, Viganò L, Selmi C, Nobile-Orazio E. Guillain-Barre syndrome associated with SARS-CoV-2 infection. A systematic review. Eur J Neurol. 2020;27:2361–2370. doi: 10.1111/ene.14462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nepal G, Rehrig JH, Shrestha GS, Shing YK, Yadav JK, Ojha R, et al. Neurological manifestations of COVID-19: a systematic review. Crit Care. 2020;24:421. doi: 10.1186/s13054-020-03121-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muscente F, De Caterina R. Causal relationship between influenza infection and risk of acute myocardial infarction: pathophysiological hypothesis and clinical implications. Eur Heart J Suppl. 2020;22:E68–E72. doi: 10.1093/eurheartj/suaa064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karchmer AW. Nosocomial bloodstream infections: organisms, risk factors, and implications. Clin Infect Dis. 2000;31:S139–S143. doi: 10.1086/314078. S139–43. [DOI] [PubMed] [Google Scholar]
- 32.Mortensen VH, Søgaard M, Kristensen B, Mygind LH, Schønheyder HC. Risk factors for hospital-acquired bacteraemia - an explorative case-control study of hospital interventions. Infect Dis (Lond) 2022;54:178–185. doi: 10.1080/23744235.2021.1994153. [DOI] [PubMed] [Google Scholar]
- 33.Jeon CY, Neidell M, Jia H, Sinisi M, Larson E. On the role of length of stay in healthcare-associated bloodstream infection. Infect Control Hosp Epidemiol. 2012;33:1213–1218. doi: 10.1086/668422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warren DK, Zack JE, Elward AM, Cox MJ, Fraser VJ. Nosocomial primary bloodstream infections in intensive care unit patients in a nonteaching community medical center: a 21-month prospective study. Clin Infect Dis. 2001;33:1329–1335. doi: 10.1086/322483. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.