Abstract
Excess nutrition causes loss of olfactory sensory neurons (OSNs) and reduces odor discrimination and odor perception in mice. To separate diet-induced obesity from the consumption of dietary fat, we designed pair-feeding experiments whereby mice were maintained on isocaloric diets for 5 months that prevented increased fat storage. To test our hypothesis that adiposity was not a prerequisite for loss of OSNs and bulbar projections, we used male and female mice with an odorant receptor-linked genetic reporter (M72tauLacZ; Olfr160) to visualize neural circuitry changes resulting from fat in the diet. Simultaneously we monitored glucose clearance (diagnostic for prediabetes), body fat deposition, ingestive behaviors, select inflammatory markers, and energy metabolism. Axonal projections to defined olfactory glomeruli were visualized in whole-mount brains and the number of OSNs were manually counted across whole olfactory epithelia. After being pair fed a moderately high-fat (MHF) diet, mice of both sexes had body weight, adipose deposits, energy expenditure, respiratory exchange ratios, and locomotor activity that were unchanged from control-fed mice. Despite this, they were still found to lose OSNs and associated bulbar projections. Even with unchanged adipocyte storage, pair-fed animals had an elevation in TNF cytokines and an intermediate ability for glucose clearance. Albeit improving health metrics, access to voluntary running while consuming an ad libitum fatty diet, still precipitated a loss of OSNs and associated axonal projections for male mice. Our results support that long-term macronutrient imbalance can drive anatomical loss in the olfactory system regardless of total energy expenditure.
Graphical Abstract Legend.
Patterns of long-term dietary consumption and exercise shape neuronal abundance and communication in the olfactory system. Design made in BioRender.

Introduction
Chemosensory ability is dependent upon nutritional health and metabolic state for both humans and rodents, demonstrating that energy homeostasis is a driver of food detection and satiation (Lacroix et al., 2015; Kolling & Fadool, 2020). In both taste and olfactory systems, diet-induced obesity (DIO) or excess nutrition causes the loss of olfactory sensory neurons (OSNs) and taste buds, ultimately causing a reduction in chemosensory ability (Tucker et al., 2012b; Thiebaud et al., 2014; Lacroix et al., 2015; Kovach et al., 2016; Kaufman et al., 2018; Fardone et al., 2018). The impact of olfactory deficits in obese mice has been well studied (Palouzier-Paulignan et al., 2012). Obese mice have a reduced density of axonal projections to the olfactory bulb, a reduction in Golf and odorant receptor proteins, and poorer odor discrimination using conditioned odor-aversion (COA) tasks, habituation/dishabituation trials, or go, no-go operant conditioning paradigms (Fadool et al., 2011; Tucker et al., 2012b; Aimé et al., 2014; Thiebaud et al., 2014; Lacroix et al., 2015; Fardone et al., 2018). Electrically, obese mice have a reduction in the receptor potential (Thiebaud et al., 2014; Lacroix et al., 2015) collectively generated from the population of OSNs, and acquired from the surface of the olfactory epithelium using a recording called an electroolfactogram (Scott & Scott-Johnson, 2002). Moreover, output neurons called mitral cells, which relay information to higher cortical regions from the olfactory bulb, have changed activity in obese rodents. In rodents with obesity, mitral cells have altered sensitivity to neurohormones such as insulin and are differentially modulated by energy important molecules (Fadool et al.,2011; Aimé et al., 2014). Low-grade, chronic inflammation brought on by DIO (Hotamisligil, 2006) has inhibited the renewal of taste buds (Kaufman et al., 2018), however, the mechanism of loss of OSNs in the olfactory system attributed to obesity remains less understood.
Two different transgenic mouse models have suggested that DIO may not be the direct or sole cause of loss of OSNs in the olfactory system. Mice with a loss of melanocortin 4 receptor (MC4R−/−) in the hypothalamus present with late-onset diabetes and associated weight gain, have hyperleptinemia, and are hyperphagic (Tucker et al., 2008). MC4R is a G-protein-coupled receptor, which when activated, reduces food intake and increases energy expenditure through leptin signaling. Despite their genetically predisposed obesity, MC4R−/− mice are not anosmic nor do they exhibit structural changes in OSN abundance or associated axonal projections (Tucker et al., 2008, 2012a; Thiebaud et al., 2014). Mice with a loss of voltage-dependent potassium channel 1.3 (Kv1. 3−/−) are thin without caloric restriction and have low levels of circulating glucose and leptin (Fadool et al., 2004; Xu et al., 2004; Tucker et al., 2008, 2012a; Thiebaud et al., 2014). Kv1.3 channels are dampeners of neuronal excitability but have also been shown to be important in control of energy homeostasis (Upadyay et al., 2013). When Kv1.3−/− mice are placed on fatty diets they are resistant to DIO and the diet does not cause a loss in olfactory discrimination despite the fact that they still exhibit structural loss of OSNs and their associated axonal projections (Tucker et al., 2008, 2012a; Thiebaud et al., 2014). Because genetically obese MC4R−/− gain adiposity on a control diet (CF), but do not lose OSNs, and the resistance of obesity in Kv1. 3−/− mice maintained on moderately high-fat diet (MHF) does not prevent loss of OSNs, we hypothesized loss of olfactory structure and function could be the result of the consumption of dietary fat or excess nutrition.
To test the idea that fat in the diet was the culprit for olfactory losses, we established a pattern of pair-feeding whereby mice were maintained on isocaloric diets for 5 months but challenged with diets consisting of different fat percentages. To test our hypothesis that adiposity was not a prerequisite for loss of sensory structures in the olfactory system, we used mice with an OR-linked genetic reporter to visualize circuitry changes in response to fat in the diet while monitoring glucose clearance ability (prediabetes), body fat deposition, ingestive behaviors, select inflammatory markers, and energy metabolism. Even though male mice that were pair-fed the MHF-diet did not gain adiposity nor total body weight over that of MHF ad libitum fed mice and were able to clear a glucose challenge the same as control-fed mice, they still exhibited loss of OSNs and associated axonal projections. Interestingly, our data showed a sexual dimorphism, in that we were unable to significantly induce DIO in female mice. Even though female mice exhibited an inability to clear glucose (prediabetes) when fed MHF ad libitum, they did not gain adiposity. Moreover, isocalorically-maintained female mice consuming excess fat via MHF diets were not prediabetic, but still retained loss of OSNs and associated axonal projections. Exercise is well-known to reduce adiposity and associated inflammation brought on by DIO. We then tested the secondary hypothesis that voluntary running could reverse the deleterious effects of the structural losses of olfactory neurons. Albeit improving other health metrics in the mice, including total energy expenditure, glucose tolerance, adipose deposition, and body weight, exercising while consuming an ad libitum fatty diet still precipitated loss of OSNs and associated axonal projections. These results suggest that the long-term macronutrient imbalance of a fatty diet can drive anatomical loss in the olfactory system regardless of total energy expenditure.
Methods
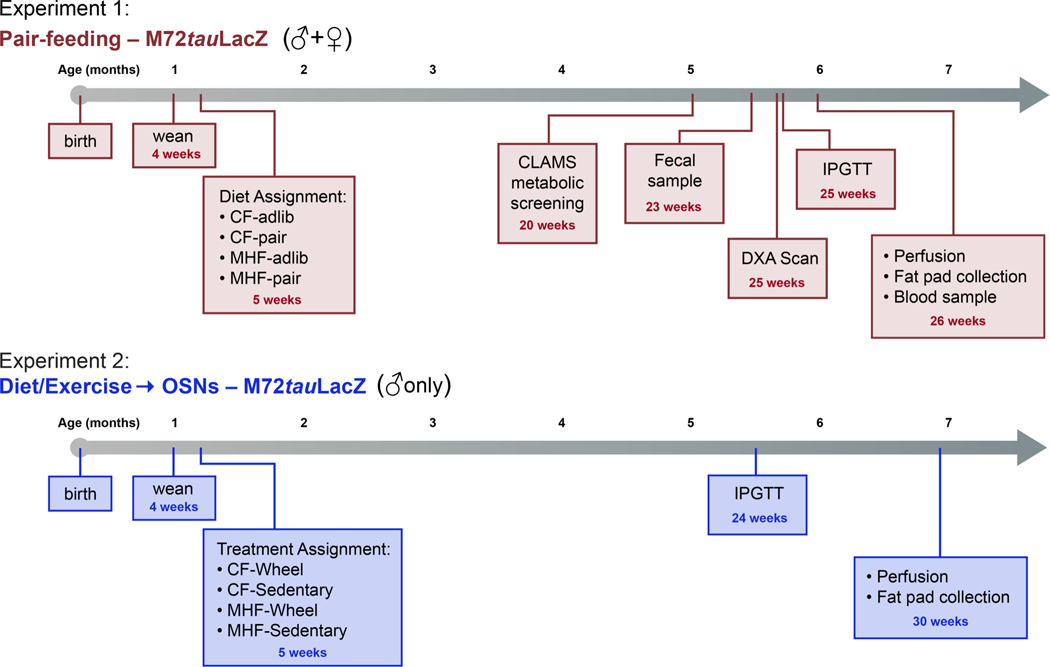
Sequence of experiments and animal care
Different series of experiments were performed on transgenic lines of mice (to include: modified diet, isocaloric feeding paradigms, serum chemistry, fat determination, glucose sensitivity, food intake, access to voluntary running, olfactory behaviors, and/or systems physiology metabolic parameters) prior to final histological processing to determine any changes in olfactory circuitry and structure. The sequence of experimental events is diagrammed in Figure 1 for ease of reference, and the associated methods are partitioned into two main cohorts or experiments, accordingly. Mice were maintained on either a control diet (CF) of Purina 5001 Rodent Chow (28.05% kCal protein, 59.81% kCal carbohydrate, and 13.5% kCal fat; https://www.labdiet.com/Products/StandardDiets/Rodents/index.html) or a Moderately High-fat Condensed Milk Diet (MHF), catalog number D12266B, from Research Diets, Inc. (16.8% kCal protein, 51.4% kCal carbohydrate, and 31.8% kCal fat; https://researchdiets.com/formulas/d12266b). Mice were weaned at 4 weeks and then randomly assigned to dietary/treatment group, which was initiated within 1 week. Littermates were assigned different dietary/treatment groups within an experiment to minimize any varying effects of maternal behavior.
Figure 1. Experimental timelines for pair-feeding treatment (Experiment 1) and voluntary exercise (Experiments 2) in transgenic lines of mice with olfactory reporters.
Experiment 1 (blue): Timeline for experiments performed using male and female M72tauLacZ mice that were maintained on isocaloric diets of differing fat content (pair fed), Experiment 2 (green): Timeline for experiments performed using male M72tauLacZ mice that were allowed access to voluntary running wheels and diets of differing fat content (diet/exercise). Histology of the main olfactory epithelium and olfactory bulb was performed for both experiments.
Ethical approval
All mice were housed in the animal vivarium at Florida State University (FSU) in conditions compliant with institutional requirements, the National Institutes of Health (NIH), and the American Veterinary Medicine Association (AVMA). All performed procedures were approved under the FSU Animal Care and Use Committee protocols #1733 and #202000036. In preparation for histology, mice were anaesthetized with a mixture of ketamine (100 mg/kg body weight) and xylazine (10 mg/kg body weight) prior to intracardial perfusion with 4% paraformaldehyde and euthanasia by decapitation (AVMA Guidelines on Euthanasia, June 2007). All authors understood the ethical principles that The Journal of Physiology operates under, and the work complied with the animal ethics checklist reported by Grundy, 2015 (Grundy, 2015).
Experiment #1: Pair Feeding
Mouse husbandry and lines.
To investigate the impact of dietary fat on the olfactory system, a transgenic line of mice with a genetic marker for OSNs expressing the odorant receptor Olfr160 was used. This mouse line (M72IRES tau LacZ (M72tauLacZ); deposited at Jackson Laboratories – stock number 006596, https://www.jax.org/strain/006596, RRID: IMSR_JAX:006596) allows visualization of the Olfr160-expressing OSNs via production of an indigo byproduct in the cells following β-galactosidase histology (Zheng et al., 2000; Zapiec & Mombaerts, 2015). Upon weaning, mice were individually housed in conventional style mouse cages with open wire bar lids. Mice were maintained on a 12h/12h light/dark cycle and allowed ad libitum access to water.
Dietary treatment.
M72tauLacZ mice of both sexes were randomly assigned to one of four treatment groups. Pair feeding, a feeding paradigm in which the food consumption for experimental groups of mice is isocalorically matched to that of a control group, was manually maintained for 5 months (Fig. 1, Experiment 1). In this design, the first group, the CF-maintained mice, was allowed food access ad libitum (CF adlib). The second group of mice were maintained on the CF diet, but each day were provided with an amount of food calorically equivalent to the food consumed by the respective matched CF adlib mouse the previous day (CF pair fed). The third group was MHF-maintained mice that were allowed food access ad libitum (MHF adlib), but of higher fat content (CF: 13.5 vs. MHF: 31.8 percent of kCal from fat). The fourth group (MHF pair fed) was similarly maintained on the MHF diet, but their daily food allotment was calorically equivalent to the food consumed by the matched CF adlib mouse. For the purposes of our study designs, an isocaloric diet is one in which mice consume equivalent daily calories (also known as yoked) but the diets contain differential fat percentages (CF and MHF pair diets), and an obesogenic diet is one in which the total amount of food leads to obesity or the deposition of excess adipose stores (MHF adlib). Daily body weights and food intake were collected over the 5-month treatment period and daily isocaloric feeding was calculated based upon company-reported kilocalories (CF = 3.35kcal/g, MHF = 4.41 kcal/g). Food consumption was carefully monitored and the isocaloric design did not induce food-restricted patterns of eating.
Indirect calorimetry.
To assess modulation of whole-body metabolism due to chronic participation in isocaloric diets of varying fat composition, the mice were temporarily transferred to individual metabolic chambers at 5 months of age (Fig. 1, Experiment 1). Mice were acclimated to metabolic chambers for 2 days prior to systems physiology measurement. Oxygen consumption (VO2; ml/kg/h), carbon dioxide production (VCO2; ml/kg/h), respiratory exchange ratio (RER), locomotor activity, food intake (kcal), and water consumption were tracked for ~5 days using the Comprehensive Laboratory Animal Monitoring System (CLAMS™; Columbus Instruments, Columbus, OH). Metabolic data were analyzed as the mean value over a continuous 72-hour interval acquired in the 5-day experimental window, sorted by light or dark cycle. During the period in the CLAMS™, mice had ad libitum access to water, but food continued to be isocalorically administered as previously described. Food and water were delivered using overhead feeders attached to specialized electronic balances that monitored both disturbance and decrease in mass. A threshold of at least 10 seconds (s) of feeder disturbance and a minimum loss of 0.03 grams (g) of chow was required for a recorded meal bout. VO2 and VCO2 were normalized with respect to body weight in kilograms, calculated and averaged over 10 days. RER was calculated as VCO2/VO2. Energy expenditure (EE) was calculated using the Lusk Graham equation (3.815 + 1.232 x RER) x VO2 (Lusk & Du Bois, 1924). Locomotor activity was continuously measured using optical beams along the X-axis of the cage (Columbus Instruments). Consecutive photo beam breaks were scored as ambulatory movement. Indirect calorimetry data were recorded in intervals using Oxymax software (Columbus Instruments). Each interval measurement represented the average value during a 30 s sampling period per cage.
Body weight and body fat.
Body weight was measured and recorded daily. To determine body composition, mice underwent a dual-energy x-ray absorptiometry (DXA) scan at ~5.5 months of age (Fig. 1, Experiment 1). Mice were anesthetized with a mixture of ketamine (100 mg/kg body weight) and xylazine (10 mg/kg body weight) and scanned whole-body in the prone position (GE Lunar iDXA; General Electric Company, Fairfield, CT). Access to the iDXA equipment was lost prior to completion of the experiment. Thus, some mice were scanned using an EchoMRI machine (EchoMRI LLC, Houston, TX) instead to determine body composition. Upon termination, we also manually excised and weighed adipose tissue from the carcasses of the mice. A researcher blinded to the treatment group collected the interscapular brown, mesenteric, retroperitoneal, subcutaneous (subsample on right side), and epididymal (male) or endometrial (female) fat pads and weighed them separately as previously performed (Tucker et al., 2012a).
Glucose tolerance.
An intraperitoneal glucose tolerance test (IPGTT) was performed on the mice between 5–6 months of age (Fig. 1, Experiment 1). Mice were fasted for 6 h starting at the beginning of the dark phase, and then were injected with a volume of 25% glucose solution equivalent to 2 g of glucose per kg of lean body mass (as determined previously via DXA or EchoMRI). A small incision was made on the tail and blood samples were collected with a Contour Next Blood Glucose Monitoring System (Ascensia Diabetes Care US, Inc.; Parsippany, NJ) paired with Contour Next Blood Glucose Test Strips (Ascensia) to determine blood glucose levels at baseline (prior to injection) and at set timepoints 15, 30, 60, 90, and 120 min following the injection. The area under the resulting curve was integrated (iAUC) per mouse and compared across treatment groups.
Solutions and reagents.
Solutions used for tissue preparation or visualization of β-galactosidase reaction product, namely, phosphate buffered saline (PBS), Buffer A, Buffer B, and Buffer C, were made as described previously (Biju et al., 2008; Thiebaud et al., 2014). Phosphate buffered saline (PBS) contained 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 1. 8 mM KH2PO4. Buffer A contained 0. 1 M phosphate buffer, 2 mM MgCl2, and 5 mM EGTA. Buffer B contained 0.1 M phosphate buffer, 2 mM MgCl2, 0. 01% sodium deoxycholate, and 0.02% Nonidet P40. Buffer C consisted of Buffer B with 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, and 1 mg/ml X-gal. Slides used for collection of tissue sections were gel coated with a solution containing 1% w/v gelatin and 2 mM chromium potassium sulfate. X-gal was purchased from Research Products International Co. (Mt. Prospect, IL). All salts were obtained from Sigma Chemical or Fisher Scientific, Inc. (Atlanta, GA).
Beta-galactosidase histology and imaging.
Mice were anesthetized using a mixture of ketamine and xylazine (as above). A toe pinch test was used to ensure the state of anesthesia prior to intracardiac perfusion with PBS followed by 4% w/v PFA/PBS for 5 minutes (min) each. Following the perfusion, the heads were removed and placed in 4% PFA/PBS at 4°C for 4 hours (h). The skulls were then rinsed in PBS for 15 min, replacing the PBS every 5 min. The skulls were next decalcified by transferring them into 0.3M EDTA in PBS for approximately 5 days. The skull and remaining tissues were carefully removed leaving the brain with the olfactory epithelium intact. Caution was used when removing the upper incisors because they run along the length of the olfactory epithelium and poor technique during their removal can damage the tissue. The exposed olfactory bulbs and olfactory epithelium were then stained to visualize beta-galactosidase expressing Olfr160-positive OSNs (Biju et al., 2008; Thiebaud et al., 2014). Briefly, the tissues were washed with Buffer A for 5 min and then again for 25 min, followed by two 5-min washes with Buffer B. The tissues were then incubated in Buffer C for 10 h followed by 0.1M phosphate buffer to stop the reaction.
The olfactory bulb whole-mounts were imaged using a Leica MZ FLIII stereomicroscope (Leica Microsystems; Buffalo Grove, IL) outfitted with a Zeiss AxioCam paired with AxioVision software (Carl Zeiss Microimaging, Jena, Germany). The pixel density of the Olfr160 glomerulus and its associated axonal projections for each bulb pair were estimated using the open-source image processing software Fiji (Schindelin et al., 2012). The whole-mount images were converted to grayscale and a threshold was applied that differentiated stained OSN projections from unstained tissue. The threshold was variable for each converted grayscale image but was adjusted to reflect the staining in the original image as closely as possible. Application of the threshold instructed the software to consider all pixels darker than the threshold as black and those lighter as white. Then the regions of interest (ROI) were drawn using the freehand selection tool around the pair of lateral glomeruli and their associated projections, quantifying the number of total black pixels for the olfactory bulb pair per mouse. The medial glomeruli were not measured due to the difficult angle of their position casting a shadow in the whole-mount images when pixel converting.
Following whole-mount imaging, the olfactory bulbs (with olfactory epithelium still attached) were cryoprotected in 10% w/v sucrose/PBS followed by 30% w/v sucrose/PBS. The tissues were incubated at 4°C in each sucrose solution until they sank to the bottom of the histology tube. The cryoprotected tissues were then frozen in O. C. T. embedding medium (Fisher HealthCare, Houston, TX) and stored at −80°C. The tissues were cryosectioned coronally from the start of the olfactory epithelium through the end of the olfactory bulbs at 16 μm thickness on a CM1860 cryostat (Leica) with the chamber temperature set at −20°C. Sections were collected on gelatin-coated Superfrost slides (CAT# 48311–601, VWR international, Radnor, PA) and stored at −20°C until use. The sectioned tissue was stained again, repeating the same procedure as described for the whole mounts. The slides were returned to room temperature, and washed with Buffer A for 5 and 25 min, and then Buffer B twice for 5 min. The slides were then incubated in Buffer C with a 2x higher concentration of x-gal for 48 h as opposed to the procedure above. This resulted in strong staining without the need for counterstaining so that the OSNs could be easily identified and manually counted. The Olfr160 expressing OSNs were visualized and enumerated manually via light microscopy with an Axiovert 135 (Zeiss). The number of Olfr160-positive OSNs was summed across the entire epithelium per mouse. Each mouse was counted with the investigator blind to the treatment of the animal.
Fecal sampling and corticosterone quantification.
Mice were provided with a clean cage at the onset of the dark phase. At the conclusion of the dark phase and the beginning of the light phase, the mice were provided with a second clean cage. At the conclusion of the light phase, the mice were transferred again to a third clean cage. The dark phase and light phase cages were emptied individually, and all fecal matter was collected via manual survey through the bedding. Each 12 h fecal sample was stored at −80°C until processing. Corticosterone was quantified using a Corticosterone Competitive ELISA Kit (Invitrogen; CAT# EIACORT). Steroid extraction, sample preparation, and the ELISA were performed according to the company’s recommended protocol.
Serum sampling and cytokine quantification.
During termination by intracardiac perfusion, a blood sample was taken from each mouse. Prior to infusion of PBS, a 20G needle was inserted into the caudal vena cava from which blood was collected. The blood was allowed to clot at room temperature for ~15 min. The samples were centrifuged at 735g at 4°C for 10 min and the supernatant was collected and stored at −20°C until processing. Interleukin 6 (IL-6) and tumor necrosis factor (TNF) were quantified using a Mouse IL-6 ELISA Kit (CAT# KMC0061; Invitrogen) and Mouse TNF ELISA Kit (CAT# 88–7324-22; Invitrogen), respectively. Sample/standard preparation and the ELISAs were performed according to the company’s recommended protocol.
Statistical analysis.
Prior to performing any statistical comparisons, data were first analyzed with the Dixon’s Q test to identify any outliers. Then data were checked for normal distribution and homogeneity of variance using the Fmax test. Analysis of data collected in Experiment 1 did not identify any outliers nor did any collected data violate homogeneity of variance (fail the Fmax test). Mean body weight, fat pad weight, food intake, iAUC for the glucose clearance curves, pixel counts for Olfr-160 glomeruli and axonal projections in whole-mount images, manual counts of Olfr-160-positive OSNs, fecal corticosterone levels, and serum IL-6 and TNF concentration were each analyzed using a one-way analysis of variance (1-way ANOVA) with dietary treatment as the factor at the 95% confidence level (α ≤ 0.05). Metabolic data from the CLAMS were analyzed using a 1-way ANOVA with dietary treatment as the factor at the 95% confidence level (α ≤ 0.05). Comparison of metabolic data across light vs. dark cycle within dietary treatment was analyzed using a one-tailed, paired t-test (α ≤ 0.05). Body weight over age measurements were analyzed with a two-way repeated measures analysis of variance (2-way RM ANOVA) using dietary treatment and time as factors. For the ordinary and RM ANOVA tests, the Tukey or Bonferroni method for multiple comparison testing was used as the post-hoc analysis to make mean-wise comparisons between treatments. Males and females were analyzed as separate cohorts. All reported values in the text and figures are mean ± standard deviation (SD). Sample sizes are reported as individual data points in the graphs and represent number of mice. Individual F statistic, and p values are reported for each experiment within the corresponding graph as described in the results section. Statistical tests were performed using GraphPad Prism 9 (GraphPad Software; San Diego, CA) while the graphs and figures were produced using Origin v8 (OriginLab Corporation; Northampton, MA), Adobe Photoshop CS4 (Adobe, Inc.; San Jose, CA), and the open-source graphics editing software Inkscape (https://inkscape.org/release/inkscape-1.1/).
Experiment #2: Voluntary exercise effect on OSNs
Mouse husbandry and lines.
To investigate the interactive effects of diet composition and wheel running on the olfactory system, M72tauLacZ male mice were weaned to larger cages (18.5 in L x 9.5 in W x 8 in H) to accommodate running wheel equipment (Fig. 1, Experiment 2). Mice were maintained on a 12/12h light/dark cycle and allowed ad libitum access to food and water.
Voluntary wheel running.
For the voluntary running experiments, M72tauLacZ mice were randomly assigned to either the CF or the MHF diet. Within each diet group, the mice were further divided into either a voluntary exercise (VEx) or a sedentary treatment (SED). Mice assigned to the VEx treatment received ad libitum access to a Vertical Wireless Running Wheel (Med Associates, Inc., St Albans, VT) as previously described (Chelette et al., 2019). Briefly, a wheel sensor was placed on top of the wire bar lid with plastic manifolds extending into the cage that held the wheel above the bedding to allow for rotation. Mice were provided with a clean running wheel biweekly that was lubricated with odorless, tasteless, non-caloric silicone oil. The wheel running data were collected using the Wheel Manager software program (Med Associates, Inc., https://www.medassociates.com/product/wheel-manager/), which archived the data every 30 seconds. Upon conclusion of data collection, the running data were exported through the Wheel Analysis software program (Med Associates, Inc.). The data were exported, organized, and analyzed using a combination of Wheel Analysis (Med Associates), Microsoft Excel, and R (R Core Team) software.
Body weight and body fat.
Body weight was measured and recorded weekly. Following termination, the mesenteric, retroperitoneal, subcutaneous, and epididymal fat pads were removed from the carcasses and weighed collectively. Brown fat was not collected.
Glucose tolerance.
An intraperitoneal glucose tolerance test (IPGTT) was performed on mice between 5–6 months of age, as in Experiment #1. The exception being that mice in this cohort were fasted for 12 h starting at the beginning of the light phase.
Termination and histology.
Termination and fixation by intracardiac perfusion as well as the beta-galactosidase staining on whole mounts and cryosectioned tissues were performed the same as in Experiment #1.
Statistical Analysis.
Prior to performing any statistical comparisons, data were first analyzed with the Dixon’s Q test to identify any outliers. Then data were checked for normal distribution and homogeneity of variance using the Fmax test. Analysis of data collected for Experiment 2 did not identify any outliers nor did collected data violate homogeneity of variance (fail the Fmax test). Mean body weight, adipose tissue weight, iAUC for the glucose clearance curves, pixel counts for whole-mount images, and Olfr-160-positive OSN counts were analyzed, and the data were presented in the same way as Experiment #1 with the notable exception that only males were used for these experiments. Running distance, time, and velocity were compared between CF and MHF maintained mice using a Student’s t-test at the 95% confidence level (α ≤ 0.05).
Results
Mice maintained on an isocaloric diet containing moderately high-fat (MHF) retain loss of axon projections
To test whether a modified diet containing fat could perturb the abundance and circuitry of olfactory sensory neurons (OSNs), M72tauLacZ mice were challenged with moderately high-fat (MHF) diet for 5 months that was either provided ad libitum (adlib) or isocalorically matched to that of a control-fed mouse (pair). The density of axonal projections observed by whole-mount imaging of the β galactosidase staining of Olfr160 axons was captured by thresholding to a fixed level - on a black and white scale - and then computing total pixel density for the lateral Olfr160 glomerulus in the right and left olfactory bulb (Fig. 2A, B). The goal of this feeding paradigm was to maintain mice on MHF diets without an induction of increased body weight or adiposity, and then observe the extent of any olfactory anatomical perturbations due to dietary content. As anticipated from earlier investigations where mice were challenged with diet-induced obesity (DIO) (Thiebaud et al., 2014), mice that had ad libitum access to the MHF diet (MHF adlib) exhibited a significant loss of Olfr160-expressing olfactory sensory axonal projections (Fig. 2) compared with those maintained on control diets (CF adlib and CF pair fed). This loss of projections was true for both male (Fig. 2A) and female (Fig. 2B) mice, the latter of which had never been examined with respect to loss of axonal projections or OSNs attributed to fatty diet. Interestingly, mice of both sexes also lost significant Olfr160-expressing axonal projections when they consumed the same daily caloric intake as CF adlib mice but did so in the form of MHF diet (Fig. 2C; one-way analysis of variance (1-way ANOVA) using dietary treatment as the factor, with a Tukey’s post-hoc test). Here, and in all subsequent analyses using the between-subjects ordinary ANOVA, the F statistic is reported with the numerator and denominator degrees of freedom (dF) as the number of (groups – 1) and number of (groups x sample size) – number of groups, respectively. The sample size (n) represents number of mice. The generated F value = F(dFnumerator, dFdenominator) and p value are reported in each graph of the figure. The post-hoc test is indicated by lower case letters; whereby different letters indicate significantly different group means at α ≤ 0.05. We examined both CF adlib and pair conditions to confirm that the CF pair treatment did not exhibit any degree of stress by being restrained to the caloric consumption of a pair fed animal, despite both treatment groups consuming an identical CF diet. Although the body weight and other noted metrics (see below) must always be restrained in the pair treatment by design, a collection of fecal pellets from mice in all 4 treatment groups failed to demonstrate significant changes in corticosterone levels (Table 1; males dark cycle - F(3, 18) = 0.9170, p = 0.4525 and males light cycle – F(3, 18) = 0.09269, p = 0.9631; females dark cycle - F(3, 18) = 0.1829, p = 0.9066 and females light cycle - F(3, 18) = 1.457, p = 0.2597), suggesting lack of measurable stress hormones induced by the pair feeding regiment.
Figure 2. Reduction of Olfr160 axonal projections in mice following consumption of high fat diet.
Representative whole-mount images of olfactory bulbs following beta-galactosidase histological staining of Olfr160-expressing olfactory sensory neurons (OSNs) from A, male and B, female M72tauLacZ mice following dietary treatments; images from left to right: control diet ad libitum (brown food symbol), control diet isocaloric pair-fed (brown food and scale symbol), moderately high-fat diet ad libitum (pink food symbol), moderately high-fat diet isocaloric pair-fed (pink food and scale symbol). Corresponding gray scale images were used for pixel determination in (C). o = olfactory bulb, g = glomerulus, and a = Olfr160 axonal projections. C, Bar graph of total Olfr160 axon projections vs diet treatment in male (left) and female (right) mice. Symbols represent individual mice sampled from left to right: CF adlib = control diet ad libitum (⬛), CF pair = control diet isocaloric pair fed (⬜), MHF adlib = moderately high-fat diet ad libitum (●), MHF pair = moderately high-fat diet isocaloric pair-fed (○). Data are reported as mean ± standard deviation in this and all subsequent figures. Sample size of number of mice from left to right, Male = 8, 8, 9, 9 | Female = 9, 9, 9, 9. One-way analysis of variance (1-way ANOVA) across treatment groups within each sex. Degrees of freedom, F test, and p value of the ANOVA are reported in each figure panel, here and in subsequent figures. Letters indicate significantly different Tukey’s post-hoc test corrected for multiple comparisons, p < 0.05.
Table 1.
Pro-inflammatory cytokine production and inhibitory regulator in response to dietary treatment.
| Assay target | CF adlib | CF pair | MHF adlib | MHF pair | p value | Units | Sample |
|---|---|---|---|---|---|---|---|
|
| |||||||
| MALES | |||||||
| TNF | 26.1 ± 20 (7) [a] | 28.4 ± 16.6 (7) [a] | 78.4 ± 42 (9) [b] | 84.2 ± 31.6 (8) [b] | 0.0005 | pg/mL | Serum |
| IL-6 | 71.4 ± 93.9 (5) | 36.8 ± 25.1 (4) | 72.4 ± 55.2 (5) | 67.8 ± 102.4 | 0.9000 | pg/mL | Serum |
| CORT | |||||||
| 12 h dark | 4301 ± 1494 (5) | 4355 ± 688 (5) | 3950 ± 678 (6) | 4901 ± 1003 (6) | 0.9200 | pg/mL | Fecal |
| 12 h light | 4342 ± 1370 (5) | 4236 ± 1493 (5) | 4130 ± 625 (6) | 3989 ± 1083 (6) | 0.9600 | ||
|
| |||||||
| FEMALES | |||||||
| TNF | 26.1 ± 11 (7) [a] | 31.1 ± 16.4 (9) [a] | 67.24 ± 29 (8) [b] | 71.2 ± 28.6 (9) [b] | 0.0003 | pg/mL | Serum |
| IL-6 | 41.6 ± 43.3 (6) | 69.3 ± 64.5 (6) | 116.5 ± 59.6 (5) | 81.4 ± 64.9 (6) | 0.2400 | pg/mL | Serum |
| CORT | |||||||
| 12 h dark | 4163 ± 1306 (6) | 3949 ± 1602 (6) | 3689 ± 380 (6) | 4206 ± 1284 (5) | 0.9100 | pg/mL | Fecal |
| 12 h light | 3701 ± 778 (6) | 3808 ± 625 (6) | 3916 ± 449 (5) | 4582 ± 1052 (5) | 0.2600 | ||
Mean ± SD (sample size); Dietary Factor = 1-way ANOVA (p value) with Tukey’s post-hoc test (α ≤ 0.05) [letters]
CF = control fed, adlib = ad libitum, MHF = moderately-high fat diet, pair = isocalorically fed to match CF
TNF = tumor necrosis factor alpha, IL-6 = interleukin 6, CORT = corticosteroid.
Mice maintained on an isocaloric diet containing MHF retain loss of olfactory sensory neurons (OSNs)
Because there was a significant loss of Olfr160-expressing axonal projections to defined glomeruli in both the MHF diet ad libitum (MHF adlib) and isocaloric conditions (MHF pair), it was anticipated that there should be a concomitant loss of Olfr160 OSNs for these same treatments at the level of the epithelium. We sectioned across entire main olfactory epithelia of differentially fed mice and found a significant loss of OSN abundance regardless of whether mice consumed the MHF diet freely (MHF adlib) or whether they were restricted to the calories consumed by CF adlib mice (MHF pair) (Fig. 3; 1-way ANOVA w/ Tukey’s post-hoc test). This loss of OSNs was not sex dependent (Fig. 3D) and occurred in female mice despite the absence of diet-induced obesity (DIO) in females (see Fig. 4 and below).
Figure 3. Reduction in number of Olfr160-expressing olfactory sensory neurons in mice following consumption of moderately high-fat diet.

A, Representative coronal tissue section of an olfactory epithelium (OE) at 50X (left), 100X (center), and 400X (right) magnification acquired from a M72tauLacZ male mouse maintained on a control diet ad libitum (CF adlib). The indigo cells are Olfr160-expressing olfactory sensory neurons (OSNs). oe = olfactory epithelium, osn = olfactory sensory neuron. B, Representative images of an OE following one of the four dietary treatments in male (top) and female (bottom) mice. C, Bar graph of the number of Olfr160-expressing OSNs vs dietary treatment in males (left) and females (right). Notations and statistical analyses as in Fig. 2. Males = 8, 8, 8, 9 | Females = 6, 7, 8, 8.
Figure 4. Male mice selectively increase body weight and food intake when dietary fat is consumed ad libitum rather than isocaloric pair-fed.
A, Line graph of body weight for male (left) and female (right) M72tauLacZ mice vs. age following dietary treatment initiated at 5 weeks of age; Two-way repeated measures ANOVA (2-way RM ANOVA); symbols indicate significant group differences for dietary factor as determined by Tukey’s post-hoc (p < 0.05) as follows: # indicates MHF adlib > CF adlib; ● indicates MHF adlib > MHF pair; ◊ indicates MHF adlib > CF pair; ⴲ indicates CF adlib > MHF pair; ♣ indicates CF adlib > CF pair. Inset: Bar graph of body weight at 26 weeks of age vs. dietary treatment group. 1-way ANOVA; letters indicate significantly different Tukey’s post-hoc test corrected for multiple comparisons, p < 0.05. Bar graph of B, daily food consumption and C, cumulative food intake over the duration of the experiment for male (left) and female (right) mice. 1-way ANOVA with Tukey’s post-hoc, p < 0.05. D, Scatterplot fitted by linear regression demonstrating degree of correlation (r2) between daily meal size and body weight at 26 weeks of age in male (left) and female (right) mice. Notations as in Fig. 2. Males = 8, 8, 9, 9 | Females = 9, 10, 9, 9.
Isocalorically maintained male mice consuming MHF have normal body weight, body fat, and caloric intake, and have improved glucose tolerance
By taking weekly body weight measurements, we observed that the mean body weight of male mice that were allowed ad libitum access to MHF diet began to diverge from other dietary treatment groups at about 2 months of treatment (Fig. 4A, left). An analysis of diet and time as factors showed a significant difference of both variables as well as a diet x time interaction (Fig 4A, left, 2-way RM ANOVA w/ Tukey’s post-hoc test). Post-hoc tests indicated an elevation of body weight in the MHF ad libitum treatment compared with all three other treatment groups starting at 13 weeks of age. We additionally performed comparisons of body weight, body fat, and adipose deposition at five months of treatment. Mice that consumed MHF ad libitum had significantly greater body weight (Fig. 4A inset, left; 1-way ANOVA w/ Tukey’s post-hoc test); an effect that was consistent with an increase in daily and cumulative food consumption (Fig. 4B, C, left; 1-way ANOVA w/ Tukey’s post-hoc test). It was noteworthy that MHF pair-fed mice were not significantly different in any of these metrics from that of CF mice (Fig. 4A–C, left). In males, there was a strong correlation between food intake and final body weight (Fig. 4D). All male treatment groups displayed similar amounts of lean body mass (Fig. 5A, left) and the surplus body weight displayed by the male MHF adlib mice correlated with significantly higher fat mass as determined by DXA and EchoMRI (Fig. 5A, left) as well as post-termination, manual excision of fat depots (Fig. 5B, left). By analyzing post mortem adipose tissue localization, we found that epididymal fat was the greatest source of adipose accumulation, but that every common source of deposition was significantly elevated in the MHF adlib diet treatment for males - [DXA/EchoMRI lean body mass: F(3,30) = 0.3 ; p = 0.86 | DXA/EchoMRI fat mass: F(3,30) = 8.1 ; p = 0.0004 | Total weight of excised fat pads: F(3,30) = 28.2 ; p < 0.0001 | Epididymal: F(3,30) = 43.8 ; p < 0.0001 | Retroperitoneal: F(3,30) = 17.3 ; p < 0.0001 | Mesenteric: F(3,30) = 8.8 ; p = 0.0002 | Subcutaneous: F(3,30) = 17.5 ; p < 0.0001 | Intrascapular Brown: F(3,27) = 9.7 ; p = 0.0002 ]. (Fig. 5A, B, left; 1-way ANOVA w/ Tukey’s post-hoc test). By comparison, fat deposition for the isocalorically, pair fed MHF dietary treatment was not significantly different than that of CF adlib controls (Fig. 5A, B, left; 1-way ANOVA w/ Tukey’s post-hoc test). In fact, none of the adipose tissue accumulations in pair fed mice (CF or MHF) were elevated above that of CF adlib controls. Male mice that consumed MHF isocalorically (pair) had body weights, food consumption, body mass, and fat depots that were not significantly different than that of CF adlib or pair treatments (Figs. 4, 5). Interestingly, despite a lack of changes in body weight, food consumption and adiposity, the MHF isocaloric treatment did elicit an intermediate change in glucose sensitivity compared with MHF adlib and CF adlib treatment conditions (Fig. 5C).
Figure 5. Male, and not female mice, increase adiposity when dietary fat is consumed ad libitum rather than isocaloric pair-fed. Both sexes demonstrate an impaired glucose clearance when dietary fat is consumed ad libitum, and an intermediate impairment when consumed isocalorically.
A, Bar graph of fat and lean tissue mass vs dietary treatment in male (left) and female (right) M72tauLacZ mice. B, Bar graph of excised fat pad weight vs dietary treatment; male (left), female (right). Epi = epididymal, Retro = retroperitoneal, Mes = mesenteric, Sub = subcutaneous, Brown = intrascapular brown, Endo = endometrial. C, Line graph of the plasma glucose concentration during the IPGTT; Inset: Bar graph of the integrated area under the curve (iAUC). Notations and statistical analyses as in Fig. 2. Males = 8, 8, 9, 9 | Females = 9, 10, 9, 9.
Female mice are not responsive to DIO, yet do exhibit glucose insensitivity with MHF adlib treatment, which improves with isocaloric diet
In contrast to male mice, female mice showed a different pattern of body weight gain with dietary treatment. An analysis of diet and time as factors did show a significant difference of both variables as well as a diet x time interaction, however post-hoc tests indicated that MHF ad libitum treatment was not different than that of CF mice (Fig. 4A; right, 2-way RM ANOVA w/ Tukey’s post-hoc test), signifying a resistance to DIO. In fact, CF adlib mice had body weights that were greater than both pair-fed treatments (CF and MHF), and only the MHF adlib mice had body weights that were greater than that of the MHF pair-fed mice. When additionally performing comparisons of body weight, body fat, and adipose deposition at five months of treatment, we found that female mice did not have significant changes in body weight for any of the dietary treatments compared with CF mice (Fig. 4A inset; right, 1-way ANOVA w/ Tukey’s post-hoc test), nor did they demonstrate any changes in food intake (Fig. 4B, C; right), correlation of body weight and meal size (Fig. 4D; right), or changes in lean/fat tissue deposition or adiposity (Fig. 5A, B; right). Nonetheless, female mice, like males, did demonstrate a failure to clear glucose following ad libitum access to MHF, which was also intermediately affected when the MHF was isocalorically consumed (Fig. 5C; right, 1-way ANOVA w/ Tukey’s post-hoc test).
Changes in serum pro-inflammatory cytokine production
Obesity is associated with low-grade inflammation, therefore, we investigated whether there might be changes in cytokines in the plasma of MHF adlib or MHF pair-fed animals. While there was not a significant change in the levels of interleukin 6 (IL6), there was a significant elevation of tumor necrosis factor alpha (TNFα) in mice administered MHF diets regardless of state of adiposity (Table 1). Elevation of this adipocyte-derived chemokine is associated with decreased glucose clearance and increased insulin resistance, so it was interesting that we found a significant elevation of TNFα in both male and female mice, especially given the resistance of DIO in the later, which retained, however, a state of prediabetes with MHF diets.
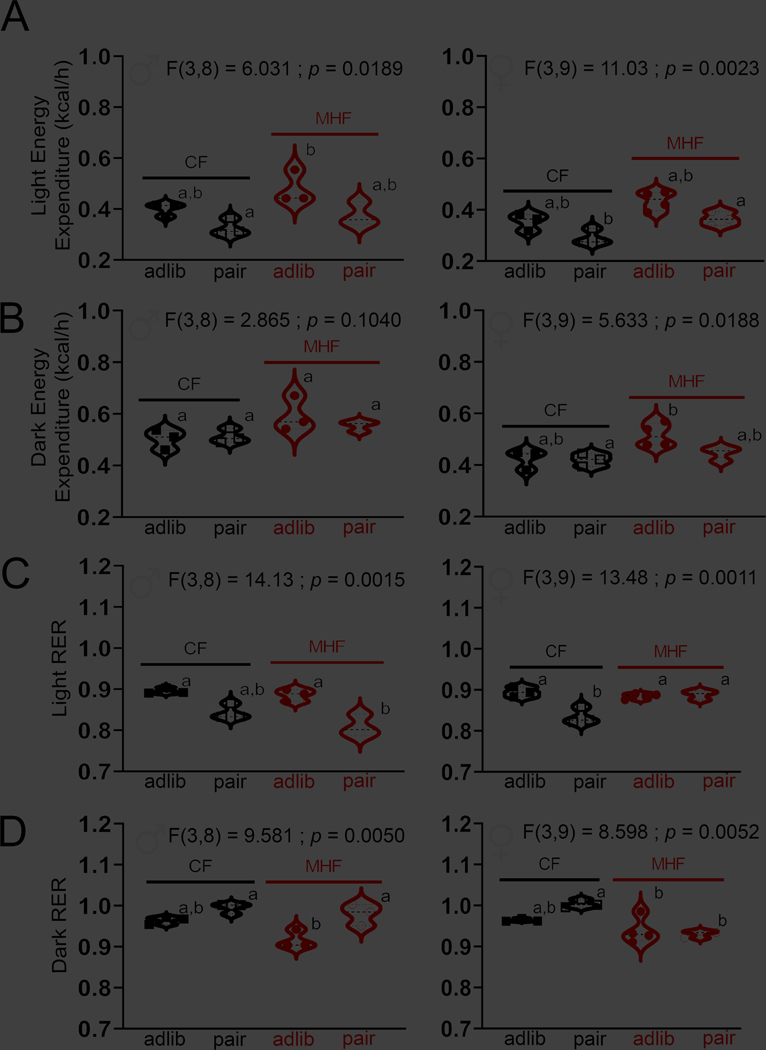
Differences in energy expenditure (EE) and respiratory exchange ratio (RER) between mice with ad libitum vs. a pair fed eating regime
Despite there being sex-specific differences in sensitivity to DIO in the mice, males and females responded similarly with respect to changes in energy expenditure (EE) and fuel utilization (respiratory exchange ratio, RER) when comparing these metrics across an ad libitum vs. a pair fed eating regime. Both male and female mice exhibited a significant difference in EE during the light cycle (Fig. 6A, 1-way ANOVA w/ Bonferroni’s post-hoc test). Mice maintained on ad libitum MHF diet had an elevated EE over that of CF pair-fed mice, independent of sex. During the dark cycle (Fig. 6B, 1-way ANOVA w/ Bonferroni’s post-hoc test), this effect was less pronounced, and did not reach significance for males. Fuel utilization, or RER, was significantly different across dietary treatments in both male and female mice, and across both light and dark cycles (Fig. 6C, D, 1-way ANOVA w/ Bonferroni’s post-hoc test). The most interesting of the post-hoc tests indicated that in females, pair-fed mice had a decreased RER during the dark cycle when consuming MHF (Fig. 6D; right) and an increase in RER when consuming MHF in the light cycle (Fig. 6C; right). In the male mice, post-hoc tests indicated that MHF-fed mice were significantly different when comparing ad libitum vs. pair fed treatments (Fig. 6C, D; left). MHF pair-fed, male mice increased their RER during the dark cycle (Fig. 6D; left) and then decreased it during the light cycle over that of ad libitum MHF-fed mice (Fig. 6C; left). Pair feeding or dietary treatment did not affect any other measured metabolic variable in the CLAMS including caloric intake, normalized VO2, normalized VCO2, or locomotor activity (non-wheel ambulatory) (Tables 2–3), with the exception of body weight, which was previously noted (Fig. 4, 5). The variable of water intake was computed in the CLAMS and reported in Tables 2–3, however, it is not as meaningful a measurement because the MHF chow contains water to promote pellet formation and thus mice drink less water when on that chow. Despite this fact, we did observe that within the CF treatment groups, in females, pair-fed mice drank significantly less in the light cycle, and in males, regardless of light cycle, the pair-fed mice drank significantly more than their CF adlib counterparts (Tables 2–3, 1-way ANOVA w/ Tukey’s post-hoc test). For all the metabolic data, it is well-known that there is a strong difference in all metrics between the light and dark cycle due to mice being nocturnal animals. An analysis across light/dark cycle within dietary treatment group supported a decrease in all metabolic variables in the light cycle (Tables 2–3, one-tail paired t-test, α = 0.05).
Figure 6. Changes in energy expenditure (EE) and respiratory exchange ratio (RER) are measured following fat and pair-fed diet treatments.
Violin plot of the energy expenditure vs. dietary treatment in male (left) and female (right) M72tauLacZ mice during the A, light and B, dark cycle. C, D Same as (A, B) but for respiratory exchange ratio (RER). Plotted are individual mice, with black dash line (median) and dotted lines (top and lower quartile). Data represent the 12 h mean of three consecutive light or dark cycles following an initial 2-day acclimation period to the CLAMS metabolic chambers. 1-way ANOVA, letters indicate significantly different Bonferroni’s post-hoc test corrected for multiple comparisons, p < 0.05.
Table 2.
Metabolic properties in male mice in response to dietary treatment.
| Metabolic Property | CF adlib | CF pair | MHF adlib | MHF pair | p value |
|---|---|---|---|---|---|
|
| |||||
| MALES | |||||
| Bodyweight (g) | 30.4 ± 1.4 (3) | 27.6 ± 1.8 (3) | 40.1 ± 11.1 (3) | 31.0 ± 3.1 (3) | 0.0745 |
| Water intake (g) | |||||
| 12 h dark | 4.4 ± 0.7 (3) [a] | 6.5 ± 0.8 (3) [b] | 2.7 ± 0.8 (3) [a] | 4.0 ± 0.7 (3) [a] | 0.0014 |
| 12 h light | 1.2 ± 0.1 (3) [a]* | 2.2 ± 0.1 (3) [b]** | 1.2 ± 0.2 (3) [a]* | 0.6 ± 0.1 (3) [c]** | <0.0001 |
| Caloric intake (kcal) | |||||
| 12 h dark | 15.7 ± 1.0 (3) | 15.0 ± 0.9 (3) | 28.6 ± 0.9 (3) | 18.7 ± 10.5 (3) | 0.7549 |
| 12 h light | 6.1 ± 2.4 (3)* | 1.6 ± 0.3 (3)*** | 10.4 ± 6.9 (3) | 5.2 (2) | 0.1844 |
| Energy expenditure (kcal/h) | |||||
| 12 h dark | 0.50 ± 0.04 (3) | 0.51 ± 0.03 (3) | 0.59 ± 0.07 (3) | 0.55 ± 0.02 (3) | 0.1040 |
| 12 h light | 0.40 ± 0.03 (3) [a,b]** | 0.33 ± 0.03 (3) [a]* | 0.48 ± 0.06 (3) [a]** | 0.38 ± 0.05 (3) [b]** | 0.0108 |
| Respiratory Exchange Ratio | |||||
| 12 h dark | 0.97 ± 0.01 (3) [a,b] | 0.98 ± 0.01 (3) [a] | 0.92 ± 0.02 (3) [b] | 0.98 ± 0.03 (3) [a] | 0.0205 |
| 12 h light | 0.90 ± 0.01 (3) [a]** | 0.84 ± 0.02 (3) [a,b]** | 0.89 ± 0.01 (3) [a]* | 0.81 ± 0.03 (3) [b]* | 0.0039 |
| Normalized VO2 (mL/kg/h) | |||||
| 12 h dark | 3347 ± 170 (3) | 3693 ± 170 (3) | 3078 ± 472 (3) | 3573 ± 251 (3) | 0.1507 |
| 12 h light | 2708 ± 78 (3)** | 2424 ± 118 (3)* | 2499 ± 385 (3)** | 2521 ± 278 (3)** | 0.4651 |
| Locomotor activity (beam breaks) | |||||
| 12 h dark | 36895 ± 11833 (3) | 47602 ± 11139 (3) | 48195 ± 18849 (3) | 64500 ± 15020 (3) | 0.2178 |
| 12 h light | 10956 ± 2645 (3)* | 16533 ± 7465 (3)* | 18110 ± 8102 (3)* | 13818 ± 2450 (3)* | 0.4894 |
Mean ± SD (sample size); Dietary Factor = 1-way ANOVA with Bonferroni post-hoc test (α ≤ 0.05) [letters].
Cycle Factor = one-tailed paired t-test
p < 0.05
p < 0.01
p < 0.001.
CF = control fed, adlib = ad libitum, MHF = moderately-high fat diet, pair = isocalorically fed to match CF
Table 3.
Metabolic properties in female mice in response to dietary treatment.
| Metabolic Property | CF adlib | CF pair | MHF adlib | MHF pair | p value |
|---|---|---|---|---|---|
|
| |||||
| FEMALES | |||||
| Bodyweight (g) | 24.4 ± 1.3 (3) | 22.7 ± 2.3 (3) | 28.5 ± 3.6 (4) | 23.9 ± 2.3 (3) | 0.0619 |
| Water intake (g) | |||||
| 12 h dark | 4.7 ± 1.2 (3) [a,b] | 5.7 ± 0.6 (3) [a] | 3.7 ± 0.9 (4) [a,b] | 2.7 ± 0.3 (3) [b] | 0.0089 |
| 12 h light | 1.7 ± 0.5 (3) [a]* | 0.2 ± 0.2 (3) [b]** | 1.5 ± 0.2 (4) [a]** | 1.6 ± 0.1 (3) [a]** | 0.0001 |
| Caloric intake (kcal) | |||||
| 12 h dark | 15.2 ± 4.7 (3) | 13.5 ± 3.2 (3) | 18.6 ± 2.8 (4) | 12.8 ± 0.1 (3) | 0.1906 |
| 12 h light | 9.0 ± 8.2 (3) | 0.7 ± 0.1 (3)** | 7.7 ± 3.6 (4)** | 3.0 ± 1.6 (3)** | 0.1380 |
| Energy expenditure (kcal/h) | |||||
| 12 h dark | 0.42 ± 0.04 (3) [a,b] | 0.42 ± 0.02 (3) [a] | 0.52 ± 0.04 (4) [b] | 0.45 ± 0.03 (3) | 0.0188 |
| 12 h light | 0.35 ± 0.03 (3) [a,b]** | 0.29 ± 0.03 (3) [a]* | 0.43 ± 0.04 (4) [a]** | 0.37 ± 0.03 (3) [b]** | 0.0023 |
| Respiratory Exchange Ratio | |||||
| 12 h dark | 0.96 ± 0.01 (3) [a,b] | 1.00 ± 0.01 (3) [a] | 0.94 ± 0.03 (4) [b] | 0.93 ± 0.01 (3) [b] | 0.0052 |
| 12 h light | 0.89 ± 0.01 (3) [a] | 0.84 ± 0.02 (3) [b]** | 0.88 ± 0.01 (4) [a]* | 0.89 ± 0.01 (3) [a]* | 0.0011 |
| Normalized VO2 (mL/kg/h) | |||||
| 12 h dark | 3587 ± 131 (3) | 3690 ± 561 (3) | 3663 ± 193 (4) | 3774 ± 241 (3) | 0.7784 |
| 12 h light | 3045 ± 135 (3)** | 2671 ± 391 (3)* | 3129 ± 226 (4)** | 3127 ± 277 (3)** | 0.2423 |
| Locomotor activity (beam breaks) | |||||
| 12 h dark | 28236 ± 10604 (3) | 40276 ± 8586 (3) | 29657 ± 2926 (4) | 33405 ± 6845 (3) | 0.0745 |
| 12 h light | 13976 ± 4230 (3)* | 17241 ± 1853 (3)* | 10903 ± 2196 (4)** | 11670 ± 7019 (3)*** | 0.3582 |
Mean ± SD (sample size); Dietary Factor = 1-way ANOVA with Bonferroni post-hoc test (α ≤ 0.05) [letters].
Cycle Factor = one-tailed paired t-test
p < 0.05
p < 0.01
p < 0.001.
CF = control fed, adlib = ad libitum, MHF = moderately-high fat diet, pair = isocalorically fed to match CF
Voluntary running does not mitigate the loss of OSNs or correlate axonal projections attributed to consumption of fatty diet
If consumption of fat causes a deleterious loss of axonal projections and associated OSNs, we questioned whether an increase in exercise could mitigate this loss. Voluntary exercise; rather than forced, weighted, or endurance exercise; is known to further increase energy expenditure and lessen general inflammation (Allen et al., 2015; Yoshimura et al., 2018). M72tauLacZ male mice that were treated with MHF ad libitum, but simultaneously allowed access to voluntary running wheels, did not exhibit a retention of Olfr160 axonal projections (Fig. 7A, B; 1-way ANOVA w/ Tukey’s post-hoc test). In fact, post-hoc analyses demonstrated that exercise alone (CF VEx) caused an intermediate loss of axonal projections, which reached statistical significance as shown in Fig. 7C, where the number of Olfr160-expressing OSNs was reduced as a result of exercise only (CF VEx treatment). Body weight remained unchanged in CF mice with access to voluntary running, and in mice treated with MHF diet and access to voluntary running, body weight was intermediate to that of heavier, MHF sedentary mice vs. lighter CF mice (Fig. 7D, E; 2-way RM and 1-way ANOVA w/ Tukey’s post-hoc test, respectively). Similar to that of body weight, the ability to clear glucose and total deposition of adipose tissue was intermediate in mice treated with MHF diet with access to voluntary running compared with that of the MHF sedentary mice vs. both CF mice conditions (Fig. 7F–H; 1-way ANOVA w/ Tukey’s post-hoc test). Despite the deleterious effects of increased body weight, loss of OSNs, increased adiposity, and poorer glucose clearance ability, male mice treated with MHF diet and allowed access to voluntary running engaged in running longer over those maintained on CF diet (Fig. 8). The mice maintained on MHF diet ran approximately twice as many kilometers (CF mice = 5.9 ± 1.7 km vs. MHF mice = 11.2 ± 4.7 km; Student’s t-test), ran at increased velocity (CF mice = 43.1 ± 3.8 rpm vs. MHF mice = 63.4 ± 21.4 rpm; Student’s t-test), and spent more time running in the dark cycle (CF mice = 182.3 ± 48.1 h vs. MHF mice = 236.9 ± 25.3 h; Student’s t-test), compared to that of CF mice.
Figure 7. Voluntary running does not mitigate the loss of Oflr160-axonal projections and expressing OSNs elicited by consumption of a fatty diet.
A, Representative whole-mount images of olfactory bulbs following beta-galactosidase histological staining of Olfr160-expressing olfactory sensory neurons (OSNs) from male M72tauLacZ mice following diet/exercise treatments; images from left to right: control diet ad libitum (brown food symbol), control diet plus running wheel (brown food and wheel symbol), moderately high-fat diet ad libitum (pink food symbol), moderately high-fat diet plus running wheel (pink food and wheel symbol). Projections were converted to pixel density and OSNs manually counted as in Figs. 2/3. o = olfactory bulb, g = glomerulus, and a = Olfr160 axonal projections. Bar graph of B, pixel density of axonal projections and C, number of Olfr160-expressing OSNs vs. diet/exercise treatment; CF Sed = control diet sedentary, CF VEx = control diet voluntary exercise, MHF = moderately high-fat diet sedentary, MHF Vex = moderately high-fat diet voluntary exercise. D, Line graph of body weight vs. age; 2-way RM ANOVA; symbols indicate significantly different for treatment factor as determined by Tukey’s post-hoc (p < 0.05) as follows: # = MHF Sed > both CF groups; ♦ = MHF VEx > both CF groups; * = MHF Sed > MHF VEx. E, Bar graph of body weight vs. diet/exercise treatment at 25 weeks of age. F, Line graph of the plasma glucose concentration and G, bar graph of the iAUC during the IPGTT. H, Bar graph of the total excised adipose tissue vs. diet/exercise treatment. Notations as in Fig. 2. (B, C, E, G, H) 1-way ANOVA, Tukey’s post-hoc, p < 0.05. Sample sizes in number of mice from left to right for whole mount: 14, 9, 12, 9 | for neuronal counts: 11, 8, 10, 8 | for body weight and IPGTT: 14, 9, 12, 9 | and for adipose tissue = 11, 9, 12, 9.
Figure 8. Mice spend more time running for greater distances and with increased velocity when provided a fatty diet.
Bar graph of the mean daily running A, distance, B, velocity, and C, duration in M72tauLacZ male mice sampled from a 28-day continuous interval within the 6-month experimental exercise block. Abbreviations and statistical notations as in Figure 6; analyzed by Student’s t-test for mean (A, B) 24 or (C) 12 h time blocks. Sample sizes in number of mice: CF VEx = 8, MHF VEx = 9.
Discussion
Our combined anatomical and physiological results demonstrate unequivocally that maintaining mice on isocaloric diets induces changes in olfactory circuitry that are dependent upon food content and now cannot be solely attributed to an obesogenic diet. In a sex independent manner, mice that consumed a moderately high-fat (MHF) chow exhibited a loss of olfactory sensory neurons (OSNs) and axonal projections to defined synaptic targets, independent of whether this consumption induced adiposity and body weight gain. Moreover, increased energy expenditure through access to voluntary running failed to mitigate the loss of the neurons and associated circuitry.
A comparison of the maintenance of OSNs in male vs. female mice challenged with different nutritional environments has not been reported previously to our knowledge. Mouse strains are differentially sensitive to obesogenic diets thus the variables of sex and age are important when selecting an appropriate model (Nishikawa et al., 2007). The fact that female C57BL6/J mice strains are resistant to diet-induced obesity (DIO) (Yang et al., 2014) provided us a secondary opportunity to test whether adiposity was necessary to induce a loss of OSNs. Both female mice and pair fed male mice failed to gain significant adipose deposits or fat tissue mass while consuming MHF diets over 5 months, yet still exhibited loss of OSNs and associated axonal projections. It is well known that an imbalance in energy homeostasis causes the development of a higher fat mass, which is associated with a low-grade inflammation (Xu et al., 2003a; Saltiel & Olefsky, 2017; Xu et al., 2021). Low grade inflammation has been reported frequently in conjunction with obese mouse models (Gregor & Hotamisligil, 2011; Kaufman et al., 2018; Duffy et al., 2019). Moreover, adipocyte-derived chemokines, such as TNFα are associated with precipitation of insulin resistance, reduced glucose clearance, and prediabetes (Hotamisligil et al., 1993; Xu et al., 2003a, 2015; Bodzin & Saltiel, 2007; Wensveen et al., 2015). Although we did not observe a significant elevation in interleukins that has been observed with the induction of obesity (Emerson et al., 2017), TNFα was elevated in a sex-independent manner in both ad libitum and pair-fed treatments, inferring the activation of chemokines with consumption of a fatty diet, and not just attributed to adiposity per se. This is consistent with our sex-independent finding of the disruption in glucose clearance in both ad libitum and pair-fed treatments. It is keenly interesting to note that DIO-resistant females retain an inability to clear glucose, an index of reduced metabolic health, despite lack of adipocyte accumulation. Because TNFα mediates apoptotic cell death (Idriss & Naismith, 2000) and its induced expression in the olfactory epithelium results in a significant loss of OSNs (Sultan et al., 2011; Sousa Garcia et al., 2017; Torabi et al., 2020) our measured elevation of this cytokine following both MHF treatments is consistent with the observed concomitant loss of OSNs. It is also conceivable, seeing as adipose tissue is not the only source of inflammation, that our observed increased TNFα is the result of an inflammatory pathway partially or entirely unrelated to adipocytes. In fact, other groups have observed increased TNFα in high fat feeding independent of obesity. For example, Delahaye et al.,2018 used a time-restricted feeding intervention with a 45% fat diet that reduced weight gain and body fat but not circulating TNFα (Delahaye et al., 2018).
Our study used the M72tauLacZ mouse line as a tractable model due to its ability to identify a specific genetic reporter for a single odorant receptor (Olfr160). The results of our study might be restricted to only a subtype of ORs, albeit the design being a helpful tool to visualize the effects of a fat diet on the structure of the olfactory system. Because the mouse olfactory gene family is estimated to have 1000 ORs, we cannot definitively conclude that fat in the diet results in significant loss of all classes of OSNs. In previous works from our laboratory, however, we have demonstrated that DIO causes loss of Gαolf protein and MOR28 (Olfr1507), as well as a reduction in olfactory marker protein positive neurons (mature OSNs) (Thiebaud et al., 2014). These parallel data suggest that it is not unlikely that the results of our current study extend beyond effects on M72-expressing OSNs.
Because isocaloric consumption of MHF diet without associated adiposity caused loss of OSNs, we wondered whether consumption of fat might also cause a reduction in odorant detection or ability. While our pair-fed data do not directly address olfactory ability, we and others have demonstrated that induction of DIO causes a loss in electroolfactogram (EOG) amplitude (Thiebaud et al., 2014; Lacroix et al., 2015; Riviere et al., 2016) signifying reduced olfactory sensitivity. Odor-evoked EOG amplitude is proportional to the number of OSNs whereas the decay time is an indication of odorant adaptation. Mice maintained on excess nutrition exhibit not only loss in olfactory structures, but loss in G-protein-coupled machinery, reductions in odorant receptors, and poorer odor discrimination using habituation/dishabituation trials, conditioned-odor aversion or go, no-go operant conditioning paradigms (Fadool et al., 2011; Tucker et al., 2012a; Aimé et al., 2014; Thiebaud et al., 2014; Lacroix et al., 2015; Fardone et al., 2018). One might therefore conjecture that the loss of OSNs in isocalorically, MHF-treated mice would lead to a loss in EOG amplitude and reduced olfactory ability, however, the type of nutrition may be an important variable. A good example is that of Riviere et al. (2016) who generated DIO in mice using 60% fructose rather than high fat, and discovered reduced EOG amplitude and reduced odorant discrimination, but curiously, an increase in OMP+ mature olfactory sensory neurons (Riviere et al., 2016). Rather than losing OSNs with poor nutrition, the mice in their experiments exhibited reduced apoptosis following the fructose supplementation. While a reduced EOG amplitude seems counterintuitive to an increase in OSNs, the authors hypothesize that the OMP+ mature neurons are aging neurons with reduced transduction properties. Therefore, a change in diet caused a change in OSN cell dynamics (newborn, mature, vs. aging) that is very interesting. Clearly future experiments should test whether pair feeding regimens cause a behavioral or electrical change in olfactory ability concurrent with a loss in olfactory structures or change in cell turnover.
In terms of metabolism, our data demonstrate changes in energy expenditure (EE) and fuel utilization (RER) associated with dietary treatment in both male and female mice. The enhanced EE we measured in MHF ad libitum treated mice would be consistent with a higher fat mass in mice following DIO. However, it is curious that increased adiposity was only observed in male mice and the increased EE was also observed in the female mice that were relatively resistant to obesity. Interestingly, utilization of fuel (RER) in MHF-treated male mice was significantly different across the ad libitum vs. pair-fed treatments. Considering that loss of olfactory structures (axonal projections and OSNs) was congruent between these two conditions, this suggests that olfactory loss is not necessarily always correlated to changes in predominant fuel utilization. When MHF was pair fed, male mice increased the use of carbohydrates for fuel during the dark cycle (RER increased) and relied more on fats during the light cycle (RER decreased), whereas that of MHF ad libitum treated mice had a stable RER regardless of light cycle. While our interpretation cannot directly include olfactory ability (cannot assume loss of OSNs = loss of olfactory ability), we and others have previously shown a relationship between metabolism and olfactory ability (Fadool et al., 2004; Riera et al., 2017). Kv1.3−/− mice are notably super-smellers in terms of olfactory discrimination and threshold, are thin, resistant to DIO (Xu et al., 2003b, 2004; Upadyay et al., 2013; Thiebaud et al., 2014; Riera et al., 2017), and blocking Kv1.3 in the olfactory bulb causes a reduction in RER in the light cycle (Schwartz et al., 2021). Mice with loss of olfactory ability due to genetic ablation of OSNs were found to have an increase in EE and be resistant to DIO (Riera et al., 2017). Pair feeding appears to uncouple these types of metabolic relationships in that loss of OSNs are retained but use of fuels and EE are uncorrelated.
Our data demonstrated that running itself can cause loss of OSNs independent of diet because mice maintained on purely CF diets and allowed access to running wheels had fewer OSNs than that of sedentary mice. Poor health factors such as reduced glucose clearance or increased adiposity were not observed for mice on CF diets, despite the loss of OSNs, suggesting that other factors can contribute to OSN loss that relate to exercise alone. Certainly, exercise inherently increases EE (Alghannam et al., 2021), but we have demonstrated that EE that becomes uncoupled during isocaloric feeding is not correlated to OSN abundance. It is known that different inflammatory signaling pathways are reduced depending upon the type and duration of exercise (Hicks et al., 2016; Yoshimura et al., 2018; Scheffer & Latina, 2020). Inflammation that is reduced with voluntary running may not be sufficient to maintain olfactory neuronal circuits and OSNs in response to a long-term consumption of a hyperlipidemic diet. While general ambulatory locomotor activity (non-wheel based) was not impacted by fat- or pair-feeding (Tables 2–3), running activity itself was significantly enhanced by increased available calories. And while running did improve health metrics of glucose clearance and adiposity in the MHF-treated mice, this was not able to rescue OSN abundance or neuronal circuits.
Exercise regimens have long been prescribed to patients suffering from DIO in attempt to reduce chronic systemic inflammation (Scheffer & Latina, 2020). It was therefore very unexpected that voluntary exercise was not protective against the obesity-induced loss of OSNs. Other studies investigating the protective potential of exercise have seen certain neuroprotective benefits from treadmill exercise and swimming (Li et al., 2017; Lu et al., 2017; Lourenco et al., 2019). It is possible that the mice in our study were not running enough voluntarily to produce these effects. It will be important to investigate other types of exercise outside of that which is entirely voluntary considering the propensity of high-fat diets to reduce spontaneous physical activity (Bjursell et al., 2008; Levine et al., 2008; Schmidt et al., 2012; Friend et al., 2017). Additionally, we know from Borg et al. (2012) that subjecting high-fat diet fed mice to endurance exercise training was not able to prevent an accumulation of lipids in the hypothalamus (Borg et al., 2012). If we surmise that neurodegeneration in olfactory structures is partially brought on by lipotoxicity, it might be logical that voluntary exercise is unable to rescue OSN abundance or associated neuronal projections.
In designing our study, we discovered that little is known about how exercise impacts the olfactory system. In trained detection dogs, a 30-min forced treadmill exercise session reduced their ability to locate a target odor (Angle et al., 2014). In humans, exercise improved inspiratory flow through the nose, but this did not result in improved olfactory detection (Marioni et al., 2010). A 10-year longitudinal study and a separate 1-year study in aged humans revealed that exercise decreased the risk of developing an olfactory impairment (Schubert et al., 2013; Zhang et al., 2020). Results from other studies investigating the effect of exercise specifically on olfactory neurons are scarce and mixed. Brown et al. 2003 showed that voluntary wheel running for ~40 days did not increase neurogenesis in the OB as measured by bromodeoxyuridine (BrdU) labeling of granule cells (Brown et al., 2003). More recent studies however have demonstrated increased neurogenesis in the subventricular zone. One group used treadmill running in a mouse model of depression generated via chronic unpredictable mild stress, preventing dopaminergic neuron loss in the glomerular layer and improving olfactory function (Tian et al., 2020). Other studies have observed rescue effects of wheel running on neural stem cells that were made defective by genetic manipulation or chronic corticosterone infusion (Brown et al., 2003; Mastrorilli et al., 2017). Chae et al. (2014) observed increased BrdU labeling and increased doublecortin (a marker for neuronal progenitors) in the subventricular zone of rats following 8 weeks of swimming exercise (Chae et al., 2014). Conversely in the OE, another group reported decreased mature OSNs (OMP-positive) and increased apoptotic OSNs following wheel running exercise in mice that they attributed to oxidative stress (Tuerdi et al., 2018). It is important to note that different exercise modalities can have different effects on a given parameter. For example, forced exercise and voluntary exercise have been shown to have opposing effects on microbiota and moderate intensity exercise decreases inflammatory cytokines, whereas high-intensity exercise increases them (Allen et al., 2015; Paolucci et al., 2018). In the olfactory system however, different research groups observed different outcomes despite similar wheel running treatments.
Given the lack of olfactory neuroprotection against DIO afforded by voluntary exercise, it will be important to investigate ways in which other diets or dietary compositions can influence sensory neuron development. Future experiments should determine the duration or critical period for exposure to dietary fat as well as recognize other mechanisms beyond inflammation for which dietary fat might trigger poor health. For example, ketogenic diets have been reported to benefit individuals suffering from epilepsy, cancer, neuronal loss, and cognitive deficits (Newman et al., 2017; Roberts et al., 2017; Li et al., 2020), but their high-fat nature may be disruptive to sensory neurons and hence satiety signals. It is important to recognize that the full macronutrient breakdown of the two diets in our study is as follows: MHF = 32% fat, 51% carbohydrate, and 17% protein; CF = 13.5% fat, 58% carbohydrate, and 28.5% protein. Therefore, an alternative interpretation is that the MHF diet could be a low protein diet and not only a moderately high-fat diet. Low protein diets have been shown to slow down cancer progression, increase longevity, and mitigate kidney disease (Roberts et al., 2017; Rhee et al., 2018; Rubio-Patino et al., 2018). It seems unlikely that the lower protein content could trigger inflammation or lead to neuronal loss in the olfactory system, but it is a possibility that should be explored. Also, the CF and MHF diets contain approximately the same amounts of saturated fat vs unsaturated, but the unsaturated fat sources are notably different. The MHF diet contains much higher levels of ω−6 fatty acids, which are associated with low-grade inflammation, and lower amounts of the ω−3 fatty acids, which have been demonstrated as anti-inflammatory (Delpech et al., 2015; Layé et al., 2018). Finally, the duration of dietary challenge is a variable that should be explored. Monteni et al.,(2002) discovered both a reduction in hippocampal plasticity and a reduced performance on the Morris Water Maze (a standard test for spatial learning and memory) following only two months on a high-fat, refined-sugar diet (Monteni et al., 2002). Another group observed impaired memory and increased anxiety-like behaviors in mice following only 1 week on a high-fat diet (Kaczmarczyk et al., 2013). It is possible that the neuronal loss observed in the olfactory system occurs much sooner than the termination points used in our pair-feeding experiments and in Thiebaud et al. (2014). Finally, fat in the diet can have deleterious effects outside of adipocyte-linked inflammation that can be investigated for effects on OSN development. For example, high-fat diets increase intestinal permeability which leads to increased levels of circulating lipopolysaccharide (LPS, a component of Gram-negative bacteria that live in the gut; an inflammatory endotoxin) (Moreira et al., 2012). Fatty diets can also increase the levels of circulating free fatty acids, which have been shown to induce inflammation through toll-like receptors (Eguchi & Nagai, 2017).
To recapitulate, our study demonstrates that a long-term macronutrient imbalance in dietary fat can cause a loss of OSNs and associated bulbar connections. Maintenance of MHF pair feeding in mice does not elicit increased deposition of adipose tissue but changes cytokine production and the animal’s ability to clear glucose, rendering the mouse in a less physiologically healthy state. Increased energy expenditure through voluntary running does not mitigate the deleterious effects of the fatty diet – mice still show a loss of OSNs and bulbar projections. Patterns of long-term dietary consumption may play a distinct role in olfactory discrimination and food choice attributed to reshaping neuronal abundance and communication in the olfactory system.
Key Points.
Obesity can disrupt the structure and function of organ systems, including the olfactory system that is important for food selection and satiety.
We designed dietary treatments in mice such that mice received fat, but the total calories provided were the same as in control diets so that they would not gain weight or increase adipose tissue.
Mice that were not obese but consumed isocaloric fatty diets still lost olfactory neuronal circuits, had fewer numbers of olfactory neurons, had an elevation in inflammatory signals, and an intermediate ability to clear glucose (prediabetes).
Mice were allowed access to running wheels while consuming fatty diets, yet still lost olfactory structures.
We conclude that a long-term imbalance in nutrition that favors fat in the diet disrupts the olfactory system of mice in the absence of obesity.
Acknowledgements:
We would like to thank Jessica Folsom, Henal Sutaria, and Nimmi Patel for their technical assistance in maintaining running wheels, measuring body weights and collection of adipose tissues. We would like to thank Jaime White-James for the oversight of our animal husbandry and routine mouse care needs. We would like to thank Charles Badland, our departmental artist, for assistance with schematics. Cover art was designed by Charles Badland, Carley Huffstetler, and Ashley Loeven; created with BioRender.
Funding:
This work was supported by National Institutes of Health (NIH) grants R01DC013080, T32DC000044, and F31DC016817 from the National Institutes of Deafness and Communication Disorders (NIDCD); and an endowment from the Robinson Family and the Tallahassee Memorial Hospital.
Footnotes
Data Availability Statement: All data referenced in this report are summarized in the primary figures contained in the publication. All photomicrographs used to generate the summarized data are stored in the Fadool Laboratory and are available upon request.
Competing Interests: The authors declare no financial or scientific conflicts of interest.
Literature Cited
- Aimé P, Palouzier-Paulignan B, Salem R, Al Koborssy D, Garcia S, Duchamp C, Romestaing C & Julliard AK (2014). Modulation of olfactory sensitivity and glucose-sensing by the feeding state in obese Zucker rats. Front Behav Neurosci 8, 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alghannam AF, Ghaith MM & Alhussain MH (2021). Regulation of energy substrate metabolism in endurance exercise. Int J Env Res Pub Heal 18, 4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JM, Berg Miller ME, Pence BD, Whitlock K, Nehra V, Gaskins HR, White BA, Fryer JD & Woods JA (2015). Voluntary and forced exercise differentially alters the gut microbiome in C57BL/6J mice. J Appl Physiol 118, 1059–1066. [DOI] [PubMed] [Google Scholar]
- Angle CT, Wakshlag JJ, Gillette RL, Steury T, Haney P, Barrett J & Fisher T (2014). The effects of exercise and diet on olfactory capability in detection dogs. J Nutr Sci 3, e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biju KC, Marks DR, Mast TG & Fadool DA (2008). Deletion of voltage-gated channel affects glomerular refinement and odorant receptor expression in the mouse olfactory system. J Comp Neurol 506, 161–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjursell M, Gerdin AK, Lelliott CJ, Egecioglu E, Elmgren A, Tornell J, Oscarsson J & Bohlooly YM (2008). Acutely reduced locomotor activity is a major contributor to Western diet-induced obesity in mice. Endocrin Metab 294, E251–E260. [DOI] [PubMed] [Google Scholar]
- Bodzin CN & Saltiel AR (2007). Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117, 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg ML, Omran SF, Weir J, Meikle PJ & Watt MJ (2012). Consumption of a high-fat diet, but not regular endurance exercise training, regulates hypothalamic lipid accumulation in mice. J Physiol 590, 4377–4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J, Cooper-Kuhn CM, Kempermann G, Van Praag H, Winkler J, Gage FH & Kuhn HG (2003). Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci 17, 2042–2046. [DOI] [PubMed] [Google Scholar]
- Chae CH, Jung SL, An SH, Park BY, Kim TW, Wang SW, Kim JH, Lee HC & Kim HT (2014). Swimming exercise stimulates neurogenesis in the subventricular zone via increase in synapsin I and nerve growth factor levels. Biol Sport 31, 309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelette BM, Thomas AM & Fadool DA (2019). Long-term obesogenic diet and targeted deletion of potassium channel Kv 1.3 have differing effects on voluntary exercise in mice. Physiol Rep 7, e14254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delahaye LB, Bloomer RJ, Butawan MB, Wyman JM, Lee HW, Liu AC, McAllan L, Han JC & van der Merwe M (2018). Time-restricted feeding of a high-fat diet in male C57BL/6 mice reduces adiposity but does not protect against increased systemic inflammation. Appl Physiol Nutr Metab 43, 1033–1042. [DOI] [PubMed] [Google Scholar]
- Delpech JC, Madore C, Joffre C, Aubert A, Kang JX, Nadjar A & Laye S (2015). Transgenic increase in n-3/n-6 fatty acid ratio protects against cognitive deficits induced by an immune challenge through decrease of neuroinflammation. Neuropsychopharm 40, 525–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy CM, Hofmeister JJ, Nixon JP & Butterick TA (2019). High fat diet increases cognitive decline and neuroinflammation in a model of orexin loss. Neurobiol Learn Mem 157, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi K & Nagai R (2017). Islet inflammation in type 2 diabetes and physiology. J Clin Invest 127, 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson SR, Kurti SP, Harms CA, Haub MD, Melgarejo T, Logan C & Rosenkranz SK (2017). Magnitude and timing of the postprandial inflammatory response to a high-fat meal in healthy adults: A systematic review. Adv Nutr 8, 213–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadool DA, Tucker K & Pedarzani P (2011). Mitral cells of the olfactory bulb perform metabolic sensing and are disrupted by obesity at the level of the Kv1.3 ion channel. PLoS One 6, e24921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadool DA, Tucker K, Perkins R, Fasciani G, Thompson RN, Parsons AD, Overton JM, Koni PA, Flavell RA & Kaczmarek LK (2004). Kv1.3 channel gene-targeted deletion produces “Super-Smeller Mice” with altered glomeruli, interacting scaffolding proteins, and biophysics. Neuron 41, 389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fardone E, Celen AB, Schreiter NA, Thiebaud N, Cooper ML & Fadool DA (2018). Loss of odor-induced c-Fos expression of juxtaglomerular activity following maintenance of mice on fatty diets. J Bioenerg Biomembr 51, 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend DM, Devarakonda K, O’Neal TJ, Skirzewski M, Papazoglou I, Kaplan AR, Liow JS, Guo J, Rane SG, Rubinstein M, Alvarez VA, Hall KD & Kravitz AV (2017). Basal ganglia dysfunction contributes to physical inactivity in obesity. Cell Metab 25, 312–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor MF & Hotamisligil GS (2011). Inflammatory mechanisms in obesity. Annu Rev Immunol 29, 445. [DOI] [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology. J Physiol 593, 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks JA, Hatzidis A, Arruda NL, Gelineau RR, De Pina IM, Adams KW & Seggio JA (2016). Voluntary wheel-running attenuates insulin and weight gain and affects anxiety-like behaviors in C57BL6/J mice exposed to a high-fat diet. Beh Brain Res 310, 10. [DOI] [PubMed] [Google Scholar]
- Hotamisligil G (2006). Inflammation and metabolic disorders. Nature 444, 860–867. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS & Spiegelman BM (1993). Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259, 87–91. [DOI] [PubMed] [Google Scholar]
- Idriss HT & Naismith JH (2000). TNF alpha and the TNF receptor superfamily: structure-function relationship(s). Microsc Res Tech 50, 184–195. [DOI] [PubMed] [Google Scholar]
- Kaczmarczyk MM, Machaj AS, Chiu GS, Lawson MA, Gainey SJ, York JM, Meling DD, Martin SA, Kwakwa KA, Newman AF, Woods JA, Kelley KW, Wang Y, Miller MJ & Freund GG (2013). Methylphenidate prevents high-fat diet (HFD)-induced learning/memory impairment in juvenile mice. . Psychoneuroendocrin 38, 1553–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman A, Choo E, Koh A & Dando R (2018). Inflammation arising from obesity reduces taste bud abundance and inhibits renewal. PLoS Biol 16, e2001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolling LJ & Fadool DA (2020). Role of olfaction for eating behavior. In The Senses: A Comprehensive Reference ,, ed. Fritzsch B& Meyerhof W, pp. 675–716. Elsevier, Academic Press. [Google Scholar]
- Kovach CP, Al KD, Huang Z, Chelette BM, Fadool JM & Fadool DA (2016). Mitochondrial ultrastructure and glucose signaling pathways attributed to the Kv1.3 ion channel. Front Physiol 7, 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix MC, Caillol M, Durieux D, Monnerie R, Grebert D, Pellerin L, Repond C, Tolle V, Zizzari P & Baly C (2015). Long-lasting metabolic imbalance related to obesity alters olfactory tissue homeostasis and impairs olfactory-driven behaviors. Chem Senses 40, 537–556. [DOI] [PubMed] [Google Scholar]
- Layé S, Nadjar A, Joffre C & Bazinet RP (2018). Anti-inflammatory effects of omega-3 fatty acids in the brain: physiological mechanisms and relevance to pharmacology. Pharm Rev 70, 12–38. [DOI] [PubMed] [Google Scholar]
- Levine JA, McCrady SK, Lanningham-Foster LM, Kane PH, Foster RC & Manohar CU (2008). The role of free-living daily walking in human weight gain and obesity. Diabetes 57, 548–554. [DOI] [PubMed] [Google Scholar]
- Li DJ, Li YH, Yuan HB, Qu LF & Wang P (2017). The novel exercise-induced hormone irisin protects against neuronal injury via activation of the Akt and ERK1/2 signaling pathways and contributes to the neuroprotection of physical exercise in cerebral ischemia. Metab Clin Exp 68, 42. [DOI] [PubMed] [Google Scholar]
- Li RJ, Liu Y, Liu HQ & Li J (2020). Ketogenic diets and protective mechanisms in epilepsy, metabolic disorders, cancer, neuronal loss, and muscle and nerve degeneration. J Food Biochem 44, e13140. [DOI] [PubMed] [Google Scholar]
- Lourenco MV et al. (2019). Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat Med 25, 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Dong Y, Tucker D, Wang R, Ahmed ME, Brann D & Zhang Q (2017). Treadmill exercise exerts neuroprotection and regulates microglial polarization and oxidative stress in a streptozotocin-induced rat model of sporadic Alzheimer’s Disease. J Alzh Dis 56, 1469–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusk G & Du Bois EF (1924). On the constancy of the basal metabolism. J Physiol 59, 213–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marioni G, Ottaviano G, Staffieri A, Zaccaria M, Lund VJ, Tognazza E, Coles S, Pavan P, Brugin E & Ermalao A (2010). Nasal functional modifications after physical exercise: olfactory threshold and peak nasal inspiratory flow. Rhinology 48, 277–280. [DOI] [PubMed] [Google Scholar]
- Mastrorilli V, Scopa C, Saraulli D, Costanzi M, Scardigli R, Rouault JP, Farioli-Vecchioli S & Tirone F (2017). Physical exercise rescues defective neural stem cells and neurogenesis in the adult subventricular zone of Btg1 knockout mice. Brain Struct Funct 222, 2855–2876. [DOI] [PubMed] [Google Scholar]
- Monteni R, Barnard RJ, Ying Z, Roberts CK & Gomez-Pinilla F (2002). A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neurosci 112, 803–814. [DOI] [PubMed] [Google Scholar]
- Moreira AP, Texeira TF, Ferreira AB, Peluzio Mdo C & Alfenas Rde C (2012). Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br J Nutr 108, 801–809. [DOI] [PubMed] [Google Scholar]
- Newman JC, Covarrubias AJ, Zhao M, Yu X, Gut P, Ng CP, Huang Y, Haldar S & Verdin E (2017). Ketogenic diet reduces midlife mortality and improves memory in aging mice. Cell Metab 26, 547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa S, Yasoshima A, Doi K, Nakayama H & Uetsuka K (2007). Involvement of sex, strain and age factors in high fat diet-induced obesity in C57BL/6J and BALB/cA mice. Exp Anim 56, 263–272. [DOI] [PubMed] [Google Scholar]
- Palouzier-Paulignan B, Lacroix M, Aimé P, Baly C, Caillol M, Congar P, Julliard A, Tucker K & Fadool D (2012). Olfaction under metabolic influences. Chem Senses 37, 769–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolucci EM, Loukov D, Bowdish D & Heisz JJ (2018). Exercise reduces depression and inflammation but intensity matters. Biol Psychol 133, 84. [DOI] [PubMed] [Google Scholar]
- Rhee CM, Ahmadi SF, Kovesdy CP & Kalantar-Zadeh K (2018). Low-protein diet for conservative management of chronic kidney disease: a systematic review and meta-analysis of controlled trials. J Cach Sarco Mus 9, 235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riera CE, Tsaousidou E, Halloran J, Follett P, Hahn O, Pereira MMA, Ruud LE, Alber J, Tharp K, Anderson CM, Bronneke H, Hampel B, Filho CDM, Stahl A, Bruning JC & Dillin A (2017). The Sense of Smell Impacts Metabolic Health and Obesity. Cell Metab 26, 198–211. [DOI] [PubMed] [Google Scholar]
- Riviere S, Soubeyre V, Jarriault D, Molinas A, Leger-Charnay E, Desmoulins L, Grebert D, Meunier N & Grosmaitre X (2016). High Fructose Diet inducing diabetes rapidly impacts olfactory epithelium and behavior in mice. Sci Rep 6, 34011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MN, Wallace MA, Tomilov AA, Zhou Z, Marcotte GR, Tran D, Perez G, Gutierrez-Casado E, Koike S, Knotts TA, Imai DM, Griffey SM, Kim K, Hagopian K, Haj FG, Baar K, Cortopassi GA, Ramsey JJ & Lopez-Dominguez JA (2017). A ketogenic diet extends longevity and healthspan in adult mice. Cell Metab 26, 539–546.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Patino C et al. (2018). Low-protein diet induces IRE1α-dependent anticancer immunosurveillance. Cell Metab 27, 828–842. [DOI] [PubMed] [Google Scholar]
- Saltiel AR & Olefsky JM (2017). Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest 127, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer D & Latina A (2020). Exercise-induced immune system response: Anti-inflammatory status on peripheral and central organs. Biochm Biophys Acta 1866, 165823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I & Frise E (2012). Fiji: An open-source platform for biological-image analysis. Nat Methods 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt SL, Harmon KA, Sharp TA, Kealey EH & Bessesen DH (2012). The effects of overfeeding on spontaneous physical activity in obesity prone and obesity resistant humans. Obesity 20, 2186–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert CR, Cruickshanks KJ, Nondahl DM, Klein BE, Klein R & Fischer ME (2013). Association of exercise with lower long-term risk of olfactory impairment in older adults. . JAMA Otolaryngol -- Head Neck Surg 139, 1061–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz AB, Kapur A, Huang Z, Anangi R, Spear JM, Stagg S, Fardone E, Dekan Z, Rosenberg JT, Grant SC, King GF, Mattoussi H & Fadool DA (2021). Olfactory bulb-targeted quantum dot (QD) bioconjugate and Kv1.3 blocking peptide improve metabolic health in obese male mice. J Neurochem 157, 1876–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J & Scott-Johnson P (2002). The electroolfactogram: a review of its history and uses. Microsc Res Tech 58, 152–160. [DOI] [PubMed] [Google Scholar]
- Sousa Garcia D, Chen M, Smith AK, Lazarini PR & Lane AP (2017). Role of the type I tumor necrosis factor receptor in inflammation-associated olfactory dysfunction. Intern Forum Allergy Rhino 7, 160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan B, May LA & Lane AP (2011). The role of TNF-α in inflammatory olfactory loss. Laryngoscope 121, 2481–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaud N, Johnson MC, Butler JL, Bell GA, Ferguson KL, Fadool AR, Fadool JC, Gale AM, Gale DS & Fadool DA (2014). Hyperlipidemic diet causes loss of olfactory sensory neurons, reduces olfactory discrimination, and disrupts odor-reversal learning. J Neurosci 34, 6970–6984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Dong J & Shi D (2020). Protection of DAergic neurons mediates treadmill running attenuated olfactory deficits and olfactory neurogenesis promotion in depression model. Biochem Biophys Res Comm 521, 725–731. [DOI] [PubMed] [Google Scholar]
- Torabi A, Mohammadbagheri E, Akbari Dilmaghani N, Bayat AH, Fathi M, Vakili K, Alizadeh R, Rezaeimirghaed O, Hajiesmaeili M, Ramezani M, Simani L & Aliaghaei A (2020). Proinflammatory cytokines in the olfactory mucosa result in COVID-19 induced anosmia. . ACS Chem Neurosci 11, 1909–1913. [DOI] [PubMed] [Google Scholar]
- Tucker K, Overton JM & Fadool DA (2008). Kv1.3 gene-targeted deletion alters longevity and reduces adiposity by increasing locomotion and metabolism in melanocortin-4 receptor-null mice. Int J Obes 32, 1222–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker K, Overton JM & Fadool DA (2012a). Diet-induced obesity resistance of Kv1.3−/− mice is olfactory bulb dependent. J Neuroendocr 24, 1087–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker KR, Godbey SJ, Thiebaud N & Fadool DA (2012b). Olfactory ability and object memory in three mouse models of varying body weight, metabolic hormones, and adiposity. Physiol Behav 107, 424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuerdi A, Kikuta S, Kinoshita M, Kamogashira T, Kondo K, Iwasaki S & Yamasoba T (2018). Dorsal-zone-specific reduction of sensory neuron density in the olfactory epithelium following long-term exercise or caloric restriction. Sci Reports 8, 17300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadyay SK, Eckel-Mahan KL, Mirbolooki MR, Tjong I, Griffey SM, Schmunk G, Koehne A, Halbout B, Iadonato S, Pedersen B, Borrelli E, Wang PH, Mukherjee J, Sassone-Corsi P & Chandy KG (2013). Selective Kv1.3 channel blocker as therapeutic for obesity and insulin resistance. Proc Natl Acad Sci 110, E2239–E2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensveen FM, Valentic S, Sestan M, Turk Wensveen T & Polic B (2015). The “Big Bang” in obese fat: Events initiating obesity-induced adipose tissue inflammation. Eur J Immunol 45, 2446–2456. [DOI] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA & Chen H (2003a). Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112, 1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Koni PA, Wang P, Li G, Kaczmarek LK, Wu Y, Li Y, Flavell RA & Desir GV (2003b). The voltage-gated potassium channel Kv1.3 regulates energy homeostasis and body weight. Hum Mol Genet 12, 551–559. [DOI] [PubMed] [Google Scholar]
- Xu J, Wang P, Li Y, Li G, Kaczmarek LK, Wu Y, Koni PA, Flavell RA & Desir GV (2004). The voltage-gated potassium channel Kv1.3 regulates peripheral insulin sensitivity. Proc Natl Acad Sci 101, 3112–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Ki D, Zhu Y, Cai S, Liang X, Tang Y, Jin S & Ding C (2021). Swertiamarin supplementation prevents obesity-related chronic inflammation and insulin resistance in mice fed a high-fat diet. Adipocyte 10, 160–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Kitade H, Ni Y & Ota T (2015). Roles of chemokines and chemokine receptors in obesity-associated insulin resistance and nonalcoholic fatty liver disease. Biomolecules 5, 1563– 1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Smith DL, Keating KD, Allison DB & Nagy TR (2014). Variations in body weight, food intake and body composition after long-term high-fat diet feeding in C57BL/6J mice. Obesity 22, 2147–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura S, Nakashima S, Tomiga Y, Kawakami S, Uehara Y & Higaki Y (2018). Short- and long-term effects of high-fat diet feeding and voluntary exercise on hepatic lipid metabolism in mice. Biochem Biophys Res Comm 507, 291–296. [DOI] [PubMed] [Google Scholar]
- Zapiec B & Mombaerts P (2015). Multiplex assessment of the positions of odorant receptor-specific glomeruli in the mouse olfactory bulb by serial two-photon tomography. Proc Natl Acad Sci 112, E5873–E5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Li D & Wang X (2020). Role of physical exercise type in olfactory deterioration in ageing. Rhinology 58, 145–150. [DOI] [PubMed] [Google Scholar]
- Zheng C, Feinstein P, Bozza T, Rodriguez I & Mombaerts P (2000). Peripheral olfactory projections are differentially affected in mice deficient in a cyclic nucleotide-gated channel 1144 subunit. Neuron 26, 81–91. [DOI] [PubMed] [Google Scholar]