Summary
Background
COVID-19 pandemic research efforts have focused on disease phenotypes in adults. A distinct spectrum of illness has been documented in paediatric populations. We aimed to review paediatric intensive care unit (ICU) admissions in Australia, across differing variant predominant phases of the pandemic.
Methods
Data reported to the Short PeRiod IncideNce sTudy of Severe Acute Respiratory Infection (SPRINT-SARI) Australia, across 49 ICUs from February 2020 to June 2022 were extracted. We defined ‘child’ as patients aged <12 years, ‘adolescent’ as patients aged 12–17 years, and ‘young adult’ as patients aged 18–25 years.
Findings
We identified 226 paediatric ICU admissions with COVID-19, representing 3.9% of ICU admissions across the study period. Comorbidity was present in 34.6% of children, 51.4% of adolescents, and 48.7% of young adults. The need for respiratory support was highest in young adults. While 28.3% of patients <18 years required invasive ventilation, in-hospital mortality in paediatric patients was 3.6%. During the Omicron period, there was an increase in the annualised incidence of age-specific COVID-19 ICU admissions per 100,000 population, albeit a decrease in the incidence per 1000 SARS-CoV-2 notifications.
Interpretation
This study demonstrated an appreciable burden of COVID-19 in paediatric patients. Adolescent patients presented phenotypically similar to young adults, however, illness severity was lower in younger cohorts. The Omicron phase of the pandemic demonstrated an increased age-specific population incidence of COVID-19 ICU admissions, albeit a reduced incidence when based on SARS-CoV-2 notifications.
Funding
SPRINT-SARI Australia is supported by the Department of Health, Commonwealth of Australia [Standing Deed SON60002733].
Keywords: SARS-CoV-2, Child, Infant, Adolescent, COVID-19, Intensive care
Research in context.
Evidence before this study
A PubMed literature search was performed on 14 November 2022, utilising search terms ((intensive care [Title/Abstract]) AND (covid-19 [Title/Abstract]) AND (paediatric [Title/Abstract])), to identify studies with a specific focus on critical paediatric SARS-CoV-2 infection requiring admission to the intensive care unit (ICU). No additional limitations were set. This initial search yielded 171 results, including: 29 cohort studies, 35 case reports or case series, 10 cross-sectional studies, 21 observational studies, 20 descriptive studies, 5 qualitative studies, 10 letters, 9 systematic reviews and 6 meta-analyses, 6 guidelines or recommendations, 3 commentary pieces and 2 mixed-methods studies, alongside 38 multicentre, 25 national and 10 multinational studies. While the clinical course and severity of paediatric COVID-19 cases requiring hospital or ICU admission has been described in detail, fewer studies outline the clinical course of patients once admitted to the ICU. Furthermore, identified studies reporting data on paediatric patients admitted to ICU less frequently compared clinical characteristics with young adult cohorts, or compared incidence of ICU admission across different variant predominant periods of the pandemic.
Added value of this study
We reviewed paediatric ICU admissions across a nationwide cohort, comparing severe or critical COVID-19 between paediatric and young adult populations. We reviewed admitted patients across the pandemic to date, and calculated the frequency of admissions throughout each variant predominant phase of the pandemic in Australia. This study had several key findings. Our data demonstrated a burden of disease most pronounced in the <5 years and 12–17 years age groups. We observed characteristics of different paediatric age groups closely, and identified similarities in clinical phenotype of disease in adolescents and young adults with respect to natural history of disease and comorbidities. While the Omicron predominant phase of the pandemic saw an increase in the annualised incidence of age-specific COVID-19 ICU admissions, there was an overall decrease per 1000 SARS-CoV-2 notifications, reflective of widespread transmission of COVID-19 alongside a reduction in disease severity associated with this variant of concern.
Implications of all the available evidence
These findings imply an ongoing burden of severe COVID-19 disease in paediatric cohorts, an important consideration as Omicron variants continue to dominate the current phase of the pandemic, and potentially new variants emerge. There has been a considerable reduction in the infection-related incidence of COVID-related ICU admission in all age groups in the Omicron period, even amongst young children who were not eligible for vaccination. The ongoing burden of disease in paediatric patients <5 years may reflect the delayed introduction of vaccination in this cohort in Australia, and should be considered in recommendations regarding further vaccine rollout nationwide. This study supports previous mortality and disease severity findings established internationally, including the need for invasive ventilation in paediatric cohorts, and demonstrates the differences in comorbidities, respiratory support requirements and natural history of disease across different age groups. These findings contribute to clinicians’ understanding of risk factors, clinical severity and disease course across the age strata in paediatric populations with severe COVID-19 disease.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic continues to contribute significant morbidity and mortality, with in excess of 500 million cases reported worldwide as of the 25th of July 2022.1 While research efforts have been predominantly focused on the phenotype of disease in adult cohorts, a distinct and generally milder spectrum of illness has been consistently documented in paediatric populations across each of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants of concern.2,3
The presentation of SARS-CoV-2 infection in patients aged <18 years old is varied and encompasses respiratory, gastrointestinal, and neurological symptoms.4 Globally, <2% of paediatric patients with a positive PCR for SARS-CoV-2 have been reported to experience severe disease, requiring some degree of respiratory support or admission to the intensive care unit (ICU).5,6 Furthermore, severe post-infectious manifestations of SARS-CoV-2 infection in paediatric patients such as Paediatric Inflammatory Multisystem Syndrome Temporally associated with SARS-CoV-2 (PIMS-TS), may also warrant admission to the ICU, usually presenting 4–6 weeks following a clinically mild acute SARS-CoV-2 infection.7
In Australia, vaccine uptake commenced in August 2021 for patients aged 12–17 years, and January 2022 for patients aged 5–11 years.8,9 Vaccination uptake in paediatric populations has been variable, demonstrating high and rapid uptake in adolescents, with >90% of patients aged 16–17 years and >80% of patients aged 12–15 years having two primary vaccine doses. Lower coverage has been achieved in patients aged 5–11 years (40%), and no COVID-19 vaccine has been routinely available in the under 5 age group to date.10 The low coverage in the 5–11 years cohort may in part have been driven by a lack of paediatric participants involved in early clinical trials, a recognition of generally mild COVID-19 disease in young children, alongside concern for the possible associated risks of vaccination in this population, such as myocarditis.11,12 There is growing recognition of the impact of immunisation in mitigating the severe disease burden in the paediatric age group.13 BNT162b2 vaccination has demonstrated a reduction in the risk of Omicron-associated hospitalisation by two thirds in patients between the ages of 5 and 11, with a vaccine effectiveness of >90% extending to 44 weeks after full vaccination in patients aged 12–18 years during the Delta phase of the pandemic.14 In addition, in August 2022, the Australian Technical Advisory Group on Immunisation (ATAGI) recommended COVID-19 vaccination in children aged 6 months to 5 years who are deemed medically high risk.15
We aimed to review ICU admissions in paediatric patients in Australia across the pandemic to date, and to compare medical risk factors and clinical care between <12 years, 12–17 years, and 18–25 years cohorts. We report our findings to inform care of paediatric patients who become critically unwell with COVID-19 in Australia.
Methods
Study design and patients
The Short PeRiod IncideNce sTudy of Severe Acute Respiratory Infection (SPRINT-SARI) Australia, supported by the Australian and New Zealand Intensive Care Society (ANZICS) Clinical Trials Group, is a multicentre, prospective, observational cohort study of patients with COVID-19 admitted to adult and paediatric ICUs within Australia. The study is coordinated by the Australian and New Zealand Intensive Care Research Centre (ANZIC-RC) at Monash University.
Consecutive patients ≤25 years admitted to participating Australian ICUs from 27 February 2020 to 27 June 2022, with polymerase chain reaction (PCR) confirmed SARS-CoV-2 infection, were eligible to be included. PCR-negative patients, and patients whose results were not available by the end of the study period, were excluded. For the purposes of comparison, patients were stratified by age group at the time of admission.
Data collection
Data were extracted on the 21st of June 2022 from the SPRINT-SARI database, with a total of 49 participating sites contributing data during the study period. Collected data from both electronic and paper medical records were entered into a database (REDCap) and all patients de-identified. The database was maintained and all analyses performed by the ANZIC-RC. Protocol standardisation and data quality assessment for the database has been previously described.16
Clinical characteristics and demographics were collected. Daily data on investigations, ICU treatments, and interventions were collected until day 28 of each patient's ICU admission. The Acute Physiology and Chronic Health Evaluation (APACHE-II) score17 was calculated within 24 h of admission to the ICU. Paediatric critical illness severity scores such as Paediatric Index of Mortality (PIM) or Paediatric RIsk of Mortality (PRISM), were not routinely collected. Data were only collected from confirmed PCR-positive patients to reduce bias.
Definitions
The term paediatric refers to all patients aged <18 years. For the purposes of comparison, we defined ‘child’ as patients aged <12 years, ‘adolescent’ as patients aged 12–17 years, and ‘young adult’ as patients aged 18–25 years. The division of age groups is based on vaccine eligible cohorts, which have been used in other studies.14
We defined the Paediatric Inflammatory Multisystem Syndrome Temporally associated with SARS-CoV-2 (PIMS-TS) as patients ≤18 years of age, with fever ≥3 days, alongside a previous PCR confirmed SARS-CoV-2 infection. The PIMS-TS is termed Multisystem Inflammatory Syndrome in Children associated with COVID-19 (MIS-C) by the United States Centers for Disease Control and World Health Organisation.7,18 In addition, patients were required to have elevated markers of inflammation (eg. Erythrocyte sedimentation rate, C-reactive protein, or procalcitonin), the exclusion of other infectious causes of inflammation such as bacterial sepsis, staphylococcal, or streptococcal toxic shock syndromes, alongside two of the following clinical criteria:
-
•
Rash or bilateral non-purulent conjunctivitis or signs of muco-cutaneous inflammation (oral, hands, or feet)
-
•
Age-specific hypotension or ‘shock’ within 24 h of presentation
-
•
Features of myocardial dysfunction, pericarditis, valvulitis, or coronary abnormalities
-
•
Evidence of coagulopathy
-
•
Acute gastrointestinal symptoms
The pre-Delta predominant phase of the pandemic encompassed all cases from January 1st 2020 to May 31st 2021, the Delta phase from June 1st 2021 to November 30th 2021, and the Omicron era from December 1st 2021 to end June 2022.
Ethics approval
Approval for data collection was granted by the Human Research Ethics Committees of Alfred Health under the National Mutual Acceptance scheme (reference: HREC/16/Alfred/59), or by application to individual sites. Research governance approvals at individual sites were granted as required. The requirement for written consent from individual patients, or their medical treatment decision maker, was waived.
Outcome measures
The primary outcome was in-hospital mortality, referring to death in hospital (either in ICU or on the ward), at any time during the index admission. Secondary outcome measures included: ICU mortality; length of stay in ICU and hospital; need for respiratory support including high flow nasal cannula, non-invasive ventilation and invasive ventilation; any interaction with pre-existing comorbidities; and time from symptom onset to hospital and ICU admission. Illness severity and comorbidities were collected as potential confounders.
Statistical analysis
All data are reported according to their relevant group. Descriptive statistics for all variables were calculated. Continuous variables are reported as medians (with interquartile ranges) and categorical variables as percentages, where appropriate. Continuous variables were compared with Kruskal–Wallis test or Wilcoxon rank-sum test, and categorical variables with Fisher exact test. All analyses were conducted in R v.4.0.3 (R Foundation), and a p < 0.05 was used as statistical significance. Missing data were excluded, without imputation.
Age-specific population and infection-related incidence of SARS-CoV-2 associated ICU admissions in paediatric patients were calculated across three SARS-CoV-2 variant eras in Australia. Age-specific mid-year population denominator data were sourced from the Australian Bureau of Statistics and adjusted for duration of surveillance in months of each variant era. Age-specific SARS-CoV-2 infection notification data to the Commonwealth National Notifiable Disease Surveillance System (NNDSS) were provided by the data custodians.
The reporting of this study adheres to the Strengthening and Reporting of Observational studies in Epidemiology (STROBE) statement.
Role of the funding source
This work and SPRINT-SARI Australia are supported by the Department of Health, Commonwealth of Australia [Standing Deed SON60002733]. The funding source had no involvement in the study design, collection, analysis or interpretation of data, writing of the report, or decision to submit this paper for publication.
Results
Across the COVID-19 pandemic in Australia to the 21st of June 2022, we identified 226 ICU admissions in patients aged <18 years with SARS-CoV-2 infection. These cases represent 3.9% (226/5749) of all ICU admissions reported to SPRINT-SARI across the study period. This contrasts with 191 admissions (3.3% of all ICU admissions) in young adults 18–25 years. Of the 226 paediatric admissions, 156 (69%) were aged <12 years (child) and 70 (31%) were aged 12–17 years (adolescent). Vaccination status varied greatly, reflecting the variation in immunisation recommendations across age groups within Australia, with 13.7% of paediatric patients having received at least one dose of SARS-CoV-2 vaccine, compared with 36.7% in the young adult cohort (p < 0.001).
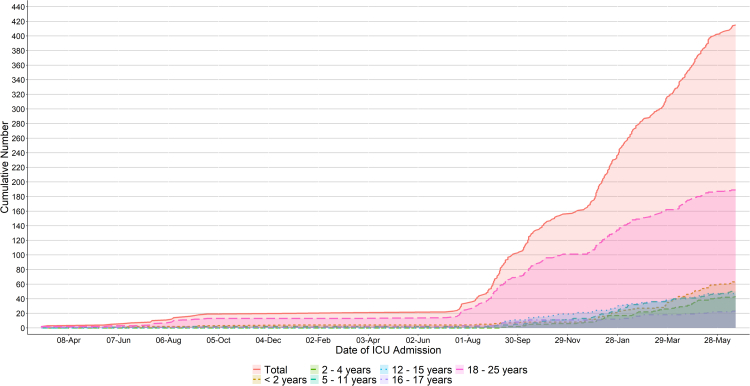
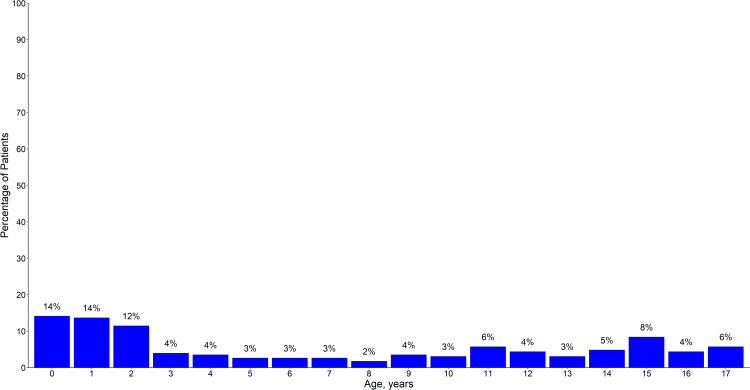
The cumulative number of cases stratified by age group is demonstrated in Fig. 1, with the distribution of ICU admissions by year of age (<18 years) shown in Fig. 2. These data demonstrate a bimodal age distribution, with patients <5 years and 12–17 years contributing the greatest proportion of paediatric cases during the study period (48% and 30% respectively). Table 1 stratifies the age-specific incidence of COVID-19 associated ICU admissions per 100,000 population, and per 1000 SARS-CoV-2 notifications, according to pre-Delta, Delta, and Omicron predominant phases of the pandemic. Comparing Delta and Omicron predominant phases, there was an increase in the age specific population incidence across all cohorts during the Omicron era. These findings were statistically significant in the <2, 2–4, and 5–11 age groups, with incidence rate ratios of 11.8, 6.0 and 5.0 respectively, and less marked or absent in those aged 12 years and over (Supplementary Table S1). In contrast, there were decreases in the age-specific incidence per 1000 SARS-CoV-2 notifications, across all age groups. These findings were statistically significant in all age groups except for those <2 years old, and most marked in those aged ≥12 years, with an IRR of ≤0.05.
Fig. 1.
Cumulative number of paediatric∗ patients admitted to the intensive care unit throughout the study period stratified by age group. Abbreviations: ICU: intensive care unit. ∗ Paediatric is < 18 years.
Fig. 2.
Distribution of paediatric∗ intensive care unit admissions by year of age. ∗ Paediatric is < 18 years.
Table 1.
Population and infection-related incidence of SARS-CoV-2 associated intensive care unit admissions in paediatric and young adult cohortsa, across three SARS-CoV-2 variant eras in Australia.
| Age group (years) | Pre-Delta (Jan 1 2020–May 31 2021) |
Delta (1 Jun 2021–30 Nov 2021) |
Omicron (1 Dec 2021–end June 2022) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| ICU admissions | Age specific annualised population incidence (per 100,000b) | Infection-related incidence (per 1000 SARS-CoV-2 notificationsd) | ICU admissions | Age specific annualised population incidence (per 100,000c) | Infection-related incidence (per 1000 SARS-CoV-2 notificationd) | ICU admissions | Age specific annualised population incidence (per 100,000c) | Infection-related incidence (per 1000 SARS-CoV-2 infection notificationsd) | |
| <2 | 4 | 0.9 (0.2–2.3) | 10.8 (2.9–27.5) | 4 | 2.6 (0.7–6.7) | 0.9 (0.2–2.2) | 55 | 30.9 (23.2–40.2) | 0.47 (0.35–0.61) |
| 2–4 | 0 | 0.0 (0–0.5) | 0.0 (0–7.3) | 5 | 2.1 (0.7–5.0) | 0.6 (0.2–1.4) | 35 | 12.7 (8.9–17.7) | 0.17 (0.12–0.24) |
| 5–11 | 2 | 0.1 (0–0.4) | 1.6 (0.2–5.7) | 7 | 1.2 (0.5–2.5) | 0.3 (0.1–0.6) | 41 | 6.0 (4.3–8.2) | 0.06 (0.04–0.08) |
| 12–15 | 0 | 0.0 (0–0.4) | 0.0 (0–4.1) | 15 | 4.6 (2.5–7.5) | 1.3 (0.8–2.2) | 30 | 7.8 (5.3–11.1) | 0.07 (0.05–0.10) |
| 16–17 | 2 | 0.5 (0.1–1.7) | 3.6 (0.4–12.8) | 9 | 5.9 (2.7–11.2) | 1.7 (0.8–3.2) | 12 | 6.8 (3.5–11.8) | 0.06 (0.03–0.11) |
| 18–25 | 13 | 0.7 (0.4–1.1) | 2.7 (1.4–4.6) | 80 | 12.2 (9.6–15.1) | 2.9 (2.3–3.6) | 96 | 12.5 (10.1–15.3) | 0.10 (0.08–0.12) |
Abbreviations: ICU: intensive care unit; NNDSS: national notifiable disease surveillance system; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
Paediatric is < 18 years and young adult is 18–25 years.
2020 mid-year population estimates from Australian Bureau of Statistics adjusted for duration of surveillance in months.
2021 mid-year population estimates from Australian Bureau of Statistics adjusted for duration of surveillance in months.
Age-specific SARS-CoV-2 infection notification data provided by NNDSS data custodians.
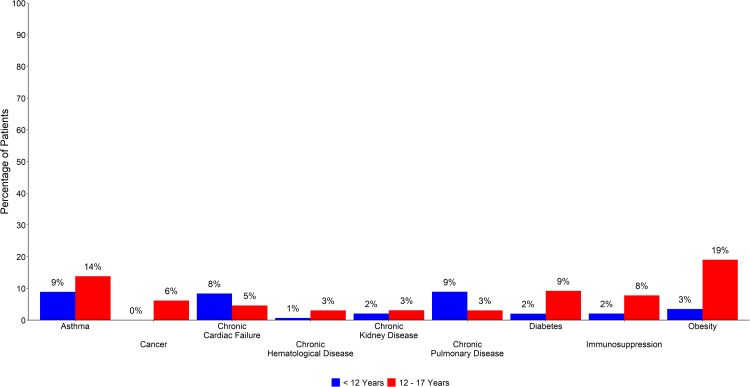
There was no statistically significant sex predominance, with 55.3% of paediatric ICU admissions being male. Medical comorbidity was present in 34.6% of children, 51.4% of those in the adolescent group, and 48.7% of young adults (Supplementary Table S2). Fig. 3 demonstrates the proportion of comorbidities in children and adolescent age groups. The most frequent comorbidities in paediatric patients were asthma (10.4%) and obesity (8.3%), with the proportion of young adults with asthma almost twice that of patients <18 years, and obesity three times as frequent (Supplementary Table S3). Both diabetes and obesity were similarly frequent in adolescents and young adults (9.2% vs 11.2%; and 19.0% vs 24.8% respectively), when compared with children <12 years of age. Patients <12 years of age had the greatest proportion of chronic cardiac failure and chronic pulmonary disease. Smoking was only reported as a risk factor in young adults.
Fig. 3.
Proportion of comorbidities in paediatric∗ patients, stratified by patients <12 years of age (Child), and patients aged 12–17 years (Adolescent). ∗ Paediatric is < 18 years.
The natural history of disease showed significant differences across the age strata. The time from symptom onset to hospital admission or ICU admission were approximately half or less in paediatric patients when compared with young adults. Furthermore, patients <12 years of age experienced the shortest duration of days from symptom onset to hospital admission, followed by adolescents, and young adults [2.0 days (IQR 0.8–4.1) vs 3.7 days (IQR 1.4–7.0) vs 5.3 days (IQR 3.0–7.2), p < 0.001].
With respect to intervention and organ support, children were least often administered COVID-19 directed treatments (Table 2). Remdesivir, tocilizumab, and baracitinib were most commonly utilised in the young adult cohort when compared with adolescents and children, and these findings were statistically significant. Steroids were used in 62.6% of children, 59.4% of adolescents, and 68.4% of young adults. A considerably higher proportion of children and adolescents did not require respiratory support during their ICU stay when compared to young adults (54.5% vs 43.9% vs 22.7%, p < 0.001). Invasive ventilation was required in 28.3% of paediatric patients and 39% of young adults (p < 0.05) (Supplementary Table S4). The use of both non-invasive ventilation and high-flow nasal cannula was also greater in the young adult cohort, and these findings were statistically significant. Inotropic or vasopressor support and the use of renal replacement therapy was similar between paediatric and young adult cohorts.
Table 2.
Interventions and development of complications in the included patients, comparing children (<12 years), adolescents (12–17 years), and young adults (18–25 years).
| <12 years (n = 156) | 12–17 years (n = 70) | 18–25 years (n = 191) | p value | |
|---|---|---|---|---|
| Interventions—no. (%) | ||||
| Drugs | ||||
| Steroids | 82/131 (62.6) | 38/64 (59.4) | 104/152 (68.4) | 0.36 |
| Remdesivir | 2 (1.3) | 4 (5.7) | 20 (10.5) | <0.01 |
| Tocilizumab | 2 (1.3) | 7 (10.0) | 22 (11.5) | <0.001 |
| Baricitinib | 5/126 (4.0) | 6/50 (12.0) | 38/132 (28.8) | <0.001 |
| Neutralising antibodies | 0/9 (0.0) | 4/13 (30.8) | 2/37 (5.4) | 0.04 |
| Organ support | ||||
| High-flow nasal cannula | 35/125 (28.0) | 20/58 (34.5) | 93/153 (60.8) | <0.001 |
| Non-invasive ventilation | 12/124 (9.7) | 11/58 (19.0) | 40/153 (26.1) | <0.01 |
| Invasive ventilation | 36/123 (29.3) | 15/57 (26.3) | 60/154 (39.0) | 0.12 |
| Inotropic or vasopressor | 26/123 (21.1) | 11/57 (19.3) | 36/152 (23.7) | 0.77 |
| Neuromuscular blocking agent | 6/11 (54.5) | 3/15 (20.0) | 8/46 (17.4) | 0.04 |
| Prone positioning | 7/123 (5.7) | 6/58 (10.3) | 51/150 (34.0) | <0.001 |
| Awake prone | 2/122 (1.6) | 5/57 (8.8) | 42/153 (27.5) | <0.001 |
| Invasive prone | 5/123 (4.1) | 2/57 (3.5) | 15/152 (9.9) | 0.11 |
| Renal replacement therapy | 1/123 (0.8) | 1/56 (1.8) | 5/151 (3.3) | 0.35 |
| Tracheostomy | 0/141 (0.0) | 1/66 (1.5) | 4/181 (2.2) | 0.16 |
| ECMO | 1/141 (0.7) | 0/66 (0.0) | 5/182 (2.7) | 0.28 |
| Highest level of respiratory support | <0.001 | |||
| No | 66/121 (54.5) | 25/57 (43.9) | 34/150 (22.7) | |
| NIV or HFNC | 20/121 (16.5) | 17/57 (29.8) | 57/150 (38.0) | |
| Invasive ventilation | 34/121 (28.1) | 15/57 (26.3) | 54/150 (36.0) | |
| ECMO | 1/121 (0.8) | 0/57 (0.0) | 5/150 (3.3) | |
| Development of complications—no. (%) | ||||
| Bacterial pneumonia | 7/133 (5.3) | 2/63 (3.2) | 27/145 (18.6) | <0.001 |
| Bacteraemia | 4/131 (3.1) | 3/62 (4.8) | 10/146 (6.8) | 0.35 |
| Stroke | 1/134 (0.7) | 1/63 (1.6) | 0/146 (0.0) | 0.18 |
| Arrhythmia | 6/134 (4.5) | 0/63 (0.0) | 5/146 (3.4) | 0.24 |
| Barotraumaa | 3/134 (2.2) | 3/64 (4.7) | 7/146 (4.8) | 0.46 |
| Cardiac arrest | 3/134 (2.2) | 1/63 (1.6) | 3/145 (2.1) | 1.0 |
| Pulmonary embolism | 0/134 (0.0) | 3/63 (4.8) | 8/145 (5.5) | <0.01 |
| Deep vein thrombosis | 0/134 (0.0) | 1/62 (1.6) | 1/144 (0.7) | 0.49 |
| Myocarditis | 5/134 (3.7) | 2/62 (3.2) | 3/144 (2.1) | 0.70 |
| PIMS-TS | 14 (9.0) | 5 (7.1) | 0 (0.0) | <0.001 |
Data are median (quartile 25%–quartile 75%) or No. (%). Percentages may not total 100 because of rounding.
Abbreviations: ECMO: extracorporeal membrane oxygenation; HFNC: high flow nasal cannula; NIV: non-invasive ventilation; PIMS-TS: Paediatric Inflammatory Multisystem Syndrome Temporally associated with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2).
Denominators vary due to the presence of missing data.
Defined as pneumothorax or pneumomediastinum or subcutaneous emphysema on chest X-ray or CT chest.
The incidence of complications differed between children, adolescents, and young adult groups (Table 2), with the development of bacterial pneumonia most common in the young adult cohort (5.3% vs 3.2% vs 18.6%, p < 0.001). PIMS-TS was most common in patients <12 years (9% vs 7.1% in the 12–17 years cohort, and 0% in the 18–25 years cohort, p = 0.001) The incidence of pulmonary embolus was more common in young adults (5.5% of patients).
Both hospital and ICU length of stay were shortest in patients <12 years, followed by the 12–17 years and 18–25 years cohorts, and these findings were statistically significant (Table 3). There was no statistically significant difference in mortality between the cohorts. In-hospital mortality was 3.9% in patients <12 years, 3.1% in those 12–17 years, and 2.8% in the young adult group. Overall paediatric in-hospital mortality was 3.6% (Supplementary Table S5).
Table 3.
Clinical outcomes in the included patients, comparing children (<12 years), adolescents (12–17 years), and young adults (18–25 years).
| <12 years (n = 156) | 12–17 years (n = 70) | 18–25 years (n = 191) | p value | |
|---|---|---|---|---|
| Duration of ventilation, days | 3.0 (2.0–6.0) | 2.0 (2.0–3.5) | 4.0 (2.0–8.2) | 0.17 |
| ICU length of stay, days | 1.8 (1.0–3.9) | 2.4 (1.1–3.5) | 3.1 (1.5–5.7) | <0.01 |
| Hospital length of stay, days | 4.7 (2.7–8.1) | 6.0 (3.8–10.9) | 7.9 (5.0–13.3) | <0.001 |
| ICU mortality—no. (%) | 5/140 (3.6) | 2/66 (3.0) | 3/187 (1.6) | 0.52 |
| In-hospital mortality—no. (%) | 5/128 (3.9) | 2/64 (3.1) | 5/181 (2.8) | 0.92 |
Data are median (quartile 25%–quartile 75%) or No. (%). Percentages may not total 100 because of rounding.
Abbreviation: ICU: intensive care unit.
Denominators vary due to the presence of missing data.
Supplementary Tables S6–S8 detail demographic data, interventions and complications, and outcomes according to <2 years, 2–4 years, 5–11 years, and 12–17 years paediatric age groups. Days from symptom onset to ICU admission was shortest in the 2–4 years cohort, followed by <2 years, 5–11 years, and 12–17 years. ICU and hospital mortality was greatest in patients <2 years, however these findings were not statistically significant. The use of invasive ventilation was also most common in this age group when compared with the 2–4, 5–11, and 12–17 years cohorts, however, the majority of patients <2 years old did not require any respiratory support.
Discussion
This nationwide, observational study compared demographics, clinical characteristics, and outcomes of paediatric patients admitted to the ICU with COVID-19 across all states and territories in Australia. Although predominantly mild or asymptomatic SARS-CoV-2 infection has been previously described in these cohorts,19, 20, 21, 22 we found patients aged <18 years made up 226/5749 (3.9%) of all SPRINT-SARI ICU admissions throughout the study period. There was a bimodal age distribution, with the majority of cases represented in those <5 years of age, followed by those aged 12–17 years. The burden of severe SARS-CoV-2 infection in younger age groups has also been examined in other studies, with severe disease seen in infants, who are not routinely eligible for vaccination.23,24
The increase in population incidence of SARS-CoV-2 associated ICU admission in the Omicron era was most pronounced in those aged <5 years, possibly reflecting a greater Omicron burden in this cohort. Importantly, no vaccine was routinely available to this age group throughout the study period. Strikingly, there has been a considerable reduction in COVID-19 ICU admissions per 1000 SARS-CoV-2 notifications in the Omicron era, when compared with the Delta predominant phase, across both paediatric and young adult cohorts. This suggests an important impact of vaccination on severe disease in Australian paediatric populations, especially in those older than 12 years, with high vaccine coverage in this age group. The reduced virulence of the Omicron variant, relative to the Delta variant, is also a likely contributor to this finding.
The burden of COVID-19 in paediatric populations has largely been limited to asymptomatic or mild infection, with a more favourable prognosis when compared with adult populations.25, 26, 27 Our nationwide data demonstrate that, while the burden of severe COVID-19 may be less prominent in paediatric populations, this cohort still contribute an appreciable proportion of ICU admissions. The literature in this area reports <2% of paediatric patients with COVID-19 to have severe disease requiring respiratory support or admission to the intensive care unit.5,6 The need for invasive ventilation in our paediatric population (28.3%) was comparable with other studies (ranging from 23.1 to 52.1%),20,26,28,29 as was the in-hospital mortality rate of 3.6% (compared with a range of 3.8–5.7% in other cohorts of paediatric ICU admissions).26,29,30
Average time from symptom onset to hospital admission has been reported between 5 and 7 days across adult populations,16,31,32 compared with a median of 2.3 days in our paediatric cohort. This may demonstrate a greater need for hospital admission at a time corresponding with the acute viral phase of the natural history of SARS-CoV-2 infection in paediatric populations, rather than the later critical illness seen in SARS-CoV-2 induced acute respiratory distress syndrome resulting from dysregulated immunity and hyper-inflammation.33,34 Symptom onset to hospital and ICU admission also varied across the age strata in our cohort, with patients <12 years old demonstrating a shorter duration when compared with adolescents and young adults. This similar history of disease seen between adolescents and young adults may reflect an ‘adult-like’ phenotype in the adolescent group, albeit less severe when compared with the young adult cohort. In addition, comorbidity data in adolescent patients were similar to young adults, with both a similar number of co-existing disorders, alongside similar rates of diabetes and obesity. This further supports the contentions of a phenotypically similar disease when comparing these groups.
Pre-hospital comorbidities have been consistently reported as factors associated with severe SARS-CoV-2 infection in paediatric populations, with 15.6–83% of paediatric patients reported to have at least one pre-existing condition across various cohorts.20,26,28,30,35,36 This was supported by our dataset, with comorbidities present in 39.8% of paediatric patients, compared with 48.7% of the young adult population. Obesity has been documented as a risk factor for severe COVID-19 in adults across multiple studies,37 and more recently in paediatric populations,38 and was the most common comorbidity in both our adolescent and young adult cohorts. Tsankov et al. report childhood obesity as likely associated with a worsened prognosis of paediatric SARS-CoV-2 infection, with the high visceral adiposity present in obese patients resulting in higher levels of inflammatory cytokines.6 These data support recommendations for optimising COVID-19 vaccine uptake in children and adolescents with risk factors for severe COVID-19 disease, including three primary vaccine doses for those individuals who are severely immunocompromised.39
Disease severity in our young adult population was comparatively more severe when compared with patients aged <18 years, with an increased use of high-flow nasal cannula, non-invasive ventilation, and invasive ventilation in the young adult cohort. This may represent an overall necessity for greater levels of respiratory support in this group. This suggests a higher relative frequency of non-pneumonitis/pneumonia presentations in paediatric groups, reflecting an apparent difference in the spectrum of complications evident in children and adolescents, with an overall milder clinical course. This is supported by additional Australian data,3 alongside national data coverage of young adults in the United States, demonstrating that of 684 young adult patients who required intensive care admission, 331 (48.4%) required mechanical ventilation.40
When comparing our cohort with older adult populations with critical SARS-CoV-2, the need for mechanical ventilation and rate of in-hospital mortality was reduced across both our paediatric and young adult groups.16,32 There are several hypotheses for this difference in severity of COVID-19 disease between age groups. These include differences in basal inflammatory states, endothelial damage and changes in clotting function associated with age, differences in angiotensin converting enzyme 2 receptors, alongside the presence of underlying disease and comorbidity in ageing populations.41,42 Zimmerman et al. propose that protective factors for children include differences in innate and adaptive immunity, increased exposure and immunity to coronaviruses, and a lower intensity of exposure to SARS-CoV-2. In addition, these data may reflect different thresholds for admission, with a less sick paediatric population being admitted to the ICU. This is supported by the shorter length of stay in our paediatric cohort, alongside a lower level of respiratory support and fewer COVID-19 directed therapies, raising the possibility of ‘prophylactic’ admission in patients <18 years of age.
Finally, the frequency of PIMS-TS or MIS-C in paediatric patients is uncertain, being variably reported in different settings, both within and external to the ICU. Indeed, this may be as high as 40–50%,29,43 in patients with severe acute COVID-19 and/or requiring ICU admission, or as low as 1–11%,20,22 in hospitalised paediatric COVID-19 patients. A separate study, in hospitalised and non-hospitalised COVID-19 patients, reported PIMS-TS/MIS-C in 2.5%.35 In our study, only 8.4% manifest this complication, likely due to Omicron being the dominant strain at the time of widespread community infection, which is associated with a relatively low frequency of PIMS-TS, as compared with earlier variants.44
Strengths and limitations
This study contributes to the existing and growing literature outlining severe or critical COVID-19 in paediatric populations across national and multinational settings.20,28,36,43 While there are other studies which describe the clinical severity of paediatric COVID-19 cases admitted to hospital, fewer explore the clinical course in paediatric patients once admitted to the ICU, or compare paediatric and young adult cohorts.
It is important to note that patients included in this study were only those managed in the ICU setting, predominantly at tertiary and quaternary institutions. This reflects a specific population and may therefore select characteristics that predispose paediatric patients to critical illness. Furthermore, disease which did not warrant ICU admission encompasses a proportion of severe infections in paediatric patients and was not captured within our dataset. In addition, paediatric critical illness severity scores such as PIM or PRISM are not routinely collected in the SPRINT-SARI Australia dataset, potentially limiting comparison with other cohorts, albeit PIM and PRISM are used variably in this setting,45 and APACHE-II has demonstrated good discrimination in smaller paediatric cohorts.46
As the standard definition of PIMS-TS encompasses patients who fulfil clinical criteria following confirmed contact with a SARS-CoV-2 infection (in the absence of PCR),18 PIMS-TS cases requiring ICU admission may be underestimated in our dataset. Furthermore, our dataset is not sufficient to provide evidence of vaccine effectiveness against PIMS-TS.
Mandatory reporting of SARS-CoV-2 infection ceased in October 2022 in Australia, resulting in an underestimation of the true incidence of disease in the community from this timepoint. Despite this, our data continue to demonstrate a decrease in age-specific ICU admissions per 1000 SARS-CoV-2 notifications across all cohorts during the Omicron predominant phase of the pandemic, when compared with the pre-Delta and Delta phases. Given the reduction in community testing, the magnitude of the fall in SARS-CoV-2 related ICU admissions during the Omicron era, is likely higher.
Finally, there are also limitations owing to the study's observational design. A number of patients remained in hospital at the time of data extraction, and there was therefore a proportion of missing outcome data, with a focus on short-term mortality.
Conclusions
This national, observational study comparing severe or critical COVID-19 disease between paediatric and young adult cohorts demonstrated that there is an appreciable burden of severe disease in paediatric cohorts, which is most pronounced in patients <5 years of age, and 12–17 years of age. The Omicron predominant phase of the pandemic saw an increased population incidence of COVID-19 ICU admissions across all age groups, most significantly in patients <5 years of age, concurrent with widespread transmission in the Australian community. However, per 1000 SARS-CoV-2 notifications, a reduced incidence was noted during the Omicron phase. Patients <12 years old demonstrated a shorter duration to hospital and ICU admission when compared with adolescents and young adults, corresponding to the acute viral phase of SARS-CoV-2 infection. Finally, while adolescent patients may present phenotypically similar to young adults with respect to comorbidity and natural history of disease, SARS-CoV-2 infection in adolescent patients remains less severe when compared with young adults.
Contributors
MO–conceptualisation; methodology; writing–original draft; writing–review and editing.
PNB–conceptualisation; formal analysis; methodology; writing–original draft; writing–review and editing.
ASN–formal analysis; methodology; visualisation; writing–review and editing.
SE–conceptualisation; writing–review and editing.
MF–conceptualisation; writing–review and editing.
NWC–conceptualisation; writing–review and editing.
AJCB–conceptualisation; methodology; writing–review and editing.
AAU–conceptualisation; methodology; supervision; writing–original draft; writing–review and editing.
PNB, ASN and AAU directly accessed and verified the underlying data reported in the manuscript.
Data sharing statement
Individual participant data will not be made available or shared.
Declaration of interests
Professor Andrew A Udy has the following disclosures: funding for SPRINT-SARI (Department of Health, Commonwealth of Australia [Standing Deed SON60002733]) is provided to Monash University as the administering institution; recipient of Grant funding (paid to Monash University) from the National Health and Medical Research Council (NHMRC) Australia, for research projects outside of the current work; recipient of Grant funding (paid to Monash University) from the Medical Research Future Fund (MRFF) Australia, for research projects outside of the current work; recipient of financial support from Integra Lifesciences for the delivery of a presentation at the Neurosurgical Society of Australasia Conference 2022; recipient of in-kind support (trial consumables) from Integra Lifesciences, for research projects outside of the current work.
Associate Professor Philip N Britton has the following disclosures: recipient of the NHMRC Early Career Fellowship – APP1145817; recipient of the Royal Australian College of Physicians Foundation Cottrell Research Establishment Fellowship 2022.
Associate Professor Nigel W Crawford has the following disclosure: member and current Chair of the National Vaccine Advisory Committee (ATAGI).
There are no further conflicts of interest to declare.
Footnotes
Please see Supplementary Notes for the full list of The SPRINT-SARI Australia Management Committee and The SPRINT-SARI Australia Investigators.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2023.100763.
Appendix A. Supplementary data
References
- 1.COVID-19 map–John Hopkins coronavirus resource center. John Hopkins University of Medicine; 2022. https://coronavirus.jhu.edu/map.html Available from: [Google Scholar]
- 2.Viner R.M., Mytton O.T., Bonell C., et al. Susceptibility to SARS-CoV-2 infection among children and adolescents compared with adults: a systematic review and meta-analysis. JAMA Pediatr. 2021;175(2):143–156. doi: 10.1001/jamapediatrics.2020.4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams P., Koirala A., Saravanos G.L., et al. COVID-19 in New South Wales children during 2021: severity and clinical spectrum. Med J Aust. 2022;217:303. doi: 10.5694/mja2.51661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark J.A., Pathan N. Hide and seek in a pandemic: review of SARS-CoV-2 infection and sequelae in children. Exp Physiol. 2022;107(7):653–664. doi: 10.1113/EP089399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailey L.C., Razzaghi H., Burrows E.K., et al. Assessment of 135794 pediatric patients tested for severe acute respiratory syndrome coronavirus 2 across the United States. JAMA Pediatr. 2021;175(2):176–184. doi: 10.1001/jamapediatrics.2020.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsankov B.K., Allaire J.M., Irvine M.A., et al. Severe COVID-19 infection and pediatric comorbidities: a systematic review and meta-analysis. Int J Infect Dis. 2021;103:246–256. doi: 10.1016/j.ijid.2020.11.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howard-Jones A.R., Burgner D.P., Crawford N.W., et al. COVID-19 in children. II: pathogenesis, disease spectrum and management. J Paediatr Child Health. 2022;58(1):46–53. doi: 10.1111/jpc.15811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Australia Technical Advisory Group on Immunisation (ATAGI) Australian Government, Department of Health and Aged Care; 2021. ATAGI recommendations on the use of COVID-19 vaccines in all young adolescents in Australia.https://www.health.gov.au/news/atagi-recommendations-on-the-use-of-covid-19-vaccines-in-all-young-adolescents-in-australia Available from: [Google Scholar]
- 9.Australia Technical Advisory Group on Immunisation (ATAGI) In: ATAGI recommendations on the use of the paediatric Pfizer COVID-19 vaccine in children aged 5 to 11 years in Australia. Health Do, editor. Australian Government, Department of Health; 2022. [Google Scholar]
- 10.2022. Department of Health and Aged Care, Australian Government, COVID-19 vaccine rollout update–21 June 2022.https://www.health.gov.au/resources/publications/covid-19-vaccine-rollout-update-21-june-2022 Available from: [Google Scholar]
- 11.Hause A.M., Gee J., Baggs J., et al. COVID-19 vaccine safety in adolescents aged 12-17 Years–United States, December 14, 2020-July 16, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(31):1053–1058. doi: 10.15585/mmwr.mm7031e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oster M.E., Shay D.K., Su J.R., et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327(4):331–340. doi: 10.1001/jama.2021.24110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang P., Wei M., Jin P., Li Z., Li J., Zhu F. COVID-19 in children: epidemic issues and candidate vaccines. Chin Med J. 2022;135:1314. doi: 10.1097/CM9.0000000000002169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price A.M., Olson S.M., Newhams M.M., et al. BNT162b2 protection against the Omicron variant in children and adolescents. N Engl J Med. 2022;386(20):1899–1909. doi: 10.1056/NEJMoa2202826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Australia Technical Advisory Group on Immunisation (ATAGI) Australian Government, Department of Health and Aged Care; 2022. ATAGI recommendations on COVID-19 vaccine use in children aged 6 months to <5 years.https://www.health.gov.au/news/atagi-recommendations-on-covid-19-vaccine-use-in-children-aged-6-months-to [Recommendations from the Australian Technical Advisory Group on Immunisation (ATAGI) COVID-19 vaccine use in children] Available from: [Google Scholar]
- 16.Burrell A.J., Pellegrini B., Salimi F., et al. Outcomes for patients with COVID-19 admitted to Australian intensive care units during the first four months of the pandemic. Med J Aust. 2021;214(1):23–30. doi: 10.5694/mja2.50883. [DOI] [PubMed] [Google Scholar]
- 17.Knaus W.A., Draper E.A., Wagner D.P., Zimmerman J.E. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 18.Britton P. 2022. Paediatric Inflammatory Multisystem Syndrome Temporally associated with SARS-COV-2 (PIMS-TS): paediatric active enhanced disease surveillance.https://paeds.org.au/surveillance-and-research/pims-ts Available from: [Google Scholar]
- 19.Bhuiyan M.U., Stiboy E., Hassan M.Z., et al. Epidemiology of COVID-19 infection in young children under five years: a systematic review and meta-analysis. Vaccine. 2021;39(4):667–677. doi: 10.1016/j.vaccine.2020.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swann O.V., Holden K.A., Turtle L., et al. Clinical characteristics of children and young people admitted to hospital with covid-19 in United Kingdom: prospective multicentre observational cohort study. BMJ. 2020;370:m3249. doi: 10.1136/bmj.m3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tosif S., Ibrahim L.F., Hughes R., et al. Characteristics and outcomes of SARS-CoV-2 infection in Victorian children at a tertiary paediatric hospital. J Paediatr Child Health. 2022;58(4):618–623. doi: 10.1111/jpc.15786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wurzel D., McMinn A., Hoq M., et al. Prospective characterisation of SARS-CoV-2 infections among children presenting to tertiary paediatric hospitals across Australia in 2020: a national cohort study. BMJ Open. 2021;11(11) doi: 10.1136/bmjopen-2021-054510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halasa N.B., Olson S.M., Staat M.A., et al. Maternal vaccination and risk of hospitalization for Covid-19 among infants. N Engl J Med. 2022;387(2):109–119. doi: 10.1056/NEJMoa2204399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hobbs C.V., Woodworth K., Young C.C., et al. Frequency, characteristics and complications of COVID-19 in hospitalized infants. Pediatr Infect Dis J. 2022;41(3):e81–e86. doi: 10.1097/INF.0000000000003435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ludvigsson J.F. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088–1095. doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shekerdemian L.S., Mahmood N.R., Wolfe K.K., et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. 2020;174(9):868–873. doi: 10.1001/jamapediatrics.2020.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu X., Zhang L., Du H., et al. SARS-CoV-2 infection in children. N Engl J Med. 2020;382(17):1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gotzinger F., Santiago-Garcia B., Noguera-Julian A., et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4(9):653–661. doi: 10.1016/S2352-4642(20)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tripathi S., Gist K.M., Bjornstad E.C., et al. Coronavirus disease 2019-associated PICU admissions: a report from the society of critical care medicine discovery network viral infection and respiratory illness universal study registry. Pediatr Crit Care Med. 2021;22(7):603–615. doi: 10.1097/PCC.0000000000002760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sachdeva R., Rice T.B., Reisner B., et al. The impact of coronavirus disease 2019 pandemic on U.S. And Canadian PICUs. Pediatr Crit Care Med. 2020;21(9):e643–e650. doi: 10.1097/PCC.0000000000002510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhatraju P.K., Ghassemieh B.J., Nichols M., et al. Covid-19 in critically ill patients in the seattle region–case series. N Engl J Med. 2020;382(21):2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cummings M.J., Baldwin M.R., Abrams D., et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gandhi R.T. The multidimensional challenge of treating coronavirus disease 2019 (COVID-19): remdesivir is a foot in the door. Clin Infect Dis. 2021;73(11):e4175–e4178. doi: 10.1093/cid/ciaa1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jose R.J., Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;8(6):E46–E47. doi: 10.1016/S2213-2600(20)30216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uka A., Buettcher M., Bernhard-Stirnemann S., et al. Factors associated with hospital and intensive care admission in paediatric SARS-CoV-2 infection: a prospective nationwide observational cohort study. Eur J Pediatr. 2022;181(3):1245–1255. doi: 10.1007/s00431-021-04276-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonzalez-Dambrauskas S., Vasquez-Hoyos P., Camporesi A., et al. Paediatric critical COVID-19 and mortality in a multinational prospective cohort. Lancet Reg Health Am. 2022;12 doi: 10.1016/j.lana.2022.100272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao M., Piernas C., Astbury N.M., et al. Associations between body-mass index and COVID-19 severity in 6.9 million people in England: a prospective, community-based, cohort study. Lancet Diabetes Endocrinol. 2021;9(6):350–359. doi: 10.1016/S2213-8587(21)00089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valenzuela G., Alarcon-Andrade G., Schulze-Schiapacasse C., et al. Short-term complications and post-acute sequelae in hospitalized paediatric patients with COVID-19 and obesity: a multicenter cohort study. Pediatr Obes. 2022;18 doi: 10.1111/ijpo.12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Australia Technical Advisory Group on Immunisation (ATAGI) Australian Government, Department of Health and Aged Care; 2022. ATAGI recommendations on the use of a third primary dose of COVID-19 vaccine in individuals who are severely immunocompromised.https://www.health.gov.au/resources/publications/atagi-recommendations-on-the-use-of-a-third-primary-dose-of-covid-19-vaccine-in-individuals-who-are-severely-immunocompromised Available from: [Google Scholar]
- 40.Cunningham J.W., Vaduganathan M., Claggett B.L., et al. Clinical outcomes in young US adults hospitalized with COVID-19. JAMA Intern Med. 2020;181:379. doi: 10.1001/jamainternmed.2020.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cusenza F., Davino G., D'Alvano T., et al. Silence of the lambs: the immunological and molecular mechanisms of COVID-19 in children in comparison with adults. Microorganisms. 2021;9(2) doi: 10.3390/microorganisms9020330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zimmermann P., Curtis N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch Dis Child. 2020 doi: 10.1136/archdischild-2020-320338. [DOI] [PubMed] [Google Scholar]
- 43.Feldstein L.R., Tenforde M.W., Friedman K.G., et al. Characteristics and outcomes of US children and adolescents with Multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA. 2021;325(11):1074–1087. doi: 10.1001/jama.2021.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lopez L., Burgner D., Glover C., et al. Lower risk of Multi-system inflammatory syndrome in children (MIS-C) with the omicron variant. Lancet Reg Health West Pac. 2022;27 doi: 10.1016/j.lanwpc.2022.100604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Keulen J.G., Polderman K.H., Gemke R.J. Reliability of PRISM and PIM scores in paediatric intensive care. Arch Dis Child. 2005;90(2):211–214. doi: 10.1136/adc.2003.046722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chhangani N.P., Amandeep M., Choudhary S., Gupta V., Goyal V. Role of acute physiology and chronic health evaluation II scoring system in determining the severity and prognosis of critically ill patients in pediatric intensive care unit. Indian J Crit Care Med. 2015;19(8):462–465. doi: 10.4103/0972-5229.162463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.