Abstract
Purpose:
In PERTAIN's primary analysis (31 months’ median follow-up), adding pertuzumab to trastuzumab and an aromatase inhibitor (AI) with/without chemotherapy significantly improved progression-free survival (PFS) in patients with previously untreated HER2-positive and hormone receptor–positive metastatic or locally advanced breast cancer (M/LABC). A potentially enhanced treatment effect was observed in patients with no induction chemotherapy. We present the final analysis (>6 years’ median follow-up).
Patients and Methods:
Patients (N = 258) were randomized 1:1 to pertuzumab (loading/maintenance: 840/420 mg) plus trastuzumab (loading/maintenance: 8/6 mg/kg) every 3 weeks and an AI (1 mg anastrozole or 2.5 mg letrozole daily; Arm A), or trastuzumab and an AI (Arm B). Induction chemotherapy was at investigator discretion. Primary endpoint: PFS. Key secondary endpoints: overall survival (OS) and safety.
Results:
Median PFS was 20.6 versus 15.8 months in Arms A and B, respectively (stratified HR, 0.67; P = 0.006). Median OS was 60.2 versus 57.2 months (stratified HR, 1.05; P = 0.78). Pertuzumab treatment effect was potentially enhanced in patients with no induction chemotherapy (26.6 vs. 12.5 months). Any-grade adverse events (AE) occurred in 122 patients per arm (96.1% vs. 98.4%); grade ≥ 3 AEs in 72 (56.7%) and 51 (41.1%); serious AEs in 46 (36.2%) and 28 (22.6%).
Conclusions:
The PFS benefit of pertuzumab was maintained and OS was similar between arms at final analysis. Adding pertuzumab may enhance activity in patients who do not require first-line chemotherapy for M/LABC. No new safety concerns were reported. These data provide additional evidence of the role of first-line pertuzumab and trastuzumab in HER2-positive M/LABC.
Translational Relevance.
On the basis of results from the phase III CLEOPATRA study, pertuzumab plus trastuzumab and chemotherapy is the first-line standard of care for patients with HER2-positive metastatic breast cancer. Bidirectional cross-talk between HER2 and estrogen receptors is known to contribute to resistance to anti-HER2 and endocrine therapies. Therefore, the PERTAIN study was designed to assess the addition of pertuzumab to trastuzumab and an aromatase inhibitor with or without induction chemotherapy for the first-line treatment of patients with HER2-positive and hormone receptor–positive metastatic or locally advanced breast cancer (M/LABC). In the final analysis at >6 years’ median follow-up, addition of pertuzumab demonstrated progression-free survival benefits and similar overall survival, with a potentially enhanced treatment effect in patients who received no induction chemotherapy prior to endocrine therapy, and no new safety concerns were reported. These findings provide additional evidence of the role of first-line pertuzumab and trastuzumab in HER2-positive M/LABC.
Introduction
The role of bidirectional cross-talk between HER2 and estrogen receptors in resistance to anti-HER2 and endocrine therapy has been widely studied (1–3). In the phase III CLEOPATRA study, significantly improved progression-free survival (PFS) and overall survival (OS) were observed when combining pertuzumab with trastuzumab and docetaxel compared with placebo plus trastuzumab and docetaxel for the first-line treatment of patients with HER2-positive metastatic breast cancer (4–7). On the basis of these results, pertuzumab plus trastuzumab and chemotherapy is the first-line standard of care for these patients (8). As CLEOPATRA did not permit patients to receive concomitant endocrine therapy (4, 5), the PERTAIN study (NCT01491737) was subsequently carried out to assess the value of adding pertuzumab to trastuzumab and an aromatase inhibitor (AI) with or without induction chemotherapy for the first-line treatment of patients with HER2-positive and hormone receptor–positive metastatic or locally advanced breast cancer (M/LABC) in the first randomized phase II trial of its kind (9). PERTAIN met its primary endpoint at 31 months’ median follow-up, showing that the addition of pertuzumab resulted in significant improvements in PFS compared with trastuzumab and an AI alone (9). In addition, subgroups of patients who did not receive induction chemotherapy or who had a disease-free interval of ≥ 12 months since adjuvant hormone therapy experienced a potentially enhanced treatment effect (9). Here, we present updated PFS, mature OS (secondary endpoint), and updated safety results from the final analysis of PERTAIN, with a median follow-up of > 6 years.
Patients and Methods
Patients
Details of the PERTAIN study have been published previously (9). Briefly, PERTAIN was a randomized, two-arm, open-label, multicenter phase II trial conducted across 71 sites in eight countries. Eligible patients were postmenopausal (fulfilling ≥ 1 National Comprehensive Cancer Network criterion; ref. 8) with previously untreated HER2-positive and hormone receptor–positive disease per local laboratory assessment, who had ≥ 1 measurable lesion, and/or nonmeasurable disease per Response Evaluation Criteria in Solid Tumors (RECIST) v1.1, Eastern Cooperative Oncology Group (ECOG) performance status 0 or 1, left ventricular ejection fraction (LVEF) ≥ 50%, and life expectancy ≥ 12 weeks. Patients who had previously received systemic nonhormonal anticancer therapy in the metastatic or locally advanced setting or approved/investigative anti-HER2 agents in any breast cancer setting, except trastuzumab and/or lapatinib in the neoadjuvant/adjuvant settings, or who had a disease-free interval < 6 months from completion of systemic nonhormonal treatment in the neoadjuvant/adjuvant settings, disease progression while receiving trastuzumab and/or lapatinib in the adjuvant setting, or uncontrolled central nervous system metastases were excluded. PERTAIN was conducted in full accordance with the guidelines for Good Clinical Practice and the Declaration of Helsinki. Each patient provided written informed consent. An independent ethics committee for each participating site provided approval of the protocol and all amendments. An independent data monitoring committee monitored safety and made recommendations regarding continuation of the study.
Procedures
Patients were randomized 1:1 to pertuzumab plus trastuzumab and an AI (anastrozole/letrozole) or trastuzumab and an AI. Pertuzumab (loading: 840 mg/maintenance: 420 mg) and trastuzumab (loading: 8 mg/kg/maintenance 6 mg/kg) were given every 3 weeks intravenously. Anastrozole (1 mg) or letrozole (2.5 mg) were administered orally once daily. Induction chemotherapy was administered at the investigator's discretion (decision made prior to randomization) and was with either docetaxel or paclitaxel, which were given intravenously per product labeling every 3 weeks or every week, respectively, for 18 to 24 weeks in combination with trastuzumab (with or without pertuzumab) and prior to starting endocrine therapy. Stratification factors were “chosen to receive induction chemotherapy” (yes/no) and “time since adjuvant hormone therapy” (<12 months/≥12 months/no prior hormone therapy).
Treatment continued until disease progression, unacceptable toxicity, withdrawal of consent, or death. Treatment with pertuzumab and trastuzumab could be discontinued or delayed due to adverse events (AE); no dose reductions were allowed. If any of the individual study medications was delayed for ≥1 day, all agents were delayed for the same timeframe. If a patient missed a dose of pertuzumab for one cycle (i.e., if the two sequential administration times were ≥6 weeks apart) or a dose of trastuzumab by ≥1 week, a reloading dose was given per the product labeling or per approved local Product Information and/or recognized clinical practice guidelines, respectively. If reloading was required for a given cycle, the three study therapies were given on the same schedule as cycle 1 and subsequent maintenance doses of pertuzumab and trastuzumab given every 3 weeks, starting 3 weeks later. Discontinuation of pertuzumab, trastuzumab, paclitaxel, and docetaxel occurred in cases of confirmed congestive heart failure. Pertuzumab and trastuzumab were also discontinued for LVEF drops to <40% (confirmed with a repeat assessment within 3 weeks of first assessment) or 40% to 45% and ≥10% below baseline. Taxane dose reductions were permitted for severe peripheral neurotoxicity.
Assessments
The primary endpoint was PFS (time from randomization until first radiographically documented progression of disease or death from any cause, whichever occurred first). Secondary endpoints reported here include OS (time from randomization to death, regardless of cause) and safety and tolerability.
Tumors were assessed per RECIST v1.1 at screening, every three cycles of anti-HER2 therapy for the first 36 months, and every six cycles (±7 days of scheduled treatment day) thereafter for patients who remained progression-free after 36 months, as well as during the safety follow-up visit (approximately 28 days after end-of-study treatment) and during 3-monthly post-treatment follow-up visits if disease progression was not established. The NCI's Common Terminology Criteria for Adverse Events Version 4.0 was used to assess AEs at screening, baseline, day –7 to day 1, during treatment, at the safety follow-up visit, and at the post-treatment follow-up visits. LVEF was assessed locally by echocardiogram or multigated acquisition scan, and change from baseline calculated for patients who were reassessed throughout the study with the same technique as that used at baseline.
Statistical methods
The updated analysis of PFS, OS, and safety data included here was planned to take place once all patients had been followed-up for ≥60 months after the last patient was randomized, unless they were lost to follow-up, withdrew consent, or died. PFS and OS were analyzed using the Kaplan–Meier approach and HRs estimated using a stratified Cox proportional hazards model, both including stratification factors (induction chemotherapy; time since adjuvant hormone therapy) from the interactive voice response system used for randomization. As the study was not adequately powered for OS analyses, estimates to assess the difference between treatment arms are provided in an exploratory manner. The HR for the pertuzumab plus trastuzumab arm versus the trastuzumab arm (reference category) was estimated from an unstratified Cox model for subgroup analyses. Patients with no PFS events were censored at the time of the last evaluable tumor assessment; patients with no tumor assessment after baseline were censored at the date of randomization. Patients with no OS event were censored at the date of last follow-up assessment; patients with no follow-up assessment were censored at the day of last study medication; and patients with no post-baseline information were censored at the date of randomization. The intention-to-treat (ITT) and safety populations were defined as all randomized patients and all patients who received ≥1 dose of study medication, respectively.
Data availability
Qualified researchers may request access to individual patient-level data through the clinical study data request platform: https://vivli.org/. Further details on Roche's criteria for eligible studies are available here: https://vivli.org/members/ourmembers/. For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here: https://www.roche.com/innovation/process/clinical-trials/data-sharing/.
Results
Population
A total of 258 patients were randomized, 129 to each arm (ITT population), between February 2012 and October 2014 (ref. 9; representativeness of study participants is described in Supplementary Table S1). Of these, 127 patients in the pertuzumab plus trastuzumab arm and 124 patients in the trastuzumab arm were included in the safety population (9). At clinical cutoff (November 14, 2019), the median follow-up was 73.2 months [95% confidence interval (CI), 68.6–75.6] and 71.1 months (95% CI, 65.5–73.9) for the pertuzumab plus trastuzumab arm and the trastuzumab arm, respectively. Baseline patient demographics and disease characteristics in the ITT population have been reported previously (9) and were generally similar between treatment arms. The number of patients chosen to receive induction chemotherapy was 146 (75 in the pertuzumab plus trastuzumab arm; 71 in the trastuzumab arm); 112 patients did not receive induction chemotherapy (54 in the pertuzumab plus trastuzumab arm; 58 in the trastuzumab arm; ref. 9). Patients who received induction chemotherapy were younger, had a lower ECOG performance status, had more stage IV disease at initial diagnosis and more visceral disease, and had a shorter time since initial diagnosis than patients who did not receive induction chemotherapy (10).
Updated PFS
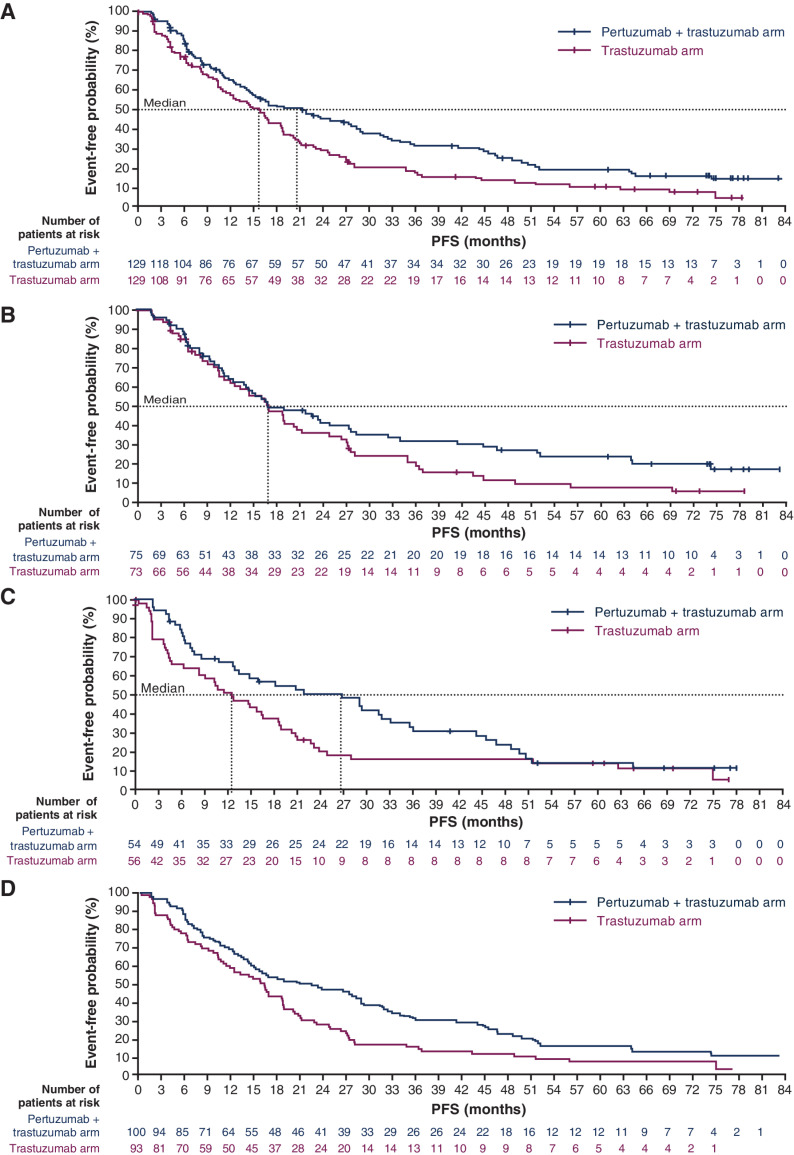
Median PFS was 20.6 months (95% CI, 14.4–28.4) in the pertuzumab plus trastuzumab arm versus 15.8 months (95% CI, 11.0–18.7) in the trastuzumab arm (stratified HR, 0.67; 95% CI, 0.50–0.89; P = 0.006; Fig. 1A). In patients who received induction chemotherapy, median PFS was 16.9 months (95% CI, 12.4–27.4) versus 16.9 months (95% CI, 11.9–20.5), respectively (unstratified HR, 0.71; 95% CI, 0.49–1.04; P = 0.076; Fig. 1B). In patients without induction chemotherapy, median PFS was 26.6 months (95% CI, 12.7–33.0) versus 12.5 months (95% CI, 6.2–18.5), respectively (unstratified HR, 0.68; 95% CI, 0.44–1.03; P = 0.067; Fig. 1C).
Figure 1.
PFS in (A) the ITT population, (B) patients who were chosen to receive induction chemotherapy, (C) patients who were not chosen to receive induction chemotherapy, and (D) patients with estrogen receptor expression ≥ 10%.
PFS was also assessed by estrogen receptor expression. In patients with estrogen receptor expression ≥ 10% (Fig. 1D), median PFS was 22.5 months (95% CI, 14.9–29.2) versus 16.4 months (95% CI, 11.9–18.8), respectively (HR, 0.66; 95% CI, 0.48–0.90; P = 0.012).
Median PFS in patients with <12 months since adjuvant hormone therapy was 14.9 months (95% CI, 9.4–22.5) in the pertuzumab plus trastuzumab arm versus 10.9 months (95% CI, 6.4–16.9) in the trastuzumab arm (unstratified HR, 0.75; 95% CI, 0.40–1.38; P = 0.352). In patients with ≥12 months since adjuvant hormone therapy, median PFS was 32.4 months (95% CI, 16.6–46.7) versus 16.5 months (95% CI, 9.7–24.7), respectively (unstratified HR, 0.60; 95% CI, 0.36–1.00; P = 0.05). In patients with no prior adjuvant hormone therapy, median PFS was 15.8 months (95% CI, 10.6–28.9) versus 16.9 months (95% CI, 11.9–20.3), respectively (unstratified HR, 0.75; 95% CI, 0.50–1.12; P = 0.155).
OS
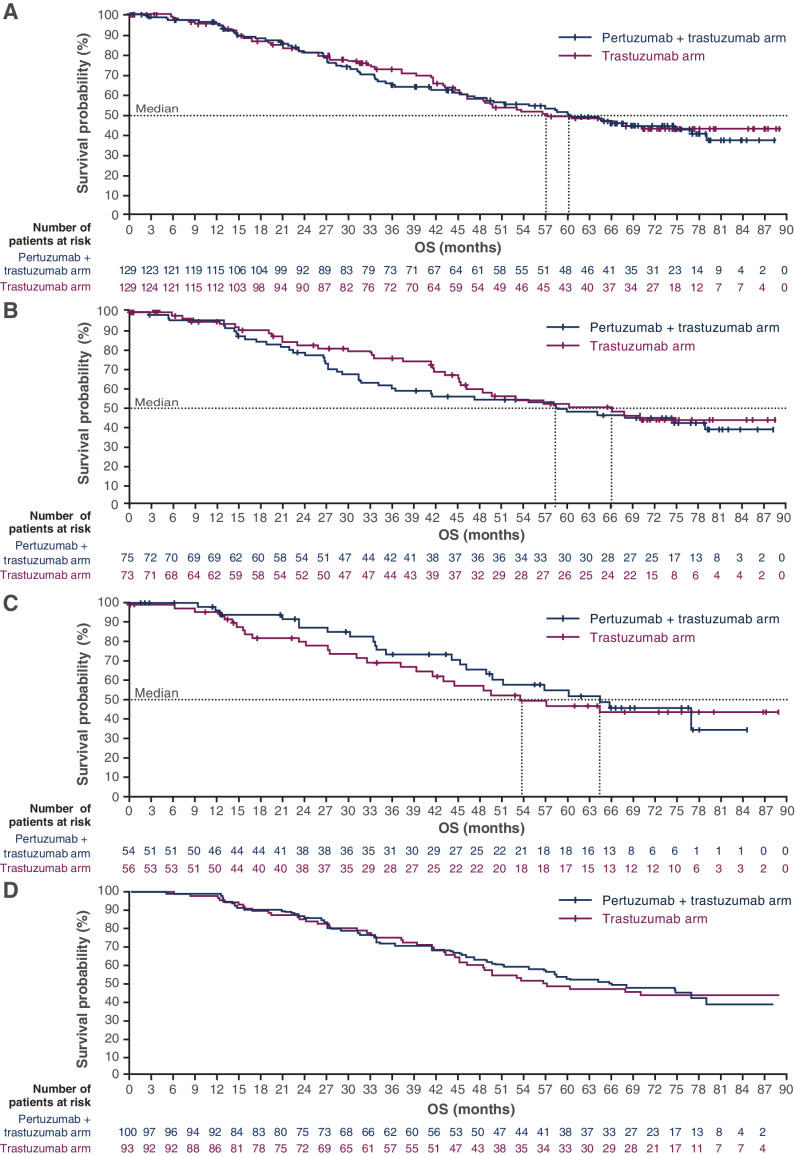
Median OS in patients treated with pertuzumab plus trastuzumab was 60.2 months (95% CI, 47.2–79.0) versus 57.2 months [95% CI, 45.4–not reached (NR)] in patients treated with trastuzumab (stratified HR, 1.05; 95% CI, 0.73–1.52; P = 0.783; Fig. 2A). In patients with induction chemotherapy, median OS was 58.5 months (95% CI, 34.0–NR) versus 66.2 months (95% CI, 45.4–NR), respectively (unstratified HR, 1.16; 95% CI, 0.73–1.85; P = 0.523; Fig. 2B). In patients without induction chemotherapy, median OS was 64.5 months (95% CI, 46.2–NR) versus 53.7 months (95% CI, 39.4–NR), respectively (unstratified HR, 0.88; 95% CI, 0.50–1.55; P = 0.654; Fig. 2C).
Figure 2.
OS in (A) the ITT population, (B) patients who were chosen to receive induction chemotherapy, (C) patients who were not chosen to receive induction chemotherapy, and (D) patients with estrogen receptor expression ≥ 10%.
When OS was assessed by estrogen receptor expression, median OS in patients with estrogen receptor expression ≥10% (Fig. 2D) was 65.8 months (95% CI, 51.2–NR) versus 57.2 months (95% CI, 46.2–NR), respectively (P = 0.900).
Median OS in patients with <12 months since adjuvant hormone therapy was 49.7 months (95% CI, 27.2–64.2) in the pertuzumab plus trastuzumab arm versus 44.6 months (95% CI, 30.0–NR) in the trastuzumab arm (unstratified HR, 1.18; 95% CI, 0.57–2.43; P = 0.656). In patients with ≥12 months since adjuvant hormone therapy, median OS was 79.0 months (95% CI, 51.2–NR) versus 45.2 months (95% CI, 41.4–NR), respectively (unstratified HR, 0.62; 95% CI, 0.32–1.23; P = 0.168). In patients with no prior adjuvant hormone therapy, median OS was 54.7 months (95% CI, 33.5–NR) versus NR (95% CI, 49.6–NR), respectively (unstratified HR, 1.36; 95% CI, 0.80–2.31; P = 0.248).
Exposure to study treatment (safety population)
In the pertuzumab plus trastuzumab arm, the median number of pertuzumab and trastuzumab cycles was 18.0 (range 1–121) and median exposure to pertuzumab and trastuzumab was 12.6 months (range 0.03–83.3). In the trastuzumab arm, the median number of trastuzumab cycles was 15.5 (range 1–116) and median exposure to trastuzumab was 10.6 months (range 0.03–79.3).
For anastrozole, the median number of treatment cycles was 13.5 (range 1–104) in the pertuzumab plus trastuzumab arm and 13.5 (range 1–110) in the trastuzumab arm, and median exposure was 9.2 months (range 0.7–76.9) and 9.6 months (range 0.7–75.2), respectively. For letrozole, the median number of treatment cycles was 18.0 (range 1–115) in the pertuzumab plus trastuzumab arm and 17.0 (range 0–108) in the trastuzumab arm, and median exposure was 12.5 months (range 0.7–79.7) and 12.1 months (range 1.0–77.0), respectively.
The median number of docetaxel and paclitaxel cycles was 6.0 (range 0–8) in each arm. Median exposure to docetaxel was 3.5 months (range 0.03–6.0) in the pertuzumab plus trastuzumab arm and 3.5 months (range 0.03–5.1) in the trastuzumab arm. Median exposure to paclitaxel was 3.9 months (range 0.03–6.0) in the pertuzumab plus trastuzumab arm and 3.9 months (range 0–15.7) in the trastuzumab arm.
Anticancer treatment following study drug discontinuation
A summary of the anticancer treatments started following discontinuation of the study drug in the ITT population and in patients by receipt of induction chemotherapy is shown in Table 1. The majority of patients started anticancer treatment following discontinuation of the study drug, with similar incidences between the arms for both the ITT population [105/129 patients (81.4%) in the pertuzumab plus trastuzumab arm vs. 109/129 patients (84.5%) in the trastuzumab arm] and patients who received induction chemotherapy [62/75 patients (82.7%) vs. 57/73 patients (78.1%)]. In patients without induction chemotherapy, the incidence of anticancer treatment following discontinuation of study drug was lower in the pertuzumab plus trastuzumab arm [43/54 patients (79.6%)] than the trastuzumab arm [52/56 patients (92.9%)]. The most commonly reported anticancer treatment in the ITT population and in patients with or without induction chemotherapy was a monoclonal antibody. Subsequent first-line treatment with pertuzumab plus trastuzumab and ado-trastuzumab emtansine was higher in the pertuzumab plus trastuzumab arm versus the trastuzumab arm in the ITT population and in patients without induction chemotherapy.
Table 1.
Anticancer treatment following study drug discontinuation in the ITT population and by receipt of induction chemotherapya.
| ITT population | With induction therapy | Without induction therapy | ||||
|---|---|---|---|---|---|---|
| Pertuzumab plus trastuzumab arm | Trastuzumab arm | Pertuzumab plus trastuzumab arm | Trastuzumab arm | Pertuzumab plus trastuzumab arm | Trastuzumab arm | |
| Patients, n (%) | (n = 129) | (n = 129) | (n = 75) | (n = 73) | (n = 54) | (n = 56) |
| Received anticancer therapy | ||||||
| Yes | 105 (81.4) | 109 (84.5) | 62 (82.7) | 57 (78.1) | 43 (79.6) | 52 (92.9) |
| No | 19 (14.7) | 19 (14.7) | 11 (14.7) | 15 (20.5) | 8 (14.8) | 4 (7.1) |
| Missing | 5 (3.9) | 1 (0.8) | 2 (2.7) | 1 (1.4) | 3 (5.6) | 0 |
| Anticancer therapies in ≥10% of patients in either arm, INN classb | ||||||
| Antiestrogens | 19 (18.1) | 27 (24.8) | 13 (21.0) | 9 (15.5) | 6 (14.0) | 18 (34.6) |
| Antimetabolites | 36 (34.3) | 27 (24.8) | 23 (37.1) | 16 (28.1) | 13 (30.2) | 11 (21.2) |
| Antineoplastic agents | 12 (11.4) | 11 (10.1) | 8 (12.9) | 7 (12.3) | 4 (9.3) | 4 (7.7) |
| AIs | 50 (47.6) | 49 (45.0) | 30 (48.4) | 27 (47.4) | 20 (46.5) | 22 (42.3) |
| Cytotoxic antibiotics | 10 (9.5) | 5 (4.6) | 8 (12.9) | 4 (7.0) | 2 (4.7) | 1 (1.9) |
| Monoclonal antibodies | 79 (75.2) | 79 (72.5) | 45 (72.6) | 42 (73.7) | 34 (79.1) | 37 (71.2) |
| Surgical and medical procedures | 19 (18.1) | 20 (18.3) | 11 (17.7) | 15 (26.3) | 8 (18.6) | 5 (9.6) |
| Taxanes | 18 (17.1) | 22 (20.2) | 8 (12.9) | 7 (12.3) | 10 (23.3) | 15 (28.8) |
| Tyrosine kinase inhibitors | 25 (23.8) | 19 (17.4) | 16 (25.8) | 13 (22.8) | 9 (20.9) | 6 (11.5) |
| Vinca alkaloids | 23 (21.9) | 24 (22.0) | 13 (21.0) | 13 (22.8) | 10 (23.3) | 11 (21.2) |
| Subsequent first-line treatment with HER2-targeted therapiesc | 61 (47.3) | 67 (51.9) | 32 (42.7) | 32 (43.8) | 29 (53.7) | 35 (62.5) |
| Pertuzumab plus trastuzumab | 20 (32.8) | 9 (13.4) | 10 (31.3) | 3 (43.8) | 10 (34.5) | 6 (17.1) |
| Ado-trastuzumab emtansine | 22 (36.1) | 11 (16.4) | 14 (43.8) | 5 (15.6) | 8 (27.6) | 6 (17.1) |
| Trastuzumab | 23 (37.7) | 50 (74.6) | 10 (31.3) | 24 (75.0) | 13 (44.8) | 26 (74.3) |
Abbreviation: INN, international nonproprietary name.
aSome therapies began before the last date of study treatment.
bA treatment may appear in more than one INN class.
cPatients may be counted in more than one category.
Safety
Any-grade AEs were reported in 122 patients in each treatment arm [122/127 patients (96.1%) in the pertuzumab plus trastuzumab arm and 122/124 (98.4%) in the trastuzumab arm]. Serious AEs (SAE) were reported in 46 patients (36.2%) in the pertuzumab plus trastuzumab arm and 28 patients (22.6%) in the trastuzumab arm, and grade ≥ 3 AEs in 72 patients (56.7%) and 51 patients (41.1%), respectively. The most common grade ≥ 3 AEs (in ≥5.0% of patients) were hypertension [15 patients (11.8%) in the pertuzumab plus trastuzumab arm and 13 (10.5%) in the trastuzumab arm], diarrhea [12 (9.4%) and 3 (2.4%), respectively], and neutropenia [4 (3.1%) and 9 (7.3%), respectively]. Discontinuation of pertuzumab as a result of AEs occurred in 16 patients. No treatment-related deaths occurred in either treatment arm.
Table 2 shows the safety profile by receipt of induction chemotherapy during the study treatment period. AEs, grade ≥ 3 AEs, and SAEs were more frequent in patients with induction chemotherapy than in those without in both treatment arms. The most common grade ≥ 3 AEs (in ≥ 3% of patients in either arm) by receipt of induction chemotherapy are shown in Table 3.
Table 2.
Safety overview by receipt of induction chemotherapy.
| With induction therapy | Without induction therapy | |||
|---|---|---|---|---|
| Pertuzumab plus trastuzumab arm | Trastuzumab arm | Pertuzumab plus trastuzumab arm | Trastuzumab arm | |
| Patients, n (%) | (n = 74) | (n = 69) | (n = 53) | (n = 55) |
| Any AE | 73 (98.6) | 69 (100) | 49 (92.5) | 53 (96.4) |
| NCI-CTCAE grade ≥3 AEs | 53 (71.6) | 34 (49.3) | 19 (35.8) | 17 (30.9) |
| AEs related to pertuzumab | 50 (67.6) | 0 | 32 (60.4) | 0 |
| SAEs | 30 (40.5) | 15 (21.7) | 16 (30.2) | 13 (23.6) |
| SAEs related to pertuzumab | 4 (5.4) | 0 | 6 (11.3) | 0 |
| AEs leading to discontinuation of pertuzumab | 11 (14.9) | 0 | 5 (9.4) | 0 |
| Number of deaths | 39 (52.7) | 31 (44.9) | 23 (43.4) | 26 (47.3) |
| Deaths due to study treatment | 0 | 0 | 0 | 0 |
Abbreviation: NCI-CTCAE, National Cancer Institute's Common Terminology Criteria for Adverse Events.
Table 3.
Grade ≥3 AEs (≥3% incidence in either arm) in the safety population, by receipt of induction chemotherapy.
| With induction therapy | Without induction therapy | |||
|---|---|---|---|---|
| Pertuzumab plus trastuzumab arm | Trastuzumab arm | Pertuzumab plus trastuzumab arm | Trastuzumab arm | |
| Patients, n (%) | (n = 74) | (n = 69) | (n = 53) | (n = 55) |
| Hypertension | 14 (18.9) | 8 (11.6) | 1 (1.9) | 5 (9.1) |
| Diarrhea | 9 (12.2) | 3 (4.3) | 3 (5.7) | — |
| Anemia | 4 (5.4) | 3 (4.3) | 2 (3.8) | — |
| Pneumonia | 4 (5.4) | 1 (1.4) | 2 (3.8) | — |
| Asthenia | 4 (5.4) | 4 (5.8) | — | — |
| Febrile neutropenia | 4 (5.4) | 2 (2.9) | — | — |
| Left ventricular dysfunction | 1 (1.4) | — | 3 (5.7) | 1 (1.8) |
| Neutropenia | 4 (5.4) | 8 (11.6) | — | 1 (1.8) |
Discussion
Patients with HER2-positive and hormone receptor–positive breast cancer have tumors with a distinctive biology and growth pattern compared with those with HER2-positive and hormone receptor–negative disease, including expression of estrogen and/or progesterone receptors, reduced HER2 enrichment, reduced proliferation rate, and fewer stromal tumor-infiltrating lymphocytes (11, 12). Results from the CLEOPATRA study showed that patients with HER2-positive and hormone receptor–positive breast cancer also had numerically higher HRs for PFS and OS when treated with HER2-targeted therapies and chemotherapy, compared with patients with hormone receptor–negative disease (4, 6, 7). Therefore, it is important to assess the best method to target the HER2 and hormone receptor axes comprehensively. Previous studies have shown that targeting HER2 and hormone receptors in HER2-positive and hormone receptor–positive breast cancer results in better clinical outcomes compared with targeting hormone receptors alone (1, 13, 14).
As the first randomized phase II trial to investigate pertuzumab plus trastuzumab and an AI for patients with HER2-positive and hormone receptor–positive M/LABC, PERTAIN met its primary objective, demonstrating significantly improved PFS with this treatment combination versus trastuzumab and an AI, as previously reported (9). These results are further supported by real-world data that suggest an association between the addition of endocrine therapy to first-line dual anti-HER2 therapy and demonstrated improved PFS and OS in patients with HER2-positive and hormone receptor–positive metastatic breast cancer (15).
This final analysis showed that the PFS benefit of pertuzumab observed at the PERTAIN primary analysis was maintained at >6 years of follow-up (20.6 months vs. 15.8 months in the trastuzumab arm). In the ALTERNATIVE clinical trial, lapatinib plus trastuzumab and an AI also showed superior PFS benefit versus trastuzumab plus an AI [median PFS: 11 vs. 5.6 months; HR, 0.62 (95% CI, 0.45–0.88); P = 0.0063] in postmenopausal patients with HER2-positive and hormone receptor–positive metastatic breast cancer previously treated with endocrine therapy and trastuzumab plus chemotherapy (16). Together, the results from PERTAIN and ALTERNATIVE demonstrate the benefit of targeting both the HER2 and estrogen receptor pathways concurrently, albeit in different settings. Pertuzumab plus trastuzumab and an AI thus remains the optimal first-line treatment for HER2-positive and hormone receptor–positive metastatic breast cancer.
In terms of median OS, there was a slight numerical increase in the pertuzumab plus trastuzumab arm in the ITT population, although Kaplan–Meier curves overlapped, as represented by the HR of 1.05; hence, there was no clinically meaningful difference. Patients in the pertuzumab plus trastuzumab arm of the ITT population were more likely to receive treatment with pertuzumab plus trastuzumab or ado-trastuzumab emtansine following discontinuation of study treatment; as such, post-study treatments do not explain the lack of meaningful survival benefit in the pertuzumab plus trastuzumab arm. The median OS for the pertuzumab plus trastuzumab arm (60.2 months) was within the range of that observed in the pertuzumab plus trastuzumab arm of the CLEOPATRA study (57.1 months; 95% CI, 50–72; ref. 5). The trastuzumab arm, on the other hand (median OS 57.2 months), appeared to be overperforming compared with CLEOPATRA (median OS 40.8 months; 95% CI, 36–48; ref. 5). However, caution should be taken when comparing results across trials due to differences in study design and patient populations (our study included only patients with hormone receptor–positive disease, whereas CLEOPATRA included patients with both hormone receptor–positive and hormone receptor–negative disease; refs. 4–7), and due to general improvements in the treatment of breast cancer over time.
Aside from the lack of power for OS and the possible confounding effects of second- and third-line treatments, one plausible hypothesis that may explain why patients in the pertuzumab plus trastuzumab arm did not demonstrate significantly improved OS compared with the trastuzumab arm is that patients in the pertuzumab plus trastuzumab arm may gain survival benefit in the first line but their disease may become more resistant afterwards. Therefore, the ground gained in the first line may be lost in subsequent lines, resulting in no long-term survival benefit for adding pertuzumab to trastuzumab and an AI. A similar phenomenon has been observed with AIs versus tamoxifen. AIs demonstrated improved response rate, as well as improved PFS and disease-free survival, respectively, versus tamoxifen in the advanced- and early-stage settings; however, demonstrating even a small OS advantage of AIs over tamoxifen took many more years (e.g., Oxford meta-analysis; ref. 17). It was thought that the reason for this was that tumors were developing more resistance after first-line AIs, possibly through ESR1 mutations. Unfortunately, tumor samples in PERTAIN were not tested for additional biomarkers, such as ESR1 mutations. Testing for ESR1 mutations in future clinical trials could indicate whether AI resistance may be a contributing factor to the observed survival outcomes. Similarly, in HER2-positive/hormone receptor–positive disease, benefit from upfront dual anti-HER2 therapy may be lost later due to more treatment resistance in tumors at the time of progression, though the biologic mechanisms are likely to be different.
An indication of a difference in treatment efficacy was observed when patients were analyzed according to whether they had received induction chemotherapy, with patients who were not chosen to receive induction chemotherapy experiencing a potentially enhanced treatment effect with the addition of pertuzumab. While PERTAIN was not powered to compare these subgroups, these results provide some insight for clinical use and would benefit from further investigation. However, it is important to note that baseline characteristics were different between patients with induction chemotherapy and those without induction chemotherapy, with patients who received induction chemotherapy having less favorable characteristics overall (lower ECOG performance status, more stage IV disease at initial diagnosis, more visceral disease, shorter time since initial diagnosis), which may have impacted the results. Recent results from the sysucc-002 clinical trial showed that trastuzumab plus endocrine therapy was noninferior to trastuzumab plus chemotherapy in patients with HER2-positive and hormone receptor–positive metastatic breast cancer (18), which provides further evidence for the positive outcome in patients not treated with chemotherapy in this setting.
Analysis of median PFS and median OS by time since adjuvant hormone therapy showed numerically longer survival outcomes in the pertuzumab plus trastuzumab arm versus the trastuzumab arm in patients with ≥12 months since adjuvant hormone therapy. There was no difference between study arms in patients with <12 months since adjuvant hormone therapy, or in patients with no prior adjuvant hormone therapy.
PFS and OS were also assessed by estrogen receptor expression. As expected, given the small numbers of patients with estrogen receptor expression <10%, the Kaplan–Meier curves for PFS and OS for patients with estrogen receptor expression ≥10% were consistent with those for the ITT population.
Safety data were in line with the primary analysis of PERTAIN, and were consistent with previous studies, including CLEOPATRA (5, 6) and PERUSE (19). While there were more grade ≥ 3 AEs and SAEs in the pertuzumab plus trastuzumab arm versus the trastuzumab arm, data were as expected and no new safety concerns were detected at final analysis compared with the primary analysis (9). In addition, no deaths were reported as being due to study treatment. With regards to safety by induction chemotherapy, patients who were not chosen to receive induction chemotherapy had a lower incidence of all-grade and grade ≥ 3 AEs, SAEs, and AEs leading to discontinuation of treatment.
Limitations of this study include the lack of power for OS and subgroup analyses, which restricts the strength of conclusions that can be drawn, including those related to OS benefit or differences between patients who were chosen to receive induction chemotherapy after randomization and those who were not. In addition to this, the fact that the choice to administer chemotherapy was at the investigator's discretion may have introduced selection bias, thus influencing these results. However, the results presented here have demonstrated the robustness of the PFS data over time in a study with a diverse patient population (9).
In conclusion, with a median follow-up of > 6 years at final analysis, adding pertuzumab to trastuzumab and an AI was shown to provide >2 years’ PFS benefit, and a similar OS was observed between treatment arms. The treatment combination also presented no new safety concerns at final analysis. Patients who were not chosen to receive induction chemotherapy after randomization experienced a potentially enhanced treatment effect through the addition of pertuzumab to trastuzumab and an AI. Overall, PERTAIN provides additional evidence for the role of pertuzumab and trastuzumab in the first-line treatment of HER2-positive M/LABC and suggests that some patients benefit from pertuzumab plus trastuzumab and an AI without induction chemotherapy.
Supplementary Material
Investigator list and Supplementary Table 1
Acknowledgments
This work was supported by F. Hoffmann-La Roche Ltd. (no grant number).
We would like to thank all the patients who participated in the trial, and their families, the investigators, clinicians, and research staff. Support for third-party writing assistance for this manuscript, furnished by Helen Keyworth, PhD, and Katie Wilson, PhD, of Health Interactions, was provided by F. Hoffmann-La Roche Ltd.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
All authors report receiving research support in the form of third-party writing assistance for this manuscript, provided by F. Hoffmann-La Roche Ltd. G. Arpino reports consulting or advisory roles for Roche, Pfizer, Lilly, Eisai, Novartis, and Amgen; research funding from Roche, Pfizer, and Novartis; and travel, accommodation, or expenses from Roche, Pfizer, Lilly, Eisai, Novartis, and Amgen. J. de la Haba Rodríguez reports consulting or advisory roles for Roche, Pfizer, Lilly, Eisai, Novartis, Amgen, and Agendia; speakers’ bureaus for Roche, Pfizer, Lilly, Eisai, Novartis, Amgen, and Agendia; research funding from Roche and Pfizer; and travel, accommodation, or expenses from Roche, Pfizer, Lilly, and Novartis. J.-M. Ferrero reports consulting or advisory roles for Roche, Pfizer, Novartis, and Eisai. S. De Placido reports consulting or advisory roles for Novartis, Roche, Celgene, AstraZeneca, Amgen, Eisai, Lilly, Pfizer, and Gentili. C.K. Osborne reports stock or other ownership in GeneTex; a consulting or advisory role for GeneTex, Lilly, and Tolmar; and book royalties from Wolters Kluwer. D. Klingbiel is an employee of and has stock or other ownership in F. Hoffmann-La Roche Ltd. V. Revelant is an employee of F. Hoffmann-La Roche Ltd. C. Wohlfarth (and spouse) is an employee of and has stock or other ownership in F. Hoffmann-La Roche Ltd. R. Poppe is an employee of F. Hoffmann-La Roche Ltd. M.F. Rimawi reports speakers’ bureaus for Genentech, Macrogenics, Daiichi Sankyo, and Seattle Genetics, as well as research funding from Pfizer.
Authors' Contributions
G. Arpino: Conceptualization, resources, data curation, supervision, investigation, visualization, writing–original draft, writing–review and editing, approval of final version of manuscript. J. de la Haba Rodríguez: Conceptualization, writing–review and editing, approval of final version of manuscript. J.-M. Ferrero: Conceptualization, formal analysis, validation, investigation, writing–review and editing, approval of final version of manuscript. S. De Placido: Conceptualization, resources, supervision, writing–original draft, writing–review and editing, approval of final version of manuscript. C.K. Osborne: Resources, writing–review and editing, approval of final version of manuscript. D. Klingbiel: Formal analysis, methodology, writing–review and editing, approval of final version of manuscript. V. Revelant: Writing–review and editing, approval of final version of manuscript. C. Wohlfarth: Data curation, writing–original draft, project administration, writing–review and editing, approval of final version of manuscript. R. Poppe: Conceptualization, formal analysis, methodology, writing–review and editing, approval of final version of manuscript. M.F. Rimawi: Conceptualization, formal analysis, supervision, investigation, visualization, methodology, writing–review and editing, approval of final version of manuscript.
References
- 1. Kaufman B, Mackey JR, Clemens MR, Bapsy PP, Vaid A, Wardley A, et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2—positive, hormone receptor–positive metastatic breast cancer: results from the randomized phase III TAnDEM study. J Clin Oncol 2009;27:5529–37. [DOI] [PubMed] [Google Scholar]
- 2. Arpino G, Gutierrez C, Weiss H, Rimawi M, Massarweh S, Bharwani L, et al. Treatment of human epidermal growth factor receptor 2–overexpressing breast cancer xenografts with multiagent HER-targeted therapy. J Natl Cancer Inst 2007;99:694–705. [DOI] [PubMed] [Google Scholar]
- 3. Arpino G, Wiechmann L, Osborne CK, Schiff R. Cross-talk between the estrogen receptor and the HER tyrosine kinase receptor family: molecular mechanism and clinical implications for endocrine therapy resistance. Endocr Rev 2008;29:217–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 2015;372:724–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Swain SM, Miles D, Kim SB, Im YH, Im SA, Semiglazov V, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomized, placebo-controlled, phase III study. Lancet Oncol 2020;21:519–30. [DOI] [PubMed] [Google Scholar]
- 6. Baselga J, Cortés J, Kim SB, Im SA, Hegg R, Im YH, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 2012;366:109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Swain SM, Kim S-B, Cortés J, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomized, double-blind, placebo-controlled, phase III study. Lancet Oncol 2013;14:461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Breast Cancer. Version 1.2022. Available from: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. [Google Scholar]
- 9. Rimawi M, Ferrero J-M, de la Haba-Rodriguez J, Poole C, De Placido S, Osborne CK, et al. First-line trastuzumab plus an aromatase inhibitor, with or without pertuzumab, in human epidermal growth factor receptor 2—positive and hormone receptor–positive metastatic or locally advanced breast cancer (PERTAIN): a randomized, open-label phase II trial. J Clin Oncol 2018;36:2826–35. [DOI] [PubMed] [Google Scholar]
- 10. Arpino G, de la Haba-Rodriguez J, Ferrero J-M, De Placido S, Klingbiel D, Revelant V, et al. Final analysis of PERTAIN: a randomized, two-arm, open-label, multicenter phase II trial assessing the efficacy and safety of first-line pertuzumab given in combination with trastuzumab plus an aromatase inhibitor in patients with HER2-positive and hormone receptor–positive metastatic or locally advanced breast cancer. Cancer Res 2021;81:Abstract PD3–02. [Google Scholar]
- 11. Rivenbank AG, O'Connor SM, Coleman WB. Molecular and cellular heterogeneity in breast cancer: challenges for personalized medicine. Am J Pathol 2013;183:1113–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cejalvo JM, Pascual T, Fernández-Martínez A, Adamo B, Chic N, Vidal M, et al. Distribution of the PAM50 breast cancer subtypes within each pathology-based group: a combined analysis of 15,339 patients across 29 studies. Ann Oncol 2017;28:Abstract 1727P. [Google Scholar]
- 13. Schwartzberg LS, Franco SX, Florance A, O'Rourke L, Maltzman J, Johnston S. Lapatinib plus letrozole as first-line therapy for HER-2+ hormone receptor–positive metastatic breast cancer. Oncologist 2010;15:122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huober J, Fasching PA, Barsoum M, Petruzelka L, Wallwiener D, Thomssen C, et al. Higher efficacy of letrozole in combination with trastuzumab compared with letrozole monotherapy as first-line treatment in patients with HER2-positive, hormone receptor–positive metastatic breast cancer: results of the eLEcTRA trial. Breast 2012;21:27–33. [DOI] [PubMed] [Google Scholar]
- 15. Loft M, Lok SW, De Boer RH, Malik L, Greenberg S, Yeo B, et al. Addition of endocrine therapy to dual anti-HER2 targeted therapy in initial treatment of HER2+/HR+ metastatic breast cancer. J Clin Oncol 2020;38:Abstract 1038. [DOI] [PubMed] [Google Scholar]
- 16. Johnston SRD, Hegg R, Im SA, Park IH, Burdaeva O, Kurteva G, et al. Phase III, randomized study of dual human epidermal growth factor receptor 2 (HER2) blockade with lapatinib plus trastuzumab in combination with an aromatase inhibitor in postmenopausal women with HER2-positive, hormone receptor–positive metastatic breast cancer: updated results of ALTERNATIVE. J Clin Oncol 2021;39:79–89. [DOI] [PubMed] [Google Scholar]
- 17. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomized trials. Lancet 2015;386:1341–52. [DOI] [PubMed] [Google Scholar]
- 18. Yuan Z, Huang JJ, Hua X, Zhao JL, Lin Y, Zhang YQ, et al. Trastuzumab plus endocrine therapy or chemotherapy as first-line treatment for metastatic breast cancer with hormone receptor–positive and HER2-positive: the sysucc-002 randomized clinical trial. J Clin Oncol 2021;39:Abstract 1003 (and oral presentation). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bachelot T, Ciruelos E, Schneeweiss A, Puglisi F, Peretz-Yablonski T, Bondarenko I, et al. Preliminary safety and efficacy of first-line pertuzumab combined with trastuzumab and taxane therapy for HER2-positive locally recurrent or metastatic breast cancer (PERUSE). Ann Oncol 2019;30:766–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Investigator list and Supplementary Table 1
Data Availability Statement
Qualified researchers may request access to individual patient-level data through the clinical study data request platform: https://vivli.org/. Further details on Roche's criteria for eligible studies are available here: https://vivli.org/members/ourmembers/. For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here: https://www.roche.com/innovation/process/clinical-trials/data-sharing/.