Abstract
Purpose:
To investigate the efficacy and safety of the novel orally active PI3Kδ inhibitor in relapsed and/or refractory patients with follicular lymphoma (FL) who had received at least two prior systemic treatments.
Patients and Methods:
Histologically confirmed relapsed and/or refractory patients with FL with disease progression after receiving second-line or greater systemic therapy were enrolled. Linperlisib was administered at 80 mg every day, orally in a 28-day cycle until disease progression or intolerable toxicity occurred. The primary outcome for the study was the objective response rate (ORR), with secondary outcomes including the duration of response (DOR), progression-free survival (PFS), overall survival (OS), disease control rate, and drug safety profile.
Results:
Of 114 screened relapsed and/or refractory patients with FL, 84 were enrolled in the full analysis set (FAS). The ORR of the 84 FAS patients was 79.8% [95% confidence interval (CI), 69.6–87.8, 67 patients], with 13 patients (15.5%) achieving a complete response and 54 patients (64.3%) with a partial response. The median DOR was 12.3 months (95% CI, 9.3–15.9). The median PFS was 13.4 months (95% CI, 11.1–16.7). The 12-month OS rate was 91.4% (95% CI, 82.7–95.8) and a median OS not reached by 42 months. The most frequent (>3%) treatment-related adverse events Grade ≥3 were infectious pneumonia (19.0%), neutropenia (15.5%), decreased lymphocyte count (4.8%), decreased leukocyte count (4.8%), increased lipase (3.6%), decreased platelet count (3.6%), hypertriglyceridemia (3.6%), and interstitial lung disease (3.6%).
Conclusions:
Linperlisib demonstrated compelling clinical activity and manageable tolerability for relapsed and/or refractory patients with FL who had received at least two prior systemic therapies.
Translational Relevance.
Pharmacologic inhibition of the δ isoform of PI3K (PI3Kδ) reduces proliferation, migration, and survival of the malignant B-cell leukemia and lymphoma cells. The novel orally active PI3Kδ inhibitor linperlisib has shown a notable efficacy in B-cell lymphomas, especially for a FL subgroup, in a previous phase I study. In this phase II trial, linperlisib demonstrated compelling efficacy and was generally well-tolerated in the treatment of relapsed or refractory patients with FL after two or more prior systemic therapies.
Introduction
There are circa 68,500 newly diagnosed non-Hodgkin lymphoma (NHL) cases per year in China, approximately 15% of the global incidence (1). As the second leading lymphoma type, follicular lymphoma (FL) is found in 10% to 20% of all NHL cases (2). FL is incurable and 20% of patients experience disease progression within 2 years of first-line treatment (3). The preferred treatment option for FL is chemo-immunotherapy, consisting of combinations of bendamustine; cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP); or cyclophosphamide, vincristine, prednisone (CVP), with CD20 antibodies such as obinutuzumab or rituximab (4) or R2 (rituximab plus lenalidomide). A limitation is the low efficacy of chemo-immunotherapy for the treatment of relapsed malignancies (5). Patients with relapsed disease continue to need subsequent effective treatments as the median overall survival (OS) and progression-free survival (PFS) are only 1.9 and 0.5 years after the sixth-line of treatment compared with 11.7 and 1.5 years after second-line treatment (6).
Currently, chimeric antigen receptor T (CAR-T) cell immunotherapies (such as CD20) have demonstrated good efficacy in relapsed and/or refractory NHL in several published studies (7), yet patients are vulnerable to toxicities associated with the treatment, and patients will progress on this form of therapy, without the capability to reintroduce the treatment. Thus, there is a continuing need in the development of oral therapies.
The δ isoform of PI3K is often dysregulated in various hematologic malignancies (8). In B-cell lymphomas, where the PI3K/AKT pathway has been demonstrated to be highly active, pharmacologic inhibition of PI3Kδ reduces proliferation, migration, and survival of malignant B-cell leukemia and lymphoma cells in the tumor microenvironment (9–11). PI3K inhibitors have been approved by the FDA for relapsed/refractory FL after two or more prior therapies (5, 12–15). Idelalisib produced an objective response rate (ORR) of 57% (16), copanlisib 58.7% (17), umbralisib 45.3% (18), and duvelisib 42.2% (19). Parsaclisib was reported to have a 77.7% ORR in a daily dosing group during a phase II study (20), and zandelisib recently showed 76.0% ORR in 25 relapsed and/or refractory FL patients in a phase Ib study (21). Despite the activity of these agents, there are tolerability issues with high frequencies of toxicities reported, particularly immune-mediated adverse events (AE), that compromise treatment. Several of the products have been removed from the market recently. Thus, a need remains for improved treatments that ideally minimize the risk for cumulative toxicities.
In this study, the efficacy and safety of the novel oral agent, linperlisib, was evaluated in heavily pretreated patients with relapsed and/or refractory FL.
Patients and Methods
Clinical trial information
A phase II, single-arm, open-label clinical trial enrolled 84 patients from 25 sites in China from April 2019 to September 2020, with an analysis cutoff date of September 30, 2021. The list of participating centers is shown in Supplementary Table S1. In a previous phase I dose escalation study, 80 mg/day was recommended as the phase II dose for linperlisib monotherapy, based on safety, tolerability, and pharmacokinetic data (22).
The study was conducted in accordance with guidelines of Declaration of Helsinki, National Medical Products Administration (NMPA) Good Clinical Practice, and was approved by the Institutional Review Boards of the Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences, and all other participating centers involved gave approval for the study protocol. Written informed consent was provided by all participating patients. The trial was registered at clinicaltrials.gov (Trial registration ID: NCT04370405).
Study participants
The detailed inclusion and exclusion criteria are provided in Supplementary Table S2. Inclusion criteria for the trial were: age >18 years, histologically confirmed relapsed and/or refractory FL, disease progression after receiving second-line or greater systemic therapies, and having a ≥4 weeks washout period from the end of any prior antitumor therapy to the start of study treatment. Exclusion criteria were: disease progression after using a PI3Kδ-targeted antineoplastic agent, histologically confirmed Grade 3b FL, patients with known FL transforming to diffuse large B-cell lymphoma, any other antitumor therapy within 4 weeks, or the use of colony-stimulating factor within 14 days before first administration of the study drug. The study participant demographics were considered to be representative for the general Chinese population (Supplementary Table S3).
Procedures
In this study, patients took 80 mg linperlisib tablets once a day with warm water having fasted for 1 hour prior or for 2 hours after administration in a 28-day cycle. Patients continued treatment until disease progression or intolerable toxicity occurred. In addition, sulfamethoxazole (SMZ) for prevention of Pneumocystis carnii pneumonia (PCP) was recommended and other infections were treated after occurrence without using any prophylactic medication. Antitumor response assessments were conducted after every 2 cycles according to the guidelines of the International Research Working Group (23) using the standard imaging modalities of contrast-enhanced CT and MRI scans of the neck, chest, abdomen, and pelvis. ORR was defined as the proportion of patients who exhibited a partial response (PR) or complete response (CR) to the study treatment. For patients with bone marrow involvement at the enrollment, bone marrow biopsies were evaluated to confirm complete responses. Duration of response (DOR) was defined as the period from the start of study treatment until disease progression or death for the patients who had achieved a response. The disease control rate (DCR) was defined as the proportion of patients with CR, PR, and stable disease (SD); PFS was defined as the period from the start of study treatment to any documented progression of the disease or death for any reason, whichever came first. Improvement or exacerbation of general status was evaluated according to changes in the Eastern Cooperative Oncology Group (ECOG) performance status (PS) before and after the study treatment. If patients benefited from therapy, patients would continue treatment until disease progression or intolerable toxicities. Per protocol, patients had a minimum of 12 months follow-up. Peripheral blood collection for serum chemokine analysis was done before taking study drug on C1D1, C1D15, C2D28, and C2D56 (± 3 days) to measure the concentrations of CCL2, CCL3, and CCL13 chemokines; Th1-related IFNγ; and IL6, IL7, and IL8 cytokines, which were reported to be related with PI3Kδ pathways (24–28).
Outcomes
The primary endpoint was ORR evaluated by an Independent Radiographic Committee (IRC). Secondary endpoints were DOR, PFS, the 6- and 12-month PFS rates, DCR, OS, and safety. The enrolled patients were further divided into progression of disease within 2 years (POD24) or non-POD24 groups (29), as well as other criteria for subgroup analyses. NCI-CTCAE 5.0 criteria were used to evaluate all AEs, serious AEs (SAE), changes in clinical laboratory results (routine blood, urine, and fecal analyses, blood biochemistry, coagulation and thyroid functions, immunology and myocardial zymogram examinations), vital signs, 12-lead electrocardiogram, and physical examinations. Safety was reviewed on C1D8, C1D15, and every 2 weeks thereafter for two cycles. Patients were subsequently followed-up every 4 weeks in clinic for safety evaluations until disease progression.
Cytokine measurements
Human plasma samples were immediately frozen and stored in a refrigerator at −80°C. A Luminex‐based multiplex system (Bio‐Rad Laboratories) was used to determine the levels of CCL2, CCL3, and CCL13 chemokines; Th1-related IFNγ; and IL6, IL7, and IL8 according to the manufacturer's instructions.
Statistical analysis
Target sample size was calculated on the assumption of the proportion of patients who would achieve an objective response with treatment of linperlisib. With the reference of historical data and study results of similar products, there was determined that an unacceptable ORR was defined as 40% ORR (null hypothesis), and the ORR for a positive endpoint determination from this study was estimated at 60%. With one-sided test of 0.025 and 90% power, using the exact binomial method, a total of 64 patients were required to achieve the 90% power for statistical significance. Considering dropouts of 20% of the patients, the enrollment of a minimum 80 subjects was planned.
The proportion of patients who achieved an objective response to linperlisib treatment and the corresponding 95% confidence interval (CI) were calculated using the Clopper–Pearson method. PFS and DOR was estimated using the Kaplan–Meier method and the 25% quantile, median, 75% quantile of PFS, and the 95% CI were determined using the Brookmeyer–Crowley method. The 6- and 12-month PFS and OS rates were estimated by Kaplan–Meier analysis and 95% CI calculated by the Greenwood method. The DCR and 95% CI was calculated using the Clopper–Pearson method.
Data availability statement
Individual participant data that underlie the results reported in this article (text, tables, figures, and supplementary data) will be shared after de-identification. Data will be available immediately following publication. Data will be shared with researchers who provide a methodologically sound proposal to achieve aims in the approved proposal. Proposals should be directed to the corresponding author, Lugui Qiu. To gain access, data requestors will need to sign a data access agreement.
Results
Patients
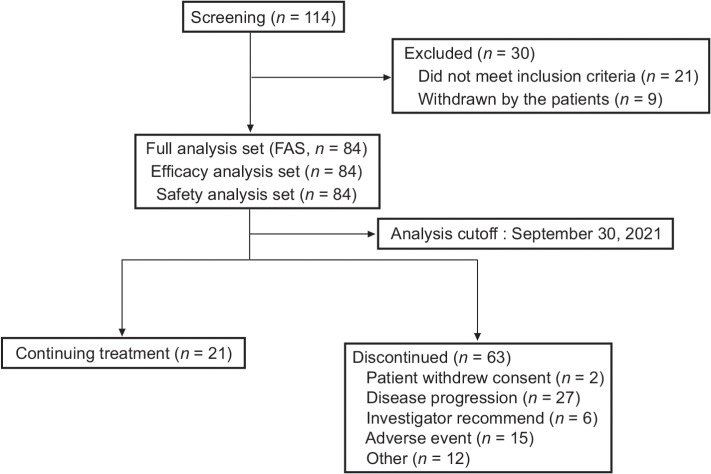
Patient demographics and baseline characteristics are summarized for the FAS population (84 patients) for both efficacy and safety outcome determinations (Fig. 1; Table 1). Patients had a median age of 51 years (range: 29–78). Fifty-four (64.3%) were males. At baseline, most patients had 0 to 1 ECOG PS [0 (53.6%), 1 (44.0%), or 2 (2.4%)]. Also, 37 (44.0%) patients had extranodal tumor involvement, including 25 with bone marrow involvement. The majority of patients had Ann Arbor staging III to IV [79 (94.0%)]. The median FL disease course was 2.7 years (range: 0.1–22.5). All patients had received previous chemoimmunotherapy; in addition, 15 patients received radiotherapy, and 7 patients underwent surgery for tumor excision. The median number of prior regimens was 4 (range: 2–18). At baseline, 39 of 84 patients (46.4%) had relapsed diseases and 14 (16.7%) had refractory (not responding to treatment) disease, whereas 31 (36.9%) had both relapsed and refractory disease (Table 1).
Figure 1.
Flowchart of the study.
Table 1.
Baseline demographic and disease history (FAS).
| Characteristic | N (%) |
|---|---|
| Number of patients | 84 |
| Age, median years (min, max) | 51 (29–78) |
| ≥65 years | 11 (13.1) |
| <65 years | 73 (86.9) |
| Gender, n (%) | |
| Male | 54 (64.3) |
| Female | 30 (35.7) |
| Han ethnicity, n (%) | 81 (96.4) |
| Allergic history, n (%) | 11 (13.1) |
| ECOG PS, n (%) | |
| 0 | 45 (53.6) |
| 1 | 37 (44.0) |
| 2 | 2 (2.4) |
| Time since diagnosis, median years (min, max) | 2.7 (0.1–22.5) |
| Ann Arbor stage, n (%) | |
| Stage II | 5 (6.0) |
| Stage III | 20 (23.8) |
| Stage IV | 59 (70.2) |
| Relapsed case, n (%) | 39 (46.4) |
| Refractory case, n (%) | 14 (16.7) |
| Both relapsed and refractory case, n (%) | 31 (36.9) |
| Median number of prior regimens, n | 4 (2–18) |
| Previous chemotherapy regimens, n (%) | 84 (100.0) |
| Previous anti-CD20 therapy, n (%) | 84 (100.0) |
| Previous radiotherapy treatment, n (%) | 15 (17.9) |
| Previous tumor surgery treatment, n (%) | 7 (8.3) |
| Hematopoietic stem cell transplant, n (%) | 2 (2.4) |
| Previous participation in other clinical trials of anti-tumor therapy, n (%) | 17 (20.2) |
Note: Data are presented as the median (min, max), n (%). The cutoff date for the analysis was September 30, 2021. Baseline was defined as the last non-null observation prior to the first drug administration.
Efficacy outcomes
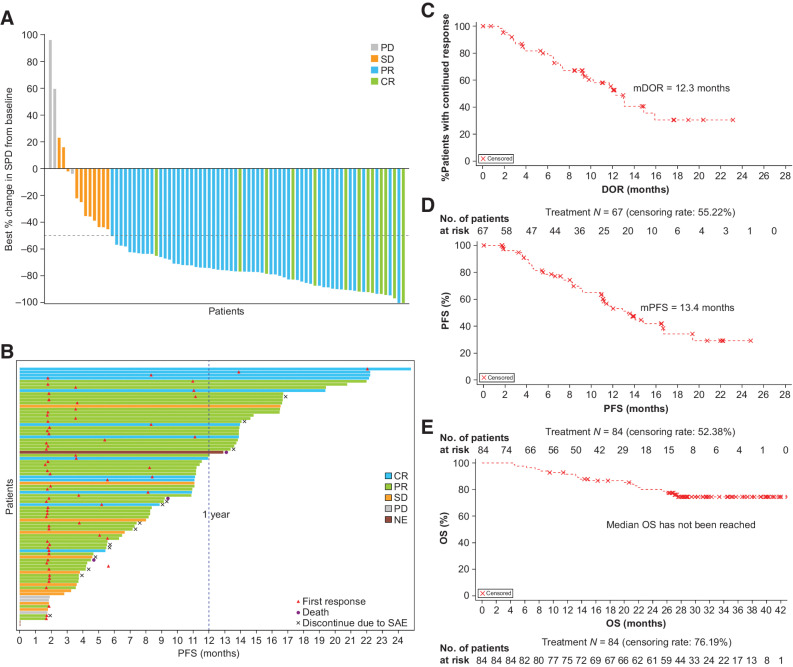
As of September 30, 2021, all patients had the opportunity to receive ≥12 months of follow-up, such that the median follow-up time for the study was 16.7 months (range: 0.9–29.3). Sixty-seven patients achieved responses, with ORR of 79.8% (95% CI, 69.6–87.8) based on IRC assessment. The study met the primary endpoint (P < 0.001) against the null hypothesis of ≤40% ORR with statistical significance. CR was achieved in 13 patients (15.5%), and PR in 54 patients (64.3%), with SD in 11 patients (13.1%) and progressive disease (PD) in 3 patients (3.6%; Table 2; Fig. 2A). Consistent with IRC, investigator assessments showed an ORR of 78.6% (95% CI, 68.3–86.8, 66 patients) was achieved (Supplementary Table S4).
Table 2.
IRC-assessed response outcomes (FAS).
| Response outcome | N (%) | 95% CI (%) |
|---|---|---|
| Objective response rate | 67 (79.8) | 69.6–87.8 |
| Best response | ||
| CR | 13 (15.5) | 8.5–25.0 |
| PR | 54 (64.3) | 53.1–74.5 |
| SD | 11 (13.1) | 6.7–22.2 |
| PD | 3 (3.6) | 0.7–10.1 |
| NA | 3 (3.6) | 0.7–10.1 |
| DCR | ||
| CR + PR + SD | 78 (92.9) | 85.1–97.3 |
| Median, months | 95% CI (%) | |
| Time to first response | 1.9 (range: 1.6–22.1) | |
| DOR | 12.3 | 9.3–15.9 |
| PFS | 13.4 | 11.1–16.7 |
| OS | NR | NR |
| % | 95% CI (%) | |
| DOR rate | ||
| 6 months | 80.0 | 67.4–88.1 |
| 12 months | 55.3 | 40.6–67.8 |
| PFS rate | ||
| 6 months | 78.7 | 67.5–86.3 |
| 12 months | 53.1 | 40.3–64.3 |
| OS rate | ||
| 6 months | 97.6 | 90.6–99.4 |
| 12 months | 91.4 | 82.7–95.8 |
| 24 monthsa | 80.2 | 69.6–87.4 |
| 36 monthsa | 74.5 | 63.3–82.8 |
Abbreviations: NA, not applicable; NR, not reached.
a24-month and 36-month OS assessments were determined from a November 25, 2022 data cutoff.
Figure 2.
Response outcomes and linperlisib treatment in relapsed and/or refractory FL. A, Waterfall plot of tumor change from baseline and best responses (CR, PR, SD, or PD); on the x-axis, each bar is an individual patient. B, A swimmer plot representing the duration of treatment, PFS for each patient on the y-axis and including best response (CR, PR, SD, or PD) to linperlisib; each bar is an individual patient. Timing of first response, death, and discontinuation are noted. A 12-month (1 year) indicator is represented with the blue dashed line. DOR (C), PFS (D), and OS (E) based on IRC assessment.
The median time to response (TTR) was 1.9 months (range: 1.6–22.1), corresponding to the time of the first radiographic assessment after linperlisib therapy was initiated (Table 2). A total of 61 patients (72.6%) who responded to linperlisib treatment had at least 12 months follow-up; 51 patients (60.7%) received the study treatment for ≥12 months (Fig. 2B) with the median DOR being 12.3 months (95% CI, 9.3–15.9; Table 2; Fig. 2C). For all of the patients having had a minimum follow-up time of 12 months, the PFS rate was 78.7% (95% CI, 67.5–86.3) at 6 months and 53.1% (95% CI, 40.3–64.3) at 12 months, with 27 patients achieving more than 1-year PFS with CR and PR (Table 2; Fig. 2B). The median PFS was 13.4 months (95% CI, 11.1–16.7; Fig. 2D). OS was initially determined with the September 30, 2021 data cutoff, and then further OS follow-up (data cutoff of November 25, 2022) demonstrated OS rates at 6, 12, 24, and 36 months that were 97.6% (95% CI, 90.6–99.4), 91.4% (95% CI, 82.7–95.8), 80.2% (95% CI, 69.6–87.4), and 74.5% (95% CI, 63.3–82.8), respectively. The median OS was not reached, and was greater than 42 months (Table 2; Fig. 2E).
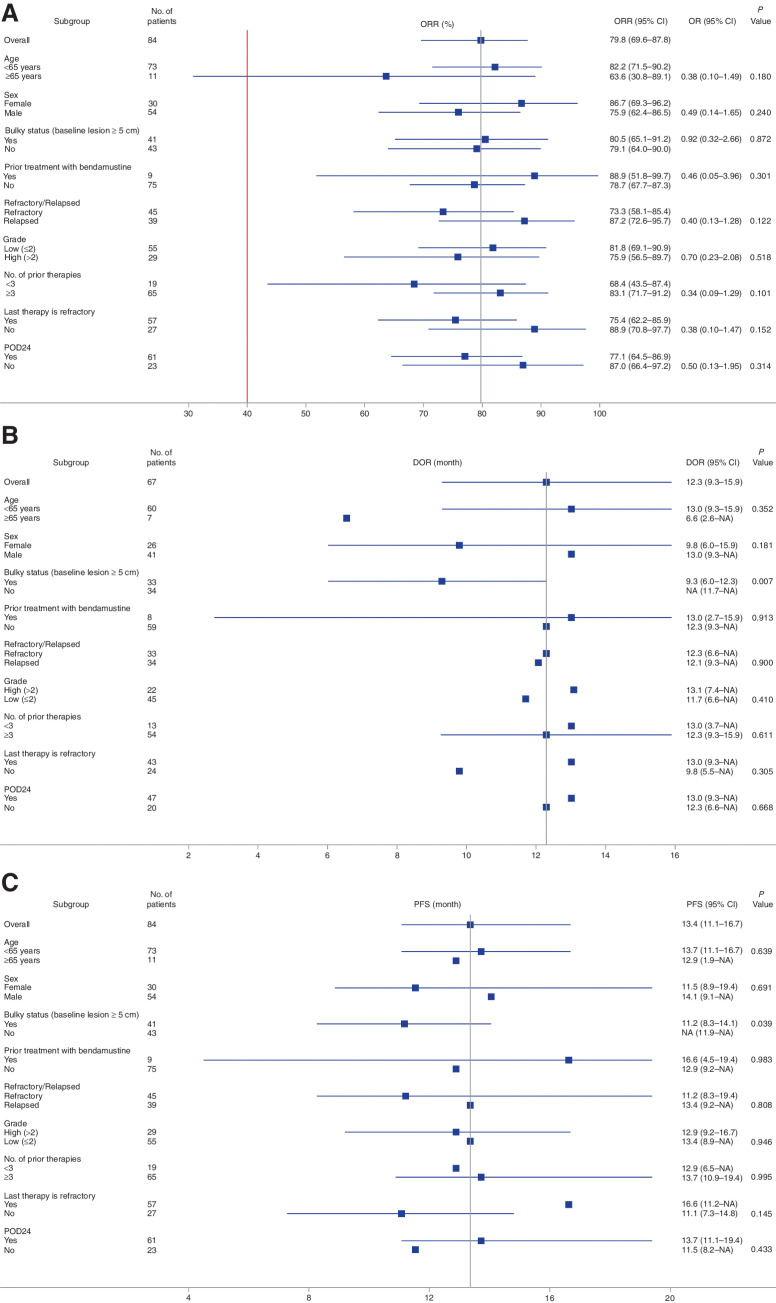
Regarding the patients with highly aggressive disease represented by the POD24 indicator, a subgroup analyses, confirmed by IRC assessment, revealed that there were no statistically significant differences in ORR (77.1% vs. 87.0%, P = 0.314), median DOR (13.0 months vs. 12.3 months, P = 0.668), and median PFS (13.7 months vs. 11.5 months, P = 0.433) between POD24 and non-POD24 patients (Fig. 3A–C); similar results are shown in the subgroup analyses confirmed by investigator assessments (Supplementary Fig. S1).
Figure 3.
Subgroup analysis of ORR, DOR, and PFS confirmed by IRC assessment for patients with FL demographic and baseline criteria. Forest plots for a variety of relapsed and/or refractory patients with FL characteristics are shown for the linperlisib phase II study. ORR (A), DOR (B), and PFS (C). Vertical line represents the median value for ORR, DOR, and PFS for all patients. P values and odds ratio (ORR only) are shown as calculated between the two subgroups for any parameter displayed. Note: In cases where patients had a censor endpoint, the confidence intervals were unable to be calculated, and only the median value is displayed for that subgroup evaluation.
Safety
The most frequent any-grade treatment-related AEs (TRAE) of abnormal laboratory tests were neutropenia (46.4%), decreased lymphocyte count (35.7%), hypertriglyceridemia (23.8%), and increased alanine aminotransferase (ALT, 22.6%; Table 3). The most frequently (>3%) Grade 3 and above TRAEs of abnormal laboratory tests were neutropenia (15.5%), decreased lymphocyte count (4.8%), decreased leukocyte count (4.8%), increased lipase (3.6%), decreased platelet count (3.6%), and hypertriglyceridemia (3.6%; Table 3).
Table 3.
Any-grade TRAEs (>10%) or ≥Grade 3 TRAEs.
| TRAE | Any-grade TRAE with >10% incidence | ≥Grade 3 TRAE |
|---|---|---|
| Laboratory test results | ||
| Neutropenia | 39 (46.4) | 13 (15.5) |
| Decreased leukocyte count | 30 (35.7) | 4 (4.8) |
| Increased ALT | 19 (22.6) | 1 (1.2) |
| Increased AST | 15 (17.9) | 1 (1.2) |
| Decreased lymphocyte count | 14 (16.7) | 4 (4.8) |
| Decreased platelet count | 13 (15.5) | 3 (3.6) |
| Increased blood lactate dehydrogenase | 14 (16.8) | 0 |
| Increased lipase | 12 (14.3) | 3 (3.6) |
| Hypertriglyceridemia | 20 (23.8) | 3 (3.6) |
| Hypercholesterolemia | 13 (15.5) | 0 |
| Hyperuricemia | 9 (10.7) | 0 |
| Hyperglycemia | 11 (13.1) | 1 (1.2) |
| Events | ||
| Diarrhea | 13 (15.5) | 1 (1.2) |
| Weight decreased | 10 (11.9) | 0 |
| Upper respiratory tract infection | 9 (10.7) | 2 (2.4) |
| Interstitial lung disease | 4 (4.8) | 3 (3.6) |
| Infectious pneumonia | 17 (20.2) | 16 (19.0) |
| Rash | 10 (11.9) | 1 (1.2) |
In addition to the laboratory test results, the most common any-grade TRAEs were infectious pneumonia (20.2%), diarrhea (15.5%), weight decrease (11.9%), rash (11.9%), upper respiratory tract infections (10.7%), and interstitial lung disease (4.8%; Table 3). Of these, Grade 3 and above TRAEs were infectious pneumonia (19.0%), interstitial lung disease (3.6%), upper respiratory tract infection (2.4%), rash (1.2%), and diarrhea (1.2%).
The 16 TRAE ≥ Grade 3 infectious pneumonia included 4 patients with bacterial infections (Streptococcus viridans, Stenotrophomonas maltophilia, Escherichia coli, and Pseudomonas aeruginosa), 5 patients with fungal infections (1 Aspergillus, 2 Pneumocystis jiroveci/carinii, 2 pulmonary fungal infection test positive cases), and 7 patients with unknown pathogen. During the enrollment period, 2 patients were diagnosed with PCP in the absence of antimicrobial prophylaxis. However, because antimicrobial prophylaxes with trimethoprim and sulfamethoxazole combination were implemented, no further PCP was reported, implying that PCP could be effectively prevented with the prophylactic management.
Dose interruptions were reported in 36 patients (42.9%), and 15 patients (17.9%) discontinued the treatment due to linperlisib-related AEs, with the highest proportion being mainly associated with infectious pneumonia (n = 9, 10.7%) and interstitial lung disease (n = 4, 4.8%; Supplementary Table S5).
Chemokine/cytokine analysis on linperlisib treatment
Levels of CCL2, CCL3, CCL13, IFNγ, IL6, IL7, and IL8 were measured in the serum of 84 patients before the first dose, and pre-dose on C1D15, C1D28, and C2D28 (±3 days) to evaluate whether blood levels were altered on linperlisib treatment, and to determine relationships between these chemokines and efficacy. The concentrations of CCL2, CCL3, CCL13, IFNγ, IL7, and IL8 were not significantly changed from baseline and did not differ whether patients were CR or PR to linperlisib treatment or did not respond to treatment while IL6 serum concentrations were especially higher at C2D28 in nonresponders than in responders (Supplementary Figs. S2 and S3).
Discussion
The phase II study of 84 relapsed and/or refractory patients with FL linperlisib monotherapy met the primary endpoint with an ORR of 79.8% with statistical significance in a heavily pretreated patient with FL population with a median of 4 prior therapies. In previous reports on relapsed and/or refractory FL or NHL clinical trials where the FL subgroup was specified, the FL ORRs were 57% (idelalisib; ref. 16), 42.2% (duvelisib; ref. 19), 59% (copanlisib; ref. 17), and 45% (umbralisib; ref. 18). Like linperlisib, parsaclisib and zandelisib treatments reported 77.7% ORR and 76.0% ORR in a daily dosing cohort of the respective phase II and phase Ib studies (20, 21). In addition, in this linperlisib study the median PFS was 13.4 months, with all of the patients having had a minimum follow-up time of 12 months, whereas the 6-month PFS rate was 78.7% and 12-month PFS rate was 53.1%. The median DOR was 12.3 months and the 6-month DOR rate was 80.0%. At the September 30, 2021, data cutoff, 21 patients were still receiving linperlisib treatment. Considering the linperlisib efficacy parameters, median PFS and median DOR are comparable with the reported values for approved PI3K inhibitors, including idelalisib (median DOR 12.5 months, median PFS 11.0 months; ref. 16), copanlisib (median DOR 16.1 months, median PFS 12.5 months; ref. 17), duvelisib (median DOR 10 months, median PFS 9.5 months; ref. 19), or umbralisib, (median DOR 11.1 months, median PFS 10.6 months; ref. 18; Supplementary Table S6).
The baseline characteristics of patients in this study were generally similar to the relapsed and/or refractory patients with FL in pivotal clinical studies of the approved PI3K inhibitors. The majority of patients had advanced stage disease at baseline with 6.0% having Ann Arbor–Cotswold's staging II and 94.0% stage III/IV FL. The ECOG PS was 0 or 1 in most patients (97.6%) and the FL histologic type indicated the disease characteristics expected of this patient population. In this study, there was a younger median age (51 years) than exhibited in relapsed and/or refractory FL trials with other PI3K inhibitors, although this age is typical for Chinese FL patients (30). Although it cannot be ruled out that younger age patients have favorable responses to linperlisib, the subgroup of 11 patients >64 years had ORR, PS, and DOR that was not statistically different from all patients in the study by IRC assessment (P ≥ 0.05; Fig. 3A–C), and investigator assessment (Supplementary Fig. S1). By all of these criteria combined, linperlisib treatment elicited significant durable responses in the relapsed and/or refractory patients with FL.
In this study, patients had received a median of four prior systemic treatments, and 77.4% had three or more prior therapies. It is important to note that a high ORR (83.1%) was observed for the linperlisib-treated patients who had at least three prior lines of therapy (Fig. 3A). Similar median prior lines of therapy were reported in clinical trials for idelalisib (four prior), duvelisib (three prior), or copanlisib (three prior, with 34% of patients having two prior lines of therapy; refs. 17, 31). Also, the percentage of patients who were refractory to their last treatment regimen or to the last anti-CD-20 immunotherapy (68% and 57%, respectively) was also typical of clinical trials with the other PI3K inhibitors. Likewise, all patients in this study had received prior rituximab and chemotherapy, although some of the patients received alternative combination therapy with rituximab. Regardless of the relapsed or refractory status and the prior treatments, linperlisib treatment resulted in ORR that was high, and not significantly different from all patients on the study (Fig. 3A). Clinical benefit was observed in 3 patients who had prior R2 (rituximab plus lenalidomide) and bendamustine-containing treatment regimens, where all 3 responded to linperlisib (one CR, two PR), suggesting that linperlisib will be a potentially effective therapy in the evolving treatment landscape, with the introduction of newly approved therapies.
It has been established that patients with FL in POD24 were generally associated with poor outcomes and unfavorable 5- or 10-year OS rates (29). In this trial, subgroup analyses revealed 77.1% ORR for POD24 patients compared with non-POD24 patients (87.0%; statistically significant difference was not found). Further, the median DOR (13.0 months vs. 12.3 months) and median PFS (13.7 months vs. 11.5 months) were numerically longer for POD24 compared with non-POD24 patients, respectively, and PD incidences were only 3.3% for POD24 and 7.2% for non-POD24 patients. Taken together, linperlisib is an effective treatment for the poor prognostic POD24 subgroup.
In previous research focusing on reasons for the high rates of infections under PI3Kδ inhibitor treatments, correlations of immune system parts with PI3Kδ inhibitor activities have been investigated. The study noted inhibitory effects of idelalisib on T-cell functions including cytokine production, migration, and proliferation, which were supposed to be responsible for viral reactivations and gastrointestinal symptoms associated with infectious pathogens (32). Later the research on PI3Kδ inhibitor toxicities related to T-cell immune response impairment has been extended to other drugs used to treat FL including copanlisib and duvelisib, and it was found that during treatments serum concentrations of various cytokines, interleukins, and other immune system–related factors were reduced. However, T-cell–related toxicities have been detected more frequently in treatment-naïve than in pretreated patients (24), which might explain the lack of changes for most of the included of cytokines. Besides, IL6 expression has been significantly higher in nonresponders than in responders at the end of the treatment, which might explain the nonresponsiveness since IL6 has been recognized as a resistance factor for PI3K-pathway–targeted lymphoma therapy (33).
The AE profile of the 84 relapsed and/or refractory patients with FL enrolled in this phase II trial indicated good tolerability and an acceptable safety profile, consistent with the safety data initially observed in the linperlisib phase I study of FL and other patients with B-cell lymphoma (22). In this study, the most common Grade 3 and above were pneumonia (19.9%) and neutropenia (15.5%), both of which were previously observed for the FL population, and have been commonly seen with other PI3K inhibitor treatments. Grade 3 and above pneumonia is seen with other PI3Kis with similar incidence (zandelisib, 16%, continuous dosing group), or potentially somewhat lower levels (idelalisb, 7%; duvelisib, 5.4%; refs. 16, 18, 19, 21). The occurrence of interstitial lung disease (n = 4) and atypical fungal pneumonia (n = 5) with linperlisib treatment should be closely monitored and further investigated and managed during treatment. The observation from this study that no PCP pneumonia was reported after the implementation of PCP prophylaxis and dose interruption and modification to prevent the occurrence and worsening of interstitial lung diseases suggests that close monitoring and early intervention could play an important role in further and better managing the treatment of disease-related toxicities. Importantly in this study, serious (Grade 3/4) diarrhea, colitis, ALT/aspartate aminotransferase (AST) elevation, rash, hyperglycemia, or hypertension were notably rare (<2%).
Previously approved PI3K inhibitors have produced toxicities that compromised the duration of treatment and were often associated with immune-mediated AEs of gastrointestinal and liver toxicities. For idelalisib and duvelisib, significant treatment-related side effects included ≥Grade 3 AE hepatotoxicity (ALT/AST elevation), diarrhea/colitis, rash, and pneumonitis, contributing to black box warnings. Consequently, these treatments were associated with high frequencies of treatment discontinuations (31.0%), dose reductions (19.4%; ref. 19), and SAEs (34). Fatal or serious infections were also reported frequently after idelalisib and duvelisib therapy. For copanlisib, 50% and 29% of patients developed hyperglycemia and hypertension, respectively, leading to dose interruptions in nearly three quarters of patients. The prevalence of hyperglycemia was likely related to copanlisib inhibition of the PI3Kα isoform. Twenty-nine percent of patients required dose reductions and 21.1% required discontinuation of therapy due to AEs (17), and their use in an elderly patient population with a high prevalence of comorbidities has been hampered. Grade ≥3 diarrhea occurred in 11.9% whereas on parsaclisib 11.9% (20), on duvelisib 14.7% (19), on copanlisib 8.5% (17), and on idelalisib 13% (16). Also, the reported Grade ≥3 ALT/AST elevation occurred in 13%/8% on idelalisib (16). The continuous dosing group of zandelisib-treated patients had ≥Grade 3 of diarrhea/colitis (24%), rash (5%), ALT or AST elevation (5%), or mucositis (3%) reported (21).
The linperlisib study had a discontinuation incidence of 17.9% overall (Supplementary Table S5). Discontinuations due to AE were mainly infectious pneumonia (10.7%), including the 2 PCP patients in the absence of prophylaxis, and interstitial lung disease (4.8%). Thus, the use of linperlisib needs to be monitored and can be further managed by prophylaxis, as there were no PCPs reported after prophylaxis was implemented, and by early intervention. It is unknown whether continuous dosing of linperlisib is required to maintain responses following the rapid occurrence of response with a median TTR of 1.9 months (Fig. 2B). Considering the appearance of AEs and the intent to treat for long duration, an evaluation of an alternative linperlisib dose schedule may be valuable to potentially reduce toxicities and maintain efficacy.
Several features of linperlisib may be contributing factors to the efficacy and tolerability observed in relapsed and/or refractory patient with FL population. The linperlisib chemical structural distinctions as a next-generation inhibitor may impart a high selectivity against PI3Kδ versus several approved PI3K drugs. Linperlisib has greater selectivity for PI3Kd over PI3Kg than idelalisib and duvelisib (100-fold), and umbralisib (48-fold) in vitro (20). Umbralisib has the additional limitation of being a dual PI3Kδ and CKε inhibitor (18). Also, copanlisib (i.v. administered) is a PI3Kα and PI3Kδ dual inhibitor, whereas linperlisib is also highly selective against PI3Kδ and linperlisib treatment did not have the associated hyperglycemia and hypertension AEs. It should be noted that linperlisib excretion is principally renal in single dose 14C-linperlisib tracing studies, whereas published information from duvelisib and idelalisib would indicate primarily excretion through the gut (35). These features potentially all contribute to the manageable safety profile of linperlisib. Along with the compelling clinical efficacy observed, linperlisib is a valuable treatment option for patients with relapsed and/or refractory FL. With the NMPA accelerated approval in China on November 8, 2022, linperlisib is available as a treatment for relapsed and/or refractory FL patients with two or more prior systemic therapies.
As a perspective, novel CAR T-cell therapies have been introduced for treatments of relapsed and/or refractory patients with FL and a bispecific antibody (BSA) therapy should be added to CAR T-cell therapy as potential therapeutic option for FL (36). For patients who relapse after those agents, linperlisib might be an option as subsequent systemic therapy.
In conclusion, linperlisib demonstrated compelling clinical efficacy and exhibited a manageable safety profile, making it a valuable treatment modality for patients with relapsed and/or refractory FL after at least two prior systemic treatments.
Supplementary Material
List of participating hospitals for the clinical trial NCT04370405
Subgroup analysis of (A) ORR, (B) DOR and (C) PFS confirmed by investigator
Detailed inclusion and exclusion criteria for the clinical trial (NCT04370405)
Serum cytokine concentrations before and during treatment
Representativeness of Study Participants
Comparison of the concentrations of serum markers measured in patients with and without a response to linperlisib
Investigator-assessed response outcomes (FAS)
Number and frequency of TRAE that led to discontinuation from the trial
Comparison of PI3K inhibitor toxicities and their efficacies for relapsed and/or refractory indolent lymphoma treatments
Acknowledgments
This trial was funded by Shanghai Yingli Pharmaceutical Co., Ltd. The funding body was involved in the design of the study, collection, analysis and interpretation of data, writing the report, and the decision to submit the manuscript for publication. This work was supported by grants from Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2022-I2M-1–022). Medical writing assistance was funded by Shanghai Yingli Pharmaceutical Co., Ltd. All authors had full access to the study data and accepted responsibility to submit the manuscript for publication.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This article is featured in Highlights of This Issue, p. 1375
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
H. Bao reports personal fees from Shanghai Yingli Pharmaceutical Co., Ltd. during the conduct of the study. Z. Xu reports personal fees from Shanghai Yingli Pharmaceutical Co., Ltd. during the conduct of the study. L. Qiu reports personal fees from Shanghai Yingli Pharmaceutical Co., Ltd. during the conduct of the study. No disclosures were reported by the other authors.
Authors' Contributions
T. Wang: Data curation, formal analysis, validation, investigation, writing–original draft, writing–review and editing. X. Sun: Data curation, formal analysis, validation, investigation, writing–original draft, writing–review and editing. L. Qiu: Data curation, formal analysis, validation, investigation, writing–review and editing. H. Su: Data curation, formal analysis, validation, investigation, writing–review and editing. J. Cao: Data curation, formal analysis, validation, investigation, writing–review and editing. Z. Li: Data curation, formal analysis, validation, investigation, writing–review and editing. Y. Song: Data curation, formal analysis, validation, investigation, writing–review and editing. L. Zhang: Data curation, formal analysis, validation, investigation, writing–review and editing. D. Li: Data curation, formal analysis, validation, investigation, writing–review and editing. H. Wu: Data curation, formal analysis, validation, investigation, writing–review and editing. W. Zhang: Data curation, formal analysis, validation, investigation, writing–review and editing. J. Li: Data curation, formal analysis, validation, investigation, writing–review and editing. K. Zhou: Data curation, formal analysis, validation, investigation, writing–review and editing. H. Zhou: Data curation, formal analysis, validation, investigation, writing–review and editing. Y. Yang: Data curation, formal analysis, validation, investigation, writing–review and editing. Z. Li: Data curation, formal analysis, validation, investigation, writing–review and editing. H. Cen: Data curation, formal analysis, validation, investigation, writing–review and editing. Z. Cai: Data curation, formal analysis, validation, investigation, writing–review and editing. Z. Zhang: Data curation, formal analysis, validation, investigation, writing–review and editing. W. Fu: Data curation, formal analysis, validation, investigation, writing–review and editing. J. Jin: Data curation, formal analysis, validation, investigation, writing–review and editing. F. Li: Data curation, formal analysis, validation, investigation, writing–review and editing. W. Wu: Data curation, formal analysis, validation, investigation, writing–review and editing. X. Gu: Data curation, formal analysis, validation, investigation, writing–review and editing. W. Zhu: Data curation, formal analysis, validation, investigation, writing–review and editing. L. Liu: Data curation, formal analysis, validation, investigation, writing–review and editing. Z. Li: Data curation, formal analysis, validation, investigation, writing–review and editing. S. Yi: Data curation, formal analysis, validation, investigation, writing–original draft, writing–review and editing. H. Bao: Data curation, formal analysis, validation, investigation, writing–review and editing. Z. Xu: Data curation, formal analysis, validation, investigation, writing–review and editing. L. Qiu: Conceptualization, data curation, formal analysis, supervision, validation, investigation, writing–original draft, writing–review and editing.
References
- 1. Liu W, Liu J, Song Y, Zeng X, Wang X, Mi L, et al. Burden of lymphoma in China, 2006–2016: an analysis of the global burden of disease study 2016. J Hematol Oncol 2019;12:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fischer T, Zing NPC, Chiattone CS, Federico M, Luminari S. Transformed follicular lymphoma. Ann Hematol 2018;97:17–29. [DOI] [PubMed] [Google Scholar]
- 3. Casulo C, Nastoupil L, Fowler NH, Friedberg JW, Flowers CR. Unmet needs in the first-line treatment of follicular lymphoma. Ann Oncol 2017;28:2094–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Luminari S, Ferrari A, Manni M, Dondi A, Chiarenza A, Merli F, et al. Long-term results of the FOLL05 trial comparing R-CVP versus R-CHOP versus R-FM for the initial treatment of patients with advanced-stage symptomatic follicular lymphoma. J Clin Oncol 2018;36:689–96. [DOI] [PubMed] [Google Scholar]
- 5. Burger JA, Okkenhaug K. Haematological cancer: idelalisib-targeting PI3Kδ in patients with B-cell malignancies. Nat Rev Clin Oncol 2014;11:184–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Batlevi CL, Sha F, Alperovich A, Ni A, Smith K, Ying Z, et al. Follicular lymphoma in the modern era: survival, treatment outcomes, and identification of high-risk subgroups. Blood Cancer J 2020;10:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Denlinger N, Bond D, Jaglowski S. CAR T-cell therapy for B-cell lymphoma. Curr Probl Cancer 2022;46:100826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carlo-Stella C, Delarue R, Scarfo L, Barde PJ, Nair A, Locatelli SL, et al. A first-in-human study of tenalisib (RP6530), a dual PI3K δ/γ inhibitor, in patients with relapsed/refractory hematologic malignancies: results from the European study. Clin Lymphoma Myeloma Leuk 2020;20:78–86. [DOI] [PubMed] [Google Scholar]
- 9. Balakrishnan K, Peluso M, Fu M, Rosin NY, Burger JA, Wierda WG, et al. The phosphoinositide-3-kinase (PI3K)-delta and gamma inhibitor, IPI-145 (duvelisib), overcomes signals from the PI3K/AKT/S6 pathway and promotes apoptosis in CLL. Leukemia 2015;29:1811–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoellenriegel J, Meadows SA, Sivina M, Wierda WG, Kantarjian H, Keating MJ, et al. The phosphoinositide 3'-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood 2011;118:3603–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peluso M, Faia K, Winkler D, Patel N, Brophy E, White K, et al. Duvelisib (IPI-145) inhibits malignant B-cell proliferation and disrupts signaling from the tumor microenvironment through mechanisms that are dependent on PI3K-δ and PI3K-γ. Blood 2014;124:328.24894774 [Google Scholar]
- 12. Bird ST, Tian F, Flowers N, Przepiorka D, Wang R, Jung TH, et al. Idelalisib for treatment of relapsed follicular lymphoma and chronic lymphocytic leukemia: a comparison of treatment outcomes in clinical trial participants vs medicare beneficiaries. JAMA Oncol 2020;6:248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brown JR. The PI3K pathway: clinical inhibition in chronic lymphocytic leukemia. Semin Oncol 2016;43:260–4. [DOI] [PubMed] [Google Scholar]
- 14. Cheah CY, Fowler NH. Idelalisib in the management of lymphoma. Blood 2016;128:331–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Magagnoli M, Carlo-Stella C, Santoro A. Copanlisib for the treatment of adults with relapsed follicular lymphoma. Expert Rev Clin Pharmacol 2020;13:813–23. [DOI] [PubMed] [Google Scholar]
- 16. Gopal AK, Kahl BS, de Vos S, Wagner-Johnston ND, Schuster SJ, Jurczak WJ, et al. PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med 2014;370:1008–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dreyling M, Santoro A, Mollica L, Leppä S, Follows G, Lenz G, et al. Long-term safety and efficacy of the PI3K inhibitor copanlisib in patients with relapsed or refractory indolent lymphoma: 2-year follow-up of the CHRONOS-1 study. Am J Hematol 2020;95:362–71. [DOI] [PubMed] [Google Scholar]
- 18. Fowler NH, Samaniego F, Jurczak W, Ghosh N, Derenzini E, Reeves JA, et al. Umbralisib, a dual PI3Kδ/CK1ε inhibitor in patients with relapsed or refractory indolent lymphoma. J Clin Oncol 2021;39:1609–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Flinn IW, Miller CB, Ardeshna KM, Tetreault S, Assouline SE, Mayer J, et al. DYNAMO: a phase II study of duvelisib (IPI-145) in patients with refractory indolent non-hodgkin lymphoma. J Clin Oncol 2019;37:912–22. [DOI] [PubMed] [Google Scholar]
- 20. Lynch RC, Avigdor A, McKinney MS, Paneesha S, Wahlin B, Hrom JS, et al. Efficacy and safety of parsaclisib in patients with relapsed or refractory follicular lymphoma: primary analysis from a phase 2 study (CITADEL-203). Blood 2021;138:813. [Google Scholar]
- 21. Pagel JM, Soumerai JD, Reddy N, Jagadeesh D, Stathis A, Asch A, et al. Zandelisib with continuous or intermittent dosing as monotherapy or in combination with rituximab in patients with relapsed or refractory B-cell malignancy: a multicentre, first-in-patient, dose-escalation and dose-expansion, phase 1b trial. Lancet Oncol 2022;23:1021–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jiang B, Qi J, Song Y, Li Z, Tu M, Ping L, et al. Phase 1 clinical trial of the PI3Kδ inhibitor YY-20394 in patients with B-cell hematological malignancies. J Hematol 2021;14:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Younes A, Hilden P, Coiffier B, Hagenbeek A, Salles G, Wilson W, et al. International working group consensus response evaluation criteria in lymphoma (RECIL 2017). Ann Oncol 2017;28:1436–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tarantelli C, Argnani L, Zinzani PL, Bertoni F. PI3Kδ inhibitors as immunomodulatory agents for the treatment of lymphoma patients. Cancers (Basel) 2021;13:5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lafarge ST, Johnston JB, Gibson SB, Marshall AJ. Adhesion of ZAP-70+ chronic lymphocytic leukemia cells to stromal cells is enhanced by cytokines and blocked by inhibitors of the PI3-kinase pathway. Leuk Res 2014;38:109–15. [DOI] [PubMed] [Google Scholar]
- 26. Flaishon L, Lantner F, Hershkoviz R, Levo Y, Shachar I. Low levels of IFN-gamma down-regulate the integrin-dependent adhesion of B cells by activating a pathway that interferes with cytoskeleton rearrangement. J Biol Chem 2001;276:46701–6. [DOI] [PubMed] [Google Scholar]
- 27. Wain JH, Kirby JA, Ali S. Leucocyte chemotaxis: examination of mitogen-activated protein kinase and phosphoinositide 3-kinase activation by monocyte chemoattractant proteins-1, -2, -3 and -4. Clin Exp Immunol 2002;127:436–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Barata JT, Silva A, Brandao JG, Nadler LM, Cardoso AA, Boussiotis VA. Activation of PI3K is indispensable for interleukin 7-mediated viability, proliferation, glucose use, and growth of T cell acute lymphoblastic leukemia cells. J Exp Med 2004;200:659–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sortais C, Lok A, Tessoulin B, Gastinne T, Mahe B, Dubruille V, et al. Progression of disease within 2 years (POD24) is a clinically relevant endpoint to identify high-risk follicular lymphoma patients in real life. Ann Hematol 2020;99:1595–604. [DOI] [PubMed] [Google Scholar]
- 30. Zha J, Fan L, Yi S, Yu H, Zheng Z, Xu W, et al. Clinical features and outcomes of 1845 patients with follicular lymphoma: a real-world multicenter experience in China. J Hematol Oncol 2021;14:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Flinn IW, O'Brien S, Kahl B, Patel M, Oki Y, Foss FF, et al. Duvelisib, a novel oral dual inhibitor of PI3K-δ,γ, is clinically active in advanced hematologic malignancies. Blood 2018;131:877–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Martinelli S, Maffei R, Fiorcari S, Quadrelli C, Zucchini P, Benatti S, et al. Idelalisib impairs T-cell-mediated immunity in chronic lymphocytic leukemia. Haematologica 2018;103:e598–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim JH, Kim WS, Park C. Interleukin-6 mediates resistance to PI3K-pathway–targeted therapy in lymphoma. BMC Cancer 2019;19:936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Patel K, Danilov AV, Pagel JM. Duvelisib for CLL/SLL and follicular non-hodgkin lymphoma. Blood 2019;134:1573–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yu J, Zhang H, Zhang Y, Zhan Y, Ma S, Hu T, et al. Absorption, metabolism, and excretion of [(14)C]YY-20394, a highly selective PI3K-Delta inhibitor in humans. Xenobiotica 2022;52:254–64. [DOI] [PubMed] [Google Scholar]
- 36. Rampotas A, Sangha G, Collins GP. Integration of cell therapies and bispecific antibodies into the treatment pathway of relapsed diffuse large B-cell lymphoma. Ther Adv Hematol 2021;12:20406207211053120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of participating hospitals for the clinical trial NCT04370405
Subgroup analysis of (A) ORR, (B) DOR and (C) PFS confirmed by investigator
Detailed inclusion and exclusion criteria for the clinical trial (NCT04370405)
Serum cytokine concentrations before and during treatment
Representativeness of Study Participants
Comparison of the concentrations of serum markers measured in patients with and without a response to linperlisib
Investigator-assessed response outcomes (FAS)
Number and frequency of TRAE that led to discontinuation from the trial
Comparison of PI3K inhibitor toxicities and their efficacies for relapsed and/or refractory indolent lymphoma treatments
Data Availability Statement
Individual participant data that underlie the results reported in this article (text, tables, figures, and supplementary data) will be shared after de-identification. Data will be available immediately following publication. Data will be shared with researchers who provide a methodologically sound proposal to achieve aims in the approved proposal. Proposals should be directed to the corresponding author, Lugui Qiu. To gain access, data requestors will need to sign a data access agreement.