Abstract
Purpose:
In the dose-expansion part of this open-label, phase I study, we explored the efficacy and safety of E7389-LF (liposomal formulation of eribulin) in Japanese patients with advanced gastric cancer.
Patients and Methods:
Patients with advanced gastric cancer who had been previously treated with ≥2 lines of chemotherapy received E7389-LF 2.0 mg/m2 every 3 weeks (the previously determined maximum tolerated dose, the primary objective of Study 114). Secondary objectives included objective response rate (ORR), progression-free survival (PFS), and safety; exploratory objectives included disease control rate (DCR) and clinical benefit rate (CBR), as well as pharmacodynamic measurements of serum biomarkers.
Results:
As of June 24, 2021, 34 patients were enrolled and treated (10 from the original dose-expansion cohort, expanded to include 24 additional patients). Six patients had partial responses, for an ORR of 17.6% [95% confidence interval (CI), 6.8–34.5], and the median PFS was 3.7 months (95% CI, 2.7–4.8). The DCR was 79.4% (95% CI, 62.1–91.3), and the CBR was 32.4% (95% CI, 17.4–50.5). Overall, 32 patients (94.1%) experienced treatment-related adverse events, and 26 patients (76.5%) experienced grade ≥3 events, most commonly neutropenia (41.2%) and leukopenia (29.4%). Of the 8 endothelial cell/vasculature markers tested in this study, 7 were significantly increased among patients treated with E7389-LF; these changes were generally consistent regardless of best overall response.
Conclusions:
E7389-LF 2.0 mg/m2 every 3 weeks was tolerable and showed preliminary activity for the treatment of patients with gastric cancer.
Translational Relevance.
This open-label expansion part of a phase I study assessed the efficacy and tolerability of a liposomal formulation of eribulin (E7389-LF) dosed at 2.0 mg/m2 once every 3 weeks in patients with advanced gastric cancer. Of the 34 patients enrolled, 6 achieved a partial response, for a response rate of 17.6%. In patients who were previously treated with anti–programmed cell death-1 (PD-1) therapies, the progression-free survival and overall survival tended to be longer in patients who had received previous anti–PD-1 therapy within the past 6.7 months versus those who had received anti–PD-1 therapy more than 6.7 months before receiving study treatment. This E7389-LF formulation was well tolerated overall. Further, endothelial cell and vasculature markers were increased after treatment; thus, eribulin may influence tumor vascular remodeling by increasing these markers. This study supports the further development of E7389-LF for patients with advanced gastric cancer.
Introduction
Stomach cancer is the fourth leading cause of cancer death in the world and the fifth most common malignant tumor type (1). Chemotherapy with or without an anti–programmed cell death-1 (PD-1) monoclonal antibody is the standard first-line treatment for patients with advanced gastric cancer (plus trastuzumab as an anti-HER2 monoclonal antibody for HER2-positive cases; ref. 2). However, the median overall survival (OS) duration is generally less than 14 months (3, 4). Therapies used in the later line tend to be less effective (5–8); thus, newer treatment options are needed.
Eribulin mesylate (eribulin) is a microtubule dynamics inhibitor approved for the treatment of patients with locally advanced or metastatic breast cancer after ≥1 prior lines of chemotherapy in the European Union (9) and for metastatic breast cancer after ≥2 prior lines of chemotherapy in the United States (10); prior treatments should include a taxane and an anthracycline. Eribulin is also approved for the treatment of inoperable or recurrent breast cancer and soft-tissue sarcoma in Japan (11) and for unresectable liposarcoma after anthracycline therapy in Europe (9). Eribulin has shown several mechanisms of vascular remodeling, including increasing vascular perfusion and permeability, promoting the epithelial state, and reducing cancer cell migration (12, 13).
E7389-LF is a liposomal formulation of eribulin developed to increase the therapeutic index of eribulin (14). A preclinical study of E7389-LF plus an anti–PD-1 therapy has suggested that E7389-LF shows unique immunomodulatory properties, including increasing the population of T cells and natural killer cells (15). In the dose-escalation part of the phase I Study 114 of E7389-LF, the recommended regimen of E7389-LF was 2.0 mg/m2 of free-base eribulin intravenously every 3 weeks (14). Notably, E7389-LF increased the expression of multiple biomarkers, including VEGFR3, intercellular adhesion molecule 1 (ICAM1), platelet and endothelial cell adhesion molecule 1, collagen IV, and TEK receptor tyrosine kinase (TEK, also known as TIE2; ref. 14). An expansion part of this study assessed the safety and efficacy of E7389-LF 2.0 mg/m2 intravenously every 3 weeks in patients with advanced HER2-negative breast cancer (16), adenoid cystic carcinoma, esophageal cancer, gastric cancer, or small cell lung cancer (17). Most notably, 2 of the 10 patients initially enrolled in the heavily pretreated gastric cancer cohort had an objective response. Thus, the gastric cancer cohort was expanded for further evaluation. Here, we report on the efficacy and safety of E7389-LF for patients with advanced gastric cancer in an expanded phase I study's dose-expansion cohort.
Patients and Methods
Study design and patients
In the dose-expansion part of Study 114, patients with advanced cancers were administered E7389-LF 2.0 mg/m2 (free-base) infused intravenously every 3 weeks (16, 17); this part included a cohort of patients with gastric cancer. This study was conducted in accordance with standard operating procedures of the sponsor, which were based on the Principles of the World Medical Association Declaration of Helsinki, all applicable Japanese Good Clinical Practices and regulations, and the Pharmaceutical and Medical Device Act for studies conducted in Japan. Ethical approval and written informed consent were granted and approved by the applicable local institutional review board; signed informed consent forms were obtained from each patient prior to enrollment.
In this expanded analysis of the gastric cancer cohort, eligible patients were diagnosed with gastric cancer that had no alternative standard or effective therapy options after ≥2 prior chemotherapy regimens (unless contraindicated). Patients were required to have an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–1; ≥1 lesion measurable by RECIST v1.1; and adequate organ function. Ten patients included in this analysis were enrolled from the initial gastric cancer dose-expansion cohort (17), in addition to approximately 22 new patients. Final analysis will occur after all patients have completed study follow-up.
In the dose-expansion cohorts of gastric cancer, esophageal cancer, and small cell lung cancer, premedication instituted on cycle 1 day 1 (C#D#) included steroids and antihistamines for the first 10 patients enrolled, antihistamines without steroids for the next 10 patients enrolled, and no premedication for any subsequently enrolled patients (17). However, premedication of antihistamines was determined to be mandatory partway through enrollment of the additional patients with gastric cancer due to the high frequency of infusion-related reactions during enrollment. In addition, all patients could also have received prophylactic pegylated granulocyte colony-stimulating factor (peg-GCSF) per investigator choice.
Objectives
The primary objective of Study 114 was to determine the maximum tolerated dose of E7389-LF (determined to be 2.0 mg/m2 of free-base eribulin every 3 weeks; ref. 14). Secondary objectives included safety assessments, objective response rate (ORR) defined by best overall response, and progression-free survival (PFS). Exploratory objectives included OS, overall disease control rate (DCR) and clinical benefit rate (CBR) defined by best overall response, as well as pharmacodynamic measurements of serum biomarkers.
Statistical analyses
The safety and efficacy analysis set included all patients who received ≥1 dose of study drug. The target total enrollment was 32 patients (10 patients in the initial gastric cancer cohort plus an additional 22 patients in the expanded cohort). As 2 partial responses (PR) were observed in the initial 10 patients, an additional sample size of 22 patients was determined necessary to further assess ORR. This was calculated using a >80% Bayesian predictive probability with the criteria by a ≥90% Bayesian posterior probability of ORR being beyond a 5% threshold. This 5% threshold was considered clinically meaningful in third or later line of gastric cancer, as tumor responses are difficult to accomplish in these patients (6, 8).
Tumor responses were assessed every 6 weeks (±1 week) by RECIST v1.1 by investigator assessment. The ORR was calculated as the proportion of patients with a best overall response of complete response (CR) or PR. The DCR was calculated as the proportion of patients with a best overall response of CR, PR, or stable disease (SD). The durable SD rate was defined as patient having an SD of ≥23 weeks’ duration, and the CBR was calculated as the proportion of patients with a best overall response of CR, PR, or durable SD. All 95% confidence intervals (CI) were calculated using the Clopper–Pearson method. The cumulative PFS rate and OS rate were calculated and plotted using the Kaplan–Meier method. The median PFS and OS and their 95% CIs were evaluated; CIs were calculated based on Greenwood formula. In a post hoc analysis, patients who received an anti–PD-1 therapy as prior therapy were analyzed by time from last anti–PD-1 therapy to receipt of study drug (either less than or equal to/greater than the median time); these subgroups were evaluated for PFS and OS. Time to first objective response was summarized using summary statistics and was calculated in the same manner as the PFS and OS analysis. A waterfall plot was created to show the percent changes over time in the sums of tumor diameters of target lesions, and a spider plot was created to show the percent changes over time in the sums of the diameters of target lesions. Toxicity, treatment-emergent adverse events (TEAE), and infusion-related reactions by premedication were assessed using Common Terminology Criteria for Adverse Events version 4.03. The neutrophil count nadirs of patients were summarized via a box plot depending on whether they had received peg-GCSF. Statistical analyses were performed using SAS software (version 9.2 or later, SAS Institute, Cary, NC).
Biomarker analyses were conducted on serum samples using a custom multiplex immunoassay panel for 76 angiogenesis-related markers or the Single Molecule Array (SiMoA) system. This latter system is a new ultrasensitive digital ELISA immunoassay that can quantify very low concentrations of protein biomarkers, for IFNγ, B lymphocyte chemoattractant, and IL13. Samples taken prior to treatment on C1D1, and prior to dosing on C2D1, C3D1, and C4D1, were analyzed. Data below the lower quantification limit were defined as out of range and were replaced by one half of the lower quantification limit. Biomarker analytes for which >20% of the samples demonstrated levels below the lower limit of quantification were excluded from biomarker analysis.
Pharmacodynamic changes of serum biomarkers from baseline were analyzed using the 1-sample Wilcoxon signed-rank test, statistical significance was determined when unadjusted P values were < 0.05. Adjusted P values for FDR control with the number of biomarkers analyzed at each time point were also calculated. Statistical analyses and plots for biomarkers were performed and generated using R (R Foundation for Statistical Computing, Vienna, Austria) version 3.6.3.
Data availability
Eisai Inc., commits to sharing data from clinical trials upon request from qualified scientific and medical researchers. Data requests are reviewed and authorized by an independent review panel on the basis of scientific merit, and data are anonymized with respect to applicable laws and regulations. Trial data availability is according to the criteria and process described on https://www.clinicalstudydatarequest.com/SearchAllPostings.aspx.
Results
Patients
A total of 34 patients were enrolled in the gastric cancer expansion cohort (Table 1); 10 patients were initially enrolled in the initial gastric cancer cohort (17), and 24 additional patients were enrolled in this expanded cohort. Two extra patients were enrolled due to operational reasons, as they gave consent and were screened before the enrollment of 20th patient. At the data cut-off date (June 24, 2021) and with a minimum follow-up of >12 months, all patients had discontinued treatment. Radiological disease progression had resulted in treatment discontinuation of 29 patients, while 2 patients discontinued owing due to clinical disease progression. Of the 3 remaining patients who discontinued treatment, adverse events were listed as the primary reason for 2 patients; the other patient withdrew consent.
Table 1.
Baseline patient demographics.
| Characteristics | Overall (N = 34) |
|---|---|
| Median age, yearsa (range) | 68.0 (42–77) |
| Interquartile range, years | 62.0–72.0 |
| Sex: male/female, n (%) | 21 (61.8)/13 (38.2) |
| ECOG PS: 0/1, n (%) | 20 (58.8)/14 (41.2) |
| Primary tumor location: gastric/GEJ, n (%) | 30 (88.2)/4 (11.8) |
| Metastatic organs, n (%) | |
| Lymph nodes | 20 (58.8) |
| Liver | 16 (47.1) |
| Peritoneum | 13 (38.2) |
| Lung | 8 (23.5) |
| Bone | 1 (2.9) |
| Previous adjuvant or neoadjuvant therapy: no/yes, n (%) | 21 (61.8)/13 (38.2) |
| Median number of previous systemic chemotherapy regimens, n | 5 |
| Number of previous regimens for systemic chemotherapy, n (%) | |
| 1 | 1 (2.9) |
| 2 | 4 (11.8) |
| ≥3 | 29 (85.3) |
| Prior anticancer medication, n (%) | |
| Fluoropyrimidines | 34 (100) |
| Taxanes | 33 (97.1) |
| Immune checkpoint inhibitors | 32 (94.1) |
| Platinum compounds | 32 (94.1) |
| Ramucirumab | 32 (94.1) |
| Irinotecan | 19 (55.9) |
| Trifluridine/tipiracil | 12 (35.3) |
Abbreviation: GEJ, gastro-esophageal junction.
aAt time of informed consent.
The median age of patients was 68.0 years (range 42–77 years), and over half of enrolled patients (n = 20; 58.8%) had an ECOG PS of 0 (Table 1). The median number of prior systemic chemotherapies was 5. Most (32) patients received a prior anti–PD-1 therapy. Of these patients, 4 (12.5%) received an anti–PD-1 therapy as first-line therapy, 3 (9.4%) received it as second-line therapy, 24 (75.0%) received it as third-line therapy or later, and 1 (3.1%) received it in a perioperative setting. The median duration of time from patients’ last dose of anti–PD-1 therapy to receiving study treatment was 6.7 months. A brief description of the baseline characteristics of patients based on their last dose of anti–PD-1 therapy is included in Supplementary Table S1. The representativeness of patients as compared with real-world populations is available as Supplementary Table S2.
Efficacy
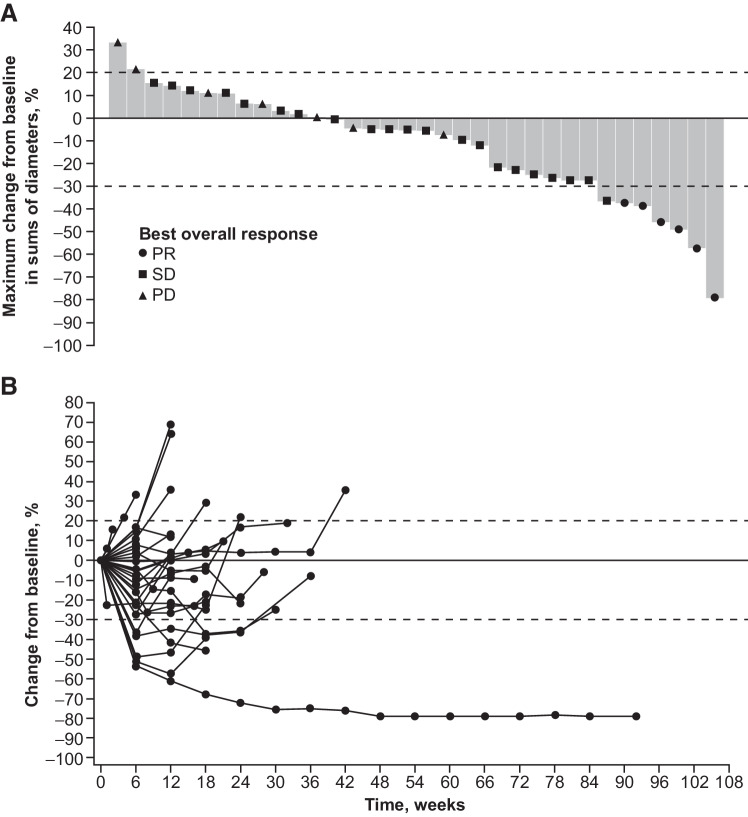
There were 4 confirmed PRs observed in the 22 patients of the expanded gastric cancer cohort. These 4 PRs were pooled along with the 2 PRs seen in the original dose-expansion cohort of 10 patients (17), resulting in a combined ORR of 6 confirmed PRs in the analysis of the planned total sample size of 32 patients (18.6%; 95% CI, 7.2–36.4). After enrollment of the last 2 additional patients, the ORR of the additional 24 patients was analyzed using Bayesian statistics, with a calculated posterior probability of 98.6% that ORR would exceed a 5% threshold. The ORR for the pooled cohort of 34 patients was 17.6% (n = 6; 95% CI, 6.8–34.5), and the DCR was 79.4% (n = 27; 95% CI, 62.1–91.3; Table 2). The CBR was 32.4% (n = 11; 95% CI, 17.4–50.5), as 5 patients (14.7%; 95% CI, 5.0–31.1) had durable SD. The median time to response was 1.45 months, and the median duration of response was 6.9 months (95% CI, 2.7–19.9). Changes in tumor size are shown in Fig. 1. All 6 PRs were seen in the 32 patients who received a prior anti–PD-1 therapy. An exploratory analysis showed that 3 PRs were seen in patients who received an anti–PD-1 therapy <6.7 months (median interval) before starting study treatment, the other 3 PRs were seen in patients who received one anti–PD-1 therapy ≥6.7 months before starting study treatment. Of these 6 patients with a PR, 2 had previously had a PR or SD while receiving anti–PD-1 therapy, and 2 previously had a PD while receiving anti–PD-1 therapy; response data were unavailable for the remaining 2 patients.
Table 2.
Summary of tumor responses.
| Characteristic | Overall (N = 34) |
|---|---|
| ORRa, n (%) | 6 (17.6) |
| 95% CI | 6.8–34.5 |
| DCRb, n (%) | 27 (79.4) |
| 95% CI | 62.1–91.3 |
| Durable SD ratec, n (%) | 5 (14.7) |
| 95% CI | 5.0–31.1 |
| CBRd, n (%) | 11 (32.4) |
| 95% CI | 17.4–50.5 |
aCR + PR.
bCR + PR + SD.
cSD of ≥23 weeks’ duration.
dCR + PR + durable SD.
Figure 1.
Change in tumor size from baseline: Maximum change to nadir (A) and change over time (B). PD, progressive disease.
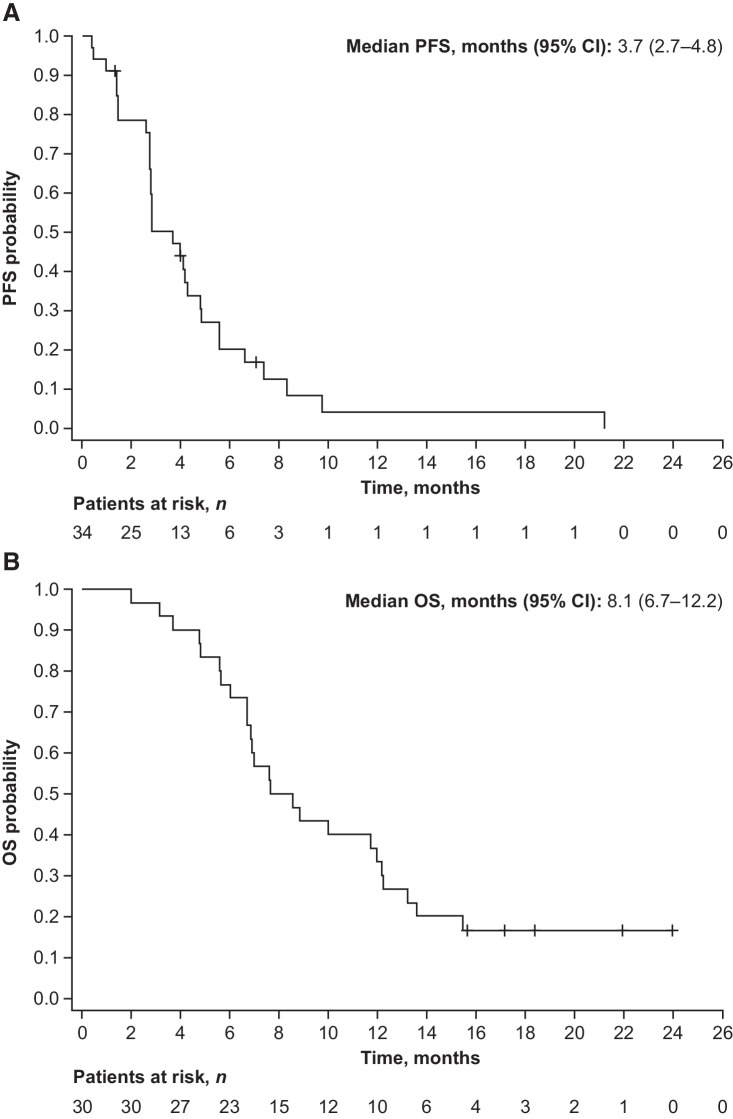
The median PFS was 3.7 months (95% CI, 2.7–4.8; Fig. 2A). In a post hoc analysis, the median PFS was 5.6 months (95% CI, 2.8–7.4) in patients who had received an anti–PD-1 therapy <6.7 months before starting study treatment, and 2.8 months (95% CI, 2.6–4.3) in those who had last received an anti–PD-1 therapy ≥6.7 months before starting study treatment (Supplementary Fig. S1A).
Figure 2.
Kaplan–Meier plot of PFS (A) and OS (B). Four patients were excluded from OS analysis as they did not agree to OS follow-up.
Among patients with gastric cancer (n = 30) who were evaluable for OS analyses, the median OS was 8.1 months (95% CI, 6.7–12.2; Fig. 2B); 4 patients did not agree to be included in survival follow-up. In a post hoc analysis, 28 patients who had received an anti–PD-1 therapy either <6.7 months (n = 15) or ≥6.7 months (n = 13) before starting study treatment were evaluable for OS (Supplementary Fig. S1B). The median OS was 12.2 months (95% CI, 4.7–15.4) in patients who had received an anti–PD-1 therapy <6.7 months before starting study treatment, and 7.0 months (95% CI, 6.0–10.0) in those who last received these therapies ≥6.7 months before starting study treatment.
Safety
Among the 34 patients enrolled and treated, 32 (94.1%) experienced treatment-related TEAEs (Table 3) and 26 patients (76.5%) experienced grade ≥3 treatment-related TEAEs. The most common grade ≥3 treatment-related TEAEs included neutropenia (41.2%), leukopenia (29.4%), anemia (14.7%), and thrombocytopenia (11.8%).
Table 3.
Treatment-related TEAEs that occurred in ≥10% of patients.
| Overall (N = 34) | |||||
|---|---|---|---|---|---|
| Highest grade | |||||
| Patients, n (%) | Any grade | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| Any treatment-related TEAEs | 32 (94.1) | 2 (5.9) | 4 (11.8) | 13 (38.2) | 13 (38.2) |
| Stomatitis | 19 (55.9) | 10 (29.4) | 8 (23.5) | 1 (2.9) | 0 |
| Decreased appetite | 18 (52.9) | 8 (23.5) | 9 (26.5) | 1 (2.9) | 0 |
| Leukopenia | 16 (47.1) | 1 (2.9) | 5 (14.7) | 8 (23.5) | 2 (5.9) |
| Neutropenia | 16 (47.1) | 0 | 2 (5.9) | 3 (8.8) | 11 (32.4) |
| Thrombocytopenia | 15 (44.1) | 5 (14.7) | 6 (17.6) | 4 (11.8) | 0 |
| Alopecia | 13 (38.2) | 7 (20.6) | 6 (17.6) | 0 | 0 |
| Anemia | 11 (32.4) | 1 (2.9) | 5 (14.7) | 5 (14.7) | 0 |
| Pyrexia | 11 (32.4) | 8 (23.5) | 3 (8.8) | 0 | 0 |
| Fatigue | 9 (26.5) | 8 (23.5) | 1 (2.9) | 0 | 0 |
| Rash | 9 (26.5) | 4 (11.8) | 4 (11.8) | 1 (2.9) | 0 |
| Nausea | 8 (23.5) | 6 (17.6) | 2 (5.9) | 0 | 0 |
| Aspartate aminotransferase increased | 7 (20.6) | 4 (11.8) | 2 (5.9) | 1 (2.9) | 0 |
| Peripheral sensory neuropathy | 7 (20.6) | 3 (8.8) | 4 (11.8) | 0 | 0 |
| Drug hypersensitivity | 6 (17.6) | 3 (8.8) | 2 (5.9) | 1 (2.9) | 0 |
| Alanine aminotransferase increased | 5 (14.7) | 3 (8.8) | 1 (2.9) | 1 (2.9) | 0 |
| Constipation | 4 (11.8) | 3 (8.8) | 1 (2.9) | 0 | 0 |
| Diarrhea | 4 (11.8) | 1 (2.9) | 3 (8.8) | 0 | 0 |
| Malaise | 4 (11.8) | 1 (2.9) | 3 (8.8) | 0 | 0 |
Note: For patients with >1 case of a treatment-related TEAE, only the highest grade was recorded here.
There were 12 patients (35.3%) who had at least 1 TEAE resulting in a dose reduction, most commonly decreased appetite (n = 5, 14.7%), febrile neutropenia, peripheral sensory neuropathy, rash, stomatitis, and thrombocytopenia (all n = 2, 5.9%). Other TEAEs resulting in dose reduction were aspartate aminotransferase increased, alanine aminotransferase increased, chronic kidney disease, dermatitis exfoliate (generalized), abnormal hepatic function, malaise, and neutropenia (all n = 1, 2.9%). Two patients had a TEAE resulting in discontinuation (bacteremia, n = 1, infusion-related reaction. n = 1). A swimmer plot of patients’ times to dose reduction and discontinuation is shown in Supplementary Fig. S2.
In this analysis, 22 patients received prophylactic peg-GCSF treatment. Rates of grade 3–4 neutropenia and febrile neutropenia were lower in those patients who had received peg-GCSF [4.5% (n = 1) and 0%, respectively] than in those who had not received peg-GCSF [83.3% (n = 10) and 16.7% (n = 2), respectively]. The change in patients’ neutrophil counts over time are shown by receipt of peg-GCSF in Supplementary Fig. S3. Of the 21 patients who received no premedication with steroid or antihistamine, 6 (28.6%) had a infusion-related reaction, of whom 1 (4.8%) had a grade 3 reaction (Supplementary Table S3). No infusion-related reactions were encountered in those who received a steroid and antihistamine (n = 3) or only an antihistamine (n = 10).
Biomarker analyses
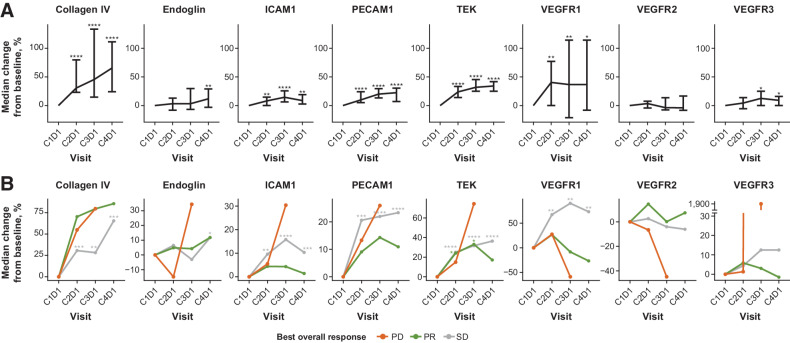
Of the 79 biomarkers assessed, 54 biomarkers were available per criteria based on the number of samples in range, including 8 vasculature biomarkers measured as soluble forms of vascular structure proteins (of these, 7 were endothelial membrane proteins, and 1 was the vasculature-specific extracellular matrix protein collagen IV). Among these 54 biomarkers, 30 were significantly (P < 0.05) altered by E7389-LF treatment from C1D1 at any time point. Of these 8 vasculature markers, 7 were significantly increased by E7389-LF in serum (Fig. 3A; Supplementary Table S4; Supplementary Fig. S4) and changes were generally consistent regardless of best overall response (Fig. 3B). These results were also generally consistent when compared with the other cancer types in Study 114, including breast cancer, adenoid cystic carcinoma, esophageal cancer, gastric cancer, and small cell lung cancer (Supplementary Fig. S5).
Figure 3.
Pharmacodynamic changes in biomarkers over time (A) and changes by best overall response (B) in the gastric cancer cohort. Serum samples were of 31 patients who had samples available at C2D1, C3D1, and C4D1; asterisks indicate results of 1-sample Wilcoxon signed-rank test results of P < 0.05 (*), P < 0.01 (**), P < 0.001 (***), or P < 0.0001 (****). In part A, vertical bars represent 95% CIs. C, cycle; D, day; PECAM, platelet and endothelial cell adhesion molecule.
Discussion
In this study, E7389-LF 2.0 mg/m2 every 3 weeks was tolerable, with a manageable safety profile and preliminary activity in patients with advanced gastric cancer. Given the observed safety profile, patients should be carefully monitored for neutropenia, and the use of prophylactic peg-GCSF may be considered.
ORR in the overall gastric cancer population was 17.6% (95% CI, 6.8–34.5). Notably, all patients enrolled were heavily pretreated — particularly, nearly all patients (97.1%) had received a taxane (another disruptor of microtubule function) as prior therapy. Thus, the response rate compared favorably to phase III studies of other treatment options received as subsequent therapy, such as TAS-102 (4%; ref. 8) and nivolumab (11%; ref. 6). However, cross trial comparisons between phase I and phase III studies should be carefully evaluated. Similarly, the median DCR (79.4%), median PFS (3.7 months), and median OS (8.1 months) compared favorably or similarly with previous studies of pretreated gastric cancer (5–8, 18–20); however, it should be noted that 4 patients were excluded from our analysis of OS because they did not agree to take part in the survival follow-up assessments.
Research has noted that prior exposure to anti–PD-1 therapies appears to improve the efficacy of chemotherapy (21–24); however, the relationship between the timing of the last anti–PD-1 therapy and subsequent efficacy is not clear. In this post hoc analysis, of the 32 patients who received an anti–PD-1 therapy, those patients who received E7389-LF less than 6.7 months after their last anti–PD-1 therapy (the median interval) appeared to have a longer median PFS (5.6 vs. 2.8 months) than those who received an anti–PD-1 therapy earlier. It should be noted that the 6 PRs observed in this expansion part occurred evenly among the “<6.7 months” group (n = 3), and the “≥6.7 months” group (n = 3), and that the median PFS of 2.8 months seen in the “≥6.7 months” group is comparable to the overall median PFS seen in other studies (6, 8). However, patients in the “<6.7 months” group versus the “≥6.7 months” group had fewer lines of prior therapies for metastatic or locally advanced disease (median 4.0 vs. 5.0, respectively) as well as having more patients with an ECOG PS of 0 (75.0% vs. 43.8%, respectively); this suggests that the former group may have had less severe disease overall. Given this possibility, the post hoc nature of this analysis, and the small sample size, these results need to be validated in a future study.
The safety profile was comparable to that of other studies of E7389-LF (14, 16, 17). Chiefly, neutropenia and leukopenia were among the most common treatment-related TEAEs. All infusion-related reactions were seen in patients with no premedication. This outcome is in contrast to the earlier analysis of the dose-expansion cohorts where no trend was seen (17), however that analysis included multiple cancer types. This implies that antihistamine premedication may help to reduce incidence of infusion-related, but these results should be validated in a larger population.
In the biomarker analyses, 7 of 8 vasculature markers of interest measured were significantly increased, which might be a surrogate for vascular remodeling effects in tumors by E7389-LF. To confirm this theory, non-clinical translational research is needed to investigate the correlation between intratumoral vascular alteration and changes in serum markers. The biomarker results were relatively consistent with those seen in all cohorts; and were comparable to the dose-escalation part (14) and the breast cancer dose-expansion cohort (16). Any slight differences observed may have been due to differences in sample sizes. In addition, biomarker changes did not depend on antitumor response.
This study has some limitations: only Japanese patients were included, which warrants further evaluation in non-Japanese populations; In addition, although this analysis assessed serum biomarkers, we did not evaluate changes in the tumor microenvironment [thus, on-treatment biopsies are being evaluated in a study of E7389-LF in combination with nivolumab in patients with gastric cancer (NCT04078295)]. In addition, the analyses of PFS and OS by time from last anti–PD-1 therapy to receipt of E7389-LF may have been affected by differences in baseline characteristics (namely lines of prior therapy for metastatic or locally advanced disease and ECOG PS value), and was post hoc in nature. Despite these limitations, these results demonstrate that E7389-LF is tolerable and has encouraging activity for patients with later-line advanced gastric cancer.
Supplementary Material
Supplementary Figure S1 shows the Kaplan–Meier plots of progression-free survival (A) and overall survival (B) by duration of time from prior anti-PD-1 treatment to start of study drug.
Supplementary Figure S2 is a swimmer plot of patients’ treatment durations.
Supplementary Figure S3 shows the neutrophil count at nadir of patients who received peg-GCSF (A) and those who did not receive peg-GCSF (B).
Supplementary Figure S4 shows the median change in biomarker expression from baseline to cycle 2 day 1 in the gastric cancer cohort (A) and all study 114 cohorts (B).
Supplementary Figure S5 shows pharmacodynamic changes in biomarkers over time (A) and changes by best overall response (B) in all study 114 cohorts.
Supplementary Table S1 shows select patient baseline characteristics by time from last anti-PD-1 therapy.
Supplementary Table S2 outlines the representativeness of study patients.
Supplementary Table S3 provides a summary of infusion-related reactions during cycle 1, per premedication.
Supplementary Table S4 provides a summary of biomarker changes in this gastric cancer cohort.
Acknowledgments
This study was funded by Eisai Co., Ltd., Tokyo, Japan. Medical writing support was provided by Michael Venditto, PharmD, of Oxford PharmaGenesis Inc., Newtown, PA, and was funded by Eisai Inc., Nutley, NJ.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
K. Shitara reports grants and personal fees from Astellas Pharma, Ono Pharmaceutical, Daiichi Sankyo, Taiho Pharmaceutical, Merck Pharmaceutical, and Amgen; personal fees from Eli Lilly and Company, Bristol Myers Squibb, Takeda Pharmaceuticals, Pfizer Inc., Novartis, AbbVie Inc., GlaxoSmithKline, Boehringer Ingelheim, Janssen, and Guardant Health Japan; and grants from Chugai Pharma, Medi Science, and Eisai outside the submitted work. S. Iwasa reports grants from Eisai during the conduct of the study as well as grants from Eisai outside the submitted work. Y. Komatsu reports grants and personal fees from Ono, Taiho, Chugai, Eli Lilly, Daiichi Sankyo, Nippon Kayaku, and MSD; personal fees from Bayer and Yakult; and grants from IQVIA, Astellas, Nippon Zoki, Shionogi, EPS, Eisai, and National Cancer Center outside the submitted work. A. Kawazoe reports personal fees from Daiichi Sankyo, Lilly, Ono, Taiho, Bristol Myers Squibb, Merck Serono biopharma, and AstraZeneca outside the submitted work. Y. Sato reports personal fees from Ono Pharmaceutical Co., Ltd., Bristol Myers Squibb Company, MSD KK, Taiho Pharmaceutical Co., Ltd., Eisai, Daiichi Sankyo Co., Ltd., and Kaken Pharmaceutical Co., Ltd. outside the submitted work. K. Yonemori reports grants from Eisai during the conduct of the study. N. Machida reports personal fees from Taiho, Bristol Myers Squibb, MSD, Ono, Lilly, Yakult, Daiichi Sankyo, Chugai, Takeda, and Nichi-Iko outside the submitted work. S. Yuki reports personal fees from Ono Pharmaceutical Co., Ltd., Bristol Myers Squibb Co., Ltd., Taiho Pharmaceutical Co. Ltd., Eli Lilly Japan K.K., Chugai Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd., Bayer Yakuhin Co. Ltd., Merck & Co., Inc., Merck Biopharma Co. Ltd., and Daiichi Sankyo Co., Ltd. outside the submitted work. T. Suzuki, S. Okumura, T. Takase, and T. Semba are employees of Eisai Co., Ltd. B. Zimmermann and A. Teng are employees of Eisai Inc. K. Yamaguchi reports grants from Eisai Co., Ltd. during the conduct of the study as well as grants and personal fees from Taiho Pharmaceutical and Ono Pharma; personal fees from Chugai Pharma, Bristol Myers Squibb Japan, Merck Biopharma, and Takeda; and grants from Yakut Honsha outside the submitted work. No disclosures were reported by the other authors.
Authors' Contributions
K. Shitara: Writing–original draft, writing–review and editing. M. Hirao: Investigation, writing–review and editing. S. Iwasa: Investigation, writing–review and editing. T. Oshima: Investigation, writing–review and editing. Y. Komatsu: Investigation, writing–review and editing. A. Kawazoe: Investigation, writing–review and editing. Y. Sato: Investigation, writing–review and editing. T. Hamakawa: Investigation, writing–review and editing. K. Yonemori: Investigation, writing–review and editing. N. Machida: Investigation, writing–review and editing. S. Yuki: Investigation, writing–review and editing. T. Suzuki: Formal analysis, validation, visualization, writing–review and editing. S. Okumura: Conceptualization, formal analysis, supervision, validation, visualization, project administration, writing–review and editing. T. Takase: Formal analysis, validation, visualization, writing–review and editing. T. Semba: Formal analysis, validation, visualization, writing–review and editing. B. Zimmermann: Formal analysis, validation, visualization, writing–review and editing. A. Teng: Formal analysis, validation, visualization, writing–review and editing. K. Yamaguchi: Investigation, writing–review and editing.
References
- 1. International Agency for Research on Cancer. Globocan 2020: Stomach fact sheet. 2020. Available from: https://gco.iarc.fr/today/data/factsheets/cancers/7-Stomach-fact-sheet.pdf.
- 2. Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2022;20:167–92. [DOI] [PubMed] [Google Scholar]
- 3. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-esophageal junction, and esophageal adenocarcinoma (CheckMate 649): a randomized, open-label, phase III trial. Lancet 2021;398:27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shitara K, Ajani JA, Moehler M, Garrido M, Gallardo C, Shen L, et al. Nivolumab plus chemotherapy or ipilimumab in gastroesophageal cancer. Nature 2022;603:942–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kang JH, Lee SI, Lim DH, Park KW, Oh SY, Kwon HC, et al. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol 2012;30:1513–8. [DOI] [PubMed] [Google Scholar]
- 6. Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastroesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538–12, ATTRACTION-2): a randomized, double-blind, placebo-controlled, phase III trial. Lancet 2017;390:2461–71. [DOI] [PubMed] [Google Scholar]
- 7. Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol 2016;34:1448–54. [DOI] [PubMed] [Google Scholar]
- 8. Shitara K, Doi T, Dvorkin M, Mansoor W, Arkenau HT, Prokharau A, et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomized, double-blind, placebo-controlled, phase III trial. Lancet Oncol 2018;19:1437–48. [DOI] [PubMed] [Google Scholar]
- 9. Halaven 0.44 mg/mL solution for injection [summary of product characteristics]. Frankfurt am Main, Germany: Eisai GmbH; 2022. [Google Scholar]
- 10. Halaven® (eribulin mesylate) [package insert]. Nutley, NJ: Eisai Inc.; 2021. [Google Scholar]
- 11. Halaven® (eribulin mesylate) [package insert]. Tokyo, Japan: Eisai Co Ltd.; 2022. [Google Scholar]
- 12. Funahashi Y, Okamoto K, Adachi Y, Semba T, Uesugi M, Ozawa Y, et al. Eribulin mesylate reduces tumor microenvironment abnormality by vascular remodeling in preclinical human breast cancer models. Cancer Sci 2014;105:1334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yoshida T, Ozawa Y, Kimura T, Sato Y, Kuznetsov G, Xu S, et al. Eribulin mesilate suppresses experimental metastasis of breast cancer cells by reversing phenotype from epithelial-mesenchymal transition (EMT) to mesenchymal-epithelial transition (MET) states. Br J Cancer 2014;110:1497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sato J, Shimizu T, Koyama T, Iwasa S, Shimomura A, Kondo S, et al. Dose escalation data from the phase I study of the liposomal formulation of eribulin (E7389-LF) in Japanese patients with advanced solid tumors. Clin Cancer Res 2022;28:1783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Niwa Y, Adachi Y, Semba T. Antitumor activity of liposomal formulation of eribulin combined with anti–PD-1 [abstract]. Cancer Res 2022;82Abstract 5584. [Google Scholar]
- 16. Masuda N, Ono M, Mukohara T, Yasojima H, Shimoi T, Kobayashi K, et al. Phase I study of the liposomal formulation of eribulin (E7389-LF): results from the breast cancer expansion cohort. Eur J Cancer 2022;168:108–18. [DOI] [PubMed] [Google Scholar]
- 17. Udagawa H, Takahashi S, Hirao M, Tahara M, Iwasa S, Sato Y, et al. Liposomal eribulin for advanced adenoid cystic carcinoma, gastric cancer, esophageal cancer, and small cell lung cancer. Cancer Med 2023;12:1269–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastroesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomized phase III trial. Lancet Oncol 2014;15:1224–35. [DOI] [PubMed] [Google Scholar]
- 19. Hironaka S, Ueda S, Yasui H, Nishina T, Tsuda M, Tsumura T, et al. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol 2013;31:4438–44. [DOI] [PubMed] [Google Scholar]
- 20. Sym SJ, Ryu MH, Lee JL, Chang HM, Kim TW, Lee SS, et al. Salvage chemotherapy with biweekly irinotecan, plus 5-fluorouracil and leucovorin in patients with advanced gastric cancer previously treated with fluoropyrimidine, platinum, and taxane. Am J Clin Oncol 2008;31:151–6. [DOI] [PubMed] [Google Scholar]
- 21. Park SE, Lee SH, Ahn JS, Ahn MJ, Park K, Sun JM. Increased response rates to salvage chemotherapy administered after PD-1/PD-L1 inhibitors in patients with non–small cell lung cancer. J Thorac Oncol 2018;13:106–11. [DOI] [PubMed] [Google Scholar]
- 22. Sagawa T, Sato Y, Hamaguchi K, Hirakawa M, Nagashima H, Waga E, et al. Improved efficacy to cytotoxic agents chemotherapy after immune checkpoint inhibitors exposure in metastatic gastric cancer [abstract]. J Clin Oncol 2020;38:Abstract 297. [Google Scholar]
- 23. Casadei B, Argnani L, Morigi A, Lolli G, Broccoli A, Pellegrini C, et al. Effectiveness of chemotherapy after anti–PD-1 blockade failure for relapsed and refractory Hodgkin lymphoma. Cancer Med 2020;9:7830–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sasaki A, Kawazoe A, Eto T, Okunaka M, Mishima S, Sawada K, et al. Improved efficacy of taxanes and ramucirumab combination chemotherapy after exposure to anti–PD-1 therapy in advanced gastric cancer. ESMO Open 2020;4:e000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1 shows the Kaplan–Meier plots of progression-free survival (A) and overall survival (B) by duration of time from prior anti-PD-1 treatment to start of study drug.
Supplementary Figure S2 is a swimmer plot of patients’ treatment durations.
Supplementary Figure S3 shows the neutrophil count at nadir of patients who received peg-GCSF (A) and those who did not receive peg-GCSF (B).
Supplementary Figure S4 shows the median change in biomarker expression from baseline to cycle 2 day 1 in the gastric cancer cohort (A) and all study 114 cohorts (B).
Supplementary Figure S5 shows pharmacodynamic changes in biomarkers over time (A) and changes by best overall response (B) in all study 114 cohorts.
Supplementary Table S1 shows select patient baseline characteristics by time from last anti-PD-1 therapy.
Supplementary Table S2 outlines the representativeness of study patients.
Supplementary Table S3 provides a summary of infusion-related reactions during cycle 1, per premedication.
Supplementary Table S4 provides a summary of biomarker changes in this gastric cancer cohort.
Data Availability Statement
Eisai Inc., commits to sharing data from clinical trials upon request from qualified scientific and medical researchers. Data requests are reviewed and authorized by an independent review panel on the basis of scientific merit, and data are anonymized with respect to applicable laws and regulations. Trial data availability is according to the criteria and process described on https://www.clinicalstudydatarequest.com/SearchAllPostings.aspx.