Key Points
Question
How often are patients diagnosed with new-onset retinal vascular occlusion (RVO) after messenger RNA (mRNA) COVID-19 vaccination compared with 2 historically used vaccinations?
Findings
In this cohort study including more than 3 million patients receiving the mRNA COVID-19 vaccine, 0.003% of patients had a new diagnosis of RVO within 21 days. The relative risk for RVO after the first mRNA COVID-19 vaccination was not significantly different from the risk with influenza vaccination and tetanus, diphtheria, pertussis (Tdap) vaccination.
Meaning
The findings of this study suggest that mRNA COVID-19 vaccination was not associated with newly diagnosed RVO.
Abstract
Importance
New-onset retinal vascular occlusion (RVO) occurring acutely after messenger RNA (mRNA) COVID-19 vaccination has been described in recent literature. Because RVO can cause vision loss or blindness, an epidemiologic investigation evaluating this potential association is of great importance to public health.
Objective
To investigate how often patients are diagnosed with new RVO acutely after the mRNA COVID-19 vaccine compared with influenza and tetanus, diphtheria, pertussis (Tdap) vaccines.
Design, Setting, and Participants
A retrospective population-based cohort design using the TriNetX Analytics platform, a federated, aggregated electronic health record (EHR) research network containing the deidentified EHR data of more than 103 million patients, was used to examine aggregate EHR data. Data were collected and analyzed on October 20, 2022. Data on patients within the TriNetX Analytics platform were searched for the presence of vaccination Common Procedural Technology codes, and instances of newly diagnosed RVO within 21 days of vaccination were recorded and reported. Propensity score matching based on demographic characteristics (age, sex, race and ethnicity) and comorbidities (diabetes, hypertension, and hyperlipidemia) was performed between vaccination groups for evaluation of relative risks (RRs).
Main Outcomes and Measures
The appearance of a new-encounter diagnosis of RVO within 21 days of the mRNA COVID-19 vaccination was the primary outcome. Historical comparison cohorts of patients receiving influenza and Tdap vaccinations allowed for evaluation of the RRs for RVO.
Results
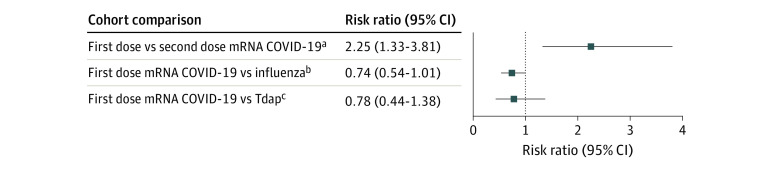
Of 3 108 829 patients (mean [SD] age at vaccination, 50.7 [20.4] years; 56.4% women) who received the mRNA COVID-19 vaccine, 104 (0.003%; 95% CI, 0.003%-0.004%) patients had a new diagnosis of RVO within 21 days of vaccination. After propensity score matching, the RR for new RVO diagnosis after the first dose of COVID-19 vaccination was not significantly different from that after influenza (RR, 0.74; 95% CI, 0.54-1.01) or Tdap (RR, 0.78; 95% CI, 0.44-1.38) vaccinations, but was greater when compared with the second dose of the COVID-19 vaccination (RR, 2.25; 95% CI, 1.33-3.81).
Conclusions and Relevance
The findings of this study suggest that RVO diagnosed acutely after mRNA COVID-19 vaccination occurs extremely rarely at rates similar to those of 2 different historically used vaccinations, the influenza and Tdap vaccines. No evidence suggesting an association between the mRNA COVID-19 vaccination and newly diagnosed RVO was found.
This cohort study examines the incidence of retinal vascular occlusion in individuals after receiving the mRNA COVID-19 vaccine.
Introduction
The messenger RNA (mRNA) COVID-19 vaccine is the first mRNA vaccine to be widely administered in humans, with more than 600 million doses given to date in the US alone.1 The mRNA COVID-19 vaccine has played a crucial role in controlling the ongoing pandemic. However, vaccine hesitancy and fear of adverse events from the vaccine remain a major national health concern.2 Thorough research into adverse events after mRNA COVID-19 vaccination is therefore of great scientific interest and importance to public health. Potential ocular adverse events after COVID-19 vaccination have been described with widely variable clinical manifestations.3,4 Recently, there has been growing interest in retinal vascular occlusion (RVO) as a possible adverse effect of mRNA COVID-19 vaccination. Retinal vascular occlusion is a serious potentially vision-threatening condition in which the vasculature of the retina becomes occluded, leading to ischemia or impaired venous drainage.5 Many case reports6,7,8,9,10,11,12,13,14,15,16,17 describe RVO occurring in patients within days of COVID-19 vaccination that manifests clinically with variable vision loss, scotomas, and blurred vision. Both retinal artery occlusion and retinal vein occlusion have been described, yet the association between COVID-19 vaccination and RVO remains unclear because the reported temporal association described in existing cases may be coincidental.
Pathophysiologic explanations for a temporary hyperviscous state after mRNA vaccination have been posited but remain largely theoretical.18 One widely cited study19 found an association between adenoviral COVID-19 vaccination and a hypercoagulable thrombocytopenic state. Another large retrospective study20 examining cerebral vascular thrombosis after both mRNA and adenoviral COVID-19 vaccination found no association between vaccination and thrombosis for either formulation of COVID-19 vaccine. An examination of more than 500 patients presenting to retina clinics with RVO found many patients had been vaccinated against COVID-19, yet no association was observed between vaccination and RVO.21 Retinal vascular occlusion has been described and reviewed as an established sequela of COVID-19 infection itself due to microvasculopathy and inflammation-induced hypercoagulability.22,23 Epidemiologic evidence for an association between mRNA COVID-19 vaccination and RVO remains scarce due to limited sample sizes in prior studies. The availability of a large population is well suited to examine this rare phenomenon and address the gaps in existing literature. Using a sample size of more than 3 million individuals receiving the mRNA COVID-19 vaccination, this study aimed to investigate the prevalence and risk of diagnosed new-onset RVO acutely after mRNA COVID-19 vaccination.
Methods
The COVID-19 Research Network within the TriNetX Analytics platform is a federated health research network that aggregates the deidentified electronic health record (EHR) data of more than 103 million patients in 77 health care organizations across 9 countries, including 54 health care organizations in the US. These data have been deemed exempt from the Western Institutional Review Board by a qualified expert as defined in Section §164.514(b)(1) of the Health Insurance Portability and Accountability Act Privacy Rule. This study used a retrospective cohort design by querying the TriNetX database for patients receiving each vaccination and examining diagnoses of RVO appearing within 21 days after each vaccination event. Data were collected and analyzed on October 20, 2022. The present study serves as a broad powerful epidemiologic investigation that uses International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) and Common Procedural Terminology codes to define patient groups and outcomes. The study methods were structured according to those in a recent study24 performed by some of us examining tinnitus after COVID-19 vaccination. This report follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for reporting observational studies in epidemiology. There were 103 079 881 million patients with any information contained in the COVID-19 Research Network of the TriNetX platform at the time of data querying. Four cohort groups were identified (exact Common Procedural Terminology code parameters detailed in the eMethods in Supplement 1): group 1 received the first dose of mRNA COVID-19 from December 15, 2020, to June 15, 2022; group 2 received the second dose of mRNA COVID-19 vaccine from December 15, 2020, to June 15, 2022; group 3 received influenza vaccine from June 1, 2018, to December 31, 2019; and group 4 received Tdap vaccine from June 1, 2018, to December 31, 2019.
Dates for the 2 COVID-19 vaccination groups span 18 months from the first day of vaccine administration in the US. The 18-month timeframe aimed to capture as many individuals receiving the COVID-19 vaccination as possible while allowing sufficient time for data in the TriNetX network to fully update before querying and analysis. Dates for the 2 other vaccination groups (influenza and Tdap) were set for the same duration (18 months) as the 2 COVID-19 groups but were queried in 2018 to 2019 to eliminate the possibility of COVID-19 vaccination within this group. Receipt of the mRNA COVID-19 vaccination was split into 2 groups (first dose and second dose) to evaluate the relative risk (RR) for RVO after the first dose compared with after 2 sequential doses. Patients with any history of encounter diagnoses of RVO any time before the vaccination event, based on ICD-10 codes, were excluded from all groups to more precisely focus on vaccine-related RVO and generate findings applicable to most of the population without a history of an encounter diagnosis of RVO.
The ICD-10 diagnostic criteria used to identify patients with RVO fell under the heading H34: RVO, which included all forms of retinal artery occlusion and retinal venous occlusion as detailed in the eMethods in Supplement 1. Each patient group was indexed to the respective vaccination event and any diagnosis of RVO within 21 days of vaccination was recorded. The 21-day timeframe was decided based on the acute nature of symptoms reported in recent literature describing RVO presenting, on average, 14 days after COVID-19 vaccination.25 The 21-day timeframe additionally made analysis of a second-dose COVID-19 vaccination group possible, as 21 days is the shortest recommended timeframe to receive a second dose of the COVID-19 vaccine after the first dose. Patients were matched demographically based on age at vaccination, sex, and race and ethnicity, and matched medically by the proportion of patients with a diagnosis of diabetes, hypertension, and hyperlipidemia (ICD-10 codes provided in the eMethods in Supplement 1), as these systemic metabolic conditions have been reported as being associated with RVO.26 Race and ethnicity were determined based on the presence of these designations within the EHR. Three distinct matching procedures were performed: first-dose COVID-19 vaccination (group 1) was matched with second-dose COVID-19 vaccination (group 2), first-dose COVID-19 vaccination (group 1) was matched with influenza vaccination (group 3), and first-dose COVID-19 vaccination (group 1) was matched with Tdap vaccination (group 4). The influenza and Tdap groups served as comparison groups for evaluating the risk of RVO acutely after the first dose of COVID-19 vaccination as the objective of the study was primarily to evaluate the adverse event profile of the COVID-19 vaccination in comparison with historically used vaccinations. A comparison of the first dose with the second dose of COVID-19 vaccination was performed to evaluate the risk profile for a new-encounter diagnosis of RVO after each dose of vaccination.
Statistical Analysis
Cohorts were propensity score matched using the TriNetX platform built-in analysis tools (1:1 matching by nearest neighbor greedy matching algorithm with a caliper of 0.25 SDs). Significance tests were 2-sided and paired. A significance threshold of P < .05 was used and 95% CIs were calculated.
Results
Of 3 108 829 patients (mean [SD] age at vaccination, 50.7 [20.4] years; 56.4% women) who received a first dose of the mRNA COVID-19 vaccine, 104 patients (0.003%; 95% CI, 0.003%-0.004%) had a new-encounter ICD-10 diagnosis of RVO within 21 days of vaccination, equating to an incidence of 3.4 individuals per 100 000 being affected. After 1:1 propensity score matching, the RR for an ICD-10 new-encounter RVO diagnoses after the first dose of mRNA COVID-19 vaccination was 0.74 (95% CI, 0.54-1.01) times the RR among those who received the influenza vaccination and 0.78 (95% CI, 0.44-1.38) times the RR among those who received the Tdap vaccination, indicating no significant differences in risk. The RR of a new-encounter ICD-10 diagnosis of RVO after the first COVID-19 vaccine was 2.25 (95% CI, 1.33-3.81) times the RR of a new-encounter ICD-10 diagnosis of RVO after the second COVID-19 vaccine. There were 3 108 829 patients who received a first dose of the mRNA COVID-19 vaccine and 1 108 006 patients who received a second dose of the mRNA COVID-19 vaccine, indicating that 1 928 823 patients with a documented first dose did not have documentation of a second dose within our data set. Results of propensity score matching with standardized mean differences for each ICD-10 covariate before and after matching are provided for all comparisons: first-dose COVID-19 vaccination compared with second-dose COVID-19 vaccination (Table 1), first-dose COVID-19 vaccination compared with influenza vaccination (Table 2), and first-dose COVID-19 compared with Tdap vaccination (Table 3). After propensity score matching, the number of patients with a new-encounter ICD-10 diagnosis of RVO and RR values for a new-encounter diagnosis of RVO within 21 days of vaccination in reference to the first dose of COVID-19 vaccination are displayed in the Figure.
Table 1. Propensity Score Matching Results Between First- and Second-Dose COVID-19 Vaccine Cohort.
| Characteristic | Before matching | After matching | ||||
|---|---|---|---|---|---|---|
| COVID-19 dose, No. (%) | SMD | COVID-19 dose, No. (%) | SMD | |||
| First (n = 3 108 829) | Second (n = 1 180 006) | First (n = 1 180 006) | Second (n = 1 180 006) | |||
| Age at vaccination, mean (SD), y | 50.7 (20.4) | 52.2 (20.6) | 0.074 | 52.2 (20.6) | 52.2 (20.6) | <0.001 |
| Sexa | ||||||
| Female | 1 753 009 (56.4) | 660 796 (56.0) | 0.008 | 660 796 (56.0) | 660 796 (56.0) | <0.001 |
| Male | 1 354 008 (43.6) | 518 105 (43.9) | 0.007 | 518 119 (43.9) | 518 3105 (43.9) | <0.001 |
| Ethnicityb | ||||||
| Hispanic or Latinx | 296 919 (9.6) | 162 950 (13.8) | 0.133 | 162 929 (13.8) | 162 950 (13.8) | <0.001 |
| Not Hispanic or Latinx | 1 812 998 (58.3) | 882 568 (74.8) | 0.355 | 882 589 (74.8) | 882 568 (74.8) | <0.001 |
| Unknown | 998 912 (32.1) | 134 488 (11.4) | 0.519 | 134 488 (11.4) | 134 488 (11.4) | <0.001 |
| Raceb | ||||||
| Asian | 136 455 (4.4) | 72 120 (6.1) | 0.077 | 72 109 (6.1) | 72 120 (6.1) | <0.001 |
| Black or African American | 370 356 (11.9) | 179 490 (15.2) | 0.096 | 179 498 (15.2) | 179 490 (15.2) | <0.001 |
| Native Hawaiian or Pacific Islander | 5053 (0.2) | 2362 (0.2) | 0.009 | 2344 (0.2) | 2362 (0.2) | <0.001 |
| White | 2 001 510 (64.4) | 773 904 (65.6) | 0.025 | 773 925 (65.6) | 773 904 (65.6) | <0.001 |
| Unknown | 578 694 (18.6) | 144 904 (12.3) | 0.176 | 144 928 (12.3) | 144 904 (12.3) | <0.001 |
| Comorbidities | ||||||
| Diabetes | 384 601 (12.4) | 147 591 (12.5) | 0.004 | 147 550 (12.5) | 147 591 (12.5) | <0.001 |
| Hypertension | 871 147 (28.0) | 332 131 (28.1) | 0.003 | 332 095 (28.1) | 332 131 (28.1) | <0.001 |
| Hyperlipidemia | 623 080 (20.0) | 220 112 (18.7) | 0.035 | 220 071 (18.6) | 220 112 (18.7) | <0.001 |
Abbreviation: SMD, standardized mean difference.
Some data not reported.
Race and ethnicity were determined based on the presence of these designations within the electronic health record.
Table 2. Propensity Score Matching Results Between First-Dose COVID-19 Vaccine Cohort and Influenza Vaccine Cohort.
| Characteristic | Before matching | After matching | ||||
|---|---|---|---|---|---|---|
| No. (%) | SMD | No. (%) | SMD | |||
| First dose COVID-19 (n = 3 108 829) | Influenza (n = 1 854 123) | First dose COVID-19 (n = 1 470 351) | Influenza (n = 1 470 351) | |||
| Age at vaccination, mean (SD), y | 50.7 (20.4) | 36.7 (27.1) | 0.582 | 47.1 (22.3) | 43.5 (25.6) | 0.151 |
| Sexa | ||||||
| Female | 1 753 009 (56.4) | 1 036 860 (55.9) | 0.009 | 862 940 (58.7) | 837 386 (57.0) | 0.035 |
| Male | 1 354 008 (43.6) | 817 020 (44.1) | 0.01 | 607 181 (41.3) | 632 722 (43.0) | 0.035 |
| Ethnicityb | ||||||
| Hispanic or Latinx | 296 919 (9.6) | 177 335 (9.6) | <0.001 | 125 288 (8.5) | 149 593 (10.2) | 0.057 |
| Not Hispanic or Latinx | 1 812 998 (58.3) | 1 312 861 (70.8) | 0.263 | 1 087 517 (74.0) | 975 831 (66.4) | 0.167 |
| Unknown | 998 912 (32.1) | 363 927 (19.6) | 0.288 | 257 546 (17.5) | 344 927 (23.5) | 0.148 |
| Raceb | ||||||
| Asian | 136 455 (4.4) | 64 344 (3.5) | 0.47 | 43 584 (3.0) | 57 762 (3.9) | 0.053 |
| Black or African American | 370 356 (11.9) | 295 251 (15.9) | 0.116 | 219 788 (14.9) | 208 124 (14.2) | 0.022 |
| Native Hawaiian or Pacific Islander | 5053 (0.2) | 2705 (0.1) | 0.004 | 1938 (0.1) | 2453 (0.2) | 0.009 |
| White | 2 001 510 (64.4) | 1 230 187 (66.3) | 0.041 | 1 057 736 (71.9) | 974 054 (66.2) | 0.123 |
| Unknown | 578 694 (18.6) | 254 269 (13.7) | 0.133 | 142 037 (9.7) | 221 064 (15.0) | 0.164 |
| Comorbidities | ||||||
| Diabetes | 384 601 (12.4) | 270 707 (14.6) | 0.074 | 286 471 (19.5) | 241 233 (16.4) | 0.08 |
| Hypertension | 871 147 (28.0) | 578 208 (31.2) | 0.069 | 614 363 (41.8) | 525 770 (35.8) | 0.124 |
| Hyperlipidemia | 623 080 (20.0) | 432 096 (23.3) | 0.079 | 462 943 (31.5) | 395 253 (26.9) | 0.101 |
Abbreviation: SMD, standardized mean difference.
Some data not reported.
Race and ethnicity were determined based on the presence of these designations within the electronic health record.
Table 3. Propensity Score Matching Results Between First-Dose COVID-19 Vaccine Cohort and Tdap Vaccine Cohort.
| Characteristic | Before matching | After matching | ||||
|---|---|---|---|---|---|---|
| No. (%) | SMD | No. (%) | SMD | |||
| First dose COVID-19 (n = 3 108 829) | Tdap (n = 743 991) | First dose COVID-19 (n = 718 400) | Tdap (n = 718 400) | |||
| Age at vaccination, mean (SD), y | 50.7 (20.4) | 38.1 (20.1) | 0.617 | 39.8 (19.1) | 39.1 (19.8) | 0.034 |
| Sexa | ||||||
| Female | 1 753 009 (56.4) | 416 429 (56.0) | 0.008 | 400 606 (55.8) | 407 135 (56.7) | 0.018 |
| Male | 1 354 008 (43.6) | 327 454 (44.0) | 0.009 | 317 639 (44.2) | 311 157 (43.3) | 0.018 |
| Ethnicityb | ||||||
| Hispanic or Latinx | 296 919 (9.6) | 68 999 (9.3) | 0.009 | 63 406 (8.8) | 68 924 (9.6) | 0.027 |
| Not Hispanic or Latinx | 1 812 998 (58.3) | 481 661 (64.7) | 0.132 | 475 534 (66.2) | 458 588 (63.8) | 0.049 |
| Unknown | 998 912 (32.1) | 193 331 (26.0) | 0.136 | 179 460 (25.0) | 190 888 (26.6) | 0.036 |
| Raceb | ||||||
| Asian | 136 455 (4.4) | 26 299 (3.5) | 0.044 | 25 590 (3.6) | 26 299 (3.7) | 0.005 |
| Black or African American | 370 356 (11.9) | 132 602 (17.8) | 0.167 | 131 764 (18.3) | 121 309 (16.9) | 0.038 |
| Native Hawaiian or Pacific Islander | 5053 (0.2) | 1574 (0.2) | 0.011 | 1669 (0.2) | 1543 (0.2) | 0.004 |
| White | 2 001 510 (64.4) | 485 322 (65.2) | 0.018 | 476 199 (66.3) | 471 055 (65.6) | 0.015 |
| Unknown | 578 694 (18.6) | 94 552 (12.7) | 0.163 | 80 111 (11.2) | 94 552 (13.2) | 0.062 |
| Comorbidities | ||||||
| Diabetes | 384 601 (12.4) | 62 119 (8.3) | 0.132 | 66 268 (9.2) | 62 071 (8.6) | 0.02 |
| Hypertension | 871 147 (28.0) | 145 859 (19.6) | 0.199 | 153 887 (21.4) | 145 716 (20.3) | 0.028 |
| Hyperlipidemia | 623 080 (20.0) | 96 179 (12.9) | 0.193 | 100 469 (14.0) | 96 120 (13.4) | 0.018 |
Abbreviations: SMD, standardized mean difference; Tdap, tetanus, diphtheria, pertussis.
Some data not reported.
Race and ethnicity were determined based on the presence of these designations within the electronic health record.
Figure. Comparisons of New International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) Diagnoses of Retinal Vein Occlusion (RVO) Occurring Within 21 Days of Vaccinations After Propensity Score Matching.
mRNA indicates messenger RNA; Tdap, tetanus, diphtheria, pertussis.
aFor the first-dose mRNA COVID-19 cohort, n = 1 180 006. There were 45 patients (0.004%) with a new RVO diagnosis. For the second-dose mRNA COVID-19 cohort, n = 1 180 006. There were 93 patients (0.006%) with a new RVO diagnosis.
bFor the first-dose mRNA COVID-19 cohort, n = 1 470 351. There were 69 patients (0.005%) with a new RVO diagnosis. For the influenza cohort, n = 1 470 351. There were 20 patients (0.002%) with a new RVO diagnosis.
cFor the first-dose mRNA COVID-19 cohort, n = 718 400. There were 27 patients (0.004%) with a new RVO diagnosis. For the Tdap cohort, n = 718 400. There were 27 patients (0.004%) with a new RVO diagnosis.
COVID-19 infection may carry a higher risk for new RVO than the mRNA vaccination: a post hoc analysis comparing the risk for new-encounter diagnoses of RVO after COVID-19 infection compared with mRNA COVID-19 vaccination was performed and showed a 4.25 (95% CI, 3.24-5.56) times higher risk after COVID-19 infection than after vaccination (eAppendix in Supplement 1).
Discussion
The risk for new-encounter diagnosis of RVO acutely after the first dose of the mRNA COVID-19 vaccine, based on ICD-10 codes, was found to be extremely low. The results of this study do not support the presence of an increased risk for diagnosed RVO occurring acutely after mRNA COVID-19 vaccination compared with 2 historically used vaccinations (influenza and Tdap). The baseline risk for developing new RVO independent of any vaccine within a 21-day timeframe must be considered with the low number of observed RVO cases. However, it remains possible that RVO is a direct adverse effect of the mRNA COVID-19 vaccine among certain patient populations that are too few to produce significant results at the population level or that EHR data limitations mask an association. The results of this study inform the scientific community about the low rates of diagnosis of this potential vision-threatening adverse event for those who have received a COVID-19 vaccination compared with influenza or Tdap vaccination. If there does exist an association between mRNA COVID-19 vaccination and new-encounter diagnoses for RVO, it occurs at similar rates experienced after influenza and Tdap vaccinations that have been administered for decades throughout the population.
The decreased risk for RVO after the second dose of the mRNA COVID-19 vaccination compared with the first dose may be explained by a true increased physiologic risk for RVO after the first dose, possibly that a naive immune response is more likely to provoke a hyperviscous state related to RVO.18 However, this finding may also be explained by a hesitancy to receive the second dose based on symptoms that occur after the first dose, uncontrolled differences in patient populations, differences in patient insurance status, or differences in recording practices between the 2 events. The number of patients who were documented to have received a second dose of the mRNA COVID-19 vaccine was less than half of those who received a first dose (1 108 006 vs 3 108 829). While the present study found a similar risk for RVO after influenza and Tdap vaccinations compared with the first dose mRNA COVID-19 vaccination, it is important to consider that influenza and Tdap vaccination queries were performed during 2018 to 2019 to eliminate the possibility of COVID-19 vaccination within this group. Differences in willingness to present to a health care professional with possible RVO must be considered as substantial changes in ophthalmology visits were observed during the COVID-19 pandemic.27 The results do not intend to belittle the experience of patients with debilitating vision loss after vaccination but aim to describe how often this phenomenon occurs as a contribution to the safety profile of the first mRNA vaccine widely administered in humans. The results of the post hoc analysis additionally suggest that the risk for a new-encounter RVO diagnosis is greater acutely after COVID-19 infection itself than after the mRNA COVID-19 vaccine (eAppendix in Supplement 1).
Limitations
This study has limitations. While large sample sizes provide power for rare outcomes such as RVO, limitations to an analysis of large sets of aggregated EHR data must be considered. The present study is limited to information that has been recorded in the EHR, which may have information bias. While the results show that ICD-10 diagnoses for RVO do not frequently occur after mRNA COVID-19 vaccination, the present study does not describe the severity of symptoms experienced or disprove a pathophysiologic connection. Although examination of 21 days after the vaccination event served as a logical design decision based on existing literature, it may be either missing occurrences of vaccine-related RVO manifesting later than 21 days after vaccination or including unrelated occurrences. Propensity score matching using the TriNetX platform was not ideal, as some covariates remained different after matching due to large differences in the original characteristics of each group. Additionally, there are uncontrolled confounding patient characteristics (eg, body mass index, history of tobacco use, and history of cerebrovascular accident) that may affect our comparisons, as demographic differences between each group were substantial before matching. Further research is necessary to explore the association between vaccination and patients who have a history of RVO. Patients who experience RVO after vaccination may have some predisposition or set of conditions that put them at higher risk for this serious condition after vaccination, which should guide further research.
Conclusions
The findings of this study suggest that, overall, new-encounter ICD-10 diagnoses for RVO acutely after mRNA COVID-19 vaccination occur extremely rarely at rates similar to those of 2 different historically used vaccines. No evidence suggesting an association between mRNA COVID-19 vaccination and new-encounter diagnoses of RVO was found. Further detailed research on patients experiencing RVO after vaccination is necessary to elucidate risk factors for this vision-threatening condition.
eMethods. Cohort Criteria and Propensity Score Matching
eAppendix. Post Hoc Analysis
Data Sharing Statement
References
- 1.Centers for Disease Control and Prevention . COVID data tracker. Accessed October 17, 2022. https://covid.cdc.gov/covid-data-tracker
- 2.Anakpo G, Mishi S. Hesitancy of COVID-19 vaccines: rapid systematic review of the measurement, predictors, and preventive strategies. Hum Vaccin Immunother. 2022;18(5):2074716. doi: 10.1080/21645515.2022.2074716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haseeb AA, Solyman O, Abushanab MM, Abo Obaia AS, Elhusseiny AM. Ocular complications following vaccination for COVID-19: a one-year retrospective. Vaccines (Basel). 2022;10(2):342. doi: 10.3390/vaccines10020342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ng XL, Betzler BK, Testi I, et al. Ocular adverse events after COVID-19 vaccination. Ocul Immunol Inflamm. 2021;29(6):1216-1224. doi: 10.1080/09273948.2021.1976221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scott IU, Campochiaro PA, Newman NJ, Biousse V. Retinal vascular occlusions. Lancet. 2020;396(10266):1927-1940. doi: 10.1016/S0140-6736(20)31559-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdin AD, Gärtner BC, Seitz B. Central retinal artery occlusion following COVID-19 vaccine administration. Am J Ophthalmol Case Rep. 2022;26:101430. doi: 10.1016/j.ajoc.2022.101430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pur DR, Catherine Danielle Bursztyn LL, Iordanous Y. Branch retinal vein occlusion in a healthy young man following mRNA COVID-19 vaccination. Am J Ophthalmol Case Rep. 2022;26:101445. doi: 10.1016/j.ajoc.2022.101445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su CK, Au SCL. Isolated and combined unilateral central retinal artery and vein occlusions after vaccination: a review of the literature. J Stroke Cerebrovasc Dis. 2022;31(8):106552. doi: 10.1016/j.jstrokecerebrovasdis.2022.106552 [DOI] [PubMed] [Google Scholar]
- 9.Endo B, Bahamon S, Martínez-Pulgarín DF. Central retinal vein occlusion after mRNA SARS-CoV-2 vaccination: a case report. Indian J Ophthalmol. 2021;69(10):2865-2866. doi: 10.4103/ijo.IJO_1477_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sacconi R, Simona F, Forte P, Querques G. Retinal vein occlusion following two doses of mRNA-1237 (Moderna) immunization for SARS-cov-2: a case report. Ophthalmol Ther. 2022;11(1):453-458. doi: 10.1007/s40123-021-00441-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takacs A, Ecsedy M, Nagy ZZ. Possible COVID-19 mRNA vaccine-induced case of unilateral central retinal vein occlusion. Ocul Immunol Inflamm. 2022:1-6. doi: 10.1080/09273948.2022.2094811 [DOI] [PubMed] [Google Scholar]
- 12.Ikegami Y, Numaga J, Okano N, Fukuda S, Yamamoto H, Terada Y. Combined central retinal artery and vein occlusion shortly after mRNA-SARS-CoV-2 vaccination. QJM. 2022;114(12):884-885. doi: 10.1093/qjmed/hcab287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sonawane NJ, Yadav D, Kota AR, Singh HV. Central retinal vein occlusion post-COVID-19 vaccination. Indian J Ophthalmol. 2022;70(1):308-309. doi: 10.4103/ijo.IJO_1757_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee S, Sankhala KK, Bose S, Gallemore RP. Combined central retinal artery and vein occlusion with ischemic optic neuropathy after COVID-19 vaccination. Int Med Case Rep J. 2022;15:7-14. doi: 10.2147/IMCRJ.S328931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugihara K, Kono M, Tanito M. Branch retinal vein occlusion after messenger RNA-based COVID-19 vaccine. Case Rep Ophthalmol. 2022;13(1):28-32. doi: 10.1159/000521838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girbardt C, Busch C, Al-Sheikh M, et al. Retinal vascular events after mRNA and adenoviral-vectored COVID-19 vaccines—a case series. Vaccines (Basel). 2021;9(11):1349. doi: 10.3390/vaccines9111349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah PP, Gelnick S, Jonisch J, Verma R. Central retinal vein occlusion following BNT162b2 (Pfizer-BioNTech) COVID-19 messenger RNA vaccine. Retin Cases Brief Rep. 2021. Published online December 1, 2021. doi: 10.1097/ICB.0000000000001214 [DOI] [PubMed] [Google Scholar]
- 18.Al-Kuraishy HM, Al-Gareeb AI, El-Bouseary MM, Sonbol FI, Batiha GE. Hyperviscosity syndrome in COVID-19 and related vaccines: exploring of uncertainties. Clin Exp Med. 2022;1-10. Published online May 24, 2022. doi: 10.1007/s10238-022-00836-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(22):2124-2130. doi: 10.1056/NEJMoa2104882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pawlowski C, Rincón-Hekking J, Awasthi S, et al. Cerebral venous sinus thrombosis is not significantly linked to COVID-19 vaccines or non-COVID vaccines in a large multi-state health system. J Stroke Cerebrovasc Dis. 2021;30(10):105923. doi: 10.1016/j.jstrokecerebrovasdis.2021.105923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feltgen N, Ach T, Ziemssen F, et al. Retinal vascular occlusion after COVID-19 vaccination: more coincidence than causal relationship? data from a retrospective multicentre study. J Clin Med. 2022;11(17):5101. doi: 10.3390/jcm11175101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Modjtahedi BS, Do D, Luong TQ, Shaw J. Changes in the incidence of retinal vascular occlusions after COVID-19 diagnosis. JAMA Ophthalmol. 2022;140(5):523-527. doi: 10.1001/jamaophthalmol.2022.0632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teo KY, Invernizzi A, Staurenghi G, Cheung CMG. COVID-19-related retinal micro-vasculopathy—a review of current evidence. Am J Ophthalmol. 2022;235:98-110. doi: 10.1016/j.ajo.2021.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dorney I, Bobak L, Otteson T, Kaelber DC. Prevalence of new-onset tinnitus after COVID-19 vaccination with comparison to other vaccinations. Laryngoscope. 2022. Published online September 13, 2022. doi: 10.1002/lary.30395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vujosevic S, Limoli C, Romano S, Vitale L, Villani E, Nucci P. Retinal vascular occlusion and SARS-CoV-2 vaccination. Graefes Arch Clin Exp Ophthalmol. 2022;260(11):3455-3464. doi: 10.1007/s00417-022-05707-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Recchia FM, Brown GC. Systemic disorders associated with retinal vascular occlusion. Curr Opin Ophthalmol. 2000;11(6):462-467. doi: 10.1097/00055735-200012000-00013 [DOI] [PubMed] [Google Scholar]
- 27.Berkenstock MK, Liberman P, McDonnell PJ, Chaon BC. Changes in patient visits and diagnoses in a large academic center during the COVID-19 pandemic. BMC Ophthalmol. 2021;21(1):139. doi: 10.1186/s12886-021-01886-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Cohort Criteria and Propensity Score Matching
eAppendix. Post Hoc Analysis
Data Sharing Statement