Abstract
Gastric cancer (GC) is one of the most common gastrointestinal tract cancers worldwide, which has high incidence and mortality rates and poor prognosis. Although multidisciplinary comprehensive therapies consisting of surgery, chemotherapy, radiotherapy, and targeted therapy have made great progress in GC treatment, a satisfactory curative effect still cannot be achieved in many circumstances, and the 5-year survival of patients with GC remains to be very low. In China, about 75% of patients with GC are diagnosed in the advanced stage and thus miss the opportunity of surgical resection. Although the conventional treatment of GC has improved the survival time of advanced patients to a certain extent, the clinical efficacy has encountered a bottleneck and cannot bring higher survival benefits to patients. With the development of immunologic and molecular biologic technologies, immunotherapy has gradually become a new essential treatment for GC, which has attracted extensive attention in the field of oncology. The US Food and Drug Administration (USFDA) and China Food and Drug Administration (CFDA) have approved a variety of immune-related drugs for the treatment of GC, and all of which have achieved good efficacy. In this review, we summarize the recent development in nonspecific enhancer therapy, adoptive immunocell therapy, tumor vaccine therapy, oncolytic virus therapy, and immune checkpoint inhibitor therapy, and their roles in the treatment of GC.
Keywords: gastric cancer, immune checkpoint inhibitor therapy, biomarkers, cancer vaccine, immunotherapy
Introduction
Gastric cancer (GC) originates from gastric mucosal epithelial cells and is a common malignancy of the digestive system. It is also one of the malignancies with the highest incidence and mortality worldwide. In 2020, more than 1 million cases were newly reported, and the death toll was estimated to be 769 000. Incidence rate and mortality rate were fifth and fourth worldwide, respectively. 1 According to national statistics, the total incidence and mortality of GC in China are second only to lung cancer. The incidence and mortality of GC in male and female patients ranked second and third, respectively. 2 There are 410 000 newly diagnosed GC patients every year. The 5-year survival rate of GC patients treated is still less than 40%, 3 which seriously threatens patients’ health and life expectancy. At present, the treatment of GC is still based on surgery, chemotherapy, radiotherapy, or combined targeted therapy. Due to atypical clinical symptoms and insidious onset of GC, most patients are already in the advanced stage when diagnosed, and existing methods cannot cure them completely, resulting in a very poor survival.4,5 Although chemotherapy regimens for advanced GC have continued to progress in the past 40 years, including 5-FU-based regimens, platinum-based regimens, and new chemotherapy drug combination regimens, the efficacy of chemotherapy is still poor, and the median survival time (mOS) is less than 1 year.6,7 Therefore, targeted drugs have become the research direction to seek therapeutic breakthroughs. In recent years, various targeted drugs are continuously conducting clinical research. The more famous clinical researches include AVAGAST, EXPAND, REAL-3, ToGA, LOGIC, etc, but the overall results are disappointing. With the exception of trastuzumab, which has improved efficacy in patients with HER-2-positive advanced GC, 6 other targeted drugs have no significant efficacy in the first-line treatment of patients with advanced GC. However, the positive rate of HER-2 is only 12% to 13% in my country's advanced GC patients, so the application scope of trastuzumab is relatively limited, and the research status of targeted drugs in the field of GC is extremely unsatisfactory. With the progress of medical technology, immunotherapy has become a new treatment modality. In recent years, immunotherapy has achieved significant efficacy in a variety of solid tumors such as malignant melanoma, nonsmall cell lung cancer, renal cancer, and head and neck cancer.8–11 Immunotherapy research on GC is actively ongoing, and a variety of treatment strategies have been published, including nonspecific enhancer therapy, adoptive immune cell therapy, tumor vaccine, oncolytic virus, and immune checkpoint inhibitor therapies. 12 These immunotherapies play a role in controlling and eliminating tumors by restarting and maintaining the tumor-immune cycle and restoring the body's normal anti-tumor immune response. 13 In particular, immune checkpoint inhibitors such as PD-1 and PD-L1 monoclonal antibodies can block the interaction between PD-1 and PD-L1, maintain the continuous activation state of T cells, and prevent the occurrence of immune escape of tumor cells, and also activate other immune cells, such as natural killer cells, to jointly attack tumor cells. At present, many new immunotherapeutic regimens are still being investigated in phase-II and III clinical trials in the treatment of advanced GC, but some clinical trials have made breakthroughs. This article reviews the current status and research progress of various immunotherapies against GC.
Immune Checkpoint Inhibitor Therapy
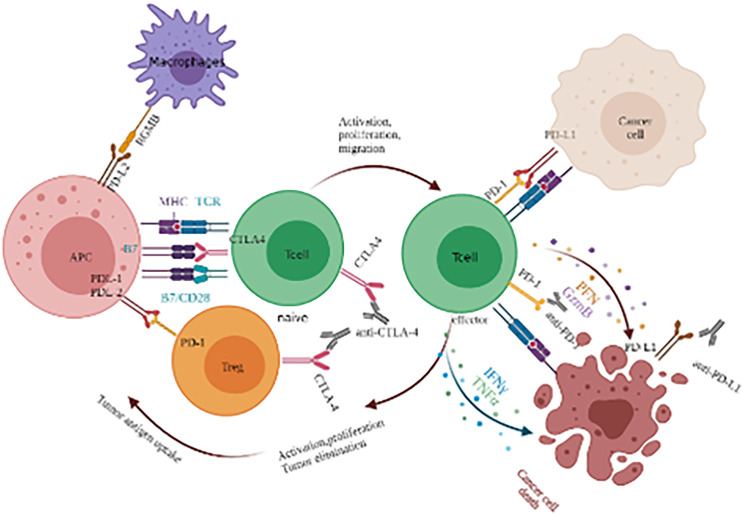
Immune checkpoints are a kind of factor that play a “brake”-like an inhibitory role in the immune response system. On the one hand, they can regulate the overactivation of immune response to prevent damage to autologous tissues. On the other hand, they can prevent the occurrence of autoimmune diseases by maintaining immune tolerance to self-antigens. Tumor cells use the characteristics of negative regulation of immune checkpoints to inhibit the activation and proliferation of immune T cells and form an immunosuppressive microenvironment, so as to escape the recognition and attack of the immune system. Immune checkpoint inhibitor therapy plays an anti-tumor role by specifically binding monoclonal antibodies to immune checkpoints on the surface of tumor cells or immune cells, blocking negative regulation and reactivating the immune system (Figure 1). Among all immune checkpoint inhibitors (ICIs), the most studied inhibitors are cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), programmed cell death 1 (PD-1), and programmed cell death-ligand 1 (PD-L1). The National Comprehensive Cancer Network (NCCN) of the United States released the 2021 clinical practice guidelines for GC, with several updates. It is recommended that all patients with newly diagnosed GC should have a microsatellite instability (MSI) test by polymerase chain reaction (PCR), or mismatch repair (MMR) test by immunohistochemistry (IHC). In previous years, the status of PD-L1 and tumor Epstein Barr virus tests have been listed in the guideline recommendations, and the FDA and CFDA approved the PD-1 inhibitor pembrolizumab as the second-line or postsecond-line and nivolumab as the first-line option for the treatment of PD-L1 CPS positive advanced GC with deficient mismatch repair (dMMR)/microsatellite instability-high (MSI-H).14,15 Since 2017, the guidelines for the treatment of GC have been updated many times. In particular, Nivolumab, the first first-line immunotherapy for GC, was approved by the FDA, which is great progress in the treatment of GC and one of the important milestones in immunotherapy, suggesting that the research focus of GC treatment has begun to change to immunotherapy. The main clinical studies of PD-1/PD-L1 and CTLA-4 targeted drugs in the treatment of advanced GC are summarized in Table 1. A summary of clinical trials of PD-1/PD-L1 in GC is shown in Table 2.
Figure 1.
Immune response mechanism of immune checkpoint inhibitors (ICIs).
Table 1.
Current Research on Anti-PD-1/PD-L1 and CTLA-4 Pathway Targeted Drugs in Gastric Cancer.
| Target | Drug | Type | Clinical Research | Reference |
|---|---|---|---|---|
| CTLA-4 inhibitor | Ipilimumab | Full humanized immunoglobulin G1 antibody | A randomized, open-label, two-arm phase II trial comparing the efficacy of sequential ipilimumab (ipi) versus best supportive care (BSC) following first-line (1L) chemotherapy in patients with unresectable, locally advanced/metastatic (A/M) gastric or gastro-esophageal junction (G/GEJ) cancer(NCT01585987) | Moehler et al 38 |
| Efficacy of Sequential Ipilimumab Monotherapy versus Best Supportive Care for Unresectable Locally Advanced/Metastatic Gastric or Gastroesophageal Junction Cancer (NCT01928394) | Bang et al23,39,42,55,56 | |||
| Tremelimumab | Fully humanized anti-CTLA-4 monoclonal IgG2 antibody | Modulation of lymphocyte regulation for cancer therapy: a phase II trial of tremelimumab in advanced gastric and esophageal adenocarcinoma | Ralph et al 58 | |
| TremelImumab and Durvalumab Combination for the Non-OperatIve Management (NOM) of Microsatellite InstabiliTY (MSI)-High Resectable Gastric or Gastroesophageal Junction Cancer: The Multicentre, Single-Arm, Multi-Cohort, Phase II INFINITY Study | Raimondi et al 59 | |||
| PD-1 inhibitor | Pembrolizumab | Humanized immunoglobulin G4 antibody | Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial | Muro et al 19 |
| Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial | Fuchs et al 14 | |||
| Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomized, open-label, controlled, phase 3 trial | Shitara et al 21 | |||
| Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy versus Chemotherapy Alone for Patients With First-line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial | Shitara et al 22 | |||
| Nivolumab | Full humanized immunoglobulin G4 antibody | Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomized, double-blind, placebo-controlled, phase 3 trial | Kang et al 25 | |
| Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: interim results of a randomized, phase II trial (ATTRACTION-4) | Boku et al 30 | |||
| CheckMate-032 Study: Efficacy and Safety of Nivolumab and Nivolumab Plus Ipilimumab in Patients With Metastatic Esophagogastric Cancer | Janjigian et al15,27,44 | |||
| An Investigator-Initiated Phase 2 Study of Nivolumab Plus Low-Dose Ipilimumab as First-Line Therapy for Microsatellite Instability-High Advanced Gastric or Esophagogastric Junction Cancer (NO LIMIT, WJOG13320G/CA209-7W7) | Kawakami et al 28 | |||
| Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer | Kelly et al 29 | |||
| Multicenter Phase I/II Study of Nivolumab Combined with Paclitaxel Plus Ramucirumab as Second-line Treatment in Patients with Advanced Gastric Cancer | Nakajima et al 31 | |||
| First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomized, open-label, phase 3 trial | Janjigian et al15,27,44 | |||
| PD-L1 inhibitor | Avelumab | Full humanized immunoglobulin G1 antibody | Phase III, randomized trial of avelumab versus physician's choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300 | Bang et al23,39,42,55 |
| Phase 1 trial of avelumab (anti-PD-L1) in Japanese patients with advanced solid tumors, including dose expansion in patients with gastric or gastroesophageal junction cancer: the JAVELIN Solid Tumor JPN trial | Doi et al 35 | |||
| Avelumab (anti-PD-L1) as first-line switch-maintenance or second-line therapy in patients with advanced gastric or gastroesophageal junction cancer: phase 1b results from the JAVELIN Solid Tumor trial | Chung et al 37 | |||
| Phase III Trial of Avelumab Maintenance After First-Line Induction Chemotherapy Versus Continuation of Chemotherapy in Patients With Gastric Cancers: Results From JAVELIN Gastric 100 | Moehler et al 38 | |||
| Atezolizumab | FC segment-modified humanized immunoglobulin G1 antibody | Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients | Herbst et al 45 | |
| Durvalumab | FC segment-modified humanized immunoglobulin G1 antibody | Ramucirumab and durvalumab for previously treated, advanced nonsmall-cell lung cancer, gastric/gastro-oesophageal junction adenocarcinoma, or hepatocellular carcinoma: An open-label, phase Ia/b study (JVDJ) | Bang et al23,39,42,55 | |
| Immune checkpoint inhibitors independently developed in China | Toripalimab | Human immunoglobulin G4 (IgG4) monoclonal antibody | Safety, efficacy, and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD-1 antibody in phase Ib/II clinical trial NCT02915432 | Wang et al60,128 |
| Camrelizumab | Human immunoglobulin G4 (IgG4) monoclonal antibody | Anti-PD-1 Antibody SHR-1210 Combined with Apatinib for Advanced Hepatocellular Carcinoma, Gastric, or Esophagogastric Junction Cancer: An Open-label, Dose Escalation, and Expansion Study | Xu et al 61 | |
| Camrelizumab Combined with Chemotherapy Followed by Camrelizumab plus Apatinib as First-line Therapy for Advanced Gastric or Gastroesophageal Junction Adenocarcinoma | Peng et al 62 | |||
| Sintilimab | Human immunoglobulin G4 (IgG4) monoclonal antibody | Safety and efficacy of sintilimab combined with oxaliplatin/capecitabine as first-line treatment in patients with locally advanced or metastatic gastric/gastroesophageal junction adenocarcinoma in a phase Ib clinical trial | Jiang et al64,88,140,144 | |
| Clinical Study of Sintilimab as Second-Line or Above Therapy in Patients With Advanced or Metastatic Gastric Cancer: A Retrospective Study | Nie et al 65 |
Table 2.
Ongoing Clinical Trials of Potential Immunotherapy Approaches of Esophageal Adenocarcinoma or Esophageal-Squamous-Cell-Carcinoma (EAC/ESCC) Patients.
| Target | Drug | Cancer Type | Intervention/Treatment | Clinical Phase | Clinical Trials.gov | Status |
|---|---|---|---|---|---|---|
| PD-1 inhibitors | Pembrolizumab | previously treated advanced G/GEJ with PD-L1 CPS ≥ 1 | Pembrolizumab or placebo Plus Chemotherapy (XP or FP) | III | NCT03221426 | Recruiting |
| Pembrolizumab | patients with potentially resectable G/GEJ | mFOLFOX6 +Pembrolizumab | II | NCT03488667 | Recruiting | |
| Pembrolizumab | metastatic gastroesophageal cancer | lenvatinib and pemrolizumab | II | NCT03321630 | Recruiting | |
| Nivolumab | advanced G/GEJC | BMS-986213 (Relatlimab and or Nivolumab) or Nivolumab only + investigator's choice chemotherapy (XELOX or FOLFOX or SOX) | II | NCT03662659 | Recruiting | |
| Nivolumab | advanced or metastatic EGA | ipilimumab or 5-FU/folinic acid and oxaliplatin (FOLFOX) in combination with nivolumab and trastuzumab | II | NCT03409848 | Recruiting | |
| Nivolumab | pStage III G/GEJC after D2 or more extensive lymph node dissection | Nivolumab or placebo + S-1 or CapeOX | III | NCT03006705 | Recruiting | |
| Camrelizumab | unresectable recurrent or metastatic Alpha-fetoprotein (AFP)-Producing G/GEJC | Cohort 1:camrelizumab and apatinib+SOX(as first-line therapy)Cohort 2:camrelizumab and apatinib (≥ 1 line) | II | NCT04609176 | Recruiting | |
| PD-L1 inhibitors | Avelumab | Her2(-), locally advanced or metastatic GC or GEJC | Avelumab maintenance versus continued chemotherapy after 12 wk of first-line induction chemotherapy | III | NCT02625610 | Recruiting |
| Avelumab | Resectable G/GEJC | avelumab+FLOT (4 cycles previous to surgery + 4 cycles of adjuvant therapy, avelumab up to one year | II | NCT03979131 | Recruiting | |
| Atezolizumab | Nonmetastatic, Resectable Gastric, and GE-junction Cancer | Atezolizumab+ chemotherapy(capecitabine, oxaliplatin and docetaxel) | II | NCT 03448835 | Recruiting | |
| Atezolizumab | Operable oesophageal and GC | Atezolizumab+ FLOT | II | NCT03399071 | Recruiting | |
| Atezolizumab | locally advanced, operable adenocarcinoma of the stomach or GEJ. | Atezolizumab+ FLOT versus FLOT alone | II | NCT03421288 | Active, not recruiting | |
| Durvalumab | Resectable GC/GEJCancer | Durvalumab or placebo combined with FLOT chemotherapy | III | NCT04592913 | Recruiting | |
| Nivolumab + ipilimumab | advanced GC | Nivolumab + ipilimumab versus Nivolumab +relatlimab versus Nivolumab + BMS-986 205 versus Nivo +rucaparib versus ipilimumab + rucaparib versus Nivolumab + ipilimumab + rucaparib | II | NCT02935634 | Recruiting |
Immune response mechanism of CTLA-4, CTLA-4 is a kind of co-stimulatory molecule expressed on the surface of T cells, which is mainly highly expressed on regulatory T cells (Treg) and activated T cells. Normally, the activation of T cell requires 2 signal pathways to be activated together. One is the combination of T cell receptor (TCR) and MHC antigen peptide complex presented by APC (signal 1), and the other is the combination of B7 molecule (B7-1 or B7-2) and costimulatory molecule CD28 on the surface of T cells (signal 2). Although the CTLA-4 molecule highly expressed after T cell activation has substantial homology with CD28, its function is the opposite, that is, the CTLA-4 molecule inhibits T cell activation after binding with the B7 molecule. Anti-CTLA-4 antibody can block CTLA-4 binding to B7 and its inhibition of T cell function.
PD-1 receptor is expressed on the surface of T cells and plays a role in the differentiation and apoptosis of T cells. PD-1 has 2 ligands, PD-L1 and PD-L2. PD-L1 protein is widely expressed in activated T cells and macrophages. PD-L1 interacts with the receptor PD-1 on T cells, which can inhibit the activation of T cells and cause T cell apoptosis. PD-L1 plays an important role in the negative regulation of immune response. PD-1/PD-L1 inhibitors can block the binding of PD-1 and PD-L1, thus blocking the negative regulatory signal, restoring the activity of T cells, and enhancing the immune response.
PD-1 Inhibitor
PD-1 is widely expressed in a variety of cells, such as T cells, B cells, monocytes, DCs, and natural killer T cells. As a ligand of PD-1, PD-L1 is expressed on antigen-presenting cells and a variety of tumor cell membranes. After binding with PD-1, PD-L1 can continuously activate the immunosuppressive signal pathway, escape its systematic surveillance and promote tumor escape. Therefore, blocking PD-1/PD-L1 binding can reverse immunosuppression and enhance the killing function of autoimmune cells against tumor cells. Some genetic studies have shown that the occurrence and development of GC are related to the polymorphism of PD-1 and CTLA-4 genes.16,17 Many studies have proved that antibodies can enhance the anti-tumor immune effect of the host by blocking the PD-1/PD-L1 pathway. So far, the FDA has approved 3 PD-1 inhibitors, pembrolizumab, nivolumab, and atezolizumab.
Pembrolizumab
Pembrolizumab is a highly specific PD-1 monoclonal antibody, which can block the interaction between PD-1 and PD-L1, promote the proliferation and activation of T cells and the production of immune cytokines, and thus to restore the immune response. 18
In a multicenter, open-label, phase 1b trial (KEYNOTE-012), 19 39 patients with PD-L1 positive advanced GC were recruited. After treatment with pembrolizumab, the median progression-free survival was 1.9 months and the median overall survival (OS) was 11.4 months. In addition, the study also showed that PD-1 expression was correlated with OS and could also be used as a clinical screening index. In another randomized phase II clinical study (KEYNOTE-059) for Japanese and European and American populations, 14 pembrolizumab was used in patients with gastric or gastroesophageal junction adenocarcinoma who had received second- or later-line chemotherapy. After 259 patients receiving pembrolizumab treatment, the ORR was 11.6% and the 6-month OS was 46.5%, the 12-month OS was 23.4%, and the ORR of PD-L1 positive and negative patients were 15.5% and 6.4%, respectively. It showed that pembrolizumab was effective and long-lasting in the treatment of advanced GC, and patients with GC with high expression of PD-L1 can obtain better clinical efficacy. As a result of this study, pembrolizumab has been approved by FDA for the third-line treatment of PD-L1 positive (CPS ≥ 1) recurrent locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma. 20
In a randomized, open-label, controlled phase III trial (KEYNOTE-061) conducted in 148 medical centers in 30 countries, 395 PD-L1 positive patients were randomly divided into 196 patients treated with pembrolizumab and 199 patients treated with paclitaxel. The results showed that the median OS was 9.1 months in the pembrolizumab group and 8.3 months in the paclitaxel group; the mPFS was 1.5 months in the pembrolizumab group and 4.1 months in the paclitaxel group. Although the overall result was negative, second-line immunotherapy alone was more effective than chemotherapy in patients with CPS ≥ 10 and patients with MSI-H. 21 As a result of this study, pembrolizumab has been approved by FDA for the second-line treatment of PD-L1 positive (CPS ≥ 1) advanced gastric or gastroesophageal junction cancer. In addition, in another phase III randomized controlled study (KEYNOTE-062), 22 763 patients with locally advanced, unresectable, or metastatic GC/esophagogastric junction cancer (HER-2 negative, PD-L1 CPS ≥ 1) were randomly divided into pembrolizumab monotherapy group, pembrolizumab combined chemotherapy group and chemotherapy group for first-line treatment. The treatment was continued until intolerable adverse reactions or disease progression occurred, or the patient was required to withdraw from the study. In terms of the improvement of OS, the first-line treatment with pembrolizumab was not inferior to standard chemotherapy (10.6 months vs 11.1 months). The 2-year survival rates of the 2 groups were 27% and 19%, respectively. In patients with PD-L1 CPS ≥ 10, the OS time of the pembrolizumab group was longer than that of the standard chemotherapy group (17.4 months vs 10.8 months), and the OS rate was higher than that of the standard chemotherapy group (39% vs 22%), but the progression-free survival time (1.5 months vs 4.1 months) and OS time (9.1 months vs 8.3 months) were not significantly improved in pembrolizumab combined with chemotherapy group compared with chemotherapy group. It suggests that pembrolizumab monotherapy or combined with chemotherapy can be used in the first-line treatment of advanced GC. However, compared with chemotherapy, pembrolizumab-based combination chemotherapy did now show clinical benefit. At present, the NCT03221426 23 clinical trial is still in progress.
Nivolumab
In addition to pembrolizumab, nivolumab is another highly humanized PD-1 monoclonal IgG4 antibody. By binding to the PD-1 receptor, nivolumab inhibits its role with PD-L1 and PD-L2 and blocks its pathway to enhance tumor immune response. 24
In 2017, ESMO first reported the results of the ATTRACTION-02 study, 25 which included 493 patients with advanced GC/gastroesophageal junction cancer who received 2 or more chemotherapy regimens in 3 places in Japan, Korea and Taiwan, China. The results of the 2-year follow-up study showed that nivolumab could reduce the risk of death by about 38% compared with the placebo group. The median OS of patients in the nivolumab group and the placebo group were 5.26 and 4.14 months, respectively, of which the OS rates in the first year were 27.3% and 11.6%, and 10.6% and 3.2% in the second year. The benefit of OS was observed regardless of tumor PD-L1 expression. Among patients with complete response (CR) and partial response (PR) in the nivolumab group, the median OS was 26.6 months. The OS rates at 1 and 2 years were 87.1% and 61.3%, respectively. 26 This shows that nivolumab has good antitumor activity in GC/GEJ patients who have been treated before, and can benefit most patients with GC. Therefore, nivolumab is also recommended as a third-line treatment for GC which is recommended in Japanese guidelines for the treatment of GC. Based on the positive results of this study, nivolumab became the first immune checkpoint inhibitor approved in Asia for the treatment of advanced GC. In a continuous, open-label, 2-stage, multicohort, phase I/II trial (CheckMate-032), nivolumab or nivolumab combined with ipilimumab showed good antitumor activity and controllable safety in the treatment of patients with refractory GC and gastroesophageal cancer. 27 Kawakami et al 28 also initiated a phase II clinical study of nivolumab plus low-dose ipilimumab in the first-line treatment of MSI-H advanced GC or esophageal gastric junction cancer. The therapeutic effect of this regimen is consistent with the previously mentioned CheckMate-032 study, reflecting good effectiveness and safety. Recently, the results of nivolumab adjuvant therapy (CheckMate-577) have also been published. 29 Among patients with esophageal or gastroesophageal junction cancer who received neoadjuvant radiotherapy and chemotherapy before surgery, the median disease-free survival of 532 patients receiving nivolumab was 22.4 months, and the median disease-free survival of 262 patients who received placebo was 11.0 months. The results showed that the disease-free survival of patients treated with nivolumab was significantly better than that of patients treated with a placebo.
PD-1 Combined Chemotherapy
ICIS combined with chemotherapy has shown a good anticancer effect in most studies. ATTRACTION-4 studies show that nivolumab combined with chemotherapy has controllable safety and clinically relevant antitumor activity. Moreover, patients with nivolumab combined with SOX and CapeOX regimen achieved higher objective remission rates and longer remission duration. 30 In a multicenter phase I/II study of nivolumab combined with paclitaxel + ramucirumab as second-line treatment for advanced GC, the ORR was 37.2%, the 6-month progression-free survival rate was 46.4%, and the median PFS was 5.1 months, 31 indicating that nivolumab combined with other therapeutic drugs may become a new way for the treatment of advanced GC. In another multicenter, randomized, open-label, phase III trial (CheckMate-649), 15 1581 unresectable patients with advanced GC/esophagogastric junction cancer who had not previously received systemic therapy and were negative for HER-2 were included. Results showed that the mOS of nivolumab combined with chemotherapy was 14.4 months and that of chemotherapy alone was 11.1 months. Nivolumab combined with chemotherapy significantly improved OS and PFS compared with chemotherapy alone, and the improvement of OS and PFS was more significant in patients with PD-L1 CPS ≥ 5. Based on this result, nivolumab combined chemotherapy has become the first-approved first-line treatment for GC worldwide. However, at the 2019 ASCO meeting, unsatisfactory results of the KEYNOTE-062 trial were reported. In patients with PD- L1 CPS ≥ 1, the total OS of patients receiving pembrolizumab was not better than chemotherapy. In patients with PD- L1 CPS ≥ 10, there was no significant difference in progression-free survival and OS compared with chemotherapy alone. 22 At present, clinical trials such as NCT03662659 and NCT03409848 32 are still recruiting subjects.
PD-L1 Inhibitor
PD-L1, a PD-1 ligand, is expressed in a variety of solid tumors. It can relieve the immunosuppressive effect by inhibiting the combination of PD-1 and PD-L1, so as to enhance the immune-mediated tumor response.
Avelumab
PD-L1 is expressed in tumor cells and tumor-infiltrating immune cells, which has a strong inhibitory effect on tumor immune response. Avelumab is a fully humanized PD-L1 IgG1 monoclonal antibody. By binding to PD-L1, avelumab can block the interaction between PD-L1 and its receptor PD-1, and thereby effectively relieving the immunosuppression. 33
In a safety study of neoadjuvant radiotherapy and chemotherapy combined with avelumab in the treatment of resectable esophageal and gastroesophageal junction cancer, 34 there was no immune-related toxicity ≥ grade 3, which suggests the therapy was well tolerated, and the pathological complete remission rate (pCR) reached 43%. At present, in order to improve the pCR rate and long-term survival effect, preoperative neoadjuvant radiotherapy and chemotherapy combined with ICIS have become a new treatment strategy. In another phase IB dose escalation trial, 35 17 patients with GC were treated with avelumab in with a dose gradient (3, 10, and 20 mg/kg). Results showed that the objective remission rate was 10.0% and the median OS was 9.1 months, suggesting that avelumab has clinical therapeutic activity and good safety in patients with advanced GC/gastroesophageal junction adenocarcinoma and disease progression after chemotherapy.
Avelumab not only shows good clinical efficacy in single-drug or combination therapy but also shows certain anticancer effects in the maintenance therapy of the later lines. In phase II clinical trial, 151 patients with GC/GEJ were divided into 2 groups. One group of patients with disease control after first-line treatment received avelumab as an alternative maintenance treatment. The other group of patients who progressed after first-line chemotherapy received avelumab as second-line treatment. The results showed that the ORR of the 2 groups were 9.0% and 9.7%, respectively, and the mPFS were 12 weeks and 6 weeks, suggesting that avelumab has a certain curative effect on GC/GEJ patients. 36 Chung et al 37 also evaluated the therapeutic effect of avelumab as first-line conversion maintenance (1L-mn) or second-line (2L) of advanced GC/gastroesophageal cancer, and the results also showed good antitumor activity and acceptable safety. Moehler et al 38 of the University of Gutenberg, Germany reported the results of a phase III trial (JAVELIN Gastric 100) and found that in the total population of advanced GC/GEJ or the predetermined PD-L1 positive population, avelumab maintenance treatment was not superior to continuous chemotherapy, despite the convenience in treatment management. Although the previous JAVELIN solid tumor study 39 and JAVELIN solid tumor Japan study35,37 showed good efficacy, and the ORR of avelumab as a second- or later-line treatment could reach 10% to 15%. However, in November 2017, Merck and Pfizer announced that avelumab monotherapy did not improve OS as a third-line treatment. The results came from the phase III clinical study of JAVELIN Gastric 300. At present, 2 phase III clinical trials (NCT02625610 and NCT02625623) are still in progress. 40
Atezolizumab
Atezolizumab is a monoclonal antibody that can bind to PD-L1 on tumor cells and tumor-infiltrating immune cells, blocks its interaction with PD-1 and B7.1 receptors, and activates T cells to destroy tumor cells by inhibiting PD-L1.
In phase I clinical study of atezolizumab on solid tumors, only 1 of 6 patients with GC had remission. 41 This study only preliminarily evaluated the antitumor activity of atezolizumab as a single drug, and there seems to be a correlation between tumor-shrinking response and PD-L1 expression. However, this study needs to expand the sample size for further verification.
Durvalumab
Durvalumab is a humanized monoclonal antibody against the PD-L1 protein. It can directly bind PD-L1 protein and inhibit its binding to PD-1 protein on the surface of T cells so that tumor cells cannot use the PD-L1/PD-1 pathway to escape the pursuit of the immune system, and thereby activating the immune system and killing tumor cells.
In a phase I a/b clinical trial of durvalumab combined with ramucirumab in the treatment of various advanced malignant tumors, 42 the initial ORR was 21% in patients with GC/gastroesophageal junction cancer who have been previously treated but cannot be removed or metastasized. The median progression-free survival and OS were 2.6 and 12.4 months, respectively, reflecting a good antitumor activity. However, the PLATFORM study gave negative results. Devalumab maintenance therapy did not significantly prolong PFS and OS in patients with advanced G/GEJ cancer who completed ≥ 18 weeks of platinum-containing induction chemotherapy. Exploratory analysis of PD-L1 expression showed a trend for improved PFS with durvalumab, but was limited by the small sample size. In addition, clinical studies of durvalumab as a first-line maintenance treatment are also recruiting (NCT02678182).
PD-1 Combined With Other Targeted Drugs
In addition to the combination of chemotherapy drugs, PD-1 also showed good research results in combination with targeted drugs. The results of the TOGA test showed that the median OS was significantly prolonged in GC patients with HER-2 overexpression after trastuzumab. 6 In 2019, the ESMO meeting updated the phase I study results of margetuximab combined with pembrolizumab. Among HER-2 and PD-L1 double-positive patients, the median OS was 20.5 months and the ORR was 48%, which is better than the first-line treatment data of the TOGA study. This joint scheme is expected to become the preferred scheme for double-positive populations. 43 In another phase II clinical study (NCT02954536) evaluating pembrolizumab combined with trastuzumab and first-line chemotherapy in the treatment of HER-2 positive metastatic gastroesophageal adenocarcinoma, the ORR was 87% and the DCR was 100%. This suggests that the combined immunization program has played an important role in the treatment of GC. 44
Herbst et al 45 also recruited and treated 92 patients, including 41 with gastric or gastroesophageal junction adenocarcinoma. The median follow-up time was 32.8 months. The results showed that ramucirumab combined with pembrolizumab had good antitumor activity and was safe and controllable. In addition, Chau et al 46 evaluated the antitumor activity and tolerance of patients with advanced cancer who had previously received treatment with ramucirumab combined with Pembrolizumab. The results showed that ORR was 25% and did not reach the response duration, PFS and OS were 5.6 and 14.6 months, respectively. It also shows the importance of combined immunal targeting in the treatment of advanced GC.
In June 2020, Lancet published the data of a clinical study led by Japanese scholars (EPOC1706). The results showed that the ORR of pembrolizumab combined with lenvatinib in the first and second-line treatments of GC was 69%, the DCR was 100%, and the median PFS of 29 patients was 7.1 months. 47
In a phase Ib trial of regorafenib combined with nivolumab in the treatment of GC and colorectal cancer, 48 25 patients with end-stage GC and 25 patients with colorectal cancer were included in the study. Except for one patient with MSI-H, the other patients had microsatellite stability. After the combined treatment of nivolumab and regorafenib, the objective remission rate of GC patients was 44% and the median progression-free survival time was 5.6 months. At present, the subsequent phase III clinical trial is still in progress.
CTLA-4 Inhibitor
CTLA-4 is a small molecule expressed on the surface of T cells. It is expressed prematurely on CD4+ and CD8+ T cells. Meanwhile, it can also negatively regulate the activation of T lymphocytes. 49 CTLA-4 inhibitors can specifically bind to CTLA-4 to disinhibit CTLA-4 on T cells, so as to restore the immune function of the body. At present, CTLA-4 inhibitors are mainly prepresented by ipilimumab and tremelimumab. Many studies have found that the expression of CTLA-4 in GC is higher than that in normal tissues, and the hypermethylation of the CTLA-4 gene promoter is a risk factor for GC. 50 Schlβer et al 51 also found from 127 GC tissues that the positive rate of CTLA-4 was up to 86.6% (110/127), and was associated with poor prognosis.
Ipilimumab
Ipilimumab is a fully humanized monoclonal antibody and an immune checkpoint protein that positively regulates T cells. Ipilimumab can specifically block the interaction between CTLA-4 and its ligands and promote the activation of T cells and subsequently enhance the tumor immune response.
In 2011, ipilimumab was approved by FDA as a drug for the treatment of advanced melanoma, but there are few clinical trials for GC. In phase II clinical trial of ipilimumab as a sequential or maintenance treatment after first-line chemotherapy for advanced GC and gastroesophageal cancer (NCT01585987), 52 no significant benefit was found in ipilimumab maintenance treatment, but the good safety profile supported the advantage of ipilimumab combination therapy. Nivolumab and ipilimumab have distinct but complementary mechanisms of action that contribute to the restoration of anti-tumor T-cell function and induction of de novo anti-tumor T-cell responses, respectively. Combination therapy demonstrated clinically meaningful anti-tumor activity with a manageable safety profile in heavily pretreated patients with advanced gastro-oesophageal cancer. 53 In another phase II clinical trial of GC (NCT01928394), the objective response rates (ORRs) of the nivolumab group and ipilimumab combined with the nivolumab group were 14% and 26%, respectively. The results showed that combined treatment with ipilimumab may have a good effect on advanced GC. Subsequently, the AIO Moonlight study showed that FOLFOX combined with Nivolumab and Ipilimumab was significantly more effective than sequential nivolumab and Ipilimumab administration after FOLFOX induction at the annual meeting of the European Society of Medical Oncology (ESMO 2022). Despite the low toxicity of sequential therapy, this study does not support the use of sequential therapy in first-line treatment. 54 However, the efficacy of ipilimumab monotherapy was unsatisfactory, since the ORR was significantly lower than that of the support treatment group, and the toxic and side effects 55 were relatively large.
Tremelimumab
Tremelimumab is a fully humanized monoclonal IgG2 antibody against CTLA-4, which can block the activity of CTLA-4, promote T cell activation, initiate the tumor immune response, and subsequently promote cancer cell death. 56
It has been confirmed by a number of clinical trials that tremelimumab has certain antitumor activity against solid tumors such as malignant melanoma, nonsmall cell lung cancer, malignant mesothelioma, and gastroesophageal cancer. 57 In phase II clinical trial, tremelimumab, as a second-line treatment for patients with metastatic GC, has a certain antitumor effect. 58 Among them, 18 patients with GC/gastroesophageal junction cancer (GC/GEJ) received tremelimumab. The median survival time was only 4.8 months, and the overall curative effect was poor. However, it is worth noting that one patient obtained a lasting and significant curative effect, and OS has been more than 32.7 months. This study suggests that tremelimumab may have a good effect on a specific subtype of gastroesophageal cancer. Therefore, screening a suitable population is of great significance for immunotherapy. Another phase IB clinical study evaluated the safety and tolerance of durvalumab and tremelimumab combined with first-line chemotherapy in the treatment of advanced solid tumors (including GC and gastroesophageal cancer). The relevant test results have not been reported. Another phase IB/II clinical trial that is recruiting evaluated the safety and tolerance of durvalumab and tremelimumab combined with first-line chemotherapy in patients with locally advanced or metastatic solid tumors, including gastric and gastroesophageal cancer. There is also a phase II, multicenter, single-arm, multicohort trial to evaluate the activity of tremelimumab combined with durvalumab as a neoadjuvant or radical treatment for patients with resectable MSI-H GC/GEJC. 59 In conclusion, tremelimumab has significant efficacy and manageable safety in the treatment of advanced GC, which is the preferred treatment for patients with advanced GC and can bring greater survival benefits.
Immune checkpoint Inhibitors Independently Developed in China
Domestic immune checkpoint inhibitors have also published some exploratory results in the third-line treatment of GC. The results of NCT02915432 showed that toripalimab showed controllable safety and promising antitumor effects in patients with refractory advanced GC (AGC). 60 In addition, domestic ICIS also evaluated the efficacy and safety of camrelizumab combined with apatinib in the second-line treatment of GC/GEJC through SHR-1210 (NCT02942329). At present, the published median OS was 11.4 months and the DCR was 78.3%. The results showed that the protocol had good patient tolerability and promising clinical efficacy. The phase III study after cohort expansion is undergoing. 61 In another multicenter, open-label, phase II trial, 62 also demonstrated that camrelizumab combined with CapeOX, and camrelizumab combined with apatinib, can be used as a first-line combination regimen for advanced or metastatic G/GEJ adenocarcinoma, showing encouraging antitumor activity and controllable toxicity.
At present, SOX combined with apatinib and camrelizumab as a treatment scheme for resectable locally AGC (NCT04208347) 63 is still in the clinical-trial phase. In 2019, Innovent Biological announced the research results of NCT02937116 at the ASCO meeting, which showed ORR of 85%, DCR of 100%, CR of 10%, good tolerance, and controllable adverse reactions. 64 Recently, in a retrospective clinical study of sintilimab as a second- or later-line treatment for patients with advanced or metastatic GC, the results showed that the median PFS was 2.5 months, which was well tolerated, and provided a feasible treatment strategy for sintilimab monotherapy or combination therapy. 65 At present, the phase III clinical of this regimen is also being carried out in China, which is expected to impact the first-line treatment of advanced GC. At the 2020 ASCO meeting, Murakami et al 66 reported on whether sequential ramucirumab or paclitaxel treatment can enhance the efficacy after the progress of ICI treatment for advanced GC. The results showed that immunotherapy might enhance the follow-up efficacy, and this sequential treatment combination could provide a new choice for patients with primary drug resistance to immunotherapy.
MSI-H
In addition to PD-L1, MSI also has certain predictive values. Studies 67 have shown that cancer patients with MSI-H who received pembrolizumab monotherapy had good clinical efficacy. For 12 different types of solid tumors, the ORR of pembrolizumab treatment was 53%, and the CR rate was 21%, including some patients with GC. Based on this, the FDA approved pembrolizumab for the treatment of patients with MSI-H solid tumors in 2017, regardless of the type of tumor.
Epstein-Barr Virus Positive
Some studies have demonstrated that Epstein-Barr virus (EBV) positivity may be used as an index to predict the efficacy of ICI in GC. In phase II clinical trial, 61 patients with metastatic GC treated with pembrolizumab were observed. It was found that EBV-positive patients responded well to pembrolizumab, with an overall remission rate of 100% and a median duration of 8.5 months. 68 Panda et al 69 found in the treatment of metastatic GC with avelumab that compared with microsatellite stability (MSS) patients, EBV-positive patients with low mutation or high mutation have greater clinical benefit and a higher proportion of tumor-infiltrating lymphocytes. This result suggests that EBV positive may also be one of the effective biomarkers for PD-1 antibody treatment.
Nonspecific Enhancer Therapy
Nonspecific enhancers are immune modulators, which typically are not used alone. They can enhance the immune response in vivo when combined with other treatment methods and thereby improving the curative effect. Nonspecific enhancers commonly used clinically are cytokines (CK), Lentinan, Streptococcus Preparation (OK-432), and Bacillus Calmette-Guerin Vaccine (BCG Vaccine).
Cytokines
CK is a kind of bioactive small molecular protein produced by immune cells stimulated by antigen, mitogen, or other factors, which can regulate the immune function of the body and promote the activation, proliferation, and differentiation of lymphocytes. 70 At present, cytokines commonly used clinically include IL-2, IFN-γ, TNF-α, GM-CSF, etc. Among them, IL-2 is a small molecular glycoprotein produced by a variety of immune cells. Its main role is to enhance the recognition ability of the body to different antigens and the immune activity of tumors to promote the proliferation and differentiation of immune cells, induce the generation of the lymphokine-activated killer (LAK) cell, promote the proliferation of natural killer cell (NK), and enhance their ability to kill tumors. 71 A previous study has shown that high-dose IL-2 or IL-2 combined with lymphokine-activated killer cells (LAK) can mediate tumor regression in patients with advanced cancers such as metastatic renal cell carcinoma, melanoma, and colorectal cancer. 72 Cesana et al 73 used low-dose IL-2 to treat patients after radical gastrectomy and postoperative adjuvant chemotherapy. They found that the counts of CD8+ T cells, CD4+ T cells, and NK cells in the periphery and adjacent to the tumor were significantly increased. In addition, animal studies have also shown that IL-7 combined with tumor vaccine or adoptive immunotherapy yielded good antitumor effects in tumor models. 74 CKs may produce certain adverse reactions when used alone, so they mostly act as adjuvants in the immunotherapy of GC, playing an auxiliary role in promoting the proliferation and differentiation of lymphocytes and the improvement of tumor microenvironment. 75
Lentinan
Lentinan is the most widely used immune adjuvant in the treatment of GC, which was reported as early as 1999. 76 It is a polysaccharide isolated from the fruiting body of high-quality Lentinus edodes. It can improve the host's homeostasis and restore and improve the host's responsiveness to lymphatic factors, hormones, and other physiologically active factors by stimulating the maturation, differentiation, and proliferation of immune cells, thus indirectly playing an anti-tumor role. 77 Ochiai et al 78 found in a controlled randomized study on advanced GC that the number of T cells in the chemotherapy combined with the lentinan group was higher than in chemotherapy alone. Moreover, It was also found that lentinan could regulate cytokines such as IL-2, IL-4, IL-6, IL-10, and TNF-α in patients with GC and improve the activity of immune cells such as lymphocytes, dendritic cells (DCs), and NK, thereby improving the killing effect of the tumor. 79 Some believe that the development of GC is related to the imbalance of Th1 and Th2 subsets of helper T cells. Yoshino et al 80 found that lentinan can improve Th1/Th2 balance, induce Th1 immune response, and regulate the secretion of immune cytokines. In addition, combined treatment has also shown good clinical efficacy. A study found that lentinan combined with S-1 has a synergistic antitumor effect. Compared with chemotherapy alone, lentinan can prolong the survival in patients with advanced GC and activate their immune activity.81,82 Oba et al 83 also found that lentinan combined with chemotherapy has a good therapeutic effect on patients with advanced GC. Compared with chemotherapy alone, lentinan can significantly prolong the OS of GC patients.
Streptococcus Preparation
Streptococcus preparation (OK-432) is a commonly used nonspecific immune enhancer, which can promote the proliferation of monocytes, enhance the activity of T lymphocytes and natural killer cells, and promote the release of a variety of cytokines. Therefore, OK-432 can prolong the survival of patients with stage III GC.84,85 It has been reported that the preparation can improve the 5- and 10-year survival in patients with stage III A and III B gastric adenocarcinoma via stimulating the release of a variety of cytokines and increasing the toxic effect of the immune system on tumor cells. Meanwhile, OK-432 has a significant difference in overall survival compared with the control group and can reduce the 3-year mortality by 13%. 86 Panzini et al 87 also found that OK-432 immunotherapy combined with chemotherapy has survival benefits for patients after radical gastrectomy, and the 3-year overall survival odds ratio was 0.81 (relative risk reduction: 13%).
Bacillus Calmette-Guerin (BCG) Vaccine
BCG vaccine is a live vaccine made from the suspension of attenuated Bovine tuberculosis bacillus that can enhance the activity of macrophages and activate various cytotoxic T cells such as CD4+ and CD8+, thereby enhancing cellular immunity. 88 The specific immune response of BCG and tumor cells can induce the transformation of Th0 cells to Th1 cells, and at the same time promote the activation and proliferation of T cells, forming a durable and effective anti-tumor immune response. 89 Early studies have shown 90 that BCG combined with FAM can significantly prolong the survival of patients with advanced GC after radical surgery.
Oncolytic Virus Therapy
Oncolytic virus therapy is a new type of tumor immunotherapy that uses natural or genetically modified viruses to selectively kill malignant cells or activate adaptive immune responses in the human body. 91 It has become a research hotspot in recent years. Oncolytic viruses can be divided into 2 categories: the first category is the inherent oncolytic wild-type strains, which have the affinity for some tumor cells and can replicate and lyse cells independently in some tumor cells and thereby achieving the specific oncolytic effects. The second type is the modification of the viral genome to make the virus tumor-selective. Then, by mutating or knocking out certain genes, loading tumor-specific promoters, and inserting tumor-targeting genes, the virus loses its ability to replicate in normal cells, but its ability to target and infect tumor cells is enhanced. And it can be replicated in a large number. Finally, it plays a role in oncolysis.92,93 At present, oncolytic virus treatment of GC is only limited to in vitro research, and there is no report of clinical studies.
Tumor Vaccine Therapy
Tumor vaccine therapy is one of the active immunotherapies, which activates the specific immune response of the body by injecting tumor antigen, inducing T lymphocytes to recognize the tumor antigen on the surface of antigen-presenting cells, and activating CD4+ helper T lymphocytes. 94 In 2 independent phase I clinical trials published in Nature in 2017, the results showed that the individualized vaccine had achieved great success in the treatment of malignant melanoma.95,96 Subsequently, Chen et al 97 comprehensively analyzed the exome sequencing data of 942 GC samples, detected 74 864 new antigens, and found significant mutant genes such as PGM5, TP53, TRIM49C, and PIK3CA, which can carry more new antigens. Because these new antigens can cover a certain proportion of the GC population, tumor vaccine therapy may have potential clinical value in the treatment of GC. Tumor vaccines mainly take the forms of DC vaccine, nucleic acid vaccine, peptide/protein vaccine, tumor cell vaccine, etc.
Dendritic Cell (DC) Vaccine
DC is a cell type different from macrophages first isolated and identified by Ralph Steinman in 1973. 98 It is the most powerful antigen-presenting cell known at present. It can produce a strong immune response and has the unique ability to activate naive T cells. 99 At present, the DC vaccine has been widely used in the treatment of a variety of tumors, 100 but its efficacy in GC is still controversial. HER-2 is an ideal target in the treatment of GC, and a variety of treatment strategies for HER-2 have achieved initial results. Kono et al 101 used the DC vaccine treated with HER-2-derived peptide pulse. Among them, 66.7% of GC patients had induced cytotoxic T lymphocytes (CTL). One patient achieved partial remission, and the other patients obtained a stable period of 3 months, which preliminarily confirmed the feasibility of targeting the HER-2/DC vaccine. Other studies have found that MAGE-3 loaded DC vaccine showed good clinical efficacy in patients with gastrointestinal tumors and can induce tumor antigen-specific T cell response, enabling patients to obtain a higher response rate. 102 In another trial using MAGE-A3/DC in the treatment of advanced GC, 103 antigen-specific T cells were detected in 4 patients, of which 3 had a certain degree of remission. Matsuda et al 104 also conducted a clinical study on 8 patients with gastrointestinal malignancies and positive CEA expression. Results showed that 4 patients with GC had CTL reactions after vaccination, and no adverse reactions related to DC vaccine injection were reported.
Nucleic Acid Vaccine
The tumor nucleic acid vaccine is an expression vector carrying an antigen gene. It can directly enter tissue cells and synthesize corresponding antigen proteins through the expression system of host cells, so as to induce a specific immune response. DNA vaccine uses plasmids or viruses as vectors to induce cellular and humoral immunity by antigen-presenting cells’ (APCs) uptake, transcription, translation, processing, and expression to immune cells. RNA vaccine can contain the coding information of multiple epitopes and simulate the expression mode of tumor antigen through in situ expression of antigens, and thereby inducing T cell response similar to that caused by immune stimulation of DNA vaccine. Since the nucleic acid vaccine does not use cells as carriers, the production and preservation conditions are relatively less strict, but its immunogenicity is lower, which is subject to the uptake and presentation efficiency of APCs. 105 Therefore, vaccines are often used in combination with cytokines such as GM-CSF and IL-10. 106 In recent years, clinical trials based on RNA vaccines have shown good safety profiles in patients with melanoma and prostatic adenoma by triggering a specific antitumor immune response.107,108 In conclusion, the safety of the nucleic acid vaccine is satisfactory, but its effectiveness needs to be tested, and there is no report of its clinical use for the treatment of GC.
Peptide/Protein Vaccine
A peptide/protein vaccine is composed of recombinant protein or purified protein with known or predicted antigen genes. The peptide is the most commonly used form of vaccine antigen. Compared with single peptide preparation, polypeptide vaccine has a better curative effect. These vaccines can activate the immune system by combining with major histocompatibility complex (MHC) molecules or T cells of APC, and thereby playing an anti-tumor role. 109 Tumor antigen peptides are easy to prepare and purify, and their immune response can be boosted by increasing their amino acid molecular weight or using immune adjuvants. 110 As for the research of GC vaccine, polypeptide vaccine is an important direction for research.
In phase I clinical study evaluating the treatment of advanced GC with the porcine parvovirus (PPV) vaccine, the survival time of 13 patients was prolonged (with mOS of about 7 months) and the cellular and humoral immune responses were enhanced. 111 In addition, the phase I/II clinical study of TS-1 combined with the PPV vaccine in the treatment of advanced GC or colorectal cancer showed that the standard dose of TS-1 combined with PPV would not curb the immune response in tumor patients, but could maintain or enhance the function of the immune system. 112 In another phase I clinical trial on patients with advanced GC inoculated with lymphocyte antigen 6 complex locus K (LY6K)-derived peptide vaccine, it was found that the expression rate of LY6K in GC was as high as 85%. Three of the 6 patients were stable, and one patient had a tumor shrinking reaction. Thus, the LY6K-derived peptide vaccine had good tolerance and safety in the treatment of advanced GC. 113
G17DT is the antigenic epitope of gastrin. There is evidence that gastrin plays an important role in stimulating the growth of GC. 114 In the G17DT clinical trial led by Anderson Cancer Center, G17DT was combined with 5-FU and cisplatin and the median survival in immune responders was significantly longer than that in nonresponders, and the median progression time was longer as well. 115 Another multicenter clinical trial 116 also evaluated the efficacy of the G17DT vaccine combined with cisplatin and fluorouracil in patients with advanced gastric or gastroesophageal adenocarcinoma. The results showed that progression-free survival (mPFS) and mOS in patients with the G17DT vaccine were longer than in those without vaccination. Successful vaccination is associated with longer mPFS and mOS, which is expected to become an effective treatment for patients with advanced GC or gastroesophageal cancer. However, the related toxic and side effects are still concerning, so it is necessary to conduct further phase III clinical verifications of the G17DT vaccine.
Recent studies have found that IMU131, a B-cell vaccine, induced specific B cells to produce corresponding antibodies to achieve an effect similar to the use of HER-2 monoclonal antibody. Wiedermann et al 117 used different doses of IMU131 to treat GC patients with HER-2/neu overexpression and found that the effective rate in 14 patients reached 54%, indicating good preliminary results. Among other types, the OTSGC-A24 binding peptide vaccine has also achieved satisfactory efficacy in the treatment of advanced GC. It not only induced a strong CTL immune response, but also had good tolerance and safety profiles. 118 Fujiwara et al 119 also found that although the HLA-A24 combined peptide vaccine did not show an obvious survival benefit in the treatment of advanced GC, it was acceptable in terms of safety.
Tumor-Related Antigen Vaccines
Tumor-associated antigens are proteins with specific amino acid sequence variations produced by tumor cells on the basis of gene mutation. These proteins do not have antigenicity under normal conditions. Once mutation takes place, they will cause a series of immune reactions. 120 This provides a new idea for the treatment of GC.
The target protein of GC immunity is the vascular endothelial growth factor receptor (VEGFR), which can activate the patient's autoimmune system and enhance the killing effect of T cells or antibodies on cancer cells. Early studies at Osaka University in Japan found that VEGFR1 and VEGFR2 polypeptide vaccines combined with first-line chemotherapy showed good prognosis and VEGFR-specific CTL response, in which the response rate could also be as high as 82%. The overall OS and time to progression (TTP) were prolonged and the therapy was well tolerated. 121 Meanwhile, the experiment also proved the clinical feasibility of the combined strategy. Subsequently, Higashihara et al 122 also carried out a phase I clinical study to observe the efficacy of HLA-A24-related URLC10 and VEGFR1 polypeptide cancer vaccines in advanced GC after chemotherapy resistance. The results also showed that the therapy had a good CTL immune response and safety profile. In addition, Iwauchi et al 123 found that ERAS can be used as a new tumor-related antigen to successfully induce CTL related to GC and enhance the immune response, which provides a theoretical basis for further clinical translational research. Since the immunogenicity of tumor antigens is relatively weak, it is often necessary to add immune adjuvants to enhance the effect. 124 The commonly used immune adjuvants for GC mainly include BCG vaccine, OK432, Corynebacterium Brevis vaccine, Levamisole, Coriolus Versicolor Polysaccharide, and Lentinan in polysaccharides. These immune adjuvants can trigger strong nonspecific immune stimulations. Macrophages in patients with GC can be activated and promote the production of a variety of cytokines, stimulate the immune system, promote the clearance of tumor cells, and reduce tumor recurrence and metastasis.
Although some clinical trials of vaccines have achieved positive results, the objective response rate of vaccines remains largely low. Individualized screening of dominant antigens, selection of the best vaccine form and adjuvants, and effective combinative treatment modalities are the direction and trend for tumor vaccine improvement in the future.
Adoptive Cellular Immunotherapy
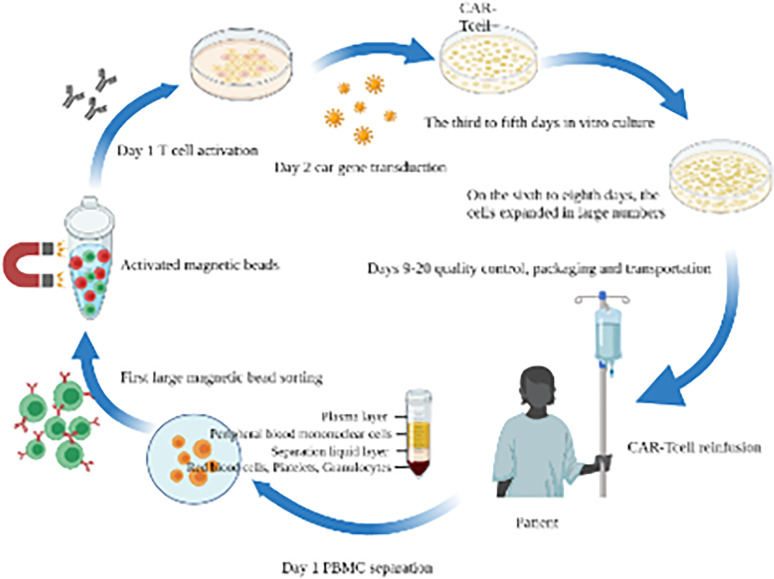
Adoptive immunotherapy is to extract the sensitized immune cells (including T cells and natural killer cells) and their products from patients or donors in vitro and reinfuse them into patients, so as to promote the proliferation of various immune cells and play the anti-tumor role. These cells can specifically recognize tumor antigens and combine with them, which can subsequently kill tumor cells. 125 At present, the commonly used adoptive T lymphocyte immunotherapy mainly includes cytokine-induced killer (CIK) cells, tumor-infiltrating lymphocyte (TIL), natural killer cell therapy, chimeric antigen receptor T-Cell (CAR-T), and T cell receptor-gene engineered T cells (TCR-T). 99 Therefore, the adoptive reinfusion therapy of immune cells has been widely concerned and recognized in the oncology community, and has achieved definite curative effects in malignant melanoma, lung cancer, kidney cancer, and other diseases. The following is the flow chart of CAR-T cell therapy (Figure 2).
Figure 2.
Chimeric antigen receptor T-cell (CAR-T Cell) therapy process.
In Figure 2, the first step in the production of CAR-T is to collect peripheral blood mononuclear cells (PBMCs) from patients (or allogeneic donors) through leukocyte separation and separation of specific T cell subsets, such as CD4+, CD8+, CD25+, or CD62L+ T cells, using magnetic bead-based technology. The isolated T cells are activated by T cell receptors and costimulatory signals such as CD28, 4-1BB, or OX40. Autologous antigen-presenting cells, such as DCs, are endogenous activators of T cell response. Enabling the expression of CAR on T cells is the core technology of CAR-T production. In this step, Lentivirus (LV) vector needs to be used to introduce the CAR gene into T cells. After obtaining stable CAR-T cells through genetic modification, large-scale in vitro expansion is also required to obtain the dose required for treatment, generally at the level of 1 billion to 10 billion. After cell expansion, it may eventually reach about 5 L in volume. In the standard process, CAR-T cells need to be concentrated to a certain volume and undergo quality control, packaging and transportation before reinfusion to patients. T cells after reinfusion will also proliferate in patients and kill tumor cells with corresponding specific antigens. CAR is a protein receptor that enables T cells to recognize specific proteins (antigens) on the surface of tumor cells. T cells expressing CAR can recognize and bind tumor antigens before attacking tumor cells.
Chimeric Antigen Receptor T-Cell Immunotherapy
CAR-T immunotherapy is an antigen receptor T cell synthesized by genetic engineering, which can target and recognize cell surface antigens and directly kill the target cells, thereby bypassing the traditional MHC immune activation pathway and enabling T lymphocytes can recognize tumor-associated antigens (TAAs) through single-chain variable regions (ScFVs), activate T lymphocytes through cell signal transduction, and release CK, thereby playing an anti-tumor therapy.126,127 Many scholars have constructed CAR-T cells related to GC immunotherapy with different vectors, aiming to further study the role and related mechanisms of CAR-T cell immunotherapy at the cellular level. 128
In a basic study, the researchers prepared a CAR-T cell targeting human epidermal growth factor receptor-2 (HER-2) by genetic engineering. The results showed that the cell could be activated by specifically recognizing the HER-2 antigen and effectively killing HER-2 positive GC cells. 129 Jung et al 130 also found that HER-2 can regulate GC stem cells (CSCs) through the Wnt/β-catenin pathway. The effect of HER-2 CAR-T cells on CSC overcomes the heterogeneity of GC to a certain extent, which can also inhibit the regions with no or low expression of HER-2. Since the release of the results of the Claudin 18.2 CAR-T cell (CT041) for solid tumors study led by Prof. Lin Shen's team at the 2021 ESMO Annual Meeting, CT041 has attracted widespread attention from within and outside the global industry. The results were striking. The objective response rate (ORR) and disease control rate (DCR) of 37 patients were 48.6% and 73.0%, respectively. The median progression-free survival (mPFS) was 3.7 months, and the OS rate at 6 months was 80.1%. Good preliminary results were achieved. 131 There is no current clinical report on the treatment of GC. There are 10 related clinical trials (NCT02744287, NCT03960060, NCT02713984, NCT03941626, NCT03941626, NCT02349724, NCT02617134, NCT03563326, NCT03890198, and NCT02725125). Most of the trials are still in the stage of subject recruitment.
T Cell Receptor-Gene Engineered T Cells Immunotherapy
TCR-T therapy is a tumor-adoptive immunotherapy based on modified T cells. At present, TCR-T immunotherapy has achieved exciting results in solid tumors such as melanoma, synovial sarcoma, esophageal cancer, and multiple myeloma.132,133 Most studies related to TCR-T are phase I or phase II trials, of which clinical studies such as NCT03941626, NCT03139370, NCT03686124, NCT02650986, and NCT03190941 are currently in the stage of patient recruitment, and there is no efficacy report available.
CIK Cell and Dendritic Cell-CIK Cell Therapy
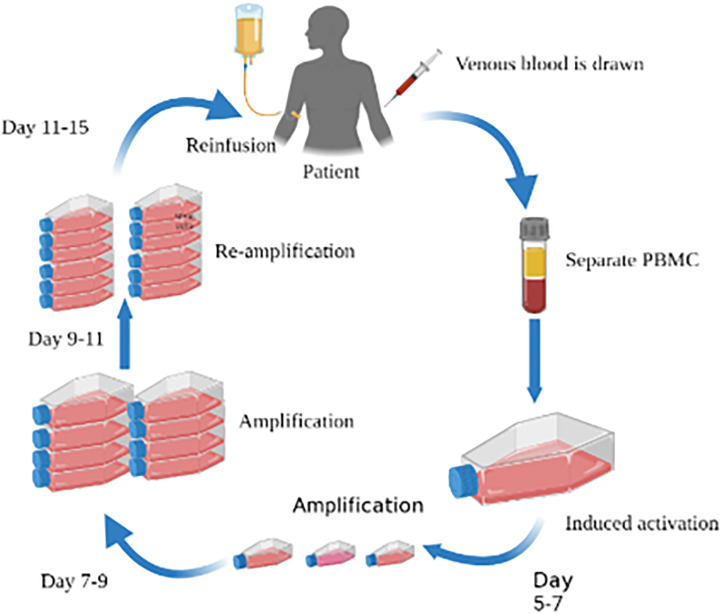
CIK cells are a group of heterogeneous cells with tumor-killing activity limited by a nonmajor histocompatibility complex, which is obtained by coculturing human peripheral blood mononuclear cells with a variety of cytokines in vitro for a period of time. It can express a variety of membrane protein molecules such as CD3+, which not only has the advantages of anti-tumor activity and tumor killing with T lymphocytes but also bears the advantages of low toxicity and high sensitivity. After cells are reinfused, they can significantly improve the immune function of the recipients (Figure 3).134,135 CIK cells have been reported as early as 2005. CIK cells can release cytokines such as interferon and tumor necrosis factor, induce apoptosis, and have an anti-proliferation effect on human MGC-803 GC cells. 136 Kim et al 137 found that after 3 million CIK cells and 10 million CIK cells were injected into mkn74 tumor-bearing nude mice, the tumor volume decreased by 58% and 78%, respectively. Shi et al 138 also published a study on CIK in the treatment of advanced GC. The results showed that CIK cell-assisted immunotherapy could prolong the disease-free survival (DFS) of patients with locally advanced GC and significantly improve the OS of patients with GC. It is suggested that this therapy may improve the distribution of T lymphocyte subsets and enhance the immune function of the host.
Figure 3.
CIK cell and DC-CIK cell treatment process.
Abbreviations: CIK, cytokine-induced killer; DC-CIK, dendritic cell-cytokine-induced killer.
The combined application also shows a good anticancer effects in a clinic. For example, an in vivo test of oxaliplatin combined with CIK cells showed that oxaliplatin could not only exert cytotoxicity by inhibiting DNA synthesis and transcription, but also induce the death of immunogenic cancer cells and enhance the further killing of CIK cells on tumor cells. Compared with chemotherapy alone or CIK cells, oxaliplatin combined with CIK cells had a more significant antitumor effect. 139 Jiang et al 140 evaluated the efficacy of CIK cells combined with chemotherapy in 32 patients with advanced GC. The results showed that compared with the chemotherapy group, the tumor markers in the CIK cells combined with the chemotherapy group were significantly reduced and the total remission rate was significantly increased. In a trial of XELOX combined with an intraperitoneal injection of CIK cells in the treatment of malignant ascites of GC, the volume of ascites and the survival rate of patients were significantly improved, and there were fewer adverse reactions. 141 These results suggest that CIK cells combined with chemotherapy can improve the survival status of patients with advanced GC. In another randomized clinical phase II study, CIK cells and DC derived from umbilical cord blood combined with chemotherapy in patients with advanced GC can significantly prolong disease-free survival, and the toxicity and side effects of this regimen are small. 142 Another prospective nonrandomized controlled study also found that the disease control rates (DCRs) of 4 groups of patients with advanced GC were 5.6%, 33.3%, 47.1%, and 76.9%, respectively, after receiving S-1 monotherapy, S-1+cisplatin (SP), dendritic cell-cytokine-induced killer (DC-CIK)+S-1, and DC-CIK+SP. The PFS and OS of patients in chemotherapy combined with the DC-CIK group were significantly better than those in the simple chemotherapy group, suggesting that cytotoxic drugs can cause changes in the tumor microenvironment after attacking cancer cells, 143 so as to increase the release of tumor antigen, promote the presentation of DC antigen and further activate the host immune response.
In addition, retrospective studies also reported good research results. In a retrospective study of 156 patients with GC who had undergone surgical resection, it was found 144 that the survival of patients receiving chemotherapy combined with CIK cells was longer than that of patients receiving chemotherapy alone, and increasing the use frequency of CIK cells seemed to be more beneficial to patients. In another retrospective analysis, compared with chemotherapy alone, CIK combined with chemotherapy can improve the 5-year survival rate and prolong the progression-free survival and overall survival of patients with postoperative GC. 145 In another retrospective study of stage II-III postresection GC, CIK cells combined with postoperative adjuvant chemotherapy were found to be able to prolong patients’ survival.
In Figure 3, mononuclear cells are isolated from human peripheral blood. After a certain period of time, DC and CIK cells with healthy activity are induced and cultured in the laboratory and proliferated. A large number of cells are then reinfused into patients. On the premise of not damaging and destroying the immune system and function of the body, they can directly identify, kill and eliminate cancer cells in human blood and lymph tissues, thereby restoring and enhancing the natural anti-cancer immune system and function.
NK Cell Therapy
NK cells are important immune cells playing an important role in the processes of anti-tumor and anti-virus reactions and immune regulation. 146 Tumor cells, virus-infected cells, aging cells, and infected parasites are the target cells of NK cells. 147 At present, studies have also found that NK cells have good antitumor activity against allogeneic or autologous solid tumors, and can effectively prevent the proliferation and metastasis of cancer cells.148,149
In a study of 146 patients with GC, CD57 was found to be a positive marker on NK cells, which was closely related to the number of NK cells, and the 5-year survival rate in patients with high NK infiltration was significantly better than those with low infiltration. The results showed that the number of NK cells was correlated with the therapeutic effect of GC and had a certain prognostic indication. 150 Wu et al 151 found that lupeol can improve the proliferation of NK cells, and subsequently increase the killing effect of NK cells on a variety of GC cell lines.
Tumor-Infiltrating Lymphocyte (TIL) Cell Therapy
TIL cell therapy is a therapy in which lymphocytes isolated from the patient's tumor are cultured and amplified in vitro and then reinfused into the patient's body. 152 In 1988, Rosenberg et al 153 first reported the animal experiment of treating the tumor with TIL cells and found that these cells contained a certain number of tumor-reactive T cells. After activation and amplification by cytokines such as interleukin-2 (IL-2), their antitumor effect is 50 to 100 times that of LAK. Another clinical literature reported that a patient with extensive metastasis of malignant melanoma received TILs treatment and achieved significant remission, 154 suggesting that TIL cells have specific recognition and killing effects on tumor cells and are a new type of anti-tumor effector cells. Kono et al 155 isolated TIL cells from primary tumors, metastatic lymph nodes, and autologous GC ascites fluids and found that the cells could specifically bind to GC antigen and play an antitumor role. Amedei et al 156 also expanded TIL cells and specific T cells from patients with GC in vitro and found increased activity and a number of helper T1 (Th1)/cytotoxic T1 (Tc1) cells. Most of the above studies are limited to laboratory experiments. Moreover, there is a phase 2 clinical trial (NCT03935893) evaluating the effectiveness and safety of TIL in the treatment of solid tumors, which is currently in the stage of patient recruitment.
Conclusion
Collectively, the current treatment methods of GC mainly include surgery, chemotherapy, radiotherapy, and molecular targeted therapy, among which medical therapy remains the mainstay for the treatment of advanced GC. Since conventional therapy cannot bring higher survival benefits to patients, immunotherapy, as a new type of tumor treatment, has great potential in clinical application, especially the anti-PD-1 monoclonal antibody. However, it is an urgent problem to evaluate the molecular characteristics of beneficiary patients. Because the specific antitumor immune response is a complex process regulated by multiple molecules and signal pathways, the combined application of immune checkpoint inhibitors may be a research direction in the future, but immune-related adverse reactions also need to be considered. The interaction among tumor cells, tumor microenvironment, and immune system contributes to tumor immune escape, which also brings challenges to the immunotherapy of GC. More understanding of the underlying mechanism is warranted for the future development of immunotherapy. In addition, previous clinical trials have shown that immunotherapy combined with standard chemotherapy can improve the therapeutic efficacy, which may be related to the fact that chemotherapy can improve the expression of tumor antigens and costimulatory molecules and down-regulate inhibitory signals. 157 However, how to achieve accurate individualized treatment needs further research.
Recent studies have shown that the expression of PD-1/PD-L1 in GC is related to a variety of tumor biology, such as proliferation and migration. Combined with PD-1/PD-L1-related immunotyping can more accurately reflect the prognosis. In addition, patients with EBV-positive and MSI-H GC have benefited from PD-1/PD-L1 inhibitors. These gratifying results open up a broad space for immunotherapy against PD-1/PD-L1 in GC. Current research on PD-1/PD-L1 mainly focus on immunotherapy. Many have found that PD-1 and PD-L1 can also promote the development of cancer independent of the PD-1/PD-L1 pathway.158,159 The immune regulation mechanism is complex, and a variety of signal pathways intersect in the process of PD-1/PD-L1 regulation. Summarizing these mechanisms can provide a theoretical basis for anti-PD-1/PD-L1 therapies. PD-1/PD-L1 in GC is mainly regulated by signal pathways at the transcription level by miRNAs and posttranslational modifications. Studies have shown that various classical signaling pathways, such as MEK-ERK and anaplastic lymphoma kinase (ALK), are also involved in the regulation of PD-1/PD-L1 in other tumors. In addition, it has also been reported that posttranslational modifications such as glycosylation, acetylation, and phosphorylation are involved in the direct regulation of PD-1/PD-L1 protein. These studies provide a new idea for exploring the regulation of PD-1/PD-L1 in GC. At present, there are still many unsolved problems in the immunotherapy of GC. For example, there are cross effects between PD-1/PD-L1 related signal pathways in GC, and the underlying mechanism is complex. Although immune checkpoint inhibitors have been used in clinical treatment, not all tumors expressing PD-1/PD-L1 respond to them. On the contrary, some patients with PD-1/PD-L1 negative tumors can respond to these drugs. Therefore, the research on the molecular mechanism of PD-1/PD-L1 in GC, especially the newly discovered relationship between posttranslational modification and PD-1/PD-L1, may contribute to the development of safer and more effective immunotherapeutic drugs for GC, bringing promises in the individualized and standardized evaluation of combined treatment methods. Ultimately, more options would be available for the treatment of GC and with more safe and effective treatment modalities.
Abbreviations
- GC
Gastric cancer
- FDA
Food and Drug Administration
- CFDA
China Food and Drug Administration
- CK
Cytokines
- BCG Vaccine
Bacillus Calmette-Guerin Vaccine
- LAK
Lymphokine-activated killer cell
- OS
Overall survival
- NK
Natural killer cell
- DC
Dendritic cells
- FAM
5-fluorouracil, adriamycin, mitomycin C
- CTL
Cytotoxic T lymphocytes
- APC
Antigen-presenting Cells
- MHC
Major histocompatibility complex
- PPV
Porcine parvovirus
- LY6K
Lymphocyte antigen 6 complex locus K
- PFS
Progression-free survival
- VEGFR
Vascular endothelial growth factor receptor
- TTP
Time to progression
- CIK
Cytokine-Induced Killer
- DFS
Disease-free survival
- TIL
Tumor infiltrating lymphocyte
- CAR-T
Chimeric Antigen Receptor T-Cell
- TCR-T
T cell receptor-gene engineered T cells
- PBMC
Peripheral blood mononuclear cell
- LV
Lentivirus
- TAA
Tumor-associated antigen
- ScFV
Single chain variable fragment
- HER-2
Human epidermalgrowth factor receptor-2
- CSC
Cancer stem cell
- DCR
Disease control rate
- SP
S-1+cisplatin
- DC-CIK
Dendritic cell-Cytokine-induced killer
- IL-2
Interleukin-2
- Th1
helper T1
- Tc1
cytotoxic T1
- ICIs
immune checkpoint inhibitors
- CTLA-4
Cytotoxic T lymphocyte-associated antigen-4
- PD-1
Programmed cell death 1
- PD-L1
Programmed cell death-ligand 1
- NCCN
National Comprehensive Cancer Network
- MSI
Microsatellite instability
- PCR
Polymerase Chain Reaction
- MMR
Mismatch repair
- IHC
Immunohistochemistry
- CPS
Combined positive score
- dMMR
Deficient Mismatch Repair
- MSI-H
Microsatellite Instability-High
- Treg
Regulatory T cell
- TCR
T cell receptor
- ORR
Objective response rates
- GEJ
Gastroesophageal junction cancer
- CR
Complete response
- PR
Partial response
- pCR
Pathological complete remission rate
- AGC
advanced gastric cancer
- MSS
Microsatellite stability
- EBV
Epstein-Barr virus
- ALK
anaplastic lymphoma kinase
Footnotes
Authors’ Contributions: NNL generated the ideas and design structure of the manuscript. ZPZ organized all figures and wrote the manuscript. NNL and MYS revised and approved the final manuscript. All authors read and approved the final manuscript.
Availability of Data and Materials: All the data obtained and/or analyzed during the current study were available from the corresponding authors on reasonable request.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Science and technology innovation action plan, the Major Project of Shanghai Municipal Science and Technology Commission (Grant number: 19401972300);“major scientific and technological innovation project”, Key R & D plan of Shandong Province (Grant number: 2021CXGC010509); The fourth batch of excellent talents in traditional Chinese medicine, the State Administration of traditional Chinese medicine (Grant number: 2017-124) Shanghai Key Laboratory of Traditional Chinese Clinical Medicine (Grant number: 20DZ2272200).
ORCID iDs: Ningning Liu https://orcid.org/0000-0002-6968-3183
Mingyu Sun https://orcid.org/0000-0001-8103-7283
References
- 1.Sung H, Ferlay J, Siegel RL, et al., Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115-132. [DOI] [PubMed] [Google Scholar]
- 3.Sun Y, Yu W, Guan W, et al. Integrated assessment of PD-L1 expression and molecular classification facilitates therapy selection and prognosis prediction in gastric cancer. Cancer Manag Res. 2019;11:6397-6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bae SH, Kim DW, Kim MS, et al. Radiotherapy for gastric mucosa-associated lymphoid tissue lymphoma: dosimetric comparison and risk assessment of solid secondary cancer. Radiat Oncol J. 2017;35(1):78-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074-2084. [DOI] [PubMed] [Google Scholar]
- 6.Ajani JA, D'Amico TA, Bentrem DJ, et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20(2):167-192. [DOI] [PubMed] [Google Scholar]
- 7.Lordick F, Carneiro F, Cascinu S, et al. Gastric cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;30(10):1005-1020. [DOI] [PubMed] [Google Scholar]
- 8.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535-1546. [DOI] [PubMed] [Google Scholar]
- 9.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823-1833. [DOI] [PubMed] [Google Scholar]
- 10.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915-1928. [DOI] [PubMed] [Google Scholar]
- 12.Matsueda S, Graham DY. Immunotherapy in gastric cancer. World J Gastroenterol. 2014;20(7):1657-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4(5):e180013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398(10294):27-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamath SD, Kalyan A, Benson AB, 3rd. Pembrolizumab for the treatment of gastric cancer. Expert Rev Anticancer Ther. 2018;18(12):1177-1187. [DOI] [PubMed] [Google Scholar]
- 19.Muro K, Chung HC, Shankaran V, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17(6):717-726. [DOI] [PubMed] [Google Scholar]
- 20.Fashoyin-Aje L, Donoghue M, Chen H, et al. FDA approval summary: pembrolizumab for recurrent locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma expressing PD-L1. Oncologist. 2019;24(1):103-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shitara K, Özgüroğlu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392(10142):123-133. [DOI] [PubMed] [Google Scholar]
- 22.Shitara K, Van Cutsem E, Bang YJ, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(10):1571-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bang YJ, Van Cutsem E, Fuchs CS, et al. KEYNOTE-585: phase III study of perioperative chemotherapy with or without pembrolizumab for gastric cancer. Future Oncol. 2019;15(9):943-952. [DOI] [PubMed] [Google Scholar]
- 24.Goodman AM, Kato S, Bazhenova L, et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther. 2017;16(11):2598-2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10111):2461-2471. [DOI] [PubMed] [Google Scholar]
- 26.Chen LT, Satoh T, Ryu MH, et al. A phase 3 study of nivolumab in previously treated advanced gastric or gastroesophageal junction cancer (012): 2-year update data. Gastric Cancer. 2020;23(3):510-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janjigian YY, Bendell J, Calvo E, et al. CheckMate-032 study: efficacy and safety of nivolumab and nivolumab plus ipilimumab in patients with metastatic esophagogastric cancer. J Clin Oncol. 2018;36(28):2836-2844. Doi: 10.1200/JCO.2017.76.6212. Epub 2018 Aug 15. Erratum in: J Clin Oncol. 2019 Feb 10;37(5):443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawakami H, Hironaka S, Esaki T, et al. An investigator-initiated phase 2 study of nivolumab Plus low-dose ipilimumab as first-line therapy for microsatellite instability-high advanced gastric or esophagogastric junction cancer (NO LIMIT, WJOG13320G/CA209-7W7). Cancers (Basel). 2021;13(4):805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelly RJ, Ajani JA, Kuzdzal J, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med. 2021;384(13):1191-1203. [DOI] [PubMed] [Google Scholar]
- 30.Boku N, Ryu MH, Kato K, et al. Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: interim results of a randomized, phase II trial (ATTRACTION-4). Ann Oncol. 2019;30(2):250-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakajima TE, Kadowaki S, Minashi K, et al. Multicenter phase I/II study of nivolumab combined with paclitaxel plus ramucirumab as second-line treatment in patients with advanced gastric cancer. Clin Cancer Res. 2021;27(4):1029-1036. [DOI] [PubMed] [Google Scholar]
- 32.Tintelnot J, Goekkurt E, Binder M, et al. Ipilimumab or FOLFOX with nivolumab and trastuzumab in previously untreated HER2-positive locally advanced or metastatic esophagogastric adenocarcinoma – the randomized phase 2 INTEGA trial (AIO STO 0217). BMC Cancer. 2020;20(1):503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li JX, Huang JM, Jiang ZB, et al. Current clinical progress of PD-1/PD-L1 immunotherapy and potential combination treatment in non-small cell lung cancer. Integr Cancer Ther. 2019;18:1534735419890020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dodbiba L, Teichman J, Fleet A, et al. Primary esophageal and gastro-esophageal junction cancer xenograft models: clinicopathological features and engraftment. Lab Invest. 2013;93(4):397-407. [DOI] [PubMed] [Google Scholar]
- 35.Doi T, Iwasa S, Muro K, et al. Phase 1 trial of avelumab (anti-PD-L1) in Japanese patients with advanced solid tumors, including dose expansion in patients with gastric or gastroesophageal junction cancer: the JAVELIN solid tumor JPN trial. Gastric Cancer. 2019;22(4):817-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oh D, Lockhart AC, Wong DJ, et al. Avelumab (MSB0010718C), an anti-PD-L1 antibody, as a third-line treatment in patients with advanced gastric or gastroesophageal junction cancer: A phase Ib JAVE LIN solid tumor trial. J Clin Oncol. 2016;34(Suppl 4):188. [Google Scholar]
- 37.Chung HC, Arkenau HT, Lee J, et al. Avelumab (anti-PD-L1) as first-line switch-maintenance or second-line therapy in patients with advanced gastric or gastroesophageal junction cancer: Phase 1b results from the JAVELIN solid tumor trial. J Immunother Cancer. 2019;7(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moehler M, Dvorkin M, Boku N, et al. Phase III trial of avelumab maintenance after first-line induction chemotherapy versus continuation of chemotherapy in patients with gastric cancers: results from JAVELIN gastric 100. J Clin Oncol. 2021;39(9):966-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bang YJ, Ruiz EY, Van Cutsem E, et al. Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN gastric 300. Ann Oncol. 2018;29(10):2052-2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roviello G, D’Angelo A, Generali D, et al. Avelumab in gastric cancer. Immunotherapy. 2019;11(9):759-768. [DOI] [PubMed] [Google Scholar]
- 41.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bang YJ, Golan T, Dahan L, et al. Ramucirumab and durvalumab for previously treated, advanced non-small-cell lung cancer, gastric/gastro-oesophageal junction adenocarcinoma, or hepatocellular carcinoma: an open-label, phase Ia/b study (JVDJ). Eur J Cancer. 2020;137:272-284. [DOI] [PubMed] [Google Scholar]
- 43.Catenacci DVT, Kang YK, Park H, et al. Margetuximab plus pembrolizumab in patients with previously treated, HER2-positive gastro-oesophageal adenocarcinoma (CP-MGAH22-05): a single-arm, phase 1b-2 trial. Lancet Oncol. 2020;21(8):1066-1076. [DOI] [PubMed] [Google Scholar]
- 44.Janjigian YY, Maron SB, Chatila WK, et al. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2020;21(6):821-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herbst RS, Arkenau HT, Santana-Davila R, et al. Ramucirumab plus pembrolizumab in patients with previously treated advanced non-small-cell lung cancer, gastro-oesophageal cancer, or urothelial carcinomas (JVDF): a multicohort, non-randomised, open-label, phase 1a/b trial. Lancet Oncol. 2019;20(8):1109-1123. [DOI] [PubMed] [Google Scholar]
- 46.Chau I, Penel N, Soriano AO, et al. Ramucirumab in combination with pembrolizumab in treatment-naïve advanced gastric or GEJ adenocarcinoma: aafety and antitumor activity from the phase 1a/b JVDF trial. Cancers (Basel). 2020;12(10):2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kawazoe A, Fukuoka S, Nakamura Y, et al. Lenvatinib plus pembrolizumab in patients with advanced gastric cancer in the first-line or second-line setting (EPOC1706): an open-label, single-arm, phase 2 trial. Lancet Oncol. 2020;21(8):1057-1065. [DOI] [PubMed] [Google Scholar]
- 48.Fukuoka S, Hara H, Takahashi N, et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase ib trial (REGONIVO, EPOC1603). J Clin Oncol. 2020;38(18):2053-2061. [DOI] [PubMed] [Google Scholar]
- 49.Kwak Y, Seo AN, Lee HE, et al. Tumor immune response and immunotherapy in gastric cancer. J Pathol Transl Med. 2020;54(1):20-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kordi-Tamandani DM, Davani SK, Baranzehi T, et al. Analysis of promoter methylation, polymorphism and expression profile of cytotoxic T-lymphocyte-associated antigen-4 in patients with gastric cancer. J Gastrointestin Liver Dis. 2014;23(3):249-253. [DOI] [PubMed] [Google Scholar]
- 51.Schlößer HA, Drebber U, Kloth M, et al. Immune checkpoints programmed death 1 ligand 1 and cytotoxic T lymphocyte associated molecule 4 in gastric adenocarcinoma. Oncoimmunology. 2015;5(5):e1100789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang X, Ge X, Zhang Y, et al. The current management and biomarkers of immunotherapy in advanced gastric cancer. Medicine (Baltimore). 2022;101(21): e29304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shitara K, Ajani JA, Moehler M, et al. Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature. 2022;603(7903):942-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stein A, Paschold L, Tintelnot J, et al. Efficacy of ipilimumab vs FOLFOX in combination with nivolumab and trastuzumab in patients with previously untreated ERBB2-positive esophagogastric adenocarcinoma: the AIO INTEGA randomized clinical trial. JAMA Oncol. 2022;8(8):1150-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bang YJ, Cho JY, Kim YH, et al. Efficacy of sequential ipilimumab monotherapy versus best supportive care for unresectable locally advanced/metastatic gastric or gastroesophageal junction cancer. Clin Cancer Res. 2017;23(19):5671-5678. [DOI] [PubMed] [Google Scholar]
- 56.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Comin-Anduix B, Escuin-Ordinas H, Ibarrondo FJ. Tremelimumab: research and clinical development. Onco Targets Ther. 2016;9:1767-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ralph C, Elkord E, Burt DJ, et al. Modulation of lymphocyte regulation for cancer therapy: a phase II trial of tremelimumab in advanced gastric and esophageal adenocarcinoma. Clin Cancer Res. 2010;16(5):1662-1672. [DOI] [PubMed] [Google Scholar]
- 59.Raimondi A, Palermo F, Prisciandaro M, et al. Tremelimumab and durvalumab combination for the non-operative management (NOM) of microsatellite instability (MSI)-high resectable gastric or gastroesophageal junction cancer: the multicentre, single-arm, multi-cohort, phase II INFINITY study. Cancers (Basel). 2021;13(11):2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang F, Wei XL, Wang FH, et al. Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD-1 antibody in phase ib/II clinical trial NCT02915432. Ann Oncol. 2019;30(9):1479-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu J, Zhang Y, Jia R, et al. Anti-PD-1 antibody SHR-1210 combined with apatinib for advanced hepatocellular carcinoma, gastric, or esophagogastric junction cancer: an open-label, dose escalation and expansion study. Clin Cancer Res. 2019;25(2):515-523. [DOI] [PubMed] [Google Scholar]
- 62.Peng Z, Wei J, Wang F, et al. Camrelizumab combined with chemotherapy followed by camrelizumab plus apatinib as first-line therapy for advanced gastric or gastroesophageal junction adenocarcinoma. Clin Cancer Res. 2021;27(11):3069-3078. [DOI] [PubMed] [Google Scholar]
- 63.Zheng Y, Wang Z, Yan C, et al. Protocol for a randomized controlled trial of perioperative S-1 plus oxaliplatin combined with apatinib and camrelizumab in patients with resectable, locally advanced gastric or gastroesophageal junction adenocarcinoma. Ann Transl Med. 2020;8(24):1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jiang H, Zheng Y, Qian J, et al. Safety and efficacy of sintilimab combined with oxaliplatin/capecitabine as first-line treatment in patients with locally advanced or metastatic gastric/gastroesophageal junction adenocarcinoma in a phase ib clinical trial. BMC Cancer. 2020;20(1):760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nie C, Lv H, Liu Y, et al. Clinical study of sintilimab as second-line or above therapy in patients with advanced or metastatic gastric cancer: a retrospective study. Front Oncol. 2021;11:741865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murakami K, Takeno A, Masuzawa T, et al. Clinical efficacy and safety of nab-paclitaxel plus ramucirumab therapy in patients with unresectable advanced or recurrent gastric cancer. Gan To Kagaku Ryoho. 2020;47(3):493-495. [PubMed] [Google Scholar]
- 67.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim ST, Cristescu R, Bass AJ, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24(9):1449-1458. [DOI] [PubMed] [Google Scholar]
- 69.Panda A, Mehnert JM, Hirshfield KM, et al. Immune activation and benefit from avelumab in EBV-positive gastric cancer. J Natl Cancer Inst. 2018;110(3):316-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Waldmann TA. Cytokines in cancer immunotherapy. Cold Spring Harb Perspect Biol. 2018;10(12):a028472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spitzer JH, Meadows GG. Modulation of perforin, granzyme A, and granzyme B in murine natural killer (NK), IL2 stimulated NK, and lymphokine-activated killer cells by alcohol consumption. Cell Immunol. 1999;194(2):205-212. [DOI] [PubMed] [Google Scholar]
- 72.Orozco Valencia A, Camargo Knirsch M, Suavinho Ferro E, et al. Interleukin-2 as immunotherapeutic in the autoimmune diseases. Int Immunopharmacol. 2020;81:106296. [DOI] [PubMed] [Google Scholar]
- 73.Cesana GC, Romano F, Piacentini G, et al. Low-dose interleukin-2 administered pre-operatively to patients with gastric cancer activates peripheral and peritumoral lymphocytes but does not affect prognosis. Ann Surg Oncol. 2007;14(4):1295-1304. [DOI] [PubMed] [Google Scholar]
- 74.Fewkes NM, Mackall CL. Novel gamma-chain cytokines as candidate immune modulators in immune therapies for cancer. Cancer J. 2010;16(4):392-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kueberuwa G, Kalaitsidou M, Cheadle E, et al. CD19 CAR T cells expressing IL-12 eradicate lymphoma in fully lymphoreplete mice through induction of host immunity. Mol Ther Oncolytics. 2017;8:41-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nakano H, Namatame K, Nemoto H, et al. A multi-institutional prospective study of lentinan in advanced gastric cancer patients with unresectable and recurrent diseases: effect on prolongation of survival and improvement of quality of life. Kanagawa lentinan research group. Hepatogastroenterology. 1999;46(28):2662-2668. [PubMed] [Google Scholar]
- 77.Brown GD, Gordon S. Fungal beta-glucans and mammalian immunity. Immunity. 2003;19(3):311-315. [DOI] [PubMed] [Google Scholar]
- 78.Ochiai T, Isono K, Suzuki T, et al. Effect of immunotherapy with lentinan on patients’ survival and immunological parameters in patients with advanced gastric cancer: results of a multi-center randomized controlled study. Int J Immunother. 1992;VIII(3):161e9. [Google Scholar]
- 79.Fukuchi M, Mochiki E, Ishiguro T, et al. Evaluation of immunity in elderly patients with unresectable gastric cancer receiving S-1/lentinan combination chemotherapy. Gan To Kagaku Ryoho. 2014;41(10):1264-1266. [PubMed] [Google Scholar]
- 80.Yoshino S, Tabata T, Hazama S, et al. Immunoregulatory effects of the antitumor polysaccharide lentinan on Th1/Th2 balance in patients with digestive cancers. Anticancer Res. 2000;20(6C):4707-4711. [PubMed] [Google Scholar]
- 81.Nimura H, Mitsumori N, Tsukagoshi S, et al. Pilot study of TS-1 combined with lentinan in patients with unresectable or recurrent advanced gastric cancer. Gan To Kagaku Ryoho. 2003;30(9):1289-1296. [PubMed] [Google Scholar]
- 82.Ina K, Furuta R, Kataoka T, et al. Lentinan prolonged survival in patients with gastric cancer receiving S-1-based chemotherapy. World J Clin Oncol. 2011;2(10):339-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oba K, Kobayashi M, Matsui T, et al. Individual patient based meta-analysis of lentinan for unresectable/recurrent gastric cancer. Anticancer Res. 2009;29(7):2739-2745. [PubMed] [Google Scholar]
- 84.Homma S, Sagawa Y, Komita H, et al. Mechanism of antitumor effect on mouse hepatocellular carcinoma by intratumoral injection of OK-432, a streptococcal preparation. Cancer Immunol Immunother. 2007;56(8):1265-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hovden AO, Karlsen M, Jonsson R, et al. Maturation of monocyte derived dendritic cells with OK432 boosts IL-12p70 secretion and conveys strong T-cell responses. BMC Immunol. 2011;12:2. Doi: 10.1186/1471-2172-12-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oba MS, Teramukai S, Ohashi Y, et al. The efficacy of adjuvant immunochemotherapy with OK-432 after curative resection of gastric cancer: an individual patient data meta-analysis of randomized controlled trials. Gastric Cancer. 2016;19(2):616-624. [DOI] [PubMed] [Google Scholar]
- 87.Panzini I, Gianni L, Tassinari D. Immunochemotherapy with OK-432 may improve survival for people with curatively resected gastric cancer. Cancer Treat Rev. 2003;29(1):73-75. [DOI] [PubMed] [Google Scholar]
- 88.Jiang S, Redelman-Sidi G. BCG in bladder cancer immunotherapy. Cancers (Basel. 2022;14(13):3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pettenati C, Ingersoll MA. Mechanisms of BCG immunotherapy and its outlook for bladder cancer. Nat Rev Urol. 2018;15(10):615-625. [DOI] [PubMed] [Google Scholar]
- 90.Popiela T, Kulig J, Czupryna A, et al. Efficiency of adjuvant immunochemotherapy following curative resection in patients with locally advanced gastric cancer. Gastric Cancer. 2004;7(4):240-245. [DOI] [PubMed] [Google Scholar]
- 91.Lawler SE, Speranza MC, Cho CF, et al. Oncolytic viruses in cancer treatment: a review. JAMA Oncol. 2017;3(6):841-849. [DOI] [PubMed] [Google Scholar]
- 92.Howells A, Marelli G, Lemoine NR, et al. Oncolytic viruses-interaction of virus and tumor cells in the battle to eliminate cancer. Front Oncol. 2017;7:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fukuhara H, Ino Y, Todo T. Oncolytic virus therapy: a new era of cancer treatment at Dawn. Cancer Sci. 2016;107(10):1373-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pan RY, Chung WH, Chu MT, et al. Recent development and clinical application of cancer vaccine: targeting neoantigens. J Immunol Res. 2018;2018:4325874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ott PA, Hu Z, Keskin DB, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547(7662):217-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sahin U, Derhovanessian E, Miller M, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547(7662):222-226. [DOI] [PubMed] [Google Scholar]
- 97.Chen C, Zhou Q, Wu R, et al. A comprehensive survey of genomic alterations in gastric cancer reveals recurrent neoantigens as potential therapeutic targets. Biomed Res Int. 2019;2019:2183510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Van Brussel I, Berneman ZN, Cools N. Optimizing dendritic cell-based immunotherapy: tackling the complexity of different arms of the immune system. Mediators Inflamm. 2012;2012:690643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Faghfuri E, Shadbad MA, Faghfouri AH, et al. Cellular immunotherapy in gastric cancer: adoptive cell therapy and dendritic cell-based vaccination. Immunotherapy. 2022;14(6):475-488. [DOI] [PubMed] [Google Scholar]
- 100.Aleixo AA, Michelin MA, Murta EF. Dendritic cell vaccine and cancer treatment: new patents. Recent Pat Endocr Metab Immune Drug Discov. 2014;8(1):26-29. [DOI] [PubMed] [Google Scholar]
- 101.Kono K, Takahashi A, Sugai H, et al. Dendritic cells pulsed with HER-2/neu-derived peptides can induce specific T-cell responses in patients with gastric cancer. Clin Cancer Res. 2002;8(11):3394-3400. [PubMed] [Google Scholar]
- 102.Tanaka F, Haraguchi N, Isikawa K, et al. Potential role of dendritic cell vaccination with MAGE peptides in gastrointestinal carcinomas. Oncol Rep. 2008;20(5):1111-1116. [PubMed] [Google Scholar]
- 103.Sadanaga N, Nagashima H, Mashino K, et al. Dendritic cell vaccination with MAGE peptide is a novel therapeutic approach for gastrointestinal carcinomas. Clin Cancer Res. 2001;7(8):2277-2284. [PubMed] [Google Scholar]
- 104.Matsuda K, Tsunoda T, Tanaka H, et al. Enhancement of cytotoxic T-lymphocyte responses in patients with gastrointestinal malignancies following vaccination with CEA peptide-pulsed dendritic cells. Cancer Immunol Immunother. 2004;53(7):609-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lopes A, Vandermeulen G, Préat V. Cancer DNA vaccines: current preclinical and clinical developments and future perspectives. J Exp Clin Cancer Res. 2019;38(1):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Al-Awadhi A, Lee Murray J, Ibrahim NK. Developing anti-HER2 vaccines: breast cancer experience. Int J Cancer. 2018;143(9):2126-2132. [DOI] [PubMed] [Google Scholar]
- 107.Bordon Y. An RNA vaccine for advanced melanoma. Nat Rev Drug Discov. 2020;19(10):671. [DOI] [PubMed] [Google Scholar]
- 108.Rausch S, Schwentner C, Stenzl A, et al. mRNA vaccine CV9103 and CV9104 for the treatment of prostate cancer. Hum Vaccin Immunother. 2014;10(11):3146-3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schumacher T, Bunse L, Pusch S, et al. A vaccine targeting mutant IDH1 induces antitumour immunity. Nature. 2014;512(7514):324-327. [DOI] [PubMed] [Google Scholar]
- 110.Kumai T, Kobayashi H, Harabuchi Y, et al. Peptide vaccines in cancer-old concept revisited. Curr Opin Immunol. 2017;45:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sato Y, Shomura H, Maeda Y, et al. Immunological evaluation of peptide vaccination for patients with gastric cancer based on pre-existing cellular response to peptide. Cancer Sci. 2003;94(9):802-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sato Y, Fujiwara T, Mine T, et al. Immunological evaluation of personalized peptide vaccination in combination with a 5-fluorouracil derivative (TS-1) for advanced gastric or colorectal carcinoma patients. Cancer Sci. 2007;98(7):1113-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ishikawa H, Imano M, Shiraishi O, et al. Phase I clinical trial of vaccination with LY6K-derived peptide in patients with advanced gastric cancer. Gastric Cancer. 2014;17(1):173-180. [DOI] [PubMed] [Google Scholar]
- 114.Smith JP, Nadella S, Osborne N. Gastrin and gastric cancer. Cell Mol Gastroenterol Hepatol. 2017;4(1):75-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gilliam AD, Watson SA, Henwood M, et al. A phase II study of G17DT in gastric carcinoma. Eur J Surg Oncol. 2004;30(5):536-543. [DOI] [PubMed] [Google Scholar]
- 116.Ajani JA, Hecht JR, Ho L, et al. An open-label, multinational, multicenter study of G17DT vaccination combined with cisplatin and 5-fluorouracil in patients with untreated, advanced gastric or gastroesophageal cancer: the GC4 study. Cancer. 2006;106(9):1908-1916. [DOI] [PubMed] [Google Scholar]
- 117.Wiedermann U, Garner-Spitzer E, Chao Y, et al. Clinical and immunologic responses to a B-cell epitope vaccine in patients with HER2/neu-overexpressing advanced gastric cancer-results from phase ib trial IMU.ACS.001. Clin Cancer Res. 2021;27(13):3649-3660. [DOI] [PubMed] [Google Scholar]
- 118.Sundar R, Rha SY, Yamaue H, et al. A phase I/ib study of OTSGC-A24 combined peptide vaccine in advanced gastric cancer. BMC Cancer. 2018;18(1):332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fujiwara Y, Okada K, Omori T, et al. Multiple therapeutic peptide vaccines for patients with advanced gastric cancer. Int J Oncol. 2017;50(5):1655-1662. [DOI] [PubMed] [Google Scholar]
- 120.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69-74. [DOI] [PubMed] [Google Scholar]
- 121.Masuzawa T, Fujiwara Y, Okada K, et al. Phase I/II study of S-1 plus cisplatin combined with peptide vaccines for human vascular endothelial growth factor receptor 1 and 2 in patients with advanced gastric cancer. Int J Oncol. 2012;41(4):1297-1304. [DOI] [PubMed] [Google Scholar]
- 122.Higashihara Y, Kato J, Nagahara A, et al. Phase I clinical trial of peptide vaccination with URLC10 and VEGFR1 epitope peptides in patients with advanced gastric cancer. Int J Oncol. 2014;44(3):662-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Iwauchi T, Tanaka H, Yamazoe S, et al. Identification of HLA-A*2402-restricted epitope peptide derived from Eras oncogene expressed in human scirrhous gastric cancer. Cancer Sci. 2011;102(4):683-689. [DOI] [PubMed] [Google Scholar]
- 124.Dubensky TW, Jr, Reed SG. Adjuvants for cancer vaccines. Semin Immunol. 2010;22(3):155-161. [DOI] [PubMed] [Google Scholar]
- 125.Ramos CA, Heslop HE, Brenner MK. CAR-T cell therapy for lymphoma. Annu Rev Med. 2016;67:165-183. Doi: 10.1146/annurev-med-051914-021702. Epub 2015 Aug 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sadelain M, Brentjens R, Rivière I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3(4):388-398. Doi: 10.1158/2159-8290.CD-12-0548. Epub 2013 Apr 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang Z, Guo Y, Han W. Current status and perspectives of chimeric antigen receptor modified T cells for cancer treatment. Protein Cell. 2017;8(12):896-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Song Y, Tong C, Wang Y, et al. Effective and persistent antitumor activity of HER2-directed CAR-T cells against gastric cancer cells in vitro and xenotransplanted tumors in vivo. Protein Cell. 2018;9(10):867-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jung DH, Bae YJ, Kim JH, et al. HER2 Regulates cancer stem cell activities via the wnt signaling pathway in gastric cancer cells. Oncology. 2019;97(5):311-318. [DOI] [PubMed] [Google Scholar]
- 131.Qi C, Gong J, Li J, et al. Claudin18.2-specific CAR T cells in gastrointestinal cancers: phase 1 trial interim results. Nat Med. 2022;28(6):1189-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314(5796):126-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Robbins PF, Kassim SH, Tran TL, et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: long-term follow-up and correlates with response. Clin Cancer Res. 2015;21(5):1019-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu G, Chen D, Zhao X, et al. Efficacy of DC-CIK immunotherapy combined with chemotherapy on locally advanced gastric cancer. J Oncol. 2022;2022:5473292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shi B, Sun A, Zhang X. Influence of different ex vivo cell culture methods on the proliferation and anti-tumor activity of cytokine-induced killer cells from gastric cancer patients. Onco Targets Ther. 2018;11:2657-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sun S, Li XM, Li XD, et al. Studies on inducing apoptosis effects and mechanism of CIK cells for MGC-803 gastric cancer cell lines. Cancer Biother Radiopharm. 2005;20(2):173-180. [DOI] [PubMed] [Google Scholar]
- 137.Kim YJ, Lim J, Kang JS, et al. Adoptive immunotherapy of human gastric cancer with ex vivo expanded T cells. Arch Pharm Res. 2010;33(11):1789-1795. [DOI] [PubMed] [Google Scholar]
- 138.Shi L, Zhou Q, Wu J, et al. Efficacy of adjuvant immunotherapy with cytokine-induced killer cells in patients with locally advanced gastric cancer. Cancer Immunol Immunother. 2012;61(12):2251-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhao Q, Zhang H, Li Y, et al. Anti-tumor effects of CIK combined with oxaliplatin in human oxaliplatin-resistant gastric cancer cells in vivo and in vitro. J Exp Clin Cancer Res. 2010;29(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jiang J, Xu N, Wu C, et al. Treatment of advanced gastric cancer by chemotherapy combined with autologous cytokine-induced killer cells. Anticancer Res. 2006;26(3B):2237-2242. [PubMed] [Google Scholar]
- 141.Jäkel CE, Vogt A, Gonzalez-Carmona MA, Schmidt-Wolf IG. Clinical studies applying cytokine-induced killer cells for the treatment of gastrointestinal tumors. J Immunol Res. 2014;2014:897214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mu Y, Wang WH, Xie JP, et al. Efficacy and safety of cord blood-derived dendritic cells plus cytokine-induced killer cells combined with chemotherapy in the treatment of patients with advanced gastric cancer: a randomized phase II study. Onco Targets Ther. 2016;9:4617-4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Qiao G, Wang X, Zhou L, et al. Autologous dendritic cell-cytokine induced killer cell immunotherapy combined with S-1 plus cisplatin in patients with advanced gastric cancer: a prospective study. Clin Cancer Res. 2019;25(5):1494-1504. [DOI] [PubMed] [Google Scholar]
- 144.Jiang JT, Shen YP, Wu CP, et al. Increasing the frequency of CIK cells adoptive immunotherapy may decrease risk of death in gastric cancer patients. World J Gastroenterol. 2010;16(48):6155-6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhao H, Fan Y, Li H, et al. Immunotherapy with cytokine-induced killer cells as an adjuvant treatment for advanced gastric carcinoma: a retrospective study of 165 patients. Cancer Biother Radiopharm. 2013;28(4):303-309. [DOI] [PubMed] [Google Scholar]
- 146.Sagebiel AF, Steinert F, Lunemann S, et al. Tissue-resident Eomes+ NK cells are the major innate lymphoid cell population in human infant intestine. Nat Commun. 2019;10(1):975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Abel AM, Yang C, Thakar MS, et al. Natural killer cells: development, maturation, and clinical utilization. Front Immunol. 2018;9:1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sakamoto N, Ishikawa T, Kokura S, et al. Phase I clinical trial of autologous NK cell therapy using novel expansion method in patients with advanced digestive cancer. J Transl Med. 2015;13:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tonn T, Schwabe D, Klingemann HG, et al. Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy. 2013;15(12):1563-1570. [DOI] [PubMed] [Google Scholar]
- 150.Ishigami S, Natsugoe S, Tokuda K, et al. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer. 2000;88(3):577-583. [PubMed] [Google Scholar]
- 151.Wu XT, Liu JQ, Lu XT, et al. The enhanced effect of lupeol on the destruction of gastric cancer cells by NK cells. Int Immunopharmacol. 2013;16(2):332-340. [DOI] [PubMed] [Google Scholar]
- 152.Kumar A, Watkins R, Vilgelm AE. Cell therapy with TILs: training and taming T cells to fight cancer. Front Immunol. 2021;12:690499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986;233(4770):1318-1321. [DOI] [PubMed] [Google Scholar]
- 154.Rosenberg SA, Packard BS, Aebersold PM, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988;319(25):1676-1680. [DOI] [PubMed] [Google Scholar]
- 155.Kono K, Ichihara F, Iizuka H, et al. Differences in the recognition of tumor-specific CD8+ T cells derived from solid tumor, metastatic lymph nodes and ascites in patients with gastric cancer. Int J Cancer. 1997;71(6):978-981. [DOI] [PubMed] [Google Scholar]
- 156.Amedei A, Niccolai E, Della Bella C, et al. Characterization of tumor antigen peptide-specific T cells isolated from the neoplastic tissue of patients with gastric adenocarcinoma. Cancer Immunol Immunother. 2009;58(11):1819-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kessels HW, de Visser KE, Tirion FH, et al. The impact of self-tolerance on the polyclonal CD8+ T cell repertoire. J Immunol. 2004;172(4):2324-2331. [DOI] [PubMed] [Google Scholar]
- 158.Clark CA, Gupta HB, Sareddy G, et al. Tumor-intrinsic PD-L1 signals regulate cell growth, pathogenesis, and autophagy in ovarian cancer and melanoma. Cancer Res. 2016;76(23):6964-6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Feng D, Qin B, Pal K, et al. BRAFV600E-induced, tumor intrinsic PD-L1 can regulate chemotherapy-induced apoptosis in human colon cancer cells and in tumor xenografts. Oncogene. 2019;38(41):6752-6766. [DOI] [PMC free article] [PubMed] [Google Scholar]