Abstract
Biofilms represent an evolutionary form of life, which translates from life in free-living cells to a community lifestyle. In natural habitats, biofilms are a multispecies complex, where synergies or antagonisms can be established. For example, Listeria monocytogenes and Pseudomonas fluorescens are associated with a dual-species biofilm that is widespread in dairy plants. In food plants, multiple strategies are devised to control biofilms, including natural compounds such as essential oils (EOs). In this respect, this study evaluated the effectiveness of Thymbra capitata (L.) Cav. (TEO) and Cinnamomum zeylanicum (CEO) against a dual-species biofilm of L. monocytogenes and P. fluorescens, mimicking dairy process conditions. Based on Minimum Inhibitory Concentrations results, the EOs concentration (10 μL/mL) was chosen for the antibiofilm assay at 12°C on polystyrene (PS), and stainless-steel surfaces for 168 h, using a Ricotta-based model system as culture medium. Biofilm biomass was assessed by crystal violet staining, and the planktonic and sessile cells were quantified in terms of Log CFU/cm2. Results showed that CEO displayed the greatest antibiofilm activity, reducing significantly (P<0.05) P. fluorescens and L. monocytogenes sessile cells of about 2.5 and 2.8 Log CFU/cm2 after 72 h, respectively. However, L. monocytogenes gained the protection of P. fluorescens, evading CEO treatment and showing a minimal sessile cell reduction of 0.7 Log CFU/cm2 after 72 h. Considering the outcome of this study, CEO might have promising perspectives for applications in dairy facilities.
Key words: Thymbra capitata (L.) Cav, Cinnamomum zeylanicum, Listeria monocytogenes, Pseudomonas fluorescens, Dairy facilities
Introduction
Microbial biofilms made of multiple bacterial species are stabler than monocultures, due to communication and interactions among cells that play a fundamental role in internal balances and the ability to resist to stressful environmental factors (Rossi et al., 2020). The various kind of foods processed in manufacturing plants involves the presence of different microbial species producing biofilms, including pathogens and spoilage microorganisms. Among these, Listeria monocytogenes colonizes and persists on several processing surfaces, as polymeric and metal surfaces (Ferreira et al., 2014), thus favoring food contamination. Ready-to-eat products are the top vehicles that cause strong-evidence outbreaks of listeriosis (EFSA, 2022), especially dairy products such as gorgonzola, camembert and Ricotta (Melo et al., 2015). On the other side, Pseudomonas fluorescens is an obligated aerobic, mesophile, psychotropic microorganism that produces thermotolerant enzymes, resistant to pasteurization or Ultra-High Temperature treatment; it causes milk protein hydrolysis, offflavors development, shelf-life reduction, and yield decrease during cheese production (Martins et al., 2015).
To prevent and control biofilms in food plants, different strategies have been adopted. Recently, to improve the sustainability and efficacy of cleaning procedures, alternative methods of natural origins, such as essential oils (EOs), have been proposed. EOs are composed of a mix of several bioactive compounds, which strike multiple cellular targets simultaneously, exerting antimicrobial activity without stimulating the development of resistance phenomena, and thus preventing the emergence of drugresistant bacteria (Rossi et al., 2020). The EOs mechanisms of antimicrobial action may involve the damage of the cytoplasmic membrane, changes in the cell membrane fatty acid profile, the disintegration of the cell structures, but also reduction of the proton motive force with depletion of ATP (Rao et al., 2019). On the other hand, the EOs antibiofilm activity strikes the pillar targets of the genetic pathways in biofilm, because it saps most of community life advantages, as it suppresses cell adhesion, inhibits EPS matrix synthesis, alters the Quorum sensing system, and softens the bacterial virulence factors. Furthermore, EOs can inhibit biofilm formation, but can also penetrate through exopolysaccharides and remove mature biofilms (Rossi et al., 2020).
Based on scientific evidence acquired in a previous study (Maggio et al., 2021), the aim of this work was to determine the effectiveness of different natural compounds against a dual-species biofilm of L. monocytogenes and P. fluorescens on polystyrene (PS) and stainless steel (SS) in a Ricottabased model system, simulating the dairy environment conditions.
Materials and methods
Bacterial strains
L. monocytogenes LM5 and P. fluorescens pf5 strains were evaluated in this study. P. fluorescens pf5 was isolated from Mozzarella cheese and chosen for its capability to form biofilm and produce blue pigment on Potato Dextrose Agar, Mascarpone Agar, and Mozzarella cheese (Rossi et al., 2018). L. monocytogenes LM5 was isolated from dairy plants and characterized and typed as described in a previous study (Maggio et al., 2021).
The strains were kept at -80°C with an anti-freezing agent (200 μL/mL of glycerol, Sigma) to preserve the cells viability during storage.
Inocula
The strains were inoculated into Tryptic Soy Broth (TSB, Liofilchem, Roseto, Italy) from fresh microbial cultures, followed by overnight incubation at 37°C (L. monocytogenes) and 30°C (P. fluorescens). The standardized inoculum was prepared by measuring the Optical Density (OD) of the bacterial suspensions with a Lambda bio 20 spectro-photometer (Perkin Elmer, Waltham, Massachusetts, USA) at 600 nm, to obtain a cell count of about 105 CFU/mL (Paparella et al., 2016) in the Ricotta-based model system, as described in the following paragraph. To maintain the same load for the mono- and dual-species inocula, the final volume was adjusted by diluting the bacterial suspension with the Ricotta-based model system (1:1) for the monoculture; instead, for the dual-species system, the bacterial suspension of one species was diluted with the other one (1:1).
Ricotta-based model system
Cow’s milk pasteurized Ricotta, made of pasteurized cow’s milk whey, pasteurized cow’s milk, and salt, was purchased from a local market. The nutritional composition was: 7.38% proteins, 8.6% fat, 3.83% carbohydrates, and 0.33% salt. The Ricottabased model system was prepared following the method described by de Carvalho et al. (2015) with some modifications, as reported in a previous study (Maggio et al., 2021). Briefly, 160 g of Ricotta were diluted in 1 L of distilled water, then heated in a water bath at 42°C for 50 min and autoclaved at 121°C for 15 min. The broth obtained was filtered by a sterile gauze and stored at 4°C.
Natural compounds
O. vulgare subsp. hirtum, Thymbra capitata (L.) Cav., C. reticulata, C. limon Cv Femminello and C. aurantium EOs were obtained from Exentiae s.r.l. (Catania, Italy), while C. zeylanicum EO was purchased from Zuccari s.r.l. (Trento, Italy). C. aurantium distilled water was obtained from Erboristeria Magentina (Torino, Italy). An initial 40 μL/mL stock of each EO was obtained by dilution in 10 mM PBS (Phosphate Buffer Saline) solution pH 7.4 and emulsifying with Tween 80 (10 μL/mL), whilst the orange distilled water was used pure. The emulsions, sterilized through filtration, were stored at 4°C.
Determination of minimum inhibitory concentration
The Minimum Inhibitory Concentration (MIC) of the EOs after 168 h at 12°C were determined by the microdilution method (CLSI, 2016), using a 96-well microtitre plate (Corning incorporated, Kennebunk, ME, USA). The MIC value was considered as the lowest concentration at which no microbial growth was observed, as a consequence of TTC (2,3,5-triphenyltetrazolium chloride, Sigma-Aldrich, Milan, Italy) red discoloration absence, previously added in a ratio of 1 μL/mL. The experiments were replicated three times. The most effective EOs, based on MICs results, were selected for this study: C. zeylanicum EO (CEO) (76.2% of trans- Cynnamaldehyde) and T. capitata (L.) Cav. EO (TEO) (73.0% of carvacrol) at the concentration of 10 μL/mL.
Biofilm formation on polystyrene surface
Two-hundred microliter of L. monocytogenes LM5 and P. fluorescens pf5 inocula were added into each well of 96-well polystyrene microplates, using the Ricottabased model system as a culture medium. The bacterial suspensions were added of 10 μL/mL of each EOs, and then incubated at 12°C for 168 h. The control samples contained untreated inocula in the Ricottabased model system. At 48, 72, 96, and 168 h of incubation, the planktonic cells were removed from each sample, and then each well was washed with 10 mM PBS pH 7.4. Total biomass was quantified by crystal violet assay at OD of 590nm, as reported by Rossi et al. (2018). L. monocytogenes LM5 and P. fluorescens pf5 in mono- and co-culture conditions were tested in five replicates.
Biofilm formation on stainless steel and enumeration of planktonic and sessile cells
To evaluate the effectiveness of CEO and TEO on biofilm formation on SS, AISI 304 coupons (2×2×0.1 cm) were used, previously cleaned according to the procedure described by Maggio et al. (2021).
Each coupon was introduced into a sterile glass container containing 5 mL monoor dual-species inocula in the Ricotta-based model system, added of 10 μL/mL EOs, and then incubated at 12°C for 168 h. Control samples with untreated inocula were also evaluated. At 48, 72, 96 and 168 h of incubation, 1 mL of the suspension was taken, and serial dilutions were performed to enumerate planktonic cells. The dilutions were distributed on selective media for enumerating Pseudomonas spp. (Pseudomonas Agar Base; Oxoid-Thermofisher, Rodano, Italy) and L. monocytogenes (Agar Listeria according to Ottaviani & Agosti; Biolife, Milano, Italy), after incubation at 30°C and 37°C for 48 h, respectively. The enumeration of sessile cells was performed by rinsing three times the SS coupons with saline solution (8.5 g/L NaCl) in sterile tubes, followed by scraping by two cotton swabs to collect the cells (Dos Santos et al., 2018). The swabs were immersed in 10 mL of saline solution and subjected to tenfold serial dilutions. The colony count was determined on the above-mentioned agar media. The assay was performed in triplicate.
Statistical analysis
The data were subjected to analysis of variance using XLSTAT ver. 2017 (Addinsoft, Paris, France), and pair comparison within the same group was achieved applying Tukey’s test procedure at P<0.05 to evaluate the EOs effectiveness on singleand dual-species results.
Results
MICs determination
According to MICs results, CEO and TEO showed good antimicrobial effectiveness. In fact, the CEO MICs were 2.5 μL/mL for L. monocytogenes LM5, and 10 μL/mL for P. fluorescens pf5 and dualspecies combination. On the other hand, the MICs observed for TEO was 5 μL/mL in all samples. However, the final concentrations of CEO and TEO were chosen in consideration of the matrix effect of the Ricottabased model system, which might reduce the EOs bioactivity, due to interactions between the hydrophobic EOs constituents and food components, such as fat, starch, and proteins (Hyldgaard et al., 2012). Therefore, the MIC selected for CEO and TEO represented the highest value detected during the assay: 10 μL/mL.
Biofilm formation on polystyrene and stainless steel surfaces
As observed by Maggio et al. (2021), L. monocytogenes LM5 didn’t adhere on PS surface (data not shown), impeding the evaluation of EOs antibiofilm activity. By contrast, on SS the biomass quantification after EOs exposure was assessed (Figure 1), showing that TEO exerted a stronger but delayed antimicrobial activity, and CEO was soon effective, but with weaker antimicrobial activity. As displayed in Figure 1, TEO could have boosted the biofilm production in monoculture of L. monocytogenes LM5 and P. fluorescens pf5 until 72 h of exposure, where the biomass produced was higher than the control samples. The TEO strong antibiofilm activity was observed from 96 h onwards for both species, where biofilm production was almost completely inhibited (almost 81% of biomass inhibition). Contrarily, CEO exhibited antibiofilm activity against the two bacterial species after 48 hours of exposure. Interesting findings were detected in dualspecies conditions, where a sudden biomass reduction was observed already after 48 hours of exposure to CEO (about 38% of biomass reduction).
Planktonic and sessile cells on stainless steel surface
The EOs effectiveness on planktonic sessile cells was assessed on L. monocytogenes LM5 and P. fluorescens pf5 alone, on L. monocytogenes LM5 in the presence of P. fluorescens pf5, and vice versa (data not shown). Remarkably, after 72 h of CEO exposure, a slight but significant reduction of 0.4 Log CFU/mL of L. monocytogenes LM5 cells alone was obtained. With respect to TEO treatment, cells decrease was observed in L. monocytogenes LM5 in coculture conditions, with a significant reduction of about 1 Log CFU/mL from 72 h. By contrast, TEO could have stimulated P. fluorescens pf5 in co-culture, with an increase of almost 1.5 Log CFU/mL after 72 h.
Regarding the sessile cells monitoring, promising outcomes were observed in the presence of CEO treatment (Figure 2), except for the slight reduction of 0.5 CFU/cm2 in P. fluorescens pf5 alone after 72 h (Figure 2A). Contrariwise, a significant (P<0.05) Log reduction of almost 2.8 CFU/cm2 was observed in L. monocytogenes LM5 monoculture after 72 h (Figure 2B). A similar result was observed in P. fluorescens pf5 in co-culture (Figure 2D) with a significant (P<0.05) Log reduction of almost 2.5 CFU/cm2 (Table 1) attained after 72 h of exposure. However, in the presence of P. fluorescens pf5, the antimicrobial activity of CEO on L. monocytogenes LM5 was reduced dramatically, with a decrease of about 0.7 Log CFU/cm2 (Table 1), suggesting a protective action derived from the co-culture.
Discussion
The EOs antimicrobial mechanisms of action gained increasing attention in scientific literature. EOs reduce the bacterial biofilm-forming ability, affecting motility through inhibition of Quorum sensing system, or destabilizing biofilm organization as an effect of interference with EPS production (Wang et al., 2020). However, the use of EOs as food preservatives is still limited due to the sensory impact and the hydrophobic nature, which complicates application in aqueous systems. However, given the multiple targets in microbial cells, EOs behave as effective antimicrobials without stimulating the development of resistance phenomena, differently from antibiotics (Rossi et al., 2020).
Figure 1.
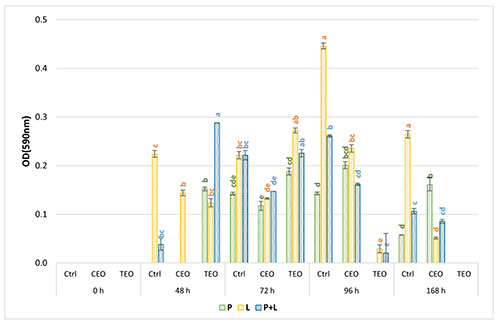
Biofilm biomass (OD590nm) of Pseudomonas fluorescens pf5 in mono- and co-culture with Listeria monocytogenes LM5 on stainless steel surface at 12°C for 168 h. The results are expressed as average of three replicates, and the bars represent the standard deviations. The colored letters indicate a statistically significant difference between EOs treatment vs. control for the same incubation time (P<0.05). Red: 48 h; Green: 72 h; Blue: 96.
Table 1.
Statistically significant differences (P<0.05) among sessile cells on stainless steel coupons of mono- and co-culture.
| Ctrl | CEO | TEO | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0h | 72h | 168h | 0h | 72h | 168h | 0h | 72h | 168h | |
| P | 0.1a | 4.2a | 4.6b | 0.1a | 3.7a* | 4.5a* | 0.1a | 5.5a* | 4.2c* |
| L | 0.1a | 6.2c | 5.7d | 0.1a | 3.4b* | 4.2b* | 0.1a | 5.5c* | 4.5b* |
| Pds | 0.1a | 5.9b | 5.3a | 0.1a | 3.4b* | 3.9c* | 0.1a | 5.1b* | 5a* |
| Lds | 0.1a | 4.3c | 4.2c | 0.1a | 3.6a* | 4c* | 0.1a | 4d* | 3.7d* |
Letters indicate the differences between different culture conditions at the same incubation time, and under the same treatment. Asterisks (*) indicate significant differences between the control and treatments of the same sample, for the different times of incubation; Ctrl, Control; CEO, C. zeylanicum EO; TEO, Thymbra capitata (L.) Cav. EO; P: P. fluorescens pf5 alone; L. monocytogenes LM5 alone; Pds: P. fluorescens pf5 coculture; Lds: L. monocytogenes LM5 in co-culture.
In this study, CEO and TEO exerted antibiofilm activity, with species-specific responses. Moreover, the Ricotta-based model system composition may have affected the EOs activity. In fact, fats and proteins might have protected microbial cells, thus decreasing the antimicrobial activity of the EOs (Burt, 2004).
Through biofilm production and sessile population monitoring, different bacterial behaviors were observed after the treatments, reflecting the different EOs composition. In detail, TEO showed a higher antimicrobial activity only against L. monocytogenes LM5 sessile cells, probably due to the main bioactive compound, carvacrol (73.0%). Carvacrol affects cell membrane permeability and disrupts the cytoplasmic membrane, damaging proteins in a concentration- dependent way (Wang et al., 2020). However, the antimicrobial activity is different against Gram-negative and -positive microorganisms. Gram-negative bacteria possess a thick layer of lipopolysaccharide outer membrane, causing them to be more resistant to hydrophobic compounds (such as carvacrol) if compared with Gram-positives, which have a single peptidoglycan layer structure (Wang et al., 2020). Therefore, the higher sensibility of L. monocytogenes LM5 cells to TEO treatment can be justified. Conversely, CEO was effective against both bacterial species. The antimicrobial activity exerted could be due to the main bioactive component, transcynnamaldehyde (76.2%), which disrupts cytoplasmatic membrane, induces reactive oxygen species production in cells, modifies gene expression, as well as undermines the multiple enzymes with ATPase activity, thus impairing different metabolic pathways (Lee et al., 2020). CEO activity is a very encouraging outcome of this research, considering that P. fluorescens is the dominant spoilage microorganism in the dairy chain and that in the three-dimensional biofilm structure resilience to external hurdles is strengthened (Rossi et al., 2020).
However, in this study, the P. fluorescens- L. monocytogenes co-culture affected the inhibitory effects of the treatments. In particular, the protection given by the combination could reduce the bioactivity of the CEO, especially towards L. monocytogenes LM5. It has been observed that in multi-species biofilms, bacterial populations can establish synergies, which increase their resistance to stressful environments (Maggio et al., 2021). For instance, P. fluorescens in community-life style would develop several advantages for L. monocytogenes. The facultative anaerobic L. monocytogenes would dwell on the lower site of the biofilm architecture, unlike the aerobe P. fluorescens (Rossi et al., 2020). Therefore, the extracellular matrix produced by Pseudomonas spp. could ease L. monocytogenes adhesion to surfaces (Aswathanarayan et al., 2014), incorporating and protecting it, and reducing the effect of exposure to antimicrobials (Puga et al., 2018). Thereby, L. monocytogenes can be positively influenced by the co-presence of P. fluorescens in different dairy productions, revealing the role of P. fluorescens not only as a spoilage organism but also as an indirect source of risk for human safety (Nava et al., 2016). Nevertheless, the strong antimicrobial activity of CEO exerted against P. fluorescens sessile cells in co-culture could be a strategy to weaken the consortium with L. monocytogenes, which could no longer take advantage of the benefits provided.
Figure 2.
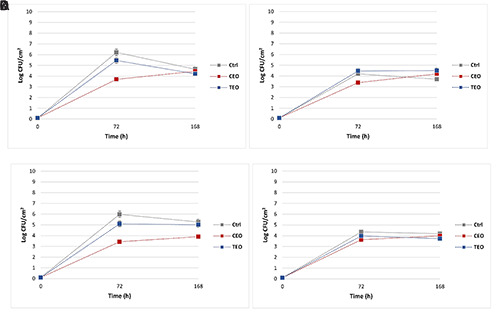
Dynamics of sessile cells on stainless steel coupons of Pseudomonas fluorescens (a) and Listeria monocytogenes in mono-culture (b), Pseudomonas fluorescens (c) and Listeria monocytogenes in co-culture (d) at 12°C for 168 h. The results represent the average values of three replicates, and the bars indicate the standard deviations. Ctrl: Control; CEO: C. zeylanicum EO; TEO: Thymbra capitata (L.) Cav. EO.
Conclusions
This study suggests that the food residues on the processing surfaces can play a key role in the effectiveness of the antimicrobial treatment, especially when natural compounds are used. In fact, the food matrix components, such as fats and proteins, could interfere with the treatment, delaying or reducing the antimicrobial activity of EOs. Nevertheless, EOs are a realistic approach to minimize the biofilm phenomenon in food plants.
In this study, the greatest antibiofilm activity was observed in CEO, and this result is very encouraging, considering the simulation of the dairy processing conditions. The CEO antimicrobial activity was higher against the sessile population of P. fluorescens when in co-culture. Considering that P. fluorescens is the dominant spoilage bacterial species in dairy plants, CEO deserves further investigation for its antimicrobial activity.
Interestingly, this study demonstrates that L. monocytogenes can be protected by P. fluorescens, and this effect might represent a risk in real conditions. In fact, in realworld conditions, the association between different species in a dual-species biofilm is a common mechanism; therefore, the protective effect of L. monocytogenes on food contact surfaces must be seriously considered for its implications for consumers and food companies.
As a matter of fact, the presence of P. fluorescens in the dairy environment should be seriously considered, not only for its spoilage potential but also for the possible protective effect on L. monocytogenes. However, the CEO antimicrobial effectiveness against P. fluorescens sessile cells in co-culture suggests a strategy to undermine the interaction with L. monocytogenes. In this respect, further investigations by multiomics approaches could improve the knowledge of the EOs mechanisms of action in dual-species biofilms.
Acknowledgement
The authors are grateful to Exentiae S.r.l. Soc. Agricola (Catania, Italy), Zuccari s.r.l. (Trento, Italy) and Erboristeria Magentina (Torino, Italy) for providing the natural compounds.
Funding Statement
Funding: None
References
- Aswathanarayan JB, Vittal RR, 2014. Attachment and biofilm formation of Pseudomonas fluorescens PSD4 isolated from a dairy processing line. Food Sci Biotechnol 23:1903–10. [Google Scholar]
- Burt S, 2004. Essential oils: their antibacterial properties and potential applications in foods-a review. Int J Food Microbiol 94:223-53. [DOI] [PubMed] [Google Scholar]
- CLSI, 2016. Performance Standards for Antimicrobial Susceptibility Testing. 26th ed. CLSI 448 supplement M100S. [Google Scholar]
- de Carvalho RJ, de Souza GT, Honório VG, de Sousa JP, da Conceição ML, Maganani M, de Souza EL, 2015. Comparative inhibitory effects of Thymus vulgaris L. essential oil against Staphylococcus aureus, Listeria monocytogenes and mesophilic starter coculture in cheese-mimicking models. Food Microbiol 52:59-65. [DOI] [PubMed] [Google Scholar]
- Dos Santos Rodrigues JB, de Souza NT, Scarano JOA, de Sousa JM, Lira MC, de Figueiredo RCBQ, Magnani M, 2018. Efficacy of using oregano essential oil and carvacrol to remove young and mature Staphylococcus aureus biofilms on food-contact surfaces of stainless steel. LWT 93:293-9. [Google Scholar]
- EFSA, 2022. The European Union One Health 2020 zoonoses report. EFSA J 19:6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira V, Wiedmann M, Teixeira P, Stasiewicz MJ, 2014. Listeria monocytogenes persistence in food-associated environments: epidemiology, strain characteristics, and implications for public health. J Food Prot 77:150-70. [DOI] [PubMed] [Google Scholar]
- Hyldgaard M, Mygind T, Meyer R, 2012. Essential Oils in Food Preservation: Mode of Action, Synergies, and Interactions with Food Matrix Components. Front Microbiol 3:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL, Jung M, Lee SC, Huh MJ, Seo SL, Park IK, 2020. Antibacterial mode of action of trans-cinnamaldehyde derived from cinnamon bark (Cinnamomum verum) essential oil against Agrobacterium tumefaciens, Pestic Biochem Phys 165:104546. [DOI] [PubMed] [Google Scholar]
- Maggio F, Rossi C, Chaves-López C, Serio A, Valbonetti L, Pomilio F, Chiavaroli AP, Paparella A, 2021. Interactions between L. monocytogenes and P. fluorescens in dual-species biofilms under simulated dairy processing conditions. Foods 10:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins M, Uelinton MP, Katharina R, Vanetti MD, 2015. Milk-deteriorating exoenzymes from Pseudomonas fluorescens 041 isolated from refrigerated raw milk. Braz J Microbiol 46:207-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo J, Andrew PW, Faleiro MI, 2015. Listeria monocytogenes in cheese and the dairy environment remains a food safety chal-lenge: the role of stress responses. Food Res Int 67:75-90. [Google Scholar]
- Nava D, Capo S, Caligiuri V, Giaccone V, Biondi L, Vaccaro GF, Guarino A, Capuano F, 2016. Study of the population dynamics of Listeria monocytogenes and Pseudomonas fluorescens in buffalo mozzarella by means of challenge testing. Ital J Food Saf 5:5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paparella A, Mazzarrino G, Chaves-López C, Rossi C, Sacchetti G, Guerrieri O, Serio A, 2016. Chitosan boosts the antimicrobial activity of Origanum vulgare essential oil in modified atmosphere packaged pork. Food Microbiol 59:23-31. [DOI] [PubMed] [Google Scholar]
- Puga CH, Dahdouh E, SanJose C, Orgaz B, 2018. Listeria monocytogenes colonizes Pseudomonas fluorescens biofilms and induces matrix over-production. Front Microbiol 9:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao J, Chen B, Julian D, Clements MC, 2019. Improving the Efficacy of Essential Oils as Antimicrobials in Foods: Mechanisms of Action. Annu Rev Food Sci T 10:365-87. [DOI] [PubMed] [Google Scholar]
- Rossi C, Chaves-López C, Serio A, Anniballi F, Valbonetti L, Paparella A, 2018. Effect of Origanum vulgare essential oil on biofilm formation and motility capacity of Pseudomonas fluorescens strains isolated from discoloured Mozzarella cheese. J Appl Microbiol 124:1220-31. [DOI] [PubMed] [Google Scholar]
- Rossi C, Chaves-López C, Serio A, Casaccia M, Maggio F, Paparella A, 2020. Effectiveness and mechanisms of essential oils for biofilm control on food-contact surfaces: An updated review. Crit Rev Food Sci Nutr 62:2172-91. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hong X, Liu J, Zhu J, Chen J, 2020. Interactions between fish isolates Pseudomonas fluorescens and Staphylococcus aureus in dual-species biofilms and sensitivity to carvacrol. Food Microbiol 91:103506. [DOI] [PubMed] [Google Scholar]
- Zygadlo J, Zunino MP, Pizzolitto R, Merlo C, Omarini A, Dambolena J, 2017. Antibacterial and anti-biofilm activities of essential oils and their components including modes of action. Essential Oils and Nanotechnology for Treatment of Microbial Diseases, Boca Raton: CRC Press; 1:112–39. [Google Scholar]


