Abstract
When there is an inadequate supply of mother’s milk, pasteurized donor human milk is preferred over formula to supplement feeds for preterm infants. Although providing donor milk helps to improve feeding tolerance and reduce necrotizing enterocolitis, changes to its composition and reductions in bioactivity during processing, are thought to contribute to the slower growth often exhibited by these infants. To improve the clinical outcomes of recipient infants by maximizing the quality of donor milk, research is currently investigating strategies to optimize all aspects of processing, including pooling, pasteurization, and freezing; however, reviews of this literature typically only summarize the impact of a processing technique on composition or bioactivity. Reviews of published research investigating the impact of donor milk processing on infant digestion/absorption are lacking and thus, was the objective for this systematic scoping review, Open Science Framework (https://doi.org/10.17605/OSF.IO/PJTMW). Databases were searched for primary research studies evaluating donor milk processing for pathogen inactivation or other rationale and subsequent effect on infant digestion/absorption. Non-human milk studies or those assessing other outcomes were excluded. Overall, 24 articles from 12,985 records screened were included. Most studied thermal methods to inactivate pathogens, predominantly Holder pasteurization (HoP) (62.5°C, 30 min) and high-temperature short-time. Heating consistently decreased lipolysis and increased proteolysis of lactoferrin and caseins; however, protein hydrolysis was unaffected from in vitro studies. The abundance and diversity of released peptides remain unclear and should be further explored. Greater investigation into less-harsh methods for pasteurization, such as high-pressure processing, is warranted. Only 1 study assessed the impact of this technique and found minimal impact on digestion outcomes compared with HoP. Fat homogenization appeared to positively impact fat digestion (n = 3 studies), and only 1 eligible study investigated freeze-thawing. Identified knowledge gaps regarding optimal methods of processing should be further explored to improve the quality and nutrition of donor milk.
Keywords: donor human milk, processing, digestion, simulated digestion, infant, milk banking
Statement of Significance
Existing reviews in the literature have only summarized the impact of processing, mainly pasteurization, on human milk components and bioactivity, while this scoping review examined all literature pertaining to any processing method (e.g., pasteurization, homogenization, freeze-thaw etc.) and systematically charted its downstream impact on nutrient digestion or absorption in the infant. The goal was to identify gaps in our knowledge to help guide future research that will optimize the production of donor human milk, to optimize its quality and nutritional benefits.
Introduction
Donor human milk is the preferred supplement to preterm formula when there is an inadequate supply of mother’s milk [1]. In North America and most countries world-wide, donor human milk undergoes Holder pasteurization (HoP) (62.5°C, 30 min) to inactivate potentially pathogenic bacteria and viruses [2, 3]. In part, because of heat processing, many essential nutrients and bioactive components are reduced or completely abolished [4, 5]. In addition, the macromolecular structure of proteins is impacted by pasteurization via the formation of high-molecular-weight aggregates including Maillard reaction by-products via protein–sugar interactions (e.g., lactuloselysine) [6]. Interestingly, protein aggregates detected in human milk following HoP have been shown to localize at the milk fat globule membrane interface where milk proteins and lipid membranes also interact [7].
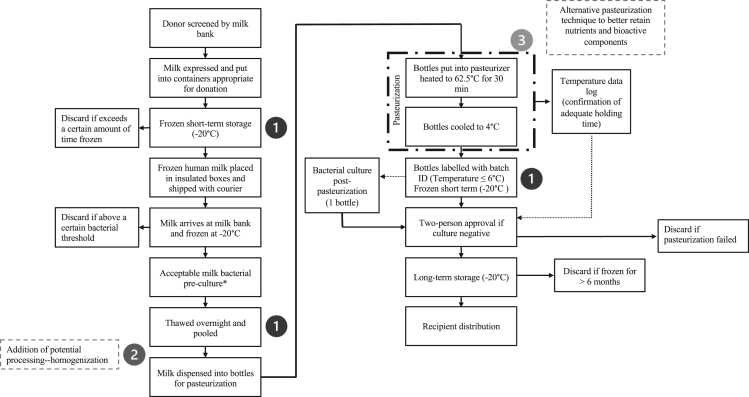
Although providing donor human milk instead of formula to vulnerable low- and very-low-birth-weight infants improves feeding tolerance and provides protection against necrotizing enterocolitis, it is also associated with a slower rate of infant growth [8]. Although this issue is likely multifactorial in nature, it may be due in part to the altered composition of the milk and loss of bioactive components and enzymatic activity as a result of processing. Figure 1 illustrates the typical processing flow of donor milk, which includes HoP, pooling, and multiple freeze-thaw cycles. The latter process can result in the formation of large ice crystals which can damage the structure of milk fat globules through shear force [9]. There is currently a push to modernize donor human milk processing to ensure that the nutrient composition and bioactive components are minimally altered, while also ensuring that the final product has greater batch-to-batch consistency in composition and remains safe for infant consumption. Research into alternative pasteurization methods, including nonthermal methods, is emerging [10]. Some of these methods, novel to human milk, include high-pressure processing (HPP), high temperature-short time (HTST), ultraviolet-C irradiation (UV-C), and ultrasonication. In particular, there is consistent evidence suggesting that nonthermal methods, such as HPP, minimally impact the bioactivity and composition of donor human milk [11]. There is also evidence suggesting that an additional homogenization step (Figure 1) could improve the milk fat globule distribution during pooling which may yield significant clinical benefit [12]. By ensuring fat droplets are well dispersed, donor human milk homogenization can reduce the amount of fat that adheres to the inner surface of plastic milk bottles during processing and once processed, may reduce the fat that sticks to the plastic tubing used for enteral feeding [13]. This would effectively maximize the delivery of fat to the infant and may result in improved weight gain given that ∼50% of the energy from human milk comes from fat [14].
FIGURE 1.
Process flow diagram depicting the typical production of donor human milk at a Canadian milk bank and areas where research into optimization is being explored. Post-pasteurized milk is stored short-term at −20°C, separate from milk ready to be dispensed while microbiological analyses are underway (∼2 d). Following approval by the medical director, milk is moved into longer-term storage at −20°C (≤ 6 mo), from where it is dispensed to hospitals. The numbers correspond to parts of the processing which can be optimized. 1) Refers to the freezing and/or thawing; 2) Refers to the potential addition of homogenization, and 3) Refers to the pasteurization process. ∗Bacterial preculture screening is only in place in select milk banks.
Existing reviews in the literature have assessed the impact of Holder and alternative pasteurization and processing methods on components and bioactivity of donor human milk [4, 10, 15]. A significant amount of research has also investigated how processed donor human milk is digested and absorbed, including simulated in vitro digestion, animal studies, and clinical trials. A systematic summary of these findings is lacking. Therefore, the objective of this scoping review is to summarize the impact of processing donor human milk on infant digestion and absorption of nutrients and bioactive components.
Methods
Search strategy and selection criteria
A scoping review was conducted to map primary original research articles, which have investigated the impact of processing on outcomes related to digestion and absorption in term- or preterm- infants. A preliminary search for existing scoping reviews was conducted on January 10, 2022, using multiple databases including Medline, Embase, Cochrane Database of Systematic Reviews, and Web of Science. The “PROSPERO” database was also checked for current reviews under the same topic. This included clinical trials of infants, in vivo animal models of infants, or in vitro simulation of infant gastrointestinal physiology. This systematic scoping review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for scoping reviews (PRISMA-ScR) [16, 17]. The scoping review protocol and meta data were uploaded to Open Science framework as a study registration before conducting the review (https://doi.org/10.17605/OSF.IO/PJTMW).
Relevant articles were identified with the assistance of a research librarian via electronic searches of online databases including Medline, Embase, and Web of Science from inception to February 4, 2022, and were screened by 2 independent reviewers. Search terms are summarized in Supplemental Table 1. The search focused on keywords and MeSH terms to identify articles related to human milk and digestion and absorption. The keywords included for all database searches were intended to capture all relevant research with respect to the first concept, human milk, and the second concept, the resultant digestion or absorption. Keywords specific to processing techniques, in addition to preterm-or term-specific digestion were not included as these articles would be captured by the search. An analysis of the text words contained in the title and abstract of retrieved papers and of the index terms used to describe the articles was also searched across the included databases. To ensure the search was comprehensive, grey literature was reviewed as per the previously published guidelines, including from dissertations and Google advanced search [18]. Moreover, references from identified articles selected for review were also examined. For full-text articles that were unable to be retrieved using institutional resources, direct contact with the author was attempted. If identified conference abstracts were reviewed by both independent reviewers and could not be linked to a published journal article, an attempt was made to contact individual authors to confirm. To guide the selection process, a criteria table was developed, clearly delineating the inclusion and exclusion criteria, summarized in Table 1. Briefly, to be included in the review, primary research articles were required to evaluate the impact of processing (thermal or nonthermal) human milk on subsequent preterm or term infant nutrient digestion or absorption. Articles that did not study human milk (e.g., infant formula) or studied human milk-derived products (e.g., human milk-based fortifiers, etc.) were excluded. Studies reporting outcomes secondary to digestion and absorption (e.g., growth, neurodevelopment) were also excluded given that these may be influenced by other factors independent of processing such as: small for gestational age at birth, morbidity, prolonged mechanical ventilation, and perinatal risk factors (e.g., exposure to corticosteroids or magnesium in utero) [19, 20]. Finally, there were no constraints on publication year or language.
TABLE 1.
Inclusion and exclusion criteria for article screening and full-text review
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
Data management and extraction
After excluding duplicate records, article screening was carried out using Covidence online software [21] by 2 independent reviewers (MAP, MRB), first by title and abstract, followed by full-text review. Inter-reviewer discrepancies were documented and resolved by consensus. To extract relevant data, a standardized spreadsheet was created to ensure both reviewers could independently extract data from studies deemed eligible. This data included: country of origin, postpartum age of human milk used in the experiments (e.g., colostrum, transitional, mature), milk type (e.g., preterm or term), the processing method (s) tested, their parameters and whether it was a nonthermal or thermal method. If the source of the donor milk was not defined, it was presumed to be from mothers who had term-born infants. The type of study used in the experiment (e.g., in vitro, in vivo [clinical/animal]), whether a term or preterm infant was being modeled, the treatment groups, the number of digestions or sample size, outcomes assessed, and their associated biochemical/analytical methods, in addition a summary of key findings was extracted. After reviewing for accuracy, the independently extracted data were combined. Common themes were extracted from the main findings of the articles and summarized by processing method.
Results
Scoping review
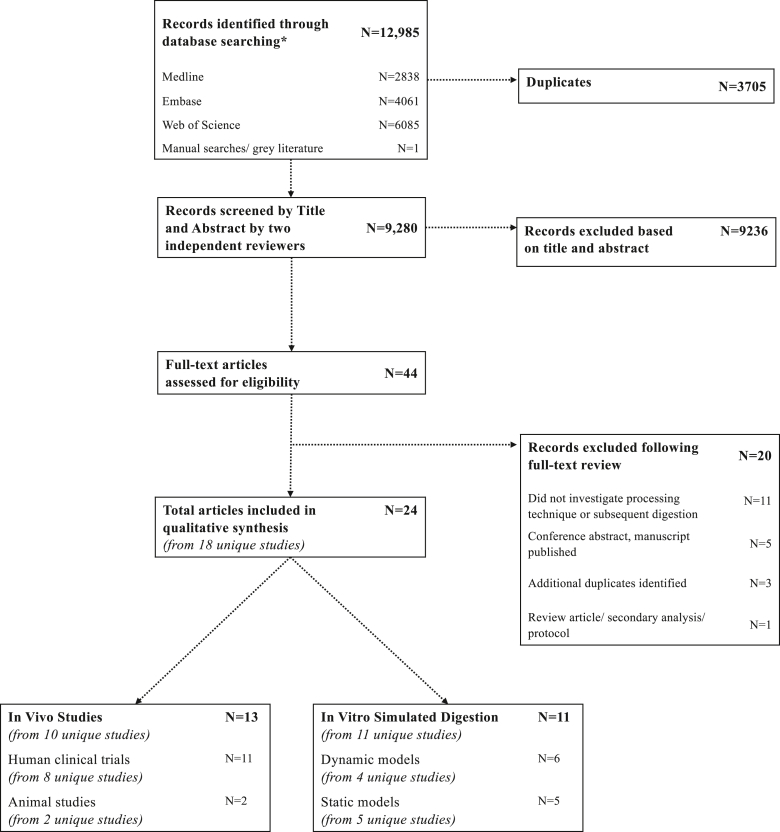
The selection of studies is summarized in Figure 2. After running the search strategy (Supplemental Table 1), a total of 12,985 records were initially identified from Medline (N = 2838), Embase (N = 4061), and Web of Science (N = 6085). One additional article was identified through manual searches. After removal of 3705 duplicates, 9280 articles were screened by title and abstract of which 9236 articles did not meet the eligibility criteria, leaving 44 articles eligible for full-text review. After full-text review, 20 articles were excluded, predominantly (N = 11) because of the article not investigating a processing technique and/or subsequent digestion; thus, a total of 24 articles (from 18 unique studies) were included in the final review. These were subsequently organized and grouped according to the study design (in vivo vs. in vitro) (TABLE 2, TABLE 3). The main findings from both in vivo and in vitro studies were grouped according to processing method and summarized in Table 4.
FIGURE 2.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram describing the selection of articles for inclusion in the scoping review and summary of included articles and unique studies stratified by in vivo study or in vitro. CFU, colony forming unit.
TABLE 2.
Summary of in vivo (human clinical and animal) studies assessing the impact of human milk processing on infant digestion
| Human milk type & processing |
Digestion-Study Design & Results |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lactational Stage (Colostrum, Mature, Transitional) | Preterm/Term milk | Processing Type and Classification | Processing Rationale | Processing parameters | Study type | Groups | Infant type | Sample size (N) | Outcomes assessed | Methods | Key findings | Ref |
| Mother’s milk (Transitional/Mature 8–9 and 21–22 d postpartum) Donor human milk (unknown, presumed mature) | Mother’s milk (preterm); Donor human milk (unknown, presumed term) | HoP1 (Thermal) | Pathogen inactivation | 62.5°C, 30 min | Clinical-Crossover (2 interventions, 8–9 wk postpartum; 21–22 d postpartum) | (1) Raw mother’s milk (2) Pasteurized donor human milk | Preterm (30 ± 3 wk GA) | N = 20 (10 male, 10 female) mother-infant pairs | Survival of anti- Bordetella pertussis filamentous hemagglutinin (FHA) and anti-pertussis toxin (PT) antibodies (IgG, IgM, IgA) in gastric samples (30 min after feed) from infants fed mother’s milk or donor human milk. | Spectrophotometric ELISAs of milk, gastric samples (30 min after feed) and stool samples | (1) Anti-PT IgA, anti-PT IgG and anti-FHA IgG in donor human milk was reduced during infant digestion at both postpartum times--anti-PT antibodies stable or increased in mother’s milk. | [29] |
| (2) Anti-FHA specific IgA and IgM higher in gastric contents from infants fed mother’s milk vs. donor human milk at 8–9 d. | ||||||||||||
| (3) Pasteurization of anti-pertussis antibodies may reduce their survival during infant digestion. | ||||||||||||
| (4) Both donor human milk and mother’s milk contain PT-specific antibodies that can survive digestion and can compensate for lower IgG transplacental transfer in preterm infants compared to term infants. | ||||||||||||
| Mother’s milk (Transitional/Mature 8–9 and 21–22 d postpartum) Donor human milk (unknown, presumed mature) | Mother’s milk(preterm); Donor human milk (unknown, presumed term) | HoP1 (Thermal) | Pathogen inactivation | 62.5°C, 30 min | Clinical-Crossover (2 interventions, 8–9 wk postpartum; 21–22 d postpartum) | (1) Raw mother’s milk (2) Pasteurized donor human milk | Preterm (30 ± 3 wk GA) | N= 20 (10 male, 10 female) | Relative abundance of anti H1N1-hemagglutinin and H3N2-specific IgG, IgM, IgA in milk, gastric contents (30 min after feed) and stool | Spectrophotometric ELISAs of milk, gastric samples (30 min after feed) and stool samples | (1) Gastric digestion reduced anti-H3N2 neuraminidase IgG from mother’s milk and from donor human milk at 21–22 d and 8–9 d, respectively. | [30] |
| (2) Anti-influenza A-specific IgM was higher in mother’s milk than donor human milk at both postnatal times in feed and gastric samples. | ||||||||||||
| (3) All influenza A antibodies were detected in stool 24h postfeeding (resisted digestion). | ||||||||||||
| Mother’s milk (Transitional/Mature 8–9 and 21–22 d postpartum) Donor human milk (unknown, presumed mature) | Mother’s milk (preterm); Donor human milk (unknown, presumed term) | HoP1 (Thermal) | Pathogen inactivation | 62.5°C, 30 min | Clinical-Crossover (2 interventions, 8–9 wk postpartum; 21–22 d postpartum) | (1) Raw mother’s milk (2) Pasteurized donor human milk |
Preterm (30 ± 3 wk GA) | N= 20 (10 male, 10 female), mother infant pairs | Concentration of secretory IgA, total IgA, total IgM, and total IgG in milk and gastric contents (30 min after feed) and stool. | Spectrophotometric ELISAs of milk, gastric samples (30 min after feed) and stool samples | (1) Total IgA, sIgA and total IgM/IgG concentrations were higher in the stomach from preterm infants fed mother’s milk vs. donor human milk. | [28] |
| (2) This could be because of initial higher concentration of maternal antibodies in mother’s milk vs. donor human milk. | ||||||||||||
| (3) sIgA and total IgM in mother’s milk were partially digested in the stomach, total IgA and IgG in mother’s milk were stable in gastric contents (none were digested). | ||||||||||||
| 4) Lower digestibility of antibodies in donor human milk could be because of changes in Ig structure after pasteurization. | ||||||||||||
| Mother’s milk (raw/pasteurized) (Transitional/Mature) | Preterm | HoP1 (Thermal) | Pathogen inactivation | 62.5°C, 30 min | Clinical-Crossover | (1) Raw mother’s milk (2) Pasteurized donor human milk |
Preterm (30 ± 1 wk GA) at 27 ± 12 d postpartum | N = 12 (6 males, 6 female) | Lipid analysis (triglycerides, diglycerides, monoglycerides, total fatty acids) and protein composition of milk and gastric contents. Free taurine (marker of meal dilution) Particle size distribution | Lipid analysis (thin layer chromatography and gas chromatography | (1) Pasteurization enhanced the proteolysis of lactoferrin, but reduced proteolysis of α-lactalbumin (after 90 min). | [27] |
| -flame ionization detection) | (2) Lipolysis (predigestion) was lower for pasteurized milk than raw milk. | |||||||||||
| Protein analysis (SDS-PAGE with semi-quantitation) | (3) Strong emulsion destabilization was observed, with smaller aggregates and a higher specific surface for pasteurized milk. | |||||||||||
| Free taurine (Cation exchange chromatography) | (4) Increase in particle size by mode and by volume in raw milk during gastric digestion vs. pasteurized milk. Differences no longer exist during intestinal digestion. | |||||||||||
| Particle size distribution (laser light scattering) | ||||||||||||
| Transitional | Preterm | HoP1 (Thermal) | Pathogen inactivation | 62.5°C, 30 min | Clinical-Crossover (One-week pasteurized mother’s milk); one-week raw mother’s milk) | (1) Raw mother’s milk (2) Pasteurized mother’s milk |
Preterm (27–30 wk) | N=5 (4 females, 1 male) | Percent fat absorption | 72h fat balance-fecal fat measured by gravimetric methods-total fat in stool at the end of each intervention week | (1) Fat balance with pasteurized milk resulted in higher fat content in the stool compared with raw milk (p=0.06). | [31] |
| (2) The mean net fat absorption coefficient was 17% higher during the balance with raw milk vs. pasteurized milk (88% [80%–92%) vs. 71% [47%–87%] p=0.06). | ||||||||||||
| Mother’s milk and pooled donor human milk (Mature/Transitional) | Preterm | HoP1 (Thermal) | Pathogen inactivation | 63°C, 30 min | Clinical-RCT | (1) Raw mother’s milk (2) Pooled pasteurized donor human milk |
Preterm (Birthweight 1000–1500 g) | N=68 (33 infants raw mother’s milk, intervention); 35 infants pooled, pasteurized donor human milk control) | Hematological and biochemical measurements (serum albumin, creatinine, sodium, potassium, vitamin E) | Serum albumin, creatinine (Technicon autoanalyzer II); Sodium, potassium (Flame spectrophotometer); Serum vitamin E (microplate, Fabianek et al.) | (1) Serum albumin, creatinine, potassium, and sodium values were similar in the two groups; this declined over the course of the study. | [32] |
| (2) Normal vitamin E levels, however; differences at 19 and 33 d after intervention (lower vitamin E in infants fed pasteurized milk vs. raw mother’s milk). | ||||||||||||
| Donor human milk (mature (2–5 mo postpartum) vs. fresh mother’s milk(transitional/mature) | Term (donor human milk); preterm (mother’s milk) | Autoclave sterilization (Thermal) | Pathogen inactivation | 100°C, 5 min | Clinical-Case Control (Matched by birthweight, within 100 g and gestational age, within 2 weeks) | (1) Raw mother’s milk (2) Sterilized donor human milk |
Preterm (low birth weight, <1300 g) | N=24 | Fat, nitrogen, and lactose balance studies- 2 weeks post intervention | 72-hour balance studies of fat (colorimetric); nitrogen (Kjeldhal); lactose (kit); | (1) Fat absorption higher in mother’s milk vs. donor human milk (90.4% vs. 69.8%) during the first week; 86% vs. 58.4% in the second week. | [25] |
| (2) Nitrogen absorption higher in mother’s milk vs. donor human milk (90.1% vs. 83.8% during first week and 86.6% vs. 82.5% in second week. | ||||||||||||
| (3) No significant differences in % carbohydrate absorbed. | ||||||||||||
| Donor human milk (mature assumed) | Term (assumed) | (1) HoP1 (Thermal) (2) Flash heat (brought to boil), rapidly cooled (Thermal) |
Pathogen inactivation | (1) 62.5°C, 30 min (2) Brought to boil | Clinical-Crossover | (1) Raw human milk (2) Pasteurized human milk (3) Boiled human milk |
Preterm (<1300g; 3–6 wk) | N = 7 (4 female, 3 male) | Fat absorption %, nitrogen balance, calcium, phosphorous and sodium balance. | 48-hour balance study, testing fecal fat by saponification and fatty acid titration with alkali (Van de Kamer); Sodium via flame emission spectrophotometry; Calcium by atomic absorption spectrophotometry; Phosphorous via colorimetric (molybdate/vanadate reagent) | (1) Fat absorption decreased by 28%–37% in boiled pasteurized and boiled milk, respectively relative to raw milk. | [24] |
| (2) No differences in nitrogen, calcium, phosphorus, or sodium balance. | ||||||||||||
| Mother’s milk and pooled donor human milk (mature assumed) | Mother’s milk (preterm); pooled donor human milk (term assumed) | HoP1 (Thermal) | Pathogen inactivation | Series A: 72°C, 5 min Series B: 62°C, 20 min; 97°C, 20 min; 100°C, 3 min | Clinical-Crossover | Series A: (1) Raw mother’s milk (2) Pasteurized milk 72°C, 5 min Series B: (1) Raw pooled milk (2) Pasteurized milk 62°C, 20 min (3) Pasteurized milk 97°C, 20 min (4) Pasteurized milk 100°C, 3 min | Series A: Preterm (960–1960 g) Series B: Preterm (1080–2220 g) | Series A:N = 8 infants (31 fat balances) Series B:N = 24 infants (67 fat balances) | Series A: Fat balances (4–5 d duration) Series B: Fat balances (4–7 d duration) | Series A: Fecal fat determined by Blix and Lindberg method. Series B: Fecal fat determined by Mojonnier extraction method | (1) No significant differences in fat absorption with heating milk (62.5°C, 30 min; 72°C for 5 min or 97°C, 3 min). | [33] |
| Mature (assumed) | Term (assumed) | (1) HoP1 (Thermal) (2) UV-C2 (Non-thermal) |
Pathogen inactivation | (1) 62.5°C, 30 min (2) 4863J/L dose |
Animal study (Preterm pigs, Large White Danish X Landrace X Duroc, 106d gestation) | (1) Pooled, Raw donor human milk (2) HoP donor human milk (3) UV-C2 treated milk |
Preterm | N=57 (N=18/19 per group) | Effect on mucosal morphology, digestive function (plasma citrulline, marker of intestinal dysfunction), intestinal permeability, NEC, gut microbiota, food transit time. | Mucosal morphology assessed by villus heigh and crypt depth via hematoxylin and eosin-stained paraformaldehyde-fixed histology slice; Plasma citrulline (marker of intestinal dysfunction); Intestinal permeability via lactulose-mannitol bolus; Gut microbiota by 16S rRNA gene MiSeq-based sequencing; Food transit time via chromium-oxide added bolus feed. | (1) No difference in food transit time, intestinal permeability, or diagnosis of necrotizing enterocolitis. | [22] |
| (2) Intestinal health was improved in pigs fed UV-C2 treated milk compared with HoP milk as indicated by a higher plasma citrulline concentration (36%) and villus height (38%). | ||||||||||||
| Mother’s milk (pasteurized/ pasteurized & homogenized) (Transitional/Mature) | Preterm | (1) HoP1 (Thermal) (2) Ultrasonic homogenization (Non-thermal) |
Pathogen inactivation & Fat homogenization | (1) 62.5°C, 30 min (2) 595 W (5min x 3) |
Clinical-Crossover | (1) Pasteurized (2) Pasteurized-Homogenized |
Preterm infants (<32 weeks GA) 29.5 ± 1.5 weeks | N=8 (3 male, 5 female) | Structural disintegration, lipolysis (fatty acid release) and proteolysis | Particle size via laser light scattering (Mastersizer) and | (1) No significant difference in surface weighted mean (D [3,2]), volumed weighted mean (D [4,3]) and the specific surface for both milks. | [34] |
| confocal laser scanning microscopy. | (2) Aggregates in pasteurized milk were formed by proteins and by milk fat globule; in pasteurized and homogenized milk, mixed aggregates were observed with co-localized proteins, lipid droplets and amphiphilic molecules. | |||||||||||
| Lipid analysis (thin layer chromatography and gas chromatography with flame ionization detection); | (3) Lipolysis was greater during digestion of pasteurized homogenized milk vs. pasteurized milk during gastric digestion; C16:0 and C18:1 were the predominant fatty acids, likely because of greater surface are for lipase adsorption. | |||||||||||
| Protein analysis (SDS-PAGE with semi-quantitation); | (4) Concentrations of major milk proteins (lactoferrin, serum albumin, α-lactalbumin and ß-casein) decreased-- no effect of homogenization. Serum albumin showed an enhanced proteolysis posthomogenization. | |||||||||||
| Mother’s milk and pooled donor human milk (Mature) | Preterm & donor human milk (Term assumed) | (1) HoP1 (Thermal) (2) Ultrasonic homogenization (Non-thermal) |
Pathogen inactivation & Fat homogenization | (1) 62.5°C, 30 min (2) Ultrasonic homogenization (10 min) |
Clinical-Crossover (Non-homogenized vs. homogenized) | (1) Non-pasteurized, homogenized (2) Non-pasteurized, non-homogenized (3) Pasteurized, homogenized (4) Pasteurized, non-homogenized |
Preterm (28–34 wk; 1000–1500g) | N=18 (N=8 Raw, unpasteurized. N=10, pasteurized mother’s milk + donor human milk) | Fat absorption % | Fecal Fat by saponification and fatty acid titration with alkali (Van de Kamer) from 2x72 h fat balance studies | (1) No difference in total fat ingestion among the groups, however; homogenization increased % fat absorption from 86.2% to 91.7% in raw milk and increased % fat absorption from 78.6% to 86.8% in pasteurized milk. | [26] |
| (2) Pasteurization of human milk decreased fat absorption--homogenization of pasteurized human milk yielded a similar fat absorption to non-homogenized raw milk. | ||||||||||||
| Donor human milk (colostrum, transitional, mature) | Term (assumed) from milk bank | (1) HoP1 (Thermal) (2) Physical homogenization (immersion blender) (Non-thermal) (3) Centrifugation (Non-thermal) |
(Pathogen inactivation & Fat homogenization | (1) 62.5°C, 30 min (2) 4800 rpm, 3 min (3) 13,000 x g for 10 min |
Animal study (Male Wistar rats) | (1) Pasteurized-homogenized (2) Homogenized-pasteurized (3) Skimmed-pasteurized and water control (4) Water-control |
Term assumed (21–51-d postpartum rat) | N=32 (8 animals per group) | Nutrient (fat) delivery and indirect measures of absorption/metabolism including total cholesterol, HDL-cholesterol, TAG, alanine aminotransferase, aspartate aminotransferase, brain unsaturated/saturated fatty acid. | Blood biochemistry (Commercial kits and spectrophotometer); Brain fatty acids (Gas chromatography, flame ionization detection) | (1) No significant differences in blood biochemistry. | [23] |
| (2) Higher concentration of C20:5n-3 (eicosapentaenoic acid); C22:6n-3 (docosahexaenoic acid) and C24:1n-9 enriched in brain in group receiving homogenized DM compared to non-homogenized. | ||||||||||||
Note.1Holder pasteurization, HoP; 2Ultraviolet C irradiation, UV-C.
TABLE 3.
Summary of simulated static and dynamic in vitro studies assessing the impact of human milk processing on infant digestion
| Human milk type & processing |
Digestion-Study Design |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lactational Stage (Colostrum, Mature, Transitional) | Preterm/Term milk | Processing Type and Classification | Processing Rationale | Processing parameters | Study type | Groups | Infant type | Sample size (N) | Outcomes assessed | Methods | Key findings | Ref |
| Pooled donor human milk (from 6 mothers) | Term (assumed) | (1) HoP1 (Thermal) (2) HPP2 (Non-thermal) | Pathogen inactivation | (1) 62.5 °C, 30 min | Static In vitro Gastrointestinal Digestion | (1) Raw human milk | Term | N=1 pool (digested in triplicate for each treatment) | Microstructure, particle size distribution and proteolysis | Microstructure via confocal laser scanning microscopy; Protein composition using SDS-PAGE; Degree of protein hydrolysis using the ortho-phthalaldehyde method; free amino acid analysis via HPLC | (1) Raw milk had a major peak size distribution of approximately 5–8 μm vs. approximately 50 μm for treated samples of HoP1 and HPP2. After 60 min of gastric digestion, HPP2 milk had a similar size distribution to raw milk. | [40] |
| (2) 400 MPa, 5 min, 25°C | (2) HoP1 human milk | (2) During gastric digestion, the protein profile of HoP1 milk began to differ from that of raw milk, with the lactoferrin band becoming fainter, while the ß-casein band was resistant to complete digestion. Lactoferrin in Raw and HPP2 milk was resistant to digestion. | ||||||||||
| (3) HPP2-treated milk | (3) Greater digestion of ß-casein in raw and HPP2 treated milk, indicative of increased hydrolysis. | |||||||||||
| (4) Overall, lower protein hydrolysis in stomach vs. intestinal. HPP2 had a slightly higher hydrolysis during the gastric phase; HoP1 milk had the highest hydrolysis during the intestinal phase. | ||||||||||||
| (5) No significant difference between raw milk and treated milk for either individual free amino acid concentrations or the total amino acid concentration after 60 min of intestinal digestion. | ||||||||||||
| Donor human milk (Mature, 5–9 mo postpartum) | Term | HoP1 (Thermal) | Pathogen inactivation | 62.5°C, 30 min | Static in vitro gastrointestinal digestion & Caco-2/TC7 in vitro absorption | (1) Raw, unpasteurized donor human milk | Preterm (4-weeks postpartum) | N=1 pool (Triplicate digestion for each treatment) | Characteristics of lipolysis (Total fatty acid analysis, triglycerides, free fatty acids, cholesterol, diacylglycerides and monoacylglycerides) and in vitro lipid uptake and gene expression | Lipid analysis (thin layer chromatography, gas chromatography-flame ionization detection); In vitro lipid uptake using Caco-2/TC7 cell culture | (1) Lipolysis occurred mainly in the intestinal phase. Triacylglycerides were hydrolysed into free fatty acids, diacylglycerol and monoacylglycerol. The extent of lipolysis was 52% lower in pasteurized milk vs. raw milk justbefore in vitro digestion. No differences were observed during gastric or intestinal digestion. | [45] |
| (2) HoP1 donor human milk | (2) No differences between pasteurized and raw milk with respect to lipid absorption using Caco2 cells. | |||||||||||
| Pooled donor human milk (mature assumed) | Term (assumed) | HoP1 (Thermal) | Pathogen inactivation | 62.5°C, 30 min | Static in vitro gastrointestinal digestion | (1) Raw, unpasteurized donor human milk | Term | N=1 pool (divided in 2) | Release of peptides from human milk proteins | Peptidomic analysis via solid-phase extraction followed by MS/MS. Characterization of peptides by computational methods. | (1) Protein-derived peptides present, most abundantly derived from ß-casein, given its high susceptibility to plasmin-mediated proteolysis. | [42] |
| (2) HoP1 donor human milk | (2) Pasteurization did not appear to alter bioactive peptide release following in vitro digestion of raw and pasteurized human milk-- many peptides from caseins and whey proteins were released. | |||||||||||
| (3) Bioactive peptides released from in vitro digestion include angiotensin I-converting inhibitory peptides, antioxidative peptides and immunomodulatory peptides. | ||||||||||||
| Mature (assumed) | Term (assumed) | HTST3 (Thermal) | Pathogen inactivation | 95°C, 1 min | Static in vitro gastrointestinal digestion | (1) Pooled, Raw donor human milk | Not defined | N=1 pool; digestion in duplicate | Protein degradation | Protein composition via SDS-PAGE and semi-quantitation; Casein micelle size via photon correlation spectroscopy (Zetasizer). | (1) Lactoferrin was digested quickly; high heat treatment of milk resulted in very little difference in protein degradation, except for α-lactoglobulin which showed a 10%–20% higher degradation compared to raw milk. | [39] |
| (2) Heat-pasteurized donor human milk | ||||||||||||
| Mature (1–3 mo postpartum) | Term | (1) HoP1 (Thermal) | Pathogen inactivation | (1) 62.5°C, 30 min | Dynamic in vitro gastrointestinal digestion system (DIDGI) | (1) Pooled, Raw donor human milk | Preterm (4 weeks postpartum) | N=1 pool; triplicate digestion for each treatment | Identification, quantification, and biochemical characteristics of peptides | Peptide identification via mass spectrometry. Computational tools for peptide characterization & quantification via label-free MS. | (1) Before digestion, identified peptides were derived from ß-casein. | [37] |
| (2) HTST3 (Thermal) | (2) 72°C, 15 sec | (2) HoP1 donor human milk | (2) Gastric digestion of HoP1 resulted in a greater number and more abundant ß-casein specific peptides. A delayed release of peptides was observed in raw milk during the intestinal phase. The effect of pasteurization was predominant during the intestinal phase--irrespective of what technology was used. | |||||||||
| (3) HTST3 Pasteurized Milk | (3) Higher intestinal digestion of lactoferrin (barely detectable in the pasteurized samples after 30 min of intestinal digestion). | |||||||||||
| (4) HTST3 pasteurization (at a gastric level), can retain a closer peptide profile compared to raw, than HoP1. Higher abundance of peptides after HTST3 digestion vs. HoP1. | ||||||||||||
| Mature (1–3 mo postpartum) | Term | (1) HoP1 (Thermal) | Pathogen inactivation | (1) 62.5°C, 30 min | Dynamic in vitro gastrointestinal digestion system (DIDGI) | (1) Pooled, Raw donor human milk | Preterm (4-weeks postpartum) | N=1 pool (digested in triplicate per treatment) | Particle size distribution, degree of protein hydrolysis, protein composition, bio-accessibility of amino acids, lipid analysis (lipid classes) | Particle size via laser light scattering (Mastersizer) and confocal laser scanning microscopy; Lipid analysis (think layer chromatography and gas chromatography with flame ionization detection); Protein analysis (SDS-PAGE); | (1) During gastric phase, formation of large aggregates in raw milk compared to HTST3 and HoP1) –Pasteurized samples did not show any modification of the particle size during gastric digestion. | [38] |
| (2) HTST3 (Thermal) | (2) 72°C, 15 sec | (2) HoP1 donor human milk | (2) Pasteurized human milk samples resulted in faster gastric proteolysis of high-molecular-weight protein bands, including lactoferrin vs. raw milk. | |||||||||
| (3) HTST3 pasteurized Milk | (3) Faster intestinal proteolysis in both pasteurized samples for high-molecular-weight bands, including native lactoferrin. Pasteurization increased the resistance of serum albumin to digestion vs. raw. No major differences between HoP1 and HTST3. | |||||||||||
| 4) No differences in release of free amino acids or triglyceride hydrolysis. | ||||||||||||
| Mature (6.6 weeks ± 2.4 weeks postpartum) | Preterm (29.8 ± 3.0 weeks) | HoP1 (Thermal) | Pathogen inactivation | 62.5°C, 30 min | Dynamic in vitro gastrointestinal digestion system (DIDGI) | (1) Raw breast milk | Preterm (28 weeks GA, 4 weeks post-natal) | N=3 per treatment group (triplicate) | Peptides released and free amino | Peptidomics (LCMS analysis w/ label-free peptide quantification); Free amino (fluorescent microplate analysis using ortho-phthalaldehyde (OPA) and ß-mercaptoethanol) | (1) Certain clusters of peptides showed a lower abundance for pasteurized vs. raw milk during the gastric phase and intestinal phase. | [44] |
| (2) Pasteurized breast milk | (2) Pasteurization impacted the human milk peptidome before digestion (from ß-casein) and induced different kinetics of peptide release during gastro-intestinal digestion mainly for heat-denatured proteins (bile salt–stimulated lipase and lactoferrin). | |||||||||||
| (3) Pasteurization impacted some peptide release during digestion (No impact on free amino) but clinical relevancy needs to be determined. | ||||||||||||
| Mature (6–14 wk postpartum) | Term | HoP1 (Thermal) | Pathogen inactivation | 62.5°C, 30 min | Dynamic in vitro gastrointestinal digestion system (DIDGI) | (1) Raw breast milk | Term (4-weeks) | N=2 for raw milk; N=3 for pasteurized milk | Peptides released and free amino | Peptidomics (LCMS analysis w/ label-free peptide quantification); Free amino (fluorescent microplate analysis using ortho-phthalaldehyde and ß-mercaptoethanol) | (1) Pasteurization impacted selectively gastric and intestinal kinetics of peptide release. | [43] |
| (2) Pasteurized breast milk | (2) Pasteurization increased the number of peptides and abundance of peptides common to both raw and pasteurized milk. Origin of peptides predominantly from ß-casein and not from other abundant proteins (lactoferrin, α-lactalbumin). | |||||||||||
| (3) Lower release of free amino for pasteurized vs.raw milk at 120 min of gastric digestion. | ||||||||||||
| (4) Unknown if changes in peptide digestion kinetics in pasteurized milk is clinically relevant. | ||||||||||||
| Donor human milk (mature, 6.6 ± 2.4 weeks postpartum) | Preterm | HoP1 (Thermal) | Pathogen inactivation | 62.5°C, 30 min | Dynamic in vitro gastrointestinal digestion system (DIDGI) | (1) Pooled, Raw donor human milk | Preterm (4-weeks postpartum) | N=1 pool (digested in triplicate per treatment) | Microstructure and particle size distribution; Lipid analysis (triglycerides, diglycerides, monoglycerides and total fatty acids) and protein composition of milk and gastric contents | Particle size via laser light scattering (Mastersizer) and confocal laser scanning microscopy; Lipid analysis (thin layer chromatography and gas chromatography with flame ionization detection); Protein analysis (SDS-PAGE with semi-quantitation). | (1) During gastric phase, formation of large aggregates in raw milk and increase in main mode diameter and in D [4,3]. Aggregates which appear postpasteurization disappeared at the beginning of gastric digestion. | [7] |
| (2) Pasteurized donor human milk | (2) Larger aggregates formed for pasteurized milk vs. raw milk during the intestinal phase. | |||||||||||
| (3) No differences in gastric lipolysis; higher lipolysis in raw vs. pasteurized milk during intestinal phase. | ||||||||||||
| (4) Gastric phase proteolysis involved predominantly lactoferrin and ß-casein, no differences by treatment. | ||||||||||||
| (5) Released phenylalanine, tyrosine, and arginine higher in pasteurized vs. raw; lower release of serine. | ||||||||||||
| Mature (11 weeks postpartum) | Term | HoP1 (Thermal) | Pathogen inactivation | 62.5°C, 30 min | Dynamic in vitro gastrointestinal digestion system (DIDGI) | (1) Pooled, Raw donor human milk | Term | N=1 pool; raw digested in duplicate; pasteurized digested in triplicate | Microstructure and particle size distribution; Lipid analysis (triglycerides, diglycerides, monoglycerides and total fatty acids) and protein composition of milk and gastric contents | Particle size via laser light scattering (Mastersizer) and confocal laser scanning microscopy; Lipid analysis (thin layer chromatography and gas chromatography with flame ionization detection); Protein analysis (SDS-PAGE with semi-quantitation). | (1) Gastric proteolysis of lactoferrin and ß-casein tended to be faster for pasteurized milk compared to raw. | [41] |
| (2) Pasteurized donor human milk | (2) Lactoferrin and ß-casein were more extensively digested than serum albumin and α-lactalbumin during gastric digestion (regardless of treatment). | |||||||||||
| (3) Pasteurization affected the intestinal release of some amino acids; effect differed according to the amino acid. Lipolysis was also lower in pasteurized milk vs. raw but no difference in fatty acid release. | ||||||||||||
| 4) Raw human milk presented a structural destabilization after 60 min of gastric digestion; no differences in particle size during intestinal phase by pasteurization. | ||||||||||||
| Pooled donor human milk (8 women, mature assumed) | Term (assumed) | Freeze-Thaw (Thermal) | Other |
Freezing: (1) Fresh milk (2) -18°C, 30 d (3) -60°C, 30 d Thawing: (1) Slow thaw (4°C, 10 h) (2) Intermediate thaw (25°C, 1 h) (3) Rapid Thaw (45°C, 1 min) |
Static in vitro gastrointestinal digestion | (1) Fresh milk (2) -18°C milk, slow thaw (3) -18°C milk, intermediate thaw (4) -18°C milk, rapid thaw (5) -60°C milk, slow thaw (6) -60°C milk, intermediate thaw (7) -60°C milk, rapid thaw |
Term | N= 1 pool (1 digestion per treatment) | Particle size distribution, microstructure, protein composition and identification, peptide molecular weight. | Particle size distribution via laser particle size analyzer; microstructure via confocal laser scanning microscope; protein composition by SDS-PAGE; protein band identification by Maldi-TOF/TOF mass spectrometry. | (1) Decrease of human milk fat globule particle size in -60°C, 45°C thaw samples during gastric digestion like fresh human milk. After intestinal digestion, more human milk fat globule particles decreased in size. | [46] |
| (2) Caseins in freeze-thaw milk were digested even faster than fresh. The 6 freeze-thawed samples showed a reduction of hydrolysis compared with fresh human milk. | ||||||||||||
| (3) Human milk frozen at -60°C and thawed at 45°C is most like fresh, including the molecular weight distribution of peptides after digestion. | ||||||||||||
Note.1Holder pasteurization, HoP; 2High pressure processing, HPP; 3High-temperature short time, HTST.
TABLE 4.
Aggregation of main findings from in vivo and in vitro digestions, organized by processing method
| Main finding of processed milk | Processing | Increased/impacted | No difference or not impacted | Decreased |
|---|---|---|---|---|
| Proteolysis of lactoferrin | 1HoP | (27,37,38,40,41) | (7) | – |
| 2HPP | – | (40) | – | |
| 3HTST | (37,38) | – | – | |
| Homogenization | – | (34) | – | |
| Freeze-thaw | – | – | – | |
| Proteolysis of caseins | 1HoP | (37,40,41) | (7,42) | – |
| 2HPP | (40) | – | – | |
| 3HTST | – | – | – | |
| Homogenization | – | (34) | – | |
| Freeze-thaw | [46] | – | – | |
| Release of free amino (Protein hydrolysis) | 1HoP | (41) | (38,40,43,44) | (43) |
| 2HPP | – | (40) | – | |
| 3HTST | – | (38) | – | |
| Homogenization | – | – | – | |
| Freeze-thaw | – | – | – | |
| Particle size distribution | 1HoP | (7) | (27,38,41) | – |
| 2HPP | – | (40) | – | |
| 3HTST | – | (38) | – | |
| Homogenization | – | (34) | – | |
| Freeze-thaw | – | [46] | – | |
| Release of peptides— abundance or diversity | 1HoP | (37) | (42,43) | (44) |
| 2HPP | – | – | – | |
| 3HTST | (37) | – | – | |
| Homogenization | – | – | – | |
| Freeze-thaw | – | – | – | |
| Lipolysis/ Fat absorption | 1HoP | – | (27,33,38) | [[7], [24], [26], [31], [41], [45]] |
| 2HPP | – | – | – | |
| 3HTST | – | (24,25) | (33,38) | |
| Homogenization | (34) | – | – | |
| Freeze-thaw | – | – | – |
HoP, Holder pasteurization
HPP, high-pressure processing;
HTST, high-temperature short-time, includes processing at 72.5°C, 15 sec; 100°C, 5 min. HoP, HTST and HPP are methods used specifically to inactivate pathogens. A dash indicates no supporting evidence available.
In vivo studies
Of the 24 articles, 13 articles were used in vivo design in which the majority (N = 11) were clinical studies exclusively examining the impact of processed milk in preterm, very-low birth weight infants Table 2. Two studies assessed the impact of donor human milk processing using preterm (White Danish X Landrace X Duroc piglets) and term (day 21–51 male Wistar rats) animal models [22, 23].
All in vivo studies included some form of thermal pasteurization treatment to inactivate pathogens. The most common thermal technique was HoP. Other thermal processing techniques tested could be classified as variants of HTST as high heat pasteurization (autoclave sterilization [100°C, 5 min] or flash heat [brought to boil]) [24, 25]. Nonthermal processing methods included UV-C irradiation for the purposes of pathogen inactivation, as well as homogenization, sonication, and centrifugation for the purposes of fat homogenization [22, 23, 26, 27].
The in vivo human studies were focused around 2 central objectives: 1) To determine the impact of processing on the digestion of protein or human milk bioactive protein components (N = 4 studies), and 2) To determine the impact of processing on fat digestion or absorption (N = 6 studies). Methodologically, protein digestion in primarily gastric samples was assessed by methods including sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with semiquantification to elucidate the digestion patterns of specific proteins, enzyme-linked immunosorbent assay for bioactive components, nitrogen balance, and particle size distribution to assess the presence and disappearance of high-molecular-weight protein aggregates (Table 2). Fat digestion was predominately executed via 72-h fat balances and analysis of fecal fat. Some articles also assessed fat lipolysis from gastric aspirates via more sophisticated methods including thin-layer chromatography to determine triacylglycerides, diglycerides, monoglycerides, and free fatty acids, and gas chromatography with flame ionization detection to determine fatty acid composition.
Processing to inactivate pathogens
For the most part, studies assessing protein digestion by nitrogen balance or by degradation of major human milk proteins consistently reported differences resulting from HoP milk compared to unpasteurized milk. Greater nitrogen absorption was reported in unpasteurized milk vs. milk heated to 100°C, 5 min in the first- and second-week following birth [25]; however, no difference was reported in nitrogen balance in infants fed Holder pasteurized or boiled milk from the weeks of age 3–6 [24]. In 1 study, immunoglobulins (Ig) from raw milk resisted digestion to a greater extent compared with milk processed by HoP. This was true for total IgA, IgG, and IgM or when antibodies specific to pertussis toxin or influenza strains were analyzed [[28], [29], [30]].
The impact of pasteurization on fat digestion was commonly assessed among in vivo studies (N = 6). Most analyses of the degree of lipolysis in relation to HoP of donor human milk (62.5°C, 30 min) reported a significant impact compared to raw milk. Overall, higher fat absorption (decreased fecal fat) was observed in infants fed raw milk compared with milk pasteurized by either Holder or other heat treatments including (100°C, 5 min or boiled) [24, 25, 31]. These findings are consistent with Stein et al. [32], where infants fed pasteurized versus raw milk had lower serum vitamin E levels, a fat-soluble vitamin. In a more recent study in preterm infants, only predigestion lipolysis appeared to be higher for raw milk [27]. Only 1 study reported no significant difference in fat absorption as a result of heating to 62.5°C for 30 min, 72°C for 5 min or 97°C for 3 min [33].
Processing to homogenize fat
The impact of homogenization was assessed by 3 in vivo studies, 2 in preterm infants (N = 26 infants total) and 1 in an animal model using term-born rats [23, 26, 34]. Overall, all 3 in vivo studies that investigated homogenization reported a positive effect on fat digestion and appeared to improve lipolysis and percent fat absorption of both raw and HoP-treated milk. This was true in clinical studies of preterm infants and in animal studies. In a rat model specifically, homogenization of pasteurized milk resulted in enriched concentrations of eicosapentaenoic acid, docosahexaenoic acid (DHA), and nervonic acid (C24: 1n-9) in the brains of male wistar rats [23]. No in vivo studies assessed the impact of freeze-thaw on the digestibility of human milk.
In vitro studies
Both static and dynamic in vitro digestion models aim to mimic the physiology of the gastrointestinal tract via regulation of pH, ionic strength of simulated digestive fluid, and concentration of digestion-specific enzymes [35]. Typically used in preclinical assessment, in vitro digestion studies help answer questions relating to biological plausibility and help to garner a better understanding of how a specific meal might be digested under simulated conditions and provide substantially quicker results compared to in vivo human studies. Static models, however, do not take into account the dynamic nature of the physicochemical conditions of digestion—whereas dynamic models are more physiologically relevant, more closely regulate pH in addition to the dynamic flows of food, and concentration of digestive enzymes in different compartments (e.g., gastric vs. intestinal) [36]. Included in the review were 11 articles reporting in vitro digestibility of processed human milk; 5 articles employed static digestion models, whereas 6 articles used dynamic digestion models. An equal number of articles modeled term and preterm infants (N = 5 each, N = 1 undefined). Except for 1 study that aimed to assess the impact of various freeze-thaw parameters, all in vitro studies tested some form of thermal pasteurization as a treatment for human milk. Of those studies testing pasteurization, all but 1 either compared alternative thermal treatments to HoP or investigated HoP solely. Additional pasteurization methods included HTST (N = 3 articles, 2/3 preterm, 1/3 undefined) [[37], [38], [39]] and HPP (N = 1, 1/1 term) [40]. No eligible in vitro studies in this review assessed the impact of processing to homogenize fat.
The most common reported outcomes of in vitro digestibility were the digestion of human milk proteins and release of peptides. Protein profile and composition were qualitatively assessed by SDS-PAGE, whereas peptide release was determined via mass spectrometry techniques and via computational tools for peptide characterization and quantification via label-free mass spectrometry. Particle size distribution was also measured in several studies to assess protein aggregation and formation of high-molecular-weight complexes. Similarly, the degree of hydrolysis was used to determine whether the overall rate of protein digestion was increased or decreased because of processing. Specifically, fluorescent microplate analysis using ortho-phthalaldehyde was typically used.
Processing to inactivate pathogens
A common finding among in vitro digestibility and absorption studies is that higher molecular weight proteins in human milk such as lactoferrin, appear to be more rapidly digested from milk subjected to thermal pasteurization (Table 3). In most of the reviewed articles, processing via HoP, high heat (95°C, 1 min) or HTST consistently reported greater lactoferrin digestibility compared to other major proteins such as α-lactalbumin—irrespective of whether a term or preterm infant was being modeled and type of in vitro model [[37], [38], [39], [40]]. However, 2 studies found no significant differences between HoP and raw milk, for which a high rate of lactoferrin digestibility was reported during simulated term and preterm digestion of HoP and raw milk [7, 41]. The impact of processing on casein digestion was reported across several studies, though there were some discordant findings, depending on the in vitro design. For example, Zhang et al. [40] using a static in vitro model of term infants, reported that there was a greater digestion of raw milk ß-casein compared to Holder pasteurized milk. In contrast, Giribaldi et al. [37] reported that HoP resulted in a greater number and more abundant release of ß-casein specific peptides. However, de Oliveira et al [41] reported no difference in casein digestion between Holder pasteurized and raw milk in a dynamic in vitro model.
Four in vitro studies assessed the impact of human milk processing on peptide release. In a study simulating term infants using a static digestion model, there was no impact of HoP on peptide release [42]. In contrast, in dynamic models of both term and preterm infants, processing by HoP or HTST resulted in an increased number or diversity of peptides released [37, 43, 44]. The concentration of free amino acids, an indicator of protein hydrolysis, was typically not impacted by HoP during dynamic preterm digestion but affected following dynamic term digestion [38, 43].
Both static and dynamic models were used to assess particle sizes in human milk. In a static model of term infant digestion, 1 article suggested that differences in initial particle size (higher for pasteurized milk) are no longer apparent upon digestion [40]. However, other dynamic models of preterm infants suggested that large aggregates are formed in raw milk upon gastric digestion, and post-pasteurization aggregates disappear upon gastric digestion [[7], [38]]. Similarly, in a dynamic model of a term infant, raw milk appeared to destabilize during gastric digestion; however, there were no reported differences during intestinal digestion [41].
In vitro studies commonly assessed lipolysis as the degree to which triglycerides are hydrolyzed, in conjunction with the appearance of free fatty acids. There appear to be discordant results regardless of digestion type (static vs. dynamic) or type of infant modeled. For example, 2 studies of simulated preterm infants using both static and dynamic models reported no differences in lipolysis during digestion of raw and pasteurized milk [38, 45]. In contrast, 2 other studies of simulated preterm and term infants using dynamic models found that raw milk yielded higher intestinal, but not gastric, lipolysis compared with pasteurized milk [7, 41]. There were no differences in the release of free fatty acids.
Freezing and thawing milk
Freezing and thawing milk was associated with increased casein proteolysis [46]. It was also reported that human milk frozen at −60°C and thawed at 45°C appeared most like fresh milk, with respect to the molecular weight distribution of peptides after digestion. No other eligible in vitro articles assessed the impact of freeze-thawing on digestion or absorption outcomes.
Overall findings grouped by digestion and absorption-related outcomes
The main findings of both in vitro and in vivo studies are summarized in Table 4 and organized by processing technique. Outcomes related to protein and fat digestion were most reported by all the included articles, including specifically the proteolysis of lactoferrin and casein, the release of free amino acids (protein hydrolysis), particle size distribution, the release of peptides, and rate of lipolysis and fat absorption.
Discussion
Although providing donor human milk instead of formula to vulnerable very-low-birth-weight infants improves feeding tolerance and provides protection against necrotizing enterocolitis, it is often associated with slower growth [8]. Alterations to human milk nutrients and bioactivity are thought to be causative agents of the slower growth observed. Therefore, optimization of donor human milk processing, along with existing efforts to ensure adequate nutrient fortification, has potential to yield direct benefits to clinical outcomes of very-low-birth-weight infants. This requires an understanding of processing methods to both minimally impact milk composition, bioactivity, and digestibility of the milk. Our scoping review aimed to both summarize what is known about the impact of various processing techniques on the digestibility and absorption of human milk and highlight research gaps in the field. Ultimately, the goal was to identify promising areas of research to incite further optimization of donor human milk processing. These identified gaps are summarized in Table 5.
TABLE 5.
Identified research gaps relating to areas of donor human milk processing and subsequent infant digestion and absorption
| Area of Donor Human Milk Processing | Identified Gap(s) | Suggested Outcomes to Investigate | Significance |
|---|---|---|---|
| Processing to inactivate pathogens |
|
|
|
| Processing to ensure homogenous donor milk fat composition |
|
|
|
| Freeze-thawing and duration of freezing |
|
|
|
Overall, the identified articles explored the impact of 3 different types of processing which can be applicable to donor human milk banking including 1) Pasteurization to inactivate bacteria and viruses; 2) fat homogenization to disperse lipid droplets; and 3) freezing and thawing, processes required for human milk banking. Overwhelmingly, eligible in vivo and in vitro studies included in this review assessed the impact of HoP as a processing method to inactivate pathogens, such as bacteria and viruses. Although investigated in only few in vitro studies, research into the digestion and absorption-related impact of alternative pasteurization processes for pathogen inactivation, including thermal (e.g., HTST) or nonthermal (e.g., HPP, UV-C) methods were lacking. Moreover, there was an overall lack of studies assessing other techniques that can be applied to donor human milk production, including fat homogenization and freeze thawing. Specifically, only 3 included studies assessed the impact of fat homogenization in vivo. Homogenization via ultrasonic or mechanical methods can be used in donor human milk production to reduce the size of the milk fat globule to prevent creaming—bovine milk homogenization in the dairy industry achieves a similar objective using different equipment. While there exists a body of literature assessing the impact of freezing on specific human milk components, only 1 in vitro study assessed the impact of various freeze-thaw parameters on subsequent digestion- and absorption-related outcomes. This area of research is understudied and reflects a major gap that should be further explored. It is also unknown in the literature how conditions or handling postprocessing impact digestibility (e.g., duration donor milk is frozen before it is thawed and mixed into feeds etc.), a topic that should also be studied.
Given the well-established fact that thermal pasteurization significantly reduces the activity of bile salt–stimulated lipase in human milk, the impact of these methods was commonly studied in vivo and in vitro [5]. As anticipated, most articles consistently agree that heat, via inactivation of lipase, inhibits or decreases the kinetics of lipolysis. Notably lacking is research into novel, nonthermal pasteurization methods (such as HPP), which have consistently reported preservation of lipase activity [[47], [48], [49]]. It would be valuable to better understand whether the preservation of lipase translates into improved lipolysis in vivo. This research is required to fully understand the total impact of novel processing methods on donor human milk. It is also important to acknowledge that advances should be concurrently made in the production of equipment scaled down for the unique requirements of donor milk banking. For example, the current available technology for HPP is designed for large food and beverage manufacturing plants. Availability of at-scale equipment will help milk banks better transition to affordable novel processing methods. Moreover, research should also investigate hurdle technology as applied to human milk where multiple processing methods (e.g., HPP combined with heat or another non-thermal method) can be combined in tandem to inactivate bacteria with greater improvements in quality [50].
Research into the impact of processing on the release of peptides, whether this is abundance or diversity, is lacking. To date, no studies have investigated resultant peptide formation from milk pasteurized by nonthermal methods (e.g., HPP) or processing techniques (such as homogenization and freezing and thawing) (Table 4). Although the same proteins (e.g., lactoferrin, ß-casein, etc.) might be digested into peptides, there may be differences in abundance and species present, highlighting the need to expand the use of peptidomics as a tool to fingerprint the protein digestion of human milk. Human trials are warranted to better understand the potential clinical implications. Although a substantial amount of research suggests that proteolysis of lactoferrin and caseins in human milk is increased following HoP, it appears this may not impact the overall rate of protein hydrolysis as assessed by the release of free amino acids [38, 40, 43, 44]. Only 1 study has assessed the impact of HPP with respect to proteolysis of lactoferrin, casein, and release of free amino acids [40]. Notably, this was a static, in vitro model of a term infant and thus, additional research is required to better understand what the outcomes might be using a more physiologically relevant dynamic model of a preterm infant. Though the implications of maintaining the concentration of native lactoferrin within the gastrointestinal tract have yet to be determined, data from supplementation trials suggest that lactoferrin may play a role in the prevention of necrotizing enterocolitis and may lower the incidence of late-onset sepsis. Proposed mechanisms include immunological actions (e.g., immune T- and B-cell proliferation, dendritic cell activation), biochemical actions (e.g., ferric iron absorption, proteinase inhibitor), host defense actions (e.g., antibacterial, antiviral, biofilm inhibitor, ferric iron sequestration), and growth actions (e.g., intestinal growth and maturation) [51].
Finally, with respect to particle size distribution, as assessed by dynamic light scattering experiments, any increase in aggregation or particle size distribution because of processing (thermal or non-thermal), is reduced during digestion. Dynamic in vitro models with distinct gastric and intestinal compartments tend to report that any differences in particle size disappear and are, therefore, irrelevant by the time intestinal digestion is complete [7, 41]. Interestingly, upon ingestion, unpasteurized milk typically resulted in the formation of large protein aggregates which are larger than those produced as a by-product of processing [38]. Since these aggregates appear to be digested, based on a reduction in particle size, it is unclear if they are physiologically relevant.
In terms of the included studies, we observed many inconsistencies in infant parameters including the type of simulated digestion (e.g., static vs. dynamic). For this reason, information from all study types should be considered when drawing conclusions with respect to digestibility and absorption. We also noticed that for several studies of in vitro digestibility, digestions were typically conducted in triplicate on a single pool of donor human milk. This might be sufficient for assessment of trends but fails to capture the considerable variation in the composition of macronutrients (fat, protein) and bioactive component composition (e.g., Ig, lactoferrin, lysozyme, etc.) that is common among donors to human milk banks [52]. Milk with differing compositions might behave differently under simulated digestion conditions and should be studied to ensure research findings are pragmatic and generalizable to typical milk banking operations.
To our knowledge, this is the first scoping review that has surveyed the literature with respect to the impact of human milk processing and subsequent nutrient digestibility and absorption. The results from this study highlight our limited understanding of the effect of human milk processing, including novel, nonthermal methods such as HPP. HPP, among other new promising techniques that could be used to pasteurize milk, has been repeatedly shown to inactivate potentially pathogenic bacteria and viruses and is superior to thermal pasteurization in terms of retaining important bioactive components [47, 53]; however, the full impact of HPP on digestibility in preterm infants must also be elucidated before large-scale implementation. Furthermore, this review also underlines the importance of optimizing other aspects throughout the production of donor human milk, including freeze and thaw cycles, and the potential addition of a homogenization step. In both in vitro and in vivo studies, ultrasonic or mechanical homogenization appears to improve fat digestion, independent of pasteurization and may help to improve the clinical use of donor human milk in the NICU. Although not assessed in any included studies in this review, ultrahigh-pressure homogenization is a relatively novel technique that combines high throughput homogenization with HPP (application of 200–400 MPa) [54], and should be further explored as a potential suitable method for producing processed and homogenized donor human milk. While additional clinical studies are warranted to better understand its benefits, homogenization technologies and their associated parameters should be optimized for application to human milk and studied in larger trials given the relatively small number of infants this has been tested on to date. This has advantages that might assist in providing some directionality and biological plausibility before larger-scale implementation. Overall, research into fat homogenization is lacking in the literature and further exploration is warranted.
Our review has many strengths. First, our search strategy ensured we conducted a comprehensive survey of the literature from several electronic databases, including studies with objective measures of digestion and absorption. This is especially relevant given the complexity surrounding the assessment of measures secondary to nutrient absorption, such as growth. Although we excluded studies of human milk-derived products or bovine-derived infant formulas, our intention was to better understand the scope of research that has been conducted with respect to processing human milk relevant to a milk bank setting.
Conclusion
A wholistic approach to optimizing processing procedures for donor human milk banking has the potential to significantly improve the outcomes of vulnerable preterm infants. Each processing step required in its production should be carefully considered. Given the absence of this research in the literature, more studies into alternative non-thermal techniques (e.g., high-pressure processing, UV-C irradiation) for processing human milk to inactivate pathogens, as well as other donor milk-related processing, such as fat homogenization and freeze-thawing, is especially warranted. This research should focus on the impact on human milk composition and bioactivity, in addition to the potential effects on downstream digestion and absorption in the recipient infant, and in particular, the proteolysis of proteins, release of peptides and digestion of fat.
Funding
Funded in part by the Hospital for Sick Children, Doctoral Restracomp Scholarship (to MAP) and the LiUNA! Fellowship for Research Innovation (to MRB). The sources of support had no role in the conception or design of this review and were not involved in the conduct of the review, interpretation of results, or writing of the manuscript.
Author disclosures
The authors report no conflicts of interest.
Data availability
Primary data extracted for the purposes of this review can be made available upon request.
Acknowledgments
The authors wish to acknowledge Glyneva Bradley-Rideout for consulting on the review search strategy. The authors’ responsibilities were as follows—MAP, MRB: conducted the research; MAP and MRB analyzed the data; MAP: wrote the first draft of the paper; SU: has primary responsibility for the final content; and all authors: conceptualized the study, helped interpret findings and read and approved the final manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.advnut.2022.11.004.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Daniels S., Corkins M., de Ferranti S., Golden N.H., Kim J.H., Magge S.N., Schwarzenberg S.J., Meek J.Y., Johnston M.G., O’Connor M.E., et al. Donor human milk for the high-risk infant: preparation, safety, and usage options in the United States. Pediatrics. 2017;139(1) doi: 10.1542/peds.2016-3440. [DOI] [PubMed] [Google Scholar]
- 2.Human Milk Banking Association of North America . 2020. HMBANA Standards for Donor Human Milk Banking: An Overview.https://www.hmbana.org/file_download/inline/95a0362a-c9f4-4f15-b9ab-cf8cf7b7b866 [Internet] Available from: [Google Scholar]
- 3.Updegrove K.H. Donor human milk banking: growth, challenges, and the role of HMBANA. Breastfeeding Med. 2013;8(5):435–437. doi: 10.1089/bfm.2013.0079. [DOI] [PubMed] [Google Scholar]
- 4.Peila C., Moro G.E., Bertino E., Cavallarin L., Giribaldi M., Giuliani F., Cresi F., Coscia A. The effect of holder pasteurization on nutrients and biologically-active components in donor human milk: a review. Nutrients. 2016;8(8):477. doi: 10.3390/nu8080477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Connor D.L., Ewaschuk J.B., Unger S. Human milk pasteurization: benefits and risks. Curr Opin Clin Nutr Metab Care. 2015;18(3):269–275. doi: 10.1097/MCO.0000000000000160. [DOI] [PubMed] [Google Scholar]
- 6.Marousez L., Sprenger N., de Lamballerie M., Jaramillo-Ortiz S., Tran L., Micours E., et al. High hydrostatic pressure processing of human milk preserves milk oligosaccharides and avoids formation of Maillard reaction products. Clin Nutr. 2022;41(1):1–8. doi: 10.1016/j.clnu.2021.11.013. [DOI] [PubMed] [Google Scholar]
- 7.de Oliveira S.C., Bourlieu C., Ménard O., Bellanger A., Henry G., Rousseau F., et al. Impact of pasteurization of human milk on preterm newborn in vitro digestion: Gastrointestinal disintegration, lipolysis and proteolysis. Food Chem. 2016;211:171–179. doi: 10.1016/j.foodchem.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 8.Quigley M., Embleton N.D., McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. 2019;7(7):CD002971. doi: 10.1002/14651858.CD002971.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Authelin J.R., Rodrigues M.A., Tchessalov S., Singh S.K., McCoy T., Wang S., et al. Freezing of biologicals revisited: scale, stability, excipients, and degradation stresses. J Pharm Sci. 2020;109(1):44–61. doi: 10.1016/j.xphs.2019.10.062. [DOI] [PubMed] [Google Scholar]
- 10.Wesolowska A., Sinkiewicz-Darol E., Barbarska O., Bernatowicz-Lojko U., Borszewska-Kornacka M.K., van Goudoever J.B. Innovative techniques of processing human milk to preserve key components. Nutrients. 2019;11(5):1169. doi: 10.3390/nu11051169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sousa S.G., Delgadillo I., Saraiva J.A. Human milk composition and preservation: evaluation of high-pressure processing as a nonthermal pasteurization technology. Crit Rev Food Sci Nutr. 2016;56(6):1043–1060. doi: 10.1080/10408398.2012.753402. [DOI] [PubMed] [Google Scholar]
- 12.Reyes S.M., Patra B., Elliott M.J. The impact of homogenization on donor human milk and human milk–based fortifiers and implications for preterm infant health. Curr Dev Nutr. 2022;6(1):nzab147. doi: 10.1093/cdn/nzab147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez F.E., Desai I.D., Davidson A.G.F., Nakai S., Radcliffe A. Ultrasonic homogenization of expressed human milk to prevent fat loss during tube feeding. J Pediatr Gastroenterol Nutr. 1987;6(4):593–597. doi: 10.1097/00005176-198707000-00018. [DOI] [PubMed] [Google Scholar]
- 14.George A.D., Gay M.C.L., Wlodek M.E., Geddes D.T. The importance of infants’ lipid intake in human milk research. Nutr Rev. 2021;79(12):1353–1361. doi: 10.1093/nutrit/nuaa141. [DOI] [PubMed] [Google Scholar]
- 15.Peila C., Emmerik N.E., Giribaldi M., Stahl B., Ruitenberg J.E., van Elburg R.M., et al. Human milk processing: a systematic review of innovative techniques to ensure the safety and quality of donor milk. J Pediatr Gastroenterol Nutr. 2017;64(3):353–361. doi: 10.1097/MPG.0000000000001435. [DOI] [PubMed] [Google Scholar]
- 16.Tricco A.C., Lillie E., Zarin W., O’Brien K.K., Colquhoun H., Levac D., et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 17.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Natal G. Searching the grey literature: a handbook for searching reports, working papers, and other unpublished research. J Med Library Assoc. 2019;107:276. [Google Scholar]
- 19.Asbury M.R., Unger S., Kiss A., Ng D.V.Y., Luk Y., Bando N., et al. Optimizing the growth of very-low-birth-weight infants requires targeting both nutritional and nonnutritional modifiable factors specific to stage of hospitalization. Am J Clin Nutr. 2019;110(6):1384–1394. doi: 10.1093/ajcn/nqz227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hee Chung E., Chou J., Brown K.A. Neurodevelopmental outcomes of preterm infants: a recent literature review. Transl Pediatr. 2020;9 doi: 10.21037/tp.2019.09.10. S3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veritas Health Innovation . 2018. Covidence Software Platform. Melbourne (Australia)http://covidence.org Version as of 16 Sept 2021. Internet: [Google Scholar]
- 22.Li Y., Nguyen D.N., de Waard M., Christensen L., Zhou P., Jiang P., et al. Pasteurization procedures for donor human milk affect body growth, intestinal structure, and resistance against bacterial infections in preterm pigs. J Nutr. 2017;147(6):1121–1130. doi: 10.3945/jn.116.244822. [DOI] [PubMed] [Google Scholar]
- 23.Correa K. de P., Silva M.E.T., Ribeiro O.S., Matta S.L.P., Peluzio M. do CG., Oliveira E.B., et al. Homogenised and pasteurised human milk: Lipid profile and effect as a supplement in the enteral diet of Wistar rats. Brit J Nutr. 2022;127(5):711–721. doi: 10.1017/S0007114521001380. [DOI] [PubMed] [Google Scholar]
- 24.Williamson S., Finucane E., Ellis H., Gamsu H.R. Effect of heat treatment of human milk on absorption of nitrogen, fat, sodium, calcium, and phosphorus by preterm infants. Arch Dis Child. 1978;53(7):555–563. doi: 10.1136/adc.53.7.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atkinson S.A., Heather Bryan M., Harvey Anderson G. Human milk feeding in premature infants: Protein, fat, and carbohydrate balances in the first two weeks of life. J Pediatr. 1981;99:617–624. doi: 10.1016/s0022-3476(81)80275-2. [DOI] [PubMed] [Google Scholar]
- 26.Thomaz A.C.P., Goncalves A.L., Martinez F.E. Effects of human milk homogenization on fat absorption in very low birth weight infants. Nutr Res. 1999;19(4):483–492. [Google Scholar]
- 27.de Oliveira S.C., Bellanger A., Ménard O., Pladys P., le Gouar Y., Dirson E., et al. Impact of human milk pasteurization on gastric digestion in preterm infants: A randomized controlled trial. Am J Clin Nutr. 2017;105(4):379–390. doi: 10.3945/ajcn.116.142539. [DOI] [PubMed] [Google Scholar]
- 28.Demers-Mathieu V., Huston R.K., Markell A.M., McCulley E.A., Martin R.L., Spooner M., Dallas D.C. Differences in maternal immunoglobulins within mother’s own breast milk and donor breast milk and across digestion in preterm infants. Nutrients. 2019;11(4):920. doi: 10.3390/nu11040920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Demers-Mathieu V., Huston R.K., Markell A.M., McCulley E.A., Martin R.L., Dallas D.C. Impact of pertussis-specific IgA, IgM, and IgG antibodies in mother’s own breast milk and donor breast milk during preterm infant digestion. Pediatr Res. 2021;89:1136–1143. doi: 10.1038/s41390-020-1031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demers-Mathieu V., Huston R.K., Markell A.M., McCulley E.A., Martin R.L., Dallas D.C. Antenatal influenza A-specific IgA, IgM, and IgG antibodies in mother’s own breast milk and donor bresst milk, and gastric contents and stools from preterm infants. Nutrients. 2019;11(7):1567. doi: 10.3390/nu11071567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andersson Y., Sävman K., Bläckberg L., Hernell O. Pasteurization of mother’s own milk reduces fat absorption and growth in preterm infants. Acta Paediatr. 2007;96(10):1445–1449. doi: 10.1111/j.1651-2227.2007.00450.x. [DOI] [PubMed] [Google Scholar]
- 32.Stein H., Cohen D., Herman A.A., Rissik J., Ellis U., Bolton K., et al. Pooled pasteurized breast milk and untreated own mother’s milk in the feeding of very low birth weight babies: a randomized controlled trial. J Pediatr Gastroenterol Nutr. 1986;5(2):242–247. [PubMed] [Google Scholar]
- 33.Söderhjelm L. Fat Absorption studies in children. Influence of heat treatment on milk on fat retention by premature infants. Acta Paediatr. 1952;41(3):207–221. doi: 10.1111/j.1651-2227.1952.tb17024.x. [DOI] [PubMed] [Google Scholar]
- 34.de Oliveira S.C., Bellanger A., Ménard O., Pladys P., le Gouar Y., Henry G., et al. Impact of homogenization of pasteurized human milk on gastric digestion in the preterm infant: A randomized controlled trial. Clin Nutr ESPEN. 2017;20:1–11. doi: 10.1016/j.clnesp.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Dupont D., Mackie A.R. Static and dynamic in vitro digestion models to study protein stability in the gastrointestinal tract. Drug Discov Today Dis Models. 2015;17(18):23–27. [Google Scholar]
- 36.Thuenemann E.C. The Impact of Food Bioactives on Health. Springer International Publishing; Cham: 2015. Dynamic Digestion Models: General Introduction; pp. 33–36. [Google Scholar]
- 37.Giribaldi M., Nebbia S., Briard-Bion V., Jardin J., Peila C., Coscia A., et al. Simulated dynamic digestion reveals different peptide releases from human milk processed by means of holder or high temperature-short time pasteurization. Food Chem. 2022;369 doi: 10.1016/j.foodchem.2021.130998. [DOI] [PubMed] [Google Scholar]
- 38.Nebbia S., Giribaldi M., Cavallarin L., Bertino E., Coscia A., Briard-Bion V., et al. Differential impact of Holder and High Temperature Short Time pasteurization on the dynamic in vitro digestion of human milk in a preterm newborn model. Food Chem. 2020;328 doi: 10.1016/j.foodchem.2020.127126. [DOI] [PubMed] [Google Scholar]
- 39.Inglingstad R.A., Devold T.G., Eriksen E.K., Holm H., Jacobsen M., Liland K.H., et al. Comparison of the digestion of caseins and whey proteins in equine, bovine, caprine and human milks by human gastrointestinal enzymes. Dairy Sci Technol. 2010;90(5):549–563. [Google Scholar]
- 40.Zhang J., Lee N.A., Duley J.A., Cowley D.M., Shaw P.N., Bansal N. Comparing the effects of hydrostatic high-pressure processing vs holder pasteurisation on the microbial, biochemical and digestion properties of donor human milk. Food Chem. 2022;373 doi: 10.1016/j.foodchem.2021.131545. [DOI] [PubMed] [Google Scholar]
- 41.de Oliveira S.C., Deglaire A., Ménard O., Bellanger A., Rousseau F., Henry G., et al. Holder pasteurization impacts the proteolysis, lipolysis and disintegration of human milk under in vitro dynamic term newborn digestion. Food Res Int. 2016;88:263–275. [Google Scholar]
- 42.Wada Y., Lönnerdal B. Bioactive peptides released from in vitro digestion of human milk with or without pasteurization. Pediatr Res Nature Publishing Group. 2015;77(4):546–553. doi: 10.1038/pr.2015.10. [DOI] [PubMed] [Google Scholar]
- 43.Deglaire A., de Oliveira S.C., Jardin J., Briard-Bion V., Emily M., Ménard O., et al. Impact of human milk pasteurization on the kinetics of peptide release during in vitro dynamic term newborn digestion. Electrophoresis. 2016;37(13):1839–1850. doi: 10.1002/elps.201500573. [DOI] [PubMed] [Google Scholar]
- 44.Deglaire A., Oliveira S de, Jardin J., Briard-Bion V., Kroell F., Emily M., et al. Impact of human milk pasteurization on the kinetics of peptide release during in vitro dynamic digestion at the preterm newborn stage. Food Chem. 2019;281:294–303. doi: 10.1016/j.foodchem.2018.12.086. [DOI] [PubMed] [Google Scholar]
- 45.Vincent M., Ménard O., Etienne J., Ossemond J., Durand A., Buffin R., et al. Human milk pasteurisation reduces pre-lipolysis but not digestive lipolysis and moderately decreases intestinal lipid uptake in a combination of preterm infant in vitro models. Food Chem. 2020;329 doi: 10.1016/j.foodchem.2020.126927. [DOI] [PubMed] [Google Scholar]
- 46.Zhang L., Qu J., Huppertz T., Liu J., Sun Z., Zhou P. Effects of different freeze-thaw processes on the bioactivity and digestibility of human milk. LWT. 2022;156 [Google Scholar]
- 47.Pitino M.A., Unger S., Doyen A., Pouliot Y., Aufreiter S., Stone D., et al. High hydrostatic pressure processing better preserves the nutrient and bioactive compound composition of human donor milk. J Nutr. 2019;149(3):497–504. doi: 10.1093/jn/nxy302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wesolowska A., Brys J., Barbarska O., Strom K., Szymanska-Majchrzak J., Karzel K., et al. Lipid profile, lipase bioactivity, and lipophilic antioxidant content in high pressure processed donor human milk. Nutrients. 2019;11(9):1972. doi: 10.3390/nu11091972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koh J., Victor A.F., Howell M.L., Yeo J.G., Qu Y., Selover B., et al. Bile salt-stimulated lipase activity in donor breast milk influenced by pasteurization techniques. Front Nutr. 2020;7 doi: 10.3389/fnut.2020.552362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xia Q., Liu Q., Denoya G.I., Yang C., Barba F.J., Yu H., et al. High hydrostatic pressure-based combination strategies for microbial inactivation of food products: the cases of emerging combination patterns. Front Nutr. 2022;9 doi: 10.3389/fnut.2022.878904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sherman M.P. Lactoferrin and necrotizing enterocolitis. Clin Perinatol. 2013;40(1):79–91. doi: 10.1016/j.clp.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Friend L.L., Perrin M.T. Fat and protein variability in donor human milk and associations with milk banking processes. Breastfeeding Med. 2020;15(6):370–376. doi: 10.1089/bfm.2020.0046. [DOI] [PubMed] [Google Scholar]
- 53.Pitino M.A., Unger S., Gill A., McGeer A.J., Doyen A., Pouliot Y., et al. High pressure processing inactivates human cytomegalovirus and hepatitis A virus while preserving macronutrients and native lactoferrin in human milk. Innov Food Sci Emerg Tech. 2022;75 [Google Scholar]
- 54.Zamora A., Guamis B. Opportunities for ultra-high-pressure homogenisation (UHPH) for the food industry. Food Eng Rev. 2015;7(2):130–142. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Primary data extracted for the purposes of this review can be made available upon request.