Abstract
Nut consumption is not associated with a higher body weight, and potential energy-regulating mechanisms may include a reduced subsequent energy intake and increased EE. The aim of this study was to examine the effect of tree nut and peanut consumption on energy intake, compensation, and expenditure. PubMed, MEDLINE, CINAHL, Cochrane, and Embase databases were searched from inception to June 2, 2021. Human studies with adults aged ≥18 y older were included. Energy intake and compensation studies were restricted to acute effects (intervention duration of ≤24 h), whereas intervention duration was not limited for EE studies. Random effects meta-analyses were conducted to explore weighted mean differences in REE. Twenty-eight articles from 27 studies (16 energy intake studies, 10 EE studies, and 1 study investigating both) with 1121 participants were included in this review, with a variety of nut types addressed (almonds, Brazil nuts, cashews, chestnuts, hazelnuts, peanuts, pistachios, walnuts, and mixed nuts). Energy compensation occurred after nut-containing loads (range: −280.5% to +176.4%) and the degree of compensation varied depending on the form (whole and chopped) and how they were consumed (alone and within a meal). The meta-analyses identified a nonsignificant increase in REE associated with nut consumption (weighted mean difference: 28.6 kcal/d; 95% CI: −10.7, 67.8 kcal/d). This study provided support for energy compensation as a potential mechanism for a lack of association between nut consumption and body weight, whereas no evidence was found for EE as an energy-regulating mechanism of nuts.
This review was registered at PROSPERO as CRD42021252292.
Keywords: nuts, tree nuts, peanuts, energy compensation, energy intake, energy expenditure, systematic review, meta-analysis
Introduction
Obesity is a prevalent issue in Australia and around the world [1,2]. Body weight is maintained by achieving energy balance, where energy intake and EE are equal. Weight gain typically occurs when energy intake exceeds EE, and the opposite is often true for weight loss. Energy intake from food and beverages is in part regulated in the body by appetite, hunger, and fullness sensations, with different foods and beverages affecting these sensations in varied ways. Daily EE comprises REE and the TEF and exercise [3]. Of these components, basal metabolic rate is the largest component of REE and is defined as the minimum energy required for vital body functions, such as respiration and circulation [4] and is usually measured during morning sleep to capture a true basal metabolic rate [3]. By contrast, REE is defined as the energy expended while resting and is measured as such [5]. Therefore, the terms basal metabolic rate and REE are often used interchangeably in the literature.
Tree nuts and peanuts are energy and nutrient dense [6]. Because of their demonstrated health benefits, regular consumption of 30 g/d of nuts is recommended [[7], [8], [9], [10], [11], [12]]. Moreover, the evidence suggests that nut consumption is associated with a lower body weight, despite their energy density [[13], [14], [15]]. Observational studies have shown that habitual nut consumption is inversely associated with weight gain and obesity risk [16,17]. A recent meta-analysis of prospective cohorts and randomized controlled trials (RCTs) combined these findings showing that nut consumption is associated with a lower incidence of overweight or obesity and a lower body weight [15]. Furthermore, the way that nuts are included in an eating pattern may affect the results. A recent systematic review has found that participants decreased their body fat in studies that instructed nuts to be substituted into the eating pattern, yet no change in participant body weight was found in studies which did not provide substitution instructions [14]. An understanding of the reasons for this difference is not well established.
Studies have been conducted to understand the underlying mechanisms responsible for the lack of association between nut intake and body weight. Nuts have a hunger-suppressing effect, yet their consumption does not promote fullness [13,18]. Hence, it is thought that the energy contained within nuts may be compensated for at later eating events [[19], [20], [21]]. That is, a reduction in energy intake may be provoked by the previous consumption of nuts. Energy compensation can be acute, with the effects measured at the next meal or for the remainder of the day, whereas chronic effects can be measured over a longer period of weeks or months.
Several studies have suggested that regular nut consumption may increase EE [[22], [23], [24], [25]], although the results are not conclusive. Mechanisms that may explain this potential increase in EE include the high protein and fat content of nuts and the fatty acid profile of nuts, consisting of mostly unsaturated fatty acids, which are preferentially oxidized over saturated fatty acids [22,23]. Further to this, the effect of nut consumption on EE can be measured over a short period (as TEF) or long period (as REE).
Although studies have examined the effects of nut consumption on energy intake compensation and EE, the studies need to be synthesized to enhance our understanding of the mechanisms of all tree nuts and peanuts. Therefore, the aim of this study was to synthesize the body of evidence on the effect of tree nut and peanut consumption on energy compensation (acute effects) and EE (acute and chronic effects).
Materials and Methods
Search strategy
This systematic review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines [26] and registered with PROSPERO (https://www.crd.york.ac.uk/prospero/, CRD42021252292). Five scientific databases—PubMed, MEDLINE (EBSCO), CINAHL (EBSCO), Cochrane CENTRAL, and Embase (Elsevier)—were searched from inception through to June 2, 2021 by 1 reviewer (CJN). Although MEDLINE is a subset of PubMed, both databases were searched to ensure that recent studies were captured, following the recommendations by Rosen and Suhami [27]. There were no restrictions to the language or date published. Alternative spelling, phrases, and truncations were included in the search strings, using both free-text search terms and relevant controlled vocabulary. The search strings were piloted, including testing whether searches identified sentinel articles that were previously recognized as being likely to be eligible for the review [19,23,25,28]. The search strings used in all 5 databases are provided in Supplementary Material 1. After the search, backward and forward citation searching of eligible articles was conducted using citationchaser [29].
Selection criteria
RCTs (including feeding studies) with either a crossover or parallel design were eligible for inclusion in this review. Studies needed to meet the following criteria: include adults aged ≥18 y; explore the consumption of almonds, Brazil nuts, cashews, chestnuts, hazelnuts, macadamias, pecans, pine nuts, pistachios, walnuts, and/or peanuts, in the form of whole nuts, chopped nuts, nut butters or nut flours, or nut-containing foods where the results could be isolated to nuts; and assess acute energy compensation or acute energy intake (in kilojoules or calories) or EE (as basal metabolic rate, REE, TEE, TEF, or diet-induced thermogenesis). Acute energy compensation and energy intake were defined as energy intake measured for a maximum of a 24-h period and could be explored at the subsequent meal after nut consumption, the remainder of the day, or during a whole 24-h period. This review was restricted to acute energy compensation and energy intake to understand the effects of nut intake in a controlled setting without the influence of other factors (such as lifestyle and overall diet) that could interfere with energy compensation/intake findings in chronic studies. Studies were excluded if they were conducted with children or animals; investigated coconuts (owing to differences in nutrient composition when compared with tree nuts and peanuts), nut oils, and nut milks; and were of a systematic review or in vitro design.
Screening and data extraction
Database searches and backward and forward citation searching were conducted by 1 reviewer (CJN). Title/abstract and full-text screening were performed independently by 2 reviewers (CJN screened all search results and YCP and S-YT, each screened half of all results) using Covidence systematic review software (Veritas Health Innovation; www.covidence.org). Conflicts that arose at either the title/abstract or full-text stages were resolved by discussion until consensus was reached among the 3 reviewers (CJN, YCP, and S-YT), with an additional reviewer (EPN) consulted as required. Separate summary tables were created for the energy compensation and EE findings and included the study details, such as study design, country, population, sample size, mean body mass index (BMI) and mean age of participants, control and interventional diets, nut type, nut form, nut dose, intervention duration, measurement of energy intake/expenditure, and outcomes (i.e., energy intake and EE). Study details were extracted by one reviewer (CJN) and source data was verified [30] by a second reviewer (EPN). WebPlotDigitizer online software [31] was used to extract numeric values of results that were presented in a graphical format only. Attempts were made to contact the study authors to retrieve data that were not provided in the published articles.
Where study authors calculated energy compensation, these values were reported in the summary tables. In case authors did not calculate energy compensation, energy compensation was calculated by the review authors. Two authors (CJN and S-YT) reviewed the energy compensation formula developed by Kirkmeyer and Mattes [18], which was applied by CJN using a Microsoft Excel (version 16, Microsoft Corporation, 2021) template (Supplementary Material 2) to calculate the energy compensation in remaining studies. The energy provided by nuts in the intervention was a required component of this calculation, and when it was not provided by the authors, the Australian Food and Nutrient Database (AUSNUT) 2011–2013 Food Nutrient Database [32] was used to determine the energy content of nuts. If the AUSNUT 2011–2013 Food Nutrient Database did not provide data for a particular nut type or form (for example, hazelnut flour), then a food product label was used to determine the energy content. Calculating energy compensation was necessary to compare the effects between studies that varied between study designs.
Based on the formula, the energy compensation values can be interpreted as the percentage of energy from nuts being compensated in subsequent energy intake. Negative values indicate not only a lack of energy compensation but also a nut-promoted energy intake. Values between 0% and +100% were deemed to indicate partial energy compensation, whereby, although the energy intake did increase, the increase was less than the energy provided by the nut addition. A value of +100% indicated complete compensation, namely no change in energy intake after nut addition. Values above +100% indicated energy compensation was beyond the energy provided by the nuts and, therefore, resulted in lower subsequent energy intake (i.e., overcompensation).
Because of variation in outcomes and measurement methods, synthesis via meta-analysis was only considered appropriate for the studies that reported the effects of nut consumption on REE or basal metabolic rate (treated as REE for the synthesis). As mentioned previously, the terms basal metabolic rate and REE are often used interchangeably. For the purpose of this review and meta-analysis, the terms were combined and treated as REE. For the meta-analysis, the mean values, standard deviation, and sample size of studies examining the effect of nut consumption on REE were extracted as recommended by the Cochrane Handbook for Systematic Reviews of Interventions [33]. Change values for REE were extracted where published; otherwise, final values were obtained. Study authors were contacted to retrieve or confirm data if the article did not provide sufficient information. Where standard error was provided instead of standard deviation, the values were transformed to standard deviation using the formula provided in the Cochrane Handbook for Systematic Reviews of Interventions (chapter 6.5.2.2) [33]. Where a study included more than 1 relevant intervention arm, data for the intervention arms were combined [33].
Statistical analyses
Random effects meta-analyses were conducted using Review Manager (RevMan Computer Program, version 5.4; the Cochrane Collaboration, 2020). Weighted mean differences (WMDs) with 95% confidence intervals were determined for change or final values for REE. Acute studies were not included in the analyses owing to the variation in outcomes. Studies were included in the meta-analyses if they measured REE in a chronic context and had sufficient data. Data from the first intervention period of crossover studies was used for comparability with parallel study data, as recommended by the Cochrane Handbook for Systematic Reviews of Interventions [33].
The proportion of total variation attributable to between-study heterogeneity was estimated using the I2 statistic, which was generated for each analysis. An I2 value of at least 75% indicates a high level of inconsistency, based on recommendations by Higgins et al. [34]. Funnel plots were deemed inappropriate owing to the low number of studies (<10) included. Sensitivity analyses were conducted to further explore the results. Where studies had several intervention arms that could be considered controls, for the purpose of this analysis, the data from the nonnut intervention arms were combined with the control arm data to observe the effect of additional controls. A leave-one-out sensitivity analysis was conducted to observe the effect of removing a single study at a time on the outcomes.
Quality assessment
Quality appraisal of the included studies was performed independently by 2 reviewers (CJN, EPN) using the Academy of Nutrition and Dietetics Quality Criteria Checklist—Primary Research [35]. Any disagreements between the reviewers were resolved by discussion to consensus.
Results
Study characteristics and quality
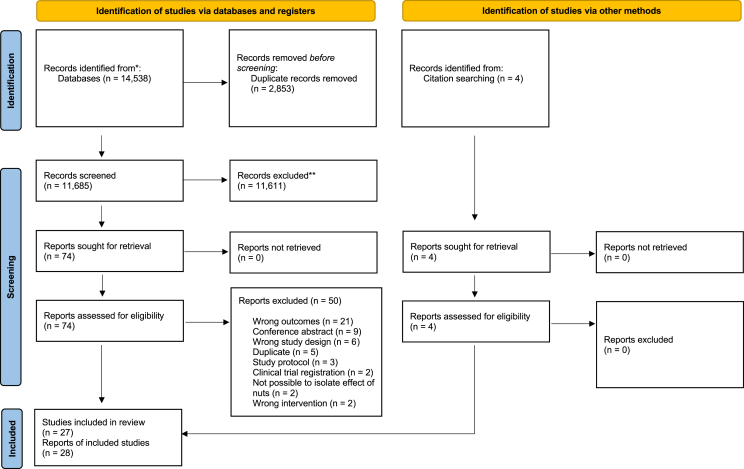
A total of 14,538 articles were identified across the 5 databases. After the removal of duplicate articles and excluded studies, 24 articles were identified as eligible. An additional 4 articles were identified after forward and backward citation searching, bringing the total number of articles included in this review to 28, derived from 27 studies [18,19,22,24,25,[36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58]]. Figure 1 shows the study selection process. Of note, 2 studies [38,59] were excluded as they measured EE through methods other than indirect calorimetry: in this case, by estimating EE using physical activity records. There were 16 studies that reported energy compensation [18,38,[40], [41], [42], [43],[45], [46], [47], [48],[50], [51], [52], [53], [54], [55]], 10 studies that reported EE [19,22,24,25,39,44,49,[56], [57], [58]], and 1 study (2 articles) that investigated both [36,37]. TABLE 1, TABLE 2 present the characteristics of the energy compensation studies and EE studies, respectively.
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of the study selection protocol.
TABLE 1.
Characteristics of the 17 included studies examining the effect of tree nut and peanut consumption on energy intake and/or energy compensation in adults aged ≥18 y
| Reference; country | Study design | Population; mean BMI (kg/m2); mean age (y) | Sample size (completers) | Control diet | Intervention diet | Nut type, form, and dose | Duration of measurement | Food intake measurement | Quality appraisal |
|---|---|---|---|---|---|---|---|---|---|
| Alves et al. [37]; Brazil | RCT | Men with overweight or obesity; 29.8; 27.1 | 71 (100% M, 0% F) | Participants consumed milkshake and biscuits (isocaloric to intervention and matched macronutrient content) | Participants consumed milkshake and peanuts | 1: High-oleic peanuts (unpeeled roasted); 56 g | 1 d | Completed food record for remainder of day. After study protocol, a 750 kcal meal was offered (sandwich, juice, and apple) | Positive |
| 2: conventional peanuts (unpeeled roasted); 56 g | |||||||||
| Barbour et al. [38]; Australia | RCT (crossover) | Healthy adults with overweight or obesity; 30 (M) and 31 (F); 62 (M) and 61 (F) | 24 (54% M, 46% F) | Participants provided with snack (15%–20% of daily energy intake): unsalted potato crisps (M: 90 g, F: 60 g) | Participants provided with snack (15%–20% of daily energy intake): high-oleic peanuts or regular peanuts | 1: High-oleic peanuts (roasted unsalted) | 180 min | Buffet meal contained preweighed food and beverages to calculate energy intake | Positive |
| 2: Regular peanuts (roasted unsalted) | |||||||||
| 84 g (M) and 56 g (F) | |||||||||
| Brown et al. [40]; New Zealand | RCT (crossover) | Healthy; 23.1; median age 29 | 100 (25% M, 75% F) | Standard breakfast (muesli, yogurt, milk) providing ∼20% of energy requirements. Snack of sweet biscuits consumed 2 h after breakfast | Standard breakfast providing ∼20% of energy requirements. Snack of almonds consumed 2 h after breakfast (isocaloric to control) | Almonds (raw): 10% of participant energy requirements or 42.5 g (1030 kJ), whichever provided more energy | 120 min; then food records for remainder of test day | Unlimited lunch of unevenly cut sandwiches. Intake calculated by subtracting leftovers from the amount presented. Participants recorded all food and drinks consumed for the remainder of the day using paper diaries (weighed records) | Positive |
| Burton-Freeman [41]; USA | RCT (crossover) | Healthy; 23; 33 (men, n = 12) and 30 (women, n = 13) | 25 (48% M, 52% F) | Consumption of either: control shake (low-fat, 4% energy as fat); or shake containing 12.5 g safflower oil (39% energy as fat) M: 300 g (1254 kJ), F: 225 g shake (941 kJ) |
1: Walnut shake (39% energy as fat) | Walnuts (finely ground, or oil, as part of a shake) | 45 min; then food records for remainder of test day | Participants provided with a preweighed lunch tray and completed food records on the test days | Positive |
| 2: Walnut oil shake (39% energy as fat) | 1: Shake containing 20 g finely ground walnuts | ||||||||
| M: 300 g (1254 kJ), F: 225 g shake (941 kJ) | 2: Shake containing 12.5 g walnut oil1 | ||||||||
| Burton-Freeman et al. [42]; USA | RCT (crossover) | Healthy; 25 (men, 8) and 23 (women, 7); 30 (M) and 35 (F) | 15 (53% M, 47% F) | Breakfast meal including muffin made with safflower and corn oil. Test meal provided approximately one-third of average daily energy of men (4.1 MJ) and women (3.2 MJ) (isocaloric to intervention) | Breakfast meal including either whole almonds or a muffin made with almond oil. Test meal provided approximately one-third of average daily energy of men (4.1 MJ) and women (3.2 MJ) | 1: Whole almonds; 40 g (men) and 28 g (women) | 6 h | Participants were offered a tray containing preweighed food (fruit, cookies, chips, nuts, drinks etc.). Participants kept half-day food records (after study period until midnight of same day) | Positive |
| 2: Almond oil; weight of muffin containing almond oil: 110 g (men) and 75 g (women) | |||||||||
| Carughi et al. [43]; France | RCT (parallel) | Healthy women; 21.6; 35 | 60 (0% M, 100% F) | Afternoon snack of 56 g of “Gouda aperitif biscuits” (isocaloric to intervention) | Afternoon snack of 56 g pistachios | Pistachios (roasted, lightly salted, in-shell); 56 g | 1 d2 | Participants kept a food diary | Positive |
| Costa et al. [45]; Brazil | RCT (crossover) | Adults with overweight or obesity; 30.9; 29.0 | 15 (33% M, 67% F) | Shake without nuts (isocaloric to intervention, matched macronutrient content) | Shake containing nuts. Nuts crushed to give similar texture to control | 30 g cashews (ground) and 15 g Brazil nuts (ground) | 4 h; then food records for remainder of test day | Served an unlimited lunch after shake, weighed food before and after lunch consumption. Intake of remaining day (after lunch) estimated through food record | Positive |
| Devi et al. [46]; New Zealand | RCT (crossover) | Healthy; 24.08; 30.2 | 32 (34% M, 66% F) | Replace normal bread consumption at breakfast with white bread | Replace normal bread consumption at breakfast with 1 of the 3 test breads: bread containing slices hazelnuts, bread containing semi-defatted hazelnut flour, or bread containing a combination of both | Hazelnuts: | 1 d3 | Participants recorded a weighed food diary on the test day | Positive |
| 1: finely sliced | |||||||||
| 2: semi-defatted flour | |||||||||
| 3: combination of the 2 | |||||||||
| 1: 30 g/120 g bread | |||||||||
| 2: 30 g/120 g bread | |||||||||
| 3: 15 g each sliced and flour per 120 g bread | |||||||||
| Devitt et al. [47]; USA, Ghana and Brazil | RCT (parallel)4 | Healthy Americans, Ghanaians, and Brazilians; 23.1 (USA n = 63), 21.9 (Ghana n = 78), and 22.9 (Brazil n = 60); 22 (USA), 25 (Ghana), and 24 (Brazil) | 201 (50% M, 50% F) | The snack mix was presented at 2 eating occasions (with a lunch meal, “meal”; or alone 120 min after the provided lunch, “snack”) on separate days | One of the 2 treatment arms: peanuts or snack mix with peanuts. Presented as per control protocol. Treatments and control isocaloric | 1: peanuts (52 g) | 1 d | Participants kept a food record for the whole of the test day | Positive |
| 2: snack mix with peanuts (containing 26 g peanuts) | |||||||||
| 1255 kJ per treatment | |||||||||
| Fantino et al. [48]; France | RCT (crossover) | Healthy premenopausal women; 23.425 and 23.606; 335 and 306 | 57 (0% M, 100% F) | No mid-morning snack | Mid-morning snack of pistachios | Pistachios (shelled, slightly salted); 44 g | 2 h; then food records for remainder of test day7 | Amounts of lunch foods were weighed and recorded, plate waste was measured. Then, participants kept food diaries for remainder of the day | Positive |
| Hollingworth et al. [50]; UK | RCT (crossover) | Healthy; 22.0; 26.0 | 42 (0% M, 100% F) | Mid-morning snack of either water or savory crackers8 | Mid-morning snack of almonds | Almonds (raw): 0.9 g per kilogram of body weight | 1 d | All foods measured preconsumption and postconsumption to determine energy intake (from unlimited lunch, dinner, and snack box) | Positive |
| Hull et al. [51]; UK | RCT (crossover) | Healthy; 22.7; 48.4 | 32 (0% M, 100% F) | Mid-morning snack of water only. | Mid-morning snack of water and almonds | Almonds (raw whole); 28 g (half-dose) or 42 g (full dose) | 1 d | Unlimited lunch and dinner weighed before and after consumption to calculate energy intake | Positive |
| Johnston and Buller [52]; USA | RCT (crossover) | Healthy; 22.7; 27.9 | 11 (9% M, 91% F) | Participants consumed first test meal (bagel and juice) with sweetened water (with or without apple cider vinegar added). This procedure was repeated for a second test meal (teriyaki chicken and rice) | As per control diet, except peanut butter on bagel and roasted peanuts in chicken meal. Added peanut butter or peanuts did not change energy content | Peanut butter and peanuts (roasted); 25 g each | 1 d | Food records for remainder of day completed by participants | Neutral |
| Johnston, Trier and Fleming [53]; USA | RCT (crossover) | Healthy; 23.1; 28.4 | 15 (13% M, 87% F) | Participants consumed either a cup of water only, or a grain bar and water, followed by a standardized meal (buttered bagel and juice) 60 min later | As per control diet, except participants consumed peanuts (isocaloric to grain bar) and water. Consumed same standardized meal 60 min later | Peanuts; 23 g | 1 d | Participants asked to record all food and beverages consumed on the test day | Positive |
| Kirkmeyer and Mattes [18]; USA | RCT (crossover): Within-subject design | Healthy; BMI not reported (normal weight, 12%–28% body fat); 22 | 24 (50% M, 50% F) | Consumed a preload of either: milk chocolate, dill pickles, salt-free and fat-free rice cakes, or no load | Consumed a preload of either: peanuts, peanut butter, almonds, or chestnuts | 1: peanuts (unsalted) | 1 d | Participants trained in diet records before the study. Participants used a standard form to record intake for 24 h before and after preload | Positive |
| 2: peanut butter (low sodium) | |||||||||
| 3: almonds (bulk, raw) | |||||||||
| 4: chestnuts (whole, in water, rinsed and baked) | |||||||||
| 500 kcal portion: 87.5 g peanuts, 70.8 g PB, 80.4 g almonds, 235.8 g chestnuts | |||||||||
| Reis et al. [54]; Brazil | RCT (crossover) | Healthy; 22.7; 28.5 | 13 (31% M, 69% F) | Control meal: cheese sandwich with 200 mL water. Matched to intervention meals for energy and macronutrient content | One of the 3 intervention meals (peanuts) with 200 mL water | 1: peanuts (raw, with skin); 63 g | 24 h after test meal | Participants kept free-feeding dietary records over the 24 h after test meal consumption | Positive |
| 2: peanuts (roasted, without skin); 63 g | |||||||||
| 3: peanuts (roasted, ground, without skin); 63 g | |||||||||
| Reis et al. [55]; Brazil | RCT (crossover) | Adults with obesity; 32.36; 35.33 | 13 (0% M, 100% F) | Breakfast contained orange juice and cereal (not isocaloric to interventions) | Breakfast with the addition of either whole peanuts or peanut butter | 1: peanuts (whole, no skins; WP); 42.5 g | 4 h; then food records for remainder of test day | Standard lunch provided. Participants were trained to record food, and recorded food intake before the study (baseline eating habits). After leaving the laboratory on test day, participants recorded all food and drink consumed for the rest of the day | Positive |
| 2: PB; 42.5 g |
F, female; M, male; PB, peanut butter; WP, whole peanuts.
Study includes additional arm of shake containing 12.5 g safflower oil, not reported here.
Part of a 4-wk study.
Part of an 8-d study.
All participants received control (isoenergetic portion of experimental lunch) and 1 of the 3 treatment arms: peanuts, snack mix, or snack mix with peanuts. Study treated as a parallel study with snack mix treated as control owing to similar macronutrient content to peanuts.
Control arm, n = 30.
Intervention arm, n = 30.
Part of a 12-wk study.
Cracker arm treated as a control because isocaloric to almonds.
TABLE 2.
Main findings of the 17 included studies examining the effect of tree nut and peanut consumption on energy intake and/or energy compensation in adults aged ≥18 y
| Reference; country | Energy intake (control diet) | Energy intake (intervention diet) | Energy compensation |
|---|---|---|---|
| Alves et al. [37]; Brazil | Data not provided | Data not provided | +102.3% (control)2; +84.0% (HOP)2; +134.2% (CVP).2 No significant difference between groups |
| Energy compensation: HOP: 84.0 (SEM: 23.2); CVP: 134.2 (SEM: 22.7). No significant difference between groups | |||
| Barbour et al. [38]; Australia | Energy intake at buffet meal 3 h after snack: 3031 kJ (SEM: 241) (P < 0.005 vs. both peanut meals) | Energy intake at buffet meal 3 h after snack: high-oleic peanut: 2380 kJ (SEM: 196) (P < 0.001 vs. control) | +39.2% (HOP)3; +30.6% (CVP)3 |
| Regular peanut snack: 2498 kJ (SEM: 186) (P < 0.005 vs. control) | |||
| Brown et al. [40]; New Zealand | Unlimited lunch intake: 2898 (SD: 1329) kJ (P > 0.05 vs. intervention) | Unlimited lunch intake: 2820 (SD: 1343) kJ (P > 0.05 vs. control) | +56.9%3 |
| Remaining energy intake (from diaries): 5200 (SD: 2676) kJ (P > 0.05 vs. intervention) | Remaining energy intake (from diaries): 4658 (SD: 2517) kJ (P > 0.05 vs. control) | ||
| Intake from lunch and remaining day combined: 8132 (SD: 3044) kJ (P < 0.05 vs. intervention) | Intake from lunch and remaining day combined: 7494 (SD: 2589) kJ (P < 0.05 vs. control) | ||
| Burton-Freeman [41]; USA | Low-fat shake | Ground walnut shake | −55.3%3 |
| Energy intake at lunch: 3014 (SEM: 134) kJ (P = 0.09 vs. ground walnut shake; P = 0.02 vs. walnut oil shake) | Energy intake at lunch: 3340 (SEM: 134) kJ (P = 0.09 vs. low-fat shake) | ||
| Total daily energy intake: 10.010 (SEM: 0.476) MJ/d (P > 0.05 vs. all shakes) | Total daily energy intake: 10.218 (SEM: 0.477) MJ/d (P > 0.05 vs. all shakes) | ||
| Safflower oil shake | Walnut oil shake | ||
| Energy intake at lunch: 3198 (SEM: 134) kJ | Energy intake at lunch: 3457 (SEM: 138) kJ (P = 0.02 vs. low-fat shake) | ||
| Total daily energy intake: 10.308 (SEM: 0.506) MJ/d (P > 0.05 vs. all shakes) | Total daily energy intake: 10.695 (SEM: 0.566) MJ/d (P > 0.05 vs. all shakes) | ||
| Burton-Freeman, Davis and Schneeman [42]; USA | Poststudy energy intake | Poststudy energy intake Whole almonds: 8.4 (SEM: 0.7) MJ Almond oil: 6.5 (SEM: 0.7) MJ NS differences among treatment and control arms |
−280.5%3 |
| 7.8 (SEM: 0.7) MJ | |||
| Carughi et al. [43]; France | Postsnack intake: 2216 (SD: 871) kJ Daily energy intake: 7383 (SD: 1510) kJ |
Postsnack intake: 2403 (SD: 941) kJ | +168.0%3 |
| Daily energy intake: 7860 (SD: 1843) kJ | |||
| Both NS vs. control | |||
| Costa et al. [45]; Brazil | Unlimited lunch intake: 923.0 (SEM: 385.0) kcal (P > 0.05 vs. intervention) | Unlimited lunch intake: 963.1 (SEM: 298.8) kcal (P > 0.05 vs. control) | −42.1%3 |
| Food intake postlunch: 1012.6 (SEM: 462.9) kcal (P > 0.05 vs. intervention) | Food intake postlunch: 1089.3 (SEM: 573.3) kcal (P > 0.05 vs. control) | ||
| Devi et al. [46]; New Zealand | Total daily energy intake: 8832 (95% CI: 7376, 10,288) kJ | Total daily energy intake | +16.5% (sliced hazelnuts)3; −11.2% (hazelnut flour)3; +149.4% (combination)3 |
| Sliced: 8699 (95% CI: 7735, 9664) kJ | |||
| Flour: 8904 (95% CI: 8034, 9773) kJ | |||
| Combination: 7749 (95% CI: 6730, 8767) kJ | |||
| NS between breads | |||
| Devitt et al. [47]; USA, Ghana and Brazil | Daily energy intake during snack mix sessions (n = 68): 10,142 (SE: 276) kJ | Daily energy intake during peanut sessions (n = 66): 10376 (SE: 276) kJ | Combining meal and snack sessions: −2.6% (peanuts)3; +20.0% (peanuts in snack mix)3 |
| Daily energy intake during snack mix with peanuts sessions (n = 67): 10293 (SE: 276) kJ | |||
| NS between arms | |||
| Fantino et al. [48]; France | Lunch intake: 677.2 (SEM: 17.3) kcal | Lunch intake: 611.6 (SEM: 17.5) kcal (P < 0.001 vs. control) | +40.5%3 |
| Dinner intake: 717.2 (SEM: 31.6) kcal | Dinner intake: 681.6 (SEM: 29.4) kcal (P > 0.05 vs. control) | ||
| Total daily intake: 1822.4 (SEM: 41.2) kcal1 | Total daily intake: 1721.1 (SEM: 39.5) kcal (P < 0.01 vs. control)1 | ||
| Hollingworth et al. [50]; UK | Water | Almonds Lunch intake: 1007.3 (SD: 299.1) kcal (P < 0.05 vs. water, P > 0.05 vs. cracker) Total daily energy intake: 2992.0 (SD: 654.2) kcal (P > 0.05 vs. water, P > 0.05 vs. cracker) |
+37.3%3 |
| Lunch intake: 1143.6 (SD: 347.4) kcal (P < 0.05 vs. crackers and almonds) | |||
| Total daily energy intake: 2797.2 (SD: 728.2) kcal (P < 0.05 vs. water; P > 0.05 vs. almonds) | |||
| Crackers | |||
| Lunch intake: 1019.6 (SD: 345.6) kcal (P < 0.05 vs. water; P > 0.05 vs. almonds) | |||
| Total daily energy intake: 2990.6 (SD: 748.9) kcal (P < 0.05 vs. water; P > 0.05 vs. almonds) | |||
| Hull et al. [51]; UK | L: 764.1 (SEM: 23.3) kcal D: 1060.0 (SEM: 41.7) kcal Total: 2168.5 (SEM: 59.7) kcal (P > 0.05 vs. interventions) |
28 g Almonds | +72.1% (28 g almonds)3; +114.7% (42 g almonds)3 |
| L: 698.0 (SEM: 31.3) kcal (P < 0.05 vs. control and 42 g) | |||
| D: 1002.1 (SEM: 36.0) kcal (P > 0.05 vs. control, P < 0.05 vs. 42 g) | |||
| Total daily EI: 2216.8 (SEM: 63.6) kcal (P > 0.05 vs. control and 42 g) | |||
| 42 g almonds | |||
| L: 622.06 (SEM: 30.2) kcal (P < 0.05 vs. control and 28 g) | |||
| D: 907.1 (SEM: 36.3) kcal (P < 0.05 vs. control and 28 g) | |||
| Total daily EI: 2130.3 (SEM: 51.5) kcal (P > 0.05 vs. control and 28 g) | |||
| Johnston and Buller [52]; USA | Data not provided | Data not provided | Data not sufficient to calculate compensation |
| Johnston, Trier and Fleming [53]; USA | Total daily energy intake: | Total daily energy intake Peanuts: 1771 (SEM: 149) kcal (P > 0.05 vs. control and grain bar) |
+176.4%3 |
| Control: 1878 (SEM: 209) kcal (P > 0.05 vs. grain bar and peanuts) | |||
| Grain bar: 1756 (SEM: 144) kcal (P > 0.05 vs. control and peanuts) | |||
| Kirkmeyer and Mattes [18]; USA | Daily energy intake (no load): 7905 (SEM: 693) kJ (P > 0.05 vs. interventions) | Daily energy intake | +104% (peanuts)2; +151% (PB)2; +57% (almonds)2; +57% (chestnuts)2 These values are not significantly different |
| Peanuts: 9372 (SEM: 591) kJ | |||
| PB: 8903 (SEM: 611) kJ | |||
| Almonds: 10,024 (SEM: 713) kJ | |||
| Chestnuts: 9616 (SEM: 673) kJ | |||
| These values are not significantly different | |||
| Reis et al. [54]; Brazil | Energy intake in 24 h after test meal: 1738.40 (SEM: 125.91) kcal NS differences among control and intervention arms |
Energy intake in 24 h after test meal | +19.2% (raw peanuts)3; +30.2% (roasted peanuts)3; +18.1% (ground roasted peanuts)3 |
| Raw: 1724.75 (SEM: 93.78) kcal | |||
| Roasted: 1684.75 (SEM: 96.58) kcal | |||
| Ground: 1728.76 (SEM: 109.59) kcal | |||
| NS differences among control and intervention arms | |||
| Reis et al. [55]; Brazil | Daily energy intake: 7842 (SD: 1696) kJ (P > 0.05 vs. interventions) | WP Daily energy intake: 8772 (SD: 2334) kJ | −76.4% (peanuts)3; −21.8% (PB)3 |
| PB Daily energy intake: 8136 (SD: 1620) kJ | |||
| Both arms P > 0.05 vs. control |
CVP, conventional peanut; D, dinner; HOP, high-oleic peanut; L, lunch; NS, not significant; PB, peanut butter; WP, whole peanuts.
Energy provided by the snacks excluded.
Compensation calculated by the author of the study.
Compensation calculated by the reviewer.
The study quality ranged from neutral (n = 2) [19,52] to positive (n = 25). The reasons for neutral quality scores included authors not discussing the study limitations and not disclosing conflicts of interests or funding sources.
Energy intake/compensation studies
All studies that examined the effect of nut consumption on energy intake or compensation (n = 17) were of an RCT design with either crossover or parallel arms and were conducted in countries including the USA [18,41,42,47,52,53], Brazil [37,45,47,54,55], France [43,48], the UK [50,51], New Zealand [40,46], Australia [38], and Ghana [47] (Table 1).
The sample size ranged from 11 to 201 participants across the 17 studies. Eleven studies [18,38,[40], [41], [42],[45], [46], [47],[52], [53], [54]] included both adult men and women, 5 studies [43,48,50,51,55] women only, and 1 study [37] men only. The types of nuts that were studied included peanuts (n = 8), almonds (n = 5), pistachios (n = 2), walnuts (n = 1), cashews (n = 1), hazelnuts (n = 1), Brazil nuts (n = 1), and chestnuts (n = 1). Notably, although most studies investigated only 1 type of nut, 2 studies investigated multiple nut types [18,45]. The physical forms of nuts included whole nuts (n = 14), ground nuts (n = 3), nut butters (n = 3), sliced nuts (n = 1), and nut flours (n = 1). Heat processing forms of nuts included raw nuts (n = 5), roasted nuts (n = 5), and baked chestnuts (n = 1). However, 3 studies [47,48,53] did not specify the form of nuts used.
Of the 17 studies, 9 [18,38,40,43,48,50,51,53,54] provided nuts alone as a snack, 7 [37,41,42,45,46,52,55] provided nuts as part of a larger snack or meal (mixed with other foods), and 1 [47] study investigated nut consumption both alone and as part of a mixed meal. The dose of nuts provided as part of a mixed meal or snack ranged from 20 to 56 g/d, and the dose of nuts when consumed alone ranged from 23 to 87.5 g/d, with the exception of 1 study [18] which provided 235.8 g of baked chestnuts as a snack. Two studies [40,50] individualized the nut dose based on the participants’ energy requirements or body weight. In 10 studies [37,38,[40], [41], [42], [43],45,47,52,54], the nut-containing intervention was compared with an isocaloric control meal. Two studies [48,51] compared nut consumption with a control of nil energy intake, and another 2 studies [46,55] compared nut consumption with a control snack that was not isocaloric to the intervention. Three studies [18,50,53] compared a nut-containing intervention with both nil energy intake and a snack (either isocaloric or of different energy content).
Energy intake was the main outcome measured in energy intake studies. However, the duration during which intake was measured varied among studies. Five studies [18,46,47,53,55] reported only the total daily energy intake (including energy intake before, during, and after the consumption of nuts), whereas another 5 studies [41,43,48,50,51] that reported total daily energy intake also separated the data into meal occasions. Five studies [37,40,42,45,52] reported only energy intake after the consumption of nuts, with or without data separated into meals. One study [38] measured energy intake only at the subsequent meal after the nut consumption, and another study [54] measured energy intake for a 24-h period after the nut consumption. The methods used to quantify energy intake include a combination of a food record dietary methodology and measuring plate waste from a preweighed buffet. Energy compensation was a secondary outcome reported in 2 studies [18,37] and was calculated by the reviewers in 14 studies [38,[40], [41], [42], [43],[45], [46], [47], [48],50,51,[53], [54], [55]].
Findings for energy intake/compensation
Next-meal energy intake
Seven of the 17 energy intake studies reported energy intake at the next meal (e.g., lunch) (Table 2). In 4 of the 7 studies, energy intake at the subsequent meal was significantly lower after consumption of nuts compared with consumption of a control snack or no snack [38,48,50,51]. Three of the 7 studies did not find any significant differences in energy intake at the subsequent meal after the nut consumption compared with that in the control arm [40,41,45].
Energy intake in subsequent meals
Nine of the 17 energy compensation studies reported energy intakes for numerous eating occasions after the intervention (e.g., lunch and dinner) (Table 2). Only 1 of these 9 studies found a significantly lower energy intake for the remainder of the day after the consumption of nuts versus a control snack [40]. Seven of the 9 studies did not report any significant differences in energy intake in subsequent meals after nut consumption [37,42,43,45,48,52,54]. One study investigated the effects of 2 doses of almonds on energy intakes and found no effect on energy intake after the 28-g dose and significantly lower energy intakes after the 42-g dose, when comparing nut consumption with water [51].
Daily energy intake
Ten of the 17 energy compensation studies reported the total daily energy intake on the day of the intervention, including energy intake before, during, and after the intervention (Table 2). One study reported a significantly lower daily energy intake in the intervention arm versus control [48]. The remaining 9 studies did not find any significant effect of nut consumption on daily energy intake [18,41,43,46,47,50,51,53,55].
Energy compensation
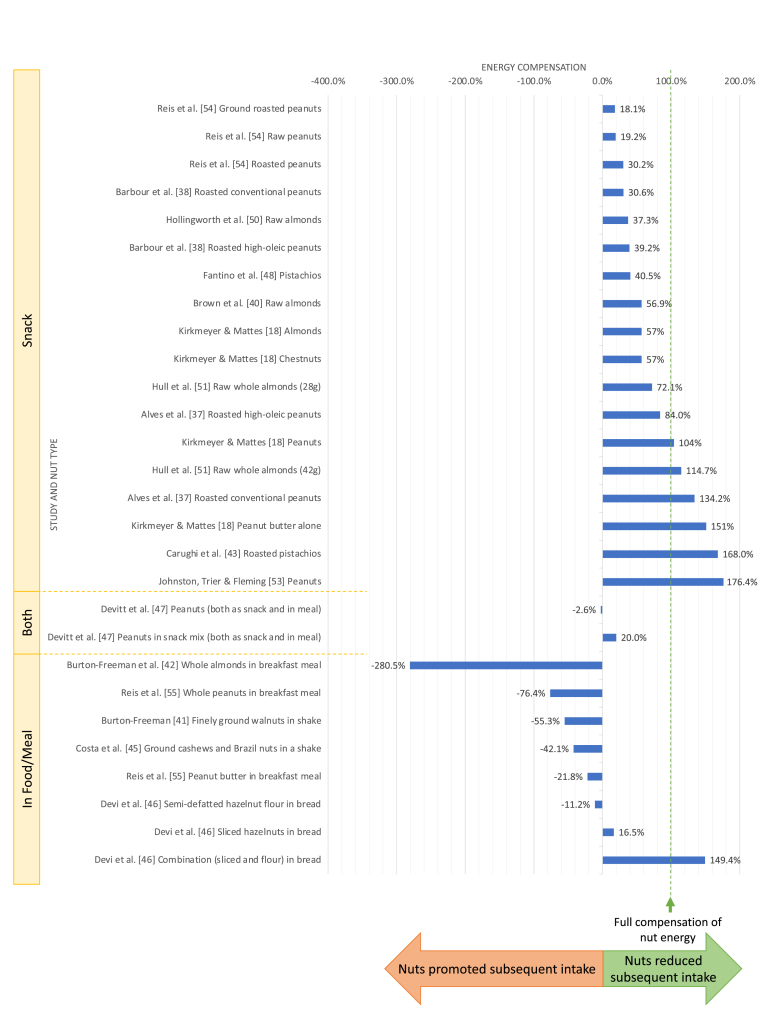
Energy compensation was calculated for 16 of the 17 energy intake studies to allow for comparison between the studies (Figure 2). In 2 of the 17 energy intake studies, energy compensation was measured and reported in the articles [18,37]. Energy compensation was calculated by reviewers in 14 of the 17 studies, whereas 1 of the 17 studies did not provide sufficient information to calculate energy compensation [52]. Therefore, the energy compensation results are provided for 16 studies.
FIGURE 2.
Energy compensation1 (%) of nut interventions categorized into subgroups based on consumption as a snack or within a meal, and sorted by nut type and study. 1Values below 0% indicate increased subsequent energy intake (more than the energy provided by nuts). Values between 0% and +100% indicate partial energy compensation (the increase in subsequent energy intake was less than the energy provided by nuts). A value of +100% indicates complete compensation (no change in subsequent energy intake). Values more than +100% indicate energy compensation was beyond the energy provided by the nuts (lower subsequent energy intake).
In 2 [43,53] of the 16 studies, energy compensation was greater than the energy provided by the nuts, that is, overcompensation, with energy compensation greater than +100%. For example, a study by Carughi et al. [43] reported a habitual energy intake of 8705 kJ and 7860 kJ on the test day when pistachios (1242 kJ) were consumed, leading to an energy compensation of +168.0%. In 5 [38,40,48,50,54] of the 16 studies, partial energy compensation occurred, indicated by a value between 0% and 100%. In 4 [41,42,45,55] of the 16 studies, energy compensation did not occur, reflected by an increase in energy intake with nut consumption.
In the remaining 5 studies, energy compensation varied because of several interventions in each study. Alves et al. [37] compared energy compensation of conventional peanuts and high-oleic peanuts and found a higher degree of compensation with conventional peanuts (+134.2% vs. +84.0%), suggesting that a difference in fatty acid profiles affects energy compensation. Kirkmeyer and Mattes [18] investigated energy compensation among various nuts and nut products. The highest energy compensation was reported for peanut butter (+151%), followed by peanuts (+104%), almonds (+57%), and chestnuts (+57%) [18]. In a study by Devi et al. [46], a bread containing semi-defatted hazelnut flour promoted subsequent energy intake (energy compensation: −11.2%), however, this effect was reversed when combining the flour with sliced hazelnuts in bread (+149.4%; overcompensation) which had a greater effect on energy compensation than that by bread containing only sliced hazelnuts (+16.5%; partial compensation). The hazelnut flour bread arm was the only arm to have an increased energy intake on the test day when compared with the control (8904 kJ vs. 8832 kJ) [46]. In a study by Devitt et al. [47], partial compensation occurred for 1 of the interventions—a snack mix containing peanuts—whereas compensation did not occur for the peanut intervention (+20.0% and −2.6%, respectively). Finally, in a study by Hull et al. [51], partial compensation occurred for the 28-g dose (+72.1%) and overcompensation occurred for the 42-g dose (+114.7%), suggesting a potential dose–response effect.
EE studies
All studies that reported on EE (n = 11) were of an RCT design (Table 3). Countries in which studies were conducted included the United States [19,25,39], Sweden [22,44], Brazil [36,37], Australia [56], New Zealand [58], Spain [24], Greece [57], and Israel [49].
TABLE 3.
Characteristics of the 11 included studies examining the effect of tree nut and peanut consumption on EE in adults aged ≥18 y
| Reference; country | Study design | Primary outcome | Population; mean BMI (kg/m2); mean age (y) | Sample size (completers) | Control diet | Intervention diet | Nut type, form, and dose | Intervention duration | EE measurement | Quality appraisal |
|---|---|---|---|---|---|---|---|---|---|---|
| Alves et al. [36,37]; Brazil | RCT (parallel) | Postprandial EE | Men with overweight or obesity. Postprandial study: 29.8; 27.1 Chronic study: 29.7; 27.4 (CT), 28.0 (CVP) and 26.8 (HOP) |
Postprandial study: 71 (100% M, 0% F) Chronic study: 65 (100% M, 0% F) |
Postprandial study: Controlled diet with the addition of 56 g biscuits (isocaloric to intervention). Chronic study: Hypocaloric diet (−250 kcal/d from requirements). Isocaloric to intervention diets. Test meal on day of measurements: strawberry milkshake and biscuits |
Postprandial study: Controlled diet with addition of 56 g of either HOPs or regular peanuts. Chronic study: Hypocaloric diet (−250 kcal/d from requirements) with the inclusion of peanuts. Test meal: strawberry milkshake and peanuts |
1: HOPs (roasted); 56 g/d 2: regular peanuts (roasted); 56 g/d |
Postprandial study: 1 d Chronic study: 4 wk |
REE obtained from respiratory gas exchange measured by indirect calorimetry using a ventilated respiratory canopy (Deltatrac II, MBM-200; Datex Instrumentarium Corporation), measured over 30 min under fasting conditions DIT and substrate oxidation were measured by gas exchange—4 times over 200 min after test meal ingestion during 20-min within 30-min intervals |
Positive |
| Chronic REE and DIT | ||||||||||
| Casas-Agustench et al. [24]; Spain | RCT (crossover) | Postprandial TEF | Healthy; 24.1; 22 | 29 (100% M, 0% F) | Morning test meal, isocaloric to each other and to intervention: 1: high MUFA (white bread, strawberry jam, apple, skim yogurt, skim cheese, virgin olive oil) 2: high SFA (white bread, strawberry jam, apple, plain yogurt, Gouda cheese, butter) |
Morning test meal: high PUFA (white bread, strawberry jam, apple, skim yogurt, walnuts) |
Walnuts; dose not reported | 1 d | Open-circuit indirect calorimetry with a canopy system (DELTATRAC II, Finland). Duration not stated. TEF measured during a 5-h period using the same indirect calorimetry system |
Positive |
| Gepner et al. [49]; Israel | RCT (crossover) | Postprandial EE | Adults with obesity; 31.1; 45.2 | 40 (100% M, 0% F) | Consumption of 5 slices wholegrain bread (150 g, isocaloric to intervention) | Consumption of walnuts (isocaloric to control) | Walnuts; 56 g | 40 min (postprandial) | Measured REE and 25-min postprandial EE for 16 min (20 min total with a 4-min adaptation phase—excluded), after a 10-min rest and a “steady state” was observed (supine position, 22°C–24°C). Measured by indirect calorimetry (QUARK REE by COSMED) with head covered with a ventilated canopy | Positive |
| Tapsell et al. [56]; Australia | RCT (crossover feeding study) | Postprandial EE | Healthy adults with overweight; 31.2; 52.8 | 16 (44% M, 56% F)1 | Controlled breakfast and lunch based on energy requirements (isocaloric to intervention diet); included olive oil at lunch | Controlled breakfast and lunch based on energy requirements; included walnuts at breakfast | Walnuts; 25–35 g (based on individual energy requirements) | 8 h | Participants entered room calorimeter while fasting in the morning and consumed breakfast and lunch during the 8-h stay in the chamber; 2 visits | Positive |
| Tentolouris et al. [57]; Greece | RCT (crossover) | Postprandial EE and MIT | Healthy females; lean participants (n = 19) 22.51 and participants with obesity (n = 22) 34.56; lean 33.2 and obese 32.3 | 41 (0% M, 100% F) | CHO-rich test meal consisting of white bread and honey | Walnuts (isocaloric to control) | Walnuts; 100 g | 3 h | Respiratory gas exchange was performed by an open-circuit ventilated hood system (Deltatrack monitor) for 30 min in the fasting state, and for 30 min every hour for a total of 3 h after meals. REE and RQ were calculated from oxygen consumption and carbon dioxide production using the Weir formula. MIT and macronutrient oxidation were calculated using formulas | Positive |
| Agebratt et al. [22]; Sweden | RCT (parallel) | Chronic BMR | Healthy non-obese; 22.3; 23.5 | 30 (60% M, 40% F) | Participants instructed to consume 7 kcal per kg body weight per day of fresh fruit in addition to habitual diet | Participants instructed to consume 7 kcal per kg body weight per day of nuts (tree nuts and peanuts accepted) in addition to habitual diet | Mixed (participants purchased tree nuts and/or peanuts) 7 kcal per kg body weight per day |
8 wk | Measured BMR by indirect calorimetry (Quark RMR, Cosmed). Participants rested lying down for 10 min before BMR measurements taken | Positive |
| Brennan et al. [39]; USA | RCT (crossover) | Chronic REE | Metabolic syndrome; 36.9; 58.0 | 15 (60% M, 40% F) | Placebo shake (isocaloric to intervention) consumed in morning, as part of a controlled isocaloric diet | Walnut-containing shake consumed in morning, as part of a controlled isocaloric diet | Walnuts; 48 g/d | 2 d (4 d apart) | REE measured using indirect calorimetry on the first and final day of each visit | Positive |
| Claesson et al. [44]; Sweden | RCT (parallel) | Chronic BMR | Healthy; age range 19–30; BMI range 19.0–26.4 | 25 (44% M, 56% F) | Regular diet with addition of candy (isocaloric to peanuts) | Regular diet with addition of peanuts | Peanuts (roasted, salted); 84 kJ/kg/d | 2 wk | BMR measured using a ventilated hood technique (Delta Trac, SensorMedics) in the fasting state in the morning. Duration of registration varied between 15 and 20 min (resting period was around 15 min before BMR recording) | Positive |
| Fraser et al. [19]; USA | RCT (crossover) | Chronic REE | Healthy; 26.7 (M) and 25.9 (F); 49.2 (M) and 49.9 (F) | 412 | Regular diet | Participants advised to consume allocated amount of almonds every day | Almonds (participant choose either raw or dry-roasted) 15% of daily energy for each individual (mean 54.3 g/d) |
6 mo | REE obtained using the Sensormedics 4400 metabolic unit. Note: a failure in the original equipment invalidated the data for groups 1 and 2. Results report groups 3 and 4 only (using new equipment) | Neutral |
| Hollis and Mattes [25]; USA | RCT (crossover) | Chronic RMR and TEF | Healthy; 25.9; 24 | 20 (0% M, 100% F) | Usual diet (no nuts) | Participants consumed almonds without advice about how to include them | Almonds (raw unsalted); 1440 kJ/d portion | 10 wk | RMR was measured by indirect calorimetry (weeks 1 and 8). Participants fasted for 12 h overnight, entered thermo-neutral laboratory and rested in supine position for 20 min. Then, ventilated hood was placed on head for 45 min, of which the last 15 min were used to determine RMR. TEF was estimated by EE measurements in 6 h after almond consumption (in supine position). TEE measured using doubly labeled water (week 8) | Positive |
| Tey et al. [58]; New Zealand | RCT (parallel) | Chronic RMR | Healthy; 23.8; 37.4 (using n = 118)3 | 493 | Habitual diet with the addition of no snacks, chocolate (50 g), or potato crisps (50 g) | Habitual diet with the addition of hazelnuts (42 g) isocaloric to chocolate and crisps. Received no dietary advice, instructed to consume full snack every day | Hazelnuts; 42 g/d (∼1100 kJ) | 12 wk | RMR measured by indirect calorimetry after 12 h overnight fasting. After a 15-min rest, gas collection was achieved for a 15-min period | Positive |
CHO, carbohydrate; CT, control; CVP, conventional peanut; DIT, diet-induced thermogenesis; F, female; HOP, high-oleic peanut; M, male; MIT, meal-induced thermogenesis.
Sample size: n = 14 for adjusted; n = 16 for nonadjusted.
Originally n = 81 (53% M, 47% F) in this study; however invalid data produced for 40 participants, so valid EE data reported for n = 41.
Sample size, n = 118 (47% M, 53% F); however, only 49 completed RMR measurements.
The sample size ranged from 15 to 71 participants, and 6 studies [19,22,39,44,56,58] included both men and women, 3 studies (from 4 articles) [24,36,37,49] men only, and 2 studies [25,57] women only. Each study investigated only 1 type of nut, and types included walnuts (n = 5), peanuts (n = 2), almonds (n = 2), hazelnuts (n = 1), and mixed nuts (n = 1). Various forms of nuts were explored, such as whole nuts, ground nuts, roasted nuts, and raw nuts. Some studies investigated multiple forms of nuts, and 7 studies [22,24,39,49,[56], [57], [58]] did not specify the forms of nuts that were used.
Of the 11 studies, 3 studies [24,39,56] investigated nuts consumed as part of a mixed meal, 2 studies [49,57] the consumption of nuts alone, and 1 study (2 articles) [36,37] both. In 5 studies [19,22,25,44,58], it was not clear how the nuts were being consumed (alone or mixed with other foods) because of the chronic nature of the studies where participants were instructed to consume nuts every day for weeks or months. The dose of nut consumption ranged from 25 to 100 g/d, although 1 study [24] did not report the dose of walnuts. Four studies [19,22,44,56] individualized the nut dose based on participants’ energy requirements or body weight. In 8 [22,24,36,37,39,44,49,56,57] of the 11 studies, nut consumption was compared with an isocaloric control snack. In 2 studies [19,25], daily nut consumption was compared with the control arm, which followed their regular diet, and in 1 study [58], participants followed their regular pattern of eating with the addition of no snacks (control) or addition of nuts, crisps, or chocolate (which were isocaloric to each other).
EE was measured both acutely (postprandial EE measured immediately after nut consumption) and chronically (REE measured after a longer-term intervention). The study duration varied from 1 d to 6 mo. Five studies [24,37,49,56,57] explored postprandial EE in an acute context. Seven studies [19,22,25,36,39,44,58] explored REE in a chronic context. EE was measured using indirect calorimetry, and in all studies that measured REE, participants were fasted and resting during the measurement, except for 2 studies [24,39], which did not specify the conditions.
Findings for EE
Studies assessing the effect of nut consumption on acute EE (<24 h)
Five of the 11 EE studies investigated outcomes in an acute context (Table 4). Three of these studies reported significant results, but these results were conflicting among the studies [24,37,49]. Gepner et al. [49] found a significantly lower postprandial EE after walnut consumption than that after wholegrain bread consumption. A study by Alves et al. [37] reported that the consumption of high-oleic peanuts elicited a higher postprandial EE that by regular peanuts and the control snack (biscuits); however, the difference was only statistically significant when comparing high-oleic and regular peanuts. Furthermore, postprandial EE was significantly lower after consuming regular peanuts than that after biscuits [37]. Casas-Agustench et al. [24] explored TEF after consuming walnuts and found increased TEF when comparing walnuts with a high-saturated fatty acid meal and a high-monounsaturated fatty acid meal; however, the difference was only statistically significant when comparing walnuts with the high-saturated fatty acid meal. The remaining 2 studies that examined acute EE did not find any significant effect of nuts on postprandial EE [56,57].
TABLE 4.
Main findings of the 11 included studies examining the effect of tree nut and peanut consumption on EE in adults aged ≥18 y
| Reference; country | Primary Outcome | EE Results (control diet) | EE Results (intervention diet) |
|---|---|---|---|
| Alves et al. [36,37]; Brazil | PP EE; chronic REE and DIT | PP study Baseline: 1930 (SEM: 41.2) kcal/d (P > 0.05 vs. both peanut meals) 200 min: 9.04% higher than baseline (P < 0.05 vs. regular peanuts) EE expressed as AUC: 582.7 (SEM: 37.7) (P > 0.05 vs. both peanut meals) Chronic study Change in REE: −21.3 (SEM: 15.4) kcal/d Baseline DIT: 3.21% (SEM: 0.25%) DIT (4 wk): 3.07% (SEM: 0.43%) (P > 0.05 vs. both peanut meals; P > 0.05 vs. control baseline) |
PP study Baseline: HOPs: 1968 (SEM: 31.3) kcal/d (P > 0.05 vs. control and regular peanuts) Regular peanuts: 1964 (SEM: 43.9) kcal/d (P > 0.05 vs. control and HOPs) 200 min: HOPs: 9.25% higher than baseline (P < 0.05 vs. regular peanuts) Regular peanuts: 6.46% higher than baseline Both P < 0.05 compared with baseline EE expressed as AUC: HOPs: 636.6 (SEM: 43.7) (P < 0.05 vs. regular peanuts; P > 0.05 vs. control) Regular peanuts: 492.9 (SEM: 35.1) (P > 0.05 vs. control) Chronic study Change in REE HOP: −0.4 (SEM: 19.5) kcal/d CVP: −26.9 (SEM: 14.1) kcal/d Baseline DIT: HOP: 3.57% (SEM: 0.26%) (P < 0.05 vs. CVP; P > 0.05 vs. control) CVP: 2.60% (SEM: 0.17%) DIT (4 wk): HOP: 2.97% (SEM: 0.21%) (P > 0.05 vs. control and vs. CVP) CVP: 2.38% (SEM: 0.24%) (P > 0.05 vs. control) Both P > 0.05 compared with baseline |
| Casas-Agustench et al. [24]; Spain | PP TEF | SFA TEF (% above RMR): 9.6% (95% CI: 7.7, 11.4) Baseline fasting RMR: 323.2 (95% CI: 302.3, 344.1) kJ/h (P > 0.05 vs. MUFA and PUFA) MUFA TEF (% above RMR): 11.8% (95% CI: 9.7, 13.9) (P < 0.05 vs. SFA) Baseline fasting RMR: 318.9 (95% CI: 298.5, 339.3) kJ/h (P > 0.05 vs. SFA and PUFA) |
PUFA TEF (% above RMR): 12.3% (95% CI: 9.7, 14.9) (P < 0.05 vs. SFA; P > 0.05 vs. MUFA) Baseline fasting RMR: 318.5 (95% CI: 298.2, 338.8) kJ/h (P > 0.05 vs. SFA and MUFA) |
| Gepner et al. [49]; Israel | PP EE | Baseline REE: 1829.4 (SD: 229.5) kcal/d (P > 0.05 vs. intervention) 40-min PP REE: 1965.4 (SD: 261.6) kcal/d (P < 0.05 vs. intervention) DIT: 14.24 (SD: 17.58) kcal/40 min (P < 0.001 vs. intervention) |
Baseline REE: 1878.9 (SD: 234.9) kcal/d (P > 0.05 vs. control) 40-min PP REE: 1860.9 (SD: 218.5) kcal/d (P < 0.05 vs. control) DIT: −2.01 (SD: 15.03) kcal/40 min (P < 0.001 vs. control) |
| Tapsell et al. [56]; Australia | PP EE | No adjustment: 677.7 (SD: 133.0) kcal Adjusted: 671.0 (SD: 104.6) kcal.1 Both not significant vs. intervention |
No adjustment: 677.8 (SD: 146.3) kcal Adjusted: 670.2 (SD: 110.0) kcal1 Both not significant vs. control |
| Tentolouris et al. [57]; Greece | PP EE and MIT | Lean participants Fasting REE: 6799.2 (SEM: 31.4) kJ/d (P < 0.05 vs. obese) 3-h PP REE: 7874.4 (SEM: 34.9) kJ/d (P < 0.001 vs. fasting; P > 0.05 vs. obese) MIT (iAUC): 128.4 (SEM: 16.8) kJ/h (P > 0.05 vs. obese; P < 0.05 vs. lean intervention) Participants with obesity Fasting REE: 8090.4 (SEM: 41.5) kJ/d (P < 0.05 vs. lean) 3-h PP REE: 8947.2 (SEM: 41.5) kJ/d (P < 0.001 vs. fasting; P > 0.05 vs. lean) MIT (iAUC): 130.8 (SEM: 11.4) kJ/h (P > 0.05 vs. lean; P < 0.05 vs. obese intervention) |
Lean participants Fasting REE: 6326.4 (SEM: 43.2) kJ/d (P < 0.05 vs. obese) 3-h PP REE: 6998.4 (SEM: 41.6) kJ/d (P < 0.001 vs. fasting; P > 0.05 vs. obese) MIT (iAUC): 74.4 (SEM: 13.2) kJ/h (P > 0.05 vs. obese; P < 0.05 vs. lean control) Participants with obesity Fasting REE: 7944.0 (SEM: 57.1) kJ/d (P < 0.05 vs. lean) 3-h PP REE: 8736.2 (SEM: 63.8) kJ/d (P < 0.001 vs. fasting; P > 0.05 vs. lean) MIT (iAUC): 89.4 (SEM: 8.4) kJ/h (P > 0.05 vs. lean; P < 0.05 vs. obese control) |
| Agebratt et al. [22]; Sweden | Chronic BMR | Baseline: 1787 (SD: 278) kcal/d 8 wk: 1845 (SD: 240) kcal/d (P = 0.52 vs. intervention) |
Baseline: 1931 (SD: 221) kcal/d 8 wk: 2031 (SD: 294) kcal/d (P = 0.52 vs. control) |
| Brennan et al. [39]; USA | Chronic REE | No values provided | “We did not find any REE changes on day 4 of admission” |
| Claesson et al. [44]; Sweden | Chronic BMR | Baseline: 6.657 (SD: 1.1) MJ/d Post: 6.762 (SD: 1.1) MJ/d (P > 0.05 vs. baseline) Change from baseline: 0.105 (SD: 0.31) MJ/2d (P > 0.05 vs. intervention change) |
Baseline: 6.896 (SD: 0.98) MJ/d Post: 7.256 (SD: 1.1) MJ/d (P < 0.05 vs. baseline; P > 0.05 vs. control post) Change from baseline: 0.360 (SD: 0.47) MJ/d (P > 0.05 vs. control change) |
| Fraser et al. [19]; USA | Chronic REE | Estimated REE: 308.8 kcal/d (n = 41) (P > 0.05 vs. intervention) | Estimated REE: 301.4 kcal/d (n = 41) (P > 0.05 vs. control) |
| Hollis and Mattes [25]; USA | Chronic RMR and TEF | RMR Baseline: 1473 (SD: 182) kcal/d Week 8: 1446 (SD: 236) kcal/d TEF (week 8): 3.1% (SD: 6.3%) above RMR No significant changes in RMR or TEF |
RMR Baseline: 1455 (SD: 200) kcal/d Week 8: 1499 (SD: 195) kcal/d TEF (week 8): 3.2% (SD: 4.6%) above RMR No significant changes in RMR or TEF |
| Tey et al. [58]; New Zealand | Chronic RMR | Baseline mean (n = 52): 1489.29 (SD: 275.82) kcal/d2 Week 12 change No snack (n = 13): −79.62 (SE: 38.39) kcal/d Chocolate (n = 12): −69.92 (SE: 89.94) kcal/d Crisps (n = 10): 11.70 (SE: 69.37) kcal/d Overall P = 0.922 among groups |
Baseline mean (n = 52): 1489.29 (SD: 275.82) kcal/d2 Week 12 change Hazelnut (n = 14): −56.86 (SE: 58.40) kcal/d Overall P = 0.922 among groups |
BMR, basal metabolic rate; CVP, conventional peanut; DIT, diet-induced thermogenesis; HOP, high-oleic peanut; iAUC, incremental area under the curve; MIT, meal-induced thermogenesis; PP, postprandial.
Sample size: n = 14 for adjusted; n = 16 for nonadjusted.
Baseline RMR for 52 participants.
Studies assessing the effect of chronic nut consumption on EE
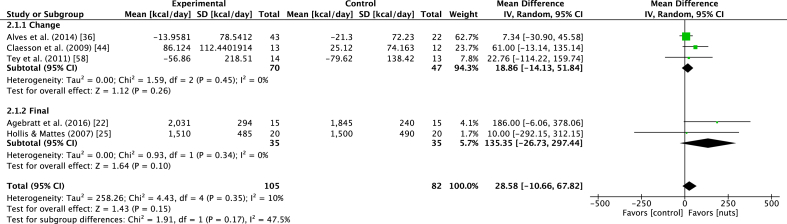
Seven of the 11 EE studies explored outcomes in a chronic context (Table 4). Of these studies, 5 studies [22,25,36,44,58] were eligible for inclusion in the meta-analyses. These studies explored nut supplementation over 2–12 wk (acute studies were excluded owing to the variation in outcomes), measured REE and had sufficient data to include in the analyses. The types of nuts included peanuts (n = 2), almonds (n = 1), hazelnuts (n = 1), and a combination of tree nuts and peanuts (n = 1). The meta-analysis showed that nut consumption was associated with a nonsignificant increase in REE, with a WMD of 28.6 kcal/d (95% CI: −10.7, 67.8 kcal/d), as presented in Figure 3. The leave-one-out sensitivity analysis showed that the removal of the study by Alves et al. [36] resulted in a significant effect (WMD: 63.9 kcal/d; 95% CI: 3.4, 124.4 kcal/d), although removal of other studies did not alter the effect (Supplementary Material 3). Sensitivity analyses examining the effect of combining multiple control arms [involving nonnut interventions by Tey et al. [58]] found similar results to the primary analysis (WMD: 27.6 kcal/d; 95% CI: −13.9, 69.1 kcal/d), presented in Supplementary Material 3.
FIGURE 3.
Forest plot of the effect of tree nut and peanut consumption on REE (in kilocalories per day), categorized into subgroups based on mean change or final values. Diamond indicates weighted mean difference with 95% CIs.
Two studies [19,39] included in the systematic review were not eligible for inclusion in the meta-analysis because they did not have sufficient data available, either in the publication or after contacting the study authors. Neither of these studies found a significant effect of nut consumption on EE, with 1 study reporting a nonsignificant decrease in REE [19], whereas the other study did not provide EE values but instead reported that they did not find any REE changes [39].
Discussion
Energy compensation is a plausible mechanism that may explain the lack of an effect of nut consumption on body weight observed in the literature [[13], [14], [15]]. The degree of energy compensation seems to be dependent on whether nuts are eaten alone as a snack or as part of a mixed meal, with stronger energy compensation observed when nuts are consumed on their own as a snack. However, the findings of this review suggest that nut consumption has no significant effect on EE. Although meta-analyses showed a small increase in REE associated with regular nut consumption, the effect is lacking statistical and clinical significance. The effect of nuts on diet-induced thermogenesis is also inconsistent and inconclusive. Although a previous systematic review explored the effect of nut consumption on energy intake [13], our review expanded this by focusing on acute energy intake compensation, in addition to examining the effect of nut intake on EE, to provide a more complete view of the effects of nuts on human energy balance regulation.
Energy compensation
In this review, energy intake after nut consumption was explored over an acute period only. The intervention duration was restricted to a 24-h period to focus on the effects of nut intake specifically, rather than other potential factors that can influence dietary behaviors over a longer duration. The nutrient profile of tree nuts and peanuts is likely a contributing factor to their energy compensation effects. Peanuts and tree nuts are nutrient dense, being high in protein, fiber, and vitamins, minerals, and phytosterols [6]. Food high in protein and fiber are known to be satiating [60] and are important to consider when choosing healthy meals and snacks. However, energy compensation ranged from −280.5% [42] to +176.4% [53], and the findings of this systematic review suggest that energy compensation is stronger when nuts are consumed alone as opposed to including nuts in a meal. The study by Burton-Freeman et al [42] found that the addition of almonds to a breakfast meal promoted subsequent food intake, with the lowest energy compensation of −280.5%. One other study [55] investigated the addition of either whole peanuts or peanut butter to a breakfast meal and found an increase in food intake.
The literature has shown that consuming healthy, whole foods as a snack increases satiety and helps regulate appetite throughout the day [61]. For this reason, when nuts are consumed as a snack, as opposed to nuts being combined with other foods in a meal, energy compensation is likely to be greater. This concept has also been explored in the literature. An RCT found that almonds were more effective at reducing hunger when they were being consumed as a snack, rather than part of a meal [62]. Similar research has been conducted in other foods. A study by Mattes and Campbell [63] explored the effect of timing and food form on appetite, with consumption of an apple (solid), apple sauce (semisolid), or apple juice (liquid) either with a meal or alone as a snack compared. The authors concluded that the apple elicited the strongest appetitive response, followed by apple sauce and apple juice, and that this effect was stronger when the apple was consumed as a snack as opposed to with a meal [63]. Therefore, consuming whole foods as snacks—particularly those that take a solid form and are high in protein or fiber—may regulate appetite throughout the day, as suggested in the literature [61], and may provoke a stronger compensatory effect than when added to meals.
Besides the timing of nut consumption in meals versus snacks, nut flours and nuts that were finely ground did not provoke energy compensation and instead resulted in an increased energy intake. Nut flours and ground nuts contain smaller particle sizes and, therefore, do not require the same level of mastication as when consuming whole nuts. The act of mastication reduces food intake and promotes satiety [64]. Cassady et al. [65] investigated the effect of mastication of almonds on appetite and found hunger suppression and increased appetite after 40 chews compared with that after 25 chews and significantly lower glucagon-like peptide 1 after 25 chews than that after 40 chews. Therefore, energy compensation may be greater with whole nuts than with ground nuts and nut flours. In addition, 2 studies [41,45] that investigated finely ground nuts mixed into shakes (beverages), which did not require mastication, did not show evidence of energy compensation. A systematic review of energy compensation after the consumption of a range of preloads (including liquid preloads such as beverages, shakes, and soups; semisolid preloads such as yogurts and chunky soups; and solid preloads such as vegetables, breads and meat, and composite meals) found that the physical form of the preloads was a contributing factor to differences in energy compensation and that solid and semisolid preloads increased energy compensation [66]. Moreover, another study found that solid foods are more satiating than liquids, which may explain why solid foods typically promote energy compensation [67]. The physical form of tree nuts and peanuts seems to affect subsequent energy intake and energy compensation effects, so a recommendation to consume nuts in their whole form would be appropriate to prevent excessive energy intake.
The effects of peanut butter consumption on energy intake and compensation varied [18,55]. One study [55] reported greater energy intake after peanut butter consumption (i.e., no compensation), whereas the other study [18] reported overcompensation with peanut butter. However, the former study included peanut butter in a breakfast meal, whereas, in the latter, peanut butter was consumed alone. This may explain the varied results for peanut butter given the findings of this review suggest that energy compensation is weaker when nuts are consumed within a meal, although more research is required to further understand this relationship. Kirkmeyer and Mattes [18] investigated both whole peanuts and peanut butter, which were both consumed alone, and found that both elicited strong energy compensatory effects. Therefore, peanut butter and whole peanuts may provoke a similar degree of energy compensation when they are consumed alone; however, future research should continue to investigate the energy compensatory effects of nut butters.
When considered separately from whether nuts are consumed as meals or snacks, the time of nut consumption (for example, morning vs. afternoon) within a single day did not seem to affect the level of energy compensation. As discussed previously, nut intake at a morning breakfast meal did not produce an energy compensation effect; however, snacking on nuts in the morning did result in partial or overcompensation in several studies. All studies instructed participants to consume nuts or nut-containing intervention in the morning, except for 2 [43,47] which investigated nut consumption in the afternoon and saw variation in results. By comparison with literature that has explored chronic effects of nuts on energy compensation, regular nut intake within a diet over a longer period shows more consistent results. A study by Sabaté et al. [21] investigated energy compensation with regular walnut consumption compared with a habitual diet. A minimally higher mean daily energy intake was observed with a walnut-supplemented diet than that with a habitual diet, but the increased energy intake in the nut-containing diet was less than expected, indicating partial compensation had occurred [21]. Another study found that regular consumption of peanuts provoked partial energy compensation, even when no dietary guidance was provided about how to incorporate the peanuts into participants’ diets [23]. Further research has identified other beneficial outcomes associated with regular nut consumption, including weight loss and improved diet quality [68,69]. Habitual nut consumption, therefore, appears to have a greater effect on energy compensation than the timing of nut consumption.
Across studies, there did not seem to be a dose–response effect of nut consumption on energy compensation. Only 1 study [51] included in this review compared 2 doses of nuts—a mid-morning snack of either 28 or 42 g of almonds. Although a higher compensatory effect was found with the higher dose in this study, owing to the variation in results across all studies, the dose of nuts did not seem to affect the degree of energy compensation. Whether nuts are consumed as a snack or part of a meal or consumed whole or finely chopped seems to be a greater predictor of energy compensation, likely because of the properties of nuts discussed previously. Future studies that investigate the energy compensation effects of nuts should include several doses to further understand whether a dose–response effect exists.
EE
The findings relating to the effect of nuts on EE were inconsistent. Studies were organized into acute (where interventions were completed within 24 h) or chronic (interventions lasting >1 d) categories. Results varied considerably among acute studies. In 1 study [37], the consumption of high-oleic peanuts increased postprandial EE when compared with regular peanut consumption. Another study included in this review found that the walnut-containing meal elicited a higher TEF than an olive oil-containing meal and a dairy-containing meal [24]. These findings suggest that the fatty acid profile of nuts affects fat oxidation and hence EE. Research has shown that in healthy male subjects, olive oil (high in oleic acid) caused an increase in postprandial EE when compared with flaxseed oil (high in linolenic acid; P < 0.0006) and sunflower oil (high in linoleic acid; P < 0.06) [70]. Another study has shown that oleic acid increases REE, whereas palmitic acid caused a decrease [71]. In this review, high-oleic peanuts were found to cause a greater increase in postprandial EE than regular peanuts. Approximately 54% of the fatty acids found in regular peanuts are monounsaturated fatty acids (includes oleic acid), and this proportion increases to 82% in high-oleic peanuts [72]. Moreover, the literature has confirmed that unsaturated fatty acids are more readily oxidized than saturated fatty acids [[73], [74], [75]], and this mechanism is likely the reason why nuts caused a greater increase in postprandial EE.
There was no evidence to suggest that the long-term consumption of nuts affects REE. A meta-analysis of 5 chronic studies found a nonsignificant increase of 28.58 kcal/d in REE, consolidating the broader findings of this systematic review. The results of this review are similar to those of 2 studies, which were excluded from this review because physical activity records, rather than indirect calorimetry, were used to estimate EE [38,59]. An increase in REE of 28.58 kcal/d is clinically insignificant. It is estimated that a deficit of at least 500 kcal/d (by removing 500 kcal/d from the diet, increasing EE by 500 kcal/d, or a combination of diet and expenditure approaches) is required for healthy weight loss of approximately 500 g/wk [76]. It is unclear why nut consumption has no effect on REE. Research has suggested that EE is higher with consumption of a high-protein diet compared with high-carbohydrate or high-fat diets [77,78]. Increases in postprandial EE is also greatest after protein consumption and lowest after fat consumption [79]. Given that nuts are high in both protein and fat, the nutrient profile of tree nuts and peanuts may explain the inconsistent findings relating to postprandial EE and the lack of an effect on REE.
Sensitivity analyses demonstrated that the removal of the study by Alves et al. [36], the highest weighted study in the meta-analysis, caused the effect of nut consumption on REE to become significant. One potential reason for this occurrence could be the differing results between the 2 intervention arms in this study, which examined the effect of high-oleic and regular peanut consumption, respectively. Although the results of both peanut interventions were combined into a single intervention for the meta-analysis, the study reported that REE was higher in the high-oleic peanut arm that in the control arm, whereas REE was lower in the regular peanut arm than in the control arm. The study population of overweight or obese male participants in the study by Alves et al. [36] may partially explain this finding because EE is typically different between male and female subjects [80].
Study strengths and limitations
The strengths of this review include the consideration of 2 energy regulation mechanisms of nuts—energy compensation and increased REE—to understand the lack of an effect of nut consumption on body weight previously reported in the literature [[13], [14], [15]]. Furthermore, in EE studies, both acute and chronic effects were explored to further understand this mechanism. A meta-analysis was conducted to explore the effect of chronic nut consumption on REE. However, this review has some limitations. Chestnuts were eligible for inclusion in this review because they are botanically considered a tree nut. However, they have a different nutrient composition to other tree nuts and peanuts, and for this reason, they are usually excluded from nut-related research. Among energy compensation studies, energy intake was measured at various time-points including at the subsequent meal, energy intake postintervention and total daily energy intake, which may have resulted in the lack of consistent results observed. As a result of the substantial variation between the types of outcomes investigated, quantitative synthesis of results via meta-analysis was not considered appropriate for energy intake and compensation studies. Circadian rhythm may potentially have an impact on REE; however, this was not accounted for among EE studies. Finally, although the studies included in this review were RCTs, there is the possibility that other factors such as a healthier lifestyle overall may have affected the findings.
Conclusion
This systematic review and meta-analysis has found evidence of energy compensation as a potential energy regulation mechanism of tree nuts and peanuts, at least in the short-term. No evidence was found for EE. Energy compensation seems to be stronger when nuts are consumed alone rather than integrated into a meal. In comparison, the consumption of nuts seemed to have no impact on REE. More research is required to better understand the effects of nut butter consumption on energy compensation, and various doses of nuts should be included in future studies. Future research should continue to explore the effect of nut consumption on postprandial EE because the results of our review were inconsistent. In addition, future studies should investigate understudied nuts, such as macadamias, pecans, and pine nuts, to better understand energy compensation and EE effects across all types of nuts. The potential effect of nutrient composition of individual nut types and the gut microbiome on energy compensation and EE should be explored in future studies.
Funding
This work was supported by Nuts for Life via a matching PhD scholarship which CJN receives (matching scholarship is equally funded by the University of Wollongong and Nuts for Life). Nuts for Life were not involved in the study design, data collection, analysis or interpretation, or writing of the report. There were no restrictions regarding publication.
Author disclosures
CJN receives a matching PhD scholarship that is equally funded by the University of Wollongong and Nuts for Life. YCP has been previously involved in clinical trials funded by and received funding from the California Walnut Commission, received funding and research support from the Almond Board of Australia, and received funding from Nuts for Life. S-YT was previously involved in clinical studies that were funded by the Almond Board of California, International Nut and Dried Fruit Council, and the California Walnut Commission. EPN has previously received research funding from Nuts for Life, the California Walnut Commission, and the International Nut and Dried Fruit Council.
Statement of Significance
This study is the first to systematically review the effects of tree nuts and peanuts on energy intake compensation and EE. The results suggest that energy compensation is a potential energy regulating mechanism of nuts; however, no evidence was found for EE.
Acknowledgments
The authors’ responsibilities were as follows – CJN, YCP, S-YT, EPN: conceived and designed the study, analyzed the data, and wrote the manuscript; CJN: collected the data; and all authors: read and approved the final manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.advnut.2022.10.006.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Australian Institute of Health and Welfare Overweight and obesity 2021. 2022. https://www.aihw.gov.au/reports/australias-health/overweight-and-obesity [Internet] July 1, Available from:
- 2.World Health Organisation Obesity and overweight 2021. 2022. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight [Internet]. July 1, Available from:
- 3.Lee R.D., Nieman D.C. 6 ed. McGraw-Hill; New York: 2013. Nutritional Assessment. [Google Scholar]
- 4.Sabounchi N.S., Rahmandad H., Ammerman A. Best-fitting prediction equations for basal metabolic rate: informing obesity interventions in diverse populations. Int J Obes. 2013;37(10):1364–1370. doi: 10.1038/ijo.2012.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMurray R.G., Soares J., Caspersen C.J., McCurdy T. Examining variations of resting metabolic rate of adults: a public health perspective. Med Sci Sports Exerc. 2014;46(7):1352–1358. doi: 10.1249/MSS.0000000000000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ros E. Health benefits of nut consumption. Nutrients. 2010;2(7):652–682. doi: 10.3390/nu2070652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Souza R.G.M., Schincaglia R.M., Pimentel G.D., Mota J.F. Nuts and human health outcomes: a systematic review. Nutrients. 2017;9(12):1311. doi: 10.3390/nu9121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long J., Ji Z., Yuan P., Long T., Liu K., Li J., et al. Nut consumption and risk of cancer: a meta-analysis of prospective studies. Cancer Epidemiol Biomarkers Prev. 2020;29(3):565–573. doi: 10.1158/1055-9965.EPI-19-1167. [DOI] [PubMed] [Google Scholar]
- 9.Luo C., Zhang Y., Ding Y., Shan Z., Chen S., Yu M., et al. Nut consumption and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a systematic review and meta-analysis. Am J Clin Nutr. 2014;100(1):256–269. doi: 10.3945/ajcn.113.076109. [DOI] [PubMed] [Google Scholar]
- 10.Mayhew A.J., De Souza R.J., Meyre D., Anand S.S., Mente A. A systematic review and meta-analysis of nut consumption and incident risk of CVD and all-cause mortality. Br J Nutr. 2016;115(2):212–225. doi: 10.1017/S0007114515004316. [DOI] [PubMed] [Google Scholar]
- 11.Neale E.P., Tapsell L.C. CRC Press; Boca Raton, FL: 2020. Nuts in healthy dietary patterns and dietary guidelines. health benefits of nuts and dried fruits; pp. 289–312. [Google Scholar]
- 12.Zhou D., Yu H., He F., Reilly K.H., Zhang J., Li S., et al. Nut consumption in relation to cardiovascular disease risk and type 2 diabetes: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr. 2014;100(1):270–277. doi: 10.3945/ajcn.113.079152. [DOI] [PubMed] [Google Scholar]
- 13.Akhlaghi M., Ghobadi S., Zare M., Foshati S. Effect of nuts on energy intake, hunger, and fullness, a systematic review and meta-analysis of randomized clinical trials. Crit Rev Food Sci Nutr. 2020;60(1):84–93. doi: 10.1080/10408398.2018.1514486. [DOI] [PubMed] [Google Scholar]
- 14.Guarneiri L.L., Cooper J.A. Intake of nuts or nut products does not lead to weight gain, independent of dietary substitution instructions: a systematic review and meta-analysis of randomized trials. Adv Nutr. 2021;12(2):384–401. doi: 10.1093/advances/nmaa113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishi S.K., Viguiliouk E., Mejia S.B., Kendall C.W.C., Bazinet R.P., Hanley A.J., et al. Are fatty nuts a weighty concern? A systematic review and meta-analysis and dose–response meta-regression of prospective cohorts and randomized controlled trials. Obes Rev. 2021;22(11) doi: 10.1111/obr.13330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bes-Rastrollo M., Wedick N.M., Martinez-Gonzalez M.A., Li T.Y., Sampson L., Hu F.B. Prospective study of nut consumption, long-term weight change, and obesity risk in women. Am J Clin Nutr. 2009;89(6):1913–1919. doi: 10.3945/ajcn.2008.27276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mozaffarian D., Hao T., Rimm E.B., Willet W.C., Hu F.B. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364(25):2392–2404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirkmeyer S.V., Mattes R.D. Effects of food attributes on hunger and food intake. Int J Obes. 2000;24(9):1167–1175. doi: 10.1038/sj.ijo.0801360. [DOI] [PubMed] [Google Scholar]
- 19.Fraser G.E., Bennett H.W., Jaceldo K.B., Sabaté J. Effect on body weight of a free 76 kilojoule (320 calorie) daily supplement of almonds for six months. J Am Coll Nutr. 2002;21(3):275–283. doi: 10.1080/07315724.2002.10719221. [DOI] [PubMed] [Google Scholar]
- 20.Mattes R.D., Kris-Etherton P.M., Foster G.D. Impact of peanuts and tree nuts on body weight and healthy weight loss in adults. J Nutr. 2008;138(9):1741S–1745S. doi: 10.1093/jn/138.9.1741S. [DOI] [PubMed] [Google Scholar]
- 21.Sabaté J., Cordero-MacIntyre Z., Siapco G., Torabian S., Haddad E. Does regular walnut consumption lead to weight gain? Br J Nutr. 2005;94(5):859–864. doi: 10.1079/bjn20051567. [DOI] [PubMed] [Google Scholar]
- 22.Agebratt C., Ström E., Romu T., Dahlqvist-Leinhard O., Borga M., Leandersson P., et al. A randomized study of the effects of additional fruit and nuts consumption on hepatic fat content, cardiovascular risk factors and basal metabolic rate. PloS One. 2016;11(1) doi: 10.1371/journal.pone.0147149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alper C., Mattes R.D. Effects of chronic peanut consumption on energy balance and hedonics. Int J Obes. 2002;26(8):1129–1137. doi: 10.1038/sj.ijo.0802050. [DOI] [PubMed] [Google Scholar]
- 24.Casas-Agustench P., López-Uriarte P., Bulló M., Ros E., Gómez-Flores A., Salas-Salvadó J. Acute effects of three high-fat meals with different fat saturations on energy expenditure, substrate oxidation and satiety. Clin Nutr. 2009;28(1):39–45. doi: 10.1016/j.clnu.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Hollis J., Mattes R. Effect of chronic consumption of almonds on body weight in healthy humans. Br J Nutr. 2007;98(3):651–656. doi: 10.1017/S0007114507734608. [DOI] [PubMed] [Google Scholar]
- 26.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosen L., Suhami R. The art and science of study identification: a comparative analysis of two systematic reviews. BMC Med Res Methodol. 2016;16(1):1–13. doi: 10.1186/s12874-016-0118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curb J.D., Wergowske G., Dobbs J.C., Abbott R.D., Huang B. Serum lipid effects of a high–monounsaturated fat diet based on macadamia nuts. Arch Intern Med. 2000;160(8):1154–1158. doi: 10.1001/archinte.160.8.1154. [DOI] [PubMed] [Google Scholar]
- 29.N.R. Haddaway, M.J. Grainger, C.T. Gray, citationchaser: an R package for forward and backward citations chasing in academic searching, 0.0.3, 2021. Available from: https://estech.shinyapps.io/citationchaser/
- 30.Houston L., Probst Y., Humphries A. Measuring data quality through a source data verification audit in a clinical research setting. Stud Health Technol Inform. 2015;214:107–113. [PubMed] [Google Scholar]
- 31.Rohatgi A. WebPlotDigitizer (version 3.9) 2015. https://automeris.io/WebPlotDigitizer/ [Internet], Available from:
- 32.Food Standards Australia New Zealand . Food Standards Australia New Zealand; Canberra, Australia: 2014. AUSNUT 2011–13 Food Nutrient Database. [Google Scholar]
- 33.Higgins J.P.T., Thomas J. The Cochrane Collaboration; London, United Kingdom: 2022. Cochrane handbook for systematic reviews of interventions. version 6.3. [Google Scholar]
- 34.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Academy of Nutrition and Dietetics Quality criteria checklist—primary research. 2022. https://www.andeal.org/evidence-analysis-manual [Internet]. Available from:
- 36.Alves R.D.M., Moreira A.P.B., Macedo V.S., de Cássia Gonçalves Alfenas R., Bressan J., Mattes R., et al. Regular intake of high-oleic peanuts improves fat oxidation and body composition in overweight/obese men pursuing a energy-restricted diet. Obesity (Silver Spring) 2014;22(6):1422–1429. doi: 10.1002/oby.20746. [DOI] [PubMed] [Google Scholar]
- 37.Alves R.D.M., Moreira A.P.B., Macedo V.S., Neuza Maria Brunoro Costa N.M.B., de Cássia Gonçalves Alfenas R., Bressan J. High-oleic peanuts increase diet-induced thermogenesis in overweight and obese men. Nutri Hosp. 2014;29(5):1024–1032. doi: 10.3305/nh.2014.29.5.7235. [DOI] [PubMed] [Google Scholar]
- 38.Barbour J.A., Howe P.R.C., Buckley J.D., Wright G.C., Bryan J., Coates A.M. Lower energy intake following consumption of Hi-oleic and regular peanuts compared with iso-energetic consumption of potato crisps. Appetite. 2014;82:124–130. doi: 10.1016/j.appet.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 39.Brennan A.M., Sweeney L.L., Liu X., Mantzoros C.S. Walnut consumption increases satiation but has no effect on insulin resistance or the metabolic profile over a 4-day period. Obesity (Silver Spring) 2010;18(6):1176–1182. doi: 10.1038/oby.2009.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown R., Ware L., Gray A.R., Chisholm A., Tey S.L. Snacking on almonds lowers glycaemia and energy intake compared to a popular high-carbohydrate snack food: an acute randomised crossover study. Int J Environ Res Public Health. 2021;18(20) doi: 10.3390/ijerph182010989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burton-Freeman B. Sex and cognitive dietary restraint influence cholecystokinin release and satiety in response to preloads varying in fatty acid composition and content. J Nutr. 2005;135(6):1407–1414. doi: 10.1093/jn/135.6.1407. [DOI] [PubMed] [Google Scholar]
- 42.Burton-Freeman B., Davis P.A., Schneeman B.O. Interaction of fat availability and sex on postprandial satiety and cholecystokinin after mixed-food meals. Am J Clin Nutr. 2004;80(5):1207–1214. doi: 10.1093/ajcn/80.5.1207. [DOI] [PubMed] [Google Scholar]
- 43.Carughi A., Bellisle F., Dougkas A., Giboreau A., Feeney M.J., Higgs J. A randomized controlled pilot study to assess effects of a daily pistachio (Pistacia vera) afternoon snack on next-meal energy intake, satiety, and anthropometry in French women. Nutrients. 2019;11(4):767. doi: 10.3390/nu11040767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Claesson A., Holm G., Ernersson Å., Lindström T., Nystrom F.H. Two weeks of overfeeding with candy, but not peanuts, increases insulin levels and body weight. Scand J Clin Lab Invest. 2009;69(5):598–605. doi: 10.1080/00365510902912754. [DOI] [PubMed] [Google Scholar]
- 45.Costa M.A.C., Hermsdorff H.H.M., Caldas A.P.S., Rocha D.M.U.P., da Silva A., de Oliveira L.L., et al. Acute consumption of a shake containing cashew and Brazil nuts did not affect appetite in overweight subjects: a randomized, cross-over study. Eur J Nutr. 2021;60(8):4321–4330. doi: 10.1007/s00394-021-02560-w. [DOI] [PubMed] [Google Scholar]
- 46.Devi A., Chisholm A., Gray A., Tey S.L., Williamson-Poutama D., Cameron S.L., et al. Nut-enriched bread is an effective and acceptable vehicle to improve regular nut consumption. Eur J Nutr. 2016;55(7):2281–2293. doi: 10.1007/s00394-015-1038-3. [DOI] [PubMed] [Google Scholar]
- 47.Devitt A.A., Kuevi A., Coelho S.B., Lartey A., Lokko P., Costa N., et al. Appetitive and dietary effects of consuming an energy-dense food (peanuts) with or between meals by snackers and nonsnackers. J Nutr Metab. 2011;2011 doi: 10.1155/2011/928352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fantino M., Bichard C., Mistretta F., Bellisle F. Daily consumption of pistachios over 12 weeks improves dietary profile without increasing body weight in healthy women: A randomized controlled intervention. Appetite. 2020;144 doi: 10.1016/j.appet.2019.104483. [DOI] [PubMed] [Google Scholar]
- 49.Gepner Y., Bril N., Shelef I., Schwarzfuchs D., Serfaty D., Rein M., et al. Higher visceral adiposity is associated with an enhanced early thermogenic response to carbohydrate-rich food. Clin Nutr. 2016;35(2):422–427. doi: 10.1016/j.clnu.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 50.Hollingworth S., Dalton M., Blundell J.E., Finlayson G. Evaluation of the influence of raw almonds on appetite control: satiation, satiety, hedonics and consumer perceptions. Nutrients. 2019;11(9):2030. doi: 10.3390/nu11092030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hull S., Re R., Chambers L., Echaniz A., Wickham M.S.J. A mid-morning snack of almonds generates satiety and appropriate adjustment of subsequent food intake in healthy women. Eur J Nutr. 2015;54(5):803–810. doi: 10.1007/s00394-014-0759-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnston C.S., Buller A.J. Vinegar and peanut products as complementary foods to reduce postprandial glycemia. J Am Diet Assoc. 2005;105(12):1939–1942. doi: 10.1016/j.jada.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 53.Johnston C.S., Trier C.M., Fleming K.R. The effect of peanut and grain bar preloads on postmeal satiety, glycemia, and weight loss in healthy individuals: an acute and a chronic randomized intervention trial. Nutr J. 2013;12(1):1–10. doi: 10.1186/1475-2891-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reis C.E.G., Bordalo L.A., Rocha A.L.C., O Freitas D.M., da Silva M.V.L., de Faria V.C., et al. Ground roasted peanuts leads to a lower post-prandial glycemic response than raw peanuts. Nutr Hosp. 2011;26(4):745–751. doi: 10.1590/S0212-16112011000400012. [DOI] [PubMed] [Google Scholar]
- 55.Reis C.E.G., Ribeiro D.N., Costa N.M.B., Bressan J., Alfenas R.C.G., Mattes R.D. Acute and second-meal effects of peanuts on glycaemic response and appetite in obese women with high type 2 diabetes risk: a randomised cross-over clinical trial. Br J Nutr. 2013;109(11):2015–2023. doi: 10.1017/S0007114512004217. [DOI] [PubMed] [Google Scholar]
- 56.Tapsell L., Batterham M., Tan S.Y., Warensjö E. The effect of a calorie controlled diet containing walnuts on substrate oxidation during 8-hours in a room calorimeter. J Am Coll Nutr. 2009;28(5):611–617. doi: 10.1080/07315724.2009.10719793. [DOI] [PubMed] [Google Scholar]
- 57.Tentolouris N., Alexiadou K., Kokkinos A., Koukou E., Perrea D., Kyriaki D., et al. Meal-induced thermogenesis and macronutrient oxidation in lean and obese women after consumption of carbohydrate-rich and fat-rich meals. Nutrition. 2011;27(3):310–315. doi: 10.1016/j.nut.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 58.Tey S.L., Brown R., Gray A., Chrisholm A., Delahunty C. Nuts improve diet quality compared to other energy-dense snacks while maintaining body weight. J Nutr Metab. 2011;2011 doi: 10.1155/2011/357350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coates A.M., Morgillo S., Yandell C., Scholey A., Buckley J.D., Dyer K.A., et al. Effect of a 12-week almond-enriched diet on biomarkers of cognitive performance, mood, and cardiometabolic health in older overweight adults. Nutrients. 2020;12(4):1180. doi: 10.3390/nu12041180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chambers L., McCrickerd K., Yeomans M.R. Optimising foods for satiety. Trends Food Sci Technol. 2015;41(2):149–160. [Google Scholar]
- 61.Njike V.Y., Smith T.M., Shuval O., Shuval K., Edshteyn I., Kalanatari V., et al. Snack food, satiety, and weight. Adv Nutr. 2016;7(5):866–878. doi: 10.3945/an.115.009340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tan S.Y., Mattes R.D. Appetitive, dietary and health effects of almonds consumed with meals or as snacks: a randomized, controlled trial. Eur J Clin Nutr. 2013;67(11):1205–1214. doi: 10.1038/ejcn.2013.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mattes R.D., Campbell W.W. Effects of food form and timing of ingestion on appetite and energy intake in lean young adults and in young adults with obesity. J Am Diet Assoc. 2009;109(3):430–437. doi: 10.1016/j.jada.2008.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miquel-Kergoat S., Azais-Braesco V., Burton-Freeman B., Hetherington M.M. Effects of chewing on appetite, food intake and gut hormones: a systematic review and meta-analysis. Physiol Behav. 2015;151:88–96. doi: 10.1016/j.physbeh.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 65.Cassady B.A., Hollis J.H., Fulford A.D., Considine R.V., Mattes R.D. Mastication of almonds: effects of lipid bioaccessibility, appetite, and hormone response. Am J Clin Nutr. 2009;89(3):794–800. doi: 10.3945/ajcn.2008.26669. [DOI] [PubMed] [Google Scholar]
- 66.Almiron-Roig E., Palla L., Guest K., Ricchiuti C., Vint N., Jebb S.A., et al. Factors that determine energy compensation: a systematic review of preload studies. Nutr Rev. 2013;71(7):458–473. doi: 10.1111/nure.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dhillon J., Running C.A., Tucker R.M., Mattes R.D. Effects of food form on appetite and energy balance. Food Qual Prefer. 2016;48:368–375. [Google Scholar]
- 68.Neale E.P., Tapsell L.C., Martin A., Batterham M.J., Wibisono C., Probst Y.C. Impact of providing walnut samples in a lifestyle intervention for weight loss: a secondary analysis of the HealthTrack trial. Food Nutr Res. 2017;61(1) doi: 10.1080/16546628.2017.1344522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Njike V.Y., Ayettey R., Petraro P., Treu J.A., Katz D.L. Walnut ingestion in adults at risk for diabetes: effects on body composition, diet quality, and cardiac risk measures. BMJ Open Diabetes Res Care. 2015;3(1) doi: 10.1136/bmjdrc-2015-000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jones P.J.H., Jew S., AbuMweis S. The effect of dietary oleic, linoleic, and linolenic acids on fat oxidation and energy expenditure in healthy men. Metabolism. 2008;57(9):1198–1203. doi: 10.1016/j.metabol.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 71.Kien C.L., Bunn J.Y., Ugrasbul F. Increasing dietary palmitic acid decreases fat oxidation and daily energy expenditure. Am J Clin Nutr. 2005;82(2):320–326. doi: 10.1093/ajcn.82.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peanut Company of Australia Hi Oleic Peanuts Are Simply The Best Peanuts Yet! 2022. https://pca.com.au/pca-profile/hi-oleic-the-best-peanuts-yet/ Internet, Available from:
- 73.Jones P.J.H., Schoeller D.A. Polyunsaturated: saturated ratio of diet fat influences energy substrate utilization in the human. Metabolism. 1988;37(2):145–151. doi: 10.1016/s0026-0495(98)90009-9. [DOI] [PubMed] [Google Scholar]
- 74.Piers L.S., Walker K.Z., Stoney R.M., Soares M.J., O'Dea K. The influence of the type of dietary fat on postprandial fat oxidation rates: monounsaturated (olive oil) vs saturated fat (cream) Int J Obes. 2002;26(6):814–821. doi: 10.1038/sj.ijo.0801993. [DOI] [PubMed] [Google Scholar]
- 75.Soares M.J., Cummings S.J., Mamo J.C.L., Kenrick M., Piers L.S. The acute effects of olive oil v. cream on postprandial thermogenesis and substrate oxidation in postmenopausal women. Br J Nutr. 2004;91(2):245–252. doi: 10.1079/BJN20031047. [DOI] [PubMed] [Google Scholar]
- 76.Raynor H.A., Champagne C.M. Position of the Academy of Nutrition and Dietetics: interventions for the treatment of overweight and obesity in adults. J Acad Nutr Diet. 2016;116(1):129–147. doi: 10.1016/j.jand.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 77.Mikkelsen P.B., Toubro S., Astrup A. Effect of fat-reduced diets on 24-h energy expenditure: comparisons between animal protein, vegetable protein, and carbohydrate. Am J Clin Nutr. 2000;72(5):1135–1141. doi: 10.1093/ajcn/72.5.1135. [DOI] [PubMed] [Google Scholar]
- 78.Whitehead J.M., McNeill G., Smith J.S. The effect of protein intake on 24-h energy expenditure during energy restriction. Int J Obes Relat Metab Disord. 1996;20(8):727–732. [PubMed] [Google Scholar]
- 79.Pesta D.H., Samuel V.T. A high-protein diet for reducing body fat: mechanisms and possible caveats. Nutr Metab. 2014;11(1):1–8. doi: 10.1186/1743-7075-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu B.N., O'Sullivan A.J. Sex differences in energy metabolism need to be considered with lifestyle modifications in humans. J Nutr Metab. 2011;2011 doi: 10.1155/2011/391809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.