Abstract
There is an equivocal and inconsistent association between legume consumption and health outcomes and longevity. The purpose of this study was to examine and quantify the potential dose–response relationship between legume consumption and all-cause and cause-specific mortality in the general population. We conducted a systematic literature search on PubMed/Medline, Scopus, ISI Web of Science, and Embase from inception to September 2022, as well as reference lists of relevant original papers and key journals. A random-effects model was used to calculate summary HRs and their 95% CIs for the highest and lowest categories, as well as for a 50 g/d increment. We also modeled curvilinear associations using a 1-stage linear mixed-effects meta-analysis. Thirty-two cohorts (31 publications) involving 1,141,793 participants and 93,373 deaths from all causes were included. Higher intakes of legumes, compared with lower intakes, were associated with a reduced risk of mortality from all causes (HR: 0.94; 95% CI: 0.91, 0.98; n = 27) and stroke (HR: 0.91; 95% CI: 0.84, 0.99; n = 5). There was no significant association for CVD mortality (HR: 0.99; 95% CI: 0.91, 1.09; n =11), CHD mortality (HR: 0.93; 95% CI: 0.78, 1.09; n = 5), or cancer mortality (HR: 0.85; 95% CI: 0.72, 1.01; n = 5). In the linear dose–response analysis, a 50 g/d increase in legume intake was associated with a 6% reduction in the risk of all-cause mortality (HR: 0.94; 95% CI: 0.89, 0.99; n = 19), but no significant association was observed for the remaining outcomes. The certainty of evidence was judged from low to moderate. A higher legume intake was associated with lower mortality from all causes and stroke, but no association was observed for CVD, CHD, and cancer mortality. These results support dietary recommendations to increase the consumption of legumes.
Keywords: legume, mortality, cardiovascular disease, stroke, cancer, all-cause mortality
Statement of Significance.
Higher legume consumption was associated with a lower incidence of all causes and stroke, but there was no association with CVD, CHD, or cancer mortality. In the linear dose–response analysis, each additional 50 g/d increase in legume consumption was associated with a 6% decrease in the risk of all-cause mortality.
Introduction
Cardiovascular diseases (CVDs) remain the leading cause of death and are a major contributor to morbidity worldwide [1]. In 2019, there were an estimated 523 million prevalent CVD cases and >18 million deaths worldwide [2]. After CVDs, cancer is the second most common cause of mortality and a major cause of morbidity globally, with 10 million deaths and 20 million incident cases observed in 2020 [3]. In 2016, dietary risk factors accounted for ∼2 million CVD deaths in the WHO European Region, ∼22% of all-cause deaths and 49% of CVD deaths [4]. Dietary risk factors for cancer have also emerged as part of public health strategies and prevention activities in an attempt to reduce the global burden of cancer [5, 6].
In addition to the well-known benefits of consumption of fruits, vegetables, nuts, and whole grains [[7], [8], [9]], the potential health impact of legume consumption needs clarification. Legumes or pulses are classified as beans, peas, and soybeans in general [10]. Legumes are renowned for their unique nutrient profile, which is high in protein, dietary fiber, B vitamins, magnesium, potassium, a variety of beneficial phytonutrients, and a low GI [11]. Because of their nutritional properties and a range of potential health benefits, legumes are considered a beneficial part of healthy diets worldwide and are recommended by several health organizations [12]. However, some concerns have been raised with regard to the phytate content of legumes, which can impair nutrient absorption [13]. Studies have shown varying results regarding the association between legume consumption and health-related outcomes. Previous meta-analyses indicated that legume consumption was associated with a decreased risk of CVD [14], several cancers [[15], [16], [17]], and obesity [18]; however, no association was observed with type 2 diabetes [19], stroke [20], or metabolic syndrome [21]. There are discrepancies in the findings of prospective studies that studied the association between legume consumption and chronic disease mortality or all-cause mortality. Several studies have found an inverse association between legume consumption and the risk of all-cause mortality [[22], [23], [24], [25]], whereas others have shown a null association [[26], [27], [28], [29]] or even positive associations [30, 31].
Although there have been meta-analyses on the association between legumes and mortality [18, 32, 33], these studies have missed several cohort studies [30, [34], [35], [36], [37]] and did not consider cause-specific deaths. In addition, 14 prospective cohort studies examining the association between legume consumption and mortality have been published recently [[25], [26], [27], [28], [29], 31, [38], [39], [40], [41], [42], [43], [44], [45]], which include over half a million participants; thus, an updated meta-analysis is warranted. Consequently, our objectives were to conduct an updated systematic review and meta-analysis of prospective observational studies in the general population to determine the association between legume consumption and the risk of mortality from all causes, as well as CVD, CHD, stroke, and cancer, and to evaluate the strength and shape of the dose–response relationships and certainty of the evidence for these associations.
Methods
This systematic review and meta-analysis was reported in compliance with the standards of the PRISMA guidelines [46]. The protocol of this survey was registered previously and is available at International Prospective Register of Systematic Reviews (http://www.crd.york.ac.uk/PROSPERO, ID = CRD42022296260).
Search strategy
A systematic literature search was carried out in PubMed/Medline, ISI Web of Science, Embase, and Scopus from inception to December 2021, and was then updated to September 2022. We combined relative keywords for the study exposure (legumes) AND outcomes (all-cause mortality and cause-specific mortality) AND study design (prospective studies). There were no date or language restrictions imposed by the search. The complete history of our search strategy for each electronic database is available in Supplemental Table 1. The reference lists of relevant original papers, meta-analyses, and reviews were reviewed as potential sources of additional eligible studies. Additionally, key journals were searched manually.
Eligibility and study selection
Two independent authors (NZ and SMM) performed full-text, title, and abstract screenings. The eligible studies were included based on the following eligibility criteria.: (1) prospective cohort studies consisting of adults (aged ≥18 y); (2) studies that reported total or subtypes of legume consumption, excluding soy foods, as the exposure or one of the exposure variables; (3) studies in which the risk of mortality from all causes and/or other causes (including CVDs, coronary heart disease, stroke, and cancer) was examined; (4) studies that reported adjusted effect estimates with corresponding 95% CIs as HRs or RRs. Any disagreements were resolved through discussion with a third reviewer (AE). For the dose–response analysis, studies had to provide the following information: a numerical quantity of legume consumption (i.e., servings per day or week, grams per day or week) for ≥2 categories with corresponding adjusted HRs and 95% CIs, total or category-specific number of cases and noncases or person-years, or risk estimates on a continuous scale. When duplicate publications from the same cohorts were published, we selected the publication with the largest number of deaths or the longest follow-up. Nevertheless, when the same study published results as categorical and continuous, the categorical model was chosen for the high and low analyses, and continuous estimates were used for linear analysis. The excluded studies and the relevant reasons for exclusion are provided in Supplemental Table 2.
Data extraction
Two researchers extracted the following data independently (NZ and SMM): first author’s name, cohort name, year of publication, study location, duration of follow-up, mean or range age at entry, total sample size, sex and the number of deaths, dietary and outcome assessment method, legume intake frequency as an amount or unit of legume consumption, the fully-adjusted risk assessments with corresponding 95% CIs, confounding variables in multivariable analysis. If a study reported age or sex-specific risk estimates, the estimates were pooled using a fixed effects model, and the pooled estimate was used in the overall analysis to include each cohort only once.
Risk of bias assessment and certainty of the evidence
The Risk Of Bias In Non-Randomized Studies - of Interventions (ROBINS-I) tool was used to assess the risk of bias [47]. It includes the risk of bias due to confounding variables, selection of participants, assessment of exposure, exposure misclassification, missing data, outcome measurement, and selective reporting of results. Two investigators (NZ and SMM) independently evaluated each study, with any disagreements being resolved by discussion with a third reviewer (AE) or mutual conversation. We also used the updated Grading of Recommendations Assessment, Development, and Evaluations (GRADE) system to assess the strength of evidence for each association, integrated with the ROBINS-I tool [48]. Unlike the previous edition [49], observational studies also commence with a high level of evidence certainty.
Data synthesis and statistical analysis
The random-effects model by DerSimonian and Laird [50], which considers both between-study and within-study variations (heterogeneity), was used to calculate the summary HRs (95% CIs) of all-cause and cause-specific mortality for the highest and lowest categories of legume consumption and for a 50 g/d increment. For studies that only reported reported risk estimates on a continuous scale, we converted the HRs per-unit increment to the highest and lowest level of intake using the average difference between the midpoints of the upper and lower categories of the remaining studies included in the analysis [51].
The linear dose–response analysis was performed using the method described by Greenland and Longnecker [52] and Orsini et al. [53]. For each study, we calculated the HRs and 95% CIs for a 50 g/d increase in legume consumption. For this purpose, studies that reported the distribution of cases, person-years, and the median or range of legume consumption with corresponding risk estimates across categories were included. We used the median of legume consumption in each category when reported directly, and we estimated the midpoint of each category by taking the mean of the lower and upper bounds when intake was reported as a range. If the highest and lowest categories were open-ended, we assumed their lengths to be the same as the adjacent intervals. Legume intake was converted from servings to grams by assuming a portion size of 100 g of legumes as 1 serving for studies that reported legume intake as servings.
We used restricted cubic splines with 3 knots at fixed percentiles (10%, 50%, and 90%) of the distribution to model the potential nonlinear association [54]. The correlation within each category of HR was considered, and the study-specific estimates were combined using a 1-stage linear mixed-effects meta-analysis [55]. Compared with the traditional 2-stage method, this approach estimates the study-specific slope lines and combines them to obtain an overall average slope in a single stage, and it is more precise, flexible, and efficient [52, 56].
We assessed the heterogeneity between studies using the Cochran’s Q test [57] and the I2 statistic [58]. Substantial heterogeneity was considered when I2 was ≥50% and P-heterogeneity was <0.10. Subgroup analyses were conducted to identify potential sources of heterogeneity and were stratified by geographical location (Europe, Asia/Australia, United States, international), gender (male, female, both), duration of follow-up (<10 y/≥10 y), dietary assessment method (FFQ/food recall), number of participants (<10,000/≥10,000), and adjustment for main covariates such as energy intake, BMI, smoking, physical activity, alcohol consumption, hypertension, diabetes, blood pressure, and serum cholesterol. To investigate the robustness of the pooled effect sizes, influence analysis was performed using the leave-one-out method [59]. When ≥10 studies were included in the analysis, we assessed publication bias by visually inspecting the funnel plot and using Egger's regression test. [60]. All statistical analyses were conducted using Stata software version 15.0 (StataCorp). P values of <0.05 were considered statistically significant.
Results
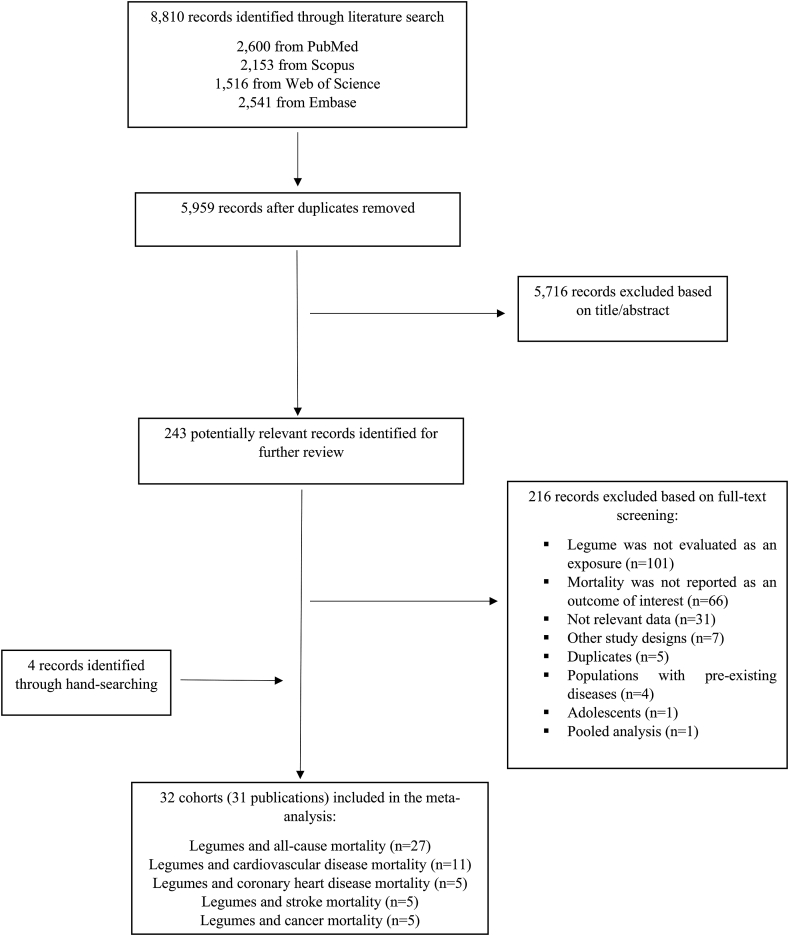
The initial database search identified 8810 papers. After the exclusion of 2851 duplicate records and 5716 irrelevant documents based on title and abstract screening, 216 potentially relevant full-text articles were considered for further review. After further exclusions were made, 32 prospective cohorts (31 publications) were included in the analyses [[22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [34], [35], [36], [38], [39], [40], [41], [42], [43], [44], [45], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70]]. Of the 31 articles, 1 included data from 2 cohort studies [24]. Twenty-seven cohorts were included in the analysis of all-cause mortality, 11 studies were included in the analysis of CVD mortality, and 5 studies were included in the analysis of CHD, stroke, and cancer mortality. Figure 1 displays the results of our literature search, screening, and selection process.
FIGURE 1.
Flow diagram of screening and selection process of the included studies.
All of the studies included were original papers that were published between 1995 and 2022. All were population-based cohort studies that were conducted in the general adult population, and studies in patients with a history of disease were excluded. Five studies were conducted in the United States [29, 40, 43, 44, 61], 14 in Europe [26, 30, 31, 34, 35, 39, 41, [64], [65], [66], [67], [68], [69], [70]], 10 in Asia [23, 24, 27, 28, 36, 38, 42, 45, 63], 1 in Australia [62], and 2 internationally [22, 25]. These studies enrolled 1,141,793 participants, ranging from 161 to 258,911. Over 3–26 y of follow-up, 93,373 deaths from all causes, 18,056 CVD deaths, 2037 CHD deaths, 2317 stroke deaths, and 12,890 cancer deaths were recorded. Of the 31 studies (32 cohorts) included, 3 were conducted exclusively among men [24, 68, 70] and 2 among women [24, 29], 2 reported separate risk estimates for men and women [63, 65], and others included both men and women. Twenty-seven cohorts used FFQs [[22], [23], [24], [25], [26], [27], [29], [30], [31], [34], [35], [36], 38, 39, 42, 44, 45, [61], [62], [63], [64], [65], [66], [67], [68], [69]] to examine dietary legume intake, whereas the remaining 5 used 24-h recalls or food records [28, 40, 41, 43, 70]. The main characteristics of the included studies are summarized in Supplemental Table 3–7, separately for each outcome. According to the ROBINS-I tool, 27 studies (85%) were judged as having a moderate risk of bias, whereas 5 studies (15%) had a serious risk of bias (Supplemental Table 8). Because there is a potential risk of bias due to residual confounding in observational studies and measurement error in dietary assessments, the risk of bias from confounding and exposure assessment will never be low.
Legumes and all-cause mortality
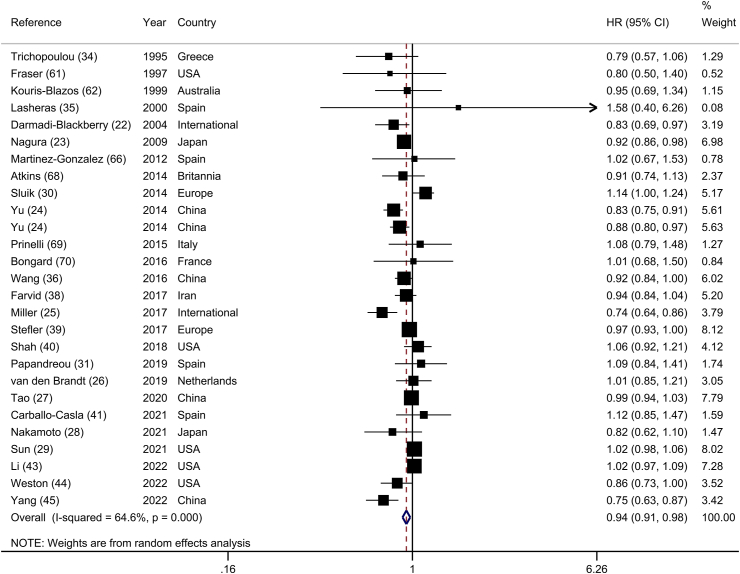
Twenty-seven prospective cohort studies (26 publications) [[22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [34], [35], [36], [38], [39], [40], [41], [43], [44], [45], 61, 62, 66, [68], [69], [70]] investigated the association between legume consumption and risk of all-cause mortality, including 989,209 participants and 93,373 deaths. Comparing the highest with the lowest categories of legume consumption, the summary HR indicated a significant inverse association between legume consumption and risk of all-cause mortality (HR: 0.94; 95% CI: 0.91, 0.98), with substantial heterogeneity between studies (I2 = 64.6%; P-heterogeneity < 0.001) (Figure 2). In the sensitivity analysis, we found that the association between legume consumption and risk of all-cause mortality did not depend on any individual study (Supplemental Figure 1). There was a significant association across subgroups in the subgroup analyses, except that the association was not significant in studies conducted in the United States (HR: 1.00; 95% CI: 0.96, 1.05; n = 5), and Europe (HR: 1.01; 95% CI: 0.95, 1.07; n = 11), those that included only women (HR: 0.95; 95% CI: 0.83, 1.10; n = 2), those with <10 y follow-up duration (HR: 0.94; 95% CI: 0.88, 1.00; n = 13), and those that used food recall or record for dietary assessment (HR: 1.01; 95% CI: 0.96, 1.07; n = 4) (Table 1). The association between legume consumption and risk of all-cause mortality was significant in studies that controlled for energy intake, smoking status, BMI, alcohol consumption, hypertension, and diabetes. Geographical location, participant number, gender, dietary assessment method, adjustment for physical activity, and diabetes were all potential sources of heterogeneity. No evidence of publication bias was found with Egger’s test (P = 0.15) or by visual inspection of the funnel plot (Supplemental Figure 2).
FIGURE 2.
Forest plot of the association between dietary intakes of legumes and risk of all-cause mortality, comparing the highest and lowest categories.
TABLE 1.
Subgroup analyses of legume consumption and risk of all-cause mortality
| Highest vs. lowest category |
Dose–response (per 50 g/d) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| n | HR (95% CI) | I2 (%) | Ph1 | n | HR (95% CI) | I2 (%) | Ph1 | ||
| All studies | 27 | 0.94 (0.91, 0.98) | 64.6 | <0.001 | 19 | 0.94 (0.89, 0.99) | 67.1 | <0.001 | |
| Geographic location | |||||||||
| United States | 5 | 1.00 (0.96, 1.05) | 28.0 | 0.23 | 3 | 0.93 (0.78, 1.11) | 61.4 | 0.07 | |
| Europe | 11 | 1.01 (0.95, 1.07) | 20.5 | 0.25 | 8 | 1.05 (0.93, 1.18) | 33.4 | 0.16 | |
| Asia and Australia | 9 | 0.90 (0.85, 0.95) | 62.9 | 0.01 | 6 | 0.89 (0.84, 0.94) | 25.4 | 0.24 | |
| International | 2 | 0.78 (0.69, 0.87) | 0.0 | 0.32 | 2 | 0.90 (0.78, 1.02) | 54.3 | 0.14 | |
| Sex | |||||||||
| Male | 3 | 0.85 (0.78, 0.93) | 0.0 | 0.51 | 1 | 0.81 (0.72, 0.89) | — | — | |
| Female | 2 | 0.95 (0.83, 1.10) | 87.1 | 0.01 | 2 | 0.94 (0.81, 1.10) | 87.9 | 0.01 | |
| Male and female | 22 | 0.95 (0.91, 0.99) | 60.2 | 0.01 | 16 | 0.95 (0.90, 0.99) | 30.2 | 0.12 | |
| Duration of follow-up, y | |||||||||
| <10 | 13 | 0.94 (0.88, 1.00) | 71.4 | <0.001 | 11 | 0.93 (0.85, 1.01) | 58.5 | 0.01 | |
| ≥10 | 14 | 0.94 (0.89, 0.99) | 58.5 | 0.01 | 8 | 0.94 (0.88, 1.00) | 67.5 | 0.01 | |
| No. of participants | |||||||||
| <10,000 | 14 | 0.89 (0.84, 0.95) | 10.3 | 0.34 | 9 | 0.88 (0.81, 0.96) | 0.0 | 0.68 | |
| ≥10,000 | 13 | 0.96 (0.92, 1.00) | 75.7 | <0.001 | 10 | 0.95 0.89, 1.00) | 78.8 | <0.001 | |
| Dietary assessment tools | |||||||||
| FFQ | 23 | 0.93 (0.89, 0.97) | 67.5 | <0.001 | 18 | 0.93 (0.88, 0.98) | 68.4 | <0.001 | |
| Food recall and record | 4 | 1.01 (0.96, 1.07) | 0.0 | 0.45 | 1 | 1.14 (0.83, 1.56) | — | — | |
| Adjustment for confounders | |||||||||
| Energy intake | Yes | 17 | 0.92 (0.88, 0.97) | 71.1 | <0.001 | 11 | 0.98 (0.87, 0.99) | 73.5 | <0.001 |
| No | 10 | 0.97 (0.91, 1.03) | 50.0 | 0.03 | 8 | 0.95 (0.86, 1.06) | 55.2 | 0.03 | |
| Smoking | Yes | 26 | 0.94 (0.90,0.98) | 65.7 | <0.001 | 18 | 0.94 (0.89, 0.99) | 68.6 | <0.001 |
| No | 1 | 1.58 (0.39, 6.25) | — | — | 1 | 1.64 (0.35, 7.67) | — | — | |
| BMI | Yes | 19 | 0.94 (0.91, 0.98) | 61.2 | 0.01 | 11 | 0.93 (0.87, 0.99) | 70.1 | <0.001 |
| No | 8 | 0.91 (0.81, 1.02) | 73.9 | <0.001 | 7 | 0.95 (0.85, 1.06) | 59.8 | 0.02 | |
| Physical activity | Yes | 22 | 0.95 (0.91, 0.99) | 68.0 | <0.001 | 15 | 0.95 (0.90, 1.00) | 70.7 | <0.001 |
| No | 5 | 0.89 (0.83, 0.95) | 0.0 | 0.69 | 4 | 0.87 (0.78, 0.96) | 0.0 | 0.79 | |
| Alcohol consumption | Yes | 16 | 0.94 (0.90, 0.99) | 70.8 | <0.001 | 10 | 0.94 (0.88, 1.01) | 78.7 | <0.001 |
| No | 11 | 0.93 (0.84, 1.03) | 53.2 | 0.02 | 9 | 0.93 (0.89, 0.97) | 0.0 | 0.73 | |
| Hypertension | Yes | 11 | 0.92 (0.86, 0.97) | 68.9 | 0.02 | 7 | 0.89 (0.83, 0.96) | 41.3 | 0.09 |
| No | 16 | 0.96 (0.91, 1.01) | 60.5 | 0.01 | 12 | 0.96 (0.91, 1.02) | 61.1 | 0.01 | |
| Blood pressure | Yes | 2 | 0.91 (0.76, 1.08) | 80.7 | <0.001 | 1 | 0.92 (0.74, 1.15) | — | — |
| No | 25 | 0.94 (0.91, 0.98) | 62.8 | <0.001 | 18 | 0.94 (0.89, 0.99) | 68.8 | <0.001 | |
| Serum cholesterol | Yes | 7 | 0.94 (0.84, 1.04) | 69.5 | 0.01 | 4 | 0.96 (0.84, 1.10) | 36.2 | 0.19 |
| No | 20 | 0.94 (0.90, 0.98) | 60.1 | <0.001 | 15 | 0.93 (0.88, 0.97) | 51.6 | 0.01 | |
| Diabetes | Yes | 12 | 0.91 (0.85, 0.97) | 79.3 | <0.001 | 9 | 0.93 (0.87, 0.99) | 78.7 | <0.001 |
| No | 15 | 0.97 (0.94, 1.01) | 27.7 | 0.15 | 10 | 0.94 (0.85, 1.04) | 44.9 | 0.06 | |
P for heterogeneity within each subgroup.
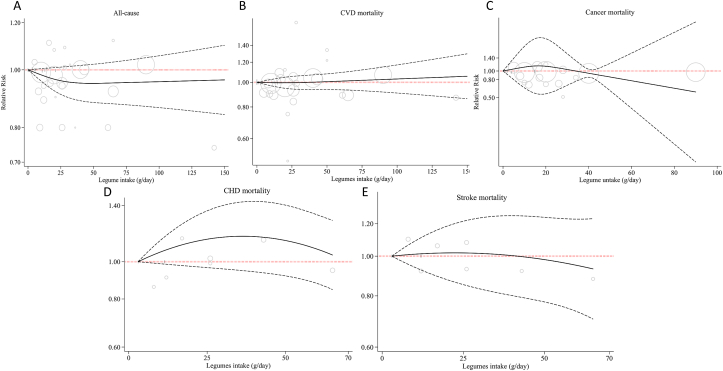
Nineteen cohorts provided sufficient data for inclusion in the linear dose–response analysis [[22], [23], [24], [25], [26], [29], [30], [31], [34], [35], [36], 38, 39, 41, 44, 61, 62, 66]. The summary estimate showed that each 50 g/d increase in legume consumption was associated with a 6% reduction in the risk of all-cause mortality (HR: 0.94; 95% CI: 0.89, 0.99; Supplemental Figure 3). Ten studies were included in the nonlinear analysis [23, 25, 26, 29, 31, 38, 41, 61, 64, 66]. However, no evidence of a nonlinear association was found (P-nonlinearity = 0.31; Figure 3 and Supplemental Table 9).
FIGURE 3.
Nonlinear dose–response association between legume consumption with risk of mortality from (A) all causes, (B) CVD, (C) cancer, (D) coronary heart disease (CHD), and (E) stroke. The solid line represents nonlinear dose–response, and dotted lines represent 95% CIs. Circles represent hazard ratio point estimates for legume consumption categories from each study, with circle size proportional to the inverse of SE. Each study’s baseline legume intake categories are indicated by small vertical black lines.
Legumes and CVD mortality
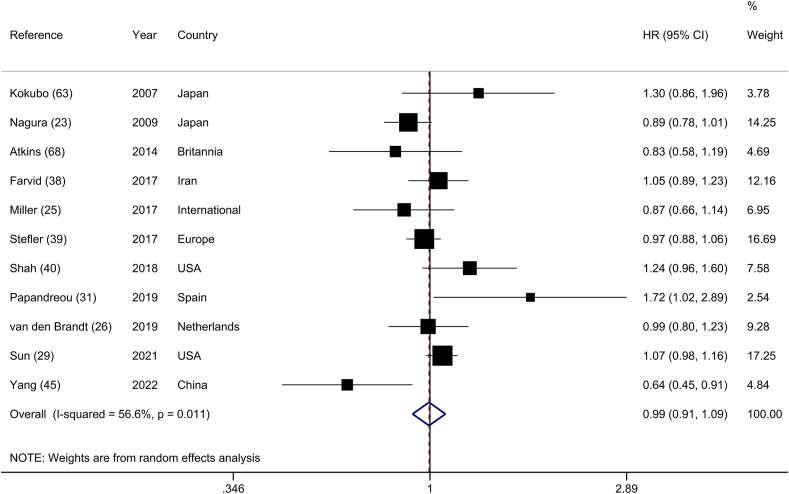
Eleven prospective cohorts [23, 25, 26, 29, 31, [38], [39], [40], 45, 63, 68], composed of 546,306 participants and 18,056 CVD deaths, were included in the analysis of the highest and lowest categories of legume intake and CVD mortality. The summary HR was 0.99 (95% CI: 0.91, 1.09), with moderate heterogeneity (I2 = 56.6%; P-heterogeneity = 0.02) (Figure 4). A sensitivity analysis excluding 1 study at a time did not materially change the summary estimate (Supplemental Figure 4). Subgroup analyses revealed that geographical location, number of participants, and adjustments for energy intake, BMI, hypertension, serum cholesterol, and diabetes were all potential sources of heterogeneity (Supplemental Table 10). Egger’s regression test (P = 0.98) and visual inspection of the funnel plot did not show any evidence of publication bias (Supplemental Figure 5).
FIGURE 4.
Forest plot of the association between dietary intakes of legumes and risk of CVD mortality, comparing the highest and lowest categories.
Eight studies contained sufficient data to be included in the linear dose–response analysis [23, 25, 26, 29, 31, 38, 39, 63]. The summary HR for CVD mortality per 50 g/d increment in legume intake was 1.01 (95% CI: 0.94, 1.08; I2 = 41.0%; Supplemental Figure 6). There was no indication of a nonlinear association (P-nonlinearity = 0.58; Figure 3 and Supplemental Table 9).
Legumes and CHD mortality
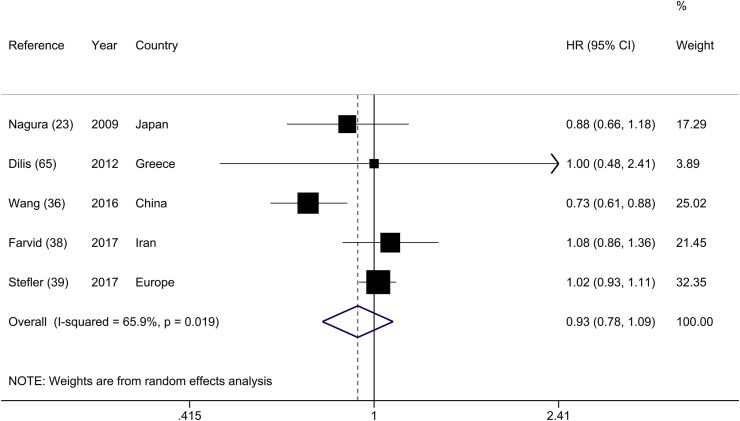
Five cohort studies [23, 36, 38, 39, 65] evaluated the relation between legume intake and CHD mortality, including 147,595 participants and 2,037 events. The summary HR showed no association between legume consumption and risk of CHD mortality (HR: 0.93; 95% CI: 0.78, 1.09), with substantial heterogeneity across studies (I2 = 65.9%; P-heterogeneity = 0.02) (Figure 5). This finding was not altered when each primary study was removed one at a time (Supplemental Figure 7). The potential sources of heterogeneity could be explained by geographical location, number of participants, and adjustments for energy intake, physical activity, and blood pressure (Supplemental Table 11).
FIGURE 5.
Forest plot of the association between dietary intakes of legumes and the risk of coronary heart disease mortality, comparing the highest and lowest categories.
All studies were included in the linear dose–response analysis. The summary HR for CHD mortality per 50 g/d increase in legume consumption was 0.90 (95% CI: 0.71, 1.13; I2 = 54.7%; Supplemental Figure 8). There was no indication of a nonlinear association between legume consumption and CHD mortality based on 2 studies [23, 38] (P-nonlinearity = 0.58; Figure 3).
Legumes and stroke mortality
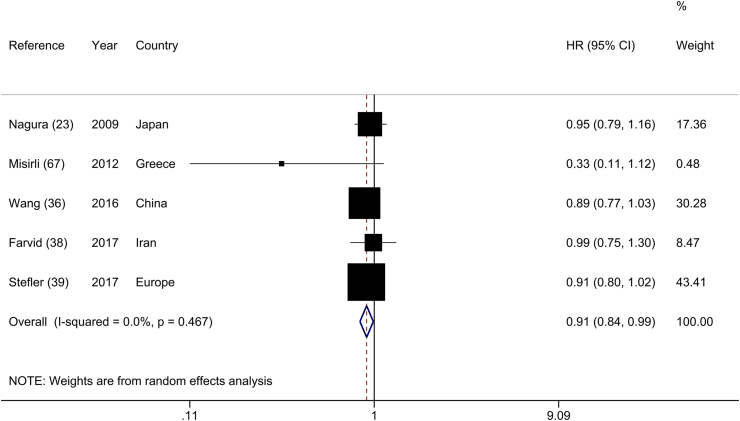
The association between the highest and lowest categories of legume consumption and stroke mortality was studied in 5 cohort studies [23, 36, 38, 39, 67], including 147,595 subjects and 2,317 stroke deaths. Stroke mortality risk was reduced by 9% when these studies were combined (HR: 0.91; 95% CI: 0.84, 0.99), and there was no evidence of heterogeneity across studies (I2 = 0.0%; P-heterogeneity = 0.46) (Figure 6). However, after the stepwise exclusion of each study in the sensitivity analysis, this association was not robust, and the results were influenced by the “Linxian Nutrition Intervention Trials” [36] and “Health Alcohol and Psychosocial Factors in Eastern Europe” [39]. When these studies were excluded from the main analysis, a nonsignificant association between legume consumption and risk of stroke mortality was observed (HR: 0.92; 95% CI: 0.82, 1.03, and HR: 0.92; 95% CI: 0.81, 1.03, respectively) (Supplemental Figure 9). The results of the subgroup analyses of legume consumption and risk of stroke mortality are presented in Supplemental Table 12.
FIGURE 6.
Forest plot of the association between dietary intakes of legumes and risk of stroke mortality, comparing the highest and lowest categories.
The linear dose–response analysis included all 5 studies [23, 36, 38, 39, 67] and showed a summary hazard ratio of 0.90 (95% CI: 0.76, 1.06; Supplemental Figure 10) per 50 g/d increase in legume intake. Furthermore, 2 studies provided sufficient data for nonlinear dose–response analysis and showed no significant association or evidence of nonlinearity (P-nonlinearity = 0.08; Figure 3).
Legumes and cancer mortality
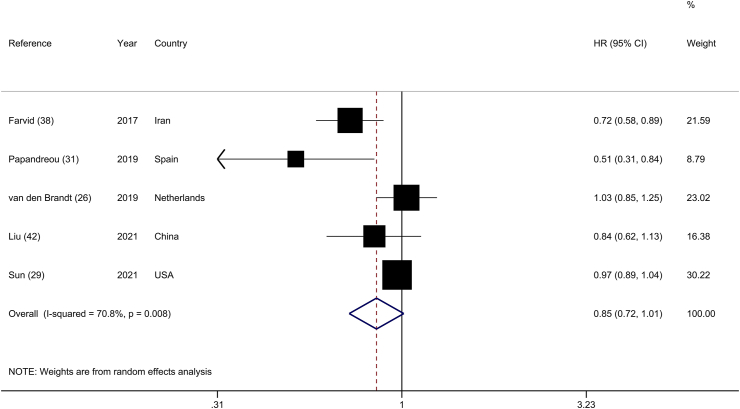
The analysis of the highest and lowest categories of legume consumption and total cancer mortality included 5 prospective cohorts [26, 29, 31, 38, 42], with 314,235 participants and 12,890 cancer deaths. High legume consumption was not significantly associated with a lower risk of cancer mortality (HR: 0.85; 95% CI: 0.72, 1.01), along with substantial heterogeneity across studies (I2 = 70.8%; P-heterogeneity = 0.01) (Figure 7). Excluding the Netherlands Cohort Study [26] in the influence analysis made the summary estimate significant, implying a 21% reduction in cancer mortality (HR: 0.79; 95% CI: 0.63, 0.99) (Supplemental Figure 11). Based on subgroup analyses, geographical location may explain the between-study heterogeneity. Stratified analysis also indicated an inverse significant association in studies that were conducted in Asia (HR: 0.76; 95% CI: 0.64, 0.90; n = 2) (Supplemental Table 13).
FIGURE 7.
Forest plot of the association between dietary intakes of legumes and risk of cancer mortality, comparing the highest and lowest categories.
The dose–response analysis was conducted on all 5 studies [26, 29, 31, 38, 42]. There was no association between every 50 g/d increment in legume consumption and cancer mortality (HR: 0.82; 95% CI: 0.61, 1.10; I2 = 72.2%; Supplemental Figure 12). We also observed no indication of a nonlinear relationship (P-nonlinearity = 0.19; Figure 3 and Supplemental Table 9).
Certainty of evidence
The GRADE system was used to assess the degree of certainty in the evidence. None of the associations had a high level of evidence certainty. However, the level of evidence for mortality from all causes, and stroke was rated “moderate,” whereas it was rated “low” for CVD, CHD, and cancer mortality (Supplemental Table 14). This judgment was chiefly influenced by concerns due to the risk of bias due to residual confounding and inconsistency.
Discussion
Principal findings
In this systematic review and dose–response meta-analysis of data from 32 prospective cohort studies, higher legume intake was associated with 6% and 9% reductions in the risk of all-cause, and stroke mortality, respectively. However, there was no significant association between legume intake and CVD, CHD, and cancer mortality. Each 50 g/d increase in legume consumption was associated with a 6% reduction in the risk of all-cause mortality. There was also no evidence of a nonlinear association between legume consumption and risk of mortality from all-cause, CVD, CHD, strokes, or cancer mortality.
Comparison with other studies
Recognized healthy eating patterns (Dietary Approaches to Stop Hypertension diet and Mediterranean diet) recommend a certain amount of legumes in addition to other foods (seeds, olive oil, dairy, fruits, etc.) that protect against overall, CVD and cancer mortality [71, 72]. A series of systematic reviews and meta-analyses involving data from 185 prospective studies and 58 clinical trials reported a 15%–30% reduction in all-cause and CVD-related mortality, as well as the incidence of CHD, stroke, and colorectal cancer when high-fiber consumers were compared with low-fiber consumers [73]. These associations were particularly noticeable for dietary fiber intakes ranging from 25 to 29 g/d, implying that increasing legume intake may be a reasonable approach to achieving this goal. In a prospective cohort study using data from the US NHANES from 1999 to 2014 (37,233 adults aged ≥20 y), the risk of total mortality was reduced by 9% (HR: 0.91; 95% CI: 0.87, 0.95) and 11% (HR: 0.89; 95% CI: 0.85, 0.93) for healthy low-carbohydrate and healthy low-fat diet scores, respectively, which were defined as a dietary pattern not only restricted in carbohydrates and fats but also rich in vegetable proteins and whereby legumes were a prevalent food item [74]. Even as carbohydrate sources, legumes can be included in low-carbohydrate diets because these eating patterns involve <45% of energy intake from carbohydrates, which differs from very-low-carbohydrate diets or ketogenic diets, which are consistent with less than 40–50 g of carbohydrates per day [75].
In the present meta-analysis, an inverse association was found between legume consumption and all-cause mortality and stroke mortality but not with other specific causes of mortality. The results for stroke mortality, however, were strongly influenced by the findings of 2 studies in the sensitivity analysis. The reported discrepancies between the risk of incidence of all-cause mortality and death from CVD, CHD, and cancer are not totally evident. The protective association is most likely because of a greater number of studies in this area and, as a result, a greater number of participants and deaths. More precise estimates are required to properly assess the association between legume consumption and CVD, CHD, and cancer mortality. Our meta-analysis provides the most up-to-date estimates of the association between legume consumption and all-cause and cause-specific mortality, and it is in line with the previous reviews and meta-analyses on the topic [18, 32, 33]. Despite this, the current meta-analysis includes 1.5–4 times the number of studies as the previously published meta-analyses, as well as roughly twice the number of participants and deaths. For instance, we included 27 cohorts (93,373 deaths) in the highest and lowest analysis of legume consumption and all-cause mortality, compared with 17 studies (53,085 deaths) [33] and 4 studies (18,408 deaths) [32] in previous meta-analyses. In the linear dose–response analysis for all-cause mortality, we included 19 cohorts as opposed to 6 studies in 1 meta-analysis [33]. In addition, we examined the certainty of evidence and dose–response relationships for cause-specific mortality that had not previously been studied.
Mechanisms
Several potential mechanisms could contribute to the beneficial associations observed with legume consumption in this meta-analysis. Because of a variety of constituent parts, legumes are thought to have cholesterol-lowering properties. Soluble fiber, in particular, has been shown to bind to bile acids in the digestive tract, preventing bile acids from being reabsorbed into the body [76]. As a result, increased bile acid synthesis decreases the liver’s cholesterol pool and increases serum cholesterol absorption, lowering blood cholesterol levels [77]. Phytosterols, a component of plant cell membranes that have been shown to lower blood cholesterol levels, are found in low to moderate concentrations in a variety of legumes, including chickpeas [78, 79]. Legumes are also high in saponins, which may help reduce cholesterol absorption from the gut [80]. Nonsoy legumes and whole soy foods have been shown to improve glycemic control indicators in various ways. Carbohydrates with a slow rate of digestion can be found in nonsoy legumes [81]. Resistant starch, which contains a higher proportion of amylose to amylopectin than other starchy carbohydrates, may be responsible for this characteristic. When combined with the high fiber content of nonsoy legumes, this characteristic lowers the GI, which may explain the beneficial effects on glucose control indicators [82]. Furthermore, because of their high fiber and protein content, as well as their low GI, nonsoy legumes may help people lose weight by increasing satiety [83] through various mechanisms [84]. Increased intraluminal viscosity reduces gastric emptying and macronutrient absorption by slowing digestion and increasing gastric distention caused by chewing effort; by influencing gut hormone secretion; and producing SCFAs (propionate, butyrate, and acetate) derived from the fermentation of fiber by colonic bacteria, which slows digestion and increases gastric distention. Foods with a low GI stimulate the digestive tract receptors for a longer time, resulting in fullness signals [85]. Because patients with CVD who are also inflamed have a poor prognosis and are more likely to relapse, the antioxidant and anti-inflammatory properties of legumes may play a role in reducing stroke mortality. The potential mechanisms underlying the inverse association between legume consumption and stroke mortality may include the following. A high concentration of phytosterols is found in legumes, and meta-analyses of randomized controlled trials have shown that they significantly reduce total cholesterol, LDL cholesterol, atherogenic apolipoprotein levels, and free fatty acids [86, 87]. These compounds might also reduce the risk of atherosclerosis [88]. Alternatively, the high fiber content of legumes, as a plant-derived food, has been associated with a reduced risk of stroke in prospective studies [89]. Fiber is believed to reduce chronic inflammation and to improve body metabolism by regulating body weight, serum cholesterol levels, blood pressure, and insulin resistance, as well as fibrinolysis and coagulation, which may be relevant in the context of existing atherosclerotic plaques [90]. Because patients with CVD who are also inflamed have a poor prognosis and are more likely to relapse [28], the antioxidant and anti-inflammatory properties of legumes may play a role in reducing stroke mortality [91].
Strengths and limitations
The primary strength of our study is that it includes prospective cohort studies and a large number of participants and deaths, providing greater statistical power to quantitatively evaluate the association between legume consumption and mortality. We also conducted linear and nonlinear dose–response analyses to elucidate the strength and shape of the observed associations. Other strengths include the use of comprehensive search strategy, extensive subgroup, sensitivity, and influence analyses, assessing the risk of bias and the certainty of evidence for each association.
Our findings should be interpreted in light of several limitations. First, because of the observational nature of the included studies, the observed associations may be influenced by residual or unmeasured confounding factors. Furthermore, causality cannot be established based only on observational data. Second, there was substantial heterogeneity between studies in the analyses of legumes and all-cause, CVD, CHD, and cancer mortality. Although we accounted for potential sources of heterogeneity, such as geographical location, participant numbers, gender, duration of follow-up, and confounding variables, studies may have also differed in the types of legumes consumed, the precision with which legume intake was measured, the cooking method, and the definition of legumes. Third, most included studies assessed legumes only once at the study’s baseline and did not account for changes in legume consumption over time, suggesting that measurement errors in legume intake may have influenced findings. Fourth, most studies lacked information on the preparation and cooking of legumes. Legumes’ nutritional value and nutrient loss vary according to their cooking method [92]. Differences in the types of legumes, as well as mixed dishes or settings in which legumes are consumed, could have influenced the observed associations. For example, beans are often eaten with bacon, sausages, and eggs in Europe and United States, which could have a different impact on health than a dish with mung dahl, vegetables, and brown rice. Whether this could explain the geographical differences in results requires further study. Fifth, the association of dietary legume consumption with stroke and cancer mortality was not stable in the influence analysis, and relatively few studies were included in the analyses of CHD and stroke mortality. Therefore, more research is required before these associations can be conclusive. Sixth, regional differences in legume intake may have influenced the highest and lowest legume intake categories, as well as the results of these comparisons. To deal with these differences and the overlap of legume intake ranges between the studies, we conducted the dose–response analysis. Finally, one of the major limitations of meta-analyses today is their inability to address the critical issue of substitution in practice. Legumes, for example, may be more beneficial than a very common starch and refined sugar breakfast, but they may not be as beneficial as a breakfast of whole grains and nuts.
Conclusions, policy implications, and future research
Altogether, higher legume intake was associated with a reduced risk of all-cause and stroke mortality; however, no association was found for CVD, CHD, and cancer mortality. Each 50-g increase in legume intake was associated with a 6% lower risk of all-cause mortality in the linear dose–response analysis. Our findings, therefore, strongly support current dietary recommendations to consume more legumes in the general population. This meta-analysis is of importance for public health globally, as increased consumption of legumes is likely to be cost-effective and bring health benefits over time. Although this meta-analysis focused on total legume intake, which is an important item to base overall conclusions on, further epidemiological research is warranted to elucidate the effects of particular types of legumes on the risk of specific chronic diseases and causes of death.
Funding
DA was funded by Olav og Gerd Meidel Raagholt’s Stiftelse for Medisinsk Forskning and the South-East Norway Regional Health Authority (grant 2017076).
Author disclosures
The authors report no conflicts of interest.
Acknowledgments
The authors’ responsibilities were as follows – NZ, AE: contributed to the study conception, literature search, screened the articles, data extraction, data analysis, and wrote the manuscript; SMM: contributed to the literature search, data extraction, data analysis, and manuscript drafting; HOS, SHR, BL: contributed to manuscript drafting and approving the final manuscript; DA: contributed to the data analysis and approving the final manuscript; and all authors: acknowledge full responsibility for the analyses and interpretation of the report and have read and approved the final manuscript. The corresponding author certifies that all of the listed authors meet the authorship criteria and that no other authors who meet the criteria have been overlooked.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.advnut.2022.10.009.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Kim H.C. Epidemiology of cardiovascular disease and its risk factors in Korea. Glob Health Med. 2021;3(3):134–141. doi: 10.35772/ghm.2021.01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roth G.A., Mensah G.A., Johnson C.O., Addolorato G., Ammirati E., Baddour L.M., et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferlay J., Colombet M., Soerjomataram I., Parkin D.M., Piñeros M., Znaor A., Bray F. Cancer statistics for the year 2020: An overview. Int J Cancer. 2021;149(4):778–789. doi: 10.1002/ijc.33588. [DOI] [PubMed] [Google Scholar]
- 4.Meier T., Gräfe K., Senn F., Sur P., Stangl G.I., Dawczynski C., et al. Cardiovascular mortality attributable to dietary risk factors in 51 countries in the WHO European Region from 1990 to 2016: a systematic analysis of the Global Burden of Disease Study. Eur J Epidemiol. 2019;34(1):37–55. doi: 10.1007/s10654-018-0473-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonzalez C.A., Riboli E. Diet and cancer prevention: contributions from the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur J Cancer. 2010;46(14):2555–2562. doi: 10.1016/j.ejca.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 6.Mousavi S.M., Zargarzadeh N., Rigi S., Persad E., Pizarro A.B., Hasani-Ranjbar S., et al. Egg consumption and risk of all-cause and cause-specific mortality: a systematic review and dose-response meta-analysis of prospective studies. Adv Nutr. 2022;13(5):1762–1773. doi: 10.1093/advances/nmac040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aune D., Keum N., Giovannucci E., Fadnes L.T., Boffetta P., Greenwood D.C., et al. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2016;353:i2716. doi: 10.1136/bmj.i2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aune D., Keum N., Giovannucci E., Fadnes L.T., Boffetta P., Greenwood D.C., et al. Nut consumption and risk of cardiovascular disease, total cancer, all-cause and cause-specific mortality: a systematic review and dose-response meta-analysis of prospective studies. BMC Med. 2016;14(1):207. doi: 10.1186/s12916-016-0730-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aune D., Giovannucci E., Boffetta P., Fadnes L.T., Keum N., Norat T., et al. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol. 2017;46(3):1029–1056. doi: 10.1093/ije/dyw319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Becerra-Tomás N., Papandreou C., Salas-Salvadó J. Legume consumption and cardiometabolic health. Adv Nutr. 2019;10(suppl 4):S437–S450. doi: 10.1093/advances/nmz003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rebello C.J., Greenway F.L., Finley J.W. A review of the nutritional value of legumes and their effects on obesity and its related co-morbidities. Obes Rev. 2014;15(5):392–407. doi: 10.1111/obr.12144. [DOI] [PubMed] [Google Scholar]
- 12.Cheng A. Canadian Diabetes Association 2013 clinical practice guidelines for the prevention and management of diabetes in Canada. Introduction. Can J Diabetes. 2013;37:S1–3. doi: 10.1016/j.jcjd.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Urbano G., López-Jurado M., Aranda P., Vidal-Valverde C., Tenorio E., Porres J. The role of phytic acid in legumes: antinutrient or beneficial function? J Physiol Biochem. 2000;56(3):283–294. doi: 10.1007/BF03179796. [DOI] [PubMed] [Google Scholar]
- 14.Marventano S., Izquierdo Pulido M., Sánchez-González C., Godos J., Speciani A., Galvano F., et al. Legume consumption and CVD risk: a systematic review and meta-analysis. Public Health Nutr. 2017;20(2):245–254. doi: 10.1017/S1368980016002299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu B., Sun Y., Qi L., Zhong R., Miao X. Dietary legume consumption reduces risk of colorectal cancer: evidence from a meta-analysis of cohort studies. Sci Rep. 2015;5:8797. doi: 10.1038/srep08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J., Mao Q.Q. Legume intake and risk of prostate cancer: a meta-analysis of prospective cohort studies. Oncotarget. 2017;8(27):44776–44784. doi: 10.18632/oncotarget.16794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhong X.S., Ge J., Chen S.W., Xiong Y.Q., Ma S.J., Chen Q. Association between dietary isoflavones in soy and legumes and endometrial cancer: a systematic review and meta-analysis. J Acad Nutr Diet. 2018;118(4):637–651. doi: 10.1016/j.jand.2016.09.036. [DOI] [PubMed] [Google Scholar]
- 18.Viguiliouk E., Glenn A.J., Nishi S.K., Chiavaroli L., Seider M., Khan T., et al. Associations between dietary pulses alone or with other legumes and cardiometabolic disease outcomes: an umbrella review and updated systematic review and meta-analysis of prospective cohort studies. Adv Nutr. 2019;10(suppl 4):S308–S319. doi: 10.1093/advances/nmz113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang J., Wan Y., Zhao M., Zhong H., Zheng J.S., Feng F. Legume and soy intake and risk of type 2 diabetes: a systematic review and meta-analysis of prospective cohort studies. Am J Clin Nutr. 2020;111(3):677–688. doi: 10.1093/ajcn/nqz338. [DOI] [PubMed] [Google Scholar]
- 20.Shi Z.Q., Tang J.J., Wu H., Xie C.Y., He Z.Z. Consumption of nuts and legumes and risk of stroke: a meta-analysis of prospective cohort studies. Nutr Metab Cardiovasc Dis. 2014;24(12):1262–1271. doi: 10.1016/j.numecd.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 21.Jiang Y.T., Zhang J.Y., Liu Y.S., Chang Q., Zhao Y.H., Wu Q.J. Relationship between legume consumption and metabolic syndrome: a systematic review and meta-analysis of observational studies. Nutr Metab Cardiovasc Dis. 2020;30(3):384–392. doi: 10.1016/j.numecd.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Darmadi-Blackberry I., Wahlqvist M.L., Kouris-Blazos A., Steen B., Lukito W., Horie Y., et al. Legumes: the most important dietary predictor of survival in older people of different ethnicities. Asia Pac J Clin Nutr. 2004;13(2):217–220. [PubMed] [Google Scholar]
- 23.Nagura J., Iso H., Watanabe Y., Maruyama K., Date C., Toyoshima H., et al. Fruit, vegetable and bean intake and mortality from cardiovascular disease among Japanese men and women: the JACC study. Br J Nutr. 2009;102(2):285–292. doi: 10.1017/S0007114508143586. [DOI] [PubMed] [Google Scholar]
- 24.Yu D.X., Zhang X.L., Xiang Y.B., Yang G., Li H.L., Gao Y.T., et al. Adherence to dietary guidelines and mortality: a report from prospective cohort studies of 134,000 Chinese adults in urban Shanghai. Am J Clin Nutr. 2014;100(2):693–700. doi: 10.3945/ajcn.113.079194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller V., Mente A., Dehghan M., Rangarajan S., Zhang X., Swaminathan S., et al. Fruit, vegetable, and legume intake, and cardiovascular disease and deaths in 18 countries (PURE): a prospective cohort study. Lancet. 2017;390(10107):2037–2049. doi: 10.1016/S0140-6736(17)32253-5. [DOI] [PubMed] [Google Scholar]
- 26.van den Brandt P.A. Red meat, processed meat, and other dietary protein sources and risk of overall and cause-specific mortality in the Netherlands Cohort Study. Eur J Epidemiol. 2019;34(4):351–369. doi: 10.1007/s10654-019-00483-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tao L., Xie Z., Huang T. Dietary diversity and all-cause mortality among Chinese adults aged 65 or older: a community-based cohort study. Asia Pac J Clin Nutr. 2020;29(1):152–160. doi: 10.6133/apjcn.202003_29(1).0020. [DOI] [PubMed] [Google Scholar]
- 28.Nakamoto M., Otsuka R., Tange C., Nishita Y., Tomida M., Imai T., et al. Intake of isoflavones reduces the risk of all-cause mortality in middle-aged Japanese. Eur J Clin Nutr. 2021;75(12):1781–1791. doi: 10.1038/s41430-021-00890-w. [DOI] [PubMed] [Google Scholar]
- 29.Sun Y., Liu B., Snetselaar L.G., Wallace R.B., Shadyab A.H., Kroenke C.H., et al. Association of major dietary protein sources with all-cause and cause-specific mortality: prospective cohort study. J Am Heart Assoc. 2021;10(5) doi: 10.1161/JAHA.119.015553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sluik D., Boeing H., Li K., Kaaks R., Johnsen N.F., Tjønneland A., et al. Lifestyle factors and mortality risk in individuals with diabetes mellitus: are the associations different from those in individuals without diabetes? Diabetologia. 2014;57(1):63–72. doi: 10.1007/s00125-013-3074-y. [DOI] [PubMed] [Google Scholar]
- 31.Papandreou C., Becerra-Tomás N., Bulló M., Martínez-González M.Á., Corella D., Estruch R., et al. Legume consumption and risk of all-cause, cardiovascular, and cancer mortality in the PREDIMED study. Clin Nutr. 2019;38(1):348–356. doi: 10.1016/j.clnu.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 32.Li H., Li J., Shen Y., Wang J., Zhou D. Legume consumption and all-cause and cardiovascular disease mortality. Biomed Res Int. 2017;2017 doi: 10.1155/2017/8450618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwingshackl L., Schwedhelm C., Hoffmann G., Lampousi A.M., Knüppel S., Iqbal K., et al. Food groups and risk of all-cause mortality: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr. 2017;105(6):1462–1473. doi: 10.3945/ajcn.117.153148. [DOI] [PubMed] [Google Scholar]
- 34.Trichopoulou A., Kouris-Blazos A., Wahlqvist M.L., Gnardellis C., Lagiou P., Polychronopoulos E., et al. Diet and overall survival in elderly people. BMJ. 1995;311(7018):1457–1460. doi: 10.1136/bmj.311.7018.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lasheras C., Fernandez S., Patterson A.M. Mediterranean diet and age with respect to overall survival in institutionalized, nonsmoking elderly people. Am J Clin Nutr. 2000;71(4):987–992. doi: 10.1093/ajcn/71.4.987. [DOI] [PubMed] [Google Scholar]
- 36.Wang J.B., Fan J.H., Dawsey S.M., Sinha R., Freedman N.D., Taylor P.R., et al. Dietary components and risk of total, cancer and cardiovascular disease mortality in the Linxian nutrition intervention trials cohort in China. Sci Rep. 2016;6 doi: 10.1038/srep22619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leo Q.J.N., Ollberding N.J., Wilkens L.R., Kolonel L.N., Henderson B.E., Le Marchand L., et al. Nutritional factors and non-Hodgkin lymphoma survival in an ethnically diverse population: the Multiethnic Cohort. Eur J Clin Nutr. 2016;70(1):41–46. doi: 10.1038/ejcn.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farvid M.S., Malekshah A.F., Pourshams A., Poustchi H., Sepanlou S.G., Sharafkhah M., et al. Dietary protein sources and all-cause and cause-specific mortality: the Golestan Cohort Study in Iran. Am J Prev Med. 2017;52(2):237–248. doi: 10.1016/j.amepre.2016.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stefler D., Malyutina S., Kubinova R., Pajak A., Peasey A., Pikhart H., et al. Mediterranean diet score and total and cardiovascular mortality in Eastern Europe: the HAPIEE study. Eur J Nutr. 2017;56(1):421–429. doi: 10.1007/s00394-015-1092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shah N.S., Leonard D., Finley C.E., Rodriguez F., Sarraju A., Barlow C.E., et al. Dietary patterns and long-term survival: a retrospective study of healthy primary care patients. Am J Med. 2018;131(1):48–55. doi: 10.1016/j.amjmed.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 41.Carballo-Casla A., Ortolá R., García-Esquinas E., Oliveira A., Sotos-Prieto M., Lopes C., et al. The Southern European Atlantic Diet and all-cause mortality in older adults. BMC Med. 2021;19(1):36. doi: 10.1186/s12916-021-01911-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu W., Hu B., Dehghan M., Mente A., Wang C., Yan R., et al. Fruit, vegetable, and legume intake and the risk of all-cause, cardiovascular, and cancer mortality: a prospective study. Clin Nutr. 2021;40(6):4316–4323. doi: 10.1016/j.clnu.2021.01.016. [DOI] [PubMed] [Google Scholar]
- 43.Li H., Zeng X., Wang Y., Zhang Z., Zhu Y., Li X., et al. A prospective study of healthful and unhealthful plant-based diet and risk of overall and cause-specific mortality. Eur J Nutr. 2022;61(1):387–398. doi: 10.1007/s00394-021-02660-7. [DOI] [PubMed] [Google Scholar]
- 44.Weston L.J., Kim H., Talegawkar S.A., Tucker K.L., Correa A., Rebholz CMJPm Plant-based diets and incident cardiovascular disease and all-cause mortality in African Americans: a cohort study. PLoS Med. 2022;19(1) doi: 10.1371/journal.pmed.1003863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang J., Yang A., Yeung S., Woo J., Lo K.J.N. Joint associations of food groups with all-cause and cause-specific mortality in the Mr. OS and Ms. OS study: a prospective cohort. Nutrients. 2022;14(19):3915. doi: 10.3390/nu14193915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jüni P., Loke Y., Pigott T., Ramsay C., Regidor D., Rothstein H., et al. Risk of bias in non-randomized studies of interventions (ROBINS-I): detailed guidance. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schünemann H.J., Cuello C., Akl E.A., Mustafa R.A., Meerpohl J.J., Thayer K., et al. GRADE guidelines: 18. How ROBINS-I and other tools to assess risk of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. J Clin Epidemiol. 2019;111:105–114. doi: 10.1016/j.jclinepi.2018.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Balshem H., Helfand M., Schünemann H.J., Oxman A.D., Kunz R., Brozek J., et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 50.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 51.Jayedi A., Rashidy-Pour A., Parohan M., Zargar M.S., Shab-Bidar S. Dietary antioxidants, circulating antioxidant concentrations, total antioxidant capacity, and risk of all-cause mortality: a systematic review and dose-response meta-analysis of prospective observational studies. Adv Nutr. 2018;9(6):701–716. doi: 10.1093/advances/nmy040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Greenland S., Longnecker M.P. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 53.Orsini N., Bellocco R., Greenland S. Generalized least squares for trend estimation of summarized dose–response data. Stata J. 2006;6(1):40–57. [Google Scholar]
- 54.Harrell Jr. F.E. Regression modeling strategies: with applications to linear models, logistic and ordinal regression, and survival analysis. 2nd Edition. Springer; New York City (USA): 2015. [Google Scholar]
- 55.Crippa A., Discacciati A., Bottai M., Spiegelman D., Orsini N. One-stage dose-response meta-analysis for aggregated data. Stat Methods Med Res. 2019;28(5):1579–1596. doi: 10.1177/0962280218773122. [DOI] [PubMed] [Google Scholar]
- 56.Orsini N., Li R., Wolk A., Khudyakov P., Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175(1):66–73. doi: 10.1093/aje/kwr265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Higgins J.P., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., et al. Cochrane handbook for systematic reviews of interventions. 2nd Edition. John Wiley & Sons; Chichester (UK): 2019. [Google Scholar]
- 58.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 59.Mousavi S.M., Jalilpiran Y., Karimi E., Aune D., Larijani B., Mozaffarian D., et al. Dietary intake of linoleic acid, its concentrations, and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of prospective cohort studies. Diabetes Care. 2021;44(9):2173–2181. doi: 10.2337/dc21-0438. [DOI] [PubMed] [Google Scholar]
- 60.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fraser G.E., Sumbureru D., Pribiš P., Neil R.L., Frankson M.A.C. Association among health habits, risk factors, and all-cause mortality in a black California population. Epidemiology. 1997;8(2):168–174. doi: 10.1097/00001648-199703000-00008. [DOI] [PubMed] [Google Scholar]
- 62.Kouris-Blazos A., Gnardellis C., Wahlqvist M.L., Trichopoulos D., Lukito W., Trichopoulou A. Are the advantages of the Mediterranean diet transferable to other populations? A cohort study in Melbourne, Australia. Br J Nutr. 1999;82(1):57–61. doi: 10.1017/s0007114599001129. [DOI] [PubMed] [Google Scholar]
- 63.Kokubo Y., Iso H., Ishihara J., Okada K., Inoue M., Tsugane S., et al. Association of dietary intake of soy, beans, and isoflavones with risk of cerebral and myocardial infarctions in Japanese populations: the Japan Public Health Center-based (JPHC) study cohort I. Circulation. 2007;116(22):2553–2562. doi: 10.1161/CIRCULATIONAHA.106.683755. [DOI] [PubMed] [Google Scholar]
- 64.Trichopoulou A., Bamia C., Trichopoulos D. Anatomy of health effects of Mediterranean diet: Greek EPIC prospective cohort study. BMJ. 2009;338:b2337. doi: 10.1136/bmj.b2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dilis V., Katsoulis M., Lagiou P., Trichopoulos D., Naska A., Trichopoulou A. Mediterranean diet and CHD: the Greek European Prospective Investigation into Cancer and Nutrition cohort. Br J Nutr. 2012;108(4):699–709. doi: 10.1017/S0007114512001821. [DOI] [PubMed] [Google Scholar]
- 66.Martínez-González M.A., Guillén-Grima F., De Irala J., Ruíz-Canela M., Bes-Rastrollo M., Beunza J.J., et al. The Mediterranean diet is associated with a reduction in premature mortality among middle-aged adults. J Nutr. 2012;142(9):1672–1678. doi: 10.3945/jn.112.162891. [DOI] [PubMed] [Google Scholar]
- 67.Misirli G., Benetou V., Lagiou P., Bamia C., Trichopoulos D., Trichopoulou A. Relation of the traditional Mediterranean diet to cerebrovascular disease in a Mediterranean population. Am J Epidemiol. 2012;176(12):1185–1192. doi: 10.1093/aje/kws205. [DOI] [PubMed] [Google Scholar]
- 68.Atkins J.L., Whincup P.H., Morris R.W., Lennon L.T., Papacosta O., Wannamethee S.G. High diet quality is associated with a lower risk of cardiovascular disease and all-cause mortality in older men. J Nutr. 2014;144(5):673–680. doi: 10.3945/jn.113.186486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Prinelli F., Yannakoulia M., Anastasiou C.A., Adorni F., Di Santo S.G., Musicco M., et al. Mediterranean diet and other lifestyle factors in relation to 20-year all-cause mortality: a cohort study in an Italian population. Br J Nutr. 2015;113(6):1003–1011. doi: 10.1017/S0007114515000318. [DOI] [PubMed] [Google Scholar]
- 70.Bongard V., Arveiler D., Dallongeville J., Ruidavets J.B., Wagner A., Simon C., et al. Food groups associated with a reduced risk of 15-year all-cause death. Eur J Clin Nutr. 2016;70(6):715–722. doi: 10.1038/ejcn.2016.19. [DOI] [PubMed] [Google Scholar]
- 71.Soltani S., Arablou T., Jayedi A., Salehi-Abargouei A. Adherence to the dietary approaches to stop hypertension (DASH) diet in relation to all-cause and cause-specific mortality: a systematic review and dose-response meta-analysis of prospective cohort studies. Nutr J. 2020;19(1):37. doi: 10.1186/s12937-020-00554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Becerra-Tomás N., Blanco Mejía S., Viguiliouk E., Khan T., Kendall C.W.C., Kahleova H., et al. Mediterranean diet, cardiovascular disease and mortality in diabetes: a systematic review and meta-analysis of prospective cohort studies and randomized clinical trials. Crit Rev Food Sci Nutr. 2020;60(7):1207–1227. doi: 10.1080/10408398.2019.1565281. [DOI] [PubMed] [Google Scholar]
- 73.Reynolds A., Mann J., Cummings J., Winter N., Mete E., Te Morenga L. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet. 2019;393(10170):434–445. doi: 10.1016/S0140-6736(18)31809-9. [DOI] [PubMed] [Google Scholar]
- 74.Shan Z., Guo Y., Hu F.B., Liu L., Qi Q. Association of low-carbohydrate and low-fat diets with mortality among US adults. JAMA Intern Med. 2020;180(4):513–523. doi: 10.1001/jamainternmed.2019.6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Macedo R.C.O., Santos H.O., Tinsley G.M., Reischak-Oliveira A. Low-carbohydrate diets: effects on metabolism and exercise–a comprehensive literature review. Clin Nutr ESPEN. 2020;40:17–26. doi: 10.1016/j.clnesp.2020.07.022. [DOI] [PubMed] [Google Scholar]
- 76.Bazzano L.A. Effects of soluble dietary fiber on low-density lipoprotein cholesterol and coronary heart disease risk. Curr Atheroscler Rep. 2008;10(6):473–477. doi: 10.1007/s11883-008-0074-3. [DOI] [PubMed] [Google Scholar]
- 77.Charlton-Menys V., Durrington P.N. Human cholesterol metabolism and therapeutic molecules. Exp Physiol. 2008;93(1):27–42. doi: 10.1113/expphysiol.2007.035147. [DOI] [PubMed] [Google Scholar]
- 78.Bazzano L.A., Thompson A.M., Tees M.T., Nguyen C.H., Winham D.M. Non-soy legume consumption lowers cholesterol levels: a meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2011;21(2):94–103. doi: 10.1016/j.numecd.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Makhmudova U., Schulze P.C., Lütjohann D., Weingärtner O. Phytosterols and cardiovascular disease. Curr Atheroscler Rep. 2021;23(11):68. doi: 10.1007/s11883-021-00964-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rochfort S., Panozzo J. Phytochemicals for health, the role of pulses. J Agric Food Chem. 2007;55(20):7981–7994. doi: 10.1021/jf071704w. [DOI] [PubMed] [Google Scholar]
- 81.Angioloni A., Collar C. High legume-wheat matrices: an alternative to promote bread nutritional value meeting dough viscoelastic restrictions. Eur Food Res Technol. 2012;234(2):273–284. [Google Scholar]
- 82.Clemente A., Olias R. Beneficial effects of legumes in gut health. Curr Opin Food Sci. 2017;14:32–36. [Google Scholar]
- 83.Abete I., Parra D., Martinez J.A. Legume-, fish-, or high-protein-based hypocaloric diets: effects on weight loss and mitochondrial oxidation in obese men. J Med Food. 2009;12(1):100–108. doi: 10.1089/jmf.2007.0700. [DOI] [PubMed] [Google Scholar]
- 84.Luo J., Qi J., Wang W., Luo Z., Liu L., Zhang G., et al. Antiobesity effect of flaxseed polysaccharide via inducing satiety due to leptin resistance removal and promoting lipid metabolism through the AMP-activated protein kinase (AMPK) signaling pathway. J Agric Food Chem. 2019;67(25):7040–7049. doi: 10.1021/acs.jafc.9b02434. [DOI] [PubMed] [Google Scholar]
- 85.Bornet F.R., Jardy-Gennetier A.E., Jacquet N., Stowell J. Glycaemic response to foods: impact on satiety and long-term weight regulation. Appetite. 2007;49(3):535–553. doi: 10.1016/j.appet.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 86.Fatahi S., Kord-Varkaneh H., Talaei S., Mardali F., Rahmani J., Ghaedi E., et al. Impact of phytosterol supplementation on plasma lipoprotein(a) and free fatty acid (FFA) concentrations: a systematic review and meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2019;29(11):1168–1175. doi: 10.1016/j.numecd.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 87.Xia W., Xiang S., Gaman M.A., Jamilian P., Prabahar K., Du G., et al. The effects of phytosterol and phytostanol supplementation on the lipid profile in postmenopausal women: a systematic review and meta-analysis of randomized controlled trials. Phytother Res. 2022 doi: 10.1002/ptr.7646. [DOI] [PubMed] [Google Scholar]
- 88.Gylling H., Plat J., Turley S., Ginsberg H.N., Ellegård L., Jessup W., et al. Plant sterols and plant stanols in the management of dyslipidaemia and prevention of cardiovascular disease. Atherosclerosis. 2014;232(2):346–360. doi: 10.1016/j.atherosclerosis.2013.11.043. [DOI] [PubMed] [Google Scholar]
- 89.Chen G.C., Lv D.B., Pang Z., Dong J.Y., Liu Q.F. Dietary fiber intake and stroke risk: a meta-analysis of prospective cohort studies. Eur J Clin Nutr. 2013;67(1):96–100. doi: 10.1038/ejcn.2012.158. [DOI] [PubMed] [Google Scholar]
- 90.GAJN Soliman. Dietary fiber, atherosclerosis, and cardiovascular disease. Nutrients. 2019;11(5):1155. doi: 10.3390/nu11051155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chmielewski P.P., Strzelec B. Elevated leukocyte count as a harbinger of systemic inflammation, disease progression, and poor prognosis: a review. Folia Morphol (Warsz). 2018;77(2):171–178. doi: 10.5603/FM.a2017.0101. [DOI] [PubMed] [Google Scholar]
- 92.Fabbri A.D.T., Crosby G.A. A review of the impact of preparation and cooking on the nutritional quality of vegetables and legumes. Int J Gastron Food Sci. 2016;3:2–11. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.