Abstract
Background
Prepulse inhibition (PPI) of startle is a sensorimotor gating phenomenon perturbed in a variety of neuropsychiatric conditions. Psychedelics disrupt PPI in rats and humans, but their effects and involvement of the serotonin 5-HT2A receptor (5-HT2AR) in mice remain unexplored.
Methods
We tested the effect of the psychedelic 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) (0.5 mg/kg, i.p.) on startle amplitude and %PPI in response to acoustic stimuli under up to four different experimental conditions that included changes in background and stimulus intensity, prepulse and pulse duration, and interstimulus interval in male and female 129S6/SvEv mice. We also evaluated the effect of the 5-HT2AR antagonist M100,907 (1 mg/kg, i.p.) on DOI-induced startle amplitude and %PPI, as well as the effect of the psychedelic LSD (0.24 mg/kg, i.p.) and the dopamine agonists apomorphine (5 mg/kg, s.c.) and SKF-82,958 (0.5 mg/kg, i.p.) in male 129S6/SvEv mice.
Results
DOI altered startle amplitude with either pulse alone or prepulse + pulse presentations in all PPI conditions, and increased %PPI in three out of four PPI conditions in male mice — an effect that was prevented by M100,907. In female mice, DOI increased %PPI without affecting startle amplitude. %PPI was positively correlated with startle amplitude in males while being negatively correlated in female mice. In male mice, LSD also increased %PPI, although it did not affect startle amplitude, whereas apomorphine and SKF-82,958 induced decreases in %PPI.
Conclusion
Our findings highlight a distinct effect of the psychedelic DOI on PPI in 129S6/SvEv mice, suggesting 5-HT2AR-dependent PPI improvement in a paradigm-dependent and sex-dependent manner.
Keywords: Serotonin 5-HT2A receptor, G protein-coupled receptor (GPCR), Psychedelics, Hallucinogens, Lysergic acid diethylamide (LSD), Psilocybin, Sensorimotor gating, Prepulse inhibition (PPI), Psychopharmacology, Sex differences
Introduction
Psychedelics, also known as classic hallucinogens, are psychoactive compounds that function primarily through the serotonergic system. Structurally, psychedelics can be classified into two main groups: phenethylamines, including mescaline and the substituted amphetamine 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI), and tryptamines, such as lysergic acid diethylamide (LSD), psilocybin, and its active compound psilocin (Glennon 1994; Hanks and Gonzalez-Maeso 2016a, 2016b; Nichols 2016). In healthy volunteers, the acute effects of psychedelics include alterations in processes related to perception, emotion, and sensory processing (Vollenweider and Preller 2020). Most of the distortions induced in humans by psychedelics such as psilocybin (Vollenweider et al. 1998) and LSD (Schmid et al. 2015) are prevented by pre-administration of the relatively selective serotonin (or 5-hydroxytryptamine) 2A receptor (5-HT2AR) antagonist ketanserin. Using rodent behavior models that predict psychedelic activity, such as head-twitch response (Canal and Morgan 2012), previous findings also demonstrated that either pharmacological blockade with the highly selective 5-HT2AR antagonist M100,907 (volinanserin) (de la Fuente Revenga et al. 2020) or deletion of the 5-HT2aR (Htr2a) gene (González-Maeso et al. 2007) prevent this murine predictor of psychedelic effects in humans. Together, these data in mice and humans suggest that the 5-HT2AR is the likely main molecular target responsible for the sensory and cognitive alterations associated with the acute administration of psychedelics.
Other acute effects of psychedelics include alterations in sensorimotor gating, which is related to the regulation of sensory information while it is transmitted to motor output systems (Halberstadt and Geyer 2018; Hanks and González-Maeso 2013; Swerdlow et al. 2016). Sensorimotor gating is an important adaptive function of the brain through which a sensory overload is prevented by filtering out (or gating) irrelevant information. This process is disrupted in multiple psychiatric disorders including schizophrenia. Prepulse inhibition (PPI) of the startle response is a measure of sensorimotor gating in which response to a startle-inducing stimulus (pulse) is diminished when the stimulus is preceded by a lesser intensity, non-startle-inducing stimulus (prepulse) (Gómez-Nieto et al. 2020). Deficits in PPI are consistent with a disruption in sensorimotor gating behavior. It is well established that other families of psychoactive drugs, such as amphetamine and phencyclidine (PCP) or the closely related dissociative dizocilpine (MK-801), lead to PPI deficits in both rat and mouse models (Allen et al. 2011; Kurita et al. 2012; Swerdlow et al. 1990). However, current findings on the effects of psychedelics on PPI in rodents remain unclear and, at times, inconsistent.
As an example, most of the previous publications using rat models suggest that psychedelics, such as DOI (Oliveras et al. 2017; Padich et al. 1996; Sipes and Geyer 1994; Varty et al. 1995), DOB or 1-(2,5-dimethoxy-4-bromophenyl)-2-aminopropane (Johansson et al. 1995), LSD (Halberstadt and Geyer 2010; Ouagazzal et al. 2001), mescaline (Páleníček et al. 2008), 2C-B or 2,5-dimethoxy-4-bromophenethylamine (Páleníček et al. 2013), and 25I-NBOMe or 4-iodo-2,5-dimethoxy-N-(2-methoxybenzyl) phenethylamine (Miliano et al. 2019), diminish PPI of the acoustic startle response. These studies also suggest that PPI deficits induced by psychedelics can be prevented by pre-administration of the 5-HT2AR antagonists M100,907 or MDL-11,939, but not by the 5-HT2CR antagonists SB-242084 or SER-082 (Ouagazzal et al. 2001; Padich et al. 1996; TE Sipes and Geyer 1995). In mouse models, DOI (1 and 5 mg/kg) produced deficits in PPI behavior in 129S6/SvEv and C57BL/6J mice (Allen et al. 2011; Halberstadt and Geyer 2018). On the other hand, DOM or 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane ranging from 0.5 to 4 mg/kg showed a lack of effect on PPI in C57BL/6J, 129Sv, and ICR mice (Dulawa and Geyer 2000). Another study also reported that DOI (1 mg/kg) had no effect on PPI in C57BL/6J × 129S6/SvEv hybrid mice (Hazama et al. 2014). Interestingly, other groups have reported that tryptamine psychedelics such as DMT, 5-MeO-DMT, and psilocin increase, rather than decrease, PPI in several strains of mice including CD-1 (Freedland and Mansbach 1999) and 129/SvEv (Halberstadt and Geyer 2011). Considering that 5-HT1AR agonists also increase PPI in mice, activation of 5-HT1AR by tryptamine psychedelics has been proposed as a potential mechanism underlying augmentation of PPI in this rodent model. This hypothesis is further supported by the partial blockade of the increase in PPI produced by 5-MeO-DMT in mice by the 5-HT1AR antagonist WAY-100635 (Dulawa et al. 2000; Gogos et al. 2008; Halberstadt and Geyer 2011). However, the exact role of 5-HT2AR in PPI alterations induced by psychedelics in mouse models remains unexplored.
Another explanation for the varying effect of psychedelics on PPI in mice might be related to the different parameters that define a PPI experiment, such as the intensities of the prepulse (s) and pulse above background noise, the duration of the prepulse and pulse, and the time between the prepulse and pulse (interstimulus interval). As it pertains to the effects of psychedelics in humans, psilocybin reduced PPI at a short interstimulus interval (30 ms) while increasing PPI at longer (>100 ms) interstimulus intervals (Gouzoulis-Mayfrank et al. 1998; Vollenweider et al. 2007). Other groups have characterized the effect of such variations in experimental conditions on PPI outcomes in different strains of mice (Aubert et al. 2006; Paylor and Crawley 1997; Varty et al. 2001), but how changes in the PPI experimental conditions affect the outcomes of psychedelics on PPI in mice has not been explored. Additionally, most of the previous preclinical findings have only been reported in male mice, whereas sex-related differences in sensorimotor gating processes have been described in humans (Swerdlow et al. 1993). Here we tested the effects of the phenethylamine psychedelic DOI on startle response and PPI of startle response under a battery of experimental conditions that included changes in background intensity, stimulus intensity, prepulse and pulse duration, and interstimulus interval in male and female 129S6/SvEv mice.
Methods
Drugs
DOI or 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane was obtained from Sigma-Aldrich (St. Louis, MO). M100,907 (volinanserin) or (R)-(+)-α-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl]-4-piperinemethanol, SKF-82,958 or 6-chloro-2,3,4,5-tetrahydro-1-phenyl-3-(2-propen-1-yl)-1H-3-benzazepin-e-7,8-diol hydrobromide, and apomorphine or (R)-5,6,6a,7-tetrahydro-6-methyl-4H-dibenzo[de,g]quinoline-10,11-diol hydrochloride were obtained from Tocris (Minneapolis, MN). LSD or lysergic acid diethylamide was obtained from Lipomed (Cambridge, MA). Drugs were dissolved in saline (0.9%). All drugs or vehicle were administered intraperitoneally (i.p.), except apomorphine, which as administered subcutaneously (s.c.). Injection volumes were determined based on body weight (0.01 ml/g).
Animals
Experiments were performed on adult (10–17 weeks old) male and female 129S6/SvEv mice (Taconic). This strain of mice was selected because extensive studies regarding cognitive and anxiety-related processes (Weisstaub et al. 2006; Morici et al. 2015; Morici et al. 2018), responses to chronic stress (Jaggar et al. 2017), and signaling mechanisms affecting gene expression and head-twitch behavior following psychedelic administration (González-Maeso et al. 2007; Moreno et al. 2011) have been performed in 129S6/SvEv mice. Additionally, 129S6/SvEv mice have previously shown high levels of PPI of both acoustic and tactile startle responses compared to other inbred strains (Paylor and Crawley 1997). Animals were housed on a 12 h light/dark cycle at 23 °C with food and water ad libitum, except during behavioral testing which took place during the light cycle. All procedures were conducted in accordance with the National Institutes of Health (NIH) guidelines and were approved by the Virginia Commonwealth University Animal Care and Use Committee. Behavioral testing took place between 9:00 a.m. and 6:00 p.m. Behavioral tests were separated by at least a 1 week wash-out period.
Head-twitch response
Detection of head-twitch responses in mice was performed as previously reported (de la Fuente Revenga et al. 2020) with additional computational (Halberstadt 2020) and visual inspection. Neodymium magnets (N50, 3 mm diameter × 1 mm height, 50 mg) were glued with cyanoacrylate to the top surface of aluminum ear tags for rodents (Las Pias Ear Tag, Stoelting Co.) with the magnetic south of the magnet in contact with the tag. Mice were ear-tagged bilaterally through the pinna antihelix under ketamine/xylazine anesthesia (120 mg/kg and 12 mg/kg, respectively) and allowed to recover before conducting behavioral testing. After treatment with DOI (0.5 mg/kg) or vehicle, ear-tagged mice were placed inside a plastic container surrounded by a coil (~500 turns 30 AWG enameled wire) of which output was amplified (Pyle PP444 phono amplifier) and recorded at 1000 Hz using a NI USB-6001 (National Instruments) data acquisition system. Testing lasted 30 min. Data acquisition was performed as previously described (de la Fuente Revenga et al. 2019). Data were processed using a previously described signal analysis protocol (de la Fuente Revenga et al. 2020). To refine head-twitch detection, the signal was also processed using a deep learning-based protocol based on scalograms (Halberstadt 2020). Mismatches between both detection methods were inspected visually without clues relative to the timestamp of the event or treatment group. After manual classification of dubious events, the final values of head-twitch counts were corrected with a custom script. The ∣V∣ threshold for the initial filter using the findpeaks function in MATLAB (Mathworks, R2019a) was halved to a more permissive (0.01 ∣V∣) threshold than previously reported (de la Fuente Revenga et al. 2020). The convolutional neural network (CNN) employed was trained using head-twitch (~1200) and non-head-twitch (~600) events at a 1:1 training and test ratio from our previous studies (de la Fuente Revenga et al. 2020) and unpublished data from experiments conducted on magnet ear-tagged mice. The combined false-positive and false-negative rate of the classifier was less than 3% in the test set.
Prepulse inhibition of startle response
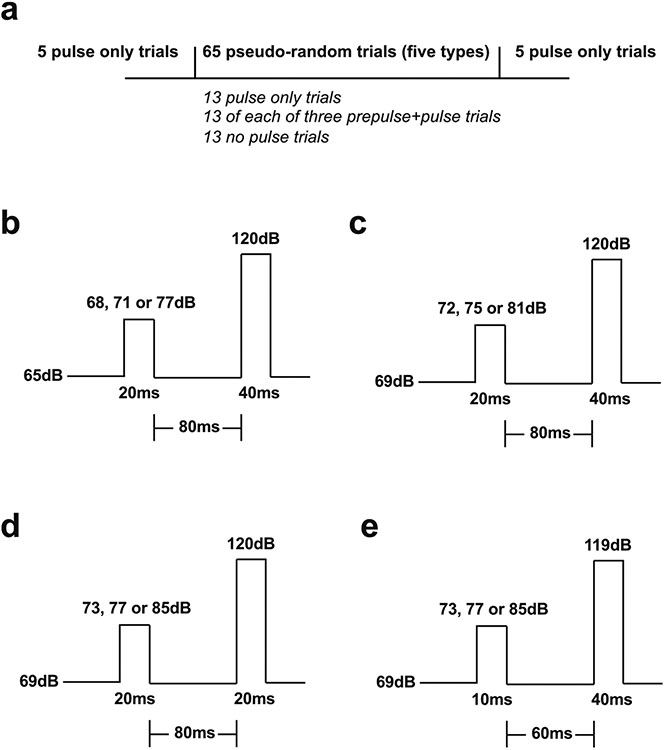
Assays were conducted as previously reported with minor modifications (Enga et al. 2017). Briefly, recording of the startle magnitude in response to acoustic stimuli was performed using the SR-LAB™ Startle Response System (San Diego Instruments). Mice were habituated to the testing room at least 1 h prior to the start of the experiment. In experiments using DOI (0.5 mg/kg) or LSD (0.24 mg/kg), treatment or vehicle was administered to subjects immediately prior to testing. The 5-HT2AR antagonist volinanserin was administered 10 min before DOI or vehicle as a pretreatment. SFK82,958 (0.5 mg/kg), apomorphine (5 mg/kg), or vehicle was injected 10 min prior to the onset of testing. The experiment proceeded as follows: Mice were placed in the testing chambers for a 5-min acclimation period at background noise, followed by the pseudo-random presentation of five trial types. These included 13 pulse-only trials, 13 of each three prepulse + pulse trials, and 13 no pulse trials for a total of 65 trials. There was an average of 15 s (range 12–18 s) between trials. In addition, the pulse-only trial was presented five times at the beginning and end of the testing session (Fig. 1a). Each session lasted about a total of 30 min.
Fig. 1.
Schematic representation of the experimental conditions within the acoustic startle reflex and PPI assays. a Mice were exposed to a pseudo-random presentation of five trial types (13 pulse-only trials, 13 of each three prepulse + pulse trials, and 13 no pulse trials for a total of 65 trials). In addition, the pulse-only trial was presented five times at the beginning and end of the testing session. b–e Mice were exposed to up to four different experimental conditions that included changes in background and stimulus intensity, prepulse and pulse duration, and interstimulus interval mean. Statistical significance of experiments involving three or more groups and two or more treatments was assessed by two-way or three-way ANOVA followed by Sidak’s multiple comparison test. Statistical significance of experiments involving time courses and two treatments was assessed by two-way repeated measures ANOVA. Statistical significance of experiments involving three or more groups was assessed by one-way ANOVA followed by Sidak’s multiple comparison test. Statistical significance of experiments involving two groups was assessed by Student’s t test. Correlation analysis was conducted using the Pearson’s r. Statistical analysis was performed with GraphPad Prism software version 9 (La Jolla, CA). The level of significance was set atp = 0.05. All values in the figure legends represent mean ± SEM.
A range of variables were studied to determine consistency of results across conditions including the background noise, pulse duration and intensity, prepulse duration and intensity, as well as the interstimulus interval. Background noise was set to either 65 dB or 69 dB. The pulse was 20 ms or 40 ms at 119 dB or 120 dB. The three prepulses were 10 ms or 20 ms at 68 dB, 71 dB, 72 dB, 73 dB, 75 dB, 77 dB, 81 dB, or 85 dB. The interstimulus interval, or time between the end of the prepulse and the onset of the pulse, was 60 ms or 80 ms (Fig. 1b, c, d, e). %PPI for each prepulse intensity was determined as a percentage using the following equation: [1 – (average startle magnitude (Vmax) of prepulse + pulse trials) / average startle magnitude (Vmax) of pulse-only trials)] × 100. The five pulse-only trials presented at the beginning and at the end of testing (see Fig. 1a) were excluded from calculating %PPI.
Statistical analysis
Animals were randomly allocated into the different experimental groups. Datapoints were excluded based on previously established criterion and were set to ± 2 s.d. from the group
Results
Effect of the psychedelic DOI on PPI in male mice
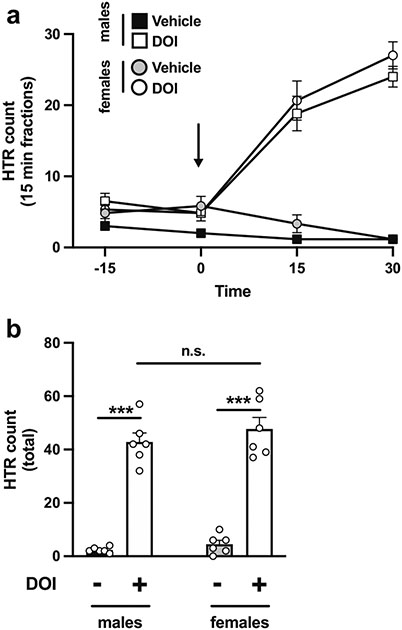
We tested the effect of DOI (0.5 mg/kg) or vehicle on PPI of startle response under four different experimental conditions. This dose of DOI was selected based on pilot dose-response assays (0.5, 1.0, 2.0, and 5.0 mg/kg, or vehicle — data not shown) and was validated on head-twitch behavior in male and female mice. Fig. 2 a shows an effect of DOI on head-twitch during the first 30 min post injection (two-way repeated measures ANOVA treatment effect: F[3,20] = 40.00, p < 0.001). Total head-twitch counts were also increased upon DOI administration (vehicle vs DOI, F[1,20] = 212.3, p < 0.001; sex-related differences, F[1,20] = 1.48, p > 0.05). Post hoc analysis showed that the head-twitch response was increased in both male (p < 0.001) and female (p < 0.001) mice injected with DOI as compared to vehicle, whereas this effect of DOI on head-twitch behavior was similar in both sexes (p > 0.05) (Fig. 2b).
Fig. 2.
a Time-course showing head-twitch (HTR) counts on 15-min blocks in male and female 129S6/SvEv mice injected (i.p.) with DOI (0.5 mg/kg) or vehicle. Black arrow indicates the administration timepoint. b Head-twitch counts corresponding to the first 30 min after injection of DOI or vehicle (n = 6 mice per group). Two-way repeated measures ANOVA (a) and two-way ANOVA (b) followed by Sidak’s multiple comparison test. ***p < 0.001, n.s., not significant
Previous results by other groups showed in rodents that alterations in PPI induced by acute psychoactive drug administration at lower prepulse intensities can be overcome by longer or more intense prepulses (Vollenweider et al. 2007). Similarly, PPI levels at lower prepulse intensities close to the background noise level can be too small or variable upon drug-induced manipulation to be detected reliably. To account for this, four PPI experimental conditions were selected with different parameters within the PPI test, specifically intensity of the background, intensities of the prepulses and pulse, prepulse and pulse duration, and interstimulus interval to maximize the ability to detect psychedelic-induced changes in both startle magnitude and %PPI (Table 1; Fig. 1).
Table 1.
Summary of the experimental conditions in the startle amplitude and PPI assays
| Sex | Psychoactive drug(s) | Background | Prepulses | Prepulse duration |
Pulse | Pulse duration |
Interstimulus interval |
|
|---|---|---|---|---|---|---|---|---|
| Fig. 3 | M | DOI (0.5 mg/kg) | 65 dB | 68, 71, 77 dB | 20 ms | 120 dB | 40 ms | 80 ms |
| Fig. 4 | M | DOI (0.5 mg/kg) | 69 dB | 72, 75, 81 dB | 20 ms | 120 dB | 40 ms | 80 ms |
| Fig. 5 | M | DOI (0.5 mg/kg) | 69 dB | 73, 77, 85 dB | 20 ms | 120 dB | 20 ms | 80 ms |
| Fig. 6 | M | DOI (0.5 mg/kg) | 69 dB | 73, 77, 85 dB | 10 ms | 119 dB | 40 ms | 60 ms |
| Fig. 7 | M | DOI (0.5 mg/kg) + M100 (1.0 mg/kg) | 69dB | 73, 77, 85 dB | 20 ms | 120 dB | 20 ms | 80 ms |
| Fig. 8 | M | DOI (0.5 mg/kg) + M100 (1.0 mg/kg) | 69 dB | 73, 77, 85 dB | 10 ms | 119 dB | 40 ms | 60 ms |
| Fig. 9 | F | DOI (0.5 mg/kg) | 69 dB | 73, 77, 85 dB | 20 ms | 120 dB | 20 ms | 80 ms |
| Fig. 10 | F | DOI (0.5 mg/kg) | 69 dB | 73, 77, 85 dB | 10 ms | 119 dB | 40 ms | 60 ms |
| Fig. 13 | M | LSD (0.24 mg/kg) | 69 dB | 73, 77, 85 dB | 20 ms | 120 dB | 20 ms | 80 ms |
| Fig. 14 | M | Apo (5 mg/kg) or SKF (0.5 mg/kg) | 69 dB | 73, 77, 85 dB | 20 ms | 120 dB | 20 ms | 80 ms |
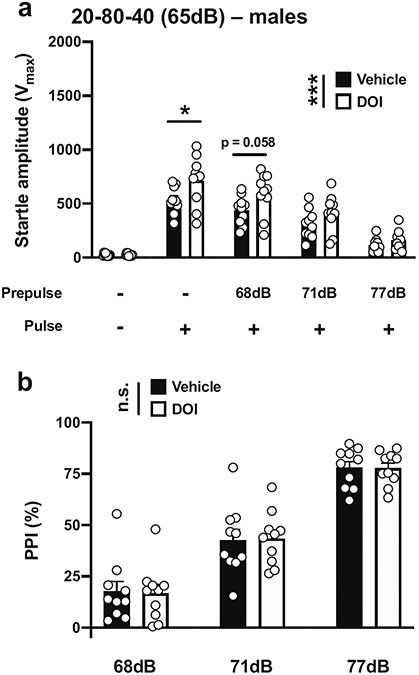
We first assessed PPI with a 40-ms pulse at 120 dB; 20 ms prepulses at 68, 71, and 77 dB; an interstimulus interval of 80 ms; and background noise set to 65 dB in male mice (Table 1; Fig. 1b). This paradigm was based on an optimal protocol suggested for PPI assays in 129Sv mice (Geyer and Dulawa 2003). Under these experimental conditions, there was a main effect of DOI on the startle amplitude (vehicle vs DOI, F[1,90] = 12.66, p < 0.001; PPI conditions, F[4,90] = 70.10, p < 0.001) (Fig. 3a). Post hoc analysis showed that the startle amplitude in response to the pulse alone was increased by DOI (p < 0.05), whereas there was a nonsignificant trend (p = 0.058) in the effect of DOI on the increase of the startle amplitude with a prepulse of 68 dB, but not with prepulses of 71 dB (p > 0.05) or 77 dB (p > 0.05) (Fig. 3a). DOI, however, did not affect %PPI under these experimental conditions (vehicle vs DOI, F[1,54] = 0.002, p > 0.05; prepulse intensity, F[2,54] = 107.5, p < 0.001) (Fig. 3b).
Fig. 3.
Effect of DOI (0.5 mg/kg) on startle amplitude (a) and %PPI (b) in male 129S6/SvEv mice. Experimental conditions included a 40 ms pulse at 120 dB, 20 ms prepulses at 68, 71 and 77 dB, an interstimulus interval of 80 ms, and background noise set to 65 dB (n = 10 mice per group). Two-way ANOVA followed by Sidak’s multiple comparison test. *p < 0.05, ***p < 0.001, n.s., not significant
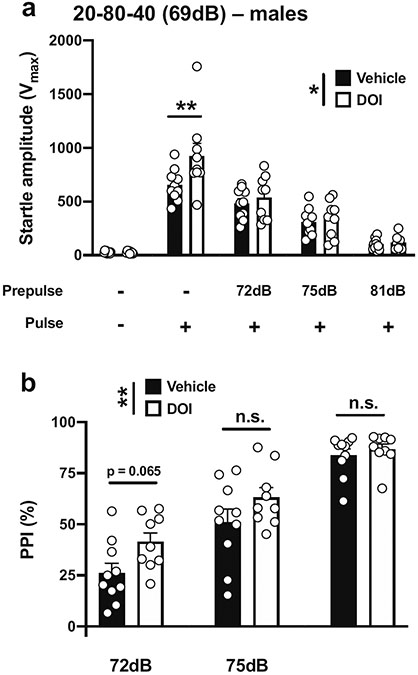
We next tested whether increasing the background noise but keeping the same difference between prepulses and background sounds (3 dB, 6 dB, and 12 dB above background) influenced the effect of DOI and on startle amplitude and % PPI in male mice. With a 40-ms pulse at 120 dB; 20 ms prepulses at increased intensities of 72, 75, and 81 dB; an interstimulus interval of 80 ms; and background noise set to 69 dB (Table 1; Fig. 1c), there was a main effect of DOI on startle amplitude (vehicle vs DOI, F[1,85] = 5.09, p < 0.05; PPI conditions, F[4,85] = 71.26, p < 0.001) (Fig. 4a). Post hoc analysis showed that DOI increased startle amplitude in the pulse-only trials (p < 0.01), whereas DOI did not affect the startle amplitudes with prepulses of 72, 75, and 81 dB (p > 0.05) (Fig. 4a). DOI affected %PPI under these experimental conditions (vehicle vs DOI, F[1,51] = 7.23, p < 0.01; prepulse intensity, F[2,51] = 63.05, p < 0.001) (Fig. 4b). Post hoc analysis showed a trend in the effect of DOI toward increased %PPI with a prepulse of 72 dB (p = 0.065), but not with prepulses of 75 or 81 dB (p > 0.05) (Fig. 4b).
Fig. 4.
Effect of DOI (0.5 mg/kg) on startle amplitude (a) and %PPI (b) in male 129S6/SvEv mice. Experimental conditions included a 40-ms pulse at 120 dB; 20 ms prepulses at 72, 75, and 81 dB; an interstimulus interval of 80 ms; and background noise set to 69 dB (n = 9–10 mice per group). Two-way ANOVA followed by Sidak’s multiple comparison test. *p < 0.05, **p < 0.01, n.s., not significant
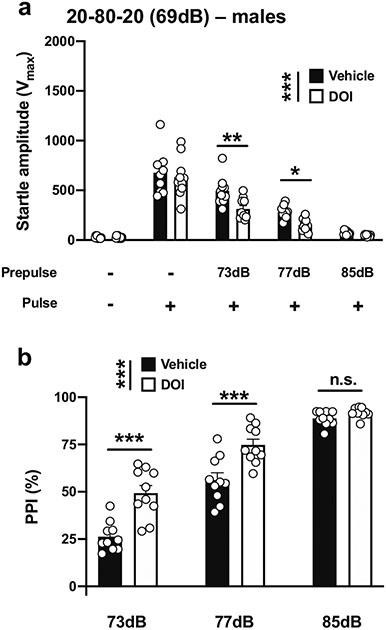
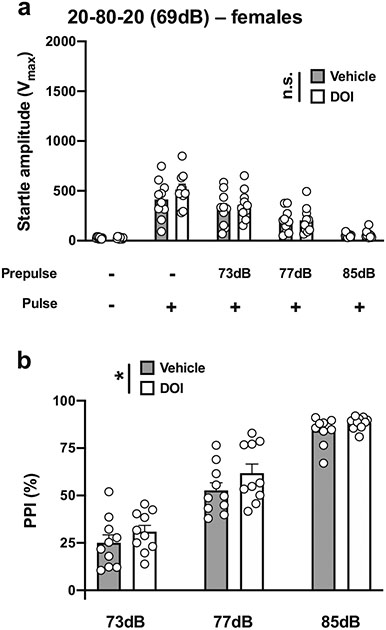
We then evaluated the effects of increasing the difference between the prepulses and background noise to 4 dB, 8 dB, and 16 dB and reducing the pulse duration using a paradigm previously used to detect PPI deficits with the dissociative ketamine in mice (Enga et al. 2017). With a 20-ms pulse at 120 dB; 20 ms prepulses at 73, 77, and 85 dB; an interstimulus interval of 80 ms; and background noise set to 69 dB (henceforth referred to as 20-80-20) (Table 1; Fig. 1d), there was a main effect of DOI on startle amplitude in male mice (vehicle vs DOI, F[1,90] = 11.73, p < 0.001; PPI conditions, F[4,90] = 110.1, p < 0.001) (Fig. 5a). Post hoc analysis showed that DOI did not affect the startle amplitude in response to the pulse alone, (p > 0.05), whereas DOI decreased the startle amplitude with a prepulse of 73 dB (p < 0.01) or 77 dB (p < 0.05), but not a prepulse of 85 dB (p > 0.05) (Fig. 5a). DOI affected %PPI under these experimental conditions (vehicle vs DOI, F[1,54] = 40.47, p < 0.001; prepulse intensity, F[2,54] = 168.9, p < 0.001) (Fig. 5b). Post hoc analysis showed that %PPI was increased by DOI with prepulses of 73 dB (p < 0.001) and 77 dB (p < 0.001), but not of 85 dB (p > 0.05) (Fig. 5b).
Fig. 5.
Effect of DOI (0.5 mg/kg) on startle amplitude (a) and %PPI (b) in male 129S6/SvEv mice. Experimental conditions included a 20-ms pulse 120 dB; 20 ms prepulses at 73, 77, and 85 dB; an interstimulus interval of 80 ms; and background noise set to 69 dB (n = 10 mice per group). Two-way ANOVA followed by Sidak’s multiple comparison test. *p < 0.05, **p < 0.01, ***p < 0.001, n.s., not significant
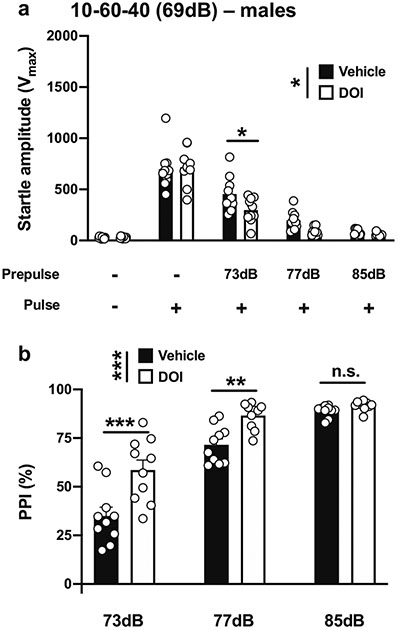
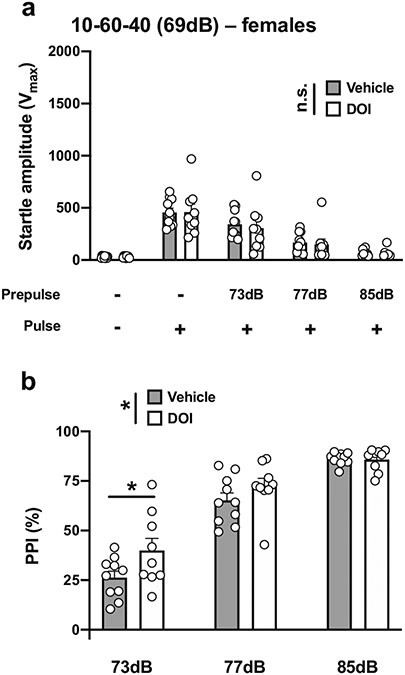
Given these unexpected increases in PPI, we then tested conditions described in a previous study that reported that DOI leads to deficits in PPI in male CD-1 mice (Ibarra-Lecue et al. 2018). We adjusted the conditions by decreasing the duration of both the prepulses and interstimulus interval while increasing the duration and decreasing the intensity of the pulse. With a 10-ms pulse at 119 dB; 10 ms prepulses at 73, 77, and 85 dB; an interstimulus interval of 60 ms; and background noise set to 69 dB (henceforth referred to as 10-60-40) (Table 1; Fig. 1e), there was a main effect of DOI on startle amplitude in male mice (vehicle vs DOI, F[1,90] = 5.68, p < 0.05; PPI conditions, F[4,90] = 128.6, p < 0.001) (Fig. 6a). Post hoc analysis showed that the startle amplitude in response to the pulse alone was not affected by DOI (p > 0.05), whereas DOI decreased the startle amplitude when preceded by a prepulse of 73 dB (p < 0.05), but not with prepulses of 77 dB (p > 0.05) or 85 dB (p > 0.05) (Fig. 6a). DOI affected %PPI under these experimental conditions (vehicle vs DOI, F[1,54] = 27.87, p < 0.001; prepulse intensity, F[2,54] = 98.94, p < 0.001) (Fig. 6b). Post hoc analysis showed that %PPI was increased by DOI with prepulses of 73 dB (p < 0.001) and 77 dB (p < 0.01), but not of 85 dB (p > 0.05) (Fig. 6b).
Fig. 6.
Effect of DOI (0.5 mg/kg) on startle amplitude (a) and %PPI (b) in male 129S6/SvEv mice. Experimental conditions included a 40-ms pulse at 119 dB; 10 ms prepulses stimulus at 73, 77, and 85 dB; an interstimulus interval of 60 ms; and background noise set to 69 dB (n = 10 mice per group). Two-way ANOVA followed by Sidak’s multiple comparison test. *p < 0.05, **p < 0.01, ***p < 0.001, n.s., not significant
M100,907 blocks the effects of DOI on PPI in male mice
Our next goal was to test the effect of the 5-HT2AR antagonist M100,907 on DOI-induced effects on PPI. Among all the experimental conditions tested in male mice (Fig. 2), we further examined those in which DOI only affected %PPI and not the startle amplitude in response to the pulse alone, with the goal of defining the role of 5-HT2AR-dependent signaling in psychedelic-induced alterations in sensorimotor gating. We therefore evaluated the effect of M100,907 on DOI-induced startle amplitude and PPI under 20-80-20 and 10-60-40 experimental conditions.
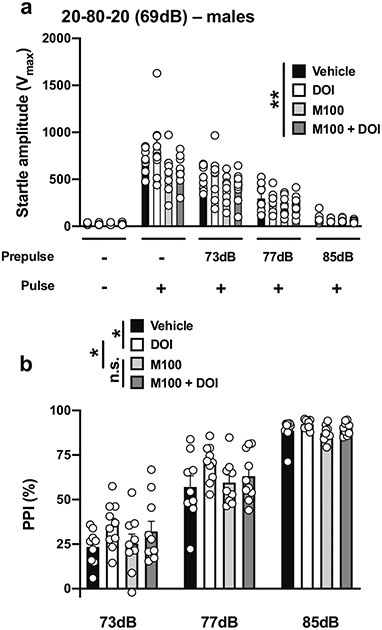
With the 20-80-20 experimental condition (Table 1; Fig. 1d), there was an effect of drug treatment (DOI and/or M100,907 or vehicle) on startle amplitude in male mice (drug treatment, F[3,171] = 4.65, p < 0.01; PPI conditions, F[4,171] = 153.0, p < 0.001) (Fig. 7a). Drug treatment affected %PPI under these experimental conditions (drug treatment, F[3,102] = 3.93, p < 0.05; prepulse intensity, F[2,102] = 242.7, p < 0.001) (Fig. 7b). Post hoc analysis showed a %PPI difference between the vehicle and DOI groups (p < 0.05) and the DOI and M100,907 groups (p < 0.05), but not between the M100,907 and M100,907 + DOI groups (p > 0.05) (Fig. 7b).
Fig. 7.
Effect of M100,907 (1.0 mg/kg) and DOI (0.5 mg/kg) on startle amplitude (a) and %PPI (b) in male 129S6/SvEv mice. Experimental conditions included a 20-ms pulse 120 dB; 20 ms prepulses at 73, 77, and 85 dB; an interstimulus interval of 80 ms; and background noise set to 69 dB (n = 9–10 mice per group). Two-way ANOVA followed by Sidak’s multiple comparison test. *p < 0.05, **p < 0.01, n.s., not significant
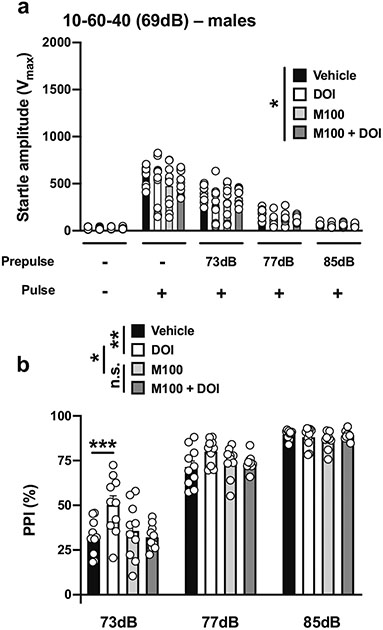
With the 10-60-40 experimental condition (Table 1; Fig. 1e), there was an effect of drug treatment (DOI and/or M100,907, or vehicle) on startle amplitude in male mice (drug treatment, F[3,180] = 2.62, p < 0.05; PPI conditions, F[4,180] = 155.8, p < 0.001) (Fig 8a). Drug treatment affected %PPI under these experimental conditions (drug treatment, F[3,108] = 5.72, p < 0.001; prepulse intensity, F[2,108] = 340.2, p < 0.001) (Fig. 8b). Post hoc analysis showed a %PPI difference between the vehicle and DOI groups (p < 0.01) and the DOI and M100,907 groups (p < 0.05), but not between the M100,907 and M100,907 + DOI groups (p > 0.05) (Fig. 8b). Post hoc analysis also showed that %PPI was increased by DOI with a prepulse of 73 dB (p < 0.001), an effect that was prevented by M100,907 (p > 0.05) and not observed with prepulses of 77 dB (p > 0.05) or 85 dB (p > 0.05) (Fig. 8b).
Fig. 8.
Effect of M100,907 (1.0 mg/kg) and DOI (0.5 mg/kg) on startle amplitude (a) and %PPI (b) in male 129S6/SvEv mice. Experimental conditions included a 40-ms pulse at 119 dB; 10 ms prepulse stimulus at 73, 77, and 85 dB; an interstimulus interval of 60 ms; and background noise set to 69 dB (n = 10 mice per group). Two-way ANOVA followed by Sidak’s multiple comparison test. *p < 0.05, **p < 0.01, ***p < 0.001, n.s., not significant
Effect of the psychedelic DOI on PPI in female mice
We next focused our attention on the effect of DOI on PPI in female mice. As before with the effect of the 5-HT2AR antagonist, we tested female mice under the two experimental conditions in which DOI only affected %PPI and not the startle amplitude in response to the pulse alone.
With the 20-80-20 experimental condition (Table 1; Fig.1d), there was no effect of DOI on startle amplitude in female mice (vehicle vs DOI, F[1,90] = 1.65, p > 0.05; PPI conditions, F[4,90] = 46.25, p < 0.001) (Fig. 9a). DOI affected % PPI under these experimental conditions (vehicle vs DOI, F[1,54] = 4.84, p < 0.05; prepulse intensity, F[2,54] = 135.7, p < 0.001) (Fig. 9b). However, this quantitatively weak effect was not corroborated by post hoc analysis since %PPI was unaffected by DOI with prepulses of 73 dB (p > 0.05), 77 dB (p > 0.05) or 85 dB (p > 0.05) (Fig. 9b).
Fig. 9.
Effect of DOI (0.5 mg/kg) on startle amplitude (a) and %PPI (b) in female 129S6/SvEv mice. Experimental conditions included a 20-ms pulse 120 dB; 20 ms prepulses at 73, 77, and 85 dB; an interstimulus interval of 80 ms; and background noise set to 69 dB (n = 10 mice per group). Two-way ANOVA followed by Sidak’s multiple comparison test. *p < 0.05, n.s., not significant
With the 10-60-40 experimental condition (Table 1; Fig.1e), there was no effect of DOI on startle amplitude in female mice (vehicle vs DOI, F[1,85] = 0.16, p < 0.05; PPI conditions, F[4,85] = 36.88, p < 0.001) (Fig. 10a). DOI affected %PPI under these experimental conditions (vehicle vs DOI, F[1,51] = 4.95, p < 0.05; prepulse intensity, F[2,51] = 107.9, p < 0.001) (Fig. 10b). Post hoc analysis showed that %PPI was increased by DOI with a prepulse of 73 dB (p < 0.05), but not of 77 dB (p > 0.05) or 81 dB (p > 0.05) (Fig. 10c).
Fig. 10.
Effect of DOI (0.5 mg/kg) on startle amplitude (a) and %PPI (b) in female 129S6/SvEv mice. Experimental conditions included a 40-ms pulse at 119 dB; 10 ms prepulse stimulus at 73, 77, and 85 dB; an interstimulus interval of 60 ms; and background noise set to 69 dB (n = 9–10 mice per group). Two-way ANOVA followed by Sidak’s multiple comparison test. *p < 0.05, n.s., not significant
Under these two PPI experimental conditions (20-80-20 and 10-60-40; see Table 1 and Fig. 1 d and e), a three-way ANOVA showed sex-related differences in the effects of DOI on PPI (Tables 2 and 3). Thus, both startle amplitude and % PPI were reduced in female mice compared to their male counterparts. There were also sex-related differences in the effects of DOI on both startle amplitude and %PPI (Tables 2 and 3).
Table 2.
Three-way ANOVA analysis of startle amplitude and PPI (%) values under 20-80-20 PPI conditions in male and female 129S6/SvEv mice
| Source of variation | Startle amplitude ANOVA |
p value | PPI (%) ANOVA |
p value |
|---|---|---|---|---|
| PPI intensity | F[4,180] = 155.1 | p < 0.001 | F[2,108] = 306.3 | p < 0.001 |
| Vehicle vs DOI | F[1,180] = 1.59 | p > 0.05 | F[1,108] = 34.37 | p < 0.001 |
| Sex | F[1,180] = 13.03 | p < 0.001 | F[1,108] = 24.98 | p < 0.001 |
| PPI intensity × drug | F[4,180] = 1.46 | p > 0.05 | F[2,108] = 3.79 | p < 0.05 |
| PPI intensity × sex | F[4,180] = 6.35 | p < 0.001 | F[2,108] = 1.71 | p > 0.05 |
| Drug × sex | F[1,180] = 10.13 | p < 0.01 | F[1, 108] = 5.38 | p < 0.05 |
| PPI int. × drug × sex | F[4,180] = 1.52 | p > 0.05 | F[2,108] = 2.15 | p > 0.05 |
Table 3.
Three-way ANOVA analysis of startle amplitude and PPI(%) values under 10-60-40 PPI conditions in male and female 129S6/SvEv mice
| Source of variation | Startle amplitude ANOVA |
p value | PPI (%) ANOVA |
p value |
|---|---|---|---|---|
| PPI intensity | F[4,175] = 158.3 | p < 0.001 | F[4,105] = 207.5 | p < 0.001 |
| Vehicle vs DOI | F[1,175] = 4.00 | p < 0.05 | F[1,105] = 22.92 | p < 0.001 |
| Sex | F[1,175] = 15.93 | p < 0.001 | F[1,105] = 36.41 | p < 0.001 |
| PPI intensity × drug | F[4,175] = 1.30 | p > 0.05 | F[2,105] = 5.69 | p < 0.01 |
| PPI intensity × sex | F[4,175] = 11.95 | p < 0.001 | F[2,105] = 3.54 | p < 0.05 |
| Drug × sex | F[1,175] = 1.88 | p > 0.05 | F[1,105] = 1.72 | p > 0.05 |
| PPI int. × drug × sex | F[4,175] = 0.50 | p > 0.05 | F[2,105] = 0.08 | p > 0.05 |
Sex differences in correlation between startle response and PPI
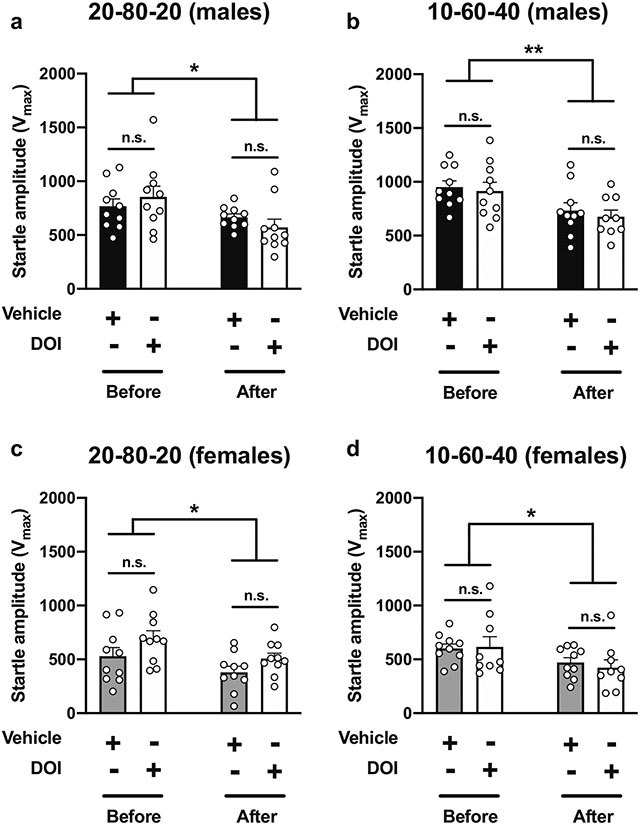
As mentioned in the “Methods” section (see also Fig. 1a), animals were presented with 5 pulse-only trials at the beginning and the end of the PPI session. Comparing the startle amplitudes in response to these trials before and after the PPI session, our data show startle habituation in both male and female mice under both the 20-80-20 and 10-60-40 experimental conditions (Table 4; Fig. 11). There were also sex-related differences between startle amplitudes (Table 4; Fig. 11), though these were not affected by DOI (Table 4; Fig. 11) as similarly noted during the combination of prepulses and pulses (see Figs. 5, 6, 9, and 10).
Table 4.
Three-way ANOVA analysis of startle amplitude within the 5 stimulus-only trials at the beginning and the end of the PPI session under 20-80-20 and 10-60-40 PPI conditions in male and female 129S6/SvEv mice
| Source of variation | 20-80-20 ANOVA |
p value | 10-60-40 ANOVA |
p value |
|---|---|---|---|---|
| Vehicle vs DOI | F[1,72] = 2.05 | p > 0.05 | F[1,69] = 0.42 | p > 0.05 |
| Before vs after | F[1,72] = 13.33 | p < 0.001 | F[1,69] = 16.61 | p < 0.001 |
| Sex | F[1,72] = 14.34 | p < 0.001 | F[1,69] =36.84 | p < 0.001 |
| Drug × bef. vs aft. | F[1,72] = 1.20 | p > 0.05 | F[1,69] = 0.18 | p > 0.05 |
| Drug × sex | F[1,72] = 2.27 | p > 0.05 | F[1,69] = 0.09 | p > 0.05 |
| Sex × bef. vs aft. | F[1,72] = 0.07 | p > 0.05 | F[1,69] = 0.48 | p > 0.05 |
| Drug × sex × bef. vs aft. | F[1,72] = 0.54 | p > 0.05 | F[1,69] = 0.05 | p > 0.05 |
Fig. 11.
Effect of DOI (0.5 mg/kg) on startle amplitude within the 5 stimulus-only trials at the beginning and the end of the PPI session at 20-80-20 (a, c) and 10-60-40 (b, c) PPI conditions in 129S6/SvEv male (a, b) and female (c, d) mice (n = 9–10 mice per group). Three-way ANOVA (see also Table 4). *p < 0.05, **p < 0.01, n.s., not significant
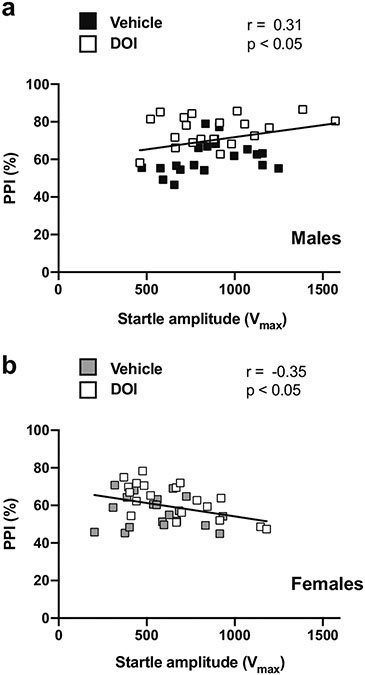
We also tested whether the magnitude of the startle amplitudes during the 5 pulse-only trials at the beginning of the PPI session correlated with average %PPI in male and female mice. Interestingly, there was a positive correlation between startle amplitudes prior to the PPI test and average %PPI in male mice (Fig. 12a), but an inverse correlation in female animals (Fig. 12b).
Fig. 12.
Pearson’s correlation between magnitudes within the 5 stimulus-only trials at the beginning of the PPI session and %PPI at 20-80-20 and 10-60-40 PPI conditions in male (a) and female (b) 129S6/SvEv mice
Effect of other psychoactive drugs on PPI in male mice
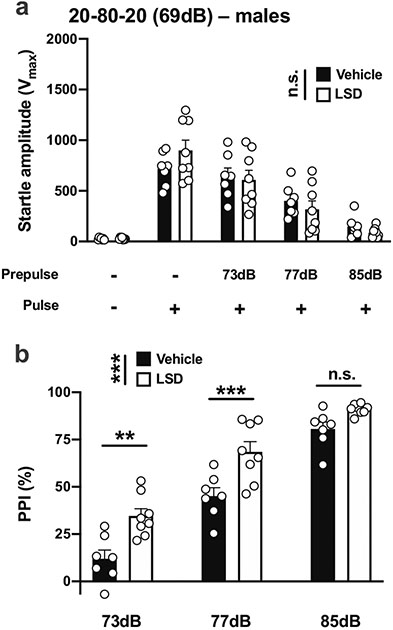
We next asked whether these effects of DOI on PPI in male 129S6/SvEv mice were also produced by the tryptamine psychedelic LSD. With the 20-80-20 experimental condition (Table 1; Fig. 1d), there was no effect of LSD on startle amplitude in male mice (vehicle vs LSD, F[1,65] = 0.0001, p > 0.05; PPI conditions, F[4,65] = 36.88, p < 0.001) (Fig. 13a). However, LSD affected %PPI under these experimental conditions (vehicle vs LSD, F[1,39] = 31.76, p < 0.001; prepulse intensity, F[2,39] = 117.6, p < 0.001) (Fig. 13b). Post hoc analysis showed that %PPI was increased by LSD with prepulses of 73 dB (p < 0.01) or 77 dB (p < 0.001), but not 81 dB (p > 0.05) (Fig. 13b).
Fig. 13.
Effect of LSD (0.24 mg/kg) on startle amplitude (a) and %PPI (b) in male 129S6/SvEv mice. Experimental conditions included a 20-ms pulse 120 dB; 20 ms prepulses at 73, 77, and 85 dB; an interstimulus interval of 80 ms; and background noise set to 69 dB (n = 7–8 mice per group). Two-way ANOVA followed by Sidak’s multiple comparison test. **p < 0.01, ***p < 0.001, n.s., not significant
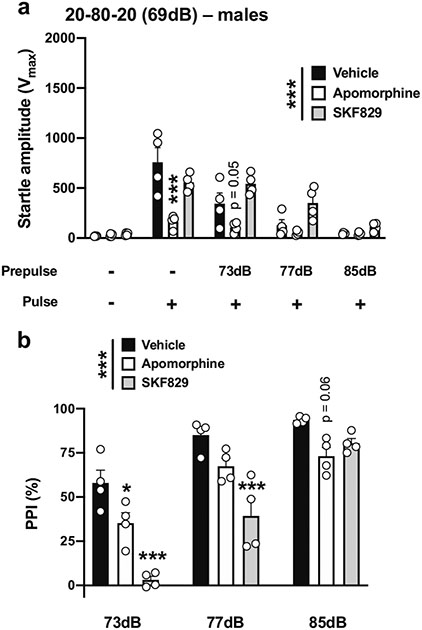
Given our relatively unexpected findings of increased PPI following DOI administration, we evaluated the effects of two additional non-psychedelic drugs: apomorphine and the dopamine D1 receptor agonist SKF-82,958, which have been reported to produce PPI deficits in male mice (Ralph-Williams et al. 2003). Using the 20-80-20 experimental condition (Table 1; Fig. 1d), there was an effect of drug treatment on startle amplitude (drug treatment, F[2,45] = 22.83, p < 0.001; PPI conditions, F[4,45] = 33.87, p < 0.001) (Fig. 14a). Post hoc analysis showed that the startle amplitude in response to the pulse alone was decreased by apomorphine (p < 0.001) but not by SKF-82,958 (p > 0.05) (Fig. 14a). Additionally, there was a trend for apomorphine to decrease the startle amplitude with a prepulse of 73 dB (p = 0.05), but not with prepulses of 77 dB (p > 0.05) or 85 dB (p > 0.05), nor with SKF-82,958 with prepulses of 73 dB (p > 0.05), 77 dB (p > 0.05) or 85 dB (p > 0.05) (Fig. 14a). Drug treatment affected %PPI under these experimental conditions (drug treatment, F[2,27] = 37.32, p < 0.001; prepulse intensity, F[2,27] = 67.13, p < 0.001) (Fig. 14b). Post hoc analysis showed that %PPI was decreased by apomorphine with prepulses of 73 dB (p < 0.05), but not of 77 dB (p > 0.05) or 85 dB (p = 0.06), and by SKF-82,958 with prepulses of 73 dB (p < 0.001) or 77 dB (p < 0.001), but not 85 dB (p > 0.05) (Fig. 14b).
Fig. 14.
Effect of apomorphine (5 mg/kg) and SKF-82,958 (0.5 mg/kg) on startle amplitude (a) and %PPI (b) in male 129S6/SvEv mice. Experimental conditions included a 20-ms pulse 120 dB; 20 ms prepulses at 73, 77, and 85 dB; an interstimulus interval of 80 ms; and background noise set to 69 dB (n = 4 mice per group). Two-way ANOVA followed by Sidak’s multiple comparison test. *p < 0.05, ***p < 0.001, n.s., not significant
Discussion
Perhaps our most compelling finding is that the phenethylamine psychedelic DOI produced an augmentation of PPI in male 129S6/SvEv mice. Most of the previous observations in rat models supported a decrease in PPI induced by different chemical families of psychedelics, but the effects reported in mice were inconsistent, with examples of augmentation, reduction, and lack of effect (Halberstadt and Geyer 2018). Our data corroborate previous observations by other groups that showed an increase in PPI in response to treatment with tryptamine psychedelics in male 129S6/SvEv mice (Halberstadt and Geyer 2011) and further support the robust nature of this phenotype since it was clearly observed among three out of the four PPI experimental conditions that we evaluated in male mice. This augmentation was prevented by the highly selective 5-HT2AR antagonist M100,907 and validated upon administration of LSD, another psychedelic structurally distinct from DOI. On the other hand, strict dopaminergic agonists apomorphine and SKF-82,958 reduced PPI in male 129S6/SvEv mice as expected, validating our experimental design.
A potential explanation for the effect of psychedelics on PPI augmentation in 129S6/SvEv mice may be related to a psychedelic-induced increase in arousal or alertness. Previous studies in humans, rats, and mice have suggested that an increase in general level of alertness might facilitate processing of the prepulse and thus lead to increased levels of PPI (Río-Álamos et al. 2019). Additionally, others have found a positive relationship between ratings of the effects of LSD and emotional arousal in healthy volunteers (Zentner et al. 2008), and controlled studies of microdosing of LSD reported objective increases in psychomotor vigilance (Bershad et al. 2019). A drug-induced enhanced state of alertness that could ultimately affect PPI manifestation is a plausible explanation further supported by the positive correlation between the startle responses obtained during the 5 stimulus-only trials — a potential index of alertness — at the beginning of the PPI session and the %PPI datasets in the same cohort of male mice. This correlation however was negative in female mice, which suggests an important sex-related difference in the underlying substrate of this effect of psychedelics. Additionally, given that LSD is also a 5-HT1AR agonist, and 5-HT1AR agonists have been found to produce PPI improvements in mice, we cannot exclude that LSD produced this behavior through an alternative mechanism in male mice (Dulawa et al. 2000; Gogos et al. 2008).
Our initial data in male mice were collected using a battery of four PPI experimental conditions. The variations within the experimental setup, including changes in background, prepulse and pulse intensities, and duration of prepulse, interstimulus interval, and pulse were selected based on previous findings by other groups showing that changes in these experimental parameters affect both startle amplitudes and %PPI outcomes (Halberstadt and Geyer 2018; Hanks and González-Maeso 2013; Swerdlow et al. 2016). We observed a DOI-dependent increase in %PPI in three out of the four PPI conditions, whereas changes in startle amplitude with either pulse alone or prepulse + pulse trials occurred across all four PPI conditions in male mice. These data suggest that the most important factor leading to increases in %PPI is a DOI-induced decrease in startle amplitude in response to prepulse + pulse trials. A secondary factor may be related to an increase in startle amplitude in the pulse-only trial, but only if the startle amplitudes in the prepulse + pulse trials are unaffected by DOI. Our observations also support the conclusion that a background intensity of 69 dB or above is necessary to expose the effect of DOI on %PPI in male 129S6/SvEv mice.
Because our goal was principally focused on sex-related differences in DOI-induced changes in %PPI, we paid special attention to the PPI conditions in which DOI affected %PPI but left startle amplitude in response to the pulse alone unaffected. This principally occurred under the 20-80-20 and 10-60-40 PPI conditions (see Table 1) in male mice. Using the same conditions, DOI also affected %PPI in female 129S6/SvEv mice without an effect of DOI on startle amplitudes with prepulse and pulse unlike in males. Although statistically significant, this effect of DOI on %PPI augmentation was reduced in female as compared to male 129S6/SvEv mice. While the behavioral effects of psychedelics between different rodent models may differ, these sex-related differences in startle magnitude and %PPI have also been reported in rats (Reilly et al. 2009) and humans (Swerdlow et al. 1993). Further work will be required to better understand the molecular and neural circuit mechanisms responsible for these sex-related differences in 129S6/SvEv mice as compared to other strains of mice.
Another important observation was that whereas DOI does not affect startle amplitude within the pulse alone trials under the 20-80-20 and 10-60-40 PPI conditions in either males or females, our data showed a statistically significant reduction in the startle amplitude during the 5 stimulus-only trials at the end of the PPI session as compared with the 5 stimulus-only trials at the beginning of the PPI session. This indicates the occurrence of startle habituation. Similar findings have been reported in rats (Farid et al. 2000), as well as alterations in the stability of the acoustic startle reflex and habituation in schizophrenia patients (Ludewig et al. 2002). From a translational perspective, we suggest the systematic inclusion of these controls in the experimental design since habituation processes may negatively bias the interpretation of PPI findings based on previously obtained startle amplitude datasets.
Brain regions involved in startle and PPI are located within the brain stem and include cochlear nuclei, caudal pontine reticular nucleus, inferior and superior colliculi, pedunculopontine and laterodorsal tegmental nuclei as well as substantia nigra. In addition, cortico-limbic areas, such as the nucleus accumbens, ventral pallidum, basolateral amygdala, septohippocampal system, mediodorsal thalamus, and medial prefrontal cortex, also modulate PPI (Fendt et al. 2001; Swerdlow et al. 2001). Brain areas enriched in 5-HT2AR expression include the frontal cortex, ventral striatum, several thalamic nuclei, and the hypothalamus (López-Giménez et al. 1997, 2001; López-Giménez et al. 1998). Considering the sex-related differences observed in both startle amplitude and %PPI as well as the effect of DOI on these behavioral changes and the sex-specific correlations between startle amplitude and %PPI, these findings provide support for the hypothesis that differences in cell-type or neural circuit-specific location of 5-HT2AR in male versus female mice may lead to distinct regulation of sensorimotor gating processes. Our results also suggest that these sex-related differences are not generalizable to other neural circuits and behavior models of psychedelic drug action since DOI-induced head-twitch behavior was comparable in male and female 129S6/SvEv mice. Additionally, generalization of our exploratory findings on sex-related differences related to DOI effects on startle reflex and augmentation of PPI in 129S6/SvEv mice should be tested in future studies with other psychedelics, especially those in advanced stages of development as potential therapeutics in psychiatric disorders such as depression.
Our findings highlight a distinct facet of psychedelic action that may advance our understanding of sex and species-specific mechanisms responsible for their unique behavioral effects. Such complex multifaceted action of psychedelics across domains, mouse strains, species, and sexes could also be exploited to ultimately reveal new molecular substrates for the development of more effective treatments for psychiatric disorders.
Funding
This work was supported in part by NIH-R01MH084894, NIH-R01MH111940, and NIHP30DA033934 (J.G.-M.), NIH-N01DA-17-8932 and NIH-N01DA-19-8949 (P.M.B.), NIH-P50AA022537 (J.T. W.), NIH-F30MH116550 (J.M.S), and NIH-T32MH020030 (M.d.l.F. R.). A.F.-T received support from grants PSI2017-82257-P (MINECO) and 2017SGR-1587.
Footnotes
Declarations
Experiments were conducted in accord with NIH guidelines and were approved by the Virginia Commonwealth University Animal Care and Use Committee.
Conflict of interest J.G.-M. has a sponsored research contract with Neuristic, and M.d.l.F.R. has a consulting agreement with Noetic Fund. The remaining authors declare that they have no conflict of interest.
References
- Allen JA, Yadav PN, Setola V, Farrell M, Roth BL (2011) Schizophrenia risk gene CAV1 is both pro-psychotic and required for atypical antipsychotic drug actions in vivo. Transl Psychiatry 1:e33. 10.1038/tp.2011.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert L, Reiss D, Ouagazzal A-M (2006) Auditory and visual prepulse inhibition in mice: parametric analysis and strain comparisons. Genes Brain Behav 5:423–431. 10.1111/j.1601-183X.2005.00178.x [DOI] [PubMed] [Google Scholar]
- Bershad AK, Schepers ST, Bremmer MP, Lee R, de Wit H (2019) Acute subjective and behavioral effects of microdoses of lysergic acid diethylamide in healthy human volunteers. Biol Psychiatry 86:792–800. 10.1016/j.biopsych.2019.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal CE, Morgan D (2012) Head-twitch response in rodents induced by the hallucinogen 2,5-dimethoxy-4-iodoamphetamine: a comprehensive history, a re-evaluation of mechanisms, and its utility as a model. Drug Test Anal 4:556–576. 10.1002/dta.1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente Revenga M, Shin JM, Vohra HZ, Hideshima KS, Schneck M, Poklis JL, González-Maeso J (2019) Fully automated head-twitch detection system for the study of 5-HT2A receptor pharmacology in vivo. Sci Rep 9:14247. 10.1038/s41598-019-49913-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente Revenga M, Vohra HZ, González-Maeso J (2020) Automated quantification of head-twitch response in mice via ear tag reporter coupled with biphasic detection. J Neurosci Methods 334:108595. 10.1016/j.jneumeth.2020.108595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulawa SC, Geyer MA (2000) Effects of strain and serotonergic agents on prepulse inhibition and habituation in mice. Neuropharmacology 39:2170–2179. 10.1016/s0028-3908(00)00030-7 [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Gross C, Stark KL, Hen R, Geyer MA (2000) Knockout mice reveal opposite roles for serotonin 1A and 1B receptors in prepulse inhibition. Neuropsychopharmacology 22:650–659. 10.1016/S0893-133X(99)00164-5 [DOI] [PubMed] [Google Scholar]
- Enga RM, Rice AC, Weller P, Subler MA, Lee D, Hall CP, Windle JJ, Beardsley PM, van den Oord EJ, McClay JL (2017) Initial characterization of behavior and ketamine response in a mouse knockout of the post-synaptic effector gene Anks1b. Neurosci Lett 641:26–32. 10.1016/j.neulet.2017.01.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farid M, Martinez ZA, Geyer MA, Swerdlow NR (2000) Regulation of sensorimotor gating of the startle reflex by serotonin 2A receptors. Ontogeny and strain differences Neuropsychopharmacology 23: 623–632. 10.1016/S0893-133X(00)00163-9 [DOI] [PubMed] [Google Scholar]
- Fendt M, Li L, Yeomans JS (2001) Brain stem circuits mediating prepulse inhibition of the startle reflex. Psychopharmacology 156:216–224. 10.1007/s002130100794 [DOI] [PubMed] [Google Scholar]
- Freedland CS, Mansbach RS (1999) Behavioral profile of constituents in ayahuasca, an Amazonian psychoactive plant mixture. Drug Alcohol Depend 54:183–194. 10.1016/s0376-8716(98)00154-9 [DOI] [PubMed] [Google Scholar]
- Geyer MA, Dulawa SC (2003) Assessment of murine startle reactivity, prepulse inhibition, and habituation. Curr Protoc Neurosci 24:8.17.1–8.17.15. 10.1002/0471142301.ns0817s24 [DOI] [PubMed] [Google Scholar]
- Glennon RA (1994) Classical hallucinogens: an introductory overview. NIDA Res Monogr 146:4–32 [PubMed] [Google Scholar]
- Gogos A, Bogeski M, van den Buuse M (2008) Role of serotonin-1A receptors in the action of antipsychotic drugs: comparison of prepulse inhibition studies in mice and rats and relevance for human pharmacology. Behav Pharmacol 19:548–561. 10.1097/FBP.0b013e32830cd822 [DOI] [PubMed] [Google Scholar]
- Gómez-Nieto R, Hormigo S, López DE (2020) Prepulse inhibition of the auditory startle reflex assessment as a hallmark of brainstem sensorimotor gating mechanisms. Brain Sciences 10:639. 10.3390/brainsci10090639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA (2007) Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron 53:439–452. 10.1016/j.neuron.2007.01.008 [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Heekeren K, Thelen B, Lindenblatt H, Kovar KA, Sass H, Geyer MA (1998) Effects of the hallucinogen psilocybin on habituation and prepulse inhibition of the startle reflex in humans. Behav Pharmacol 9:561–566. 10.1097/00008877-199811000-00011 [DOI] [PubMed] [Google Scholar]
- Halberstadt AL (2020) Automated detection of the head-twitch response using wavelet scalograms and a deep convolutional neural network. Sci Rep 10:8344. 10.1038/s41598-020-65264-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA (2010) LSD but not lisuride disrupts prepulse inhibition in rats by activating the 5-HT(2A) receptor. Psychopharmacology 208:179–189. 10.1007/s00213-009-1718-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA (2011) Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens. Neuropharmacology 61:364–381. 10.1016/j.neuropharm.2011.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA (2018) Effect of hallucinogens on unconditioned behavior. Curr Top Behav Neurosci 36:159–199. 10.1007/7854_2016_466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks JB, González-Maeso J (2013) Animal models of serotonergic psychedelics. ACS Chem Neurosci 4:33–42. 10.1021/cn300138m [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks JB, González-Maeso J (2016a) Hallucinogens: circuits, behavior and translational models. In: Preedy VR (ed) Neuropathology of drug addictions and substance misuse, volume 2. Elsevier, pp 813–820 [Google Scholar]
- Hanks JB, González-Maeso J (2016b) Molecular and cellular basis of hallucinogenic drug action. In: Preedy VR (ed) Neuropathology of drug addictions and substance misuse, volume 2. Elsevier, pp 803–812 [Google Scholar]
- Hazama K, Hayata-Takano A, Uetsuki K, Kasai A, Encho N, Shintani N, Nagayasu K, Hashimoto R, Reglodi D, Miyakawa T, Nakazawa T, Baba A, Hashimoto H (2014) Increased behavioral and neuronal responses to a hallucinogenic drug in PACAP heterozygous mutant mice. PLoS One 9:e89153. 10.1371/journal.pone.0089153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarra-Lecue I, Mollinedo-Gajate I, Meana JJ, Callado LF, Diez-Alarcia R, Urigüen L (2018) Chronic cannabis promotes pro-hallucinogenic signaling of 5-HT2A receptors through Akt/mTOR pathway. Neuropsychopharmacology 43:2028–2035. 10.1038/s41386-018-0076-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggar M, Weisstaub N, Gingrich JA, Vaidya VA (2017) 5-HT2A receptor deficiency alters the metabolic and transcriptional, but not the behavioral, consequences of chronic unpredictable stress. Neurobiol Stress 7:89–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson C, Jackson DM, Zhang J, Svensson L (1995) Prepulse inhibition of acoustic startle, a measure of sensorimotor gating: effects of antipsychotics and other agents in rats. Pharmacol Biochem Behav 52:649–654. 10.1016/0091-3057(95)00160-x [DOI] [PubMed] [Google Scholar]
- Kurita M, Holloway T, García-Bea A, Kozlenkov A, Friedman AK, Moreno JL, Heshmati M, Golden SA, Kennedy PJ, Takahashi N, Dietz DM, Mocci G, Gabilondo AM, Hanks J, Umali A, Callado LF, Gallitano AL, Neve RL, Shen L, Buxbaum JD, Han M-H, Nestler EJ, Meana JJ, Russ SJ, González-Maeso J (2012) HDAC2 regulates atypical antipsychotic responses through the modulation of mGlu2 promoter activity. Nat Neurosci 15:1245–1254. 10.1038/nn.3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Giménez JF, Mengod G, Palacios JM, Vilaró MT (1997) Selective visualization of rat brain 5-HT2A receptors by autoradiography with [3H]MDL 100,907. Naunyn Schmiedeberg's Arch Pharmacol 356: 446–454. 10.1007/pl00005075 [DOI] [PubMed] [Google Scholar]
- López-Giménez JF, Vilaró MT, Palacios JM, Mengod G (1998) [3H] MDL100,907 labels 5-HT2A serotonin receptors selectively in primate brain. Neuropharmacology 37:1147–1158. 10.1016/S0028-3908(98)00102-6 [DOI] [PubMed] [Google Scholar]
- López-Giménez JF, Vilaró MT, Palacios JM, Mengod G (2001) Mapping of 5-HT2A receptors and their mRNA in monkey brain: [3H] MDL100,907 autoradiography and in situ hybridization studies. The J of Comparative Neurology 429:571–589. [DOI] [PubMed] [Google Scholar]
- Ludewig K, Geyer MA, Etzensberger M, Vollenweider FX (2002) Stability of the acoustic startle reflex, prepulse inhibition, and habituation in schizophrenia. Schizophr Res 55:129–137. 10.1016/s0920-9964(01)00198-0 [DOI] [PubMed] [Google Scholar]
- Miliano C, Marti M, Pintori N, Castelli MP, Tirri M, Arfe R, De Luca MA (2019) Neurochemical and behavioral profiling in male and female rats of the psychedelic agent 25I-NBOMe. Front Pharmacol 10:1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JL, Holloway T, Albizu L, Sealfon SC, Gonzalez-Maeso J (2011) Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists. Neurosci Lett 493:76–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morici JF, Ciccia L, Malleret G, Gingrich JA, Bekinschtein P, Weisstaub NV (2015) Serotonin 2a receptor and serotonin 1a receptor interact within the medial prefrontal cortex during recognition memory in mice. Front Pharmacol 6:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morici JF, Miranda M, Gallo FT, Zanoni B, Bekinschtein P, Weisstaub NV (2018) 5-HT2a receptor in mPFC influences context-guided reconsolidation of object memory in perirhinal cortex. Elife 7:e33746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols DE (2016) Psychedelics. Pharmacol Rev 68:264–355. 10.1124/pr.115.011478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveras I, Sánchez-González A, Sampedro-Viana D, Piludu MA, Río-Alamos C, Giorgi O, Corda MG, Aznar S, González-Maeso J, Gerbolés C, Blázquez G, Cañete T, Tobeña A, Fernández-Teruel A (2017) Differential effects of antipsychotic and propsychotic drugs on prepulse inhibition and locomotor activity in Roman high- (RHA) and low-avoidance (RLA) rats. Psychopharmacology 234:957–975. 10.1007/s00213-017-4534-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouagazzal A, Grottick AJ, Moreau J, Higgins GA (2001) Effect of LSD on prepulse inhibition and spontaneous behavior in the rat. A pharmacological analysis and comparison between two rat strains. Neuropsychopharmacology 25:565–575. 10.1016/S0893-133X(01)00282-2 [DOI] [PubMed] [Google Scholar]
- Padich RA, McCloskey TC, Kehne JH (1996) 5-HT modulation of auditory and visual sensorimotor gating: II. Effects of the 5-HT2A antagonist MDL 100,907 on disruption of sound and light prepulse inhibition produced by 5-HT agonists in Wistar rats. Psychopharmacology 124:107–116. 10.1007/BF02245610 [DOI] [PubMed] [Google Scholar]
- Páleníček T, Balíková M, Bubeníková-Valesová V, Horácek J (2008) Mescaline effects on rat behavior and its time profile in serum and brain tissue after a single subcutaneous dose. Psychopharmacology 196:51–62. 10.1007/s00213-007-0926-5 [DOI] [PubMed] [Google Scholar]
- Páleníček T, Fujáková M, Brunovský M, Horáček J, Gorman I, Balíková M, Rambousek L, Syslová K, Kačer P, Zach P, Bubeníková-Valešová V, Tylš F, Kubešová A, Puskarčíková J, Höschl C (2013) Behavioral, neurochemical and pharmaco-EEG profiles of the psychedelic drug 4-bromo-2,5-dimethoxyphenethylamine (2C-B) in rats. Psychopharmacology 225:75–93. 10.1007/s00213-012-2797-7 [DOI] [PubMed] [Google Scholar]
- Paylor R, Crawley JN (1997) Inbred strain differences in prepulse inhibition of the mouse startle response. Psychopharmacology 132:169–180. 10.1007/s002130050333 [DOI] [PubMed] [Google Scholar]
- Ralph-Williams RJ, Lehmann-Masten V, Geyer MA (2003) Dopamine D1 rather than D2 receptor agonists disrupt prepulse inhibition of startle in mice. Neuropsychopharmacology 28:108–118. 10.1038/sj.npp.1300017 [DOI] [PubMed] [Google Scholar]
- Reilly W, Koirala B, Devaud LL (2009) Sex differences in acoustic startle responses and seizure thresholds between ethanol-withdrawn male and female rats. Alcohol Alcohol 44:561–566. 10.1093/alcalc/agp049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Río-Álamos C, Piludu MA, Gerbolés C, Barroso D, Oliveras I, Sánchez-González A, Cañete T, Tapias-Espinosa C, Sampedro-Viana D, Torrubia R, Tobeña A, Fernández-Teruel A (2019) Volumetric brain differences between the Roman rat strains: neonatal handling effects, sensorimotor gating and working memory. Behav Brain Res 361:74–85. 10.1016/j.bbr.2018.12.033 [DOI] [PubMed] [Google Scholar]
- Schmid Y, Enzler F, Gasser P, Grouzmann E, Preller KH, Vollenweider FX, Brenneisen R, Müller F, Borgwardt S, Liechti ME (2015) Acute effects of lysergic acid diethylamide in healthy subjects. Biol Psychiatry 78:544–553. 10.1016/j.biopsych.2014.11.015 [DOI] [PubMed] [Google Scholar]
- Sipes TA, Geyer MA (1994) Multiple serotonin receptor subtypes modulate prepulse inhibition of the startle response in rats. Neuropharmacology 33:441–448. 10.1016/0028-3908(94)90074-4 [DOI] [PubMed] [Google Scholar]
- Sipes TE, Geyer MA (1995) DOI disruption of prepulse inhibition of startle in the rat is mediated by 5-HT(2A) and not by 5-HT(2C) receptors. Behav Pharmacol 6:839–842 [PubMed] [Google Scholar]
- Swerdlow NR, Mansbach RS, Geyer MA, Pulvirenti L, Koob GF, Braff DL (1990) Amphetamine disruption of prepulse inhibition of acoustic startle is reversed by depletion of mesolimbic dopamine. Psychopharmacology 100:413–416. 10.1007/BF02244616 [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Auerbach P, Monroe SM, Hartston H, Geyer MA, Braff DL (1993) Men are more inhibited than women by weak prepulses. Biol Psychiatry 34:253–260. 10.1016/0006-3223(93)90079-S [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL (2001) Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology 156:194–215. 10.1007/s002130100799 [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Geyer MA (2016) Sensorimotor gating of the startle reflex: what we said 25 years ago, what has happened since then, and what comes next. J Psychopharmacol 30:1072–1081. 10.1177/0269881116661075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varty GB, Higgins GA, Higgins GA (1995) Examination of drug-induced and isolation-induced disruptions of prepulse inhibition as models to screen antipsychotic drugs. Psychopharmacology 122: 15–26. 10.1007/BF02246437 [DOI] [PubMed] [Google Scholar]
- Varty GB, Walters N, Cohen-Williams M, Carey GJ (2001) Comparison of apomorphine, amphetamine and dizocilpine disruptions of prepulse inhibition in inbred and outbred mice strains. Eur J Pharmacol 424:27–36. 10.1016/s0014-2999(01)01115-3 [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Preller KH (2020) Psychedelic drugs: neurobiology and potential for treatment of psychiatric disorders. Nat Rev Neurosci 21:611–624. 10.1038/s41583-020-0367-2 [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Bäbler A, Vogel H, Hell D (1998) Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport 9:3897–3902. 10.1097/00001756-199812010-00024 [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Csomor PA, Knappe B, Geyer MA, Quednow BB (2007) The effects of the preferential 5-HT2A agonist psilocybin on prepulse inhibition of startle in healthy human volunteers depend on interstimulus interval. Neuropsychopharmacology 32:1876–1887. 10.1038/sj.npp.1301324 [DOI] [PubMed] [Google Scholar]
- Weisstaub NV, Zhou M, Lira A, Lambe E, Gonzalez-Maeso J, Hornung JP, Sibille E, Underwood M, Itohara S, Dauer WT, Ansorge MS, Morelli E, Mann JJ, Toth M, Aghajanian G, Sealfon SC, Hen R, Gingrich JA (2006) Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science 313:536–540 [DOI] [PubMed] [Google Scholar]
- Zentner M, Grandjean D, Scherer KR (2008) Emotions evoked by the sound of music: characterization, classification, and measurement. Emotion 8:494–521. 10.1037/1528-3542.8A494 [DOI] [PubMed] [Google Scholar]