Abstract
Schizophrenia is a severe brain disorder that usually produces a lifetime of disability. First generation or typical such as haloperidol and second generation or atypical such as clozapine and risperidone remain the current standard for schizophrenia treatment. In some patients with schizophrenia, antipsychotics produce complete remission of positive symptoms, such as hallucinations and delusions. However, antipsychotic drugs are ineffective against cognitive deficits and indeed treated schizophrenia patients have small improvements or even deterioration in several cognitive domains. This underlines the need for novel and more efficient therapeutic targets for schizophrenia treatment. Serotonin and glutamate have been identified as key parts of two neurotransmitter systems involved in fundamental brain processes. Serotonin (or 5-hydroxytryptamine) 5-HT2A receptor (5-HT2AR) and metabotropic glutamate 2 receptor (mGluR2) are G protein-coupled receptors (GPCRs) that interact at epigenetic and functional levels. These two receptors can form GPCR heteromeric complexes through which their pharmacology, function and trafficking becomes affected. Here we review past and current research on the 5-HT2AR-mGluR2 heterocomplex and its potential implication in schizophrenia and antipsychotic drug action.
Keywords: Serotonin 5-HT2A receptor, metabotropic glutamate 2 receptor, G protein-coupled receptor (GPCR), GPCR dimerization, psychedelics, antipsychotics, schizophrenia, epigenetics
Introduction
Schizophrenia is a chronic debilitating mental disorder that affects approximately 1% of the general population (Sawa and Snyder, 2002; Freedman, 2003). The symptoms generally include positive such as hallucinations and delusions, negative such as deficits of motivation and social withdrawal, and cognitive such as attention deficit and impaired memory. Although currently used antipsychotic medications may reduce hallucinations and delusions in certain groups of schizophrenia patients, their clinical efficacy in negative and cognitive symptoms remains modest (Lieberman et al., 2008; Ebdrup et al., 2011; Miyamoto et al., 2012; Meltzer, 2013). Identifying and testing new molecular targets for the treatment of schizophrenia is therefore crucial to optimally manage this and other psychiatric disorders.
Over the past several decades, the most common neurotransmitter potentially linked to schizophrenia has been the monoaminergic dopamine, and particularly a potential excess of dopamine transmission in certain subcortical limbic areas such as the nucleus accumbens (NAc), amygdala and hippocampus (Seeman and Lee, 1975; Meltzer and Stahl, 1976; Seeman et al., 1976). This notion stemmed from pharmacological evidences that demonstrated that drugs such as phenothiazine, which decrease dopamine activity, showed antipsychotic properties, whereas drugs such as amphetamine, which increase dopamine release, were considered as psychotomimetic (Meltzer and Stahl, 1976). However, more recent findings have implicated additional neurotransmitter systems in psychotic disorders and their treatment. As an example, psychosis in patients with Parkinson’s disease (PD) has been proposed to be caused by a serotonin-dopamine imbalance (Stahl, 2016), and accordingly, the relatively selective serotonin (or 5-hydroxytryptamine) 5-HT2A receptor (5-HT2AR) antagonist pimavanserin, which also shows a negligible affinity against the dopamine D2 receptor (Casey et al., 2022), has proven antipsychotic activity in PD patients without an effect on motor function when compared with placebo (Meltzer et al., 2010).
The glutamate theory of psychosis, and particularly the N-methyl-D-aspartate (NMDA) receptor hypoactivity theory, proposes that hypofunction of the NMDA receptor in brain regions such as the prefrontal cortex may be responsible for some of the behavioral deficits observed in schizophrenia patients (Moghaddam, 2004; Stahl, 2018). In healthy volunteers, non-competitive NMDA antagonists such as phencyclidine (PCP) (Morris et al., 2005), also known as the angel dust, impair cognitive functioning in a manner similar to that encountered in schizophrenia patients (Kristiansen et al., 2007). Preclinical studies in rodent models suggest that targeting two subtypes of metabotropic glutamate receptors (mGluRs) has the capability to improve cognitive deficits resulting from dysfunction of NMDA receptor. These include mGluR5, which can directly modulate the function of NMDA channel, and mGluR2/3s, which regulate the release of glutamate principally via presynaptic mechanisms (Moghaddam, 2004).
The potential involvement of serotonin in schizophrenia was hypothesized in the 1950s. It was based on the structural similarities between serotonin and psychedelics such as lysergic acid diethylamide (LSD) and psilocin (active metabolite of psilocybin), and the findings that psychedelics caused hallucinations and emotional disturbances similar to those observed in schizophrenia patients (Woolley and Shaw, 1954). Several clinical studies indicate that a portion of schizophrenia patients respond to selective blockade of 5-HT2ARs (Geyer and Vollenweider, 2008; Meltzer, 2013). Psychosis caused by agonism at 5-HT2ARs upon psychedelic administration resembles some of the symptoms in first-episode schizophrenia patients (Vollenweider et al., 1998; Schmid et al., 2015). Additionally, the 5-HT2AR (HTR2A) gene ranks in the top 12 of the more than 1,000 genes tested for genetic association with schizophrenia, and genome-wide association studies focused on the de novo mutations showed that the HTR2A gene might potentially be implicated in schizophrenia-related networks (Fromer et al., 2014).
This review attempts to explore recent advances in the interactions between serotonergic and glutamatergic systems, particularly 5-HT2AR and mGluR2, in preclinical models of psychosis, neuropsychiatric disorders and antipsychotic drug action.
Structure and function of GPCRs
G protein-coupled receptors (GPCRs) comprise the largest superfamily of membrane proteins in the mammalian genome (Thomsen et al., 2005; Rosenbaum et al., 2009; Katritch et al., 2013; Lee et al., 2018), and are targets of around 700 approved drugs (Sriram and Insel, 2018). They enable communication between the extracellular and intracellular milieu of the cell through mediators that include a large variety of hormones, neurotransmitters, metabolites, cytokines, photons, ions and other stimuli essential for normal physiological functions (Rosenbaum et al., 2009). Based on function and primary sequence, the GPCR superfamily is broadly classified into six families, namely class A – rhodopsin-like receptors, class B – secretin family, class C – metabotropic glutamate receptors, class D – fungal mating pheromone receptors, class E – cAMP receptors, class F – frizzled (FZD) and smoothened (SMO) receptors. This receptor superfamily comprises of more than 800 genes which modulate several signaling pathways involved in regulation of biological processes that include blood pressure, behavior, cognition, immune response, smell, taste and mood, among many others. All members of this superfamily share several structural motifs, mainly the seven transmembrane domains interconnected by loops with extracellular N-terminus and intracellular C-terminus. However, they have low sequence identity, and possess different extracellular N-terminal domains and diverse ligand-binding pockets. For class A GPCRs, the ligand-binding site is within the transmembrane (TM) region. For class B GPCRs, ligand binding involves both extracellular and TM domains. For class C GPCRs, the ligand-binding pocket is found in the extracellular domain (ECD) that contains a Venus flytrap (VFT) domain. In the case of class F GPCRs, their ECD comprises of an extracellular cysteine-rich domain (CRD) and an ECD linker domain (Basith et al., 2018).
GPCRs were traditionally thought to transduce extracellular stimuli to intracellular second messengers by acting as ligand-regulated guanine nucleotide exchange factors for heterotrimeric (α-, β-, and γ-subunit) GTP-binding proteins (G proteins) (Gilman, 1987). According to this model, an agonist-bound GPCR adopts an active conformation that causes exchange of GDP for GTP on the Gα-subunit. This causes dissociation of Gα from Gβγ subunit. Subsequently, the activated G protein subunits are capable of either activating or inhibiting downstream targets such as ion channels, phospholipases and adenylyl cyclases (ACs). Regulation of GPCR signaling is accomplished by a conserved two-step mechanism that involves receptor phosphorylation by GPCR kinases (GRKs) followed by arrestin recruitment to phosphorylated receptors (Gurevich and Gurevich, 2019). More recent studies highlighted the ability of GPCRs to signal via G protein-independent mechanisms like the β-arrestin signaling pathway. They also act as scaffolds for a large number of GPCR-interacting proteins which in turn regulate their function, localization and trafficking (Magalhaes et al., 2012).
GPCR complexes (dimers and oligomers)
Initially, GPCRs were considered to function as monomeric structural units. However, in the last three decades, several techniques including crystallographic studies have provided substantial evidence supporting the existence of GPCR homomers and/or heteromers as functional signaling units (Gonzalez-Maeso, 2011; Gonzalez-Maeso, 2014; Gaitonde and Gonzalez-Maeso, 2017; Ferre et al., 2022). The concept of receptor dimerization/oligomerization traces back to the 1980/90s, and these include the study that reported reduction in the affinity of dopamine D2 agonist binding in plasma membrane preparations of rat striatal neurons upon stimulation of adenosine A2A receptors (Ferre et al., 1991). However, it was only in 1993 that the first insight into the role dimerization in GPCR function was established at the molecular level (Maggio et al., 1993). They generated chimeras of the muscarinic M3 and α2C-adrenergic receptors and tested the functional response elicited by these chimeric constructs: chimeric M3/α2C receptor was comprised of TM domains 1–5 of the muscarinic M3 receptor and the last two TM domains of the α2C-adrenergic receptor, whereas chimeric α2C/M3 receptor was generated by fusing TM domains 1–5 of the α2C-adrenergic receptor and the last two TM domains of the muscarinic M3 receptor. In these two chimeric constructs, the intracellular loop 3 of the muscarinic M3 receptor was kept unaltered because this region mediates G protein coupling. In the presence of the muscarinic receptor agonist carbachol, signaling was abolished when either of these two chimeric constructs were expressed alone. However, when the α2C/M3 and M3/α2C chimeric constructs were co-expressed together, carbachol-induced signaling was rescued.
The first example that heterodimerization was fully required for class C GPCR function was that of the GABAB receptor. Expression of a fully functional receptor required heterodimerization between two distinct and separate gene products, namely, GABAB-R1 and GABAB-R2 (Jones et al., 1998; Kaupmann et al., 1998; White et al., 1998). Another key example of how molecular proximity affects functional and ligand binding properties is the heteromerization of the δ and κ opioid receptors (Jordan and Devi, 1999). More recently, fluorescence resonance energy transfer (FRET) studies demonstrated that within an heteromeric complex formed between α2A-adrenergic and μ-opioid receptors, binding of morphine to the μ-opioid receptor triggers a conformational change in the α2A-adrenergic receptor bound to norepinephrine that inhibits Gi protein-dependent signaling and subsequently the downstream MAPK signaling cascade (Vilardaga et al., 2008).
It has also been proposed the existence of GPCR-effector macromolecular membrane assemblies (GEMMAs) comprised of specific GPCR combinations, G proteins, effectors and other associated plasma membrane-localized proteins (Ferre et al., 2022). GEMMAs harbor distinct functional and pharmacological characteristics thereby making them unique drug targets. For example, mutants of the small GTPase Sar1 or Rab1 prevented anterograde trafficking to the plasma membrane and diminished plasma membrane localization of β2-adreneregic receptor but did not interfere with the interactions between β2-adrenergic receptor and Gβ1/Gγ2 subunits, AC2 and β2-adrenergic receptor or AC2 and Gβ1 or Gγ2 fused to bioluminescence resonance energy transfer (BRET) chromophores (Ebdrup et al., 2011), indirectly pointing towards the pre-assembly of β2-adrenergic receptor Gβ1γ2-AC2 complexes.
Serotonin and 5-HT2ARs
Serotonin is one of the main neurotransmitters that has been detected in the nervous systemsof virtually all metazoan phyla. It functions as a crucial regulator of memory, cognition, sleep, mood, appetite, learning, social behaviors and whole-body homeostasis. In humans, it exerts its physiological effects through 14 distinct receptors, out of which 13 are GPCRs, and one is a ligand-gated cation channel (McCorvy and Roth, 2015; Sharp and Barnes, 2020). The International Union of Basic and Clinical Pharmacology (IUPHAR) has classified serotonin receptors into 5-HT1 (5-HT1A, 5-HT1B, 5-HT1D, 5-ht1e, and 5-HT1F), 5-HT2 (5-HT2A, 5-HT2B, and 5-HT2C), 5-HT3, 5-HT4, 5-HT5 (5-HT5A, 5-ht5b), 5-HT6, and 5-HT7 receptors (McCorvy and Roth, 2015; Barnes et al., 2021). This classification was based on their primary sequence, intracellular transduction mechanisms, selective agonists and antagonists, and ligand binding affinities criteria. It is currently accepted that the CNS expresses seven types of serotonin receptors (5-HT1–7). However, 5-ht1e and 5-ht5b retain low case letters since their functional response remains unknown. All 5-HT receptor subtypes have been reported in both brain and peripheral tissues with the exception of 5-ht1e, 5-HT2C and 5-HT6 which show limited expression outside the CNS. However, 95% of 5-HT in human body is synthesized, stored and released in the gut, where its function is focused on either contraction of smooth muscles or depolarization of the enteric nerve (McCorvy and Roth, 2015).
The serotonin 5-HT2AR is the main excitatory GPCR of the 5-HT receptor family. In humans and rodent models, this receptor subtype is primarily expressed in the frontal cortex, whereas it is also detectable at relatively lower densities in other brain regions such as hippocampus, thalamus and basal ganglia. Within the cortex, they are densely populated on the dendrites of excitatory glutamatergic pyramidal neurons, particularly in layer V (Lopez-Gimenez et al., 1997; Jakab and Goldman-Rakic, 1998; Lopez-Gimenez et al., 2001).
The three 5-HT2R subtypes, namely, 5-HT2AR, 5-HT2BR and 5-HT2CR, couple to Gq/11 proteins and activate a cytoplasmic protein phospholipase C (PLC). Activated PLC cleaves phosphatidylinositol 4,5-bisphosphate (PIP2), a phospholipid of the plasma membrane, followed by generation of diacylglycerol (DAG) and inositol triphosphate (IP3). Ultimately, IP3 induces release of Ca2+ from intracellular stores (Leysen, 2004). These three 5-HT2R subtypes can also activate phospholipase A2 (PLA2), which thereby causes the release of arachidonic acid (AA). Interestingly, previous studies have shown that 5-HT2AR-induced PLA2 activation does not depend upon PLC activation (Kurrasch-Orbaugh et al., 2003b). Rather, it has been observed that NIH3T3 cells stably expressing 5-HT2AR causes PLA2 activation though a complex signaling mechanism involving both Gi/o-associated Gβγ-mediated activation of extracellular signal-regulated kinase, p44/p42 (ERK1,2) and Gα12/13-coupled, Rho-mediated p38 activation (Kurrasch-Orbaugh et al., 2003a).
Apart from endogenous 5-HT, several antipsychotics, psychedelics and antidepressants function at least in part by targeting the 5-HT2ARs. Preclinical and clinical studies suggest that 5-HT2AR antagonists, such as mirtazapine, mianserin and trazodone, have antidepressant properties (Marek et al., 2005), whereas its agonists possess cognition-enhancing and psychedelic characteristics. Abnormality in 5-HT2AR activity is associated with a number of psychiatric conditions, including schizophrenia, depression, anxiety, and substance use disorders. Pharmacological studies indicate that high-affinity antagonists of 5-HT2ARs such as clozapine and risperidone are effective atypical antipsychotics (Hanks and Gonzalez-Maeso, 2013; Hanks and González-Maeso, 2016b; Hanks and González-Maeso, 2016a; Lopez-Gimenez and Gonzalez-Maeso, 2018; Jaster et al., 2021).
Classical psychedelics are compounds that evoke profound alterations in perception, cognition and mood, and have been used recreationally and for religious and spiritual purposes for centuries (Nichols, 2016). Richard Glennon, Milt Titeler and their teams were among the first to propose the potential role of 5-HT2Rs in the mechanism of action of psychedelics (Glennon et al., 1984). Later, experiments with knockout (KO) mice (Gonzalez-Maeso et al., 2003; Gonzalez-Maeso et al., 2007) and 5-HT2AR antagonists such as M100907 (volinanserin) (Halberstadt and Geyer, 2018; de la Fuente Revenga et al., 2019) corroborated the role of 5-HT2AR-dependent signaling in the cellular and behavioral effects of psychedelics. These studies in rodent models were further supported by data from humans which demonstrated that the psychosis-like effects of psilocybin and LSD in healthy volunteers were reversed by the 5-HT2R antagonist ketanserin (Vollenweider et al., 1998; Schmid et al., 2015; Preller et al., 2016; Preller et al., 2017).
All psychedelics such as LSD, psilocin and mescaline are potent 5-HT2AR agonists but not all 5-HT2AR agonists are psychedelics – these include non-psychedelic 5-HT2AR agonists such as lisuride and ergotamine which are currently used in the treatment of neuropsychiatric conditions such as PD or migraine, respectively (Silbergeld and Hruska, 1979; Mokler et al., 1983; Adams and Geyer, 1985; Marona-Lewicka et al., 2002). One of the potential mechanisms behind the behavioral differences between psychedelic and non-psychedelic 5-HT2AR agonists is the concept of biased agonism, which describes the ability of ligands acting at the same GPCR to elicit distinct cellular signaling profiles (Urban et al., 2007; Gonzalez-Maeso and Sealfon, 2009a; Gonzalez-Maeso and Sealfon, 2009b). Recent findings in rodent models suggests that psychedelic 5-HT2AR agonists may represent a new approach to treat psychiatric conditions including depression and substance use disorders (Ly et al., 2018; de la Fuente Revenga et al., 2021; Jaster et al., 2021; Shao et al., 2021; Cameron et al., 2023), an effect that is also induced by the non-psychedelic 5-HT2AR agonist lisuride (Nakamura et al., 1989; Qu et al., 2022). Although this topic goes beyond the scope of this review, psychedelic and non-psychedelic 5-HT2AR agonists are currently being used as a pharmacological tool to investigate the signaling mechanisms that allow chemically similar agonists to target the same receptor population but induce different signaling and, potentially, therapeutic-like effects in rodent models of depression and substance use disorder (Cameron et al., 2021; Cao et al., 2022; Kaplan et al., 2022). An interesting question for additional studies seems to be how both receptor activation via psychedelic 5-HT2AR agonists and receptor blockade via either traditional antidepressants such as mirtazapine (see above) or more selective 5-HT2AR (M100907 and ketanserin) and 5-HT2CR (SB242084) antagonists lead to antidepressant-like effect in rodents (Kaplan et al., 2022; Pacheco et al., 2023).
Previous review articles have emphasized that LSD, a classical psychedelic, appears to exert its psychoactive properties principally via the 5-HT2-like receptors, particularly through the 5-HT2AR (Hanks and Gonzalez-Maeso, 2013; Hanks and González-Maeso, 2016b; Hanks and González-Maeso, 2016a; Lopez-Gimenez and Gonzalez-Maeso, 2018; Jaster et al., 2021). The first attempt to provide structural insights into the binding mode of LSD to a 5-HT2R was the crystal structure of the human 5-HT2BR bound to LSD, which was resolved at 2.9 Å (Wacker et al., 2017). This study shows that LSD has an exceptionally slow dissociation rate from both 5-HT2BR and 5-HT2AR. Molecular dynamics (MD) simulation studies revealed that LSD’s slow binding kinetics might be due to the formation of a “lid” by the extracellular loop 2 (EL2) at the entrance of the binding pocket. Substitution of a key residue (L209) in EL2 increases the mobility of this lid and augments LSD’s binding kinetics. This selectively hampers LSD-mediated β-arrestin-2 recruitment yet negligibly affects Gq signaling. The crystal structures of the human 5-HT2AR complexed with the antipsychotic drugs risperidone and zotepine were resolved at 3.0/3.6 Å and at 2.9/3.3 Å resolution, respectively (Kimura et al., 2019). Several other structural analyses were conducted to gain molecular insights into actions of psychedelics at the serotonin receptors. The active-state structure of the human 5-HT2AR bound to 25-CN-NBOH (a prototypical psychedelic and selective 5-HT2AR agonist) complexed with an engineered Gαq heterotrimer was determined by cryo-electron microscopy (cryo-EM) at a resolution of 3.27 Å. X-ray crystal structures of the human 5-HT2AR complexed with LSD or the inverse agonist methiothepin were obtained at a resolution of around 3.4 Å (Kim et al., 2020). These findings could accelerate the discovery of more selective 5-HT2AR agonists and antagonists for the treatment of a variety of psychiatric conditions.
Related to the effect of psychedelic drug administration on gene expression, previous studies using traditional gene-expression assays such as DNA microarrays reported that a single administration of psychedelic and non-psychedelic 5-HT2AR agonists induced the expression of INBD and c-Fos genes in brain regions such as the mouse somatosensory cortex (Nichols and Sanders-Bush, 2002; Gonzalez-Maeso et al., 2003; Nichols et al., 2003; Gonzalez-Maeso et al., 2007). However, expression of Egr-1 and Egr-2 was only escalated upon administration of psychedelics such as DOI, DOM, DOB, mescaline, LSD, and psilocin, an effect that was not observed by non-psychedelic 5-HT2AR agonists such as lisuride and ergotamine. Also, with the exception of INBD, the effect of psychedelic and non-psychedelic 5-HT2AR agonists on gene expression was abolished in 5-HT2AR-KO mice. These alterations in gene expression showed maximal changes at ~60 min and returned to basal levels at ~120 min after drug exposure. one marks and DNA methylation affect chromatin organization and DNA accessibility (Ibi and Gonzalez-Maeso, 2015). Recent findings reported long-lasting (up to 7 days) changes on chromatin organization in mouse frontal cortex, particularly at enhancer regions of genes involved in synaptic plasticity, following a single administration of the psychedelic DOI (de la Fuente Revenga et al., 2021). Repeated LSD administration (7 days) also affects DNA methylation in CpG sites of mouse frontal cortex samples (Inserra et al., 2022). Additional investigation is required to characterize the functional relevance of these prolonged transcriptomic and epigenomic alterations induced by classical psychedelics.
Metabotropic glutamate receptors and mGluR2
Glutamate is one of the most functionally relevant neurotransmitters of the CNS. It executes its functions principally via two main classes of receptors: ionotropic glutamate receptors, which are multimeric ion channels conducting fast synaptic responses, and mGluRs, which are GPCRs modulating slow synaptic transmission via intracellular second messengers (Hollmann and Heinemann, 1994). There are 8 members within the mGluR family which have been subdivided into 3 groups on the basis of G protein coupling, sequence homology and ligand selectivity. Group I includes mGluRs 1 and 5, group II includes mGluRs 2 and 3, and group III includes mGluRs, 4, 6, 7, and 8 (Conn and Pin, 1997; Niswender and Conn, 2010; Nicoletti et al., 2011).
Generally, group I mGluRs activate PLCβ by coupling to Gq/11 proteins. Apart from this signaling pathway, group I mGluRs can also activate a plethora of downstream effectors, including phospholipase D, cyclin-dependent protein kinase 5, casein kinase 1, c-Jun kinase, components of MAPK/ERK pathway, and the MTOR/p70 S6 pathway. Group II and III mGluRs are predominantly coupled to Gi/o proteins, and also couple to other signaling pathways, including activation of phosphatidyl inositol 3-kinase PI3 kinase and MAPK pathways (Conn and Pin, 1997; Niswender and Conn, 2010; Nicoletti et al., 2011).
Several members of the mGluR family undergo alternative splicing. mGluR1 has 5 splice variants mGluR1a, b, c, d, and e. mGluR5 has two main splice variants, namely, mGluR5a and mGluR5b. Amongst group II mGluRs, mGluR2 does not have any reported splice variant but mGluR3, mGluR6, mGluR7 and mGluR8 have 4, 3, 5 and 3 splice variants, respectively (Conn and Pin, 1997; Niswender and Conn, 2010; Nicoletti et al., 2011).
Members of the class C GPCR tend to form constitutive homo and heterodimers amongst themselves as well as with other GPCR classes, and dimerization is a prerequisite process for glutamate-induced signaling through the ECD to the mGluR TM core (Conn and Pin, 1997; Niswender and Conn, 2010). It has been demonstrated that heterodimerization between members of the mGluR family is selective. Thus, group I members only heterodimerize with members belonging to their group, whereas group II and III members can form heterodimers with each other (Doumazane et al., 2011). Examples of mGluR heterodimers include mGluR2/mGluR4, mGluR2/mGluR3, mGluR2/mGluR7, and mGluR1/mGluR5 (Levitz et al., 2016; Pandya et al., 2016; Habrian et al., 2019; Lee et al., 2020).
Related to their functional properties, although binding of one agonist to either of the subunits in an mGluR homodimer is able to induce G protein coupling, agonist occupation of both ECDs is required for full receptor activation (Kniazeff et al., 2004). Ligand binding to mGluR homodimers recruits G protein to only one of the two subunits (Hlavackova et al., 2005). Assays combining single-molecule pull-down (SiMPull) with single-molecule total internal reflection fluorescence (smTIRF) microscopy revealed that homo- and heteromerization of mGluRs is primarily mediated via interactions at a hydrophobic interface in the upper lobe of the ligand binding domain (LBD), followed by secondary sites of interaction that include the inter-subunit disulfide bridge and TM domains (Levitz et al., 2016). FRET measurements also revealed that conformational rearrangements underlying activation of mGluRs may be controlled by interactions between ligand binding domains (Levitz et al., 2016).
More recent cryo-EM studies determined the structures of mGluR2 homodimers complexed with Gi1, and mGluR4 homodimers complexed with Gi3 at resolutions of 3.1 Å (extracellular domains of mGluR2) and 3.5 Å (Gi1 and the TM domains of mGluR2), and 3.1 Å (extracellular domains of mGluR4) and 4.0 Å (mGluR4–Gi3). In both mGluR2 and mGluR4, the C-terminal region of the Gαi subunit binds to a groove formed by ICL1, ICL2 and ICL3 and the intracellular regions of TM 3 and 5. In the mGluR2–Gi1 complex, the mGluR2 positive allosteric modulator (PAM) JNJ-40411813 binds at a site formed by TMs 3, 5, 6 and 7 of the G protein-bound subunit. In the inactive state, the interface for homodimerization is TM6. Agonist binding induces a conformational change that alters the dimerization interface to TM6. Additionally, in order to facilitate coupling of G proteins, the TM domains undergo a clockwise rotation and formation of an asymmetric interface between TM6 of the mGluR2-G protein-bound subunit and TM7 of the mGluR2-G protein free subunit (Lin et al., 2021).
Another study solved the cryo-EM structures of inactive mGluR2, inactive mGluR7, heterodimer of mGluR2–mGluR7 and the PAM- and agonist-bound mGluR2 structures at overall resolutions of 3.6 Å, 4.0 Å, 3.9 Å and 3.1 Å, respectively. The extracellular domains of mGluR7 and the mGluR2–mGluR7 heterodimer were further resolved to 3.6 Å and 3.5 Å, respectively. The crystal structures of the transmembrane domains of mGluR2 bound to two negative allosteric modulators (NAM), namely, NAM563 and NAM59710, were resolved at 2.5 Å and 2.7 Å, respectively (Du et al., 2021). This study confirmed TM4 as the symmertric dimeric interface between two mGluR2 subunits in inactive state and TM6 as the interface when bound to an agonist and PAM. However, in the case of the inactive mGluR7 homodimer and mGluR2-mGluR7 heterodimer, the interface is formed by TM5 (Du et al., 2021). These studies also suggest that it is the TM4 domain as the major responsible for the difference in dimerization between full length mGluR2 and mGluR3, given that the sequence indentity between these two receptors is nearly 70%. Othosteric agonist activation of group II mGluRs is influenced by inter-TM4 interactions. Ligand-coupled SiMPull assays strongly support a rearrangement from TM4 to TM6, with agonists and PAMs favoring a TM6-TM6 interface while antagonists and NAMs favoring a TM4-TM4 interface (Thibado et al., 2021). Some of these cryo-EM and SiMPull employed mGluR2 constructs with a C121A mutation, which may have an impact on the relative location of the two subunits within the mGluR2 homodimer. This is because previous studies suggested that the mGluR2-C121A construct alters homodimerization and dynamics (Levitz et al., 2016; Koehl et al., 2019; Shah et al., 2020).
5-HT2AR-mGluR2 heteromeric complex
Class A Gq/11-coupled 5-HT2AR and class C Gi/o-coupled mGluR2 are able to interact physically to form a GPCR complex (Gonzalez-Maeso et al., 2008). Assembly of 5-HT2AR and mGluR2 as a GPCR complex has been validated by several independent in vitro methods, such as co-immunoprecipitation, FRET, BRET, bimolecular fluorescence complementation (BiFC), and proximity ligation assay (PLA) (Gonzalez-Maeso et al., 2008; Moreno et al., 2012; Moreno et al., 2016; Toneatti et al., 2020). Most of these studies were conducted in vitro in HEK293 cells.
Three amino acids located at the intracellular region of the TM4 of mGluR2, namely, Ala-677, Ala-681, and Ala-685, have been proposed to be necessary for the association with 5-HT2AR as a GPCR heterocomplex (Gonzalez-Maeso et al., 2008; Moreno et al., 2012). Substitution of these three residues in mGluR2 with the corresponding residues from mGluR3 disrupted 5-HT2AR-mGluR2 heteromerization. By introducing the photoactivatable unnatural amino acid p-azido-L-phenylalanine (azF) at selected individual positions along the TM segments of mGluR2, it has also been suggested that 5-HT2AR crosslinked with azF incorporated at the intracellular end of mGluR2’s TM4 (Shah et al., 2020). It remains unknown whether 5-HT2AR assembly affects the structural arrangement of the two protomers within the mGluR2 homodimer under apo- and agonist-bound states, and further cryo-EM and X-ray assays are needed to provide additional structural insights into the interaction of 5-HT2AR and mGluR2.
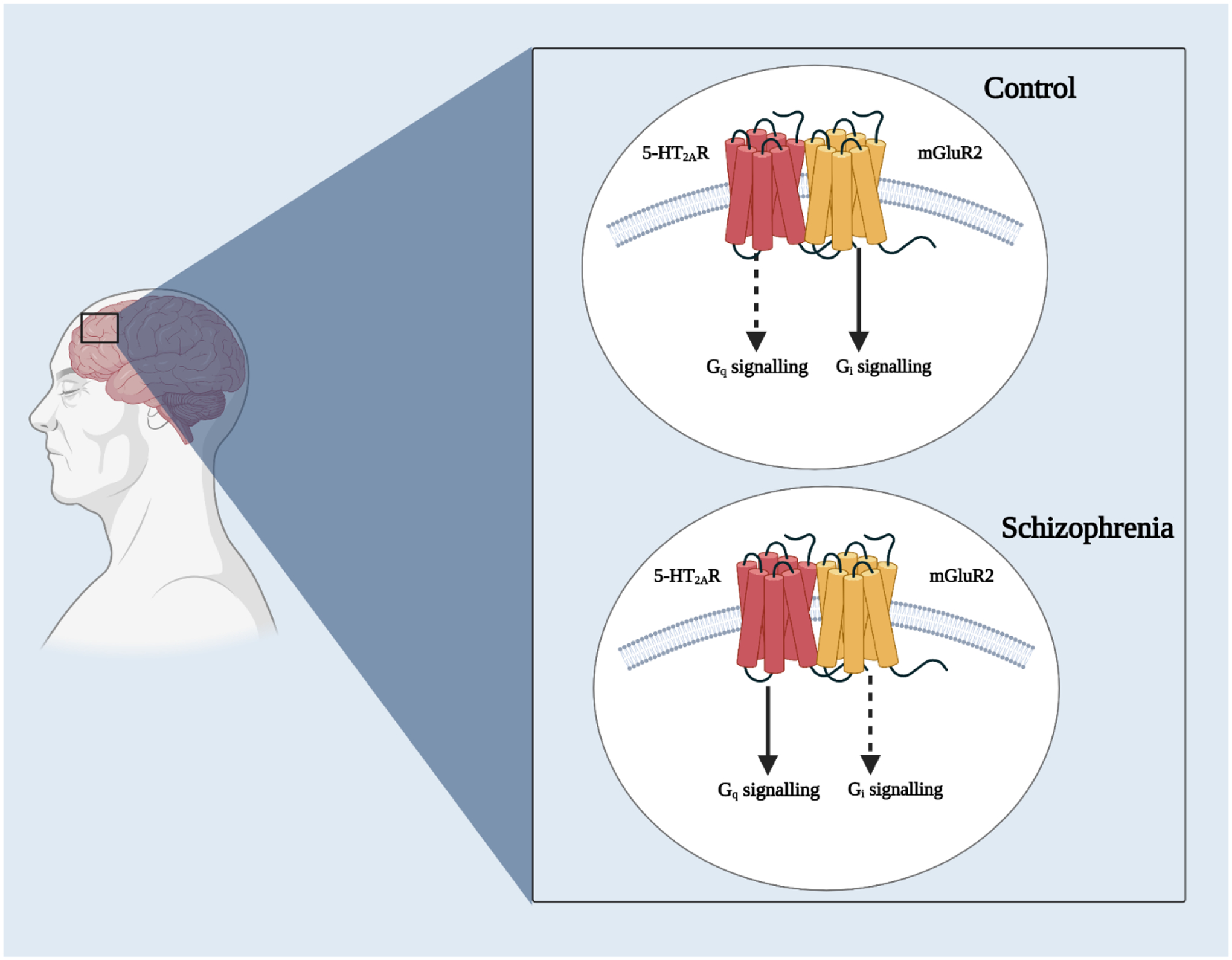
Orthosteric agonists usually present a higher affinity to the active conformation of the GPCR, whereas neutral antagonists cannot distinguish between the active and inactive structural states (Strange, 1998; Strange, 1999). Consequently, competitive binding displacement of a radiolabeled antagonist by an unlabeled (cold) agonist usually leads to a biphasic displacement curve with high affinity (Ki-high) and low affinity (Ki-low) binding sites. This particular pharmacological profile has been reported in HEK293 cells expressing 5-HT2AR alone (displacement of [3H]ketanserin by DOI and other 5-HT2AR agonists) and mGluR2 alone (displacement of [3H]LY341495 by LY379268 and other mGluR2/3 agonists) (Gonzalez-Maeso et al., 2007; Gonzalez-Maeso et al., 2008; Moreno et al., 2012). Related to the allosteric crosstalk between 5-HT2AR and mGluR2, it was also shown that the biphasic displacement curve of [3H]ketanserin by DOI in cells expressing 5-HT2AR alone became monophasic in cells co-expressing 5-HT2AR and mGluR2, and that the mGluR2/3 agonist LY379268 concentration-dependently increased the affinity of DOI displacing [3H]ketanserin in cells co-expressing 5-HT2AR and mGluR2. As expected, cold GTPγS shifted the displacement curve of [3H]ketanserin with DOI to the right. Importantly, it was reported that this allosteric crosstalk between 5-HT2AR and mGluR2 required GPCR heteromerization since it was absent in cells co-expressing 5-HT2AR and mGluR3, and prevented in cells co-expressing 5-HT2AR and mGluR2ΔTM4 (an mGluR2/mGluR3 chimeric construct that, according to previous in vitro findings, is not able to from the 5-HT2AR-mGluR2 complex) (Gonzalez-Maeso et al., 2007; Gonzalez-Maeso et al., 2008; Moreno et al., 2012).
Previous data using orthosteric agonists and/or antagonists suggested a functional crosstalk that requires assembly of 5-HT2AR and mGluR2 as a GPCR heterocomplex. As one example, it was demonstrated that stimulation of cells expressing 5-HT2AR-mGluR2 heteromers with an mGluR2 agonist led to activation of Gq/11 proteins by the 5-HT2AR in HEK293 cells (Moreno et al., 2016). Additionally, in Xenopus oocytes, using inhibition of the Kir2.3 channel current as a readout of Gq activity together with activation of Kir3.4 channel current as a readout of Gi activity, it was observed that co-expression of 5-HT2AR and mGluR2 reduced Gq activity induced by the endogenous neurotransmitter 5-HT to almost half of the activity of the 5-HT2AR construst when expressed alone (Fribourg et al., 2011). In contrast to Gq activity, the Gi activity induced by the endogenous neurotransmitter glutamate was increased in cells co-expressing mGluR2 and 5-HT2AR. This gave rise to the concept of balance index (BI), which measures the change in activity of Gi and Gq such that BI= ΔGi - ΔGq. Methysergide, DOI and clozapine function as antagonist, agonist and inverse agonist of the 5-HT2AR, respectively. Upon co-expression of both receptors and in the presence of glutamate, methysergide had no effect on Gi signaling, DOI decreased Gi activity, while clozapine significantly escalated Gi signaling. (2S)-α-Ethylglutamic acid (eGlu), LY379268 and LY341495 function as antagonist, agonist and inverse agonist of mGluR2, respectively (Conn and Pin, 1997; Niswender and Conn, 2010; Nicoletti et al., 2011). Upon expression of the heteromer and in the presence of serotonin, eGlu did not affect serotonin-elicited Gq signaling, LY379268 decreased Gq signaling to around 35%, while LY341495 increased it to around 83% (Fribourg et al., 2011). This cross-signaling between 5-HT2AR and mGluR2 was also observed in mammalian HEK293 cells stably transfected with these two receptors (Baki et al., 2016).
Published reports by other groups have validated the formation of the 5-HT2AR-mGluR2 complex in vitro (Rives et al., 2009; Delille et al., 2012; Murat et al., 2019) and ex vivo (Hamor et al., 2018; Olivero et al., 2018; Banerjee and Vaidya, 2020; Taddeucci et al., 2022). However, some (Olivero et al., 2018; Murat et al., 2019; Poulie et al., 2020; Taddeucci et al., 2022) but not all (Delille et al., 2012) of the previous studies reported functional crosstalk between these two receptors. A potential explanation of this lack of functional crosstalk in cells co-expressing 5-HT2AR and mGluR2 may be related to variations in densities of 5-HT2AR and/or mGluR2, as it has been suggested in HEK293 cells either co-transfected with different ratios of plasmids encoding 5-HT2AR and mGluR2 (Moreno et al., 2016), or stably expressing various levels of the two GPCRs (Baki et al., 2016).
Some of the conclusions obtained from in vitro biochemical and biophysical studies have been supported by assays in whole animal models. As an example, 5-HT2AR and mGluR2 are part of the same protein complex in native tissue since co-immunoprecipiation has been reported in mouse (Fribourg et al., 2011; Hamor et al., 2018) rat (Taddeucci et al., 2022) and postmortem human brain frontal cortex samples (Gonzalez-Maeso et al., 2008), as well as in synaptosomal lysates from rat spinal cord (Olivero et al., 2018). Head-twitch behavior – a mouse behavioral proxy of human psychedelic activity (Hanks and Gonzalez-Maeso, 2013) – was significantly diminished in mGluR2-KO mice (Moreno et al., 2011). This intriguing phenotype has been validated by other groups (Benvenga et al., 2018). Using a virally (HSV)-mediated over-expression approach, it was also demonstrated that this behavior phenotype is rescued in mGluR2-KO mice stereotaxically injected with HSV-mGluR2 in the frontal cortex (Moreno et al., 2012). This, however, did not occur upon HSV-mediated expression of mGluR2ΔTM4. The ability of clozapine to reduce hyperlocomotor activity induced by dissociative drugs such as MK801 and PCP was also diminished in mGluR2-KO as compared to wild-type controls (Hideshima et al., 2018). These findings suggest a potential role of this GPCR heterocomplex in the cortical processes affected by psychedelics and antipsychotic medications.
A key molecular event affecting the crosstalk between 5-HT2AR and mGluR2 was found to be the phosphorylation of Ser843 in mGluR2 in HEK293 cells co-expressing 5-HT2AR (Murat et al., 2019). Only in cells co-expressing these two receptors, phosphorylation of Ser843 in mGluR2 was augmented upon application of mGluR2 agonist LY379268. This phenonemom was corroborated in frontal cortex samples from wild-type mice, and absent in the same tissue samples of 5-HT2AR-KO animals. Increase in Ser843 phosphorylation was also observed upon 5-HT2AR stimulation by 5-HT in cells co-expressing both mGluR2 and 5-HT2AR. In both mouse frontal cortex tissue samples and HEK293 cells, the mGluR2 antagonist LY341495 prevented Ser843 phosphorylation elicited by 5-HT2AR stimulation, while the LY379268-induced effect was also blocked by the 5-HT2AR receptor antagonist M100907. Additionally, G protein-dependent signaling elicited by mGluR2 or 5-HT2AR receptor stimulation in cells co-expressing both receptors was strongly reduced upon mutation of Ser843 into Ala.
One of the most commonly abused synthetic drugs worldwide is methamphetamine. Its chronic usage often turns into a compulsive drug-taking disorder accompanied by cognitive deficits and recurring psychosis. Recent findings reported dysregulation of 5-HT2AR and mGluR2 expression in brain regions including medial prefrontal cortex, dorsal hippocampus, and perirhinal cortex upon 14 days of repeated treatment with methamphetamine as compared to vehicle and 7 days of withdrawal (Hamor et al., 2018). As compared with other psychostimulants, these findings also reported that there were alterations in the expression ratio of 5-HT2AR and mGluR2 in medial prefrontal cortex (methamphetamine, PCP and MK801), dorsal hippocampus (methamphetamine and PCP), and perirhinal cortex (MK801). Repeated administration of methamphetamine also led to an increase in Gq activity and a decrease in Gi activity in the medial prefrontal cortex. These data suggest that alrerations in G protein-coupling via cortical 5-HT2AR vs mGluR2 may be reponsible for some of the common signaling mechanisms underlying psychosis and cognitive alterations in subjects with methamphetamine use disorder.
Cellular and sub-cellular colocalization of 5-HT2AR and mGluR2
Autoradiography with [3H]LY354740 (a selective mGluR2/3 receptor agonist) and [125I]DOI (a potent partial 5-HT2AR agonist) demonstrated an overlapping distribution of mGluR2/3 and 5-HT2AR in rat medial prefrontal cortex samples (Marek et al., 2000). Subsequent FISH assays were used to suggest co-localization of 5-HT2AR and mGluR2 in mouse frontal cortex both at cellular (FISH and immunocytochemistry assays) (Gonzalez-Maeso et al., 2008; Fribourg et al., 2011) and sub-cellular (electron microscopy) (Moreno et al., 2012) levels. This co-localization occurred particularly within layer V cortical pyramidal neurons (Gonzalez-Maeso et al., 2008). However, in frontal cortex pyramidal neurons, the expression of mGluR3 mRNA was modest and it barely co-localized with 5-HT2AR mRNA (Gonzalez-Maeso et al., 2008). Using a subcellular fractionation approach to purify fractions enriched in presynaptic active zone (PAZ) and post-synaptic density (PSD) proteins in mouse frontal cortex samples, it was observed that 5-HT2AR was detected in the PSD, whereas mGluR2 was detected in both the PSD and PAZ fractions (Moreno et al., 2016). This suggests that the 5-HT2AR-mGluR2 complex is located mostly postsynaptically in frontal cortex pyramidal neurons. However, the potential role of presynaptic 5-HT2AR modulating plasticity via presynaptic thalamocortical circuits has also been suggested (Marek et al., 2001; Barre et al., 2016). As an example, there is a presynaptic crosstalk between 5-HT2AR and mGluR2/3 in the frontal cortex where the 5-HT2AR antagonist MDL11939 augments the inhibitory effect induced by LY379268 through a mechanism that may not require GPCR heteromerization, since it was reported that 5-HT2AR and mGluR2 co-immunoprecipitate in homogenates but not in subcellular fractions isolated from synaptic terminals in rat frontal cortex (Taddeucci et al., 2022).
Presynaptic mGluR2/3 autoreceptors control glutamate exocytosis in spinal cord endings. Interestingly, recent findings reported that glutamatergic presynaptic terminals of rat spinal cord show strong colocalization of 5-HT2AR and mGluR2/3, where they crosstalk with each other in an antagonistic fashion. Thus, antagonists of the 5-HT2AR act as indirect positive allosteric modulators of mGluR2/3 autoreceptors and regulate the exocytosis of glutamate (Olivero et al., 2018). Further studies are required to better understand the pattern of localization and co-localization of 5-HT2AR and mGluR2 in different central and peripheral tissues as well as the mechanisms that regulate their function.
GPCR activity is regulated by membrane trafficking processes (Dong et al., 2007; Magalhaes et al., 2012; von Zastrow and Williams, 2012; Pavlos and Friedman, 2017; Thomsen et al., 2018). In mammalian HEK293 cells, 5-HT2ARs affected the trafficking and localization of mGluR2 via a mechanism that required their assembly as a GPCR heteromeric complex. In resting state (i.e., absence of agonists), the 5-HT2AR was mainly localized in intracellular vesicles whereas mGluR2 showed surface localization (Toneatti et al., 2020). However, co-expression of both receptors increased the intracellular localization of the otherwise membrane-localized mGluR2. Co-localization of mGluR2 with Rab5, Rab7 and transferrin receptor (all markers of endocytic vesicles) increased upon co-expression with 5-HT2AR. Additionally, the presence of DOI as a 5-HT2AR agonist or LY379268 as an mGluR2/3 agonist differentially affected the density and localization of mGluR2 with endosomal markers in cells expressing mGluR2 alone or co-expressing mGluR2 and 5-HT2AR. The antipsychotic clozapine but not the 5-HT2AR antagonist M100907 reduced the sub-cellular localization of 5-HT2AR and mGluR2 through a mechanism that required their molecular proximity. Ex vivo in mouse frontal cortex samples, expression of 5-HT2AR also augmented the intracellular localization of mGluR2. Thus, there was a significantly greater proportion of mGluR2 at the plasma membrane in neurons from 5-HT2AR-KO mice as compared to wild-type controls (Toneatti et al., 2020). Together, these data indicate that heteromerization of 5-HT2AR-mGluR2 plays a plausible role in receptor trafficking and sorting.
Epigenetic crosstalk between 5-HT2AR and mGluR2
Expression of 5-HT2AR indirectly affects mGluR2 transcription since 5-HT2AR-KO animals exhibited reduced mGluR2/3 binding and mGluR2, but not mGluR3, mRNA expression in frontal cortex samples (Gonzalez-Maeso et al., 2008; Kurita et al., 2013). Additionally, Cre-mediated 5-HT2AR transcription in the frontal cortex of flox-STOP-flox mice restored mGluR2 expression to control levels (Gonzalez-Maeso et al., 2008). Covalent histone modifications and DNA methylation act cooperatively to regulate gene expression in a dynamic and reversible fashion (Ibi and Gonzalez-Maeso, 2015). Chromatin immunoprecipitation (ChIP) followed by qPCR techniques revealed that acetylation of histone H3 (H3ac) and histone H4 (H4ac), markers of transcriptional activation, were diminished at the promoter region of the mGluR2 gene in the frontal cortex of 5-HT2AR-KO mice (Kurita et al., 2013). Neither methylation of histone H3 at lysine 4 (H3K4me1/2/3) nor trimethylation of histone H3 at lysine 9 (H3K9me3) were affected. However, trimethylation of histone H3 at lysine 27 (H3K27me3), a marker for transcriptional repression, was considerably augmented at the mGluR2 promoter in the frontal cortex of 5-HT2AR-KO mice as compared to wild-type controls. Similar ChIP-qPCR assays also revealed that binding of the transcription factor Egr1 at the mGluR2 promoter was reduced in the frontal cortex of 5-HT2AR-KO mice. Additionally, immunoreactivity of mGluR2 was increased upon viral (HSV)-mediated over-expression of Egr1 in the mouse frontal cortex (Kurita et al., 2013). These findings suggesting that 5-HT2AR-dependent signaling epigenetically affects mGluR2 transcription have been supported by studies testing the effect of chronic antipsychotic treatment.
Thus, chronic treatment with the atypical antipsychotic clozapine and risperidone, but not with the typical antipsychotic haloperidol, strongly decreased H3ac binding at the mGluR2 promoter in both mouse and human frontal cortex (Kurita et al., 2012; de la Fuente Revenga et al., 2018a). A similar epigenetic phenotype was observed in schizophrenia subjects since H3ac binding at the mGluR2 promoter was reduced in frontal cortex samples of antipsychotic treated, but not antipsychotic free, schizophrenic subjects (Kurita et al., 2012). This epigenetic modification at the mGluR2 promoter region was 5-HT2AR-dependent since it was absent in 5-HT2AR-KO mice, and was associated with an increased binding of histone deacetylase 2 (HDAC2) to the mGluR2 promoter. Conversely, co-administration of the class I and II HDAC inhibitor SAHA (vorinostat) prevented the antipsychotic profile of the mGluR2/3 agonist LY379269 and downregulation of frontal cortex mGluR2/3 density via HDAC2 (de la Fuente Revenga et al., 2018a).
Based on this preclinical work, it was proposed that repeated administration of atypical antipsychotics would consequently restrict the clinical effects of the antipsychotic LY2140023 (pomaglumetad), an oral prodrug of the mGluR2/3 agonist LY404039 (Patil et al., 2007). Importantly, this hypothesis was validated by clinical results from a post-hoc analysis where the antipsychotic effects of LY2140023 were comparable to those induced by risperidone in schizophrenia patients previously treated with typical antipsychotics (i.e., haloperidol) whereas previous exposure to atypical antipsychotics (e.g., clozapine and olanzapine) led to an effect of LY2140023 that did not separate from placebo (Kinon et al., 2015).
Antipsychotic drugs including both typical (e.g., haloperidol) and atypical (e.g., clozapine and risperidone) were serendipitously discovered in the mid-twentieth century (Crilly, 2007). Although chronic administration of these agents produces significant reduction or even complete remission of psychotic symptoms, such as hallucinations and delusions (Lieberman et al., 2008; Ebdrup et al., 2011; Miyamoto et al., 2012; Meltzer, 2013), the majority of schizophrenia patients have cognitive deficits that often do not improve upon antipsychotic drug treatment – some patients even deteriorate (Goldberg et al., 1993; Husa et al., 2014; Fervaha et al., 2015; Nielsen et al., 2015; Husa et al., 2016). This decline in cognitive abilities upon long-term exposure to antipsychotics has been corroborated in animal models (Rosengarten and Quartermain, 2002; Schroder et al., 2005). Related to this, it was reported that chronic treatment with clozapine decreases the density of 5-HT2AR in the frontal cortex, which in turn reduces the impact of 5-HT2AR-dependent signaling on the MAPK-ERK cascade (Ibi et al., 2017). This leads to downregulation of the NF-κB repressor IκBα, which consequently augments both nuclear translocation of NF-κB and its binding to the Hdac2 promoter in frontal cortex pyramidal neurons – an atypical antipsychotic-induced epigenetic event that occurred in association with up-regulation of HDAC2 in CaMKIIα-positive frontal cortex pyramidal neurons (Fig. 1). As previous data also suggested that chronic treatment with the atypical antipsychotic clozapine reduces the density of mature spines, and impairs cognition through a HDAC2-dependent mechanism (Ibi et al., 2017; de la Fuente Revenga et al., 2018b), together, these observations suggest that the NF-κB→HDA2 pathway via 5-HT2AR may be responsible for the deleterious effects of these drugs in terms of synaptic frontal cortex synaptic remodeling and cognitive function, and that HDAC2 may represent a novel target to improve synaptic plasticity and cognition in treated schizophrenia patients.
Fig. 1.
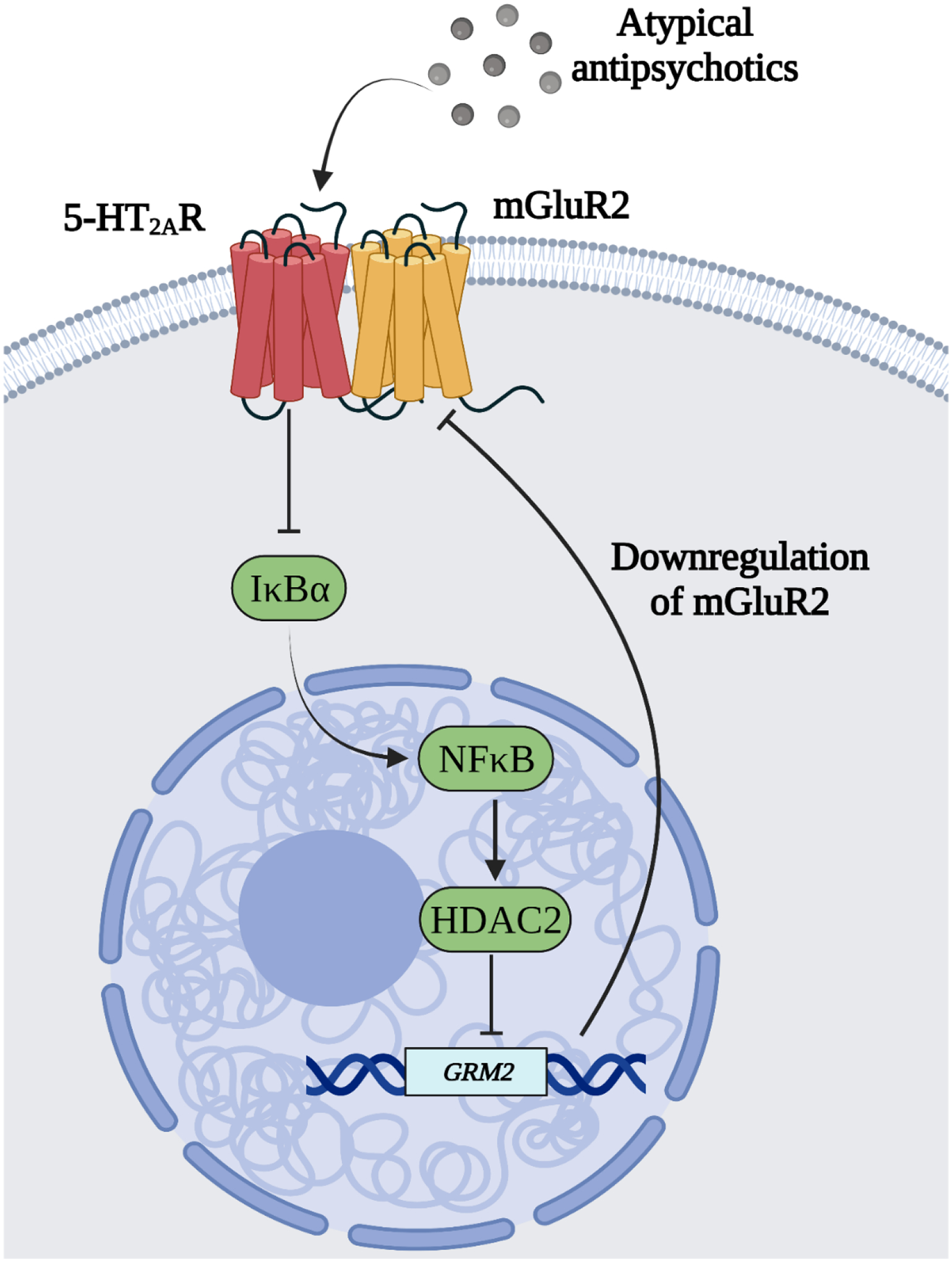
HDAC2-dependent regulation of mGluR2 transcription upon chronic atypical antipsychotic treatment. Chronic atypical antipsychotic treatment decreases density of 5-HT2AR in the frontal cortex, which also leads to down-regulation of the NF-κB repressor IκBα. This pathway induces NF-κB-dependent augmentation of HDAC2 expression and, consequently, increased binding of HDAC2 to the mGluR2 promoter. Together, these findings suggest that disinhibition of the NF-κB pathway by chronic treatment with atypical, but not typical, antipsychotics induces HDAC2-dependent repressive histone modifications at the mGluR2 promoter, which consequently restricts the antipsychotic effects of mGluR2/3 agonists.
Bivalent ligands for the 5-HT2AR-mGluR2 complex
An attractive strategy to enable selective targeting of a GPCR heteromeric complex over its two parent receptors is the design of a heterobivalent ligand, which comprises two receptor-selective “head” groups separated by a chemical spacer of suitable length and flexibility (Berque-Bestel et al., 2008; Shonberg et al., 2011; Huang et al., 2021a). In theory, bivalent ligands successfully bridging two binding sites of adjacent protomers should confer extremely high affinity (resulting from the total binding energy of two recognition elements) and thus selectivity for the GPCR heteromer. The bivalent ligand strategy was pioneered by Portoghese and colleagues in their development of selective ligands for studies of opioid receptor dimerization (Erez et al., 1982; Portoghese et al., 1982; Portoghese et al., 1986). Because of their selective recognition properties, bivalent ligands could be eventually used for a tissue-specific targeting of cells expressing an individual GPCR dimer. Toward this goal, numerous studies have explored the binding and signaling properties of new bivalent ligands targeting putative GPCR heterodimers/heterocomplexes, providing valuable insights into the quaternary structure of a GPCR dimer and the functional relevance of GPCR dimerization (Akgun et al., 2013; Le Naour et al., 2013; Hubner et al., 2016; Poulie et al., 2020; Huang et al., 2021b; Romantini et al., 2021; Pulido et al., 2022).
Among these, synthesis of a series of heterobivalent ligands targeting the heteromeric 5-HT2AR-mGluR2 complex based on the 5-HT2AR antagonist M100907 and the mGluR2 PAM JNJ-42491293 deserved special attention (Poulie et al., 2020). This combination of an orthosteric 5-HT2AR antagonist with an mGluR2 PAM was selected because of the distance between the orthosteric binding sites within the VFT domain of the mGluR2 and the TM core of the 5-HT2AR. In this paper, the authors showed a functional crosstalk between the two receptors in HEK293 cells co-expressing 5-HT2AR and mGluR2. This was validated in cells co-expressing 5-HT2AR and mGluR2 and the chimeric Gα protein Gqo5 that directs Gαi/o-signaling of mGluR2 into the Gαq/11-dependent pathway and intracellular Ca2+ mobilization. However, as for the seven bivalent ligands designed for the putative 5-HT2AR-mGluR2 complex, the authors did not observe a definitive correlation between the functional potency and spacer length of the ligands (Poulie et al., 2020). The most obvious molecular reason behind this lack of simultaneous interaction with the two different binding sites is their use of short spacer with lengths ranging from 3 to 12 polyethylene glycol (PEG) units. It also remains unknown whether these bivalent ligands truly act by simultaneously targeting binding sites in both receptors of the 5-HT2AR-mGluR2 heteromer, or whether they have preference for the orthosteric 5-HT2AR and/or the allosteric mGluR2 sites and thus mediate their functional outcomes exclusively through binding to one of the two receptors. An additional alternative molecular mechanism to explain crosstalk phenotypes observed upon bivalent ligand administration in cells co-expressing two GPCRs may not require GPCR heteromerization but binding of each of the two pharmacophoric heads of the bivalent ligand to two GPCRs that are not in close molecular proximity. This is an important point to address experimentally since, as an example, previous findings reported that agonists targeting GABABR or mGluR1 lead to crosstalk between these two class C GPCR through a mechanism that does not require their heteromerization (Rives et al., 2009).
5-HT2AR-mGluR2 in postmortem schizophrenia brain samples
Several previous studies have tested the level of expression and density of the 5-HT2AR in postmortem human brain samples of schizophrenia subjects and controls, particularly in the frontal cortex. Most of these assays were carried out with radioligands including the 5-HT2AR antagonist [3H]ketanserin (Reynolds et al., 1983; Mita et al., 1986; Laruelle et al., 1993; Burnet et al., 1996; Dean and Hayes, 1996; Dean et al., 1996; Dean et al., 1998; Dean et al., 1999; Marazziti et al., 2003; Matsumoto et al., 2005; Dean et al., 2008; Gonzalez-Maeso et al., 2008; Kang et al., 2009; Muguruza et al., 2013) and the 5-HT2AR agonist [125I]LSD (Bennett et al., 1979; Whitaker et al., 1981; Joyce et al., 1993; Gurevich and Joyce, 1997). More recent findings also evaluated 5-HT2AR density by positron emission tomography (PET) with [18F]altanserin in drug-naïve first-episode schizophrenia patients (Rasmussen et al., 2010). Intriguingly, there are obvious differences in the results obtained: some studies suggested up-regulation of 5-HT2AR density whereas other groups proposed absence of alterations or down-regulation in the number of 5-HT2AR binding sites. One of the principal reasons behind these differences may be related to demographic conditions such as age, since 5-HT2AR density correlates negatively with aging (Gonzalez-Maeso et al., 2008; Muguruza et al., 2013), and some of the studies in postmortem human brain samples included control subjects that were not individually matched by age with the schizophrenia group. Additionally, chronic treatment with atypical antipsychotics down-regulates 5-HT2AR receptor density in brain regions such as the frontal cortex (Kurita et al., 2012; Moreno et al., 2013), and some of the studies in postmortem human brain samples did not evaluate the presence of antipsychotic medications in postmortem toxicological analysis as an independent experimental variable (for a review article, see (Gonzalez-Maeso and Sealfon, 2009b)). In suicide victims with other psychiatric conditions including dysthymia, alcohol use disorder and anorexia nervosa, 5-HT2AR density did not differ as compared to controls (Muguruza et al., 2013)
Consequently, a potential pharmacological explanation related to these differences in 5-HT2AR density in frontal cortex samples from schizophrenia subjects as compared to controls is related to the functional properties of the radioligand used to assess 5-HT2AR. As mentioned above, agonists present a higher affinity for the active conformation of the receptor, whereas inverse agonists bind with higher affinity to the inactive conformation of the receptor. Recent findings tested 5-HT2AR density in the same cohort of postmortem human frontal cortex samples of schizophrenia subjects and controls individually matched by sex and age using three separate 5-HT R radioligands: [18F]altanserin (inverse agonist), [3H]LSD (agonist) and [3H]MDL100907 (antagonist) (Diez-Alarcia et al., 2021). The authors reported that 5-HT2AR density (Bmax) was reduced, increased, and unaffected in schizophrenia subjects when binding assays were carried out with [18F]altanserin, [3H]LSD, and [3H]MDL100907, respectively (Fig. 2). Based on these findings, it was proposed that the fraction of the active conformation of the 5-HT2AR could be increased in the frontal cortex of schizophrenic subjects as compared to controls, which would also provide the basis for the differences in 5-HT2AR densities upon binding with radioligands of different functional characteristics. This concept of alterations in the functional properties of the 5-HT2AR in schizophrenia subjects is further supported by findings suggesting a higher stimulation of G proteins by the 5-HT2AR agonist DOI (Garcia-Bea et al., 2019) (Fig. 3), as well as the increase in the fraction of high-affinity binding sites of DOI displacing [3H]ketanserin binding in postmortem frontal cortex samples of antipsychotic-free schizophrenia subjects (Muguruza et al., 2013).
Fig. 2.
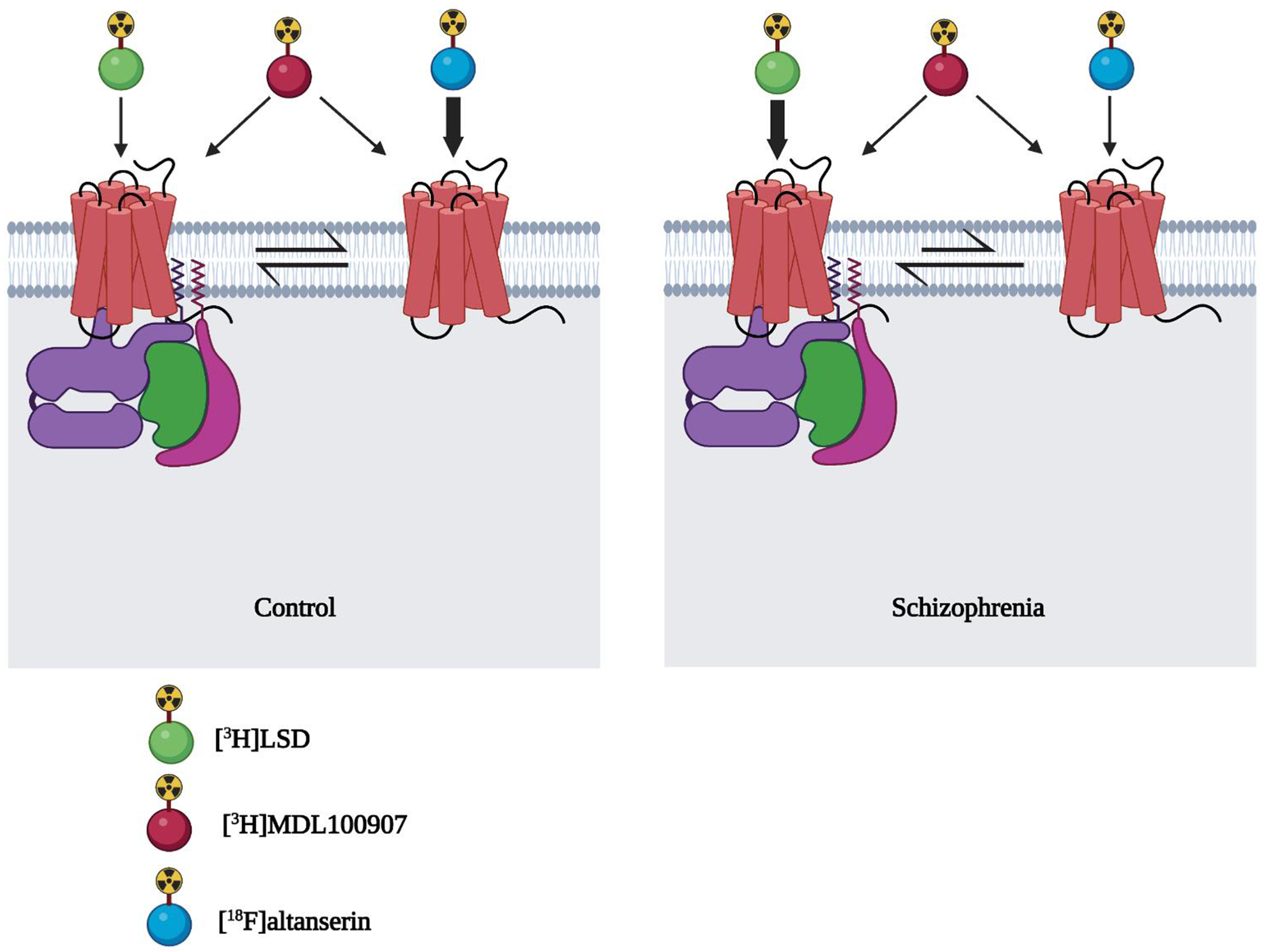
Binding assays with the 5-HT2AR agonist [3H]LSD, antagonist [3H]M100907 and inverse agonist [18F]altanserin show increased, unaffected and decreased 5-HT2AR densities, respectively, in frontal cortex samples of schizophrenia subjects. These divergent receptor density values may be explained by their preferences in terms of affinity for the active vs inactive conformations of the 5-HT2AR.
Fig. 3.
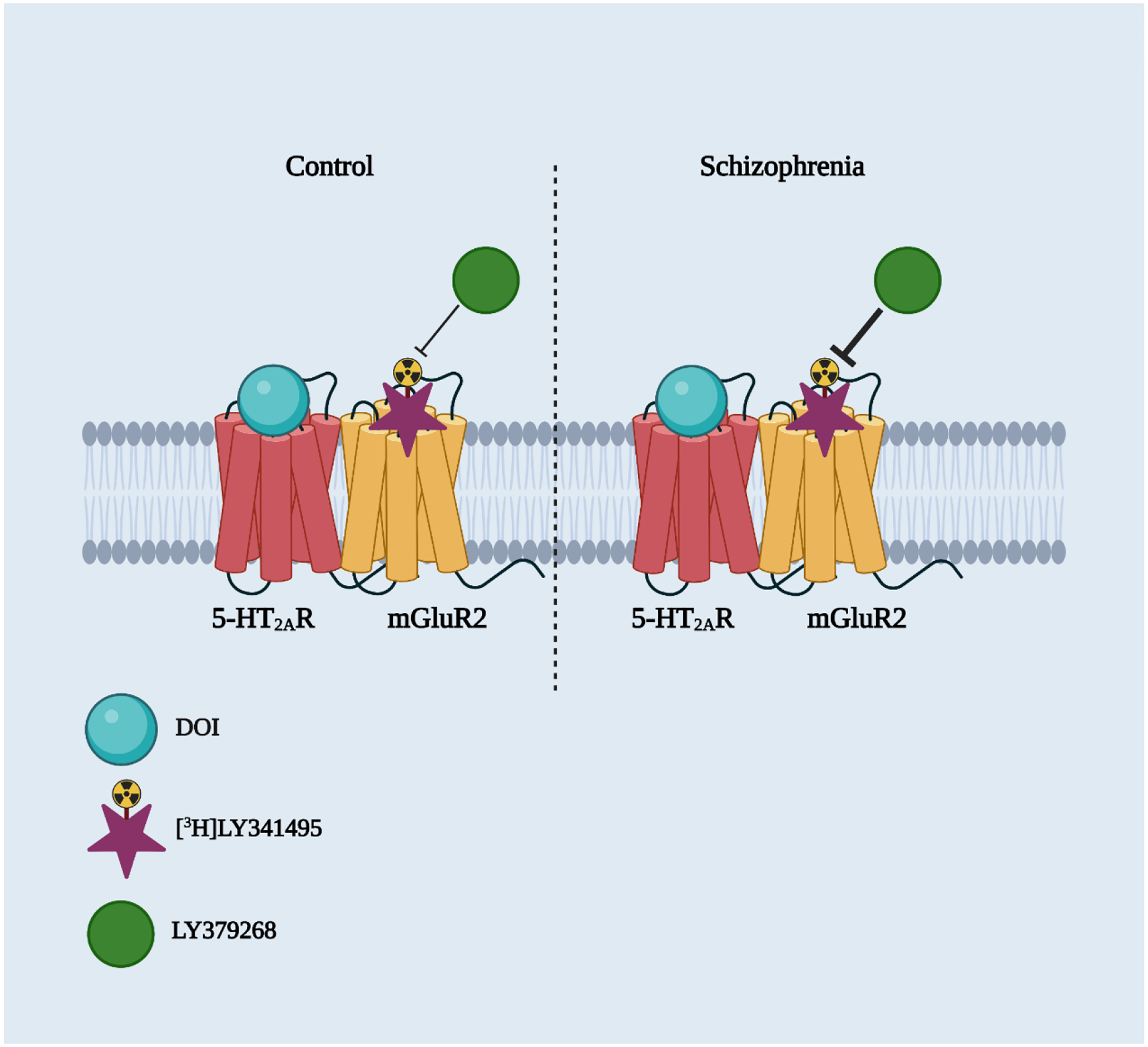
The allosteric effect of the 5-HT2AR agonist DOI on the affinity of LY379268 displacing the mGluR2/3 antagonist [3H]LY341495 is increased in frontal cortex samples of schizophrenia subjects. This suggests that the functional crosstalk between 5-HT2AR and mGluR2 as a GPCR heterocomplex may be involved in the altered cortical processes in schizophrenia patients.
Potential alterations in the 5-HT2AR-mGluR2 complex have also been observed in postmortem frontal cortex samples of schizophrenia subjects. The authors compared the effect of DOI on displacement of [3H]LY341495 binding by LY379268 in postmortem frontal cortex samples of schizophrenia subjects and controls (Moreno et al., 2012). As expected, competition of[3H]LY341495 binding by LY379268 was best described by a two-site model. Additionally, and supporting previous findings in vitro in tissue culture and in mouse frontal cortex samples (Gonzalez-Maeso et al., 2008), DOI decreased the high affinity component of LY379268 displacing [3H]LY341495 in both schizophrenia subjects and controls. Interestingly, it was also reported that the difference between the high affinities of LY379268 in the presence and in the absence of DOI was increased in postmortem frontal cortex samples of schizophrenia subjects as compared to controls (Moreno et al., 2012) (Fig. 4). Related to the functional crosstalk between 5-HT2AR and mGluR2 in native tissue, it was previously reported that LY379268 was able to activate both Gq/11 and Gi1,2,3 proteins in mouse frontal cortex samples. However, when this functional outcome was tested in the frontal cortex of 5-HT2AR-KO animals, LY379268 induced [35S]GTPγS binding via Gi1,2,3 whereas the effect of LY379268 on Gq/11-dependent[35S]GTPγS binding was eliminated (Moreno et al., 2016). These findings suggest that mGluR2 activates Gq/11-dependent signaling pathways via 5-HT2AR. This signaling crosstalk between mGluR2 and 5-HT2AR was also observed in frontal cortex samples of controls subjects – LY379268 induced both Gq/11 - and Gi1,2,3-dependent [S]GTPγS binding. However, the effect of LY379268 on Gq/11 activation was reduced in frontal cortex samples from schizophrenia subjects, whereas the LY379268-dependent effect on Gi1,2,3 coupling was unaffected (Moreno et al., 2016). These findings could be relevant for the elucidation of mechanisms underlying psychosis and antipsychotic treatment response in schizophrenia patients.
Fig. 4.
Alterations of G protein coupling to the 5-HT2AR-mGluR2 complex in the frontal cortex of schizophrenia subjects. Activation of Gq/11 proteins by the mGluR2/3 agonist LY379268 potentially via 5-HT2AR is reduced in postmortem frontal cortex membrane preparations from schizophrenia subjects, whereas the LY379268-dependent activation of Gi1,2,3 proteins is unaffected.
Concluding remarks and future directions
Several lines of evidence suggest the existence of a close functional interplay between 5-HT2AR and mGluR2 receptors (Fig. 5). They regulate each other at an epigenetic level and via postsynaptic mechanisms. The heteromeric GPCR complex formed by 5-HT2AR and mGluR2 has also been involved in signaling and neural circuit processes related to clinically relevant effects of psychedelics and antipsychotics. Several questions remain open for further study including the molecular stability of these two GPCRs as a heterocomplex, and the subcellular location where the two protomers of the 5-HT2AR-mGluR2 complex come to a stage of physical proximity. Similarly, additional investigation will be required to determine the potential role of the 5-HT2AR-NF-κB-HDAC2 pathway in the epigenetic control of mGluR2-dependent clinical outcomes. The majority of the preclinical studies focused on 5-HT2AR-dependent mechanisms were carried out almost exclusively in male rodent models, yet recent findings indicate the importance of studying sex as a biological variable (Vohra et al., 2021; Jaster et al., 2022; Sierra et al., 2022; Alper et al., 2023). Although it is beyond the scope of this review, it is worth mentioning that accumulating data mainly from the area of placebo analgesia within the opioid-induced anti-nociception field clearly shows that the effectiveness of a placebo is often related to a subject’s expectations for pain relief (Pecina and Zubieta, 2015). This placebo effect has also been observed in the effect of the deliriant drug scopolamine on disruption of learned behavior in rats (Herrnstein, 1962). The lack of a valid placebo group in all the clinical studies testing the effects of classical psychedelics in patients with psychiatric conditions particularly depression – together with expectancy effects (Butler et al., 2022) and the wish of some of the participants to experience a psychedelic trip – leaves open the question of the effects of psychedelics by themselves on therapeutic outcomes.
Fig. 5.
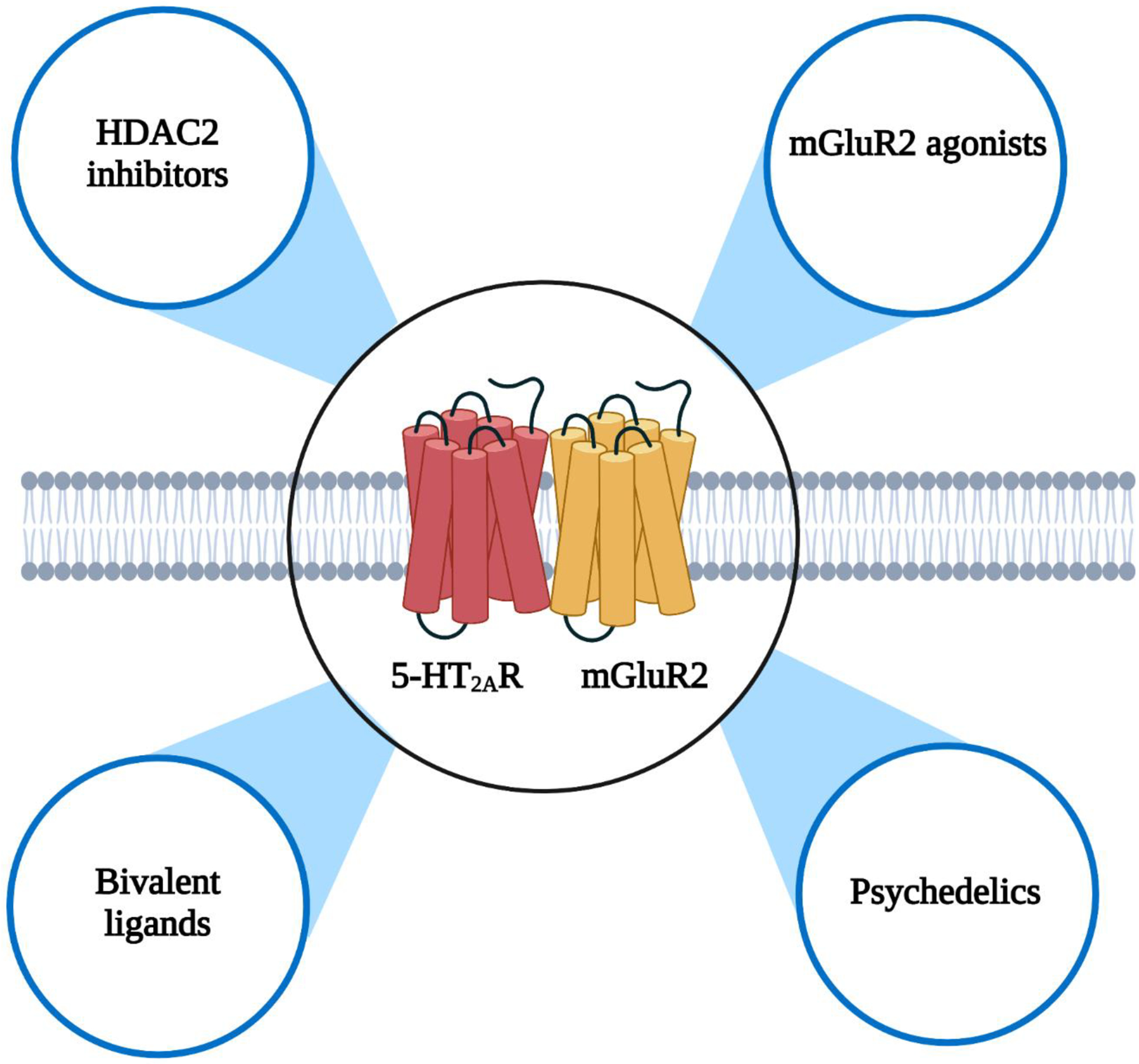
Pharmacological strategies to target the 5-HT2AR-mGluR2 complex. Potential routes to affect the crosstalk between 5-HT2AR and mGluR2 include i) HDAC2 inhibitors to prevent the 5-HT2AR-dependent epigenetic modifications at the mGluR2 promoter upon chronic antipsychotic treatment, ii) orthosteric and allosteric mGluR2 agonists as antipsychotics, iii) classical psychedelics as fast-acting antidepressants, and iv) bivalent ligands composed of 5-HT2AR antagonists and mGluR2 PAMs as a pharmacological tool to treat schizophrenia.
Highlights:
5-HT2AR-mGluR2 heteromeric complex
5-HT2AR-mGluR2 in postmortem schizophrenia brain samples
Epigenetic crosstalk between 5-HT2AR and mGluR2
Bivalent ligands for the 5-HT2AR-mGluR2 complex
Funding and disclosure
NIH R01MH084894 and P30DA033934 participated in the funding of this study. Figures were created using biorender.com.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT authors contribution statement
Somdatta Saha: Conceptualization, Writing – original draft. Javier González-Maeso: Conceptualization, Writing – review and editing, Supervision, Funding acquisition.
Declaration of competing interest
J.G.-M. has or has had sponsored research contracts with Terran Biosciences and Noetic Fund, and serves on scientific advisory boards for Adelia Therapeutics and Cognesy Therapeutics. S.S. declares no conflict of interest.
Bibliography
- Adams LM, Geyer MA (1985). Patterns of exploration in rats distinguish lisuride from lysergic acid diethylamide. Pharmacol Biochem Behav 23: 461–468. [DOI] [PubMed] [Google Scholar]
- Akgun E, Javed MI, Lunzer MM, Smeester BA, Beitz AJ, Portoghese PS (2013). Ligands that interact with putative MOR-mGluR5 heteromer in mice with inflammatory pain produce potent antinociception. Proc Natl Acad Sci USA 110: 11595–11599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper K, Cange J, Sah R, Schreiber-Gregory D, Sershen H, Yaragudri Vinod K (2023). Psilocybin sex-dependently reduces alcohol consumption in C57BL/6J mice. Frontiers in Pharmacology 13: 1074633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baki L, Fribourg M, Younkin J, Eltit JM, Moreno JL, Park G et al. (2016). Cross-signaling in metabotropic glutamate 2 and serotonin 2A receptor heteromers in mammalian cells. Pflugers Arch 468: 775–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee AA, Vaidya VA (2020). Differential signaling signatures evoked by DOI versus lisuride stimulation of the 5-HT(2A) receptor. Biochem Biophys Res Commun 531: 609–614. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Ahern GP, Becamel C, Bockaert J, Camilleri M, Chaumont-Dubel S et al. (2021). International Union of Basic and Clinical Pharmacology. CX. Classification of Receptors for 5-hydroxytryptamine; Pharmacology and Function. Pharmacol Rev 73: 310–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barre A, Berthoux C, De Bundel D, Valjent E, Bockaert J, Marin P et al. (2016). Presynaptic serotonin 2A receptors modulate thalamocortical plasticity and associative learning. Proc Natl Acad Sci U S A 113: E1382–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basith S, Cui M, Macalino SJY, Park J, Clavio NAB, Kang S et al. (2018). Exploring G Protein-Coupled Receptors (GPCRs) Ligand Space via Cheminformatics Approaches: Impact on Rational Drug Design. Front Pharmacol 9: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett JP Jr., Enna SJ, Bylund DB, Gillin JC, Wyatt RJ, Snyder SH (1979). Neurotransmitter receptors in frontal cortex of schizophrenics. Arch Gen Psychiatry 36: 927–934. [DOI] [PubMed] [Google Scholar]
- Benvenga MJ, Chaney SF, Baez M, Britton TC, Hornback WJ, Monn JA et al. (2018). Metabotropic Glutamate2 Receptors Play a Key Role in Modulating Head Twitches Induced by a Serotonergic Hallucinogen in Mice. Front Pharmacol 9: 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berque-Bestel I, Lezoualc’h F, Jockers R (2008). Bivalent ligands as specific pharmacological tools for G protein-coupled receptor dimers. Curr Drug Discov Technol 5: 312–318. [DOI] [PubMed] [Google Scholar]
- Burnet PW, Eastwood SL, Harrison PJ (1996). 5-HT1A and 5-HT2A receptor mRNAs and binding site densities are differentially altered in schizophrenia. Neuropsychopharmacology 15: 442–455. [DOI] [PubMed] [Google Scholar]
- Butler M, Jelen L, Rucker J (2022). Expectancy in placebo-controlled trials of psychedelics: if so, so what? Psychopharmacology (Berl) 239: 3047–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron LP, Patel SD, Vargas MV, Barragan EV, Saeger HN, Warren HT et al. (2023). 5-HT2ARs Mediate Therapeutic Behavioral Effects of Psychedelic Tryptamines. ACS Chem Neurosci 14: 351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron LP, Tombari RJ, Lu J, Pell AJ, Hurley ZQ, Ehinger Y et al. (2021). A non-hallucinogenic psychedelic analogue with therapeutic potential. Nature 589: 474–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D, Yu J, Wang H, Luo Z, Liu X, He L et al. (2022). Structure-based discovery of nonhallucinogenic psychedelic analogs. Science 375: 403–411. [DOI] [PubMed] [Google Scholar]
- Casey AB, Cui M, Booth RG, Canal CE (2022). “Selective” serotonin 5-HT2A receptor antagonists. Biochem Pharmacol 200: 115028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Pin JP (1997). Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol 37: 205–237. [DOI] [PubMed] [Google Scholar]
- Crilly J (2007). The history of clozapine and its emergence in the US market: a review and analysis. History of Psychiatry 18: 30–60. [DOI] [PubMed] [Google Scholar]
- de la Fuente Revenga M, Ibi D, Cuddy T, Toneatti R, Kurita M, Ijaz MK et al. (2018a). Chronic clozapine treatment restrains via HDAC2 the performance of mGlu2 receptor agonism in a rodent model of antipsychotic activity. Neuropsychopharmacology 44: 443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente Revenga M, Ibi D, Saunders JM, Cuddy T, Ijaz MK, Toneatti R et al. (2018b). HDAC2-dependent Antipsychotic-like Effects of Chronic Treatment with the HDAC Inhibitor SAHA in Mice. Neuroscience 388: 102–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente Revenga M, Shin JM, Vohra HZ, Hideshima KS, Schneck M, Poklis JL et al. (2019). Fully automated head-twitch detection system for the study of 5-HT2A receptor pharmacology in vivo. Sci Rep 9: 14247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente Revenga M, Zhu B, Guevara GA, Naler LB, Saunders JM, Zhou Z et al. (2021). Prolonged epigenomic and synaptic plasticity alterations following single exposure to a psychedelic in mice. Cell Rep 37: 109836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean B, Crossland N, Boer S, Scarr E (2008). Evidence for altered post-receptor modulation of the serotonin 2a receptor in schizophrenia. Schizophr Res 104: 185–197. [DOI] [PubMed] [Google Scholar]
- Dean B, Hayes W (1996). Decreased frontal cortical serotonin2A receptors in schizophrenia. Schizophr Res 21: 133–139. [DOI] [PubMed] [Google Scholar]
- Dean B, Hayes W, Hill C, Copolov D (1998). Decreased serotonin2A receptors in Brodmann’s area 9 from schizophrenic subjects. A pathological or pharmacological phenomenon? Mol Chem Neuropathol 34: 133–145. [DOI] [PubMed] [Google Scholar]
- Dean B, Hayes W, Opeskin K, Naylor L, Pavey G, Hill C et al. (1996). Serotonin2 receptors and the serotonin transporter in the schizophrenic brain. Behav Brain Res 73: 169–175. [DOI] [PubMed] [Google Scholar]
- Dean B, Hussain T, Hayes W, Scarr E, Kitsoulis S, Hill C et al. (1999). Changes in serotonin2A and GABA(A) receptors in schizophrenia: studies on the human dorsolateral prefrontal cortex. J Neurochem 72: 1593–1599. [DOI] [PubMed] [Google Scholar]
- Delille HK, Becker JM, Burkhardt S, Bleher B, Terstappen GC, Schmidt M et al. (2012). Heterocomplex formation of 5-HT(2A)-mGlu(2) and its relevance for cellular signaling cascades. Neuropharmacology 62: 2184–2191. [DOI] [PubMed] [Google Scholar]
- Diez-Alarcia R, Muguruza C, Rivero G, Garcia-Bea A, Gomez-Vallejo V, Callado LF et al. (2021). Opposite alterations of 5-HT(2A) receptor brain density in subjects with schizophrenia: relevance of radiotracers pharmacological profile. Transl Psychiatry 11: 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Filipeanu CM, Duvernay MT, Wu G (2007). Regulation of G protein-coupled receptor export trafficking. Biochim Biophys Acta 1768: 853–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doumazane E, Scholler P, Zwier JM, Trinquet E, Rondard P, Pin JP (2011). A new approach to analyze cell surface protein complexes reveals specific heterodimeric metabotropic glutamate receptors. Faseb J 25: 66–77. [DOI] [PubMed] [Google Scholar]
- Du J, Wang D, Fan H, Xu C, Tai L, Lin S et al. (2021). Structures of human mGlu2 and mGlu7 homo- and heterodimers. Nature 594: 589–593. [DOI] [PubMed] [Google Scholar]
- Ebdrup BH, Rasmussen H, Arnt J, Glenthoj B (2011). Serotonin 2A receptor antagonists for treatment of schizophrenia. Expert Opin Investig Drugs 20: 1211–1223. [DOI] [PubMed] [Google Scholar]
- Erez M, Takemori AE, Portoghese PS (1982). Narcotic antagonistic potency of bivalent ligands which contain beta-naltrexamine. Evidence for bridging between proximal recognition sites. J Med Chem 25: 847–849. [DOI] [PubMed] [Google Scholar]
- Ferre S, Ciruela F, Dessauer CW, Gonzalez-Maeso J, Hebert TE, Jockers R et al. (2022). G protein-coupled receptor-effector macromolecular membrane assemblies (GEMMAs). Pharmacol Ther 231: 107977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre S, von Euler G, Johansson B, Fredholm BB, Fuxe K (1991). Stimulation of high-affinity adenosine A2 receptors decreases the affinity of dopamine D2 receptors in rat striatal membranes. Proc Natl Acad Sci USA 88: 7238–7241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fervaha G, Takeuchi H, Lee J, Foussias G, Fletcher PJ, Agid O et al. (2015). Antipsychotics and amotivation. Neuropsychopharmacology 40: 1539–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R (2003). Schizophrenia. N Engl J Med 349: 1738–1749. [DOI] [PubMed] [Google Scholar]
- Fribourg M, Moreno JL, Holloway T, Provasi D, Baki L, Mahajan R et al. (2011). Decoding the Signaling of a GPCR Heteromeric Complex Reveals a Unifying Mechanism of Action of Antipsychotic Drugs. Cell 147: 1011–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromer M, Pocklington AJ, Kavanagh DH, Williams HJ, Dwyer S, Gormley P et al. (2014). De novo mutations in schizophrenia implicate synaptic networks. Nature 506: 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaitonde SA, Gonzalez-Maeso J (2017). Contribution of heteromerization to G protein-coupled receptor function. Curr Opin Pharmacol 32: 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Bea A, Miranda-Azpiazu P, Muguruza C, Marmolejo-Martinez-Artesero S, Diez-Alarcia R, Gabilondo AM et al. (2019). Serotonin 5-HT2A receptor expression and functionality in postmortem frontal cortex of subjects with schizophrenia: Selective biased agonism via Galphai1-proteins. Eur Neuropsychopharmacol 29: 1453–1463. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Vollenweider FX (2008). Serotonin research: contributions to understanding psychoses. Trends Pharmacol Sci 29: 445–453. [DOI] [PubMed] [Google Scholar]
- Gilman AG (1987). G proteins: transducers of receptor-generated signals. Annu Rev Biochem 56: 615–649. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Titeler M, McKenney JD (1984). Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sci 35: 2505–2511. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Greenberg RD, Griffin SJ, Gold JM, Kleinman JE, Pickar D et al. (1993). The effect of clozapine on cognition and psychiatric symptoms in patients with schizophrenia. Br J Psychiatry 162: 43–48. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J (2011). GPCR oligomers in pharmacology and signaling. Mol Brain 4: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Maeso J (2014). Family A GPCR heteromers in animal models. Front Pharmacol 5: 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, Lopez-Gimenez JF et al. (2008). Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature 452: 93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Sealfon SC (2009a). Agonist-trafficking and hallucinogens. Curr Med Chem 16: 1017–1027. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Sealfon SC (2009b). Psychedelics and schizophrenia. Trends Neurosci 32: 225–232. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R et al. (2007). Hallucinogens Recruit Specific Cortical 5-HT(2A) Receptor-Mediated Signaling Pathways to Affect Behavior. Neuron 53: 439–452. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Yuen T, Ebersole BJ, Wurmbach E, Lira A, Zhou M et al. (2003). Transcriptome fingerprints distinguish hallucinogenic and nonhallucinogenic 5-hydroxytryptamine 2A receptor agonist effects in mouse somatosensory cortex. J Neurosci 23: 8836–8843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich EV, Joyce JN (1997). Alterations in the cortical serotonergic system in schizophrenia: a postmortem study. Biol Psychiatry 42: 529–545. [DOI] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV (2019). GPCR Signaling Regulation: The Role of GRKs and Arrestins. Front Pharmacol 10: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habrian CH, Levitz J, Vyklicky V, Fu Z, Hoagland A, McCort-Tranchepain I et al. (2019). Conformational pathway provides unique sensitivity to a synaptic mGluR. Nat Commun 10: 5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA (2018). Effect of Hallucinogens on Unconditioned Behavior. Curr Top Behav Neurosci 36: 159–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamor PU, Sirova J, Palenicek T, Zaniewska M, Bubenikova-Valesova V, Schwendt M (2018). Chronic methamphetamine self-administration dysregulates 5-HT2A and mGlu2 receptor expression in the rat prefrontal and perirhinal cortex: Comparison to chronic phencyclidine and MK-801. Pharmacol Biochem Behav 175: 89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks JB, Gonzalez-Maeso J (2013). Animal models of serotonergic psychedelics. ACS Chem Neurosci 4: 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks JB, González-Maeso J, 2016a. Hallucinogens: Circuits, behavior and translational models. In: Preedy VR, (Ed), The Neuropathology of drug addiction and substance misuse. Elsevier. [Google Scholar]
- Hanks JB, González-Maeso J, 2016b. Molecular and cellular basis of hallucinogenic drug action. In: Preedy VR, (Ed), The Neuropathology of drug addiction and substance misuse. Elsevier. [Google Scholar]
- Herrnstein RJ (1962). Placebo effect in the rat. Science 138: 677–678. [DOI] [PubMed] [Google Scholar]
- Hideshima KS, Hojati A, Saunders JM, On DM, de la Fuente Revenga M, Shin JM et al. (2018). Role of mGlu2 in the 5-HT2A receptor-dependent antipsychotic activity of clozapine in mice. Psychopharmacology (Berl) 235: 3149–3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlavackova V, Goudet C, Kniazeff J, Zikova A, Maurel D, Vol C et al. (2005). Evidence for a single heptahelical domain being turned on upon activation of a dimeric GPCR. Embo J 24: 499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S (1994). Cloned glutamate receptors. Annu Rev Neurosci 17: 31–108. [DOI] [PubMed] [Google Scholar]
- Huang B, St Onge CM, Ma H, Zhang Y (2021a). Design of bivalent ligands targeting putative GPCR dimers. Drug Discov Today 26: 189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Wang H, Zheng Y, Li M, Kang G, Barreto-de-Souza V et al. (2021b). Structure-Based Design and Development of Chemical Probes Targeting Putative MOR-CCR5 Heterodimers to Inhibit Opioid Exacerbated HIV-1 Infectivity. J Med Chem 64: 7702–7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubner H, Schellhorn T, Gienger M, Schaab C, Kaindl J, Leeb L et al. (2016). Structure-guided development of heterodimer-selective GPCR ligands. Nat Commun 7: 12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husa AP, Moilanen J, Murray GK, Marttila R, Haapea M, Rannikko I et al. (2016). Lifetime antipsychotic medication and cognitive performance in schizophrenia at age 43 years in a general population birth cohort. Psychiatry Res 247: 130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husa AP, Rannikko I, Moilanen J, Haapea M, Murray GK, Barnett J et al. (2014). Lifetime use of antipsychotic medication and its relation to change of verbal learning and memory in midlife schizophrenia - An observational 9-year follow-up study. Schizophr Res 158: 134–141. [DOI] [PubMed] [Google Scholar]
- Ibi D, de la Fuente Revenga M, Kezunovic N, Muguruza C, Saunders JM, Gaitonde SA et al. (2017). Antipsychotic-induced Hdac2 transcription via NF-kappaB leads to synaptic and cognitive side effects. Nat Neurosci 20: 1247–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibi D, Gonzalez-Maeso J (2015). Epigenetic signaling in schizophrenia. Cell Signal 27: 2131–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inserra A, Campanale A, Cheishvili D, Dymov S, Wong A, Marcal N et al. (2022). Modulation of DNA methylation and protein expression in the prefrontal cortex by repeated administration of D-lysergic acid diethylamide (LSD): Impact on neurotropic, neurotrophic, and neuroplasticity signaling. Prog Neuropsychopharmacol Biol Psychiatry 119: 110594. [DOI] [PubMed] [Google Scholar]
- Jakab RL, Goldman-Rakic PS (1998). 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc Natl Acad Sci USA 95: 735–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaster AM, de la Fuente Revenga M, Gonzalez-Maeso J (2021). Molecular targets of psychedelic-induced plasticity. J Neurochem 162: 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaster AM, Younkin J, Cuddy T, de la Fuente Revenga M, Poklis JL, Dozmorov MG et al. (2022). Differences across sexes on head-twitch behavior and 5-HT2A receptor signaling in C57BL/6J mice. Neurosci Lett 788: 136836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KA, Borowsky B, Tamm JA, Craig DA, Durkin MM, Dai M et al. (1998). GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA(B)R2. Nature 396: 674–679. [DOI] [PubMed] [Google Scholar]
- Jordan BA, Devi LA (1999). G-protein-coupled receptor heterodimerization modulates receptor function. Nature 399: 697–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce JN, Shane A, Lexow N, Winokur A, Casanova MF, Kleinman JE (1993). Serotonin uptake sites and serotonin receptors are altered in the limbic system of schizophrenics. Neuropsychopharmacology 8: 315–336. [DOI] [PubMed] [Google Scholar]
- Kang K, Huang XF, Wang Q, Deng C (2009). Decreased density of serotonin 2A receptors in the superior temporal gyrus in schizophrenia--a postmortem study. Prog Neuropsychopharmacol Biol Psychiatry 33: 867–871. [DOI] [PubMed] [Google Scholar]
- Kaplan AL, Confair DN, Kim K, Barros-Alvarez X, Rodriguiz RM, Yang Y et al. (2022). Bespoke library docking for 5-HT(2A) receptor agonists with antidepressant activity. Nature 610: 582–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katritch V, Cherezov V, Stevens RC (2013). Structure-function of the G protein-coupled receptor superfamily. Annu Rev Pharmacol Toxicol 53: 531–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupmann K, Malitschek B, Schuler V, Heid J, Froestl W, Beck P et al. (1998). GABA(B)-receptor subtypes assemble into functional heteromeric complexes. Nature 396: 683–687. [DOI] [PubMed] [Google Scholar]
- Kim K, Che T, Panova O, DiBerto JF, Lyu J, Krumm BE et al. (2020). Structure of a Hallucinogen-Activated Gq-Coupled 5-HT2A Serotonin Receptor. Cell 182: 1574–1588 e1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura KT, Asada H, Inoue A, Kadji FMN, Im D, Mori C et al. (2019). Structures of the 5-HT2A receptor in complex with the antipsychotics risperidone and zotepine. Nat Struct Mol Biol 26: 121–128. [DOI] [PubMed] [Google Scholar]
- Kinon BJ, Millen BA, Zhang L, McKinzie DL (2015). Exploratory Analysis for a Targeted Patient Population Responsive to the Metabotropic Glutamate 2/3 Receptor Agonist Pomaglumetad Methionil in Schizophrenia. Biol Psychiatry 78: 754–762. [DOI] [PubMed] [Google Scholar]
- Kniazeff J, Bessis AS, Maurel D, Ansanay H, Prezeau L, Pin JP (2004). Closed state of both binding domains of homodimeric mGlu receptors is required for full activity. Nat Struct Mol Biol 11: 706–713. [DOI] [PubMed] [Google Scholar]
- Koehl A, Hu H, Feng D, Sun B, Zhang Y, Robertson MJ et al. (2019). Structural insights into the activation of metabotropic glutamate receptors. Nature 566: 79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen LV, Huerta I, Beneyto M, Meador-Woodruff JH (2007). NMDA receptors and schizophrenia. Curr Opin Pharmacol 7: 48–55. [DOI] [PubMed] [Google Scholar]
- Kurita M, Holloway T, Garcia-Bea A, Kozlenkov A, Friedman AK, Moreno JL et al. (2012). HDAC2 regulates atypical antipsychotic responses through the modulation of mGlu2 promoter activity. Nat Neurosci 15: 1245–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita M, Moreno JL, Holloway T, Kozlenkov A, Mocci G, Garcia-Bea A et al. (2013). Repressive Epigenetic Changes at the mGlu2 Promoter in Frontal Cortex of 5-HT2A Knockout Mice. Mol Pharmacol 83: 1166–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurrasch-Orbaugh DM, Parrish JC, Watts VJ, Nichols DE (2003a). A complex signaling cascade links the serotonin2A receptor to phospholipase A2 activation: the involvement of MAP kinases. J Neurochem 86: 980–991. [DOI] [PubMed] [Google Scholar]
- Kurrasch-Orbaugh DM, Watts VJ, Barker EL, Nichols DE (2003b). Serotonin 5-hydroxytryptamine 2A receptor-coupled phospholipase C and phospholipase A2 signaling pathways have different receptor reserves. J Pharmacol Exp Ther 304: 229–237. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, Casanova MF, Toti R, Weinberger DR, Kleinman JE (1993). Selective abnormalities of prefrontal serotonergic receptors in schizophrenia. A postmortem study. Arch Gen Psychiatry 50: 810–818. [DOI] [PubMed] [Google Scholar]
- Le Naour M, Akgun E, Yekkirala A, Lunzer MM, Powers MD, Kalyuzhny AE et al. (2013). Bivalent ligands that target mu opioid (MOP) and cannabinoid1 (CB1) receptors are potent analgesics devoid of tolerance. J Med Chem 56: 5505–5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Munguba H, Gutzeit VA, Singh DR, Kristt M, Dittman JS et al. (2020). Defining the Homo- and Heterodimerization Propensities of Metabotropic Glutamate Receptors. Cell Rep 31: 107605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Basith S, Choi S (2018). Recent Advances in Structure-Based Drug Design Targeting Class A G Protein-Coupled Receptors Utilizing Crystal Structures and Computational Simulations. J Med Chem 61: 1–46. [DOI] [PubMed] [Google Scholar]
- Levitz J, Habrian C, Bharill S, Fu Z, Vafabakhsh R, Isacoff EY (2016). Mechanism of Assembly and Cooperativity of Homomeric and Heteromeric Metabotropic Glutamate Receptors. Neuron 92: 143–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leysen JE (2004). 5-HT2 receptors. Curr Drug Targets CNS Neurol Disord 3: 11–26. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Bymaster FP, Meltzer HY, Deutch AY, Duncan GE, Marx CE et al. (2008). Antipsychotic drugs: comparison in animal models of efficacy, neurotransmitter regulation, and neuroprotection. Pharmacol Rev 60: 358–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Han S, Cai X, Tan Q, Zhou K, Wang D et al. (2021). Structures of Gi-bound metabotropic glutamate receptors mGlu2 and mGlu4. Nature 594: 583–588. [DOI] [PubMed] [Google Scholar]
- Lopez-Gimenez JF, Gonzalez-Maeso J (2018). Hallucinogens and Serotonin 5-HT2A Receptor-Mediated Signaling Pathways. Curr Top Behav Neurosci 36: 45–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Gimenez JF, Mengod G, Palacios JM, Vilaro MT (1997). Selective visualization of rat brain 5-HT2A receptors by autoradiography with [3H]MDL 100,907. Naunyn Schmiedebergs Arch Pharmacol 356: 446–454. [DOI] [PubMed] [Google Scholar]
- Lopez-Gimenez JF, Vilaro MT, Palacios JM, Mengod G (2001). Mapping of 5-HT2A receptors and their mRNA in monkey brain: [3H]MDL100,907 autoradiography and in situ hybridization studies. J Comp Neurol 429: 571–589. [DOI] [PubMed] [Google Scholar]
- Ly C, Greb AC, Cameron LP, Wong JM, Barragan EV, Wilson PC et al. (2018). Psychedelics Promote Structural and Functional Neural Plasticity. Cell Rep 23: 3170–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes AC, Dunn H, Ferguson SS (2012). Regulation of GPCR activity, trafficking and localization by GPCR-interacting proteins. Br J Pharmacol 165: 1717–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio R, Vogel Z, Wess J (1993). Coexpression studies with mutant muscarinic/adrenergic receptors provide evidence for intermolecular “cross-talk” between G-protein-linked receptors. Proc Natl Acad Sci U S A 90: 3103–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazziti D, Giannaccini G, Giromella A, Betti L, Pesce D, Nardi I et al. (2003). [3H]-ketanserin binding sites in different psychiatric disorders. Neurochem Int 42: 511–516. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Martin-Ruiz R, Abo A, Artigas F (2005). The selective 5-HT2A receptor antagonist M100907 enhances antidepressant-like behavioral effects of the SSRI fluoxetine. Neuropsychopharmacology 30: 2205–2215. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Wright RA, Gewirtz JC, Schoepp DD (2001). A major role for thalamocortical afferents in serotonergic hallucinogen receptor function in the rat neocortex. Neuroscience 105: 379–392. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Wright RA, Schoepp DD, Monn JA, Aghajanian GK (2000). Physiological antagonism between 5-hydroxytryptamine(2A) and group II metabotropic glutamate receptors in prefrontal cortex. J Pharmacol Exp Ther 292: 76–87. [PubMed] [Google Scholar]
- Marona-Lewicka D, Kurrasch-Orbaugh DM, Selken JR, Cumbay MG, Lisnicchia JG, Nichols DE (2002). Re-evaluation of lisuride pharmacology: 5-hydroxytryptamine1A receptor-mediated behavioral effects overlap its other properties in rats. Psychopharmacology (Berl) 164: 93–107. [DOI] [PubMed] [Google Scholar]
- Matsumoto I, Inoue Y, Iwazaki T, Pavey G, Dean B (2005). 5-HT2A and muscarinic receptors in schizophrenia: a postmortem study. Neurosci Lett 379: 164–168. [DOI] [PubMed] [Google Scholar]
- McCorvy JD, Roth BL (2015). Structure and function of serotonin G protein-coupled receptors. Pharmacol Ther 150: 129–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HY (2013). Update on typical and atypical antipsychotic drugs. Annu Rev Med 64: 393–406. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Mills R, Revell S, Williams H, Johnson A, Bahr D et al. (2010). Pimavanserin, a serotonin(2A) receptor inverse agonist, for the treatment of parkinson’s disease psychosis. Neuropsychopharmacology 35: 881–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HY, Stahl SM (1976). The dopamine hypothesis of schizophrenia: a review. Schizophr Bull 2: 19–76. [DOI] [PubMed] [Google Scholar]
- Mita T, Hanada S, Nishino N, Kuno T, Nakai H, Yamadori T et al. (1986). Decreased serotonin S2 and increased dopamine D2 receptors in chronic schizophrenics. Biol Psychiatry 21: 1407–1414. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Miyake N, Jarskog LF, Fleischhacker WW, Lieberman JA (2012). Pharmacological treatment of schizophrenia: a critical review of the pharmacology and clinical effects of current and future therapeutic agents. Mol Psychiatry 17: 1206–1227. [DOI] [PubMed] [Google Scholar]
- Moghaddam B (2004). Targeting metabotropic glutamate receptors for treatment of the cognitive symptoms of schizophrenia. Psychopharmacology (Berl) 174: 39–44. [DOI] [PubMed] [Google Scholar]
- Mokler DJ, Commissaris RL, Warner MR, Rech RH (1983). Blockade of the behavioral effects of lysergic acid diethylamide, 2,5-dimethoxy-4-methylamphetamine, quipazine and lisuride by 5-hydroxytryptamine antagonists. J Pharmacol Exp Ther 227: 557–562. [PubMed] [Google Scholar]
- Moreno JL, Holloway T, Albizu L, Sealfon SC, Gonzalez-Maeso J (2011). Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists. Neurosci Lett 493: 76–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JL, Holloway T, Umali A, Rayannavar V, Sealfon SC, Gonzalez-Maeso J (2013). Persistent effects of chronic clozapine on the cellular and behavioral responses to LSD in mice. Psychopharmacology (Berl) 225: 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JL, Miranda-Azpiazu P, Garcia-Bea A, Younkin J, Cui M, Kozlenkov A et al. (2016). Allosteric signaling through an mGlu2 and 5-HT2A heteromeric receptor complex and its potential contribution to schizophrenia. Sci Signal 9: ra5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JL, Muguruza C, Umali A, Mortillo S, Holloway T, Pilar-Cuellar F et al. (2012). Identification of three residues essential for 5-hydroxytryptamine 2A-metabotropic glutamate 2 (5-HT2A.mGlu2) receptor heteromerization and its psychoactive behavioral function. J Biol Chem 287: 44301–44319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris BJ, Cochran SM, Pratt JA (2005). PCP: from pharmacology to modelling schizophrenia. Curr Opin Pharmacol 5: 101–106. [DOI] [PubMed] [Google Scholar]
- Muguruza C, Moreno JL, Umali A, Callado LF, Meana JJ, Gonzalez-Maeso J (2013). Dysregulated 5-HT(2A) receptor binding in postmortem frontal cortex of schizophrenic subjects. Eur Neuropsychopharmacol 23: 852–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murat S, Bigot M, Chapron J, Konig GM, Kostenis E, Battaglia G et al. (2019). 5-HT(2A) receptor-dependent phosphorylation of mGlu(2) receptor at Serine 843 promotes mGlu(2) receptor-operated G(i/o) signaling. Mol Psychiatry 24: 1610–1626. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Ikoma Y, Kimura K, Nakada Y, Kobayashi S, Yamaguchi M et al. (1989). [Effects in animal models of depression of lisuride alone and upon coadministration with antidepressants]. Nihon Yakurigaku Zasshi 94: 81–89. [DOI] [PubMed] [Google Scholar]
- Nichols CD, Garcia EE, Sanders-Bush E (2003). Dynamic changes in prefrontal cortex gene expression following lysergic acid diethylamide administration. Brain Res Mol Brain Res 111: 182–188. [DOI] [PubMed] [Google Scholar]
- Nichols CD, Sanders-Bush E (2002). A single dose of lysergic acid diethylamide influences gene expression patterns within the mammalian brain. Neuropsychopharmacology 26: 634–642. [DOI] [PubMed] [Google Scholar]
- Nichols DE (2016). Psychedelics. Pharmacol Rev 68: 264–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti F, Bockaert J, Collingridge GL, Conn PJ, Ferraguti F, Schoepp DD et al. (2011). Metabotropic glutamate receptors: from the workbench to the bedside. Neuropharmacology 60: 1017–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen RE, Levander S, Kjaersdam Telleus G, Jensen SO, Ostergaard Christensen T, Leucht S (2015). Second-generation antipsychotic effect on cognition in patients with schizophrenia-a meta-analysis of randomized clinical trials. Acta Psychiatr Scand [DOI] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ (2010). Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol 50: 295–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivero G, Grilli M, Vergassola M, Bonfiglio T, Padolecchia C, Garrone B et al. (2018). 5-HT2A-mGlu2/3 receptor complex in rat spinal cord glutamatergic nerve endings: A 5-HT2A to mGlu2/3 signalling to amplify presynaptic mechanism of auto-control of glutamate exocytosis. Neuropharmacology 133: 429–439. [DOI] [PubMed] [Google Scholar]
- Pacheco AT, Olson RJ, Garza G, Moghaddam B (2023). Acute psilocybin enhances cognitive flexibility in rats. Neuropharmacology (Online ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya NJ, Klaassen RV, van der Schors RC, Slotman JA, Houtsmuller A, Smit AB et al. (2016). Group 1 metabotropic glutamate receptors 1 and 5 form a protein complex in mouse hippocampus and cortex. Proteomics 16: 2698–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, Andreev BV et al. (2007). Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med 13: 1102–1107. [DOI] [PubMed] [Google Scholar]
- Pavlos NJ, Friedman PA (2017). GPCR Signaling and Trafficking: The Long and Short of It. Trends Endocrinol Metab 28: 213–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina M, Zubieta JK (2015). Molecular mechanisms of placebo responses in humans. Mol Psychiatry 20: 416–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portoghese PS, Ronsisvalle G, Larson DL, Takemori AE (1986). Synthesis and opioid antagonist potencies of naltrexamine bivalent ligands with conformationally restricted spacers. J Med Chem 29: 1650–1653. [DOI] [PubMed] [Google Scholar]
- Portoghese PS, Ronsisvalle G, Larson DL, Yim CB, Sayre LM, Takemori AE (1982). Opioid agonist and antagonist bivalent ligands as receptor probes. Life Sci 31: 1283–1286. [DOI] [PubMed] [Google Scholar]
- Poulie CBM, Liu N, Jensen AA, Bunch L (2020). Design, Synthesis, and Pharmacological Characterization of Heterobivalent Ligands for the Putative 5-HT2A/mGlu2 Receptor Complex. J Med Chem 63: 9928–9949. [DOI] [PubMed] [Google Scholar]
- Preller KH, Herdener M, Pokorny T, Planzer A, Kraehenmann R, Stampfli P et al. (2017). The Fabric of Meaning and Subjective Effects in LSD-Induced States Depend on Serotonin 2A Receptor Activation. Curr Biol 27: 451–457. [DOI] [PubMed] [Google Scholar]
- Preller KH, Pokorny T, Hock A, Kraehenmann R, Stampfli P, Seifritz E et al. (2016). Effects of serotonin 2A/1A receptor stimulation on social exclusion processing. Proc Natl Acad Sci USA 113: 5119–5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulido D, Casado-Anguera V, Gomez-Autet M, Llopart N, Moreno E, Casajuana-Martin N et al. (2022). Heterobivalent Ligand for the Adenosine A2A-Dopamine D2 Receptor Heteromer. J Med Chem 65: 616–632. [DOI] [PubMed] [Google Scholar]
- Qu Y, Chang L, Ma L, Wan X, Hashimoto K (2022). Rapid antidepressant-like effect of non-hallucinogenic psychedelic analog lisuride, but not hallucinogenic psychedelic DOI, in lipopolysaccharide-treated mice. Pharmacol Biochem Behav 222: 173500. [DOI] [PubMed] [Google Scholar]
- Rasmussen H, Erritzoe D, Andersen R, Ebdrup BH, Aggernaes B, Oranje B et al. (2010). Decreased frontal serotonin2A receptor binding in antipsychotic-naive patients with first-episode schizophrenia. Arch Gen Psychiatry 67: 9–16. [DOI] [PubMed] [Google Scholar]
- Reynolds GP, Rossor MN, Iversen LL (1983). Preliminary studies of human cortical 5-HT2 receptors and their involvement in schizophrenia and neuroleptic drug action. J Neural Transm Suppl 18: 273–277. [PubMed] [Google Scholar]
- Rives ML, Vol C, Fukazawa Y, Tinel N, Trinquet E, Ayoub MA et al. (2009). Crosstalk between GABAB and mGlu1a receptors reveals new insight into GPCR signal integration. Embo J 28: 2195–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romantini N, Alam S, Dobitz S, Spillmann M, De Foresta M, Schibli R et al. (2021). Exploring the signaling space of a GPCR using bivalent ligands with a rigid oligoproline backbone. Proc Natl Acad Sci USA 118: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum DM, Rasmussen SG, Kobilka BK (2009). The structure and function of G-protein-coupled receptors. Nature 459: 356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengarten H, Quartermain D (2002). The effect of chronic treatment with typical and atypical antipsychotics on working memory and jaw movements in three- and eighteen-month-old rats. Prog Neuropsychopharmacol Biol Psychiatry 26: 1047–1054. [DOI] [PubMed] [Google Scholar]
- Sawa A, Snyder SH (2002). Schizophrenia: diverse approaches to a complex disease. Science 296: 692–695. [DOI] [PubMed] [Google Scholar]
- Schmid Y, Enzler F, Gasser P, Grouzmann E, Preller KH, Vollenweider FX et al. (2015). Acute Effects of Lysergic Acid Diethylamide in Healthy Subjects. Biol Psychiatry 78: 544–553. [DOI] [PubMed] [Google Scholar]
- Schroder N, de Lima MN, Quevedo J, Dal Pizzol F, Roesler R (2005). Impairing effects of chronic haloperidol and clozapine treatment on recognition memory: possible relation to oxidative stress. Schizophr Res 73: 377–378. [DOI] [PubMed] [Google Scholar]
- Seeman P, Lee T (1975). Antipsychotic drugs: direct correlation between clinical potency and presynaptic action on dopamine neurons. Science 188: 1217–1219. [DOI] [PubMed] [Google Scholar]
- Seeman P, Lee T, Chau-Wong M, Wong K (1976). Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature 261: 717–719. [DOI] [PubMed] [Google Scholar]
- Shah UH, Toneatti R, Gaitonde SA, Shin JM, Gonzalez-Maeso J (2020). Site-Specific Incorporation of Genetically Encoded Photo-Crosslinkers Locates the Heteromeric Interface of a GPCR Complex in Living Cells. Cell Chem Biol 27: 1308–1317 e1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao LX, Liao C, Gregg I, Davoudian PA, Savalia NK, Delagarza K et al. (2021). Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo. Neuron 109: 2535–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp T, Barnes NM (2020). Central 5-HT receptors and their function; present and future. Neuropharmacology 177: 108155. [DOI] [PubMed] [Google Scholar]
- Shonberg J, Scammells PJ, Capuano B (2011). Design strategies for bivalent ligands targeting GPCRs. ChemMedChem 6: 963–974. [DOI] [PubMed] [Google Scholar]
- Sierra S, Muchhala KH, Jessup DK, Contreras KM, Shah UH, Stevens DL et al. (2022). Sex-specific role for serotonin 5-HT2A receptor in modulation of opioid-induced antinociception and reward in mice. Neuropharmacology 209: 108988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbergeld EK, Hruska RE (1979). Lisuride and LSD: dopaminergic and serotonergic interactions in the “serotonin syndrome”. Psychopharmacology (Berl) 65: 233–237. [DOI] [PubMed] [Google Scholar]
- Sriram K, Insel PA (2018). G Protein-Coupled Receptors as Targets for Approved Drugs: How Many Targets and How Many Drugs? Mol Pharmacol 93: 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl SM (2016). Parkinson’s disease psychosis as a serotonin-dopamine imbalance syndrome. CNS Spectr 21: 355–359. [DOI] [PubMed] [Google Scholar]
- Stahl SM (2018). Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: dopamine, serotonin, and glutamate. CNS Spectr 23: 187–191. [DOI] [PubMed] [Google Scholar]
- Strange PG (1998). Three-state and two-state models. Trends Pharmacol Sci 19: 85–86. [DOI] [PubMed] [Google Scholar]
- Strange PG (1999). G-protein coupled receptors: conformations and states. Biochem Pharmacol 58: 1081–1088. [DOI] [PubMed] [Google Scholar]
- Taddeucci A, Olivero G, Roggeri A, Milanese C, Giorgio FPD, Grilli M et al. (2022). Presynaptic 5-HT2A-mGlu2/3 Receptor-Receptor Crosstalk in the Prefrontal Cortex: Metamodulation of Glutamate Exocytosis. Cells 11: 3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibado JK, Tano JY, Lee J, Salas-Estrada L, Provasi D, Strauss A et al. (2021). Differences in interactions between transmembrane domains tune the activation of metabotropic glutamate receptors. Elife 10: e67027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen ARB, Jensen DD, Hicks GA, Bunnett NW (2018). Therapeutic Targeting of Endosomal G-Protein-Coupled Receptors. Trends Pharmacol Sci 39: 879–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen W, Frazer J, Unett D (2005). Functional assays for screening GPCR targets. Curr Opin Biotechnol 16: 655–665. [DOI] [PubMed] [Google Scholar]
- Toneatti R, Shin JM, Shah UH, Mayer CR, Saunders JM, Fribourg M et al. (2020). Interclass GPCR heteromerization affects localization and trafficking. Sci Signal 13: eaaw3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H et al. (2007). Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther 320: 1–13. [DOI] [PubMed] [Google Scholar]
- Vilardaga JP, Nikolaev VO, Lorenz K, Ferrandon S, Zhuang Z, Lohse MJ (2008). Conformational cross-talk between alpha2A-adrenergic and mu-opioid receptors controls cell signaling. Nat Chem Biol 4: 126–131. [DOI] [PubMed] [Google Scholar]
- Vohra HZ, Saunders JM, Jaster AM, de la Fuente Revenga M, Jimenez J, Fernandez-Teruel A et al. (2021). Sex-specific effects of psychedelics on prepulse inhibition of startle in 129S6/SvEv mice. Psychopharmacology (Berl) 239: 1649–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Babler A, Vogel H, Hell D (1998). Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport 9: 3897–3902. [DOI] [PubMed] [Google Scholar]
- von Zastrow M, Williams JT (2012). Modulating neuromodulation by receptor membrane traffic in the endocytic pathway. Neuron 76: 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker D, Wang S, McCorvy JD, Betz RM, Venkatakrishnan AJ, Levit A et al. (2017). Crystal Structure of an LSD-Bound Human Serotonin Receptor. Cell 168: 377–389 e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker PM, Crow TJ, Ferrier IN (1981). Tritiated LSD binding in frontal cortex in schizophrenia. Arch Gen Psychiatry 38: 278–280. [DOI] [PubMed] [Google Scholar]
- White JH, Wise A, Main MJ, Green A, Fraser NJ, Disney GH et al. (1998). Heterodimerization is required for the formation of a functional GABA(B) receptor. Nature 396: 679–682. [DOI] [PubMed] [Google Scholar]
- Woolley DW, Shaw E (1954). Some neurophysiological aspects of serotonin. Br Med J 2: 122–126. [DOI] [PMC free article] [PubMed] [Google Scholar]


