Abstract
Purpose of Review
The response to natural stressors involves both cardiac stimulation and vascular changes, primarily triggered by increases in sympathetic activity. These effects lead to immediate flow redistribution that provides metabolic support to priority target organs combined with other key physiological responses and cognitive strategies, against stressor challenges. This extremely well-orchestrated response that was developed over millions of years of evolution is presently being challenged, over a short period of time. In this short review, we discuss the neurogenic background for the origin of emotional stress-induced hypertension, focusing on sympathetic pathways from related findings in humans and animals.
Recent Findings
The urban environment offers a variety of psychological stressors. Real or anticipatory, emotional stressors may increase baseline sympathetic activity. From routine day-to-day traffic stress to job-related anxiety, chronic or abnormal increases in sympathetic activity caused by emotional stressors can lead to cardiovascular events, including cardiac arrhythmias, increases in blood pressure and even sudden death. Among the various alterations proposed, chronic stress could modify neuroglial circuits or compromise antioxidant systems that may alter the responsiveness of neurons to stressful stimuli. These phenomena lead to increases in sympathetic activity, hypertension and consequent cardiovascular diseases.
Summary
The link between anxiety, emotional stress, and hypertension may result from an altered neuronal firing rate in central pathways controlling sympathetic activity. The participation of neuroglial and oxidative mechanisms in altered neuronal function is primarily involved in enhanced sympathetic outflow. The significance of the insular cortex-dorsomedial hypothalamic pathway in the evolution of enhanced overall sympathetic outflow is discussed.
Keywords: Emotional stress, Anxiety, Autonomic nervous system, Sympathetic, Hypertension
Introduction
Hypertension is now recognized as the most important risk factor in cardiovascular diseases, ranking first among global causes of death. Strikingly, this silent disease is considered the leading cause of years of life lost due to premature death [1].
According to a recent United Nations report, in the past few decades, the world has been urbanizing rapidly. In 1950, only 30% of the world’s population lived in urban areas, a proportion that grew to 55% by 2018 [2]. Urbanization offers new opportunities, but it has also some adverse impacts, such as health risks, including sedentarism, unhealthy diet, substance abuse (mainly alcohol and tobacco), anxiety, and emotional stress [3]. Poor mental health contributes to loss of quality of life and generally leads to numerous other unhealthy habits leading to disease. In this regard, it is interesting to observe that the percentage of the population with hypertension is positively correlated with the increase in growth of the urban population in previous decades (Fig. 1) [2, 4].
Fig. 1.
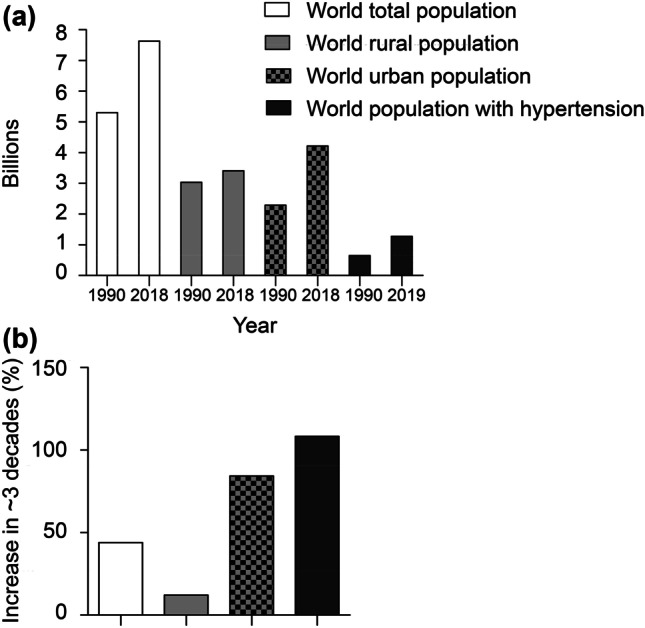
Total world population, world urban population, world rural population and world population with hypertension; rates of change over approximately three decades (a). Approximate percentage of growth for each population in ~ 30 years interval (b). Note that the percentage of the population with hypertension in the past 3 decades grows accordingly to the growth of the urban population. Data extracted from World Urbanization Prospects: The 2018 Revision [2] and Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019 [4]
Emotional stress is merely considered a risk factor in most cases, however it is a global health problem, and indeed is the hidden force behind innumerous diseases, including hypertension and cardiac arrhythmias [5, 6•, 7]. Genetic and other risk factors do not fully explain the development of hypertension, and there is increasing evidence suggesting that psychosocial stress may also play an important role [8]. This picture became clear with the uncertainty and isolation caused by the COVID-19 pandemic that led to different mood disorders [9]. Indeed, the incidence of cardiovascular complications has increased during the COVID-19 pandemic. The proposed mechanisms include generalized increases in psychological distress and sympathetic hyperactivity [9].
Psychological or emotional stress can be defined as the subjective perception of a potential or real environmental threat or challenge. This perception triggers an organized set of physiological responses that aim to improve fight or flight efficiency and chances of survival. The word stress can assume different forms. Sometimes it is used to describe the stimulus or situation (the stressor), whereas sometimes it is used to describe the physiological responses and the cognitive-behavioral strategies that are used to face that stimulus or condition (the stress or body reaction). More simply, stress involves the stressor and the stress reaction. However, it is necessary to consider that the stressor does not always need to be physically present, but it can be mentally present, assuming the form of anxiety, in anticipation of a real stress situation. In this regard, the urbanized and competitive modern life has created powerful, often invisible, new enemies or emotional stressors. The variety of psychological stressors are almost always present and usually are found in combination. Indeed, evidence points to a higher prevalence of anxiety disorders in urban environments [10, 11]. Anxiety disorders often co-occur with major depression and are associated with the development of other physical illnesses [12]. Real or anticipatory (anxiety) emotional stressors trigger physiological responses that, when abnormal (chronic or exaggerated), can be deleterious for health. In this regard, current evidence indicates that there is a positive association between comorbid anxiety and hypertension [6•].
Sympathetic activation is a hallmark in anxiety and emotional stress, leading to increased cardiac output and peripheral resistance [13, 14]. Importantly, stress biomarkers are present in patients with essential hypertension [5]. Therefore, at least part of the mechanism contributing to this enormous and growing number of hypertensive patients certainly result from sustained and abnormal increases in sympathetic activity due to anxiety and emotional stress. As a quotidian and simple example, it is known that sustained and abnormal increase in sympathetic activity triggered by workplace mental stressors or urban noise can lead to cardiac arrhythmias and hypertension [15–17]. For all reasons described above, the understanding of the mechanisms underlying emotional stress-associated responses and disease, needs to advance. This review discusses neural mechanisms contributing to emotional stress-associated hypertension.
Neurogenic Background for Emotional Stress-Associated Hypertension
The response to stress, whether real or anticipatory, leads to activation of the hypothalamic–pituitary–adrenal (HPA) axis and a sympathoadrenal response that involves rapid activation of sympathoneural and adrenomedullary components. In humans and animals, baseline sympathetic activity or sympathetic reactivity can be evaluated by direct nerve recording (sympathoneural component) or catecholamine spillover (humoral component) [18]. The first evaluates bursts of neural activity in sympathetic nerves [19], the second evaluates endogenous release of catecholamines, usually noradrenaline [18]. Increase in sympathetic activity is associated with increased heart rate, cardiac output, peripheral resistance, plasma and urinary noradrenaline levels and regional noradrenaline changes, for example in the cardiac tissue [20].
Importantly, strong evidence indicates that the pattern of sympathetic response during mental stress preferentially involves the heart [5], specifically through increasing heart rate and stroke volume. Long term, these are contributing factors for raising systolic blood pressure. In addition, in abnormal conditions, the altered pattern of sympathetic activity to the heart can lead to cardiac arrhythmias and, under severe emotional stress, takotsubo syndrome, characterized by acute transient left ventricular dysfunction. Indeed, a recent study indicated that a substantial number of patients with takotsubo cardiomyopathy were also afflicted with hypertension (64.2%) [7]. The latter finding suggests that the progressive increase in ventricular after-load (peripheral resistance) together with sudden stress-associated cardiac sympathetic overactivity may contribute to the ventricular dysfunction observed in takotsubo episodes.
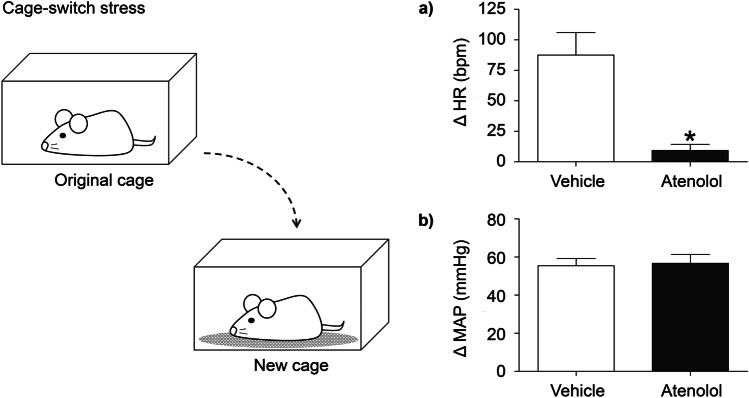
From a pharmacological standpoint, after blockade of beta 1 adrenergic receptors it is expected that heart rate and blood pressure do not increase during stressful situations. Following this rationale, it is presumed that by preventing the stress-associated cardiac changes, one could avoid a chain reaction of other physical anxiety symptoms, attenuating anxiety [21•] and thus offer cardiovascular protection. However, a previous study by Lee and colleagues using mice showed that atenolol 10 mg in the drinking water for 7 days before cage-switch stress (CSS), a form of acute psychosocial stress, only moderately attenuated the tachycardic effect to acute stress and had no effect to blunt the initial increase in MAP [22•]. Indeed, the peak pressor response in the acute phase of CSS was enormous, approximately 40 mmHg, likely an α1-receptor mediated vasoconstrictor response. The authors questioned why heart rate increased at all during stress if there was significant β-receptor blockade and raised the possibility of parasympathetic tone withdrawal. Recent experiments from our laboratory using a different route of administration showed that a single and lower dose of atenolol in rats (2 mg/Kg; intraperitoneal) resulted in almost complete blockade of the tachycardic response in the acute phase of CSS (Fig. 2). Based on these findings, the route of administration and absorption appears to explain the different responses. However, as Lee and colleagues have previously observed [22•], we also found a large pressor response (approximately 55 mmHg) that was completely preserved even with an almost complete blockade of the tachycardic response. Therefore, these findings in rodents reveal new insights regarding beta-blockers and stress cardiovascular response. First, in the acute phase of stress, it seems that the vascular and cardiac responses are completely dissociated since a large pressor response is present, even in the presence of atenolol. Second, as demonstrated previously by Esler and colleagues [5], these experiments show that the heart is the main target during acute stress responses and the powerful contribution of cardiac sympathetic output to the tachycardic response during the acute phase of stress. Finally, this observation may have a clinical implication, as well. Blockade of inotropic and chronotropic cardiac changes with atenolol may help to relieve anxiety symptoms because this specific visceral response is not perceived by the patient; however, cardiovascular protection is not guaranteed given that a large pressor response may still be present when facing stressful situations.
Fig. 2.
Mean maximum changes in heart rate (HR) (a) and mean arterial pressure (MAP) (b) caused by environment change stress in male Wistar rats (n = 6) treated with vehicle (white bars) or atenolol (black bars) during cage-switch stress (left drawing). In the experiment illustrated, the new cage was previously occupied by another male rat. Tachycardia is completely abolished in the group of rats that received previous treatment with beta-adrenergic antagonist, atenolol (intraperitoneal; 2 mg/kg). Note that the pressor response is completely preserved. See main text for discussion. (Ethics approval protocol number: 278/76/2017); t test, *P < 0.05
It is important to clarify that the neurogenic background for stress-associated hypertension may not result from isolated stress episodes but from chronic sympathetic overactivity triggered by long-term stressful situations and abnormal anxiety. Direct recording of sympathetic nerve fiber activity in humans (clinical microneurography) has supported this correlation. Lambert and colleagues demonstrated a correlation between anxiety and greater firing rate of individual sympathetic fibers during bursts of muscle sympathetic nerve activity (MSNA) [23]. Holwerda and colleagues showed that individuals with chronic anxiety demonstrate selective augmentation in relative MSNA burst amplitude, indicating enhanced sympathetic drive in a population with higher risk of cardiovascular disease [24]. An interesting study by El Sayed and colleagues indicated that the reactivity of blood pressure at the onset of mental stress dictates the direction of change in MSNA, a phenomenon related to baroreflex sensitivity. In some individuals a gradual rise in pressure enables baroreflex resetting without substantial baroreceptor loading, allowing MSNA to become elevated during mental stress [25]. White coat hypertension is a clear example that some people are simply more autonomic-reactive in anxiety situations and this phenotype is already present in children [26]. Central sympathetic hyperactivity exists in white coat hypertension, even though to a lesser degree than essential hypertension [27]. These findings indicate that stress and anxiety modulate the pattern of sympathetic activity, which, in turn, may confer greater cardiovascular risk. Stress, anxiety and sympathetic overactivity is a problem that needs to be more completely addressed. To understand the neurogenic background for stress-associated hypertension, there are two necessary steps: (i) to identify the central nuclei involved in organizing or modulating the autonomic responses to stress and (ii) to understand the influence each has on the other. These steps are crucial and may permit the discovery of specific dysregulations associated with chronic stress and anxiety.
Sympathetic Pathways Mediating the Cardiovascular Response During Stressful Events
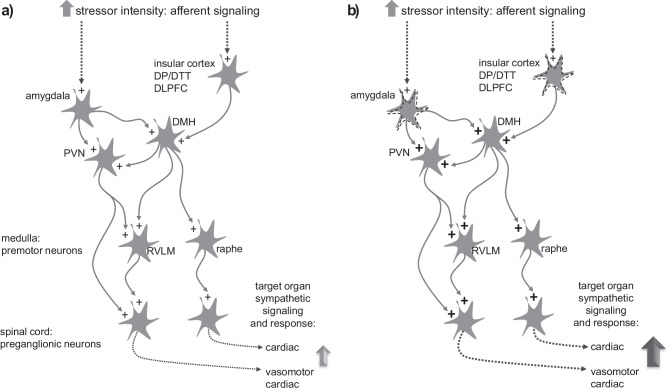
Determining the type and intensity of environmental stimuli is the key to trigger defense reactions. Firstly, pathways conducting exteroceptive inputs would constitute a setpoint. These potentially stressful sensory stimuli may be luminous, auditive, motor, vibrotactile, olfactive, thermal, and others. Despite having a generalized and unspecified component, the intensity and harmful potential of these environmental stimuli manifests uniquely within the brain of each individual, which is mainly based on genetic background and previous learned experiences. Exteroceptive stimuli generated from sensory organs are conducted to the central nervous system by sensory nerves reaching specific thalamic and cortical areas. Simultaneous activation of amygdala subregions determines whether a stimulus is considered a threat, or not, and adjusts the perception of intensity [28, 29]. If a risk situation is detected, baseline efferent sympathetic activity will increase accordingly (Fig. 3). The central command driven from the cortex and the amygdala will then organize the adequate response. Part of this response depends on projections reaching the dorsomedial hypothalamus (DMH). The DMH is currently considered a key region that controls the autonomic, respiratory, and neuroendocrine responses to psychological stress, as demonstrated in animals [30] and humans [31]. The DMH, through sympathetic premotor neuron projections, distributes the sympathetic discharge to target organs in response to the stimuli [30]. Premotor neurons are involved in the maintenance of sympathetic tone to target organs. Three groups of sympathetic premotor neurons have mostly been studied in the context of cardiovascular responses to stress, which include: the rostral ventrolateral medulla (RVLM), the paraventricular hypothalamus (PVN), and medullary raphe.
Fig. 3.
Top-down control of sympathetic responses in the presence of an emotional stressor. In this hypothetical scheme, the presence of a stressor stimuli evokes a prompt sympathetic response evoking cardiac, vasomotor, and renal changes that are necessary for an optimal functional defensive reaction (a). In the abnormal circuit (b), note that the hypothetical stressor of same magnitude now evokes an enhanced sympathetic response given that the stimulus is boosted by hyperreactive circuits in emotion related regions (here represented by amygdala and/or cortical regions), enhancing excitatory activity in downstream pathways. “Plus” symbols ( +) indicate excitatory activity; Dashed arrows represent indirect projections. DMH, dorsomedial hypothalamus; PVN, paraventricular hypothalamus; RVLM, rostral ventrolateral medulla; Raphe: medullary raphe neurons; DP/DTT dorsal peduncular cortex and dorsal tenia tecta; DLPFC, dorsolateral prefrontal cortex. See main text for details
Neurons located within the RVLM region are well known for their pivotal role in the generation of sympathetic tone controlling vasomotion and renal function [32, 33]. The PVN is a diencephalic region that contains neurons projecting to the RVLM and to sympathetic preganglionic neurons in the intermediolateral cell column (IML) [34]. Therefore, PVN tonic activity would increase noradrenergic-mediated vasoconstriction either directly via IML neurons or through its excitatory projections innervating the RVLM, respectively [35]. GABA is reported to be a dominant inhibitory neurotransmitter in the PVN. Studies indicated that hyperactivation resulting from reductions in gabaergic activity that controls PVN excitability are part of the puzzling combination resulting in essential hypertension [36]. Finally, evidence indicates that medullary raphe neurons are responsible for adjusting the cardiac and thermoregulatory responses to emotional stress [37•]. Whether or not raphe neurons may present dysregulated activity in hypertensive states remains to be determined.
In abnormal conditions, as a strengthened learning circuit, it is likely that some regions of the brain may become dysregulated with chronic stress and anxiety. Consequently, in such a condition, the sympathetic output to a stressor of a hypothetical stress of the same magnitude may result in an enhanced response to target organs when compared to a normal response (Fig. 3). This increased activation is probably modulated by a top-down processes, which are initiated in emotion related regions. It is important to observe that several brain regions may be involved in such phenomena; however, for clarity of discussion, we indicate some potential candidates, which include; amygdala, cortical regions, the PVN and the DMH.
Several studies suggest that abnormal amygdala responses could lead to abnormal sympathetic responses to different physiological systems, including the cardiovascular system [24, 38]. Amygdala is activated in response to the intensity of negative stimuli [39, 40], working as an “intensity detector” [28]. Studies using neuroimaging and physiological measurements showed that individuals with post-traumatic stress disorder (PTSD) present significantly more amygdala activation in response to negative emotional stimuli and greater sympathetic responses when compared to control individuals [41•]. Corroborating these findings, people with PTSD present greater risk for developing hypertension [42]. Interestingly, resting amygdalar metabolic activity is associated with abnormal cardiac function and perfusion in women, suggesting a closer link between emotional stress and cardiovascular disease in women [43]. This is quite relevant, considering that previous evidence indicates that women are more susceptible than men to emotional stress [44]. Therefore, anxiety or intense stress can establish a new baseline abnormal firing rate in amygdala neuronal circuits, thus leading to altered (enhanced) sympathetic responses. It is quite important to remember that the amygdala sends projections to different hypothalamic nuclei, including the PVN and the DMH. Evidence indicates that these are pathways in which the amygdala activates sympathetic outflow to the cardiovascular system during stressful events [45].
In addition to amygdala, fear and anxiety circuits within cortical regions are also involved in mediating sympathetic responses [46] and evidence indicates that specific regions may regulate chronic stress-induced cardiovascular susceptibility [47]. Kataoka and colleagues showed that psychosocial stress signals from emotion-related forebrain regions activate an excitatory (glutamatergic) pathway from the dorsal peduncular cortex and dorsal tenia tecta (DP/DTT), a medial prefrontal cortical area, to the DMH [37•]. The DP/DTT—DMH pathway is referred to as a central master neural pathway that drives autonomic and behavioral stress responses [37•]. Another potential cortical region involved is the dorsolateral prefrontal cortex (dlPFC). This region is primarily responsible for social and executive functioning [48, 49] and appears to be critical for on-going mood regulation with negative emotional judgment and major depressive disorders [50–52]. A recent study by Sesa-Ashton and colleagues showed that stimulation of dlPFC, either left or right, in awake humans resulted in cyclic modulation of MSNA, BP and heart rate, and a significant increase in BP [53]. It is suggested that the effects of dlPFC on MSNA could be potentially mediated through the DMH via connections through the insula [53], however functionally, this projection needs to be further studied.
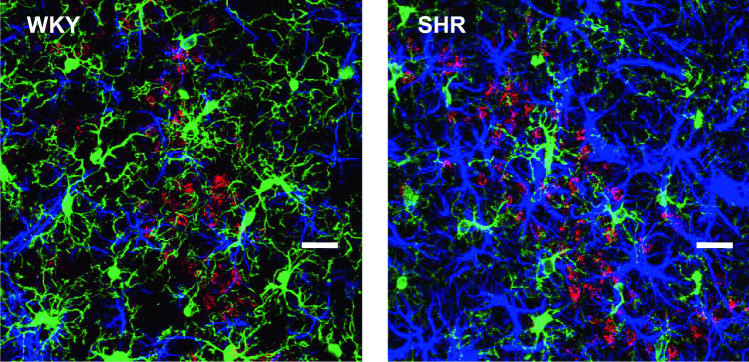
The insular cortex (IC) plays an important role in integrating emotional, memory and autonomic activity [54]. Human and animal studies have implicated the insula in processing positive and negative emotions, risk evaluation and probability of expected outcomes of an action [55, 56]. Neuroimaging studies have demonstrated that abnormal gray matter activity levels in the insula have been associated with anxiety and mood disorders [57]. Therefore, disorganized neuronal activity in the insula could enhance sympathetic activity. In this regard, we have recently shown that, in spontaneously hypertensive rats (SHR), the increased activity of the sympathetic nervous system may be related to alterations in the neurovascular unit of the IC, leading to neurogenic hypertension [58]. An increased density of astrocytes and de-ramified microglia is present in the IC of SHR. In addition, the densest number of neurons projecting to the DMH is also observed (Fig. 4). Interestingly, SHR are well known for their aggressive and anxious behavior and for the highest levels of physiological arousal to environmental stimuli [59], suggesting a direct correlation between anxious behavior and hypertension. Whether these IC neuroglial alterations are linked to SHR behavior and higher blood pressure levels, remains to be further confirmed. However, it is important to highlight that such alterations involve two regions that are directly involved in emotional behavior and autonomic cardiovascular control, the IC and the DMH (see below). It is also interesting to note that Wistar-Kyoto strain, from which SHR rats are derived and are considered the appropriate control group for SHR [59], are naturally more responsive to stress when compared to other strains, even though they present normal blood pressure levels [60]. These observations strongly indicate that a stressful behavioral profile composes the genetic basis for the development of hypertension in SHR rats. Whether or not chronic stress can also modify such neuroglial circuits in normal individuals, leading to altered or hyperactive responses to environment stressors remains to be determined.
Fig. 4.
SHR rats present marked alterations to the neurovascular unit within the IC (blue: astrocytes; green: microglia) and enhanced NMDA-mediated anatomical projections to the DMH (red: neurons retrogradely labeled from the DMH). These alterations may contribute to the maintenance of hypertension and altered behavior in SHR. See text for details. Scale bar: 30 µm. Experiments were conducted in accordance with NIH guidelines and carried out in agreement with Augusta University Institutional Animal Care and Use Committee Guidelines
Emotions processed in the IC can affect sympathetic activity through different pathways. The first includes direct projections to the RVLM [61]. The RVLM provides sympathoexcitatory drive to the heart, kidney, and is involved in vascular resistance. The second alternative could be via the amygdala. The IC and the amygdala are closely integrated with dense reciprocal connections [56]. An additional pathway could be via direct projections to the hypothalamus. In this regard, we have recently demonstrated that the IC sends dense excitatory glutamatergic projections to the DMH (Fig. 3). The IC-DMH signaling may be enhanced in specific conditions, leading to abnormal increases in cardiac sympathetic output [62]. Several previous studies demonstrated functional and anatomical connections between the DMH and RVLM [63]. Using a sophisticated neuroimaging approach in rats, Kono and colleagues found that the connection between the DMH and the RVLM serves as a chronological amplifier of stress-induced sympathetic excitation [64]. The study proposes a basic mechanism to understand the sustained augmentation of sympathetic activity following stressful life events [64].
Triggered by external stressors or internal stressful states, the increase in sympathetic activity is modulated by a top-down processes initiated in emotion related regions. Specifically, the amygdala and cortical regions detect stimuli intensity and type, resulting in the modulation of the autonomic response via the PVN and DMH. These hypothalamic regions then adjust the sympathetic output to the cardiovascular system via descending excitatory projections to downstream regions, including the RVLM and raphe (Fig. 3a). In the abnormal brain, the stimuli magnitude, type and intensity are magnified in some circuits, leading to an enhanced and sustained sympathetic response (Fig. 3b).
Role of ROS-Mediated Activation of Sympathetic Pathways During Stress
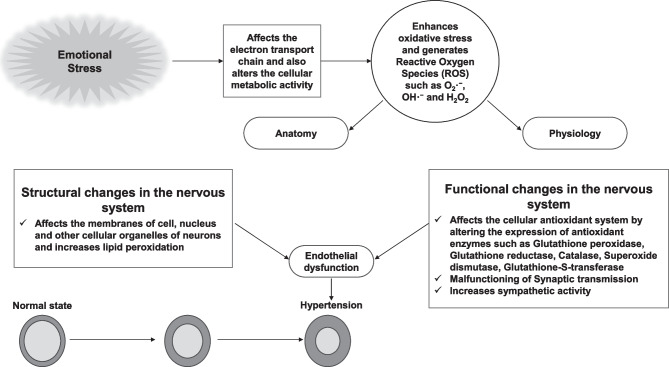
Emotional stress is a condition caused by various factors such as anxiety, anger, and sadness. Emotional stressors trigger many physiological alterations in the body (anatomically and physiologically) which can be deleterious for physical and mental health. Current cross-sectional studies indicate that there is a strong connection between anxiety and hypertension. When an individual is suffering from emotional stress, such as anxiety, the autonomic nervous system (ANS) is enhanced via the hypothalamic-pituitary axis, as well as emotional stressors that affect the physiology of the hypothalamus [6•, 65]. Malfunctioning of the hypothalamus affects the hypothalamic–pituitary–adrenal (HPA) axis. Stressful conditions also activate the sympathetic-adreno-medullar (SAM) axis. [66–68]. Emotional stress affects motor coordination, as well as other cognitive functions. It also alters the communication process in various regions of the brain [69]. The electron transport chain or respiratory chain located in the mitochondria pumps protons (H+) to generate membrane potential. During this process, a small quantity of reactive oxygen species (ROS) are generated, which are detoxified by specific antioxidant enzymes. During stressful conditions, neural mitochondria are negatively impacted, which in turn generates excessive free radicals or ROS. ROS generated during stressful conditions may cause neuronal degeneration and/or neuronal dysfunction in the brain [70–73]. The specific pathways that are vulnerable and affected by stress are illustrated in Fig. 3 and discussed in the previous section above. The majority of neuronal cell membrane is composed of polyunsaturated fatty acids, which are key substrates for ROS. Hence, during stressful conditions, neurons are highly vulnerable to lipid peroxidation via free radicals. Chronic stress enhances lipid peroxidation, while reducing the activities of antioxidant enzymes, such as glutathione peroxidase and catalase in rat brain [74]. A compromised antioxidant system negatively affects normal neuronal metabolic activity. The presence of free radicals or ROS during stressful conditions leads to vascular damage and endothelial dysfunction [75]. Stress-mediated enhanced sympathetic activity also contributes to the development of hypertension and other cardiovascular diseases [76, 77] (Fig. 5). Vascular damage, endothelial dysfunction, and elevated sympathetic activity are the major factors for the development of hypertension mediated by ROS that is generated during heightened stressful events.
Fig. 5.
Schematic diagram for the effects of stress on ROS-mediated changes in neural pathways related to enhanced sympathetic outflow. Emotional stress increases ROS levels in neural pathways while reducing the activities of antioxidant enzymes resulting in enhanced activation of neurons in the amygdala, paraventricular nucleus, and rostral ventrolateral medulla (see Fig. 3), overall resulting in increased sympathetic outflow and concomitant hypertension
Conclusions
Acute psychological stress induces elevation of blood pressure, which persists after the stress is relieved. There is increasing evidence of a positive association between comorbid anxiety and hypertension [6•]. The impact of modern urban stress on health is clear and research that addresses stress and anxiety needs strong support. There are numerous aspects that require further understanding, including stressors to emotional reactivity and cognitive coupling. Particularly in regard to hypertension, understanding how anxiety and stress determines the autonomic response in abnormal conditions is a critical part of the process. Among different aspects that may be involved, the participation of neuroglial and oxidative mechanisms in altered neuronal function is likely to be involved in enhanced sympathetic outflow. The significance of insular cortex-dorsomedial hypothalamic pathway in the evolution of enhanced overall sympathetic outflow seems relevant and deserves additional investigation. By identifying the nuclei, pathways, and the neurochemical peculiarities that are involved, new discoveries into the mechanisms of action, new molecular targets and drug development may be reached. These discoveries could provide alternatives that may help many patients that cannot tolerate side effects of the current drugs available.
Funding
The authors received funding from Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG APQ-01128–21), Conselho Nacional de Desenvolvimento Científico e Tecnológico do Brasil, CNPq, INCT NanoBiofar (PQ MAPF 308923/2021–9; CHXC 404079/2021–0) and PRPq UFMG. This work was also supported by grants NIH 5R01NS097818-04 (J. A. Filosa), NIH R01DK114663 and R01DK129311 and endowed McIntyre Professorship to Dr. Kaushik P. Patel.
Compliance with Ethical Standards
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Secondary Hypertension: Nervous System Mechanisms
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. Heart disease and stroke statistics—2022 update: a report from the American Heart Association. Circulation. 2022;145(8):e153–639. doi: 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 2.Nations U. revision of world urbanization prospects. United Nations Department of Economic and Social Affairs. 2018;
- 3.Szabo CP. Urbanization and mental health: a developing world perspective. Curr Opin Psychiatry. 2018;31(3):256–257. doi: 10.1097/YCO.0000000000000414. [DOI] [PubMed] [Google Scholar]
- 4.Zhou B, Carrillo-Larco RM, Danaei G, Riley LM, Paciorek CJ, Stevens GA, et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. The Lancet. 2021;398(10304):957–980. doi: 10.1016/S0140-6736(21)01330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esler M. Mental stress and human cardiovascular disease. Neurosci Biobehav Rev. 2017;74:269–276. doi: 10.1016/j.neubiorev.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Johnson HM. Anxiety and hypertension: is there a link? A literature review of the comorbidity relationship between anxiety and hypertension. Curr Hypertens Rep. 2019;21(9):1–7. doi: 10.1007/s11906-019-0972-5. [DOI] [PubMed] [Google Scholar]
- 7.Khera R, Light-McGroary K, Zahr F, Horwitz PA, Girotra S. Trends in hospitalization for takotsubo cardiomyopathy in the United States. Am Heart J. 2016;172:53–63. doi: 10.1016/j.ahj.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spruill TM. Chronic psychosocial stress and hypertension. Curr Hypertens Rep. 2010;12(1):10–16. doi: 10.1007/s11906-009-0084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah SMA, Mohammad D, Qureshi MFH, Abbas MZ, Aleem S. Prevalence, Psychological Responses and associated correlates of depression, anxiety and stress in a global population, during the coronavirus disease (COVID-19) pandemic. Community Ment Health J. 2021;57(1):101–110. doi: 10.1007/s10597-020-00728-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borg BA, Rabinak CA, Marusak HA. Violence exposure and mental health consequences among urban youth. Curr Psychol. 2021;1–10. [DOI] [PMC free article] [PubMed]
- 11.Ventimiglia I, Seedat S. Current evidence on urbanicity and the impact of neighbourhoods on anxiety and stress-related disorders. Curr Opin Psychiatry. 2019;32(3):248–253. doi: 10.1097/YCO.0000000000000496. [DOI] [PubMed] [Google Scholar]
- 12.Bandelow B, Michaelis S, Wedekind D. Treatment of anxiety disorders. Dialogues Clin Neurosci. 2017;19(2):93. doi: 10.31887/DCNS.2017.19.2/bbandelow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Silva T, Cosentino G, Ganji S, Riera-Gonzalez A, Hsia DS. Endocrine causes of hypertension. Curr Hypertens Rep. 2020;22(11):1–13. doi: 10.1007/s11906-020-01108-3. [DOI] [PubMed] [Google Scholar]
- 14.Saxena T, Ali AO, Saxena M. Pathophysiology of essential hypertension: an update. Expert Rev Cardiovasc Ther. 2018;16(12):879–887. doi: 10.1080/14779072.2018.1540301. [DOI] [PubMed] [Google Scholar]
- 15.Hahad O, Beutel M, Gori T, Schulz A, Blettner M, Pfeiffer N, et al. Annoyance to different noise sources is associated with atrial fibrillation in the Gutenberg Health Study. Int J Cardiol. 2018;264:79–84. doi: 10.1016/j.ijcard.2018.03.126. [DOI] [PubMed] [Google Scholar]
- 16.Trudel X, Brisson C, Gilbert-Ouimet M, Milot A. Psychosocial stressors at work and ambulatory blood pressure. Curr Cardiol Rep. 2018;20(12):1–9. doi: 10.1007/s11886-018-1070-z. [DOI] [PubMed] [Google Scholar]
- 17.Zeeb H, Hegewald J, Schubert M, Wagner M, Dröge P, Swart E, et al. Traffic noise and hypertension–results from a large case-control study. Environ Res. 2017;157:110–117. doi: 10.1016/j.envres.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 18.Carter JR, Goldstein DS. Sympathoneural and adrenomedullary responses to mental stress. Compr Physiol. 2015;5(1):119. doi: 10.1002/cphy.c140030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.da Silva AQG, Xavier CH, Campagnole-Santos MJ, Caligiorne SM, Baltatu OC, Bader M, et al. Cardiovascular responses evoked by activation or blockade of GABAA receptors in the hypothalamic PVN are attenuated in transgenic rats with low brain angiotensinogen. Brain Res. 2012;1448:101–110. doi: 10.1016/j.brainres.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 20.Saavedra JM, Benicky J. Brain and peripheral angiotensin II play a major role in stress. Stress. 2007;10(2):185–193. doi: 10.1080/10253890701350735. [DOI] [PubMed] [Google Scholar]
- 21.• Armstrong C, Kapolowicz MR. A preliminary investigation on the effects of atenolol for treating symptoms of anxiety. Mil Med. 2020;185(11–12):e1954–60. This study suggests that the beta-blocker atenolol may be well-tolerated and effective among persons with anxiety disorders. [DOI] [PubMed]
- 22.• Lee DL, Webb RC, Brands MW. Sympathetic and angiotensin-dependent hypertension during cage-switch stress in mice. Am J Physiol-Regul Integrat Compar Physiol. 2004;287(6):R1394–8. (This study shows that atenolol only moderately attenuates the tachycardic effect to acute stress and had no effect to blunt the initial increase in MAP.) [DOI] [PubMed]
- 23.Lambert E, Dawood T, Straznicky N, Sari C, Schlaich M, Esler M, et al. Association between the sympathetic firing pattern and anxiety level in patients with the metabolic syndrome and elevated blood pressure. J Hypertens. 2010;28(3):543–550. doi: 10.1097/HJH.0b013e3283350ea4. [DOI] [PubMed] [Google Scholar]
- 24.Holwerda SW, Luehrs RE, Gremaud AL, Wooldridge NA, Stroud AK, Fiedorowicz JG, et al. Relative burst amplitude of muscle sympathetic nerve activity is an indicator of altered sympathetic outflow in chronic anxiety. J Neurophysiol. 2018;120(1):11–22. doi: 10.1152/jn.00064.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El Sayed K, Macefield VG, Hissen SL, Joyner MJ, Taylor CE. Blood pressure reactivity at onset of mental stress determines sympathetic vascular response in young adults. Physiol Rep. 2018;6(24):e13944. doi: 10.14814/phy2.13944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krmar RT. White-coat hypertension from a paediatric perspective. Acta Paediatr. 2019;108(1):44–49. doi: 10.1111/apa.14416. [DOI] [PubMed] [Google Scholar]
- 27.Smith PA, Graham LN, Mackintosh AF, Stoker JB, Mary DA. Sympathetic neural mechanisms in white-coat hypertension. J Am Coll Cardiol. 2002;40(1):126–132. doi: 10.1016/S0735-1097(02)01931-9. [DOI] [PubMed] [Google Scholar]
- 28.Bonnet L, Comte A, Tatu L, Millot JL, Moulin T, Medeiros de Bustos E. The role of the amygdala in the perception of positive emotions: an “intensity detector”. Front Behav Neurosci. 2015;9:178. [DOI] [PMC free article] [PubMed]
- 29.Namburi P, Al-Hasani R, Calhoon GG, Bruchas MR, Tye KM. Architectural representation of valence in the limbic system. Neuropsychopharmacology. 2016;41(7):1697–1715. doi: 10.1038/npp.2015.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fontes MAP, Filho ML, Machado NLS, Paula CA de, Cordeiro LMS, Xavier CH, et al. Asymmetric sympathetic output: The dorsomedial hypothalamus as a potential link between emotional stress and cardiac arrhythmias. Autonomic Neuroscience: Basic and Clinical. 1° de novembro de 2017;207:22–7. [DOI] [PubMed]
- 31.Henderson LA, Macefield VG. The role of the dorsomedial and ventromedial hypothalamus in regulating behaviorally coupled and resting autonomic drive. Em: Handbook of Clinical Neurology. Elsevier. 2021;187–200. [DOI] [PubMed]
- 32.Dampney RAL, Coleman MJ, Fontes MAP, Hirooka Y, Horiuchi J, Li YW, et al. Central mechanisms underlying short-and long-term regulation of the cardiovascular system. Clin Exp Pharmacol Physiol. 2002;29(4):261–268. doi: 10.1046/j.1440-1681.2002.03640.x. [DOI] [PubMed] [Google Scholar]
- 33.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7(5):335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 34.Shafton AD, Ryan A, Badoer E. Neurons in the hypothalamic paraventricular nucleus send collaterals to the spinal cord and to the rostral ventrolateral medulla in the rat. Brain Res. 1998;801(1–2):239–243. doi: 10.1016/S0006-8993(98)00587-3. [DOI] [PubMed] [Google Scholar]
- 35.Zheng H, Katsurada K, Nandi S, Li Y, Patel KP. A critical role for the paraventricular nucleus of the hypothalamus in the regulation of the volume reflex in normal and various cardiovascular disease states. Curr Hyperten Rep. 2022;1–12. [DOI] [PMC free article] [PubMed]
- 36.Zhang K, Li YF, Patel KP. Reduced endogenous GABA-mediated inhibition in the PVN on renal nerve discharge in rats with heart failure. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2002;282(4):R1006–R1015. doi: 10.1152/ajpregu.00241.2001. [DOI] [PubMed] [Google Scholar]
- 37.• Kataoka N, Shima Y, Nakajima K, Nakamura K. A central master driver of psychosocial stress responses in the rat. Science. 2020;367(6482):1105–12. Points out the DMH as a key relay of a central master neural pathway that drives autonomic and behavioral stress responses. [DOI] [PubMed]
- 38.Calhoon GG, Tye KM. Resolving the neural circuits of anxiety. Nat Neurosci. 2015;18(10):1394–1404. doi: 10.1038/nn.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Canli T, Zhao Z, Brewer J, Gabrieli JD, Cahill L. Event-related activation in the human amygdala associates with later memory for individual emotional experience. J Neurosci. 2000;20(19):RC99–RC99. [DOI] [PMC free article] [PubMed]
- 40.Taylor SF, Liberzon I, Koeppe RA. The effect of graded aversive stimuli on limbic and visual activation. Neuropsychologia. 2000;38(10):1415–1425. doi: 10.1016/S0028-3932(00)00032-4. [DOI] [PubMed] [Google Scholar]
- 41.• Ressler K, Berretta S, Bolshakov VY, Rosso IM, Meloni EG, Rauch SL, et al. Post-traumatic stress disorder: clinical and translational neuroscience from cells to circuits. Nat Rev Neurol. 2022;18(5):273–88. Shows that individuals with PTSD present significantly more amygdala activation in response to negative emotional stimuli and greater sympathetic responses when compared to control individuals. [DOI] [PMC free article] [PubMed]
- 42.Brudey C, Park J, Wiaderkiewicz J, Kobayashi I, Mellman TA, Marvar PJ. Autonomic and inflammatory consequences of posttraumatic stress disorder and the link to cardiovascular disease. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2015;309(4):R315–R321. doi: 10.1152/ajpregu.00343.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fiechter M, Roggo A, Burger IA, Bengs S, Treyer V, Becker A, et al. Association between resting amygdalar activity and abnormal cardiac function in women and men: a retrospective cohort study. European Heart Journal-Cardiovascular Imaging. 2019;20(6):625–632. doi: 10.1093/ehjci/jez047. [DOI] [PubMed] [Google Scholar]
- 44.Vaccarino V, Bremner JD. Behavioral, emotional and neurobiological determinants of coronary heart disease risk in women. Neurosci Biobehav Rev. 2017;74:297–309. doi: 10.1016/j.neubiorev.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Z, Liu N, Xiao J, Wang Y, Dong R. The amygdala via the paraventricular nucleus regulates asthma attack in rats. CNS Neurosci Ther. 2020;26(7):730–740. doi: 10.1111/cns.13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tovote P, Fadok JP, Lüthi A. Neuronal circuits for fear and anxiety. Nat Rev Neurosci. 2015;16(6):317–331. doi: 10.1038/nrn3945. [DOI] [PubMed] [Google Scholar]
- 47.Schaeuble D, Myers B. Cortical–hypothalamic integration of autonomic and endocrine stress responses. Front Physiol. 2022;151. [DOI] [PMC free article] [PubMed]
- 48.Holper L, Burke CJ, Fausch C, Seifritz E, Tobler PN. Inequality signals in dorsolateral prefrontal cortex inform social preference models. Social Cognitive and Affective Neuroscience. 2018;13(5):513–524. doi: 10.1093/scan/nsy020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuan P, Raz N. Prefrontal cortex and executive functions in healthy adults: a meta-analysis of structural neuroimaging studies. Neurosci Biobehav Rev. 2014;42:180–192. doi: 10.1016/j.neubiorev.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bench C, Frackowiak R, Dolan RJ. Changes in regional cerebral blood flow on recovery from depression. Psychol Med. 1995;25(2):247–261. doi: 10.1017/S0033291700036151. [DOI] [PubMed] [Google Scholar]
- 51.Grimm S, Beck J, Schuepbach D, Hell D, Boesiger P, Bermpohl F, et al. Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: an fMRI study in severe major depressive disorder. Biol Psychiat. 2008;63(4):369–376. doi: 10.1016/j.biopsych.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 52.Zhong M, Wang X, Xiao J, Yi J, Zhu X, Liao J, et al. Amygdala hyperactivation and prefrontal hypoactivation in subjects with cognitive vulnerability to depression. Biol Psychol. 2011;88(2–3):233–242. doi: 10.1016/j.biopsycho.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 53.Sesa-Ashton G, Wong R, McCarthy B, Datta S, Henderson LA, Dawood T, et al. Stimulation of the dorsolateral prefrontal cortex modulates muscle sympathetic nerve activity and blood pressure in humans. Cereb Cortex Commun. 2022;3(2):tgac017. [DOI] [PMC free article] [PubMed]
- 54.Oppenheimer S, Cechetto D. The insular cortex and the regulation of cardiac function. Compr Physiol. 2016;6:1081–1133. doi: 10.1002/cphy.c140076. [DOI] [PubMed] [Google Scholar]
- 55.Gogolla N. The insular cortex. Curr Biol. 2017;27(12):R580–R586. doi: 10.1016/j.cub.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 56.Moraga-Amaro R, Stehberg J. The insular cortex and the amygdala: shared functions and interactions. The amygdala: a discrete multitasking manager. 2012;231–56.
- 57.Gasquoine PG. Contributions of the insula to cognition and emotion. Neuropsychol Rev. 2014;24(2):77–87. doi: 10.1007/s11065-014-9246-9. [DOI] [PubMed] [Google Scholar]
- 58.Marins FR, Iddings JA, Fontes MA, Filosa JA. Evidence that remodeling of insular cortex neurovascular unit contributes to hypertension-related sympathoexcitation. Physiol Rep. 2017;5(5):e13156. doi: 10.14814/phy2.13156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tucker DC, Johnson AK. Behavioral correlates of spontaneous hypertension. Neurosci Biobehav Rev. 1981;5(4):463–471. doi: 10.1016/0149-7634(81)90016-6. [DOI] [PubMed] [Google Scholar]
- 60.Paré WP. The performance of WKY rats on three tests of emotional behavior. Physiol Behav. 1992;51(5):1051–1056. doi: 10.1016/0031-9384(92)90091-F. [DOI] [PubMed] [Google Scholar]
- 61.Marins FR, Limborço-Filho M, Xavier CH, Biancardi VC, Vaz GC, Stern JE, et al. Functional topography of cardiovascular regulation along the rostrocaudal axis of the rat posterior insular cortex. Clin Exp Pharmacol Physiol. 2016;43(4):484–493. doi: 10.1111/1440-1681.12542. [DOI] [PubMed] [Google Scholar]
- 62.Marins FR, Limborço‐Filho M, Iddings JA, Xavier CH, Biancardi VC, Stern JE, et al. Tachycardia evoked from insular stroke in rats is dependent on glutamatergic neurotransmission in the dorsomedial hypothalamus. Eur J Neurol. 2021;28(11):3640–49. [DOI] [PubMed]
- 63.Fontes MAP, Xavier CH, Marins FR, Limborço-Filho M, Vaz GC, Müller-Ribeiro FC, et al. Emotional stress and sympathetic activity: contribution of dorsomedial hypothalamus to cardiac arrhythmias. Brain Research março de. 2014;1554:49–58. doi: 10.1016/j.brainres.2014.01.043. [DOI] [PubMed] [Google Scholar]
- 64.Kono Y, Yokota S, Fukushi I, Arima Y, Onimaru H, Okazaki S, et al. Structural and functional connectivity from the dorsomedial hypothalamus to the ventral medulla as a chronological amplifier of sympathetic outflow. Sci Rep. 2020;10(1):1–11. doi: 10.1038/s41598-020-70234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grimsrud A, Stein DJ, Seedat S, Williams D, Myer L. The association between hypertension and depression and anxiety disorders: results from a nationally-representative sample of South African adults. PLoS ONE. 2009;4(5):e5552. doi: 10.1371/journal.pone.0005552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.DiMicco JA, Samuels BC, Zaretskaia MV, Zaretsky DV. The dorsomedial hypothalamus and the response to stress: part renaissance, part revolution. Pharmacol Biochem Behav. 2002;71(3):469–480. doi: 10.1016/S0091-3057(01)00689-X. [DOI] [PubMed] [Google Scholar]
- 67.Mifsud KR, Reul JM. Mineralocorticoid and glucocorticoid receptor-mediated control of genomic responses to stress in the brain. Stress. 2018;21(5):389–402. doi: 10.1080/10253890.2018.1456526. [DOI] [PubMed] [Google Scholar]
- 68.Pruessner JC, Dedovic K, Pruessner M, Lord C, Buss C, Collins L, et al. Stress regulation in the central nervous system: evidence from structural and functional neuroimaging studies in human populations-2008 Curt Richter Award Winner. Psychoneuroendocrinology. 2010;35(1):179–191. doi: 10.1016/j.psyneuen.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 69.Savtchouk I, Liu SJ. Remodeling of synaptic AMPA receptor subtype alters the probability and pattern of action potential firing. J Neurosci. 2011;31(2):501–511. doi: 10.1523/JNEUROSCI.2608-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hovatta I, Juhila J, Donner J. Oxidative stress in anxiety and comorbid disorders. Neurosci Res. 2010;68(4):261–275. doi: 10.1016/j.neures.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 71.Lambert AJ, Brand MD. Reactive oxygen species production by mitochondria. Mitochondrial DNA. 2009;165–81. [DOI] [PubMed]
- 72.Picard M, McEwen BS. Psychological stress and mitochondria: a systematic review. Psychosom Med. 2018;80(2):141. doi: 10.1097/PSY.0000000000000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kumar A, Rinwa P, Kaur G, Machawal L. Stress: Neurobiology, consequences and management. Journal of pharmacy & bioallied sciences. 2013;5(2):91. doi: 10.4103/0975-7406.111818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Che Y, Zhou Z, Shu Y, Zhai C, Zhu Y, Gong S, et al. Chronic unpredictable stress impairs endogenous antioxidant defense in rat brain. Neurosci Lett. 2015;584:208–213. doi: 10.1016/j.neulet.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 75.Montezano AC, Dulak-Lis M, Tsiropoulou S, Harvey A, Briones AM, Touyz RM. Oxidative stress and human hypertension: vascular mechanisms, biomarkers, and novel therapies. Can J Cardiol. 2015;31(5):631–641. doi: 10.1016/j.cjca.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 76.Hering D, Lachowska K, Schlaich M. Role of the sympathetic nervous system in stress-mediated cardiovascular disease. Curr Hypertens Rep. 2015;17(10):1–9. doi: 10.1007/s11906-015-0594-5. [DOI] [PubMed] [Google Scholar]
- 77.Lambert EA, Lambert GW. Stress and its role in sympathetic nervous system activation in hypertension and the metabolic syndrome. Curr Hypertens Rep. 2011;13(3):244–248. doi: 10.1007/s11906-011-0186-y. [DOI] [PubMed] [Google Scholar]