Abstract
Purpose
Periodontitis and coronavirus disease (COVID-19) share risk factors and activate similar immunopathological pathways, intensifying systemic inflammation. This study investigated the clinical, immunological and microbiological parameters in individuals with COVID-19 and controls, exploring whether periodontitis-driven inflammation contributes to worsening COVID-19 endpoints.
Methods
Case (positive RT-PCR for SARS-CoV-2) and control (negative RT-PCR) individuals underwent clinical and periodontal assessments. Salivary levels of TNF-α, IL-6, IL-1β, IL-10, OPG, RANKL, neutrophil extracellular traps, and subgingival biofilm were analyzed at two timepoints. Data on COVID-19-related outcomes and comorbidity information were evaluated from medical records.
Results
Ninety-nine cases of COVID-19 and 182 controls were included for analysis. Periodontitis was associated with more hospitalization (p = 0.009), more days in the intensive care unit (ICU) (p = 0.042), admission to the semi-ICU (p = 0.047), and greater need for oxygen therapy (p = 0.042). After adjustment for confounders, periodontitis resulted in a 1.13-fold increase in the chance of hospitalization. Salivary IL-6 levels (p = 0.010) were increased in individuals with COVID-19 and periodontitis. Periodontitis was associated with increased RANKL and IL-1β after COVID-19. No significant changes were observed in the bacterial loads of the periodontopathogens Porphyromona gingivalis, Aggregatibacter actinomycetemcomitans, Tanerella forsythia, and Treponema denticola.
Conclusions
Periodontitis was associated with worse COVID-19 outcomes, suggesting the relevance of periodontal care to reduce the burden of overall inflammation. Understanding the crosstalk between SARS-CoV-2 infection and chronic conditions such as periodontitis that can influence disease outcome is important to potentially prevent complications of COVID-19.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10266-023-00811-2.
Keywords: COVID-19, Cytokines, Periodontal diseases, Periodontitis, SARS-CoV-2
Introduction
The SARS-CoV-2 outbreak has set up an unprecedented pandemic, causing the collapse of healthcare systems across the world due to the high number of hospitalizations and deaths [1]. Although high vaccination rates appear to be leading to disease control in some countries, inequities in vaccine distribution worldwide, combined with the virus ability to mutate, indicate that the emergence of new variants and outbreaks cannot be ruled out [2]. Multiple factors (e.g., senility, obesity, and underlying chronic conditions) have been associated with greater odds of worse COVID-19 endpoints [3]. However, crosstalk with other inflammatory and infectious diseases can boost the systemic inflammatory burden and modify the course of infection, resulting in a variety of infection phenotypes [4].
Periodontitis is a highly prevalent infectious-inflammatory oral disease [5]. Periodontal pockets serve as a reservoir of microorganisms, including viruses, fungi, and bacteria that can enter the bloodstream and/or be aspirated [6]. Furthermore, individuals with periodontitis are threefold more likely to develop nosocomial pneumonia than those who are periodontally healthy [7].
Poor oral health status and periodontitis were linked to worse outcomes of COVID-19 [8–12]. A possible explanation is that SARS-CoV-2 infects and replicates in oral tissues that express angiotensin converting enzyme-2 (ACE-2) such as the periodontium [13], epithelium, salivary glands [14], and a pre-existing pathological condition may exacerbate these processes. Moreover, periodontitis and COVID-19 activate similar immunopathological pathways, including the overexpression of cytokines and neutrophil extracellular traps (NET), intensifying systemic inflammation [15–19]. Nevertheless, the literature on the issue is sparse and the pathophysiological mechanisms of the COVID-19-periodontitis association have not yet been defined. Our hypothesis is that periodontitis-driven inflammation contributes to worse COVID-19 endpoints. Herein, we investigated the clinical, immunological and microbiological parameters of individuals with COVID-19 and controls.
Materials and methods
Study design, sample characteristics, and ethical clearance
A population-based case control study was carried out in the city of Belo Horizonte, Brazil, during the COVID-19 outbreak, from September 2020 to May 2021. Individuals examined in the special wards for COVID-19 of two referral services participated in this study: Hospital das Clínicas of the Federal University of Minas Gerais and Hospital Eduardo de Menezes. Subjects who tested positive for SARS-CoV-2 in RT-PCR by nasal swab were included in the case group. The control group consisted of individuals who had negative salivary RT-PCR for SARS-CoV-2. In this group, individuals had attended the Dental Urgency Department of the School of Dentistry at the Federal University of Minas Gerais from January 2021 to March 2021. The report of this study conformed to the STROBE statement. The study was approved by the Ethics Committee of the Institution (#37273320.0.0000.5149; #37273320.0.3002.5124).
Sample size calculation and eligibility criteria
The two groups were matched for age and sex at a proportion of 1.8 control for every case. Inclusion criteria were participants of any age group, with at least six teeth, and no contraindication for periodontal evaluation [7, 20–22]. Individuals were excluded in case of pregnancy, and if they had been treated for periodontal disease in the last 3 months. In addition, in the COVID-19 group patients were also excluded if they did not have a positive RT-PCR determined by a nasal swab, or if they were under mechanical ventilation or using non-rebreather masks.
Demographic and behavioral characteristics
Participants provided information on schooling, family income, habits (i.e., smoking and alcohol consumption), height and weight, oral hygiene habits, previous dental treatments and, in the case of those infected with COVID-19, signs and symptoms presented. Information regarding comorbidities and medication were also obtained from medical records. Data on serum levels of lymphocytes, neutrophils, eosinophils, C-reactive protein, and D-dimer during hospitalization were collected from the medical records.
Clinical and periodontal assessment—definition of periodontitis and staging
Clinical and periodontal examinations were performed by a single calibrated examiner (L.M.B.). Third molars, teeth with extensive dental caries, absence of a clear cemento-enamel junction, severe gingival clinical alterations, iatrogenic restorative procedures, and excessive presence of calculus precluding periodontal probing were excluded [23].
Clinical evaluation was undertaken using a head light, clinical mirror, explorer, periodontal probe (UNC-15, Hu-Friedy, Chicago, IL, USA), and gauze. Initially, the plaque index [24] and the Decayed, Missing, and Filled Teeth (DMFT) index were recorded. The following periodontal parameters were assessed on all included teeth: probing depth (PD), clinical attachment level (CAL), and bleeding on probing (BOP). Six measurements were performed per tooth (circumferential probing) and the highest value for each site (mesial, buccal, distal, and lingual) was recorded, for a total of four values for each tooth. Individuals were classified according to the 2018 classification for periodontitis as healthy and stages I, II, III, or IV [25]. For data analysis, participants of both groups (cases and controls) were further classified into individuals without periodontitis [periodontal health and stage I (borderline between gingivitis and periodontitis) were grouped] and individuals with periodontitis (stages II, III or IV).
COVID-19-related outcomes
COVID-19 severity was estimated using the following surrogate clinical endpoints: use of oxygen, hospitalization, admission to the intensive care unit (ICU), admission to the semi-intensive care unit (SICU), use of mechanical ventilation, length of hospitalization, length of ICU admission, length of mechanical ventilation, and death [8–12].
Saliva sample collection
In both groups, saliva samples were collected by the unstimulated technique for 5 min into sterile 50 mL tubes during evaluation (T1) [26]. At a second timepoint (T2), saliva samples were collected from individuals who had had COVID-19 (mean 106.2 days after initial diagnosis). Saliva samples were stored at − 80 °C.
SARS-CoV-2 RNA extraction and RT-PCR assay
For SARS-CoV-2 RNA detection, RT-PCR assay was performed using saliva samples diluted in viral transport medium, as described previously [27]. Briefly, viral RNA extraction was executed using the Quick-RNA™ Viral Kit (cat#R1035, ZYMO Research, Irvine, CA USA), and later amplified using the Multiplex Luna® Universal Probe One-Step RT-PCR Kit (New England Biolabs, Bioscience, Ipswich, MA, USA). Reactions were analyzed using the Applied ABI 7500 equipment (Applied Biosystems™, Foster City, CA, USA) at 55 °C for 1 min (45 cycles). The diagnosis of COVID-19 was performed according to CDC recommendations [28].
Quantification of molecules involved in inflammation and NET
Salivary concentrations of tumor necrosis factor-alpha (TNF-α), interleukin (IL)-6, IL-1β, IL-10, osteoprotegerin (OPG), and receptor activator of nuclear factor Kappa-B ligand (RANKL) were measured by an ELISA-based capture assay using DuoSet® ELISA kits—R&D Systems, Minneapolis, MN, USA). Optical density was determined at 450 nm using a FlexStation 3 Microplate Reader (Molecular Devices, San Jose, CA, USA). The Quant-iT™ PicoGreen® dsDNA Assay Kit (Invitrogen, Carlsbad, CA, USA) was used to quantify NET levels by identifying the myeloperoxidase (MPO)–DNA complex in saliva [29]. Fluorescence intensity (excitation at 488 nm and emission at 525 nm wavelength) was determined with a FlexStation 3 Microplate Reader.
Biofilm collection and bacterial DNA quantification
Subgingival biofilm samples were collected from the five sites with the highest PD using sterile absorbent paper cones (#30, Dentsply®, New York, NY, USA) introduced into each periodontal site for 60 s. The cones were then placed in a sterile microtube and stored at − 80 °C. A second sample of biofilm was collected from the COVID-19 patients at T2. Bacterial DNA quantification procedures were performed according to previously published methods [30, 31]. Briefly, bacterial DNA from periodontal biofilm samples was extracted by the DNA Purification System (QIAamp UCP Pathogen, Qiagen, Hilden, Germany). Total bacterial load was quantified using the microbial DNA qPCR assay kit (QIAgen, Hilden, Germany) for the 16S rRNA gene. RT-PCR analyses were performed in a MiniOpticon system (BioRad) using SybrGreen MasterMix (Invitrogen), specific primers (Porphyromonas gingivalis: sense TGCAACTTGCCTTACAGAGGG, antisense ACTCGTATCGCCCGTTATTC; Tanerella forsythia: sense GGGTGAGTAACGCGTATGTAACCT, antisense ACCCATCCGCAACCAATAAA; Treponema denticola: sense AGAGCAAGCTCTCCCTTACCGT, antisense TAAGGGCGGCTTGAAATAATGA; Aggregatibacter actinomycetemcomitans: sense ATGCCAACTTGACGTTAAAT, antisense AAACCCATCTCTGAGTTCTTCT), and 5 ng of DNA in each reaction. Calculations for determining each target microbe level were made from triplicate measurements of the target gene in comparison with standard curves for each target.
Data analysis
The Statistical Package for the Social Sciences software (version 22.0, IBM Inc., New Armonk, NJ, USA) and GraphPad Prism software (version 7.00, Graph-Pad software, La Jolla, CA, USA) were used for the description and statistics of the case and control groups. Intragroup comparisons between individuals with or without periodontitis were performed. The chi-square test was performed to analyze categorical variables and the Student T test was performed for quantitative variables. In the COVID-19 group, comparisons (with the chi-square test and the Student T test) between individuals with and without periodontitis were performed for COVID-19-related outcomes. A binary regression was performed to examine the influence of periodontitis on COVID-19-related outcomes, controlling for confounding variables. Comparisons of cytokine concentrations between individuals with or without periodontitis were performed with Student T tests. The differences (ΔT2–T1) for the variables cytokine concentrations and bacterial loads during (T1) and after (T2) COVID-19 infection (cytokine variations and bacterial load variations) were calculated with Paired T tests. Before the regression models, an explanatory analysis was conducted to identify confounders for each outcome. Variables with p value ≤ 0.20 were included in the models. For the regressions, the significance level was set at p value ≤ 0.05.
Results
Demographic characteristics, oral health-related behaviors and oral health outcomes
A total of 221 individuals on the COVID-19 wards were assessed for eligibility, 99 participants met the inclusion criteria and were included in the case group; age ranged from 16 to 74 years. Two hundred-and-forty-two patients were assessed to compose the control group and 182 were included; age ranged from 20 to 74 years. There were no differences between groups regarding age, sex, family income, schooling, oral hygiene habits, and body mass index (BMI). Smoking and alcoholism were more prevalent in the control group. Individuals in the control group had worse oral and periodontal status (higher DMFT index, higher number of tooth losses, as well as higher mean PD, CAL, and BOP) than individuals in the COVID-19 group. However, no difference between groups was observed for the prevalence of periodontitis (Supplementary Table 1).
In both groups, individuals with periodontitis were middle-aged/older adults and had increased BMI compared to individuals with no periodontitis. In the COVID-19 group, individuals with periodontitis had a lower family income and lower schooling than those without periodontitis. In the control group, most participants with periodontitis were males and most participants without periodontitis were women. There was no difference in tooth brushing and flossing frequency between patients with and without periodontitis in both groups (p > 0.05) (Table 1).
Table 1.
Demographic characteristics and oral health-related behaviours of COVID-19 and control participants with and without periodontitis
| Variables | Control | COVID-19 | ||||
|---|---|---|---|---|---|---|
| No periodontitis | Periodontitis | p value | No periodontitis | Periodontitis | p value | |
| Age, n (%) | ||||||
| < 49 years | 56 (58.9) | 36 (41.4) | 0.026* | 34 (65.4) | 15 (31.9) | 0.001* |
| ≥ 50 years | 39 (41.1) | 51 (58.6) | 18 (34.6) | 32 (68.1) | ||
| Sex, n (%) | ||||||
| Male | 40 (42.1) | 56 (64.4) | 0.003* | 28 (53.8) | 24 (51.1) | 0.842* |
| Female | 55 (57.9) | 31 (35.6) | 24 (46.2) | 23 (48.9) | ||
| Family income, n (%) | ||||||
| ≤ 2 MWs | 31 (40.3) | 36 (45.6) | 0.237** | 20 (38.5) | 32 (68.1) | 0.003** |
| 3–5 MWs | 25 (32.5) | 29 (36.7) | 22 (42.3) | 12 (25.5) | ||
| ≥ 5 MWs | 21 (27.3) | 14 (17.7) | 10 (19.2) | 3 (6.4) | ||
| Schooling, n (%) | ||||||
| ≤ 8 years | 24 (31.2) | 34 (42.5) | 0.186* | 11 (21.2) | 25 (53.2) | 0.002* |
| > 8 years | 53 (68.8) | 46 (57.5) | 41 (78.8) | 22 (46.8) | ||
| Tooth brushing, n (%) | ||||||
| Once/day | 2 (2.6) | 3 (3.8) | 0.390** | 3 (5.8) | 3 (6.4) | 0.821** |
| Twice/day | 22 (28.6) | 36 (45.0) | 15 (28.8) | 10 (21.3) | ||
| 3 times/day | 53 (68.8) | 33 (41.3) | 28 (53.8) | 30 (63.8) | ||
| > 3 times/day | 0 (0.0) | 8 (10.0) | 6 (11.5) | 4 (8.5) | ||
| Flossing, n (%) | ||||||
| No | 28 (39.4) | 38 (52.8) | 0.132* | 22 (42.3) | 24 (51.1) | 0.424* |
| Yes | 43 (60.6) | 34 (47.2) | 30 (57.7) | 23 (48.9) | ||
| Smoking, n (%) | ||||||
| No | 55 (87.3) | 52 (76.5) | 0.111** | 40 (76.9) | 33 (70.2) | 0.262** |
| Yes | 6 (9.5) | 11 (16.2) | 3 (5.8) | 0 (0.0) | ||
| Ex-smoker | 2 (3.2) | 5 (7.4) | 9 (17.3) | 14 (29.8) | ||
| BMI (kg/m2) (median, min–max) | 24.60 (21.72–31.47) | 29.29 (20.38–41.21) | 0.086**** | 25.78 (14.35–45.72) | 29.72 (17.9–57.14) | 0.001**** |
BMI body mass index, max maximum, min minimum, MW minimum wage
*Pearson chi-square test; **Linear by linear; ***Fisher’s Exact; ****Mann–Whitney test. Bold means statistically significant at p ≤ 0.05
Control group: age (n = 182); sex (n = 182); family income (n = 156); schooling (n = 157); tooth brushing (n = 157); flossing (n = 143); smoking (n = 125); and BMI (n = 92)
COVID-19 group: age (n = 99); sex (n = 99); family income (n = 99); schooling (n = 99); tooth brushing (n = 99); flossing (n = 99); smoking (n = 96); and BMI (n = 99)
The prevalence of depression, pulmonary and kidney diseases was higher among individuals in the COVID-19 group compared to those in the control group (Supplementary Table 2). The main symptoms of COVID-19 reported were respiratory symptoms (76.8%), fever (54.5%), ageusia (52.5%), anosmia (49.5%), gastrointestinal symptoms (40.4%), tiredness/malaise (21.2%), and tachycardia (2.0%).
Periodontal and clinical status
In both groups, patients with periodontitis had worse oral health status, as the measurements of DMFT index, PD, and CAL among them were higher than among individuals without periodontitis. Tooth loss and BOP were also increased in individuals with periodontitis in both groups. In the COVID-19 group, plaque index was worse in patients with periodontitis than in patients without periodontitis (p < 0.001) (Table 2).
Table 2.
Clinical characteristics of COVID-19 and control participants with and without periodontitis
| Variables | Control | COVID-19 | ||||
|---|---|---|---|---|---|---|
| No periodontitis | Periodontitis | p value | No periodontitis | Periodontitis | p value | |
| DMFT index (median, min–max) | 19 (4–25) | 18 (8–24) | 0.003* | 10 (0–25) | 18 (0–27) | < 0.001* |
| Tooth loss (median, min–max) | 2 (0–10) | 5 (0–19) | < 0.001* | 1 (0–22) | 10 (0–22) | < 0.001* |
| PD (mm) (median, min–max) | 1.93 (1.59–2.33) | 2.43 (1.96–7.11) | < 0.001* | 1.79 (1.32–2.41) | 2.44 (1.65–3.48) | < 0.001* |
| CAL (mm) (median, min–max) | 1.98 (1.51–2.33) | 2.49 (2.01–9.50) | < 0.001* | 1.86 (1.35–3.01) | 2.55 (1.75–7.45) | < 0.001* |
| BOP, % (median, min–max) | 3.85 (0–19) | 8.6 (0–33) | < 0.001* | 0 (0–8) | 1.67 (0–28) | < 0.001* |
| Salivary flow (ml/min) (median, min–max) | 0 (0–2) | 0 (0–2) | 0.098* | 0.65 (0.10–1.9) | 0.80 (0.10–1.64) | 0.716* |
| Plaque index, n (%) | ||||||
| Excellent/good | 18 (94.7) | 16 (76.2) | 0.186*** | 46 (88.5) | 26 (55.3) | < 0.001** |
| Poor/terrible | 1 (5.3) | 5 (23.8) | 6 (11.5) | 21 (44.7) | ||
BOP bleeding on probing, CAL clinical attachment level, max maximum, DMFT decayed, missing, and filled teeth, min minimum, ml/min milliliters per minute, mm millimeter, PD probing depth
*Mann–Whitney test; **Pearson chi-square test; ***Fisher’s Exact. Bold means statistically significant at p ≤ 0.05
Control group (no periodontitis/periodontitis): DMFT (n = 53/n = 40); tooth loss (n = 95/n = 87); PD (n = 95/n = 87); CAL (n = 95/n = 87), BOP (n = 77/n = 80); salivary flow (n = 74/n = 66). COVID-19 group (no periodontitis/periodontitis): all variables (n = 52/n = 47)
Periodontitis and COVID-19-related outcomes
Among individuals with COVID-19, periodontitis was associated with more hospitalization (p = 0.009), more days in the ICU (p = 0.042), admission to the SICU (p = 0.047), and higher need for oxygen therapy (p = 0.042). However, there was no difference in total hospitalization length, need for ICU, need for mechanical ventilation, or death between individuals with and without periodontitis in the COVID-19 group (Table 3).
Table 3.
COVID-19-related outcomes of patients with and without periodontitis
| Variables | COVID-19 | ||
|---|---|---|---|
| No periodontitis | Periodontitis | p value | |
| Hospitalization, n (%) | |||
| No | 10 (19.2) | 1 (2.1) | 0.009* |
| Yes | 42 (80.8) | 46 (97.9) | |
| Hospitalization length (mean ± SD) | 16.04 ± 35.84 | 16.26 ± 15.53 | 0.969*** |
| ICU, n (%) | |||
| No | 43 (82.7) | 32 (68.1) | 0.105* |
| Yes | 9 (17.3) | 15 (31.9) | |
| Change ICU to SICU, n (%) | |||
| No | 52 (100.0) | 43 (91.5) | 0.047** |
| Yes | 0 (0.0) | 4 (8.5) | |
| Days on ICU (mean ± SD) | 0.83 ± 2.11 | 2.23 ± 4.20 | 0.042*** |
| Mechanical ventilation, n (%) | |||
| No | 50 (96.2) | 44 (93.6) | 0.666** |
| Yes | 2 (3.8) | 3 (6.4) | |
| Days on mechanical ventilation (mean ± SD) | 0.19 ± 0.97 | 0.43 ± 1.76 | 0.412*** |
| Death, n (%) | |||
| No | 51 (98.1) | 47 (100.0) | 1.000** |
| Yes | 1 (1.9) | 0 (0.0) | |
| Oxygen therapy, n (%) | |||
| No | 19 (36.5) | 8 (17.0) | 0.042* |
| Yes | 33 (63.5) | 39 (83.0) | |
ICU intensive care unit, SD standard deviation, SICU semi-intensive care unit
*Pearson chi-square test; **Fisher’s exact; ***Student t test. Bold means statistically significant at p ≤ 0.05
For the COVID-19 group, binary regression models evaluated the association of periodontitis with hospitalization and use of oxygen, controlled for the confounders BMI, cardiovascular alteration, and age (Table 4). After adjustment, individuals with periodontitis were 1.13 times more likely to be hospitalized than individuals without periodontitis (CI = 1.01–1.26; p = 0.028). The association between periodontitis and use of oxygen, controlled for the confounders was not significant.
Table 4.
Binary regression of factors influencing hospitalization and use of oxygen in COVID-19 patients
| Hospitalization | Use of oxygen | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Periodontitis | ||||||
| No | 1 | – | 0.028 | 1 | 0.149 | |
| Yes | 1.13 | 1.01–1.26 | 1.19 | 0.938–1.519 | ||
| BMI | ||||||
| Underweight | 1 | – | – | 1 | – | – |
| Normal weight | 1.01 | 0.881–1.144 | 0.493 | 1.14 | 0.747–1.260 | 0.273 |
| Overweight | 1.03 | 0.874–1.233 | 0.665 | 1.03 | 0.793–1.338 | 0.820 |
| Obese | 1.19 | 0.721–1.960 | 0.945 | 2.69 | 0.457–15.873 | 0.310 |
| Cardiovascular alteration | ||||||
| No | 1 | – | 0.026 | 1 | – | 0.183 |
| Yes | 1.16 | 1.018–1.322 | 1.18 | 0.924–1.510 | ||
| Age | ||||||
| < 49 years | 1 | 0.285 | 1 | – | 0.557 | |
| ≥ 50 years | 1.06 | 0.947–1.200 | 1.07 | 0.841–1.379 | ||
BMI body mass index, CI confidence interval, OR odds ratio. Bold means statistically significant at p ≤ 0.05
The association of periodontitis and COVID-19 outcomes was also analyzed by comparing healthy, stage I, II, III and IV groups. The more severe the periodontitis, the higher was the percentage of hospitalization. No specific stage of periodontitis or health was associated with more need of hospitalization, ICU, mechanical ventilation, death, oxygen therapy, or with lengthier hospitalization, days in the ICU or days under mechanical ventilation (Supplementary Table 3). The association of PD, CAL and BOP with the COVID-19 outcomes was not significant. In addition, there was no correlation between these variables and the duration of hospitalization, days in the ICU, and days of use of mechanical ventilation. (Supplementary Table 4).
Cytokine analysis in periodontitis and no periodontitis patients
The concentration of IL-6 was higher in patients with periodontitis than in patients without periodontitis in the COVID-19 group (p = 0.010). Patients with COVID-19 and periodontitis also had a higher salivary concentration of IL-6 than control individuals with periodontitis (p = 0.009). The concentration of IL-1β was increased in individuals with periodontitis when compared to individuals without periodontitis in the COVID-19 group (p = 0.028) and control (p = 0.050). No statistical differences were identified for the concentrations of IL-10, TNF-α, OPG, RANKL, and NET (Fig. 1).
Fig. 1.
Cytokine and neutrophil extracellular traps (NET) concentration on the saliva of individuals of group COVID-19 and control with and without periodontitis. (A) Interleukin (IL)-6, (B) IL-1β, (C) IL-10, (D) tumoral necrosis factor alpha (TNF-α), (E) osteoprotegerin (OPG) (F), receptor activator of nuclear factor Kappa-B ligand (RANKL), (G) RANKL/OPG, and (H) NET. *Statistically significant at p ≤ 0.05
A weak positive correlation was observed between the salivary concentration of NET and PD (β = 0.319; p = 0.001), CAL (β = 0.302; p = 0.003) and BOP (β = 0.237; p = 0.019). A weak correlation was also observed between PD and IL-1β (β = 0.361; p < 0.001) (Supplementary Table 5).
Cytokine and C-reactive protein variation during and after COVID-19 infection
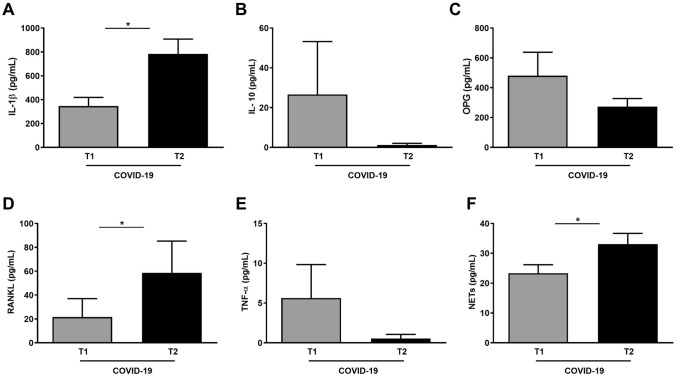
When analyzing cytokine variation (ΔT2–T1), significant increases in IL-1β (p = 0.005), RANKL (p = 0.010), and NET (p = 0.039) concentration were observed from T1 to T2 (≈ 100 days after initial diagnosis of SARS-CoV-2 infection). The concentrations of IL-10, OPG and TNF-α at T2 decreased compared to T1, without statistical significance (Fig. 2). Cytokine variation observed during and after the infection was not associated with the serum levels of C-reactive protein, lymphocytes, neutrophils, eosinophils, and D-dimer (data not shown). A weak positive correlation between the serum levels of C-reactive protein at T1 and the salivary levels of RANKL (β = 0.234; p = 0.032), NET (β = 0.224; p = 0.041), and RANKL/OPG (β = 0.222; p = 0.045) at T1 was observed (Supplementary Table 6).
Fig. 2.
Cytokine and neutrophil extracellular trap concentration in the saliva of individuals with COVID-19 during and after the infection. (A) Interleukin (IL)-1β, (B) IL-10, (C) osteoprotegerin (OPG), (D) receptor activator of nuclear factor Kappa-B ligand (RANKL), (E) tumoral necrosis factor alpha (TNF-α), and (F) neutrophil extracellular traps. *Statistically significant at p ≤ 0.05
Regression models were constructed with factors (ICU need, BMI, intubation need, periodontitis, smoking, and oxygen use) that could potentially explain the observed variation in cytokine concentration. Periodontitis was associated with a significant increase in levels of RANKL (p = 0.001) and IL-1β (p = 0.016). Individuals who did not need oxygen therapy exhibited a reduction in the concentration of RANKL (p = 0.002). The variation of NET concentration during and after the infection, as well as the variation in C-reactive protein at the beginning and at the end of the infection, were not associated with the variables included in the multivariate model (Table 5).
Table 5.
Factors influencing the cytokine concentration change during and after COVID-19 infection
| Variables | OPG | RANKL | IL-10 | IL-1β | TNF-α | NET | CRP | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | p value | Mean ± SD | p value | Mean ± SD | p value | Mean ± SD | p value | Mean ± SD | p value | Mean ± SD | p value | Mean ± SD | p value | ||
| ICU | |||||||||||||||
| No | − 230.31 ± 973.05 | 0.713** | − 35.33 ± 112.18 | 0.142** | − 28.41 ± 123.05 | 0.834** | 439.34 ± 592.28 | 0.960* | − 6.29 ± 19.34 | 0.618** | 8.72 ± 19.87 | 0.519* | − 68.04 ± 126.48 | 0.073** | |
| Yes | − 686.83 ± 950.83 | 0.00 ± 0.00 | 0.00 ± 0.00 | 416.02 ± 955.27 | 0.00 ± 0.00 | 18.21 ± 2.89 | − 85.94 ± 62.59 | ||||||||
| BMI | |||||||||||||||
| Under/normal weight | − 710.11 ± 1132.63 | 0.406** | − 66.99 ± 150.91 | 0.491** | − 56.19 ± 168.57 | 0.095** | 249.64 ± 472.18 | 0.207* | − 11.87 ± 25.95 | 0.126** | 13.41 ± 24.02 | 0.438* | − 80.64 ± 109.82 | 0.881** | |
| Overweight/obese | 110.19 ± 575.04 | 0.23 ± 0.72 | 2.26 ± 5.04 | 605.39 ± 678.49 | 0.00 ± 0.00 | 6.40 ± 13.48 | − 69.83 ± 112.80 | ||||||||
| Intubation | |||||||||||||||
| No | − 218.32 ± 945.37 | 0.561** | − 33.37 ± 109.15 | 0.894** | − 26.84 ± 119.57 | 0.886** | 475.57 ± 594.80 | 0.246* | − 5.94 ± 18.82 | 0.732** | 9.14 ± 19.36 | 0.583* | − 72.09 ± 114.69 | 0.332** | |
| Yes | − 662.32 ± 985.50 | 23.4 ± 33.09 | 0.00 ± 0.00 | − 259.46 ± 0.00 | 0.00 ± 0.00 | 20.25 ± 0.00 | − 89.44 ± 52.81 | ||||||||
| Periodontitis | |||||||||||||||
| No | − 402.99 ± 981.38 | 0.352** | − 43.13 ± 117.33 | 0.001** | − 32.73 ± 130.89 | 0.306** | 271.99 ± 549.07 | 0.016* | − 7.12 ± 20.52 | 0.453** | 12.74 ± 20.10 | 0.188* | − 63.58 ± 113.55 | 0.469** | |
| Yes | 188.97 ± 782.33 | 11.61 ± 21.72 | 1.96 ± 3.92 | 1055.22 ± 350.46 | 0.00 ± 0.00 | − 1.58 ± 7.81 | − 81.33 ± 110.02 | ||||||||
| Smoking | |||||||||||||||
| No | − 281.76 ± 982.86 | 0.453** | − 33.37 ± 109.15 | 0.497** | − 26.83 ± 119.57 | 0.886** | 459.76 ± 611.04 | 0.498* | − 5.94 ± 18.82 | 0.732** | 7.96 ± 17.85 | 0.085* | − 73.38 ± 111.12 | NA | |
| Yes | 16.685 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 25.19 ± 0.00 | 0.00 ± 0.00 | 41.53 ± 0.00 | NA | ||||||||
| Use of oxygen | |||||||||||||||
| No | − 405.84 ± 958.34 | 0.272** | − 42.90 ± 123.04 | 0.002** | − 34.51 ± 135.69 | 0.716** | 344.67 ± 559.67 | 0.276* | − 7.63 ± 21.20 | 0.385** | 7.97 ± 21.87 | 0.308* | − 61.09 ± 166.09 | 0.204** | |
| Yes | 78.55 ± 951.65 | 0.00 ± 0.00 | 0.00 ± 0.00 | 695.07 ± 707.46 | 0.00 ± 0.00 | 14.61 ± 5.20 | − 76.34 ± 95.31 | ||||||||
BMI body mass index, CRP C-reactive protein, ICU intensive care unit, IL interleukin, NA not available, NET neutrophil extracellular traps, OPG osteoprotegerin, RANKL receptor activator of nuclear factor Kappa-B ligand, SD standard deviation, TNF tumoral necrosis factor
*Student t test; **Mann–Whitney test. Bold means statistically significant at p ≤ 0.05
Microbiological profile during and after COVID-19 infection
The bacterial loads of P. gingivalis and A. actinomycetemcomitans during the COVID-19 infection (T1) were higher, albeit not significant, when compared to T2 (≅ 100 days after infection). The bacterial loads of T. forsythia and T. denticola at T2 were higher, albeit not significant than at T1 (Supplementary Fig. 1). There was no difference in bacterial load between patients with and without periodontitis at the two timepoints analyzed (data not shown).
Discussion
The present study investigated the potential association between the periodontal status and the severity of SARS-CoV-2 infection through clinical, inflammatory, and microbiological parameters. Our findings demonstrated that periodontitis was associated with higher rates of hospitalization, lengthier stay in the ICU, admission to the SICU, and higher need for oxygen therapy. After adjustment for confounders such as BMI, cardiovascular alteration and age, periodontitis resulted in a 1.13-fold increased chance of hospitalization. Increased salivary levels of IL-6 were seen in patients with COVID-19 and periodontitis. The presence of periodontitis was also related to a positive variation of RANKL and IL-1β after COVID-19, even though no significant changes in the bacterial loads of periodontopathogens P. gingivalis, A. actinomycetemcomitans, T. forsythia, and T. denticola were detected.
In 2019, there were 1.1 billion prevalent cases of severe periodontitis worldwide [5], making this one of the most prevalent diseases in the world and a public health problem, since it can lead to tooth loss, masticatory impairment, and worse oral health-related quality of life, with a negative impact on several systemic diseases [7, 31–34]. Our clinical evaluation revealed that individuals with periodontitis had higher rates of hospitalization and oxygen therapy requirements, a longer stay in the ICU and higher SICU admission, in agreement with the data of Gupta et al. [11]. Although there was no association between periodontitis and the total hospitalization period, we observed that patients with periodontitis stayed longer in the ICU. In contrast, we did not find an association of periodontitis with mechanical ventilation and ICU admission or with death [9, 11, 12]. This was possibly due to the low mortality rate observed in our sample (1%) compared to others (2.4%, 9.7% and 24.2%, respectively) [9, 11, 12]. Further dissimilarities with other studies may be related to study design, clinical examination, periodontitis classification, and time of examination. In our study, periodontal examination was performed during the infection and the diagnosis of periodontitis was based on PD, CAL, and tooth loss; moreover, a matched control group was included. In contrast, some studies did not perform clinical periodontal examination [8, 9], did not include a control group [12, 35], and only evaluated patients after the infection [10]. Multivariate analysis showed that even after the removal of confounders, periodontitis persisted as a significant factor regarding the need of hospitalization. However, caution is needed when analyzing these data, because a statistical significance does not necessarily translate into clinical significance [36].
The potential association of COVID-19 and periodontitis is based on biological plausibility, since the two conditions appear to have similar pathological immune-mediated mechanisms [18, 19] and share closely similar risk factors [37, 38], and the periodontal pockets may act as a reservoir of SARS-CoV-2 [13]. Periodontitis is characterized by loss of periodontal attachment due to microbiological challenge and is associated with host-mediated inflammation [25], with the identification of an increased number of proinflammatory markers such as IL-1β, IL-6, IL-10, TNF-α, OPG, RANKL, and NET that are representative of the periodontal disease status [17, 39, 40].
While the IL-6 was proposed in few studies as a marker of periodontitis [16, 17, 41], the increase in IL-6 was not consistently shown when a heterogeneous group (patients exhibiting distinct periodontal status) was compared to a control group (periodontal health group) [42, 43]. Furthermore, salivary IL-6 concentration was not modified by periodontal therapy [26, 44]. On the other hand, in a homogeneous group of patients with stage IV periodontitis, a robust increase in IL-6 was detected compared to controls [45]. In our study, IL-6 concentration did not consistently discriminate individuals with and without periodontitis in the control group. However, the COVID-19 group with periodontitis showed a significant increase of IL-6 compared to controls with periodontitis and to COVID-19 individuals without periodontitis. This strongly suggests a contribution of both periodontitis and COVID-19 to salivary IL-6 levels and supports the hypothesis that periodontitis-induced local and systemic inflammation results in worse COVID-19 outcomes. In turn, COVID-19-induced systemic inflammation may impact periodontitis contributing to the IL-6 levels in saliva, an assumption that deserves further investigation.
Noteworthy, patients with COVID-19 had higher serum levels of cytokines including IL-6 and this increase was significantly higher in critically ill patients [15]. Even though we did not observe significant changes in the target periodontal bacteria, it has been recently shown that periodontopathogens induce the expression of ACE-2, IL-6, and IL-8 in alveolar epithelial cells, suggesting that the aspiration of these bacteria regulates the inflammatory response of the lower respiratory tract [46]. Therefore, we hypothesize that periodontitis-induced local and systemic inflammation contributes to the deterioration of clinical condition and to more severe outcomes during COVID-19.
We also analyzed cytokine production after COVID-19 infection, detecting significant increases of IL-1β, RANKL, and NET. A regression model showed that the presence of periodontitis explained IL-1β and RANKL changes. Overall, these data raise concerns about the persistence of the virus at inflamed periodontal sites even after infection. The presence of virus in periodontal pockets may inhibit the macrophage response to the bacterial challenge, reducing cytokine production [47]. Thus, by these mechanisms, SARS-CoV-2 could lead to changes in bacterial load and periodontal status, a hypothesis that needs to be further examined. Alternatively, the exacerbated systemic inflammation during COVID-19 could lead to a deterioration of periodontal inflammation, thus resulting in an increase of those markers even after the infection. Consistently, the impact of systemic inflammation on periodontal tissues has been observed in chronic diseases, such as rheumatoid arthritis [32] and diabetes [34].
A significant increase in the formation of NET was observed in individuals with periodontal diseases [40], in contrast to our findings, in which patients with periodontitis of both groups showed higher but statistically nonsignificant NET concentration. Conversely, exacerbated formation of NET has been reported in individuals with severe COVID-19 [48], while in our study salivary levels of NET increased only after infection, and this trend was not explained when correlated with confounders, including periodontitis.
The strengths of this study include the representative sample of unvaccinated patients from two reference centers, extensive data collection on cytokine, and bacterial load measurements during and after the infection. To the best of our knowledge, this is the first study to evaluate cytokines and microorganisms in patients with and without COVID-19 and to associate the findings with periodontal status. Nonetheless, a limitation of this report is that the clinical evaluation of ICU patients was not possible because of the use of a mask with a reservoir and the mechanical ventilation tube, which precluded the assessment of highly severe patients. This study was carried out at the beginning of the pandemic, and at that time protocols/guidelines for the management of affected individuals were still being elaborated, a fact that certainly resulted in non-standardized clinical decisions. Taken together, the present data suggest that improvement in oral hygiene and periodontal treatment could potentially prevent complications of COVID-19 by reducing the possibilities of nosocomial pneumonia and overall inflammation, as well as preventing the flow of microorganisms to the bloodstream through the periodontal pocket ulcerated epithelium.
In summary, worse periodontal status was associated with increased hospitalization, oxygen therapy, ICU length, and SICU admission. Periodontitis patients had augmented salivary levels of IL-6 during COVID-19 infection. The presence of periodontitis was also related to an increase in RANKL and IL-1β after infection, although there were no significant changes in the bacterial loads of the periodontopathogens P. gingivalis, A. actinomycetemcomitans, T. forsythia, and T. denticola. The data reinforce the relevance of oral and periodontal care in patients with COVID-19 as a potential strategy to reduce overall inflammation and aspiration of microorganisms, thus preventing complications.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to extend our thanks to Dr. Fabiana Kakehasi, Dr. Vandack Nobre (Hospital das Clínicas, Universidade Federal de Minas Gerais), and Dr. Ester Grassi (Hospital Eduardo de Menezes, Fundação Hospitalar do Estado de Minas Gerais) for the attention given throughout the project. The authors also thank Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG; #APQ-00706-21), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; #88887.649872/2021-00; Finance code #001) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; #305077/2021-0), Brazil, for financial support. L.M.B., S.R.O., J.A.A.A., L.M., A.C.V.P.S., and A.H.S. are the recipients of fellowships.
Author contribution
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by LMB, SRO, JAAdA, FPDC, LM, ALMB, ACVPdS, AFdS, DFM, AHS, MdCSA, DVT, GPG, FdQC, RSdA, RPdS, RSG, AS, FOM, LGA, FOC, and TAS. The first draft of the manuscript was written by LMB, FOC, and TAS and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG; #APQ-00706-21), the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; #88887.649872/2021-00; Finance code #001) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; #305077/2021-0).
Data availability
All data supporting the findings of this study are available within the paper and its Supplementary Information.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.COVID-19 Cumulative Infection Collaborators Estimating global, regional, and national daily and cumulative infections with SARS-CoV-2 through Nov 14, 2021: a statistical analysis. Lancet. 2022;399:2351–2380. doi: 10.2196/24248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flanagan KL, MacIntyre CR, McIntyre PB, Nelson MR. SARS-CoV-2 vaccines: where are we now? J Allergy Clin Immunol Pract. 2021;9:3535–3543. doi: 10.1016/j.jaip.2021.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ejaz H, Alsrhani A, Zafar A, Javed H, Junaid K, Abdalla AE, et al. COVID-19 and comorbidities: deleterious impact on infected patients. J Infect Public Health. 2020;13:1833–1839. doi: 10.1016/j.jiph.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fagni F, Simon D, Tascilar K, Schoenau V, Sticherling M, Neurath MF, et al. COVID-19 and immune-mediated inflammatory diseases: effect of disease and treatment on COVID-19 outcomes and vaccine responses. Lancet Rheumatol. 2021;3:e724–e736. doi: 10.1016/S2665-9913(21)00247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen MX, Zhong YJ, Dong QQ, Wong HM, Wen YF. Global, regional, and national burden of severe periodontitis, 1990–2019: an analysis of the Global Burden of Disease Study 2019. J Clin Periodontol. 2021;48:1165–1188. doi: 10.1111/jcpe.13506. [DOI] [PubMed] [Google Scholar]
- 6.Dong J, Li W, Wang Q, Chen J, Zu Y, Zhou X, et al. Relationships between oral microecosystem and respiratory diseases. Front Mol Biosci. 2022;8:718222. doi: 10.3389/fmolb.2021.718222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomes-Filho IS, de Oliveira TF, da Cruz SS, Passos-Soares Jde S, Trindade SC, Oliveira MT, et al. Influence of periodontitis in the development of nosocomial pneumonia: a case control study. J Periodontol. 2014;85:e82–90. doi: 10.1902/jop.2013.130369. [DOI] [PubMed] [Google Scholar]
- 8.Larvin H, Wilmott S, Wu J, Kang J. The impact of periodontal disease on hospital admission and mortality during COVID-19 pandemic. Front Med (Lausanne). 2020;7:604980. doi: 10.3389/fmed.2020.604980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marouf N, Cai W, Said KN, Daas H, Diab H, Chinta VR, et al. Association between periodontitis and severity of COVID-19 infection: a case-control study. J Clin Periodontol. 2021;48:483–491. doi: 10.1111/jcpe.13435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anand PS, Jadhav P, Kamath KP, Kumar SR, Vijayalaxmi S, Anil S. A case-control study on the association between periodontitis and coronavirus disease (COVID-19) J Periodontol. 2022;93:584–590. doi: 10.1002/JPER.21-0272. [DOI] [PubMed] [Google Scholar]
- 11.Gupta S, Mohindra R, Singla M, Khera S, Sahni V, Kanta P, et al. The clinical association between periodontitis and COVID-19. Clin Oral Investig. 2022;26:1361–1374. doi: 10.1007/s00784-021-04111-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costa CA, Vilela ACS, Oliveira SA, Gomes TD, Andrade AAC, Leles CR, et al. Poor oral health status and adverse COVID-19 outcomes: a preliminary study in hospitalized patients. J Periodontol. 2022;93:1889–1901. doi: 10.1002/JPER.21-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandes Matuck B, Dolhnikoff M, Maia GVA, Isaac Sendyk D, Zarpellon A, Costa Gomes S, et al. Periodontal tissues are targets for Sars-Cov-2: a post-mortem study. J Oral Microbiol. 2020;13:1848135. doi: 10.1080/20002297.2020.1848135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang N, Pérez P, Kato T, Mikami Y, Okuda K, Gilmore RC, et al. SARS-CoV-2 infection of the oral cavity and saliva. Nat Med. 2021;27:892–903. doi: 10.1038/s41591-021-01296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han H, Ma Q, Li C, Liu R, Zhao L, Wang W, et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect. 2020;9:1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawamoto D, Amado PPL, Albuquerque-Souza E, Bueno MR, Vale GC, Saraiva L, et al. Chemokines and cytokines profile in whole saliva of patients with periodontitis. Cytokine. 2020;135:155197. doi: 10.1016/j.cyto.2020.155197. [DOI] [PubMed] [Google Scholar]
- 17.Pan W, Wang Q, Chen Q. The cytokine network involved in the host immune response to periodontitis. Int J Oral Sci. 2019;11:30. doi: 10.1038/s41368-019-0064-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta S, Sahni V. The intriguing commonality of NETosis between COVID-19 & periodontal disease. Med Hypotheses. 2020;144:109968. doi: 10.1016/j.mehy.2020.109968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sahni V, Gupta S. COVID-19 & periodontitis: the cytokine connection. Med Hypotheses. 2020;144:109908. doi: 10.1016/j.mehy.2020.109908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wagner MC, Haas AN, Oppermann RV, Rosing CK, Albandar JM, Susin C. Effect of alcohol consumption on clinical attachment loss progression in an urban population from south Brazil: a 5-year longitudinal study. J Periodontol. 2017;88(12):1271–1280. doi: 10.1902/jop.2017.170231. [DOI] [PubMed] [Google Scholar]
- 21.Alves RC, Félix SA, Rodriguez-Archilla A, Oliveira P, Brito J, Dos Santos JM. Relationship between menopause and periodontal disease: a cross-sectional study in a Portuguese population. Int J Clin Exp Med. 2015;8(7):11412–11419. [PMC free article] [PubMed] [Google Scholar]
- 22.Eke PI, Borgnakke WS, Genco RJ. Recent epidemiologic trends in periodontitis in the USA. Periodontol. 2020;82(1):257–267. doi: 10.1111/prd.12323. [DOI] [PubMed] [Google Scholar]
- 23.Costa FO, Guimarães AN, Cota LO, Pataro AL, Segundo TK, Cortelli SC, et al. Impact of different periodontitis case definitions on periodontal research. J Oral Sci. 2009;51:199–206. doi: 10.2334/josnusd.51.199. [DOI] [PubMed] [Google Scholar]
- 24.Silness J, Loe H. Periodontal disease in pregnancy. Ii. Correlation between oral hygiene and periodontal condtion. Acta Odontol Scand. 1969;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 25.Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J Clin Periodontol. 2018;45:S149–S161. doi: 10.1111/jcpe.12945. [DOI] [PubMed] [Google Scholar]
- 26.Khurshid Z, Zohaib S, Najeeb S, Zafar MS, Slowey PD, Almas K. Human saliva collection devices for proteomics: an update. Int J Mol Sci. 2016;17:846. doi: 10.3390/ijms17060846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miguita L, Martins-Chaves RR, Geddes VEV, Mendes SDR, Costa SFDS, Fonseca PLC, et al. Biosafety in dental health care during the COVID-19 pandemic: a longitudinal study. Front Oral Health. 2022;3:871107. doi: 10.3389/froh.2022.871107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Center for Disease Control and Prevention (CDC) 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel. Centers for Disease Control and Prevention, Division of Viral Diseases, Atlanta, USA. 2020;CDC-006–00006.
- 29.Oliveira SR, de Arruda JAA, Schneider AH, Carvalho VF, Machado CC, Corrêa JD, et al. Are neutrophil extracellular traps the link for the cross-talk between periodontitis and rheumatoid arthritis physiopathology? Rheumatology (Oxford) 2021;61:174–184. doi: 10.1093/rheumatology/keab289. [DOI] [PubMed] [Google Scholar]
- 30.Nonnenmacher C, Dalpke A, Mutters R, Heeg K. Quantitative detection of periodontopathogens by real-time PCR. J Microbiol Methods. 2004;59:117–125. doi: 10.1016/j.mimet.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Cavalla F, Biguetti CC, Colavite PM, Silveira EV, Martins W, Jr, Letra A, et al. TBX21-1993T/C (rs4794067) polymorphism is associated with increased risk of chronic periodontitis and increased T-bet expression in periodontal lesions, but does not significantly impact the IFN-g transcriptional level or the pattern of periodontophatic bacterial infection. Virulence. 2015;6:293–304. doi: 10.1080/21505594.2015.1029828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartold PM, Lopez-Oliva I. Periodontitis and rheumatoid arthritis: an update 2012–2017. Periodontol. 2000;2020(83):189–212. doi: 10.1111/prd.12300. [DOI] [PubMed] [Google Scholar]
- 33.Teeuw WJ, Slot DE, Susanto H, Gerdes VE, Abbas F, D'Aiuto F, et al. Treatment of periodontitis improves the atherosclerotic profile: a systematic review and meta-analysis. J Clin Periodontol. 2014;41:70–79. doi: 10.1111/jcpe.12171. [DOI] [PubMed] [Google Scholar]
- 34.Preshaw PM, Taylor JJ, Jaedicke KM, De Jager M, Bikker JW, Selten W, et al. Treatment of periodontitis reduces systemic inflammation in type 2 diabetes. J Clin Periodontol. 2020;47:737–746. doi: 10.1111/jcpe.13274. [DOI] [PubMed] [Google Scholar]
- 35.Gomes SC, da Fonseca JG, Miller LM, Manenti L, Angst PDM, Lamers ML, et al. SARS-CoV-2 RNA in dental biofilms: supragingival and subgingival findings from inpatients in a COVID-19 intensive care unit. J Periodontol. 2022;93:1476–1485. doi: 10.1002/JPER.21-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris JD, Brand JC, Cote MP, Faucett SC, Dhawan A. Research pearls: the significance of statistics and perils of pooling. Part 1: Clinical versus statistical significance. Arthroscopy. 2017;33(6):1102–1112. doi: 10.1016/j.arthro.2017.01.053. [DOI] [PubMed] [Google Scholar]
- 37.Reynolds MA. Modifiable risk factors in periodontitis: at the intersection of aging and disease. Periodontol. 2000;2014(64):7–19. doi: 10.1111/prd.12047. [DOI] [PubMed] [Google Scholar]
- 38.Gao YD, Ding M, Dong X, Zhang JJ, Kursat Azkur A, Azkur D, et al. Risk factors for severe and critically ill COVID-19 patients: a review. Allergy. 2021;76:428–455. doi: 10.1111/all.14657. [DOI] [PubMed] [Google Scholar]
- 39.Hienz SA, Paliwal S, Ivanovski S. Mechanisms of bone resorption in periodontitis. J Immunol Res. 2015;2015:615486. doi: 10.1155/2015/615486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Magán-Fernández A, Rasheed Al-Bakri SM, O'Valle F, Benavides-Reyes C, Abadía-Molina F, Mesa F. Neutrophil extracellular traps in periodontitis. Cells. 2020;9:1494. doi: 10.3390/cells9061494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reddahi S, Bouziane A, Rida S, Tligui H, Ennibi O. Salivary biomarkers in periodontitis patients: a pilot study. Int J Dent. 2022 doi: 10.1155/2022/3664516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rathnayake N, Akerman S, Klinge B, et al. Salivary biomarkers of oral health: a cross-sectional study. J Clin Periodontol. 2013;40(2):140–147. doi: 10.1111/jcpe.12038. [DOI] [PubMed] [Google Scholar]
- 43.Miller CS, Ding X, Dawson DR, 3rd, Ebersole JL. Salivary biomarkers for discriminating periodontitis in the presence of diabetes. J Clin Periodontol. 2021;48(2):216–225. doi: 10.1111/jcpe.13393. [DOI] [PubMed] [Google Scholar]
- 44.Aljateeli M, Koticha T, Bashutski J, et al. Surgical periodontal therapy with and without initial scaling and root planing in the management of chronic periodontitis: a randomized clinical trial. J Clin Periodontol. 2014;41(7):693–700. doi: 10.1111/jcpe.12259. [DOI] [PubMed] [Google Scholar]
- 45.Hadzic Z, Pasic E, Hukic M, Vukelic MG, Hadzic S. Salivary interleukin-6 levels patients with periodontitis stage IV. Meandros Med Dent J. 2021;22:140–147. doi: 10.4274/meandros.galenos.2021.05945. [DOI] [Google Scholar]
- 46.Takahashi Y, Watanabe N, Kamio N, Yokoe S, Suzuki R, Sato S, et al. Expression of the SARS-CoV-2 receptor ACE2 and proinflammatory cytokines induced by the periodontopathic bacterium Fusobacterium nucleatum in human respiratory epithelial cells. Int J Mol Sci. 2022;22:1352. doi: 10.3390/ijms22031352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin YL, Li M. Human cytomegalovirus and Epstein-Barr virus inhibit oral bacteria-induced macrophage activation and phagocytosis. Oral Microbiol Immunol. 2009;24:243–248. doi: 10.1111/j.1399-302X.2009.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Masso-Silva JA, Moshensky A, Lam MTY, Odish MF, Patel A, Xu L, et al. Increased peripheral blood neutrophil activation phenotypes and neutrophil extracellular trap formation in critically ill coronavirus disease 2019 (COVID-19) patients: a case series and review of the literature. Clin Infect Dis. 2022;74:479–489. doi: 10.1093/cid/ciab437. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are available within the paper and its Supplementary Information.