Abstract
To reduce the use of antibiotics, research into nutritional strategies designed to improve the gut health of weaned pigs is underway. This study sought to examine the effects of reducing dietary crude protein (CP) and/or supplementing the feed with sodium butyrate protected by the sodium salts of medium-chain fatty acids on the growth performance and gut health of weaned piglets. Ninety-six weaned piglets (Landrace × large white, 21 days of age) were allotted to four experimental treatments for 14 d. The experimental design was factorial with 2 CP levels and 2 feed-additive doses (0 vs. 1 kg/t). Results showed that reducing CP from 22.2% to 18.8% diet had no effect on piglet growth performance parameters during the first post-weaning week (P > 0.05), but did compromise growth in the second week (P = 0.011), impacting overall growth performance results (P = 0.019). Nonetheless, dietary CP level reduction led reducing crypt depth (P = 0.03657). In addition, Lactobacillus counts that were increased in the ileum (P = 0.032) and reduced in the colon (P = 0.032). Furthermore, apparent ileal digestibility of organic matter (P = 0.026) and fecal consistency (P < 0.05) were improved throughout the experiment. Moreover, in piglets fed diets containing 22.2% CP, the use of the feed-additive tended to improve the gain-to-feed ratio (P = 0.091) compared to those fed supplemented diets containing 18.8% CP. In addition, feed supplementation increased ileal numbers of goblet cells (P = 0.036), as well as apparent ileal digestibility of dry matter (P = 0.057) and organic matter (P = 0.003). Supplementation also had beneficial effects on the microbiota of the colon, increasing Lactobacillus counts (P = 0.006) and diminishing Enterobacteriaceae counts (P = 0.003), as well as affecting microbial metabolite profiles in that acetic acid concentrations tended to be increased (P = 0.088) and valeric acid concentrations were reduced (P = 0.002). These findings support the use of both strategies can improve the gut health of weaned piglets and prompt further research into the possible benefits of combining these two nutritional strategies on gut health and growth performance.
Keywords: butyric acid, feed-additive, gut health, low protein diet, medium-chain fatty acid, weaned pig
Reducing dietary crude protein and supplementing the feed with sodium butyrate protected by medium-chain fatty acid sodium salts in weaned pigs are nutritional strategies with impacts on the gut barrier. The results of this study provide direction for further research on how to improve the gut health and growth performance of piglets.
Introduction
Modern intensive pig production is characterized by early weaning around 3 to 4 wk of age when the intestinal function of piglets is still developing (Lallès et al., 2007; Moeser et al., 2017). Weaning has been recognized to be one of the most critical phases disrupting gut health and increasing susceptibility to diseases in piglets (Xiong et al., 2019). Antibiotics and pharmacological levels of zinc oxide have been extensively used for years until the European Union’s ban (European Parliament and European Council, 2018). Accordingly, several nutritional strategies have been considered to maintain and improve piglet gut health during the postweaning period (Celi et al., 2017; Rattingan et al., 2020).
As piglets cannot digest high dietary crude protein (CP) levels, one strategy is to reduce its levels. This strategy accompanied by supplementation with synthetic amino acids (AA) may restrict the proliferation of proteolytic bacteria and the production of toxic metabolites in the gut, thus reducing post-weaning diarrhea (Opapeju et al., 2009; de Lange et al., 2010). However, data arising from studies examining the effects on the gut barrier of reducing dietary CP have been inconsistent, and some authors argue that this strategy can compromise the growth performance parameters. In fact, some authors attributed this growth impairment to reductions in intestinal absorptive surface area by observing reduced intestinal villus heights (Nyachoti et al., 2006; Pierce et al., 2007; Limbach et al., 2021; Lynegaard et al., 2021). Thus, using feed-additives that may have beneficial effects on the intestinal barrier and, consequently, growth performance, appears to be a promising strategy in piglets receiving reduced CP diets.
In this context, natural-source derived compounds such as oil refining by-products have also been under research to supplement piglet diets (Celi et al., 2017; López-Colom et al., 2020). Some studies have explored the gut health-promoting effects of butyric acid and medium-chain fatty acids (MCFA). They are a source of energy for intestinal cells promoting their proliferation and differentiation as well as having antibacterial effects (Lu et al., 2008; Jackman et al., 2020). Likewise, salt forms and their combinations have been proposed to increase their impacts on the gut barrier and thereby target postweaning problems such as diarrhea and delayed growth (Tsiloyiannis et al., 2001; López-Colom et al., 2020).
To the knowledge of the present authors, however, no study has assessed the joint impacts of these two strategies. The main hypothesis of the present study was that reducing CP and supplementing the diet with sodium butyrate protected by MCFA can be two strategies that, in combination, improve the gut health of piglets. In fact, a room to elucidate is the efficacy of butyric acid and MFCA supplementation to mitigate the potential negative impact on productive parameters due to CP reduction and, in addition, to promote the beneficial effects on the gut barrier. Therefore, the aim of this study was to test the effects of reducing dietary CP levels from 22% to 18.5% and of the feed-additive sodium butyrate protected by MCFA salts (BM) on gut health and growth performance parameters in weaned piglets.
Materials and Methods
Animals, housing, and experimental design
The trial was carried out at the Servei de Granges i Camps Experimentals of the Universitat Autònoma de Barcelona (UAB) and received prior approval (permit no. CEAAH 1619/HR-10-13) from the Animal and Human Experimental Ethics Committee of this institution. The treatment, management, housing, husbandry, and slaughtering conditions complied with European Union Guidelines (European Parliament, 2010). All efforts were made to minimize animal suffering.
Ninety-six male piglets (Landrace × large white) weaned at 21 d of age at a commercial farm with high health status were transported to the UAB’s animal facilities. The pigs were raised in a controlled environment with automatic heating and forced ventilation. Average room temperature was 30 ± 2 °C. Throughout the study (14 d), the light program was set at 13 h light/11 h dark.
Upon arrival, animals were distributed in four boxes with eight pens each (32 pens and 3 animals per pen). Considering initial BW, a low- (5.11 ± 0.38 kg), intermediate- (5.87 ± 0.33 kg), and high-weight (6.76 ± 0.38 kg) piglet were housed in each pen to obtain a similar average BW (5.93 ± 0.01 kg) among pens, which are randomly distributed into the experimental treatments (two replicates pens/treatment in each box).
Four experimental treatments (n = 8) were prepared according to a 2 × 2 factorial arrangement, with two dietary CP levels (standard, S: 22%, and low, L: 18.5%) and two levels of BM feed-additive (0 and 1 kg/t). Thus, the experimental treatments were: standard CP level non-supplemented (S0), standard CP level supplemented with BM at 1 kg/t (S1), low CP level non-supplemented (L0), and low CP supplemented with BM at 1 kg/t (L1).
Diets and experimental product
The basal diets (Table 1) were formulated to satisfy nutrient requirements standards for pigs (FEDNA, 2013). According to the calculated CP level, the standard diet (S) containing 22% CP meets the minimum recommended values (19.4% CP) according to FEDNA (2013) for 5 to 7 kg BW pigs. In contrast, the low protein diet (L) contained 18.5% CP and was supplemented with the synthetic AA lysine, methionine, threonine, tryptophan, and valine to meet FEDNA (2013) recommendations: 1.39% SID lysine; 0.42% SID methionine; 0.90% SID threonine; 0.25% SID tryptophan; and 0.96% SID valine. Each experimental diet was prepared in a particular batch. For the BM-supplemented treatments, the BM additive (DICOSAN, Norel S.A., Madrid, Spain) contains 50% sodium butyrate protected by a mixture of sodium salts of fatty acids obtained from coconut fatty acid (FA) distillates (minimum 40% crude fat, whose FA profile comprised 48.0% lauric acid (C12:0), 16.0% myristic acid (C14:0), 15.5% oleic acid (C18:1), 8.5% palmitic acid (C16:0), 3.5% caprylic acid (C8:0), 3.5% capric acid (C10:0), 2.5% stearic acid (C18:0), and 2.5% linoleic acid (C18:2)). The targeted level of butyric acid was 0.4 kg/t and was analytically confirmed in the diet by HPLC chromatograph (Agilent 1200) with detector UV-DAD (Agilent). Titanium dioxide (TiO2) was included at 0.5% in the experimental treatments. Mashed feed and water were available ad libitum.
Table 1.
Ingredients and nutritional composition of the basal diets, as-fed basis
| S | L | |
|---|---|---|
| Ingredients, % | ||
| Corn | 1.62 | 12.99 |
| Wheat | 34.99 | 34.97 |
| Barley | 18.00 | 17.98 |
| Soybean meal 47% | 23.37 | 12.30 |
| Soybean oil | 5.00 | 5.00 |
| Spray dried porcine plasma | 2.50 | 2.48 |
| Hydrolyzed porcine mucosa | 1.00 | 1.00 |
| Skimmed milk 70% | 6.25 | 6.24 |
| Animal fat | 4.22 | 3.03 |
| l-lysine HCl | 0.77 | 0.77 |
| dl-methionine | 0.23 | 0.33 |
| l-tryptophan | 0.06 | 0.12 |
| l-threonine | 0.28 | 0.37 |
| l-valine | 0.03 | 0.22 |
| Choline chloride | 0.16 | 0.13 |
| Calcium carbonate | 0.44 | 0.44 |
| Bicalcium phosphate | 0.75 | 0.94 |
| Salt | 0.20 | 0.20 |
| Vitamin and mineral premix1 | 0.50 | 0.50 |
| Titanium dioxide | 0.50 | 0.50 |
| Analyzed gross energy, kcal/kg | 4,203 | 4,104 |
| Analyzed chemical composition, % | ||
| Dry matter | 90.34 | 90.05 |
| Ash | 5.36 | 4.98 |
| Crude protein | 22.2 | 18.8 |
| Ether extract | 6.63 | 5.64 |
| Neutral detergent fiber | 9.41 | 8.91 |
| Acid detergent fiber | 3.24 | 2.68 |
| Calculated composition, % | ||
| Crude protein | 22.0 | 18.5 |
| Standardized ileal digestible AA | ||
| Lysine | 1.47 | 1.47 |
| Valine | 1.00 | 1.00 |
| Threonine | 1.06 | 0.99 |
| Methionine plus cysteine | 0.88 | 0.87 |
| Isoleucine | 0.84 | 0.64 |
| Histidine | 0.51 | 0.40 |
| Tryptophan | 0.32 | 0.32 |
| Lactose | 4.50 | 4.50 |
1Provided per kilogram of complete diet: 7,800 IU vitamin A, 15 μg 25-hidroxycolecalciferol, 600 IU vitamin D3, 60 mg vitamin E, 3 mg vitamin K, 1.8 mg vitamin B1, 4.8 mg vitamin B2, 3.6 mg vitamin B6, 0.03 mg vitamin B12, 0.9 mg folic acid, 33 mg niacin, 18 mg calcium panthotenate, 0.18 mg biotin, 75.6 mg Fe, 0.6 mg I, 60 mg Cu, 72.6 mg Zn, 36.6 mg Mn, 0.096 mg methionine-Se produced by Saccharomyces cerevisiae NCYc, 0.084 Se, 37.8 mg BHT, 4.536 mg propyl gallate 86.4 mg sodium saccharine, 2.739 mg neohesperidina dihidrocha, 900 FYT phytase, 1,057.5 U β-glucanase, 8,100 U celulase, 180 U α-amylase, 765 U protease, 15,750 U xylanase, 12.2 mg.
L, low-protein diet (18.8% CP); S, standard-protein diet (22.2% CP).
Experimental and analytical procedures
Diets were characterized according to dry matter (DM), ash, CP contents of diethyl ether extracts following Association of Official Agricultural Chemists standard procedures (AOAC, 2005). Neutral detergent fibers and acid detergent fibers were determined according to the method of Van Soest et al. (1991). Gross energy (GE) was analyzed by adiabatic bomb calorimetry (IKA-Kalorimeter system C4000; Staufen, Germany).
Growth performance parameters and fecal consistency
Feed consumption (pen basis) and individual BW of the animals were recorded weekly. Feed intake was calculated based on the feed weight reduction of the feeders. In addition, daily feed waste was monitored to correct pen consumption. Thus, average daily feed intake (ADFI), average daily gain (ADG), and gain: feed efficiency (G:F) per pen were calculated for each period and for the overall study. During the experiment, the health status of the animals was daily checked and the mortality rate was recorded. No antibiotic treatment was given. Fecal consistency was assessed daily using the score system described by Espinosa et al. (2017): 0 = normal feces; 1 = moist feces; 2 = mild diarrhea; 3 = severe diarrhea; and 4 = watery diarrhea, always by a single trained technician who assigned an overall pen score.
On day 14, one pig per pen was selected based on the mean BW within the pen (8.73 ± 0.88 kg) to take samples to evaluate the effects on ileal morphometry, microbial counts, and fermentation. Furthermore, the remaining piglet with the heaviest BW per pen (9.95 ± 1.33 kg) was selected to collect sufficient ileum digesta and perform a digestibility study. The animals were intramuscular sedated with xylacin (2 mg/kg BW) combined with ketamine (20 mg/kg BW), and euthanized with a lethal dose injection of sodium pentobarbital (140 mg/kg BW). Once euthanized, the animals were bled, the abdomen was immediately opened, and the intestinal tract was extracted.
Ileal histomorphometry
Ileum (from Meckel’s diverticulum to the ileocecal junction) was divided into two equal sections, named as proximal and distal. From the middle of the distal ileum, 2-cm long sections were harvested, opened longitudinally, washed thoroughly in sterile PBS, and fixed by immersion in a 4% formaldehyde solution (Panreac; Castellar del Vallès, Spain) for the histological study. Then, tissue samples were dehydrated and embedded in paraffin, sectioned at 4-µm thickness, and stained with hematoxylin and eosin. Morphological measurements (villus height, crypt depth, and counts of intraepithelial lymphocytes (IEL) and goblet cells in the villi, and mitoses in the crypts) were made on ten well-oriented villus-crypt pairs with a light microscope (BHS, Olympus, Tokyo, Japan) according to the technique described by Nofrarías et al. (2006).
Microbiological analysis
To ensure the provenience of the digesta contents (2 g), ileum and proximal colon (5 cm from the ceco-colic junction) were attached by surgical clamps. Digesta samples were collected and kept immediately on ice for microbial counts. Total Lactobacillus and Enterobacteriaceae were determined through traditional counting. Content samples were 6-fold serial diluted (1:10) in lactated Ringer’s solution (Sigma–Aldrich Co.; St. Louis, MO, USA) and inoculated on MRS agar or MacConkey for 48 h. To quantitatively determine Lactobacilli, culture plates were incubated under aerobic, microaerophilic (5% CO2), and anaerobic conditions at 37 ºC. For Enterobacteriaceae counts, plates were incubated in aerobic conditions at 37 ºC and 42 ºC. The Lactobacillus:Enterobacteriaceae ratio was calculated to evaluate the possible related modulation between the two populations. Among all Enterobacteriaceae, coliforms and Escherichia coli were isolated. Counts were expressed as colony-forming units per gram of fresh matter.
Intestinal fermentation
Samples from ileum and proximal colon were also separately homogenized, and pH was immediately determined with a pH-meter (Sension+ pH1, 5050T electrode, Hatch Co., Loveland, USA) calibrated on the same day of use. In addition, after scoring the digesta according to the following consistency scale: 1 = liquid; 2 = liquid with some formed material; 3 = thick; 4 = semi-solid, different aliquots of homogenized digesta content were sampled. Samples of approximately 5 g were collected and kept immediately at −20 ºC to determine short-chain fatty acids (SCFA) and lactic acid following the method described by Richardson et al. (1989) and modified by Jensen et al. (1995) using gas chromatography. Samples were subjected to an acid–base treatment followed by diethyl ether extraction and derivation with the agent N-(tertbutyldimethyilsilyl)-N-methyl-trifluoroacetamide plus 1% tert-butyldimethylchlorosilane (Sigma–Aldrich Co.; St. Louis, MO, USA).
Another set of 3-mL samples was preserved in a 0.2 M H2SO4 solution (1:1) and also kept at −20 ºC for subsequent ammonia (NH3) determination using a gas-sensitive electrode (Hatch Co., Loveland, USA) combined with a digital voltmeter (Crison GLP 22, Crison Instruments S.A., Barcelona, Spain) according to the method modified by Barba-Vidal et al. (2017). Collected samples were centrifuged at 1,372 x g for 10 min and the resultant supernatant was neutralized with 1 mL of 10 M NaOH to reach a pH of 11, while stirring and measuring the ammonia released as different voltages in millivolts.
Digestibility study
Ileum content from the heaviest BW animal from each pen was collected. The samples were homogenized and stored at −20 ºC until freeze-dried, ground, and kept at 5 ºC until determining apparent ileal digestibility (AID). Following the same procedures as for the feed, DM, OM (organic matter), and CP were determined in the ileal contents. In addition, the inert marker, ingested during the entire study, was determined in the feed and ileal content by spectrophotometry ICP-OES (Optima 3200 RL, Perkin Elmer; Waltham, MA, USA). The digestibility coefficients of DM, OM, and CP were determined using the TiO2 ratio in the diet and ileal digesta according to the following equation:
where [TiO2]d and [N]d are the concentrations of the inert marker and the nutrient, respectively, in the diet, and [TiO2]f and [N]f are the concentrations of each one of them in the ileal digesta.
Statistical Analysis
Results are provided as means and their residual standard errors. Microbiological counts were log10-transformed. Data were analyzed in R software version 3.6.0 (R Core Team, 2013) using the lme4 package (Bates et al., 2015), lmer() function for adjusted linear mixed model. The model included the fixed effects of CP level, BM inclusion, and their interaction. Furthermore, the room was considered as random effect. Due to factorial arrangement, the main effects are discussed for responses in which the interaction between CP level and BM inclusion was not significant. For daily fecal consistency, data were subjected to frequency analysis using the fisher.test function in the same package.
For all analyzed data, the experimental unit was the pen (n = 8). Significance was set at P < 0.05, and P ≤ 0.10 was taken to denote a statistical trend. When treatment effects were established, Tukey’s test was used to determine whether means were significantly different.
Results
Upon arrival at the animal house, all piglets were in good health and adapted well to the new facilities and feed. The analyzed CP levels were 22.2% in S diets and 18.8% in L diets and a mild episode of diarrhea was noted with the S diet. Five casualties were produced (on days 4, 6, 8, and 12) in the absence of pharmacological support. Mortality during the study was under 6%: three humane euthanasias were indicated (1 L0; 1 S0; and 1 L1), and there were two spontaneous casualties (1 L0; and 1 L1). In one of the latter showing immobility of the limbs before death, a necropsy was performed and Streptococcus suis type II infection was confirmed by PCR on joint swabs. There were no differences in the number of causalities produced in each of the treatment groups (P > 0.05).
Growth performance parameters and fecal consistency
Initial BW was similar among treatments. The effects of the experimental treatments on BW, ADG, ADFI, and G:F are shown in Table 2. In the first week post-weaning, no significant effects were found. However, during the second week, the S diets had higher ADG (P = 0.011) leading to greater final BW (P = 0.019) and tended to improve G:F (P = 0.060) than the L diets. For the whole 2 weeks period after weaning, ADG was also higher in the animals fed the S diet (P = 0.019). An interaction effect of CP and BM levels was observed on G:F (P = 0.091) such that the S diet with BM supplementation tended to show a synergistic impact increasing this ratio.
Table 2.
Growth performance of weaned piglets (0 to 14 d post-weaning) according to dietary CP level and BM supplementation
| Treatment1 | CP level2 | BM suplementation2 | P-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S0 | S1 | L0 | L1 | S | L | 0 | 1 | RSE | CP level | BM supplementation | Interaction | |
| Initial BW, kg | 5.94 | 5.92 | 5.93 | 5.93 | 5.93 | 5.93 | 5.94 | 5.93 | 0.022 | 0.999 | 0.409 | 0.240 |
| Final BW, kg | 9.03 | 9.15 | 8.62 | 8.29 | 9.09 | 8.45 | 8.83 | 8.72 | 0.761 | 0.019 | 0.669 | 0.385 |
| ADG, g | ||||||||||||
| Week 1 | 130 | 136 | 109 | 92.0 | 133 | 100 | 119 | 114 | 44.38 | 0.152 | 0.800 | 0.625 |
| Week 2 | 313 | 328 | 279 | 252 | 321 | 265 | 296 | 290 | 40.40 | 0.011 | 0.771 | 0.306 |
| Overall | 222 | 232 | 194 | 172 | 227 | 183 | 208 | 202 | 35.67 | 0.019 | 0.747 | 0.376 |
| ADFI, g | ||||||||||||
| Week 1 | 301 | 224 | 236 | 252 | 263 | 244 | 268 | 238 | 48.04 | 0.441 | 0.217 | 0.062 |
| Week 2 | 542 | 442 | 513 | 470 | 492 | 492 | 528 | 456 | 79.98 | 0.217 | 0.997 | 0.624 |
| Overall | 386 | 333 | 375 | 361 | 359 | 368 | 380 | 347 | 59.22 | 0.774 | 0.280 | 0.521 |
| G:F, g/g | ||||||||||||
| Week 1 | 0.45 | 0.62 | 0.41 | 0.36 | 0.54 | 0.39 | 0.43 | 0.49 | 0.19 | 0.117 | 0.537 | 0.231 |
| Week 2 | 0.63 | 0.77 | 0.60 | 0.55 | 0.70 | 0.58 | 0.62 | 0.66 | 0.13 | 0.060 | 0.420 | 0.150 |
| Overall | 0.56xy | 0.72x | 0.54xy | 0.49y | 0.64 | 0.52 | 0.55 | 0.61 | 0.12 | 0.046 | 0.329 | 0.091 |
1Values are means for 8 pens.
2Values are means for 16 pens.
x-y Means within a row lacking a common superscript differ (P ≤ 0.10).
0, non-supplemented diet; 1, diet supplemented with BM at 1 kg/t; S0, S diet not supplemented; ADFI, average daily feed intake; ADG, average daily gain; BM, sodium butyrate protected by medium-chain fatty acid salts, DICOSAN (Norel S.A., Madrid, Spain); BW, body weight; CP, crude protein; G:F, gain:feed ratio.; L, low dietary CP level (18.8%); L0, L diet not supplemented; L1, L diet supplemented with BM at 1 kg/t. RSE, residual standard error; S, standard dietary CP level (22.2%); S1, S diet supplemented with BM at 1 kg/t
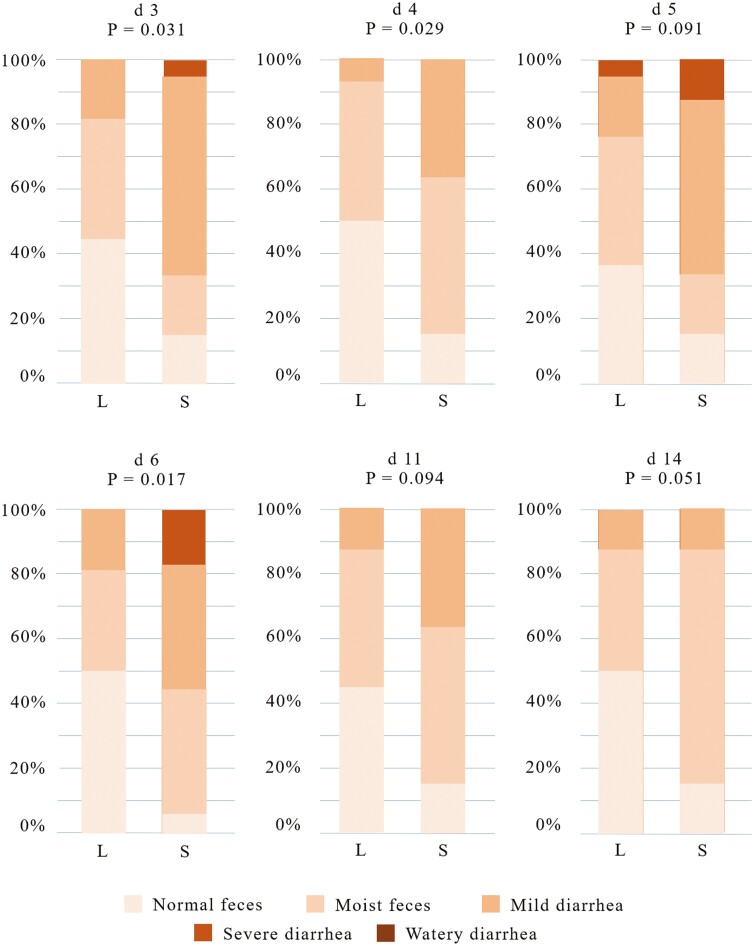
Regarding fecal consistency, an enhancement of consistency was observed during the first post-weaning week on days 3 (P = 0.031), 4 (P = 0.029), 5 (P = 0.091), and 6 (P = 0.017) and a tendency to improve fecal consistency on days 11 (P = 0.094) and 14 (P = 0.051) post-weaning was observed with the L diet (Figure 1). No differences were found related to dietary BM supplementation or interaction (data not shown).
Figure 1.
Percentage of animals included in the different fecal scores. S, standard dietary CP level (22.2%); L, low dietary CP level (18.8%). N = 8. P-values were obtained using Fisher’s Exact Test on R software.
Ileal histomorphometry
The effects of the experimental treatments on ileal histomorphometry are summarized in Table 3. No interactions between the main factors were observed. In the animals receiving S diets, a trend toward increased crypt depth was observed (P = 0.057). Furthermore, BM supplementation increased total goblet cell counts (P = 0.036).
Table 3.
Ileal histomorphometry of weaned piglets (day 14 post-weaning) according to dietary CP level and BM supplementation
| Treatment1 | CP level2 | BM suplementation2 | P-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ileum | S0 | S1 | L0 | L1 | S | L | 0 | 1 | RSE | CP level | BM supplementation | Interaction |
| Villus | ||||||||||||
| Height, µm | 268 | 286 | 266 | 299 | 277 | 283 | 267 | 293 | 46.01 | 0.798 | 0.276 | 0.757 |
| Goblet cells | 9.71 | 12.8 | 9.25 | 10.9 | 11.3 | 10.1 | 9.48 | 11.9 | 2.170 | 0.283 | 0.036 | 0.509 |
| IEL | 7.21 | 9.10 | 7.73 | 9.28 | 8.16 | 8.51 | 7.47 | 9.19 | 2.320 | 0.683 | 0.134 | 0.849 |
| Crypt | ||||||||||||
| Depth, µm | 226 | 218 | 213 | 204 | 223 | 209 | 220 | 211 | 13.46 | 0.057 | 0.198 | 0.956 |
| Mitosis | 0.69 | 0.51 | 0.69 | 0.59 | 0.60 | 0.64 | 0.69 | 0.55 | 0.030 | 0.750 | 0.248 | 0.750 |
| Villus:crypt ratio | 1.18 | 1.32 | 1.26 | 1.47 | 1.25 | 1.37 | 1.22 | 1.40 | 0.225 | 0.324 | 0.121 | 0.761 |
1Values are means for 8 pens.
2Values are means for 16 pens.
0, non-supplemented diet; 1, diet supplemented with BM at 1 kg/t; S0, S diet not supplemented; BM, sodium butyrate protected by medium-chain fatty acid salts, DICOSAN (Norel S.A., Madrid, Spain); L, low dietary CP level (18.8%); IEL, intraepithelial lymphocytes; L0, L diet not supplemented; L1, L diet supplemented with BM at 1 kg/t.; RSE, residual standard error; S, standard dietary CP level (22.2%); S1, S diet supplemented with BM at 1kg/t
Microbiological analysis
Microbial counts recorded in the ileum and colon are provided in Table 4. No interaction effect was detected between CP level and BM supplementation. However, CP level affected the Lactobacillus population such that animals receiving the L diets had higher counts of this bacterium in the ileum (P = 0.032) and lower counts in the colon (P = 0.032). The supplementation with BM was only noted to modulate the microbial counts in the colonic. Accordingly, animals fed diets that were supplemented with BM had higher Lactobacillus counts (P = 0.006) and lower counts of Enterobacteriaceae (P = 0.003), including coliforms (P = 0.003) and Escherichia coli (P = 0.022) in the colon, determining a significantly higher Lactobacillus:Enterobacteriaceae ratio (P = 0.007).
Table 4.
Ileal and colonic microbiota of weaned piglets (day 14 post-weaning) according to dietary CP level and BM supplementation
| Treatment1 | CP level2 | BM suplementation2 | P-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S0 | S1 | L0 | L1 | S | L | 0 | 1 | RSE | CP level | BM supplementation | Interaction | |
| Ileum | ||||||||||||
| Lactobacillus, log cfu/g FM | 8.64 | 8.11 | 8.79 | 8.89 | 8.38 | 8.84 | 8.72 | 8.50 | 0.407 | 0.032 | 0.300 | 0.135 |
| Enterobacteriaceae, log cfu/g FM | 5.72 | 5.27 | 4.86 | 4.85 | 5.50 | 4.86 | 5.29 | 5.06 | 1.094 | 0.252 | 0.676 | 0.700 |
| Total coliforms, log cfu/g FM | 5.34 | 4.34 | 4.48 | 4.1 | 4.84 | 4.29 | 4.91 | 4.22 | 1.194 | 0.365 | 0.255 | 0.610 |
| Escherichia coli, log cfu/g FM | 4.5 | 3.16 | 3.67 | 2.96 | 3.83 | 3.32 | 4.09 | 3.06 | 1.429 | 0.477 | 0.164 | 0.657 |
| Lactobacillus:Enterobacteriaceae ratio | 1.75 | 1.6 | 1.95 | 2.17 | 1.68 | 2.06 | 1.85 | 1.89 | 0.842 | 0.183 | 0.911 | 0.623 |
| Colon, log cfu/g FM | ||||||||||||
| Lactobacillus, log cfu/g FM | 9.10 | 9.31 | 9.05 | 9.14 | 9.21 | 9.10 | 9.08 | 9.23 | 0.098 | 0.032 | 0.006 | 0.250 |
| Enterobacteriaceae, log cfu/g FM | 6.81 | 5.35 | 7.93 | 5.64 | 6.08 | 6.79 | 7.37 | 5.50 | 1.555 | 0.236 | 0.003 | 0.480 |
| Total coliforms, log cfu/g FM | 6.51 | 5.14 | 7.73 | 5.46 | 5.83 | 6.60 | 7.12 | 5.30 | 1.074 | 0.180 | 0.003 | 0.424 |
| Escherichia coli, log cfu/g FM | 5.66 | 4.52 | 7.19 | 4.75 | 5.09 | 5.97 | 6.43 | 4.64 | 1.462 | 0.165 | 0.022 | 0.407 |
| Lactobacillus:Enterobacteriaceae ratio | 1.48 | 1.81 | 1.17 | 1.75 | 1.65 | 1.46 | 1.33 | 1.78 | 0.362 | 0.253 | 0.007 | 0.419 |
1Values are means for 8 pens.
2Values are means for 16 pens.
0, non-supplemented diet; 1, diet supplemented with BM at 1 kg/t; S0, S diet not supplemented; BM: sodium butyrate protected by medium-chain fatty acid salts, DICOSAN (Norel S.A., Madrid, Spain); L, low dietary CP level (18.8%); L0, L diet not supplemented; L1, L diet supplemented with BM at 1 kg/t; RSE, residual standard error; S, standard dietary CP level (22.2%); S1, S diet supplemented with BM at 1kg/t.
Intestinal fermentation
All the changes induced by the experimental treatments on the pH and fermentation products in the ileum and colon are summarized in Table 5. According to the experimental treatment, pH, ammonia concentration and digesta consistency did not vary in the ileum and colon (data not shown). CP level had no effect on SCFA. In the groups receiving the BM additive in the feed, there was a trend toward an increase in the molar concentration of acetic acid (P = 0.088) and a decrease in valeric acid (P = 0.002) in the colon.
Table 5.
pH and fermentation products in the ileum and colon of weaned piglets (day 14 post-weaning) according to dietary CP level and BM supplementation
| Treatment1 | CP level2 | BM suplementation2 | P-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S0 | S1 | L0 | L1 | S | L | 0 | 1 | RSE | CP level | BM supplementation | Interaction | |
| Ileum | ||||||||||||
| pH | 6.47 | 6.79 | 6.63 | 6.54 | 6.59 | 6.59 | 6.55 | 6.62 | 0.285 | 0.976 | 0.643 | 0.269 |
| NH3, μm/g | 1.43 | 1.69 | 1.21 | 1.80 | 1.56 | 1.51 | 1.32 | 1.75 | 0.720 | 0.912 | 0.235 | 0.693 |
| Total lactate, µmol/g | 35.7 | 25.8 | 37.1 | 30.0 | 30.8 | 33.5 | 36.4 | 27.9 | 1.761 | 0.755 | 0.342 | 0.876 |
| Total SCFA, µmol/g | 5.40 | 8.34 | 5.57 | 5.93 | 6.87 | 5.75 | 5.49 | 7.14 | 2.646 | 0.405 | 0.223 | 0.340 |
| Total acetate, % | 86.8 | 98.6 | 98.7 | 73.1 | 92.6 | 85.9 | 92.6 | 85.8 | 20.06 | 0.510 | 0.503 | 0.072 |
| Total butyrate, % | 0.93 | 1.42 | 1.31 | 1.94 | 1.18 | 1.63 | 1.12 | 1.68 | 2.023 | 0.658 | 0.581 | 0.948 |
| Colon | ||||||||||||
| pH | 6.17 | 6.09 | 6.09 | 5.9 | 6.13 | 6.00 | 6.13 | 6.00 | 0.209 | 0.194 | 0.219 | 0.570 |
| NH3, μm/g | 13.6 | 12.3 | 7.10 | 12.9 | 12.9 | 9.98 | 10.3 | 12.6 | 9.63 | 0.484 | 0.722 | 0.411 |
| Total lactate, µmol/g | 5.56 | 4.04 | 4.34 | 3.33 | 4.80 | 3.84 | 4.95 | 3.69 | 4.542 | 0.673 | 0.581 | 0.911 |
| Total SCFA, µmol/g | 118 | 130 | 127 | 126 | 124 | 126 | 122 | 128 | 13.28 | 0.694 | 0.397 | 0.291 |
| Total acetate, % | 55.3 | 59.2 | 55.9 | 57.3 | 57.2 | 56.6 | 55.6 | 58.2 | 3.004 | 0.661 | 0.088 | 0.407 |
| Total propionate, % | 24.5 | 22.4 | 23.7 | 24.7 | 23.4 | 24.2 | 24.1 | 23.5 | 1.856 | 0.411 | 0.555 | 0.102 |
| Total butyrate, % | 14.6 | 14.3 | 14.9 | 14.3 | 14.5 | 14.6 | 14.8 | 14.3 | 2.095 | 0.878 | 0.634 | 0.892 |
| Total valerate, % | 3.41 | 2.26 | 3.41 | 2.42 | 2.84 | 2.92 | 3.41 | 2.34 | 0.625 | 0.811 | 0.002 | 0.804 |
| Total BCFA, % | 2.22 | 1.93 | 2.12 | 1.34 | 2.08 | 1.73 | 2.17 | 1.64 | 0.676 | 0.313 | 0.125 | 0.478 |
0, non-supplemented diet; 1, diet supplemented with BM at 1 kg/t; S0, S diet not supplemented; BCFA, branched chain fatty acids; BM: sodium butyrate protected by medium-chain fatty acid salts, DICOSAN (Norel S.A., Madrid, Spain); L, low dietary CP level (18.8%); L0, L diet not supplemented; L1, L diet supplemented with BM at 1 kg/t; NH3, ammonia; RSE, residual standard error; S, standard dietary CP level (22.2%); S1, S diet supplemented with BM at 1kg/t; SCFA, short-chain fatty acids;.
1Values are means for 8 pens.
2Values are means for 16 pens.
Digestibility balances
The effects of the experimental treatments on ileal digestibility are provided in Table 6. No interaction effects between the main factors were detected. The L diets resulted in higher OM digestibility (P = 0.026). In addition, BM supplementation tended to increase DM digestibility (P = 0.057) and animals fed diets supplemented with BM showed higher OM digestibility (P = 0.003). There were no significant differences in CP digestibility between treatments.
Table 6.
Dry matter, organic matter, and crude protein apparent ileal digestibility (AID) coefficients of weaned piglets (day 14 post-weaning) according to dietary CP level and BM supplementation
| Treatment1 | CP level2 | BM suplementation2 | P-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AID, % | S0 | S1 | L0 | L1 | S | L | 0 | 1 | RSE | CP level | BM supplementation | Interaction |
| Dry matter | 93.13 | 94.78 | 93.37 | 94.81 | 93.96 | 94.09 | 93.25 | 94.80 | 1.556 | 0.861 | 0.057 | 0.890 |
| Organic matter | 38.27 | 56.51 | 53.79 | 59.3 | 47.39 | 56.55 | 46.03 | 57.91 | 6.436 | 0.026 | 0.003 | 0.153 |
| Crude protein | 50.14 | 63.35 | 59.99 | 65.72 | 56.75 | 62.86 | 55.07 | 64.54 | 8.581 | 0.417 | 0.119 | 0.787 |
0, non-supplemented diet; 1, diet supplemented with BM at 1 kg/t; S0, S diet not supplemented; BM: sodium butyrate protected by medium-chain fatty acid salts, DICOSAN (Norel S.A., Madrid, Spain); L, low dietary CP level (18.8%); L0, L diet not supplemented; L1, L diet supplemented with BM at 1 kg/t; RSE, residual standard error; S, standard dietary CP level (22.2%); S1, S diet supplemented with BM at 1kg/t.
1Values are means for 8 pens.
2Values are means for 16 pens.
Discussion
Effects of dietary CP level
The reduction of dietary CP level has been suggested as a potential strategy to enhance the gut health of weaned piglets, whose gut function is still not fully developed (Xiong et al., 2019). However, the question remains at what level of CP is limiting in terms of compromising the growth performance parameters (Millet et al., 2018). In this context, the lack of an impact of CP level on piglet growth performance observed during the first-week post-weaning in the present study is in agreement with previous reports indicating that growth performance is not reduced during the immediate postweaning period when the dietary CP is reduced by 2 or up to 4% to feed piglet with 18 or 19% CP diets (Manzanilla et al. 2009; Wu et al. 2015; Tang et al., 2019; Limbach et al., 2021). Nonetheless, during the second-week post-weaning, lower ADG and G:F were observed in piglets fed the L diets (18.8% CP), which contained 3.4% less CP than the S diets, affecting the overall period data. These findings agree with previous authors who observed that growth performance can begin to be compromised when dietary CP levels were reduced to 19% (Nyachoti et al., 2006; Pierce et al., 2007; Limbach et al., 2021) but disagree with other authors who did not observe impairment on growth performance parameters (Yue and Qiao, 2008; Wu et al., 2015; Lee et al., 2017). Furthermore, Toledo et al. (2014) evaluated further reductions to obtain diets from 20% up to 15% CP levels and no differences in growth performance parameters were found. The results variability by reducing dietary CP to feed piglets with 18% to 19% CP can be explained by the age of the piglets and the AA content of the diet (Nyachoti et al., 2006; Toledo et al., 2014). Indeed, reduction of soybean meal in the diet has been described to decrease isoleucine and histidine levels below FEDNA (2013) recommendations (Gloaguen et al. 2013). In this context, previous authors suggested that piglets can receive lower levels of isoleucine or histidine than recommended without compromising their growth performance (James et al., 2000; Toledo et al., 2014). However, the impairment of growth performance parameters observed by reducing dietary CP from 22.2% to 18.8% in the present study may be due to further limiting isoleucine levels alone or when combined with limiting histidine levels for piglets weaned at 21 days of age at an average weight of 5.9 kg. Further studies are needed to establish histidine and isoleucine requirements of piglets to reduce dietary CP without compromising growth performance and to promote intestinal health.
One of the impacts on the gut barrier of the reduced-CP diets tested in the present study was a lower fecal consistency score observed for 14 d post-weaning. In fact, considering that the first postweaning week is usually the stage during which most diarrhea is observed (Rodríguez-Sorrento et al., 2022), the significant improvement in fecal consistency during the first postweaning week in piglets that received reduced-CP diets indicates that this nutritional strategy is useful in mitigating postweaning diarrhea (Heo et al., 2008; Wang et al., 2018; Rodríguez-Sorrento et al., 2022). Previous authors attributed this improvement to increased expression of tight junctions and effects on intestinal morphology, such as increased villus height and reduced crypt depth by diets with reduced CP level by 2% to 6% to 19%, 18%, or 17% (Manzanilla et al., 2009; Wu et al., 2015; Limbach et al. 2021). In fact, the present study corroborates that piglets receiving a reduced-CP diet had a lower crypt depth and less diarrhea (Zhang et al., 2020). Furthermore, the higher Lactobacillus counts observed in the ileum of piglets fed reduced-CP diets agree with previous authors who observed less postweaning diarrhea (Wellock et al., 2008; Manzanilla et al., 2009; Tang et al., 2019). Thus, reducing dietary CP levels from 22.2% to 18.8% seems to allow Lactobacillus proliferation at the expense of minority bacteria in the ileum, while limiting the presence of this bacterial population in the colon.
With regard to microbiota, intestinal fermentation was also assessed. There were no differences in pH or microbial metabolites (NH3 and total VFA) among CP levels tested, as reported by other authors (Bikker et al., 2006; Htoo et al., 2007; Opapeju et al., 2009). However, some authors observed that as dietary CP levels increase, weaned piglets cannot produce enough hydrochloric acid, promoting bacteria proliferation as well as fermentation and microbial metabolites in the ileum and large intestine (Proházska and Baron, 1980; Nyachoti et al., 2006; Wang et al., 2018; Limbach et al., 2021). Therefore, the present reduction from 22.2% to 18.8% of dietary CP, seemed not to modify pH or microbial metabolite concentrations, although Lactobacillus counts were affected and fecal consistency improved.
The possible benefits for gut health of reducing CP intake from 22.2% to 18.8% in terms of AID were also examined. The working hypothesis was that this strategy could improve the digestion capacity of weaned piglets. The improved digestibility of OM observed could be related to the higher Lactobacillus counts observed in the ileum of piglets fed L diets which, as mentioned above, could thwart the growth of pathogenic bacteria (Manzanilla et al., 2009). Further, the improvement in fecal consistency noted supports the ability of this nutritional strategy to reduce the negative impact on the ileum of the post-weaning stress that disrupts digestive and absorptive capacity (Xiong et al., 2019). In addition, some authors (Fang et al., 2019; Yu et al., 2019) described improved CP digestibility in response to 2% to 6% reductions in dietary CP levels in piglets (to 21.7%, 17.7%, and 14%) and hypothesized that it could be due to a reduction in intestinal pH of piglets receiving a CP-reduced diet, improving proteolytic digestion and limiting the growth of some bacteria. It should be emphasized that the authors reporting improved CP digestibility worked with pigs with a more mature intestinal tract than in the present study. On the contrary, some authors (Bikker et al., 2006; Wellock et al., 2006) observed lower CP digestibility when reducing CP levels and comparing them to higher levels (from 23% and 22% to 18% and 17%, respectively). Nonetheless, other authors (Heo et al., 2008; Manzanilla et al., 2009) noted no differences in CP digestibility in response to CP reductions of 2 to 7 points to obtain diets with 16% to 19% for more immature pigs as those forming the present study population. Hence, reducing dietary CP from 22.2% to 18.8% emerged as a strategy to optimize the amount of CP in the feed of postweaning piglets without compromising its digestibility yet improving OM digestibility.
Thus, it seems that CP level reduction from 22.2% to 18.8% is a nutritional strategy that successfully impacts the gut barrier, improving fecal consistency throughout the post-weaning period, increasing Lactobacillus counts and OM digestibility in the ileum. In addition, although growth performance and gain:feed efficiency were reduced probably because isoleucine alone or isoleucine and histidine requirements were unlikely satisfied, the digestibility of DM and CP were not affected. Collectively, these observations suggest that limiting dietary CP levels could be an interesting strategy to reinforce the gut health of weaned piglets.
Effects of BM supplementation
Previous authors described improvement in growth performance in response to the supplementation with sodium butyrate or MCFA in free or protected forms at 1 to 3 kg/t in the diets of weaned piglets (Lu et al., 2008; Hanczakowska et al., 2013; Chiofalo et al., 2014). However, little is known about the impacts of this strategy when combined with different dietary CP levels. The trend observed here was better growth efficiency in piglets fed a standard diet supplemented with BM (S1). In contrast, Lee et al. (2021) described improved feed efficiency in piglets fed low-protein diets supplemented with protected organic acids (fumaric, citric, malic, and phosphoric acids) at 3 kg/t. These contradictory results may be due to the different FA included in the feed-additive used. The particular composition of the feed-additive tested in the present study may increase digestive enzyme secretion which, in turn, is more intense in heavier piglets with a larger pancreas (Lindemann et al., 1986; Amer et al., 2021). Thus, the interaction observed in the present study suggests that supplementation with BM in the S diets could increase the enzyme activity of piglets and, consequently, improve the gain:feed efficiency. Conversely, the growth of animals receiving the L diets was restricted so the effect of the additive to increase enzyme activity may have been limited.
As butyric acid and MCFA can potentially increase the intestinal absorption surface area (Lu et al., 2008; Hanczakowska et al., 2013; Chiofalo et al., 2014), dietary supplementation with BM was expected to improve fecal consistency. However, BM supplementation did not affect fecal score during the two weeks post-weaning. According to previous authors, sodium butyrate or MCFA may increase villus height and up-regulate the expression of tight junctions in the ileum resulting in lower diarrhea incidence (Lu et al., 2008; Hanczakowska et al., 2011; Huang et al., 2015). However, no effect on ileal villus height:crypt depth ratio was observed in the present study. This observation is in line with the results of studies examining the use of butyric acid or MCFA at 1 to 3 kg/t (Biagi et al., 2007; Hanczakowska et al., 2016). Nonetheless, other studies have showed beneficial effects by using sodium butyrate in the free form at 0.5 to 1 kg/t, or in the protected form at 0.5 to 3 kg/t, or of MCFA combined with other organic acids at 2 and 4 kg/t (Hanczakowska et al., 2013; Lei et al., 2017; Feng et al., 2018; Upadhaya et al., 2020). Thus, the dose of BM additive used in this study (50% sodium butyrate) of 1 kg/t may be too low for butyric acid and MCFA to affect fecal consistency and the ileal absorptive surface area.
Feed supplementation with BM at 1 kg/t was, nevertheless, able to increase the number of goblet cells in the ileal mucosa. This result agrees with previous studies that evaluated butyric acid and MCFA showing an increased proliferation of these mucin-secretory cells and higher mucin expression that reinforce the mucus layer as a chemical barrier (Burger-van Paassen et al., 2009). Nonetheless, this increase in the number of goblet cells has been observed in the jejunum of piglets fed MCFA as glycerides or in the ileum when butyric acid is directly infused (Diao et al., 2017; De Keyser et al., 2019). Thus, the impact observed in the present study on the ileum may be due to the protection of butyric acid in the BM additive in agreement with López-Colom et al. (2020). Hence, consistent with prior work, supplementation with BM by increasing goblet cell count suggests increased gut protection in piglets.
In addition, the antibacterial activity of butyric acid and MCFA (Lei et al., 2017; Tugnoli et al., 2020) could lead to improved gut development and a lower incidence of diarrhea in weaned piglets. However, no effects were observed here in the ileum while the antibacterial activity of the BM additive was confirmed in the colon increasing Lactobacillus and decreasing Enterobacteriaceae counts including Escherichia coli. This result agrees with different authors who described effects on distal intestine sections attributable to the slow release of acid from protected acids in sodium salt forms (Piva et al., 2007; Mallo et al., 2012). In fact, the protection of FA in the BM additive seems to reach impact distal segments of the intestinal tract. Previous authors suggested that the reduction of non-favorable microbial populations, such as Escherichia coli, and the increase of beneficial microorganisms may be attributed to the ability of fatty acids to regulate intestinal pH or diffuse into the bacterial cells (Hanczakowska et al., 2016; Jackman et al., 2020; Upadhaya et al., 2020). The results of the present study motivate the study of butyric acid and MCFA as a feed-additive to modulate intestinal microbiota that plays a crucial role in the intestinal health of weaned piglets (Celi et al., 2017).
Concerning microbial metabolites, although no differences in NH3 concentrations were observed, acetic acid levels rose in parallel with a drop in the levels of valeric acid in the colon of piglets supplemented. This increase in acetic acid content may be due to the selective promotion of acetogenic bacteria, alongside the butyrate itself included in the additive can be converted into acetic acid (Manzanilla et al., 2006; López-Colom et al., 2019). The reduction in valeric acid content may result from MCFA contained in the feed-additive with an inhibition of the production of protein-fermentative end products or the bacterial population responsible for their synthesis (Pierce et al., 2007; Światkiewicz et al. 2020). Hence, it seems that BM is able to modulate microbial counts and fermentation in the colon, although this was not reflected in fecal consistency.
In addition, an improvement in DM (tendency) and OM (significantly) AID were observed, according to previous studies that described higher nutrient digestibility by feed-supplementation with protected sodium butyrate and MCFA at 0.5 to 3 kg/t (Hanczakowska et al., 2011; Upadhaya et al., 2020). Such beneficial effects on nutrient digestibility have been attributed to the role of these FA in reinforcing the gut barrier by increasing villus height and minimizing pathogen proliferation. On the contrary, Manzanilla et al. (2006) and Le Gall et al. (2009) described the reduced ileal and total digestibility of DM and nitrogen associated with free sodium butyrate supplementation at 3 kg/t in weaned pig diets. These authors hypothesized that sodium butyrate has an inhibiting effect on amylolytic bacteria, diminishing starch utilization. Therefore, the present study shows that sodium butyrate (50%) protected by the salts of MCFA at 1 kg/t provided a sufficient amount of butyric acid and MCFA to improve DM (trend) and OM (significant) AID, and this may be attributed to the increased number of goblet cells in the ileal mucosa.
In conclusion, the present findings demonstrate that reduce dietary CP (18.8%) in weaned piglets promotes a reduction in the crypt depth, as well as beneficial microbial population in the ileum, and improves OM digestibility and fecal consistency. Also, the compromise of growth performance by reducing CP to 18.8% encourages further research to accurately limiting isoleucine and histidine requirements for weaned piglets. In turn, supplementation of the feed with sodium butyrate protected by MCFA salts at 1 kg/t may increase the layer of mucus by increasing the number of goblet cells, simultaneously modulating colonic microbial counts and enhancing dry matter and organic matter digestibility. Thus, both strategies may be considered to improve the gut health of weaned piglets. In fact, as effects emerged on different parameters of gut health, the present study provides direction for further studies designed to examine how these strategies could reduce the use of antibiotics and promote the well-being, health, and improved growth performance of piglets.
Acknowledgments
We thank Ana Burton BSc, a native English-speaking, for language and editorial assistance. This work was co-financed by the European Fund of Regional Development of the European Union within the framework of the FEDER operating program of Catalunya 2014-2020 (project COMRDI16-1-0033) and managed by ACCIÓ.
Glossary
Abbreviations
- 0
non-supplemented diet
- 1
diet supplemented with sodium butyrate protected by the sodium salts of medium-chain fatty acids at 1 kg/t
- AA
amino acid
- ADG
average daily gain
- ADFI
average daily feed intake
- G:F
gain:feed ratio
- AID
apparent ileal digestibility
- FA
fatty acids
- FEDNA
Federación Española para el desarrollo de la Nutrición Animal
- L
low protein
- MCFA
medium-chain fatty acids
- S
standard protein
- TiO2
titanium dioxide
- UAB
Universitat Autònoma de Barcelona
Contributor Information
Meritxell Sadurní, Animal Nutrition and Welfare Service, Department of Animal and Food Science, Universitat Autònoma de Barcelona, 08193 Bellaterra, Spain.
Ana Cristina Barroeta, Animal Nutrition and Welfare Service, Department of Animal and Food Science, Universitat Autònoma de Barcelona, 08193 Bellaterra, Spain.
Cinta Sol, Norel S.A., 28007 Madrid, Spain.
Mónica Puyalto, Norel S.A., 28007 Madrid, Spain.
Lorena Castillejos, Animal Nutrition and Welfare Service, Department of Animal and Food Science, Universitat Autònoma de Barcelona, 08193 Bellaterra, Spain.
Conflict of Interest Statement
The authors declare no real or perceived conflict of interest. M. P. and C.S. were employees of Norel S. A. As a collaborator with the project COMRDI16-1-033, Norel S. A. did not influence the data selection, interpretation, conclusions, or the decision on how or what to publish.
Literature Cited
- Amer, S. A., A-Nasser A., Al-Khalaifah H. S., AlSadek D. M. M., Abdel Fattah D. M., Roushdy E. M., Sherief W. R. I. A., Farag M. F. M., Altohamy D. E., . et al. 2021. Effect of dietary medium-chain α-monoglycerides on the growth performance, intestinal histomorphology, amino acid digestibility, and broiler chickens’ blood biochemical parameters. Animals. 11:57. doi: 10.3390/ani11010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC International. 2005. Official Methods of Analysis of AOAC International. 18th ed. AOAC International, Gaithersburg, MD. [Google Scholar]
- Barba-Vidal, E., Castillejos L., López-Colom P., Rivero Urgell M., Moreno Muñoz J. A., and Martín-Orúe S. M.. . 2017. Evaluation of the probiotic strain Bifidobacterium longum subsp. Infantis CECT 7210 capacities to improve health status and fight digestive pathogens in a piglet model. Front. Microbiol. 8:533. doi: 10.3389/fmicb.2017.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates, D., Mächler M., Bolker B., and Walker S.. . 2015. Fitting linear mixed-effects models using lme4. J Stat Software 67:1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- Biagi, G., Piva A., Moschini M., Vezzali E., and Roth F. X.. . 2007. Performance, intestinal microflora, and wall morphology of weanling pigs fed sodium butyrate. J. Anim. Sci. 85:1184–1191. doi: 10.2527/jas.2006-378. [DOI] [PubMed] [Google Scholar]
- Bikker, P., Dirkzwager A., Fledderus J., Trevisi P., le Huërou-Luron I., Lallès J. P., and Awati A.. . 2006. The effect of dietary protein and fermentable carbohydrates levels on growth performance and intestinal characteristics in newly weaned piglets. J. Anim. Sci. 84:3337–3345. doi: 10.2527/jas.2006-076. [DOI] [PubMed] [Google Scholar]
- Burger-van Paassen, N., Vincent A., Puiman P. J., van der Sluis M., Bouma J., Boehm G., van Goudoever J. B., van Seuningen I., and Renes I. B.. . 2009. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: implications for epithelial protection. Biochem. J. 420:211–219. doi: 10.1042/BJ20082222. [DOI] [PubMed] [Google Scholar]
- Celi, P., Cowieson A. J., Fru-Nji F., Steinert R. E., Kluenter A. M., and Verlhac V.. . 2017. Gastrointestinal functionality in animal nutrition and health: new opportunities for sustainable animal production. Anim. Feed Sci. Technol. 234:88–100. doi: 10.1016/j.anifeedsci.2017.09.012. [DOI] [Google Scholar]
- Chiofalo, B., Liotta L., Lo Presti V., Agnello A., Montalbano G., Marino A., and Chiofalo V.. . 2014. Dietary supplementation of free or microencapsulated sodium butyrate on weaned piglet performance. J. Nutr. Ecol. Food Res. 2:1–8. doi: 10.1166/jnef.2014.1053. [DOI] [Google Scholar]
- De Keyser, K., Dierick N., Kanto U., Hongsapak T., Buyens G., Kuterna L., and Vanderbeke E.. . 2019. Medium-chain glycerides affect gut morphology, immune and goblet cells in post-weaning piglets: in vitro fatty acid screening with Escherichia coli and in vivo consolidation with LPS challenge. J. Anim Physiol Anim Nutr. 103:221–230. doi: 10.1111/jpn.12998. [DOI] [PubMed] [Google Scholar]
- Diao, H., Jiao R., Yu B., He J., Yu J., Zheng P., Huang Z. Q., Luo Y. H., Luo J. Q., Mao X. B., . et al. 2017. Stimulation of intestinal growth with distal ileal infusion of short-chain fatty acid: a reevaluation in a pig model. RSC Adv. 7:30792–30806. doi: 10.1039/c7ra03730a. [DOI] [Google Scholar]
- Espinosa, C. D., Fry R. S., Usry J. L., and Stein H. H.. . 2017. Copper hydroxychloride improves growth performance and reduces diarrhea frequency of weanling pigs fed a corn–soybean meal diet but does not change apparent total tract digestibility of energy and acid hydrolyzed ether extract. J. Anim. Sci. 95:5447–5454. doi: 10.2527/jas2017.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Parliament and European Council. 2018. Regulation (EC) No. 2019/6 of the European Parliament and of the Council of 11 December 2018 on veterinary medicinal products and repealing Directive 2001/83/EC Off. J. Eur. Union L:4–43. [Google Scholar]
- European Parliament. 2010. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. L:276-33. [Google Scholar]
- Fang, L. H., Jin Y. H., Do S. H., Hong J. S., Kim B. O., Han T. H., and Kim Y. Y.. . 2019. Effects of dietary energy and crude protein levels on growth performance, blood profiles, and nutrient digestibility in weaning pigs. Asian-Australas. J. Anim. Sci. 32:566–563. doi: 10.5713/ajas.18.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, W., Wu Y., Chen G., Fu S., Li B., Wang B. D., Wang W., and Liu J.. . 2018. Sodium butyrate attenuates diarrhea in weaned piglets and promotes tight junction protein expression in colon in a GPR109A-dependent manner. Cell. Physiol. Biochem. 47:1617–1629. doi: 10.1159/000490981. [DOI] [PubMed] [Google Scholar]
- Fundación Española para el Desarrollo de la Nutrición Animal. 2013. Necesidades nutricionales para ganado porcino: NORMAS FEDNA, Madrid, Spain. [Google Scholar]
- Gloaguen, M., Le Floc’h N., Primot Y., Corrent E., and van Milgen J.. . 2013. Response of piglets to the standardized ileal digestible isoleucine, histidine and leucine supply in cereal-soybean meal-based diets. Animal 7:901–908. doi: 10.1017/S1751731112002339. [DOI] [PubMed] [Google Scholar]
- Hanczakowska, E., Szewczyk A., and Okon K.. . 2011. Effects of dietary caprylic and capric acids on piglet performance and mucosal epithelium structure of the ileum. J. Anim. Feed Sci. 20:556545–556565. doi: 10.22358/jafs/66213/2011. [DOI] [Google Scholar]
- Hanczakowska, E., Szewczyk A., Świątkiewicz M., and Okoń K.. . 2013. Short- and medium- chain fatty acids as a feed supplement for weaning and nursery pigs. Pol. J. Vet. Sci. 16:647–654. doi: 10.2478/pjvs-2013-0092. [DOI] [PubMed] [Google Scholar]
- Hanczakowska, E., Światkiewicz M., Natonek-Wiśniewska M., and Okoń K.. . 2016. Medium chain fatty acids (MCFA) and/or probiotic Enterococcus faecium as a feed supplement for piglets. Livest. Sci. 192:1–7. doi: 10.1016/j.livsci.2016.08.002. [DOI] [Google Scholar]
- Heo, J. M., Kim J. C., Hansen C. F., Mullan B. P., Hampson D. J., and Pluske J. R.. . 2008. Effects of feeding low protein diets to piglets on plasma urea nitrogen, faecal ammonia nitrogen, the incidence of diarrhea and performance after weaning. Arch. Anim. Nutr. 62:343–358. doi: 10.1080/17450390802327811. [DOI] [PubMed] [Google Scholar]
- Htoo, J. K., Araiza B. A., Sauer W. C., Rademacher M., Zhang Y., Cervantes M., and Zijlstra R. T.. . 2007. Effect of dietary protein content on ileal amino acid digestibility, growth performance, and formation of microbial metabolites in ileal and cecal digesta of early-weaned pigs. J. Anim. Sci. 85:3303–3312. doi: 10.2527/jas.2007-0105. [DOI] [PubMed] [Google Scholar]
- Huang, C., Song P., Fan P., Hou C., Thacker P., and Ma X.. . 2015. Dietary sodium butyrate decreases postweaning diarrhea by modulating intestinal permeability and changing the bacterial communities in weaned piglets. J. Nutr. Immunol. 145:2774–2780. doi: 10.3945/jn.115.217406. [DOI] [PubMed] [Google Scholar]
- Jackman, J. A., Boyd R. D., and Elrod C. C.. . 2020. Medium-chain fatty acids and monoglycerides as feed additives for pig production: towards gut health improvement and feed pathogen mitigation. J. Anim. Sci. Biotechnol. 11:44. doi: 10.1186/s40104-020-00446-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, B. W., Goodband R. D., Tokach M. D., Nelssen J. L., and DeRouchey J. M.. . 2000. The optimum isoleucine:lysine ratio in starter diets to maximize growth performance of the early-weaned pig. Kans. Agric. Exp. Stn. Res. Rep. 0:20–26. doi: 10.4148/2378-5977.6677. [DOI] [Google Scholar]
- Jensen, M. T., Cox R. P., and Jensen B. B.. . 1995. Microbial production of skatole in the hind gut of pigs given different diets and its relation to skatole deposition in backfat. Anim. Sci. 61:293–304. doi: 10.1017/s1357729800013837. [DOI] [Google Scholar]
- Lallès, J. P., Bosi P., Smidt H., and Stokes C. R.. . 2007. Nutritional management of gut health in pigs around weaning. Proc. Nutr. Soc. 66:260–268. doi: 10.1017/S0029665107005484. [DOI] [PubMed] [Google Scholar]
- de Lange, C. F. M., Pluske J. R., Gong J., and Nyachoti C. M.. . 2010. Strategic use of feed ingredients and feed additives to stimulate gut health and development in young pigs. Livest. Sci. 134:124–134. doi: 10.1016/j.livsci.2010.06.117. [DOI] [Google Scholar]
- Lee, J. H., Kim H. B., Yun W., Kwak W. G., Lee C. H., Oh H. J., Kim D. W., Song M. H., and Cho J. H.. . 2017. Effects of reducing dietary crude protein and metabolic energy in weaned piglets. S. Afr. J. Anim. Sci. 47:574–581. doi: 10.4314/sajas.v47i4.16. [DOI] [Google Scholar]
- Lee, J. S., Kim T. H., Song M. H., Oh H. J., Yun W., Lee J. H., Kim Y. J., Lee B. K., Kim H. B., and Cho J. H.. . 2021. Effects of microencapsulated organic acids on growth performance, nutrient digestibility, fecal microbial counts, and blood profiles in weaning pigs. J. Anim. Sci. Technol. 63:104–113. doi: 10.5187/jast.2021.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall, M., Gallois M., Sève B., Louveau I., Holst J. J., Oswald I. P., Lallès J. P., and Guilloteau P.. . 2009. Comparative effect of orally administered sodium butyrate before or after weaning on growth and several indices of gastrointestinal biology of piglets. Br. J. Nutr. 102:1285–1296. doi: 10.1017/S0007114509990213. [DOI] [PubMed] [Google Scholar]
- Lei, X. J., Park J. W., Baek D. H., Kim J. K., and Kim I. H.. . 2017. Feeding the blend of organic acid and medium chain fatty acids reduces the diarrhea in piglets orally challenged with enterotoxigenic Escherichia coli K88. Anim. Feed Sci. Technol. 224:46–51. doi: 10.1016/j.anifeedsci.2016.11.016. [DOI] [Google Scholar]
- Limbach, J. R., Espinosa C. D., Perez-Calvo E., and Stein H. H.. . 2021. Effect of dietary crude protein level on growth performance, blood characteristics and indicators of intestinal health in weanling pigs. J. Anim. Sci. 99:skab166. doi: 10.1093/jas/skab166/6279783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann, M. D., Cornelius S. G., El Kandelgy S. M., Moser R. L., and Pettigrew J. E.. . 1986. Effect of age, weaning and diet on digestive enzyme levels in the piglet. J. Anim. Sci. 62:1298–1307. doi: 10.2527/jas1986.6251298x. [DOI] [PubMed] [Google Scholar]
- López-Colom, P., Castillejos L., Barba-Vidal E., Zhu Y., Puyalto M., Mallo J. J., and Martín-Orúe S. M.. . 2019. Response of gastrointestinal fermentative activity and colonic microbiota to protected sodium butyrate and protected sodium heptanoate in weaned piglets challenged with ETEC F4+. Arch. Anim. Nutr. 73:339–359. doi: 10.1080/1745039X.2019.1641376. [DOI] [PubMed] [Google Scholar]
- López-Colom, P., Castillejos L., Rodríguez-Sorrento A., Puyalto M., Mallo J. J., and Martín-Orúe S. M.. . 2020. Impact of in-feed sodium butyrate or sodium heptanoate protected with medium-chain fatty acids on gut health in weaned piglets challenged with Escherichia coli F44+. Arch. Anim. Nutr. 74:271–295. doi: 10.1080/1745039X.2020.1726719. [DOI] [PubMed] [Google Scholar]
- Lu, J. J., Zou X. T., and Wang Y. M.. . 2008. Effects of sodium butyrate on the growth performance, intestinal microflora and morphology of weanling pigs. J. Anim. Feed Sci. 17:568–578. doi: 10.22358/jafs/66685/2008. [DOI] [Google Scholar]
- Lynegaard, J. C., Kjeldsen N. J., Bache J. K., Weber N. R., Hansen C. F., Nielsen J. P., and Amdi C.. . 2021. Low protein diets without medicinal zinc oxide for weaned pigs reduced diarrhea treatments and average daily gain. Animal. 15:100075. doi: 10.1016/j.animal.2020.100075. [DOI] [PubMed] [Google Scholar]
- Mallo, J. J., Balfagón A., Gracia M. I., Honrubia P., and Puyalto M.. . 2012. Evaluation of different protections of butyric acid aiming for release in the last part of the gastrointestinal tract of piglets. J. Anim. Sci. 90:227–229. doi: 10.2527/jas.53959. [DOI] [PubMed] [Google Scholar]
- Manzanilla, E. G., Nofrarías M., Anguita M., Castillo M., Pérez J. F., Martín-Orúe S. M., Kamel C., and Gasa J.. . 2006. Effects of butyrate, avilamycin, and a plant extract combination on the intestinal equilibrium of early-weaned pigs. J. Anim. Sci. 84:2743–2751. doi: 10.2527/jas.2005-509. [DOI] [PubMed] [Google Scholar]
- Manzanilla, E. G., Pérez J. F., Martín M., Blandón J. C., Baucells F., Kamel C., and Gasa J.. . 2009. Dietary protein modifies effects of plant extracts in the intestinal ecosystem of the pig at weaning. J. Anim. Sci. 87:2029–2037. doi: 10.2527/jas.2008-1210. [DOI] [PubMed] [Google Scholar]
- Millet, S., Aluwé M., De Boewer J., De Witte B., Douidah L., Broeke A. V., Leen F., De Cuyper C., Amper B., and De Campeneere S.. . 2018. The effect of crude protein reduction on performance and nitrogen metabolism in piglets (four to nine weeks of age) fed two dietary lysine levels. J. Anim. Sci. 96:3824–3836. doi: 10.1093/jas/sky254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeser, A. J., Pohl C. S., and Rajput M.. . 2017. Weaning stress and gastrointestinal barrier development: Implications for lifelong gut health in pigs. Anim. Nutr 3:313–321. doi: 10.1016/j.aninu.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nofrarías, M., Manzanilla E. G., Pujols J., Gibert X., Majó N., Segalés J., and Gasa J.. . 2006. Effect of spray-dried porcine plasma and plant extracts on intestinal morphology and on leukocyte cell subsets of weaned pigs. J. Anim. Sci. 84:2735–2742. doi: 10.2527/jas.2005-414. [DOI] [PubMed] [Google Scholar]
- Nyachoti, C. M., Omogbenigun F. O., Rademacher M., and Blank G.. . 2006. Performance responses and indicators of gastrointestinal health in early-weaned pigs fed low-protein amino acid-supplemented diets. J. Anim. Sci. 84:125–134. doi: 10.2527/2006.841125x. [DOI] [PubMed] [Google Scholar]
- Opapeju, F. O., Krause D. O., Payne R. L., Rademacher M., and Nyachoti C. M.. . 2009. Effect of dietary protein level on growth performance, indicators of enteric health, and gastrointestinal microbial ecology of weaned pigs induced with postweaning colibacillosis. J. Anim. Sci. 87:2635–2643. doi: 10.2527/jas.2008-1310. [DOI] [PubMed] [Google Scholar]
- Pierce, K. M., Callan J. J., McCarthy P., and O’Doherty J. V.. . 2007. The interaction between lactose level and crude protein concentration on piglet post-weaning performance, nitrogen metabolism, selected faecal microbial populations and faecal volatile fatty acid concentrations. Anim. Feed Sci. Technol. 132:267–282. doi: 10.1016/j.anifeedsci.2006.02.010. [DOI] [Google Scholar]
- Piva, A., Pizzamiglio V., Morlacchini M., Tedeschi M., and Piva G.. . 2007. Lipid microencapsulation allows slow release of organic acids and natural identical flavors along the swine intestine. J. Anim. Sci. 85:486–493. doi: 10.2527/jas.2006-323. [DOI] [PubMed] [Google Scholar]
- Prohászka, L., and Baron F.. . 1980. The predisposing role of high dietary protein supplies in enteropathogenic E. coli infections of weaned pigs Zbl.Vet. Med. 27:222–232. doi: 10.1111/j.1439-0450.1980.tb01908.x. [DOI] [PubMed] [Google Scholar]
- R Core Team. 2013. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Rattingan, R., Sweeney T., Maher S., Ryan M. T., Thornton K., and O’Doherty J. V.. . 2020. Effects of reducing crude protein concentration and supplementation with either laminarin or zinc oxide on the growth performance and intestinal health of newly weaned pigs. Anim. Feed Sci. Technol. 270:114693. doi: 10.1111/jpn.13428. [DOI] [Google Scholar]
- Richardson, A. J., Calder A. G., Stewart C. S., and Smith A.. . 1989. Simultaneous determination of volatile and non-volatile acidic fermentation products of anaerobes by capillary gas chromatography. Lett. Appl. Microbiol. 9:5–8. doi: 10.1111/j.1472-765X.1989.tb00278.x. [DOI] [Google Scholar]
- Rodríguez-Sorrento, A., Castillejos L., López-Colom P., Cifuentes-Orjuela G., Moreno-Muñoz J. A., and Martín-Orúe S. M.. . 2022. Assessment of the effects of the synbiotic combination of Bifidobacterium longum subsp. infantis CECT 7210 and oligofructose-enriched inulin against digestive bacterial infections in a piglet model. Front. Microbiol. 8:13–831737. doi: 10.3389/fmicb.2022.831737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Światkiewicz, M., Hanczakowska E., Okoń K., Kowalczyk P., and Grela E. R.. . 2020. Effect of maternal diet and medium chain fatty acids supplementation for piglets on their digestive tract development, structure, and chime acidity as well as performance and health status. Animals 10:834. doi: 10.3390/ani10050834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, W., Qian Y., Yu B., Zhang T., Gao J., He J., Huang Z., Zheng P., Mao X., Luo J., . et al. 2019. Effects of Bacillus subtilis DSM32315 supplementation and dietary crude protein level on performance, gut barrier function and microbiota profile in weaned piglets. J. Anim. Sci. 97:2125–2138. doi: 10.1093/jas/skz090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo, J. B., Furlan A. C., Pozza P. C., Carvalho P. L. O., Peñuela-Sierra L. M., and Huepa L. M. D.. . 2014. Effect of the reduction of the crude protein content of diets supplemented with essential amino acids on the performance of piglets weighing 6-15 kg. Livest. Sci. 168:94–101. doi: 10.1016/j.livsci.2014.07.006. [DOI] [Google Scholar]
- Tsiloyiannis, V. K., Kyriakis S. C., Vlemmas J., and Sarris K.. . 2001. The effect of organic acids on the control of porcine post-weaning diarrhoea. Res. Vet. Sci. 70:287–293. doi: 10.1053/rvsc.2001.0476. [DOI] [PubMed] [Google Scholar]
- Tugnoli, B., Giovagnoni G., Piva A., and Grilli E.. . 2020. From acidifiers to intestinal health enhancers: How organic acids can improve growth efficiency of pigs. Animals 10:134. doi: 10.3390/ani10010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhaya, S. D., Jiao Y., Kim Y. M., Lee K. Y., and Kim I. H.. . 2020. Coated sodium butyrate supplementation to a reduced nutrient diet enhanced the performance and positively impacted villus height and faecal and digesta bacterial composition in weaner pigs. Anim. Feed.Sci. Technol. 265:114534. doi: 10.1016/j.anifeedsci.2020.114534. [DOI] [Google Scholar]
- Van Soest, P. V., Robertson J. B., and Lewis B. A.. . 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. Dairy Sci. 74:3583–3597. doi: 10.3168/JDS.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- Wang, Y., Zhou J., Wang G., Cai S., Zeng X., and Qiao S.. . 2018. Advances in low-protein diets for swine. J. Anim. Sci. Biotechnol. 9:60. doi: 10.1186/s40104-018-0276-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellock, I. J., Fortomaris P. D., Houdijk J. G. M., and Kyriazakis I.. . 2006. The effect of dietary protein supply on the performance risk of post-weaning enteric disorders in newly weaned pigs. Anim. Sci. 82:327–335. doi: 10.1079/ASC200643. [DOI] [Google Scholar]
- Wellock, I. J., Fortomaris P. D., Houdijk J. G. M., and Kyriazakis I.. . 2008. Effects of dietary protein supply, weaning age and experimental enterotoxigenic Escherichia coli infection on newly weaned pigs: health. Animal 2:825–833. doi: 10.1017/S1751731108001559. [DOI] [PubMed] [Google Scholar]
- Wu, Y., Jian Z., Zheng C., Wang L., Zhu C., Yang X., Wen X., and Ma X.. . 2015. Effects of protein sources and level in antibiotic-free diets on diarrhea, intestinal morphology, and expression of tight junction in weaned piglets. Anim. Nutr. 1:170–176. doi: 10.1016/j.aninu.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, X., Tan B., Song M., Ji P., Kim K., Yin Y., and Liu Y.. . 2019. Nutritional intervention for the intestinal development and health of weaned pigs. Front. Vet. Sci. 6:46. doi: 10.3389/fvets.2019.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, D., Zhu W., and Hang S.. . 2019. Effects of low-protein diet on the intestinal morphology, digestive enzyme activity, blood urea nitrogen, and gut microbiota and metabolites in weaned pigs. Arch. Anim. Nutr. 73:287–305. doi: 10.1080/1745039X.2019.1614849. [DOI] [PubMed] [Google Scholar]
- Yue, L. Y., and Qiao S. Y.. . 2008. Effects of low-protein diets supplemented with crystalline amino acids on performance and intestinal development in piglets over the first 2 weeks after weaning. Livest. Sci. 115:144–152. doi: 10.1016/j.livsci.2007.06.018. [DOI] [Google Scholar]
- Zhang, W. X., Zhang Y., Deng X. W., Liu J. X., He M. L., and Wang H. F.. . 2020. Effects of dietary supplementation of tribuytirin and essential oils on gut health and microbiota of weaned piglets. Animals, 10:180. doi: 10.3390/ani10020180. [DOI] [PMC free article] [PubMed] [Google Scholar]