Keywords: glomerular filtration rate, clinical epidemiology, chronic kidney disease
Abstract
Significance Statement
A national task force convened by the NKF-ASN recently recommended a new race-free creatinine equation for calculating eGFR. Although this equation is expected to be widely adopted, its broad effect on recommended clinical care across the eGFR spectrum and across different racial and ethnic groups is not known. The authors used nationally representative data from 44,360 participants in NHANES to quantify expected changes to recommended care. They found that nationwide implementation of the new creatinine-based eGFR equation may affect recommended care for hundreds of thousands of Black adults and millions of non-Black adults, including new CKD diagnoses and reversals, CKD stage reclassifications, and changes in kidney donation eligibility, nephrologist referral, and medication dosing.
Background
The National Kidney Foundation and American Society of Nephrology Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease recently recommended a new race-free creatinine-based equation for eGFR. The effect on recommended clinical care across race and ethnicity groups is unknown.
Methods
We analyzed nationally representative cross-sectional questionnaires and medical examinations from 44,360 participants collected between 2001 and 2018 by the National Health and Nutrition Examination Survey. We quantified the number and proportion of Black, White, Hispanic, and Asian/Other adults with guideline-recommended changes in care.
Results
The new equation, if applied nationally, could assign new CKD diagnoses to 434,000 (95% confidence interval [CI], 350,000 to 517,000) Black adults, reclassify 584,000 (95% CI, 508,000 to 667,000) to more advanced stages of CKD, restrict kidney donation eligibility for 246,000 (95% CI, 189,000 to 303,000), expand nephrologist referrals for 41,800 (95% CI, 19,800 to 63,800), and reduce medication dosing for 222,000 (95% CI, 169,000 to 275,000). Among non-Black adults, these changes may undo CKD diagnoses for 5.51 million (95% CI, 4.86 million to 6.16 million), reclassify 4.59 million (95% CI, 4.28 million to 4.92 million) to less advanced stages of CKD, expand kidney donation eligibility for 3.96 million (95% CI, 3.46 million to 4.46 million), reverse nephrologist referral for 75,800 (95% CI, 35,400 to 116,000), and reverse medication dose reductions for 1.47 million (95% CI, 1.22 million to 1.73 million). The racial and ethnic mix of the populations used to develop eGFR equations has a substantial effect on potential care changes.
Conclusion
The newly recommended 2021 CKD-EPI creatinine-based eGFR equation may result in substantial changes to recommended care for US patients of all racial and ethnic groups.
For decades, clinical laboratories have used race in estimates of kidney function.1 In response to calls to reconsider this practice and growing recognition of race as a social and not biologic construct,2–4 the National Kidney Foundation (NKF) and American Society of Nephrology (ASN) Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease recommended nationwide implementation of a new creatinine-based equation developed by the CKD Epidemiology Collaboration (CKD-EPI) for the eGFR.5,6 The new 2021 CKD-EPI eGFR equation is based on serum creatinine, was developed in a population with 31.5% Black adults, and does not use race. Given the central role of eGFR in diagnosing and staging CKD, informing medication selection and dosing, and determining eligibility for kidney donation, kidney transplantation, specialist referral, and clinical trials, data are needed to understand and inform changes to care that may result from implementing the new equation.
The new equation is expected to be adopted widely. Although expected redistribution across GFR categories and comparisons to measured GFR have been reported,6–8 the broad implications for clinical care remain unclear. We use nearly two decades of data from a nationally representative sample of US adults to quantify changes to recommended care after nationwide implementation of the new eGFR equation on Black, White, Hispanic, and Asian/Other adults. We estimate changes relative to the 2006 Modification of Diet in Renal Disease (MDRD) equation,9 the most commonly used equation in the United States, and to the 2009 CKD-EPI equation,10 the more accurate and more recently recommended equation. We also show how outcomes are sensitive to the race and ethnicity mix of the study population used to develop new equations.
Methods
Setting and Participants
We conducted cross-sectional analyses using questionnaire, laboratory, and drug prescription data from nine survey cycles of the National Health and Nutrition Examination Survey (NHANES) conducted by the Centers for Disease Control and Prevention.11 Our study population constitutes a nationally representative sample of the noninstitutionalized civilian US population spanning 2001–2018. All study participants provided written informed consent following a research protocol approved by the National Center for Health Statistics institutional review board.
Of 91,351 total participants, we removed 40,606 participants (44.5%) with censored age or age <18 years, an additional 1353 participants (1.5%) with self-reported pregnancy or positive urine pregnancy test, and an additional 5032 participants (5.5%) with missing measurements for serum creatinine. Of 44,360 remaining participants, 18,598 (41.9%) self-identified as “non-Hispanic White,” 11,812 (26.6%) self-identified as “Mexican American” or “Other Hispanic,” 9522 (21.5%) self-identified as “non-Hispanic Black,” and 4428 (9.98%) self-identified as “Other Race—Including Multi-Racial” (Supplemental Table 1A). Given that 77.2% of “Other Race—Including Multi-Racial” adults further self-identified as “non-Hispanic Asian” in NHANES 2011–2018, we refer to this group hereafter as “Asian/Other.” We will also omit the “non-Hispanic” modifier for simplicity. Serum creatinine values were corrected to standardized measurements when appropriate, in accordance with the NHANES analytic notes.12
Statistical Analysis
We calculated the number and proportion of adults with revised disease classifications and clinical recommendations on the basis of eGFR computed using prior (2006 MDRD and 2009 CKD-EPI) and revised (2021 CKD-EPI) creatinine-based equations, including absolute and relative changes. CKD was defined as eGFR<60 ml/min/1.73 m2 or urine albumin-creatinine ratio >30 mg/g without the chronicity requirement. GFR thresholds for staging and clinical recommendations were assigned using the 2012 Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline for the Evaluation and Management of CKD13 and the 2007 American Association of Family Physicians (AAFP) Annual Clinical Focus on Management of Chronic Illness.14 Cases of dose reduction were identified on the basis of GFR thresholds among participants documented as taking those drugs. We used the provided survey weights and design variables to account for undersampling, nonresponse, and the NHANES survey structure.15 To account for missing serum creatinine values, we redistributed weights from participants with missing values to participants of the same race and ethnicity, gender, and age strata with recorded values. Summary statistics on relevant demographic and medical characteristics were not significantly different between adults with and without serum creatinine values (Supplemental Table 1B). A separate analysis of CKD prevalence and medication dosing changes was conducted using four alternative equations derived by CKD-EPI6 using study populations resampled to have different proportions of Black participants in the development data. Proportions were chosen to reflect recently proposed or implemented approaches to remove race from eGFR, ranging from 0% Black (similar to direct elimination of the race adjustment) to 100% Black (equivalent to universalizing race adjustment); intermediate are proportional representation (13% Black) and even representation (50% Black). All analyses were performed using R version 4.0.0.16
Outcomes
Study outcomes included changes in eGFR distribution, eGFR category, CKD diagnosis, eligibility for kidney donation and kidney transplantation, coverage by Medicare for medical nutrition therapy and kidney disease education, and recommendations for nephrologist referral, pre-emptive arteriovenous fistula placement, medication dose reductions, and drug contraindications. Drug classes included angiotensin-converting enzyme (ACE) inhibitors, angiotensin-II receptor blockers (ARBs), antibiotics, anticoagulants, anticonvulsants, antihyperglycemics, beta-blockers, diuretics, opioids, and statins (Supplemental Table 2). GFR-dependent recommendations for each drug class were derived from KDIGO or AAFP guidelines.13,14 In the one patient with conflicting recommendations (glipizide), the more recent KDIGO guideline was preferred. When KDIGO guidelines specified a drug class and AAFP guidelines specified individual drugs, only the individual drugs listed by AAFP were included; otherwise, all applicable entries in the NHANES prescription drug tables were included (Supplemental Table 2).
Results
Changes to eGFR: Decreased in Black Adults and Increased in Non-Black Adults
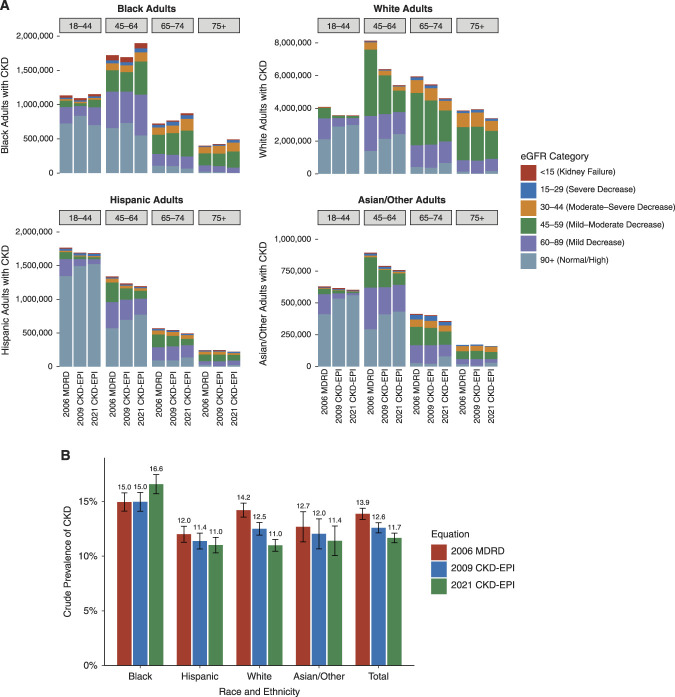
Relative to the 2006 MDRD equation, the 2021 CKD-EPI creatinine-based equation generally produced similar eGFR values for Black adults and higher eGFR values for White, Hispanic, and Asian/Other adults, with median changes of −3.53, +11.8, +10.1, and +11.4 ml/min per 1.73 m2, respectively (Figure 1, A and B). Relative to the 2009 CKD-EPI equation, the new equation generally produced lower eGFR values for Black adults and higher eGFR values for White, Hispanic, and Asian/Other adults, with median changes of −10.5, +3.95, +3.27, and +3.63 ml/min per 1.73 m2 (Figure 1, C and D). Changes varied significantly across age groups and at various ends of the GFR spectrum (Figure 2A). In general, changing from the 2009 CKD-EPI creatinine-based equation affected a similar number of Black adults but fewer non-Black adults compared with changing from the 2006 MDRD equation. Unless specified, all results hereafter pertain to changes with respect to the 2006 MDRD equation.
Figure 1.
Distribution of eGFR in US adults by race and ethnicity with new and prior equations.
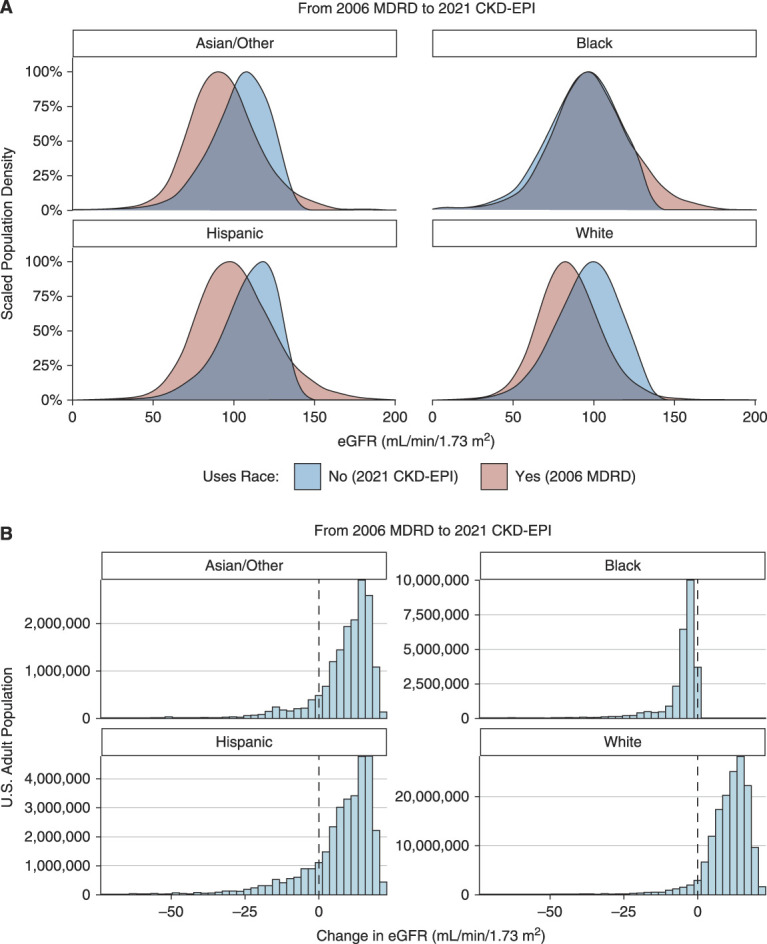
(A) Density functions for the eGFR in US adults when computed using the 2021 CKD-EPI equation and the 2006 MDRD equation. Estimates were survey weighted to be representative of the 2001–2018 US population. (B) Histogram for the change in estimated GFR in US adults when switching from the 2006 MDRD equation to the 2021 CKD-EPI equation.
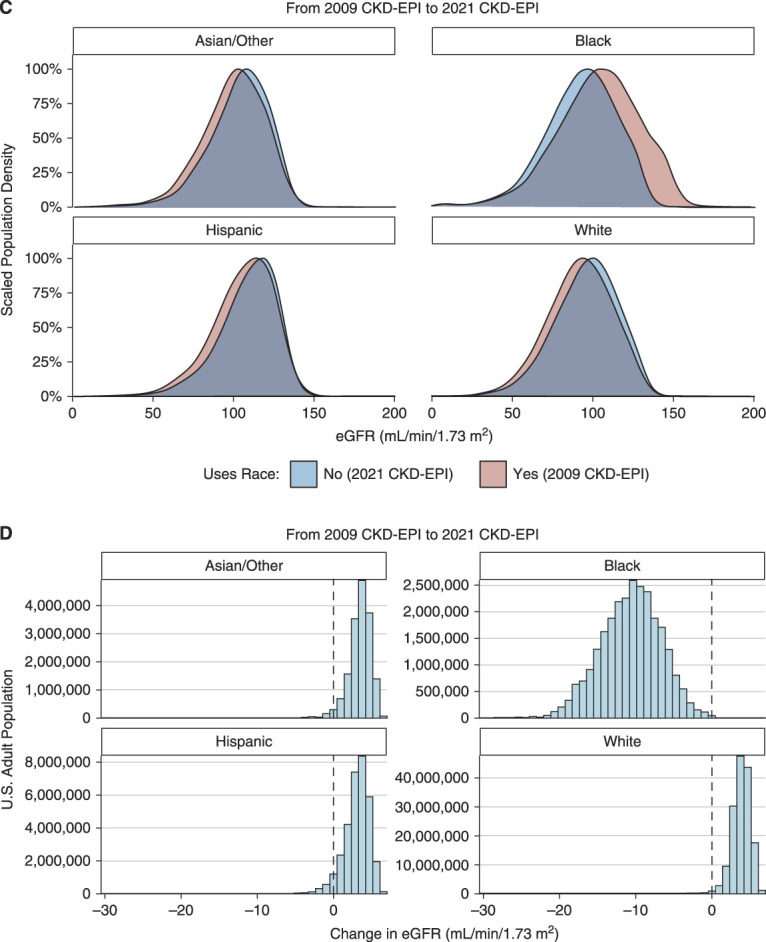
(C) and (D) Same as (A) and (B), but modeling a switch from the 2009 CKD-EPI equation to the 2021 CKD-EPI equation.
Figure 2.
Differences in CKD status and stage by race and ethnicity with new and prior GFR estimating equations. (A) Change in CKD disease burden by age group colored by eGFR category when switching from the 2009 CKD-EPI and 2006 MDRD equations to the 2021 CKD-EPI equation. CKD is defined as eGFR<60 ml/min per 1.73 m2 or urine albumin-creatinine ratio >30 mg/g without the chronicity requirement. (B) Crude prevalence of CKD derived using the 2021 CKD-EPI equation, the 2009 CKD-EPI equation, and the 2006 MDRD equation. Error bars represent 95% CIs. Estimates from NHANES were survey weighted to be representative of the 2001–2018 US population.
Changes to CKD Classification: New Diagnoses and More Advanced Stages in Black Adults and the Reverse in Non-Black Adults
After survey adjustment, 1.63% (95% confidence interval [CI], 1.38 to 1.91) of Black adults were projected to have a new CKD diagnosis. This amounts to 434,000 (95% CI, 350,000 to 517,000) new diagnoses nationally, a relative increase of 10.9%. Of the Black adults already diagnosed with CKD, 14.7% (95% CI, 12.8 to 16.8) could be reclassified to a more advanced stage of disease, translating to 584,000 (95% CI, 508,000 to 667,000) reclassifications nationally. Among non-Black adults, 2.71% (95% CI, 2.44 to 3.00) were projected to have a CKD diagnosis reversed. This amounts to 5.51 million (95% CI, 4.86 million to 6.16 million) reversed diagnoses nationally, a relative decrease of 19.7%. Of the non-Black adults originally diagnosed with CKD, 16.4% (95% CI, 15.3 to 17.6) could be reclassified to a less advanced stage of disease, translating to 4.59 million (95% CI, 4.28 million to 4.92 million) reclassifications across the United States. The survey-weighted crude prevalence of CKD increased by 1.63% among Black adults and decreased for other groups: −1.00% for Hispanic adults, −3.22% for White adults, and −1.28% for Asian/Other adults, resulting in an overall decrease of 2.21% (Figure 2B). These effects were robust across survey cycles and with age adjustment (Supplemental Figure 1).
Changes to Medication Dose Adjustments: Increased in Black Adults and Decreased in Non-Black Adults
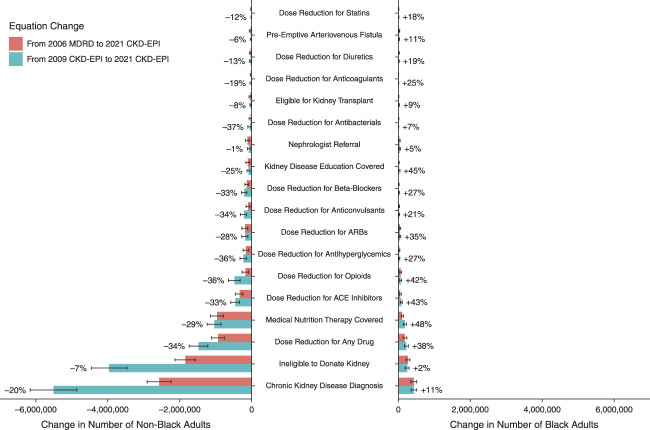
Changes in eGFR and CKD stage have substantial effects on recommended drug dosing and contraindications. Compared with 2006 MDRD, the new equation may increase the number of Black adults with GFR-based dose reductions by 38.1%, affecting 222,000 (95% CI, 169,000 to 275,000) individuals nationally (Figure 3). Conversely, the number of non-Black adults with dose reductions may decrease by 34.0%, affecting 1.47 million (95% CI, 1.22 million to 1.73 million) individuals nationally. Observed changes in NHANES participant counts are listed in Supplemental Table 3.
Figure 3.
Changes in clinical recommendations for US adults with new and prior GFR estimating equations. The projected number of US adults who would be recommended for changes in clinical care after adoption of the 2021 CKD-EPI equation when switching from the 2009 CKD-EPI equation or the 2006 MDRD equation. Drug classes are defined in the Methods. Percentage labels, shown for changes from 2006 MDRD only, reflect the relative increase or decrease in the number of adults with recommended contraindications or dose reductions in these drug classes, namely, (# with new equation − # with prior equation)/(# with prior equation). Recommendations are from KDIGO or the AAFP. Pharmacologic recommendations are only computed for patients who are currently taking that medication. Data from NHANES from 2001 to 2018; estimates, and their confidence intervals were adjusted using NHANES design and weights to be representative of the US population.
After adoption of the new equation, the number of Black adults taking ACE inhibitors, ARBs, opioids, antihyperglycemics (including metformin, sulfonylureas, and SGLT2 inhibitors), beta blockers, and diuretics who are recommended for dose reductions may increase by 85,400 (95% CI, 58,000 to 113,000), 46,400 (95% CI, 26,500 to 66,200), 61,200 (95% CI, 36,700 to 85,700), 26,900 (95% CI, 12,100 to 41,700), 16,200 (95% CI, 2940 to 29,400), and 11,000 (95% CI, 1140 to 20,900), respectively (Figure 3, Supplemental Table 4). These changes amount to relative increases of 42.6%, 35.1%, 42.1%, 26.9%, 27.0%, and 19.2%.
Conversely, the number of non-Black adults currently taking ACE inhibitors, ARBs, opioids, antihyperglycemics, betablockers, and diuretics who are recommended for dose reductions may decrease by 455,000 (95% CI, 335,000 to 575,000), 184,000 (95% CI, 97,000 to 272,000), 478,000 (95% CI, 317,000 to 639,000), 232,000 (95% CI, 144,000 to 320,000), 205,000 (95% CI, 129,000 to 280,000), and 31,100 (95% CI, 6440 to 55,700) respectively. These are relative increases of 33.0%, 28.2%, 36.4%, 36.4%, 33.0%, and 12.7%. Similar patterns of increases and decreases were observed for antibacterials, anticoagulation, and anticonvulsants (Figure 3, Supplemental Table 4).
Eligibility and Coverage for Kidney Disease Services: Increased in Black Adults and Decreased in Non-Black Adults
Among Black adults, ineligibility for kidney donation and eligibility for kidney transplant listing may increase by 2.40% and 9.36% respectively, with 246,000 (95% CI, 189,000 to 303,000) and 15,800 (95% CI, 3780 to 24,800) individuals affected nationally (Supplemental Table 4A). Recommendations for nephrologist referral and arteriovenous fistula placement may increase by 4.93% and 10.9% respectively, affecting 41,800 (95% CI, 19,800 to 63,800) and 17,200 (95% CI, 4,100 to 30,300) individuals. Medicare coverage of medical nutrition therapy and kidney disease education may increase by 48.5% and 45.3%, respectively, with 184,000 (95% CI, 145,000 to 223,000) and 22,200 (95% CI, 2,920 to 41,500) individuals affected.
Conversely, ineligibility for kidney donation and eligibility for kidney transplant listing among non-Black adults may decrease by 6.76% and 8.14%, respectively, with 3.96 million (95% CI, 3.46 million to 4.46 million) and 25,000 (95% CI, 6,170 to 43,700) individuals affected nationally (Supplemental Table 4C). Recommendations for nephrologist referral and fistula placement may decrease by 1.32% and 6.35%, respectively, affecting 75,800 (95% CI, 35,400 to 116,000) and 16,200 (95% CI, 2,260 to 34,600) individuals. Medicare coverage of medical nutrition therapy and kidney disease education may decrease by 29.4% and 25.3%, with 1.04 million (95% CI, 0.85 million to 1.23 million) and 87,000 (95% CI, 43,000 to 131,000) individuals affected.
Decreases in Prevalence of CKD and Medication Adjustments Disaggregated across Hispanic, White, and Asian/Other Adults
Due to the binary race coefficient in prior equations, median eGFR changes were similar across non-Black populations, including Hispanic, White, and Asian/Other adults (Figure 1). However, due to differences in age and eGFR distributions, substantial differences were observed in the estimated number and proportion of individuals potentially affected by implementation of the new equation (Supplemental Table 4).
The 2021 CKD-EPI creatinine-based equation may result in reversal of CKD diagnosis among 3.22% (95% CI, 2.88 to 3.59) of White adults, 1.00% (95% CI, 0.79 to 1.24) of Hispanic adults, and 1.28% (95% CI, 0.86 to 1.82) of Asian/Other adults, amounting to 4.98 million White, 325,000 Hispanic, and 211,000 Asian/Other adults nationwide (Table 1). After adoption of the new equation, 16.2% (95% CI, 14.8 to 17.7) of White adults, 14.9% (95% CI, 13.2 to 16.8) of Hispanic adults, and 21.0% (95% CI, 15.8 to 27.0) of Asian/Other adults may be reclassified to less advanced stages of CKD, translating to 3.56 million White, 585,000 Hispanic, and 438,000 Asian/Other adults (Table 1). The 2.20% difference in CKD crude prevalence between White and Hispanic adults reduced to −0.02% with the new equation (Figure 2B), but the difference in age-adjusted CKD prevalence remained (Supplemental Figure 1).
Table 1.
Changes in CKD stage and status for US adults with new and prior GFR estimating equations
| For Black Adults | ||||
|---|---|---|---|---|
| Population | New CKD | More Advanced CKD | ||
| Reclassified (no.) |
Proportion of Total [% (95% CI)] | Reclassified (no.) |
Proportion of CKD [% (95% CI)] | |
| From 2006 MDRD | ||||
| In NHANES | 205 | 2.15 (1.87 to 2.46) | 283 | 17.3 (15.5 to 19.2) |
| In the US (per 100,000) | 1630 | 1.63 (1.38 to 1.91) | 2194 | 14.7 (12.8 to 16.8) |
| In the US (total) | 433,524 | 1.63 (1.38 to 1.91) | 583,777 | 14.7 (12.8 to 16.8) |
| From 2009 CKD-EPI | ||||
| In NHANES | 185 | 1.94 (1.68 to 2.24) | 280 | 16.9 (15.1 to 18.8) |
| In the US (per 100,000) | 1618 | 1.62 (1.36 to 1.91) | 2537 | 17.0 (14.7 to 19.4) |
| In the US (total) | 430,411 | 1.62 (1.36 to 1.91) | 674,989 | 17.0 (14.7 to 19.4) |
| For White Adults | ||||
|---|---|---|---|---|
| Population | No Longer Has CKD | Less Advanced CKD | ||
|
Reclassified
(no.) |
Proportion of Total [% (95% CI)] |
Reclassified
(no.) |
Proportion of CKD [% (95% CI)] | |
| From 2006 MDRD | ||||
| In NHANES | 649 | 3.49 (3.23 to 3.76) | 509 | 15.3 (14.1 to 16.6) |
| In the US (per 100,000) | 3223 | 3.22 (2.88 to 3.59) | 2308 | 16.2 (14.8 to 17.7) |
| In the US (total) | 4,976,659 | 3.22 (2.88 to 3.59) | 3,563,646 | 16.2 (14.8 to 17.7) |
| From 2009 CKD-EPI | ||||
| In NHANES | 383 | 2.06 (1.86 to 2.27) | 356 | 11.7 (10.5 to 12.8) |
| In the US (per 100,000) | 1518 | 1.52 (1.33 to 1.72) | 1,212 | 9.7 (8.58 to 10.9) |
| In the US (total) | 2,343,760 | 1.52 (1.33 to 1.72) | 1,872,175 | 9.7 (8.58 to 10.9) |
| For Hispanic Adults | ||||
|---|---|---|---|---|
| Population | No Longer Has CKD | Less Advanced CKD | ||
| Reclassified (no.) | Proportion of Total [% (95% CI)] | Reclassified (no.) | Proportion of CKD [% (95% CI)] | |
| From 2006 MDRD | ||||
| In NHANES | 156 | 1.32 (1.12 to 1.54) | 265 | 15.2 (13.6 to 17) |
| In the US (Per 100,000) | 996 | 0.99 (0.79 to 1.24) | 1793 | 14.9 (13.2 to 16.8) |
| In the US (Total) | 324,700 | 0.99 (0.79 to 1.24) | 584,718 | 14.9 (13.2 to 16.8) |
| From 2009 CKD-EPI | ||||
| In NHANES | 76 | 0.64 (0.5 to 0.80) | 140 | 8.43 (7.14 to 9.87) |
| In the US (Per 100,000) | 374 | 0.37 (0.26 to 0.51) | 796 | 6.99 (5.64 to 8.54) |
| In the US (Total) | 121,843 | 0.37 (0.26 to 0.51) | 259,549 | 6.99 (5.64 to 8.54) |
| For Asian/Other Adults | ||||
|---|---|---|---|---|
| Population | No Longer Has CKD | Less Advanced CKD | ||
| Reclassified (no.) | Proportion of Total [% (95% CI)] | Reclassified (no.) | Proportion of CKD [% (95% CI)] | |
| From 2006 MDRD | ||||
| In NHANES | 63 | 1.42 (1.09 to 1.82) | 101 | 17.0 (14.0 to 20.2) |
| In the US (Per 100,000) | 1277 | 1.28 (0.86 to 1.82) | 2659 | 21.0 (15.8 to 27) |
| In the US (Total) | 210,535 | 1.28 (0.86 to 1.82) | 438,390 | 21.0 (15.8 to 27) |
| From 2009 CKD-EPI | ||||
| In NHANES | 33 | 0.75 (0.51 to 1.05) | 62 | 11.0 (8.52 to 13.8) |
| In the US (per 100,000) | 638 | 0.64 (0.41 to 0.95) | 1292 | 10.7 (7.21 to 15.2) |
| In the US (total) | 105,168 | 0.64 (0.41 to 0.95) | 213,026 | 10.7 (7.21 to 15.2) |
eGFR-based reclassifications leading to new diagnoses of CKD or more advanced stages of CKD among Black adults when switching from the 2009 CKD-EPI and 2006 MDRD equations to the 2021 CKD-EPI equation. Reclassifications are reported for observations in NHANES (not survey adjusted), per 100,000 Black adults in the United States (survey adjusted), and among all Black adults in the United States (survey adjusted). Although it is possible for Black adults switching to the 2021 CKD-EPI equation to qualify for a less advanced stage of CKD or no longer qualify as having CKD, no cases were observed. eGFR-based reclassifications leading to reversal of CKD diagnoses or less advanced stages of CKD among non-Hispanic White adults when switching from the 2009 CKD-EPI and 2006 MDRD equations to the 2021 CKD-EPI equation. Six cases of White adults qualifying for more advanced stages of CKD were observed (not shown), all when switching from the 2006 MDRD equation. No cases were observed of White adults newly qualifying as having CKD when switching from either equation. Hispanic adults. Four cases of Hispanic adults qualifying for more advanced stages of CKD were observed (not shown), all when switching from the 2006 MDRD equation. No cases were observed of Hispanic adults newly qualifying as having CKD when switching from either equation. Asian/Other adults. No cases of Asian/Other adults newly qualifying as having CKD or qualifying for more advanced stages of CKD were observed when switching from either equation.
After implementation of the new equation, the number of White adults with dose adjustments for any drug may decrease by 34.5% (95% CI, 30.0 to 39.0) compared with reductions of 32.3% (95% CI, 23.9 to 40.8) for Hispanic adults and 29.1% (95% CI, 17.0 to 41.3) for Asian/Other adults (Supplemental Table 4). These equate to 1.29 million (95% CI, 1.04 to 1.54 million) White adults, 112,000 (95% CI, 73,500 to 159,000) Hispanic adults, and 75,000 (95% CI, 34,800 to 115,000) Asian/Other adults nationally (Supplemental Table 4). Relative changes for dose reductions of individual drug classes varied widely, with substantially more precise estimates for White and Hispanic adults than for Asian/Other adults (Supplemental Table 4).
Representation in the Development Population Affects Estimated Prevalence of CKD and Medication Adjustments
The choice of the race and ethnicity mix of the population used to develop the 2021 CKD-EPI creatinine-based equation directly affects the prevalence of CKD (Figure 4A), recommended dose reductions (Figure 4B), and other GFR-dependent decisions in both Black and non-Black individuals (Supplemental Figure 2). Using the 2021 CKD-EPI equation, CKD prevalence was estimated to be 11.7% among US adults. Using alternative creatinine-based equations developed by CKD-EPI on study populations of 0% or 100% Black participants, estimated CKD prevalence shifted to 12.8% or 10.6%, respectively. The 2021 CKD-EPI equation was estimated to result in dose reductions for approximately 3.66 million US adults overall. Using alternative equations developed on study populations of 0% or 100% Black participants, dose reduction estimates shifted to 4.66 million and 2.71 million US adults, respectively. Alternative eGFR equations developed by CKD-EPI from study populations with nationally representative (13%) and evenly represented proportions of Black participants (50%) resulted in greater and fewer individuals estimated to have CKD or medication adjustments, respectively, compared with the 2021 CKD-EPI equation (31.5% Black participants).
Figure 4.
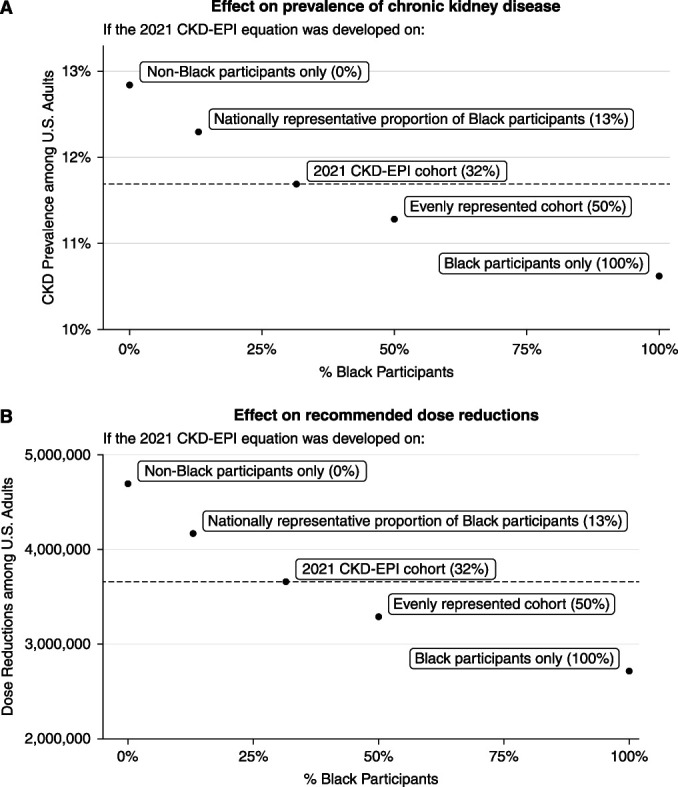
Enrichment of Black participants in development data for eGFR equations affects prevalence of CKD and pharmacologic recommendations. (A) Prevalence of CKD. (B) Number of individuals with recommended dose reduction for a drug they are currently taking, shown against proportion of Black participants for five race-free equations. Candidate equations were developed using the CKD-EPI cohort with non-Black individuals resampled to the given proportion of Black participants. Equations developed using greater proportions of Black participants resulted in lower prevalence of CKD and fewer individuals with recommended dose reductions among all race and ethnicity groups.
Discussion
The NKF-ASN Task Force recommendations follow decisions by major medical centers to remove race from eGFR reporting, most often by direct removal of the coefficient for Black versus non-Black race.17 These changes were prompted by concerns2,3,18,19 regarding race-based medicine and structural racism, recognizing the ways that race stratification may influence access to care for Black adults and reinforce misconceptions of intrinsic biologic difference dating back to the 18th century.4,20–24 However, direct removal also led to significant underestimation of the measured GFR in Black patients.5,17 Unlike direct removal of the race coefficient, which affected only Black individuals,21 the new equation affects care for all patients and was shown to produce smaller systematic errors across groups.6 Our study demonstrates that the new eGFR creatinine equation may still lead to wide-ranging changes in diagnosing, staging, and managing kidney disease that differ substantially across race and ethnicity groups.
For Black adults, nationwide implementation of the 2021 CKD-EPI creatinine-based equation may result in 434,000 new diagnoses of CKD and 584,000 reclassifications to more advanced stages of CKD. Conversely, the new equation may lead to 5.51 million non-Black adults no longer having a CKD diagnosis and 4.59 million reclassifications to less advanced stages of CKD. These estimates are on the basis of comparison with the 2006 MDRD equation used by the majority (70%) of laboratories as of 2018.25,26 Because some of the largest laboratories use the 2009 CKD-EPI equation, we also estimated outcomes for this equation. Our data are consistent with prior studies of the potential effect of the 2021 CKD-EPI creatinine-based equation with respect to its 2009 counterpart, including analyses of NHANES 1999–20036 and NHANES 2011–2018,27 and studies using data from the UK Biobank,28 Veterans Affairs national health system,7 and various institutional laboratory record systems.8,29 Our study may provide better estimates given its larger sample size, national representation, analysis of both the 2006 MDRD and 2009 CKD-EPI equations, and most notably, consideration of broad implications, including medication changes estimated using the prescription drug data from NHANES. In addition, unlike prior studies, we separately quantify clinical implications for Hispanic and Asian/Other adults, who are also affected by disparities compared with White adults.
Although predicting physician and patient behavior from changes in eGFR reporting is difficult, changes in CKD prevalence and stage could lead to substantial changes in guideline-recommended care. In Black patients, decreased eGFR introduced by the 2021 CKD-EPI creatinine-based equation could potentially provide a more useful gauge of clinical risks and earlier attention to CKD by increasing referrals to specialist care, evaluations for kidney transplantation, and coverage by Medicare and other insurance. In addition, more Black adults may become eligible for trials studying CKD progression,30 which may help uncover factors related to documented findings of rapid kidney function decline among Black adults.31,32 Conversely, potential incidents of overdiagnosis may lead to excess health care utilization and adverse outcomes among Black patients,33,34 whereas potential incidents of underdiagnosis may decrease CKD care and reimbursement for White, Hispanic, and Asian/Other patients with kidney disease. The net effects on certain outcomes including access to kidney transplantation are unclear because, although the new equation is likely to increase the number of Black adults evaluated for transplantation, it may simultaneously reduce the pool of eligible donors. Moreover, programs may begin to evaluate patients for transplantation at less stringent GFR thresholds (e.g., <25 ml/min per 1.73 m2), resulting in variability of expected changes.
The new equation may result in substantial changes to medication selection and dosing. Should the new equation be implemented nationally, an estimated 222,000 Black adults (a relative increase of 38.1%) may be recommended for dose reductions or contraindications across a broad range of medications, including those used to treat hypertension, cardiovascular disease, diabetes, chronic pain, seizure disorders, and bacterial infections. Conversely, an estimated 1.47 million non-Black adults (a relative decrease of 34.0%) may no longer be recommended for the same medication adjustments. These changes bring potential benefits and harms. For Black adults, decreased use of evidence-based medications such as ACE inhibitors, beta blockers, metformin, and opioids are concerning given documented disparities in kidney disease,35 cardiovascular disease,36,37 diabetes,38 and pain control39,40 between Black and White adults. More Black patients may also be excluded from pivotal trials with GFR-based inclusion or exclusion criteria, which may compound existing gaps in representation.41 For White, Hispanic, and Asian/Other patients, higher eGFR values may increase therapeutic options, but also increase the risk of kidney toxicity or toxic accumulation of medications.
The choice of race and ethnicity mix of the population used to develop eGFR equations affects the magnitude of potential care changes. The 2021 CKD-EPI creatinine-based equation was derived from a study population with 31.5% of Black adults, which approximates the national burden of kidney failure among Black adults (37%)42 rather than their share of the general population (13%).43 As a result, this equation reduces systematic bias in Black adults as compared with the previous approach of directly eliminating race adjustment without refitting.6,44 This choice is consistent with a core value of the NKF-ASN Task Force to promote equity, as opposed to equality, in kidney health, because this composition of the equation development population incorporates data from Black adults in proportion to disease burden. As with other clinical equations,45 the eGFR equations reflect the demographics, clinical characteristics, genetics, and environmental and social histories of the study participants in the development data of the individual cohorts comprising the “pooled cohort” equation.46 Further research quantifying the variability of the eGFR equation as a function of which cohorts and populations are included would be useful. Such an understanding is a prerequisite for making unbiased comparisons of existing and future approaches to estimating GFR.
The potential benefits should motivate continued adoption of the 2021 CKD-EPI creatinine-based equation. In our view, the aforementioned concerns also do not preclude moving forward; mitigating responses may include increased use of confirmatory testing with measured GFR or creatinine clearance or cystatin C,47 adaptation of dosing guidelines, shared decision making, and replacement of GFR threshold criteria with risk-based criteria for care-based decisions.13 Greater attention to GFR has increased awareness of the variability and individual-level inaccuracy48,49 of eGFR. For example, a recent analysis of four prospective cohort studies49 found that among individuals with a creatinine-based eGFR of 60 ml/min per 1.73 m2, half of measured GFR values varied from 52 to 67 ml/min per 1.73 m2. Beyond creatinine-based GFR estimates, the NKF-ASN Task Force recommends increased use of cystatin C, including the 2012 CKD-EPI eGFRcys equation and 2021 CKD-EPI eGFRcr-cys equations, to increase the accuracy of eGFR used in clinical practice.6
Our study has several limitations. First, not all physicians and medical institutions follow the studied guidelines.50 Second, NHANES does not contain longitudinal serum creatinine measurements to assess chronicity and may misrepresent some medical conditions. Third, affected numbers may be lowered by underutilization of eGFR or slow adoption of the new equation. Fourth, changes in care may be attenuated by confirmatory testing or more detailed clinical evaluation. Indeed, physicians incorporate many factors when making patient decisions; we expect individual decisions to deviate from guidelines in both directions and that our estimates are robust for population-level changes to recommended care. As science, values, and evidence inform changes to abolish misuses of race in medicine,3,4,51 it is important to assess the potential effects and develop strategies to protect against unintended consequences. Ultimately, achieving equity in kidney health will require the discovery of improved race-independent biomarkers and urgently addressing the pervasive and systemic drivers of health care disparities.52,53
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Disclosures
A.S. Levey reports receiving research funding from grants and contracts paid to Tufts Medical Center from NIH and NKF; reports contracts to paid from AstraZeneca (Data and Safety Monitoring Board (DSMB) for dapagliflozin trials); and reports receiving honoraria from academic medical centers for visiting professorships. H.A. Taylor reports having consultancy agreements with Pfizer, Novartis, and United Health Group (UHG); reports receiving research funding from 23andMe and UHG; and reports receiving honoraria from Pfizer, Novartis, and UHG. I.S. Kohane reports having consultancy agreements with Danaher; reports having an ownership interest in Activate Care, Canary Medical, and Inovalon; reports receiving honoraria from Janssen; and reports having an advisory or leadership role with Activate Care, Canary Medical, and Inovalon. J.K. Wang reports employment with PathAI; and having consultancy agreements with Prometheus Biosciences. L.A. Inker reports having consultancy agreements with Diamtrix; receiving research funding from to institute for research and contracts with the Chinnocks, NIH, NKF, Omeros, and Reata Pharmaceuticals; reports having consulting agreements with Tricida Inc.; reports having an advisory or leadership role with the Alport Foundation Medical Advisory Council and the NKF Scientific Advisory Board; and reports having other interests or relationships as an American Society of Nephrology member and an NKF member. N.R. Powe reports having an advisory or leadership role with Hennepin Health Care Research Institute, Patient Centered Outcomes Research Institute, Portland VA Research Foundation, Robert Wood Johnson Foundation, University of Washington, and Vanderbilt University. Because N.R. Powe is an associate editor of the Journal of the American Society of Nephrology, he was not involved in the peer review process for this manuscript. A guest editor oversaw the peer review and decision-making process for this manuscript. All remaining authors have nothing to disclose.
Funding
This work was supported by the National Heart, Lung, and Blood Institute (K01HL138259) and the National Institute of Environmental Health Sciences (R01ES032470).
Author Contributions
J.A. Diao, A.K. Manrai, and N.R. Powe and conceptualized the study; J.A. Diao, A.K. Manrai, and G.J. Wu were responsible for the data curation; J.A. Diao, L.A. Inker, I.S. Kohane, A.S. Levey, N.R. Powe, A.K. Manrai, H.A. Taylor, H. Tighiouart, J.K. Wang, and G.J. Wu were responsible for the formal analysis; A.K. Manrai was responsible for the funding acquisition; J.A. Diao, L.A. Inker, I.S. Kohane, A.S. Levey, N.R. Powe, A.K. Manrai, H.A. Taylor, H. Tighiouart, J.K. Wang, and G.J. Wu were responsible for the investigation; J.A. Diao, L.A. Inker, A.S. Levey, A.K. Manrai, and N.R. Powe were responsible for the methodology; A.K. Manrai and N.R. Powe provided supervision; J.A. Diao and G.J. Wu were responsible for the visualization; J.A. Diao and A.K. Manrai wrote the original draft; and J.A. Diao, L.A. Inker, I.S. Kohane, A.S. Levey, A.K. Manrai, N.R. Powe, H.A. Taylor, H. Tighiouart, J.K. Wang, and G.J. Wu reviewed and edited the manuscript.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/JSN/D724.
Supplemental Table 1. Population characteristics of non pregnant US adults.
Supplemental Table 2. GFR-dependent drug dosing adjustments for CKD.
Supplemental Table 3. Observed changes to GFR-dependent clinical decisions in NHANES (2001–2018).
Supplemental Table 4. Survey adjusted national projections of changes to GFR-dependent clinical decisions in the United States, 2001–2018.
Supplemental Figure 1. Secular trends in CKD prevalence by race and ethnicity with new and prior GFR estimating equations, 2001–2018.
Supplemental Figure 2. Enrichment of Black participants in eGFR development data affects CKD prevalence and pharmacologic recommendations for both Black and non-Black individuals.
References
- 1.Levey AS, Titan SM, Powe NR, Coresh J, Inker LA: Kidney disease, race, and GFR estimation. Clin J Am Soc Nephrol 15: 1203–1212, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eneanya ND, Yang W, Reese PP: Reconsidering the consequences of using race to estimate kidney function. JAMA 322: 113–114, 2019 [DOI] [PubMed] [Google Scholar]
- 3.Vyas DA, Eisenstein LG, Jones DS: Hidden in plain sight - Reconsidering the use of race correction in clinical algorithms. N Engl J Med 383: 874–882, 2020 [DOI] [PubMed] [Google Scholar]
- 4.Roberts DE: Abolish race correction. Lancet 397: 17–18, 2021 [DOI] [PubMed] [Google Scholar]
- 5.Delgado C, Baweja M, Crews DC, Eneanya ND, Gadegbeku CA, Inker LA, et al. : A unifying approach for GFR estimation: Recommendations of the NKF-ASN Task Force on reassessing the inclusion of race in diagnosing kidney disease. Am J Kidney Dis 79: 268–288.e1, 2022 [DOI] [PubMed] [Google Scholar]
- 6.Inker LA Eneanya ND Coresh J Tighiouart H Wang D Sang Y et al. ; Chronic Kidney Disease Epidemiology Collaboration : New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med 385: 1737–1749, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gregg LP, Richardson PA, Akeroyd J, Matheny ME, Virani SS, Navaneethan SD: Effects of the 2021 CKD-EPI creatinine eGFR equation among a national US Veteran Cohort. Clin J Am Soc Nephrol 17: 283–285, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meeusen JW, Kasozi RN, Larson TS, Lieske JC: Clinical impact of the refit CKD-EPI 2021 creatinine-based eGFR equation. Clin Chem 68: 534–539, 2022 [DOI] [PubMed] [Google Scholar]
- 9.Levey AS Coresh J Greene T Stevens LA Zhang YL Hendriksen S et al. ; Chronic Kidney Disease Epidemiology Collaboration : Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Levey AS Stevens LA Schmid CH Zhang YL Castro AF 3rd Feldman HI et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC) : National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data (Internet). Available at: http://www.cdc.gov/nchs/nhanes/
- 12.Selvin E, Manzi J, Stevens LA, Van Lente F, Lacher DA, Levey AS, et al. : Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988–1994, 1999–2004. Am J Kidney Dis 50: 918–926, 2007 [DOI] [PubMed] [Google Scholar]
- 13.KDIGO CKD Work Group : KDIGO 2012 Clinical Practice Guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3: 1–150, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Munar MY, Singh H: Drug dosing adjustments in patients with chronic kidney disease. Am Fam Physician. 2007. May 15;75(10):1487–96. [PubMed] [Google Scholar]
- 15.Lumley T. (2004). Analysis of Complex Survey Samples. Journal of Statistical Software, 9(8), 1–19. [Google Scholar]
- 16.Ihaka R, Gentleman RR: A Language for Data Analysis and Graphics. J Comput Graph Stat (Internet) 2012. Available at: http://www-tandfonline-com.ezp-prod1.hul.harvard.edu/doi/abs/10.1080/10618600.1996.10474713
- 17.Powe NR: Black kidney function matters: Use or misuse of race? JAMA 324: 737–738, 2020 [DOI] [PubMed] [Google Scholar]
- 18.Grubbs V: Precision in GFR reporting: Let’s stop playing the race card. Clin J Am Soc Nephrol 15: 1201–1202, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heffron AS, Khazanchi R, Nkinsi N, Bervell JA, Cerdeña JP, Diao JA, et al. : Trainee perspectives on race, antiracism, and the path toward justice in kidney care. Clin J Am Soc Nephrol 17: 1251–1254, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmed S, Nutt CT, Eneanya ND, Reese PP, Sivashanker K, Morse M, et al. : Examining the potential impact of race multiplier utilization in estimated glomerular filtration rate calculation on African-American care outcomes. J Gen Intern Med 36: 464–471, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diao JA, Wu GJ, Taylor HA, Tucker JK, Powe NR, Kohane IS, et al. : Clinical implications of removing race from estimates of kidney function. JAMA 325: 184–186, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinchen KS, Sadler J, Fink N, Brookmeyer R, Klag MJ, Levey AS, et al. : The timing of specialist evaluation in chronic kidney disease and mortality. Ann Intern Med 137: 479–486, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Powe NR: Let’s get serious about racial and ethnic disparities. J Am Soc Nephrol 19: 1271–1275, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Eneanya ND, Boulware LE, Tsai J, Bruce MA, Ford CL, Harris C, et al. : Health inequities and the inappropriate use of race in nephrology. Nat Rev Nephrol 18: 84–94, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller G: Educational Discussion: Practices and recommendations for reporting estimated glomerular filtration rate (eGFR) from the College of American Pathologists (Internet). 2018. Available at: https://documents.cap.org/documents/2018-current-status-reporting-egfr.pdf
- 26.Tsai JW, Cerdeña JP, Goedel WC, Asch WS, Grubbs V, Mendu ML, et al. : Evaluating the impact and rationale of race-specific estimations of kidney function: Estimations from US NHANES, 2015–2018. EClinicalMedicine 42: 101197, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walther CP, Winkelmayer WC, Navaneethan SD: Updated US prevalence estimates for chronic kidney disease stage and complications using the new race-free equation to estimate glomerular filtration rate. JAMA Netw Open 5: e220460, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneider CV, Schneider KM: New equations for estimating the GFR without race. N Engl J Med 386: 1671–1672, 2022 [DOI] [PubMed] [Google Scholar]
- 29.Ghuman JK, Shi J, Zelnick LR, Hoofnagle AN, Mehrotra R, Bansal N: Impact of removing race variable on CKD classification using the creatinine-based 2021 CKD-EPI equation. Kidney Med 4: 100471, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walther CP, Winkelmayer WC, Navaneethan SD: Black race coefficient in GFR estimation and diabetes medications in CKD: National estimates. J Am Soc Nephrol 32: 1319–1321, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peralta CA, Vittinghoff E, Bansal N, Jacobs D, Jr, Muntner P, Kestenbaum B, et al. : Trajectories of kidney function decline in young black and white adults with preserved GFR: results from the Coronary Artery Risk Development in Young Adults (CARDIA) study. Am J Kidney Dis 62: 261–266, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Young BA, Katz R, Boulware LE, Kestenbaum B, de Boer IH, Wang W, et al. : Risk factors for rapid kidney function decline among African Americans: The Jackson Heart Study (JHS). Am J Kidney Dis 68: 229–239, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilbert Welch H, Schwartz L, Woloshin S: Overdiagnosed: Making People Sick in the Pursuit of Health, Boston, MA, Beacon Press, 2012 [Google Scholar]
- 34.Kale MS, Korenstein D: Overdiagnosis in primary care: framing the problem and finding solutions. BMJ 362: k2820, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Norris K, Nissenson AR: Race, gender, and socioeconomic disparities in CKD in the United States. J Am Soc Nephrol 19: 1261–1270, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Carnethon MR Pu J Howard G Albert MA Anderson CAM Bertoni AG et al. ; American Heart Association Council on Epidemiology and Prevention; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Functional Genomics and Translational Biology; and Stroke Council : Cardiovascular Health in African Americans: A scientific statement from the American Heart Association. Circulation 136: e393–e423, 2017 [DOI] [PubMed] [Google Scholar]
- 37.Nanna MG, Navar AM, Zakroysky P, Xiang Q, Goldberg AC, Robinson J, et al. : Association of patient perceptions of cardiovascular risk and beliefs on statin drugs with racial differences in statin use: Insights from the patient and provider…. JAMA Cardiol 3: 739–748, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chow EA, Foster H, Gonzalez V, McIver L: The disparate impact of diabetes on racial/ethnic minority populations. Clin Diabetes 30: 130–133, 2012 [Google Scholar]
- 39.Hoffman KM, Trawalter S, Axt JR, Oliver MN: Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proc Natl Acad Sci USA 113: 4296–4301, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dominick KL, Bosworth HB, Jeffreys AS. Racial/ethnic variations in non‐steroidal anti‐ inflammatory drug (NSAID) use among patients with osteoarthritis and drug safety. Pharmacoepidemiol Drug Saf 2004. 13: 683–694. [DOI] [PubMed] [Google Scholar]
- 41.Charytan DM Yu J Jardine MJ Cannon CP Agarwal R Bakris G et al. ; CREDENCE study investigators : Potential effects of elimination of the black race coefficient in eGFR calculations in the CREDENCE Trial. Clin J Am Soc Nephrol 17: 361–373, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saran R, Robinson B, Abbott KC, Bragg-Gresham J, Chen X, Gipson D, et al. : US Renal Data System 2019 Annual Data Report: Epidemiology of kidney disease in the United States. Am J Kidney Dis 75(Suppl 1): A6–A7, 2020 [DOI] [PubMed] [Google Scholar]
- 43.US Census Bureau : Race and ethnicity in the United States: 2010 Census and 2020 Census. 2022. (cited 2022 Feb 27). Available at: https://www.census.gov/library/visualizations/interactive/race-and-ethnicity-in-the-united-state-2010-and-2020-census.html
- 44.Powe NR: Race and kidney function: The facts and fix amidst the fuss, fuzziness, and fiction. Med (N Y) 3: 93–97, 2022 [DOI] [PubMed] [Google Scholar]
- 45.Goff DC Jr Lloyd-Jones DM Bennett G Coady S D’Agostino RB Gibbons R et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines : 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 129(Suppl 2): S49–S73, 2014 [DOI] [PubMed] [Google Scholar]
- 46.Alvarado F, Aklilu A, Powe NR, Vart P, Delgado C: Diversity in studies developing kidney function estimating equations: Improving representation, interpretation, and utility of clinical research [published online ahead of print]. Am J Kidney Dis 2022 [DOI] [PubMed] [Google Scholar]
- 47.Inker LA, Titan S: Measurement and estimation of GFR for use in clinical practice: Core curriculum 2021. Am J Kidney Dis 78: 736–749, 2021 [DOI] [PubMed] [Google Scholar]
- 48.Sehgal AR: Race and the false precision of glomerular filtration rate estimates. Ann Intern Med 173: 1008–1009, 2020 [DOI] [PubMed] [Google Scholar]
- 49.Shafi T, Zhu X, Lirette ST, Rule AD, Mosley T, Butler KR, et al. : Quantifying individual-level inaccuracy in glomerular filtration rate estimation: A cross-sectional study. Ann Intern Med 175: 1073–1082, 2022 [DOI] [PubMed] [Google Scholar]
- 50.Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA, et al. : Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA 282: 1458–1465, 1999 [DOI] [PubMed] [Google Scholar]
- 51.Cerdeña JP, Plaisime MV, Tsai J: From race-based to race-conscious medicine: How anti-racist uprisings call us to act. Lancet 396: 1125–1128, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bailey ZD, Feldman JM, Bassett MT: How structural racism works - Racist policies as a root cause of US racial health inequities. N Engl J Med 384: 768–773, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Powe NR: The pathogenesis of race and ethnic disparities: Targets for achieving health equity. Clin J Am Soc Nephrol 16: 806–808, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]