Abstract
Background and Objective
The objective of this study was to evaluate the short-term physiologic effect and one-year functional effect of a 12-week inspiratory and expiratory respiratory strength training (RST) program in individuals with amyotrophic lateral sclerosis (ALS).
Methods
A double-blinded, randomized, sham-controlled trial was conducted in 45 individuals with early-stage ALS. Participants were randomized into 12 weeks of active RST (30% load, n = 23) or sham RST (0% load, n = 22). An intent-to-treat analysis was conducted. Linear regression of pre-post change with group status and pretest scores as predictors was conducted. Primary outcomes included maximum expiratory and inspiratory pressure (MEP, MIP), and secondary outcomes were cough spirometry and forced vital capacity. Exploratory follow-up outcomes included one-year global and bulbar decline (ALS Functional Rating Scale-Revised [ALSFRS-R] total and bulbar subscale slope), oral intake status, and time to noninvasive ventilation (NIV).
Results
TheRST completion rate was 91% with no RST-related adverse events. A 12-week RST program led to increases in MEP (p = 0.004), but not MIP (p = 0.33). On average, MEP increased by 20.8 cm H2O after active RST (95% CI +7.6 to +33.9) and decreased by 1.0 cm H2O (95% CI −9.1 to +7.2) after sham RST. Mean MIP increased by 8.9 cm H2O (95% CI +1.5 to +16.3) and 4.8 cm H2O (95% CI −0.6 to +10.2) for the active and sham groups, respectively. Regarding secondary outcomes, RST led to significant increases in cough peak inspiratory flow (p = 0.02); however, it did not affect cough expiratory flow (p = 0.06) or FVC (p = 0.60). Regarding 12-month outcomes, a significant difference in the ALSFRS-R bulbar subscale slope was observed across treatment groups, with a more than two-fold faster rate of bulbar decline in the sham vs active RST groups observed (−0.29 vs −0.12 points/month, p = 0.02). Total ALSFRS-R slope, feeding status, and time to NIV did not differ across treatment groups (p > 0.05).
Discussion
RST was well tolerated and led to improvements in some, but not all, short and long-term outcomes. RST represents a proactive rehabilitative intervention that could increase physiologic capacity of specific breathing and airway clearance functions during the early stages of ALS. Further work is needed to determine optimal training intensity, resistance load specifications, and potential long-term functional outcomes.
Classification of Evidence
This study provides Class II evidence that a mild-intensity respiratory strength training program improves maximum expiratory pressure, but not maximum inspiratory pressure, in patients with early-stage ALS.
Amyotrophic lateral sclerosis (ALS) is a fatal neuromuscular disease characterized by rapid degeneration of upper and lower motor neurons and progressive muscle weakness in the spinal, axial, and bulbar regions. Regardless of the symptom onset site, development of restrictive breathing patterns and hypoventilation occur in individuals with ALS, leading to eventual respiratory failure in most patients.1 Diaphragm weakness from underlying lower motor neuron degeneration in ALS leads to reduced intrathoracic pressure-generating capacity, reduced chest expansion during inhalation, and diminished elastic recoil forces during exhalation.2 Upper motor neuron involvement in ALS leads to chest wall rigidity, resistance, and reduced range of motion that functionally increase the mechanical forces required to maintain adequate ventilation.2,3 Finally, reduced speed; range of motion; and weakness of respiratory, laryngeal, and bulbar muscles further affect airway clearance abilities in individuals with ALS, clinically manifesting as increased difficulties in managing secretions, in defending the airway, and in effectively ejecting tracheal aspirate from the airway.4-8
Although progressive muscle weakness is a cardinal feature of ALS, use of strength-based interventions have been historically discouraged because of concerns of muscle overuse and exacerbation of physical decline.9,10 Traditional ALS models of care have, therefore ,been primarily palliative in nature.11 However, emerging evidence suggests that early mild-moderate intensity exercise programs may increase physiologic capacity and strength, prevent the development of disuse atrophy and deconditioning, mitigate pain and spasticity, and improve overall psychological well-being in individuals with ALS.10,12-19 Preliminary studies demonstrate that a moderate-intensity program targeting expiratory musculature (expiratory strength training) was safe and led to short-term improvements in airway clearance physiologic capacity (maximum expiratory pressure and peak expiratory cough flow) in mildly affected individuals with ALS.20,21 Given that both expiratory and inspiratory systems are affected in ALS, we sought to extend our earlier work and examine the potential synergistic effect of a strength training program targeting both ventilatory (inspiratory) and airway clearance (expiratory) functions. Specifically, we evaluated the short-term physiologic effect and 12-month functional effect of an in-home inspiratory and expiratory respiratory strength training (RST) program in mildly affected individuals with ALS.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
This randomized, double-blinded, sham-controlled trial included 2 US participating sites, the University of Florida (Gainesville) and the University of South Florida (Tampa). This study was approved by the appropriate university institutional review boards and conducted in accordance with the Declaration of Helsinki and in good clinical practice compliance (ClinicalTrials.gov identifier: NCT02710110). All included participants provided informed written consent. The study protocol and statistical analysis plan are found in eSAP 1, links.lww.com/WNL/C659.
Study Overview
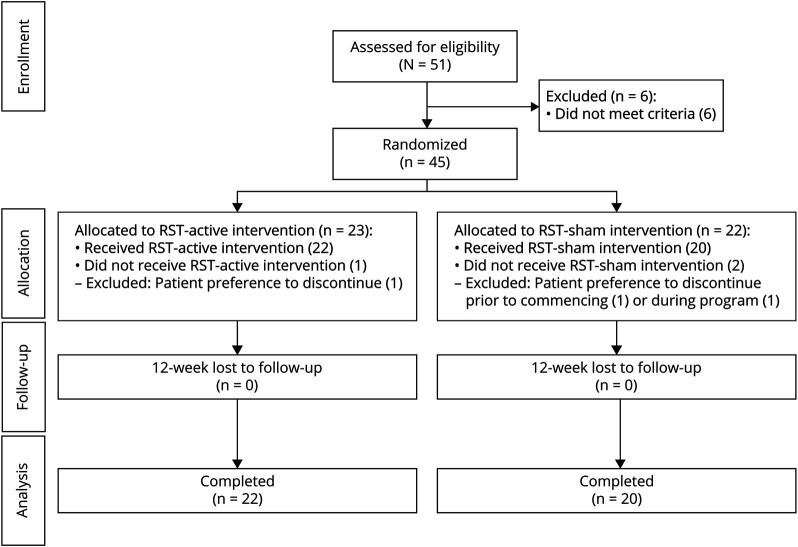
This study is a prospective, double-blinded, randomized, sham-controlled clinical trial. Once enrolled, participants were assigned a study number and randomized to either the experimental (active RST) or control (sham RST) group. All baseline assessments were performed in the aerodigestive research core laboratory, after which participants commenced the randomly assigned 12-week treatment program in their home. Within 2 days of completion of the assigned RST protocol, participants returned to the same laboratory to be reassessed. Twelve-month follow-up data included the ALSFRS-R total and bulbar subscale scores, feeding status (oral vs nil per oral), and noninvasive ventilation (NIV) status. Figure 1 illustrates this time line and the number of participants included in each analysis.
Figure 1. CONSORT Flow Diagram.
.
Participant Selection Criteria
Fifty-one individuals with a diagnosis of probable or definite ALS (revised El Escorial criteria)22 and interest in participation were screened across the 2 sites. Forty-five individuals met criteria and were enrolled in the study by a research evaluator at either the University of Florida site (n = 23) or the University of South Florida (n = 22). Diagnosis was confirmed by academic neuromuscular neurologists specializing in ALS before screening for enrollment. Inclusion criteria were (1) confirmed ALS diagnosis, (2) FVC ≥70% predicted, (3) ALSFRS-R23 score greater than 34, (4) ability to follow multistep commands and competency in RST techniques, and (5) willing to not commence any new pharmacologic or behavioral treatments during the 12-week assigned RST program or on a stable dose of any ALS medications for >60 days. Individuals with ALS who had known allergies to barium, a tracheostomy, a diaphragmatic pacer, or who were on mechanical ventilation were excluded from this study.
Randomization and Blinding
Participants were assigned to the experimental (active RST, n = 23) or control (sham RST, n = 22) group by the statistician using a permuted block (block size = 6) randomization schedule, stratified by site and controlling for disease severity (defined by the ALSFRS-R) to ensure an approximately equal balance across both groups for disease severity. Participants, investigators, clinical researchers performing and/or interpreting evaluations, and clinical care providers of enrolled participants were masked to treatment assignment. A research assistant, who did not interact with the study participants and was not involved in data analysis, was responsible for preparing participant packets containing the assigned training devices (sham or active trainers), a nose clip, and a flanged rubber mouthpiece, which were provided to the treating home therapist. Cough spirometry data were deidentified and coded to blind participant identification and evaluation testing time point.
Interventions
Active RST Protocol
RST was conducted at home using handheld, one-way, spring-loaded, calibrated resistance trainers. Inspiratory breath sets were completed using a Threshold Inspiratory Muscle Trainer (IMT, Phillips Respironics, Murrysville, PA), and expiratory repetitions were completed using the Expiratory Muscle Strength Trainer (EMST 150, Aspire Products, LLC, Cedar Point, NC) with nose clips in place. Given that labial weakness is typical in participants with ALS, a flanged rubber mouthpiece was attached to the opening of the expiratory trainer to facilitate labial seal during exercises (MTH6400, MD Spiro, Lewiston, ME). On the first training session of each week, a research therapist conducted a home visit to reassess MEP and MIP using a handheld digital manometer (Micro Respiratory Pressure Meter, MP01, Micro Direct Inc., Lewiston, ME) and recalibrated trainers to represent 30% of the current MEP/MIP values, reflecting a mild training load. Participants were instructed to complete 25 inspiratory and 25 expiratory repetitions (5 sets of 5 repetitions), 5 days per week, resulting in 50 daily repetitions, 250 weekly repetitions, and a total of 3,000 repetitions throughout the twelve-week training program. Participants were instructed to rest between each 5-repetition set, and a typical training session lasted approximately 30 minutes. To encourage compliance and accountability, participants and their caregivers were provided with a home therapy log and asked to track daily therapy sessions by marking off each exercise set performed. These logs were collected weekly.
Sham Training Protocol
Participants assigned to the sham RST group completed a twelve-week training protocol with trainers that looked identical to the active trainers, however, with the internal spring removed (expiratory trainer) or cut (inspiratory trainer). Therefore, these participants performed exercises against no physiologic load. The training and home therapist protocols were identical in every other aspect to the active RST group.
Short-term Physiologic Outcomes
Maximum Expiratory and Inspiratory Pressure
MEP and MIP were the primary outcome metrics and were assessed using a handheld digital manometer (Micro Respiratory Pressure Meter, MP01, Micro Direct Inc., Lewiston, ME). To minimize labial leakage in participants who were unable to create a tight lip seal because of facial weakness, a flanged rubber mouthpiece was attached to the manometer and manual labial support was provided by the research clinician. The participant was seated with a nose clip in place during testing to reduce nasal emissions. Participants were instructed to place their lips around the mouthpiece and blow out or suck in as forcefully as possible, after a deep breath in or out, for MEP and MIP testing, respectively. Three trials were collected, and the highest obtained MEP and MIP were used in the analysis.
Voluntary Cough Spirometry
Cough function was assessed using an oral pneumotachograph (MLT 1000, ADInstruments, Inc.; Colorado Springs, CO), connected to a spirometry mouthpiece filter (MQ 304 Spirometer Filter, VacuMed; Ventura, CA) during voluntary cough production. The participant was seated with nose clips in place as the examiner held the mouthpiece filter securely. The participant was instructed to “cough hard like something is stuck in your throat.” The examiner demonstrated the procedure and ensured the participant understood the requirements of this task, and 3 maximum voluntary cough trials were obtained. The airflow signal was measured, low pass filtered at 60 Hz, digitized at 1,000 Hz, and displayed on a portable laptop computer using Lab Chart Version 7 (Microsoft Corp; Redmond, WA). Physiologic voluntary cough airflow measures deduced from the cough flow waveforms included peak inspiratory and expiratory flow rates (PIF, PEF, L/minute). Cough spirometry measures were assessed on the initial cough epoch across the 3 trials (i.e., the first cough attempt for each of the 3 trials) by a blinded rater.
Forced Vital Capacity
FVC was assessed using a Micro I handheld spirometer (CareFusion, Yorba Linda, CA) with the participant seated and in accordance with the American Thoracic Society guidelines. Percent predicted values for FVC were recorded and used for statistical analysis.
Twelve-Month Functional Follow-up Outcomes
The ALSFRS-R (total and bulbar subscale), oral-intake status (oral = consuming oral intake; nil per oral/non-oral = not consuming any oral intake), NIV utilization (yes/no), and time to NIV referral (months from symptom onset) were indexed twelve months after trial completion. Follow-up analysis was performed by a research assistant blinded to study group allocation who indexed electronic medical records (EMRs) and multidisciplinary clinic notes to derive exploratory metrics of interest. After data extraction, the research assistant met with the attending neurologists at both sites to review obtained data given that some individuals with ALS chose to undergo early prophylactic feeding tube placement (while still able to consume oral intake) and to mitigate any clinical record errors. For participants who had records indicating a gastrostomy tube placement or NIV referral, the research assistant additionally contacted the participant and/or caregiver to confirm the use of either device. Oral feeding status was operationally defined as nil per oral: not consuming any foods or liquids by mouth, and oral feeding: still consuming some oral intake by mouth. Date of NIV referral was verified through chart records, and the duration in months from ALS symptom onset was calculated. Given that clinical prescribers were blinded to treatment allocation and all followed the state of Florida insurance requirements (FVC <50%, NIF <40 cm H2O), the date of referral was used to capture this event end point. A participant was additionally coded as a “yes” to NIV if a documented referral was placed in the EMR that was confirmed by the participant or caregiver as being initiated and in use.
Sample Size Determination
A priori power and sample size calculation was performed using means and standard deviations obtained from individuals with ALS in our previous clinical trial. Using this information, we determined that a total sample of 38 (19 per group) will be required to detect within-group pre-post changes of 13 points in MEPs (SD = 55) at 80% power and alpha = 0.025 (assuming r = 0.90 between pre and post measurements, based on preliminary data). In addition, differences in maximum MEP change of 23 points (SD = 22) between groups were able to be detected with this sample.
Statistical Analysis
Measures were summarized as means and standard deviations (continuous) and percentages (categorical). An intent-to-treat analysis was followed, with missing data imputed using multivariate normal imputation for continuous measures and with most common class used for categorical measures. Linear regression models were used to assess group differences in pre-to-post change for the primary outcomes of interest (MEP, MIP). These models included post-test scores as the outcome with group status and pre-test scores as predictors. Using pre-test scores as a predictor creates a residual change score relative to the outcome and controls for potential baseline performance differences. If the effect of group status is statistically significant (p < 0.05), then it can be interpreted that the groups differed in their change from pre to post. This approach was also used for secondary outcomes that were continuously measured, and logistic regression analyses were used for categorical outcomes (oral intake status). p < 0.025 was considered statistically significant for 2 primary outcomes of MEP and MIP after adjustment for multiple comparisons using Bonferroni correction.24
Twelve-month follow-up analyses compared group differences in slope for both the ALSFRS-R total and bulbar subscale scores (t-test). Slope is an indicator of the average monthly rate of decline, that is, per month change in total ALSFRS-R and bulbar subscale scores. Analyses for group differences in 12-month change in oral feeding status were assessed using the χ2 test. A Kaplan-Meier survival analysis (with censoring) was used to compare time to NIV referral between groups. A Cox proportional hazard model was run to estimate hazard ratios between groups for time to NIV (with 95% confidence intervals). p < 0.05 was considered statistically significant for secondary and exploratory analyses. All analyses were conducted by the study biostatistician using JMP Pro 14.0 (SAS Institute, Cary, NC).
Data Availability
Appropriate anonymized data can be made available to qualified investigators on submission of an acceptable analysis plan. Proposals should be directed to eplowman@ufl.edu. To gain access, data requesters will need to sign a data access agreement.
Results
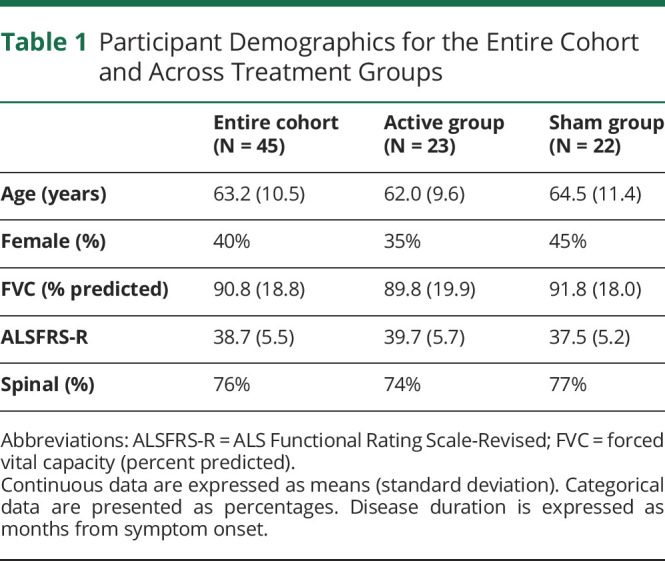
Forty-five individuals met inclusion criteria and were enrolled in this study between September 2016 and May 2018, with follow-up data completed 12 months after the last participant's final evaluation. Demographic details are summarized in Table 1 and show participant characteristics to be well-balanced across groups. Sex distribution in ALS onset type was well balanced, with a spinal onset disease type noted in 78% and 74% of male and female patients, respectively. As is indicated in Figure 1, of the 45 participants commencing, 42 completed the prescribed RST regimen (i.e., 6.7% attrition rate). In these participants, exercise adherence was excellent and ranged between 90 and 100 percent (between 2,700 and 3,000 completed repetitions).
Table 1.
Participant Demographics for the Entire Cohort and Across Treatment Groups
Primary Short-term Physiologic Outcomes
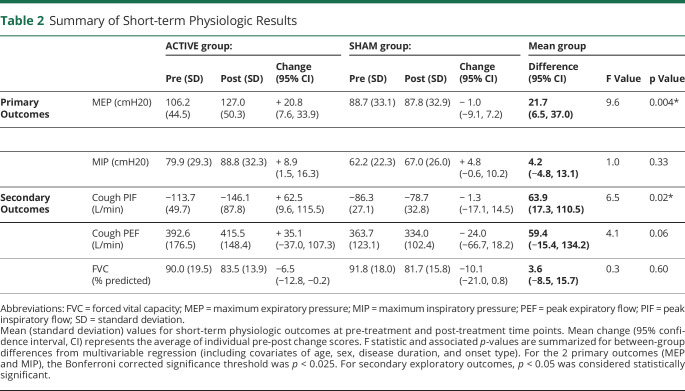
Data are presented in Table 2 and denote a change in MEP between pre-treatment and post-treatment time points across groups. Participants in the active RST group demonstrated a mean MEP increase of 20.8 cm H2O after treatment (95% CI 7.6 to 33.9), compared with a mean decrease in MEP of 1.0 cm H2O for individuals in the sham group (95% CI −9.1 to 7.2). Mean MEP pre-post group difference was 21.7 cm H2O (95% CI 6.5 to 37.0, p = 0.004). No MIP group differences were observed (mean pre-post group difference = 4.2, 95% CI −4.8 to 13.1, p = 0.33). On average, MIP increased by 8.9 cm H2O (95% CI 1.5 to 16.3) vs 4.8 cm H2O (95% CI −0.6 to 10.2) in the active and sham groups, respectively.
Table 2.
Summary of Short-term Physiologic Results
Secondary Short-term Physiologic Outcomes
Change in voluntary cough PIF between pre-test and post-test time points differed by treatment group (Table 2). On average, cough PIF increased by 62.5 L/min (95% CI 9.6 to 115.5) in the active RST group compared with a 1.3 L/min (95% CI −17.1 to 14.5) decrease in the sham RST group, with a mean group difference of 63.9 L/min (95% CI 17.3 to 110.5; p = 0.02). The active RST group demonstrated a mean increase in cough PEF of 35.1 L/min (95% CI −37.0 to 107.3), and the sham RST group had a mean cough PEF decrease of 24.0 L/min (95% CI −66.7 to 18.2). Mean cough PEF group difference was 59.4 L/min (95% CI −15.4 to 134.2, p = 0.06). FVC change did not differ across groups (Table 2) with a mean pre-post group difference of 3.6% predicted (95% CI −8.5 to 15.7, p = 0.60).
Twelve-Month Functional Follow-up Data
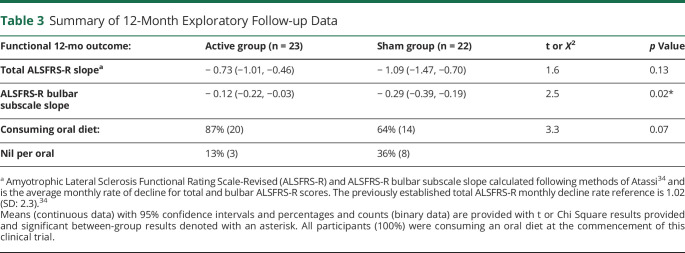
Twelve-month group follow-up data are summarized in Table 3.
Table 3.
Summary of 12-Month Exploratory Follow-up Data
ALSFRS-R Slope
Mean ALSFRS-R slope did not differ across treatment groups and was −0.73 (95% CI −1.01 to −0.46) in the active group compared with −1.09 points/month (95% CI −1.47 to −0.70) in the sham group (p = 0.13).
ALSFRS-R Bulbar Subscale Slope
ALSFRS-R bulbar slope was significantly lower in the active RST group (mean slope: −0.12, 95% CI −0.22 to −0.03) compared with the sham RST group (mean slope: −0.29, 95% CI −0.39 to −0.19, p = 0.02).
NIV
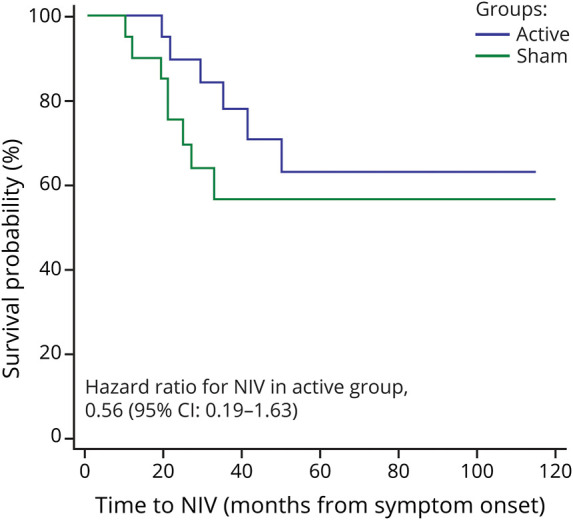
Survival curves for time to NIV referral (from ALS symptom onset) are depicted in Figure 2. The hazard ratio for NIV initiation in the active RST group was 0.56 (95% CI 0.19 to 1.63, p = 0.29).
Figure 2. Survival Curves Comparing Time to Noninvasive Ventilation (NIV) Referral Between the Active (Blue) and Sham (Green) Treatment Groups.
.
Oral Intake Status
All participants were consuming an oral diet at the commencement of this study. One-year follow-up data revealed that 13% of active (n = 3) and 36% of sham (n = 8) group participants could no longer eat by mouth (χ2 = 3.3, df = 1, p = 0.07).
Discussion
RST was well tolerated with no study-related adverse events and minimal attrition. RST led to immediate physiologic improvements in MEP and cough function; however, it did not affect MIP or FVC. One-year ALSFRS-R bulbar subscale decline rates were attenuated for individuals in the active RST group, with other functional 12-month end points not noted to significantly differ across treatment groups.
Earlier works have studied the short-term effect of a 5 and 8-week expiratory strength training regimen at a 50% training load in individuals with ALS.20,21 In these studies, expiratory strength training led to an average increase in MEP of 25%20 and 29%.20,21 This study provides additional support that an active expiratory strength-based program is feasible and well-tolerated and can increase subglottic air pressure physiologic capacity in individuals with early-stage ALS. A comparison of gains across studies suggests potential load-dependent training outcomes for MEP. This study used a 30% training load and induced, on average, a 20% gain in MEP. Comparatively, our previous intervention studies using a 50% training load on the expiratory system were associated with higher gains in MEP ranging from 25% to 30%.20,21 The decision to reduce resistance training loads by 20% in this study was motivated by a desire to avoid fatigue and overtraining, given that both inspiratory and expiratory exercises were included with a higher number of repetitions (from 25 to 50 repetitions). Although improvement in MEP was noted, it was smaller than that in our previous studies and our additional clinical observations in patients with ALS who have trained at loads of 50% or higher.
The lack of observed improvement in MIP may relate to our decision to reduce the training load intensity to 30%, which may not be of sufficient load to the inspiratory system to alter pressure-generating capacities (MIP). Our interpretations are supported, in part, by several previous reports that inspiratory strength programs using progressive loads of up to 60% MIP confer a survival benefit in ALS.13,17,18 This is also plausible considering the overload principle of strength training, which states that a physiologic load must be sufficient to induce neuromuscular adaptations and increased force-generating capacity.25,26 Indeed, typical strength-based programs begin with a resistance load of 60% that progressively increases to 80% of an individual's one-repetition-maximum.25,26 Although we were cautious in our RST protocol to not overly tax individuals with ALS, it seems that our chosen training load of 30% may have been below the optimal intensity to induce adaptation for inspiratory musculature.
Furthermore, although all participants assigned to the active RST group commenced both expiratory and inspiratory training at a 30% load of individualized MEP and MIP, a ceiling effect for inspiratory training loads was encountered in 2 participants who completed the past several weeks' inspiratory exercises at a 25% MIP load. This unexpected issue was due to the available training range of the inspiratory trainers used in this study, which went up to 41 cm H2O (and thus could only train at a 30% load for individuals whose MIP was ≤138 cm H2O). Training at an even lower load in these 2 participants likely affected their potential MIP gains and could have influenced, in part, the observed differential group findings across MEP and MIP primary outcomes. There is also the possibility that, regardless of load, RST may not be capable of improving MIP and diaphragm strength in this progressive neurogenic disease. Since the completion of this trial, inspiratory trainers are now available with training loads of up to 150 cm H2O that we intend to evaluate in our future studies. Although more work is needed to better understand optimal RST training loads and intensity in ALS, current data would suggest that a 50% load is more suitable for mildly affected patients with ALS.
Similar to our most recent randomized control trial of expiratory strength training in ALS,19 FVC was not affected by RST. We had speculated in our previous study that a combined inspiratory and expiratory training program might be needed to translate respiratory strength gains to improved lung capacity (i.e., FVC).21 Although it appears this was not the case, an alternative interpretation is that the training load of 30% was insufficient to induce a transferrable change in lung capacity and FVC. Another explanation for the noted lack of change in FVC may be rooted in the specificity of the strength-based training task itself. In accordance with the specificity principle of plasticity,27 targeted expiratory and inspiratory muscle strength training would induce increased pressure-generation capabilities (i.e., MEP, MIP). By contrast, a program targeting increased lung volume recruitment, such as breath stacking or lung volume recruitment therapy, would increase lung capacity and FVC. Indeed, similar reports of increased respiratory strength without change in FVC or lung capacity after RST have been noted in both multiple sclerosis and ALS,13,15,17,28-30 suggesting that the type of training of the respiratory muscles will dictate the specific type of gains.27 It will be of great interest to examine potential synergistic effects of training programs incorporating both respiratory strength and lung volume recruitment in this patient population.
Consistent with our previous expiratory strength training randomized control trial,21 we noted meaningful improvements in cough spirometry outcomes. Given that peak cough flow depends on the strength and coordination of inspiratory and expiratory musculature, it is plausible that RST facilitated the noted gains in cough flow. A recent study noted that in ALS, cough PEF values decline rapidly at a rate of 124.8 L/minute annually, further highlighting the potential clinical effect of these results and potential translation to improved secretion management and airway defense physiologic capacity.31 The attenuated one-year ALSFRS-R bulbar subscale decline results may be related to the noted cough improvements, given that it includes a secretion management item. In addition, oral intake was preserved in 86% of active RST participants, compared with only 64% in the sham RST group. The latter finding corroborates our previous report that expiratory training affected aspects of oral intake.21 Given the importance of nutrition on ALS disease progression and survival,32,33 these exploratory preliminary data warrant further investigation.
Although the survival analysis did not denote significant differences in the timing of NIV referral across groups, inspection of data in the 14 participants who commenced NIV revealed a 12-month delay for the active RST participants who started using NIV, suggesting that respiratory function was preserved for a relatively longer period in these participants. Furthermore, the active RST group experienced a 46% decreased rate of NIV, as denoted by the obtained hazard ratio of 0.56. Additional work is needed to confirm these long-term data and to further delineate the nature of these functional changes.
Several inherent limitations of this study need to be highlighted. First, although this work represents one of the largest RST studies performed in ALS to date, it was conducted in a small number of participants with a clear need to examine RST in larger cohorts. Second, this work was performed in mildly affected individuals with ALS who were early in ALS disease progression. Therefore, the results cannot be generalized to individuals with ALS whose disease is further progressed. Future work is needed to examine the threshold for safely incorporating active respiratory training programs across the continuum of ALS disease progression. We suspect that a critical window of opportunity exists during the milder stages of the disease to mitigate decline and the inevitable need for invasive and/or assistive respiratory equipment (e.g., cough assist, NIV, insufflation therapy) when active interventions may likely be counterproductive. Third, although participants and examiners were blinded to the assigned treatment condition, it was not feasible to blind the home therapist who performed the weekly home visit. Although the devices looked identical, the home therapist noted that group assignment was discernible because of the inherent differences in air pressure generation required to break the pressure threshold and observable differences in air pressure sound and perceived effort during training. Related to training devices was the inherent limitation for loading the inspiratory system as previously discussed. Future studies should use trainers that are now available with a more extensive training range. Fourth, given that it would be unethical to control for participant activity during the follow-up period after completion of training, there is the potential for long-term data to be influenced by other variables (e.g., use of new medications, alternative off-label treatments). Finally, follow-up exploratory data were derived primarily by medical chart review and confirmed by both the attending neurologist and patients/caregivers. Future work examining long-term outcomes with the inclusion of additional meaningful end points, including incidence of hospitalization and aspiration pneumonia, would be a valuable contribution. Future studies could also include a validated patient-reported quality-of-life outcome to index patient perceptions of the RST program.
A mild-intensity, twelve-week RST program was well tolerated in early affected individuals with ALS and led to improvement in MEP and cough PIF, but not in MIP or FVC. Specificity of training and training intensity seem to be important factors that warrant further examination. One-year follow-up data indicated no group differences in ALSFRS-R scores; however, attenuated bulbar subscale decline with potentially clinically relevant preservation of eating and breathing functions needs to be more closely studied. Future work is needed to discern the optimal type and intensity of training regimens and to further understand timing of training along the ALS disease continuum.
Glossary
- ALS
amyotrophic lateral sclerosis
- ALSFRS-R
Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised
- EMR
electronic medical record
- FVC
forced vital capacity
- MEP
maximum expiratory pressure
- MIP
maximum inspiratory pressure
- NIV
noninvasive ventilation
- PEG
percutaneous endoscopic gastrostomy
- PEF
peak expiratory flow
- PIF
peak inspiratory flow
- RST
respiratory strength training
- SD
standard deviation
Appendix. Authors
Footnotes
Class of Evidence: NPub.org/coe
Study Funding
This study was funded by a ALS Association Clinical Management Grant.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Yang R, Huang R, Chen D, et al. Causes and places of death of patients with amyotrophic lateral sclerosis in south-west China. Amyotroph Lateral Scler. 2011;12(3):206-209. [DOI] [PubMed] [Google Scholar]
- 2.Cleary S, Richman-Eisenstat J. An overview of respiratory treatments for individuals with amyotrophic lateral sclerosis 1. Perspect Swallowing Swallowing Disord (Dysphagia). 2013;22(1):17-25. [Google Scholar]
- 3.Plowman EK, Watts SA, Tabor L, et al. Impact of expiratory strength training in amyotrophic lateral sclerosis. Muscle Nerve. 2016;54(1):48-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plowman EK, Watts SA, Robison R, et al. Voluntary cough airflow differentiates safe versus unsafe swallowing in amyotrophic lateral sclerosis. Dysphagia. 2016;31(3):383-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robison R, DiBiase L, Ashley A, et al. Swallowing safety and efficiency impairment profiles in individuals with amyotrophic lateral sclerosis. Dysphagia. 2022;37(3):644-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tabor-Gray LC, Gallestagui A, Vasilopoulos T, Plowman EK. Characteristics of impaired voluntary cough function in individuals with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20(1-2):37-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tabor-Gray L, Vasilopoulos T, Plowman EK. Differences in voluntary and reflexive cough strength in individuals with amyotrophic lateral sclerosis and healthy adults. Muscle Nerve. 2020;62(5):597-600. [DOI] [PubMed] [Google Scholar]
- 8.Gaziano J, Tabor L, Watts SR, Plowman EK. Prevalence, Timing and Source of Aspiration in Individuals with ALS. Dysphagia Research Society.; 2015. [Google Scholar]
- 9.Shefner JM. Effects of strength training in amyotrophic lateral sclerosis: how much do we know?. Muscle Nerve. 2019;59(1):6-7. [DOI] [PubMed] [Google Scholar]
- 10.Dalbello-Haas V, Florence JM, Krivickas LS. Therapeutic exercise for people with amyotrophic lateral sclerosis or motor neuron disease. Cochrane Database Syst Rev. 2008;16(2):CD005229. [DOI] [PubMed] [Google Scholar]
- 11.Britton D, Cleary S, Miller R. What is ALS and what is the Philosophy of Care? Perspect Swallowing Swallowing Disord (Dysphagia). 2013;22(1):4-11. [Google Scholar]
- 12.Bohannon RW. Results of resistance exercise on a patient with amyotrophic lateral sclerosis. Phys Ther. 1983;63(6):965-968. [DOI] [PubMed] [Google Scholar]
- 13.Cheah BC, Boland RA, Brodaty NE, et al. INSPIRATIonAL--INSPIRAtory muscle training in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2009;10(5-6):384-392. [DOI] [PubMed] [Google Scholar]
- 14.Drory VE, Goltsman E, Goldman Reznik J, Mosek A, Korczyn AD. The value of muscle exercise in patients with amyotrophic lateral sclerosis. J Neurol Sci. 2001;191(1-2):133-137. [DOI] [PubMed] [Google Scholar]
- 15.Ferreira GD, Costa ACC, Plentz RDM, Coronel CC, Sbruzzi G. Respiratory training improved ventilatory function and respiratory muscle strength in patients with multiple sclerosis and lateral amyotrophic sclerosis: systematic review and meta-analysis. Physiotherapy. 2016;102(3):221-228. [DOI] [PubMed] [Google Scholar]
- 16.Pinto AC, Alves M, Nogueira A, et al. Can amyotrophic lateral sclerosis patients with respiratory insufficiency exercise? J Neurol Sci. 1999;169(1-2):69-75. [DOI] [PubMed] [Google Scholar]
- 17.Pinto S, de Carvalho M. Can inspiratory muscle training increase survival in early-affected amyotrophic lateral sclerosis patients? Amyotroph Lateral Scler Frontotemporal Degener. 2013;14(2):124-126. [DOI] [PubMed] [Google Scholar]
- 18.Pinto S, Swash M, de Carvalho M. Respiratory exercise in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2012;13(1):33-43. [DOI] [PubMed] [Google Scholar]
- 19.Robison R, Tabor-Gray LC, Wymer JP, Plowman EK. Combined respiratory training in an individual with C9orf72 amyotrophic lateral sclerosis. Ann Clin Transl Neurol. 2018;5(9):1134-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plowman EK, Watts SA, Tabor L, et al. Impact of expiratory strength training in amyotrophic lateral sclerosis. Muscle Nerve 2016;54(1):48-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plowman EK, Tabor-Gray L, Rosado KM, et al. Impact of expiratory strength training in amyotrophic lateral sclerosis: results of a randomized, sham-controlled trial. Muscle Nerve. 2019;59(1):40-46. [DOI] [PubMed] [Google Scholar]
- 22.Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(5):293-299. [DOI] [PubMed] [Google Scholar]
- 23.Cedarbaum JM, Stambler N, Malta E, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci. 1999;169(1-2):13-21. [DOI] [PubMed] [Google Scholar]
- 24.Vasilopoulos T, Morey TE, Dhatariya KRMJ, Rice MJ. Limitations of significance testing in clinical research: a review of multiple comparison corrections and effect size calculations with correlated measures. Anesth Analgesia. 2016;122(3):825-830. [DOI] [PubMed] [Google Scholar]
- 25.Burkhead LM, Sapienza CM, Rosenbek JC. Strength-training exercise in dysphagia rehabilitation: principles, procedures, and directions for future research. Dysphagia. 2007;22(3):251-265. [DOI] [PubMed] [Google Scholar]
- 26.Deschenes MR, Kraemer WJ. Performance and physiologic adaptations to resistance training. Am J Phys Med Rehabil. 2002;81(Supplement):S3-S16. [DOI] [PubMed] [Google Scholar]
- 27.Kleim JAJTA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res. 2008;51(1):S225-S239. [DOI] [PubMed] [Google Scholar]
- 28.Fry DK, Pfalzer LA, Chokshi AR, Wagner MT, Jackson ES. Randomized control trial of effects of a 10-week inspiratory muscle training program on measures of pulmonary function in persons with multiple sclerosis. J Neurol Phys Ther. 2007;31(4):162-172. [DOI] [PubMed] [Google Scholar]
- 29.Pfalzer L, Fry D. Effects of a 10-week inspiratory muscle training program on lower-extremity mobility in people with multiple sclerosis: a randomized controlled trial. Int J MS Care. 2011;13(1):32-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ray AD, Udhoji S, Mashtare TL, Fisher NM. A combined inspiratory and expiratory muscle training program improves respiratory muscle strength and fatigue in multiple sclerosis. Arch Phys Med Rehabil. 2013;94(10):1964-1970. [DOI] [PubMed] [Google Scholar]
- 31.Tattersall R, Murray D, Heverin M, et al. Respiratory measurements and airway clearance device prescription over one year in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2020;21(1-2):70-77. [DOI] [PubMed] [Google Scholar]
- 32.Desport JC, Preux PM, Truong CT, Courat L, Vallat JM, Couratier P. Nutritional assessment and survival in ALS patients. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(2):91-96. [DOI] [PubMed] [Google Scholar]
- 33.López-Gómez JJ, Ballesteros-Pomar MD, Torres-Torres B, et al. Malnutrition at diagnosis in amyotrophic lateral sclerosis (als) and its influence on survival: using glim criteria. Clin Nutr. 2021;40(1):237-244. [DOI] [PubMed] [Google Scholar]
- 34.Atassi N, Berry J, Shui A, et al. The PRO-ACT database: design, initial analyses, and predictive features. Neurology. 2014;83(19):1719-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rooney J, Burke T, Vajda A, Heverin M, Hardiman O. What does the ALSFRS-R really measure? A longitudinal and survival analysis of functional dimension subscores in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2017;88(5):381-385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Appropriate anonymized data can be made available to qualified investigators on submission of an acceptable analysis plan. Proposals should be directed to eplowman@ufl.edu. To gain access, data requesters will need to sign a data access agreement.