Abstract
Background and Objectives
Idiopathic intracranial hypertension (IIH) most typically occurs in women of childbearing age with increased weight as a key risk factor for development or exacerbation of the disease. Pregnancy is common in this group of patients. The longer-term effect of pregnancy on IIH has not been established and was the aim of this study.
Methods
A prospective cohort study (IIH Life) recruited consecutive patients with IIH between 2012 and 2021 and evaluated outcomes including vision (logarithm of the minimum angle of resolution visual acuity, Humphrey visual field perimetric mean deviation, and optical coherence tomography [OCT] imaging) and headache. Four cohorts were evaluated: those with IIH diagnosed for the first time while pregnant, those with established IIH who became pregnant, those with a pregnancy prior to their diagnosis of IIH, and those with IIH who never became pregnant.
Results
Three hundred seventy-seven people with IIH agreed to participate in the IIH Life maternal health study. Mean follow-up was 17.5 months (SD 20.5). IIH diagnosed in pregnancy was rare. Patients diagnosed with IIH while pregnant had greater papilledema (mean OCT total retinal thickness +11.59 µm/mo [95% CI 1.25–21.93]), although they had comparable visual field and acuity measures compared with those with established IIH who became pregnant during their disease course (−1.2 µm/mo [95% CI −2.6 to 0.21]). In those with established IIH, pregnancy did not adversely affect visual or headache outcomes over time, and the trajectory was akin to those with IIH who never had a pregnancy. Headache outcomes showed variability reflecting the IIH cohort as a whole.
Discussion
A diagnosis of IIH while pregnant was rare but associated with more severe papilledema. Long-term visual outcomes in IIH were analogous irrespective of the timing of the pregnancy. These data are reassuring; however, close vigilance of IIH clinical features during pregnancy is recommended.
Idiopathic intracranial hypertension (IIH) is a disease characterized by elevated intracranial pressure (ICP) associated with increased total body weight.1 IIH incidence is rising commensurate with global obesity rates in women2,3 and occurs most typically in women of childbearing age with increased bodyweight.4,5 Weight gain can exacerbate disease,6-8 and weight loss has been shown to induce remission.9-11 One of the most common reasons why a woman of childbearing age gains body weight is pregnancy.12 There is consequently a concern that pregnancy could induce a diagnosis of IIH or those with an existing diagnosis of IIH could undergo an exacerbation of their disease secondary to becoming pregnant.
Approximately 38% of normal pregnancy weight gain is due to the baby, amniotic fluid, and placenta.13 In healthy pregnant women, the location of the weight gain is predominantly distributed in the hips, back, and thighs,13 whereas in those with IIH, the adipose distribution has been shown to be in the truncal region.14 In women with obesity, there is a lower fat mass gain during pregnancy than in other body mass index (BMI) groups.15,16 The avoidance of excessive gestational weight gain is important due to the risk of IIH exacerbation, higher postpartum fat mass retention, and both maternal and fetal complications.12,15,17
The etiology of IIH is not fully understood, but evidence is emerging to suggest that IIH is not exclusively a disease of the neuro-ophthalmic axis but a systemic metabolic disease.18-24 IIH is noted in association with truncal obesity, increased risk of type 2 diabetes mellitus, greater insulin resistance, and doubled the risk of cardiovascular disease compared with age-, sex-, and BMI-matched individuals.14,19,25-27 In addition, adipose tissue in IIH is metabolically distinct being transcriptionally primed for increased calorie intake with a unique depot-specific lipogenic profile.19 Patients with IIH also demonstrate altered hormonal metabolism with a unique phenotype of serum and CSF, androgen excess, and dysregulated glucocorticoid metabolism.24 The features of systemic metabolic dysregulation in IIH are likely relevant to the reduced fertility rates and an increased risk of gestational complications including preeclampsia and gestational diabetes mellitus.28
There are very few studies of IIH in pregnancy, with only 2 retrospective studies. The first study evaluated 24 patients with IIH with 36 pregnancies, and the latter assessed 12 patients with IIH with 16 pregnancies.29,30 Consequently, there are limited data on which to guide treatment in this area, especially in women who are pregnant at the time of IIH diagnosis. Concern remains that patients with IIH could experience disease exacerbation in pregnancy due to weight gain and hormonal fluctuation. Indeed, historically, patients with IIH were often counseled not to become pregnant. The long-term effect of pregnancy on the disease course of IIH also lacks evidence. This study aimed to find whether patients with IIH diagnosed during pregnancy had a more severe disease course and adverse longer-term outcomes. In addition, the aim was to determine whether people with an existing diagnosis of IIH, who subsequently become pregnant, had worse long-term outcomes compared with those who never became pregnant.
Methods
Study Design
A prospective observational cohort study (IIH: Life) was conducted to evaluate outcomes in women with IIH over time. The patients attended a tertiary referral neuro-ophthalmology clinic at a single neuroscience center in Birmingham, United Kingdom (University Hospitals Birmingham [UHB] NHS Foundation Trust). Data were prospectively collected over a 9-year period, between April 23, 2012, and September 8, 2021, at the time of their routine clinical visits. All sequential patients were included with written informed consent. Recruits included people who were either initially diagnosed at UHB or at other referring hospitals within the United Kingdom and subsequently transferred to UHB. The follow-up visits occurred according to clinical practice, and data from each clinic attendance were recorded.
Study Population
All participants had a confirmed diagnosis of IIH using the modified Dandy criteria.31 The criteria required the presence of papilledema, normal neuroimaging (except for signs of raised ICP), lumbar puncture opening pressure >25 cmCSF in a properly performed procedure, and all secondary causes of raised ICP are excluded. All those included provided written informed consent to take part and completed the IIH Life maternal health study questionnaire. Those who were referred with a potential diagnosis of IIH, but in whom the diagnosis was not confirmed, those with a secondary cause of intracranial hypertension, and those with IIH without papilledema were excluded from the study. In addition, male patients were excluded.
Data Collection
The following data were collected: clinical history, BMI, weight, date of visits, diagnostic lumbar puncture date for disease duration (surrogate measure defined as the time from the first diagnostic lumbar puncture to baseline visit), diagnostic lumbar puncture opening pressure, ICP medication prescription (acetazolamide, topiramate, and other diuretics [furosemide and amiloride]), and details of IIH-related surgery. The self-reported maternal health questionnaire data (see eAppendix 1, links.lww.com/WNL/C626) and the routinely collected clinical visit data determined whether patients were pregnant at the initial IIH diagnosis or had been prior to their diagnosis or became pregnant during follow-up. Within the maternal health questionnaire, patients are asked how many pregnancies and miscarriages they have had.
Visual outcomes were assessed by logarithm of the minimum angle of resolution (logMAR) visual acuity, Humphrey visual field perimetric mean deviation (PMD; 24-2 Swedish Interactive Testing Algorithm central threshold), and optical coherence tomography (OCT) measures (using Heidelberg Spectralis) of global peripapillary retinal nerve fiber layer thickness (RNFL), global peripapillary total retinal thickness (TRT), and macular ganglion cell layer (GCL) volume (1, 2.22, 3.45 mm volume scan). Frisén papilledema grading (0–5 where 0 equates to no papilledema)32,33 was established by a suitably trained neuro-ophthalmology specialist following a dilated slit-lamp examination. Headache outcomes were assessed by headache frequency (monthly headache days), migraine-like headache frequency (monthly migraine-like days), headache severity (0–10 numerical rating scale, where 0 is no pain, and 10 is the most severe pain), and headache disability using the Headache Impact Test 6 (HIT-6) test.34
Statistical Analysis
Continuous and categorical variables were reported as mean (SD) and number (percentage), respectively. Statistical analysis was performed using R (v4.1.0).35 Missing data due to patient choice or lost to follow-up were excluded.
Four groups of patients were defined according to the timing of pregnancy in relation to IIH diagnosis or disease course. These were (1) IIH diagnosed in pregnancy, (2) those with established IIH who became pregnant, (3) pregnancy exclusively occurring prior to IIH diagnosis, and (4) patients with IIH who were never pregnant. The effect of these timings was compared for visual and headache outcomes. The disease course was compared between the 4 groups.
Further analyses explored disease factors that were hypothesized to affect long-term outcomes, including the effect disease duration, diagnostic lumbar puncture opening pressure (both continuously and categorized <25, 25–29.9, 30–39.9, or 40+ cmCSF), BMI at the first visit, BMI at each visit, body weight at the first visit, body weight at each visit, change in gestational weight (for groups 1 and 2), any surgical intervention, and age at the first visit. Models were developed independently for each visual outcome using forward stepwise regression, with null models adjusting for pregnancy timing.
Linear mixed-effects model (lme4) fitting was used for regression models.36 A continuous form of our dependent variables was assumed for all outcomes. The average response value, an adjustment for time from registration to outcome measure, was estimated using population-level terms and an interaction between variables of interest and time point. Patient-level intercepts addressed serial correlation in responses and the nesting of measurements from eyes within patients (as modeling included data from both eyes, where available). Where covariates were added to models, they were transformed or centered around the mean value as appropriate. Each visual outcome had independent modeling.
For headache outcomes, initially, the effect of pregnancy timing was explored. Further analyses explored the effect of previous personal migraine history, family history of migraine, BMI, and daily headache at registration. Models were developed independently for each headache outcome.
Standard Protocol Approvals, Registrations, and Patient Consents
The study was ethically approved by the National Health Service National Research Ethics Committee (14/LO/1208). Written informed consent was obtained from all participants in the study.
Patient and Public Involvement
IIHUK, a national patient charity (Registered Charity in England and Wales no 1143522 and Scotland SCO43294) that supports carers and people with IIH, endorsed and helped developed the IIH Life concept and questionnaire. IIHUK contributed to funding the project. The Medical Research Council, National Institute of Health Research Healthcare Quality Improvement Partnership grant, and the Sir Jules Thorne Award for biomedical science contributed to this longitudinal project.
Data Availability
The corresponding author takes full responsibility for the data, the analyses and interpretation, and the conduct of the research. The corresponding author has full access to all the data and has the right to publish any and all data separate and apart from any sponsor. Proposals for data access should be made to the corresponding author. Reasonable scientifically sound proposals, from appropriately qualified research groups, will provide data beginning 12 months and ending 3 years after the publication of this article to researchers whose proposed use of the data is approved by the corresponding author. Requesters will need to sign a data access agreement, which will cover the terms and conditions of the release of data and will include publication requirements, authorship, acknowledgments, and obligations for the responsible use of data.
Results
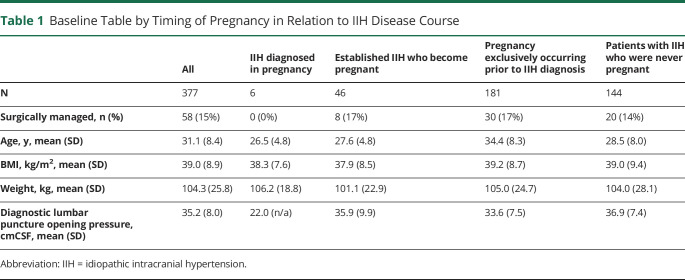
The analysis included 377 women who had a confirmed diagnosis of IIH and had completed the maternal health questionnaire. As categorized, 4 groups consisted of group 1 in whom IIH was diagnosed in pregnancy (2%, 6/377 patients); group 2 were participants with established IIH who became pregnant during their disease course (12%, 46/377 patients); group 3 were individuals who only had pregnancies prior to their diagnosis of IIH (48%, 181/377 patients); and group 4 were patients with IIH who were never pregnant either prior to their disease or during the course of this study (38%, 144/377 patients; Table 1). Miscarriage data were also sought and found rare, with 1 patient in group 2 reporting 1 miscarriage during the IIH disease course, with a subsequent successful pregnancy during which data were recorded.
Table 1.
Baseline Table by Timing of Pregnancy in Relation to IIH Disease Course
Among the entire cohort, 15% (58) of patients required surgical intervention for the management of sight-threatening papilledema during their disease course but none while pregnant. None of the IIH diagnosed in the pregnancy group (group 1) required surgical intervention for sight-threatening papilledema. The proportion of patients undergoing surgical intervention for sight-threatening papilledema at any point was comparable between the 3 remaining groups (those with a pregnancy during the IIH disease course [8, 17%], pregnancy prior to IIH diagnosis but not during the IIH disease course [30, 17%], and those with IIH but never pregnant [20, 14%]).
Baseline characteristics for the 4 groups did differ (Table 1); however, baseline BMI was similar between the groups. No patients were treated with acetazolamide, topiramate, or diuretics during pregnancy, with 3 cases stopping when pregnancy was confirmed. Time from diagnostic LP to conception, an estimate of disease duration before pregnancy, was 26.6 (19.3) months for those pregnant during their IIH disease course. Mean follow-up was 17.5 (SD 20.5) months for all patients, although this was 25.2 (SD 20.3) months if excluded those with a single visit.
Self-Reported Maternal Health Questionnaire
The questionnaire revealed that 16% (61/370) postponed pregnancy plans due to having a diagnosis of IIH, 22% (78/350) reported difficulties getting pregnant, 31% (113/360) reported that IIH had affected their choice of contraception, and 7% (22/329) stated that a pregnancy resulted from inadequate contraception due to a change made due to having IIH.
Visual Prognosis in Those Who Became Pregnant During the IIH Disease Course
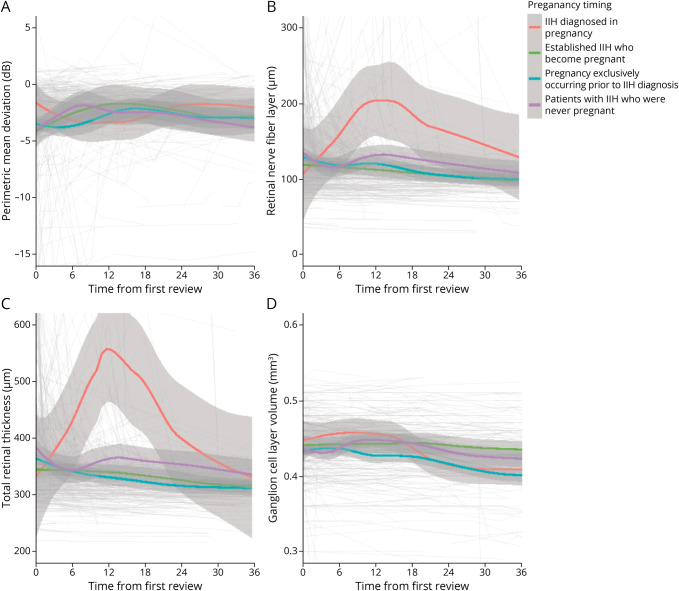
In group 2 (established IIH who became pregnant), the visual outcomes were comparable to those in group 4 (IIH who never had a pregnancy; Figure 1; Table 2). The logMAR visual acuity and Humphrey visual field PMD outcomes were similar for the 2 groups, 2 and 4 (Figure 1A; eFigure 1A, links.lww.com/WNL/C627). OCT measures showed similar baseline values, although the trajectory of improvement was slower for TRT in those with a pregnancy during the IIH disease course, −1.2 (95% CI −2.60 to 0.21) µm/mo vs −3.19 (95% CI −4.09 to −2.28) µm/mo (for those never pregnant; Figure 1C; Table 2) with the difference of 1.99 (95% CI 0.32 to 3.67) µm/mo. RNFL showed a similar trajectory to TRT (Figure 1B; Table 2). GCL volume only showed a slight decrease over time for those with established IIH who became pregnant (Figure 1D; Table 2).
Figure 1. Longitudinal Visual Data From Baseline Visit for Patients With IIH Categorized by the Timing of Pregnancy in Relation to IIH Diagnosis/Disease and LOESS Smoothers Added to Show Trends Across the Categories.
(A) Perimetric mean deviation measured by Humphrey visual field 24-2 testing (dB). (B) Retinal nerve fiber layer thickness measured on OCT (µm). (C) Total retinal thickness of the optic nerve head measured on OCT (µm). (D) Macular ganglion cell layer volume measured on OCT (mm3). IIH = idiopathic intracranial hypertension; OCT = optical coherence tomography.
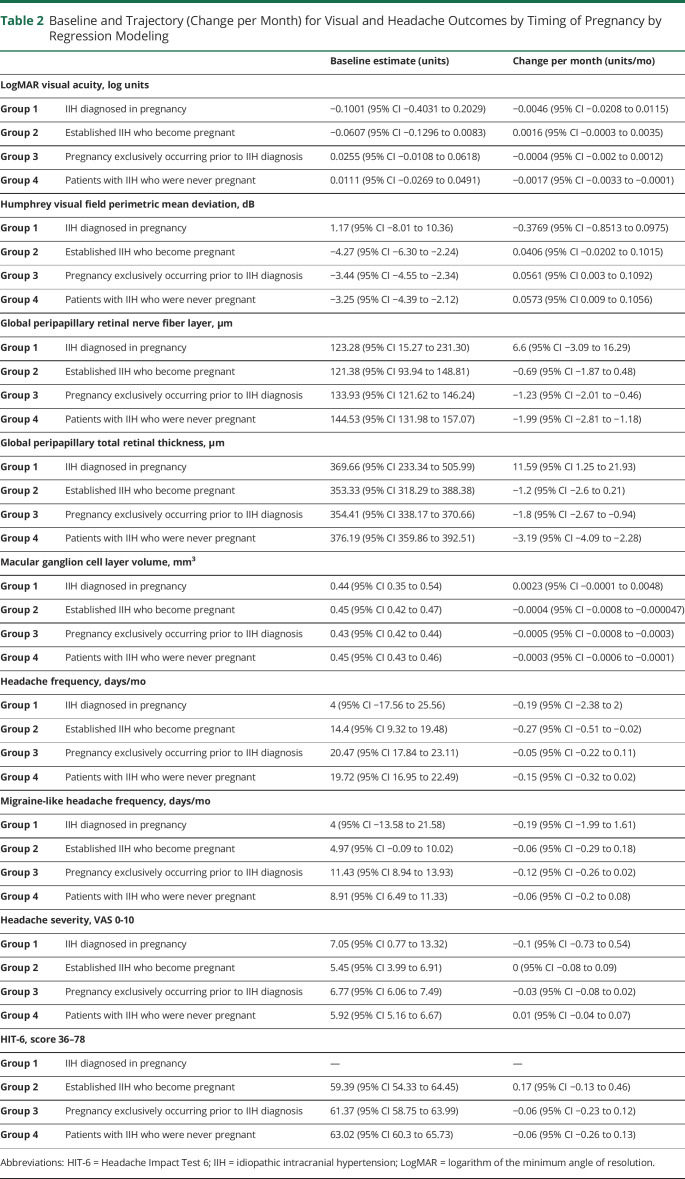
Table 2.
Baseline and Trajectory (Change per Month) for Visual and Headache Outcomes by Timing of Pregnancy by Regression Modeling
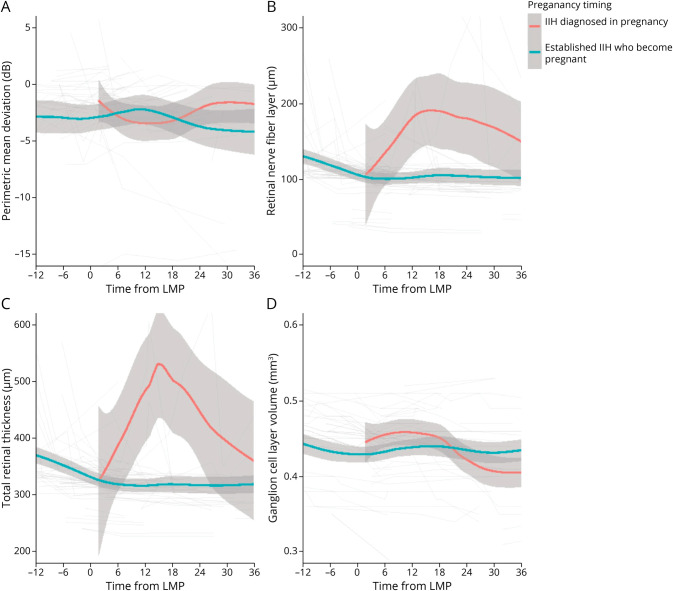
To determine outcomes according to the timing of the pregnancy, the data were evaluated from the timing of the last menstrual period. Visual function (logMAR visual acuity and Humphrey visual field PMD) was stable in the patients with pregnancy occurring during their IIH disease course (Figure 2A; eFigure 1B; Table 3). Papilledema measurements using OCT also remained stable following conception (Figure 2, B–D). In group 2 patients (became pregnant during the IIH disease course), there was recovery of papilledema (measured by RNFL) in the 12 months prior to pregnancy (improvement of 22.32 µm [95% CI 11.77–32.88], from 129.15 to 106.83 µm). Macular GCL volume remained stable during pregnancy in those with a pregnancy during the IIH disease course (group 2; Figure 2D; Table 3).
Figure 2. Longitudinal Visual Data From Estimated LMP for Patients With IIH Categorized by Whether Pregnant at the Time of IIH Diagnosis or Established IIH Who Become Pregnant and LOESS Smoothers Added to Show Trends Across the Categories.
(A) Perimetric mean deviation measured by Humphrey visual field 24-2 testing (dB). (B) Retinal nerve fiber layer thickness measured on OCT (µm). (C) Total retinal thickness of the optic nerve head measured on OCT (µm). (D) Macular ganglion cell layer volume measured on OCT (mm3). IIH = idiopathic intracranial hypertension; LMP = last menstrual period; OCT = optical coherence tomography.
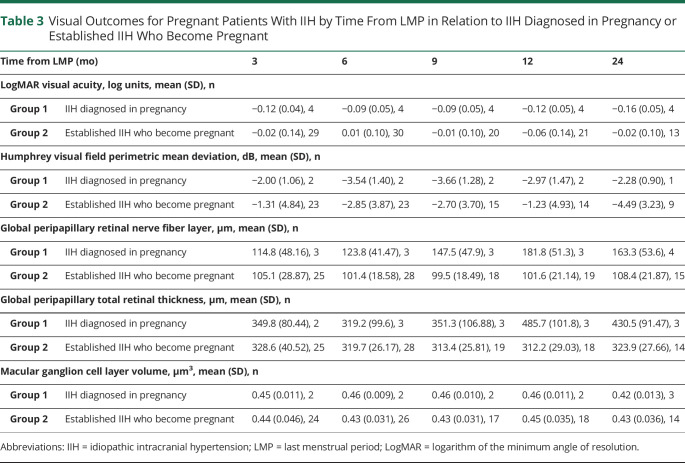
Table 3.
Visual Outcomes for Pregnant Patients With IIH by Time From LMP in Relation to IIH Diagnosed in Pregnancy or Established IIH Who Become Pregnant
Visual Prognosis in Those Diagnosed With IIH in Pregnancy
IIH was rarely diagnosed for the first time in pregnancy (n = 6, 1.6%). Although the numbers are small, these patients demonstrated a deterioration in PMD, OCT RNFL, and TRT following enrollment (Figure 1, A–C; Table 3). Outcomes recovered over time. These patients' baseline visits were also while pregnant, unlike group 2 where the pregnancy occurred during follow-up. There was a statistically significant worse trajectory, toward greater papilledema, in this cohort (group 1; as measured by the TRT on OCT) +11.59 µm/mo (95% CI 1.25–21.93; Figure 1C; Table 2), compared with the other groups (groups 2–4; −1.2 [95% CI −2.6 to 0.21], −1.8 [95% CI −2.67 to −0.94] and −3.19 [95% CI −4.09 to −2.28], respectively), and during pregnancy (Figure 2C) compared with group 2. Other measures were relatively stable over time (Figures 1 and 2; eFigure 1; Table 2).
Visual Prognostic Factors in Pregnancy
Prognosis for visual outcomes was principally determined by the duration of disease prior to pregnancy. The longer the disease duration, the greater the reduction in papilledema (RNFL change at baseline of −0.31 [95% CI −0.53 to −0.093] µm per month since diagnostic lumbar puncture and TRT change −0.49 [95% CI −0.77 to −0.21] µm per month).
Baseline BMI was also important, with higher BMI indicating a poorer prognosis for visual field PMD (0.092 [0.0083, 0.18] dB per 1 kg/m2). Prognosis was not affected by weight gain during pregnancy, change in BMI during pregnancy, or diagnostic lumbar puncture opening pressure.
Headache Prognosis in IIH According to Pregnancy Timing
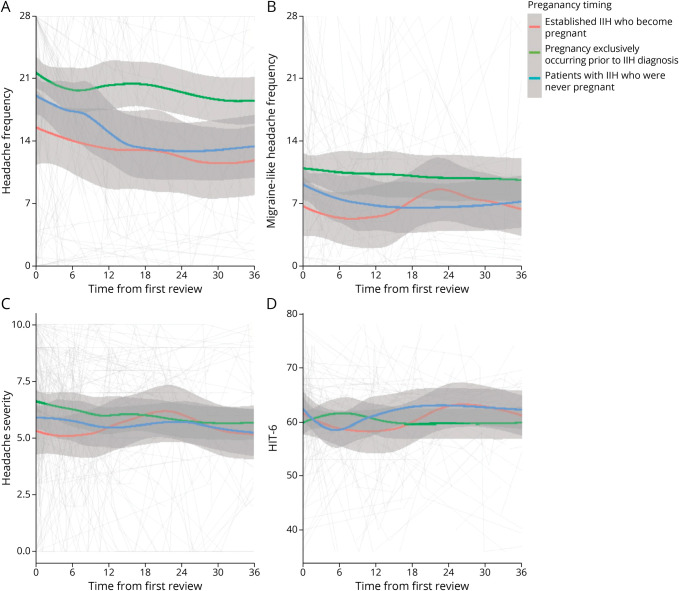
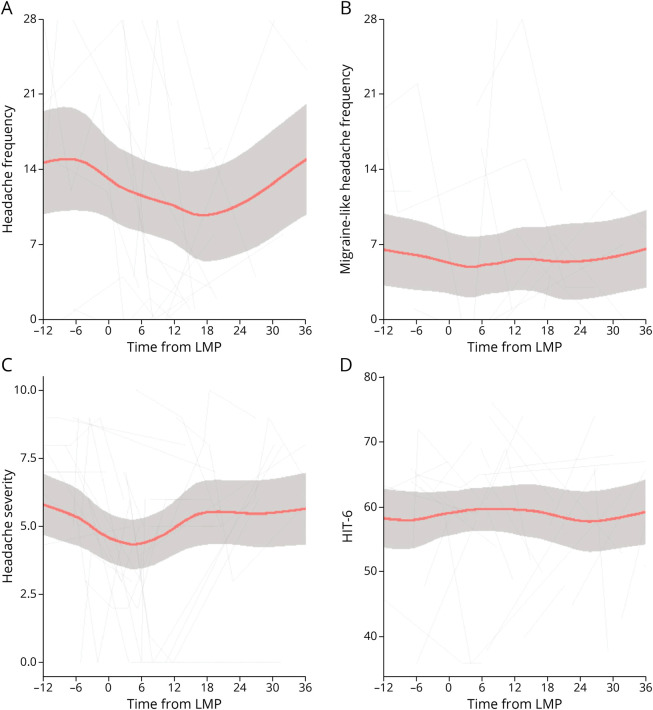
The entire cohort had a high headache morbidity but with marked variability over time. The baseline mean headache frequency (headache days per month) was 14.4 (95% CI 9.32–19.48) for those with established IIH who become pregnant (group 2), 20.5 (95% CI 17.84–23.11) for those with pregnancy only prior to IIH diagnosis (group 3), and 19.7 (95% CI 16.95–22.49) for those never pregnant (group 4; Figure 3; Table 2). There were insufficient data to comment on group 1, those with IIH diagnosed while pregnant (Figure 3; Table 2). Headache frequency improved during pregnancy for those pregnant during the IIH disease course (Figure 4A), whereas the migraine-like headache frequency remained stable (Figure 4B).
Figure 3. Longitudinal Headache Data From Baseline Visit for Patients With IIH Categorized by the Timing of Pregnancy in Relationship to IIH Disease Course and LOESS Smoothers Added to Show Trends Across the Categories.
(A) Headache frequency (days per month). (B) Migraine-like headache frequency (days per month). (C) Headache mean severity of predominant headache (0–10 numerical rating scale). (D) HIT-6 (quality of life measure score 36–78). HIT-6 = Headache Impact Test 6; IIH = idiopathic intracranial hypertension.
Figure 4. Longitudinal Headache Data From Estimated LMP for Patients With Established IIH Who Become Pregnant and LOESS Smoothers Added to Show Trends Across the Categories.
(A) Headache frequency (days per month). (B) Migraine-like headache frequency (days per month). (C) Headache mean severity of predominant headache (0–10 numerical rating scale). (D) HIT-6 (quality of life measure score 36–78). HIT-6 = Headache Impact Test 6; IIH = idiopathic intracranial hypertension; LMP = last menstrual period.
Headache severity was moderate intensity, rated 5.5 of 10 (95% CI 3.99–6.91) for those pregnant during their IIH disease course (group 2), 6.8 (95% CI 6.06–7.49) for those with pregnancy only prior to IIH diagnosis (group 3), and 5.9 (95% CI 5.16–6.67) for those never pregnant (group 4; Figure 3C; Table 2). Severity appeared to improve prior to pregnancy slightly with relative stability during pregnancy (Figure 4C).
Headache burden, measured by the HIT-6 score, was comparable between these 3 groups (Figure 3D; Table 2) with a moderate to severe impact (59–63 of 78), with relative stability during pregnancy (group 2; Figure 4D). There was no difference in trajectory (Table 2) between any of the pregnancy groups, and it remained stable. Daily headache at baseline visit was the main prognostic factor for headache frequency and severity outcomes, with previous migraine history and disease duration having a lesser but statistically significant effect on headache frequency.
Discussion
This prospective study assessed the effect of pregnancy on IIH outcomes. Pregnancy at the time of IIH diagnosis is rare but characterized by worsening of visual field function and papilledema measured by OCT imaging during the pregnancy with subsequent recovery after the pregnancy. Despite a seemingly more severe disease course, none of this subset required surgical intervention for sight-threatening papilledema. For those with established IIH, becoming pregnant did not adversely affect visual outcomes. Headache outcomes in the long term showed marked variability, and no relationship was found to the timing of pregnancy.
Self-reported fertility problems were reported by 22% of our cohort. It is noteworthy that 31% of participants reported that IIH had affected their contraception choice and a concern that 7% stated that a pregnancy resulted from inadequate contraception due to changes made as a result of their IIH diagnosis. This observation highlights the need to modernize the education of physicians, particularly for those who manage contraceptive prescriptions as there are no contraindicated contraceptives in IIH.12
The proportion of patients diagnosed with IIH while pregnant is lower (1.6%) than previously reported in other smaller studies 3.3%–8.4%.29,30 This could be due to variation between global IIH populations and sample sizes. Although a diagnosis of IIH diagnosed in pregnancy (group 1) was a rare occurrence, the disease should be closely monitored throughout pregnancy considering the more severe papilledema here when diagnosed during pregnancy. In group 1, we also noted a trend toward declining PMD and worsening papilledema measured by OCT imaging (Figure 2). We have previously shown that acute IIH has more severe papilledema than chronic IIH,37 so there is the possibility that newly diagnosed IIH in pregnancy would have more severe papilledema than those with more chronic IIH who become pregnant. Those patients with IIH diagnosed in pregnancy (group 1) were interesting in that papilledema worsened during the 12 months following diagnosis. This contrasts with patients with newly diagnosed IIH who are not pregnant who gradually improve over time.37 This poses a significant treatment dilemma as in pregnancy there are more limited treatment options, including the potential teratogenicity of carbonic anhydrase inhibitors,12 although 1 study showed the safety of acetazolamide in pregnancy.38 The mechanism to explain why new-onset IIH may deteriorate during pregnancy is not known. Hypertestosteronism has been demonstrated in IIH and linked to driving CSF secretion and ICP elevation.24 It is also known that during pregnancy testosterone levels increase.39 Thus, a potential mechanism might be the increased testosterone in pregnancy upregulating CSF secretion causing increased ICP and a worsening of the papilledema observed here during pregnancy.
The longitudinal outcomes for patients with established IIH who became pregnant (group 2) are comparable to those patients with IIH who were never pregnant (group 4) and those with pregnancies only prior to an IIH diagnosis (group 3). This is a constructive result and highlights that pregnancy should not be avoided in all patients with IIH, although control of the disease should be optimized before planned pregnancies where possible.29,30 Papilledema improved in the 12 months before conception (Figure 2, B–C) reflecting disease optimization targeting ocular remission before planned pregnancies, although it could also reflect the natural disease course seen in patients with IIH with active disease.37 The current recommendation is to optimize IIH control prior to pregnancy,12 and this may additionally help reduce the previously demonstrated increased risk of gestational diabetes mellitus and preeclampsia.28 This may also reflect the worse visual outcomes seen in those diagnosed in pregnancy (group 1) as they may have not had the opportunity to have the disease optimized before conception. For this group, avoidance of excessive gestational weight gain would be considered important.12,17
This study did not demonstrate pregnancy-relevant modifiable visual prognostic factors, of either gestational bodyweight or BMI gain, although this may reflect the difficulty in differentiating normal gestational weight gain from excessive gestational weight gain. Weight management is the only disease-modifying therapy in IIH5,9,10; however, this is more challenging in pregnancy, especially given the differing distributions of adiposity seen in pregnancy and IIH.13,14 Routine weight monitoring during pregnancy is not presently recommended in the United Kingdom according to the National Institute for Heath and Care Excellence, although IIH would a condition in which it should be monitored due to the risks of high gestational weight gain.40 An individualized approach is recommended, discussed in a sensitive manner avoiding obesity stigmatizing language.12 Disease duration was a prognostic marker likely reflecting a longer time from elevated ICP detected and therefore more likely to be in ocular remission. This highlights that targeting disease remission before pregnancy has improved long-term outcomes.
Headache burden in IIH is a significant problem even after the disease comes into ocular remission41 and while recently headache morbidity appears to be correlated with raised ICP, the mechanisms driving headache once the ICP has settled remain unclear.42,43 These data show marked variability in headache outcomes, which reflects what has been found in the IIH population as a whole.37 Headache frequency was high at baseline (Figure 3) in groups 2, 3, and 4 with either mild improvement or stability over time (Table 2). This plateau was also seen for migraine-like headache frequency, headache severity, and HIT-6 score and highlights the need for new treatment options for the management of persistent post-IIH headache, which has been called for by patients and physicians.41,44 Headache treatments are limited in pregnancy, and these are mainly based around acute medications rather than prophylactics.12 The prognostic factors in this cohort were daily headache at diagnosis and a personal migraine history. This is helpful clinically as it could signpost those people who require escalation of headache treatment and referral to specialist services.
This was a prospectively collected real-world clinical practice study and has inherent limitations such as missing data. For example, missing data exist for those patients seen at other institutions before referral to UHB. Attempts were made to minimize this by obtaining prior clinical noting; however, some data were omitted. As a tertiary neuro-ophthalmology referral center, initial visits may have occurred at other hospitals in the country before subsequent referral, and therefore, baseline data could have been at a different point in their disease course. To reduce this bias, we defined disease duration as being the time from the first diagnostic lumbar puncture to the first encounter in our clinic (baseline visit); however, lead-time bias may have an effect on the accuracy of these data. There are currently no studies evaluating the exact start of symptoms that confirm a diagnosis of IIH, as defining disease onset. The maternal health questionnaire was self-reported and recall bias could exist here. An unavoidable limitation is the sample size for those diagnosed with IIH in pregnancy, as it is a rare occurrence, however our results highlight that this should be studied further. Use of medications is limited in pregnancy and therefore may partially explain some of the worsening in papilledema seen in those diagnosed in pregnancy, although would not explain fully the differences as those with subsequent pregnancies did not deteriorate. Some outcomes were missing due to patient preference and others as OCT imaging protocols changed after the start of the study. Some patients were in ocular remission from an IIH perspective when pregnancy occurred, and therefore, the changes seen reflect a real-world cohort rather than what occurs in active IIH during pregnancy. As per clinical practice, some individuals were discharged to other institutions for local follow-up, whereas others in remission were discharged completely, leading to reduced numbers longitudinally followed up. In this analysis, we also did not have a control group without IIH, although to control for pregnancy, we included patients who had never been pregnant.
Diagnosing IIH in pregnancy is rare with worsening of PMD and papilledema measurements noted during pregnancy. Established IIH with subsequent pregnancies occurs more frequently and has a good visual outcome. Headache outcomes showed marked variability with similar long-term outcome between all groups. Monitoring of people with IIH during their pregnancy is important and particularly needed in those diagnosed with IIH at the time of pregnancy. Our data suggest that in those with well-controlled IIH prior to pregnancy, the pregnancy did not exacerbate IIH.
Acknowledgment
The authors thank all the patients with IIH who have contributed to this study and the patient charity IIHUK for their continued support of our research.
Glossary
- BMI
body mass index
- GCL
ganglion cell layer
- HIT-6
Headache Impact Test 6
- ICP
intracranial pressure
- IIH
idiopathic intracranial hypertension
- logMAR
logarithm of the minimum angle of resolution
- OCT
optical coherence tomography
- PMD
perimetric mean deviation
- RNFL
retinal nerve fiber layer thickness
- TRT
total retinal thickness
- UHB
University Hospitals Birmingham
Appendix. Authors

Footnotes
CME Course: NPub.org/cmelist
Study Funding
IIH:Life database is funded by the Healthcare Quality Improvement Partnership, and IIHUK, a registered patient charity (number 1143522), supported this work. AJS has been funded during this study by Medical Research Council grant number MR/KO15184/1, National Institute of Health Research grant NIHR-CS-011-028 (clinician scientist fellowship), and Sir Jules Thorne Award for Biomedical Science (no grant number). The study sponsor and funders had no role in the study design, interpretation of the data, data collection, or drafting of the manuscript.
Disclosure
M. Thaller and V. Homer report no disclosures relevant to the manuscript. S. P. Mollan reports consultancy fees from Invex Therapeutics, advisory board fees from Invex Therapeutics and GenSight, and speaker fees from Heidelberg Engineering, Chugai-Roche Ltd, Allergan, Santen, Teva UK, Chiesi, and Santhera. A. J. Sinclair reports consulting fees from Allergan, Chiesi, Novartis, and Lundbeck, personal fees from Invex Therapeutics during the conduct of the study as well as share option and shareholdings, and speaker fees from Novartis, Allergan, and Teva UK. Go to Neurology.org/N for full disclosures.
References
- 1.Mollan SP, Grech O, Alimajstorovic Z, Wakerley BR, Sinclair AJ. New horizons for idiopathic intracranial hypertension: advances and challenges. Br Med Bull. 2020;136(1):118-126. doi: 10.1093/bmb/ldaa034. [DOI] [PubMed] [Google Scholar]
- 2.Mollan SP, Aguiar M, Evison F, Frew E, Sinclair AJ. The expanding burden of idiopathic intracranial hypertension. Eye 2019;33(3):478-485. doi: 10.1038/s41433-018-0238-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mollan SP, Mytton J, Tsermoulas G, Sinclair AJ. Idiopathic intracranial hypertension: evaluation of admissions and emergency readmissions through the hospital episode statistic dataset between 2002-2020. Life 2021;11(5):417. doi: 10.3390/life11050417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mollan SP, Davies B, Silver NC, et al. Idiopathic intracranial hypertension: consensus guidelines on management. J Neurol Neurosurg Psychiatry 2018;89(10):1088-1100. doi: 10.1136/jnnp-2017-317440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mollan SP, Tahrani AA, Sinclair AJ. The potentially modifiable risk factor in idiopathic intracranial hypertension: body weight. Neurol Clin Pract. 2021;11(4):e504-e507. doi: 10.1212/CPJ.0000000000001063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thaller M, Tsermoulas G, Sun R, Mollan SP, Sinclair AJ. Negative impact of COVID-19 lockdown on papilloedema and idiopathic intracranial hypertension. J Neurol Neurosurg Psychiatry 2021;92(7):795-797. doi: 10.1136/jnnp-2020-325519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniels AB, Liu GT, Volpe NJ, et al. Profiles of obesity, weight gain, and quality of life in idiopathic intracranial hypertension (pseudotumor cerebri). Am J Ophthalmol. 2007;143(4):635-641.e1. doi: 10.1016/j.ajo.2006.12.040. [DOI] [PubMed] [Google Scholar]
- 8.Ko MW, Chang SC, Ridha MA, et al. Weight gain and recurrence in idiopathic intracranial hypertension: a case-control study. Neurology 2011;76(18):1564-1567. doi: 10.1212/WNL.0b013e3182190f51. [DOI] [PubMed] [Google Scholar]
- 9.Sinclair AJ, Burdon MA, Nightingale PG, et al. Low energy diet and intracranial pressure in women with idiopathic intracranial hypertension: prospective cohort study. BMJ 2010;341:c2701. doi: 10.1136/bmj.c2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mollan SP, Mitchell JL, Ottridge RS, et al. Effectiveness of bariatric surgery vs community weight management intervention for the treatment of idiopathic intracranial hypertension: a randomized clinical trial. JAMA Neurol. 2021;78(6):678-686. doi: 10.1001/jamaneurol.2021.0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mollan SP, Mitchell JL, Yiangou A, et al. Association of amount of weight lost after bariatric surgery with intracranial pressure in women with idiopathic intracranial hypertension. Neurology 2022;99(11):e1090-e1099. doi: 10.1212/WNL.000000000020083910.1212/wnl.0000000000200839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thaller M, Wakerley BR, Abbott S, Tahrani AA, Mollan SP, Sinclair AJ. Managing idiopathic intracranial hypertension in pregnancy: practical advice. Pract Neurol. 2022;22(4):295-300. doi: 10.1136/practneurol-2021-003152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donangelo CM, Bezerra FF. Pregnancy: metabolic adaptations and nutritional requirements. In: Caballero B, Finglas PM, Toldrá F, eds. Encyclopedia of Food and Health. Academic Press; 2016:484-490. [Google Scholar]
- 14.Hornby C, Botfield H, O'Reilly MW, et al. Evaluating the fat distribution in idiopathic intracranial hypertension using dual-energy X-ray absorptiometry scanning. Neuroophthalmology 2018;42(2):99-104. doi: 10.1080/01658107.2017.1334218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Subhan FB, Shulman L, Yuan Y, McCargar LJ, Kong L, Bell RC. Association of pre-pregnancy BMI and gestational weight gain with fat mass distribution and accretion during pregnancy and early postpartum: a prospective study of Albertan women. BMJ Open 2019;9(7):e026908. doi: 10.1136/bmjopen-2018-026908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Most J, Altazan AD, Hsia DS, Beyl RA, Redman LM. Body composition during pregnancy differs by obesity class. Obesity (Silver Spring) 2020;28(2):268-276. doi: 10.1002/oby.22699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santos S, Voerman E, Amiano P, et al. Impact of maternal body mass index and gestational weight gain on pregnancy complications: an individual participant data meta-analysis of European, North American and Australian cohorts. BJOG 2019;126(8):984-995. doi: 10.1111/1471-0528.15661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Markey K, Mitchell J, Botfield H, et al. 11β-Hydroxysteroid dehydrogenase type 1 inhibition in idiopathic intracranial hypertension: a double-blind randomized controlled trial. Brain Commun. 2020;2(1):fcz050. doi: 10.1093/braincomms/fcz050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westgate CSJ, Botfield HF, Alimajstorovic Z, et al. Systemic and adipocyte transcriptional and metabolic dysregulation in Idiopathic Intracranial Hypertension. JCI Insight 2021;6(10):e145346. doi: 10.1172/jci.insight.145346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Westgate CSJ, Markey K, Mitchell JL, et al. Increased systemic and adipose 11β-HSD1 activity in idiopathic intracranial hypertension. Eur J Endocrinol. 2022;187(2):323-333. doi: 10.1530/EJE-22-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinclair AJ, Onyimba CU, Khosla P, et al. Corticosteroids, 11beta-hydroxysteroid dehydrogenase isozymes and the rabbit choroid plexus. J Neuroendocrinol 2007;19(8):614-620. doi: 10.1111/j.1365-2826.2007.01569.x. [DOI] [PubMed] [Google Scholar]
- 22.Sinclair AJ, Walker EA, Burdon MA, et al. Cerebrospinal fluid corticosteroid levels and cortisol metabolism in patients with idiopathic intracranial hypertension: a link between 11beta-HSD1 and intracranial pressure regulation? J Clin Endocrinol Metab. 2010;95(12):5348-5356. doi: 10.1210/jc.2010-0729. [DOI] [PubMed] [Google Scholar]
- 23.Hardy RS, Botfield H, Markey K, et al. 11βHSD1 inhibition with AZD4017 improves lipid profiles and lean muscle mass in idiopathic intracranial hypertension. J Clin Endocrinol Metab. 2021;106(1):174-187. doi: 10.1210/clinem/dgaa766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Reilly MW, Westgate CS, Hornby C, et al. A unique androgen excess signature in idiopathic intracranial hypertension is linked to cerebrospinal fluid dynamics. JCI Insight 2019;4(6):e125348. doi: 10.1172/jci.insight.125348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hornby C, Mollan SP, Botfield H, O'Reilly MW, Sinclair AJ. Metabolic concepts in idiopathic intracranial hypertension and their potential for therapeutic intervention. J Neuroophthalmol 2018;38(4):522-530. doi: 10.1097/WNO.0000000000000684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adderley NJ, Subramanian A, Nirantharakumar K, et al. Association between idiopathic intracranial hypertension and risk of cardiovascular diseases in women in the United Kingdom. JAMA Neurol. 2019;76(9):1088. doi: 10.1001/jamaneurol.2019.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grech O, Mollan SP, Wakerley BR, Alimajstorovic Z, Lavery GG, Sinclair AJ. Emerging themes in idiopathic intracranial hypertension. J Neurol. 2020;267(12):3776-3784. doi: 10.1007/s00415-020-10090-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thaller M, Mytton J, Wakerley BR, Mollan SP, Sinclair AJ. Idiopathic intracranial hypertension: evaluation of births and fertility through the Hospital Episode Statistics dataset. BJOG 2022;129(12):2019-2027. doi: 10.1111/1471-0528.17241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Digre KB, Varner MW, Corbett JJ. Pseudoturnor cerebri and pregnancy. Neurology 1984;34(6):721-729. doi: 10.1212/wnl.34.6.721. [DOI] [PubMed] [Google Scholar]
- 30.Huna-Baron R, Kupersmith MJ. Idiopathic intracranial hypertension in pregnancy. J Neurol. 2002;249(8):1078-1081. doi: 10.1007/s00415-002-0791-4. [DOI] [PubMed] [Google Scholar]
- 31.Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology 2013;81(13):1159-1165. doi: 10.1212/WNL.0b013e3182a55f17. [DOI] [PubMed] [Google Scholar]
- 32.Frisen L. Swelling of the optic nerve head: a staging scheme. J Neurol Neurosurg Psychiatry 1982;45(1):13-18. doi: 10.1136/jnnp.45.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vijay V, Mollan SP, Mitchell JL, et al. Using optical coherence tomography as a surrogate of measurements of intracranial pressure in idiopathic intracranial hypertension. JAMA Ophthalmol. 2020;138(12):1264-1271. doi: 10.1001/jamaophthalmol.2020.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kosinski M, Bayliss MS, Bjorner JB, et al. A six-item short-form survey for measuring headache impact: the HIT-6™. Qual Life Res. 2003;12(8):963-974. doi: 10.1023/A:1026119331193. [DOI] [PubMed] [Google Scholar]
- 35.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2018. Available at: R-project.org/. [Google Scholar]
- 36.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1):1-48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 37.Thaller M, Homer V, Hyder Y, et al. The idiopathic intracranial hypertension prospective cohort study: evaluation of prognostic factors and outcomes. J Neurol. 2022;270(2):851-863. doi: 10.1007/s00415-022-11402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Falardeau J, Lobb BM, Golden S, Maxfield SD, Tanne E. The use of acetazolamide during pregnancy in intracranial hypertension patients. J Neuroophthalmol 2013;33(1):9-12. doi: 10.1097/WNO.0b013e3182594001. [DOI] [PubMed] [Google Scholar]
- 39.O'Leary P, Boyne P, Flett P, Beilby J, James I. Longitudinal assessment of changes in reproductive hormones during normal pregnancy. Clin Chem. 1991;37(5):667-672. doi: 10.1093/clinchem/37.5.667. [DOI] [PubMed] [Google Scholar]
- 40.National Institute for Health and Care Excellence. Weight management before, during and after Pregnancy [PH27]. 2010. https://www.nice.org.uk/guidance/ph27 [Google Scholar]
- 41.Mollan SP, Grech O, Sinclair AJ. Headache attributed to idiopathic intracranial hypertension and persistent post-idiopathic intracranial hypertension headache: a narrative review. Headache 2021;61(6):808-816. doi: 10.1111/head.14125. [DOI] [PubMed] [Google Scholar]
- 42.Mollan SP, Wakerley BR, Alimajstorovic Z, et al. Intracranial pressure directly predicts headache morbidity in idiopathic intracranial hypertension. J Headache Pain 2021;22(1):118. doi: 10.1186/s10194-021-01321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grech O, Mollan SP, Wakerley BR, Fulton D, Lavery GG, Sinclair AJ. The role of metabolism in migraine pathophysiology and susceptibility. Life (Basel) 2021;11(5):415. doi: 10.3390/life11050415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mollan S, Hemmings K, Herd CP, Denton A, Williamson S, Sinclair AJ. What are the research priorities for idiopathic intracranial hypertension? A priority setting partnership between patients and healthcare professionals. BMJ Open 2019;9(3):e026573. doi: 10.1136/bmjopen-2018-026573. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The corresponding author takes full responsibility for the data, the analyses and interpretation, and the conduct of the research. The corresponding author has full access to all the data and has the right to publish any and all data separate and apart from any sponsor. Proposals for data access should be made to the corresponding author. Reasonable scientifically sound proposals, from appropriately qualified research groups, will provide data beginning 12 months and ending 3 years after the publication of this article to researchers whose proposed use of the data is approved by the corresponding author. Requesters will need to sign a data access agreement, which will cover the terms and conditions of the release of data and will include publication requirements, authorship, acknowledgments, and obligations for the responsible use of data.