Abstract
Background and Objectives
There is a rising incidence of infective endocarditis–related stroke (IERS) in the United States attributed to the opioid epidemic. A contemporary epidemiologic description is necessary to understand the impact of the opioid epidemic on clinical characteristics of IERS. We describe and analyze trends in the demographics, risk factors, and clinical features of IERS.
Methods
This is a retrospective cohort study within a biracial population of 1.3 million in the Greater Cincinnati/Northern Kentucky region. All hospitalized patients with hemorrhagic or ischemic stroke were identified and physician verified from the 2005, 2010, and 2015 calendar years using ICD-9 and ICD-10 codes. IERS was defined as an acute stroke attributed to infective endocarditis meeting modified Duke Criteria for possible or definite endocarditis. Unadjusted comparison of demographics, risk factors, outcome, and clinical characteristics was performed between each study period for IERS and non-IERS. An adjusted model to compare trends used the Cochran-Armitage test for categorical variables and a general linear model or Kruskal-Wallis test for numerical variables. Examination for interaction of endocarditis status in trends was performed using a general linear or logistic model.
Results
A total of 54 patients with IERS and 8,204 without IERS were identified during the study periods. Between 2005 and 2015, there was a decline in rates of hypertension (91.7% vs 36.0%; p = 0.0005) and increased intravenous drug users (8.3% vs 44.0%; p = 0.02) in the IERS cohort. The remainder of the stroke population demonstrated a significant rise in hypertension, diabetes, atrial fibrillation, and perioperative stroke. Infective endocarditis status significantly interacted with the trend in hypertension prevalence (p = 0.001).
Discussion
From 2005 to 2015, IERS was increasingly associated with intravenous drug use and fewer risk factors, specifically hypertension. These trends likely reflect the demographics of the opioid epidemic, which has affected younger patients with fewer comorbidities.
Ischemic stroke is the most common neurologic complication of infective endocarditis, afflicting 20%–40% of cases.1,2 Risk factors of infective endocarditis–related stroke (IERS) are vegetation size, Staphylococcus aureus, and aortic or mitral valve involvement.3,4 IERS frequently occurs in the first week of illness and is often the impetus for patients to seek initial medical care.5,6 Early identification of IERS is paramount because initiation of antibiotic therapy is effective in reducing further embolization, and inadvertent administration of thrombolytic therapy carries an excessive risk of hemorrhagic transformation.5,7,8
Over the past decade, North America has seen a surge of opioid use and a rise in intravenous drug use.9 The age-adjusted incidence rate of opioid drug–related deaths in the United States has risen alarmingly from 6.1 per 100,000 in 1999 to 21.7 per 100,000 in 2017.10 A study of the national inpatient sample data from 1993 to 2015 found an increase in rates of hospitalization for patients with stroke associated with infective endocarditis and opioid use using ICD-9 codes, correlating temporally with the rates of national opioid use.11 However, specific clinical characteristics were unavailable to describe the evolving phenotype of the disease.12 Furthermore, the impact of concurrent changes in other subgroups at high risk for infective endocarditis remains uncertain. Population-based studies conducted within high-income nations of the 21st century have described an increase in rates of infective endocarditis associated with valvular heart disease, malignancy, indwelling catheters, hemodialysis, and implanted cardiac devices. Nosocomial infections accounted for approximately 25%–30% of these cases, and the average age of patients had shifted to older than 70 years.13,14 Most of these studies were conducted before the current opioid epidemic, and there is scant data on population-based trends in stroke secondary to infective endocarditis.15,16 Given the changing epidemiology of infective endocarditis, a contemporary description of the unique clinical characteristics of IERS is needed.17,18
There have been few population-based studies of infective endocarditis in the United States with most occurring before the current opioid epidemic and scant data on population-based trends in stroke secondary to infective endocarditis.15,16 Ohio and Kentucky were greatly affected by the opioid epidemic with the third and fourth highest national rates of age-adjusted drug overdose in 2015, respectively.19,20 We sought to compare temporal trends in the demographics and risk factor profile of IERS with the overall stroke population and describe trends in the clinical features of IERS in the Greater Cincinnati/Northern Kentucky region of the United States from 2005 to 2015.
Methods
Population
The Greater Cincinnati/Northern Kentucky Stroke Study (GCNKSS) is a population-based epidemiologic study including all inpatient strokes conducted every 5 years in the Greater Cincinnati/Northern Kentucky region. This population is largely representative of the United States regarding median age, percent Black, and socioeconomic indicators.21 In brief, all residents in the 5-county region admitted to local acute-care hospitals with ischemic or hemorrhagic stroke in 2005, 2010, and 2015 were identified by a study nurse through ICD-9 codes 430–436 or ICD-10 codes G45, G46, H34, and I60-I69 from hospital and emergency department discharge lists. Double counting was prevented by cross-checking of event sources. All participating sites in each study period had IRB approval. Prior studies have described the details of patient inclusion and data acquisitions.22 Potential cases of IERS were identified by ICD-9 and ICD-10 codes of hospitalized patients and adjudicated by study physicians. IERS was defined by modified Duke criteria for possible or definite infective endocarditis.23
Data Collection/Measurements
Age, sex, race, and medical history were abstracted for all hospitalized stroke patients in the population to compare temporal trends in demographic and risk factors between patients with IERS and all non-IERS patients in the population. Active intravenous drug use was not identified for all stroke patients and was specifically extracted from chart review of the IERS group. Heroin constituted most of the intravenous drug users (IVDU). End-stage renal disease was not collected in 2005 and was specifically extracted from the charts of the IERS.
To describe temporal trends in the clinical features of IERS, presenting symptoms, initial laboratory work, initial National Institute of Health Stroke Scale, remote history of infective endocarditis, and tissue plasminogen activator (tPA) or endovascular therapy administration were abstracted. A remote history of infective endocarditis was defined as occurring more than 6 months before the pertinent hospitalization. The location of initial medical contact with stroke symptoms, specifically emergency department vs inpatient, was included to identify patients who had stroke as the primary presenting feature of infective endocarditis. The time of last known well (LKW) was determined from study nurse estimation from review of clinical records into increments of 0–6 hours, 7–12 hours, 13–18 hours, 19–24 hours, and beyond 24 hours. Patients presenting to the emergency department with an unknown LKW time were categorized as greater than 24 hours. Patients who were already hospitalized during stroke onset without a documented LKW time were categorized as 0–6 hours. Neurologic deterioration was defined as any documented clinical worsening of neurologic symptoms over the course of the hospitalization. Fever was defined as a recorded temperature of >100.5 F, and sepsis was determined by the presence of at least 2 systemic inflammatory response syndrome criteria during stroke. Blood culture results were obtained from discharge summaries from the case records. Study charts included the radiologist interpretation of brain imaging and echocardiogram results. Ischemic and hemorrhagic stroke subtyping was adjudicated by physician reviewers. Neurologic outcomes at discharge were abstracted from records, and information on death was obtained from the Centers for Disease Control and Prevention National Death Index.
Statistical Analysis
SAS software, version 9.4 (SAS Institute, Cary, NC) was used for data management and analysis. Descriptive statistics of baseline demographic information, medical history, laboratory data, presenting clinical features, and blood culture results was performed for each of the 3 study periods and overall. Categorical variables were described using frequency and percent; continuous variables were described as mean and associated SD or median with interquartile range and range.
Comparison of demographics, risk factors, outcome, and clinical features between study periods for IERS and non-IERS was performed through the χ2 or Fisher exact test for categorical variables with an associated Cochran-Armitage test for trend over the study periods; a general linear model or Kruskal-Wallis test was performed for numerical variables. Examination for interaction of endocarditis status for demographics, risk factor, and outcome trend between study periods used a general linear or logistic model, as appropriate.
Using census data of the region for each respective year of the study and the US census data for 2010, the estimated rate of stroke associated with endocarditis was calculated and age, sex, and race adjusted to the 2010 US population. The 95% confidence intervals were estimated using a Poisson distribution. Examination of temporal trends in the rate of non-IERS between the 3 years 2005, 2010, and 2015 used the t test with a Bonferroni correction. Due to the small number of events, statistical examination of the change in rate over time in the IERS was not performed. Evaluation of statistical significance was set at a critical value of <0.05.
A secondary analysis compared demographic and clinical characteristics between IVDU and non-IVDU. Comparison between groups used the Fisher exact test for categorical variables and the t test or Wilcoxon rank sum test for numerical variables. An exploratory analysis compared trends in demographics and risk factors in IERS without IVDU over the study periods using the χ2 test for categorical variables with an associated Cochran-Armitage test for trend and a general linear model for numerical variables.
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by the institutional review boards at all participating hospitals, and informed consent was waived.
Data Availability
Anonymized data may be shared at the request to any qualified investigator for purposes of replicating procedures and results.
Results
Trends in IERS Compared With the Stroke Population
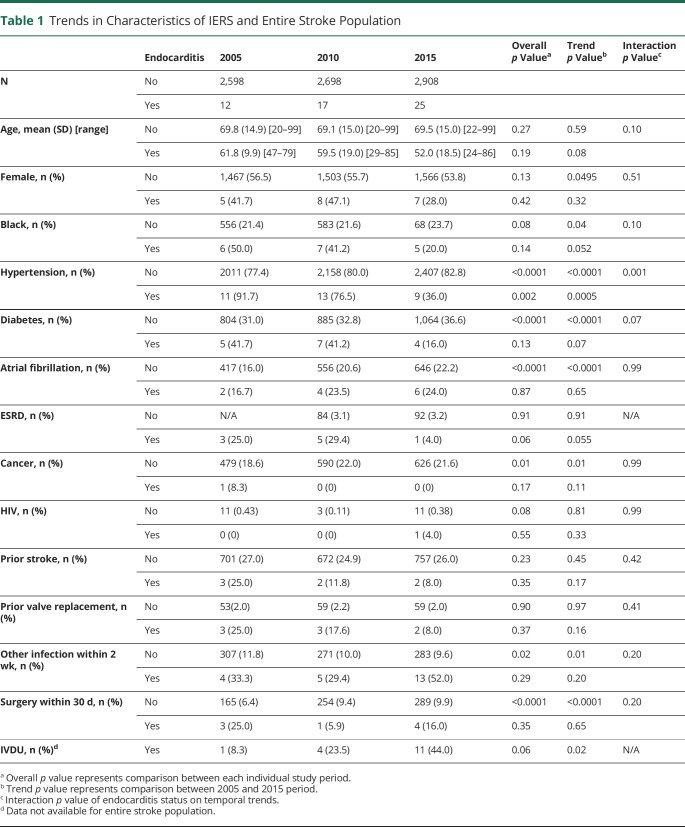
A total of 54 cases of IERS and 8,205 non-IERS were identified over the study periods. There were no missing demographic or comorbidity data in both groups. Among those with IERS, there were significantly fewer patients with hypertension (91.7% in 2005, 76.5% in 2010, and 36.0% in 2015) and significantly more IVDU (8.3% in 2005, 23.5% in 2010, and 44.0% in 2015). There also was a nonsignificant trend among patients with IERS toward increased proportions of White individuals, younger age, and a lower proportion of other traditional vascular risk factors including diabetes, prior heart valve replacement, end-stage renal disease, and prior stroke. In the non-IERS cohort, there was an increased prevalence of male individuals, Black individuals, and hypertension, diabetes, atrial fibrillation, malignancy, and perioperative stroke. Test of interaction of endocarditis status on the temporal trends in demographic and risk factors revealed endocarditis status significantly interacted with hypertension rates (Table 1).
Table 1.
Trends in Characteristics of IERS and Entire Stroke Population
Description of Clinical Features of IERS Cohort
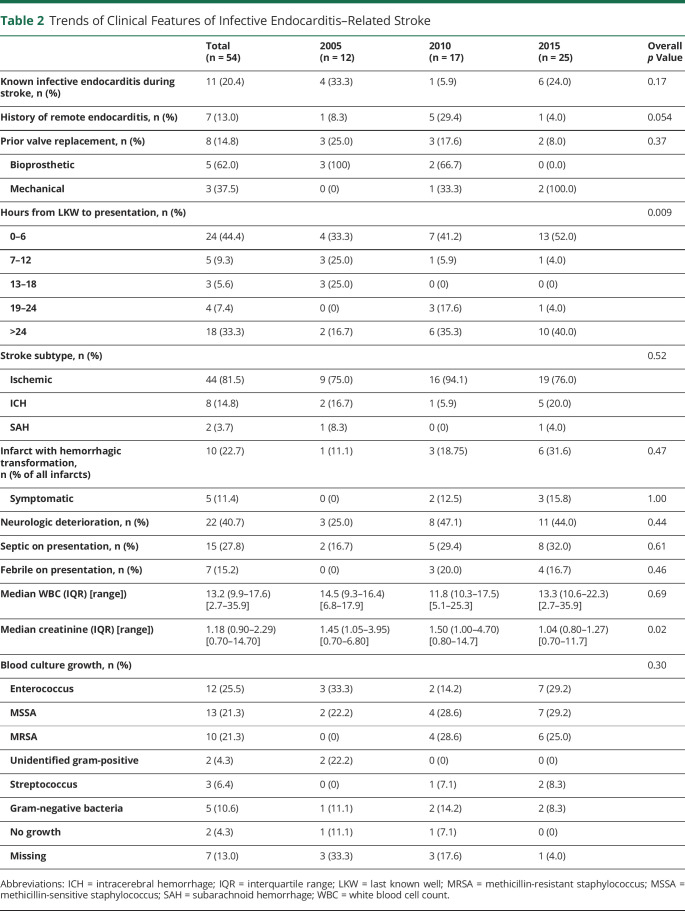
Among patients with IERS, ischemic stroke accounted for 81.5% of all patients, intraparenchymal hemorrhage composed 14.8%, and subarachnoid hemorrhage was 3.7%. Approximately 74.1% of patients had manifest stroke symptoms on initial presentation to the emergency department. Only 20.4% of patients were known to have active infective endocarditis during stroke recognition. Clinical criteria of sepsis were evident in 27.8% during stroke recognition. Among patients presenting initially with stroke symptoms in the emergency department, only 15.2% had fever. All patients with IERS had a complete blood count and basic metabolic panel. Median white blood cell counts were mildly elevated at 13.2 cells × 109/L. There was a significant decline in initial median creatinine over the study period from 1.45 (interquartile range [IQR] 9.3–16.4) in 2005, to 1.5 (IQR 1.00–4.70) in 2010, to 1.04 (IQR 0.80–1.27) in 2015 (p = 0.02). Neurologic decline during hospitalization occurred in 40.7% of cases. Hemorrhagic transformation occurred in 22.7% of all infarcts, and symptomatic hemorrhagic transformation occurred in 11.4% (Table 2). A significant change in distribution of time from LKW to presentation was seen over the study period, with a trend for patients to present either beyond 24 hours or within the first 6 hours. Although 44.4% of patients arrived within the first 6 hours from the time last seen well, one patient received tPA and one patient underwent endovascular therapy during the study time frame. Vascular imaging was performed in 51.8% of cases; 28.6% of cases with vascular imaging identified mycotic aneurysms (eTable 1, links.lww.com/WNL/C624).
Table 2.
Trends of Clinical Features of Infective Endocarditis–Related Stroke
The most common bacteria identified on blood culture, comprising 3-quarters of the cases, were enterococcus, methicillin-sensitive S aureus, and methicillin-resistant S aureus. All cases secondary to methicillin-resistant S aureus occurred in the 2010 and 2015 cohort with no documented occurrences in 2005 (Table 2). More than half of all vegetations were located on the mitral valve, and more than one-third had aortic valve vegetations. Valvular regurgitation was the most common finding on echocardiogram other than vegetation with mitral regurgitation in 58.5% and aortic regurgitation in 28.3% (eTable 1, links.lww.com/WNL/C624). Data were missing for 13% of blood culture results and 9.3% of vegetation location.
Trends in Rate and Outcomes of IERS
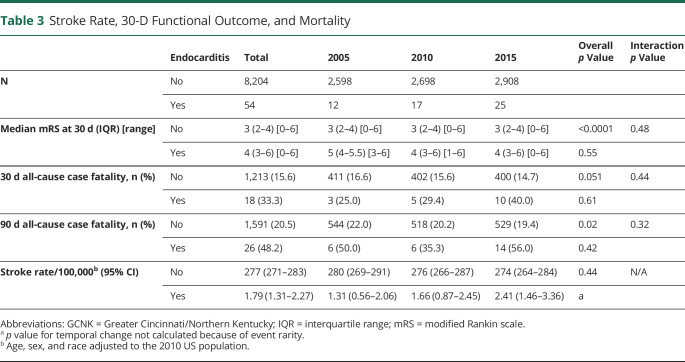
The rate of IERS increased from 1.31 per 100,000 (CI 0.56–2.06) in 2005 to 1.66 per 100,000 (CI 0.87–2.45) in 2010 to 2.41 per 100,000 (CI 1.46–3.36) in 2015 (Table 3). Thirty-day modified Rankin scale was missing in 5.67% of the stroke population without IERS. There were no missing outcome data in the IERS cohort. Thirty-day and 90-day mortality data were complete in the entire stroke population. Functional outcome and mortality rates were poor with no significant improvements over the study periods. The median modified Rankin scale at 30 days was 4 (IQR 3–6), and 90-day case fatality was 48.3%. The non-IERS stroke population experienced a significant improvement in ninety-day mortality over time from 22.0% in 2005 to 19.4% in 2015 (p = 0.02). There was no statistical interaction of endocarditis status on functional outcome or mortality trend (Table 3).
Table 3.
Stroke Rate, 30-D Functional Outcome, and Mortality
Characteristics of Intravenous Drug Users With IERS
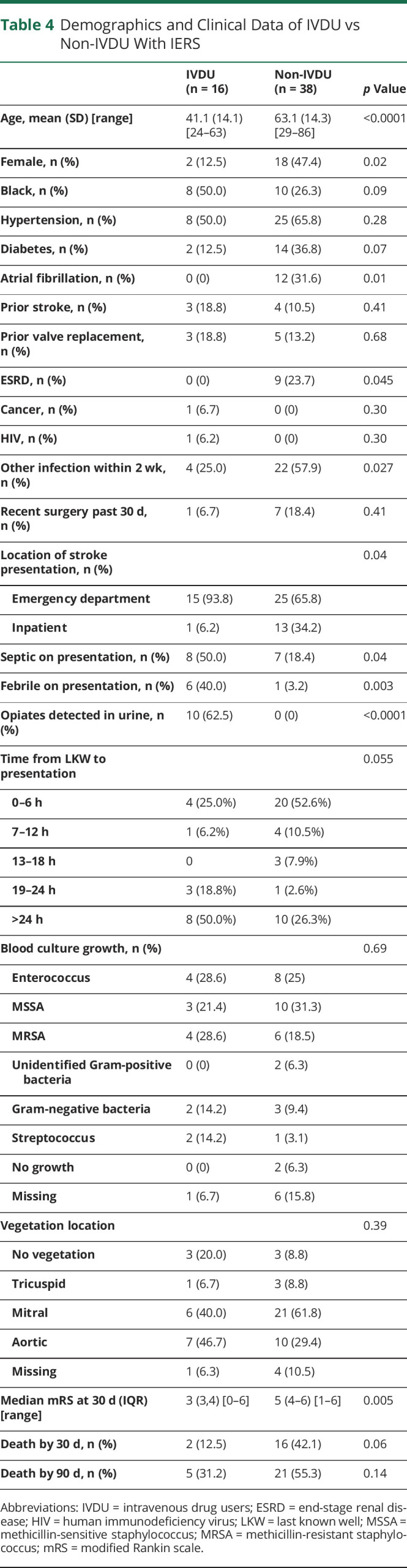
Compared with non-IVDU with endocarditis, active IVDU were significantly younger, male, and without history of atrial fibrillation or end-stage renal disease (Table 4). The location of stroke symptom recognition was in the emergency department for 93.8% of IVDU compared with 65.8% of non-IVDU (p = 0.04). Urine drug testing on admission was missing in 0% of IVDU with IERS and 10.5% of non-IVDU with IERS. Among patients with IERS, urine testing for opioids was positive in 62.5% of IVDU compared to 0% of non-IVDU (p ≤ 0.0001). IVDU compared with non-IVDU were more likely to have evidence of sepsis (50.0% vs 18.4%, p = 0.04) and fevers (40.0% vs 3.2%, p = 0.003) on presentation. There were significantly higher rates of preceding separate infections among non-IVDU compared with IVDU (57.9% vs 25.0%, p = 0.027), and there was a significant rise in preceding infections among non-IVDU over the study period (eTable 2, links.lww.com/WNL/C624). There was a nonsignificant trend for a difference in time to presentation with 50.0% of IVDU presenting more than 24 hours from LKW. By contrast, 52.6% of non-IVDU were identified within 6 hours from LKW. Enterococcus and methicillin-resistant S aureus were the most common microbial isolates from blood cultures among IVDU while methicillin-sensitive S aureus was the most common in non-IVDU. IVDU achieved a significantly better functional outcome at 30 days with a median modified Rankin scale of 3 compared with 5 for non-IVDU (Table 4).
Table 4.
Demographics and Clinical Data of IVDU vs Non-IVDU With IERS
Discussion
In this population-based stroke study of the Greater Cincinnati/Northern Kentucky region, the demographics of patients with IERS has shifted toward younger ages, increased intravenous drug use, and reduced rates of hypertension. By contrast, the remainder of the stroke population demonstrated increased rates of traditional vascular risk factors over time. This lends further support to the literature on the impact of the opioid epidemic on the epidemiology of infective endocarditis. Prior studies of the National Inpatient Sample of hospitalizations have found an increased incidence of infective endocarditis associated with drug use across the United States.18,24 Epidemiologic studies of infective endocarditis in developed nations before the opioid crisis had described declines in rates of Streptococci viridans and rheumatic heart disease, while the frequency S aureus, advanced age, prosthetic valve replacement, degenerative valvular disease, and hemodialysis had risen.15,16,25–29 By contrast, patients with intravenous drug use–related infective endocarditis tend to be younger, from lower socioeconomic status, and with fewer medical comorbidities.18,24
Over the 15-year study period in this study, we see a trend toward an increased rate of IERS. The increase was primarily driven by a rise in cases associated with intravenous drug use and temporally coincides with the national opioid epidemic.30 Although the increase was not formally statistically tested because of the small number of cases, the number of cases doubled over the decade, confirming the previously reported increase in IERS from the national inpatient sample.11 The midwestern region of the United States has been particularly afflicted with the greatest increases in rates of infective endocarditis and IERS.11,18 In Ohio, the rapid increase in heroin-related deaths began in 2008 and peaked in 2016. The state has the fourth highest rate of opioid-related death in the United States with 29.6/100,000 in 2018.10 The southwestern portion of the state, including the Greater Cincinnati region, has particularly high rates with opioid-related death rates of 43.4/100,00 in Hamilton County.31
Neurologic deficits are a common initial manifestation of infective endocarditis before its diagnosis.6 For most patients with IERS in our population, neurologic deficits attributed to stroke were present on first arrival at the emergency department; however, less than half of patients had fever, sepsis, or a known infection. This highlights the lack of consistent historical and clinical biomarkers to aid clinicians in accurate identification in the acute setting. Biomarkers such as C-reactive protein, white blood cell count, procalcitonin, and D-dimer have been investigated in infective endocarditis with limited utility in prognosis and poor diagnostic precision.32,33 Patients with IERS in our study had only a modest elevation in white blood cell count, limiting its practical reliability in diagnostic and therapeutic decision-making. This poses a challenge for clinicians treating patients with acute ischemic stroke because active infective endocarditis is a contraindication to tPA due to high rates of hemorrhagic transformation.34 Our cohort validates this contraindication with 22.7% experiencing hemorrhagic transformation and 11.7% being symptomatic. Only 1 patient with IERS received tPA during this study; the patient experienced a fatal symptomatic hemorrhagic transformation.
Changes in presentation features of IERS also highlights important alterations in the population composition of this disease. Over the study period, patients with IERS were more likely to present either within the initial 6 hours from LKW or beyond 24 hours. Half of IVDU presented beyond 24 hours, whereas most of the non-IVDU presented within 6 hours. IVDU were also significantly more likely to have neurologic deficits identified in the emergency department, whereas more than one-third of non-IVDU developed stroke symptoms during hospitalization. Contemporary cohort studies before the opioid epidemic estimated 25%–30% of infective endocarditis to be health care associated and nosocomial acquired, with a rising proportion of healthcare-associated cases.14,16 Patients with healthcare-acquired or nosocomial-acquired IERS may have stroke symptoms identified sooner while in the hospital or due to greater awareness and access to medical care, whereas IVDU may be reluctant to seek medical care urgently. Prior studies have supported that younger ages and drug use is associated with prolonged time presentation in acute stroke, possibly due to lack of medical literacy or social support.35,36 Non-IVDU also were significantly more likely to have a separate proceeding infection within the prior 2 weeks and less likely to present with other indications of infection such as fever or sepsis potentially due to older age and medical comorbidities, resulting in an impaired host immune response.
Despite advances in medical care over the study period, neurologic and functional outcomes are poor in patients with IERS.34 Nearly one-third of patients were dead at 30 days, and approximately 48.2% of patients in our cohort died within 3 months. IVDU were found to have a significantly better functional outcome, likely related to younger age and fewer medical comorbidities. In prior studies, IVDU with infective endocarditis have been shown to have a lower hospital mortality compared with non-IVDU; however, long-term outcomes remain poor primarily due to relapse in substance use. A high proportion experienced neurologic decline after their initial stroke, and among survivors, most were left with moderate to severe disability. Recurrent stroke, worsening sepsis, cardiac decompensation, and other medical complications contribute to this decline.17,24,37 While early initiation of antibiotic therapy is known to dramatically reduce the risk of recurrent embolic events, patients with infective endocarditis remain at an elevated risk of recurrent stroke for up to 2 years.8,38–40
This study suggests that the modern epidemiology of infective endocarditis and its complications such as stroke may be dichotomized based on IV drug use. IVDUs have divergent outcomes, comorbidities, and social challenges compared with non-IVDU who have more complex comorbidities, different mechanisms of disease acquisition such as from indwelling catheters or devices, and worse outcome. These differences will likely only continue to exacerbate as the opioid epidemic continues and survival improves in specific patient populations such as those with left ventricular assist devices and end-stage renal disease.41,42
The primary limitation of the study was the small number of IERS with resultant difficulty in detecting statistically significant changes in stroke rate or clinical features. Although the GCNKSS collects detailed patient-level data, features specific to infective endocarditis were not consistently collected such as blood culture data or vegetation location. Interpretation of specific microbial trends in IERS over time may be limited. Although the use of IV drugs was documented by study nurses, data extraction required manual review from study charts. Thus, it was not able to be extracted from the entire stroke cohort, and it is uncertain whether the rise in IVDU occurred in the remainder stroke population as well. Due to the stigma associated with drug use and insensitivity of urine drug testing, underreporting of intravenous drug use is possible. Furthermore, several patients did not have completion of all diagnostic testing because testing was not proscribed because this was a retrospective epidemiology study. However, one of the strengths of the GCNKSS is the independent review of medical records and determination of stroke etiology of all patients by a vascular neurology–trained study physician. It is possible that hospitalized patients with IERS were not captured because of missed diagnosis from symptom recognition failure or illness severity, leading to death before evaluation. Individuals diagnosed with stroke in the outpatient setting were not included in this study, which may underestimate the rate of IERS; however, because active infective endocarditis typically requires hospitalization, this was not felt to pose a significant limitation. Lastly, because the GCNKSS captures only patients with stroke, we did not have access to data on all cases with infective endocarditis in the population and were unable to determine whether changes in rate of IERS are solely attributable to changes in overall rate of infective endocarditis in the population.
The demographic and clinical features of stroke due to infective endocarditis have changed from 2005 to 2015. The impact of the opioid epidemic is reflected in the transforming phenotype of IERS. Cerebrovascular complications remain a serious manifestation of the disease and require a high index of suspicion. The poor functional outcomes and mortality have not improved over the decade study period, emphasizing the need for tailored innovation in this pathology.
Glossary
- ICD-9
International Classification of Diseases–9
- IERS9
infective endocarditis–related stroke
- IQR
interquartile range
- IVDU9
intravenous drug users
- GCNKSS9
Greater Cincinnati Northern Kentucky Stroke Study
- LKW
last known well
- tPA9
tissue plasminogen activator
Appendix. Authors
Study Funding
The GCNKSS (Greater Cincinnati/Northern Kentucky Stroke Study) was funded by a grant from the National Institutes of Neurologic Disorders and Stroke (R01 NS 30678).
Disclosure
M. Ridha reports no disclosures relevant to the manuscript; M.L. Flaherty reports funding from the National Institute of Neurologic Disorders and Stroke (R01NS030678); Y.N. Aziz and L.M.C. Ades report no disclosures relevant to the manuscript; K. Alwell reports funding from the National Institute of Neurologic Disorders and Stroke (R01NS030678); J. Khoury reports funding from the National Institute of Neurologic Disorders and Stroke (R01NS030678); D. Woo reports funding from the National Institute of Neurologic Disorders and Stroke (R01NS030678); S. Ferioli reports funding from the National Institute of Neurologic Disorders and Stroke (R01NS030678); O. Adeoye receives support from Sense Diagnostics, Inc.; P. Khatri reports funding from the National Institute of Neurologic Disorders and Stroke (R01NS030678); F.D.L.R.L Rosa reports no disclosures relevant to the manuscript; E. Mistry reports funding from the National Institute of Neurologic Disorders and Stroke (R01NS030678) and from National Institute of Neurological Disorders and Stroke (K23NS113858); S.L. Demel reports no disclosures relevant to the manuscript; J. Mackey reports funding from the National Institute of Neurologic Disorders and Stroke (R01NS030678); S. Martini, E. Coleman, and A.S. Jasne report no disclosures relevant to the manuscript; S. Slavin reports funding from the National Institute of Neurologic Disorders and Stroke (R01NS030678); K. Walsh reports funding from the National Institute of Neurologic Disorders and Stroke (R01NS030678); M. Star reports no disclosures relevant to the manuscript; M. Haverbusch reports funding from the National Institute of Neurologic Disorders and Stroke (R01NS030678); T.E. Madsen is funded by the National Heart, Lung, and Blood Institute (K23HL140081); J. Broderick reports no disclosures relevant to the manuscript; B. Kissela reports funding from the National Institute of Neurologic Disorders and Stroke (R01NS030678); D. Kleindorfer reports funding from the National Institute of Neurologic Disorders and Stroke (R01NS030678). Go to Neurology.org/N for full disclosures.
References
- 1.Morris NA, Matiello M, Lyons JL, Samuels MA. Neurologic complications in infective endocarditis. Neurohospitalist. 2014;4(4):213-222. doi: 10.1177/1941874414537077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper HA, Thompson EC, Laureno R, et al. Subclinical brain embolization in left-sided infective endocarditis. Circulation. 2009;120(7):585-591. doi: 10.1161/CIRCULATIONAHA.108.834432. [DOI] [PubMed] [Google Scholar]
- 3.Iung B, Tubiana S, Klein I, et al. Determinants of cerebral lesions in endocarditis on systematic cerebral magnetic resonance imaging: a prospective study. Stroke. 2013;44(11):3056-3062. doi: 10.1161/STROKEAHA.113.001470. [DOI] [PubMed] [Google Scholar]
- 4.Mohananey D, Mohadjer A, Pettersson G, et al. Association of vegetation size with embolic risk in patients with infective endocarditis. JAMA Intern Med. 2018;178(4):502-510. doi: 10.1001/jamainternmed.2017.8653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hart RG, Foster JW, Luther MF, Kanter MC. Stroke in infective endocarditis. Stroke. 1990;21(5):695-700. doi: 10.1161/01.STR.21.5.695. [DOI] [PubMed] [Google Scholar]
- 6.Epaulard O, Roch N, Potton L, Pavese P, Brion JP, Stahl JP. Infective endocarditis-related stroke: diagnostic delay and prognostic factors. Scand J Infect Dis. 2009;41(8):558-562. doi: 10.1080/00365540902984701. [DOI] [PubMed] [Google Scholar]
- 7.Walker KA, Sampson JB, Skalabrin EJ, Majersik JJ. Clinical characteristics and thrombolytic outcomes of infective endocarditis-associated stroke. Neurohospitalist. 2012;2(3):87-91. doi: 10.1177/1941874412446199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dickerman SA, Abrutyn E, Barsic B, et al. The relationship between the initiation of antimicrobial therapy and the incidence of stroke in infective endocarditis: an analysis from the ICE Prospective Cohort Study (ICE-PCS). Am Heart J. 2007;154(6):1086-1094. doi: 10.1016/j.ahj.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 9.Products–Data Briefs–Number 356–January 2020. 2020. Accessed September 7, 2020. cdc.gov/nchs/products/databriefs/db356.htm. [Google Scholar]
- 10.National Institute on Drug Abuse. Ohio: Opioid-Involved Deaths and Related Harms. National Institute on Drug Abuse. 2020. Accessed May 13, 2021. drugabuse.gov/drug-topics/opioids/opioid-summaries-by-state/ohio-opioid-involved-deaths-related-harms. [Google Scholar]
- 11.Salehi Omran S, Chatterjee A, Chen ML, Lerario MP, Merkler AE, Kamel H. National trends in hospitalizations for stroke associated with infective endocarditis and opioid use between 1993 and 2015. Stroke. 2019;50(3):577-582. doi: 10.1161/STROKEAHA.118.024436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thakkar S, Doshi R. Letter by thakkar and doshi regarding article, “national trends in hospitalizations for stroke associated with infective endocarditis and opioid use between 1993 and 2015.” Stroke. 2019;50(6):e162. doi: 10.1161/STROKEAHA.119.025317. [DOI] [PubMed] [Google Scholar]
- 13.Labriola L, Jadoul M. Haemodialysis is a major risk factor for infective endocarditis. Lancet. 2016;388(10042):339-340. doi: 10.1016/S0140-6736(16)31105-9. [DOI] [PubMed] [Google Scholar]
- 14.Cahill TJ, Prendergast BD. Infective endocarditis. Lancet. 2016;387(10021):882-893. doi: 10.1016/S0140-6736(15)00067-7. [DOI] [PubMed] [Google Scholar]
- 15.de Sa DDC, Tleyjeh IM, Anavekar NS, et al. Epidemiological trends of infective endocarditis: a population-based study in Olmsted County, Minnesota. Mayo Clin Proc. 2010;85(5):422-426. doi: 10.4065/mcp.2009.0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toyoda N, Chikwe J, Itagaki S, Gelijns AC, Adams DH, Egorova NN. Trends in infective endocarditis in California and New York state. JAMA. 1998-20132017;317(16):1652-1660. doi: 10.1001/jama.2017.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mori M, Brown KJ, Bin Mahmood SU, Geirsson A, Mangi AA. Trends in infective endocarditis hospitalizations, characteristics, and valve operations in patients with opioid use Disorders in the United States: 2005-2014. J Am Heart Assoc. 2020;9(6):e012465. doi: 10.1161/JAHA.119.012465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kadri AN, Wilner B, Hernandez AV, et al. Geographic trends, patient characteristics, and outcomes of infective endocarditis associated with drug abuse in the United States from 2002 to 2016. J Am Heart Assoc. 2019;8(19):e012969. doi: 10.1161/JAHA.119.012969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths–United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(5051):1445-1452. doi: 10.15585/mmwr.mm655051e1. [DOI] [PubMed] [Google Scholar]
- 20.2015. Drug overdose death rates|drug overdose|CDC injury center. 2021. Accessed June 17, 2021. cdc.gov/drugoverdose/data/statedeaths/drug-overdose-death-2015.html.
- 21.Broderick J, Brott T, Kothari R, et al. The Greater Cincinnati/Northern Kentucky Stroke Study: preliminary first-ever and total incidence rates of stroke among blacks. Stroke. 1998;29(2):415-421. doi: 10.1161/01.str.29.2.415. [DOI] [PubMed] [Google Scholar]
- 22.Broderick J, Brott T, Kothari R, et al. The greater Cincinnati/Northern Kentucky stroke study. Stroke. 1998;29(2):415-421. doi: 10.1161/01.STR.29.2.415. [DOI] [PubMed] [Google Scholar]
- 23.Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30(4):633-638. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 24.Rudasill SE, Sanaiha Y, Mardock AL, et al. Clinical outcomes of infective endocarditis in injection drug users. J Am Coll Cardiol. 2019;73(5):559-570. doi: 10.1016/j.jacc.2018.10.082. [DOI] [PubMed] [Google Scholar]
- 25.Ferraris L, Milazzo L, Rimoldi SG, et al. Epidemiological trends of infective endocarditis in a single center in Italy between 2003-2015. Infect Dis (Lond). 2018;50(10):749-756. doi: 10.1080/23744235.2018.1472806. [DOI] [PubMed] [Google Scholar]
- 26.Walls G, McBride S, Raymond N, et al. Infective endocarditis in New Zealand: data from the international collaboration on endocarditis prospective cohort study. N Z Med J. 2014;127(1391):38-51. [PubMed] [Google Scholar]
- 27.Bin Abdulhak AA, Baddour LM, Erwin PJ, et al. Global and regional burden of infective endocarditis, 1990-2010: a systematic review of the literature. Glob Heart. 2014;9(1):131-143. doi: 10.1016/j.gheart.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Tleyjeh IM, Abdel-Latif A, Rahbi H, et al. A systematic review of population-based studies of infective endocarditis. Chest. 2007;132(3):1025-1035. doi: 10.1378/chest.06-2048. [DOI] [PubMed] [Google Scholar]
- 29.Pant S, Patel NJ, Deshmukh A, et al. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol. 2015;65(19):2070-2076. doi: 10.1016/j.jacc.2015.03.518. [DOI] [PubMed] [Google Scholar]
- 30.Hedegaard H, Curtin SC, Warner M. Suicide mortality in the United States, 1999-2017. NCHS Data Brief. 2018(330):1-8. [PubMed] [Google Scholar]
- 31.Ohio Department of Health, Bureau of Vital Statistics. 2019 OH Drug Overdose Data; 2020:12. odh.ohio.gov/wps/wcm/connect/gov/0a7bdcd9-b8d5-4193-a1af-e711be4ef541/2019_OhioDrugOverdoseReport_Final_11.06.20.pdf?MOD=AJPERES&CONVERT_TO=url&CACHEID=ROOTWORKSPACE.Z18_M1HGGIK0N0JO00QO9DDDDM3000-0a7bdcd9-b8d5-4193-a1af-e711be4ef541-nmv3qSt. [Google Scholar]
- 32.Cornelissen CG, Frechen DA, Schreiner K, Marx N, Krüger S. Inflammatory parameters and prediction of prognosis in infective endocarditis. BMC Infect Dis. 2013;13(1):272. doi: 10.1186/1471-2334-13-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu N, Fu Y, Wang S, Li S, Cai D. High level of D-dimer predicts ischemic stroke in patients with infective endocarditis. J Clin Lab Anal. 2020;34(5):e23206. doi: 10.1002/jcla.23206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asaithambi G, Adil MM, Qureshi AI. Thrombolysis for ischemic stroke associated with infective endocarditis. Stroke. 2013;44(10):2917-2919. doi: 10.1161/STROKEAHA.113.001602. [DOI] [PubMed] [Google Scholar]
- 35.Le SM, Copeland LA, Zeber JE, et al. Factors affecting time between symptom onset and emergency department arrival in stroke patients. eNeurologicalSci. 2020;21:100285. doi: 10.1016/j.ensci.2020.100285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lacy CR, Suh DC, Bueno M, Kostis JB. Delay in presentation and evaluation for acute stroke. Stroke. 2001;32(1):63-69. doi: 10.1161/01.STR.32.1.63. [DOI] [PubMed] [Google Scholar]
- 37.Nguemeni Tiako MJ, Mori M, Bin Mahmood SU, et al. Recidivism is the leading cause of death among intravenous drug users who underwent cardiac surgery for infective endocarditis. Semin Thorac Cardiovasc Surg. 2019;31(1):40-45. doi: 10.1053/j.semtcvs.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 38.Vilacosta I, Graupner C, SanRomán J, et al. Risk of embolization after institution of antibiotic therapy for infective endocarditis. J Am Coll Cardiol. 2002;39(9):1489-1495. doi: 10.1016/S0735-1097(02)01790-4. [DOI] [PubMed] [Google Scholar]
- 39.Østergaard L, Andersson NW, Kristensen SL, et al. Risk of stroke subsequent to infective endocarditis: a nationwide study. Am Heart J. 2019;212:144-151. doi: 10.1016/j.ahj.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 40.Merkler AE, Chu SY, Lerario MP, Navi BB, Kamel H. Temporal relationship between infective endocarditis and stroke. Neurology. 2015;85(6):512-516. doi: 10.1212/WNL.0000000000001835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shah S, Leonard AC, Meganathan K, Christianson AL, Thakar CV. Temporal trends in incident mortality in dialysis patients: focus on sex and racial disparities. Am J Nephrol. 2019;49(3):241-253. doi: 10.1159/000497446. [DOI] [PubMed] [Google Scholar]
- 42.Varshney AS, DeFilippis EM, Cowger JA, Netuka I, Pinney SP, Givertz MM. Trends and outcomes of left ventricular assist device therapy: JACC focus seminar. J Am Coll Cardiol. 2022;79(11):1092-1107. doi: 10.1016/j.jacc.2022.01.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data may be shared at the request to any qualified investigator for purposes of replicating procedures and results.