Abstract
Background
Pulmonary embolism (PE) is a potentially life‐threatening condition in which a clot can migrate from the deep veins, most commonly in the leg, to the lungs. Conventional treatment of PE used unfractionated heparin (UFH), low molecular weight heparin (LMWH), fondaparinux, and vitamin K antagonists (VKAs). Recently, two forms of direct oral anticoagulants (DOACs) have been developed: oral direct thrombin inhibitors (DTIs) and oral factor Xa inhibitors. DOACs have characteristics that may be favourable to conventional treatment, including oral administration, a predictable effect, no need for frequent monitoring or re‐dosing, and few known drug interactions. This review reports the efficacy and safety of these drugs in the long‐term treatment of PE (minimum duration of three months). This is an update of a Cochrane Review first published in 2015.
Objectives
To assess the efficacy and safety of oral DTIs and oral factor Xa inhibitors versus conventional anticoagulants for the long‐term treatment of PE.
Search methods
The Cochrane Vascular Information Specialist searched the Cochrane Vascular Specialised Register, CENTRAL, MEDLINE, Embase and CINAHL databases, the World Health Organization International Clinical Trials Registry Platform and the ClinicalTrials.gov trials registers to 2 March 2022. We checked the reference lists of relevant articles for additional studies.
Selection criteria
We included randomised controlled trials (RCTs) in which people with a PE confirmed by standard imaging techniques were allocated to receive an oral DTI or an oral factor Xa inhibitor compared with a conventional anticoagulant or compared with each other for the long‐term treatment of PE (minimum duration three months).
Data collection and analysis
We used standard Cochrane methods. Our primary outcomes were recurrent PE, recurrent venous thromboembolism (VTE), and deep vein thrombosis (DVT). Secondary outcomes were all‐cause mortality, major bleeding, and health‐related quality of life. We used GRADE to assess the certainty of evidence for each outcome.
Main results
We identified five additional RCTs with 1484 participants for this update. Together with the previously included trials, we have included ten RCTs with a total of 13,073 participants. Two studies investigated an oral DTI (dabigatran) and eight studies investigated oral factor Xa inhibitors (three rivaroxaban, three apixaban, and two edoxaban). The studies were of good methodological quality overall.
Meta‐analysis showed no clear difference in the efficacy and safety of oral DTI compared with conventional anticoagulation in preventing recurrent PE (odds ratio (OR) 1.02, 95% confidence interval (CI) 0.50 to 2.04; 2 studies, 1602 participants; moderate‐certainty evidence), recurrent VTE (OR 0.93, 95% CI 0.52 to 1.66; 2 studies, 1602 participants; moderate‐certainty evidence), DVT (OR 0.79, 95% CI 0.29 to 2.13; 2 studies, 1602 participants; moderate‐certainty evidence), and major bleeding (OR 0.50, 95% CI 0.15 to 1.68; 2 studies, 1527 participants; moderate‐certainty evidence). We downgraded the certainty of evidence by one level for imprecision due to the low number of events.
There was also no clear difference between the oral factor Xa inhibitors and conventional anticoagulation in the prevention of recurrent PE (OR 0.92, 95% CI 0.66 to 1.29; 3 studies, 8186 participants; moderate‐certainty evidence), recurrent VTE (OR 0.83, 95% CI 0.66 to 1.03; 8 studies, 11,416 participants; moderate‐certainty evidence), DVT (OR 0.77, 95% CI 0.48 to 1.25; 2 studies, 8151 participants; moderate‐certainty evidence), all‐cause mortality (OR 1.16, 95% CI 0.79 to 1.70; 1 study, 4817 participants; moderate‐certainty evidence) and major bleeding (OR 0.71, 95% CI 0.36 to 1.41; 8 studies, 11,447 participants; low‐certainty evidence); the heterogeneity for major bleeding was significant (I2 = 79%). We downgraded the certainty of the evidence to moderate and low because of imprecision due to the low number of events and inconsistency due to clinical heterogeneity. None of the included studies measured health‐related quality of life.
Authors' conclusions
Available evidence shows there is probably little or no difference between DOACs and conventional anticoagulation in the prevention of recurrent PE, recurrent VTE, DVT, all‐cause mortality, and major bleeding. The certainty of evidence was moderate or low. Future large clinical trials are required to identify if individual drugs differ in effectiveness and bleeding risk, and to explore effect differences in subgroups, including people with cancer and obesity.
Keywords: Humans; Anticoagulants; Anticoagulants/therapeutic use; Antithrombins; Antithrombins/therapeutic use; Factor Xa Inhibitors; Factor Xa Inhibitors/therapeutic use; Hemorrhage; Hemorrhage/chemically induced; Neoplasm Recurrence, Local; Neoplasm Recurrence, Local/drug therapy; Pulmonary Embolism; Pulmonary Embolism/drug therapy; Pulmonary Embolism/prevention & control; Venous Thromboembolism; Venous Thromboembolism/prevention & control
Plain language summary
Are direct oral anticoagulants (a type of 'blood thinner') better than traditional anticoagulants for treating a pulmonary embolism (a blood clot in the lung)?
What is a pulmonary embolism?
A pulmonary embolism occurs when a piece of blood clot breaks off from a clot somewhere else in the body and travels in the blood to the lungs. This can be life‐threatening and occurs in approximately 4 to 12 per 10,000 people. The chances of getting a pulmonary embolism can increase with risk factors, including previous clots, prolonged periods of immobility (such as travelling on aeroplanes or taking bed rest), cancer, exposure to oestrogens (pregnancy, oral contraceptives, or hormone replacement therapy), blood disorders (thrombophilia), and trauma.
How is a pulmonary embolism treated?
Until recently, the standard treatment for a pulmonary embolism was an anticoagulant: a medicine that either treats or prevents blood clots, often called a 'blood thinner'. Conventional anticoagulants include heparin, fondaparinux, and vitamin K antagonists. However, these drugs can cause side effects and have limitations.
Two types of anticoagulant have been developed: direct thrombin inhibitors (DTIs) and factor Xa inhibitors. These anticoagulants are given orally (that is, by mouth, in the form of a pill), have a predictable effect, do not require frequent monitoring or re‐dosing (taking multiple doses), and have few known interactions with other medicines. For these reasons, direct oral anticoagulants have become the medicines of choice for treating DVT.
What did we want to find out?
We wanted to find out if direct oral anticoagulants are useful and safe for treating people with a pulmonary embolism, compared with conventional anticoagulants. We looked at whether 3 months' treatment or longer prevented further blood clots (recurrent deep vein thrombosis (DVT), when a clot forms in a deep vein, usually in the leg), recurrent pulmonary embolism, and pulmonary embolisms. The main safety outcomes included death and unwanted, harmful adverse events, such as major bleeding.
What did we do?
We searched for studies in which people with a pulmonary embolism confirmed by standard imaging techniques were randomly allocated to one of two treatment groups. These types of studies give the most reliable evidence about treatment effects. People in the experimental groups received an oral DTI or an oral factor Xa inhibitor, and their results were compared to the results of people given conventional anticoagulation. All participants were given long‐term treatment of pulmonary embolism (a minimum duration of 3 months).
What did we find?
After searching for relevant studies, we included 10 studies with a combined total of 13,073 participants. Studies compared oral DTIs and factor Xa inhibitors with conventional anticoagulation. We combined the data from the studies and found that there was no clear difference in the incidence of:
‐ recurrent pulmonary embolism; ‐ recurrent deep vein thrombosis (DVT: when a blood clot forms, usually in a deep vein of the leg or pelvis); ‐ recurrent venous thromboembolism (when DVT and pulmonary embolism occur together); ‐ death; ‐ major bleeding
This review showed that there was no clear difference between the direct oral anticoagulants and conventional treatment in preventing recurrent PE, recurrent VTE, DVT, mortality, and major bleeding. No study measured health‐related quality of life.
What are the limitations of the evidence?
We are moderately confident in this evidence. This was because the number of events involved in the studies was small and there were differences in how individual studies were carried out.
How up to date is this evidence?
This review updates a previous Cochrane Review. The evidence is up to date to March 2022.
Key messages
Current evidence shows there is probably little or no difference between direct oral anticoagulants and conventional anticoagulation for preventing recurrent pulmonary embolism, recurrent venous thromboembolism (VTE), deep vein thrombosis (DVT), all‐cause mortality, and major bleeding in people who are being treated for a pulmonary embolism.
Summary of findings
Summary of findings 1. Oral direct thrombin inhibitors (DTIs) versus conventional anticoagulation for the treatment of pulmonary embolism.
| Oral direct thrombin inhibitors (DTIs) versus conventional anticoagulation for the treatment of pulmonary embolism | ||||||
| Patient or population: people with a pulmonary embolism, confirmed by standard imaging techniques Setting: hospital Intervention: oral DTIs Comparison: conventional anticoagulation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with conventional anticoagulation | Risk with oral DTI | |||||
|
Recurrent PEa Follow‐up: 6 months |
Study population | OR 1.02 (0.50 to 2.04) | 1602 (1 RCT) | ⊕⊕⊕⊝ Moderateb | The data from RE‐COVER 2009 and RE‐COVER II 2014 were taken from one pooled analysis and are therefore shown as one study in our analyses. | |
| 20 per 1000 | 20 per 1000 (10 to 40) | |||||
|
Recurrent VTEc Follow‐up: 6 months |
Study population | OR 0.93 (0.52 to 1.66) | 1602 (1 RCT) | ⊕⊕⊕⊝ Moderateb | The data from RE‐COVER 2009 and RE‐COVER II 2014 were taken from one pooled analysis and are therefore shown as one study in our analyses. | |
| 31 per 1000 | 29 per 1000 (16 to 50) | |||||
|
DVTd Follow‐up: 6 months |
Study population | OR 0.79 (0.29 to 2.13) | 1602 (1 RCT) | ⊕⊕⊕⊝ Moderateb | The data from RE‐COVER 2009 and RE‐COVER II 2014 were taken from one pooled analysis and are therefore shown as one study in our analyses. | |
| 11 per 1000 | 9 per 1000 (3 to 23) | |||||
| All‐cause mortality | See comment | See comment | See comment | ‐ | RE‐COVER 2009 and RE‐COVER II 2014 did not report on all‐cause mortality. | |
|
Major bleedinge Follow‐up: 6 months |
Study population | OR 0.50 (0.15 to 1.68) | 1527 (1 RCT) | ⊕⊕⊕⊝ Moderateb | The data from RE‐COVER 2009 and RE‐COVER II 2014 were taken from one pooled analysis and are therefore shown as one study in our analyses. | |
| 10 per 1000 | 5 per 1000 (2 to 17) | |||||
| Health‐related quality of life | See comment | See comment | See comment | ‐ | RE‐COVER 2009 and RE‐COVER II 2014 did not measure health‐related quality of life. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CTPA: computed tomographic pulmonary angiography; DVT: deep vein thrombosis; ISTH: International Society on Thrombosis and Haemostasis; OR: odds ratio; PE: pulmonary embolism; RCT: randomised controlled trial; V/Q: ventilation/perfusion; VTE: venous thromboembolism | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate, the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited, the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aConfirmed by V/Q lung scanning, pulmonary angiography, or CTPA bWe downgraded one level for imprecision due to the low number of events. The possibility of publication bias is not excluded but we did not consider it sufficient to downgrade the certainty of evidence. cVTE includes clinically overt DVT and PE. Clinically overt DVT, confirmed by standard imaging techniques (venography, impedance plethysmography, whole‐leg compression ultrasound, proximal compression ultrasound); or clinically overt PE, confirmed by V/Q lung scanning, pulmonary angiography, or CTPA. dClinically overt DVT confirmed by standard imaging techniques (venography, impedance plethysmography, whole‐leg compression ultrasound, proximal compression ultrasound). eAs defined by the ISTH (Schulman 2005): 1. Fatal bleeding, and/or 2. symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intra‐articular or pericardial, or intramuscular with compartment syndrome, and/or 3. bleeding causing a fall in haemoglobin level of 20 g/L (1.24 mmol/L) or more, or leading to transfusion of two or more units of whole blood or packed red cells.
Summary of findings 2. Oral factor Xa inhibitors versus conventional anticoagulation for the treatment of pulmonary embolism.
| Oral factor Xa inhibitors versus conventional anticoagulation for the treatment of pulmonary embolism | ||||||
| Patient or population: people with a pulmonary embolism, confirmed by standard imaging techniques Setting: hospital Intervention: oral factor Xa inhibitors Comparison: conventional anticoagulation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with conventional anticoagulation | Risk with oral factor Xa inhibitors | |||||
|
Recurrent PEa Follow‐up: 0 to 12 months |
Study population | OR 0.92 (0.66 to 1.29) | 8186 (3 RCTs) | ⊕⊕⊕⊝ Moderateb |
‐ | |
| 18 per 1000 | 16 per 1000 (12 to 23) | |||||
|
Recurrent VTEc Follow‐up: 0 to 12 months |
Study population | OR 0.83 (0.66 to 1.03) | 11,416 (8 RCTs) | ⊕⊕⊕⊝
Moderateb |
2 of 8 studies reported no events | |
| 32 per 1000 | 26 per 1000 (21 to 33) | |||||
|
DVTd Follow‐up: 5 days to 12 months |
Study population | OR 0.77 (0.48 to 1.25) | 8151 (2 RCTs) | ⊕⊕⊕⊝ Moderateb | ‐ | |
| 10 per 1000 | 7 per 1000 (5 to 12) | |||||
|
All‐cause mortality Follow‐up: 0 to 12 months |
Study population | OR 1.16 (0.79 to 1.70) | 4817 (1 RCT) | ⊕⊕⊕⊝ Moderateb | ‐ | |
| 21 per 1000 | 24 per 1000 (16 to 35) | |||||
|
Major bleedinge Follow‐up: 0 to 12 months |
Study population | OR 0.71 (0.36 to 1.41) | 11,447 (8 RCTs) | ⊕⊕⊝⊝ Lowb,f | 2 of 8 studies reported no events | |
| 23 per 1000 | 16 per 1000 (8 to 32) | |||||
| Health‐related quality of life | See comment | See comment | See comment | ‐ | The studies did not measure health‐related quality of life. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CTPA: computed tomographic pulmonary angiography; DVT: deep vein thrombosis; ISTH: International Society on Thrombosis and Haemostasis; OR: odds ratio; PE: pulmonary embolism; RCT: randomised controlled trial; V/Q: ventilation/perfusion; VTE: venous thromboembolism | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate, the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited, the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate, the true effect is likely to be substantially different from the estimate of effect | ||||||
aConfirmed by V/Q lung scanning, pulmonary angiography, or CTPA. bWe downgraded one level for imprecision due to the low number of events. The possibility of publication bias is not excluded but we did not consider it sufficient to downgrade the certainty of evidence. cVTE includes clinically overt DVT and PE. Clinically overt DVT, confirmed by standard imaging techniques (venography, impedance plethysmography, whole‐leg compression ultrasound, proximal compression ultrasound); or clinically overt PE, confirmed by V/Q lung scanning, pulmonary angiography, or CTPA. dClinically overt DVT confirmed by standard imaging techniques (venography, impedance plethysmography, whole‐leg compression ultrasound, proximal compression ultrasound). eAs defined by the ISTH (Schulman 2005): 1. Fatal bleeding, and/or 2. symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intra‐articular or pericardial, or intramuscular with compartment syndrome, and/or 3. bleeding causing a fall in haemoglobin level of 20 g/L (1.24 mmol/L) or more, or leading to transfusion of two or more units of whole blood or packed red cells. fWe downgraded one level for serious inconsistency (I2 = 79% due to clinical heterogeneity).
Background
Description of the condition
Pulmonary embolism (PE) is a potentially life‐threatening condition in which a blood clot blocks the supply of blood to the lungs. PE is often a consequence of a thrombus in the deep veins of the legs (deep vein thrombosis, DVT) that dislodges and migrates in the blood to the pulmonary arteries. The incidence of PE has been estimated as 4 to 12 per 10,000 people annually (Keller 2020; Konstantinides 2020; Wendelboe 2016), and longitudinal studies have revealed that the annual PE prevalence rates tend to rise over time (Dentali 2016; de Miguel‐Diez 2014; Keller 2020; Lehnert 2018). DVT is present in approximately 70% to 80% of people with PE, yet only 15% of PE cases have symptoms of DVT (Huerta 2007). One long‐term complication of PE is chronic thromboembolic pulmonary hypertension (CTPH). CTPH occurs when the clot obstructs the pulmonary arteries, causing excessive pressure in the pulmonary artery and stress to the right ventricle. CTPH is uncommon but it can result in heart failure (NICE 2020). It is estimated that up to a quarter of all PE patients present with sudden death (Heit 2015). In the USA, PE is one of the leading causes of cardiovascular mortality, contributing to nearly 300,000 deaths annually (Wendelboe 2016).
Risk factors for PE are similar to those for DVT and are classified as provoked or unprovoked (Kearon 2012). For unprovoked PE, no clear precipitating risk factor can be identified; risk factors are either hereditary or more often acquired. For provoked PE, risk factors include cancer, acute medical illness, surgery, trauma, immobility (often in hospital and lasting at least three days), obesity, inflammatory diseases/infection, hormone therapy (oestrogen‐containing), pregnancy (particularly the postpartum period), long‐distance travel, recent hospitalisation, and antiphospholipid syndrome (APS) (Kakkos 2021).
People presenting with signs or symptoms of PE, such as chest pain, shortness of breath, or coughing up blood, will have their general medical history assessed, undergo a physical examination, and may be offered a chest X‑ray to exclude other causes (NICE 2020). However, it can be particularly challenging to diagnose PE as the symptoms (dyspnoea, pleuritic chest pain, retrosternal chest pain, cough, and haemoptysis) are not specific (NICE 2020). In severe cases, right ventricle failure leads to dizziness, syncope, tachypnoea, tachycardia, hypoxia, elevated jugular venous pressure, systemic hypotension, and cardiogenic shock (NICE 2020). The UK National Institute for Health and Care Excellence (NICE) recommends that people presenting with suspected PE should be assessed using a two‐level PE Wells score (NICE 2020; Wells 2000). Points are awarded for the presence of clinical features, including clinical signs and symptoms of DVT (minimum of leg swelling and pain with palpation of the deep veins), heart rate greater than 100 beats per minute, immobilisation for more than three days or surgery in the previous four weeks, previous DVT/PE, haemoptysis, and malignancy (on treatment, treated in the last six months, or palliative) (NICE 2020). For people with a low pre‐test probability, the use of a D‐dimer assay combined with a clinical prediction rule has a high negative predictive value and avoids the need for unnecessary imaging (Qaseem 2007). However, for people who have an intermediate or high pre‐test probability of PE, imaging is essential. People with a score of greater than four are judged to be likely to have had a PE and should undergo immediate diagnostic imaging. If this cannot be performed immediately, patients should be given immediate interim parenteral anticoagulant therapy until the imaging test is done. Those with a negative diagnosis in whom a DVT is likely should be given a proximal leg vein ultrasound scan (NICE 2020).
There are three types of imaging techniques used to diagnose PE: computed tomography pulmonary angiogram (CTPA), ventilation‐perfusion (V/Q) scan, and pulmonary angiography.
CTPA involves injecting a contrast agent intravenously and performing a computed tomography (CT) scan of the chest to visualise the pulmonary arteries and detect any thrombi in the pulmonary arteries down to the subsegmental branches. The procedure has over 90% specificity and sensitivity in diagnosing PE in the main, lobar, and segmental pulmonary arteries (Riedel 2004). However, the radiation dose administered to the individual is much larger than that of a V/Q scan, and thus people who have a CTPA may be at an increased lifetime risk of cancer (Anderson 2009). CTPA use is limited in people with iodine allergy, hyperthyroidism, those who are pregnant and breastfeeding, and is contraindicated in people with severe renal failure (Konstantinides 2020).
A V/Q scan comprises two parts: the ventilation part, where the patient breathes in a radioisotope (in the form of a gas or an aerosol) and the perfusion part, where the patient is given an intravenous injection of the isotope. A gamma camera is used to detect where the isotopes are in the lungs and the images show which areas of the lungs are ventilated but not perfused (NICE 2020). Another version of this test, the V/Q single photon emission computed tomography (V/Q SPECT) has been developed. The camera is rotated around the patient, thus generating three‐dimensional images and leading to a more accurate diagnosis (Laurence 2012).
Pulmonary angiography is an imaging method that uses x‐rays and a special dye to see how blood flows through the lung. The procedure is invasive, with related risks of mortality and complications, especially for people with haemodynamic compromise or respiratory failure (Stein 1992). For several decades, pulmonary angiography was the "gold standard" for diagnosing or ruling out acute PE, but it is now rarely used because less‐invasive CTPA provides comparable diagnostic accuracy (Konstantinides 2020).
Description of the intervention
Conventional treatment of PE used unfractionated heparin (UFH), low molecular weight heparin (LMWH), fondaparinux, or vitamin K antagonists (VKAs). These drugs block the action of thrombin either by "activating naturally occurring thrombin inhibitors or by inhibiting specific factors in the coagulation system that subsequently impact on thrombin generation or activity" (Weitz 2003). Although heparins and VKAs are effective anticoagulants, there are limitations associated with each. LMWH must be administered parenterally and may be associated with an increased risk of bleeding, loss of bone strength, elevated liver enzymes, and heparin‐induced thrombocytopenia (Konstantinides 2020). Meanwhile, VKAs have a narrow therapeutic window, require frequent monitoring and dosage adjustments, and can have multiple interactions with other drugs (Ageno 2012).
Two further classes of oral anticoagulants have been developed: direct thrombin inhibitors (DTIs) and factor Xa inhibitors. DTIs and factor Xa inhibitors have characteristics that may be favourable to heparin and VKAs, including ease of oral administration, a predictable effect, lack of frequent monitoring or re‐dosing, and fewer known drug interactions (compared with VKA) (Fox 2012).
The latest American College of Chest Physicians (ACCP) guidelines recommended apixaban, dabigatran, edoxaban, or rivaroxaban over VKAs as treatment‐phase (first three months) anticoagulant therapy for people with VTE (DVT of the leg or PE); and recommended an oral Xa inhibitor (apixaban, edoxaban, rivaroxaban) over LMWH for the initiation and treatment phases of therapy for acute VTE associated with cancer (Kearon 2016; Stevens 2021). Similarly, the 2019 European Society of Cardiology (ESC) guidelines recommended DOACs in preference to VKAs in eligible individuals ready for an oral anticoagulant (Konstantinides 2020). The NICE 2020 guidelines recommend offering either apixaban or rivaroxaban as initial choices, and suggested other regimens only for people suitable for neither: consider a DOAC for people with active cancer and confirmed proximal DVT or PE, and consider other strategies when DOAC is unsuitable.
According to research by Lutsey and colleagues, the use of DOACs (especially rivaroxaban and apixaban) to treat VTE has increased dramatically in the USA since the US Food and Drug Administration (FDA) approved them for this application (Lutsey 2019). Drawing on individual health insurance data for 2012 to 2017, they found that DOACs accounted for less than 2% of the prescriptions for VTE treatment at the beginning of 2012 and increased to more than 80% by the fourth quarter of 2017 (Lutsey 2019).
How the intervention might work
Oral direct thrombin inhibitors
DTIs work by binding directly to the enzyme thrombin without the need for a co‐factor, such as antithrombin. Unlike heparins and VKAs, DTIs can inhibit both soluble thrombin and fibrin‐bound thrombin (Kam 2005). Other advantages include a more predictable anticoagulant effect because of their lack of binding to other proteins, lack of an antiplatelet effect, and no suspected concern of heparin‐induced thrombocytopenia (HIT) (Lee 2011). There are several types of DTIs.
Dabigatran
Dabigatran etexilate is a reversible oral DTI that is metabolised to its active ingredient, dabigatran, in the gastrointestinal tract (Ageno 2012). It does not require anticoagulation monitoring, is excreted by the kidneys, and has a half‐life of 12 to 17 hours. As well as a treatment for venous thrombosis, this drug has been involved in many large randomised studies of atrial fibrillation (Connolly 2009), acute coronary syndromes (Oldgren 2011), prevention of thrombosis following orthopaedic surgery (Eriksson 2007), and in people with mechanical heart valves (Van de Werf 2012). In common with the other DOACs, dabigatran is associated with a lower incidence of intracranial haemorrhage (compared with VKAs). However, again compared with VKAs, dabigatran showed a higher incidence of indigestion and heartburn and a higher incidence of gastrointestinal bleeding (Baetz 2008). For the treatment of PE in people with creatinine clearance (CrCL) of more than 30 mL/min, the recommended dosage of dabigatran is 150 mg twice daily following at least five days of initial therapy with a parenteral anticoagulant. For people aged 80 years or older and for people having verapamil, the recommended dose is 110 mg twice daily. In people aged 75 to 80 years, people with moderately reduced kidney function, people with gastritis, oesophagitis or gastro‐oesophageal reflux, and people at increased risk of bleeding, either dose (300 mg or 220 mg) can be given, based on an individual assessment. Dabigatran is not recommended in people with CrCL of less than 30 mL/min (Konstantinides 2020; NICE 2020).
Ximelagatran
Ximelagatran is a prodrug that is metabolised to melagatran as it is better absorbed from the gastrointestinal tract (Kam 2005). It has a plasma half‐life of three hours, has a predictable response after oral administration, and does not require coagulation monitoring. Ximelagatran was found to be effective in the treatment of venous thromboembolism but caused unacceptable liver toxicity (Boudes 2006), and, therefore, was never licensed. For the treatment of PE, the usual dosing of ximelagatran for adults is 48 mg twice daily (Ho 2006).
Oral factor Xa inhibitors
Factor Xa inhibitors bind directly to the active site of factor Xa, thus blocking the activity of the clotting factor. Unlike indirect factor Xa inhibitors such as fondaparinux, direct factor Xa inhibitors "inactivate free FXa and FXa incorporated with the prothrombinase complex equally well" and do not require interaction with the inhibitor antithrombin (Eriksson 2009). They have been shown to be non‐inferior to VKAs but without the need for regular blood test monitoring. They appear to have fewer drug interactions (compared with VKAs) and no food or alcohol interactions.
Rivaroxaban
Rivaroxaban is a reversible direct factor Xa inhibitor. The plasma half‐life is estimated to be eight to 10 hours if renal function is normal (Spyropoulos 2012). For the treatment of PE, the recommended dosage of rivaroxaban is 15 mg twice daily for the first 21 days followed by 15 mg once daily in people with moderate (CrCL 30 mL/min to 49 mL/min) or severe (CrCL 15 mL/min to 29 ml/min) renal impairment, if their risk of bleeding outweighs the risk of recurrent DVT or PE (NICE 2020).
Apixaban
Apixaban is an oral, small molecule, reversible inhibitor of factor Xa with a plasma half‐life of eight to 15 hours. For the treatment of PE, the recommended dosage of apixaban is 10 mg twice daily for the first seven days followed by 5 mg twice daily for continued treatment (NICE 2020). For people with severe renal impairment (CrCL 15 mL/min to 29 mL/min), apixaban should be used with caution (Eriksson 2009).
Betrixaban
Betrixaban is an orally administered direct factor Xa inhibitor. It has a half‐life of 19 to 27 hours (Palladino 2013). For the prophylaxis of PE, the recommended dose of betrixaban is an initial single dose of 160 mg starting on day 1, followed by 80 mg once daily, taken for 35 to 42 days at the same time each day with food. For people with severe renal impairment (CrCL 15 mL/min to 30 mL/min computed by Cockcroft‐Gault using actual body weight), the recommended dose of betrixaban is an initial single dose of 80 mg followed by 40 mg once daily (FDA 2017).
Edoxaban
Edoxaban is an oral direct inhibitor of activated factor X that is rapidly absorbed with a half‐life of nine to 11 hours. Edoxaban has a dual mechanism of elimination with one‐third eliminated via the kidneys and the remainder excreted in the faeces (Eikelboom 2010). For the treatment of PE, the recommended dose of edoxaban is 60 mg taken orally once daily following at least five days of initial therapy with a parenteral anticoagulant. The edoxaban dose should be reduced to 30 mg once daily in people with CrCL 15 mL/min to 50 mL/min, those who weigh less than or equal to 60 kg, or those who are taking certain concomitant P‐glycoprotein inhibitor medications (NICE 2020).
Why it is important to do this review
The efficacy and safety of oral DTIs and oral factor Xa inhibitors for the treatment of VTE has been examined in several randomised controlled trials (the EINSTEIN‐PE study (EINSTEIN‐PE 2012), the ODIXa‐DVT study (Agnelli 2007), the Botticelli study (Botticelli Investigators 2008), the AMPLIFY study (Agnelli 2013), the RE‐COVER II study (Schulman 2011), and the THRIVE studies (Eriksson 2003)). Several non‐Cochrane systematic reviews have examined the benefits and harms of DTIs and factor Xa inhibitors versus VKAs in the treatment of acute VTE (Fox 2012; Samaranayake 2022; Song 2021; Wu 2022). However, specific data on people with PE were not reported, and appropriate subgroup analyses that considered some clinical factors (such as the history of VTE, age, and active cancer) were lacking. It is important to assess the efficacy and safety of oral DTIs and oral factor Xa inhibitors for the treatment of PE to help both healthcare professionals and people with PE choose the most appropriate treatment. Since the first version of this review was completed (Robertson 2015a), several new RCTs have been published (AMPLIFY‐J 2015; Caravaggio 2020; Hokusai VTE Cancer 2018; J‐EINSTEIN DVT and PE 2015; MERCURY PE 2018). Thus, it is necessary to update the review and present the most up‐to‐date evidence to aid decision‐making in clinical practice. For this update, due to the limited number of included studies, subgroup analyses were only possible for active cancer and the different types of factor Xa inhibitors.
Objectives
To assess the efficacy and safety of oral DTIs and oral factor Xa inhibitors versus conventional anticoagulants for the long‐term treatment of PE.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) in which participants with a confirmed pulmonary embolism (PE) were allocated to receive an oral direct thrombin inhibitor (DTI) or an oral factor Xa inhibitor for the treatment of PE. We included studies published and in progress if preliminary results were available. We placed no restrictions on publication status and non‐English studies were eligible for inclusion in the review. We excluded DTIs and factor Xa inhibitors that were not given by the oral route.
Types of participants
We included participants with a PE, confirmed by standard imaging techniques (CTPA, V/Q scan, or pulmonary angiography).
Types of interventions
We included the following interventions:
oral DTIs (e.g. dabigatran, ximelagatran) (although ximelagatran was withdrawn from the market in 2006 due to safety issues, we included it in the review to make the results as comprehensive as possible);
oral factor Xa inhibitors (e.g. rivaroxaban, apixaban, betrixaban, edoxaban);
other anticoagulants (e.g. LMWH, UFH, VKAs).
We included the following comparisons:
one oral DTI versus another oral DTI;
one oral factor Xa inhibitor versus another oral factor Xa inhibitor;
oral DTI versus oral factor Xa inhibitor;
oral DTI or oral factor Xa inhibitor versus another anticoagulant.
Treatment had to be for a minimum duration of three months as this is conventional anticoagulation practice for a PE.
Types of outcome measures
Primary outcomes
Recurrent PE, confirmed by standard imaging techniques (CTPA, V/Q scan, or pulmonary angiography)
Recurrent venous thromboembolism (VTE, clinically overt DVT, confirmed by standard imaging techniques, including venography, impedance plethysmography, whole‐leg compression ultrasound, or proximal compression ultrasound; or clinically overt PE, confirmed by CTPA, V/Q scan, or pulmonary angiography)
Clinically overt DVT, confirmed by standard imaging techniques (venography, impedance plethysmography, whole‐leg compression ultrasound, or proximal compression ultrasound).
Secondary outcomes
All‐cause mortality
-
Adverse effects of treatment, including major bleeding (as defined by the International Society on Thrombosis and Haemostasis (ISTH); Schulman 2005):
fatal bleeding;
symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intra‐articular or pericardial, or intramuscular with compartment syndrome;
bleeding causing a fall in haemoglobin level of 20 g/L (1.24 mmol/L) or more, or leading to transfusion of two or more units of whole blood or packed red cells;
any combination of the three points above.
Health‐related quality of life, as reported in included studies
Search methods for identification of studies
Electronic searches
The Cochrane Vascular Information Specialist conducted systematic searches of the following databases, from inception to 2 March 2022, for RCTs and controlled clinical trials without language, publication year, or publication status restrictions:
the Cochrane Vascular Specialised Register via the Cochrane Register of Studies (CRS‐Web);
the Cochrane Central Register of Controlled Trials (CENTRAL; 2022 via the Cochrane Register of Studies Online (CRSO);
MEDLINE (Ovid MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE);
Embase Ovid;
CINAHL (Cumulative Index to Nursing and Allied Health Literature) Ebsco.
We developed search strategies for other databases from the search strategy designed for MEDLINE. Where appropriate, they were combined with adaptations of the highly sensitive search strategy designed by Cochrane for identifying RCTs and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 4, Lefebvre 2021). Search strategies for major databases are provided in Appendix 1.
We searched the following trials registries:
the World Health Organization International Clinical Trials Registry Platform (who.int/trialsearch);
ClinicalTrials.gov (clinicaltrials.gov).
The most recent searches were carried out on 2 March 2022.
Searching other resources
We reviewed the reference lists of relevant articles for potential additional citations.
Data collection and analysis
Selection of studies
We used Covidence to screen retrieved records (Covidence). Seven review authors (ML, JL, XH, LY, XW, QW, SX), working in pairs, independently screened all titles and abstracts based on the specified inclusion and exclusion criteria, and then reviewed full‐text records of all potentially eligible articles. We linked together multiple reports of the same study and used the most comprehensive report as the primary reference. We resolved any disagreements by discussion. We illustrated the study selection process in a PRISMA diagram (Page 2021). We listed studies excluded after full‐text assessment in the Characteristics of excluded studies table, and provided the reasons for exclusion.
Data extraction and management
Six review authors (ML, JL, XW, XH, QW, SX) independently extracted the data from the included studies, using a modified version of the Cochrane Vascular standard data extraction form. We resolved any disagreements in data extraction and management through discussion and we sought the opinion of a third author (LY or KY), when necessary. We collected the following data:
study details (e.g. trial name, year, country, authors);
methods (e.g. study design, number of participants and study centres, withdrawals and drop‐outs, treatment duration, follow‐up time);
participant characteristics (e.g. country, setting, age, sex, inclusion and exclusion criteria, history of VTE, previous major surgery, active cancer, pregnancy, recent period of immobility, hereditary or acquired thrombophilia);
interventions (e.g. oral DTIs, oral factor Xa inhibitors);
comparisons (e.g. other anticoagulants, i.e. LMWH, UFH, VKAs);
outcomes (e.g. recurrent PE, recurrent VTE, clinically overt DVT, all‐cause mortality, major bleeding, health‐related quality of life);
funding source;
declarations of interest of the study authors.
Assessment of risk of bias in included studies
Pairs of reviewers (ML, JL, XW, XH) independently assessed the risk of bias using the Cochrane risk of bias tool, as described in section 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). The tool provides a protocol for judgements on sequence generation, allocation methods, blinding, incomplete outcome data, selective outcome reporting, and any other relevant biases. We judged each of these domains as either high, low, or unclear risk of bias according to Higgins 2017 and provided support for each judgement. We presented the conclusions in a Risk of bias in included studies table. We resolved any disagreements through discussions with a third author (LY or KY).
Measures of treatment effect
For dichotomous outcomes, we used odds ratios (ORs) as the effect measure, with 95% confidence intervals (CIs). For continuous data, we planned to calculate mean differences (MDs) with 95% CIs. If similar outcomes were measured using different scales, we planned to calculate the standardised mean difference (SMD).
Unit of analysis issues
The unit of analysis in this review was each participant recruited into each included study.
Dealing with missing data
We based the analysis on intention‐to‐treat (ITT) data from the individual clinical trials whenever possible. We adopted the 'available‐case analysis' if the primary studies did not use ITT analysis. We contacted the study authors for additional information where there were missing or unavailable data. One study author provided missing outcome data when requested (Hokusai‐VTE 2013). In addition, some studies only reported overall data about VTE, with no specific data on PE. We contacted these study authors for detailed information. When we did not get a response within one month, we excluded these studies for the reason "unable to obtain specific outcome data for people with a pulmonary embolism" (see Characteristics of excluded studies table).
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity by checking the study details. We assessed statistical heterogeneity between the trials: by visually examining the forest plots to check for overlapping CIs; with the Chi2 test for homogeneity with a 10% level of significance; and by using the I2 statistic to measure the degree of inconsistency between the studies (Higgins 2022). We interpreted I² as described in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2022):
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: may represent considerable heterogeneity.
We planned to investigate the reasons for significant heterogeneity where I2 was greater than 50% by examining the characteristics of included articles, including participants, interventions, comparisons, outcomes, and study design.
Assessment of reporting biases
We planned to assess publication bias by funnel plots if a sufficient number of studies (10 or more) were available in the meta‐analyses. In order to investigate any potential selective reporting bias, we compared the full‐text reports of the included studies with the registration information and protocols, and we also searched for eligible studies that had been registered and should have been completed, but were without available published data.
Data synthesis
We used Review Manager Web (RevMan Web) to synthesise the data with a meta‐analysis, following the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022; RevMan Web 2022). One review author (ML) entered the data into RevMan Web, and the second review author (JL) cross‐checked the data entry. We resolved any discrepancies by consulting the source publication. We used a random‐effects model to synthesise the data, even with a small I², as we expected clinical heterogeneity across studies to exist, due to different oral factor Xa inhibitors (e.g. apixaban, rivaroxaban, edoxaban), different conventional thrombin inhibitors in the control group (e.g. warfarin, dalteparin), different treatment durations (e.g. three, six, and 12 months). If meta‐analysis was not appropriate or possible, we planned to report the results using a narrative synthesis (McKenzie 2002).
Subgroup analysis and investigation of heterogeneity
We planned to conduct subgroup analysis for the factors below if there were two or more studies in each subgroup. We planned to analyse the subgroup differences using the Chi2 test, set at a P value of 0.05.
History of VTE.
Age.
Active cancer (treatment within the last six months or palliative).
Pregnancy.
Major surgery requiring general or regional anaesthesia in the previous 12 weeks.
Recent period of immobility (bedridden for three or more days in the previous 12 weeks).
Thrombophilia (hereditary or acquired).
Treatment duration (three months or more than three months).
Types of factor Xa inhibitors.
For this update, due to the limited number of included studies, subgroup analyses were only possible for active cancer and the types of factor Xa inhibitors.
Sensitivity analysis
We planned to perform sensitivity analyses to explore the robustness of the results. We intended to exclude studies that we judged to be at high risk of bias (defined as high risk in any domain). We also planned to perform sensitivity analyses with and without studies in ximelagatran, given that this drug is no longer available. However, we found no studies that tested ximelagatran in people with PE.
Summary of findings and assessment of the certainty of the evidence
We developed summary of findings tables, using GRADEpro GDT software, to present the results of this review (GRADEpro GDT; Schünemann 2022a). We created one table each for our two comparisons: 'Oral DTIs versus conventional anticoagulation for the treatment of PE' and 'Oral factor Xa inhibitors versus conventional anticoagulation for the treatment of PE'. We assessed the certainty of the body of evidence for each outcome as high, moderate, low, or very low by considering the risk of bias, inconsistency, indirectness, imprecision, and publication bias according to the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2022b). We assessed the certainty of evidence for the following outcomes: recurrent PE, recurrent VTE, DVT, all‐cause mortality, major bleeding, and health‐related quality of life, as described in the Types of outcome measures section.
Results
Description of studies
See Figure 1.
1.
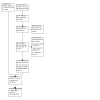
Study flow diagram
Results of the search
For this update, the searches identified 13,412 records, leaving 10,797 records after deduplication. We assessed 10,611 records as not relevant, based on the title and abstract screening. We assessed 186 potentially relevant records by full text. We identified five new included studies (24 reports) and 39 additional reports to already included studies. We excluded 29 studies (90 reports) with reasons, and discarded 20 further reports as not relevant. We identified one study (one report) as awaiting classification, and 10 ongoing studies (12 reports). The previous version of this review included five studies (22 reports). Thus, a total of 10 studies (85 reports) are now included in this review. If an article reported on two studies, we listed the article as an additional publication under each study, which makes both the counts of total reports in included and excluded studies 91. See Figure 1, Characteristics of included studies, Characteristics of excluded studies, and Characteristics of ongoing studies.
Included studies
Ten RCTs involving 13,073 adults met the inclusion criteria for this review (AMPLIFY 2013; AMPLIFY‐J 2015; Caravaggio 2020; EINSTEIN‐PE 2012; Hokusai VTE Cancer 2018; Hokusai‐VTE 2013; J‐EINSTEIN DVT and PE 2015; MERCURY PE 2018; RE‐COVER 2009; RE‐COVER II 2014). See Characteristics of included studies.
AMPLIFY 2013 was a double‐blind study in which 5395 participants with a deep vein thrombosis (DVT) or pulmonary embolism (PE) were randomised to receive oral apixaban 10 mg twice daily for the first seven days, followed by 5 mg twice daily for six months or enoxaparin 1 mg/kg of body weight every 12 hours for at least five days and warfarin concomitantly for six months. Participants were followed up for six months. Outcomes included a composite of recurrent symptomatic venous thromboembolism (VTE) (fatal or non‐fatal PE and DVT), mortality related to VTE, major bleeding, and clinically relevant non‐major bleeding.
AMPLIFY‐J 2015 was a randomised, active‐controlled, open‐label study in which 80 participants with a DVT or PE were randomised to receive oral apixaban 10 mg for seven days as an initial therapy, followed by 5 mg for 23 weeks (n = 40) or continuous infusion of unfractionated heparin (UFH) for at least five days and warfarin concomitantly for 24 weeks (n = 40). Participants were followed up at zero, two, 12, 24, and 28 weeks. Outcomes included major bleeding, clinically relevant non‐major bleeding, all bleeding events, adjudicated recurrent symptomatic VTE (non‐fatal DVT or non‐fatal PE) or VTE‐related death during 24 weeks, and thrombotic burden deterioration at two, 12, and 24 weeks.
Caravaggio 2020 was a multinational, randomised, controlled, investigator‐initiated, open‐label, non‐inferiority trial in which 1155 people with a cancer‐associated DVT or PE were randomised to receive oral apixaban 10 mg twice daily for the first seven days, followed by 5 mg twice daily for six months (n = 576) or dalteparin 200 IU/kg of body weight once daily for the first month, followed by 150 IU/kg of body weight daily for six months (n = 579). Participants were followed up at enrolment and at four weeks, then at three, six, and seven months after randomisation. Outcomes included recurrent VTE, recurrent DVT, recurrent PE, fatal PE, major bleeding, major gastrointestinal bleeding, major non‐gastrointestinal bleeding, clinically relevant non‐major bleeding, death from any cause, and event‐free survival.
EINSTEIN‐PE 2012 was an open‐label study in which 4832 participants were randomised to receive oral rivaroxaban 15 mg twice daily for the first three weeks, followed by 20 mg once daily (n = 2419), or enoxaparin 1.0 mg/kg of body weight twice daily and either warfarin or acenocoumarol, started within 48 hours of randomisation (n = 2413). Participants were followed up at three, six, and 12 months. Outcomes included recurrent PE, recurrent DVT, major bleeding, and all‐cause mortality.
Hokusai VTE Cancer 2018 was a multinational, prospective, randomised, open‐label, blinded endpoint, non‐inferiority study in which 1046 participants with a cancer‐associated DVT or PE were randomised to receive oral edoxaban at a fixed dose of 60 mg or 30 mg once daily for six to 12 months (n = 522) or dalteparin 200 IU/kg of body weight once daily for 30 days, with a maximum daily dose of 18,000 IU, followed by dalteparin 150 IU/kg of body weight once daily for six to 12 months (n = 524). Participants were followed up on day 31 after randomisation and at three, six, nine, and 12 months. Outcomes included a composite of recurrent VTE, major bleeding, clinically relevant non‐major bleeding, event‐free survival, VTE‐related death, mortality from all causes, recurrent DVT, and recurrent PE.
Hokusai‐VTE 2013 was a double‐blind study in which 3319 participants were randomised to receive 60 mg oral edoxaban once daily (n = 1650) or dose‐adjusted warfarin therapy and edoxaban‐like placebo (n = 1669). Outcomes were measured monthly for one year. Results were presented for all participants with VTE but specific outcome data for the subset of participants with PE were obtained through communication with the author.
J‐EINSTEIN DVT and PE 2015 was an open‐label, randomised trial in which 40 participants with a DVT or PE were randomised to receive oral rivaroxaban 15 mg twice daily for a total of three weeks in a double‐blind fashion, followed by open‐label rivaroxaban 15 mg once daily for three, six, or 12 months (n = 33) or UFH for at least five days, overlapping with and followed by an international normalised ratio (INR) (range 1.5 to 2.5) titrated warfarin for three, six, or 12 months (n = 7). Participants were followed up on day 22 and at the end of the three, six, or 12 months' intended treatment period. Outcomes included the occurrence of symptomatic recurrent VTE or asymptomatic deterioration, venous ultrasound and spiral CT, major bleeding, and clinically relevant non‐major bleeding.
MERCURY PE 2018 was a randomised, open‐label, parallel‐group, multicenter study in which 114 participants with PE were randomised to receive rivaroxaban with food, 15 mg twice daily for 21 days and then 20 mg once daily to study completion for three months (n = 51), or standard of care (any Food and Drug Administration (FDA)‐approved anticoagulant strategy) for three months (n = 63). Participants were followed up at on day 30 and day 90. Outcomes included the total amount of time spent in the hospital, VTE, bleeding events, major bleeding, recurrent VTE, VTE‐related death, satisfaction with anticlot treatment, total costs of care, and clinically relevant non‐major bleeding.
RE‐COVER 2009 was a phase III, non‐inferiority, double‐blind, double‐dummy trial in which people with VTE (n = 2539) were given 150 mg dabigatran twice daily or warfarin. In addition, initial treatment with an approved parenteral anticoagulant (UFH administered intravenously or low molecular weight heparin (LMWH) administered subcutaneously) was started before participants were randomised. Treatment was for a period of six months and included sham monitoring of INR and sham titration of warfarin in the control group. To gain regulatory approval, the study was repeated with an identical design (RE‐COVER II 2014). RE‐COVER 2009 and RE‐COVER II 2014 only reported outcome data of VTE, not separate data for PE, so we took data from a pooled analysis published in an additional report (Goldhaber 2016).
Excluded studies
See Characteristics of excluded studies.
We excluded 29 studies (ADAM VTE trial 2020; AMPLIFY Extended 2013; Borsi 2021; Botticelli DVT 2008; CASTA DIVA Trial 2022; COBRRA pilot feasibility study 2017; CONKO‐011 2015; de Athayde Soares 2019; DIVERSITY trial 2021; EINSTEIN‐CHOICE trial 2017; Einstein‐DVT Dose 2008; Einstein DVT 2013; EINSTEIN Extension 2007; EINSTEIN‐Jr Trial 2020; Farhan 2019; IRIVASC‐Trial 2022; Mokadem 2021; ODIXa‐DVT 2007; Ohmori 2018; Piazza 2014; PRAIS trial 2019; PRIORITY 2022; REMEDY 2013; RE‐SONATE 2013; SELECT‐D 2018; Sukovatykh 2017; THRIVE 2005; THRIVE I 2003; THRIVE III 2003). We excluded 11 studies as participants had DVT only (Botticelli DVT 2008; de Athayde Soares 2019; Einstein‐DVT Dose 2008; Einstein DVT 2013; Farhan 2019; Mokadem 2021; ODIXa‐DVT 2007; Ohmori 2018; Piazza 2014; PRAIS trial 2019; Sukovatykh 2017). We excluded 11 studies as although all participants had a VTE, specific data on the subgroup with PE were not published (ADAM VTE trial 2020; Borsi 2021; CASTA DIVA Trial 2022; COBRRA pilot feasibility study 2017; CONKO‐011 2015; DIVERSITY trial 2021; EINSTEIN‐Jr Trial 2020; IRIVASC‐Trial 2022; PRIORITY 2022; SELECT‐D 2018; THRIVE 2005). We made attempts to contact the authors for these data but were unsuccessful. We excluded three studies as they were extended studies testing the effectiveness of DOACs as prophylaxis rather than the treatment of PE (AMPLIFY Extended 2013; EINSTEIN Extension 2007; REMEDY 2013). We excluded the THRIVE I 2003 study as treatment was for less than three months, and the THRIVE III 2003 study as the control arm was a placebo. We excluded EINSTEIN‐CHOICE trial 2017 as the control arm was aspirin. Finally, we excluded the REMEDY 2013 study from this review as participants were already included in the RE‐COVER 2009 and RE‐COVER II 2014 studies.
Studies awaiting classification
One trial record is awaiting classification as there are currently insufficient details to assess its eligibility for inclusion (NCT01780987).
Ongoing studies
Ten trials are ongoing and there are currently no suitable data available for including in this review (EudraCT 2014‐002606‐20; NCT02464969; NCT02664155; NCT02744092; NCT02798471; NCT03129555; NCT03266783; NCT05171049; Pettit 2018; UMIN000020069). See Characteristics of ongoing studies.
Risk of bias in included studies
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
All 10 included studies stated that they used a computerised system to generate the random sequence, so we deemed the risk of selection bias relating to random sequence generation to be low. All 10 included studies reported that treatment allocation was concealed with the use of a computerised system, so we judged them to be at low risk of selection bias for allocation concealment (AMPLIFY 2013; AMPLIFY‐J 2015; Caravaggio 2020; EINSTEIN‐PE 2012; Hokusai VTE Cancer 2018; Hokusai‐VTE 2013; J‐EINSTEIN DVT and PE 2015; MERCURY PE 2018; RE‐COVER 2009; RE‐COVER II 2014).
Blinding
The AMPLIFY‐J 2015, Caravaggio 2020, EINSTEIN‐PE 2012, Hokusai VTE Cancer 2018, J‐EINSTEIN DVT and PE 2015, and MERCURY PE 2018 studies were open‐label: blinding of participants and personnel was not conducted. However, we judged that the lack of blinding in the control group was unlikely to have affected the outcome, and we thus judged these studies to have a low risk of performance bias. The AMPLIFY 2013, RE‐COVER 2009, RE‐COVER II 2014, and Hokusai‐VTE 2013 studies were double‐blind and therefore we judged them to be at low risk of performance bias.
All studies blinded outcome assessors to treatment; therefore, we judged them to be at low risk of detection bias.
Incomplete outcome data
Nine studies sufficiently reported that fewer than 20% of participants dropped out or withdrew, and these numbers were balanced across treatment groups. Therefore, we judged them to be at low risk of attrition bias (AMPLIFY‐J 2015; Caravaggio 2020; EINSTEIN‐PE 2012; Hokusai VTE Cancer 2018; Hokusai‐VTE 2013; J‐EINSTEIN DVT and PE 2015; MERCURY PE 2018; RE‐COVER 2009; RE‐COVER II 2014). The AMPLIFY 2013 study inappropriately excluded a number of randomised participants from the ITT analysis. Furthermore, a large number of participants within each treatment group were classified as discontinuing the study for "other reasons" with no explanations given, and we therefore deemed the risk of attrition bias to be unclear.
Selective reporting
Eight studies clearly pre‐specified the study outcomes and data on the expected outcomes were presented (AMPLIFY‐J 2015; EINSTEIN‐PE 2012; Hokusai VTE Cancer 2018; Hokusai‐VTE 2013; J‐EINSTEIN DVT and PE 2015; MERCURY PE 2018; RE‐COVER 2009; RE‐COVER II 2014); we judged these studies to be at low risk of reporting bias. The AMPLIFY 2013 study pre‐defined minor bleeding as a secondary outcome but data were not reported in the paper, and therefore we deemed the risk of reporting bias in this study to be high. The Caravaggio 2020 study pre‐defined quality of life as a secondary outcome in the protocol but the data on this outcome were not reported in the paper; therefore we deemed the risk of reporting bias to be high.
Other potential sources of bias
We did not find any methodological issues that might directly lead to a risk of bias; therefore, we deemed the risk of other bias to be low in all 10 included studies.
Effects of interventions
We identified two studies that compared an oral DTI versus conventional anticoagulation with warfarin (RE‐COVER 2009; RE‐COVER II 2014), and eight studies that compared an oral factor Xa inhibitor versus conventional anticoagulation with warfarin or dalteparin (AMPLIFY‐J 2015; AMPLIFY 2013; Caravaggio 2020; EINSTEIN‐PE 2012; Hokusai VTE Cancer 2018; Hokusai‐VTE 2013; J‐EINSTEIN DVT and PE 2015; MERCURY PE 2018). We did not find any studies comparing one DTI with another DTI, one factor Xa inhibitor with another factor Xa inhibitor, or an oral DTI with a factor Xa inhibitor. We used a random‐effects model for all analyses due to clinical heterogeneity across studies (different oral factor Xa inhibitors, different conventional thrombin inhibitors in the control group, and different treatment durations).
Oral direct thrombin inhibitor versus conventional anticoagulation
In the meta‐analysis of oral DTIs versus conventional anticoagulation, we used data from Goldhaber 2016, which reported combined data from the RE‐COVER 2009 and RE‐COVER II 2014 studies. This is reflected in the data analysis tables and Table 1 by showing only one study for this comparison.
Recurrent pulmonary embolism
Two studies with a combined total of 1602 participants measured recurrent pulmonary embolism (PE) (RE‐COVER 2009; RE‐COVER II 2014). There was no clear difference in the rate of recurrent PE between participants treated with dabigatran (16 events/795 participants) and those treated with conventional anticoagulation (16 events/807 participants) leading to an odds ratio (OR) of 1.02 (95% confidence interval (CI) 0.50 to 2.04; 2 studies, 1602 participants; moderate‐certainty evidence; Analysis 1.1).
1.1. Analysis.

Comparison 1: Oral DTIs versus conventional anticoagulation, Outcome 1: Recurrent pulmonary embolism
Recurrent venous thromboembolism
Two studies with a combined total of 1602 participants measured recurrent venous thromboembolism (VTE) (RE‐COVER 2009; RE‐COVER II 2014). There was no clear difference in the rate of recurrent VTE between participants treated with dabigatran (23 events/795 participants) and those treated with conventional anticoagulation (25 events/807 participants) leading to an OR of 0.93 (95% CI 0.52 to 1.66; 2 studies, 1602 participants; moderate‐certainty evidence; Analysis 1.2).
1.2. Analysis.

Comparison 1: Oral DTIs versus conventional anticoagulation, Outcome 2: Recurrent venous thromboembolism
Deep vein thrombosis
Two studies with a combined total of 1602 participants measured deep vein thrombosis (DVT) (RE‐COVER 2009; RE‐COVER II 2014). There was no clear difference in the rate of DVT between participants treated with dabigatran (7 events/795 participants) and those treated with conventional anticoagulation (9 events/807 participants) leading to an OR of 0.79 (95% CI 0.29 to 2.13; 2 studies, 1602 participants; moderate‐certainty evidence; Analysis 1.3).
1.3. Analysis.

Comparison 1: Oral DTIs versus conventional anticoagulation, Outcome 3: Deep vein thrombosis
All‐cause mortality
Neither study presented results on all‐cause mortality for the specific group of participants with PE.
Adverse effects of treatment
Both RE‐COVER 2009 and RE‐COVER II 2014 measured major bleeding (as defined by the International Society on Thrombosis and Haemostasis (ISTH); Schulman 2005). There was no clear difference in the rate of major bleeding between participants treated with oral DTIs (4 events/759 participants) and those treated with conventional anticoagulation (8 events/768 participants) leading to an OR of 0.50 (95% CI 0.15 to 1.68; 2 studies, 1602 participants; moderate‐certainty evidence; Analysis 1.4).
1.4. Analysis.

Comparison 1: Oral DTIs versus conventional anticoagulation, Outcome 4: Major bleeding
Health‐related quality of life
Health‐related quality of life was not a reported outcome in either RE‐COVER 2009 or RE‐COVER II 2014.
Oral factor Xa inhibitor versus conventional anticoagulation
See Table 2.
Recurrent pulmonary embolism
Data on recurrent PE was available in three studies with 8186 participants (AMPLIFY‐J 2015; EINSTEIN‐PE 2012; Hokusai‐VTE 2013). The duration of treatment in all three studies was longer than three months. Meta‐analysis showed no clear difference in the rate of recurrent PE between participants treated with oral factor Xa inhibitors (67 events/4087 participants) and those treated with conventional anticoagulation (73 events/4099 participants), leading to an OR of 0.92 (95% CI 0.66 to 1.29; I2 = 0; 3 studies, 8186 participants; moderate‐certainty evidence; Analysis 2.1).
2.1. Analysis.

Comparison 2: Oral factor Xa inhibitors versus conventional anticoagulation, Outcome 1: Recurrent pulmonary embolism
Recurrent venous thromboembolism
We included eight studies with a combined total of 11,416 participants in a meta‐analysis (AMPLIFY‐J 2015; AMPLIFY 2013; Caravaggio 2020; EINSTEIN‐PE 2012; Hokusai VTE Cancer 2018; Hokusai‐VTE 2013; J‐EINSTEIN DVT and PE 2015; MERCURY PE 2018). The treatment duration of MERCURY PE 2018 was three months, and the events in both groups were 0, so it was not estimated in the meta‐analysis. The treatment duration of the other seven studies was longer than three months. Meta‐analysis showed no clear difference in the rate of recurrent VTE between participants treated with oral factor Xa inhibitors (150 events/5698 participants) and those treated with conventional anticoagulation (183 events/5718 participants), leading to an OR of 0.83 (95% CI 0.66 to 1.03; I2 = 0%; 8 studies, 11,416 participants; moderate‐certainty evidence; Analysis 2.2).
2.2. Analysis.

Comparison 2: Oral factor Xa inhibitors versus conventional anticoagulation, Outcome 2: Recurrent venous thromboembolism
Deep vein thrombosis
We included two studies with a combined total of 8151 participants in a meta‐analysis (EINSTEIN‐PE 2012; Hokusai‐VTE 2013). There was no clear difference in the rate of recurrent DVT between participants treated with oral factor Xa inhibitors (30 events/4069 participants) and those treated with conventional anticoagulation (39 events/4082 participants), leading to an OR of 0.77 (95% CI 0.48 to 1.25; I2 = 0; 2 studies, 8151 participants; moderate‐certainty evidence; Analysis 2.3). The AMPLIFY 2013 study did not present DVT data for the subgroup of participants with a PE and therefore we did not include it in this meta‐analysis.
2.3. Analysis.

Comparison 2: Oral factor Xa inhibitors versus conventional anticoagulation, Outcome 3: Deep vein thrombosis
All‐cause mortality
Three studies measured all‐cause mortality (AMPLIFY‐J 2015; EINSTEIN‐PE 2012; MERCURY PE 2018). In the AMPLIFY‐J 2015 and MERCURY PE 2018 studies, the events in both groups were 0, so they were not estimated in the meta‐analysis. There was no clear difference in the rate of all‐cause mortality between participants treated with the oral factor Xa inhibitor rivaroxaban (2.40%; 58 events/2412 participants) and those treated with conventional anticoagulation (50 events/2405 participants), leading to an OR of 1.16 (95% CI 0.79 to 1.70; 1 study, 4817 participants; moderate‐certainty evidence; Analysis 2.4).
2.4. Analysis.

Comparison 2: Oral factor Xa inhibitors versus conventional anticoagulation, Outcome 4: All‐cause mortality
Adverse effects of treatment
Eight studies measured major bleeding (as defined by ISTH, Schulman 2005) (AMPLIFY‐J 2015; AMPLIFY 2013; Caravaggio 2020; EINSTEIN‐PE 2012; Hokusai VTE Cancer 2018; Hokusai‐VTE 2013; J‐EINSTEIN DVT and PE 2015; MERCURY PE 2018). There was no clear difference in the rate of major bleeding between participants treated with oral factor Xa inhibitors (95 events/5721 participants) and those treated with conventional anticoagulation (130 events/5726 participants) leading to an OR of 0.71 (95% CI 0.36 to 1.41; 8 studies, 11,447 participants; low‐certainty evidence; Analysis 2.5). Considerable heterogeneity was detected between the studies (I2 = 79%), likely due to clinical heterogeneity.
2.5. Analysis.

Comparison 2: Oral factor Xa inhibitors versus conventional anticoagulation, Outcome 5: Major bleeding
Health‐related quality of life
Health‐related quality of life was not reported in any of the included studies.
Subgroup analysis
For this update, due to the limited number of included studies, subgroup analyses were only possible for participants who received oral factor Xa inhibitors. We performed subgroup analysis by active cancer and different types of factor Xa inhibitors for the outcome of recurrent VTE and major bleeding.
Results of subgroup analyses showed no clear difference in the incidence of recurrent VTE in participants without cancer (OR 0.89, 95% CI 0.68 to 1.17; 6 studies, 9898 participants; moderate‐certainty evidence; Analysis 2.2) compared to participants with cancer (OR 0.65, 95% CI 0.42 to 1.01; 3 studies, 1518 participants; high‐certainty evidence; Analysis 2.2). The test for subgroup differences did not indicate a difference (P = 0.23). For the subgroup analysis based on the different types of factor Xa inhibitors, there was also no clear difference in the incidence of recurrent VTE when participants received treatment with apixaban (OR 0.86, 95% CI 0.54 to 1.35; 3 studies, 2459 participants; moderate‐certainty evidence; Analysis 2.6), rivaroxaban (OR 1.14, 95% CI 0.75 to 1.71; 3 studies, 4981 participants; moderate‐certainty evidence; Analysis 2.6), and edoxaban (OR 0.66, 95% CI 0.48 to 0.92; 2 studies, 3976 participants; moderate‐certainty evidence; Analysis 2.6) compared with conventional anticoagulation. The test for subgroup differences did not indicate a difference (P = 0.13).
2.6. Analysis.

Comparison 2: Oral factor Xa inhibitors versus conventional anticoagulation, Outcome 6: Recurrent venous thromboembolism (subgroup analysis based on different types of factor Xa inhibitors)
Subgroup analysis of the incidence of major bleeding in participants without cancer (OR 0.45, 95% CI 0.19 to 1.06; 6 studies, 10,152 participants; low‐certainty evidence; Analysis 2.5) versus participants with cancer (OR 1.51, 95% CI 0.84 to 2.71; 2 studies, 1295 participants; low‐certainty evidence; Analysis 2.5) suggested a possible subgroup difference (test for subgroup differences: P = 0.02). Due to the limited number of studies in this analysis, and as CIs overlap, this should be interpreted with caution. When analysed according to different types of factor Xa inhibitors, there was no clear difference in the incidence of major bleeding between apixaban and conventional anticoagulation (OR 0.36, 95% CI 0.07 to 1.91; 3 studies, 2503 participants; low‐certainty evidence; Analysis 2.7), or between edoxaban and conventional anticoagulation (OR 1.44, 95% CI 0.80 to 2.58; 2 studies, 3976 participants; low‐certainty evidence; Analysis 2.7). However, when treated with rivaroxaban, participants had a lower incidence of major bleeding compared to those treated with conventional anticoagulation (OR 0.49, 95% CI 0.31 to 0.79; 3 studies, 4968 participants; low‐certainty evidence; Analysis 2.7). The test for subgroup differences indicated a possible difference (P = 0.01). Due to the limited number of studies in this analysis, and as CIs overlap, this should be interpreted with caution.
2.7. Analysis.

Comparison 2: Oral factor Xa inhibitors versus conventional anticoagulation, Outcome 7: Major bleeding (subgroup analysis based on different types of factor Xa inhibitors)
Sensitivity analysis
We performed sensitivity analysis by excluding studies we judged to be at high risk of bias (AMPLIFY 2013; Caravaggio 2020). Based on the available data, only sensitivity analyses of oral factor Xa inhibitors for PE in the incidence of recurrent VTE and major bleeding were possible. The results showed that oral factor Xa inhibitors versus conventional anticoagulation made no clear difference in reducing the incidence of recurrent VTE (OR 0.78, 95% CI 0.54 to 1.14; 6 studies, 8992 participants; moderate‐certainty evidence; Analysis 2.8) and major bleeding (OR 0.91, 95% CI 0.42 to 1.97; 6 studies, 8979 participants; low‐certainty evidence; Analysis 2.9). The results of the sensitivity analyses are consistent with the results of Analysis 2.2 and Analysis 2.5, which indicates that the results are robust.
2.8. Analysis.

Comparison 2: Oral factor Xa inhibitors versus conventional anticoagulation, Outcome 8: Recurrent venous thromboembolism (sensitivity analysis by including only studies at low risk of bias)
2.9. Analysis.

Comparison 2: Oral factor Xa inhibitors versus conventional anticoagulation, Outcome 9: Major bleeding (sensitivity analysis by including only studies at low risk of bias)
Discussion
We included an additional five studies for this update, bringing the total to 10 included studies involving 13,073 participants. Two studies investigated an oral DTI (dabigatran) and eight studies investigated oral factor Xa inhibitors (three rivaroxaban, three apixaban, and two edoxaban). Overall the studies were of good methodological quality. The certainty of the evidence was moderate or low.
Summary of main results
Recurrent pulmonary embolism
Moderate‐certainty evidence showed no clear difference between both oral DTIs and factor Xa inhibitors compared to conventional anticoagulation in preventing recurrent pulmonary embolism (PE). We downgraded the certainty of the evidence by one level for imprecision.
Recurrent venous thromboembolism
Moderate‐certainty evidence showed no clear difference between oral DTIs and conventional anticoagulation in the prevention of recurrent venous thromboembolism (VTE) during treatment, indicating that neither was more nor less effective. We downgraded the certainty of the evidence by one level for imprecision.
Similarly, for factor Xa inhibitors, moderate‐certainty evidence showed no clear difference between factor Xa inhibitors and conventional anticoagulation in preventing recurrent VTE, indicating that neither was more nor less effective. We downgraded the certainty of the evidence by one level for imprecision.
Subgroup analyses showed no clear difference in the incidence of recurrent VTE between participants with and without cancer, and between participants who received different types of factor Xa inhibitors. Given the limited number of studies providing data in each subgroup, this may reflect a lack of information. More included studies may change the evidence in the future.
Deep vein thrombosis
Moderate‐certainty evidence showed no clear difference between both oral DTIs and factor Xa inhibitors compared to conventional anticoagulation in preventing deep vein thrombosis (DVT). We downgraded the certainty of the evidence by one level for imprecision.
All‐cause mortality
No study measured all‐cause mortality in participants treated with oral DTIs. Moderate‐certainty evidence showed no clear difference between oral factor Xa inhibitors and conventional therapy in preventing all‐cause mortality. We downgraded the certainty of the evidence by one level for imprecision.
Major bleeding
Moderate‐certainty evidence indicated no clear difference between oral DTIs and conventional anticoagulation in major bleeding. We downgraded the certainty of the evidence by one level for imprecision. For factor Xa inhibitors, low‐certainty evidence showed no clear difference between factor Xa inhibitors and conventional anticoagulation in preventing major bleeding. We downgraded the certainty of the evidence by one level for serious inconsistency due to clinical heterogeneity and by one level for imprecision.
Subgroup analyses suggested possible subgroup differences in the incidence of major bleeding between participants without cancer versus those with cancer, and between participants receiving different types of factor Xa inhibitors. Due to the limited number of studies in the subgroups, and as CIs overlap, this should be interpreted with caution. More included studies may change the evidence in the future.
Health‐related quality of life was not reported in the included studies.
Overall completeness and applicability of evidence
This review assessed whether long‐term treatment with oral anticoagulants – DTIs and factor Xa inhibitors – reduced the rate of recurrent PE, recurrent VTE, DVT, all‐cause mortality, and major bleeding in people with PE. Two studies tested DTIs and eight studies tested factor Xa inhibitors within similar study populations. The included studies analysed and reported all of our outcomes of interest, except for health‐related quality of life. Heterogeneity was high for major bleeding in the studies testing factor Xa inhibitors. As expected, this is likely due to clinical heterogeneity, because included studies varied in factor Xa inhibitors (three apixaban, three rivaroxaban, two edoxaban), comparators (six warfarin, two dalteparin), and treatment duration (three months (one study), five and a half months (one study), six months (two studies), 12 months (four studies)).
For this update, we performed subgroup analyses for active cancer and different types of factor Xa inhibitors. Limited data indicated no clear difference in the incidence of recurrent VTE between participants with and without cancer, and between participants who received different types of factor Xa inhibitors. For major bleeding, both subgroup analyses (i.e. of people experiencing PE with versus without cancer, and subgroups of different types of factor Xa inhibitors) suggested possible subgroup differences. Bleeding risk is an important consideration when using DOACs for cancer patients, particularly in patients with gastrointestinal malignancies (Thapa 2019). A systematic review and meta‐analysis of the Hokusai VTE Cancer 2018 and SELECT‐D 2018 studies suggested that the risk of major bleeding in cancer patients was approximately doubled when using DOACs compared with LMWH (Li 2019). For factor Xa inhibitors, while efficacy with apixaban, edoxaban, and rivaroxaban versus dalteparin has been consistent in the treatment of cancer‐associated VTE, one study showed heterogeneity is evident with respect to major gastrointestinal bleeding, with an increased risk with edoxaban and rivaroxaban but not apixaban (Athanazio 2022). All of these results are based on limited evidence; more evidence is needed to confirm these conclusions in the future.
We were unable to perform subgroup analyses for other factors because of the lack of participant‐level data. More evidence is needed, as these analyses might be important to guide clinical management in people with different risk factors for PE.
Although many consider DVT and PE to be manifestations of the same disorder, we elected to study these two conditions separately as there is evidence of clinically significant differences between them. First, the majority of recurrent events occur at the same site as the original thrombosis (in other words, in people presenting with a PE, a recurrent event after treatment is much more likely to be another PE). Second, both oral contraceptive use and Factor V Leiden mutation are more likely to be associated with DVT than PE. Third, lung disease is much more likely to be associated with PE. Consequently, a review on the effectiveness of oral DTIs and factor Xa inhibitors for the long‐term treatment of DVT was published at the same time as the previous version of this review (Robertson 2015b). It has also now been updated (Wang 2023).
We did not find any studies comparing:
one oral DTI versus another oral DTI;
one oral factor Xa inhibitor versus another oral factor Xa inhibitor;
oral DTI versus oral factor Xa inhibitor.
We identified no additional studies investigating DTIs for this update; therefore, this review cannot provide more evidence for this intervention. Given the recent clinical thinking that DOACs have a class effect, we will pool the results of all DTIs and factor Xa inhibitors in future updates. This may increase the power of the studies, reduce type II errors, and allow any effects to be determined clearly.
In 2020, the UK's National Institute for Health and Care Excellence (NICE) measured the cost‐effectiveness of DOACs versus conventional anticoagulation for the treatment of DVT and PE (NICE 2020). Assuming that people remain on the same drug in the initial and extended phases of treatment, apixaban was highly cost‐effective both in people with a DVT and people with a PE. Rivaroxaban had the next most favourable effect on major bleeding and generated the second‐highest total quality‐adjusted life years (QALYs). The cost of the two drugs was similar and the difference in total costs was mainly led by differences in the number of bleeding events.
Certainty of the evidence
We assessed the overall risk of bias as low in eight of 10 included studies, reflecting good methodological quality. We judged two studies to have a high risk of reporting bias. Six of the 10 included studies were open‐label because of the complexity of monitoring international normalised ratio (INR) in the conventional anticoagulation arm. However, all outcomes were assessed by observers who were blinded to the treatment and all safety outcomes were adjudicated by a central independent committee in each included study. We could not investigate publication bias because we could not assess asymmetry in a funnel plot with the limited number of studies included in the meta‐analysis.
For the comparison of oral DTIs versus conventional anticoagulation, we graded the certainty of the evidence as moderate, downgrading by one level for imprecision due to the low number of events. For oral factor Xa inhibitors versus conventional anticoagulation, we downgraded the evidence for recurrent PE, recurrent VTE, DVT, and all‐cause mortality to moderate due to the low number of events. For this comparison, we downgraded the evidence for major bleeding by two levels to low, due to the low number of events and substantial clinical heterogeneity. See Table 1; Table 2.
Potential biases in the review process
The search was as comprehensive as possible, and we are confident that we have included all relevant studies. However, the possibility remains that some relevant trials, particularly in the 'grey' literature (for example, conference proceedings), have been missed. Eight review authors independently performed study selection and data extraction in order to minimise bias in the review process. We strictly adhered to the inclusion and exclusion criteria set out in the protocol in order to limit subjectivity. We performed data collection according to the process suggested by Cochrane. We contacted study authors in an attempt to obtain PE‐specific data. We also followed Cochrane processes as described by Higgins 2017 for assessing the risk of bias. Two of the included studies, RE‐COVER 2009 and RE‐COVER II 2014, only reported outcome data of VTE, not separate data for PE, so we took data from a pooled analysis published in an additional report (Goldhaber 2016). This was the best available evidence.
Agreements and disagreements with other studies or reviews
This review assesses the efficacy and safety of oral DTIs and oral factor Xa inhibitors for the long‐term treatment of PE. A similar review by Dentali 2015 evaluated the difference in the safety and efficacy of DOACs compared to conventional treatment in participants presenting with DVT and with PE. The Dentali 2015 review included six studies (27,023 participants) and indicated that DOACs appear to have similar efficacy and safety profiles compared to VKAs for people with DVT and PE. Dentali 2015 focused on both PE and DVT, but did not include some more recent RCTs that are included in this update (AMPLIFY‐J 2015; Caravaggio 2020; Hokusai VTE Cancer 2018; J‐EINSTEIN DVT and PE 2015; MERCURY PE 2018).
Fourteen other systematic reviews have assessed the same oral anticoagulants (Antoniazzi 2013; Castellucci 2013; Cohen 2015; Fox 2012; Gómez‐Outes 2014; Gómez‐Outes 2015; Hirschl 2014; Kang 2014; Moik 2020; Samaranayake 2022; Sardar 2014; Song 2021; Van der Huille 2014; Wu 2022). However, these reviews focused on people with a VTE, and did not report specific data on the subgroup with a PE. Four reviews indicated that DOACs can decrease the risk of recurrent VTE compared to conventional treatment (Moik 2020; Samaranayake 2022; Song 2021; Wu 2022); seven reviews found that DOACs are associated with less bleeding than conventional treatment (Antoniazzi 2013; Fox 2012; Gómez‐Outes 2014; Gómez‐Outes 2015; Hirschl 2014; Van der Huille 2014; Wu 2022); one review found that DOACs are associated with lower all‐cause mortality compared with those receiving conventional anticoagulants in people with venous thromboembolism (Wu 2022).
The Fox 2012 review performed meta‐analysis by brand rather than class of drug and found no difference in recurrent VTE between the two treatment groups. Rivaroxaban was the only drug found to be significantly associated with fewer major bleeding episodes (odds ratio (OR) 0.57, 95% confidence interval (CI) 0.39 to 0.84). All‐cause mortality did not differ between the two treatment groups.
The Van der Huille 2014 review showed no difference between the two treatment groups in terms of recurrent VTE, fatal PE, and all‐cause mortality. However, DOACs were associated with a significantly reduced risk of major bleeding (relative risk (RR) 0.60, 95% CI 0.41 to 0.88) and fatal bleeding (RR 0.36, 95% CI 0.15 to 0.87).
Hirschl 2014 found no differences between DOACs and conventional treatment regarding recurrent VTE and death. However, bleeding was reduced by rivaroxaban (RR 0.55, 95% CI 0.38 to 0.81), apixaban (RR 0.31, 95% CI 0.17 to 0.55), and edoxaban (RR 0.81, 95% CI 0.71 to 0.93).
The Gómez‐Outes 2014 review found no clear difference in the risk of recurrent VTE between the two treatment groups (RR 0.91, 95% CI 0.79 to 1.06), but DOACs were associated with reduced major bleeding (absolute risk difference of ‐0.6%, 95% CI ‐1.0% to ‐0.3%).
A network meta‐analysis by Kang 2014 found that DOACs did not differ in the risk of mortality or recurrent VTE. However, dabigatran was associated with increased major bleeding compared to apixaban (RR 2.69, 95% CI 1.19 to 6.07), and edoxaban also had a higher bleeding rate compared to apixaban (RR 2.74, 95% CI 1.40 to 5.39).
The Wu 2022 review included 65 real‐world evidence studies and found that, in people with VTE, rivaroxaban and apixaban are associated with a lower risk of recurrent VTE (rivaroxaban: HR 0.68, 95% CI 0.60 to 0.76; apixaban: HR 0.83, 95% CI 0.73 to 0.93), major bleeding events (rivaroxaban: HR 0.73, 95% CI 0.65 to 0.81; apixaban: HR 0.76, 95% CI 0.68 to 0.85), and all‐cause mortality (HR 0.56, 95% CI 0.39 to 0.80) compared with those receiving conventional anticoagulants.
The Song 2021 review included four RCTs and 14 retrospective studies and found that DOACs decreased the risk of recurrent VTE (RCTs: OR 0.60, 95% CI 0.45 to 0.80; retrospective studies: OR 0.73, 95% CI 0.59 to 0.90) and recurrent DVT (RCTs: OR 0.54, 95% CI 0.36 to 0.80; retrospective studies: OR 0.20, 95% CI 0.06 to 0.63), but not PE recurrence (OR 0.75, 95% CI 0.53 to 1.05), fatal PE (OR 0.87, 95% CI 0.33 to 2.26), and major bleeding events (OR 1.04, 95% CI 0.84 to 1.28) compared with LMWHs in people with cancer.
The Samaranayake 2022 review conducted a network meta‐analysis including four RCTs with 2907 participants with venous thromboembolism. They found that the overall risk of recurrent VTE was lower in the DOACs group compared to the dalteparin group (RR 0.63, 95% CI 0.44 to 0.91). There was no statistically significant difference in risk of major bleeding (RR 1.31, 95% CI 0.83 to 2.07) and all‐cause mortality (RR 1.0, 95% CI 0.84 to 1.18) at six months' follow‐up between DOACs and LMWH.
A review by Moik 2020 that included four RCTs (with 2894 participants with cancer‐associated VTE) found that DOACs significantly reduced recurrent VTE compared to LMWHs (RR 0.62, 95% CI 0.43 to 0.91), but were associated with a non‐significant increase in major bleeding (RR 1.31, 95% CI 0.83 to 2.08). Mortality risks were comparable between groups (RR 0.99, 95% CI 0.83 to 1.18).
The Gómez‐Outes 2015 review found that DOACs were associated with lower major bleeding (RR 0.62, 95% CI 0.45 to 0.85), while there was no clear difference in recurrent VTE rate in people receiving DOACs or standard therapy (RR 0.91, 95% CI 0.79 to 1.05).
A review by Cohen 2015 conducted a network meta‐analysis to compare the efficacy and safety of DOACs for the initial and long‐term treatment of VTE. They found that apixaban was associated with a statistically significantly reduced risk of major bleeding compared with rivaroxaban (RR 0.47, 95% CI 0.36 to 0.61), dabigatran (RR 0.69, 95% CI 0.51 to 0.94), and edoxaban (RR 0.54, 95% CI 0.41 to 0.69). Dabigatran was associated with a significantly lower risk of major bleeding compared with rivaroxaban (RR 0.68, 95% CI 0.53 to 0.87) and edoxaban (RR 0.77, 95% CI 0.60 to 0.99). There were no clear differences between the DOACs with regard to the risk of VTE and related death.
The Antoniazzi 2013 review included participants with VTE and atrial fibrillation. Eight studies were included and results showed that the risk of major bleeding was lower in participants treated with dabigatran (RR 0.83, 95% CI 0.78 to 0.97) compared with the control group.
The reviews by Castellucci 2013, Cohen 2016, Sardar 2014, and Wang 2018 compared oral anticoagulants and antiplatelet drugs, but the focus was on the secondary prevention of venous thromboembolism rather than treatment.
Authors' conclusions
Implications for practice.
Available evidence shows there is little or no difference between direct oral anticoagulants (DOACs) and conventional anticoagulation in the prevention of recurrent pulmonary embolism (PE), recurrent venous thromboembolism (VTE), deep vein thrombosis (DVT), all‐cause mortality, and major bleeding. According to GRADE criteria, the certainty of evidence was moderate to low. DOACs, such as direct thrombin inhibitors (DTIs) and factor Xa inhibitors, may therefore be an alternative to conventional anticoagulation treatment for acute pulmonary embolism. One potential practical advantage of DOACs over conventional anticoagulants is their ease of use due to fixed doses and no need for routine monitoring with blood tests.
Implications for research.
There is some evidence of wide inter‐individual variation in anticoagulant effect from the fixed doses of DOACs as currently prescribed. This may be of clinical importance, and further research is needed to compare DOACs directly with one another to see which one is most effective and safe, specifically in relation to bleeding risk in various subgroups, such as in people with cancer or obesity. Such studies would need to be very large and may not be considered financially viable to sponsoring drug companies. Any impact on the decision to use extended phase anticoagulation and interruption of procedures with DOAC use should also be investigated. Further research is also required to establish other factors associated with the use of DOACs, such as adherence, quality of life, cost‐effectiveness, and tolerability. Finally, research is required in categories of venous thrombosis not specifically examined in the studies included here, such as in people with malignancy or a thrombophilic abnormality, as well as travel‐associated venous thrombosis.
Feedback
Feedback February 2016,
Summary
Email received from Dr Lynda Ware.
1. The Hokusai‐VTE study. In both the narrative paragraph describing the study (page 11) and in the “characteristics of included studies” section (page 31) you describe a 'dabigatran‐like placebo'. This study was looking at edoxaban and the original paper speaks of an edoxaban placebo. I think therefore that there is a possible transcription error and I suggest that you actually meant “edoxaban‐like placebo” on pages 11 and 31.
Authors' response: Thank you for pointing this out. This is a transcription error and has now been changed.
2. Recurrent pulmonary embolism. In the summary of main results in the main body text you conclude that 'for Factor Xa inhibitors, there was substantial heterogeneity when we combined data from the two studies in a meta‐analysis. Therefore no meaningful conclusions can be drawn from this analysis'. The heterogeneity is measured as 58% using the I2 statistic and I can understand why you therefore decided not to combine the studies. When you say “no meaningful conclusions can be drawn from this analysis” do you mean the pooled analysis? Or the individual studies? Do you in fact draw some conclusions from those individual studies (see below)?
Authors' response: We meant to say "no meaningful conclusions can be drawn from the pooled analysis” and this has been been amended.
However, in the authors' conclusions in the abstract it states that 'moderate to high quality evidence suggests that there are no differences between DOACs and standard anticoagulation for the long‐term treatment of pulmonary embolism for the outcome(s) recurrent pulmonary embolism ....'. Your use of the term 'DOACs' implies both DTIs (represented in the data by dabigatran) and the Factor Xa inhibitors. In other words, you appear to be saying that (a) there is “moderate to high quality evidence that there is no difference between DTIs and standard anticoagulation for the long‐term treatment etc…”, and (b) there is “moderate to high quality evidence that there is no difference between the Factor Xa inhibitors and standard anticoagulation for the long‐term treatment etc…'
There are two problems I believe with this. A specific one is that for the Factor Xa inhibitors you have stated that “no meaningful conclusions can be drawn from this analysis”. How can you reconcile this with the above?
Authors' response: We agree with your point and have reworded the paragraph drawing conclusions for the separate drug classes rather than grouping them together.
Secondly, “the absence of evidence is not evidence of absence”. Can you really say that there is “moderate to high quality evidence that there is no difference between” the alternatives? Based on three trials? Or do you mean that “there is no evidence of a difference between the two treatments. Using the GRADE criteria, the quality of evidence supporting that statement is moderate to high” or “there is no evidence of a difference between the two treatments. The studies assessing this were at low risk of bias”.
Authors' response: Despite there being few studies for any of the comparisons, there is a relatively large number of patients. However, we can see your point and have amended accordingly.
Reply
The authors of the review have responded to this feedback giving a point by point response to the comments above.
Contributors
Feedback: Dr Lynda Ware, Cochrane UK Response: Dr Lindsay Robertson on behalf of the review authors
What's new
| Date | Event | Description |
|---|---|---|
| 14 April 2023 | New search has been performed | New search run. Five new studies included, 17 new studies excluded and 14 ongoing studies identified. |
| 14 April 2023 | New citation required but conclusions have not changed | New search run. Five new studies included, 17 new studies excluded and 14 ongoing studies identified. New author team involved. Text has been revised to reflect current Cochrane standards. No change to conclusions. |
History
Protocol first published: Issue 2, 2014 Review first published: Issue 12, 2015
| Date | Event | Description |
|---|---|---|
| 16 December 2016 | Feedback has been incorporated | Review amended following comments received |
Acknowledgements
The review authors would like to thank Lindsay Robertson, James McCaslin, and colleagues for their contributions to previous versions of this review.
The review authors would like to thank Dr Cathryn Broderick, Dr Marlene Stewart and other Cochrane Vascular editors for their assistance and advice in completing this review. We thank Candida Fenton for designing and running the searches. We thank Yanfang Ma for helping select studies for inclusion.
The review authors would like to thank Faith Armitage for copy editing the review.
The review authors and the Cochrane Vascular editorial base are grateful to the following peer reviewers, and those who wished to remain anonymous, for their time and comments: Martin H Ellis MD, Hematology Institute and Blood Bank, Meir Medical Center, Kfar Saba and Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel; Daniel Schimmel MD, Northwestern University, Feinberg School of Medicine, Chicago, IL, USA.
Appendices
Appendix 1. Sources searched and search strategies
| Source | Search strategy | Hits retrieved |
| 1. Medline (Ovid MEDLINE® Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE® Daily and Ovid MEDLINE®) 1946 to present (Date of most recent search: 2 March 2022) |
1 exp Pulmonary Embolism/ 2 exp THROMBOEMBOLISM/ 3 (emboli* adj4 pulmonary).ti,ab. 4 thromboemboli*.ti,ab. 5 (PE or VTE).ti,ab. 6 (Pulmonary adj4 clot).ti,ab. 7 (lung adj4 clot).ti,ab. 8 or/1‐7 9 exp ANTITHROMBINS/ 10 Hirudin Therapy/ 11 (thrombin adj3 inhib*).ti,ab. 12 hirudin*.ti,ab. 13 (dabigatran or Pradaxa or Rendix).ti,ab. 14 (BIBR‐953* or BIBR953* or BIBR‐1048 or BIBR1048).ti,ab. 15 (ximelagatran or Exanta or Exarta or melagatran).ti,ab. 16 (AZD0837 or AZD‐0837).ti,ab. 17 (S35972 or S‐35972).ti,ab. 18 Factor Xa Inhibitors/ 19 (Factor X* adj4 (antag* or inhib* or block*)).ti,ab. 20 (FX* adj4 (antag* or inhib* or block*)).ti,ab. 21 (FX* adj4 (antag* or inhib* or block*)).ti,ab. 22 (10* adj4 (antag* or inhib* or block*)).ti,ab. 23 (rivaroxaban or Xarelto).ti,ab. 24 (Bay‐597939 or Bay597939).ti,ab. 25 (betrixaban or PRT054021).ti,ab. 26 apixaban.ti,ab. 27 (BMS‐562247 or BMS‐562247 or ELIQUIS).ti,ab. 28 (DU‐176b or DU176b).ti,ab. 29 (PRT‐054021 or PRT054021).ti,ab. 30 (YM150 or YM‐150 or LY517717 or LY‐517717 or DU‐176b or DU176*).ti,ab. 31 (GW813893 or "Tak 442" or TAK442 or PD0348292 or GSK‐813893 or GSK813893).ti,ab. 32 (edoxaban or lixiana).ti,ab. 33 or/9‐32 34 8 and 33 35 randomized controlled trial.pt. 36 controlled clinical trial.pt. 37 randomized.ab. 38 placebo.ab. 39 drug therapy.fs. 40 randomly.ab. 41 trial.ab. 42 groups.ab. 43 or/35‐42 44 exp animals/ not humans.sh. 45 43 not 44 46 34 and 45 |
March 2022: 2641 |
| 2. EMBASE via Ovid (Date of most recent search: 2 March 2022) |
1 exp lung embolism/ 2 exp thromboembolism/ 3 (emboli* adj4 pulmonary).ti,ab. 4 thromboemboli*.ti,ab. 5 (PE or VTE).ti,ab. 6 (Pulmonary adj4 clot).ti,ab. 7 (lung adj4 clot).ti,ab. 8 or/1‐7 9 exp antithrombin/ 10 anticoagulant therapy/ 11 (thrombin adj3 inhib*).ti,ab. 12 hirudin*.ti,ab. 13 (dabigatran or Pradaxa or Rendix).ti,ab. 14 (BIBR‐953* or BIBR953* or BIBR‐1048 or BIBR1048).ti,ab. 15 (ximelagatran or Exanta or Exarta or melagatran).ti,ab. 16 (AZD0837 or AZD‐0837).ti,ab. 17 (S35972 or S‐35972).ti,ab. 18 blood clotting factor 10a inhibitor/ 19 (Factor X* adj4 (antag* or inhib* or block*)).ti,ab. 20 (FX* adj4 (antag* or inhib* or block*)).ti,ab. 21 (10* adj4 (antag* or inhib* or block*)).ti,ab. 22 (rivaroxaban or Xarelto).ti,ab. 23 (Bay‐597939 or Bay597939).ti,ab. 24 (betrixaban or PRT054021).ti,ab. 25 apixaban.ti,ab. 26 (BMS‐562247 or BMS‐562247 or ELIQUIS).ti,ab. 27 (DU‐176b or DU176b).ti,ab. 28 (PRT‐054021 or PRT054021).ti,ab. 29 (YM150 or YM‐150 or LY517717 or LY‐517717 or DU‐176b or DU176*).ti,ab. 30 (GW813893 or "Tak 442" or TAK442 or PD0348292 or GSK‐813893 or GSK813893).ti,ab. 31 (edoxaban or lixiana).ti,ab. 32 or/9‐31 33 8 and 32 34 randomized controlled trial/ 35 controlled clinical trial/ 36 random$.ti,ab. 37 randomization/ 38 intermethod comparison/ 39 placebo.ti,ab. 40 (compare or compared or comparison).ti. 41 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 42 (open adj label).ti,ab. 43 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 44 double blind procedure/ 45 parallel group$1.ti,ab. 46 (crossover or cross over).ti,ab. 47 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 48 (assigned or allocated).ti,ab. 49 (controlled adj7 (study or design or trial)).ti,ab. 50 (volunteer or volunteers).ti,ab. 51 trial.ti. 52 or/34‐51 53 33 and 52 |
March 2022: 7892 |
| 3. CINAHL via Ebsco (Date of most recent search: 2 March 2022) |
S47 S31 AND S46 S46 S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 S45 MH "Random Assignment" S44 MH "Triple‐Blind Studies" S43 MH "Double‐Blind Studies" S42 MH "Single‐Blind Studies" S41 MH "Crossover Design" S40 MH "Factorial Design" S39 MH "Placebos" S38 MH "Clinical Trials" S37 TX "multi‐centre study" OR "multi‐center study" OR "multicentre study" OR "multicenter study" OR "multi‐site study" S36 TX crossover OR "cross‐over" S35 AB placebo* S34 TX random* S33 TX trial* S32 TX "latin square" S31 S8 AND S30 S30 S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 S29 TX apixaban S28 TX edoxaban or lixiana S27 TX GW813893 or "Tak 442" or TAK442 or PD0348292 or GSK‐813893 or GSK813893 S26 TX YM150 or YM‐150 or LY517717 or LY‐517717 or DU‐176b or DU176* S25 TX PRT‐054021 or PRT054021 S24 TX DU‐176b or DU176b S23 TX BMS‐562247 or BMS‐562247 or ELIQUIS S22 TX betrixaban or PRT054021 S21 TX Bay‐597939 or Bay597939 S20 TX rivaroxaban or Xarelto S19 TX (10* n4 (antag* or inhib* or block*) ) S18 TX (FX* n4 (antag* or inhib* or block*)) S17 TX (Factor X* n4 (antag* or inhib* or block*)) S16 TX S35972 or S‐35972 S15 TX AZD0837 or AZD‐0837 S14 TX ximelagatran or Exanta or Exarta or melagatran S13 TX BIBR‐953* or BIBR953* or BIBR‐1048 or BIBR1048 S12 TX Antithrombins S11 TX dabigatran or Pradaxa or Rendix S10 TX hirudin* S9 TX thrombin n3 inhib* S8 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 S7 TX PE or VTE S6 TX lung N4 clot S5 TX Pulmonary N4 clot S4 TX thromboemboli* S3 TX emboli* N4 pulmonary S2 (MH "Thromboembolism+") S1 (MH "Pulmonary Embolism") |
March 2022: 473 |
| 4. VASCULAR REGISTER IN CRSW (Date of most recent search: 2 March 2022) |
rivaroxaban OR apixaban OR dabigatran OR ximelagatran OR betrixaban OR edoxaban | March 2022: 1568 |
| 5. CENTRAL via CRSO (Date of most recent search: 2 March 2022) |
#1 MESH DESCRIPTOR Pulmonary Embolism EXPLODE ALL TREES 1057 #2 MESH DESCRIPTOR THROMBOEMBOLISM EXPLODE ALL TREES 2166 #3 (emboli* adj4 pulmonary):TI,AB,KY 345 #4 thromboemboli*:TI,AB,KY 10025 #5 (PE or VTE):TI,AB,KY 7326 #6 (Pulmonary adj4 clot):TI,AB,KY 14 #7 (lung adj4 clot):TI,AB,KY 1 #8 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 15873 #9 MESH DESCRIPTOR ANTITHROMBINS EXPLODE ALL TREES 2289 #10 MESH DESCRIPTOR Hirudin Therapy EXPLODE ALL TREES 75 #11 (thrombin adj3 inhib*):TI,AB,KY 698 #12 hirudin*:TI,AB,KY 483 #13 (dabigatran or Pradaxa or Rendix):TI,AB,KY 1068 #14 (BIBR‐953* or BIBR953* or BIBR‐1048 or BIBR1048):TI,AB,KY 48 #15 (ximelagatran or Exanta or Exarta or melagatran):TI,AB,KY 182 #16 (AZD0837 or AZD‐0837):TI,AB,KY 22 #17 (S35972 or S‐35972):TI,AB,KY 0 #18 MESH DESCRIPTOR Factor Xa Inhibitors EXPLODE ALL TREES 1091 #19 (Factor X* adj4 (antag* or inhib* or block*)):TI,AB,KY 1113 #20 (FX* adj4 (antag* or inhib* or block*)):TI,AB,KY 107 #21 (FX* adj4 (antag* or inhib* or block*)):TI,AB,KY 107 #22 (10* adj4 (antag* or inhib* or block*)):TI,AB,KY 1677 #23 (rivaroxaban or Xarelto):TI,AB,KY 1898 #24 (Bay‐597939 or Bay597939):TI,AB,KY 0 #25 (betrixaban or PRT054021):TI,AB,KY 93 #26 apixaban:TI,AB,KY 1030 #27 (BMS‐562247 or BMS‐562247 or ELIQUIS):TI,AB,KY 48 #28 (DU‐176b or DU176b):TI,AB,KY 53 #29 (PRT‐054021 or PRT054021):TI,AB,KY 3 #30 (YM150 or YM‐150 or LY517717 or LY‐517717 or DU‐176b or DU176*):TI,AB,KY 108 #31 (GW813893 or "Tak 442" or TAK442 or PD0348292 or GSK‐813893 or GSK813893):TI,AB,KY 7 #32 (edoxaban or lixiana):TI,AB,KY 620 #33 #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 7475 #34 #8 AND #33 2079 |
March 2022: 533 |
| 6. Clinicaltrials.gov (Date of most recent search: 2 March 2022) |
Pulmonary Embolism OR thromboembolism | rivaroxaban OR apixaban OR dabigatran OR ximelagatran OR betrixaban OR edoxaban | March 2022: 146 |
| 7. ICTRP Search Portal (Date of most recent search: 2 March 2022) |
Pulmonary Embolism OR thromboembolism | rivaroxaban OR apixaban OR dabigatran OR ximelagatran OR betrixaban OR edoxaban | March 2022: 159 |
| TOTAL before de‐duplication | March 2022: 13412 | |
| TOTAL after de‐duplication | March 2022: 10797 | |
Appendix 2. CRS search strategy 2015
| Search run on Wednesday 28 January 2015 | ||
| #1 | MESH DESCRIPTOR Antithrombins EXPLODE ALL TREES | 790 |
| #2 | MESH DESCRIPTOR Hirudin Therapy | 75 |
| #3 | (thrombin near3 inhib*):TI,AB,KY | 444 |
| #4 | hirudin*:TI,AB,KY | 327 |
| #5 | (dabigatran or Pradaxa or Rendix):TI,AB,KY | 199 |
| #6 | (BIBR‐953* or BIBR953* or BIBR‐1048 or BIBR1048):TI,AB,KY | 9 |
| #7 | (ximelagatran or Exanta or Exarta or melagatran):TI,AB,KY | 147 |
| #8 | (AZD0837 or AZD‐0837):TI,AB,KY | 12 |
| #9 | (S35972 or S‐35972):TI,AB,KY | 0 |
| #10 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 | 1387 |
| #11 | MESH DESCRIPTOR Factor Xa Inhibitors | 1 |
| #12 | (Factor X* near4 (antag* or inhib* or block*)):TI,AB,KY | 415 |
| #13 | (FX* near4 (antag* or inhib* or block*)):TI,AB,KY | 33 |
| #14 | (10* near4 (antag* or inhib* or block*) ):TI,AB,KY | 842 |
| #15 | #11 OR #12 OR #13 OR #14 | 1237 |
| #16 | (rivaroxaban or Xarelto):TI,AB,KY | 251 |
| #17 | (Bay‐597939 or Bay597939):TI,AB,KY | 0 |
| #18 | (betrixaban or PRT054021):TI,AB,KY | 14 |
| #19 | apixaban:TI,AB,KY | 134 |
| #20 | (BMS‐562247 or BMS‐562247 or ELIQUIS):TI,AB,KY | 0 |
| #21 | (DU‐176b or DU176b):TI,AB,KY | 11 |
| #22 | (PRT‐054021 or PRT054021):TI,AB,KY | 1 |
| #23 | (YM150 or YM‐150 or LY517717 or LY‐517717 or DU‐176b or DU176*):TI,AB,KY | 38 |
| #24 | (GW813893 or "Tak 442" or TAK442 or PD0348292 or GSK‐813893 or GSK813893):TI,AB,KY | 3 |
| #25 | edoxaban or lixiana | 51 |
| #26 | #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 | 456 |
| #27 | #10 OR #15 OR #26 | 2793 |
| #28 | MESH DESCRIPTOR Thrombosis | 1133 |
| #29 | MESH DESCRIPTOR Thromboembolism | 841 |
| #30 | MESH DESCRIPTOR Venous Thromboembolism | 159 |
| #31 | MESH DESCRIPTOR Venous Thrombosis EXPLODE ALL TREES | 1857 |
| #32 | (thrombus* or thrombotic* or thrombolic* or thromboemboli* or thrombos* or embol*):TI,AB,KY | 13382 |
| #33 | MESH DESCRIPTOR Pulmonary Embolism EXPLODE ALL TREES | 676 |
| #34 | (PE or DVT or VTE):TI,AB,KY | 3057 |
| #35 | ((vein* or ven*) near thromb*):TI,AB,KY | 5003 |
| #36 | (blood near3 clot*):TI,AB,KY | 1305 |
| #37 | (pulmonary near3 clot*):TI,AB,KY | 5 |
| #38 | (lung near3 clot*):TI,AB,KY | 3 |
| #39 | #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 | 16505 |
| #40 | #27 AND #39 | 1026 |
Data and analyses
Comparison 1. Oral DTIs versus conventional anticoagulation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Recurrent pulmonary embolism | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.2 Recurrent venous thromboembolism | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.3 Deep vein thrombosis | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.4 Major bleeding | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 2. Oral factor Xa inhibitors versus conventional anticoagulation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Recurrent pulmonary embolism | 3 | 8186 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.66, 1.29] |
| 2.2 Recurrent venous thromboembolism | 8 | 11416 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.66, 1.03] |
| 2.2.1 Non‐cancer associated pulmonary embolism | 6 | 9898 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.68, 1.17] |
| 2.2.2 Cancer associated pulmonary embolism | 3 | 1518 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.42, 1.01] |
| 2.3 Deep vein thrombosis | 2 | 8151 | Odds Ratio (M‐H, Random, 95% CI) | 0.77 [0.48, 1.25] |
| 2.4 All‐cause mortality | 3 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.5 Major bleeding | 8 | 11447 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.36, 1.41] |
| 2.5.1 Non‐cancer associated pulmonary embolism | 6 | 10152 | Odds Ratio (M‐H, Random, 95% CI) | 0.45 [0.19, 1.06] |
| 2.5.2 Cancer associated pulmonary embolism | 2 | 1295 | Odds Ratio (M‐H, Random, 95% CI) | 1.51 [0.84, 2.71] |
| 2.6 Recurrent venous thromboembolism (subgroup analysis based on different types of factor Xa inhibitors) | 8 | 11416 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.66, 1.04] |
| 2.6.1 Apixaban | 3 | 2459 | Odds Ratio (M‐H, Random, 95% CI) | 0.86 [0.54, 1.35] |
| 2.6.2 Rivaroxaban | 3 | 4981 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.75, 1.71] |
| 2.6.3 Edoxaban | 2 | 3976 | Odds Ratio (M‐H, Random, 95% CI) | 0.66 [0.48, 0.92] |
| 2.7 Major bleeding (subgroup analysis based on different types of factor Xa inhibitors) | 8 | 11447 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.36, 1.41] |
| 2.7.1 Apixaban | 3 | 2503 | Odds Ratio (M‐H, Random, 95% CI) | 0.36 [0.07, 1.91] |
| 2.7.2 Rivaroxaban | 3 | 4968 | Odds Ratio (M‐H, Random, 95% CI) | 0.49 [0.31, 0.79] |
| 2.7.3 Edoxaban | 2 | 3976 | Odds Ratio (M‐H, Random, 95% CI) | 1.44 [0.80, 2.58] |
| 2.8 Recurrent venous thromboembolism (sensitivity analysis by including only studies at low risk of bias) | 6 | 8992 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.54, 1.14] |
| 2.9 Major bleeding (sensitivity analysis by including only studies at low risk of bias) | 6 | 8979 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.42, 1.97] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
AMPLIFY 2013.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind trial Duration of study: 6 months | |
| Participants | Setting: hospital Country: multinational (358 centres in 28 countries: United States, Argentina, Australia, Austria, Brazil, Canada, China, Czech Republic, Denmark, France, Germany, Hungary, India, Israel, Italy, Korea, Malaysia, Mexico, Norway, Poland, Portugal, Puerto Rico, Romania, Russia, Singapore, South Africa, Spain, Ukraine) Number of participants: 5395 (PE 1836, other VTE 3559); apixaban 2691 (PE 930, other VTE 1761), enoxaparin + warfarin 2704 (PE 906, DVT 1798) Age, mean (SD) years: apixaban 57.2 (16.0) years, enoxaparin + warfarin 56.7 (16.0) years Sex: apixaban 1569 M/1122 F; enoxaparin + warfarin 1598 M/1106 F Inclusion criteria: people ≥ 18 years of age with an objectively confirmed, symptomatic proximal DVT or PE (with or without DVT) Exclusion criteria: active bleeding, a high risk of bleeding, or other contraindications to treatment with enoxaparin and warfarin; if they had cancer and long‐term treatment with LMWH was planned; if their DVT or PE was provoked in the absence of a persistent risk factor for recurrence; if < 6 months of anticoagulant treatment was planned; or if they had another indication for long‐term anticoagulation therapy, dual antiplatelet therapy, treatment with aspirin at a dose > 165 mg daily, or treatment with potent inhibitors of cytochrome P‐450 3A4; if they had received > 2 doses of a once‐daily LMWH regimen, fondaparinux, or a VKA; > 3 doses of a twice daily LMWH regimen; or more than 36 hours of continuous IV heparin. Additional exclusion criteria were a haemoglobin level < 9 mg/dL, a platelet count < 100,000/mm3, a serum creatinine level > 2.5 mg/dL (220 μmol/L), or a calculated creatinine clearance < 25 mL/minute | |
| Interventions | Intervention 1: oral apixaban 10 mg twice daily for the first 7 days, followed by 5 mg twice daily for 6 months Intervention 2: enoxaparin 1 mg/kg body weight every 12 hours for at least 5 days and warfarin concomitantly for 6 months. Warfarin dose was adjusted to maintain the INR 2.0 to 3.0. Enoxaparin or placebo was discontinued when a blinded INR of ≥ 2.0 was achieved Follow‐up: weeks 2, 4, 8, 12, 16, 20, and 24 after randomisation and 30 days after the end of the intended treatment period | |
| Outcomes | Primary: composite of recurrent symptomatic VTE (fatal or non‐fatal PE and DVT), and mortality related to VTE; major bleeding Secondary: recurrent symptomatic VTE, mortality related to VTE, mortality from cardiovascular causes, mortality from any cause and the composite of major bleeding and clinically relevant non‐major bleeding | |
| Funding | Quote: "Supported by Pfizer and Bristol‐Myers Squibb." Comment: Pfizer Inc and Bristol‐Myers Squibb were the pharmaceutical companies that developed apixaban. It is possible that this may have influenced the report of outcomes. |
|
| Declarations of interest | Quote: "Dr. Agnelli reports receiving personal fees from Boehringer Ingelheim, Sanofi, Daiichi‐Sankyo, and Bayer. Dr. Buller reports receiving grant support from Bayer, Sanofi, and Daiichi‐Sankyo. Dr. Cohen reports receiving payment for board membership from Bayer, Bristol‐Myers Squibb, Daiichi‐Sankyo, Johnson & Johnson, Pfizer, Portola Pharmaceuticals, and Sanofi, and consulting fees, lecture fees, travel support, and payment for the development of educational presentations from Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Daiichi‐Sankyo, Glaxo‐SmithKline, Johnson & Johnson, Mitsubishi Pharma, Pfizer, Portola Pharmaceuticals, Sanofi, Schering‐Plough, and Takeda. Drs. Curto, Johnson, Masiukiewicz, Pak, and Thompson report being employees of Pfizer. Dr. Gallus reports receiving consulting fees from Pfizer, Bristol‐Myers Squibb, Daiichi‐Sankyo, Bayer, and Boehringer Ingelheim. Dr. Raskob reports receiving consulting fees and travel support from Bayer, Janssen Pharmaceuticals, Daiichi‐Sankyo, and Quintiles. Dr. Weitz reports receiving consulting fees from Boehringer Ingelheim, Daiichi‐Sankyo, Bayer, Pfizer, Bristol‐Myers Squibb, Merck, Janssen Pharmaceuticals, and Portola Pharmaceuticals. No other potential conflict of interest relevant to this article was reported. | |
| Notes | Results were presented for all participants with a VTE but specific recurrent VTE data for the subset of participants with a PE were available in the supplementary material | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed with the use of an interactive voice‐response system" Comment: study judged at low risk of selection bias. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was performed with the use of an interactive voice‐response system" Comment: study judged at low risk of selection bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double blind. Patients were assigned to receive apixaban tablets plus placebo enoxaparin injections and placebo warfarin tablets or conventional therapy with enoxaparin injections and warfarin tablets plus placebo apixaban tablets. The study used blinded INR monitoring with a point‐of‐care device that generated an encrypted code for INR results. Investigators reported the code to the interactive voice‐response system and received either an actual INR value (for patients assigned to warfarin) or a sham INR value (for patients receiving apixaban)" Comment: study judged at low risk of performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "An independent committee, whose members were unaware of the study‐group assignments, adjudicated the qualifying diagnosis, the anatomical extent of the initial deep vein thrombosis or pulmonary embolism, and all suspected outcomes." Comment: study judged at low risk of detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | A number of randomised participants were inappropriately excluded from the ITT analysis. Additionally, 144/377 of apixaban participants and 142/413 participants given conventional treatment were classified as discontinuing for "other reasons", with no explanations given. Therefore we deemed the risk of attrition bias to be unclear. |
| Selective reporting (reporting bias) | High risk | Study protocol was available. Minor bleeding was a pre‐defined secondary outcome but the data on this outcome were not reported in the paper. Therefore we deemed the risk of reporting bias to be high. |
| Other bias | Low risk | We did not find any methodological issues that might directly lead to a risk of bias. |
AMPLIFY‐J 2015.
| Study characteristics | ||
| Methods | Study design: a randomised, active‐controlled, open‐label study Duration of study: 5.5 months | |
| Participants | Setting: hospital Country: Japan Number of participants: 80 (PE 35, other VTE 45); apixaban 40 (PE 18, other VTE 22), UFH/warfarin 40 (PE 17, other VTE 23) Age, mean (SD) years: apixaban 64.3 (13.40) years, UFH/warfarin 66.1 (17.72) years Sex: apixaban: 22 M/18 F, UFH/warfarin: 17 M/23 F Inclusion criteria: Japanese patients, ≥ 20 years of age and who had objectively confirmed, symptomatic proximal DVT or PE (with or without DVT). Proximal DVT was defined as thrombosis involving at least the popliteal vein or a more proximal vein. Exclusion criteria: people were excluded if they had thrombectomy or used fibrinolytic agent, had active bleeding, a high risk of bleeding, or other contraindications to treatment with UFH and warfarin; if they had another indication for long‐term anticoagulation therapy, dual antiplatelet therapy, or treatment with aspirin > 165 mg daily. Other key exclusion criteria were > 2 doses of fondaparinux, or continuous infusion of UFH > 36 hours, and > 2 doses of oral VKA before first administration of the study drug. Additional exclusion criteria were haemoglobin < 9 g/dL, platelet count < 100,000/mm3, and creatinine clearance < 25 mL/min. | |
| Interventions | Intervention 1: received apixaban 10 mg twice daily for 7 days as an initial therapy, followed by apixaban 5 mg twice daily for 23 weeks as long‐term therapy Intervention 2: given a continuous IV infusion of UFH to maintain the activated partial thromboplastin time in the range 1.5–2.5‐fold the control value. Warfarin was also concomitantly administered. UFH was continuously given until the effect of warfarin was stabilised; after which, participants were given warfarin alone. UFH was given for at least 5 days consecutively and was discontinued at once if the PT‐INR was ≥ 1.5. If PT‐INR exceeded 2.0 within the initial 5 days of administration, UFH could be discontinued based on the investigator’s judgment. The warfarin dose was adjusted to maintain INR between 1.5 and 2.5 in accordance with Japan PE/DVT treatment guidelines. Treatment was administered for 24 weeks (5.5 months). Follow‐up: 0, 2, 12, 24 and 28 weeks | |
| Outcomes | Primary: the incidence of the adjudicated composite of ISTH‐defined major bleeding and CRNM bleeding during the treatment period. Secondary: the incidence of the adjudicated ISTH major bleeding events and all bleeding events (ISTH major, CRNM, and minor) during the treatment period, composite endpoint of adjudicated recurrent symptomatic VTE (non‐fatal DVT or non‐fatal PE) or VTE‐related death during 24 weeks, thrombotic burden deterioration at 2, 12 and 24 weeks | |
| Funding | Quote: "This study was funded by Pfizer Inc and Bristol‐Myers Squibb." Comment: Pfizer Inc and Bristol‐Myers Squibb were the pharmaceutical companies that developed apixaban, and the results of the primary outcome favoured the apixaban group. It is possible that this may have influenced the report of outcomes. |
|
| Declarations of interest | Quote: "M. Nakamura has received remuneration from Daiichi Sankyo, Bayer Yakuhin. M. Nishikawa has received remuneration and research funds from Daiichi Sankyo. I. Komuro has received remuneration from Daiichi Sankyo, Nippon Boehringer Ingelheim, and scholarship funds from Astellas Pharma, Daiichi Sankyo, Takeda Pharmaceutical, Nippon Boehringer Ingelheim, Bristol‐Myers Squibb. I. Kitajima has received remuneration from Nippon Boehringer Ingelheim. H.O. has received remuneration from AstraZeneca, Bayer Yakuhin, Boehringer Ingelheim Japan, Bristol‐Myers Squibb, Daiichi Sankyo, Mitsubishi Tanabe Pharma, MSD, Pfizer Japan, Sanofi, Takeda Pharmaceutical and Teijin Pharma, and has received research funds from Bayer Yakuhin, Daiichi Sankyo and Novartis Pharma, and has received scholarship funds from Astellas Pharma, AstraZeneca, Bristol‐Myers Squibb, Chugai Pharmaceutical, Daiichi Sankyo, Dainippon Sumitomo Pharma, Kowa, MSD, Otsuka Pharmaceutical, Pfizer Japan, Sanofi, Shionogi and Takeda Pharmaceutical. Y.U., T.Y., H.M., R.Y. have no conflict of interest." | |
| Notes | Study characteristics were presented for all participants with a VTE but specific recurrent PE, recurrent VTE, all‐cause mortality, and major bleeding with a PE were available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An interactive voice response system was used for randomisation" Comment: study judged at low risk of selection bias. |
| Allocation concealment (selection bias) | Low risk | Quote: "An interactive voice response system was used for randomisation" Comment: study judged at low risk of selection bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Open label" Comment: blinding of participants and personnel was not conducted. However, review authors judged that the lack of blinding was unlikely to have affected the outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "all outcome events were adjudicated by an event adjudication committee in a blinded manner so as to maintain validity of assessment" Comment: blinding was performed adequately; study judged at low risk of detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In the apixaban group, 3 did not complete the overall trial period (2 no longer willing to participant in study, 1 had other reason). In the UFH/warfarin group, 2 did not complete the overall trial period (1 withdrew from the study prior to the initiation of study treatment, 1 had other reason). Comment: fewer than 20% of participants dropped out or withdrew and the study author performed ITT analysis; study judged at low risk of attrition bias. |
| Selective reporting (reporting bias) | Low risk | All the study's pre‐specified outcomes were reported in the pre‐specified way. Study judged at low risk of reporting bias. |
| Other bias | Low risk | We did not find any methodological issues that might directly lead to a risk of bias. |
Caravaggio 2020.
| Study characteristics | ||
| Methods | Study design: multinational, randomised, controlled, investigator‐initiated, open‐label, non‐inferiority trial Duration of study: 6 months | |
| Participants | Setting: multicentre Country: multinational (119 centres in 11 countries: Belgium, France, Germany, Israel, Italy, Poland, Portugal, Spain, the Netherlands, United Kingdom, United States of America) Number of participants: 1155 (PE 638, other VTE 517); apixaban 576 (PE 304, other VTE 272), dalteparin 579 (PE 334, other VTE 245) Age, mean (SD) years: apixaban 67.2 (11.3 ) years, dalteparin 67.2 (10.9) years Sex: apixaban: 292 M/284 F; dalteparin: 276 M/303 F Inclusion criteria: consecutive adults with cancer who had a newly diagnosed symptomatic or incidental proximal lower‐limb DVT or PE were eligible to participate in the trial. People with confirmed cancer other than basal‐cell or squamous‐cell carcinoma of the skin, primary brain tumour, known intracerebral metastases, or acute leukaemia were eligible to participate in the trial. Exclusion criteria: patients’ clinical characteristics, issues related to anticoagulant treatment, bleeding risk, and standard issues from clinical trials of anticoagulant agents | |
| Interventions | Intervention 1: apixaban was given orally at a dose of 10 mg twice daily for the first 7 days and 5 mg twice daily thereafter. Treatment was administered for 6 months. Intervention 2: dalteparin was given subcutaneously at a dose of 200 IU/kg of body weight once daily for the first month, after which the dose was reduced to 150 IU/kg daily. The maximum daily dose allowed for dalteparin was 18,000 IU. Treatment was administered for 6 months Follow‐up: trial visits were scheduled at enrolment and at 4 weeks, 3 months, 6 months, and 7 months after randomisation | |
| Outcomes | Primary: recurrent VTE, recurrent DVT, recurrent PE, fatal PE, major bleeding, major gastrointestinal bleeding, major non‐gastrointestinal bleeding Secondary: recurrent VTE or major bleeding, clinically relevant non‐major bleeding, major or clinically relevant non‐major bleeding, death from any cause, event‐free survival | |
| Funding | Quote: "Supported by the Bristol‐Myers Squibb–Pfizer Alliance." Comment: the study was supported by the Bristol‐Myers Squibb–Pfizer Alliance, the pharmaceutical companies that developed apixaban and dalteparin respectively, and the results of the primary outcome supported the non‐inferiority hypothesis of apixaban. It is possible that this may have influenced the report of outcomes. |
|
| Declarations of interest | Quote: "Dr. Agnelli reports receiving lecture fees from Pfizer and Bayer Healthcare and serving as chair of a registry for Daiichi Sankyo; Dr. Becattini, receiving lecture fees and consulting fees from Bayer Healthcare, Bristol‐Myers Squibb, and Daiichi Sankyo; Dr. Meyer, receiving grant support and travel support from Leo Pharma, Bristol‐Myers Squibb–Pfizer, Stago, and Bayer Healthcare; Dr. Muñoz, receiving grant support, consulting fees, lecture fees, advisory board fees, and travel support from Sanofi and Celgene, lecture fees and advisory board fees from AstraZeneca, Servier, Bristol‐Myers Squibb–Pfizer, Daiichi Sankyo, Bayer, and Merck Sharp & Dohme, lecture fees, advisory board fees, and travel support from Roche, grant support, lecture fees, and advisory board fees from Leo Pharma, advisory" | |
| Notes | Results were presented for all participants with a VTE, but specific recurrent VTE and major bleeding data for the subset of participants with a PE were available in the supplementary material. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was centrally performed through an interactive online system" Comment: study judged at low risk of selection bias. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was centrally performed through an interactive online system" Comment: study judged at low risk of selection bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Open label" Comment: blinding of participants and personnel was not conducted. However, review authors judged that the lack of blinding was unlikely to have affected the outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "blinded adjudication of the outcomes" Comment: blinding was performed adequately, study judged at low risk of detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In the apixaban group, 177 did not complete the overall trial period (137 died, 12 were lost to follow‐up, 28 had other reasons). In the dalteparin group, 197 did not complete the overall trial period (149 died, 8 were lost to follow‐up, 40 had other reasons). Comment: fewer than 20% of participants dropped out or withdrew, and the study author performed ITT analysis. Study judged at low risk of attrition bias. |
| Selective reporting (reporting bias) | High risk | Study protocol was available. Quality of life was a predefined secondary outcome, but the data on this outcome were not reported in the paper. In addition, it was stated that a "significant interaction was noted between age subgroups and treatment for recurrent venous thromboembolism" but no result was found in the paper and appendix. Therefore, the risk of reporting bias was deemed to be high. |
| Other bias | Low risk | We did not find any methodological issues that might directly lead to a risk of bias. |
EINSTEIN‐PE 2012.
| Study characteristics | ||
| Methods | Study design: randomised, open‐label, event‐driven, non‐inferiority trial Duration of study: 12 months | |
| Participants | Setting: hospital Country: multinational (263 centres in 36 countries: Andorra, Australia, Austria, Belgium, Brazil, Canada, China, Czech Republic, Denmark, Estonia, Finland, France, Germany, China Hong Kong, Hungary, India, Indonesia, Ireland, Israel, Italy, Korea, Latvia, Lithuania, Malaysia, the Netherlands, New Zealand, Norway, Philippines, Poland, Singapore, South Africa, Spain, Sweden, Switzerland, China Taiwan, Thailand, United Kingdom, USA) Number of participants: 4832 (all are PE); rivaroxaban 2419, warfarin 2413 Age, mean (SD) years: rivaroxaban 57.9 (7.3) years, warfarin 57.5 (7.2) years Sex: rivaroxaban 1309 M/1110 F, warfarin 1247 M/1166 F Inclusion criteria: aged 18 or older with an acute, symptomatic PE with objective confirmation, with or without symptomatic DVT Exclusion criteria: contraindications to heparin or warfarin, had received treatment for more than 48 hours with therapeutic doses of heparin, had received more than one dose of a VKA, had cancer for which long‐term treatment with LMWH was anticipated, had another indication for warfarin therapy, continued to receive treatment with aspirin at a dose of more than 100 mg daily or dual antiplatelet therapy, or had a creatinine clearance of less than 30 mL per minute | |
| Interventions | Intervention 1: oral rivaroxaban 15 mg twice daily for the first 3 weeks, followed by 20 mg once daily Intervention 2: enoxaparin 1.0 mg/kg of body weight twice daily and either warfarin or acenocoumarol, started within 48 hours of randomisation. Enoxaparin was discontinued when the INR was 2.0 or more for 2 consecutive days and the participants had received at least 5 days of enoxaparin treatment. The dose of VKA was adjusted to maintain an INR of 2.0 to 3.0, determined at least once a month Follow‐up: 3, 6, and 12 months | |
| Outcomes | Primary: "symptomatic recurrent VTE, defined as a composite of DVT or fatal or non‐fatal PE and clinically relevant bleeding, defined as a composite of major or clinically relevant non‐major bleeding. Death was classified as PE, bleeding or other established diagnoses. PE was considered the cause of death if there was objective documentation of the condition or if death could not be attributed to a documented cause and PE could not be confidently ruled out. Bleeding was defined as major if it was clinically overt and associated with a decrease in the haemoglobin level of 2.0 g per decilitre or more, if bleeding led to the transfusion of 2 or more units of red blood cells, or if bleeding was intracranial or retroperitoneal, occurred in another critical site, or contributed to death. CRNM bleeding was defined as overt bleeding that did not meet the criteria for major bleeding but was associated with medical intervention, unscheduled contact with a physician, interruption or discontinuation of a study drug, or discomfort or impairment of activities of daily life" Secondary: major bleeding, death from any cause, vascular events (acute coronary syndrome, ischaemic stroke, transient ischaemic attack or systemic embolism) and net clinical benefit (defined as a composite of the primary efficacy outcome and major bleeding, as assessed in the intention‐to‐treat population) | |
| Funding | Quote: "Supported by Bayer HealthCare and Janssen Pharmaceuticals." Comment: the study was funded by Bayer HealthCare, the pharmaceutical company that developed rivaroxaban. It is possible that this may have influenced the timeframe of reported safety outcomes. |
|
| Declarations of interest | "Dr. Agnelli reports receiving consulting fee, travel support, payment for writing or reviewing the manuscript, and lecture fees from Ictom/Bayer Healthcare. Dr. Berkowitz reports receiving travel support and employment from Bayer HealthCare Pharmaceuticals. Dr. Bounameaux reports receiving consulting fee, travel support, fees for participation in review activities, lecture fees and board membership from ICTOM/Bayer Healthcare, Pfizer, sanofi‐aventis, GlaxoSmithKline, Boehringer‐Ingelheim, and Daiichi‐Sankyo. Dr. Buller reports receiving consulting fee, travel support, fees for participation in review activities, payment for writing or reviewing the manuscript, and Board membership from ICTOM/Bayer Healthcare, Pfizer, sanofi‐aventis, GlaxoSmithKline, Boehringer‐Ingelheim, and Daiichi‐Sankyo. Dr. Chlumsky reports receiving consulting fee, travel support, fees for participation in review activities, and other fee from ICTOM/Bayer Healthcare. Dr. Cohen reports receiving consulting fee, travel support, fees for participation in review activities, payment for writing or reviewing the manuscript, and lecture fees from ICTOM/Bayer Healthcare, Astellas,AstraZeneca, Daiichi, Johnson and Johnson, Pfizer and other companies. Dr. Davidson reports receiving travel support, and fees for participation in review activities from Bayer. Dr. Decousus reports receiving fees for participation in review activities, payment for writing or reviewing the manuscript from Bayer. Dr. Gallus reports receiving consulting fee, travel support, fees for participation in review activities, payment for writing or reviewing the manuscript, from ICTOM/Bayer Health Care AG. Dr. Gallus reports receiving consulting fee, travel support, fees for participation in review activities, payment for writing or reviewing the manuscript from ICTOM/Bayer Health Care AG. Dr. Jacobson reports receiving consulting fee, travel support, fees for participation in review activities, payment for writing or reviewing the manuscript from Ictom/Bayer HealthCare AG. Dr. Lensing reports receiving travel support and employment from Bayer Healthcare. Dr. Minar reports receiving consulting fee, travel support, fees for participation in review activities, and lecture fees from BayerHealthcare/ ICTOM. Dr. Misselwitz is an employee of Bayer, the sponsor of the trial. Dr. Prins reports receiving consulting fee, travel support, payment for writing or reviewing the manuscript from ICTOM/BayerHeatltcare. Dr. Raskob reports receiving consulting fee, travel support, fees for participation in review activities, payment for writing or reviewing the manuscript, lecture fees, and payment for manuscript preparation from ICTOM/Bayer Healthcare, Daiichi Sankyo, BMS, and Pfizer. Dr. Schellong reports receiving consulting fee, travel support, fees for participation in review activities, payment for writing or reviewing the manuscript, lecture fees, and payment for development of educational presentations from ICTOM/Bayer Healthcare, Boehringer Ingelheim, Daiichi Sankyo, and other companies. Dr. Segers reports receiving consulting fee, travel support, fees for participation in review activities, payment for writing or reviewing the manuscript from Bayer Healthcare. Dr. Verhamme reports receiving consulting fee, travel support, fees for participation in review activities, payment for writing or reviewing the manuscript, lecture fees from ICTOM/Bayer Healthcare, Boehringer Ingelheim, Sanofi‐Aventis, and other companies. Dr. Verhamme reports receiving consulting fee, travel support, fees for participation in review activities, payment for writing or reviewing the manuscript, lecture fees from ICTOM/Bayer Healthcare, Boehringer Ingelheim, Pfizer." Disclosure forms provided by the authors are available at: https://www.nejm.org/doi/suppl/10.1056/NEJMoa1113572/suppl_file/nejmoa1113572_disclosures.pdf |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed with the use of a computerised voice‐response system" Comment: study judged at low risk of selection bias. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was performed with the use of a computerised voice‐response system" Comment: study judged to be at a low risk of selection bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Open‐label" Comment: only one dose of rivaroxaban was given and as the comparison was enoxaparin/VKA, blinding of participants and personnel was not possible. However, we judged that the lack of blinding in the control group was unlikely to have affected the outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A central committee whose members were unaware of the study‐group assignments adjudicated the results of all baseline lung‐imaging tests and all suspected outcome events" Comment: study judged to be at low risk of detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In the rivaroxaban group, 1 was excluded because of invalid informed consent, 7 did not receive rivaroxaban. In the standard therapy group, 8 did not receive standard therapy. Comment: fewer than 20% of participants dropped out or withdrew, and the study author performed ITT analysis. Study judged at low risk of attrition bias. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study's pre‐specified outcomes have been reported in the pre‐specified way. Study judged at low risk of reporting bias. |
| Other bias | Low risk | We did not find any methodological issues that might directly lead to a risk of bias. |
Hokusai VTE Cancer 2018.
| Study characteristics | ||
| Methods | Study design: a multinational, prospective, randomised, open‐label, blinded endpoint, non‐inferiority study Duration of study: 6 to 12 months | |
| Participants | Setting: multicentre Country: multinational (114 centres in 13 countries: Australia, Austria, Belgium, Canada, Czech Republic, France, Germany, Hungary, Italy, Netherlands, New Zealand, Spain, USA) Number of participants: 1046 (PE 657, other VTE 389); edoxaban 522 (PE 328, other VTE 194), dalteparin 524 (PE 329, other VTE 195) Age, mean (SD) years: edoxaban 64.3 (11.0) years, dalteparin 63.7 (11.7) years Sex: edoxaban: 277 M/245 F, dalteparin: 263 M/261 F Inclusion criteria: adults with cancer were eligible for inclusion in the trial if they had acute symptomatic or incidentally detected DVT involving the popliteal, femoral, or iliac vein or the inferior vena cava; acute symptomatic PE that was confirmed by means of diagnostic imaging; or incidentally detected PE involving segmental or more proximal pulmonary arteries Exclusion criteria: people who met any of the following criteria were not eligible for enrolment: thrombectomy, insertion of a caval filter, or use of a fibrinolytic agent to treat the current (index) episode of DVT and/or PE; more than 72 hours pre‐treatment with therapeutic dosages of anticoagulant treatment (LMWH, UFH, and fondaparinux per local labelling), oral direct anticoagulants or VKA prior to randomisation to treat the current (index) episode; treatment with therapeutic doses of an anticoagulant including dalteparin for an indication other than VTE prior to randomisation; active bleeding or any contraindication for treatment with LMWH/dalteparin or edoxaban; an ECOG Performance Status of 3 or 4 at the time of randomisation; calculated CrCL < 30 mL/min; history of heparin‐associated thrombocytopenia; acute hepatitis, chronic active hepatitis, liver cirrhosis; hepatocellular injury with concurrent transaminase (ALT/AST > 3 x ULN) and bilirubin (> 2 x ULN) elevations in the absence of a clinical explanation; life expectancy < 3 months; platelet count < 50,000/mL; uncontrolled hypertension as judged by the investigator (e.g. systolic BP > 170 mmHg or diastolic BP > 100 mmHg despite antihypertensive treatment); women of childbearing potential without proper contraceptive measures, and women who are pregnant or breastfeeding. | |
| Interventions | Intervention 1: edoxaban was administered orally at a fixed dose of 60 mg once daily, with or without food. It was administered at a lower dose (30 mg once daily) in participants with a creatinine clearance of 30 mL to 50 mL per minute or a body weight of 60 kg or less or in those receiving concomitant treatment with potent P‐glycoprotein inhibitors. Treatment was administered for 6 to 12 months; median duration was 7 months. Intervention 2: dalteparin was given subcutaneously at a dose of 200 IU/kg of body weight once daily for 30 days, with a maximum daily dose of 18,000 IU. Thereafter, dalteparin was given at a dose of 150 IU/kg once daily. If the platelet count declined to less than 100,000 per μL during treatment, the dose of dalteparin was temporarily reduced. Treatment was administered for 6 to 12 months; median duration was 6 months. Follow‐up: participants underwent assessment, in the clinic or by telephone, on day 31 after randomisation and at months 3, 6, 9, and 12. | |
| Outcomes |
Primary: a composite of recurrent venous thromboembolism or major bleeding; death Secondary: recurrent VTE; major bleeding; clinically relevant non‐major (CRNM) bleeding; major + CRNM bleeding; event‐free survival, VTE‐related death, mortality from all causes, recurrent DVT, recurrent PE |
|
| Funding | Quote: "Funded by Daiichi Sankyo." Comment: this study was funded by Daiichi Sankyo, the pharmaceutical company that developed edoxaban, and the result of the primary outcome supported the non‐inferiority hypothesis of edoxaban. It is possible that this may have influenced the report of outcomes. |
|
| Declarations of interest | Quote: "Dr. Buller reports personal fees from Daiichi‐Sankyo, during the conduct of the study; personal fees from Bayer Healthcare, personal fees from BMS/Pfizer, personal fees from Boehringer‐Ingelheim, personal fees from Portola, personal fees from Medscape, personal fees from Eli Lilly, personal fees from Sanofi Aventis, and personal fees from Ionis outside the submitted work. Dr. Carrier reports personal fees from Daiichi Sankyo during the conduct of the study; grants and personal fees from BMS, grants and personal fees from LEO Pharma, personal fees from Pfizer, personal fees from Sanofi, and personal fees from Bayer outside the submitted work. Dr. Di Nisio reports personal fees from Daiichi Sankyo outside the submitted work. Dr. Garcia reports grants and personal fees from Daiichi Sankyo during the conduct of the study; personal fees from BMS, personal fees from Boehringer‐Ingelheim, grants and personal fees from Janssen, personal fees from Pfizer, personal fees from Medscape, and grants and personal fees from Incyte outside the submitted work. Dr. Garcia reports grants and personal fees from Daiichi Sankyo during the conduct of the study; personal fees from BMS, personal fees from Boehringer‐Ingelheim, grants and personal fees from Janssen, personal fees from Pfizer, personal fees from Medscape, and grants and personal fees from Incyte outside the submitted work. Dr. Kakkar reports personal fees from Daiichi Sankyo during the conduct of the study; grants and personal fees from Bayer AG, personal fees from Boehringer‐Ingelheim, personal fees from Janssen Pharma, personal fees from Sanofi SA, and personal fees from Verseon outside the submitted work. Dr. Kovacs reports grants and personal fees from Pfizer, grants and personal fees from Bayer, grants from Daiichi Sankyo Pharma, and grants from Bristol Meyers Squibb outside the submitted work. Dr. Mercuri reports personal fees from Daiichi‐Sankyo, outside the submitted work. Dr. Meyer reports non‐financial support from Leo Pharma, grants and non‐financial support from BMS‐Pfizer, non‐financial support from Stago, and non‐financial support from Bayer Healthcare outside the submitted work. Dr. Raskob reports personal fees from Daiichi Sankyo during the conduct of the study; personal fees from Bayer Healthcare, personal fees from BMS, personal fees from Boehringer‐Ingelheim, personal fees from Eli Lilly, personal fees from Janssen, personal fees from Johnson and Johnson, personal fees from Pfizer, personal fees from Portola, personal fees from Merck, and personal fees from Medscape outside the submitted work. Dr. Segers reports grants from Daiichi Sankyo during the conduct of the study; grants from IONIS Pharmaceuticals, grants from Daiichi Sankyo, and grants from Janssen Pharmaceuticals outside the submitted work. Dr. Shi reports personal fees from Daiichi Sankyo outside the submitted work. Dr. Verhamme reports grants and personal fees from Daiichi Sankyo, during the conduct of the study; grants and personal fees from Bayer Healthcare, personal fees from BMS, grants and personal fees from Boehringer‐Ingelheim, personal fees from Portola, personal fees from Medscape, grants and personal fees from LeoPharma, grants from Sanofi, personal fees from Medtronic, personal fees from Pfizer, outside the submitted work. Dr. Wang reports non‐financial support from Daiichi Sankyo during the conduct of the study. Dr. Weitz reports personal fees from Daiichi‐Sankyo, during the conduct of the study; personal fees from Bayer Healthcare, personal fees from BMS, personal fees from Boehringer‐Ingelheim, personal fees from Ionis Pharmaceuticals, personal fees from Janssen, personal fees from Johnson and Johnson, personal fees from Pfizer, personal fees from Portola, personal fees from Medscape, personal fees from Novartis outside the submitted work. Dr. Yeo reports grants and personal fees from Daiichi Sankyo during the conduct of the study; personal fees from Bayer Healthcare, personal fees from Pfizer, personal fees from Boerhringer Ingelheim, personal fees from Sanofi, and personal fees from Leo Pharma outside the submitted work. Dr. Zhang reports personal fees from Daiichi Sankyo outside the submitted work. Dr. Zhang reports personal fees from Daiichi Sankyo outside the submitted work." Disclosure forms provided by the authors are available at: www.nejm.org/doi/suppl/10.1056/NEJMoa1711948/suppl_file/nejmoa1711948_disclosures.pdf |
|
| Notes | Study characteristics were presented for all participants with a VTE, but specific recurrent VTE and major bleeding with a PE were available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed with the use of an interactive Web‐based system" Comment: study judged at low risk of selection bias. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed with the use of an interactive Web‐based system" Comment: study judged at low risk of selection bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Open label" Comment: blinding of participants and personnel was not conducted. However, review authors judged that the lack of blinding was unlikely to have affected the objective outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "all events were adjudicated by a committee whose members were unaware of the treatment assignments" Comment: blinding was performed adequately; study judged at low risk of detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In the edoxaban group, 16 did not complete the overall trial period (3 did not receive the assigned treatment, 10 withdrew consent, 3 were lost to follow‐up). In the dalteparin group, 18 did not complete the overall trial period (1 did not receive the assigned treatment, 12 withdrew consent, 5 were lost to follow‐up). Comment: fewer than 20% of participants dropped out or withdrew, and the study author performed ITT analysis. Study judged at low risk of attrition bias. |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes were reported in the pre‐specified way. Study judged at low risk of reporting bias. |
| Other bias | Low risk | We did not find any methodological issues that might directly lead to a risk of bias. |
Hokusai‐VTE 2013.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, non‐inferiority study Duration of study: 12 months | |
| Participants | Setting: multicentre Country: multinational (439 centres in 36 countries: Argentina, Australia, Austria, Belarus, Belgium, Brazil, Canada, China, Czech Republic, Denmark, Estonia, France, Germany, Hungary, India, Israel, Italy, Japan, Korea, Mexico, the Netherlands, New Zealand, Norway, Philippines, Poland, Russia, Singapore, South Africa, Spain, Sweden, Switzerland, China Taiwan, Thailand, Turkey, Ukraine, United Kingdom, USA) Number of participants: 3319 (all are PE); edoxaban 1650, warfarin 1669 Age, mean (SD) years: edoxaban 57.1 (16.6) years, warfarin 57.4 (16.5) years Sex: edoxaban 863 M/787 F, warfarin 875 M/794 F Inclusion criteria: people aged 18 or older who had objectively diagnosed, acute, symptomatic DVT involving the popliteal, femoral or iliac veins or acute, symptomatic PE (with or without DVT) Exclusion criteria: contraindications to heparin or warfarin, had received treatment for more than 48 hours with therapeutic doses of heparin, had received more than one dose of a VKA, had cancer for which long‐term treatment with LMWH was anticipated, had another indication for warfarin therapy, continued to receive treatment with aspirin at a dose of more than 100 mg daily or dual antiplatelet therapy, or had a creatinine clearance of less than 30 mL per minute | |
| Interventions | Intervention 1: oral edoxaban 60 mg once daily or 30 mg once daily in participants with a creatinine clearance of 30 mL to 50 mL per minute or a body weight of 60 kg or less or in participants who were receiving concomitant treatment with potent P‐glycoprotein inhibitors Intervention 2: dose‐adjusted warfarin therapy to achieve an INR of 2.0 to 3.0 and edoxaban‐like placebo Follow‐up: days 5, 12, 30, and 60 after randomisation, monthly while on study drug or every 3 months after discontinuing the study drug and finally at 12 months | |
| Outcomes | Primary: incidence of symptomatic recurrent VTE (DVT and fatal or non‐fatal PE), clinically relevant bleeding (major or clinically relevant non major) Secondary: none | |
| Funding | Quote: "Funded by Daiichi‐Sankyo." Comment: the study was funded by Daiichi‐Sankyo, the pharmaceutical company that developed edoxaban. It is possible that this may have influenced the timeframe of reported safety outcomes. |
|
| Declarations of interest | Quote: "Dr. Büller reports receiving consulting fees from Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Isis Pharmaceuticals, and ThromboGenics, and grant support from Bayer and Pfizer. Dr. Decousus reports receiving fees for board membership from Bayer and Daiichi Sankyo, lecture fees from GlaxoSmithKline, and grant support from Bayer, Bristol‐Myers Squibb–Pfizer, Boehringer Ingelheim, and Portola. Drs. Grosso, Mercuri, Schwocho, and Shi report being employees of Daiichi Sankyo. Dr. Middeldorp reports receiving consulting fees from Bayer and Bristol‐Myers Squibb–Pfizer, lecture fees from Bayer, GlaxoSmithKline, Bristol‐Myers Squibb–Pfizer, and Boehringer Ingelheim, and grant support from GlaxoSmithKline, Bristol‐Myers Squibb–Pfizer, and Sanquin. Dr. Prins reports receiving consulting fees from Bayer, Pfizer, and Boehringer Ingelheim, and lecture fees from Bayer. Dr. Raskob reports receiving consulting fees and travel support from Bayer, Bristol‐Myers Squibb, Janssen, Johnson & Johnson, Pfizer, Sanofi‐Aventis, and Takeda. Dr. Schellong reports receiving consulting fees from Bayer and Boehringer Ingelheim, and lecture fees from Bayer, Boehringer Ingelheim, and Bristol‐Myers Squibb–Pfizer. Dr. Segers reports receiving fees for the scientific management of the studies as director of the International Clinical Trial Organization and Management (ICTOM) academic research organization from Bayer, Isis Pharmaceuticals, and Pfizer. Dr. Verhamme reports receiving consulting fees from Bayer, Boehringer Ingelheim, ThromboGenics, and Pfizer, lecture fees from Bayer, Boehringer Ingelheim, Leo Pharma, Sanofi‐Aventis, and Pfizer, and grant support from Bayer, Boehringer Ingelheim, Leo Pharma, and Sanofi‐Aventis. Dr. Wells reports receiving lecture fees from Bayer, Boehringer Ingelheim, Biomerieux, and Bristol‐Myers Squibb–Pfizer. No other potential conflict of interest relevant to this article was reported." | |
| Notes | We successfully contacted study authors for outcome data, including the rate of recurrent pulmonary embolism, deep vein thrombosis, and major bleeding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed with the use of an interactive Web‐base system" Comment: study judged at low risk of selection bias. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was performed with the use of an interactive Web‐base system" Comment: study judged to be at a low risk of selection bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Edoxaban or warfarin was administered in a double‐blind fashion" Comment: study judged to be at a low risk of performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "An independent committee, whose members were unaware of the study‐group assignments, adjudicated all suspected outcome and the results of baseline imaging tests and assessed the anatomical extent of thrombosis" Comment: study judged to be at a low risk of performance bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In the edoxaban group, 25 did not receive heparin–edoxaban, 181 did not complete the overall study period, 132 died, 36 withdrew consent, 7 were lost to follow‐up, 6 had other reasons. In the warfarin group, 27 did not receive heparin–warfarin, 167 did not complete the overall study period, 126 died, 34 withdrew consent, 4 were lost to follow‐up, 3 had other reasons. Comment: fewer than 20% of participants dropped out or withdrew, and the study author performed ITT analysis. Study judged at low risk of attrition bias. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study's pre‐specified outcomes have been reported in the pre‐specified way. Study judged at low risk of reporting bias. |
| Other bias | Low risk | We did not find any methodological issues that might directly lead to a risk of bias. |
J‐EINSTEIN DVT and PE 2015.
| Study characteristics | ||
| Methods | Study design: an open‐label, randomised trial Duration of study: 6.5 months | |
| Participants | Setting: multicentre Country: Japan (30 centres) Number of participants: 97 (PE 40, other VTE 57); rivaroxaban 78 (PE 33, other VTE 45), UFH (UFH)/warfarin 19 (PE 7, other VTE 12) Age, mean (SD) years: rivaroxaban (10 mg twice daily/15 mg once daily): 65.0 (9.9) years; rivaroxaban (15 mg twice daily/15 mg once daily): 68.8 (12.2) years; UFH/warfarin: 63.4 (18.3) years Sex: rivaroxaban (10 mg twice daily/15 mg once daily): 16 M/7 F; rivaroxaban (15 mg twice daily/15 mg once daily): 25 M/30 F; UFH/warfarin: 10 M/9 F Inclusion criteria: people older than 20 years who had acute, objectively confirmed symptomatic proximal DVT and/or PE Exclusion criteria: people were excluded if they had received heparin or fondaparinux treatment for longer than 48 hours or more than a single dose of warfarin. Other exclusion criteria were: thrombectomy, insertion of a caval filter, or use of a fibrinolytic agent for the current episode; any contraindication listed in the local labelling of UFH or warfarin or another indication for the use of UFH or warfarin; a creatinine clearance < 30 mL/min; significant hepatic disease or ALT > 3 times ULN; bacterial endocarditis; active bleeding or a high risk of bleeding contraindicating treatment with UFH or warfarin; a systolic BP of more than 180 mm Hg or a diastolic BP of more than 110 mm Hg; childbearing potential without proper contraceptive measures, pregnancy, or breast‐feeding; concomitant use of strong cytochrome P450 3A4 inhibitors (i.e. azole‐antimycotics or HIV protease inhibitors); and a life expectancy of fewer than 3 months. | |
| Interventions | Intervention 1: participants received rivaroxaban 15 mg twice daily for a total of 3 weeks in a double‐blind fashion, followed by open‐label rivaroxaban 15 mg once daily. Treatment was continued for 3, 6, or 12 months, as decided by the treating physician. The mean treatment duration was 195 days. Intervention 2: participants assigned to control treatment received IV UFH, with the dose adjusted to prolong the APPT to 1.5–2.5‐fold that of controls, for at least 5 days, overlapping with and followed by INR (range 1.5–2.5)‐titrated warfarin. UFH was discontinued when the INR was 1.5 or more for 2 consecutive measurements at least 24 hours apart. Initially, the INR was measured every 2 to 3 days and, when stable, at least once per month. Treatment was continued for 3, 6, or 12 months, as decided by the treating physician. The mean treatment duration was 200 days. Follow‐up: day 22 and at the end of the 3, 6, or 12 months' intended treatment period. | |
| Outcomes | Primary: the occurrence of symptomatic recurrent VTE or asymptomatic deterioration Secondary: venous ultrasound and spiral CT, major bleeding, CRNM bleeding | |
| Funding | Quote: "The program was sponsored by Bayer Yakuhin Ltd." Comment: Bayer Yakuhin Ltd, Japan developed rivaroxaban. Some authors received funding from some pharmaceutical companies. The results showed a similar efficacy and safety profile with rivaroxaban and control treatment. It is possible that this may have influenced the report of outcomes. |
|
| Declarations of interest | Quote: "Bayer Yakuhin supported this study, was involved in the design of the trial, and collected and analysed the data. MHP has received research support and honoraria, and has participated in advisory boards for Bayer HealthCare, Sanofi‐Aventis, Boehringer Ingelheim, GlaxoSmithKline, Daiichi Sankyo, LEO Pharma, ThromboGenics, and Pfizer. AWAL, MKato, JO, YM, KI, and MKajikawa are employees of Bayer HealthCare Pharmaceuticals. NY has received honoraria for oral presentations from Daiichi Sankyo. AH has received research grants from Astellas Pharmaceuticals, AstraZeneka, MSD, Otsuka Pharmaceutical, Kissei Pharmaceutical, Kyowa Hakko Kirin, Kowa Pharmaceuticals, Sanofi, Daiichi Sankyo, Takeda Pharmaceuticals, Mitsubishi Tanabe Pharma, Boehringer Ingelheim, Nihon Medi‐Physics, and Bayer Yakuhin, and has received funding from Sanofi, Daiichi Sankyo, Toa Eiyo, Novartis, and Bayer Yakuhin for participation in clinical trials. AH has received funding for endowed courses from Otsuka Pharmaceutical, Fukuda Denshi, Hokushin Medical, Boston Scientific, and Vega Life Corporation. SS has received funding from Bayer Yakuhin, Daiichi Sankyo, Takeda Pharmaceuticals, Otsuka Pharmaceutical, Novartis Pharma, and Boehringer Ingelheim for participation in clinical trials. The other authors declare that they have no competing interests." | |
| Notes | Results were presented for all participants with a VTE but specific recurrent VTE data for the subset of participants with a PE were available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done centrally, using an interactive web response system" Comment: study judged at low risk of selection bias. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was done centrally, using an interactive web response system" Comment: study judged at low risk of selection bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Open label" Comment: blinding of participants and personnel was not conducted. However, review authors judged that the lack of blinding was unlikely to have affected the objective outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "use objective and validated tests to confirm suspected recurrent VTE and the use of an independent committee, whose members were blinded to treatment assignment to adjudicate outcome events" Comment: blinding was performed adequately; study judged at low risk of detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three participants from a single site were excluded from all analyses because of serious non‐compliance with the protocol/Good Clinical Practice guidelines. Comment: fewer than 20% of participants dropped out or withdrew, and the study author performed ITT analysis. Study judged at low risk of attrition bias. |
| Selective reporting (reporting bias) | Low risk | All the study's pre‐specified outcomes were reported in the pre‐specified way. Study judged at low risk of reporting bias. |
| Other bias | Low risk | We did not find any methodological issues that might directly lead to a risk of bias. |
MERCURY PE 2018.
| Study characteristics | ||
| Methods | Study design: randomised, open‐label, parallel‐group, multicenter study Duration of study: 3 months | |
| Participants | Setting: multicentre (35 hospitals) Country: USA Number of participants: 114 (all were PE); rivaroxaban 51, standard of care 63 Age, mean (SD) years: rivaroxaban 49.14 (13.3) years, standard of care 47.56 (17.2) years Sex: rivaroxaban 24M/27F, standard of care 31M/32F Inclusion criteria: adults presenting to the emergency department (ED) with objectively confirmed, low‐risk PE were eligible for enrolment. Low‐risk PE was defined by the absence of any Hestia criteria, adapted for emergency medicine by removing 24‐hour requirements. Exclusion criteria: people were excluded for a troponin level above the institutional upper reference level, contraindications to anticoagulation, or by investigator determination of barriers to treatment or follow‐up. Although the Hestia criteria exclude haemodynamically unstable patients, instability is not defined and was determined per the physician's judgment. | |
| Interventions | Intervention 1: participants randomised to early discharge on rivaroxaban were discharged within 24 hours of ED triage and were instructed to take rivaroxaban with food, 15 mg twice daily for 21 days and then 20 mg once daily to study completion. Treatment for 3 months Intervention 2: standard of care participants were treated per local protocol, which could include hospitalisation and any Food and Drug Administration (FDA)‐approved anticoagulant strategy, including rivaroxaban. If receiving warfarin, the target INR was 2.0 to 3.0, with testing per local protocol. Follow‐up: 30 days, 90 days | |
| Outcomes | Primary: total amount of time spent in the hospital, expressed in hours for venous thromboembolic or bleeding events, in the 30 days after randomisation. The primary safety outcome was major bleeding within 90 days. Secondary: prespecified secondary efficacy endpoints included 90‐day rates of new/recurrent VTE, VTE‐related death, unplanned hospital or physician office visits for VTE | |
| Funding | Quote: "Funding for this research was provided by Janssen Pharmaceuticals, Raritan, NJ" Comment: Janssen Pharmaceuticals, Raritan, NJ, manufactures rivaroxaban. The results showed a similar efficacy and safety profile with rivaroxaban and control treatment. It is possible that this may have influenced the report of outcomes. |
|
| Declarations of interest | Quote: "Consulting for commercial interests, including advisory board work‐WFP, CC, DD, and AS have received funding personally from Janssen for consulting. WFP, and CC have received funding personally from Bayer, AG. JK has received grant funding from Janssen for a separate study. Payment for writing independent of grant funding—No author received payment from for writing any part of this manuscript. Employment—PW and JX are employed by Janssen, which manufactures rivaroxaban. Institutional Grant Receipt—WFP, CC, DD, SF, CKa, CKe, JM, and AS’s institution has received funding from Janssen for this investigator‐initiated research. Miscellaneous—DD has been a member of the SAEM board of directors. Author Contributions: WFP—study concept and design, acquisition of the data, analysis and interpretation of the data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical expertise, and acquisition of funding. CC—acquisition of the data, analysis and interpretation of the data, critical revision of the manuscript for important intellectual content, and statistical expertise. DD—study concept and design, acquisition of the data, and critical revision of the manuscript for important intellectual content. SK—acquisition of the data and critical revision of the manuscript for important intellectual content. CKa—study concept and design, analysis and interpretation of the data, and critical revision of the manuscript for important intellectual content. CKe—acquisition of the data and critical revision of the manuscript for important intellectual content. JK—study concept and design and critical revision of the manuscript for important intellectual content. JM—acquisition of the data and critical revision of the manuscript for important intellectual content. PW—study concept and design, analysis and interpretation of the data, critical revision of the manuscript for important intellectual content, statistical expertise, and acquisition of funding. JX—analysis and interpretation of the data, critical revision of the manuscript for important intellectual content, and statistical expertise. AS—study concept and design, acquisition of the data, analysis and interpretation of the data, critical revision of the manuscript for important intellectual content, statistical expertise, and acquisition of funding." | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned in a 1:1 ratio to emergency department discharge on open‐label rivaroxaban or standard care (as determined by the attending physician) by an interactive Web within 12 hours of diagnosis." Comment: study judged at low risk of selection bias. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was centrally performed through an interactive online system." Comment: study judged at low risk of selection bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Open label, as participants could not be blinded to their own hospitalisation, to reduce observer bias clinical and safety endpoints were adjudicated by a panel blinded to treatment allocation." Comment: lack of blinding was unlikely to have affected the outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "As participants could not be blinded to their own hospitalisation, to reduce observer bias clinical and safety endpoints were adjudicated by a panel blinded to treatment allocation." Comment: blinding was performed adequately; study judged at low risk of detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In the rivaroxaban group, 7 dropouts (3 lost to follow‐up, 0 withdrew consent, 4 other reasons). In the standard of care group: 8 dropouts (4 lost to follow‐up, 2 withdrew consent, 2 other reasons). Comment: fewer than 20% of participants dropped out or withdrew, and the study author performed ITT analysis. Study judged at low risk of attrition bias. |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes were reported in the pre‐specified way. Study judged at low risk of reporting bias. |
| Other bias | Low risk | We did not find any methodological issues that might directly lead to a risk of bias. |
RE‐COVER 2009.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, double‐dummy non‐inferiority trial Duration of study: 6 months | |
| Participants | Setting: multicentre Country: multinational (228 clinical centres in 29 countries: USA, Argentina, Australia, Austria, Belgium, Brazil, Canada, Czech Republic, Denmark, France, Germany, Greece, Hungary, India, Israel, Italy, Mexico, the Netherlands, New Zealand, Norway, Portugal, Russian Federation, Slovakia, South Africa, Spain, Sweden, Turkey, Ukraine, United Kingdom) Number of participants: 2539 (PE 786, other VTE 1753); dabigatran 1273 (PE 391, other VTE 882), warfarin 1266 (PE 395, other VTE 871) Age, mean (range) years: dabigatran 55 (15.8) years, warfarin 54.4 (16.2) years Sex: dabigatran 738 M/535 F, warfarin 746 M/520 F Inclusion criteria: people aged ≥ 18 years who had acute, symptomatic, objectively verified proximal DVT of the legs or PE and for whom 6 months of anticoagulant therapy was considered an appropriate treatment Exclusion criteria: duration of symptoms > 14 days, PE with haemodynamic instability or requiring thrombolytic therapy, another indication for warfarin therapy, recent unstable cardiovascular disease, a high risk of bleeding, liver disease with an ALT level that was 2 x ULN range, an estimated creatinine clearance < 20 mL/minute, a life expectancy < 6 months, contraindication to heparin or to radiographic contrast material, pregnancy or risk of becoming pregnant, requirement for long‐term anticoagulant therapy | |
| Interventions | Intervention 1: oral dabigatran 150 mg twice daily and warfarin‐like placebo Intervention 2: dose‐adjusted warfarin therapy to achieve an INR of 2.0 to 3.0 and dabigatran‐like placebo Follow‐up: 6 months | |
| Outcomes | Primary: recurrent VTE evaluated using the same diagnostic methods used for the initial diagnosis Secondary: bleeding that was defined as major if it was clinically overt and if it was associated with a fall in the haemoglobin level ≥ 20 g/L, resulted in the need for transfusion of ≥ 2 units of red cells, involved a critical site, or was fatal. | |
| Funding | Quote: "Supported by Boehringer Ingelheim." Comment: the study was funded by Boehringer‐Ingelheim, the pharmaceutical company that developed dabigatran. It is possible that this may have influenced the timeframe of reported safety outcomes. |
|
| Declarations of interest | Quote: "Dr. Schulman reports receiving consulting fees from AstraZeneca, Bayer HealthCare, Boehringer Ingelheim, GlaxoSmithKline, and Sanofi‐Aventis, lecture fees from LEO Pharma, Sanofi‐Aventis, and Boehringer Ingelheim, and grant support from Bayer HealthCare; Dr. Kearon, consulting fees from Boehringer Ingelheim; Dr. Kakkar, consulting fees and honoraria from Boehringer Ingelheim, Bayer Schering Pharma, Sanofi‐Aventis, Bristol‐Myers Squibb, Pfizer, ARYx Therapeutics, Canyon Pharmaceuticals, and Eisai, lecture fees from Sanofi‐Aventis, Bayer Schering Pharma, Boehringer Ingelheim, GlaxoSmithKline, Eisai, and Pfizer, and grant support from Sanofi‐Aventis, Boehringer Ingelheim, Pfizer, and Bayer Schering Pharma; Dr. Mismetti, consulting fees and lecture fees from Boehringer Ingelheim, Sanofi‐Aventis, and GlaxoSmithKline; Dr. Schellong, lecture fees and consulting fees from Bayer HealthCare, Boehringer Ingelheim, and GlaxoSmithKline and consulting fees from Sanofi‐Aventis; Dr. Eriksson, consulting fees and lecture fees from Boehringer Ingelheim, Pfizer, AstraZeneca, Bayer HealthCare, LEO Pharma, and Sanofi‐Aventis; and Dr. Goldhaber, clinical research support from Sanofi‐Aventis, Bristol‐Myers Squibb, and Boehringer Ingelheim, and consulting fees from Sanofi‐Aventis, Boehringer Ingelheim, Merck, MEDRAD Interventional/Possis, Bristol‐Myers Squibb, Genentech, and Medscape. Mr. Baanstra and Dr. Schnee report being employees of Boehringer Ingelheim. No other potential conflict of interest relevant to this article was reported." | |
| Notes | 2539 participants were recruited into the trial but only 1602 had a PE and were included in the analysis of this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer generated randomisation scheme" Comment: study judged at low risk of selection bias. |
| Allocation concealment (selection bias) | Low risk | Quote: "Staff members at the clinical centres called an interactive voice‐response system that randomly assigned subjects to one of the supplied medication kits. The treatment‐group assignment was concealed from all the investigators and their staff at the coordinating centre and the clinical centres and from the clinical monitors" Comment: study judged to be at low risk of selection bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double blind. The treatment‐group assignment was concealed from all the investigators and their staff at the coordinating centre and the clinical centres and from the clinical monitors. Warfarin or a placebo that looked identical to warfarin.... Administration of dabigatran or a placebo that looked identical to dabigatran" Comment: study judged to be at low risk of performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All suspected outcome events and deaths were classified by central adjudication committees, whose members were unaware of the treatment assignments" Comment: study judged to be at low risk of detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The study drug was stopped before 6 months in 204 participants (16%) in the dabigatran group (126 because of an adverse event, 21 because of non‐adherence, 9 because of loss to follow‐up, 39 because of withdrawal of consent, and 9 for other reasons) and in 183 participants (14.5%) in the warfarin group (102 because of an adverse event, 35 because of non‐adherence, 6 because of loss to follow‐up, 36 because of withdrawal of consent, and 4 for other reasons). Comment: fewer than 20% of participants dropped out or withdrew, and the study author performed ITT analysis. Study judged at low risk of attrition bias. |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes have been reported in the pre‐specified way. Study judged at low risk of reporting bias. |
| Other bias | Low risk | We did not find any methodological issues that might directly lead to a risk of bias. |
RE‐COVER II 2014.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, double‐dummy trial Duration of study: 6 months | |
| Participants | Setting: 208 study sites Country: multinational (228 clinical centres in 30 countries: USA, Australia, Brazil, Bulgaria, Canada, China, Czech Republic, Denmark, France, Hungary, India, Israel, Italy, Korea, Malaysia, the Netherlands, New Zealand, Norway, Philippines, Poland, Russian Federation, Singapore, Slovakia, South Africa, Spain, Sweden, China Taiwan, Thailand, Turkey, Ukraine, United Kingdom) Number of participants: 2568 (PE 816, other VTE 1752): dabigatran 1280 (PE 402, other VTE 878), warfarin 1288 (PE 414, other VTE 874) Age, mean (SD) years: dabigatran 54.7 (16.2) years, warfarin 55.1 (16.3) years Sex: dabigatran 781 M/499 F, warfarin 776 M/512 F Inclusion criteria: people aged 18 or older who had acute, symptomatic, objectively verified proximal DVT of the legs or PE and for whom 6 months of anticoagulant therapy was considered to be an appropriate treatment Exclusion criteria: duration of symptoms longer than 14 days; PE with haemodynamic instability or requiring thrombolytic therapy; another indication for warfarin therapy; recent unstable cardiovascular disease; a high risk of bleeding; liver disease with an aminotransferase level that was 3 times the ULN range; an estimated creatinine clearance of less than 20 mL per minute; a life expectancy of less than 6 months; a contraindication to heparin or to radiographic contrast material; pregnancy or risk of becoming pregnant; requirement for long‐term anticoagulant therapy | |
| Interventions | Intervention 1: oral dabigatran 150 mg twice daily and warfarin‐like placebo for 6 months Intervention 2: active warfarin adjusted to achieve an INR of 2.0 to 3.0 and dabigatran‐like placebo for 6 months | |
| Outcomes | Primary: recurrent VTE objectively verified, preferably with the same method as for the index event Secondary: major bleeding defined according to the ISTH criteria | |
| Funding | Quote: "The study was funded by Boehringer‐Ingelheim." Comment: Boehringer‐Ingelheim is the pharmaceutical company that developed dabigatran. It is possible that this may have influenced the timeframe of reported safety outcomes. | |
| Declarations of interest | None | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised by use of an interactive voice response system and a computer‐generated randomisation scheme in blocks of 4" Comment: study judged at low risk of selection bias. |
| Allocation concealment (selection bias) | Low risk | Comment: no information given about how treatment allocation was concealed but study authors state that "the design of the trial was essentially identical to that of the first study with dabigatran for the treatment of acute VTE" (RE‐COVER 2009), which we judged to be at low risk of selection bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double‐blind" Comment: stated as double‐blind. No other information given about how blinding was maintained but study authors state that "the design of the trial was essentially identical to that of the first study with dabigatran for the treatment of acute VTE", which we judged to be at low risk of performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A central adjudication committee, the members of which were unaware of the treatment assignments, classified all suspected outcome events, bleeding events, and deaths" Comment: study judged to be at low risk of detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 14 participants in the dabigatran group and 7 in the warfarin group did not receive any study medication (10 did not meet the inclusion criteria or met the exclusion criteria, 9 withdrew consent, and 2 had an adverse event). Comment: fewer than 20% of participants dropped out or withdrew, and the study author performed ITT analysis. Study judged at low risk of attrition bias. |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes have been reported in the pre‐specified way. Study judged at low risk of reporting bias. |
| Other bias | Low risk | We did not find any methodological issues that might directly lead to a risk of bias. |
ALT/AST: alanine transaminase/aspartate aminotransferase APTT: activated partial thromboplastin time BP: blood pressure CRNM: clinically relevant non‐major CT: computed tomography DVT: deep vein thrombosis ECOG: Eastern Cooperative Oncology Group F: female INR: international normalised ratio ISTH: International Society on Thrombosis and Haemostasis ITT: intention‐to‐treat IV: intravenous LMWH: low molecular weight heparin M: male PE: pulmonary embolism PT‐INR: prothrombin time‐international normalised ratio SD: standard deviation UFH: unfractionated heparin ULN: upper limit of normal VKA: vitamin K antagonist VTE: venous thromboembolism
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ADAM VTE trial 2020 | Unable to obtain specific outcome data for people with a pulmonary embolism |
| AMPLIFY Extended 2013 | Extended study testing prophylaxis rather than treatment |
| Borsi 2021 | Unable to obtain specific outcome data for people with a pulmonary embolism |
| Botticelli DVT 2008 | People with a pulmonary embolism were excluded from the study |
| CASTA DIVA Trial 2022 | Unable to obtain specific outcome data for people with a pulmonary embolism |
| COBRRA pilot feasibility study 2017 | Unable to obtain specific outcome data for people with a pulmonary embolism |
| CONKO‐011 2015 | Unable to obtain specific outcome data for people with a pulmonary embolism |
| de Athayde Soares 2019 | People with a pulmonary embolism were excluded from the study |
| DIVERSITY trial 2021 | Unable to obtain specific outcome data for people with a pulmonary embolism |
| Einstein DVT 2013 | People with a pulmonary embolism were excluded from the study |
| EINSTEIN Extension 2007 | Extended study testing prophylaxis rather than treatment |
| EINSTEIN‐CHOICE trial 2017 | Comparator was aspirin |
| Einstein‐DVT Dose 2008 | People with a pulmonary embolism were excluded from the study |
| EINSTEIN‐Jr Trial 2020 | Unable to obtain specific outcome data for people with a pulmonary embolism |
| Farhan 2019 | People with a pulmonary embolism were excluded from the study |
| IRIVASC‐Trial 2022 | Unable to obtain specific outcome data for people with a pulmonary embolism |
| Mokadem 2021 | People with a pulmonary embolism were excluded from the study |
| ODIXa‐DVT 2007 | People with a pulmonary embolism were excluded from the study |
| Ohmori 2018 | People with a pulmonary embolism were excluded from the study |
| Piazza 2014 | People with a pulmonary embolism were excluded from the study |
| PRAIS trial 2019 | People with a pulmonary embolism were excluded from the study |
| PRIORITY 2022 | Unable to obtain specific outcome data for people with a pulmonary embolism |
| RE‐SONATE 2013 | People were already included in the RE‐COVER I and RE‐COVER II studies |
| REMEDY 2013 | Extended study testing prophylaxis rather than treatment |
| SELECT‐D 2018 | Unable to obtain specific outcome data for people with a pulmonary embolism |
| Sukovatykh 2017 | People with a pulmonary embolism were excluded from the study |
| THRIVE 2005 | Unable to obtain specific outcome data for people with a pulmonary embolism |
| THRIVE I 2003 | Treatment was for fewer than 3 months |
| THRIVE III 2003 | Participants in control group were given a placebo |
Characteristics of studies awaiting classification [ordered by study ID]
NCT01780987.
| Methods | Study design: randomised, multicentre, open‐label study |
| Participants | Setting: 20 hospitals Country: Japan Inclusion criteria: men or women aged ≥ 20 years with acute symptomatic proximal DVT with evidence of proximal thrombosis or acute symptomatic PE with evidence of thrombosis in segmental or more proximal branches Exclusion criteria: active bleeding or high risk for bleeding contraindicating treatment with UFH and a VKA, uncontrolled hypertension (systolic BP > 180 mm Hg or diastolic BP > 110 mm Hg) and people requiring dual anti‐platelet therapy |
| Interventions | Intervention 1: apixaban 10 mg twice a day for 7 days followed by 5 mg twice a day for 23 weeks Intervention 2: UFH, dose adjustment based on APTT 1.5 to 2.5 times the control value, and until INR ≥ 1.5 for 5 days or more plus warfarin for 24 weeks at a dose to target INR range between 1.5 to 2.5 |
| Outcomes | Primary: major bleeding and clinically relevant non‐major bleeding Secondary: symptomatic VTE or VTE‐related death, major bleeding and all bleeding |
| Notes |
APTT: activated partial thromboplastin time BP: blood pressure DVT: deep vein thrombosis INR: international normalised ratio PE: pulmonary embolism UFH: unfractionated heparin VKA: vitamin K antagonist VTE: venous thromboembolism
Characteristics of ongoing studies [ordered by study ID]
EudraCT 2014‐002606‐20.
| Study name | A randomised, open‐label, active controlled, safety and descriptive efficacy study in paediatric subjects requiring anticoagulation for the treatment of a venous thromboembolic event |
| Methods | Study design: randomised, open‐label, active controlled study |
| Participants |
Setting: hospitals Country: Austria, Canada, Germany, Italy, Russian Federation, Ukraine, USA Inclusion criteria: "1. Children 12 to < 18 years of age at the time of consent (Age Group 1). An approved amended protocol will be implemented prior to enrolment of each subsequent age group (Age Groups 2, 3, and 4). 2. Presence of an index VTE which is confirmed by imaging. Index VTE include, but are not limited to, DVT, PE, cerebral sinovenous thrombosis, renal vein thrombosis, portal vein thrombosis, and splanchnic thrombosis. 3. Intention to manage the index VTE with anticoagulation treatment for at least 12 weeks or intention to manage the index VTE with anticoagulation treatment in neonates for at least 6 weeks. 4. Evidence of a personally signed and dated informed consent document indicating that the subject (or a legally acceptable representative) has been informed of all pertinent aspects of the study. Depending on local regulations, whenever the minor is able to give assent, the minor’s assent must also be obtained. 5. Subjects/legally acceptable representatives who are willing and able to comply with scheduled visits, treatment plan, laboratory tests, and other study procedures. 6. Female subjects who, in the opinion of the investigator, are biologically capable of having children and are sexually active and at risk for pregnancy must agree to use a highly effective method of contraception throughout the study and for 28 days after the last dose of assigned treatment." Exclusion criteria: "1. Anticoagulant treatment for the index VTE for greater than 7 days prior to randomisation. 2. Thrombectomy, thrombolytic therapy, or insertion of a caval filter to treat the index VTE. 3. A mechanical heart valve. 4. Active bleeding or high risk of bleeding (e.g. CNS tumours) at the time of randomisation. 5. Intracranial bleed, including intraventricular haemorrhage, within 3 months prior to randomisation. 6. Abnormal baseline liver function (ALT > 3 x ULN or conjugated bilirubin > 2x ULN) at randomisation. 7. At the time of randomisation, inadequate renal function as defined in Section 7.2.2 Estimated Glomerular Filtration Rate Assessment of the protocol. 8. Platelet count < 50×10^9 per L at randomisation. 9. At the time of randomisation, uncontrolled severe hypertension as defined in Section 7.1 Physical Examination of the protocol. 10. At the time of randomisation, use of prohibited concomitant medication as listed for apixaban in Section 5.5 Concomitant Medication of the protocol. 11. Known allergy to apixaban. 12. Female subjects who are either pregnant or breastfeeding a child. 13. Geographically unavailable for follow‐up. 14. Family members who are either investigational site staff members directly involved in the conduct of this trial or site staff members otherwise supervised by the Investigator. Family members who are Pfizer or Bristol Myers Squibb (BMS) employees directly involved in the conduct of this trial. 15.Taking an investigational drug in other studies within 30 days before the first dose of apixaban and/or during study participation. N.B. using marketed medications commonly used in usual and customary practice, though not labelled for use in children, is acceptable. 16. Other severe acute or chronic medical or psychiatric condition or laboratory abnormality that may increase the risk associated with study participation or investigational product administration or may interfere with the interpretation of study results and, in the judgment of the investigator, would make the subject inappropriate for entry into this study." |
| Interventions |
Intervention 1: oral apixaban Intervention 2: not specified |
| Outcomes |
Primary: the composite of major and clinically relevant non‐major bleeding; all image‐confirmed and adjudicated symptomatic and asymptomatic recurrent VTE defined as either contiguous progression or non‐contiguous new thrombus and including DVT, PE, and paradoxical embolism and VTE‐related mortality Secondary: all‐cause death, index VTE status (e.g. progression, regression, or resolution), stroke, new symptomatic or asymptomatic DVT, new symptomatic PE, apixaban concentrations, anti‐FXa activity |
| Starting date | April 2015 |
| Contact information | Bristol‐Myers Squibb International Corporation, clinical.trials@bms.com |
| Notes |
NCT02464969.
| Study name | Apixaban for the acute treatment of VTE in children |
| Methods | Study design: random, open‐label, parallel, active controlled study |
| Participants |
Setting: hospital, clinic, research centre Country: USA, Australia, Canada, France, Germany, Israel, Mexico, Russian Federation, Spain, Turkey, Ukraine, United Kingdom Inclusion criteria: "1.Birth to < 18 years of age with a minimum weight of 2.6 kg at the time of randomisation. 2.Presence of an index VTE which is confirmed by imaging. 3.Intention to manage the index VTE with anticoagulation treatment for at least 6 to 12 weeks. Subjects able to tolerate oral feeding, nasogastric, gastric feeding for at least 5 days." Exclusion criteria: "1.Anticoagulant treatment for the index VTE for greater than 14 days prior to randomisation. Neonates that are enrolled into the PK cohort must be on a minimum of 5 days and a maximum of 14 days SOC anticoagulation prior to randomisation. Neonates that are enrolled into the post PK cohort may receive SOC anticoagulation for up to 14 days prior to randomisation. 2. Thrombectomy, thrombolytic therapy, or insertion of a caval filter to treat the index VTE. 3. A mechanical heart valve. 4. Active bleeding or high risk of bleeding at the time of randomisation. 5. Intracranial bleed, including intraventricular haemorrhage, within 3 months prior to randomisation. 6. Abnormal baseline liver function at randomisation. 7. Inadequate renal function at the time of randomisation. 8. Platelet count < 50 x 109 per L at randomisation. 9. Uncontrolled severe hypertension at the time of randomisation. 10. Use of prohibited concomitant medication at the time of randomisation. 11. Female subjects who are either pregnant or breastfeeding a child. 12. Use of aggressive life‐saving therapies such as ventricular assist devices or extracorporeal membrane oxygenation at the time of enrolments. 13. Unable to take oral or enteric medication via the nasogastric or gastric tube. 14. Known inherited or acquired antiphospholipid syndrome. 15. Known inherited bleeding disorder or coagulopathy with increased bleeding risk (e.g., haemophilia, von Willebrand disease, etc.)." |
| Interventions |
Intervention 1: "oral apixaban ‐ subjects between birth to < 18 years will be dosed on a body weight tiered regimen. Subjects ≥ 35kg will receive 10mg twice daily for 7 days followed by 5mg twice daily thereafter;< 35kg to 25kg will receive 8mg twice daily for 7 days followed by 4mg twice daily thereafter; <25 to 18kg will receive 6mg twice daily for 7 days and then 3mg twice daily thereafter; <18 to 12kg will receive 4mg twice daily for 7 days and then 2mg twice daily thereafter; <12 to 9kg will receive 3mg twice daily for 7 days and then 1.5mg twice daily thereafter; < 9kg to 6kg will receive 2 mg twice daily for 7 days and 1mg twice daily thereafter; <6kg to 5kg will receive 1mg twice daily for 7 days and 0.5mg twice daily thereafter; <5kg to 4kg will receive 0.6mg twice daily for 7 days and 0.3mg twice daily thereafter; PK cohort neonates ≥ 2.6kg will receive 0.1mg twice daily. Dose will be adjusted as determined by PK measurements (i.e., to 0.2mg twice daily, 0.1mg daily or dose will stay the same). For the post PK cohort neonates ˂4kg to 2.6kg, if confirmed by PK sub analysis, subjects will receive 0.2mg twice daily for 7 days and 0.1mg twice daily thereafter." Intervention 2: "SOC ‐ UFH, LMWH, and/or a VKA. For subjects under 2 years of age, SOC will be limited to UFH or LMWH" |
| Outcomes |
Primary: the composite of major and clinically relevant non‐major bleeding; a composite of all image‐confirmed and adjudicated symptomatic and asymptomatic recurrent VTE‐ and VTE‐related mortality Secondary: apixaban concentration; anti‐Xa levels |
| Starting date | November 2015 |
| Contact information | Pfizer CT.gov Call Center 1‐800‐718‐1021 ClinicalTrials.gov_Inquiries@pfizer.com |
| Notes |
NCT02664155.
| Study name | VTE in renally impaired patients and direct oral anticoagulants |
| Methods | Study design: randomised, open‐label, parallel controlled trial |
| Participants |
Setting: hospital Country: France Inclusion criteria: people with a moderate renal insufficiency defined by a creatinine clearance between 30 mL to 50 mL/min (Cockcroft and Gault formulae) or a severe renal insufficiency (between 15 mL to 29 mL/min); people with acute, objectively‐confirmed symptomatic proximal DVT or PE (with or without DVT), planned to be treated for at least 3 months; > 18 years; life expectancy more than 3 months; social security affiliation; signed informed consent Exclusion criteria: indication for anticoagulants other than VTE; active bleeding or a high risk of bleeding contraindicating anticoagulant treatment; a systolic blood pressure of more than 180 mm Hg or a diastolic blood pressure of more than 110 mm Hg; anticoagulation for more than 72 hours prior to randomisation; chronic liver disease or chronic hepatitis; people at high risk of bleeding; creatinine clearance < 15 mL/min or end stage renal disease or indication for extra‐renal dialysis; need for concomitant anti‐platelet therapy other than aspirin 75 mg to 325 mg per day. However concomitant treatment with aspirin is discouraged in this population at bleeding risk; concomitant use of a strong inhibitor of CYP3A4 (e.g. a protease inhibitor for human immunodeficiency virus infection or azole‐antimycotics agents ketoconazole, itraconazole, voriconazole, posaconazole) or a CYP3A4 inducer (e.g. rifampin, carbamazepine, or phenytoin); active pregnancy or expected pregnancy or no effective contraception; any contraindication listed in the local labelling of UFH, LMWH, or VKA or oral anticoagulant; cancer‐associated VTE requiring long‐term treatment with LMWH; life expectancy of fewer than 3 months |
| Interventions |
Intervention 1: oral apixaban and rivaroxaban ‐ apixaban (Eliquis tablet) 10 mg twice daily for 7 days then 2.5 mg twice daily for 3 months; rivaroxaban (Xarelto tablet) 15 mg twice daily for 21 days then 15 mg once daily for 3 months. Intervention 2: the control group receiving the SOC, i.e. heparins/VKA regimen. Participants will receive the current recommended therapy: subcutaneous or intravenous UFH/VKA in case of severe renal insufficiency and subcutaneous LMWH/VKA in case of moderate renal insufficiency for at least 5 days. VKA will begin concomitantly and continue for 3 months. |
| Outcomes |
Primary: non‐inferiority of reduced doses of DOACs Secondary: bleeding events; VTE events |
| Starting date | October 2016 |
| Contact information | Centre Hospitalier Universitaire de Saint Etienne; Ministry of Health, France |
| Notes |
NCT02744092.
| Study name | DOACs versus LMWH with or without warfarin for VTE in cancer |
| Methods | Study design: randomised, open‐label, parallel study |
| Participants |
Setting: hospital Country: USA Inclusion criteria: diagnosis of advanced solid tumour cancer, lymphoma, or myeloma (no time restrictions or limitations) or diagnosis of early stage solid tumour cancer, lymphoma, or myeloma ≤ 12 months prior to study enrolment; diagnosis of VTE ≤ 30 days prior to study enrolment for which potential benefits of anticoagulation therapy to prevent recurrence of VTE are felt by the treating physician to exceed the potential harms; any anticoagulation drug/strategy may be used to treat the index VTE; protocol treatment will begin ≤ 30 days after the index VTE diagnosis date. Treating physician intends to put participant on anticoagulation therapy for at least 3 months; age ≥ 18 years; platelet count is ≥ 50,000/mm3 (≤ 7 days prior to enrolment); CrCL (creatinine clearance) is ≥ 15 mL/min (≤ 7 days prior to enrolment) Exclusion criteria: diagnosis of acute leukaemia; has ever received or is scheduled to receive an allogeneic haematopoietic stem cell transplantation; people who have ever received an autologous haematopoietic stem cell transplantation are eligible; people who are scheduled to receive an autologous haematopoietic stem cell transplantation (autoHSCT) are not eligible; ongoing, clinically significant bleeding (Common Terminology Criteria for Adverse Events (CTCAE) grade 3 or 4); ongoing therapy with a P‐gp inhibitor (e.g. nelfinavir, indinavir, or saquinavir‐protease inhibitors for HIV) as these drugs interact with the factor Xa inhibitors; therapy with any azole antifungals (e.g. itraconazole, ketaconazole, voriconazole) at the time of enrolment |
| Interventions |
Intervention 1: "randomised arm 1 will get anticoagulation therapy with a DOAC. There are 4 FDA‐approved DOAC drugs that may be used for this study: Rivaroxaban, Apixaban, Edoxaban, or Dabigatran. The treatment (including dosage form, dosage, frequency and duration) should be administered in accordance with the drug's FDA package insert, and all modifications are at the discretion of the treating investigator." Intervention 2: "randomised arm 2 will get anticoagulation therapy with LMWH with or without a transition to warfarin. There are 3 FDA‐approved LMWH drugs that may be used for this study: Dalteparin, Enoxaparin, or Fondaparinux. The treatment (including dosage form, dosage, frequency and duration) should be administered in accordance with the drug's FDA package insert, and all modifications are at the discretion of the treating investigator." |
| Outcomes |
Primary: cumulative VTE recurrence reported by participants (via study‐specific questionnaire) or clinicians (via study‐specific case report form) Secondary: cumulative rates of major bleeding reported by participants (via study‐specific questionnaire) or clinicians (via study‐specific case report form); health‐related quality of life reported by participants via the Optum Short Form (SF)‐12v2 Health Survey questionnaire; burden of anticoagulation therapy reported by participants via the Anti‐Clot Treatment Scale (ACTS) questionnaire; mortality reported by participants' surrogates (via study‐specific questionnaire) or clinicians (via study‐specific case report form). |
| Starting date | December 2016 |
| Contact information | CANVAS@AllianceFoundationTrials.org |
| Notes |
NCT02798471.
| Study name | Hokusai study in paediatric patients with confirmed venous thromboembolism |
| Methods | Study design: randomised, open‐label, parallel, multicentre study |
| Participants |
Setting: hospital Country: USA, Argentina, Brazil, Bulgaria, Canada, Chile, Croatia, Czechia, Denmark, El Salvador, France, Germany, Guatemala, Hungary, India, Israel, Kenya, Korea, Lebanon, Malaysia, the Netherlands, Norway, Panama, Portugal, Romania, Russian Federation, Serbia, Singapore, Slovenia, Spain, China, Thailand, Turkey, Ukraine Inclusion criteria: "male or female paediatric subjects between birth (defined as 38 weeks gestational age) and less than 18 years of age at the time of consent; paediatric subjects with the presence of documented VTE confirmed by appropriate diagnostic imaging and requiring anticoagulant therapy for at least 90 days; subjects must have received at least 5 days of heparin therapy prior to randomisation to treat the newly identified index VTE. In addition, prior to being randomised to edoxaban or SOC, subjects initially treated with VKA are recommended to have an INR < 2.0; subject and/or parent(s)/legal guardian(s) or legally acceptable representative is informed and provides signed consent for the child to participate in the study; female subjects who have menarche must test negative for pregnancy at screening and must consent to avoid becoming pregnant by using an approved contraception method throughout the study." Exclusion criteria: "subjects with active bleeding or high risk of bleeding contraindicating treatment with LMWH, SP Xa inhibitors, VKAs, or DOACs; identified high risk of bleeding during prior experimental administration of DOACs; subjects who have been or are being treated with thrombolytic agents, thrombectomy or insertion of a caval filter for the newly identified index VTE; administration of antiplatelet therapy is contraindicated in both arms except for low dose aspirin defined as 1‐5 mg/Kg/day with maximum of 100 mg/day; administration of rifampin is prohibited during the study and subjects on concomitant use of rifampin are excluded; subjects with hepatic disease associated with coagulopathy leading to a clinically relevant bleeding risk (aPTT > 50 seconds or INR > 2.0 not related to anticoagulation therapy) or ALT > 5 x the ULN or total bilirubin > 2 × ULN with direct bilirubin > 20% of the total at screening visit; subjects with GFR < 30% of normal for age and size as determined by the Schwartz formula; subjects with stage 2 hypertension defined as blood pressure systolic and/or diastolic confirmed > 99th percentile + 5 mmHg; subject with thrombocytopenia < 50 × 109/L at screening visit. Subjects with a history of heparin‐induced thrombocytopenia may be enrolled in the study at the Investigator's discretion; life expectancy less than the expected study treatment duration (3 months); subjects who are known to be pregnant or breastfeeding; subjects with any condition that, as judged by the Investigator, would place the subject at increased risk of harm if he/she participated in the study, including contraindicated medications; subjects who participated in another clinical study or treated with an experimental therapy with less than a 30 day washout period prior to identifying the qualifying index VTE." |
| Interventions | Intervention 1: 15 mg or 30 mg tablets for participants 12 years of age to < 18 years, and 60 mg edoxaban suspension for oral administration to participants under 12 years of age Intervention 2: SOC could include LMWH, VKA, or synthetic pentasaccharide Xa inhibitors |
| Outcomes | Primary: symptomatic recurrent VTE; death as a result of VTE; no change or extension of thrombotic burden Secondary: major bleeding; clinically relevant non‐major bleeding; symptomatic recurrent VTE, death as a result of VTE and major and clinically relevant non‐major bleeding; peak plasma concentration; area under the plasma concentration versus time curve (AUC); apparent systemic clearance (CL/F); apparent volume of distribution (V/F); prothrombin time (PT); aPTT; anti‐activated factor X (Anti‐FXa). |
| Starting date | March 2017 |
| Contact information | Daiichi Sankyo Contact for Clinical Trial Information, 908‐992‐6400, CTRinfo@dsi.com |
| Notes |
NCT03129555.
| Study name | The Danish non‐vitamin K antagonist oral anticoagulation study in patients with VTE (DANNOAC‐VTE) |
| Methods | Study design: cluster‐randomised cross‐over study |
| Participants |
Setting: hospital Country: Denmark Inclusion criteria: diagnosis of VTE in outpatient clinic or as discharge diagnosis after hospitalisation; a claimed prescription of a NOAC from a Danish pharmacy within 14 days of discharge or outpatient clinic visit Exclusion criteria: a prescription of a NOAC within 90 days prior to hospitalisation or outpatient clinic visit for VTE; patients with NOAC preference apart from preference consistent with current cluster‐randomised NOAC; other contraindications mentioned in the "Summary of Product Characteristics" for the respective NOAC |
| Interventions |
Intervention 1: dabigatran etexilate oral capsule. After cluster randomisation, dabigatran will be given to all participants with VTE when possible for 6 months. Hereafter the cluster will use the other 3 NOACs for 6 months one at the time. Intervention 2: rivaroxaban oral tablet. After cluster randomisation, rivaroxaban will be given to all participants with VTE when possible for 6 months. Hereafter the cluster will use the other 3 NOACs for 6 months one at the time. Intervention 3: edoxaban oral tablet. After cluster randomisation, edoxaban will be given to all participants with VTE when possible for 6 months. Hereafter the cluster will use the other 3 NOACs for 6 months one at the time. Intervention 4: apixaban oral tablet. After cluster randomisation, apixaban will be given to all participants with VTE when possible for 6 months. Hereafter the cluster will use the other 3 NOACs for 6 months one at the time. |
| Outcomes |
Primary: a composite endpoint of new VTE or all‐cause death Secondary: new VTE; all‐cause death; bleeding requiring hospitalisation Other outcome measures: discontinuation of therapy; adherence to therapy |
| Starting date | May 2017 |
| Contact information | Casper N Bang, MD, PhD +4570250000 caspernfb@hjerteforeningen.dk Gunnar H Gislason, MD, PhD +4570250000 gunnar.gislason@hjerteforeningen.dk |
| Notes |
NCT03266783.
| Study name | Comparison of bleeding risk between rivaroxaban and apixaban for the treatment of acute VTE |
| Methods | Study design: randomised, open‐label, parallel study |
| Participants |
Setting: hospital
Country: Canada
Inclusion criteria: confirmed newly diagnosed symptomatic acute VTE (proximal power extremity DVT or segmental or greater PE); age ≥ 18 years old; informed consent obtained Exclusion criteria: have received > 72 hours of therapeutic anticoagulation; creatinine clearance < 3 mL/min calculated with the Cockcroft‐Gault formula; any contraindication for anticoagulation with apixaban or rivaroxaban as determined by the treating physician such as, but not limited to: active bleeding; active malignancy, defined as a) diagnosed with cancer within the past 6 months; or b) recurrent, regionally advanced or metastatic disease; or c) currently receiving treatment or have received any treatment for cancer during the 6 months prior to randomisation; or d) a hematologic malignancy not in complete remission; weight > 120 kg; liver disease (Child‐Pugh Class B or C); use of contraindicated medications; another indication for long‐term anticoagulation (e.g. atrial fibrillation); pregnant (note below) or breastfeeding (Note: as reported by the patient or a pregnancy test will be ordered at the discretion of the treating physician for women of childbearing potential as per standard of care). |
| Interventions |
Intervention 1: apixaban, 10 mg orally twice daily for 1 week, then 5 mg orally twice daily for 3 months of treatment Intervention 2: rivaroxaban, 15 mg orally twice daily for 3 weeks, then 20 mg orally twice daily for 3 months of treatment |
| Outcomes |
Primary: the rate of adjudicated clinically relevant bleeding events Secondary: adjudicated major bleeding events; adjudicated major bleeding events; adjudicated recurrent VTE events; adjudicated recurrent VTE events; all‐cause mortality; medication adherence; QALYs gained; impact of verbal consent on patient participation in comparison with participants from sites using written informed consent |
| Starting date | December 2017 |
| Contact information | Lana Castellucci, MD, FRCPC 613‐737‐8899 ext,74641 lcastellucci@toh.ca Veronica Bates, BSc, CCRP 613‐737‐8899 ext, 71068 vebates@ohri.ca |
| Notes |
NCT05171049.
| Study name | A study comparing abelacimab to apixaban in the treatment of cancer‐associated VTE |
| Methods | Study design: randomised, multicenter, open‐label, parallel study |
| Participants | Setting: hospital Country: USA Inclusion criteria: male or female participants ≥18 years old or other legal maturity age according to the country of residence; confirmed diagnosis of cancer (by histology, adequate imaging modality), other than basal‐cell or squamous‐cell carcinoma of the skin alone with one of the following: active cancer, defined as either locally active, regionally invasive, or metastatic cancer at the time of randomisation and/or currently receiving or having received anticancer therapy (radiotherapy, chemotherapy, hormonal therapy, any kind of targeted therapy or any other anticancer therapy) in the last 6 months; confirmed symptomatic or incidental proximal lower limb acute DVT (i.e. popliteal, femoral, iliac, and/or inferior vena cava thrombosis) and/or a confirmed symptomatic PE, or an incidental PE in a segmental, or larger pulmonary artery. Patients are eligible within 72 hours from diagnosis of the qualifying VTE. Anticoagulation therapy with a therapeutic dose of DOAC for at least 6 months is indicated. Able to provide written informed consent. Exclusion criteria: thrombectomy, insertion of a caval filter or use of a fibrinolytic agent to treat the current (index) DVT and/or PE; more than 72 hours of pre‐treatment with therapeutic doses of UFH, LMWH, fondaparinux, DOAC, or other anticoagulants; an indication to continue treatment with therapeutic doses of an anticoagulant other than that VTE treatment prior to randomisation (e.g. atrial fibrillation, mechanical heart valve, prior VTE); platelet count <50,000/mm3; PE leading to haemodynamic instability (blood pressure < 90 mmHg or shock); acute ischaemic or hemorrhagic stroke or intracranial haemorrhage within the 4 weeks preceding screening; brain trauma or a cerebral or spinal cord surgery within 4 weeks of screening; need for aspirin in a dosage of > 100 mg/day or any other antiplatelet agent alone or in combination with aspirin; primary brain cancer or untreated intracranial metastases at baseline; acute myeloid or lymphoid leukaemia; bleeding requiring medical attention at the time of randomisation or in the preceding 4 weeks; planned major surgery at baseline; Eastern Cooperative Oncology Group (ECOG) performance status of 3 or 4 at screening; life expectancy < 3 months at randomisation; calculated creatinine clearance < 30 mL/min (Cockcroft‐Gault equation); haemoglobin < 8 g/dL; acute hepatitis, chronic active hepatitis, liver cirrhosis; or an ALT ≥ 3 x and/or bilirubin ≥ 2 x ULN in absence of clinical explanation; uncontrolled hypertension (systolic BP >180 mm Hg or diastolic BP >100 mm Hg despite antihypertensive treatment); women of child‐bearing potential who are unwilling or unable to use highly effective contraceptive measures during the study from screening up to 3 days after last treatment of apixaban or 100 days after administration of abelacimab; sexually active males with sexual partners of childbearing potential must agree to use a condom or other reliable contraceptive measure up to 3 days after last treatment of apixaban or 100 days after administration of abelacimab; pregnant or breastfeeding women; people known to be receiving strong dual inducers or inhibitors of both CYP3A4 and P gp; history of hypersensitivity to any of the study drugs (including apixaban) or excipients, to drugs of similar chemical classes, or any contraindication listed in the label for apixaban; people with any condition that in the investigator's judgement would place them at increased risk of harm if he/she participated in the study; use of other investigational (not registered) drugs within 5 half‐lives prior to enrolment or until the expected pharmacodynamic(s) effect has returned to baseline, whichever is longer. Participation in academic non‐interventional studies or interventional studies, comprising testing different strategies or different combinations of registered drugs is permitted. |
| Interventions |
Intervention 1: apixaban administered orally twice a day, 10 mg followed by 5 mg Intervenyion 2: abelacimab intravenous administration followed by monthly administration of the same dose subcutaneously |
| Outcomes |
Primary: time to first event of centrally‐adjudicated VTE recurrence consisting of new proximal DVT, new PE or fatal PE, including unexplained death for which PE cannot be ruled out Secondary: time to first event of ISTH‐adjudicated major or clinically relevant non‐major bleeding events; net clinical benefit defined as survival without VTE recurrence, or major or clinically relevant non‐major bleeding |
| Starting date | May 2022 |
| Contact information | Nancy Widener 239‐284‐3741,Nancy.w@anthostherapeutics.com Deb Freedholm 609‐439‐8246, Deb.f@anthostherapeutics.com |
| Notes |
Pettit 2018.
| Study name | High treatment failure rates with rivaroxaban and apixaban in a randomised controlled trial of young women with VTE |
| Methods | Study design: randomised, parallel, open‐label study |
| Participants |
Setting: hospital
Country: USA
Inclusion criteria: non‐pregnant women, aged 18 to 50. For study purposes, evidence of negative pregnancy is accounted for by the treating physician's initiation of treatment with oral anticoagulants; objectively diagnosed VTE or atrial fibrillation/flutter; patient‐reported active menstruation (does not apply to women who were recently pregnant); clinical plan and patient agreement to treat with oral anticoagulation for 3 months or longer; participants must have a working telephone Exclusion criteria: package insert exclusions for Eliquis (apixaban) or Xarelto (rivaroxaban): active pathological bleeding or severe hypersensitivity reaction to Xarelto or Eliquis (e.g. anaphylactic reactions); plan to become pregnant in the next 3 months; concomitant prescribed use of aspirin or thienopyridines or other platelet‐inhibiting drugs; plan for surgical hysterectomy or endometrial ablation; known uterine cancer; Von Willebrand's disease, or haemophilia; known coagulopathy from liver disease; conditions likely to preclude adherence to study procedures: active intravenous drug use, known alcoholism, homelessness, or uncontrolled psychiatric illness |
| Interventions |
Intrvention 1: rivaroxaban, 15 mg twice daily for 7 days, then 20 mg daily for 3 months Intervention 2: apixaban, 10 mg twice daily for 7 days, then 5 mg twice daily for 3 months |
| Outcomes |
Primary: PBAC scores (< 100 normal) Secondary: rate of discontinuation; number of participants that held drug for menorrhagia; rate of major haemorrhage; rate of recurrent VTE; rate of cross‐over to another anticoagulant; rate of clinically relevant non‐major bleeding; haemoglobin concentration; physical component summary of standard from 36 [sic]. |
| Starting date | September 2016 |
| Contact information | Patti Hogan, 317‐962‐1190, hoganpr@iu.edu Kate Pettit, 317‐880‐3870, klpettit@iu.edu |
| Notes |
UMIN000020069.
| Study name | Comparison of efficacy and safety between warfarin, rivaroxaban and edoxaban in patients with acute PE in Showa University |
| Methods | Study design: randomised, parallel, open‐label study (assessor(s) blinded) |
| Participants | Setting: hospital Country: Japan Inclusion criteria: people with acute PE who are hospitalised in Showa University Hospital, Showa University Fujigaoka Hospital and Showa University Koto Toyosu Hospital and needed intensive care, 20 to 90 years old Exclusion criteria: creatinine clearance less than 30 mL/min; people with acute bleeding; people with active malignancy; people needing PCPS, IABP, and aortic sheathes; contraindication for each drug |
| Interventions |
Intervention 1: rivaroxaban administration at the dose of 15 mg twice a day in the first 3 weeks. Subsequently, rivaroxaban administration at the dose of 15 mg once a day for 6 months. Intervention 2: warfarin administration for 6 months with prothrombin time INR between 2.00 and 3.00. Intervention 3: edoxaban administration at the dose of 60 mg (30 mg if participants meet the reduced criteria of edoxaban) once a day for 6 months |
| Outcomes |
Primary: central disease score and thrombus volume in pulmonary vein measured by CT (if possible) Secondary: CT index score; coagulation test; change in UCG*; length of hospital stay and coronary care unit; length of disappearance of PE from an initial day *We are unsure what UCG is and have requested information from study authors |
| Starting date | December 2015 |
| Contact information | Norikazu Watanabe, Division of Cardiology, Department of Medicine, Showa University, Shinagawaku Tokyo, Email: n‐watanabe@med.showa‐u.ac.jp |
| Notes |
ALT: alanine transaminase APTT: activated partial thromboplastin time CNS: central nervous system CYP3A4: cytochrome P‐450 3A4 DOACs: direct oral anticoagulantsDVT: deep vein thrombosis GFR: glomerular filtration rate IABP: intra‐aortic balloon pumping INR: international normalised ratio LMWH: low molecular weight heparin NOAC: non‐vitamin K antagonist oral anticoagulation PBAC: pictorial blood loss assessment chart PCPS: percutaneous cardiopulmonary support PE: pulmonary embolism PK: pharmacokinetics QALYs: quality‐adjusted life years SOC: standard of care UFH: unfractionated heparin ULN: upper limit of normal VKA: vitamin K antagonist VTE: venous thromboembolism
Differences between protocol and review
2023 version For this update, we used a random‐effects model for all analyses as we expected clinical heterogeneity across studies. This may be due to different oral factor Xa inhibitors (e.g. apixaban, rivaroxaban, edoxaban), different conventional thrombin inhibitors in the control group (e.g. warfarin, dalteparin), different treatment durations (e.g. three, six, or 12 months). We amended 'standard anticoagulation' to 'conventional anticoagulation' throughout for consistency. In response to peer reviewer comments, we carried out an additional subgroup analysis based on the different types of factor Xa inhibitors.
2015 version In a change from the protocol (Robertson 2014b), we excluded studies where treatment lasted fewer than three months. This was because a meta‐analysis of venous thromboembolism treatment strategies demonstrated an increased rate of recurrence after fewer than three months of anticoagulation but no significant difference with various longer periods of treatment (Boutitie 2011).
Contributions of authors
ML: selected studies for inclusion, extracted data, assessed the risk of bias of studies, contacted authors for missing data, performed data analysis and interpretation, created the summary of findings tables using the GRADE approach, and updated the review. JL: selected studies for inclusion, extracted data, assessed the risk of bias of the studies, performed data analysis and interpretation, and commented on the review. XW: selected studies for inclusion, extracted data, assessed the risk of bias of the studies, contacted authors for missing data, and commented on the review. XH: selected studies for inclusion, extracted data, assessed the risk of bias of the studies and commented on the review. QW: selected studies for inclusion, extracted data, and commented on the review. SX: selected studies for inclusion, extracted data, and commented on the review. PY: commented on the review. JT: commented on the review. JFL: provided clinical consultation on the whole process. PX: provided clinical consultation on the whole process. KY: commented on the review, and provided methodological guidance on the whole process. LY: selected studies for inclusion, commented on the review, and provided methodological guidance on the whole process.
Sources of support
Internal sources
-
‐, Other
No internal sources of support
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
Declarations of interest
ML: none known. JL: none known. XW: none known. XH: none known. QW: none known. SX: none known. PY: none known. JT: none known. JFL: none known. PX: none known. KY: none known. LY: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
AMPLIFY 2013 {published data only}
- Agnelli G, Buller H, Cohen A, Curto M, Gallus AS, Johnson M, et al. Apixaban for the treatment of symptomatic deep-vein thrombosis and pulmonary embolism: a randomized, double-blind trial (AMPLIFY). Journal of Thrombosis and Haemostasis 2013;11 (Suppl 2):18. [Google Scholar]
- Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, et al. Oral apixaban for the treatment of acute venous thromboembolism. New England Journal of Medicine 2013;369(9):799-808. [DOI] [PubMed] [Google Scholar]
- Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Pak R, et al. Apixaban for the treatment of venous thromboembolism in cancer patients: data from the AMPLIFY trial. Canadian Journal of Cardiology 2014;30(10):S278. [Google Scholar]
- Agnelli G, Buller HR, Cohen A, Gallus AS, Lee TC, Pak R, et al. Oral apixaban for the treatment of venous thromboembolism in cancer patients: results from the AMPLIFY trial. Journal of Thrombosis and Haemostasis 2015;13(12):2187-91. [DOI] [PubMed] [Google Scholar]
- Agnelli G. Apixaban was noninferior to enoxaparin plus warfarin in patients with acute venous thromboembolism. Annals of Internal Medicine 2013;159(8):JC2. [DOI] [PubMed] [Google Scholar]
- Bleker SM, Cohen AT, Büller HR, Agnelli G, Gallus AS, Raskob GE, et al. Clinical presentation and course of bleeding events in patients with venous thromboembolism, treated with apixaban or enoxaparin and warfarin. Thrombosis and Haemostasis 2016;116(6):1159-64. [DOI] [PubMed] [Google Scholar]
- Brekelmans M, Scheres L, Bleker S, Hutten B, Timmermans A, Büller H, et al. Abnormal vaginal bleeding in women with venous thromboembolism treated with apixaban or warfarin. Thrombosis and Haemostasis 2017;117(04):809-15. [DOI] [PubMed] [Google Scholar]
- Cohen A, Agnelli G, Buller H, Gallus A, Raskob G, Sanders P, et al. Characteristics and outcomes in patients with venous thromboembolism taking concomitant anti-platelet agents and anticoagulants in the AMPLIFY trial. Thrombosis and Haemostasis 2019;119(3):461-6. [DOI] [PubMed] [Google Scholar]
- Cohen A, Agnelli G, Buller HR, Chaudhuri S, Gallus AS, Raskob GE, et al. Analysis of the bleeding and thromboembolic risk with concomitant use of antiplatelet treatment in the AMPLIFY trial. In: Canadian Journal of Cardiology. Vol. 30. 2014:S272.
- Cohen A, Gallus AS, Agnelli G, Buller HR, Pak R, Porcari AR, et al. Time in therapeutic range (TTR) and relative efficacy and safety of treatment with apixaban or enoxaparin/warfarin for acute symptomatic venous thromboembolism: an analysis of the AMPLIFY trial data. Blood 2014;124(21):1543. [Google Scholar]
- Cohen AT, Pan S, Byon W, Ilyas BS, Lee TC. Efficacy, safety, and exposure of apixaban in patients with high body weight or obesity and venous thromboembolism: insights from AMPLIFY. Advances in Therapy 2021;38(6):3003-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EUCTR2007-007867-25-PT. A safety and efficacy trial evaluating the use of apixaban in the treatment of symptomatic deep vein thrombosis and pulmonary embolism. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2007%E2%80%90007867%E2%80%9025%E2%80%90PT (first received 14 July 2008).
- Gallus AS, Agnelli G, Buller HR, Cohen A, Lee TC, Pak R, et al. Apixaban for treatment of venous thromboembolism in patients from study centres in Asia: a subgroup analysis of the amplify trial. Journal of Thrombosis and Haemostasis 2015;13:727. [DOI] [PubMed] [Google Scholar]
- Lee T, Pan S, Byon W, Ilyas BS. Safety and efficacy of apixaban versus enoxaparin/warfarin in patients with extremes of body weight: post-hoc analysis of the AMPLIFY trial. Blood 2019;134 (Suppl 1):1152. [Google Scholar]
- Liu X, Johnson M, Mardekian J, Phatak H, Thompson J, Cohen AT. Apixaban reduces hospitalizations in patients with venous thromboembolism: an analysis of the apixaban for the Initial management of pulmonary embolism and deep‐vein thrombosis as first‐line therapy (AMPLIFY) trial. Journal of the American Heart Association 2015;4(12):e002340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00633893. Efficacy and safety study of apixaban for the treatment of deep vein thrombosis or pulmonary embolism. clinicaltrials.gov/ct2/show/NCT00633893 (first received 12 March 2008).
- NCT00643201. Efficacy and safety study of apixaban for the treatment of deep vein thrombosis or pulmonary embolism. clinicaltrials.gov/ct2/show/NCT00643201 (first received 26 March 2008).
- Raskob GE, Gallus AS, Sanders P, Thompson JR, Agnelli G, Buller HR, et al. Early time courses of recurrent thromboembolism and bleeding during apixaban or enoxaparin/warfarin therapy. A sub-analysis of the AMPLIFY trial. Thrombosis and Haemostasis 2016;115(4):809-16. [DOI] [PubMed] [Google Scholar]
AMPLIFY‐J 2015 {published data only}
- Nakamura M, Nishikawa M, Komuro I, Kitajima I, Uetsuka Y, Yamagami T, et al. Apixaban for the treatment of Japanese subjects with acute venous thromboembolism (AMPLIFY-J Study). Circulation Journal: Official Journal of the Japanese Circulation Society 2015;79(6):1230–6. [DOI] [PubMed] [Google Scholar]
Caravaggio 2020 {published data only}
- Ageno W, Vedovati MC, Cohen A, Huisman M, Bauersachs R, Gussoni G, et al. Bleeding with apixaban and dalteparin in patients with cancer-associated venous thromboembolism: results from the Caravaggio study. Thrombosis and Haemostasis 2021;121(05):616-24. [DOI] [PubMed] [Google Scholar]
- Agnelli G, Becattini C, Bauersachs R, Brenner B, Campanini M, Cohen A, et al. Apixaban versus dalteparin for the treatment of acute venous thromboembolism in patients with cancer: the Caravaggio study. Thrombosis and Haemostasis 2018;118(9):1668-78. [DOI] [PubMed] [Google Scholar]
- Agnelli G, Becattini C, Meyer G, Muoz A, Verso M. Apixaban for the treatment of venous thromboembolism associated with cancer. New England Journal of Medicine 2020;382(17):1599-607. [DOI] [PubMed] [Google Scholar]
- Agnelli G, Muoz A, Franco L, Mahé I, Brenner B, Connors JM, et al. Apixaban and dalteparin for the treatment of venous thromboembolism in patients with different sites of cancer. Thrombosis and Haemostasis 2022;122(5):796-807. [DOI] [PubMed] [Google Scholar]
- Becattini C, Bauersachs R, Maraziti G, Bertoletti L, Cohen A, Connors JM, et al. Renal function and clinical outcome of patients with cancer-associated venous thromboembolism randomized to receive apixaban or dalteparin. Results from the Caravaggio trial. Haematologica 2022;107(7):1567-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giustozzi M, Connors JM, Ruperez Blanco AB, Szmit S, Falvo N, Cohen AT, et al. Clinical characteristics and outcomes of incidental venous thromboembolism in cancer patients: insights from the Caravaggio study. Journal of Thrombosis and Haemostasis 2021;19(11):2751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03045406. Apixaban for the treatment of venous thromboembolism in patients with cancer (CARAVAGGIO). clinicaltrials.gov/ct2/show/NCT03045406 (first received 7 February 2017).
- Verso M, Munoz A, Bauersachs R, Huisman MV, Agnelli G. Effects of concomitant administration of anticancer agents and apixaban or dalteparin on recurrence and bleeding in patients with cancer-associated venous thromboembolism. European Journal of Cancer 2021;148(Suppl C):371-81. [DOI] [PubMed] [Google Scholar]
EINSTEIN‐PE 2012 {published data only}
- Buller HR, Prins MH, Lensin AW, Decousus H, Jacobson BF, Minar E, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. New England Journal of Medicine 2012;366(14):1287-97. [DOI] [PubMed] [Google Scholar]
- Fermann GJ, Erkens PM, Prins MH, Wells PS, Pap ÁF, Lensing AW, et al. Treatment of pulmonary embolism with rivaroxaban: outcomes by simplified pulmonary embolism severity index score from a post hoc analysis of the EINSTEIN PE study. Academic Emergency Medicine 2015;22(3):299-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00439777. Oral direct factor Xa inhibitor rivaroxaban In patients with acute symptomatic pulmonary embolism with or without symptomatic deep-vein thrombosis: Einstein-PE evaluation. clinicaltrials.gov/ct2/show/NCT00439777 (first received 26 February 2007).
- Prins M, Bamber L, Cano S, Wang M, Lensing AWA, Bauersachs R. Patient-reported treatment satisfaction with oral rivaroxaban versus standard therapy in the treatment of acute symptomatic pulmonary embolism. Blood 2012;120(21):1163. [Google Scholar]
- Prins MH, Bamber L, Cano SJ, Wang MY, Erkens P, Bauersachs R, et al. Patient-reported treatment satisfaction with oral rivaroxaban versus standard therapy in the treatment of pulmonary embolism; results from the EINSTEIN PE trial. Thrombosis Research 2015;135(2):281-8. [DOI] [PubMed] [Google Scholar]
- Prins MH, Erkens PG, Lensing AW. Incidence of recurrent venous thromboembolism in patients following completion of the EINSTEIN DVT and EINSTEIN PE studies. Journal of Thrombosis and Haemostasis 2013;11(Suppl 2):257. [Google Scholar]
- Prins MH, Lensing AW, Bauersachs R, Van Bellen B, Bounameaux H, Brighton TA, et al. Oral rivaroxaban versus standard therapy for the treatment of symptomatic venous thromboembolism: a pooled analysis of the EINSTEIN-DVT and PE randomized studies. Thrombosis Journal 2013;11(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bellen B, Bamber L, Correa De Carvalho F, Prins M, Wang M, Lensing AWA. Reduction in the length of stay with rivaroxaban as a single-drug regimen for the treatment of deep vein thrombosis and pulmonary embolism. Current Medical Research and Opinion 2014;30(5):829-37. [DOI] [PubMed] [Google Scholar]
- Van Bellen B, Prins M, Bamber L, Wang M, Lensing AWA. Reduction in initial length of stay with rivaroxaban single-drug regimen versus LMWH-VKA standard of care: findings from the Einstein trial program. Blood 2012;120(21):3419. [Google Scholar]
- Wang Y, Wang C, Chen Z, Zhang J, Liu Z, Jin B, et al. Rivaroxaban for the treatment of symptomatic deep-vein thrombosis and pulmonary embolism in Chinese patients: a subgroup analysis of the EINSTEIN DVT and PE studies. Thrombosis Journal 2013;11(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang C. Rivaroxaban for the treatment of symptomatic deep vein thrombosis and/or pulmonary embolism in Chinese patients: a subgroup analysis of the EINSTEIN DVT and PE studies. Journal of Thrombosis and Haemostasis 2013;11(Suppl 2):694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hokusai‐VTE 2013 {published data only}
- Brekelmans MP, Ageno W, Beenen LF, Brenner B, Buller HR, Chen CZ, et al. Recurrent venous thromboembolism in patients with pulmonary embolism and right ventricular dysfunction: a post-hoc analysis of the Hokusai-VTE study. Lancet Haematology 2016;3(9):e437-45. [DOI] [PubMed] [Google Scholar]
- Brekelmans MP, Bleker SM, Bauersachs R, Boda Z, Büller HR, Choi Y, et al. Clinical impact and course of major bleeding with edoxaban versus vitamin K antagonists. Thrombosis and Haemostasis 2016;116(1):155-61. [DOI] [PubMed] [Google Scholar]
- Di Nisio M, Van Es N, Carrier M, Wang TF, Garcia D, Segers A, et al. Extended treatment with edoxaban in cancer patients with venous thromboembolism: a post-hoc analysis of the Hokusai-VTE Cancer study. Journal of Thrombosis and Haemostasis 2019;17:1866–74. [DOI] [PubMed] [Google Scholar]
- EUCTR2009-014290-40-SE. A phase 3, randomized, double-blind, double-dummy, parallel group, multi-center, multi-national study for evaluation of efficacy and safety of DU-176b versus warfarin in subjects with atrial fibrillation - effective anticoagulation with factor Xa next generation in atrial fibrillation (ENGAGE-AF TIMI-48). trialsearch.who.int/?trialid=EUCTR2009-014290-40-SE (first received 30 November 2009).
- Eichinger S, Lin M, Kyrle PA, Grosso MA. Recurrent venous thromboembolism during anticoagulation: an investigator-initiated post-hoc analysis of the hokusai-VTE trial. Blood 2018;132 (Suppl 1):2539. [DOI] [PubMed] [Google Scholar]
- Klok FA, Barco S, Konstantinides SV. External validation of the VTE-BLEED score for predicting major bleeding in stable anticoagulated patients with venous thromboembolism. Thrombosis and Haemostasis 2017;117(6):1164-70. [DOI] [PubMed] [Google Scholar]
- Kraaijpoel N, Van Es N, Raskob GE, Büller HR, Carrier M, Zhang G, et al. Risk scores for occult cancer in patients with venous thromboembolism: a post hoc analysis of the Hokusai-VTE study. Thrombosis and Haemostasis 2018;118(7):1270-8. [DOI] [PubMed] [Google Scholar]
- Medina A, Raskob G, Ageno W, Cohen AT, Brekelmans MPA, Chen CZ, et al. Outpatient management in patients with venous thromboembolism with edoxaban: a post hoc analysis of the Hokusai-VTE study. Thrombosis and Haemostasis 2017;117(12):2406-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina A, Raskob G, Ageno W, Cohen AT, Brekelmans MPA, Chen CZ, et al. Safety and efficacy of edoxaban compared with warfarin for the treatment of acute symptomatic deep-vein thrombosis in the outpatient setting. Research and Practice in Thrombosis and Haemostasis 2017;1:913. [Google Scholar]
- Mulder FI, Es N, Kraaijpoel N, Di Nisio M, Carrier M, Duggal A, et al. Edoxaban for treatment of venous thromboembolism in patient groups with different types of cancer: results from the Hokusai VTE Cancer study. Thrombosis Research 2020;185:13-9. [DOI] [PubMed] [Google Scholar]
- NCT00986154. Comparative investigation of Low Molecular Weight (LMW) heparin/edoxaban tosylate (DU176b) versus (LMW) heparin/warfarin in the treatment of symptomatic deep-vein blood clots and/or lung blood clots. (The Edoxaban Hokusai-VTE Study). clinicaltrials.gov/ct2/show/NCT00986154 (first received 29 September 2009).
- Nakamura M, Wang YQ, Wang C, Oh D, Yin WH, Kimura T, et al. Efficacy and safety of edoxaban for treatment of venous thromboembolism: a subanalysis of East Asian patients in the Hokusai-VTE trial. Journal of Thrombosis and Haemostasis 2015;13(9):1606-14. [DOI] [PubMed] [Google Scholar]
- Nyberg J, Karlsson KE, Jönsson S, Yin O, Miller R, Karlsson MO, et al. Edoxaban exposure-response analysis and clinical utility index assessment in patients with symptomatic deep-vein thrombosis or pulmonary embolism. CPT: Pharmacometrics and Systems Pharmacology 2016;5(4):222-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskob G, Ageno W, Cohen AT, Brekelmans MP, Grosso MA, Segers A, et al. Extended duration of anticoagulation with edoxaban in patients with venous thromboembolism: a post-hoc analysis of the Hokusai-VTE study. Lancet Haematology 2016;3(5):e228-36. [DOI] [PubMed] [Google Scholar]
- Raskob G, Buller H, Prins M, Segers A, Shi M, Schwocho L, et al. Edoxaban for the long-term treatment of venous thromboembolism: rationale and design of the Hokusai-venous thromboembolism study-methodological implications for clinical trials. Journal of Thrombosis and Haemostasis 2013;11(7):1287-94. [DOI] [PubMed] [Google Scholar]
- Raskob GE, Buller H, Angchaisuksiri P, Oh D, Boda Z, Lyons RM, et al. Edoxaban for long-term treatment of venous thromboembolism in cancer patients. Blood 2013;122(21):211. [Google Scholar]
- Raskob GE, Es N, Segers A, Angchaisuksiri P, Oh D, Boda Z, et al. Edoxaban for venous thromboembolism in patients with cancer: results from a non-inferiority subgroup analysis of the Hokusai-VTE randomised, double-blind, double-dummy trial. Lancet Haematology 2016;3(8):e379-87. [DOI] [PubMed] [Google Scholar]
- Scheres LJ, Brekelmans MP, Walter A, Cihan A, Büller HR, Sabine E, et al. Abnormal vaginal bleeding in women of reproductive age treated with edoxaban or warfarin for venous thromboembolism: a post hoc analysis of the Hokusai-VTE study. British Journal of Obstetrics and Gynaecology 2018;125(12):1581-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Hokusai-VTE Investigators. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. New England Journal of Medicine 2013;369(15):1406-15. [DOI] [PubMed] [Google Scholar]
- Vanassche T, Verhamme P, Wells PS, Segers A, Ageno W, Brekelmans MP, et al. Impact of age, comorbidity, and polypharmacy on the efficacy and safety of edoxaban for the treatment of venous thromboembolism: an analysis of the randomized, double-blind Hokusai-VTE trial. Thrombosis Research 2018;162:7-14. [DOI] [PubMed] [Google Scholar]
- Verhamme P, Wells PS, Segers A, Ageno W, Brekelmans MP, Cohen AT, et al. Dose reduction of edoxaban preserves efficacy and safety for the treatment of venous thromboembolism. An analysis of the randomised, double-blind HOKUSAI VTE trial. Thrombosis and Haemostasis 2016;116(4):747-53. [DOI] [PubMed] [Google Scholar]
Hokusai VTE Cancer 2018 {published data only}
- Amaya N, Uzui H, Hisazaki K, Hasegwa K, Kaseno K, Tada H. Edoxaban normalizes the elevated D-dimer levels potently and promptly in patients with venous thromboembolisms: a comparison with traditional anticoagulant therapy. European Heart Journal 2016;37:278. [Google Scholar]
- Bosch FT, Van Es N, Di Nisio M, Carrier M, Segers A, Grosso MA, et al. The Ottawa score does not predict recurrent venous thromboembolism in cancer patients: results from the Hokusai-VTE cancer study. Research and Practice in Thrombosis and Haemostasis 2019;3(S1):717-8. [Google Scholar]
- Kraaijpoel N, Nisio MD, Mulder FI, Es NV, Raskob GE. Clinical impact of bleeding in cancer-associated venous thromboembolism: results from the Hokusai VTE Cancer randomized trial. Thrombosis Research 2018;164:S223. [DOI] [PubMed] [Google Scholar]
- Mulder FI, Di Nisio M, Ay C, Carrier M, Bosch FTM, Segers A, et al. Clinical implications of incidental venous thromboembolism in cancer patients. In: European Respiratory Journal. Vol. 55. 2020:1901697. [DOI] [PubMed]
- Mulder FI, Van EN, Kraaijpoel N, Di NM, Carrier M, Garcia D, et al. Efficacy and safety of edoxaban in clinically relevant subgroups: results from the Hokusai VTE Cancer randomized trial. Thrombosis Research 2018;164:S194. [Google Scholar]
- NCT02073682. Cancer venous thromboembolism (VTE). clinicaltrials.gov/ct2/show/NCT02073682 (first received 27 February 2014).
- Raskob G, Van Es N, Verhamme P, Carrier M, Di Nisio M, Garcia D, et al. Edoxaban versus dalteparin for treatment of venous thromboembolism (VTE) associated with cancer: Hokusai VTE-cancer randomized trial. Supportive Care in Cancer 2018;26(2):S316-7. [Google Scholar]
- Raskob GE, Van Es N, Verhamme P, Carrier M, Di Nisio M, Garcia D, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. New England Journal of Medicine 2018;378(7):615-24. [DOI] [PubMed] [Google Scholar]
- Raskob GE, Van Es N, Verhamme P, Carrier M, Di Nisio M, Garcia DA, et al. A randomized, open-label, blinded outcome assessment trial evaluating the efficacy and safety of LMWH/Edoxaban versus dalteparin for venous thromboembolism associated with cancer: Hokusai VTE-cancer study. Blood 2017;130 (Suppl 1):[no pagination]. [Google Scholar]
J‐EINSTEIN DVT and PE 2015 {published data only}
- Matsuo H, Prins M, Lensing AW, Fujinuma EW, Miyamoto Y, Kajikawa M. Shortened length of hospital stay with rivaroxaban in patients with symptomatic venous thromboembolism in Japan: the J-EINSTEIN pulmonary embolism and deep vein thrombosis program. Current Medical Research and Opinion 2015;31(6):1057-61. [DOI] [PubMed] [Google Scholar]
- NCT01516814. Venous thromboembolism (VTE) treatment study in Japanese pulmonary embolism (PE) patients. clinicaltrials.gov/ct2/show/NCT01516814 (first received 25 January 2012).
- Yamada N, Hirayama A, Maeda H, Sakagami S, Shikata H, Prins MH, et al. Oral rivaroxaban for Japanese patients with symptomatic venous thromboembolism-the J-EINSTEIN DVT and PE program. Thrombosis Journal 2015;13:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
MERCURY PE 2018 {published data only}
- Frank PW, Coleman CI, Diercks DB, Francis S, Kabrhel C, Keay C, et al. Emergency department discharge of pulmonary embolus patients. Academic Emergency Medicine 2018;25(9):995-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02584660. A study of rivaroxaban for early discharge of low risk pulmonary embolism from the emergency department. clinicaltrials.gov/ct2/show/NCT02584660 (first received 22 October 2015).
- Peacock W, Diercks D, Francis S, Kabrhel C, Keay C, Kline J, et al. Multicenter trial of rivaroxaban for early discharge of pulmonary embolism from the emergency department. Annals of Emergency Medicine 2017;70(4):S29-S30. [Google Scholar]
RE‐COVER 2009 {published data only}
- Goldhaber SZ, Schellong S, Kakkar A, Eriksson H, Feuring M, Kreuzer J, et al. Treatment of acute pulmonary embolism with dabigatran versus warfarin: a pooled analysis of data from RE-COVER and RE-COVER II. Thrombosis and Haemostasis 2016;116(4):714-21. [DOI] [PubMed] [Google Scholar]
- NCT00291330. Efficacy and safety of dabigatran compared to warfarin for 6 month treatment of acute symptomatic venous thromboembolism (RE-COVER I). clinicaltrials.gov/ct/show/NCT00291330 (first received 14 February 2006).
- Schulman S, Eriksson H, Feuring M, Hantel S. Efficacy of dabigatran versus warfarin in patients with acute venous thromboembolism and thrombophilia: a pooled analysis of RE-COVER and RE-COVER II. Circulation 2014;130 (Suppl 2):A18594. [Google Scholar]
- Schulman S, Eriksson H, Goldhaber SZ, Kakkar A, Kearon C, Mismetti P, et al. Safety of dabigatran vs. warfarin for acute venous thromboembolism: pooled analyses of RE-COVER and RE-COVER II. Journal of Thrombosis and Haemostasis 2013;11:225-6. [Google Scholar]
- Schulman S, Eriksson H, Goldhaber SZ, Kakkar A, Kearon C, Mismetti P, et al. Treatment of acute pulmonary embolism with dabigatran or warfarin: a pooled analysis of efficacy data from RE-COVER and RE-COVER II. European Heart Journal 2014;35 (Suppl 1):990. [Google Scholar]
- Schulman S, Eriksson H, Goldhaber SZ, Kakkar A, Kearon C, Schellong SM, et al. Influence of age on the efficacy and safety of dabigatran versus warfarin for the treatment of acute venous thromboembolism: a pooled analysis of RE-cover and RE-cover II. Blood 2013;122(21):2375. [Google Scholar]
- Schulman S, Eriksson H, Goldhaber SZ, Kakkar A, Kearon C, Schellong SM, et al. Influence of concomitant NSAID or ASA on the efficacy and safety of dabigatran versus warfarin for the treatment of acute venous thromboembolism: a pooled analysis from RE-COVER and RE-COVER II. Blood 2013;122(21):212. [Google Scholar]
- Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. New England Journal of Medicine 2009;361(24):2342-52. [DOI] [PubMed] [Google Scholar]
RE‐COVER II 2014 {published data only}
- Goldhaber SZ, Schellong S, Kakkar A, Eriksson H, Feuring M, Kreuzer J, et al. Treatment of acute pulmonary embolism with dabigatran versus warfarin: a pooled analysis of data from RE-COVER and RE-COVER II. Thrombosis and Haemostasis 2016;116(4):714-21. [DOI] [PubMed] [Google Scholar]
- NCT00680186. Phase III study testing efficacy & safety of oral dabigatran etexilate vs warfarin for 6 m treatment for acute symp venous thromboembolism (VTE) (RE-COVER II). clinicaltrials.gov/ct2/show/NCT00680186 (first received 20 May 2008).
- Schulman S, Eriksson H, Feuring M, Hantel S. Efficacy of dabigatran versus warfarin in patients with acute venous thromboembolism and thrombophilia: a pooled analysis of RE-COVER and RE-COVER II. Circulation 2014;130 (Suppl 2):A18594. [Google Scholar]
- Schulman S, Eriksson H, Goldhaber SZ, Kakkar A, Kearon C, Mismetti P, et al. Safety of dabigatran vs. warfarin for acute venous thromboembolism: pooled analyses of RE-COVER and RE-COVER II. Journal of Thrombosis and Haemostasis 2013;11:225-6. [Google Scholar]
- Schulman S, Eriksson H, Goldhaber SZ, Kakkar A, Kearon C, Mismetti P, et al. Treatment of acute pulmonary embolism with dabigatran or warfarin: a pooled analysis of efficacy data from RE-COVER and RE-COVER II. European Heart Journal 2014;35 (Suppl 1):990. [Google Scholar]
- Schulman S, Eriksson H, Goldhaber SZ, Kakkar A, Kearon C, Schellong SM, et al. Influence of age on the efficacy and safety of dabigatran versus warfarin for the treatment of acute venous thromboembolism: a pooled analysis of RE-cover and RE-cover II. Blood 2013;122(21):2375. [Google Scholar]
- Schulman S, Eriksson H, Goldhaber SZ, Kakkar A, Kearon C, Schellong SM, et al. Influence of concomitant NSAID or ASA on the efficacy and safety of dabigatran versus warfarin for the treatment of acute venous thromboembolism: a pooled analysis from RE-COVER and RE-COVER II. Blood 2013;122(21):212. [Google Scholar]
- Schulman S, Kakkar AK, Goldhaber SZ, Schellong S, Eriksson H, Mismetti P, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation 2014;129:764-72. [DOI] [PubMed] [Google Scholar]
- Schulman S, Kakkar AK, Schellong SM, Goldhaber SZ, Henry E, Mismetti P, et al. A randomized trial of dabigatran versus warfarin in the treatment of acute venous thromboembolism (RE-COVER II). Blood 2011;118(21):Abstract 205. [Google Scholar]
References to studies excluded from this review
ADAM VTE trial 2020 {published data only}
- McBane RD, McBane LR, Loprinzi CL, Ashrani A, Perez-Botero J, Ferre Ra Leon, et al. Apixaban and dalteparin in active malignancy associated venous thromboembolism: the ADAM VTE trial. Thrombosis and Haemostasis 2017;117(10):1952-61. [DOI] [PubMed] [Google Scholar]
- McBane RD, Wysokinski WE, Le-Rademacher JG, Zemla T, Ashrani A, Tafur A, et al. Apixaban and dalteparin in active malignancy-associated venous thromboembolism: the ADAM VTE trial. Journal of Thrombosis and Haemostasis 2020;18(2):411-21. [DOI] [PubMed] [Google Scholar]
- NCT02585713. Apixaban or dalteparin in reducing blood clots in patients with cancer related venous thromboembolism. clinicaltrials.gov/ct2/show/NCT02585713 (first received 20 November 2015).
AMPLIFY Extended 2013 {published data only}
- Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, et al. Apixaban for extended treatment of venous thromboembolism. New England Journal of Medicine 2013;368(8):699-708. [DOI] [PubMed] [Google Scholar]
- Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson MR, et al. Two doses of apixaban for the extended treatment of venous thromboembolism. Blood 2012;120(21):LBA-1. [Google Scholar]
- Liu X, Thompson J, Phatak H, Mardekian J, Porcari AR, Johnson MR. Apixaban reduces hospitalization in patients with venous thromboembolism: an analysis of the AMPLIFY-EXT trial. Blood 2013;122(21):[no pagination]. [DOI] [PubMed] [Google Scholar]
Borsi 2021 {published data only}
- Borsi SH, Raji H, Dargahi Malamir M, Nokhostin F, Kargaran A. Rivaroxaban versus enoxaparin for treatment of patients with nonhematologic cancer with venous thromboembolism: a randomized clinial trial. Tehran University Medical Journal Sciences Journals 2021;79(4):281-9. [Google Scholar]
Botticelli DVT 2008 {published data only}
- Barrett YC, Wang J, Knabb R, Mohan P. Apixaban decreases coagulation activity in patients with acute deep-vein thrombosis. Thrombosis and Haemostasis 2011;105:181-9. [DOI] [PubMed] [Google Scholar]
- Botticelli IWC, Buller H, Deitchman D, Prins M, Segers A. Efficacy and safety of the oral direct factor Xa inhibitor apixaban for symptomatic deep vein thrombosis. The Botticelli DVT dose-ranging study. Journal of Thrombosis and Haemostasis 2008;6(8):1313-8. [DOI] [PubMed] [Google Scholar]
- Buller HR. A dose finding study of the oral direct factor Xa inhibitor apixaban in the treatment of patients with acute symptomatic deep vein thrombosis-The Botticelli Investigators. In: XXIst Congress of the International Society on Thrombosis and Haemostasis. Geneva, 2007.
- NCT00252005. Oral direct factor Xa-inhibitor apixaban in patients with acute symptomatic deep-vein thrombosis - the Botticelli DVT study. clinicaltrials.gov/ct/show/NCT00252005 (first received November 2005).
CASTA DIVA Trial 2022 {published data only}
- NCT02746185. Cancer associated thrombosis, a pilot treatment study using rivaroxaban (CASTA-DIVA). clinicaltrials.gov/ct2/show/NCT02746185 (first received 21 April 2016).
- Planquette B, Bertoletti L, Charles-Nelson A, Laporte S, Grange C, Mahé I, et al. Rivaroxaban vs dalteparin in cancer-associated thromboembolism: a randomized trial. Chest 2022;161(3):781-90. [DOI] [PubMed] [Google Scholar]
COBRRA pilot feasibility study 2017 {published data only}
- Castellucci LA, Hogg K, Chiang P, Wu CM, Templier GL, Gal GL, et al. Comparison of bleeding risk between rivaroxaban and apixaban: a pilot feasibility study. Blood 2017;130 (Suppl 1):1108. [Google Scholar]
CONKO‐011 2015 {published data only}
- EUCTR2015-001478-16-DE. The role of rivaroxaban in the treatment of tumor patients with thrombosis. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2015-001478-16-DE (first received 9 September 2015).
- NCT02583191. Rivaroxaban in the treatment of venous thromboembolism (VTE) in cancer patients. clinicaltrials.gov/ct2/show/NCT02583191 (first received 22 October 2015).
- Riess H, Sinn M, Kreher S. CONKO-011: Evaluation of patient satisfaction with the treatment of acute venous thromboembolism with rivaroxaban or low molecular weight heparin in cancer patients. A randomized phase III study [CONKO-011: Evaluation der Patientenzufriedenheit bei der Behandlung akuter venöser Thromboembolien mit Rivaroxaban oder niedermolekularem Heparin bei Krebspatienten]. Deutsche Medizinische Wochenschrift 2015;140 (Suppl 1):S22-3. [DOI] [PubMed] [Google Scholar]
- Riess H, Sinn M, Lohneis A, Hellmann M, Striefler J, Südhoff T, et al. Improved patient-reported treatment satisfaction with rivaroxaban as compared to low molecular weight heparins for cancer patients with acute venous thromboembolism. Research and Practice in Thrombosis and Haemostasis 2021;5 (Suppl 2):[no pagination]. [Google Scholar]
- Sinn M, Juhling A, Hellmann M, Omar M, Sudhoff T, Stahl M, et al. Patient-reported treatment satisfaction with rivaroxaban in cancer patients with acute venous thromboembolism - Results from the CONKO-011 trial. Oncology Research and Treatment 2021;44 (Suppl 2):276-7. [Google Scholar]
de Athayde Soares 2019 {published data only}
- NCT02704598. Comparison between xarelto versus warfarin in the recanalization rate of deep venous thrombosis in patients Legs. (DVT). clinicaltrials.gov/ct2/show/NCT02704598 (first received 10 March 2016).
- Soares R, Matielo MF, Neto F, Nogueira MP, Sacilotto R. Comparison of the recanalization rate and postthrombotic syndrome in patients with deep venous thrombosis treated with rivaroxaban or warfarin. Surgery 2019;166(6):1076-83. [DOI] [PubMed] [Google Scholar]
- Athayde Soares R, Matielo MF, Brochado Neto FC, Almeida RD, Sacilotto R. Comparison of the recanalization rate and post-thrombotic syndrome in patients with deep venous thrombosis treated with rivaroxaban or warfarin. Journal of Vascular Surgery 2019;70(5):e169-e170. [DOI] [PubMed] [Google Scholar]
DIVERSITY trial 2021 {published data only}
- Albisetti M, Biss B, Bomgaars L, Brandão LR, Brueckmann M, Chalmers E, et al. Design and rationale for the DIVERSITY study: an open-label, randomized study of dabigatran etexilate for pediatric venous thromboembolism. Research and Practice in Thrombosis and Haemostasis 2018;2(2):347-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albisetti M, Brandão L, Bomgaars L, Chalmers E, Luciani M, Mitchell L, et al. Efficacy and safety of dabigatran etexilate for treatment of venous thromboembolism in paediatric patients - results of the DIVERSITY trial. Research and Practice in Thrombosis and Haemostasis 2019;3:139-40. [Google Scholar]
- Albisetti M, Tartakovsky I, Halton J, Bomgaars L, Chalmers E, Mitchell L, et al. Efficacy and safety of dabigatran in the treatment and secondary prevention of venous thromboembolism in children with central line or implantable device–related thrombosis. Research and Practice in Thrombosis and Haemostasis 2021;5 (Suppl 2):[no pagination]. [Google Scholar]
- Brandão L, Tartakovsky I, Halton J, Bomgaars L, Chalmers E, Mitchell L, et al. Efficacy and safety of dabigatran in the treatment and secondary prevention of venous thromboembolism in children with cerebral venous and sinus thrombosis. Research and Practice in Thrombosis and Haemostasis 2021;5 (Suppl 2):[no pagination]. [Google Scholar]
- EUCTR2013-002114-12. Open label study comparing efficacy and safety of dabigatran etexilate to standard of care in paediatric patients with VTE. trialsearch.who.int/?TrialID=EUCTR2013%E2%80%90002114%E2%80%9012%E2%80%90Outside%E2%80%90EU/EEA (first received 18 Feburary 2014).
- Halton J, Brando LR, Luciani M, Bomgaars L, Woods-Swafford W, Mitchell LG, et al. Dabigatran etexilate for the treatment of acute venous thromboembolism in children (DIVERSITY): a randomised, controlled, open-label, phase 2b/3, non-inferiority trial. Lancet Haematology 2021;8(1):E22-E33. [DOI] [PubMed] [Google Scholar]
- Halton J, Brandão L, Luciani M, Bomgaars L, Chalmers E, Mitchell L, et al. Efficacy and safety of dabigatran etexilate for treatment of venous thromboembolism in paediatric patients aged from birth to < 2 years: results of the DIVERSITY Trial. Research and Practice in Thrombosis and Haemostasis 2020;4(Suppl 1):35. [Google Scholar]
- NCT01895777. Open label study comparing efficacy and safety of dabigatran etexilate to standard of care in paediatric patients with venous thromboembolism (VTE). clinicaltrials.gov/ct2/show/NCT01895777 (first received 11 July 2013).
EINSTEIN‐CHOICE trial 2017 {published data only}
- Prandoni P, Lensing AW, Prins MH, Gebel M, Pap AF, Homering M, et al. Benefits and risks of extended treatment of venous thromboembolism with rivaroxaban or with aspirin. Thrombosis Research 2018;168:121-9. [DOI] [PubMed] [Google Scholar]
- Weitz JI, Lensing AW, Prins MH, Bauersachs R, Beyer-Westendorf J, Bounameaux H, et al. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. New England Journal of Medicine 2017;376(13):1211-22. [DOI] [PubMed] [Google Scholar]
Einstein DVT 2013 {published data only}
- Bamber L, Wang MY, Prins MH, Ciniglio C, Bauersachs R, Lensing AW, et al. Patient-reported treatment satisfaction with oral rivaroxaban versus standard therapy in the treatment of acute symptomatic deep-vein thrombosis. Thrombosis and Haemostasis 2013;110(4):732-41. [DOI] [PubMed] [Google Scholar]
- Bauersachs R, Lensing AW, Pap A, Decousus H. No need for a rivaroxaban dose reduction in renally impaired patients with symptomatic venous thromboembolism. Journal of Thrombosis and Haemostasis 2013;11:30-1. [Google Scholar]
- Bistervels IM, Bavalia R, Gebel M, Lensing AW, Middeldorp S, Prins MH, et al. Effect of polypharmacy on bleeding with rivaroxaban versus vitamin K antagonist for treatment of venous thromboembolism. Journal of Thrombosis and Haemostasis 2022;20(6):1376-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookhart BK, Haskell L, Bamber L, Wang M, Schein J, Mody SH. Length of stay and economic consequences with rivaroxaban vs enoxaparin/vitamin K antagonist in patients with DVT and PE: findings from the North American EINSTEIN clinical trial program. Journal of Medical Economics 2014;17(10):691-5. [DOI] [PubMed] [Google Scholar]
- Buller HR. Oral rivaroxaban for the acute and continued treatment of symptomatic venous thromboembolism. The Einstein-DVT and Einstein-Extension study. Blood 2010;116(21):187. [Google Scholar]
- Cheung W, Middeldorp S, Prins MP, Pap AF, Lensing AW, Hoek-ten CAJ, et al. Post thrombotic syndrome in patients treated with rivaroxaban or enoxaparin/vitamin K antagonists for acute deep vein thrombosis. Journal of Thrombosis and Haemostasis 2015;13(S2):219-20. [DOI] [PubMed] [Google Scholar]
- Cheung YW, Middeldorp S, Prins MH, Pap AF, Prandoni P. Post-thrombotic syndrome in patients treated with rivaroxaban or enoxaparin/vitamin K antagonists for acute deep-vein thrombosis. A post-hoc analysis. Thrombosis and Haemostasis 2016;116(4):733-8. [DOI] [PubMed] [Google Scholar]
- EUCTR2004-002171-16-IT. Once-daily oral direct factor Xa inhibitor BAY 59-7939 in patients with acute symptomatic deep-vein thrombosis. The EINSTEIN-DVT dose-finding study. clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2004-002171-16 (first received 15 October 2008).
- EUCTR2006-004495-13-DK. Oral direct factor Xa inhibitor rivaroxaban in patients with acute symptomatic deep-vein thrombosis or pulmonary embolism. clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2006-004495-13 (first received 29 March 2007).
- Eerenberg ES, Middeldorp S, Levi M, Lensing AW, Büller HR. Clinical impact and course of major bleeding with rivaroxaban and vitamin K antagonists. Journal of Thrombosis and Haemostasis 2015;13(9):1590-6. [DOI] [PubMed] [Google Scholar]
- Kline JA, Jimenez D, Courtney DM, Ianus J, Cao L, Wells PS. Use of the riete 2008 bleeding score to identify patients at low risk for major bleeding in patients treated with rivaroxaban. Academic Emergency Medicine 2015;22(5):S162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00440193. Oral direct factor Xa inhibitor rivaroxaban in patients with acute symptomatic deep vein thrombosis - the EINSTEIN DVT study. clinicaltrials.gov/ct2/show/NCT00440193 (first received 26 February 2007).
- Prandoni P. Treatment of patients with acute deep vein thrombosis and/or pulmonary embolism: efficacy and safety of non-VKA oral anticoagulants in selected populations. Thrombosis Research 2014;134(2):227-33. [DOI] [PubMed] [Google Scholar]
Einstein‐DVT Dose 2008 {published data only}
- Buller H, Darius H. EINSTEIN DVT: Oral rivaroxaban versus standard therapy in the initial treatment of symptomatic deep vein thrombosis and long-term prevention of recurrent venous thromboembolism. escardio.org/congresses/esc-2010/congress-reports/Pages/708-4-EINSTEIN-DVT.aspx#.UvNXl03itMs 2010.
- Buller HR, Agnelli G. Once-or twice-daily rivaroxaban for the treatment of proximal deep vein thrombosis: similar efficacy and safety to standard therapy in dose-ranging studies. Blood 2006;108(11 Pt 1):172-3. [Google Scholar]
- Buller HR, Lensing AW, Prins MH, Agnelli G, Cohen A, Gallus AS, et al. A dose-ranging study evaluating once-daily oral administration of the factor Xa inhibitor rivaroxaban in the treatment of patients with acute symptomatic deep vein thrombosis: the Einstein-DVT dose-ranging study. Blood 2008;112(6):2242-7. [DOI] [PubMed] [Google Scholar]
- NCT00395772. Once-daily oral direct factor XA inhibitor bay59-7939 in patients with acute symptomatic deep-vein thrombosis. clinicaltrials.gov/ct2/show/NCT00395772 (first received December 2004).
EINSTEIN Extension 2007 {published data only}
- NCT00439725. Once - daily oral direct factor Xa inhibitor rivaroxaban In the long-term prevention of recurrent symptomatic venous thromboembolism in patients with symptomatic deep-vein thrombosis or pulmonary embolism. The Einstein-Extension study. clinicaltrials.gov/ct2/show/NCT00439725 (first received 26 February 2007).
EINSTEIN‐Jr Trial 2020 {published data only}
- Lensing AW, Male C, Young G, Kubitza D, Kenet G, Patricia MM, et al. Rivaroxaban versus standard anticoagulation for acute venous thromboembolism in childhood. Design of the EINSTEIN-Jr phase III study. Thrombosis Journal 2018;16:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Male C, Lensing AW, Palumbo JS, Kumar R, Nurmeev I, Hege K, et al. Rivaroxaban compared with standard anticoagulants for the treatment of acute venous thromboembolism in children: a randomised, controlled, phase 3 trial. Lancet Haematology 2020;7(1):e18-e27. [DOI] [PubMed] [Google Scholar]
- NCT02234843. EINSTEIN junior: oral rivaroxaban in children with venous thrombosis (EINSTEIN Jr). clinicaltrials.gov/ct2/show/NCT02234843 (first received 13 November 2014).
- Thom K, Lensing AW, Nurmeev I, Bajolle F, Bonnet D, Kenet G, et al. Safety and efficacy of anticoagulant therapy in pediatric catheter-related venous thrombosis (EINSTEIN-Jr CVC-VTE). Blood Advances 2020;4(19):4632-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Farhan 2019 {published data only}
- Farhan A, Bukhari M, Umar J, Raza MA. Oral rivaroxaban in symptomatic deep vein thrombosis. Journal of the College of Physicians and Surgeons Pakistan 2019;29(9):814-8. [DOI] [PubMed] [Google Scholar]
IRIVASC‐Trial 2022 {published data only}
- NCT02066662. Rivaroxaban compared to vitamin K antagonist upon development of cardiovascular calcification. clinicaltrials.gov/ct2/show/NCT02066662 (first received 19 February 2014).
- Stöhr R, Dirrichs T, Kneizeh K, Reinartz S, Frank D, Brachmann J, et al. Influence of rivaroxaban compared to vitamin K antagonist treatment upon development of cardiovascular calcification in patients with atrial fibrillation and/or pulmonary embolism. Clinical Cardiology 2022;45(4):352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mokadem 2021 {published data only}
- Mokadem ME, Hassan A, Algaby AZ. Efficacy and safety of apixaban in patients with active malignancy and acute deep venous thrombosis. Vascular 2021;29(5):745-50. [DOI] [PubMed] [Google Scholar]
- NCT04462003. Efficacy of apixaban in malignancy with deep venous thrombosis (DVT). clinicaltrials.gov/ct2/show/NCT04462003 (first received 3 July 2019).
ODIXa‐DVT 2007 {published data only}
- Agnelli G, Gallus A, Goldhaber SZ, Haas S, Huisman MV, Hull RD, et al. Treatment of proximal deep-vein thrombosis with the oral direct factor Xa inhibitor rivaroxaban (BAY 59-7939): the ODIXa-DVT (oral direct factor Xa inhibitor BAY 59-7939 in patients with acute symptomatic deep-vein thrombosis) study. Circulation 2007;116(2):180-7. [DOI] [PubMed] [Google Scholar]
- NCT00839163. Oral direct factor Xa inhibitor BAY 59-7939 in patients with acute symptomatic proximal deep vein thrombosis (ODIXa-DVT). clinicaltrials.gov/ct2/show/NCT00839163 (first received 9 Febuary 2009).
Ohmori 2018 {published data only}
- Ohmori H, Kada A, Nakamura M, Saito AM, Sanayama Y, Shinagawa T, et al. Deep vein thrombosis in severe motor and intellectual disabilities patients and its treatment by anticoagulants of warfarin versus edoxaban. Annals of Vascular Diseases 2019;12(3):372-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H, Nakamura M, Kada A, Saito AM, Sanayama Y, Shinagawa T, et al. Multicenter, open-label, randomized controlled trial of warfarin and edoxaban tosilate hydrate for the treatment of deep vein thrombosis in persons with severe motor intellectual Disabilities. Kurume Medical Journal 2018;65(1):11-6. [DOI] [PubMed] [Google Scholar]
Piazza 2014 {published data only}
- NCT01662908. A randomized, open-label, parallel-group, multi-center study for the evaluation of efficacy and safety of edoxaban monotherapy versus low molecular weight (LMW) heparin/warfarin in subjects with symptomatic deep-vein thrombosis (eTRIS). clinicaltrials.gov/ct2/show/NCT01662908 (first received 13 August 2012).
- Piazza G, Mani V, Grosso M, Mercuri M, Lanz H, Schussler S, et al. A randomized, open-label, multicenter study of the efficacy and safety of edoxaban monotherapy versus low-molecular weight heparin/warfarin in patients with symptomatic deep vein thrombosis-edoxaban thrombus reduction imaging study (eTRIS). Circulation 2014;130(2):A12074. [Google Scholar]
PRAIS trial 2019 {published data only}
- Kang JiM, Park KH, Ahn S, Cho S, Min SK. Rivaroxaban after thrombolysis in acute Iliofemoral venous thrombosis: a randomized, open-labeled, multicenter trial. Scientific Reports 2019;9(1):20356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min SK, Ahn S, Park KH, Kang JM, Kim JY. Prevention of recurrence after thrombolysis in acute iliofemoral Venous thrombosis with rivaroxaban (Prais Study): a prospective, randomized, open label, multicenter trial. European Journal of Vascular and Endovascular Surgery 2019;58(6):e516. [Google Scholar]
PRIORITY 2022 {published data only}
- Kim JH, Yoo C, Seo S, Jeong JH, Ryoo BY, Kim KP, et al. A phase II study to compare the safety and efficacy of direct oral anticoagulants versus subcutaneous dalteparin for cancer-associated venous thromboembolism in patients with advanced upper gastrointestinal, hepatobiliary and pancreatic cancer: PRIORITY. Cancers (Basel) 2022;14(3):559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03139487. A randomized phase II open label study to compare the safety and iefficacy of subcutaneous dalteparin versus direct oral anticoagulants for cancer-associated venous thromboembolism. clinicaltrials.gov/ct2/show/NCT03139487 (first received 4 May 2017).
REMEDY 2013 {published data only}
- Liem TK, DeLoughery TG. Randomised controlled trial: extended-duration dabigatran is non-inferior to warfarin and more effective than placebo for symptomatic VTE. Evidence Based Medicine 2014;19(1):29. [DOI] [PubMed] [Google Scholar]
- Schulman S, Kearon C, Kakkar AK, Schellong S, Eriksson H, Baanstra D, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. New England Journal of Medicine 2013;368(8):709-18. [DOI] [PubMed] [Google Scholar]
RE‐SONATE 2013 {published data only}
- Schulman S, Baanstra D, Eriksson H, Goldhaber S, Kakkar A, Kearon C, et al. Dabigatran vs. placebo for extended maintenance therapy of venous thromboembolism. Journal of Thrombosis and Haemostasis 2011;9 (Suppl 2):22. [Google Scholar]
- Schulman S, Baanstra D, Eriksson H, Goldhaber SZ, Kakkar A, Kearon C, et al. Benefit of extended maintenance therapy for venous thromboembolism with dabigatran etexilate is maintained over 1 year of post-treatment follow-up. Blood (ASH Annual Meeting Abstracts) 2012;120 (21):Abstract 332. [Google Scholar]
- Schulman S, Kearon C, Kakkar AK, Schellong S, Eriksson H, Baanstra D, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. New England Journal of Medicine 2013;368(8):709-18. [DOI] [PubMed] [Google Scholar]
SELECT‐D 2018 {published data only}
- EUCTR2012-005589-37-GB. Anticoagulation therapy in selected cancer patients at risk of recurrence of venous thromboembolism. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2012-005589-37-GB (first received 8 February 2013).
- Young A, Dunn J, Chapman O, Grumett J, Marshall A, Phillips J, et al. SELECT-D: Anticoagulation therapy in selected cancer patients at risk of recurrence of venous thromboembolism. Journal of Clinical Oncology 2014;32(32):Suppl 1. [DOI] [PubMed] [Google Scholar]
- Young A, Marshall A, Thirlwall J, Chapman O, Lokare A, Hill C, et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D). Journal of Clinical Oncology 2018;36(20):2017-23. [DOI] [PubMed] [Google Scholar]
- Young A, Marshall A, Thirlwall J, Hill C, Hale D, Dunn J, et al. Anticoagulation therapy in selected cancer patients at risk of recurrence of venous thromboembolism: results of the Select-D™ pilot trial. Blood 2017;130 (Suppl 1):[no pagination]. [DOI] [PubMed] [Google Scholar]
- Young A, Phillips J, Hancocks H, Hill C, Joshi N, Marshall A, et al. OC-11 - Anticoagulation therapy in selected cancer patients at risk of recurrence of venous thromboembolism. Thrombosis Research 2016;140(Suppl 1):S172-3. [DOI] [PubMed] [Google Scholar]
Sukovatykh 2017 {published data only}
- Sukovatykh BS, Sereditskiĭ AV, Muradian VF, Belikov LN, Gerasimova OF. Results of administering oral anticoagulants for treatment of patients with venous thromboembolic complications. Angiology and Vascular Surgery 2017;23(2):82. [PubMed] [Google Scholar]
THRIVE 2005 {published data only}
- Fiessinger JN, Huisman MV, Davidson BL, Bounameaux H, Francis CW, Eriksson H, et al. Ximelagatran vs low-molecular-weight heparin and warfarin for the treatment of deep vein thrombosis: a randomized trial. JAMA 2005;293(6):681-9. [DOI] [PubMed] [Google Scholar]
- Francis CW, Ginsberg JS, Berkowitz SD, Bounameaux H, Davidson BL, Eriksson H, et al. Efficacy and safety of the oral direct thrombin inhibitor ximelagatran compared with current therapy for acute, symptomatic deep vein thrombosis, with or without pulmonary embolus: the THRIVE treatment study. Blood 2003;102(11):Abstract 7. [Google Scholar]
- Harenberg J, Ingrid J, Tivadar F. Treatment of venous thromboembolism with the oral thrombin inhibitor, ximelagatran. Israel Medical Association Journal 2002;4(11):1003-5. [PubMed] [Google Scholar]
- Harenberg J, Joerg I, Weiss C. Incidence of recurrent venous thromboembolism of patients after termination of treatment with ximelagatran. European Journal of Clinical Pharmacology 2006;62(3):173-7. [DOI] [PubMed] [Google Scholar]
- Huisman MV, The THRIVE Treatment Study Investigators. Efficacy and safety of the oral direct thrombin inhibitor ximelagatran compared with current standard therapy for acute symptomatic deep vein thrombosis, with or without pulmonary embolism: a randomized, double-blind, multinational study. Journal of Thrombosis and Haemostasis 2003;1 (Suppl 1):[no pagination]. [Google Scholar]
- Wimperis J, Fiessinger JN, Huisman MV, Davidson BL, Bounameaux H, Francis CW, et al. Ximelagatran, an oral direct thrombin inhibitor, compared with current standard therapy for acute, symptomatic deep vein thrombosis, with or without pulmonary embolism: the THRIVE treatment study. British Journal of Haematology 2004;125 (Suppl 1):66. [Google Scholar]
THRIVE I 2003 {published data only}
- Eriksson H, Wahlander K, Gustafsson D, Welin LT, Frison L, Schulman S, et al. A randomized, controlled, dose-guiding study of the oral direct thrombin inhibitor ximelagatran compared with standard therapy for the treatment of acute deep vein thrombosis: THRIVE I. Journal of Thrombosis and Haemostasis 2003;1(1):41-7. [DOI] [PubMed] [Google Scholar]
THRIVE III 2003 {published data only}
- Eriksson H, Lundstrom T, Wahlander K, Clason SB, Schulman S. Prognostic factors for recurrence of venous thromboembolism (VTE) or bleeding during long-term secondary prevention of VTE with ximelagatran. Thrombosis and Haemostasis 2005;94(3):522-7. [PubMed] [Google Scholar]
- Eriksson H, Wahlander K, Lundstrom T, Billing Clason S, Schulman S. Extended secondary prevention with the oral direct thrombin inhibitor ximelagatran for 18 months after 6 months of anticoagulation in patients with venous thromboembolism: a randomized, placebo-controlled trial. Blood 2002;100:81a. [Google Scholar]
- Harenberg J, Jorg I, Weiss C, Harenberg J, Jorg I, Weiss C. Observations of alanine aminotransferase and aspartate aminotransferase in THRIVE studies treated orally with ximelagatran. International Journal of Toxicology 2006;25(3):165-9. [DOI] [PubMed] [Google Scholar]
- Schulman S, Wahlander K, Lundstrom T, Clason SB, Eriksson H. Secondary prevention of venous thromboembolism with the oral direct thrombin inhibitor ximelagatran. New England Journal of Medicine 2003;349(18):1713-21. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT01780987 {published data only}
- NCT01780987. A study to evaluate safety and efficacy of apixaban in Japanese acute deep vein thrombosis (DVT) and pulmonary embolism (PE) patients. clinicaltrials.gov/show/NCT01780987 (first received 30 July 2022).
References to ongoing studies
EudraCT 2014‐002606‐20 {published data only}
- EudraCT 2014-002606-20. A randomized, open-label, active controlled, safety and descriptive efficacy study in pediatric subjects requiring anticoagulation for the treatment of a venous thromboembolic event. clinicaltrialsregister.eu/ctr-search/trial/2014-002606-20/3rd (first received 8 June 2015).
NCT02464969 {published data only}
- NCT02464969. Apixaban for the acute treatment of venous thromboembolism in children. clinicaltrials.gov/ct2/show/NCT02464969 (first received 8 June 2015).
NCT02664155 {published data only}
- NCT02664155. Venous thromboembolism in renally impaired patients and direct oral anticoagulants. clinicaltrials.gov/ct2/show/NCT02664155 (first received 26 January 2016).
NCT02744092 {published data only}
- NCT02744092. Direct oral anticoagulants (DOACs) versus LMWH +/- warfarin for VTE in cancer. clinicaltrials.gov/ct2/show/NCT02744092 (first received 20 April 2016).
- Schrag D, Uno H, Rosovsky RP, Rutherford CJ, Sanfilippo KM, Villano JL, et al. The comparative effectiveness of direct oral anti-coagulants and low molecular weight heparins for prevention of recurrent venous thromboembolism in cancer: the CANVAS pragmatic randomized trial. Journal of Clinical Oncology 2021;39(Suppl 15):12020. [Google Scholar]
NCT02798471 {published data only}
- NCT02798471. Hokusai study in pediatric patients with confirmed venous thromboembolism (VTE). clinicaltrials.gov/ct2/show/NCT02798471 (first received 14 June 2016).
- Van Ommen CH, Albisetti M, Chan AK, Estepp J, Jaffray J, Kenet G, et al. The Edoxaban Hokusai VTE PEDIATRICS Study: an open-label, multicenter, randomized study of edoxaban for pediatric venous thromboembolic disease. Research and Practice in Thrombosis and Haemostasis 2020;4(5):886-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT03129555 {published data only}
- NCT03129555. The Danish non-vitamin K antagonist oral anticoagulation study in patients with venous thromboembolism (DANNOAC-VTE). clinicaltrials.gov/ct2/show/NCT03129555 (first received 26 April 2017).
NCT03266783 {published data only}
- NCT03266783. Comparison of bleeding risk between rivaroxaban and apixaban for the treatment of acute venous thromboembolism (COBRRA). clinicaltrials.gov/ct2/show/NCT03266783 (first received 30 August 2017).
NCT05171049 {published data only}
- NCT05171049. A study comparing abelacimab to apixaban in the treatment of cancer-associated VTE (ASTER). clinicaltrials.gov/ct2/show/NCT05171049 (first received 28 December 2021).
Pettit 2018 {published data only}
- Pettit KL, Kline JA. High treatment failure rates with rivaroxaban and apixaban in a randomized controlled trial of young women with venous thromboembolism. Academic Emergency Medicine 2018;25(Suppl 1):S263-4. [Google Scholar]
UMIN000020069 {published data only}
- UMIN000020069. Comparison of efficacy and safety between warfarin, rivaroxaban and edoxaban in patients with acute pulmonary embolism in showa university. center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000023184 (first received 10 December 2015).
Additional references
Ageno 2012
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G, et al. Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141(Suppl 2):e44S-88S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Agnelli 2007
- Agnelli G, Gallus A, Goldhaber SZ, Haas S, Huisman MV, Hull RD, et al. Treatment of proximal deep-vein thrombosis with the oral direct factor Xa inhibitor rivaroxaban (BAY 59-7939): the ODIXa-DVT (Oral direct factor Xa inhibitor BAY 59-7939 in patients with acute symptomatic deep-vein thrombosis) study. Circulation 2007;116(2):180-7. [DOI] [PubMed] [Google Scholar]
Agnelli 2013
- Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, et al. Oral apixaban for the treatment of acute venous thromboembolism. New England Journal of Medicine 2013;369(9):799-808. [DOI] [PubMed] [Google Scholar]
Anderson 2009
- Anderson DR, Barnes DC. Computerized tomographic pulmonary angiography versus ventilation perfusion lung scanning for the diagnosis of pulmonary embolism. Current Opinion in Pulmonary Medicine 2009;15(5):425-9. [DOI] [PubMed] [Google Scholar]
Antoniazzi 2013
- Antoniazzi S, Berdai D, Conti V, Robinson P, Radice S, Clementi E, et al. Risk of major bleeding with dabigatran versus active controls: a systematic review and meta-analysis. Drug Safety 2013;36:818. [Google Scholar]
Athanazio 2022
- Athanazio RA, Ceresetto JM, Marfil Rivera LJ, Cesarman-Maus G, Galvez K, Marques MA, et al. Direct oral anticoagulants for the treatment of cancer-associated venous thromboembolism: a Latin American perspective. Clinical and Applied Thrombosis/Hemostasis 2022;28:10760296221082988. [DOI: 10.1177/10760296221082988] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baetz 2008
- Baetz BE, Spinler SA. Dabigatran etexilate: an oral direct thrombin inhibitor for prophylaxis and treatment of thromboembolic diseases. Pharmacotherapy 2008;28(11):1354-73. [DOI] [PubMed] [Google Scholar]
Botticelli Investigators 2008
- Botticelli Investigators, Writing Committee, Büller H, Deitchman D, Prins M, Segers A, et al. Efficacy and safety of the oral direct factor Xa inhibitor apixaban for symptomatic deep vein thrombosis. The Botticelli DVT dose-ranging study. Journal of Thrombosis and Haemostasis 2008;6(8):1313-8. [DOI] [PubMed] [Google Scholar]
Boudes 2006
- Boudes PF. The challenges of new drugs benefits and risks analysis: lessons from the ximelagatran FDA Cardiovascular Advisory Committee. Contemporary Clinical Trials 2006;27(5):432-40. [DOI] [PubMed] [Google Scholar]
Boutitie 2011
- Boutitie F, Pinede L, Schulman S, Agnelli G, Raskob G, Julian J, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants' data from seven trials. BMJ 2011;342:d3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Castellucci 2013
- Castellucci LA, Cameron C, Le Gal G, Rodger MA, Coyle D, Wells PS, et al. Efficacy and safety outcomes of oral anticoagulants and antiplatelet drugs in the secondary prevention of venous thromboembolism: systematic review and network meta-analysis. BMJ 2013;347:f5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 2015
- Cohen AT, Hamilton M, Mitchell SA, Phatak H, Liu X, Bird A et al. Comparison of the novel oral anticoagulants apixaban, dabigatran, edoxaban, and rivaroxaban in the initial and long-term treatment and prevention of venous thromboembolism: systematic review and network meta-analysis. PLoS One 2015;10(12):e0144856. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 2016
- Cohen AT, Hamilton M, Bird A, Mitchell SA, Li S, Horblyuk R, et al. Comparison of the non-VKA oral anticoagulants apixaban, dabigatran, and rivaroxaban in the extended treatment and prevention of venous thromboembolism: systematic review and network meta-analysis. PLoS One 2016;11(9):e0163386. [DOI] [PMC free article] [PubMed] [Google Scholar]
Connolly 2009
- Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. New England Journal of Medicine 2009;361(12):1139-51. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Version accessed 23 March 2022. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
de Miguel‐Diez 2014
- Miguel-Diez J, Jimenez-Garcia R, Jimenez D, Monreal M, Guijarro R, Otero R, et al. Trends in hospital admissions for pulmonary embolism in Spain from 2002 to 2011. European Respiratory Journal 2014;44:942-50. [DOI] [PubMed] [Google Scholar]
Deeks 2022
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Available from training.cochrane.org/handbook.
Dentali 2015
- Dentali F, Di Minno MN, Gianni M, Ambrosino P, Squizzato A, Ageno W. Non-vitamin K oral anticoagulants in patients with pulmonary embolism: a systematic review and meta-analysis of the literature. Internal and Emergency Medicine 2015;10(4):507-14. [DOI] [PubMed] [Google Scholar]
Dentali 2016
- Dentali F, Ageno W, Pomero F, Fenoglio L, Squizzato A, Bonzini M. Timetrends and case fatality rate of in-hospital treated pulmonary embolism during11 years of observation in Northwestern Italy. Thrombosis and Haemostasis 2016;115:399-405. [DOI] [PubMed] [Google Scholar]
Eikelboom 2010
- Eikelboom JW, Weitz JI. Update on antithrombotic therapy: new anticoagulants. Circulation 2010;121(13):1523-32. [DOI] [PubMed] [Google Scholar]
Eriksson 2003
- Eriksson H, Wåhlander K, Gustafsson D, Welin LT, Frison L, Schulman S, et al. A randomized, controlled, dose-guiding study of the oral direct thrombin inhibitor ximelagatran compared with standard therapy for the treatment of acute deep vein thrombosis: THRIVE I. Journal of Thrombosis and Haemostasis 2003;1(1):41-7. [DOI] [PubMed] [Google Scholar]
Eriksson 2007
- Eriksson BI, Dahl OE, Rosenecher N, Kurtha AA, Dijk CN, Frostick SP, et al. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. Journal of Thrombosis and Haemostasis 2007;5(11):2178-85. [DOI] [PubMed] [Google Scholar]
Eriksson 2009
- Eriksson BI, Quinlan DJ, Weitz JI. Comparative pharmacodynamics and pharmacokinetics of oral direct thrombin and factor Xa inhibitors in development. Clinical Pharmacokinetics 2009;48(1):1-22. [DOI] [PubMed] [Google Scholar]
FDA 2017
- FDA approved betrixaban (BEVYXXA, Portola) for the prophylaxis of venous thromboembolism (VTE) in adult patients. fda.gov/drugs/resources-information-approved-drugs/fda-approved-betrixaban-bevyxxa-portola-prophylaxis-venous-thromboembolism-vte-adult-patients (accessed 13 December 2022).
Fox 2012
- Fox BD, Kahn SR, Langleben D, Eisenberg MJ, Shimony A. Efficacy and safety of novel oral anticoagulants for treatment of acute venous thromboembolism: direct and adjusted indirect meta-analysis of randomised controlled trials. BMJ 2012;345:e7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldhaber 2016
- Goldhaber SZ, Schellong S, Kakkar A, Eriksson H, Feuring M, Kreuzer J, et al. Treatment of acute pulmonary embolism with dabigatran versus warfarin. A pooled analysis of data from RE-COVER and RE-COVER II. Thrombosis and Haemostasis 2016;116(4):714-21. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 6 July 2022. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Gómez‐Outes 2014
- Gomez-Outes A, Terleira-Fernandez AI, Lecumberri R, Suarez-Gea ML, Vargas-Castrillon E. Direct oral anticoagulants in the treatment of acute venous thromboembolism: a systematic review and meta-analysis. Thrombosis Research 2014;134(4):774-82. [DOI] [PubMed] [Google Scholar]
Gómez‐Outes 2015
- Gómez-Outes A, Lecumberri R, Suárez-Gea ML, Terleira-Fernández AI, Monreal M, Vargas-Castrillón E. Case fatality rates of recurrent thromboembolism and bleeding in patients receiving direct oral anticoagulants for the initial and extended treatment of venous thromboembolism: a systematic review. Journal of Cardiovascular Pharmacology and Therapeutics 2015;20(5):490-500. [DOI] [PubMed] [Google Scholar]
Heit 2015
- Heit JA. Epidemiology of venous thromboembolism. Nature Reviews Cardiology 2015;12(8):464-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Available from training.cochrane.org/handbook/archive/v5.2.
Higgins 2022
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Available from training.cochrane.org/handbook.
Hirschl 2014
- Hirschl M, Kundi M. New oral anticoagulants in the treatment of acute venous thromboembolism: a systematic review with indirect comparisons. Vasa 2014;43(5):353-64. [DOI] [PubMed] [Google Scholar]
Ho 2006
- Ho SJ, Brighton TA. Ximelagatran: direct thrombin inhibitor. Vascular Health and Risk Management 2006;2(1):49-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huerta 2007
- Huerta C, Johansson S, Wallander MA, Garcia Rodriguez LA. Risk factors and short-term mortality of venous thromboembolism diagnosed in the primary care setting in the United Kingdom. Archives of Internal Medicine 2007;167(9):935-43. [DOI] [PubMed] [Google Scholar]
Kakkos 2021
- Kakkos SK, Gohel M, Baekgaard N, Bauersachs R, Bellmunt-Montoya S, Black SA, et al. European Society for Vascular Surgery (ESVS) 2021 Clinical Practice Guidelines on the Management of Venous Thrombosis. European Journal of Vascular and Endovascular Surgery 2021;61(1):9-82. [DOI] [PubMed] [Google Scholar]
Kam 2005
- Kam PC, Kaur N, Thong CL. Direct thrombin inhibitors: pharmacology and clinical relevance. Anaesthesia 2005;60(6):565-74. [DOI] [PubMed] [Google Scholar]
Kang 2014
- Kang N, Sobieraj DM. Indirect treatment comparison of new oral anticoagulants for the treatment of acute venous thromboembolism. Thrombosis Research 2014;133:1145-51. [DOI] [PubMed] [Google Scholar]
Kearon 2012
- Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141(2 Suppl):e419S-94S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kearon 2016
- Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest 2016;149(2):315-52. [DOI] [PubMed] [Google Scholar]
Keller 2020
- Keller K, Hobohm L, Ebner M, Kresoja KP, Munzel T, Konstantinides SV, et al. Trends in thrombolytic treatment and outcomes of acute pulmonary embolismin Germany. European Heart Journal 2020;41:522-9. [DOI] [PubMed] [Google Scholar]
Konstantinides 2020
- Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, et al. ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). European Heart Journal 2020;41(4):543-603. [DOI] [PubMed] [Google Scholar]
Laurence 2012
- Laurence IJ, Redman SL, Corrigan AJ, Graham RN. V/Q SPECT imaging of acute pulmonary embolus - a practical perspective. Clinical Radiology 2012;67(10):941-8. [DOI] [PubMed] [Google Scholar]
Lee 2011
- Lee CJ, Ansell JE. Direct thrombin inhibitors. British Journal of Clinical Pharmacology 2011;72(4):581-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2021
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf MI, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Available from training.cochrane.org/handbook.
Lehnert 2018
- Lehnert P, Lange T, Moller CH, Olsen PS, Carlsen J. Acute pulmonary embolismin a national Danish cohort: increasing incidence and decreasing mortality. Thrombosis and Haemostasis 2018;118:539-46. [DOI] [PubMed] [Google Scholar]
Li 2019
- Li A, Garcia DA, Lyman GH, Carrier M. Direct oral anticoagulant (DOAC) versus low-molecular-weight heparin (LMWH) for treatment of cancer-associated thrombosis (CAT): a systematic review and meta-analysis. Thrombosis Research 2019;173:158-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lutsey 2019
- Lutsey PL, Walker RF, MacLehose RF, Alonso A, Adam TJ, Zakai NA. Direct oral anticoagulants and warfarin for venous thromboembolism treatment: trends from 2012 to 2017. Research and Practice in Thrombosis and Haemostasis 2019;3(4):668-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
McKenzie 2002
- McKenzie JE, Brennan SE. Chapter 12: Synthesizing and presenting findings using other methods. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Moik 2020
- Moik F, Posch F, Zielinski C, Pabinger I, Ay C. Direct oral anticoagulants compared to low-molecular-weight heparin for the treatment of cancer-associated thrombosis: updated systematic review and meta-analysis of randomized controlled trials. Research and Practice in Thrombosis and Haemostasis 2020;4(4):550-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2020
- National Institute for Health and Care Excellence. Venous thromboembolic diseases: diagnosis, management and thrombophilia testing, 2020. nice.org.uk/guidance/ng158 (accessed 29 July 2022).
Oldgren 2011
- Oldgren J, Budaj A, Granger CB, Khder Y, Roberts J, Siegbahn A, et al. Dabigatran vs. placebo in patients with acute coronary syndromes on dual antiplatelet therapy: a randomized, double-blind, phase II trial. European Heart Journal 2011;32(22):2781-9. [DOI] [PubMed] [Google Scholar]
Page 2021
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Palladino 2013
- Palladino M, Merli G, Thomson L. Evaluation of the oral direct factor Xa inhibitor - betrixaban. Expert Opinion on Investigational Drugs 2013;22(11):1465-72. [DOI] [PubMed] [Google Scholar]
Qaseem 2007
- Qaseem A, Snow V, Barry PE, Hornbake R, Rodnick JE, Tobolic T, et al. Current diagnosis of venous thromboembolism in primary care: a clinical practice guideline from the American Academy of Family Physicians and the American College of Physicians. Annals of Internal Medicine 2007;146(6):454-8. [DOI] [PubMed] [Google Scholar]
RevMan Web 2022 [Computer program]
- Review Manager Web (RevMan Web). Version 4.12.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Riedel 2004
- Riedel M. Diagnosing pulmonary embolism. Postgraduate Medicine Journal 2004;80(944):309-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Robertson 2015b
- Robertson L, Kesteven P. Oral direct thrombin inhibitors or oral factor Xa inhibitors for the treatment of deep vein thrombosis. Cochrane Database of Systematic Reviews 2015, Issue 6. Art. No: CD010956. [DOI: 10.1002/14651858.CD010956.pub2] [DOI] [PubMed] [Google Scholar]
Samaranayake 2022
- Samaranayake CB, Anderson J, McCabe C, Zahir SF, W Upham J, Keir G. Direct oral anticoagulants for cancer-associated venous thromboembolisms: a systematic review and network meta-analysis. Internal Medicine Journal 2022;52(2):272-81. [DOI] [PubMed] [Google Scholar]
Sardar 2014
- Sardar P, Chatterjee S, Mukherjee D. Efficacy and safety or new oral anticoagulants for extended treatment of venous thromboembolism: systematic review and meta-analyses of randomised controlled trials. Drugs 2013;73:1171-82. [DOI] [PubMed] [Google Scholar]
Schulman 2005
- Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. Journal of Thrombosis and Haemostasis 2005;3(4):692-4. [DOI] [PubMed] [Google Scholar]
Schulman 2011
- Schulman S, Kakkar AK, Schellong SM, Goldhaber SZ, Henry E, Mismetti P, et al. A randomized trial of dabigatran versus warfarin in the treatment of acute venous thromboembolism (RE-COVER II). Blood 2011;118:Abstract 205. [Google Scholar]
Schünemann 2022a
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Available from training.cochrane.org/handbook 2022.
Schünemann 2022b
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Available from training.cochrane.org/handbook.
Song 2021
- Song X, Liu Z, Zeng R, Shao J, Liu B, Zheng Y, et al. Treatment of venous thromboembolism in cancer patients: a systematic review and meta-analysis on the efficacy and safety of different direct oral anticoagulants (DOACs). Annals of Translational Medicine 2021;9(2):162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spyropoulos 2012
- Spyropoulos AC, Douketis JD. How I treat anticoagulated patients undergoing an elective procedure or surgery. Blood 2012;120(15):2954-62. [DOI] [PubMed] [Google Scholar]
Stein 1992
- Stein PD, Athanasoulis C, Alavi A, Greenspan RH, Hales CA, Saltzman HA, et al. Complications and validity of pulmonary angiography in acute pulmonary embolism. Circulation 1992;85(2):462-8. [DOI] [PubMed] [Google Scholar]
Stevens 2021
- Stevens SM, Woller SC, Baumann Kreuziger L, Bounameaux H, Doerschug K, Geersing GJ, et al. Executive summary: antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest 2021;160(6):2247-59. [DOI] [PubMed] [Google Scholar]
Thapa 2019
- Thapa N, Shatzel J, Deloughery TG, Olson SR. Direct oral anticoagulants in gastrointestinal malignancies: is the convenience worth the risk? Journal of Gastrointestinal Oncology 2019;10(4):807-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van de Werf 2012
- Van de Werf F, Brueckmann M, Connolly SJ, Friedman J, Granger CB, Hartter S, et al. A comparison of dabigatran etexilate with warfarin in patients with mechanical heart valves: the randomized, phase II study to evaluate the safety and pharmacokinetics of oral dabigatran etexilate in patients after heart valve replacement (RE-ALIGN). American Heart Journal 2012;163(6):931-7. [DOI] [PubMed] [Google Scholar]
Van der Huille 2014
- Van der Huille T, Den Exter PL, Dekkers OM, Klok FA. Effectiveness and safety of novel anticoagulants as compared with vitamin K antagonists in the treatment of acute symptomatic venous thromboembolism: a systematic review and meta-analysis. Journal of Thrombosis and Haemostasis 2014;12:320-8. [DOI] [PubMed] [Google Scholar]
Wang 2018
- Wang KL, Van Es N, Cameron C, Castellucci LA, Büller HR, Carrier M. Extended treatment of venous thromboembolism: a systematic review and network meta-analysis. Heart 2019;105(7):545-52. [DOI] [PubMed] [Google Scholar]
Wang 2023
- Wang X, Ma Y, Hui X, Li M, Li J, Tian J, et al. Oral direct thrombin inhibitors or oral factor Xa inhibitors versus conventional anticoagulants for the treatment of deep vein thrombosis. Cochrane Database of Systematic Reviews 2023, Issue 4. Art. No: CD010956. [DOI: 10.1002/14651858.CD010956.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weitz 2003
- Weitz JI. A novel approach to thrombin inhibition. Thrombosis Research 2003;109(Suppl 1):S17-22. [DOI] [PubMed] [Google Scholar]
Wells 2000
- Wells PS, Anderson DR, Rodger M, Ginsberg JS, Kearon C, Gent M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thrombosis and Haemostasis 2000;83(3):416-20. [PubMed] [Google Scholar]
Wendelboe 2016
- Wendelboe AM, Raskob GE. Global burden of thrombosis: epidemiologic aspects. Circulation Research 2016;118:1340-7. [DOI] [PubMed] [Google Scholar]
Wu 2022
- Wu O, Morris S, Larsen TB, Skjøth F, Evans A, Bowrin K, et al. Effectiveness and safety of nonvitamin K oral anticoagulants rivaroxaban and apixaban in patients with venous thromboembolism: a meta-analysis of real-world studies. Cardiovascular Therapeutics 2022;2022:2756682. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Robertson 2014b
- Robertson L, Kesteven P. Oral direct thrombin inhibitors or oral factor Xa inhibitors for the treatment of pulmonary embolism. Cochrane Database of Systematic Reviews 2014, Issue 2. Art. No: CD010957. [DOI: 10.1002/14651858.CD010957] [DOI] [PMC free article] [PubMed] [Google Scholar]
Robertson 2015a
- Robertson L, Kesteven P, McCaslin JE. Oral direct thrombin inhibitors or oral factor Xa inhibitors for the treatment of pulmonary embolism. Cochrane Database of Systematic Reviews 2015, Issue 12. Art. No: CD010957. [DOI: 10.1002/14651858.CD010957.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


