Abstract
OBJECTIVE: To determine whether synovial macrophages and monocytes isolated from patients with rheumatoid arthritis patients are capable of differentiating into osteoclastic bone resorbing cells; and the cellular and humoral conditions required for this to occur. METHODS: Macrophages isolated from the synovium and monocytes from the peripheral blood of rheumatoid arthritis patients were cultured on bone slices and coverslips, in the presence and absence of UMR 106 rat osteoblast-like cells, 1,25-dihydroxy vitamin D3 (1,25(OH)2D3) and macrophage colony stimulating factor (M-CSF), and assessed for cytochemical and functional evidence of osteoclast differentiation. RESULTS: Isolated calcitonin receptor (CTR), tartrate resistant acid phosphatase (TRAP), and vitronectin receptor (VNR) negative, CD11b and CD14 positive monocytes and macrophages differentiated into CTR, TRAP, and VNR positive multinucleated cells capable of extensive lacunar bone resorption when co-cultured for 14 d with UMR 106 cells in the presence 1,25(OH)2D3 and M-CSF. CONCLUSIONS: Mononuclear phagocytes (monocytes and macrophages) from rheumatoid arthritis patients are capable of differentiating into multinucleated cells showing all the cytochemical and functional criteria of mature osteoclasts. Synovial macrophage-osteoclast differentiation may represent an important cellular mechanism in the bone destruction associated with rheumatoid arthritis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Hamilton T. A. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- Allard S. A., Bayliss M. T., Maini R. N. The synovium-cartilage junction of the normal human knee. Implications for joint destruction and repair. Arthritis Rheum. 1990 Aug;33(8):1170–1179. doi: 10.1002/art.1780330818. [DOI] [PubMed] [Google Scholar]
- Allen C. A., Highton J., Palmer D. G. Increased expression of p150,95 and CR3 leukocyte adhesion molecules by mononuclear phagocytes in rheumatoid synovial membranes. Comparison with osteoarthritic and normal synovial membranes. Arthritis Rheum. 1989 Aug;32(8):947–954. doi: 10.1002/anr.1780320803. [DOI] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Athanasou N. A., Quinn J., Heryet A., Puddle B., Woods C. G., McGee J. O. The immunohistology of synovial lining cells in normal and inflamed synovium. J Pathol. 1988 Jun;155(2):133–142. doi: 10.1002/path.1711550210. [DOI] [PubMed] [Google Scholar]
- Athanasou N. A., Quinn J. Immunophenotypic differences between osteoclasts and macrophage polykaryons: immunohistological distinction and implications for osteoclast ontogeny and function. J Clin Pathol. 1990 Dec;43(12):997–1003. doi: 10.1136/jcp.43.12.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasou N. A., Wells C. A., Quinn J., Ferguson D. P., Heryet A., McGee J. O. The origin and nature of stromal osteoclast-like multinucleated giant cells in breast carcinoma: implications for tumour osteolysis and macrophage biology. Br J Cancer. 1989 Apr;59(4):491–498. doi: 10.1038/bjc.1989.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R., Neff L., Tran Van P., Nefussi J. R., Vignery A. Kinetic and cytochemical identification of osteoclast precursors and their differentiation into multinucleated osteoclasts. Am J Pathol. 1986 Feb;122(2):363–378. [PMC free article] [PubMed] [Google Scholar]
- Brennan F. M., Field M., Chu C. Q., Feldmann M., Maini R. N. Cytokine expression in rheumatoid arthritis. Br J Rheumatol. 1991;30 (Suppl 1):76–80. [PubMed] [Google Scholar]
- Bromley M., Woolley D. E. Histopathology of the rheumatoid lesion. Identification of cell types at sites of cartilage erosion. Arthritis Rheum. 1984 Aug;27(8):857–863. doi: 10.1002/art.1780270804. [DOI] [PubMed] [Google Scholar]
- Bromley M., Woolley D. E. Histopathology of the rheumatoid lesion. Identification of cell types at sites of cartilage erosion. Arthritis Rheum. 1984 Aug;27(8):857–863. doi: 10.1002/art.1780270804. [DOI] [PubMed] [Google Scholar]
- Bröker B. M., Edwards J. C., Fanger M. W., Lydyard P. M. The prevalence and distribution of macrophages bearing Fc gamma R I, Fc gamma R II, and Fc gamma R III in synovium. Scand J Rheumatol. 1990;19(2):123–135. doi: 10.3109/03009749009102116. [DOI] [PubMed] [Google Scholar]
- Burmester G. R., Locher P., Koch B., Winchester R. J., Dimitriu-Bona A., Kalden J. R., Mohr W. The tissue architecture of synovial membranes in inflammatory and non-inflammatory joint diseases. I. The localization of the major synovial cell populations as detected by monoclonal reagents directed towards Ia and monocyte-macrophage antigens. Rheumatol Int. 1983;3(4):173–181. doi: 10.1007/BF00541597. [DOI] [PubMed] [Google Scholar]
- Chambers T. J., Horton M. A. Failure of cells of the mononuclear phagocyte series to resorb bone. Calcif Tissue Int. 1984 Sep;36(5):556–558. doi: 10.1007/BF02405365. [DOI] [PubMed] [Google Scholar]
- Chambers T. J., Revell P. A., Fuller K., Athanasou N. A. Resorption of bone by isolated rabbit osteoclasts. J Cell Sci. 1984 Mar;66:383–399. doi: 10.1242/jcs.66.1.383. [DOI] [PubMed] [Google Scholar]
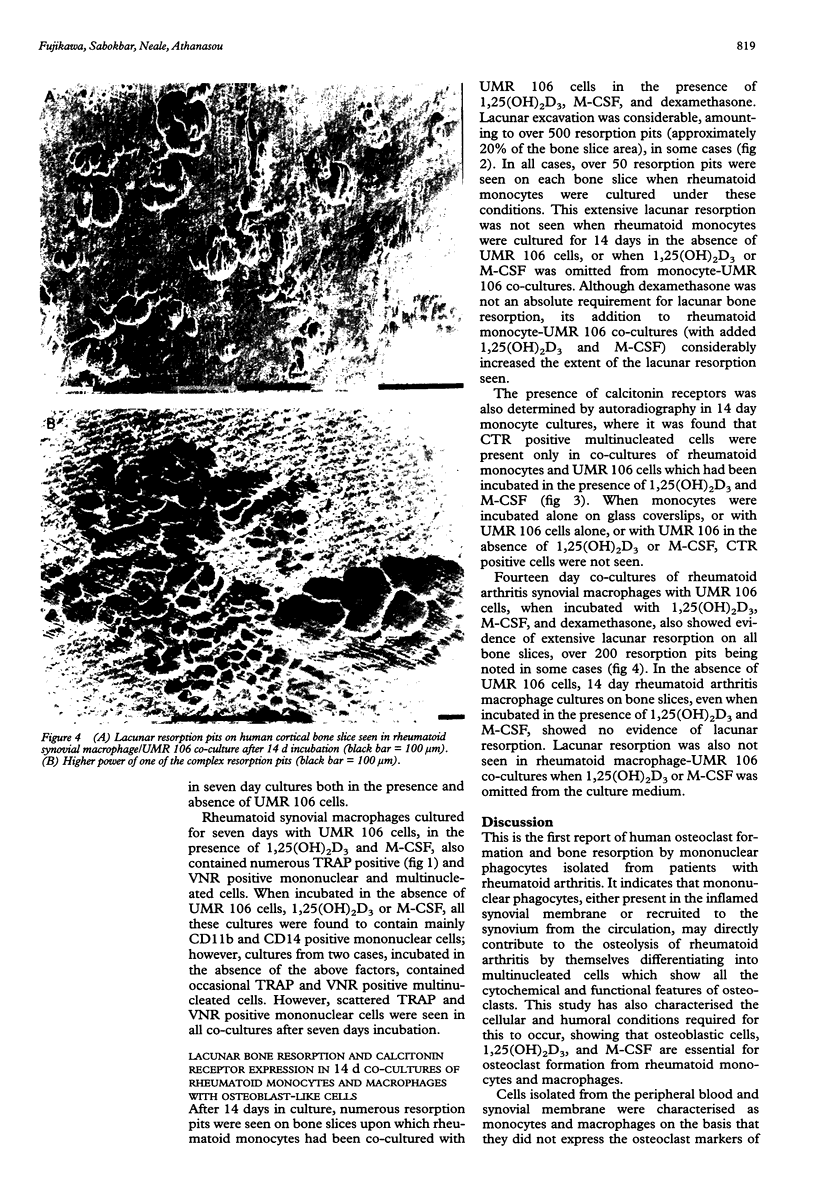
- Chang J. S., Quinn J. M., Demaziere A., Bulstrode C. J., Francis M. J., Duthie R. B., Athanasou N. A. Bone resorption by cells isolated from rheumatoid synovium. Ann Rheum Dis. 1992 Nov;51(11):1223–1229. doi: 10.1136/ard.51.11.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle J. T. The role of cellular interactions in joint erosions. Clin Orthop Relat Res. 1984 Jan-Feb;(182):24–30. [PubMed] [Google Scholar]
- Felix R., Cecchini M. G., Fleisch H. Macrophage colony stimulating factor restores in vivo bone resorption in the op/op osteopetrotic mouse. Endocrinology. 1990 Nov;127(5):2592–2594. doi: 10.1210/endo-127-5-2592. [DOI] [PubMed] [Google Scholar]
- Felix R., Cecchini M. G., Hofstetter W., Elford P. R., Stutzer A., Fleisch H. Impairment of macrophage colony-stimulating factor production and lack of resident bone marrow macrophages in the osteopetrotic op/op mouse. J Bone Miner Res. 1990 Jul;5(7):781–789. doi: 10.1002/jbmr.5650050716. [DOI] [PubMed] [Google Scholar]
- Fujii I., Shingu M., Nobunaga M. Monocyte activation in early onset rheumatoid arthritis. Ann Rheum Dis. 1990 Jul;49(7):497–503. doi: 10.1136/ard.49.7.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerne P. A., Zuraw B. L., Vaughan J. H., Carson D. A., Lotz M. Synovium as a source of interleukin 6 in vitro. Contribution to local and systemic manifestations of arthritis. J Clin Invest. 1989 Feb;83(2):585–592. doi: 10.1172/JCI113921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale L. P., Martin M. E., McCollum D. E., Nunley J. A., Springer T. A., Singer K. H., Haynes B. F. Immunohistologic analysis of the distribution of cell adhesion molecules within the inflammatory synovial microenvironment. Arthritis Rheum. 1989 Jan;32(1):22–30. doi: 10.1002/anr.1780320105. [DOI] [PubMed] [Google Scholar]
- Hayes M. E., Denton J., Freemont A. J., Mawer E. B. Synthesis of the active metabolite of vitamin D, 1,25(OH)2D3, by synovial fluid macrophages in arthritic diseases. Ann Rheum Dis. 1989 Sep;48(9):723–729. doi: 10.1136/ard.48.9.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson B., Revell P. A., Edwards J. C. Synovial lining cell hyperplasia in rheumatoid arthritis: dogma and fact. Ann Rheum Dis. 1988 Apr;47(4):348–349. doi: 10.1136/ard.47.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg N., Palmer D. G., Revell P. A. Mononuclear phagocytes of normal and rheumatoid synovial membrane identified by monoclonal antibodies. Immunology. 1985 Dec;56(4):673–681. [PMC free article] [PubMed] [Google Scholar]
- Horton M. A., Lewis D., McNulty K., Pringle J. A., Chambers T. J. Monoclonal antibodies to osteoclastomas (giant cell bone tumors): definition of osteoclast-specific cellular antigens. Cancer Res. 1985 Nov;45(11 Pt 2):5663–5669. [PubMed] [Google Scholar]
- Kotake S., Sato K., Kim K. J., Takahashi N., Udagawa N., Nakamura I., Yamaguchi A., Kishimoto T., Suda T., Kashiwazaki S. Interleukin-6 and soluble interleukin-6 receptors in the synovial fluids from rheumatoid arthritis patients are responsible for osteoclast-like cell formation. J Bone Miner Res. 1996 Jan;11(1):88–95. doi: 10.1002/jbmr.5650110113. [DOI] [PubMed] [Google Scholar]
- McCarthy D., Taylor M. J., Bernhagen J., Perry J. D., Hamblin A. S. Leucocyte integrin and CR1 expression on peripheral blood leucocytes of patients with rheumatoid arthritis. Ann Rheum Dis. 1992 Mar;51(3):307–312. doi: 10.1136/ard.51.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy G. R., Varani J., Orr W., Gondek M. D., Ward P. A. Resorbing bone is chemotactic for monocytes. Nature. 1978 Sep 14;275(5676):132–135. doi: 10.1038/275132a0. [DOI] [PubMed] [Google Scholar]
- Nicholson G. C., Moseley J. M., Sexton P. M., Mendelsohn F. A., Martin T. J. Abundant calcitonin receptors in isolated rat osteoclasts. Biochemical and autoradiographic characterization. J Clin Invest. 1986 Aug;78(2):355–360. doi: 10.1172/JCI112584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge N. C., Alcorn D., Michelangeli V. P., Kemp B. E., Ryan G. B., Martin T. J. Functional properties of hormonally responsive cultured normal and malignant rat osteoblastic cells. Endocrinology. 1981 Jan;108(1):213–219. doi: 10.1210/endo-108-1-213. [DOI] [PubMed] [Google Scholar]
- Quinn J. M., Athanasou N. A. Tumour infiltrating macrophages are capable of bone resorption. J Cell Sci. 1992 Mar;101(Pt 3):681–686. doi: 10.1242/jcs.101.3.681. [DOI] [PubMed] [Google Scholar]
- Quinn J. M., McGee J. O., Athanasou N. A. Cellular and hormonal factors influencing monocyte differentiation to osteoclastic bone-resorbing cells. Endocrinology. 1994 Jun;134(6):2416–2423. doi: 10.1210/endo.134.6.8194468. [DOI] [PubMed] [Google Scholar]
- Quinn J. M., Sabokbar A., Athanasou N. A. Cells of the mononuclear phagocyte series differentiate into osteoclastic lacunar bone resorbing cells. J Pathol. 1996 May;179(1):106–111. doi: 10.1002/(SICI)1096-9896(199605)179:1<106::AID-PATH535>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Quinn J., Joyner C., Triffitt J. T., Athanasou N. A. Polymethylmethacrylate-induced inflammatory macrophages resorb bone. J Bone Joint Surg Br. 1992 Sep;74(5):652–658. doi: 10.1302/0301-620X.74B5.1527108. [DOI] [PubMed] [Google Scholar]
- Roussel M. F., Downing J. R., Rettenmier C. W., Sherr C. J. A point mutation in the extracellular domain of the human CSF-1 receptor (c-fms proto-oncogene product) activates its transforming potential. Cell. 1988 Dec 23;55(6):979–988. doi: 10.1016/0092-8674(88)90243-7. [DOI] [PubMed] [Google Scholar]
- Seitz M., Loetscher P., Fey M. F., Tobler A. Constitutive mRNA and protein production of macrophage colony-stimulating factor but not of other cytokines by synovial fibroblasts from rheumatoid arthritis and osteoarthritis patients. Br J Rheumatol. 1994 Jul;33(7):613–619. doi: 10.1093/rheumatology/33.7.613. [DOI] [PubMed] [Google Scholar]
- Sherr C. J. Colony-stimulating factor-1 receptor. Blood. 1990 Jan 1;75(1):1–12. [PubMed] [Google Scholar]
- Smith J. B., Bocchieri M. H., Smith J. B., Jr, Sherbin-Allen L., Abruzzo J. L. Colony stimulating factor occurs in both inflammatory and noninflammatory synovial fluids. Rheumatol Int. 1990;10(3):131–134. doi: 10.1007/BF02274828. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Udagawa N., Tanaka S., Murakami H., Owan I., Tamura T., Suda T. Postmitotic osteoclast precursors are mononuclear cells which express macrophage-associated phenotypes. Dev Biol. 1994 May;163(1):212–221. doi: 10.1006/dbio.1994.1137. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Yamana H., Yoshiki S., Roodman G. D., Mundy G. R., Jones S. J., Boyde A., Suda T. Osteoclast-like cell formation and its regulation by osteotropic hormones in mouse bone marrow cultures. Endocrinology. 1988 Apr;122(4):1373–1382. doi: 10.1210/endo-122-4-1373. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Takahashi N., Udagawa N., Tamura T., Akatsu T., Stanley E. R., Kurokawa T., Suda T. Macrophage colony-stimulating factor is indispensable for both proliferation and differentiation of osteoclast progenitors. J Clin Invest. 1993 Jan;91(1):257–263. doi: 10.1172/JCI116179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udagawa N., Takahashi N., Akatsu T., Tanaka H., Sasaki T., Nishihara T., Koga T., Martin T. J., Suda T. Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7260–7264. doi: 10.1073/pnas.87.18.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir E. C., Horowitz M. C., Baron R., Centrella M., Kacinski B. M., Insogna K. L. Macrophage colony-stimulating factor release and receptor expression in bone cells. J Bone Miner Res. 1993 Dec;8(12):1507–1518. doi: 10.1002/jbmr.5650081214. [DOI] [PubMed] [Google Scholar]
- Xu W. D., Firestein G. S., Taetle R., Kaushansky K., Zvaifler N. J. Cytokines in chronic inflammatory arthritis. II. Granulocyte-macrophage colony-stimulating factor in rheumatoid synovial effusions. J Clin Invest. 1989 Mar;83(3):876–882. doi: 10.1172/JCI113971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanni G., Whelan A., Feighery C., Bresnihan B. Synovial tissue macrophages and joint erosion in rheumatoid arthritis. Ann Rheum Dis. 1994 Jan;53(1):39–44. doi: 10.1136/ard.53.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Hayashi S., Kunisada T., Ogawa M., Nishikawa S., Okamura H., Sudo T., Shultz L. D., Nishikawa S. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990 May 31;345(6274):442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- Ziff M. Pathways of mononuclear cell infiltration in rheumatoid synovitis. Rheumatol Int. 1989;9(3-5):97–103. doi: 10.1007/BF00271865. [DOI] [PubMed] [Google Scholar]
- Zvaifler N. J., Firestein G. S. Pannus and pannocytes. Alternative models of joint destruction in rheumatoid arthritis. Arthritis Rheum. 1994 Jun;37(6):783–789. doi: 10.1002/art.1780370601. [DOI] [PubMed] [Google Scholar]
- van Furth R. Origin and turnover of monocytes and macrophages. Curr Top Pathol. 1989;79:125–150. [PubMed] [Google Scholar]