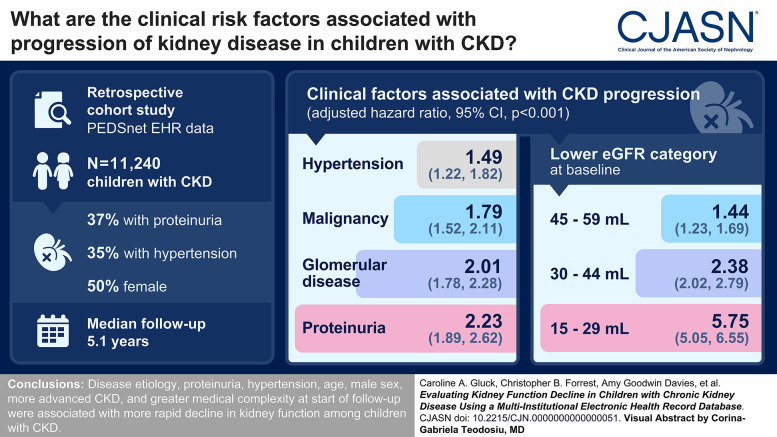
Visual Abstract
Keywords: children, chronic kidney disease, clinical nephrology, ESRD, progression of chronic renal failure, hematuria
Abstract
Background
The objectives of this study were to use electronic health record data from a US national multicenter pediatric network to identify a large cohort of children with CKD, evaluate CKD progression, and examine clinical risk factors for kidney function decline.
Methods
This retrospective cohort study identified children seen between January 1, 2009, to February 28, 2022. Data were from six pediatric health systems in PEDSnet. We identified children aged 18 months to 18 years who met criteria for CKD: two eGFR values <90 and ≥15 ml/min per 1.73 m2 separated by ≥90 days without an intervening value ≥90. CKD progression was defined as a composite outcome: eGFR <15 ml/min per 1.73 m2, ≥50% eGFR decline, long-term dialysis, or kidney transplant. Subcohorts were defined based on CKD etiology: glomerular, nonglomerular, or malignancy. We assessed the association of hypertension (≥2 visits with hypertension diagnosis code) and proteinuria (≥1 urinalysis with ≥1+ protein) within 2 years of cohort entrance on the composite outcome.
Results
Among 7,148,875 children, we identified 11,240 (15.7 per 10,000) with CKD (median age 11 years, 50% female). The median follow-up was 5.1 (interquartile range 2.8–8.3) years, the median initial eGFR was 75.3 (interquartile range 61–83) ml/min per 1.73 m2, 37% had proteinuria, and 35% had hypertension. The following were associated with CKD progression: lower eGFR category (adjusted hazard ratio [aHR] 1.44 [95% confidence interval (95% CI), 1.23 to 1.69], aHR 2.38 [95% CI, 2.02 to 2.79], aHR 5.75 [95% CI, 5.05 to 6.55] for eGFR 45–59 ml/min per 1.73 m2, 30–44 ml/min per 1.73 m2, 15–29 ml/min per 1.73 m2 at cohort entrance, respectively, when compared with eGFR 60–89 ml/min per 1.73 m2), glomerular disease (aHR 2.01 [95% CI, 1.78 to 2.28]), malignancy (aHR 1.79 [95% CI, 1.52 to 2.11]), proteinuria (aHR 2.23 [95% CI, 1.89 to 2.62]), hypertension (aHR 1.49 [95% CI, 1.22 to 1.82]), proteinuria and hypertension together (aHR 3.98 [95% CI, 3.40 to 4.68]), count of complex chronic comorbidities (aHR 1.07 [95% CI, 1.05 to 1.10] per additional comorbid body system), male sex (aHR 1.16 [95% CI, 1.05 to 1.28]), and younger age at cohort entrance (aHR 0.95 [95% CI, 0.94 to 0.96] per year older).
Conclusions
In large-scale real-world data for children with CKD, disease etiology, albuminuria, hypertension, age, male sex, lower eGFR, and greater medical complexity at start of follow-up were associated with more rapid decline in kidney function.
Introduction
CKD in children is rare, leading to major gaps in the quantity and quality of evidence that informs clinical decision making in pediatric CKD. CKD in children may occur as a result of congenital anomalies of the kidney and urinary tract (CAKUT) or acquired disease.1 The most common form of acquired pediatric CKD that leads to kidney failure is glomerular disease.2 In addition, repeated AKI episodes have been associated with higher risk for the development and progression of CKD.3,4 Importantly, children with CKD may suffer from cardiovascular disease, metabolic bone disease, and neurocognitive dysfunction.5–9 Cardiovascular disease in particular is increased in this population 10- to 100-fold as compared with the general population, and cardiovascular disease in children with kidney failure accounts for 25% of mortality.10 For children who require long-term dialysis in childhood, the average life expectancy is 38 years; for those children who receive a kidney transplant, the average life expectancy is 63 years.11
The most comprehensive epidemiologic data about progression of CKD in children come from the Chronic Kidney Disease in Children (CKiD) study.12 CKiD is an ongoing prospective cohort study that has enrolled >1000 children (over three recruitment waves during a 15-year period) from >50 centers across North America and followed them annually.12,13 CKiD has identified predictors of disease progression among children with mild-to-moderate CKD, revealing the important associations of glomerular disease etiology,5,14 hypertension,15–17 and proteinuria14,16,18 with the risk for kidney function decline. Data from CKiD have been invaluable. However, it is still limited by relatively small sample size, particularly for studying specific CKD etiologies, and potential selection bias in the recruited cohort. CKiD also excludes children with other comorbid conditions, such as malignancy. Although the detailed, granular data obtained in a prospective cohort study cannot be replicated using electronic health record (EHR) data obtained in routine clinical care, it is time- and resource-intensive, and participants may be less representative of the source population.
A major advantage of EHR data collected in real-world clinic settings is that selection bias is minimized, it directly reflects clinical practice, and the breadth of longitudinal data capture provides unprecedented opportunities for health outcomes and comparative effectiveness research, particularly in rare diseases. Within pediatrics, PEDSnet (pedsnet.org) was formed to leverage multi-institutional EHR data for clinical research.19 The objective of this study was to use real-world data from PEDSnet to identify children with CKD, evaluate CKD progression, and stratify clinical risk factors for kidney function decline. We hypothesized that large-scale real-world data could recapitulate factors associated with CKD progression demonstrated in prospective cohort studies. Therefore, we evaluated well-established risk factors for CKD progression, including glomerular disease etiology, hypertension, and proteinuria. Our secondary objective was to evaluate CKD progression in populations of children with CKD underrepresented in prior studies, particularly those with a history of malignancy.
Methods
Study Setting and Data Source
This study used data from PEDSnet, a clinical research network that has aggregated EHR data from inpatient and outpatient settings in the United States for over 7 million patients across six children's health care organizations (Institutional Review Board protocol #20-017298): Children's Hospital of Philadelphia (CHOP); Children's Hospital Colorado; Nationwide Children's Hospital; Nemours Children's Health System (a Delaware and Florida health system); Seattle Children's Hospital; and Cincinnati Children's Hospital Medical Center.20 Children in PEDSnet are from all 50 states; the states with the greatest concentration of patients are Colorado, Delaware, Florida, Illinois, Indiana, Kentucky, New Jersey, Ohio, Pennsylvania, and Washington.
PEDSnet standardizes EHR data across institutions to the PEDSnet common data model, which is based on the observational medical outcomes partnership common data model version 5.19 For this study, we used the PEDSnet database version 4.6, which spanned from January 2009 to February 2022. Data were extracted in August 2022. This analysis was determined to not meet the criteria for human subjects research by the CHOP Institutional Review Board, which was accepted by all participating PEDSnet sites.
Study Sample
Children with CKD were identified using the following criteria: two eGFR values <90 ml/min per 1.73 m2 and ≥15 ml/min per 1.73 m2 separated by at least 90 days without an intervening value ≥90 ml/min per 1.73 m2. As described by previous CKiD studies,12 it was expected that most children with CKD would have underlying CAKUT. To capture the association of risk factors with CKD progression early in disease course, an eGFR value <90 ml/min per 1.73 m2 was chosen. However, we additionally performed a sensitivity analysis for children with qualifying eGFR values <60 ml/min per 1.73 m2. eGFR was calculated using the creatinine-based and sex-specific estimating equation developed and validated in CKiD, CKiD under 25.21 eGFR was calculated using the closest height measurement within 180 days of the creatinine measurement; in cases with multiple eGFR values on the same day, the smallest value was used for this analysis. As a data quality measure, we excluded implausible data: creatinine (≤0 or ≥50) or height (Centers for Disease Control and Prevention [CDC] height z-score ≥3 or ≤−5). Children entered the cohort on the date of the first qualifying eGFR value <90 ml/min per 1.73 m2 and ≥15 ml/min per 1.73 m2. Children were excluded for age <18 months or >18 years at cohort entrance. They were also excluded if they had long-term dialysis or a kidney transplant procedure before cohort entrance. Dialysis and kidney transplant status were determined using EHR procedure codes (all codesets used are available at https://github.com/PEDSnet/ckd_progression). Finally, to ensure that we captured clinically significant CKD (for example due to CAKUT) and not recurrent episodes of AKI, we required patients to have been seen by a nephrologist in an outpatient setting on or after cohort entry. Of note, in contrast to adult practice, children with mildly impaired GFR are routinely referred to pediatric nephrologists for care. The overwhelming majority of pediatric nephrologists practice within large children’s hospitals that provide dialysis and kidney transplantation for this population.22
Three subcohorts of children with CKD were identified on the basis of CKD etiology: glomerular, nonglomerular, and malignancy associated. The first subcohort comprised children with a history of malignancy, defined as having at least two physician visits (inpatient, outpatient, or emergency department visits) on different days at which at least one diagnosis code for malignancy was used. Children without a history of malignancy were further categorized as having glomerular versus nonglomerular CKD using a computable phenotype algorithm for glomerular disease developed and evaluated in PEDSnet.23 As described by Denburg et al., the glomerular disease computable phenotype requires two occurrences of glomerular diagnosis codes or one occurrence of a glomerular diagnosis code and a native kidney biopsy.
Outcomes
CKD progression was defined by the composite kidney failure outcome of eGFR <15 ml/min per 1.73 m2, ≥50% decline in eGFR, long-term dialysis, or kidney transplant. Long-term dialysis was defined as the occurrence of two dialysis codes separated by at least 90 days (to avoid capture of acute dialysis). This composite outcome was used to be consistent with prior studies of CKD progression in CKiD.14,16
Covariates
We assessed the association of hypertension and proteinuria with the composite outcomes overall and across subcohorts. Hypertension was defined by the presence of a diagnosis code associated with at least two physician visits (inpatient, outpatient, or emergency department visits) within 2 years of cohort entrance. The hypertension codeset was developed based on clinician consensus. Because the majority of our cohort was expected to have nonglomerular CKD (due to congenital anomalies of the kidney or urinary tract or tubulointerstitial disease), proteinuria was defined as having at least one urinalysis with at least 1+ proteinuria within 2 years of cohort entrance to maximize our sensitivity. We additionally performed a sensitivity analysis that required patients to have two urinalyses each with 1+ or more proteinuria within 2 years of cohort entrance. Variables included age at cohort entrance, sex, race/ethnicity (on the basis of self-report in EHR), duration of follow-up, eGFR and eGFR category at cohort entrance, and health system. To account for chronic disease burden, we used the numeric count of body systems with chronic conditions in the 3 years before cohort entrance using International Classification of Diseases (ICD) 9/10 codes as specified by the pediatric medical complexity algorithm (PMCA)24; because many patients in our pediatric cohort had CKD due to genitourinary disease, the PMCA count was filtered to exclude kidney and genitourinary disease from the count.
Statistical Analyses
Descriptive statistics were used to summarize cohort characteristics using medians and interquartile ranges (IQRs) for continuous variables and counts and frequencies for categorical variables. Cumulative incidence analysis of the time to progression to the composite kidney failure outcome was performed by subcohort of CKD etiology (malignancy, glomerular, and nonglomerular) and by clinical risk factors of hypertension and proteinuria. We additionally completed a sensitivity analysis with death as a competing risk. Multivariable Cox proportional hazards and accelerated failure time (Weibull) models were used to analyze the association of clinical covariates with the time to reaching the composite outcome using software packages in R version 4.2.1. In performing these models, all variables were tested for collinearity. The final models include the following variables: age, sex, CKD etiology, proteinuria and/or hypertension, eGFR category at cohort entrance, and PMCA count (without kidney or genitourinary categories). Missing data were treated as true missing for these analyses. In performing the Cox proportional hazards regression analysis, we noted that the data violated the assumption for proportional hazards. Therefore, we additionally used an accelerated failure time model to estimate the time to composite outcome across these groups, whereby the estimate represents survival time ratios, which are smaller when time to the outcome is accelerated.
Results
Figure 1 shows the study sample derivation, which yielded a cohort of 11,240 children (final sample 15.7 per 10,000 children in PEDSnet). Descriptive characteristics of this cohort and subcohorts are shown in Table 1. Overall, the median age at cohort entrance was 11 years with a follow-up time from cohort entrance of 5.1 (IQR 2.8–8.3) years. Children in the nonglomerular CKD cohort included children with CAKUT, such as hydronephrosis, renal dysplasia, renal agenesis, and obstructive and reflux nephropathy; see Supplemental Table 1 showing the 25 most frequent PMCA kidney and genitourinary diagnosis codes for this subcohort on or after cohort entry. Children with a history of malignancy were younger at cohort entrance and had longer follow-up time than the other subcohorts. There were no significant differences in sex across cohorts. Most children had eGFR 60–89 ml/min per 1.73 m2 at cohort entrance; the glomerular CKD subcohort had lower median eGFR and a greater proportion of eGFR 15–59 ml/min per 1.73 m2 than the other subcohorts. Overall and across subcohorts, at least 31% had proteinuria and at least 32% had hypertension within 2 years of cohort entrance. There were 2408 children (21%) missing proteinuria measurement. The burden of proteinuria and hypertension was highest among children with glomerular CKD. We examined treatment with antihypertensives and in particular renin-angiotensin-aldosterone system blockade for patients with a diagnosis of hypertension and/or proteinuria (Supplemental Table 2). Sixty-one percent of patients with hypertension were treated with any antihypertensive within the first 2 years, and 59% of patients with both hypertension and proteinuria were treated with renin-angiotensin-aldosterone system blockade. The proportion of children with a cardiac diagnosis at cohort entrance was 16% to 26% across subcohorts. Descriptive characteristics for the sensitivity analysis that assessed stricter definitions for cohort entry (eGFR <60 ml/min per 1.73 m2) and proteinuria (two values) are included in Supplemental Tables 3 and 4, respectively. Compared with the original analysis, in the cohort requiring eGFR <60 ml/min per 1.73 m2, we noted the overall cohort was smaller (3210 children) with more male participants in the overall cohort (55%). As expected, a larger proportion of the eGFR <60 cohort had hypertension (48%) and proteinuria (57%). Using a stricter definition for proteinuria, the percentage of children with proteinuria in the nonglomerular and glomerular subcohorts decreased from 31% and 81%, respectively, to 17% and 69% in the sensitivity analysis.
Figure 1.
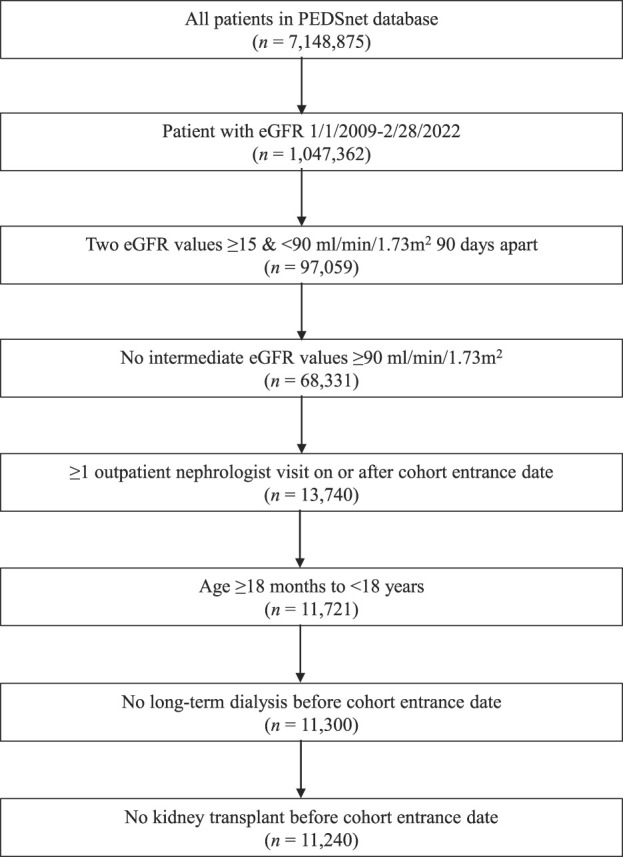
Study sample derivation.
Table 1.
Characteristics of children with CKD in PEDSnet at the time of cohort entry
| Characteristic | Nonglomerular | Glomerular | Malignancy | Overall |
|---|---|---|---|---|
| N | 8987 | 1314 | 939 | 11,240 |
| Age at cohort entrance (yr) | 10 [5–14] | 13 [9–15] | 8 [4–13] | 11 [5–15] |
| Follow-up (yr) | 5.0 [2.7–8.2] | 4.9 [2.8–7.7] | 6.7 [4.2–10.2] | 5.1 [2.8–8.3] |
| Sex, n (%) | ||||
| Female | 4408 (49) | 689 (52) | 492 (52) | 5589 (50) |
| Male | 4579 (51) | 625 (48) | 447 (48) | 5651 (50) |
| Race/ethnicity,a n (%) | ||||
| Hispanic or Latino | 1139 (13) | 250 (19) | 104 (11) | 1493 (13) |
| Non-Hispanic Asian or Pacific Islander | 299 (3) | 65 (5) | 39 (4) | 403 (4) |
| Non-Hispanic Black or African American | 1899 (21) | 306 (23) | 133 (14) | 2338 (21) |
| Non-Hispanic White | 4934 (55) | 591 (45) | 593 (63) | 6118 (54) |
| Multiple/other/missing | 716 (8) | 102 (8) | 70 (7) | 888 (8) |
| eGFR at cohort entrance (ml/min per 1.73 m2) | 76 [63–83] | 69 [45–81] | 77 [66–84] | 75 [61–83] |
| eGFR category at cohort entrance, n (%) | ||||
| eGFR 60–89 ml/min per 1.73 m2 | 7011 (78) | 813 (62) | 780 (83) | 8604 (77) |
| eGFR 45–59 ml/min per 1.73 m2 | 960 (11) | 171 (13) | 88 (9) | 1219 (11) |
| eGFR 30–44 ml/min per 1.73 m2 | 514 (6) | 134 (10) | 39 (4) | 687 (6) |
| eGFR 15–29 ml/min per 1.73 m2 | 502 (6) | 196 (15) | 32 (3) | 730 (7) |
| No proteinuria,b n (%) | 4108 (46) | 137 (10) | 421 (45) | 4666 (42) |
| Proteinuria, n (%) | 2818 (31) | 1059 (81) | 289 (31) | 4166 (37) |
| Missing proteinuria, n (%) | 2061 (23) | 118 (9) | 229 (24) | 2408 (21) |
| Hypertension,c n (%) | 2854 (32) | 783 (60) | 347 (37) | 3984 (35) |
| History of cardiac disease,d n (%) | 1728 (19) | 206 (16) | 241 (26) | 2175 (19) |
Continuous data are presented as median and interquartile range and categorical data as N (%).
Race/ethnicity on the basis of self-report in electronic health record.
Proteinuria defined as ≥1 urinalysis with ≥1+ protein within 2 years of cohort entrance.
Hypertension defined as ≥2 visits with hypertension diagnosis code within 2 years of cohort entrance.
Cardiac diagnosis within 3 years before cohort entrance determined using International Classification of Diseases (ICD) 9/10 codes provided by the pediatric medical complexity algorithm.23
Overall, 1874 children (17%) progressed to the composite kidney failure outcome of eGFR <15 ml/min per 1.73 m2, ≥50% decline in eGFR, long-term dialysis, or kidney transplant. A ≥50% decline in eGFR occurred in 1633 (15%) children, while 1135 (10%) of the cohort reached eGFR <15 ml/min per 1.73 m2. Long-term dialysis or kidney transplant occurred in 448 (4%) and 465 (4%) of the cohort, respectively. Children with glomerular CKD were more likely to reach the composite outcome (40%) than those with nonglomerular CKD (13%) and those with a history of malignancy (23%). Frequencies of the component end points of the composite outcome by cohort are shown in Supplemental Table 5. We additionally examined frequencies of reaching the composite end point by eGFR category at cohort entrance and the presence of proteinuria as shown in Supplemental Table 6.
Children with glomerular CKD experienced the most rapid progression to kidney failure, and children with malignancy-associated CKD progressed more rapidly than those with nonglomerular CKD; however, this effect of disease etiology was dampened by worse eGFR category on cohort entrance (Figure 2). Hypertension and proteinuria were associated with more rapid decline in kidney function (Figure 3). Children with CKD and both hypertension and proteinuria experienced the fastest progression to kidney failure. Figures for sensitivity analyses (eGFR <60 ml/min per 1.73 m2 for cohort entrance, strict proteinuria definition, and death as competing risk) are included in Supplemental Figures 1–4. In the sensitivity analysis for eGFR <60 cohort, CKD associated with glomerular disease and malignancy were again associated with higher risk for kidney disease progression. When death was analyzed as a competing risk, there was no significant effect on cumulative incidence of composite kidney failure outcomes in the main analysis or sensitivity analysis requiring eGFR <60 ml/min per 1.73 m2 for cohort entry. Proteinuria and hypertension remained robust risk factors associated with the composite kidney failure outcome across all sensitivity analyses.
Figure 2.
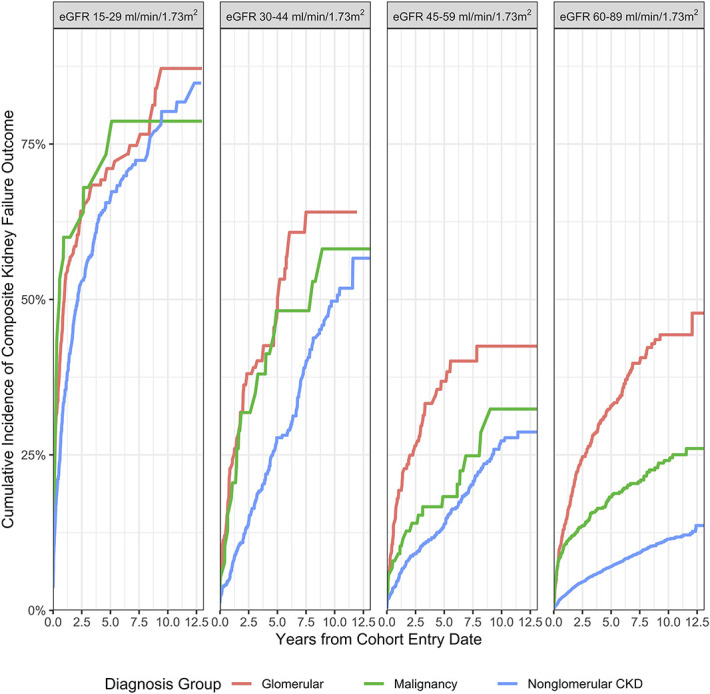
Cumulative incidence of composite kidney failure outcome by entry eGFR category and cohort.
Figure 3.
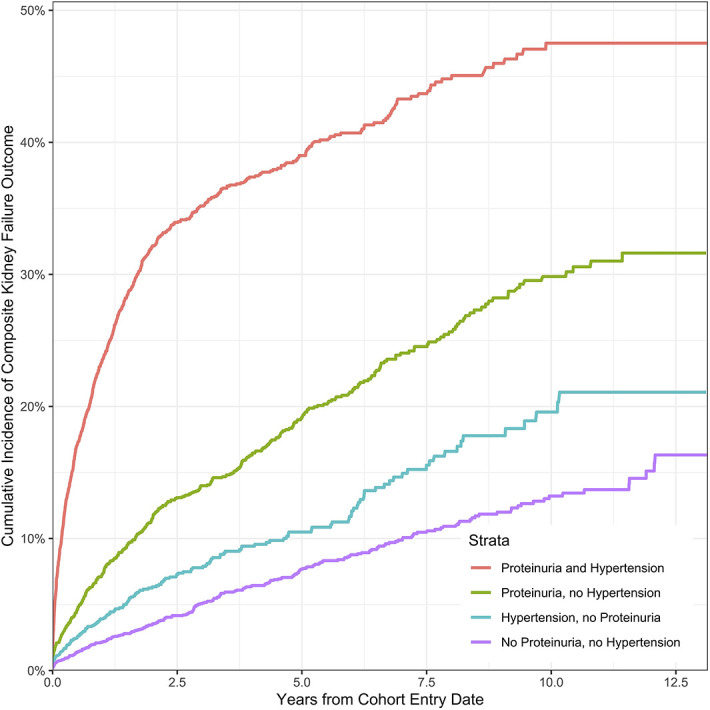
Cumulative incidence of composite kidney failure outcome by proteinuria and hypertension.
The multivariable Cox model (Table 2) demonstrated the significant and independent effects of hypertension and proteinuria on CKD progression: adjusted hazard ratio (aHR) associated with hypertension 1.49 (95% confidence interval [95% CI], 1.22 to 1.82) and proteinuria 2.23 (95% CI, 1.89 to 2.62). Children with glomerular disease and a history of malignancy as compared with the nonglomerular CKD subcohort had higher risks of CKD progression (aHR 2.01 [95% CI, 1.78 to 2.28] and aHR 1.79 [95% CI, 1.52 to 2.11], respectively). Children with both proteinuria and hypertension as compared with children without proteinuria or hypertension had higher risk of CKD progression aHR 3.98 (95% CI, 3.40 to 4.68). Children with greater CKD severity (lower eGFR category) at cohort entrance had higher risk of reaching the composite outcome; the highest risk occurred with children entering with eGFR 15–29 ml/min per 1.73 m2 with an aHR of 5.75 (95% CI, 5.05 to 6.55). Finally, children with higher degrees of medical complexity, male sex, and younger age at cohort entrance experienced higher risk of CKD progression. In addition, we assessed the speed at which patients reached the composite outcome using the accelerated failure time model. In the accelerated failure time model, patients who reach the outcome faster have a smaller coefficient. The results from the accelerated failure time model mirrored those from the Cox model because variables associated with higher aHRs in the Cox model had lower risk ratios in the accelerated failure time models. Models for sensitivity analyses are included in Supplemental Tables 7 and 8. For the eGFR <60 sensitivity analysis, the effects of proteinuria and hypertension remained associated with kidney failure outcomes, although the total effect size was slightly lower than in the main analysis (hypertension and proteinuria aHR 2.98 versus aHR 3.98). Effect size for glomerular disease also decreased from an aHR of 2.01 in the original analysis to 1.72 in the sensitivity analysis. This is likely due to interaction of disease etiology with eGFR category risk at the time of cohort entry. For the stricter proteinuria sensitivity analysis, effect size of proteinuria and hypertension together actually increased from aHR 3.98 in the original analysis to 4.45 in the sensitivity analysis. This is likely due to improved specificity of this definition.
Table 2.
Multivariable Cox proportional hazards model and Weibull accelerated failure time model evaluating the association of clinical factors with progression to the composite kidney failure outcome
| Exposurea | N (%) of Children with Event | Cox Model (Adjusted Hazard Ratio) | Weilbull Accelerated Failure Time Model (Log) (Ratio of Survival Times) | ||
|---|---|---|---|---|---|
| Estimates | Confidence Interval | Ratios | Confidence Interval | ||
| Age at cohort entrance (yr) | NA | 0.95 | 0.94 to 0.96 | 1.09 | 1.07 to 1.11 |
| Sex | |||||
| Female | 729 (16) | Reference | Reference | ||
| Male | 837 (19) | 1.16 | 1.05 to 1.28 | 0.80 | 0.67 to 0.96 |
| Proteinuria/hypertension | |||||
| No proteinuria, no hypertension | 252 (8) | Reference | Reference | ||
| Hypertension, no proteinuria | 156 (10) | 1.49 | 1.22 to 1.82 | 0.52 | 0.37 to 0.72 |
| Proteinuria, no hypertension | 410 (19) | 2.23 | 1.89 to 2.62 | 0.29 | 0.22 to 0.39 |
| Proteinuria, hypertension | 784 (38) | 3.98 | 3.40 to 4.68 | 0.08 | 0.06 to 0.11 |
| CKD etiology | |||||
| Nonglomerular | 918 (13) | Reference | Reference | ||
| Glomerular | 467 (39) | 2.01 | 1.78 to 2.28 | 0.23 | 0.18 to 0.29 |
| Malignancy | 181 (26) | 1.79 | 1.52 to 2.11 | 0.31 | 0.23 to 0.42 |
| eGFR category | |||||
| 60–89 ml/min per 1.73 m2 | 767 (12) | Reference | Reference | ||
| 45–59 ml/min per 1.73 m2 | 201 (20) | 1.44 | 1.23 to 1.69 | 0.51 | 0.39 to 0.67 |
| 30–44 ml/min per 1.73 m2 | 192 (34) | 2.38 | 2.02 to 2.79 | 0.23 | 0.17 to 0.30 |
| 15–29 ml/min per 1.73 m2 | 406 (65) | 5.75 | 5.05 to 6.55 | 0.03 | 0.02 to 0.04 |
| Medical complexityb | NA | 1.07 | 1.05 to 1.10 | 0.85 | 0.82 to 0.89 |
Exposures evaluated but not shown: clinical sites.
Numerical count of pediatric medical complexity algorithm diagnosis categories (excluding kidney and genitourinary categories).
Discussion
These data demonstrate that in children with CKD of glomerular or malignancy-associated origins, proteinuria, hypertension, younger age, more advanced CKD, male sex, and greater medical complexity at start of follow-up were associated with more rapid decline in kidney function. Children with these risk factors are of particular concern for CKD progression and may be targeted for comparative effectiveness studies to preserve kidney function.
This study used a large real-world EHR data resource to study progression of CKD in children. Among over 7.1 million children, we identified 11,240 (15.7 per 10,000) with CKD. This pragmatic study replicated key findings from the CKiD prospective cohort study that demonstrated the association of glomerular disease, hypertension, proteinuria, and eGFR category with kidney function decline. Effect sizes for these variables were similar when compared between EHR data and data from the CKiD cohort.
For example, in our real-world data, 13% of patients with nonglomerular CKD and 40% of patients with glomerular CKD reached the composite end point. Glomerular CKD as compared with nonglomerular CKD accelerated failure time as indicated by a smaller survival time ratio (0.23). In similar analyses using CKiD cohort data from 398 children, 29% of patients with nonglomerular CKD and 41% of patients with glomerular CKD reached a similar composite end point (long-term dialysis, transplant, or ≥50% reduction of GFR).16 Glomerular disease decreased time to the composite event by 43%.14 In our CKD cohort, patients with hypertension, proteinuria, and hypertension and proteinuria had an accelerated failure time (0.52, 0.29, and 0.08, respectively) compared with children with CKD without hypertension or proteinuria. This is comparable with the shortened time to composite event using a combination of CKiD cohort data and data from the Effect of Strict Blood Pressure Control and ACE Inhibition on Chronic Renal Failure Progression in Pediatric Patients clinical trial25; in 1232 children, the composite end point was reached in 41% of children with proteinuria and 79% of children with nephrotic range proteinuria when analyzed using lognormal regression methods.16
Our results are also comparable with adult cohort studies that assess kidney disease progression. For example, the Chronic Renal Insufficiency Cohort (CRIC) study of adults with CKD showed that albuminuria is associated with higher risk for progression.26 Risk tables for CKD progression on the basis of eGFR category and albuminuria were recapitulated by CKiD studies.14 Although our real-world data did not capture albuminuria, we similarly showed higher rates of composite kidney outcomes in patients with proteinuria and lower eGFR category at cohort entry. In particular, approximately 70% and 39% of patients entering our cohort with proteinuria and initial eGFR 15–29 ml/min per 1.73 m2 and 30–44 ml/min per 1.73 m2, respectively, reached the composite kidney outcome, compared with 44% and 26% without proteinuria. We additionally showed that hypertension diagnosis was associated with a higher risk for reaching a kidney outcome and that proteinuria and hypertension together showed interaction in increasing this risk. In the CRIC cohort, improved hypertension control was associated with slowing of CKD progression.27 In our cohort, 61% of children with a diagnosis of hypertension had exposure to an antihypertensive medication. Assessment of hypertension control was beyond the scope of this investigation but will be important to consider in future studies. Finally, we did note higher risk of CKD progression with male sex, as was noted in adult studies.27
A major strength of this study is that it included an unselected sample of all patients cared for at six major pediatric medical centers. Further contributing to generalizability of our findings, this study also includes children from populations that experience a high burden of CKD but have been excluded from or underrepresented in prior studies, namely children with cancer.
This study does have several limitations. Determination of CKD etiology and comorbid conditions was based on diagnosis codes only. Although children with glomerular CKD were identified using a previously published computable phenotype algorithm23 and medical complexity was also defined based on a validated pediatric algorithm,24 there may have been some misclassification. Sensitivity for determining hypertension may be lowered because it is frequently underdiagnosed, and we required this diagnosis to be present at two visits; this analysis is unable to determine whether the hypertension was controlled or not, which has been shown to influence progression of CKD.17 Our definition of proteinuria on the basis of one urinalysis, although sensitive, may not have been specific for clinically significant or persistent proteinuria. To mitigate this, we performed a sensitivity analysis that required two urine samples with at least 1+ proteinuria with similar results. We have additionally provided a sensitivity analysis for patients with eGFR < 60 ml/min per 1.73 m2 to address CKD misclassification in the higher eGFR range and found largely similar results with respect to risk factors for progression. Finally, we have addressed data quality issues, such as implausible creatinine or height values in the EHR. However, we do not have information on the method of creatinine measurement and cannot account for these differences across laboratories because of limitations inherent to EHR data.
Overall, this study leverages large-scale multi-institutional EHR data collected in real-world settings to study a rare disease, pediatric CKD, over an extended time period and includes a previously underrepresented population, children with a history of cancer. Our findings confirm several previously established risk factors for CKD progression from prior observational studies (glomerular disease, proteinuria, and hypertension) and provide face validity to our novel approach for studying pediatric CKD. This study may serve as the foundation for future pragmatic clinical trials in children with CKD and as a roadmap for use of EHR data networks to adequately power the study of rare disease.
Supplementary Material
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We also acknowledge the contributions of Julia Schuchard and Jordan Musante.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Leveraging Electronic Health Record to Monitor Progression of Kidney Disease in Children,” on pages 152–153.
Disclosures
S. Breitenstein reports employment with, and patents or royalties from, Bayer AG. B.P. Dixon reports consultancy agreements with Alexion Pharmaceuticals, Apellis Pharmaceuticals, and Novartis Pharmaceuticals; honoraria from Alexion Pharmaceuticals, Apellis Pharmaceuticals, and Novartis Pharmaceuticals; and an advisory or leadership role for aHUS Action Network (unpaid). T. Eissing reports employment with and ownership interest in Bayer AG. M.R. Denburg reports employment with The Children's Hospital of Philadelphia, Perelman School of Medicine at the University of Pennsylvania; research funding from Mallinckrodt; patents or royalties from InBore LLC; advisory or leadership role on the Editorial Board of Kidney International Reports and the NKF Delaware Valley Medical Advisory Board; and other interests or relationships with American Society of Pediatric Nephrology Research and Program Committees. M.R. Denburg's spouse reports consultancy agreements with Trisalus Life Sciences, ownership interest in Precision Guided Interventions LLC and In-Bore LLC, research funding from Instylla, and an advisory or leadership role for Trisalus Life Sciences Scientific Advisory Board. V.R. Dharnidharka reports consultancy agreements with Atara Biotherapeutics and Medincell; research funding from CareDx; honoraria from CareDx and Sanofi; advisory or leadership roles for North American Pediatric Renal Trials and Collaborative Studies (unpaid) and the Council for American Society of Pediatric Nephrology (unpaid); and other interests or relationships with Independent Data Safety Monitoring Committee, Akebia/MedPace. J.T. Flynn reports serving as Editor-in-Chief of Pediatric Nephrology; serving as an Editorial Board member of Blood Pressure Monitoring, Hypertension, and Journal of Pediatrics; serving as a Board Member of Renal Physicians Association; and reports other interests or relationships with American Society of Pediatric Nephrology, International Pediatric Nephrology Association, and Renal Physicians Association. C.B. Forrest reports employment with Children's Hospital of Philadelphia and patents or royalties from Johns Hopkins University; C.B. Forrest does not receive any personal funding, but the Children's Hospital of Philadelphia receives funding that C.B. Forrest oversees from UCB, Bayer, Lily, and Sanofi. S.L. Furth, A. Goodwin Davies, M. Maltenfort, and J.R. Mcdonald report employment with Children's Hospital of Philadelphia. C.A. Gluck reports employment with Nemours Children's Health, honoraria from Travere, and an advisory or leadership role for Travere. C.A. Hovinga reports employment with Critical Path Institute and an advisory or leadership role for the FDA. A. Kaiser reports employment with, ownership interest in, and patents or royalties with Bayer AG and reports other interests or relationships with EFSPI SIG “Small populations.” M. Mitsnefes reports consultancy agreements with KBP BIOSCIENCES. A. Neu reports employment with Johns Hopkins University School of Medicine; consultancy agreements with Akebia; research funding from Alexion, AstraZeneca, Bayer, Bayer, BMS, CareDx, Covis (AMAG), Horizon, Leadiant, Otsuka, Reata, Relypsa, and Retrophin; honoraria from American Board of Pediatrics; advisory or leadership roles as President of the Board of Directors of The North American Pediatric Renal Trials and Collaborative Studies, a member of the Project Committee of ASN's NTDS, and Co-Medical Editor of American Board of Pediatrics Nephrology Subboard; and other interests or relationships as faculty member for the Children's Hospital Association's Standardizing Care to Improve Outcomes Collaborative. Johns Hopkins has a joint venture with Fresenius Medical Care, which funds the medical director position for the pediatric dialysis unit. A.L. Skversky reports employment with and ownership interest in Bayer Pharmaceuticals. W.E. Smoyer reports consultancy agreements with Visterra, Otsuka, and Vertex; ownership interest in NephKey Therapeutics; research funding from Aurinia; honoraria from USC for CTSA External Advisory Committee and from UCLA for CTSA External Advisory Committee; royalties from UpToDate; and advisory or leadership roles as member of the Board of Directors of Pediatric Nephrology Research Consortium (PNRC), member of the Board of Directors of NephCure Kidney International (NKI), and a member of the Coordinating Committee of the Institute for the Advancement of Clinical Trials in Children (I-ACT). M.J. Somers reports consultancy agreements with Alnylam, Dicerna, Horizon, Orfan Biotech, and Precision Biosciences; research funding from BioPorto and Dicerna; and advisory or leadership role for NAPRTCS Board of Directors and as ASPN Past President.
Funding
This study was supported by grant U18FD006297 from the Institute for Advanced Clinical Trials for Children (iactc.org) under FDA and by The Children's Hospital of Philadelphia Pediatric Center of Excellence in Nephrology and the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number 5P50DK114786-05. The research reported was conducted using PEDSnet, A National Pediatric Learning Health System, supported by PCORI Award RI-CHOP-01-PS1. This study was partially funded through PCORI Award #RD-2020C2-20338.
Author Contributions
S. Breitenstein, A.G. Davies, R.M. Denburg, T. Eissing, C. Forrest, A.C. Gluck, A.C. Hovinga, A. Kaiser, M. Maltenfort, R.J. McDonald, and L.A. Skversky conceptualized the study; A.G. Davies and M. Maltenfort were responsible for data curation; A.G. Davies, R.M. Denburg, C. Forrest, A.C. Gluck, M. Maltenfort, and R.J. McDonald were responsible for formal analysis, project administration, software, and validation and wrote the original draft; R.M. Denburg and C. Forrest were responsible for funding acquisition and resources; R.M. Denburg, C. Forrest, A.C. Gluck, and R.J. McDonald provided supervision; A.G. Davies, R.M. Denburg, C. Forrest, A.C. Gluck, M. Maltenfort, and R.J. McDonald were responsible for methodology; and all authors were responsible for investigation and reviewed and edited the manuscript.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/CJN/B561.
Supplemental Figure 1. Cumulative incidence of composite kidney failure outcome by entry eGFR category and subcohort for sensitivity analysis eGFR <60.
Supplemental Figure 2. Cumulative incidence of composite kidney failure outcome by hypertension and proteinuria for sensitivity analysis eGFR <60.
Supplemental Figure 3. Cumulative incidence of composite kidney failure outcome by hypertension and proteinuria for sensitivity analysis stricter proteinuria.
Supplemental Figure 4. Cumulative incidence of composite kidney failure outcome by cohort with competing risk of death.
Supplemental Table 1. Top 25 most frequent kidney and genitourinary diagnoses (from PMCA) CKD nonglomerular subcohort at or after cohort entry.
Supplemental Table 2. Antihypertensive treatment exposure within the first 2 years of cohort entry for children with hypertension and/or proteinuria.
Supplemental Table 3. Characteristics of children with chronic kidney disease in PEDSnet at the time of cohort entry for sensitivity analysis eGFR <60.
Supplemental Table 4. Characteristics of children with chronic kidney disease in PEDSnet at the time of cohort entry for sensitivity analysis stricter proteinuria definition.
Supplemental Table 5. Composite kidney failure outcome and component outcomes overall and by subcohort of chronic kidney disease.
Supplemental Table 6. Frequency of composite kidney failure outcome by starting eGFR category at cohort entrance and proteinuria.
Supplemental Table 7. Multivariable Cox proportional hazards model and Weibull accelerated failure time model evaluating the association of clinical factors with progression to the composite kidney failure outcome for sensitivity analysis eGFR <60.
Supplemental Table 8. Multivariable Cox proportional hazards model and Weibull accelerated failure time model evaluating the association of clinical factors with progression to the composite kidney failure outcome for sensitivity analysis using stricter proteinuria definition.
References
- 1.Harambat J, van Stralen KJ, Kim JJ, Tizard EJ. Epidemiology of chronic kidney disease in children. Pediatr Nephrol. 2012;27(3):363-373. doi: 10.1007/s00467-011-1939-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS): NAPRTCS 2011 Annual Dialysis Report, 2011. Available at: https://naprtcs.org/system/files/2011_Annual_Dialysis_Report.pdf. [Google Scholar]
- 3.Melhem N, Rasmussen P, Joyce T, et al. Acute kidney injury in children with chronic kidney disease is associated with faster decline in kidney function. Pediatr Nephrol. 2021;36(5):1279-1288. doi: 10.1007/s00467-020-04777-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371(1):58-66. doi: 10.1056/nejmra1214243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furth SL, Abraham AG, Jerry-Fluker J, et al. Metabolic abnormalities, cardiovascular disease risk factors, and GFR decline in children with chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(9):2132-2140. doi: 10.2215/CJN.07100810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson RJ, Harshman LA. Neurocognition in pediatric chronic kidney disease: a review of data from the chronic kidney disease in children (CKiD) study. Semin Nephrol. 2021;41(5):446-454. doi: 10.1016/j.semnephrol.2021.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hooper SR, Gerson AC, Butler RW, et al. Neurocognitive functioning of children and adolescents with mild-to-moderate chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(8):1824-1830. doi: 10.2215/CJN.09751110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brady TM, Roem J, Cox C, et al. Adiposity, sex, and cardiovascular disease risk in children with CKD: a longitudinal study of youth enrolled in the chronic kidney disease in children (CKiD) study. Am J Kidney Dis. 2020;76(2):166-173. doi: 10.1053/j.ajkd.2020.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denburg MR, Kumar J, Jemielita T, et al. Fracture burden and risk factors in childhood CKD: results from the CKiD cohort study. J Am Soc Nephrol. 2016;27(2):543-550. doi: 10.1681/ASN.2015020152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.System USRD: 2021 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2021. [Google Scholar]
- 11.System USRD: 2018 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2021. [Google Scholar]
- 12.Atkinson MA, Ng DK, Warady BA, Furth SL, Flynn JT. The CKiD study: overview and summary of findings related to kidney disease progression. Pediatr Nephrol. 2021;36(3):527-538. doi: 10.1007/s00467-019-04458-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furth SL, Cole SR, Moxey-Mims M, et al. Design and methods of the chronic kidney disease in children (CKiD) prospective cohort study. Clin J Am Soc Nephrol. 2006;1(5):1006-1015. doi: 10.2215/CJN.01941205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furth SL, Pierce C, Hui WF, et al. Estimating time to ESRD in children with CKD. Am J Kidney Dis. 2018;71(6):783-792. doi: 10.1053/j.ajkd.2017.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reynolds BC, Roem JL, Ng DKS, et al. Association of time-varying blood pressure with chronic kidney disease progression in children. JAMA Netw Open. 2020;3(2):e1921213. doi: 10.1001/jamanetworkopen.2019.21213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warady BA, Abraham AG, Schwartz GJ, et al. Predictors of rapid progression of glomerular and nonglomerular kidney disease in children and adolescents: the chronic kidney disease in children (CKiD) cohort. Am J Kidney Dis. 2015;65(6):878-888. doi: 10.1053/j.ajkd.2015.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flynn JT, Carroll MK, Ng DK, Furth SL, Warady BA. Achieved clinic blood pressure level and chronic kidney disease progression in children: a report from the chronic kidney disease in children cohort. Pediatr Nephrol. 2021;36(6):1551-1559. doi: 10.1007/s00467-020-04833-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong CS, Pierce CB, Cole SR, et al. Association of proteinuria with race, cause of chronic kidney disease, and glomerular filtration rate in the chronic kidney disease in children study. Clin J Am Soc Nephrol. 2009;4(4):812-819. doi: 10.2215/CJN.01780408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forrest CB, Margolis PA, Bailey LC, et al. PEDSnet: a national pediatric learning health system. J Am Med Inform Assoc. 2014;21(4):602-606. doi: 10.1136/amiajnl-2014-002743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forrest CB, Margolis P, Seid M, Colletti RB. PEDSnet: how a prototype pediatric learning health system is being expanded into a national network. Health Aff. 2014;33(7):1171-1177. doi: 10.1377/hlthaff.2014.0127 [DOI] [PubMed] [Google Scholar]
- 21.Pierce CB, Munoz A, Ng DK, Warady BA, Furth SL, Schwartz GJ. Age- and sex-dependent clinical equations to estimate glomerular filtration rates in children and young adults with chronic kidney disease. Kidney Int. 2021;99(4):948-956. doi: 10.1016/j.kint.2020.10.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashoor I, Weidemann D, Elenberg E, et al. The pediatric nephrology workforce crisis: a call to action. J Pediatr. 2021;239:5-10.e4. doi: 10.1016/j.jpeds.2021.03.033 [DOI] [PubMed] [Google Scholar]
- 23.Denburg MR, Razzaghi H, Bailey LC, et al. Using electronic health record data to rapidly identify children with glomerular disease for clinical research. J Am Soc Nephrol. 2019;30(12):2427-2435. doi: 10.1681/ASN.2019040365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simon TD, Haaland W, Hawley K, Lambka K, Mangione-Smith R. Development and validation of the pediatric medical complexity algorithm (PMCA) version 3.0. Acad Pediatr. 2018;18(5):577-580. doi: 10.1016/j.acap.2018.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ESCAPE Trial Group, Wühl E, Trivelli A, et al. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361(17):1639-1650. doi: 10.1056/NEJMoa0902066 [DOI] [PubMed] [Google Scholar]
- 26.Koye DN, Magliano DJ, Reid CM, et al. Risk of progression of nonalbuminuric CKD to end-stage kidney disease in people with diabetes: the CRIC (chronic renal insufficiency cohort) study. Am J Kidney Dis. 2018;72(5):653-661. doi: 10.1053/j.ajkd.2018.02.364 [DOI] [PubMed] [Google Scholar]
- 27.Hannan M, Ansari S, Meza N, et al. Risk factors for CKD progression: overview of findings from the CRIC study. Clin J Am Soc Nephrol. 2021;16(4):648-659. doi: 10.2215/CJN.07830520 [DOI] [PMC free article] [PubMed] [Google Scholar]