Abstract
AKI is a common complication of critical illness and is associated with substantial morbidity and risk of death. Continuous KRT comprises a spectrum of dialysis modalities preferably used to provide kidney support to patients with AKI who are hemodynamically unstable and critically ill. The various continuous KRT modalities are distinguished by different mechanisms of solute transport and use of dialysate and/or replacement solutions. Considerable variation exists in the application of continuous KRT due to a lack of standardization in how the treatments are prescribed, delivered, and optimized to improve patient outcomes. In this manuscript, we present an overview of the therapy, recent clinical trials, and outcome studies. We review the indications for continuous KRT and the technical aspects of the treatment, including continuous KRT modality, vascular access, dosing of continuous KRT, anticoagulation, volume management, nutrition, and continuous KRT complications. Finally, we highlight the need for close collaboration of a multidisciplinary team and development of quality assurance programs for the provision of high-quality and effective continuous KRT.
Keywords: Critical Care Nephrology and Acute Kidney Injury Series, CRRT, AKI, ICU, renal replacement therapy, continuous KRT, acute kidney injury
Introduction
AKI is a common complication of critical illness. In contemporary international cohorts, the incidence of AKI ranges from 20% to ≥50% of all intensive care unit (ICU) admissions, with 5%–15% developing AKI requiring KRT (AKI-KRT).1,2 The incidence of AKI and AKI-KRT in the ICU has been increasing over the last 20 years,3 likely due to a combination of improved AKI recognition and documentation, ongoing expansion of critical care services and organ support therapies, and increasing age and comorbidity of ICU populations. AKI-KRT specifically carries a very high short-term mortality of >50% across diverse ICU populations, including both prepandemic and coronavirus disease 2019 (COVID-19) cohorts.3–8
Continuous KRT (CKRT) has become the modality of choice in many ICUs for patients with AKI or kidney failure who are hemodynamically unstable.1–3 The use of CKRT is likely to continue to grow because sepsis is the most common cause of AKI in the ICU and its incidence in the United States has continued to rise since 2000.4,9 Furthermore, the COVID-19 pandemic has contributed to increased KRT use in the ICU, with AKI complicating 25%–40% of all COVID-19 admissions, and AKI-KRT developing in 20%–45% of patients who are critically ill with COVID-19.7,8 Every nephrologist and intensivist should therefore be adept at prescribing, monitoring, and adjusting CKRT. In this manuscript, we review the key operational principles of CKRT that the practicing ICU clinician will regularly use.
Choice of KRT Modality and Indications for CKRT
Although the following concepts regarding indications for KRT and comparisons of modality are useful, fundamentally, the decision to initiate KRT in the ICU or select a KRT modality should be carried out in the context of the patient’s clinical condition and their specific solute, fluid, and metabolic goals. Additionally, KRT prescriptions should adapt to the dynamic nature of acute illness and therefore must be iteratively reassessed and adjusted.
Choice of Modality: CKRT versus Intermittent Modalities
The available KRT modalities in the ICU include CKRT, prolonged intermittent KRT (PIKRT), intermittent hemodialysis (HD), and peritoneal dialysis (PD). PIKRT is a hybrid form of KRT, with blood and dialysate flow rates intermediate between intermittent HD and CKRT and treatment times between 6 and 18 hours. PD has become a viable KRT modality in the ICU for AKI, especially in resource-poor settings or during pandemic-induced shortages of KRT machines or supplies, but prospective data to compare its use with CKRT in the ICU are limited.10–12
Although CKRT is often considered standard of care for patients who are hemodynamically unstable and requiring KRT, data to prove superiority of CKRT over intermittent HD or PIKRT have been elusive. CKRT has been compared with intermittent HD in observational studies, randomized controlled trials (RCTs), and meta-analyses with largely equivalent outcomes, including mortality and kidney recovery.13–17 Although some trials demonstrated improvement in hemodynamic tolerability with CKRT over intermittent HD, other trials failed to show any significant difference in rates of hypotension or need for vasoactive support.15–17 However, most trials comparing intermittent HD and CKRT, including the three largest RCTs,14–16 tailored the intermittent HD prescription by increasing treatment durations beyond 4 hours and using decreased blood and/or dialysate flow rates to try to improve hemodynamic tolerability. Likewise, some trials excluded severe hemodynamic instability.14 As such, it is reasonable to consider KRT in the ICU as a spectrum of therapy that can be adapted to a given patient’s clinical condition, with intermittent HD, PIKRT, and CKRT having similar clinical outcomes in patients capable of tolerating all three modalities.
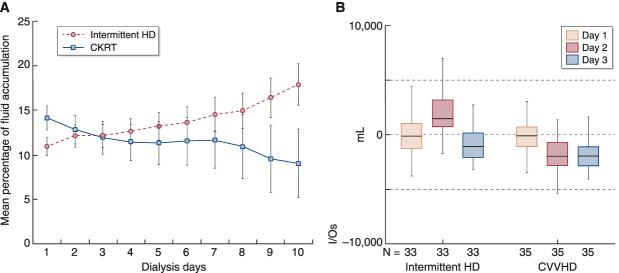
Despite generally equivalent outcomes, CKRT does have significant practical advantages over intermittent HD (Table 1). Chief among these advantages is superiority in preventing or treating volume overload (Figure 1).17,18 An overwhelming amount of observational data has shown that volume overload is independently and strongly associated with mortality and decreased kidney recovery in patients with AKI in the ICU.18,19 Continuous therapy allows for gradual volume removal, mitigating the risk of hypotension caused by ultrafiltration. In contrast, patients in the ICU who are oliguric and receive intermittent HD typically have a net positive fluid balance between treatments and require relatively rapid ultrafiltration during treatment to achieve target fluid balance. The ability to control volume balance precisely makes CKRT the preferred KRT modality in patients at highest risk of harm from volume overload, namely patients with cardiac and/or pulmonary failure requiring mechanical circulatory support, including extracorporeal membrane oxygenation.
Table 1.
Potential advantages and disadvantages of continuous KRT (relative to intermittent hemodialysis or prolonged intermittent KRT)
| Advantages | Disadvantages |
|---|---|
|
ICU, intensive care unit; CKRT, continuous KRT.
Although one single-center observational study39 suggests using arteriovenous fistulae/grafts for CKRT may be safe and feasible, doing so is not standard of care in most institutions.
Note that CKRT is not a contraindication to early mobilization per se because observational data suggest that physical rehabilitation is feasible and safe in patients on CKRT,85 but many providers perceive CKRT to be a barrier to physical therapy.
Relative amounts of dialysis versus ICU nurse support will depend on local staffing models.
Figure 1.
CKRT achieves superior volume control than intermittent HD in patients with critical illness. (A) Fluid accumulation over time in patients on CKRT and on intermittent HD. Percentage of fluid accumulation was calculated as fluid balance (fluid intake in liters minus total output in liters) divided by baseline body weight (in kilograms). Data from the Program to Improve Care in Acute Renal Disease (PICARD) study.18 (B) Twenty-four–hour total fluid balance (I/Os, in milliliters) during the first 3 days of KRT in patients on intermittent HD and CVVHD therapy. Shown are median values with interquartile range (box borders) and extreme values (whiskers). CKRT, continuous KRT; CVVHD, continuous venovenous hemodialysis; HD, hemodialysis. (A) Reprinted from ref. 18, with permission. (B) Reprinted from ref. 17, with permission.
In addition, CKRT is preferred in patients with or at risk of intracranial hypertension.20,21 Rapid clearance of urea from the extracellular compartment with intermittent HD can lead to a shift of water into the brain. In patients with CKD who are dialysis naive, this may lead to cerebral edema (i.e., dialysis disequilibrium syndrome). Similarly, osmotic shifts can precipitate or aggravate intracranial hypertension in patients in the ICU who are at risk, such as those with brain injury or acute liver failure (Figure 2). In these patients, CKRT is preferred over intermittent HD.
Figure 2.
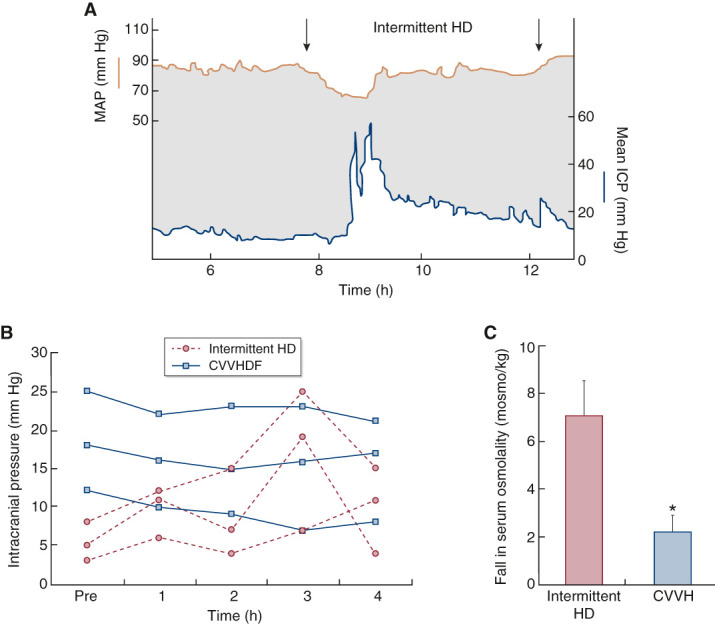
CKRT is less likely than intermittent HD to exacerbate intracranial hypertension or to decrease cerebral perfusion pressure. (A) In a patient with kidney failure after neurosurgical evacuation of a subdural hematoma, the start of intermittent HD is associated with a significant fall in mean arterial BP (MAP) and a significant rise in intracranial pressure (ICP). Cerebral perfusion pressure (CPP) in patients with intracranial hypertension is the difference between MAP and ICP (i.e., CPP=MAP–ICP) and is represented in this figure by the gray shaded area. The dual effects of decreased MAP and increased ICP with intermittent HD initiation can combine to produce a dramatic reduction in CPP. (B) Changes in mean ICP during intermittent HD and continuous venovenous hemodiafiltration (CVVHDF). In contrast to intermittent HD, CKRT, due to slower solute removal and more gradual changes in plasma osmolality, has minimal effect on ICP. (C) Reduction in plasma osmolality after 1 hour of treatment with intermittent HD and continuous venovenous hemofiltration (CVVH) at a total effluent dose of approximately 2 L/h. Shown are means (bars) and SEM (whiskers). *P<0.05 for comparison with intermittent HD. Reprinted or adapted from refs. 20 and 21, with permission.
Finally, total daily dosage of clearance provided by CKRT is typically higher than standard intermittent HD due to its continuous nature (Table 2).22–24 Therefore, once initial metabolic control is achieved, patients on CKRT can usually be provided full-dose nutrition without restriction of protein, phosphate, or potassium.
Table 2.
Comparison of modalities by theoretic maximal clearances and estimated weekly dosages
| Modality | Maximal Theoretic Clearance (ml/min) | Approximate Total Weekly Dose (standardized Kt/Vurea) |
|---|---|---|
| Intermittent HD, 3 times/wk | 280 | 2 |
| Intermittent HD, 7 times/wk | 280 | 5 |
| CKRT, postfilter CVVH, 25 ml/kg per hour | 25 | 7 |
| CKRT, prefilter CVVH, 35 ml/kg per hour | 28 | 8 |
| CKRT, postfilter CVVH, 35 ml/kg per hour | 35 | 10 |
| CKRT, CVVHD, 35 ml/kg per hour | 35 | 10 |
| Normal kidney | 90–140 | 16 |
Clearance values represent theoretic maximums, assuming hematocrit of 30%, body weight of 60 kg, body water of 36 L, intermittent HD blood flow rate of 400 ml/min, CKRT blood flow rate of 200 ml/min, sieving coefficient of one, and dialysate saturation (i.e., extraction ratio) of 100%. Estimated Kt/V values for CKRT similarly assume theoretic maximal values, with no downtime, filter efficiency loss, or patient fluid overload. Standardized Kt/V values for intermittent HD assume single-pool Kt/V of 1.3, representing typical goal clearance rather than maximal theoretic achievable clearance. HD, hemodialysis; CKRT, continuous KRT; CVVH, continuous venovenous hemofiltration; CVVHD, continuous venovenous hemodialysis. Adapted from refs. 22, 23, and 24, with permission.
Indications for CKRT
Indications for CKRT include all of the traditional indications for KRT applied to patients with hemodynamic instability and/or multiorgan failure (Table 3). These indications include AKI complicated by hyperkalemia, diuretic-resistant volume overload, severe uremia, severe metabolic acidosis, and persistent oliguria or anuria. Other relative indications include conditions that may require continuous solute control, such as severe rhabdomyolysis and tumor lysis syndrome. CKRT is also preferred for AKI complicated by acute brain injury, acute liver failure, or cardiopulmonary failure requiring extracorporeal membrane oxygenation. Similarly, CKRT may be used as a bridge to organ transplantation in patients with AKI-complicating advanced heart or liver disease who are often intolerant to intermittent HD due to low effective circulating volumes. In patients with AKI complicated by acute brain injury or acute liver failure, CKRT may be used to safely induce steady-state therapeutic hypernatremia to help reduce cerebral edema. Likewise, CKRT may be preferred over intermittent HD in AKI complicated by severe dysnatremias because the dialysate sodium concentration is usually limited to a range of 130–155 mEq/L in intermittent HD, which may lead to overcorrection. The slower dialysance of CKRT and the ability to customize the circuit and/or sodium concentration of CKRT solutions can prevent overly rapid correction of dysnatremias, mitigating the risk of osmotic demyelination or cerebral edema.25,26 Ultimately, the decision to initiate CKRT must be made in view of the patient’s clinical context, including nonkidney organ function, and according to the goals of CKRT.
Table 3.
Potential indications for continuous KRT in patients who are critically ill with AKI
| Potential Indication | |
|---|---|
| Classic indications for KRT in the setting of hemodynamic instability | |
| Hyperkalemia | |
| Severe metabolic acidosis | |
| Diuretic-resistant volume overload | |
| Life-threatening or severe complications of uremia (e.g., bleeding in the setting of uremic platelet dysfunction, pericarditis) | |
| Poisoning with dialyzable toxins (e.g., toxic alcohols, salicylates, lithium)a | |
| Persistent oliguria or anuria | |
| CKRT-specific indications | |
| Gradual correction of severe dysnatremia (e.g., serum [Na+] <120 mEq/L or >165 mEq/L) | |
| Intracranial hypertension or conditions associated with high risk of intracranial hypertension or requiring maintenance of therapeutic hypernatremia (e.g., acute liver failure, acute brain injury) | |
| Cardiopulmonary failure requiring ECMO or other mechanical circulatory support | |
| Organ support in patients with advanced heart or liver disease unable to tolerate intermittent HD, especially when used as a bridge to transplantation or other destination therapy | |
| Conditions requiring continuous solute removal due to high cell turnover or cell lysis (e.g., rhabdomyolysis or tumor lysis syndrome) |
CKRT, continuous KRT; [Na+], sodium concentration; ECMO, extracorporeal membrane oxygenation; HD, hemodialysis.
Intermittent HD, given its higher clearance, is generally preferred over CKRT in the treatment of poisonings, but CKRT can be considered in patients with severe hemodynamic instability.
Choice of CKRT Modality
Solute clearance with CKRT can be achieved via diffusion in continuous venovenous HD (CVVHD), convection in continuous venovenous hemofiltration (CVVH), or both in continuous venovenous hemodiafiltration (CVVHDF) (Figure 3). Although CVVHD is considered a form of predominantly diffusive clearance, the use of high-flux dialyzers allows for convective flow via filtration and back-filtration between the dialysate and blood compartments as the result of pressure gradient changes along the length of the membrane.27 As a result, CVVHD may also attain clearance of higher molecular weight solutes than expected with just diffusion alone. Hemofiltration can be achieved with replacement fluid administered either prefilter, postfilter, or both. CVVH with 100% postfilter replacement fluid is most efficient but increases the risk of filter clotting due to hemoconcentration and increased filtration fraction (FF); whereas prefilter replacement fluid dilutes the blood before hemofiltration, resulting in less-efficient small molecule clearance but a decreased tendency to promote filter clotting.28 FF is the proportion of plasma water entering the filter that is removed by ultrafiltration and is inversely proportional to blood flow rate. In practice, most centers use a mixture of pre- and postfilter replacement fluids to achieve an FF of ≤20%–25%. High-flux hemodialyzers have become the standard for CKRT and can, theoretically, remove water-soluble substances up to 10,000 Da, whereas hemofiltration can remove substances up to approximately 40,000 Da.23 However, in vivo size cutoffs are thought to be smaller, approximately 2000 Da for HD and 15,000 Da for hemofiltration, due to decreased effective pore size resulting from the adsorption of plasma proteins onto hemofilters.29
Figure 3.
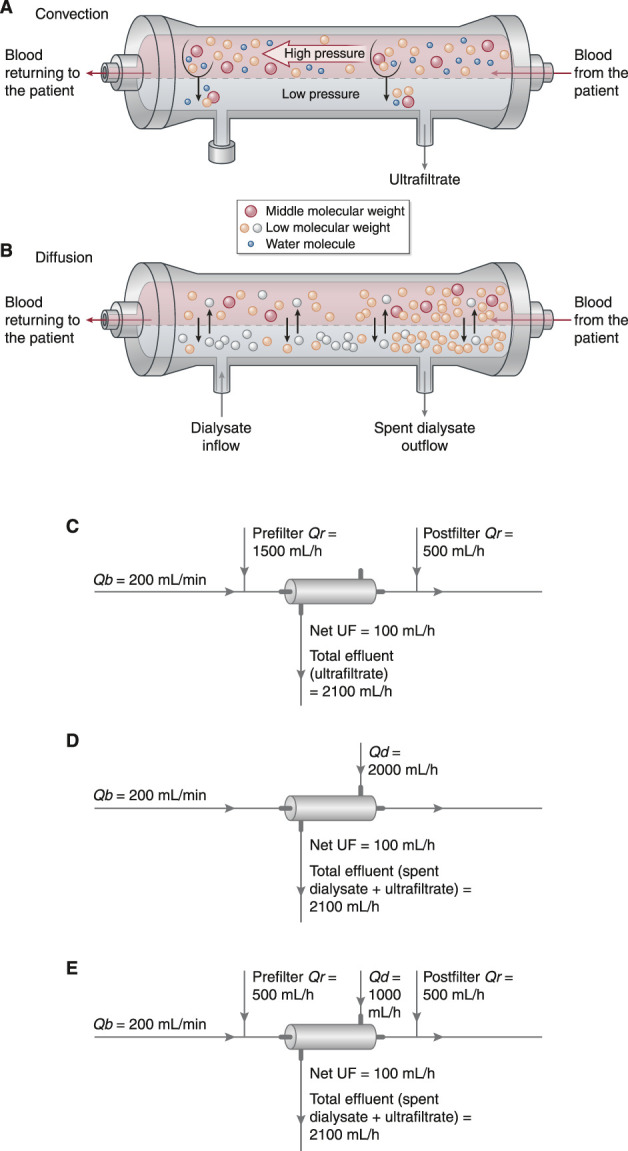
Modalities of CKRT utilize primarily convective clearance (hemofiltration), diffusive clearance (hemodialysis), or both. (A) In hemofiltration, solute clearance occurs primarily by convection. In convection, solutes are transported across the hemofilter membrane along with plasma water as a result of a hydrostatic pressure (i.e., transmembrane pressure) generated on the blood side of the membrane. Solutes cleared by convection include urea and other small molecules along with larger “middle molecules.” (B) In HD, solute clearance occurs primarily by diffusion, which is driven by a concentration gradient across the semipermeable membrane. Small solutes in high concentration in the blood diffuse across the membrane into the dialysate, which contains either little (e.g., potassium) or none (e.g., urea) of the solutes being cleared. Small solutes in higher concentration in the dialysate (e.g., bicarbonate) diffuse into the blood. Dialysate runs across the HD membrane countercurrent to the direction of blood flow to maintain a concentration gradient for removal of small solutes along the entire length of the semipermeable membrane. Modern hemodialyzers are virtually all “high-flux” dialyzers, which clear substances larger than historical low-flux dialyzers. However, unlike hemofiltration, HD does not effectively clear larger middle molecules. Ultrafiltration can be performed with HD by applying a transmembrane pressure across the membrane, but, in contrast to the high volume of ultrafiltration used to achieve significant solute clearance in hemofiltration, the volumes of ultrafiltration performed in HD are relatively small, contribute little to solute clearance, and are instead used only to achieve net volume removal. (C) In CVVH, a high volume of ultrafiltrate is generated and is replaced with an equal or (if net volume removal is desired) a somewhat smaller amount of physiologic crystalloid solution to effect net solute removal. The physiologic solution may be infused before the hemofilter (prefilter replacement fluid), into the return line (postfilter replacement fluid), or both. The net ultrafiltration rate (UF) is equal to the difference between the effluent rate and the replacement fluid rates (Qr), and it is adjusted to achieve net volume removal as desired. A typical CVVH prescription is shown, which, for a 70-kg patient, would provide a total dose of 30 ml/kg per hour and a net ultrafiltration rate of 100 ml/h (1.4 ml/kg per hour). To maintain efficient solute clearance in CVVH, blood flow rate (Qb) should be kept approximately five to six times higher than the replacement fluid rates. (D) In CVVHD, dialysate is driven through the dialyzer across the membrane from the blood flow in a direction countercurrent to blood flow. In most settings, the dialysate solution used in CVVHD is very similar or identical to the replacement fluid used in CVVH. In contrast to CVVH, ultrafiltration in CVVHD makes only minor contribution to solute removal but is performed primarily for the purposes of volume management, with ultrafiltrate generated at a rate equal to the desired rate of fluid removal. The effluent consists of both the spent dialysate and ultrafiltrate, and the net ultrafiltration rate is equal to the difference between the total effluent flow rate and the dialysate flow rate (Qd). A typical CVVHD prescription is shown, which, for a 70-kg patient, again provides a total dose of 30 ml/kg per hour and a net ultrafiltration rate of 100 ml/h (1.4 ml/kg per hour). To maintain efficient solute clearance in CVVHD, blood flow rate should be kept approximately 2.5 times higher than the dialysate flow rate. (E) CVVHDF combines a high volume of ultrafiltration coupled with replacement fluid (to achieve solute clearance by convection) with dialysate perfused across the membrane countercurrent to blood flow (to achieve solute clearance by diffusion). As in CVVH, ultrafiltrate volume in excess of the desired rate of fluid removal is replaced with a physiologic crystalloid solution that may be infused before the hemofilter (prefilter replacement fluid), into the return line (postfilter replacement fluid), or both. The effluent consists of both the spent dialysate and ultrafiltrate with the net ultrafiltration rate equal to the difference between the total effluent flow rate and the sum of dialysate and total replacement fluid flow rates. A typical CVVHD prescription is shown, which, for a 70-kg patient, again provides a total dose of 30 ml/kg per hour and a net ultrafiltration rate of 100 ml/h (1.4 ml/kg per hour). Adapted from refs. 88 and 89, with permission.
Despite the small differences in efficiency of solute removal and in the size of solutes removed, no convincing evidence exists to suggest that a specific CKRT modality influences clinical outcomes,30 and the choice is typically made on the basis of local practice patterns, logistics, and provider preference. Although the convective removal of middle-sized molecules, such as cytokines and inflammatory mediators, has been hypothesized to be beneficial in patients with AKI, sepsis, or shock after cardiac surgery, clinical trials have largely shown no clinical benefit.31,32 In addition, studies evaluating the effect of the choice of diffusion and/or convection on circuit patency suggest longer circuit life with CVVHD or CVVHDF over CVVH, but comparisons have been limited by heterogeneous study designs, variable use of anticoagulation, small sample size, and inconsistent results, precluding definitive conclusions.33,34
Prescribing CKRT
Vascular Access
Poor vascular access is a frequent source of CKRT circuit malfunction. The Kidney Disease Improving Global Outcomes (KDIGO) 2012 AKI guidelines recommend placement of temporary dialysis catheters preferentially in the right internal jugular (IJ) vein, followed by either femoral vein, followed by the left IJ, with use of the subclavian veins as last resort.35 Although there is general consensus that the right IJ is preferred, others suggest the left IJ may be preferred over femoral placement as long as care is taken to ensure the catheter tip reaches the cavoatrial junction.36 Preferential use of the left IJ over the femoral veins may be especially prudent in patients with obesity, which is associated with higher risk of femoral catheter complication or malfunction.37
In the largest RCT of access site for temporary dialysis catheter placement, 750 patients were randomized to femoral or jugular catheter placement. The rates of catheter-related bacteremia or colonization were similar except for patients in the highest body mass index tertile (>28.4 kg/m2), in which bacteremia or colonization was higher in the femoral group.37 In a post hoc analysis, catheter malfunction was least common with right IJ placement, followed by femoral placement, with the highest rates of malfunction at the left IJ; however, the left IJ catheters were all 16 cm long, which would generally be inadequate to ensure catheter tip placement near the cavoatrial junction.36,38
Although one single-center retrospective study suggests that needle cannulation of preexisting arteriovenous fistulae or grafts may be safely used for CKRT in patients with kidney failure,39 the standard of care in most institutions is to perform CKRT with only catheters due to concerns over needle dislodgement or damage to the fistulae/grafts caused by prolonged cannulation. Depending on the anticipated duration of shock, patients with kidney failure, hypotension, and preexisting vascular access may be best served by intermittent HD or PIKRT with vasopressor support to avoid catheter placement.
Dose
The clearance of urea and other low molecular weight solutes in CKRT is approximately equal to effluent flow rate. Therefore, the effluent rate is a surrogate of solute clearance, or dose, provided by CKRT and is reported in milliliters per kilogram per hour. Dosing of CKRT has been studied in two large seminal RCTs: the Veterans Affairs/National Institutes of Health Acute Renal Failure Trial Network (ATN) study and the Randomized Evaluation of Normal versus Augmented Level Replacement Therapy (RENAL) study.5,6 Collectively, they randomized >2000 patients with AKI in the ICU to higher-dose (effluent dose of 35–40 ml/kg per hour) versus lower-dose (effluent dose of 20–25 ml/kg per hour) CKRT and found no difference in mortality or kidney recovery. This led to the KDIGO recommendation that an average of 20–25 ml/kg per hour of total effluent be delivered to patients with AKI requiring CKRT.35 However, CKRT treatment interruptions are common due to circuit clotting, circuit changes, procedures, and radiologic studies. As a result of such circuit “downtime,” delivered dose is typically lower—up to 20% lower in some studies—than prescribed dose.40 Furthermore, CKRT filter efficiency can be lost over time due to decreased effective surface area, permeability, and concentration polarization related to clotting, clogging, or protein adsorption.41 To ensure adequate delivered dose, KDIGO guidelines suggest prescribing 25–30 ml/kg per hour.35 Higher CKRT doses may be needed transiently for severe or refractory hyperkalemia, severe acidosis, hyperammonemia, or intoxications complicated by severe hemodynamic instability. Importantly, once adequate solute control is achieved, the dose should be readjusted to the standard range to mitigate nonselective clearance of nutrients and medications. Therefore, CKRT dosing should be dynamic, undergoing iterative adaption to the changing clearance needs of patients who are critically ill.
Some experts suggest that CKRT doses <20 ml/kg per hour may confer higher mortality risk on the basis of historical observational data or extrapolation of kidney failure studies, but prospective data showing that lower CKRT doses are harmful are lacking.42,43 Doses <20 ml/kg per hour can be considered in specific situations, such as in the setting of shortages of premanufactured CKRT fluid or for patients with severe obesity. Notably, for patients with obesity, the ATN trial did employ adjusted body weight (ideal body weight plus 25% of the difference between ideal and actual weight). However, the ATN and RENAL trials excluded patients >128.5 and >100 kg, respectively.5,6 As such, prospective data to guide CKRT dosing in severe obesity are lacking. Reasonable approaches include dosing by actual body weight but capping the total dose (e.g., at 5000 ml/h), initially using actual body weight and subsequently decreasing the dose on serial assessment, or initially using adjusted body weight. Observational data from Japan suggest that doses as low as 15 ml/kg per hour are safe,44 but no prospective data exist to support this practice, which cannot be recommended for routine use.
Blood Flow Rate
Blood flow in CKRT is prescribed at a rate lower than that used for intermittent HD, typically in the range of 100–300 ml/min. The primary determinant of clearance in CKRT is the total effluent rate rather than blood flow rate. The effluent rate is the sum of the dialysate rate and total ultrafiltration rate, whereas the total ultrafiltration is the sum of the net ultrafiltration rate and replacement fluid rate (Figure 3).
In contrast to a typical intermittent HD prescription, CKRT blood flow rate (100–300 ml/min) is usually several times higher than the effluent rate (30–50 ml/min), effectively making the effluent rate the dose-limiting element of the prescription. Therefore, changes in blood flow rate have little effect on small molecule clearance in CKRT. However, at very high CKRT doses, blood flow may begin to significantly influence clearance. For diffusion-based CKRT modalities (e.g., CVVHD), the blood flow rate should be at least 2.5 times the dialysate rate to allow for near-complete saturation of the dialysate.45 For convective CKRT modalities, the greater the prefilter replacement fluid rate relative to blood flow rate, the larger the loss of efficiency of solute clearance compared with postfilter replacement fluid at the same rate due to the predilution effect. In contrast, the greater the postfilter replacement fluid rate relative to blood flow rate, the greater the FF and risk of circuit clotting. As a general rule, in CVVH, blood flow rate ≥200–250 ml/min (i.e., five or more times the prefilter replacement fluid rate) is recommended to maximize clearance.46
Notably, decreased circuit life span with low blood flow rate has not been observed consistently in human studies.34,47,48 In the only RCT comparing differences in blood flow rate alone, 100 patients treated with CVVH or CVVHDF (with most receiving no anticoagulation and a minority receiving heparin) were randomized to blood flow of 150 or 250 ml/min, with no effect on rates of filter clotting.47 Although, in theory, high blood flow rate limits clotting by decreasing the FF, higher blood flow rate may predispose to access or circuit pressure alarms, which, especially if triggering repeated reductions in blood flow or blood pump stoppages, may also predispose to circuit clotting.49 Although FF is the classic parameter used to assess the effect of hemoconcentration on the risk of filter clotting, postfilter hematocrit has recently been proposed as a potentially more useful metric.50
Finally, despite the common perception that blood flow contributes to hypotension in CKRT, decreasing blood flow rate to mitigate KRT-induced hypotension, especially when using modern hollow-fiber dialyzers, is typically ineffective in improving hemodynamics.51
Anticoagulation
Owing to its continuous nature, anticoagulation is often required to prevent circuit clotting in CKRT. Options include preemptive use of either regional citrate anticoagulation or heparin or initiating CKRT without anticoagulation and starting anticoagulation if clotting is encountered. Although CKRT without anticoagulation remains common in practice, the KDIGO AKI guidelines suggest, in patients without contraindications, the preemptive use of regional citrate.5,6,35 The use of anticoagulation appears especially important in patients with COVID-19–associated AKI receiving CKRT, who appear to be at very high risk of filter clotting, with some requiring dual anticoagulation with regional citrate and systemic heparin to maintain filter patency.52
In regional citrate anticoagulation, citrate is infused prefilter (generating a blood citrate concentration of 3–6 mmol/L) to produce a low intrafilter ionized calcium (iCa) level (typically targeted to <0.4 mmol/L), which prevents clotting in the filter. Intravenous calcium is infused either in the postfilter limb of the CKRT circuit before return of blood to the patient, or via a separate systemic infusion to prevent hypocalcemia in the patient. A variable portion of citrate, typically >50%, is removed in the CKRT effluent, with the rest being delivered to the patient and metabolized primarily by the liver. In contrast to heparin, which is not dialyzable and produces systemic anticoagulation, regional citrate has the advantage of no systemic anticoagulant effect. Regional citrate anticoagulation has repeatedly been shown to be superior to systemic heparin in prolonging filter life and decreasing bleeding complications.53 This finding was recently revalidated in the 600-patient multicenter Effect of Regional Citrate Anticoagulation versus Systemic Heparin Anticoagulation During Continuous KRT on Dialysis Filter Life Span and Mortality Among Critically Ill Patients With Acute Kidney Injury (RICH) trial, the largest RCT of citrate anticoagulation to date.54
Regional citrate anticoagulation does come at the expense of increased complexity and the need to monitor for metabolic complications. The most feared complication is citrate accumulation, which is also known as citrate toxicity or citrate lock but which should be differentiated from citrate excess (Table 4).55 Citrate excess causes metabolic alkalosis because each citrate molecule is metabolized to three molecules of bicarbonate, whereas citrate toxicity can result in dangerously low systemic iCa levels along with (due to accumulating calcium-citrate complexes) increased total calcium (tCa) levels, escalating intravenous calcium requirements, and anion gap metabolic acidosis.56 The normal tCa/iCa ratio is approximately 2.0, and a tCa/iCa ratio >2.5 has been traditionally used to detect significant citrate accumulation.56,57 Correcting tCa for hypoalbuminemia is not generally recommended,56 and one small study suggests it is not necessary.58 Elevated lactate appears to be the best predictor of citrate accumulation,59 with hepatic dysfunction and shock states being chief among the clinical risk factors for citrate toxicity. However, emerging data suggest that regional citrate anticoagulation can be used cautiously with close monitoring in patients with advanced liver disease.48,60 This is especially relevant because the coagulopathy of liver disease increases risks of bleeding and clotting, often precluding the safe use of systemic heparin but also causing decreased CKRT filter life.61
Table 4.
Metabolic complications of regional citrate anticoagulation related to excess or inadequate citrate
| Complication | Mechanism | Diagnostic Features | Management Options |
|---|---|---|---|
| Excess buffer from citrate | Excess citrate supply, resulting in excessive metabolic conversion of citrate to bicarbonate and excess buffer |
|
|
| Citrate accumulationb | Impaired metabolic conversion of citrate resulting in accumulation of citrate-calcium complexes in blood |
|
|
| Insufficient buffer from citrate | Inadequate citrate supply, resulting in insufficient conversion of citrate to bicarbonate and insufficient buffer |
|
|
tCa, total calcium; iCa, ionized calcium; CKRT, continuous KRT. Adapted from ref 55, with permission.
These changes in blood flow and citrate rate are done proportionally to increase or decrease the delivery of citrate while maintaining the same ratio of citrate infusion rate to blood flow rate and thus similar intrafilter ionized calcium concentration.
Citrate accumulation is also known as citrate toxicity or citrate lock.
Other metabolic complications of regional citrate anticoagulation include isolated hyper- or hypocalcemia (from inappropriate calcium supplementation), hypernatremia (with hypertonic citrate formulations), and hypomagnesemia (as citrate weakly chelates magnesium).56 In addition, exploratory secondary outcomes of the RICH trial suggested citrate may increase the risk of hypophosphatemia or infection,54 although these unexpected findings may not be directly due to citrate exposure. For example, a recent post hoc analysis of the RICH trial found filter life span >48 hours to be independently associated with an increased rate of new infection, whereas the choice of anticoagulation was not.62
Net Ultrafiltration
Data to guide the prescription of volume removal (net ultrafiltration) in CKRT are limited. Although ultrafiltration rate is the primary modifiable risk factor for hypotension during KRT, emerging data suggest nonmodifiable risk factors (e.g., increased vascular permeability or impaired vascular refilling) may mediate the risk of hypotension in patients in the ICU undergoing KRT, implying that some patients may potentially benefit from ongoing net ultrafiltration despite some hypotension requiring vasopressor support.63,64 Management of volume status in patients receiving CKRT ultimately requires integration of a multitude of data points and careful, serial reassessment of ultrafiltration goals and tolerance, optimally done in collaboration between the nephrology and critical care teams.
It is recommended that net ultrafiltration, like total effluent dose, be prescribed in a weight-based manner (ml/kg per hour).65 Accumulating observational data indicate that the relationship between net ultrafiltration and mortality has a U-shaped curve (Figure 4), with the lowest mortality associated with net ultrafiltration rates of 1.0–1.75 ml/kg per hour,65,66 but whether proactively targeting such intermediate net ultrafiltration rates improves mortality requires validation in interventional studies.
Figure 4.
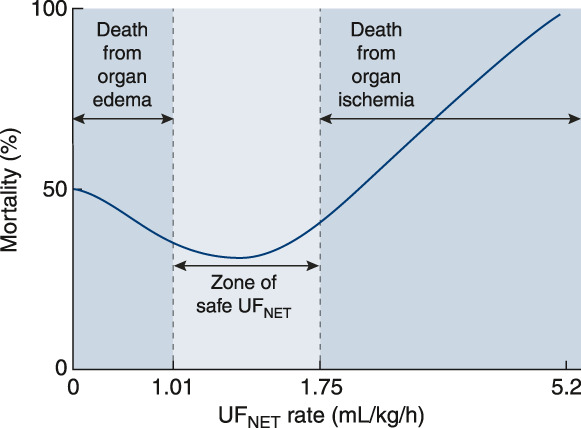
Net ultrafiltration rate is independently associated with mortality in patients treated with CKRT. The relationship between mortality and net ultrafiltration (UFNET) in patients in the intensive care unit (ICU) with AKI is J shaped. In a post hoc analysis of >1400 patients from the Randomized Evaluation of Normal versus Augmented Level Replacement Therapy study comparing high versus low dose of CKRT for AKI in the ICU,6 low UFNET rates <1.01 ml/kg per hour and high UFNET rates >1.75 ml/kg per hour were associated with higher risk-adjusted 90-day mortality compared with UFNET rates in a middle range of 1.01–1.75 ml/kg per hour. It has been proposed that these effects are mediated by harms of organ edema in those treated with low UFNET rates and by organ ischemia in those treated with high UFNET rates, but no prospective trial data yet exist to demonstrate that targeting a moderate rate of UFNET improves outcomes. Data from Murugan et al.66 Reprinted from ref. 65, with permission.
Complications of CKRT
Other complications of CKRT include vascular access dysfunction and air embolization (Table 5). Rare serious allergic reactions to the hemofilter have also been reported. Although hypotension is universally considered a possible complication of CKRT, many other factors may contribute to hypotension in patients with critical illness requiring CKRT beyond net ultrafiltration or CKRT itself. As such, comprehensive hemodynamic assessment and serial re-evaluation are paramount because some patients with volume excess may benefit from ongoing net ultrafiltration despite increased vasopressor requirements.
Table 5.
Overview of continuous KRT complications
| Complications |
|---|
| Catheter-related complications |
| Hematoma, hemorrhage, or traumatic arteriovenous fistula |
| Infection (catheter-associated blood stream infection or local soft-tissue infection) |
| Vein thrombosis or stenosis |
| Pneumothorax or hemothorax |
| Air embolism |
| Visceral injury |
| Complications related to extracorporeal circuit |
| Allergic reaction to dialyzer/hemofilter or circuit tubing |
| Circuit thrombosis |
| Hemolysis |
| Air embolism |
| Hypothermia |
| Hypotension |
| Metabolic disturbances |
| Complications of regional citrate anticoagulation |
| Citrate accumulation, i.e., citrate toxicity or citrate lock (see above) |
| Citrate/buffer excess or citrate/buffer deficit (see above) |
| Isolated hypo- or hypercalcemia |
| Hypernatremia (if using formulation containing trisodium citrate) |
| Hypomagnesemia |
| Hypophosphatemia, possibly aggravating respiratory muscle weakness |
| Others: hypokalemia, hypocalcemia, hypomagnesemia |
| Hypoglycemia (when using dextrose-free CKRT solutions) |
| Euglycemic ketoacidosis (when using dextrose-free CKRT solutions) |
| Inappropriate (excess or inadequate) medication dosing |
| Inadequate nutrition due to nonselective clearance of key nutrients |
CKRT, continuous KRT.
Hypophosphatemia warrants specific attention because emerging data suggest it may contribute to poor outcomes in critical illness. Because phosphate is primarily intracellular and slowly re-equilibrates with the extracellular space, the primary determinant of phosphate clearance with KRT is duration of therapy. A single session of intermittent HD will effectively clear phosphate from the extracellular compartment but remove only a modest amount of total body phosphate. CKRT, being continuous, overcomes the slow transcellular shift of phosphate and, if done with phosphate-free solutions, often results in hypophosphatemia within 48–72 hours of therapy. Large observational studies have associated CKRT-induced hypophosphatemia with higher risks of prolonged mechanical ventilation and/or need for tracheostomy, an effect presumed to be mediated by hypophosphatemia-induced respiratory muscle weakness.67–69 Hypophosphatemia can be prevented or mitigated by either proactive repletion (i.e., scheduling phosphate supplementation as soon as initial AKI-induced hyperphosphatemia corrects) or by using locally compounded or premanufactured CKRT solutions containing phosphate. In a single-center, before-and-after study, use of phosphate-containing (versus nonphosphate-containing) CKRT solutions was independently associated with a lower rate of incident hypophosphatemia and decreased duration of mechanical ventilation,70,71 but interventional studies are needed to validate these findings.
Notably, the commercially available phosphate-containing CKRT solutions are formulated without dextrose. Although observational data suggest the risk of severe hypoglycemia is not significantly higher with these solutions compared with standard phosphate-free solutions with dextrose,71,72 additional glucose monitoring may be prudent. Additionally, the prolonged use of glucose-free CKRT solutions in patients not receiving nutrition can predispose to the development of euglycemic ketoacidosis, which manifests as unexplained anion gap metabolic acidosis, normal serum glucose, and ketonemia and corrects with the provision of insulin with glucose.73
Unlike phosphate, most standard CKRT solutions contain physiologic or near-physiologic concentrations of potassium, calcium, and magnesium. As a result, hypokalemia, hypocalcemia, and hypomagnesemia develop less frequently than hypophosphatemia, and fewer data exist on the effect of depletion of these electrolytes on CKRT outcomes.5,6,74,75 In particular, hypomagnesemia has been linked with skeletal muscle weakness in small human studies, and some limited data have associated hypomagnesemia in general ICU populations with higher rates of mechanical ventilation, prolonged ICU stay, and mortality.76,77 However, prospective data on the benefits of treating or preventing CKRT-induced hypomagnesemia are lacking.78
Finally, effective quality assurance programs and reliable multidisciplinary communication are needed to prevent avoidable complications, such as errors in prescription or delivery of CKRT, over- or undershooting ultrafiltration targets, or miscommunication between services.79,80 Local protocols—typically consisting of continuous or frequent vital sign assessment and serial (e.g., every 6–12 hours) measurement of basic chemistries, iCa, magnesium, and phosphate—should be implemented and strictly observed to aid in early detection of complications.
Multidisciplinary Collaboration for Delivering High-Quality CKRT
Optimal provision of CKRT requires collaboration with the multidisciplinary ICU team, including intensivists, nurses, nutritionists, pharmacists, physical and occupational therapists, and ICU leadership.
Because CKRT nonselectively removes small water-soluble solutes, inadvertent or excess removal of beneficial substances may occur. In addition to phosphate, this includes a multitude of water-soluble nutrients, prominently including small proteins and amino acids, which can significantly aggravate the negative nitrogen balance typical of critical illness and associated systemic inflammation. Care must be taken to provide adequate nutrition, with recommended daily targets of 25–35 kcal/kg total calories and 1.5–2.5 g/kg of protein.81
Likewise, excess drug removal by CKRT may potentially cause harm. Data to guide drug dosing in CKRT are relatively limited, but observational data suggest that both over- and underdosing of antibiotics in patients on CKRT is common and that, along with HD or hemofiltration, hemofilter adsorption may significantly contribute to drug removal.82–84 Given the importance of appropriate antibiotic therapy to sepsis outcomes, special care must be taken to ensure adequate dosing of antibiotics despite drug clearance by CKRT, with therapeutic drug monitoring performed when feasible.
Although observational studies have shown that physical rehabilitation is feasible and safe in patients undergoing CKRT, many providers perceive CKRT to be a contraindication to early mobilization.85 As such, nephrologists must advocate that patients receiving CKRT who are otherwise appropriate candidates for early mobilization receive the physical therapy they need.
Finally, CKRT should be provided within the context of a robust quality assurance program to standardize best local practices with institutional protocols, design and regularly execute training sessions to build and maintain staff competence, and monitor CKRT quality indicators and clinical and patient-centered outcomes.79,80 An essential element of such programs is cooperation with information technology services to develop dedicated order sets and autopopulating CKRT flow sheets within the electronic health record (EHR). These EHR features can optimize the prescription and monitoring of CKRT and facilitate automatic transfer of data collected by CKRT machines or charted in the EHR into a CKRT dashboard that allows for real-time tracking of CKRT metrics.80,86,87 Importantly, there is a dire need to validate CKRT quality indicators and benchmarks that can be feasibly implemented and facilitate CKRT standardization.
Areas of Future Research and Conclusion
Table 6 lists additional CKRT-related research questions that remain to be investigated. With the continued rise in the incidence of AKI-KRT, nephrologists and intensivists are spending increasing amounts of time caring for patients receiving CKRT. The knowledge and skills to provide high-quality CKRT remain as vital as ever to the daily practice of critical care nephrology.
Table 6.
Areas of ongoing controversy and need for more prospective research in continuous KRT
| CKRT-Related Topics Requiring Additional Future Research |
|---|
| Evaluation of optimal approaches to volume management and net ultrafiltration in CKRT |
| Evaluation of strategies for optimal discontinuation of CKRT and optimal transitioning from CKRT to intermittent HD or PIKRT |
| CKRT dosing |
| Validation of approaches to patients with obesity |
| Determination of safe lower dose limit to better guide dosing in pandemic or resource-limited settings |
| Determination of dosing of other solute targets besides urea |
| Evaluation of whether phosphate-containing CKRT solutions improve clinical outcomes beyond correction of hypophosphatemia, such as decreased duration of mechanical ventilation |
| Evaluation of use of tunneled catheters (rather than nontunneled catheters) for AKI requiring CKRT |
| Conduction of interventional studies to best validate the following approaches |
| Precision fluid and solute management |
| Optimal integration of CKRT into ECMO and other extracorporeal organ support systems (e.g., ECCO2R, blood purification devices) |
| Nutritional support in CKRT, including how to best mitigate the effects of nonselective removal of amino acids, phosphate, and other micronutrients |
| Optimal drug dosing in CKRT, especially of antibiotics in the setting of sepsis |
| Physical therapy/early mobilization in CKRT |
| Development and validation of risk classification and/or clinical decision support systems to optimize patient selection for CKRT, CKRT delivery, and timing of CKRT initiation and discontinuation |
| Validation of CKRT quality indicators and benchmarks that could affect clinical and patient-centered outcomes |
| Evidence-based standardization of CKRT practice and education |
| Evaluation of equity in CKRT access and delivery to minority populations and in resource-limited settings |
CKRT, continuous KRT; HD, hemodialysis; PIKRT, prolonged intermittent KRT; ECMO, extracorporeal membrane oxygenation; ECCO2R, extracorporeal carbon dioxide removal.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Disclosures
J.A. Neyra reports serving on the editorial boards of Advances in Chronic Kidney Disease, American Journal of Kidney Diseases, and Kidney360; serving as a guest editor for critical care nephrology in Advances in Chronic Kidney Disease and a section editor for Clinical Nephrology; and having consultancy agreements with Baxter Healthcare Inc., Biomedical Insights, and Leadiant Biosciences. J.P. Teixeira reports receiving research funding from Astute Medical/bioMérieux, La Jolla Pharmaceutical Company, Pfizer, Rediscovery Life Sciences LLC, and Sentien Biotechnologies Inc.; having ownership interest via current or previous stocks and/or options (in the last 24 months) in Clever Leaves Holdings, Flora Growth Corp., Green Thumb Industries Inc., Juva Life Inc., NeoGenomics Inc., Novocure Ltd., and Novo Nordisk A/S; and having consultancy agreements with, and serving on a speakers bureau for, Outset Medical. J.P. Teixeira's spouse reports honoraria from Cara Therapeutics. A. Tolwani reports serving on a speakers bureau for Baxter; having a patent on 0.5% trisodium citrate solution for CRRT anticoagulation (the license has been bought by Baxter); having consultancy agreements with Baxter Healthcare; serving on the editorial boards of CJASN and Kidney International; and receiving honoraria from UpToDate.
Funding
None.
Author Contributions
J.A. Neyra, J.P. Teixeira, and A. Tolwani wrote the original draft and reviewed and edited the manuscript.
References
- 1.Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, Edipidis K, Forni LG, Gomersall CD, Govil D, Honoré PM, Joannes-Boyau O, Joannidis M, Korhonen AM, Lavrentieva A, Mehta RL, Palevsky P, Roessler E, Ronco C, Uchino S, Vazquez JA, Vidal Andrade E, Webb S, Kellum JA: Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med 41: 1411–1423, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Bouchard J, Acharya A, Cerda J, Maccariello ER, Madarasu RC, Tolwani AJ, Liang X, Fu P, Liu ZH, Mehta RL: A prospective international multicenter study of AKI in the intensive care unit. Clin J Am Soc Nephrol 10: 1324–1331, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wald R, McArthur E, Adhikari NK, Bagshaw SM, Burns KE, Garg AX, Harel Z, Kitchlu A, Mazer CD, Nash DM, Scales DC, Silver SA, Ray JG, Friedrich JO: Changing incidence and outcomes following dialysis-requiring acute kidney injury among critically ill adults: A population-based cohort study. Am J Kidney Dis 65: 870–877, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Uchino S Kellum JA Bellomo R Doig GS Morimatsu H Morgera S Schetz M Tan I Bouman C Macedo E Gibney N Tolwani A Ronco C; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators : Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 294: 813–818, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Palevsky PM Zhang JH O’Connor TZ Chertow GM Crowley ST Choudhury D Finkel K Kellum JA Paganini E Schein RM Smith MW Swanson KM Thompson BT Vijayan A Watnick S Star RA Peduzzi P; VA/NIH Acute Renal Failure Trial Network : Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 359: 7–20, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellomo R Cass A Cole L Finfer S Gallagher M Lo S McArthur C McGuinness S Myburgh J Norton R Scheinkestel C Su S; RENAL Replacement Therapy Study Investigators : Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med 361: 1627–1638, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Chan L Chaudhary K Saha A Chauhan K Vaid A Zhao S Paranjpe I Somani S Richter F Miotto R Lala A Kia A Timsina P Li L Freeman R Chen R Narula J Just AC Horowitz C Fayad Z Cordon-Cardo C Schadt E Levin MA Reich DL Fuster V Murphy B He JC Charney AW Böttinger EP Glicksberg BS Coca SG Nadkarni GN; Mount Sinai COVID Informatics Center (MSCIC) : AKI in hospitalized patients with COVID-19. J Am Soc Nephrol 32: 151–160, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta S Coca SG Chan L Melamed ML Brenner SK Hayek SS Sutherland A Puri S Srivastava A Leonberg-Yoo A Shehata AM Flythe JE Rashidi A Schenck EJ Goyal N Hedayati SS Dy R Bansal A Athavale A Nguyen HB Vijayan A Charytan DM Schulze CE Joo MJ Friedman AN Zhang J Sosa MA Judd E Velez JCQ Mallappallil M Redfern RE Bansal AD Neyra JA Liu KD Renaghan AD Christov M Molnar MZ Sharma S Kamal O Boateng JO Short SAP Admon AJ Sise ME Wang W Parikh CR Leaf DE; STOP-COVID Investigators : AKI treated with renal replacement therapy in critically ill patients with COVID-19. J Am Soc Nephrol 32: 161–176, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaieski DF, Edwards JM, Kallan MJ, Carr BG: Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med 41: 1167–1174, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Ponce D, Balbi A, Cullis B: Acute PD: Evidence, guidelines, and controversies. Semin Nephrol 37: 103–112, 2017 [DOI] [PubMed] [Google Scholar]
- 11.Shimonov D, Srivatana V: Peritoneal dialysis for acute kidney injury during the COVID-19 pandemic. Clin J Am Soc Nephrol 15: 1829–1831, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chionh CY, Soni SS, Finkelstein FO, Ronco C, Cruz DN: Use of peritoneal dialysis in AKI: A systematic review. Clin J Am Soc Nephrol 8: 1649–1660, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nash DM, Przech S, Wald R, O’Reilly D: Systematic review and meta-analysis of renal replacement therapy modalities for acute kidney injury in the intensive care unit. J Crit Care 41: 138–144, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Lins RL Elseviers MM Van der Niepen P Hoste E Malbrain ML Damas P Devriendt J; SHARF investigators : Intermittent versus continuous renal replacement therapy for acute kidney injury patients admitted to the intensive care unit: Results of a randomized clinical trial. Nephrol Dial Transplant 24: 512–518, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Vinsonneau C Camus C Combes A Costa de Beauregard MA Klouche K Boulain T Pallot JL Chiche JD Taupin P Landais P Dhainaut JF; Hemodiafe Study Group : Continuous venovenous haemodiafiltration versus intermittent haemodialysis for acute renal failure in patients with multiple-organ dysfunction syndrome: A multicentre randomised trial. Lancet 368: 379–385, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Schefold JC, von Haehling S, Pschowski R, Bender T, Berkmann C, Briegel S, Hasper D, Jörres A: The effect of continuous versus intermittent renal replacement therapy on the outcome of critically ill patients with acute renal failure (CONVINT): A prospective randomized controlled trial. Crit Care 18: R11, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Augustine JJ, Sandy D, Seifert TH, Paganini EP: A randomized controlled trial comparing intermittent with continuous dialysis in patients with ARF. Am J Kidney Dis 44: 1000–1007, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Bouchard J Soroko SB Chertow GM Himmelfarb J Ikizler TA Paganini EP Mehta RL; Program to Improve Care in Acute Renal Disease (PICARD) Study Group : Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int 76: 422–427, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Woodward CW, Lambert J, Ortiz-Soriano V, Li Y, Ruiz-Conejo M, Bissell BD, Kelly A, Adams P, Yessayan L, Morris PE, Neyra JA: Fluid overload associates with major adverse kidney events in critically ill patients with acute kidney injury requiring continuous renal replacement therapy. Crit Care Med 47: e753–e760, 2019 [DOI] [PubMed] [Google Scholar]
- 20.Davenport A: Practical guidance for dialyzing a hemodialysis patient following acute brain injury. Hemodial Int 12: 307–312, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Davenport A: Continuous renal replacement therapies in patients with acute neurological injury. Semin Dial 22: 165–168, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Diaz-Buxo JA, Loredo JP: Standard Kt/V: Comparison of calculation methods. Artif Organs 30: 178–185, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Ghannoum M, Roberts DM, Hoffman RS, Ouellet G, Roy L, Decker BS, Bouchard J: A stepwise approach for the management of poisoning with extracorporeal treatments. Semin Dial 27: 362–370, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Clark WR, Leblanc M, Ricci Z, Ronco C: Quantification and dosing of renal replacement therapy in acute kidney injury: A reappraisal. Blood Purif 44: 140–155, 2017 [DOI] [PubMed] [Google Scholar]
- 25.Rosner MH, Connor MJ, Jr: Management of severe hyponatremia with continuous renal replacement therapies. Clin J Am Soc Nephrol 13: 787–789, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yessayan LT, Szamosfalvi B, Rosner MH: Management of dysnatremias with continuous renal replacement therapy. Semin Dial 34: 472–479, 2021 [DOI] [PubMed] [Google Scholar]
- 27.Clark WR, Soltys PJ: Hemodialysis systems for intermittent, semi-continuous, and continuous therapies in acute renal failure. Home Hemodial Int (1997) 2: 30–33, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Uchino S, Fealy N, Baldwin I, Morimatsu H, Bellomo R: Pre-dilution vs. post-dilution during continuous veno-venous hemofiltration: Impact on filter life and azotemic control. Nephron Clin Pract 94: c94–c98, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Feldhoff P, Turnham T, Klein E: Effect of plasma proteins on the sieving spectra of hemofilters. Artif Organs 8: 186–192, 1984 [DOI] [PubMed] [Google Scholar]
- 30.Friedrich JO, Wald R, Bagshaw SM, Burns KE, Adhikari NK: Hemofiltration compared to hemodialysis for acute kidney injury: Systematic review and meta-analysis. Crit Care 16: R146, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Combes A, Bréchot N, Amour J, Cozic N, Lebreton G, Guidon C, Zogheib E, Thiranos JC, Rigal JC, Bastien O, Benhaoua H, Abry B, Ouattara A, Trouillet JL, Mallet A, Chastre J, Leprince P, Luyt CE: Early high-volume hemofiltration versus standard care for post-cardiac surgery shock. The HEROICS Study. Am J Respir Crit Care Med 192: 1179–1190, 2015 [DOI] [PubMed] [Google Scholar]
- 32.Borthwick EM, Hill CJ, Rabindranath KS, Maxwell AP, McAuley DF, Blackwood B: High-volume haemofiltration for sepsis in adults. Cochrane Database Syst Rev 1: CD008075, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Califano AM, Bitker L, Baldwin I, Fealy N, Bellomo R: Circuit survival during continuous venovenous hemodialysis versus continuous venovenous hemofiltration. Blood Purif 49: 281–288, 2020 [DOI] [PubMed] [Google Scholar]
- 34.Brain M, Winson E, Roodenburg O, McNeil J: Non anti-coagulant factors associated with filter life in continuous renal replacement therapy (CRRT): A systematic review and meta-analysis. BMC Nephrol 18: 69, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kidney Disease Improving Global Outcomes (KDIGO) Acute Kideny Injury Work Group : KDIGO clinical practice guideline for acute kidney injury. Available at: https://kdigo.org/guidelines/acute-kidney-injury/. Accessed April 11, 2022 [Google Scholar]
- 36.Morgan D, Ho K, Murray C, Davies H, Louw J: A randomized trial of catheters of different lengths to achieve right atrium versus superior vena cava placement for continuous renal replacement therapy. Am J Kidney Dis 60: 272–279, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Parienti JJ Thirion M Mégarbane B Souweine B Ouchikhe A Polito A Forel JM Marqué S Misset B Airapetian N Daurel C Mira JP Ramakers M du Cheyron D Le Coutour X Daubin C Charbonneau P; Members of the Cathedia Study Group : Femoral vs jugular venous catheterization and risk of nosocomial events in adults requiring acute renal replacement therapy: A randomized controlled trial. JAMA 299: 2413–2422, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Parienti JJ Mégarbane B Fischer MO Lautrette A Gazui N Marin N Hanouz JL Ramakers M Daubin C Mira JP Charbonneau P du Cheyron D; Cathedia Study Group : Catheter dysfunction and dialysis performance according to vascular access among 736 critically ill adults requiring renal replacement therapy: A randomized controlled study. Crit Care Med 38: 1118–1125, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Al Rifai A, Sukul N, Wonnacott R, Heung M: Safety of arteriovenous fistulae and grafts for continuous renal replacement therapy: The Michigan experience. Hemodial Int 22: 50–55, 2018 [DOI] [PubMed] [Google Scholar]
- 40.Claure-Del Granado R, Macedo E, Chertow GM, Soroko S, Himmelfarb J, Ikizler TA, Paganini EP, Mehta RL: Effluent volume in continuous renal replacement therapy overestimates the delivered dose of dialysis. Clin J Am Soc Nephrol 6: 467–475, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neyra JA, Tolwani A: CRRT prescription and delivery of dose. Semin Dial 34: 432–439, 2021 [DOI] [PubMed] [Google Scholar]
- 42.Prowle JR, Schneider A, Bellomo R: Clinical review: Optimal dose of continuous renal replacement therapy in acute kidney injury. Crit Care 15: 207, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clark EG, Vijayan A: Intensive RRT for AKI: Dial down your enthusiasm! [published online ahead of print June 3, 2022]. Kidney360 10.34067/KID.0000972022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uchino S Toki N Takeda K Ohnuma T Namba Y Katayama S Kawarazaki H Yasuda H Izawa J Uji M Tokuhira N Nagata I; Japanese Society for Physicians and Trainees in Intensive Care (JSEPTIC) Clinical Trial Group : Validity of low-intensity continuous renal replacement therapy. Crit Care Med 41: 2584–2591, 2013 [DOI] [PubMed] [Google Scholar]
- 45.Leypoldt JK, Kamerath CD, Gilson JF, Friederichs G: Dialyzer clearances and mass transfer-area coefficients for small solutes at low dialysate flow rates. ASAIO J 52: 404–409, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Clark WR, Turk JE, Kraus MA, Gao D: Dose determinants in continuous renal replacement therapy. Artif Organs 27: 815–820, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Fealy N, Aitken L, du Toit E, Lo S, Baldwin I: Faster blood flow rate does not improve circuit life in continuous renal replacement therapy: A randomized controlled trial. Crit Care Med 45: e1018–e1025, 2017 [DOI] [PubMed] [Google Scholar]
- 48.Szamosfalvi B, Puri V, Sohaney R, Wagner B, Riddle A, Dickinson S, Napolitano L, Heung M, Humes D, Yessayan L: Regional citrate anticoagulation protocol for patients with presumed absent citrate metabolism. Kidney360 2: 192–204, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baldwin I, Bellomo R, Koch B: Blood flow reductions during continuous renal replacement therapy and circuit life. Intensive Care Med 30: 2074–2079, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Hatamizadeh P, Tolwani A, Palevsky P: Revisiting filtration fraction as an index of the risk of hemofilter clotting in continuous venovenous hemofiltration. Clin J Am Soc Nephrol 15: 1660–1662, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eastwood GM, Peck L, Young H, Bailey M, Reade MC, Baldwin I, Bellomo R: Haemodynamic impact of a slower pump speed at start of continuous renal replacement therapy in critically Ill adults with acute kidney injury: A prospective before-and-after study. Blood Purif 33: 52–58, 2012 [DOI] [PubMed] [Google Scholar]
- 52.Valle EO, Cabrera CPS, Albuquerque CCC, Silva GVD, Oliveira MFA, Sales GTM, Smolentzov I, Reichert BV, Andrade L, Seabra VF, Lins PRG, Rodrigues CE: Continuous renal replacement therapy in COVID-19-associated AKI: Adding heparin to citrate to extend filter life-A retrospective cohort study. Crit Care 25: 299, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu C, Mao Z, Kang H, Hu J, Zhou F: Regional citrate versus heparin anticoagulation for continuous renal replacement therapy in critically ill patients: A meta-analysis with trial sequential analysis of randomized controlled trials. Crit Care 20: 144, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zarbock A Küllmar M Kindgen-Milles D Wempe C Gerss J Brandenburger T Dimski T Tyczynski B Jahn M Mülling N Mehrländer M Rosenberger P Marx G Simon TP Jaschinski U Deetjen P Putensen C Schewe JC Kluge S Jarczak D Slowinski T Bodenstein M Meybohm P Wirtz S Moerer O Kortgen A Simon P Bagshaw SM Kellum JA Meersch M; RICH Investigators and the Sepnet Trial Group : Effect of regional citrate anticoagulation vs systemic heparin anticoagulation during continuous kidney replacement therapy on dialysis filter life span and mortality among critically ill patients with acute kidney injury: A randomized clinical trial. JAMA 324: 1629–1639, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neyra JA, Yessayan L, Thompson Bastin ML, Wille KM, Tolwani AJ: How to prescribe and troubleshoot continuous renal replacement therapy: A case-based review. Kidney360 2: 371–384, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Davenport A, Tolwani A: Citrate anticoagulation for continuous renal replacement therapy (CRRT) in patients with acute kidney injury admitted to the intensive care unit. NDT Plus 2: 439–447, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meier-Kriesche HU, Gitomer J, Finkel K, DuBose T: Increased total to ionized calcium ratio during continuous venovenous hemodialysis with regional citrate anticoagulation. Crit Care Med 29: 748–752, 2001 [DOI] [PubMed] [Google Scholar]
- 58.Zheng Y, Zhuang F, Zhu Q, Ma S, Xu Y, Lu J, Hao G, Gu Y, Hao C, Zhu M, Ding F: Albumin-corrected total/ionized calcium ratio is not superior to total/ionized calcium ratio as an indicator of citrate accumulation. Int J Artif Organs 40: 602–606, 2017 [DOI] [PubMed] [Google Scholar]
- 59.Tan JN, Haroon SWP, Mukhopadhyay A, Lau T, Murali TM, Phua J, Tan ZY, Lee N, Chua HR: Hyperlactatemia predicts citrate intolerance with regional citrate anticoagulation during continuous renal replacement therapy. J Intensive Care Med 34: 418–425, 2019 [DOI] [PubMed] [Google Scholar]
- 60.Zhang W, Bai M, Yu Y, Li L, Zhao L, Sun S, Chen X: Safety and efficacy of regional citrate anticoagulation for continuous renal replacement therapy in liver failure patients: A systematic review and meta-analysis. Crit Care 23: 22, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chua HR, Baldwin I, Bailey M, Subramaniam A, Bellomo R: Circuit lifespan during continuous renal replacement therapy for combined liver and kidney failure. J Crit Care 27: 744.e7–744.e15, 2012 [DOI] [PubMed] [Google Scholar]
- 62.Gerss J Meersch M Kindgen-Milles D Brandenburger T Willam C Kellum JA Zarbock A; RICH investigators : The effect of filter lifespan during continuous renal replacement therapy in critically ill patients with AKI on the rate of new onset infection: Analysis from the RICH Randomized Controlled Trial [published online ahead of print May 13, 2022]. Am J Respir Crit Care Med 10.1164/rccm.202201-0063LE [DOI] [PubMed] [Google Scholar]
- 63.Bitker L, Bayle F, Yonis H, Gobert F, Leray V, Taponnier R, Debord S, Stoian-Cividjian A, Guérin C, Richard JC: Prevalence and risk factors of hypotension associated with preload-dependence during intermittent hemodialysis in critically ill patients. Crit Care 20: 44, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Douvris A, Zeid K, Hiremath S, Bagshaw SM, Wald R, Beaubien-Souligny W, Kong J, Ronco C, Clark EG: Mechanisms for hemodynamic instability related to renal replacement therapy: A narrative review. Intensive Care Med 45: 1333–1346, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Murugan R, Bellomo R, Palevsky PM, Kellum JA: Ultrafiltration in critically ill patients treated with kidney replacement therapy. Nat Rev Nephrol 17: 262–276, 2021 [DOI] [PubMed] [Google Scholar]
- 66.Murugan R, Kerti SJ, Chang CH, Gallagher M, Clermont G, Palevsky PM, Kellum JA, Bellomo R: Association of net ultrafiltration rate with mortality among critically ill adults with acute kidney injury receiving continuous venovenous hemodiafiltration: A secondary analysis of the Randomized Evaluation of Normal vs Augmented Level (RENAL) of Renal Replacement Therapy Trial. JAMA Netw Open 2: e195418, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Demirjian S, Teo BW, Guzman JA, Heyka RJ, Paganini EP, Fissell WH, Schold JD, Schreiber MJ: Hypophosphatemia during continuous hemodialysis is associated with prolonged respiratory failure in patients with acute kidney injury. Nephrol Dial Transplant 26: 3508–3514, 2011 [DOI] [PubMed] [Google Scholar]
- 68.Sharma S, Kelly YP, Palevsky PM, Waikar SS: Intensity of renal replacement therapy and duration of mechanical ventilation: Secondary analysis of the Acute Renal Failure Trial Network Study. Chest 158: 1473–1481, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aubier M, Murciano D, Lecocguic Y, Viires N, Jacquens Y, Squara P, Pariente R: Effect of hypophosphatemia on diaphragmatic contractility in patients with acute respiratory failure. N Engl J Med 313: 420–424, 1985 [DOI] [PubMed] [Google Scholar]
- 70.Thompson Bastin ML, Stromberg AJ, Nerusu SN, Liu LJ, Mayer KP, Liu KD, Bagshaw SM, Wald R, Morris PE, Neyra JA: Association of phosphate-containing versus phosphate-free solutions on ventilator days in patients requiring continuous kidney replacement therapy. Clin J Am Soc Nephrol 17: 634–642, 2022 10.2215/CJN.12410921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thompson Bastin ML, Adams PM, Nerusu S, Morris PE, Mayer KP, Neyra JA: Association of phosphate containing solutions with incident hypophosphatemia in critically ill patients requiring continuous renal replacement therapy. Blood Purif 51: 122–129, 2022 [DOI] [PubMed] [Google Scholar]
- 72.Crowley KE, DeGrado JR, Charytan DM: Serum glucose and phosphorus concentrations during continuous renal replacement therapy using commercial replacement solutions with or without phosphorus. Hemodial Int 24: 330–334, 2020 [DOI] [PubMed] [Google Scholar]
- 73.Coutrot M, Hékimian G, Moulin T, Bréchot N, Schmidt M, Besset S, Nieszkowska A, Franchineau G, Bourcier S, Bourron O, Luyt CE, Combes A: Euglycemic ketoacidosis, a common and underecognized complication of continuous renal replacement therapy using glucose-free solutions. Intensive Care Med 44: 1185–1186, 2018 [DOI] [PubMed] [Google Scholar]
- 74.Afshinnia F, Belanger K, Palevsky PM, Young EW: Effect of ionized serum calcium on outcomes in acute kidney injury needing renal replacement therapy: Secondary analysis of the acute renal failure trial network study. Ren Fail 35: 1310–1318, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thongprayoon C, Cheungpasitporn W, Radhakrishnan Y, Zabala Genovez JL, Petnak T, Shawwa K, Qureshi F, Mao MA, Kashani KB: Association of serum potassium derangements with mortality among patients requiring continuous renal replacement therapy [published online ahead of print January 22, 2022]. Ther Apher Dial 10.1111/1744-9987.13804 [DOI] [PubMed] [Google Scholar]
- 76.Dhingra S, Solven F, Wilson A, McCarthy DS: Hypomagnesemia and respiratory muscle power. Am Rev Respir Dis 129: 497–498, 1984 [DOI] [PubMed] [Google Scholar]
- 77.Jiang P, Lv Q, Lai T, Xu F: Does hypomagnesemia impact on the outcome of patients admitted to the intensive care unit? A systematic review and meta-analysis. Shock 47: 288–295, 2017 [DOI] [PubMed] [Google Scholar]
- 78.Zakharchenko M, Los F, Brodska H, Balik M: The effects of high level magnesium dialysis/substitution fluid on magnesium homeostasis under regional citrate anticoagulation in critically ill. PLoS One 11: e0158179, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ruiz EF Ortiz-Soriano VM Talbott M Klein BA Thompson Bastin ML Mayer KP Price EB Dorfman R Adams BN Fryman L Neyra JA; University of Kentucky CRRT Quality Assurance Group : Development, implementation and outcomes of a quality assurance system for the provision of continuous renal replacement therapy in the intensive care unit. Sci Rep 10: 20616, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rewa OG Tolwani A Mottes T Juncos LA Ronco C Kashani K Rosner M Haase M Kellum J Bagshaw SM; ADQI Consensus Meeting Members on behalf of ADQI XXII : Quality of care and safety measures of acute renal replacement therapy: Workgroup statements from the 22nd acute disease quality initiative (ADQI) consensus conference. J Crit Care 54: 52–57, 2019 [DOI] [PubMed] [Google Scholar]
- 81.McClave SA Taylor BE Martindale RG Warren MM Johnson DR Braunschweig C McCarthy MS Davanos E Rice TW Cresci GA Gervasio JM Sacks GS Roberts PR Compher C; Society of Critical Care Medicine; American Society for Parenteral and Enteral Nutrition : Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr 40: 159–211, 2016 [DOI] [PubMed] [Google Scholar]
- 82.Beumier M, Casu GS, Hites M, Seyler L, Cotton F, Vincent JL, Jacobs F, Taccone FS: β-lactam antibiotic concentrations during continuous renal replacement therapy. Crit Care 18: R105, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roberts DM Roberts JA Roberts MS Liu X Nair P Cole L Lipman J Bellomo R; RENAL Replacement Therapy Study Investigators : Variability of antibiotic concentrations in critically ill patients receiving continuous renal replacement therapy: A multicentre pharmacokinetic study. Crit Care Med 40: 1523–1528, 2012 [DOI] [PubMed] [Google Scholar]
- 84.Onichimowski D, Ziółkowski H, Nosek K, Jaroszewski J, Rypulak E, Czuczwar M: Comparison of adsorption of selected antibiotics on the filters in continuous renal replacement therapy circuits: In vitro studies. J Artif Organs 23: 163–170, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mayer KP, Joseph-Isang E, Robinson LE, Parry SM, Morris PE, Neyra JA: Safety and feasibility of physical rehabilitation and active mobilization in patients requiring continuous renal replacement therapy: A systematic review. Crit Care Med 48: e1112–e1120, 2020 [DOI] [PubMed] [Google Scholar]
- 86.Neyra JA, Kashani K: Improving the quality of care for patients requiring continuous renal replacement therapy. Semin Dial 34: 501–509, 2021 [DOI] [PubMed] [Google Scholar]
- 87.Opgenorth D, Reil E, Lau V, Fraser N, Zuege D, Wang X, Bagshaw SM, Rewa O: Improving the quality of the performance and delivery of continuous renal replacement therapy (CRRT) to critically ill patients across a healthcare system: QUALITY CRRT: A study protocol. BMJ Open 12: e054583, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tolwani A: Continuous renal-replacement therapy for acute kidney injury. N Engl J Med 367: 2505–2514, 2012 [DOI] [PubMed] [Google Scholar]
- 89.Verma S, Palevsky PM: Prescribing continuous kidney replacement therapy in acute kidney injury: A narrative review. Kidney Med 3: 827–836, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]