Abstract
Kidney replacement therapy (KRT) is a vital, supportive treatment for patients with critical illness and severe AKI. The optimal timing, dose, and modality of KRT have been studied extensively, but gaps in knowledge remain. With respect to modalities, continuous KRT and intermittent hemodialysis are well-established options, but prolonged intermittent KRT is becoming more prevalent worldwide, particularly in emerging countries. Compared with continuous KRT, prolonged intermittent KRT offers similar hemodynamic stability and overall cost savings, and its intermittent nature allows patients time off therapy for mobilization and procedures. When compared with intermittent hemodialysis, prolonged intermittent KRT offers more hemodynamic stability, particularly in patients who remain highly vulnerable to hypotension from aggressive ultrafiltration over a shorter duration of treatment. The prescription of prolonged intermittent KRT can be tailored to patients’ progression in their recovery from critical illness, and the frequency, flow rates, and duration of treatment can be modified to avert hemodynamic instability during de-escalation of care. Dosing of prolonged intermittent KRT can be extrapolated from urea kinetics used to calculate clearance for continuous KRT and intermittent hemodialysis. Practice variations across institutions with respect to terminology, prescription, and dosing of prolonged intermittent KRT create significant challenges, especially in creating specific drug dosing recommendations during prolonged intermittent KRT. During the coronavirus disease 2019 pandemic, prolonged intermittent KRT was rapidly implemented to meet the KRT demands during patient surges in some of the medical centers overwhelmed by sheer volume of patients with AKI. Ideally, implementation of prolonged intermittent KRT at any institution should be conducted in a timely manner, with judicious planning and collaboration among nephrology, critical care, dialysis and intensive care nursing, and pharmacy leadership. Future analyses and clinical trials with respect to prescription and delivery of prolonged intermittent KRT and clinical outcomes will help to guide standardization of practice.
Keywords: critical care nephrology and AKI series, intermittent RRT, PIRRT
Introduction
Patients with AKI in the hospital, especially in the intensive care unit (ICU), require kidney replacement therapy (KRT) when metabolic demands exceed the renal capacity and conservative measures to control complications, such as hyperkalemia, metabolic acidosis, and volume overload, become ineffective. The timing, modality, and dose of KRT are based on the clinical status of the patient, local expertise, and availability of equipment at the institution. In this review, we discuss the option of prolonged intermittent KRT (PIKRT) in the management of patients with kidney failure in the ICU. The use of PIKRT has increased rapidly across the globe over the last 1–2 decades, and PIKRT came to the forefront during the coronavirus disease 2019 (COVID-19) pandemic as nephrologists struggled to maximize the precious KRT resources available at their institution during patient surges. In this review, we explore the differences in the technology, terminology, and prescription of PIKRT with the use of two clinical vignettes that illustrate how PIKRT may be utilized at different stages of critical illness and AKI (Boxes 1 and 2). We also summarize the advantages and disadvantages of hybrid therapies.
Box 1. Clinical vignette one.
A 67-year-old man with a past medical history of stage 3 CKD and heart failure with reduced ejection fraction presented to the emergency department with worsening shortness of breath and lethargy over the past several days. Physical examination on arrival revealed the following: heart rate 122/min, BP 96/57 mm Hg, respiratory rate 24/min, 1+ bilateral lower extremity edema, and bilateral crackles in both lung bases. Laboratory data were notable for serum potassium 6.1 mmol/L, CO2 11 mmol/L, BUN 76 mg/dl, serum creatinine 5.5 mg/dl (elevated from baseline of 1.3 mg/dl), and serum lactate 6 mmol/L. A COVID-19 RNA PCR test was positive. A chest X-ray revealed bilateral multifocal opacities, concerning for pneumonia. He was intubated shortly after presentation, transferred to the ICU, and placed on a ventilator. His fraction of inspired oxygen was 0.9 and positive end-expiratory pressure was 10. He also developed profound hypotension, necessitating the initiation of two vasoactive medications for hypotension. Immediately after admission to the ICU, the patient developed profound oliguria, and nephrology was consulted for management of AKI with refractory hyperkalemia, hypoxia, and metabolic acidosis. The nephrologist decided to initiate KRT.
Box 2. Clinical vignette two.
A 55-year-old male with a history of ventricular tachycardia and indwelling automated implantable cardioverter defibrillator was admitted to the hospital with bacteremia. His hospital course was complicated by nonoliguric KDIGO stage 3 AKI, requiring initiation of intermittent hemodialysis, three times a week. Due to impending explant of the automated implantable cardioverter defibrillator and need for close hemodynamic and telemetry monitoring, he was transferred to the ICU. The patient is hemodynamically stable without need for vasoactive medications. He does not require ventilator support.
Evolution of PIKRT
PIKRT is an umbrella term used to describe a hybrid form of KRT that offers either diffusive or convective clearance that is considerably longer than a standard hemodialysis session of 3–4 hours, but not 24-hour duration like continuous KRT (CKRT). In the seminal description of the first hemodialysis treatment by W.J. Kolff, the duration of the treatment was 11.5 hours, with a blood flow of 116 ml/min (1,2). A dialysis treatment with these parameters (blood flow rate and duration) would now be considered as a hybrid therapy and labeled PIKRT. After the original description, technology to provide safe and effective dialysis rapidly improved over the next 2–3 decades, and by the 1970s, duration of maintenance hemodialysis in the outpatient setting was shortened to approximately 3–4 hours. Similar intermittent hemodialysis protocols were also implemented for patients with AKI in the hospital, but physicians realized that patients who were critically ill with severe volume overload and hemodynamic instability were not receiving optimal clearance and ultrafiltration by a 4-hour dialysis treatment, three times per week. Although peritoneal dialysis was being utilized for patients with AKI, especially those with hemodynamic instability, it did not offer any option for controlled ultrafiltration. In 1977, continuous arteriovenous hemofiltration was introduced by Kramer as a KRT modality for patients who were critically ill (3), and over the last four decades, use of CKRT for patients who were hemodynamically unstable expanded rapidly across major medical centers, primarily in developed countries (4). In 2000, Kumar and colleagues described a hybrid therapy—extended daily dialysis—in the ICU for patients with AKI, and this publication propelled the current era of hybrid KRT (5,6). Over the last two decades, numerous publications have outlined the various technologies and terminologies used in medical centers all over the world to describe hybrid therapies (7). Until recently, the use of PIKRT was more prevalent in emerging countries (8), but during the COVID-19 pandemic, PIKRT was utilized in numerous centers that experienced patient surges, especially in the United States and Europe (9). A recent paper also described the growing use of PIKRT among the Veteran Administration hospitals in the United States (10).
PIKRT Nomenclature and Prescription
The term PIKRT (previously known as PIRRT) was coined in 2002 to describe any form of hybrid KRT in the acute care setting (11). One of the challenges of adapting PIKRT at an institution is the heterogeneity in terminology, modality, and equipment used to describe the various hybrid forms of KRT. Although initial reports described a less-efficient hemodialysis treatment of longer duration (diffusive mode of clearance), for example, sustained low-efficiency dialysis (SLED) (12,13), centers have also used convective clearance with high ultrafiltration rates, such as accelerated venovenous hemofiltration (14,15). Others have described PIKRT utilizing a combination of diffusion and convection, for example, sustained hemodiafiltration (16). PIKRT has been performed using hemodialysis machines, but CKRT machines can also be utilized to provide PIKRT, utilizing either convective or diffusive clearance (7,17). To be used for PIKRT, ideally, the CKRT machine should have an effluent line that will allow for ultrafiltrate and spent dialysate to be discarded directly into the toilet, therefore alleviating the nursing labor required to empty a 5-L effluent bag every 40–60 minutes. Similar to CKRT, there are no data demonstrating the benefit of diffusion over convection and vice versa, and using one mode over the other is on the basis of physician preference and equipment availability. Table 1 outlines the various terminologies and technology that have been coined in the literature to describe hybrid KRT therapies in different countries and medical centers. The dialysate (or replacement fluid) flow rates, blood flow rates, efficacy of clearance, duration, and frequency are intermediate between intermittent hemodialysis and CKRT, as shown in Figure 1. The two patient scenarios noted in this review highlight how PIKRT may be used as a substitute for CKRT or intermittent hemodialysis, or as a transition therapy from CKRT to intermittent hemodialysis, depending on the hemodynamic stability of the patient in the ICU. When implementing PIKRT, the goal should be to provide clearance that is dose equivalent to, or intermediate between, CKRT and intermittent hemodialysis, depending on the clinical scenario. Additional considerations when choosing PIKRT would be nursing labor, cost of equipment and disposables, and patient convenience. The differences in prescription when utilizing CKRT equipment versus hemodialysis equipment are shown in Table 2.
Table 1.
Prolonged intermittent KRT: Nomenclature and commonly used KRT machines
| Acronym | Procedure | KRT Machines |
|---|---|---|
| Diffusion only | ||
| SLED (12,13,47) | Sustained low-efficiency dialysis | Fresenius 2008, Gambro AK200S, Gambro Integra |
| ED (48), EDD (5) | Extended dialysis or Extended daily dialysis | Fresenius 2008, Fresenius Genius |
| SLED-BD (23) | Sustained low-efficiency dialysis with batched dialysate | Fresenius Genius |
| E-HFD (49) | Extended high-flux hemodialysis | Fresenius Genius |
| Convection only | ||
| AVVH (14,15) | Accelerated venovenous hemofiltration | NxStage System One |
| Diffusion and convection | ||
| S-HDF (16) | Sustained hemodiafiltration | Nikkiso DBB-02 |
| SLEDD-f (50) | Sustained low-efficiency daily diafiltration | Fresenius 4008S ArRT-Plus |
| PDIKRT (51) | Prolonged daily intermittent KRT | Fresenius 4008S ArRT-Plus |
Terminology and equipment shown above reflect published data. Other types of machines may be used for a particular modality. For example, any CKRT machine with an effluent line can be used for AVVH or PIKRT with diffusive clearance. SLED, sustained low-efficiency dialysis; ED, extended dialysis; EDD, extended daily dialysis; SLED-BD, sustained low-efficiency dialysis with batched dialysate; E-HFD, extended high-flux hemodialysis; AVVH, accelerated venovenous hemofiltration; S-HDF, sustained hemodiafiltration; SLEDD-f, sustained low-efficiency daily diafiltration; PDIKRT, prolonged daily intermittent KRT; CKRT, continuous KRT; PIKRT, prolonged intermittent KRT.
Figure 1.
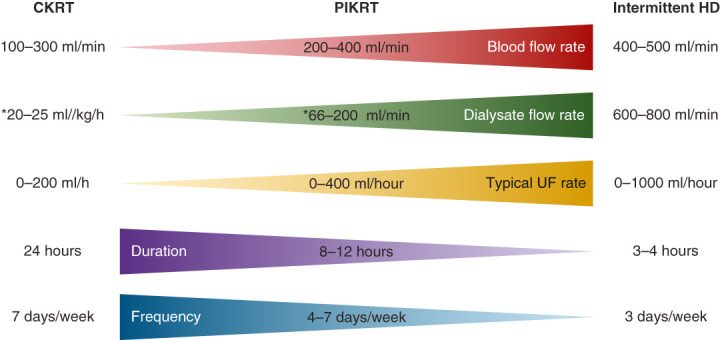
Treatment parameters during continuous KRT (CKRT), prolonged intermittent KRT (PIKRT), and intermittent hemodialysis (HD). The blood flow rates, dialysate flow rates, and ultrafiltration rates may vary across centers. Kidney Disease Improving Global Outcomes (KDIGO) recommends effluent flow rate of 20–25 ml/kg per hour for CKRT, and intermittent HD to be provided three times a week. *For CKRT and PIKRT, this number may represent dialysate flow rate, replacement fluid flow rate, or a combination, on the basis of institutional practices.
Table 2.
Standard prolonged intermittent KRT prescriptions with continuous KRT and hemodialysis machines
| Variable | CKRT Machines | Hemodialysis Machines |
|---|---|---|
| Duration of treatment | 8–12 hr | 8–12 hr |
| Frequency of treatment | 4–7 d/wka | 3–7 d/wk |
| Dialysate flow rateb | 66–133 ml/minc | 200 ml/min |
| Blood flow rate | 200–400 ml/min | 200–400 ml/min |
| Ultrafiltration rate | Depending on volume and hemodynamic status | Depending on volume and hemodynamic status |
CKRT, continuous KRT; PIKRT, prolonged intermittent KRT.
Clearance using CKRT equipment is lower than with intermittent HD equipment; 3 days per week will not provide adequate clearance.
During PIKRT with CKRT equipment, therapy fluid can be delivered as either dialysate (diffusive clearance) or replacement fluid (convective clearance).
Dialysate flow rate calculated on the basis of CKRT flow rates. Recommended CKRT flow rate is 20–25 ml/kg per hour. For example, for 100 kg, 20 ml/kg per hour = 48,000 ml in 24 hours. For 8-hour PIKRT, divide 48,000 ml by 8 hours = 6000 ml/hour = 100 ml/min.
PIKRT as a Substitute for CKRT
How can nephrologists and medical centers utilize PIKRT at their institutions? In the first clinical vignette (Box 1), we describe a patient who is critically ill and hemodynamically unstable and on vasoactive medications with AKI. In most institutions, CKRT (if available) will be the modality of choice on the basis of 2012 Kidney Disease Improving Global Outcomes (KDIGO) recommendations (18). However, PIKRT is definitely a consideration for these patients. In a retrospective cohort study, Marshall and colleagues demonstrated that ICU mortality at three different medical centers (one each in New Zealand, Australia, and Italy) was not altered after the institutions changed their primary KRT modality in patients who were hemodynamically unstable from CKRT to PIKRT (19). Other smaller single-center studies had demonstrated that PIKRT was well tolerated with insignificant cardiovascular instability (20). On the basis of these data, the 2013 National Kidney Foundation Kidney Disease Outcomes Quality Initiative and the Canadian Society of Nephrology commentaries on the KDIGO guidelines noted that PIKRT can be considered as an alternative modality to CKRT in the patient who is hemodynamically unstable (21,22). In 2012, Schwenger published the first randomized controlled trial that evaluated mortality and other clinical outcomes in patients who were critically ill and treated with PIKRT (as sustained low-efficiency dialysis with batched dialysate) and those treated with continuous venovenous hemofiltration (23). There was no difference in hemodynamic instability between the two groups, and the PIKRT group had significantly fewer days of mechanical ventilation. The primary outcome of 90-day mortality was similar between the two groups. Other observational studies have also supported the interchangeability between CKRT and PIKRT when evaluating clinical outcomes such as mortality, ventilator dependence, and length of hospital stay (19,24). It should be noted that KDIGO and other society guidelines recommend CKRT as the modality of choice in patients with raised intracranial pressure based on data demonstrating rise in intracranial pressure during hemodialysis treatments (25). At this time, there are insufficient data to determine whether PIKRT can be safely implemented in those with traumatic brain injury and/or intracranial hypertension from other causes, and CKRT should remain the preferred modality for this high-risk group of patients.
PIKRT as a Substitute for Intermittent Hemodialysis
In the second clinical vignette (Box 2), the patient is hemodynamically stable and does not require vasopressor or ventilator support. For patients in this scenario, intermittent hemodialysis is the modality of choice at most centers. It offers the patient the option of getting adequate clearance three times a week, with a relatively short duration of treatment, compared with CKRT. However, provision of intermittent hemodialysis in the ICU requires one-on-one hemodialysis nursing support. To offset the progressive shortage of dialysis nurses in both the outpatient and inpatient setting (26), institutions such as ours have implemented PIKRT for use in patients who are hemodynamically stable and in the ICU. The nursing oversight of PIKRT is institution dependent. Some institutions may require dialysis nursing presence in the unit for the duration of treatment, but the dialysis nurse may oversee two or more patients at a time, in the same ICU (Figure 2). Alternatively, PIKRT can be performed as a collaborative effort between dialysis and critical care, thus alleviating the burden on just one group of nurses (27). PIKRT can be performed either during daytime or at night, and nocturnal PIKRT is an excellent option for the patient in the second clinical vignette (Box 2) (7).
Figure 2.
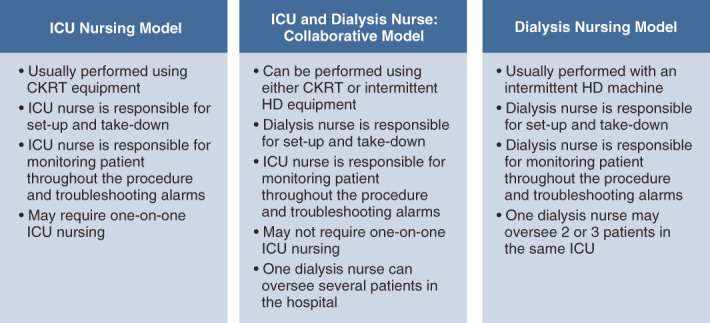
Models of nursing support for PIKRT in the intensive care unit (ICU). This figure shows the different nursing models used at various institutions to provide prolonged KRT in the ICU.
PIKRT for De-Escalation of Care
In addition to being used as a substitute for CKRT and intermittent hemodialysis, PIKRT can also be utilized as a transition therapy between CKRT and intermittent hemodialysis (Figure 3). Hemodynamic improvement will gradually lead to cessation of vasoactive agents in patients who are critically ill, and nephrologists will attempt to treat such patients with intermittent hemodialysis as care is slowly de-escalated to allow transfer out of the ICU. In the Veterans Affairs/National Institutes of Health Acute Renal Failure Trial Network study, patients with hemodynamic stability as defined by Cardiovascular Sequential Organ Failure Assessment score of 0–1 were primarily treated with intermittent hemodialysis, and those with Sequential Organ Failure Assessment score of 3–4 were primarily treated with CKRT (28). However, the patients who were critically ill with improving Cardiovascular Sequential Organ Failure Assessment scores remain highly vulnerable to volume shifts, blood loss, cardiac dysfunction, etc., all which may result in hypotension during hemodialysis sessions, especially when high ultrafiltration rates are attempted. Hypotension during KRT is a risk factor for further ischemic tubular injury and has been hypothesized as a major factor leading to delay in recovery of kidney function (29,30). Two studies, each with a different definition of hypotension, have reported the incidence of hypotension during hemodialysis at 30% and 57% (31,32), which is significantly higher when compared with CKRT (33). Therefore, as part of de-escalation of care in the ICU, PIKRT can be used as a “shift” or transition therapy because hemodynamic stability with PIKRT is generally comparable to that of CKRT. PIKRT provided as 6–12 hour therapy, 5–6 days a week, will allow the patient to have sufficient clearance and ultrafiltration. At our institution, PIKRT is primarily used as transition therapy and usually provided at night. This allows patients to undergo physical therapy, radiology exams, and procedures during the day, when appropriate staff are available (7). Therefore, for the patient noted in the first clinical vignette (Box 1), as the patient’s hemodynamic stability improves to the point of cessation of vasoactive agents or requiring only a low dose of a single agent, that patient can be transitioned to PIKRT, preferably at night. PIKRT, when utilized as a transition therapy from CKRT to intermittent hemodialysis, has been associated with lower ICU readmission rates (15).
Figure 3.
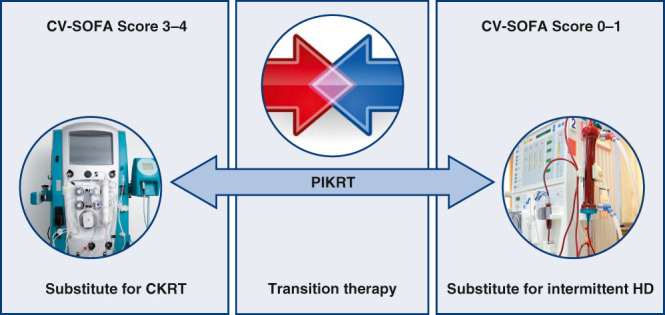
Use of PIKRT in the ICU. PIKRT can be used as a substitute for CKRT or intermittent HD, or as a transition between CKRT and intermittent HD during de-escalation of care in the ICU. The Cardiovascular Sequential Organ Failure Assessment (CV-SOFA) score is one of the many tools used to determine hemodynamic stability of the patient. CV-intermittent HD SOFA SCORE: mean arterial pressure (MAP) >70=0, MAP <70 mm Hg =1, dopamine ≤5 or dobutamine (any dose) =2, dopamine >5, epinephrine ≤0.1, or norepinephrine ≤0.1=3, dopamine >15, epinephrine >0.1, or norepinephrine >0.1=4.
Dosing of PIKRT
Urea clearance, albeit an imperfect marker, is the primary method used to assess the dose of KRT. Middle molecular clearance is important, but there are no guidelines supporting the use of middle molecular clearance to estimate dosing of CKRT or PIKRT. KDIGO and the National Kidney Foundation Kidney Disease Outcomes Quality Initiative recommend the provision of CKRT at a dose of 20–25 ml/kg per hour and intermittent hemodialysis, three times per week, with a single pool Kt/Vurea (spKt/Vurea) of 1.3 per treatment. This is equivalent to a standard weekly Kt/Vurea of 6–7 for CKRT and 2 for intermittent hemodialysis (34) (Figure 4). There are no recommendations regarding dosing of PIKRT. So how do we ensure adequate clearance with a hybrid therapy? Studies have shown that PIKRT will provide urea clearance comparable to CKRT (12,20). In one study, CKRT at 25 ml/kg per hour yielded a weekly StdKt/Vurea of 7.1 when compared with 8.4 with sustained low-efficiency dialysis (8-hour sessions, 6 days per week, blood flow 350 ml/min, and dialysate flow 200 ml/min) (12). Because we primarily utilize PIKRT as a transition therapy at our institution, our PIKRT prescription is extrapolated from the typical CKRT dose. For example, a 100-kg patient who is prescribed CKRT at 20 ml/kg per hour will require 48 L of fluid (replacement fluid, dialysate, or combination) in 24 hours. If PIKRT is prescribed for 8 hours, the patient will require 6 L/hour of substitution fluid or dialysate, depending on whether therapy is provided as convection or diffusion. In addition, because PIKRT is not provided as continuous therapy, we measure pre- and post-BUN to calculate urea reduction ratio, and spKt/Vurea. This allows us to tailor dosing of subsequent treatments and determine frequency of sessions. A spKt/Vurea of approximately 0.7–0.9 per treatment is equivalent to a weekly standard Kt/Vurea of approximately 2.5–4.0, depending on a treatment frequency of 5–7 days per week. This ensures that dosing of PIKRT is approximately midway between the recommended doses for CKRT and intermittent hemodialysis. PIKRT dosing can be adjusted either by increasing the frequency and/or dialysate flow rate if higher clearances are required. We do not recommend decreasing the dose of PIKRT below the recommended urea clearance for intermittent hemodialysis.
Figure 4.
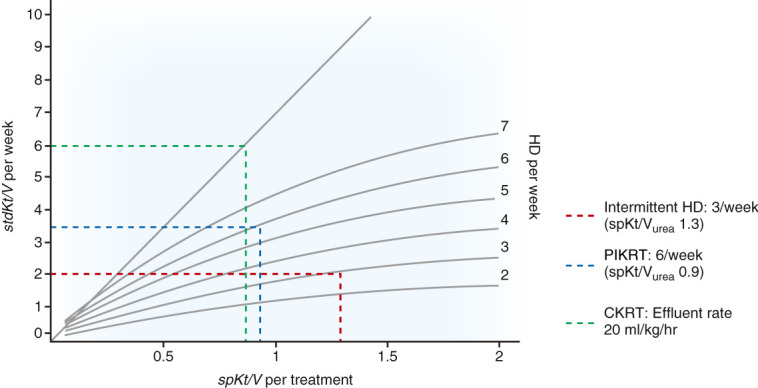
Urea kinetics during different modalities of KRT. Urea kinetics are a suboptimal marker for assessing adequacy of KRT. However, current guidelines for adequacy of hemodialysis and CKRT utilize urea kinetics and Kt/Vurea. Standard Kt/Vurea (StdKt/Vurea) may be utilized to compare weekly clearances among different modalities. CKRT with an effluent flow rate of 20 ml/kg per hour provides StdKt/Vurea of 6 (assuming no interruptions of therapy), and intermittent HD, three times per week, with a single pool Kt/Vurea of 1.3 per treatment provides a weekly clearance of 2.0. PIKRT protocol at our institution usually provides a single pool Kt/Vurea of approximately 0.9 per session and, if given 6 days a week, will provide a weekly StdKt/Vurea of 3.5. Adapted from ref. 34, with permission.
Advantages of PIKRT
PIKRT offers certain advantages over CKRT and over intermittent hemodialysis (Table 3). Provision of noncontinuous, but adequate, solute clearance in patients who are hemodynamically unstable offers them the opportunity of machine-free time for physical therapy and ambulation, and may even lead to more ventilator-free days (23). Early mobilization in the ICU has been demonstrated to be associated with greater muscle strength and improved mobility at hospital discharge (35). Nocturnal PIKRT in particular offers a significant advantage over CKRT because patients usually sleep through the procedure with minimal interruption. Nocturnal PIKRT also allows for the utilization of the entire daytime for radiology and surgical procedures and uninterrupted physical therapy and ambulation, when maximum personnel are available (7). When compared with CKRT, PIKRT with hemodialysis equipment has been shown to be less expensive because the expense of CKRT is driven by consumables, particularly the commercially prepared, bagged, sterile solutions (12,23). Unlike CKRT, which typically requires anticoagulation for maximum filter survival, PIKRT can be performed without anticoagulation, especially when utilizing CKRT equipment (14,15). If anticoagulation is required, either heparin or citrate can be used during PIKRT, similar to many existing anticoagulation protocols for CKRT (36,37). The utilization of CKRT equipment for PIKRT also allows for the treatment of multiple patients with the same machine in the course of 24 hours. This feature became particularly important during the COVID-19 pandemic when hospitals utilized PIKRT to maximize the number of patients treated during hospital surges (38). Depending on the duration of PIKRT, one CKRT machine can be used to treat up to three patients in the same 24-hour period. This requires careful planning, coordination, sharing protocol, and adequate time for takedown, disinfection, and set-up for the next patient (38). Similarly, when PIKRT is used as a substitute for intermittent hemodialysis in the ICU, in most centers, this eliminates 1:1 ratio for hemodialysis nurses, thereby offsetting labor costs. During the pandemic, PIKRT, instead of intermittent hemodialysis, was frequently used in the ICU for patients who were hemodynamically stable because this reduced potential exposure of the hemodialysis nurses who would otherwise need to be present in the room for several hours to provide therapy. We also utilize nocturnal PIKRT instead of intermittent hemodialysis in our heart failure/left-ventricular assist device step-down floor because these patients also typically require 1:1 bedside nursing for intermittent hemodialysis.
Table 3.
Strengths and limitations of prolonged KRT
| Strengths | Limitations |
|---|---|
| PIKRT can be used as a substitute for CKRT or intermittent HD PIKRT can be used as transition therapy from CKRT to intermittent HD as hemodynamic status improves Nocturnal PIKRT allows for machine-free time daily for early mobilization and procedures PIKRT is cost-effective compared with CKRT and intermittent HD Unlike intermittent HD in the ICU, one-on-one dialysis nursing support may not be required, depending on type of PIKRT |
PIKRT prescriptions vary widely and no guidelines have established the appropriate dosing regimen or frequency |
| PIKRT with intermittent HD equipment as a substitute for CKRT may lead to low-level endotoxin exposure, with resultant inflammation and oxidative stress | |
| Medication dosing varies significantly on the basis of equipment and flow rates and, therefore, there is risk for underdosing and overdosing of various drugs, especially antibiotics There is higher risk for hypophosphatemia and hypokalemia compared with intermittent HD | |
| PIKRT can be performed without anticoagulation, with less risk for filter clotting when compared with CKRT | |
| PIKRT allows for utilization of one CKRT machine for up to three patients per day |
PIKRT, prolonged intermittent KRT; CKRT, continuous KRT; HD, hemodialysis; ICU, intensive care unit.
Limitations of PIKRT
When used as a substitute for CKRT, PIKRT with a hemodialysis machine subjects the patient to nonultrapure dialysate, leading to low-dose endotoxin exposure, with resultant exacerbation of inflammation and oxidative stress (39) (Table 3). At this time, there are no data to support the use of an endotoxin filter with hemodialysis equipment for PIKRT. One of the major limitations with PIKRT is medication dosing and adjustment. Medication dosing in patients who are critically ill is extremely challenging because the dose of a drug depends on drug factors (e.g., volume of distribution, protein binding, and molecular weight) and patient factors, especially kidney and liver function. Medications that are cleared by extracorporeal therapy will need to be adjusted on the basis of the modality of KRT. The heterogeneity in practice of PIKRT has impeded the development of any uniform dosing recommendations. The daily clearances achieved with a sustained low-efficiency treatment with standard hemodialysis machine are different from that achieved during PIKRT with CKRT equipment, thereby leading to significant differences in the serum concentrations of commonly used medications (40). Pharmacokinetic modeling studies using Monte Carlo simulations offer some guidance on drug dosing during various forms of hybrid dialysis therapies (41). Lack of familiarity with PIKRT modality by physicians, nurses, and pharmacists can lead to insufficient dosing of medications, especially antibiotics (42). It is imperative that any changes in the weekly prescription of PIKRT are communicated to the primary treating physician and pharmacist so that dosing of medications can be adjusted accordingly. Therapeutic drug monitoring, whenever available, is crucial in patients undergoing KRT, and this should be obtained when patients are also undergoing PIKRT. In addition, because clearance during PIKRT is higher than during CKRT, medications such as antimicrobials and antiseizure agents may have to be dosed immediately before and after PIKRT to ensure that drug concentrations do not decrease to a therapeutically ineffective level during treatment. Another factor to consider when implementing PIKRT is the nursing support required to provide the treatment. Delivery models of KRT vary across institutions, and introduction of PIKRT may lead to conflicts regarding the supervision and nursing support of PIKRT especially in those centers that (1) have exclusively utilized ICU nursing for CKRT and/or (2) exclusively utilized dialysis nursing oversight for intermittent hemodialysis. In patients with end-stage kidney failure in the ICU, provision of PIKRT may require placement of a catheter because use of arteriovenous fistula or graft is generally not recommended for PIKRT due to risk of needle dislodgement and exsanguination during prolonged treatment sessions without immediate dialysis nursing support (7). This would increase the risk for catheter-associated blood stream infections, with adverse outcomes. Medical centers with one-on-one ICU nursing during CKRT have reported successful and safe treatments with arteriovenous fistula and arteriovenous graft, and this may be a consideration for PIKRT if one-on-one nursing is available (43). Hypophosphatemia is a major complication in patients who are critically ill in the ICU, particularly in those undergoing CKRT. Similarly, patients undergoing PIKRT with high clearances are vulnerable to severe hypophosphatemia, which can lead to severe muscle weakness and prolonged ventilator dependence (44). Close monitoring of serum phosphate and a preemptive treatment plan is essential for management of hypophosphatemia during PIKRT. Similarly, hypokalemia can also occur more frequently when compared with intermittent hemodialysis, and higher potassium concentration in the dialysate and/or replacement fluid solutions will be required (45).
Summary
In conclusion, PIKRT is an excellent option for the provision of KRT in the ICU. PIKRT can be utilized as a substitute for CKRT, a model that has been successfully implemented in various institutions across the world. However, PIKRT can also be utilized as a transition modality during the de-escalation phase in the ICU, especially in patients whose hemodynamic status has sufficiently improved to be weaned off their high vasopressor requirements. Nocturnal PIKRT offers the advantage of adequate KRT dosing at night, leaving machine-free time during the day for rehabilitation therapy and ambulation and imaging and surgical procedures. PIKRT is also an option in acute patient surges during natural and anthropogenic disasters and pandemics, when the supply of CKRT equipment at an individual institution may not allow a dedicated machine for a single patient for 24 hours. Electrolyte disturbances, in particular hypophosphatemia, are a major concern, and protocol for monitoring and preemptive treatment should be established (46). Implementation of PIKRT at an institution requires careful planning and multidisciplinary (nephrologists, intensivists, dialysis nursing, ICU nurses, pharmacists, infectious disease specialists) collaboration to ensure maximum success. In particular, nursing and pharmacy buy-in is crucial to ensure appropriate nursing support during treatments and to safeguard against therapeutic under- and overdosing of various medications. The recommendations in this review are primarily on the basis of opinion because PIKRT remains a relatively newer modality and literature is limited. Large randomized controlled trials are required to determine if PIKRT is as beneficial and cost effective as CKRT and intermittent hemodialysis, and to establish appropriate drug dosing during treatments. Future research and guidelines should also focus on standardizing the terminology and dosing of PIKRT to ensure adequate delivery of therapy.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Disclosures
A. Vijayan reports having consultancy agreements with Astute Inc. and NxStage; having stock in Outset; receiving research funding from Astellas and Spectral; receiving honoraria from ASN, Baxter, Medscape, and NxStage; having an advisory or leadership role for NxStage; and other interests or relationships as a member of the National Kidney Foundation. The remaining author has nothing to disclose.
Funding
None.
Author Contributions
A. Vijayan conceptualized the study; Z. Levine was responsible for the data curation; A. Vijayan was responsible for the project administration; A. Vijayan provided supervision; and Z. Levine and A. Vijayan wrote the original draft and reviewed and edited the manuscript.
References
- 1.Himmelfarb J, Ikizler TA: Quantitating urea removal in patients with acute renal failure: Lost art or forgotten science? Semin Dial 13: 147–149, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Kolff WJ: Dialysis in treatment of uremia: Artificial kidney and peritoneal lavage. AMA Arch Intern Med 94: 142–160, 1954 [DOI] [PubMed] [Google Scholar]
- 3.Kramer P, Schrader J, Bohnsack W, Grieben G, Gröne HJ, Scheler F: Continuous arteriovenous haemofiltration: A new kidney replacement therapy. Proc Eur Dial Transplant Assoc 18: 743–749, 1981 [PubMed] [Google Scholar]
- 4.Ronco C: Continuous renal replacement therapy: Forty-year anniversary. Int J Artif Organs 40: 257–264, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar VA, Craig M, Depner TA, Yeun JY: Extended daily dialysis: A new approach to renal replacement for acute renal failure in the intensive care unit. Am J Kidney Dis 36: 294–300, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Overberger P Pesacreta M Palevsky PM; VA/NIH Acute Renal Failure Trial Network : Management of renal replacement therapy in acute kidney injury: A survey of practitioner prescribing practices. Clin J Am Soc Nephrol 2: 623–630, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edrees F, Li T, Vijayan A: Prolonged intermittent renal replacement therapy. Adv Chronic Kidney Dis 23: 195–202, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Bouchard J, Acharya A, Cerda J, Maccariello ER, Madarasu RC, Tolwani AJ, Liang X, Fu P, Liu ZH, Mehta RL: A prospective international multicenter study of AKI in the intensive care unit. Clin J Am Soc Nephrol 10: 1324–1331, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldfarb DS, Benstein JA, Zhdanova O, Hammer E, Block CA, Caplin NJ, Thompson N, Charytan DM: Impending shortages of kidney replacement therapy for COVID-19 patients. Clin J Am Soc Nephrol 15: 880–882, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vangala C, Shah M, Dave NN, Attar LA, Navaneethan SD, Ramanathan V, Crowley S, Winkelmayer WC: The landscape of renal replacement therapy in Veterans Affairs Medical Center intensive care units. Ren Fail 43: 1146–1154, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellomo R, Baldwin I, Fealy N: Prolonged intermittent renal replacement therapy in the intensive care unit. Crit Care Resusc 4: 281–290, 2002 [PubMed] [Google Scholar]
- 12.Berbece AN, Richardson RM: Sustained low-efficiency dialysis in the ICU: Cost, anticoagulation, and solute removal. Kidney Int 70: 963–968, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Clark JA, Schulman G, Golper TA: Safety and efficacy of regional citrate anticoagulation during 8-hour sustained low-efficiency dialysis. Clin J Am Soc Nephrol 3: 736–742, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gashti CN, Salcedo S, Robinson V, Rodby RA: Accelerated venovenous hemofiltration: Early technical and clinical experience. Am J Kidney Dis 51: 804–810, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Allegretti AS, Endres P, Parris T, Zhao S, May M, Sylvia-Reardon M, Bezreh N, Culbert-Costley R, Ananian L, Roberts RJ, Lopez N, Charytan DM, Tolkoff-Rubin N: Accelerated venovenous hemofiltration as a transitional renal replacement therapy in the intensive care unit. Am J Nephrol 51: 318–326, 2020 [DOI] [PubMed] [Google Scholar]
- 16.Abe M, Okada K, Suzuki M, Nagura C, Ishihara Y, Fujii Y, Ikeda K, Kaizu K, Matsumoto K: Comparison of sustained hemodiafiltration with continuous venovenous hemodiafiltration for the treatment of critically ill patients with acute kidney injury. Artif Organs 34: 331–338, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Duran P, Concepcion LA: Usefulness of prolonged renal replacement therapy in patients with acute kidney injury requiring dialysis. Proc Bayl Univ Med Cent 33: 322–325, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kidney Disease: Improving Global Outcomes (KDIGO) Work Group : KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2: 8–12, 2012 [Google Scholar]
- 19.Marshall MR, Creamer JM, Foster M, Ma TM, Mann SL, Fiaccadori E, Maggiore U, Richards B, Wilson VL, Williams AB, Rankin AP: Mortality rate comparison after switching from continuous to prolonged intermittent renal replacement for acute kidney injury in three intensive care units from different countries. Nephrol Dial Transplant 26: 2169–2175, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Kielstein JT, Kretschmer U, Ernst T, Hafer C, Bahr MJ, Haller H, Fliser D: Efficacy and cardiovascular tolerability of extended dialysis in critically ill patients: A randomized controlled study. Am J Kidney Dis 43: 342–349, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Palevsky PM, Liu KD, Brophy PD, Chawla LS, Parikh CR, Thakar CV, Tolwani AJ, Waikar SS, Weisbord SD: KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis 61: 649–672, 2013 [DOI] [PubMed] [Google Scholar]
- 22.James M, Bouchard J, Ho J, Klarenbach S, LaFrance JP, Rigatto C, Wald R, Zappitelli M, Pannu N: Canadian Society of Nephrology commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis 61: 673–685, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Schwenger V, Weigand MA, Hoffmann O, Dikow R, Kihm LP, Seckinger J, Miftari N, Schaier M, Hofer S, Haar C, Nawroth PP, Zeier M, Martin E, Morath C: Sustained low efficiency dialysis using a single-pass batch system in acute kidney injury - A randomized interventional trial: The REnal Replacement Therapy Study in Intensive Care Unit PatiEnts. Crit Care 16: R140, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitchlu A, Adhikari N, Burns KE, Friedrich JO, Garg AX, Klein D, Richardson RM, Wald R: Outcomes of sustained low efficiency dialysis versus continuous renal replacement therapy in critically ill adults with acute kidney injury: A cohort study. BMC Nephrol 16: 127, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lund A, Damholt MB, Wiis J, Kelsen J, Strange DG, Møller K: Intracranial pressure during hemodialysis in patients with acute brain injury. Acta Anaesthesiol Scand 63: 493–499, 2019 [DOI] [PubMed] [Google Scholar]
- 26.Boyle SM, Washington R, McCann P, Koul S, McLarney B, Gadegbeku CA: The nephrology nursing shortage: Insights from a pandemic. Am J Kidney Dis 79: 113–116, 2022 [DOI] [PubMed] [Google Scholar]
- 27.Burgner A, Golper T: Walkaway PIRRT (as SLED) for acute kidney injury. Clin J Am Soc Nephrol 16: 138–140, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palevsky PM Zhang JH O’Connor TZ Chertow GM Crowley ST Choudhury D Finkel K Kellum JA Paganini E Schein RM Smith MW Swanson KM Thompson BT Vijayan A Watnick S Star RA Peduzzi P Peduzzi P; VA/NIH Acute Renal Failure Trial Network : Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 359: 7–20, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conger JD: Does hemodialysis delay recovery from acute renal failure? Semin Dial 3: 146–148, 1990 [Google Scholar]
- 30.Manns M, Sigler MH, Teehan BP: Intradialytic renal haemodynamics--potential consequences for the management of the patient with acute renal failure. Nephrol Dial Transplant 12: 870–872, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Tonelli M, Astephen P, Andreou P, Beed S, Lundrigan P, Jindal K: Blood volume monitoring in intermittent hemodialysis for acute renal failure. Kidney Int 62: 1075–1080, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Bitker L, Bayle F, Yonis H, Gobert F, Leray V, Taponnier R, Debord S, Stoian-Cividjian A, Guérin C, Richard JC: Prevalence and risk factors of hypotension associated with preload-dependence during intermittent hemodialysis in critically ill patients. Crit Care 20: 44, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bagshaw SM, Berthiaume LR, Delaney A, Bellomo R: Continuous versus intermittent renal replacement therapy for critically ill patients with acute kidney injury: A meta-analysis. Crit Care Med 36: 610–617, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Diaz-Buxo JA, Loredo JP: Standard Kt/V: Comparison of calculation methods. Artif Organs 30: 178–185, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Tipping CJ, Harrold M, Holland A, Romero L, Nisbet T, Hodgson CL: The effects of active mobilisation and rehabilitation in ICU on mortality and function: A systematic review. Intensive Care Med 43: 171–183, 2017 [DOI] [PubMed] [Google Scholar]
- 36.Lahmer T, Messer M, Rasch S, Beitz A, Schnappauf C, Schmid RM, Huber W: Sustained low-efficiency dialysis with regional citrate anticoagulation in medical intensive care unit patients with liver failure: A prospective study. J Crit Care 30: 1096–1100, 2015 [DOI] [PubMed] [Google Scholar]
- 37.Kumar VA, Yeun JY, Depner TA, Don BR: Extended daily dialysis vs. continuous hemodialysis for ICU patients with acute renal failure: A two-year single center report. Int J Artif Organs 27: 371–379, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Division of Nephrology, Columbia University Vagelos College of Physicians : Disaster response to the COVID-19 pandemic for patients with kidney disease in New York City. J Am Soc Nephrol 31: 1371–1379, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Susantitaphong P, Riella C, Jaber BL: Effect of ultrapure dialysate on markers of inflammation, oxidative stress, nutrition and anemia parameters: A meta-analysis. Nephrol Dial Transplant 28: 438–446, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Hoff BM, Maker JH, Dager WE, Heintz BH: Antibiotic dosing for critically ill adult patients receiving intermittent hemodialysis, prolonged intermittent renal replacement therapy, and continuous renal replacement therapy: An update. Ann Pharmacother 54: 43–55, 2020 [DOI] [PubMed] [Google Scholar]
- 41.Lewis SJ, Kays MB, Mueller BA: Use of Monte Carlo simulations to determine optimal carbapenem dosing in critically ill patients receiving prolonged intermittent renal replacement therapy. J Clin Pharmacol 56: 1277–1287, 2016 [DOI] [PubMed] [Google Scholar]
- 42.Keough LA, Krauss A, Hudson JQ: Inadequate antibiotic dosing in patients receiving sustained low efficiency dialysis. Int J Clin Pharm 40: 1250–1256, 2018 [DOI] [PubMed] [Google Scholar]
- 43.Al Rifai A, Sukul N, Wonnacott R, Heung M: Safety of arteriovenous fistulae and grafts for continuous renal replacement therapy: The Michigan experience. Hemodial Int 22: 50–55, 2018 [DOI] [PubMed] [Google Scholar]
- 44.Thompson Bastin ML, Stromberg AJ, Nerusu SN, Liu JL, Mayer KP, Liu KD, Bagshaw SM, Wald R, Morris PE, Neyra JA: Association of phosphatecontaining versus phosphate-free solutions on ventilator days in critically ill patients requiring continuous kidney replacement therapy. Clin J Am Soc Nephrol 17: 634–642, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marshall MR, Golper TA, Shaver MJ, Alam MG, Chatoth DK: Sustained low-efficiency dialysis for critically ill patients requiring renal replacement therapy. Kidney Int 60: 777–785, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Vijayan A: CRRT fluid choices: A solution for a common problem? Clin J Am Soc Nephrol 17: 631–633, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fiaccadori E, Regolisti G, Cademartiri C, Cabassi A, Picetti E, Barbagallo M, Gherli T, Castellano G, Morabito S, Maggiore U: Efficacy and safety of a citrate-based protocol for sustained low-efficiency dialysis in AKI using standard dialysis equipment. Clin J Am Soc Nephrol 8: 1670–1678, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kielstein JT, Schiffer M, Hafer C: Back to the future: Extended dialysis for treatment of acute kidney injury in the intensive care unit. J Nephrol 23: 494–501, 2010 [PubMed] [Google Scholar]
- 49.Lonnemann G, Floege J, Kliem V, Brunkhorst R, Koch KM: Extended daily veno-venous high-flux haemodialysis in patients with acute renal failure and multiple organ dysfunction syndrome using a single path batch dialysis system. Nephrol Dial Transplant 15: 1189–1193, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Marshall MR, Ma T, Galler D, Rankin AP, Williams AB: Sustained low-efficiency daily diafiltration (SLEDD-f) for critically ill patients requiring renal replacement therapy: Towards an adequate therapy. Nephrol Dial Transplant 19: 877–884, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Naka T, Baldwin I, Bellomo R, Fealy N, Wan L: Prolonged daily intermittent renal replacement therapy in ICU patients by ICU nurses and ICU physicians. Int J Artif Organs 27: 380–387, 2004 [DOI] [PubMed] [Google Scholar]


