Abstract
This report describes vedolizumab’s clinical efficacy and safety in a patient with severe ulcerative colitis on haemodialysis for an end-stage kidney failure. The patient was a 75-year-old man on long-standing chronic diffusive three times per week haemodialytic treatment due to vascular nephropathy. At the presentation, the patient had severe bloody diarrhoea treated with a steroid cycle with temporary benefits and then developed steroid dependence. Upon remission, the patient started vedolizumab (Entivyo®) as maintenance therapy. After 6 weeks of induction, patient started the maintenance therapy with an infusion every 8 weeks. After the sixth infusion, the interval was prolonged to 9 weeks because of the good and fast response. Vedolizumab treatment proceeded without adverse events. However, no changes in renal function were noted during the same period, no complications were reported, and the patient regularly continued haemodialysis. At the second induction infusion (week 2) and the second maintenance infusion (week 22), we measured vedolizumab serum level before and after haemodialysis, observing no significant changes. Our case is the first report about using vedolizumab in a patient under haemodialysis, showing that vedolizumab can be safe, well tolerated, and effective in patients undergoing haemodialysis. However, more extensive trials are needed to prove its use in these patients.
Keywords: Vedolizumab, haemodialysis, ulcerative colitis, inflammatory bowel disease
Introduction
Integrins are involved in tissue immune cell recruitment under homeostatic and pathological conditions. Among them, heterodimeric integrin α4β7, a cell surface molecule expressed on T cells, is involved in the recruitment of T cells to the intestinal mucosa upon its interaction with its ligand mucosal vascular addressin cell adhesion molecule-1 (MAdCAM-1).1,2 Pharmacological intervention in tissue-specific recruitment of T cells has the potential advantage of precise therapeutic intervention at the site of inflammation. Vedolizumab (VDZ) is one of the biological drugs approved for both Crohn’s disease (CD) and ulcerative colitis (UC) with this specific mechanism of action. The active molecule is a humanized monoclonal IgG1 antibody specific for integrin α4β7.
Due to the unique gut selectivity and the favourable benefit–risk profile, VDZ nowadays is the most extensively used in older patients with moderate to severe UC with multiple comorbidities.2,3 Herein, we describe the case of a patient with severe UC in haemodialysis for an end-stage kidney failure (chronic kidney disease (CKD)) due to vascular nephropathy that was successfully treated with VDZ and represents, to our knowledge, the first report about the use of VDZ in a patient under haemodialysis. Blood samples for VDZ levels assessment were collected pre-VDZ infusion and pre-haemodialysis and 1 h post-haemodialysis end of treatment at the beginning of induction phase (2 weeks) and at the beginning of maintenance phase (22 weeks).
Case report
We present a case of a 75-year-old male patient referred to IRCCS Policlinico San Donato, Gastroenterology Unit in March 2019 because of proctorrhagia and anaemia. The patient was on chronic bicarbonate diffusive haemodialysis performed three times a week and erythropoietin supplementation due to vascular nephropathy, and he encountered other comorbidities such as arterial hypertension, severe right carotid stenosis, and previous myocardial infarction.
The patient underwent a colonoscopy that showed marked erythema, absent vascular pattern, and some erosions confined to the caecum and sigmoid colon (Figure 1); his histopathological analysis report showed oedema, hyperemia, and eosinophilic infiltration in the lamina propria with regular glandular architecture. Endoscopic and histopathological features were conclusive for moderate inflammatory bowel disease (IBD) type unclassified. The patient was treated with a steroid cycle with clinical remission and maintenance therapy with mesalazine (5-ASA) 3.2 g per day.
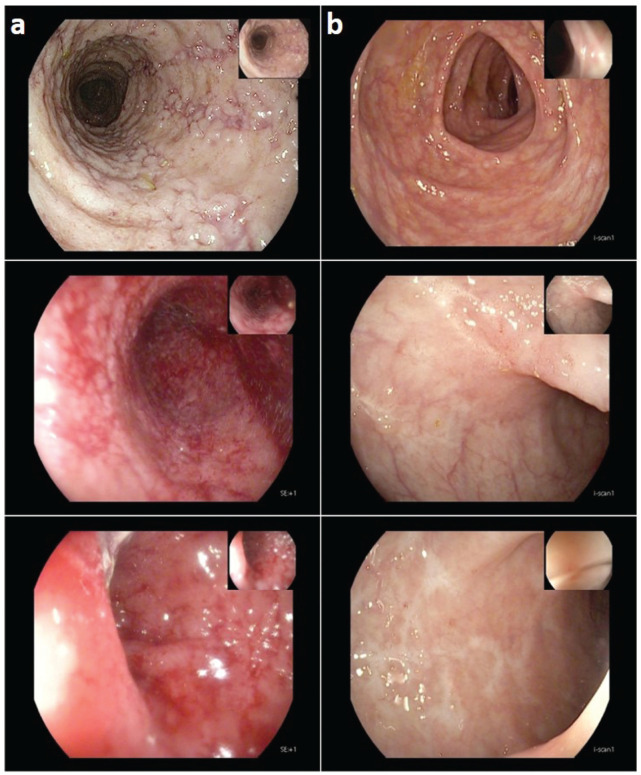
Figure 1.
Endoscopic images (a) initial colonoscopy images at the time of diagnosis shows marked erythema, absent vascular pattern, and some erosions confined to the caecum and sigmoid colon and (b) after 1-year VDZ therapy with no evidence of active disease.
In August 2020, the patient was readmitted to our emergency room (ER) for acute severe bloody diarrhoea. On admission, the patient was alert. His blood pressure was 150/75 mm Hg, heart rate was 72 bpm, respiration rate was 15/min, and his status was apyretic. Physical examination showed tenderness all over the abdomen and increased bowel sounds. No sign of peritonitis was determined. Lab data reported white blood cells 12 × 109/L, haemoglobin 8.6 g/L, and platelets 328 × 109/L. Serum C-reactive protein was increased 27 mg/L (normal range ⩽ 5 mg/L), creatinine 4.53 mg/dL, and albumin 2.3 g/dL. The patient liver and coagulation functions were normal.
During the subsequent hospital admission, a rectosigmoidscopy was performed, which showed severe proctosigmoiditis. After carefully investigating any concomitant infectious disease, we re-started steroid therapy with methylprednisolone 1 mg/kg and enema therapy with 5-ASA, thus obtaining an improvement in diarrhoea and observing a consistent decrease of serum inflammatory markers. Nevertheless, the patient required regular blood transfusions and increased erythropoietin supplementation due to progressive anemization.
On the fourth day after the introduction of steroid therapy, a complete ileocolonoscopy was performed, which displayed evidence of severe colitis with diffuse oedema, erythema, and small ulcers involving mainly the transverse colon, sparing the ileal mucosa. The histopathological examination showed oedema, hyperemia, and inflammatory cell infiltration in the lamina propria associated with signs of cryptitis and cryptic abscesses with glandular distortion in the left colon (Grade 4 Geboes score). 4 Immunoreactivity for Cytomegalovirus (CMV) was negative.
Based on these findings, the patient has diagnosed an ulcerative pancolitis with Mayo endoscopic score 35,6 and disease activity index (DAI) score 10. 7 This change in diagnosis is not uncommon given the fact that 3%–10% of patients with colonic inflammation have overlapping clinical and pathological features, making it difficult to distinguish between IBD resulting in the diagnosis of IBD type unclassified. 8 Furthermore, a change in diagnosis was reported after re-evaluation during follow-up in 10% of patients, highlighting the heterogeneity of these diseases and the difficulty of ascertaining their correct diagnoses. 9
After the initiation of the steroid therapy, the patient yielded clinical remission; we considered the patient for biological treatment with VDZ rather than continue steroid therapy based on the severe features and extension of the colitis despite the previous steroid treatment and the ongoing maintenance therapy with 5-ASA, besides the multiple comorbidities. Also, the well-demonstrated efficacy of VDZ as maintenance therapy in UC and reports of the safety of infliximab in patients on haemodialysis10–12 encouraged us to use VDZ rather than continue steroid therapy. From September 2020, the patient received infusions of 300 mg VDZ 24 h before the start of haemodialysis, at 0, 2, 6 weeks, and every 8 weeks after that; the treatment was prolonged to 9 weeks after the sixth infusion because of the good and rapid response and clinical improvement. The interval between VDZ infusion and haemodialysis was the same during all VDZ treatments (i.e. at week 2 and at week 22).
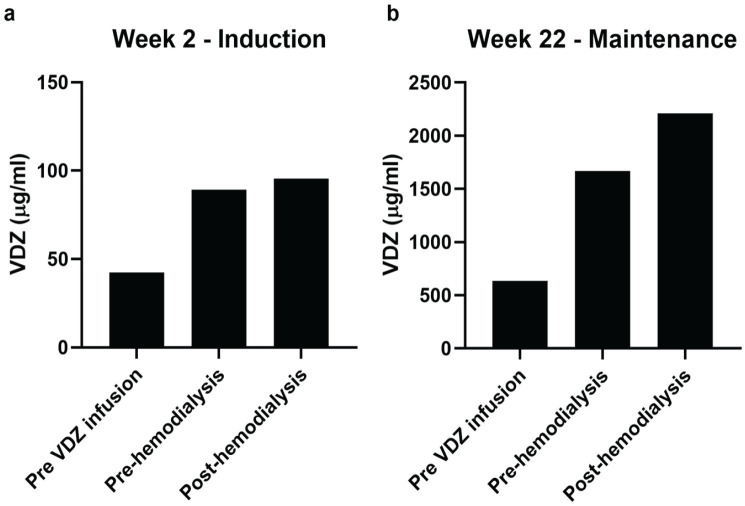
After the fourth VDZ infusion, the steroid therapy was carefully tapered, and there was no relapse. During follow-up, serum levels of VDZ were measured pre-VDZ infusion pre-haemodialysis and 24 h after VDZ infusion immediately before haemodialysis and 1- post-haemodialysis end of treatment using RIDASCREEN® VDZ Monitoring ELISA kit (R-Biopharm AG, Italy) at week 2 and at week 22. Several studies assessed trough levels after induction treatment or during maintenance therapy as predictors of sustained clinical response and showed a significant correlation between low trough levels and decreased clinical response in CD and UC adult patients. We, therefore, hypothesize these could be critical time points for this study. 13 We observed a slight increase in the VDZ concentration rather than a reduction, both at the second induction infusion (week 2) and at the second maintenance infusion (week 22) (Figure 2). Perhaps, because of the modification of the distribution volume of the drug due to the overall reduction of patient’s volemia and the electrolytic changes caused by the haemodialysis.
Figure 2.
Quantification of serum vedolizumab (VDZ) in (a) induction period and (b) maintenance period. Data are presented in pre-VDZ infusion and in pre- and post-haemodialysis.
The VDZ treatment proceeded without adverse effects. Patient’s conditions improved with no diarrhoea, no other blood transfusion was performed, and patient stopped erythropoietin supplementation.
After 1 year of treatment, the patient underwent an ileocolonoscopy that displayed evidence of marked improvement of inflammation with residual oedema and erythematous pattern confined in the left colon. On pathological examination, the right colon achieved mucosal healing, and a significant improvement of diffuse inflammatory cell infiltration was observed in the left colon (Grade 1 of Geboes). 4
Discussion
Biologic drugs have entirely changed the treatment and prognosis of IBD. In elderly patients, VDZ seems to have the best balance between efficacy and safety profile. There are very few reports10–12 only on tumour necrosis factor (TNF)-alpha antagonists in patients on dialysis, and there is no report about the use of VDZ in such patients.
Kume et al. 14 described a similar case treated with infliximab. They measured the serum concentration of infliximab before and after haemodialysis and found that it was essentially unchanged by the haemodialysis. We repeated the same procedure for our patient with VDZ, confirming the same conclusions.
As for IFX (MW 149 kDa), a high molecular weight of therapeutic monoclonal antibodies as VDZ (MW 147 kDa), preserve from the direct excretion in urine; instead, they are degraded to peptides and amino acids that are either reused by the body or excreted by the kidneys. Moreover, dialytic filters are usually selective for molecules with a molecular weight under 70 kDa. However, its pharmacokinetics in patients on dialysis are not known.
Nevertheless, some questions remain regarding introducing these drugs to patients with end-stage kidney failure. First, as demonstrated with infliximab, the serum albumin concentration, which is typically low in chronic kidney disease patients, could predict the clinical response in patients with UC. 15 Moreover, it was estimated that the albumin concentration modifies VDZ clearance and is a predictor of favourable response to VDZ in UC.2,16,17 Second, it is not established which could be the correct timing of the infusion in such patients, considering the unknown elimination pharmacokinetics in haemodialytic patients. However, it should be considered that alternative treatment protocols such as steroids or other immunosuppressant drugs for patients suffering from severe UC and CKD represent a worse benefit–risk profile and may have more contraindications compared to immunomodulatory treatment.
Conclusion
In our knowledge, this is the first case report illustrating the administration of VDZ for treating severe, steroid-dependent ulcerative pancolitis in a patient suffering from CKD on haemodialysis. Since the patient is in long-term clinical and endoscopic remission, it may be asserted that VDZ can be safely administered in this peculiar clinical scenario. As reported in other studies, the absence of unexpected adverse events suggests that patients with end-stage kidney failure could tolerate biologic drugs such as VDZ. The drug maintains its efficacy despite the haemodialytic treatment. However, considering the steadily increasing number of patients on regular dialysis treatment in Italy, more extensive studies are needed to assess the safety and the correct timing of VDZ administration in such a setting.
Supplemental Material
Supplemental material, sj-docx-1-sco-10.1177_2050313X231165641 for Efficacy of vedolizumab as maintenance therapy in a patient with ulcerative colitis receiving haemodialysis in end-stage kidney failure: A case report by Guglielmo Albertini Petroni, Laura Francesca Pisani, Edoardo Borsotti, Maria Doria and Maria Laura Annunziata in SAGE Open Medical Case Reports
Acknowledgments
The authors thank the nurses of IRCCS Policlinico San Donato of the Gastroenterology and Endoscopy Unit and of the Nephrology and Dialysis Unit.
Footnotes
Author contributions: G.A.P. contributed to writing – original draft preparation and study design. E.B. critically revised the paper and patient’s clinical management. L.F.P. contributed to conceptualization, methodology, investigation, and writing – review, and editing. M.D. contributed to writing – review and editing and patient’s clinical management. M.L.A. contributed to conceptualization, investigation, and writing – review and editing. G.A.P. and L.F.P equally contribute to the paper and are co-first authors.
Data availability statement: The data presented in this study are available within this article and methods protocol details are available in Supplemental Materials.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by Italy’s Ministero Italiano della Salute (Italian Ministry oh Health Grant) and IRCCS Policlinico San Donato.
Ethical approval: Ethical approval to report this case was obtained from local Ethics Committee of San Raffaele Hospital, Milan, Italy (protocol no. GR2016-IBD) on 9 February 2018.
Informed consent: Written informed consent was obtained from the patient for its anonymized information to be published in this article.
ORCID iD: Laura Francesca Pisani  https://orcid.org/0000-0002-9490-3723
https://orcid.org/0000-0002-9490-3723
Supplemental material: Supplemental material for this article is available online.
References
- 1.Mora JR, Von Andrian UH. Specificity and plasticity of memory lymphocyte migration. Curr Top Microbiol Immunol 2006; 308: 83–116. [PubMed] [Google Scholar]
- 2.Rosario M, Dirks NL, Milch C, et al. A review of the clinical pharmacokinetics, pharmacodynamics, and immunogenicity of vedolizumab. Clin Pharmacokinet 2017; 56(11): 1287–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soler D, Chapman T, Yang LL, et al. The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther 2009; 330(3): 864–875. [DOI] [PubMed] [Google Scholar]
- 4.Geboes K, Riddell R, Ost A, et al. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut 2000; 47(3): 404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khanna R, Ma C, Jairath V, et al. Endoscopic assessment of inflammatory bowel disease activity in clinical trials. Clin Gastroenterol Hepatol 2022; 20(4): 727.e2–736.e2. [DOI] [PubMed] [Google Scholar]
- 6.Ket SN, Palmer R, Travis S. Endoscopic disease activity in inflammatory bowel disease. Curr Gastroenterol Rep 2015; 17(12): 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med 1987; 317(26): 1625–1629. [DOI] [PubMed] [Google Scholar]
- 8.Matsui T, Yao T, Sakurai T, et al. Clinical features and pattern of indeterminate colitis: Crohn’s disease with ulcerative colitis-like clinical presentation. J Gastroenterol 2003; 38(7): 647–655. [DOI] [PubMed] [Google Scholar]
- 9.Lee HS, Choe J, Lee HJ, et al. Change in the diagnosis of inflammatory bowel disease: a hospital-based cohort study from Korea. Intest Res 2016; 14(3): 258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiba M, Tsuda S, Tsuji T, et al. Crohn’s disease successfully treated with infliximab in a patient receiving hemodialysis: case report and review of the literature. Medicine 2014; 93(7): e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh R, Cuchacovich R, Huang W, et al. Infliximab treatment in a patient with rheumatoid arthritis on hemodialysis. J Rheumatol 2002; 29(3): 636–637. [PubMed] [Google Scholar]
- 12.Hammoudeh M. Infliximab treatment in a patient with rheumatoid arthritis on haemodialysis. Rheumatology 2006; 45(3): 357–359. [DOI] [PubMed] [Google Scholar]
- 13.Altwegg R, Vincent T. TNF blocking therapies and immunomonitoring in patients with inflammatory bowel disease. Mediators Inflamm 2014; 2014: 172821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kume K, Yamasaki M, Yoshikawa I, et al. Infliximab treatment in a patient with Crohn’s disease on haemodialysis. Colorectal Dis 2011; 13(3): 341. [DOI] [PubMed] [Google Scholar]
- 15.Fasanmade AA, Adedokun OJ, Olson A, et al. Serum albumin concentration: a predictive factor of infliximab pharmacokinetics and clinical response in patients with ulcerative colitis. Int J Clin Pharmacol Ther 2010; 48(5): 297–308. [DOI] [PubMed] [Google Scholar]
- 16.Colombel JF, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut 2017; 66(5): 839–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gisbert JP, Chaparro M. Predictors of primary response to biologic treatment [anti-TNF, vedolizumab, and ustekinumab] in patients with inflammatory bowel disease: from basic science to clinical practice. J Crohns Colitis 2020; 14(5): 694–709. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-sco-10.1177_2050313X231165641 for Efficacy of vedolizumab as maintenance therapy in a patient with ulcerative colitis receiving haemodialysis in end-stage kidney failure: A case report by Guglielmo Albertini Petroni, Laura Francesca Pisani, Edoardo Borsotti, Maria Doria and Maria Laura Annunziata in SAGE Open Medical Case Reports