Abstract
Objective: Women with locally advanced breast cancer (LABC) or inoperable local recurrence often suffer from a significantly reduced quality of life (QOL) due to local tumor-associated pain, bleeding, exulceration, or malodorous discharge. We aimed to further investigate the benefit of radiotherapy (RT) for symptom relief while weighing the side-effects. Materials and methods: Patients who received symptom-oriented RT for palliative therapy of their LABC or local recurrence in the Department of Radiation Oncology at Heidelberg University Hospital between 2012 and 2021 were recorded. Clinical, pathological, and therapeutic data were collected and the oncological and symptomatic responses as well as therapy-associated toxicities were analyzed. Results: We retrospectively identified 26 consecutive women who received palliative RT with a median total dose of 39 Gy or single dose of 3 Gy in 13 fractions due to (impending) exulceration, pain, local hemorrhage, and/or vascular or plexus compression. With a median follow-up of 6.5 months after initiation of RT, overall survival at 6 and 12 months was 60.0% and 31.7%, and local control was 75.0% and 47.6%, respectively. Radiation had to be discontinued in 4 patients due to oncological clinical deterioration or death. When completed as initially planned, symptom improvement was achieved in 95% and WHO level reduction of analgesics in 28.6% of patients. In 36% (16%) of patients, local RT had already been indicated >3 months (>6 months) before the actual start of RT, but was delayed or not initiated among others in favor of drug alternatives or systemic therapies. RT-associated toxicities included only low-grade side-effects (CTCAE I°-II°) with predominantly skin erythema and fatigue even in the context of re-RT. Conclusion: Palliative RT in symptomatic LABC or locoregional recurrence is an effective treatment option for controlling local symptoms with only mild toxicity. It may thus improve QOL and should be considered early in palliative patient care management.
Keywords: palliative care, radiation therapy, LABC, exulceration, pain, hemorrhage
Introduction
According to estimates by the International Agency for Research on Cancer (IARC), breast cancer was responsible for 24.5% of all annual tumor diseases in women worldwide in 2020, hence being the most frequent cancer in women. 1 Routine screening can lead to an incidence reduction of advanced-stage breast cancer 2 as well as early detection of locoregional tumor recurrences. Nonetheless, at initial diagnosis, around 10% to 30% of patients present with locally advanced breast cancer (LABC)3,4 and locoregional recurrences occur in approximately 5% to 15% of patients after standard treatment with breast-conserving surgery followed by adjuvant radiotherapy (RT) or even mastectomy.5–8
In LABC or metastatic breast cancer, patients may develop fungating or ulcerating breast tumors with associated symptoms including chronic pain, malodourous discharges, bleeding, or local wound infections.9,10 It has been shown that these symptomatic lesions may lead to physical or psychosocial implications 11 and ultimately impact patients’ quality of life (QOL).
Palliative locoregional therapy comprises surgery, which is however not always feasible in advanced stages due to expansive tumor infiltration into the surrounding tissue (eg, the thoracic wall or the adjacent bones).4,9 In some cases, neither surgery nor systemic treatment can be performed due to patients’ unsuitability or refusal. Since shortly following the discovery of X-rays in 1896, RT has been widely used as a non-invasive palliative treatment option for local symptom relief, including relief of pain and cessation of hemorrhage.12–15 Being a treatment option with a limited treatment burden with regard to side-effects and the short treatment period, palliative RT has been shown to improve patient's QOL. 16
Surprisingly, there is a paucity of literature describing palliative RT for ulcerating/fungating breast lesions. More than 700 patients undergo RT for breast cancer each year at our institution, but less than 1% of them receive locoregional symptomatic RT. In the current study, we report our experience of symptom-based palliative RT for LABC or locoregional recurrence and further investigate the benefit of RT for symptom relief while weighing the risks.
Materials and Methods
Patient Selection
In this retrospective study, we identified all consecutive patients having received palliative local RT for symptomatic locally advanced or recurrent breast cancer between 2012 and 2021 at the University Hospital in Heidelberg. The analysis was approved by the ethical review board (S-535/2021), and the requirement for written informed consent from each individual was waived by the appropriate institutional review board.
Information was obtained from the patients’ medical and RT records. Data collected included patient demographics, tumor characteristics, RT parameters, as well as oncological and symptomatic treatment response. All patient details and personally identifiable information were de-identified. The reporting of this study conforms to STROBE guidelines. 17
Radiotherapy
Treatments of patients were discussed interdisciplinarily by gynecologists, medical oncologists, radiologists, pathologists, and radiation oncologists. In all but one patient, RT was administered using 6MV photons with treatment planning being performed by computed tomography. Radiation techniques included three-dimensional conformal RT (3D-CRT) or intensity-modulated radiotherapy (IMRT) encompassing volumetric-modulated arc therapy (VMAT) and helical radiotherapy (HT). Due to extensive skin infiltrations, electron irradiation was applied in 2 patients: 1 patient received treatment administered by 6MV photons combined with 18 MeV electrons; 1 patient was treated with 18 MeV electrons only, in this case treatment planning was performed clinically. As patients were treated symptomatically with palliative intent, target volumes were defined individually. The clinical target volume comprised the tumor lesions as well as the cutaneous infiltration responsible for breast symptoms. A planning target volume (PTV) margin of 7 to 10 mm was added depending on the applied technique.
Biologically effective dose (BED) and equivalent dose in 2Gy fractions (EQD2) were calculated using the following formulae derived from the linear-quadratic model:
with n = number of treatment fractions, d = dose per fraction in Gray (Gy), = dose at which the linear and quadratic components of cell kill are equal (Gy). An ratio of 4 Gy was assumed for breast cancer in our study. 18
Follow-up and Statistical Analysis
Overall survival (OS) was calculated in months from the start of RT until the date of death or censorship. Local control (LC) of the treated lesion was calculated from the start of RT until the first diagnosis of recurrent disease or censorship. Treatment response was assessed by physician observation at follow-up visits including regular staging according to current guidelines. Outcomes reported encompassed wound healing, reduction in mass size, pain control, bleeding cessation, and improvement of motor function or lymphedema in the case of plexus and vessel affection.
Common Terminology Criteria for Adverse Events (CTCAE; version 5.0) were used for the grading of acute and late toxicity.
OS and LC were analyzed during follow-up period and calculated using the Kaplan-Meier method. Survival curves were further compared between subgroups in univariate analysis applying the log-rank test or cox regression, with statistical significance set at p < .05. Statistical analyses were performed using SPSS (version 28.0.1.1; Chicago, Illinois).
Results
Patient Characteristics
A total of 26 consecutive patients were identified and analyzed in the present study. Patient and tumor characteristics are summarized in Table 1. Median age was 61 years (range 25-83). The prevalent Eastern Cooperative Oncology Group (ECOG) score was 1 (52.2%). Median time from initial diagnosis of breast cancer to the beginning of palliative RT was 33.4 months (range 4.9-353.5). At presentation, 88.0% had distant metastases. Symptoms requiring palliative RT were (impending) exulceration (n = 16), pain (n = 9), local bleeding (n = 4), and/or compression of vessels or plexus (n = 2).
Table 1.
Patient and Tumor Characteristics.
| Patient and Tumor Characteristics | |
|---|---|
| Median age | 61 years (range 25-83) |
| ECOG | |
| 0 | 17.4% |
| 1 | 52.2% |
| 2 | 17.4% |
| 3 | 8.7% |
| 4 | 4.3% |
| Initial tumor stage | |
| T-stage | |
| T1 | 26.1% |
| T2 | 26.1% |
| T3 | 13.0% |
| T4 | 34.8% |
| N-stage | |
| N0 | 28.0% |
| N1-3 | 72.0% |
| M-stage | |
| M0 | 37.5% |
| M1 | 62.5% |
| Tumor grade | |
| G1 | 3.8% |
| G2 | 38.5% |
| G3 | 57.7% |
| Receptor status | |
| HR+, HER2- | 44.0% |
| HR+, HER2+ | 12.0% |
| HR−, HER2+ | 12.0% |
| HR−, HER2− | 32.0% |
| Tumor stage at palliative irradiation | |
| Localized | 12.0% |
| Metastatic | 88.0% |
| Systemic treatment before presentation | |
| Endocrine therapy | 46.2% |
| Chemotherapy | 76.9% |
| Anti-HER2 therapy | 19.2% |
| Immunotherapy | 15.4% |
| Other targeted therapy | 38.5% |
| No prior systemic treatment | 3.8% |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; HR, hormone receptor; HER2, human epidermal growth factor receptor 2.
Eleven patients (42.3%) had prior irradiation history, with 1 patient having undergone irradiation to the same site twice before. Prior RT had been performed as intraoperative radiotherapy (n = 1) or adjuvant RT (n = 10) with a median BED of 88.1 Gy (range 66.8-161.2) and EQD2 of 54.4 Gy (range 44.5-106.4). Median time from initial (adjuvant) RT to palliative RT was 53 months (range 11-360).
Treatment
Depending on the size and extent of the tumor, palliative RT was delivered by IMRT (65.4%) including VMAT (50.0%) and HT (15.4%), by 3D-CRT (30.8%) or by using electrons (3.8%). Median cumulative RT dose was 39 Gy (range 9-54), delivered in 13 fractions (range 3-26) of 3 Gy (range 1.8-4), which was at the same time the most prevalent fractionation scheme (n = 7). Radiation had to be discontinued in 4 patients due to dehiscence of surgical scar (n = 1), clinical deterioration (n = 2), or death (n = 1). Among the patients who completed RT as initially planned, median BED was 68.3 Gy (range 40.0-94.5) and EQD2 was 45.5 Gy (range 26.7-63.0), respectively. PTV volume ranged from 242.4 to 4112.4 cm3 with a median PTV volume of 1206.8 cm3. Treatment-related parameters are summarized in Table 2.
Table 2.
Treatment-Related Parameters.
| Treatment-Rrelated Parameters | |
|---|---|
| Prior irradiation to target site | 42.3% |
| Median BED of prior RT | 88.1 Gy (range 66.8-161.2) |
| Median EQD2 of prior RT | 54.4 Gy (range 44.5-106.4) |
| Treatment technique of palliative RT | |
| VMAT | 50.0% |
| 3D-RT | 30.8% |
| HT | 15.4% |
| Electrons | 3.8% |
| Applied dose of palliative RT (total dose/dose per fraction) | |
| 39 Gy / 3 Gy | 26.9% |
| 45 Gy / 3 Gy | 23.1% |
| 36 Gy / 3 Gy | 11.5% |
| 30 Gy / 3 Gy | 7.7% |
| 51 Gy / 3 Gy | 7.7% |
| 9 Gy / 3 Gy | 3.8% |
| 20 Gy / 4 Gy | 3.8% |
| 38 Gy / 2 Gy | 3.8% |
| 40.05 Gy / 2.67 Gy | 3.8% |
| 46.8 Gy / 1.8 Gy | 3.8% |
| 54 Gy / 3 Gy | 3.8% |
| Median PTV volume | 1206.8 cm3 (range 242.4-4112.4) |
| Systemic treatment during RT | |
| Chemotherapy | 7.7% |
| Immunotherapy | 3.8% |
Abbreviations: RT, radiotherapy; PTV, planning target volume; VMAT, volumetric modulated arc therapy; 3D-CRT, three-dimensional conformal radiotherapy; HT, helical radiotherapy; BED, biologically effective dose.
Outcomes and Symptom Relief
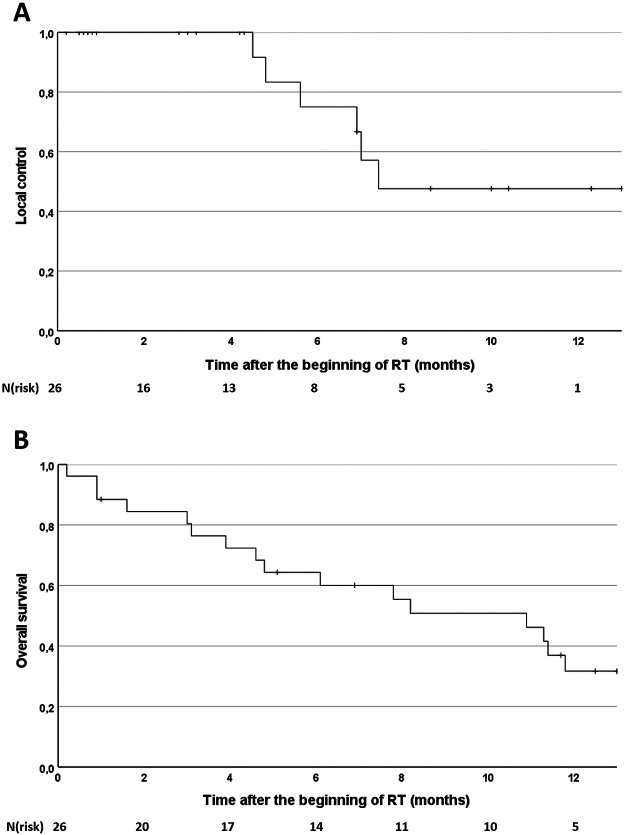
Median follow-up was 6.5 months after the beginning of RT. After 6 and 12 months OS was 60.0% and 31.7% and LC 75.0% and 47.6%, respectively (Figure 1). Median OS was 10.9 months and median LC was 7.4 months. OS and LC did not significantly differ between patients receiving BED ≥ 68.3 Gy or BED < 68.3 Gy (p = .68 and p = .94, respectively). Further analysis did not show any significant impact of analyzed patient characteristics, initial tumor characteristics, or radiation parameters on LC or OS.
Figure 1.
Kaplan-Meier curves for local control rate (A) and overall survival rate (B).
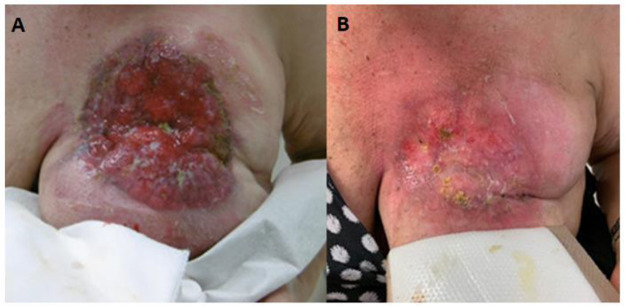
RT led to symptom palliation in 95% of patients. The absence of symptom palliation in 5% of the patients was not associated with a clinically relevant difference in BED compared to the median BED (68.3 Gy) administrated (maximum difference of 1.5 Gy). One patient needed re-irradiation approximately 10 months after initial analgetic RT due to renewed, intermittently bleeding local recurrence. A level reduction of analgetics according to the WHO analgesic ladder was achieved in 28.6% of patients. In 36% of patients, local RT had already been indicated >3 months before the actual start of RT, while 16% of patients presented with a RT indication as long as 6 months before the initiation of RT. However, RT was delayed or not initiated among others in favor of drug alternatives. An example of the evolution of an initially exulcerating LABC after palliative RT with 39 Gy in 3 Gy per fraction is shown in Figure 2.
Figure 2.
Exulcerating locally advanced breast carcinoma before (A) and after (B) palliative radiotherapy with 39 Gy in 3 Gy per fraction.
Regarding acute toxicities, no high-grade toxicities have been reported (> CTCAE II°). Treatment-related toxicity comprised solely low-grade side-effects (CTCAE I°-II°) with predominantly erythema and fatigue. Radiation-induced skin toxicity did not significantly depend on PTV volume. Three patients (11.3%) underwent simultaneous chemo- or immunotherapy with no increased toxicities being reported. Toxicity was not aggravated in the context of re-irradiation. Late RT-associated side-effects could not be observed in any patient.
Discussion
Patients referred for palliative treatment due to LABC or advanced-stage locoregional recurrence are often unsuitable for surgery or systemic treatment due to, for example, expansive unresectable tumor infiltration or a poor performance score.4,9 Sometimes palliative systemic treatment alone may not adequately address the desired symptom palliation. 19 Furthermore, women with LABC or inoperable local recurrence often suffer from a significantly reduced QOL due to local tumor-associated pain, bleeding, exulceration, or malodorous discharge.9–11
Palliative RT plays an important role in symptom management and might become even more required as a response to the relative aging of the populations.20,21 Still there is surprisingly scarce evidence regarding palliative RT in symptomatic LABC or locoregional recurrence. In this regard, this single-center study aimed to characterize palliative RT in symptomatic LABC or locoregional recurrence at our institution and further examine the benefits while weighing the risks.
Symptom palliation has been met in 95% of our patient cohort, with no correlation between BED and symptom relief. Previously, Vempati et al recommended a dose of >30 Gy in standard fractionation or equivalent BED for adequate symptom palliation, since a lower dose did not lead to any symptom relief. In their cohort, for patients receiving >30 Gy corresponding to a BED of approximately >44 to 47 Gy, symptom palliation was achieved in only 69%. 22 In our study, among the patients who completed RT as initially planned, BED ranged from 52.0 to 94.5 Gy for all but 1 patient. However, this patient still experienced symptom relief. Similarly, in a cohort of 44 patients, Jacobson et al did not detect a threshold dose for a clinical benefit from fractionated palliative RT with 100% symptom relief when applying sufficiently high BEDs ranging from 68 to 79 Gy. 4 Choi et al further reported that 90.9% of patients treated with a BED ranging from 69.1 to 89.4 Gy for symptomatic incurable inflammatory breast cancer (IBC) showed >30% of symptom relief compared to pre-treatment symptoms. 23 In line with the above studies, single fraction RT with 8 Gy (BED 24 Gy) applied in patients with a poor performance score and dismal prognosis is known to be less beneficial in terms of symptom palliation leading to an increased need for re-irradiation. 4
These data together with our findings suggest that palliative RT can effectively achieve symptom palliation especially when applied with higher doses.
In patients being treated for painful tumor lesions, palliative RT led to a level reduction of analgetics according to the WHO analgesic ladder in 28.6% of patients. Hence analgetic RT may further reduce potential side-effects of analgetics including opioids and may alleviate the burden of polypharmacy. Several studies have indeed described the benefit of reducing polypharmacy especially in patients with limited life expectancies due to its association with poorer health-related QOL.8,24,25
Palliative treatment was well tolerated. Treatment-related toxicity comprised solely low-grade acute side-effects (CTCAE I°-II°). This is consistent with previous reports.9,22,23 In a study analyzing symptomatic RT for patients with IBC, the RT field was shown to be a significant predictor for skin toxicity. 23 In our collective, we did not observe a significant correlation between radiation-induced toxicities and PTV or BED.
In line with previous publications, prior RT to the same site did not relevantly increase treatment-related toxicities.4,22 This also applies to 1 patient in our cohort having undergone irradiation to the same site twice before with a cumulative BED of 146.2 Gy. This may partly be due to the relatively long period between the initial RT and the palliative treatment, with a median time interval of 53 months between radiation treatments. Late RT-associated side-effects were not observed in any patient within the follow-up period, possibly due to the relatively short follow-up period and the observed limited OS following RT in our study.
In our series simultaneous chemo- or immunotherapy has been applied in 3 patients without observing any increased treatment-related toxicities. Depending on the substance, concomitant treatment is generally very carefully considered or refrained from in order to avoid potentially increased undesirable treatment-induced toxicity. Although a local palliative RT indication had already existed >3 months respective >6 months prior to the start of RT in 36% respective 16% of patients in our study, RT was delayed or even not initiated among others in favor of drug alternatives. However, the benefit of palliative RT depends to a great extent on timing. It has been previously shown that a significant number of patients die shortly after receiving palliative RT,26,27 inherently experiencing less benefit. Given its effectiveness and high tolerability, symptomatic RT may be considered earlier depending on the actual palliative setting to allow durable benefit with minimal toxicity for patients with an often limited life expectancy.
Indeed, OS after 6 and 12 months was limited with only 60.0% and 31.7%, respectively, consistent with the high number of patients with distant metastases (88.0%). LC was 47.6% after 12 months with a median LC of 7.4 months, which is in line with previously reported LC rates.9,28 Other studies reported a correlation between LC and the applied dose or the tumor volume.29–31 Although we did observe a trend between LC and the irradiated tumor volume, this correlation was not significant. Nevertheless, the rather low LC rates in our study may be due to the relatively large PTVs of most of the patients with a median volume of 1206.8 cm3. As we did not detect any correlation between BED and LC, the relatively small dose level span used in our study might be not sufficient to confirm such an effect due to the small patient cohort.
Our study has several limitations such as its retrospective nature that may impact the findings due to potential confounding factors or possible methodological issues. Another limitation arises from the relatively short follow-up, which is nevertheless mainly a result of the limited survival of our patients after completion of palliative RT. Furthermore, the reported results and statistical measurements need to be evaluated with caution not least due to the small sample size. This is however inevitable due to the small percentage of breast cancer patients that are in general referred for symptomatic RT (<1%) at our institution, ranging in the same order of magnitude than those reported from other centers. 4 Nevertheless, this study further characterizes and describes the effectiveness and tolerability of palliative RT in LABC or locoregional recurrence.
Conclusion
Palliative RT in symptomatic LABC or locoregional recurrence is an effective treatment option for controlling local symptoms with only mild toxicity. It may thus improve QOL and should be considered early in palliative patient care management.
Abbreviations
- 3D-CRT
three-dimensional conformal radiotherapy
- BED
biologically effective dose
- CTCAE
Common Terminology Criteria for ADVERSE events
- EQD2
equivalent dose in 2 Gy fractions
- ECOG
Eastern Cooperative Oncology Group
- Gy
Gray
- HER2
human epidermal growth factor receptor 2
- HT
helical radiotherapy
- HR
hormone receptor
- IARC
International Agency for Research on Cancer
- IBC
inflammatory breast cancer
- IORT
intraoperative radiotherapy
- IMRT
intensity-modulated radiotherapy
- LABC
locally advanced breast cancer
- LC
local control
- OS
overall survival
- PTV
planning target volume
- QOL
quality of life
- RT
radiotherapy
- VMAT
volumetric modulated arc therapy.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: F.W. received speaker fees from AstraZeneca and Merck Sharp & Dohme outside the submitted work. M.W. received speaker fees from Novartis, Roche, MSD and Grants from Celgene outside the submitted work. J.D. received grants from CRI—The Clinical Research Institute GmbH, Accuray Incorporated, Accuray International Sàrl, RaySearch Laboratories AB, Vision RT limited, Astellas Pharma GmbH, Astra Zeneca GmbH, Solution Akademie GmbH, Ergomed PLC Surrey Research Park, Merck Serono GmbH, Siemens Healthcare GmbH, Quintiles GmbH, Pharmaceutecal Research Associates GmbH, Boehringer Ingelheim Pharma GmbH Co, PTW-Freiburg Dr. Pychlau GmbH, Nanobiotix A.A., IntraOP Medical and Varian Medical Systems outside the submitted work. J.HR. received speaker fees from ViewRay Inc. and Pfizer Inc., travel reimbursement from ViewRay Inc., IntraOP Medical and Elekta Instrument AB as well as grants from IntraOP Medical and Varian Medical Systems outside the submitted work.
Funding: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The authors received no financial support for the research, authorship, and/or publication of this article. PH and TF are funded by the Physician-Scientist Program of Heidelberg University, Faculty of Medicine.
Institutional Review Board Statement: The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the local ethical review board of the University of Heidelberg (approval number: S-535/2021, approval date: July 05, 2021). Individual written informed consent from all subjects involved in the study was not necessary to obtain according to the local ethics committee approval.
ORCID iDs: Line Hoeltgen https://orcid.org/0000-0002-3433-2455
Eva Meixner https://orcid.org/0000-0001-7087-9581
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Khil L, Heidrich J, Wellmann I, et al. Incidence of advanced-stage breast cancer in regular participants of a mammography screening program: a prospective register-based study. BMC Cancer. 2020;20(1):174. doi: 10.1186/s12885-020-6646-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30. doi: 10.3322/caac.21332 [DOI] [PubMed] [Google Scholar]
- 4.Jacobson G, Kaidar-Person O, Haisraely O, et al. Palliative radiation therapy for symptomatic advance breast cancer. Sci Rep. 2021;11(1):5282. doi: 10.1038/s41598-021-84872-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Early Breast Cancer Trialists’ Collaborative G, Darby S, McGale P, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804):1707-1716. doi: 10.1016/S0140-6736(11)61629-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Early Breast Cancer Trialists’ Collaborative G, McGale P, Taylor C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomised trials. The Lancet. 2014;383(9935):2127-2135. doi: 10.1016/S0140-6736(14)60488-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christiansen P, Al-Suliman N, Bjerre K, Moller S. Danish breast cancer cooperative G. Recurrence pattern and prognosis in low-risk breast cancer patients–data from the DBCG 89-A programme. Acta Oncol. 2008;47(4):691-703. doi: 10.1080/02841860802056594 [DOI] [PubMed] [Google Scholar]
- 8.Bartelink H, Maingon P, Poortmans P, et al. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol. 2015;16(1):47-56. doi: 10.1016/S1470-2045(14)71156-8 [DOI] [PubMed] [Google Scholar]
- 9.Chia D, Tan E, Lu J, et al. Clinical outcomes of fungating breast cancer treated with palliative radiotherapy. J Radiat Oncol. 2016;5(4):411-416. doi: 10.1007/s13566-016-0278-z [DOI] [Google Scholar]
- 10.Jacobson G, Galper S, Shahadi ID, Symon Z, Rabin T, Ben-David M. Palliative breast radiation—effectiveness, fractionation, and toxicity. Int J Radiat Oncol Biol Phys. 2017;99(2):S6-S7. doi: 10.1016/j.ijrobp.2017.06.031 [DOI] [Google Scholar]
- 11.Tryfonidis K, Senkus E, Cardoso MJ, Cardoso F. Management of locally advanced breast cancer-perspectives and future directions. Nat Rev Clin Oncol. 2015;12(3):147-162. doi: 10.1038/nrclinonc.2015.13 [DOI] [PubMed] [Google Scholar]
- 12.Jones J. A brief history of palliative radiation oncology. Radiat Oncol Palliative Cancer Care. 2013;1:3-14. doi: 10.1002/9781118607152.ch1 [DOI] [Google Scholar]
- 13.Grubbe EH. X-rays in the treatment of cancer and other malignant diseases. Medical Record (1866-1922). 1902;62(18):692. [Google Scholar]
- 14.Zhu YJ. Palliative radiotherapy for painful bone metastases: Short-course or long-course? Ann Palliat Med. Apr 2012;1(1):78-80. doi: 10.3978/j.issn.2224-5820.2011.10.03 [DOI] [PubMed] [Google Scholar]
- 15.Canon V, Gomez-Iturriaga A, Casquero F, et al. Quality of life improvement in patients with bone metastases undergoing palliative radiotherapy. Rep Pract Oncol Radiother. 2022;27(3):428-439. doi: 10.5603/RPOR.a2022.0048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spencer K, Parrish R, Barton R, Henry A. Palliative radiotherapy. Br Med J. 2018;360:k821. doi: 10.1136/bmj.k821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495-1499. doi: 10.1016/j.ijsu.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 18.van Leeuwen CM, Oei AL, Crezee J, et al. The alfa and beta of tumours: a review of parameters of the linear-quadratic model, derived from clinical radiotherapy studies. Radiat Oncol. 2018;13(1):96. doi: 10.1186/s13014-018-1040-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prigerson HG, Bao Y, Shah MA, et al. Chemotherapy use, performance Status, and quality of life at the end of life. JAMA Oncol. 2015;1(6):778-784. doi: 10.1001/jamaoncol.2015.2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Donovan A, Morris L. Palliative radiation therapy in older adults with cancer: Age-related considerations. Clin Oncol (R Coll Radiol). 2020;32(11):766-774. doi: 10.1016/j.clon.2020.06.011 [DOI] [PubMed] [Google Scholar]
- 21.Lutz ST. Palliative radiotherapy: history, recent advances, and future directions. Ann Palliat Med. 2019;8(3):240-245. doi: 10.21037/apm.2019.03.02 [DOI] [PubMed] [Google Scholar]
- 22.Vempati P, Knoll MA, Dharmarajan K, Green S, Tiersten A, Bakst RL. Palliation of ulcerative breast lesions with radiation. Anticancer Res. 2016;36(9):4701-4705. doi: 10.21873/anticanres.11024 [DOI] [PubMed] [Google Scholar]
- 23.Choi HS, Jang HS, Kang KM, Choi BO. Symptom palliation of hypofractionated radiotherapy for patients with incurable inflammatory breast cancer. Radiat Oncol. 2019;14(1):110. doi: 10.1186/s13014-019-1320-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Machon M, Larranaga I, Dorronsoro M, Vrotsou K, Vergara I. Health-related quality of life and associated factors in functionally independent older people. BMC Geriatr. 2017;17(1):19. doi: 10.1186/s12877-016-0410-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schenker Y, Park SY, Jeong K, et al. Associations between polypharmacy, symptom burden, and quality of life in patients with advanced, life-limiting illness. J Gen Intern Med. 2019;34(4):559-566. doi: 10.1007/s11606-019-04837-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy JD, Nelson LM, Chang DT, Mell LK, Le QT. Patterns of care in palliative radiotherapy: A population-based study. J Oncol Pract. 2013;9(5):e220-e227. doi: 10.1200/JOP.2012.000835 [DOI] [PubMed] [Google Scholar]
- 27.Park KR, Lee CG, Tseng YD, et al. Palliative radiation therapy in the last 30 days of life: A systematic review. Radiother Oncol. 2017;125(2):193-199. doi: 10.1016/j.radonc.2017.09.016 [DOI] [PubMed] [Google Scholar]
- 28.Badwe R, Hawaldar R, Nair N, et al. Locoregional treatment versus no treatment of the primary tumour in metastatic breast cancer: An open-label randomised controlled trial. Lancet Oncol. 2015;16(13):1380-1388. doi: 10.1016/S1470-2045(15)00135-7 [DOI] [PubMed] [Google Scholar]
- 29.Borger JH, van Tienhoven G, Passchier DH, et al. Primary radiotherapy of breast cancer: Treatment results in locally advanced breast cancer and in operable patients selected by positive axillary apex biopsy. Radiother Oncol. 1992;25(1):1-11. doi: 10.1016/0167-8140(92)90188-z [DOI] [PubMed] [Google Scholar]
- 30.Arriagada R, Mouriesse H, Sarrazin D, Clark RM, Deboer G. Radiotherapy alone in breast cancer. I. Analysis of tumor parameters, tumor dose and local control: The experience of the Gustave-Roussy Institute and the Princess Margaret Hospital. Int J Radiat Oncol Biol Phys. 1985;11(10):1751-1757. doi: 10.1016/0360-3016(85)90027-6 [DOI] [PubMed] [Google Scholar]
- 31.Chu AM, Cope O, Doucette J, Curran B. Non-metastatic locally advanced cancer of the breast treated with radiation. Int J Radiat Oncol Biol Phys. 1984;10(12):2299-2304. doi: 10.1016/0360-3016(84)90236-0 [DOI] [PubMed] [Google Scholar]