Abstract
Background
Polycystic ovarian syndrome (PCOS) is an endocrine metabolic disorder of women.
Purpose
This study aimed to explore the potential of aqueous extract of Garcinia cambogia Desr. (AEGC) in PCOS.
Methodology
The HPLC was used to determine the phytoconstituents present in Garcinia cambogia. Thirty adult female albino rats were divided into 6 groups: Normal control (NC) disease Control (PCOS; letrozole 1 mg/kg), plant extract (AEGC 100, 300, 500 mg/kg) and standard (metformin; 20 mg/kg). Disease was confirmed by vaginal smear cytology. After 10 weeks, animals were euthanized, ovaries dissected for histopathology, blood collected for hormonal and biochemical analysis.
Results
HPLC analysis showed the presence of phenolic contents; chlorogenic acid, gallic acid, coumaric acid while flavonoid contents were quercetin, kaempferol, and rutin. After treatment, there was dose dependent reduction of weight, ovarian cysts, improvement of follicle growth. DPPH radical scavenging percentage was 67.89%. Hormonal analysis showed a significant improvement (P < .05) in follicle stimulating hormone (FSH), estrogen, and progesterone while a reduction in testosterone, luteinizing hormone (LH) and insulin level. Antioxidant enzymatic markers were significantly (P < .05) increased. Lipid profile and LFTs were also improved.
Conclusions
The study validated the potential of Garcinia cambogia in the management of PCOS.
Keywords: Polycystic ovarian syndrome, Garcinia cambogia, ovarian cyst, follicles, antioxidant, hormonal level
Introduction
Polycystic ovarian syndrome (PCOS) is a complicated metabolic and endocrine abnormality that is associated with hyperandrogenism, hyperinsulinemia, hirsutism, acne, psychological issue, and weight gain. Adolescent women with PCOS have an approximate count of about 50–80 million globally. 1 The occurrence of PCOS range from 2.2 to 26% world widely, depending on the environment and descriptive variations in the definition of PCOS.1,2 This disease is identified by the formation of poly (more than 1) cysts on the surface of the ovaries and can be detected by ultrasound. 3 Obesity and Insulin resistance additionally aggravates the metabolic states. PCOS is a diverse disease which is also characterized by anovulation (lack of ovulation) chronically. 4
The high frequency GnRH pulse may increase LH and decreases FSH, disrupting the LH/FSH ratio in women with PCOS. This leads to the onset of metabolic and reproductive complaints such as more production of androgen in the ovarian theca cells with the inability of the ovaries to produce estrogen and FSH. 5 As a result of all these changes, the formation of follicular cysts occurs in the ovaries because the follicles unable to reach the mature stage.6,7 Obesity and PCOS are also closely associated and both aggravate each other. Obesity may lead to insulin resistance that results in anovulation. If obesity is reversed, insulin sensitivity might be improved. Both these aspects have a considerable effect on the pituitary-ovarian axis. PCOS is also caused by other issues such as genetics, eating disorders like bulimia nervosa, and binge eating. 8 PCOS is a syndrome that may cause endometrial cancer, hirsutism, acne, acanthosis nigricans, alopecia, infertility, preeclampsia, hyperlipidemia, glucose intolerance, oligomenorrhea, and cardiovascular abnormalities.9,10
At present, interventions for the PCOS include Clomiphene citrate and letrozole to improve fertility, metformin, antiandrogens, flutamide, OCP (oral contraceptives that are estrogen-progestin combinations), pioglitazone, spironolactone, GnRH agonists, and myo-inositol to manage the associated symptoms. 3 However, a lot of adverse effects are associated with the use of these medicines that limit their use for a short duration. For example, metformin causes lactic acidosis (both fatal and nonfatal) in 1–17 of every 100,000 individuals per year. 1 Weight gain, thromboembolism, and cardiovascular events have all been linked to oral contraceptive pills (OCPs). Some adverse effects are more prevalent among OCP users, including stomach discomfort, headache, dysmenorrhea, backache, nausea, and dizziness, whereas antiandrogens are hepatotoxic and can be lethal. 5
People are interested in herbal therapies due to aforementioned negative effects of conventional therapies.11-13 Pharmacological and therapeutic efficiency of herbal drugs is attributable due to the existence of many phytoconstituents, which may be utilized to treat a variety of ailments.14-16 Herbal therapies for PCOS have been reported to be quite effective in reducing hyperandrogenism, obesity and improving ovulation and insulin sensitivity with no major side effects. 17
Various herbal, alternative medicines and food supplements have shown potential against PCOS.1,18Garcinia cambogia Desr. also known as Garcinia gummi gutta (L.) Roxb. is member of family Clusiaceae is used to give flavor or aroma in fish curries. 19 This fruit is a popular nutritional supplement for weight loss. The extract and its constituent such as hydroxy citric acid (HCA) have been demonstrated to have anti-obesity action, markedly reduced food consumption and body fat growth through modulating serotonin levels, increased fat burning, and lower de novo lipogenesis.5,20,21 This study aimed to explore the therapeutic potential of Garcinia cambogia against letrozole induced PCOS, as well as to provide the rationale for the folkloric uses at scientific grounds with possible mechanism exploration.
Material and Methods
Chemicals and Apparatus
Chemicals, reagents, and solvents used for this research work were all of analytical grade. Chemicals and solvents used were distilled water, normal saline (Immunasol NS, A.Z. Pharmaceuticals Co.), carboxymethyl cellulose (CMC), absolute ethanol (British drug house), metformin (Martin Dow Company), Formalin, methylene blue (Fisher chemicals), eosin and hematoxylin dye (Cosmos Biomedical Ltd.) were purchased for slide preparation.
Apparatuses used was glass beakers, micropipette, stirrer, magnetic stirrer, microscope slides with coverslips, spatula, pestle and mortar, maceration bottle, Eppendorf tubes, Whatman filter paper, dissection box. Instruments used for the experiment were electrical weighing balance (Adventurer TM OHAUS), hot plate, UV spectrophotometer ELISA reader (DIA Source), Accu-scope HD, water bath, centrifuge machine (DLAB).
Acquisition of Aqueous Extract of Garcinia cambogia
Garcinia cambogia aqueous extract was acquired from Miksons healthcare SMS (Pvt.) Ltd. Rawat Rawalpindi, which was imported from Venkatesh Food Industries, India (An FSSC 22000 certified company). HPLC analysis report was observed for purity and authentication of the aqueous extract.
In-Vitro Studies
HPLC Analysis
The phenolic and flavonoid contents of an aqueous extract of Garcinia cambogia (AEGC) were determined using HPLC. AEGC samples were analyzed in HPLC on a phase C18 column (5 m, 250 4.6 mm) using an oven set at 30°C. Chromera HPLC system (Perkin Elmer, USA) connected to Flexer Binary LC pump and UV/VIS Liquid chromatography sensor (Shelton CT, 06 484 USA).
Assessment of Total Phenolic Content (TPC)
The TPC of AEGC was measured by using procedure described by A shukla et al., 22 First of all 0.5 mL solution of AEGC prepared in falcon tube by adding .05 g of extract in 5 mL of distilled water. In this solution, 0.5 mL of Folin-ciocalteu reagent (1:1 with water) was added, followed by 7.5 mL of deionized water. After gentle shaking, the mixture was left uninterrupted at room temperature for 10 minutes before adding 1.5 mL of NA2CO3 (20% w/v) solution. This combination is then placed in a dark area at room temperature for 40 minutes. In addition, the absorbance was measured at 725 nm in a spectrophotometer against the blank reagent. Gallic acid was utilized to create a reference curve, and the TPC was measured using the gallic acid standard curve (100–1300 ppm). TPC was quantified as Gallic acid equivalent (GAE) and the findings were represented in mg/g of the extract.23,24 The TPC of each sample was calculated using the formula
where: V = volume of extract in mL
M = mass of extract in grams
C = total phenolic content, mg GAE/g dry extract
C = gallic acid concentration calculated from the calibration curve (mg/mL)
Total Flavonoid Content (TFC) Estimation
Total flavonoids contents were measured as quercetin equivalent by making its standard curve (10–130 ppm). First of all .5 mL of GC solution was prepared and kept at room temperature for 5 minutes. It was then treated with .6 mL of 10% aluminum chloride (AlCl3) for 5 minutes at room temperature. Then 2 mL of 1M NaOH was added sequentially and made up the final volume by adding 2.4 mL of distilled water. Absorbance was measured at 510 nm by using a UV spectrophotometer. TFC was measured as quercetin equivalent (CE).23,24
Determination of Anti-oxidant Activity
Evaluation of Oxidative Stress Markers
Patients with PCOS express a decreased level of antioxidant enzymes such as catalase (CAT), reduced glutathione (GSH), superoxide dismutase (SOD). Tissue homogenate was made by combining 0.1 M phosphate buffer (pH 7.4) with 1g of liver tissue and grinding it with a tissue homogenizer. Phosphate buffer was made up of 10 mM potassium chloride, 1 mmol ethylene diamine tetra acetic acid (EDTA), .25 M sucrose, and 1 mM phenylmethylsulfonylfluoride. The mixture was centrifuged at 800 rpm at 4°C for 30 minutes to get supernatant. The supernatant solution was separated and the antioxidant markers were determined. 25
DPPH (2, 2-diphenylpicrylhydrazyl) Radical Scavenging Assay
Stock solution Of DPPH (2.4 mg/100 mL methanol) was made by adding 2.4 mg DPPH in a small volume of methanol and then the volume was made up to 100 mL. After that, the stock solvent solution was prepared by using ethanol and distilled water in 20:80. Stock solution of Garcinia cambogia was also prepared by adding .05 g of GC in a 0.5 mL of distilled water. .5 μL GC extract solution was taken in a falcon tube to prepare 5 different concentrations (20, 40, 60, 80, 100 ppm). Then a 200 mg/L ascorbic acid stock solution was made. Similarly, from this stock solution of ascorbic acid, samples of 5 different concentrations were generated (20, 40, 60, 80, 100 ppm). Finally, both the AEGC and ascorbic acid samples received 4 mL of DPPH solution, the resulting mixture was stored in the darkness at room temperature (30 min), and the absorbance at 515 nm was observed using UV spectrophotometer. Absorbance of DPPH solution without antioxidant (used as blank reagent) was also recorded.23,24 The following equation was used to calculate the capacity to scavenge DPPH radicles
AB: Absorbance of blank at 0 min
AA: Absorbance of the sample to be tested at 30 min
In-Vivo Studies
Housing of Experimental Animals
Thirty healthy female wistar albino rats (100–150 g) were acquired and housed at the animal house of the department of Pharmacology, Government College University, Faisalabad, Pakistan. All rats were accommodated in stainless steel cages (5 rats/cage). Standardized laboratory conditions were followed (10/14-hour light/dark cycle at 25–30°C with 45–55 percent of overall humidity). A standard laboratory diet and water at libitum were given to rats. The animal’s study was conducted under the pre-approval from Ethical Review committee (ERC) of Government College University, Faisalabad, Pakistan. The reference number of the approval was GCUF/ERC/52.
Disease Induction in Female Rats
Letrozole at a dose of (1 mg/kg p. o.) was used to induce PCOS in rats after 1 week of adaptation period. 26 Letrozole suspension was developed by suspending letrozole in .5% of CMC carboxymethyl cellulose solution. To confirm the disease induction estrous cycle was monitored daily and weight changes were recorded weekly. Vaginal smear cytology performed on daily basis to find out the estrous cycle stages. Estrus cycle stages were confirmed by the presence of relative proportion of leukocytes, cornified cells, and epithelial cells in the vaginal smear when observed under the light microscopy.
Experimental Design
Animals were divided by completely randomized design in 6 groups (n = 5). Groups were categorized as Normal control (N.C), diseased (PCOS), standard (metformin), GC (100 mg/kg), GC (300 mg/kg), and GC (500 mg/kg). For identification of different groups their tails were labeled by using different permanent colored markers. The treatment period for all groups was 5 weeks, doses were administered orally through gavage. Vaginal smear was observed daily and weight changes recorded weekly.
Subsequently, after 10 weeks of dosing and after 24 hours of the last dose, the animals were euthanized. Blood was collected by cardiac puncture. After that centrifugation of the blood samples was carried out to separate the serum and frozen at −20°C for hormonal assessment and biochemical analysis. Ovaries were dissected and preserved in 10% buffered formalin solution to perform the histopathology analysis. Livers were preserved in 10% buffered formalin solution for the assessment of anti-oxidant status. Experimental design and dosage schedule are given in Table 1.
Table 1.
Experimental Design.
| Groups | Dose Schedule |
|---|---|
| Normal control group (N.C) | .5% w/v CMC, 1 mL/100 kg/day p.o |
| a Disease induced group (PCOS) | Letrozole in .5% w/v in CMC, 1 mg/kg/day p.o |
| Standard (metformin) group | Metformin in .5% w/v in CMC 20 mg/kg/day p.o |
| Garcinia cambogia treatment group GC (100 mg/Kg) | Garcinia cambogia in .5% w/v in CMC 100 mg/kg/day p.o |
| Garcinia cambogia treatment group GC (300 mg/Kg) | Garcinia cambogia in .5% w/v in CMC 300 mg/kg/day p.o |
| Garcinia cambogia treatment group GC (500 mg/Kg) | Garcinia cambogia in .5% w/v in CMC500 mg/kg/day p.o |
aDisease induction period was 5 weeks and then treatment period was also 5 weeks. Total duration of the study was 10 weeks.
Determination of Estrous Cycle Staging
The estrous cycle was divided into 4 stages: proestrus, estrus, metestrus, and diestrus. Estrous cycle was identified by adopting a method defined previously. 25 To obtain a vaginal smear of rats, each animals were taken out from the cage and opened the vaginal hole carefully by grasping the tail in 1 hand. A cotton swab moistened with distilled water was placed into the vagina of the rat. Rotate the cotton swab softly for a while and remove out. This cotton swab was used to create a smear on the cleaned, grease-free glass slide. After that air-dried the slide and wash it with 10% methanol solution and place with methylene blue stain on smear slide. Wash it with distilled water and wait for the slides to get air dried the slide and a binocular microscope was used to detect distinct phases of the estrous cycle.5,25
Histopathological Examination of Ovarian Tissues
Following dissection, Rat ovaries from all groups were isolated and fixed in a 10% solution of buffer formalin. Upon fixing, paraffin blocks were created, and 5 μM thick slices were cut with a microtome. Eosin and Hematoxylin was used to stain the slides.
Serum Hormonal Estimation
For Hormonal analysis, Rat blood serum was tested using radioimmunoassay (RIA) and Enzyme-Linked Immune-Sorbent Essay (ELISA) kits. After obtaining a blood sample through heart puncture, the serum was separated by cold centrifugation at 3000 rpm for 5 minutes. The level of follicle stimulating hormone (FSH), progesterone testosterone was determined by using Radioimmunoassay (RIA) kit (Beckman Coulter, Inc. USA). While luteinizing hormone (LH), estrogen and insulin levels were determined by ELISA (Pointe Scientific Inc. USA). 5
Lipid Profile and Liver Function Test (LFTs) Analysis
All the lipid profile indicators such as total cholesterol, triglyceride (TG), low density lipoprotein (LDL) and high-density lipoprotein (HDL were determined. Likewise, different liver function indicators, such as alanine amino transferase (ALT), aspartate amino transferase (AST), and alkaline phosphatase (ALP) levels, as well as total and direct bilirubin levels, were also analyzed, on semi-automated chemistry analyzer (microlab-300). 5
Statistical Analysis
The results were depicted as Mean ± SEM. Results were analyzed by analysis of variance, that is, one-way and two-way ANOVA followed by the Tukey’s multiple comparisons test using graph pad prism version 8.0.2. The results were considered significant if the P value less than .05 (P < .05)
Results
In Vitro Studies
HPLC Analysis
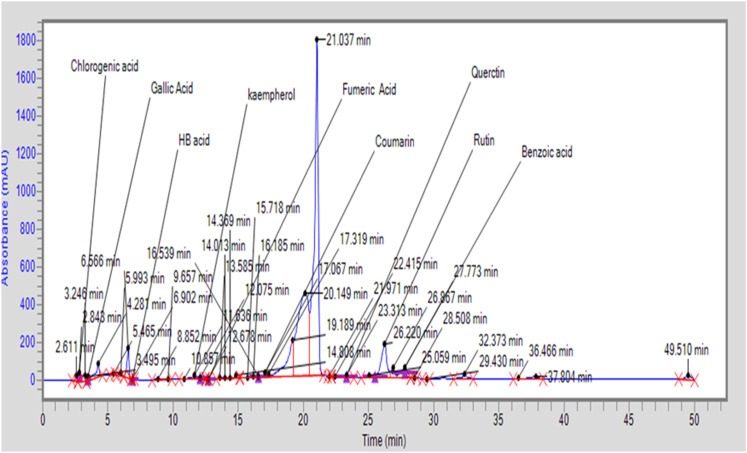
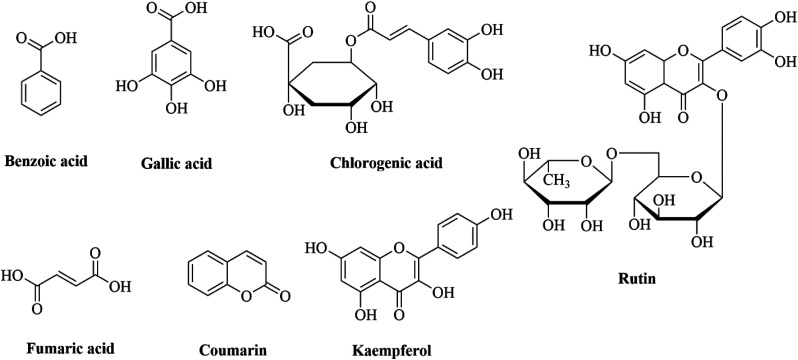
Phenolic contents present in aqueous extract of Garcinia cambogia (AEGC) were chlorogenic acid (6.517 μg/gm), gallic acid (2.114 μg/gm), HB acid (3.515 μg/gm), fumaric acid (.363 μg/gm), coumaric acid (505.91 μg/gm), and benzoic acid (57.53 μg/gm). Flavonoid contents in AEGC included quercetin (251.44 μg/gm), kaempferol (17.03 μg/gm), and rutin (637.78 μg/gm) (Table 2). HPLC chromatogram is given in Figure 1 while the structure of phytoconstituents are given in Figure 2.
Table 2.
HPLC analysis of phenolic and flavonoids contents of Garcinia cambogia aqueous extract (AEGC).
| Phenolic Contents | (μg/Gm) | Flavonoids Contents | (μg/Gm) | |
|---|---|---|---|---|
| Chlorogenic acid | 6.517 | Quercetin | 251.44 | |
| Gallic acid | 2.114 | Rutin | 637.78 | |
| HB acid | 3.515 | Kaempferol | 17.03 | |
| Coumaric acid | 505.91 | |||
| Benzoic acid | 57.53 | |||
| Fumaric acid | .363 | |||
Figure 1.
HPLC chromatogram of aqueous extract of Garcinia cambogia.
Figure 2.
HPLC determined phytoconstituents structure.
Total Phenolic Contents (TFC)
The TPC of the aqueous extract of Garcinia cambogia was 75 ± .124 (μg/gm). TPC is measured as gallic acid equivalents (GAE).
Total Flavonoids Contents (TPC)
The total flavonoid content (TFC) of the aqueous extract of Garcinia cambogia was 45.10 ± .08 (μg/gm). TFC is expressed as quercetin equivalent (QE).
DPPH Radical Scavenging Assay
DPPH radical scavenging potential of AEGC was determined and is obtained in parts per million (ppm). The findings were compared with ascorbic acid as a reference. The IC50 value of Garcinia cambogia was also calculated and it was (71.90 μg/mL) in comparison to ascorbic acid (31.62 μg/mL). The results are shown in Table 3.
Table 3.
DPPH Radical Scavenging Potential of Aqueous Extract of Garcinia cambogia (AEGC).
| Ascorbic Acid Standard | Aqueous Extract of Garcinia cambogia | ||
|---|---|---|---|
| Sample ppm | Scavenging % | Sample ppm | Scavenging % |
| 20 | 42.71 | 20 | 38.98 |
| 40 | 58.46 | 40 | 50.11 |
| 60 | 65. | 60 | 58.02 |
| 80 | 69.46 | 80 | 62.84 |
| 100 | 75.78 | 100 | 67.89 |
In Vivo Studies
Determination of Changes in Body Weight of Female Rats
Body Weight Changes during Disease (PCOS) Induction
After Continuous administration (for 5 weeks) of letrozole to the disease control group, a significant increase P < .05 was detected in the body weight of rats as compared to the normal control group. Body weight of the control rat was improved by 13.27% of its original weight whereas PCOS rats' weight was increased by 20%. The results are shown in Table 4.
Table 4.
Body Weight changes during disease (PCOS) induction.
| Weeks | Normal Control Group (Weight/G) | Diseased (PCOS) Group (Weight/G) |
|---|---|---|
| 1st | 113.6 ± 1.8 | 105.0 ± 4.2 |
| 2nd | 117.4 ± 1.9 | 110.4 ± 4.4 |
| 3rd | 119.4 ± 2.2 | 115.6 ± 4.8 |
| 4th | 124.0 ± 2.09 | 121.2 ± 4.6 |
| 5th | 128.0 ± 2.168 | 126.2 ± 4.4 |
| Increase in body weight | 13.27% | 20% |
Effect on Body Weight of Female Rats During Treatment Period
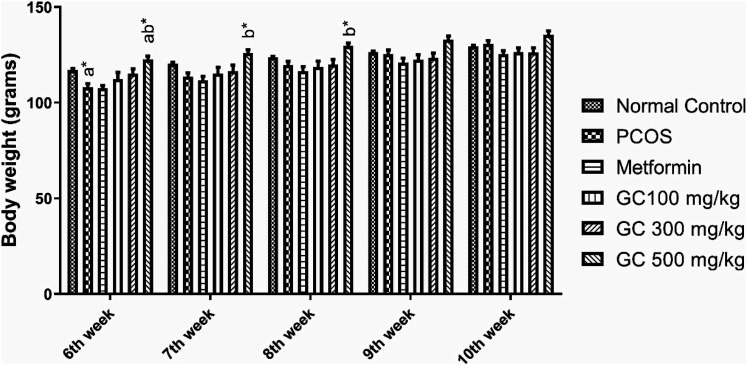
At the end of the study period, rats from PCOS group gained more weight as compared to rats of normal control group. But the weight gain of the metformin group and GC (100 mg/kg) group was increased in a much similar way as that of PCOS group. While GC group (300 mg/kg) and GC (500 mg/kg) rats showed a decrease in body weight as compared to PCOS group. Change in body weights of rats throughout treatment period are depicted in Figure 3.
Figure 3.
Effect of Aqueous Extract of Garcinia cambogia (AEGC) on body weight of female rats. Values are expressed as mean ± SEM (n = 5). adenotes significant difference from Normal Control; bdenotes significant difference from Disease Control (PCOS); results were compared by one-way ANOVA followed by Tukey’s multiple comparisons test. *P < .05, **P < .01.
Determination of Estrous Cycle Staging or Vaginal Smear Cytology
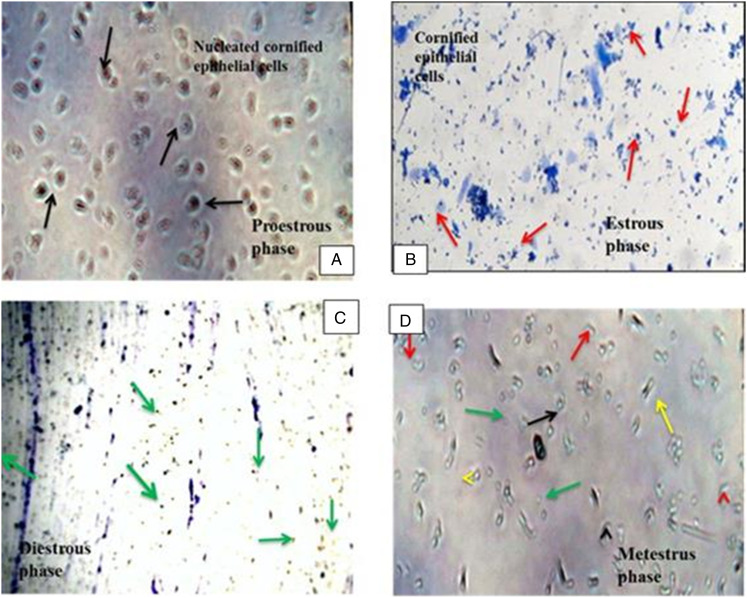
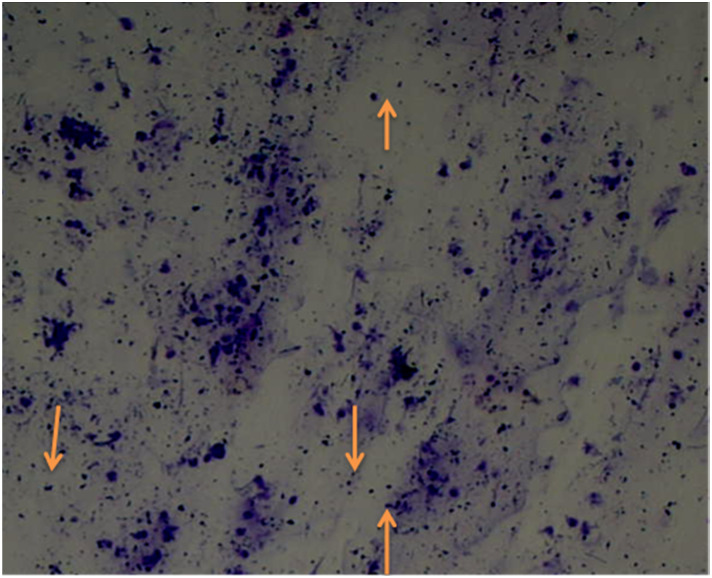
All phases of the estrous cycle of a normal control (healthy) rat are shown in Figure 4. In the pro-estrous phase, cornified epithelial cells were predominately nucleated while estrous phase has predominant Cornified epithelial cells. Diestrus phase primarily consists of leukocytes and cornified epithelial cells and nucleated cornified cells are all existed in the metestrus stage. Rats with PCOS shows delay in their normal estrous cycle, as they remain in the diestrus phase for prolong time. Vaginal cytology pictures of PCOS rats are given in Figure 5.
Figure 4.
Vaginal cytology of rats showing different stages of the estrous cycle. Vaginal smear of rats’ estrous cycle presented different estrous phases with their existing type of cells (A) Pro-estrous stage presented dominantly nucleated cornified epithelial cells ( ). (B) Estrous stage categorized with cornified epithelial cells (
). (B) Estrous stage categorized with cornified epithelial cells ( ). (C) Diestrus stage contains mostly leukocytes (
). (C) Diestrus stage contains mostly leukocytes ( ). (D) Metestrus stage, in this phase, leukocytes, cornified epithelial cells, and nucleated cornified cells are present.
). (D) Metestrus stage, in this phase, leukocytes, cornified epithelial cells, and nucleated cornified cells are present.
Figure 5.
Vaginal cytology of diseased rat (PCOS) with diestrus phase (predominant leukocytes).
Histopathological Changes in Ovarian Cross Section
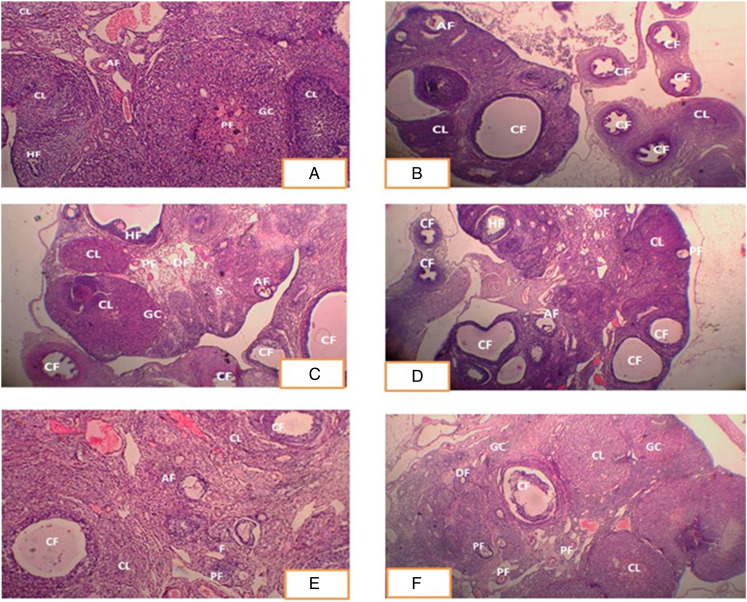
Histopathological slices of normal control group ovaries revealed a typical architecture, including tiny to medium follicles, numerous corpus luteum, granulosa cells, and follicles at several levels of development (Figure 6A). Ovarian sections from the PCOS group showed cystic follicles and a disordered granulosa cell compartment with variable granulosa cell thickness, which is diagnostic of atretic antral follicles. Ovaries of rats treated with letrozole had a high number of cystic follicles but no corpus luteum. These alterations can be explained by lower FSH and higher testosterone levels in rats treated with letrozole (Figure 6B). However, a prominent decrease in the number and size of cystic follicles occurred after the administration of metformin, AEGC (100, 300, and 500 mg/kg) as shown in Figure 6C-F). The difference between the group is in dose dependent manner, the most prominent effects seen at dose 500 mg/Kg of AEGC and the least effect at 100 mg/kg.
Figure 6.
Histopathological Changes in Ovarian Cross Section (A) Ovarian section of control group exhibited normal morphology of rat ovary with different stages of ovarian follicles. (B) PCOS group showed typical cystic follicles and disorganized granulosa cell. (C) Ovarian section of metformin treated rats showed primary follicles, developing follicles and healthy follicles and lower no. of cystic follicles (D) Ovarian section of rat treated with GC (100 mg/kg) showed lower no. of cystic follicles, granulosa cells, and developing follicles but in lower number. (E) Section from rat ovaries treated with GC (300 mg/kg) showed more developing and primary follicles, granulosa cells. (F) Ovarian section of rat treated with GC (500 mg/kg) showed granulosa cells, developing follicles, primary follicles, and corpus luteum, more similarity with normal histopathology. CL: Corpus luteum, CF: Cystic follicles, AF: atretic follicles, DF: developing follicles, PF: primary follicles, GC: granulosa cells, HF: healthy follicles. Hematoxylin and eosin stained cross sections of female rat’s ovary.
Effect on Hormonal Profile
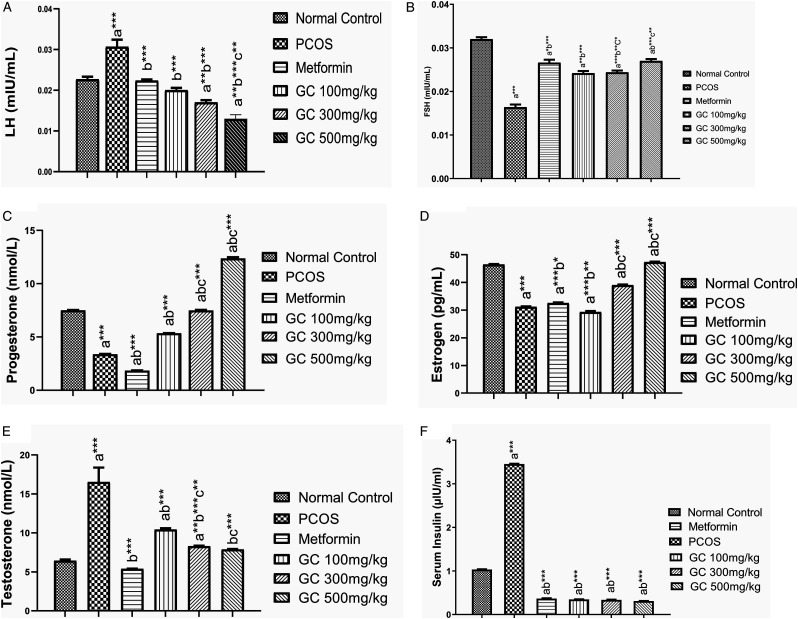
Hormone analysis showed that luteinizing hormone (LH) level was increased in PCOS group significantly (P < .001) as compared to control group. AEGC at all dose 100, 300, and 500 mg/kg significantly (P < .001) reduced the LH surge. The most significant effect was observed at 300 mg/kg and lowest effect was at 100 mg/kg (Figure 7(a)). PCOS had a bad impact on follicle growth that is depicted with lower level of FSH; similar results were obtained in our study. But AEGC significantly (P < .001) enhanced the FSH level in a dose dependent fashion, while metformin that is also improved FSH level (Figure 7B). Progesterone level was reduced in PCOS rats but AEGC increased it significantly (P < .001) in a dose dependent manner (Figure 7C).
Figure 7.
Effect on hormonal profile (A) LH (B) FSH (C) Progesterone (D) Estrogen (E) Testosterone (F) Insulin.
Values are expressed as mean ± SEM (n = 5). adenotes significant difference from Normal Control; bdenotes significant difference from Disease Control (PCOS); cdenotes significant difference from AEGC 100 mg/kg; results were compared by one-way ANOVA followed by Tukey’s multiple comparisons test. *P < .05, **P < .01, ***P < .001.
Estrogen level was lowered in PCOS group while AEGC and metformin have improved it and the most significant (P < .001) effect was noted in AEGC 300 mg/kg group female rats (Figure 7D). One of the most important androgens involved in PCOS symptomatology is testosterone, its level almost triplicate in PCOS while there was significant (P < .001) detrimental effects observed under the influence of AEGC in a dose dependent manner (Figure 7E). Insulin resistance is one of the diagnostic features of the PCOS, similarly, Insulin level was significantly (P < .001) higher in PCOS rats but it was reduced with metformin and AEGC 5 weeks treatment. Results are shown in Figure 7F
Effect on Lipid Profile
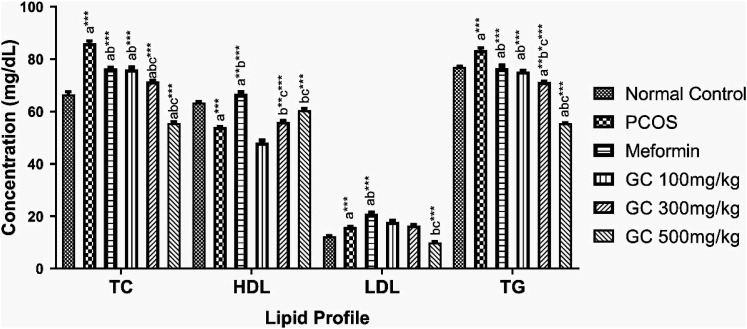
It elevated the level of total cholesterol (TC), LDL cholesterol, and triglycerides (TG) significantly (p < .001) while HDL (p < .001) level was deceased, as shown in Figure 8. Metformin being an insulin sensitizer had good impact on lipid profile. The extract in a dose dependent fashion (500 mg/kg > 300 mg/kg > 100 mg/kg) lowered the level of TC, LDL and TG significantly (P < .001), while increasing LDL level also significantly (P < .001). All the results are shown in Figure 8.
Figure 8.
Effect on lipid profile.
Values are expressed as mean ± SEM (n = 5). adenotes significant difference from Normal Control; bdenotes significant difference from Disease Control (PCOS); cdenotes significant difference from AEGC 100 mg/kg; results were compared by one-way ANOVA followed by Tukey’s multiple comparisons test. * p < .05, ** p < .01, *** p < .001.
Effect on Oxidative Stress Markers
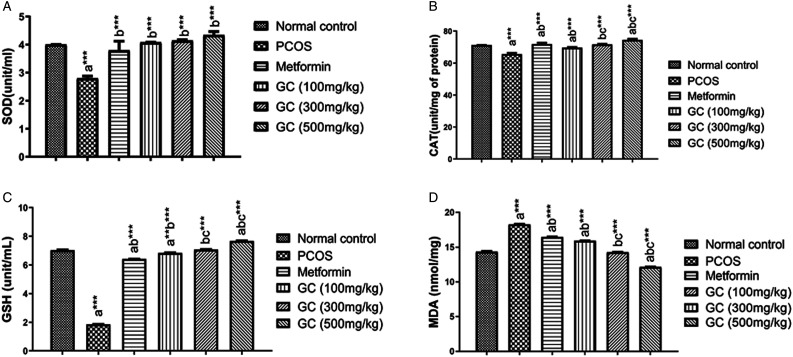
When the level of various oxidative stress markers such as superoxide dismutase (SOD), catalase (CAT), and glutathione (GSH) was measured, the levels of all these antioxidant enzymes such as CAT, SOD, and GSH were decreased significantly (P < .001) in PCOS group (letrozole treated). While the metformin and AEGC at all doses improved the oxidative stress marker, the most prominent effect being observed on GSH. AEGC reduced SOD, CAT, and GSH significantly (P < .001) in a dose dependent manner (500 >300 > 100 mg/kg). Results are shown in Figure 9A-C.
Figure 9.
Effect on oxidative stress marker.
Values are expressed as mean ± SEM (n=5). adenotes significant difference from Normal Control; bdenotes significant difference from Disease Control (PCOS); cdenotes significant difference from AEGC 100 mg/kg; results were compared by one-way ANOVA followed by Tukey’s multiple comparisons test. * p < .05, ** p < .01, *** p < .001.
MDA is very common marker of oxidative stress. 3 PCOS induction caused a substantial rise (P < .001) in MDA level in female rat liver tissues as found in results. While the treatment with AEGC all doses reduced the level of lipid per oxidation in the lever and hence decreased MDA level significantly (P < .001) especially at higher dose 500 mg/kg. Metformin has no effect on MDA level might be due to its ineffectiveness against the lipid peroxidation. Results are depicted in Figure 9D.
Effect on Liver Function Test (LFTs)
Liver is a vital organ and any abnormality that has broad aspects always effects liver. The toxicity of drugs and chemicals on the liver are tested by liver function tests. The levels of the enzymes aspartate aminotransferase (AST), alanine transaminase (ALT), and alkaline phosphatase (ALP) are very important to determine the toxicity of chemicals and disease. AST, ALT, and ALP were significantly higher (P < .001) in the PCOS rats when as compared to the normal control group. However, AEGC at all the doses significantly (P < .001) lowered the level of ALT, AST, and ALP in a dose dependent manner (Table 5). Moreover, the total, direct, and indirect bilirubin level was also measured and it was found that PCOS significantly (p < .001) disturbed their levels. But with treatment of AEGC especially at higher dose significantly reduced their concentration as depicted by the results found in Table 5.
Table 5.
Effect of Aqueous Extract of Garcinia cambogia on Liver Function Markers.
| Parameters | Normal control | PCOS | Metformin | G.C 100 mg/kg | G.C 300 mg/kg | G.C 500 mg/kg |
|---|---|---|---|---|---|---|
| (ALT) (U/L) | 50.57 ± 1.11 | 73.00 ± 2.309 a*** | 81.66 ± 1.45 a***b** | 63.33 ± 1.9 ab*** | 56.66 ± .2 b*** | 48.000 ± .7 bc*** |
| (AST) (U/L) | 257.2 ± 11.39 | 298.8 ± 2.02 a*** | 428.00 ± 4.05 ab*** | 290.00 ± 1.63 a***b** | 261.2 ± 1.4 bc** | 185.000 ± .7 abc*** |
| Alkaline phosphatase (U/l) | 376.00 ± .33 | 450.80 ± 2.08 a*** | 429.00 ± 1.45 ab*** | 440.8 ± 1.5 ab*** | 421.88 ± 1.158 abc*** | 307.66 ± 1.4 abc*** |
| Total Bilirubin (mg/dL) | .734 ± .00 | .806 ± .00 a*** | .704 ± .003 ab*** | .708 ± .003 ab*** | .710 ± .005 ab*** | .508 ± .0006 abc*** |
| Direct Bilirubin (mg/dL) | .198 ± .00 | .304 ± .00 a*** | 302 ± .00 a*** | .304 ± .006 a*** | 0.280 ± .01 bc*** | .210 ± .00A abc*** |
| Indirect Bilirubin (mg/dL) | .402 ± .00 | .502 ± .00 a*** | 402 ± .003 b*** | .402 ± .003 b*** | .308 ± .005 abc*** | .302 ± .003 abc*** |
Values are expressed as mean ± SEM (n = 5). adenotes significant difference from Normal Control; bdenotes significant difference from Disease Control (PCOS); cdenotes significant difference from AEGC 100 mg/kg; results were compared by one-way ANOVA followed by Tukey’s multiple comparisons test. *P < .05, **P < .01, ***P < .001.
Discussion
Female sex hormones such as estrogen, progesterone, and eggs (oocytes) are produced from ovaries. 27 The ovaries are well structured reproductive organ consisting of theca cells, stromal cells, and granulosa cells which interacted to form oocytes containing follicles and cause ovulation (release of eggs). After ovulation, these oocytes containing follicles got converted into corpus luteum (an endocrine structure) needed for the development and maintenance of pregnancy. The hypothalamic-pituitary-ovarian axis (HPO) through GnRH controls the release of FSH and LH, which regulate the menstrual cycle and encourage the development of the oocyte in the ovaries. 28
One in ten women worldwide are affected by polycystic ovarian syndrome (PCOS), a common hormonal illness in the female population of adult age. 29 PCOS is increasingly being identified in adolescent girls seeking therapy for hyper androgenic symptoms and indications (abnormal number of androgens i.e., male hormones). PCOS is a prevalent endocrine disorder that raises the risk of type 2 diabetes, insulin resistance, dyslipidemia, obesity, anovulation, oligomenorrhea, and cardiovascular hazards, among other illnesses. 1
The PCOS rat model used in this study was established by various researchers.26,30 For the purpose of inducing disease in rats, letrozole, a non-steroidal aromatase inhibitor chemically known as 4,40-[(1H-1,2,4-triazol-1-yl) methylene] bis-benzonitrile, is utilized. 31 Letrozole inhibited ovarian function by inhibiting the paracrine signaling, whole body aromatization, and folliculogenesis. 31 After 5 weeks of letrozole administration, disease induction was confirmed by the vaginal smear analysis, body weight changes. A comparison of the body weight of normal control groups (13.4%) with the PCOS group (20%) demonstrates that rats of PCOS group gained more weight (Table 4). Vaginal smear cytology of letrozole-treated rats exhibits that these rats stayed in the diestrus phase for a long period (Figures 4 and 5). Histopathological evaluation of slides also shows the presence of cystic follicles that can be seen in PCOS groups (Figure 6). The weight changes were continuously examined during treatment phase in all the groups. There was consistent weight gain in PCOS group but the metformin and AEGC especially at higher doses (500 mg/kg) reduced the weight, that shows the better prognosis. These findings are also persistent with the previous studies. 1 Hydroxy citric acid (HCA) is one of the major phytoconstituents of AEGC and has anti-obesity action. 32 HCA weight loss effect might be due to reduced food consumption and body fat growth through modulating serotonin levels, increased fat burning, and lower de novo lipogenesis.5,20,21
The pathophysiology of PCOS includes innate ovarian dysfunction that is heavily impacted by extrinsic variables such as hypothalamic-pituitary-ovarian axis (HPA) abnormalities and hyperinsulinemia. Excessive gonadotrophin releasing hormone (GnRH) causes luteinizing hormone (LH) hypersecretion, which affects both ovarian androgen production and oocyte development.5,18 The similar phenomenon, we observed in PCOS rats with higher level of LH and significant low level of FSH, thus disrobing FSH/LH ratio. But after treatment of AEGC LH surge was controlled and FSH levels were significantly improved in a dose dependent manner (Figure 7A and B). The gonadotrophin anomalies are exacerbated by disrupted ovarian-pituitary and hypothalamic feedback. HPA also triggers the PI3K/Akt pathway and causes the overexpression of the ovarian CYP17A1 gene and levels of the 17-hydroxylase enzyme, which synthesize androgens from progesterone.33,34 Thus androgen level disturbed and their assessment crucial during any PCOS model development and treatment period. In the recent study, estrogen and testosterone level was also disturbed in PCOS rats. 5 There was over production of testosterone and under production of estrogen. But after treatment with AEGC, we found a significant normalization in both the androgen serum concentration (Figure 7D and E). Similarly, there was also improvement in progesterone level with AEGC treatment (Figure 7C).
Hyperinsulinemia is caused by both peripheral insulin resistance and impaired pancreatic beta cell activity.1,5 PCOS runs in families, and a range of genetic defects tend to result in syndrome traits and account for the symptoms' variability. Environmental factors, such as diet, culture, and lifestyle, also have an impact on this syndrome’s manifestation.1,35 When we measured the serum insulin level, there was significant rise in serum insulin that depicted insulin resistance (IR). Metformin is the most commonly used drug that has very significant improvement of insulin resistance that is observed with lower level of serum insulin in metformin treated rats. Similarly, AEGC reduced the insulin level significantly (Figure 7F). In this study, metformin (20 mg/kg) was used as standard treatment for PCOS. It improves insulin sensitivity by reducing glucose intolerance and insulin resistance hence aiding in the management of PCOS symptoms.
Histopathological analysis of the ovarian cross section was also consistent with serum hormone level determination. Ovarian sections from the PCOS group showed cystic follicles and a disordered granulosa cell compartment with variable granulosa cell thickness, which is diagnostic of atretic antral follicles. Ovaries of rats treated with letrozole had a high number of cystic follicles but no corpus luteum. These alterations can be explained by lower FSH and higher testosterone levels in rats treated with letrozole (Figure 6B). However, a prominent decrease in the number and size of cystic follicles occurred after the administration of metformin, AEGC (100, 300, and 500 mg/kg) as shown in Figure 6C-F). The difference between the group is in dose dependent manner, the most prominent effects seen at dose 500 mg/Kg of AEGC and the least effect at 100 mg/kg.
PCOS being a metabolic abnormality always disturbed total cholesterol, TG, LDL, and HDL status. All of these lipid parameters were perturbed in PCOS rats, while metformin as well as AEGC restored lipid profile in all the treatment groups. But AEGC beneficial effects were in a dose dependent fashion (Figure 8). Furthermore, PCOS induction has a strong association with hyper oxidation status of the ovaries and is evident with lower ovarian tissue concentration of SOD, CAT, GSH in PCOS female rats. While higher level of MDA showing higher lipid per oxidation status of ovaries of PCOS rats. AEGC reduced the inflammatory conditions of the ovaries by enhancing the levels of all antioxidation enzymes and reduced the lipid peroxidation (Figure 9A-D). Liver is a vital organ and also disturbed by metabolic abnormalities. PCOS disturbed the liver function that is seen elevated level liver function markers (LFTs). AEGC ameliorated these changes with passage of time and LFTs gets normal (Table 5).
Garcinia cambogia a member of the family Clusiaceae has established therapeutic effects as an anti-diabetic, anti-oxidant, anti-inflammatory, anti-obesity, and hypolipidemic agent. Aqueous extract of Garcinia Garcinia cambogia has a very high concentration of flavonoids such as quercetin (251.44 μg/g), rutin (637.78 μg/g), kaempferol (17.03 μg/g), and have 67.89% DPPH inhibition at 100 ppm (Figure 1, Tables 2 and 3). All the disease management effects of AEGC might be due to presence of all these above discussed phytoconstituents. Quercetin is an anti-inflammatory, anti-apoptotic, antioxidant, and anticancer agent. Quercetin improves the antioxidant level by increasing the total plasma antioxidant concentration. The proposed mechanism behind this antioxidant effect is that quercetin supplementation decreases the level of anti-inflammatory cytokines TNFα (IL-10) and IL-8:IL-10 ratios. The fact is that more the level of basal cytokines, the more prominent inhibiting effect of cytokines will be observed. 36 Quercetin may be used for the regulation of reproductive system functions, including folliculogenesis, maturation of the oocyte, and ovulation. Quercetin can improve the antioxidant ability of the ovary by up-regulating the oxidative stress-related genes in rat. 37 In addition, Quercetin activates the nuclear factor Nrf2-ARE (erythroid-derived 2) like 2/Antioxidant response element path and induces the activity of phase II anti-oxidant enzymes like glutathione s-transferase, catalase, superoxide dismutase, NAD(P)H: quinone oxidoreductase 1, glutathione peroxidase (GPx), and thioredoxin. 38 Data from the earlier studies showed quercetin’s capacity to block the route of luteinizing hormone -derived androgen production. 33
Free fatty acids produce ROS due to their oxidation and are responsible for mitochondrial damage. While quercetin lessens the ability of mitochondria to oxidize (producing -oxidation and ATP). 32 Actually, quercetin reduces lipid peroxidation brought on by free fatty acids and increases mitochondrial oxidative capability. In this study, Garcinia cambogia raises HDL levels while lowering serum levels of TGs, LDL, and total cholesterol possibly due to the presence of quercetin.
Rutin, a naturally occurring flavone derivative, has significant anti-inflammatory properties. The aglycone of rutin is thought to be the mechanism by which it inhibits the activity of induced NO synthase, which prevents lipid peroxidation (iNOs). The active production of NO and superoxide anions is started by the action of iNOs on macrophages. Peroxynitrites are created when iNOs and free radicals react (high damage potential). They are successful in preventing low-density lipoprotein oxidation, which results in permanent damage to cell membranes. By binding NO, the rutin aglycone can stop this series of events. 39 Rutin also endorses ovarian cell steroidogenesis and overcomes their viability, propagation, and apoptosis. 40 PCOS patients have larger ovarian follicles with a thick layer of theca cells and fragmented granulosa cells that develop the cysts. Morphological changes in ovarian follicles appeared due to the higher level of testosterone and disturbed folliculogenesis.
Rutin decreased the testosterone level in serum and restored the folliculogenesis. 41 Apoptosis, angiogenesis, inflammation, and metastasis are all influenced by kaempferol. Thus, kaempferol is a flavonoid lipid molecule that also decreases lipid peroxidation. 42 In this study, aqueous extract of Garcinia cambogia increased CAT, GSH, and SOD levels and decreased MDA levels in treated rats, demonstrating the GC plant potential as an antioxidant possibly due to the presence of quercetin, rutin, and kaempferol. All the other phytochemicals except quercetin, rutin, and kaempferol present in AEGC such as benzoic acid, fumaric acid, chlorogenic also have a role in reducing lipid per oxidation status of ovaries, follicle development, serum androgens balance, insulin resistance, and lipid profile regulation. This study revealed that aqueous extract of Garcinia cambogia has the potential to manage polycystic ovarian syndrome in a dose dependent manner. It also opens a new era in which more research can be initiated on molecular level and further mechanistic studies can be initiated.
Conclusion
These findings of this study suggested that aqueous extract of Garcinia cambogia (AEGC) could ameliorate the PCOS in a dose dependent fashion. These beneficial effects of AEGC were also statistically comparable with metformin. Phytochemical present in AEGC have a role in producing these effects and the mechanism behind this phenomenon might be cure of lipid peroxidation, hormonal balance, improvement of insulin sensitivity, and lipid profile regulation.
Acknowledgments
We are thankful to Miksons healthcare SMS (Pvt.) Ltd. Rawat Rawalpindi, Pakistan providing aqueous extract of Garcinia cambogia for this research.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Saba Rana https://orcid.org/0000-0001-7719-9247
Liaqat Hussain https://orcid.org/0000-0001-7171-5917
Uzma Saleem https://orcid.org/0000-0002-1541-4236
References
- 1.Zeng L-H, Rana S, Hussain L, et al. Polycystic ovary syndrome: a disorder of reproductive age, its pathogenesis, and a discussion on the emerging role of herbal remedies. Front Pharmacol. 2022;13:874914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolf WM, Wattick RA, Kinkade ON, Olfert MD. Geographical prevalence of polycystic ovary syndrome as determined by region and race/ethnicity. Int J Environ Res Publ Health. 2018;15(11):2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hussain L, Aamir N, Hussain M, Asif M, Chauhdary Z, Manzoor F, Siddique R, Riaz M. Therapeutic Investigation of Standardized Aqueous Methanolic Extract of Bitter Melon (Momordica charantia L.) for Its Potential against Polycystic Ovarian Syndrome in Experimental Animals’ Model: In Vitro and In Vivo Studies. Evidence-Based Complementary and Alternative Medicine. 2002;1-14, 5143653. 10.1155/2022/5143653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nett TM, Turzillo AM, Baratta M, Rispoli LA. Pituitary effects of steroid hormones on secretion of follicle-stimulating hormone and luteinizing hormone. Domest Anim Endocrinol. 2002;23(1-2):33-42. [DOI] [PubMed] [Google Scholar]
- 5.Younas A, Hussain L, Shabbir A, Asif M, Hussain M, Manzoor F. Effects of Fagonia indica on Letrozole-Induced Polycystic Ovarian Syndrome (PCOS) in Young Adult Female Rats. Evid Based Complement Alternat Med. 2022;2022:1397060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel S. Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J Steroid Biochem Mol Biol. 2018;182:27-36. [DOI] [PubMed] [Google Scholar]
- 7.Coss D. Regulation of reproduction via tight control of gonadotropin hormone levels. Mol Cell Endocrinol. 2018;463:116-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diamanti-Kandarakis E, Kandarakis H, Legro RS. The role of genes and environment in the etiology of PCOS. Endocrine. 2006;30(1):19-26. [DOI] [PubMed] [Google Scholar]
- 9.Legro RS, Feingold KR, Anawalt B, et al. Evaluation and Treatment of Polycystic Ovary Syndrome. South Dartmouth (MA); Endocrine. 2015. [Google Scholar]
- 10.Norman RJ, Davies MJ, Lord J, Moran LJ. The role of lifestyle modification in polycystic ovary syndrome. Trends Endocrinol Metab. 2002;13(6):251-257. [DOI] [PubMed] [Google Scholar]
- 11.Iqbal SM, Hussain L, Hussain M, et al. Nephroprotective Potential of a Standardized Extract of Bambusa arundinacea: In Vitro and In Vivo Studies. ACS Omega. 2022;7(21):18159-18167. doi: 10.1021/acsomega.2c02047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bashir A, Asif M, Saadullah M, et al. Therapeutic Potential of Standardized Extract of Melilotus indicus (L.) All. and Its Phytochemicals against Skin Cancer in Animal Model: In Vitro, In Vivo, and In Silico Studies. ACS Omega. 2022;7(29):25772-25782. doi: 10.1021/acsomega.2c03053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hussain L, Akash MSH, Ain N, Qadir MI. Analgesic, anti-inflammatory and antipyretic activity of Salvia moorcroftiana. Pak J Pharm Sci. 2017;30(2):481-486. [PubMed] [Google Scholar]
- 14.Saadullah M, Arif S, Hussain L, Asif M, Khurshid U. Dose Dependent Effects of Breynia cernua Against the Paraquat Induced Parkinsonism like Symptoms in Animals’ Model: In Vitro, In Vivo and Mechanistic Studies. Dose Response. 2022;20(3):15593258221125478. doi: 10.1177/15593258221125478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asif M, Zafar M, Saleem M, et al. Evaluation of antidiabetic and wound healing properties of ethanol extract of Hedera nepalensis in alloxan-induced diabetic rats. South Afr J Bot. 2022;146:118-126. [Google Scholar]
- 16.Hussain L, Ikram J, Hanif M, et al. The effect of Argyrolobium roseum (Camb.) Jaub&Spach on some liver function biochemical parameters. Romanian Biotechnological Letters. 2014;19(6):10007. [Google Scholar]
- 17.Kashani L, Akhondzadeh S. Herbal medicine in the treatment of polycystic ovary syndrome. 2016;15(59):1-5. [Google Scholar]
- 18.Manzoor F, Nisa M-U, Shakoor A, Hussain L, Mahmood A, Younas A. Effect of sodium alginate supplementation on weight management and reproductive hormones in polycystic females. Food Funct. 2022;13:9847-9855. doi: 10.1039/D2FO01539K [DOI] [PubMed] [Google Scholar]
- 19.Semwal RB, Semwal DK, Vermaak I, Viljoen A. A comprehensive scientific overview of Garcinia cambogia. Fitoterapia. 2015;102:134-148. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed AH, Korkor AM, Mansour AM, Abbass HS, Mohammed Ael-S. Evaluation of antidiabetic and anti-obesity potential and safety of a polyherbal remedy. Al-Azhar J Pharm Sci. 2022;65(1):229-245. [Google Scholar]
- 21.Haber SL, Awwad O, Phillips A, Park AE, Pham TM. Garcinia cambogia for weight loss. Am J Health Syst Pharm. 2018;75(2):17-22. [DOI] [PubMed] [Google Scholar]
- 22.Shukla A, Shukla R, Pandey V, Golhani D, Jain C. In vitro antioxidant activity of Garcinia cambogia fruit. J Med Pharm Allied Sci. 2014;3(01):67-73. [Google Scholar]
- 23.Kiani R, Arzani A, Mirmohammady Maibody SAM. Polyphenols, flavonoids, and antioxidant activity involved in salt tolerance in wheat, Aegilops cylindrica and their amphidiploids. Front Plant Sci. 2021;12:646221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anokwuru C, Anyasor G, Ajibaye O, Fakoya O, Okebugwu P. Effect of extraction solvents on phenolic, flavonoid and antioxidant activities of three nigerian medicinal plants. Nature. 2011;9(7):53-61. [Google Scholar]
- 25.Nallathambi A, Bhargavan RJA. Regulation of estrous cycle by Cynodon dactylon in letrozole induced polycystic ovarian syndrome in Wistars albino rats. Anat Cell Biol. 2019;52(4):511-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kafali H, Iriadam M, Ozardalı I, Demir N. Letrozole-induced polycystic ovaries in the rat: a new model for cystic ovarian disease. Arch Med Res. 2004;35(2):103-108. [DOI] [PubMed] [Google Scholar]
- 27.Pineda M. Female reproductive system. McDonald's veterinary endocrinology and reproduction. 2003(5):283-340. [Google Scholar]
- 28.Rashidi Z, Khosravizadeh Z, Talebi A, Khodamoradi K, Ebrahimi R, Amidi F. Overview of biological effects of Quercetin on ovary. Phytother Res. 2021;35(1):33-49. [DOI] [PubMed] [Google Scholar]
- 29.Artini PG, Di Berardino OM, Simi G, et al. Best methods for identification and treatment of PCOS. Minerva Ginecol. 2010;62(1):33-48. [PubMed] [Google Scholar]
- 30.Kafali H, Iriadam M, Ozardalı I, Demir N. Letrozole-induced polycystic ovaries in the rat: a new model for cystic ovarian disease. Arch Med Res. 2004;35(2):103-108. [DOI] [PubMed] [Google Scholar]
- 31.Shi D, Vine DF. Animal models of polycystic ovary syndrome: a focused review of rodent models in relationship to clinical phenotypes and cardiometabolic risk. Fertil Steril. 2012;98(1):185-193. [DOI] [PubMed] [Google Scholar]
- 32.Kim C-S, Kwon Y, Choe SY, et al. Quercetin reduces obesity-induced hepatosteatosis by enhancing mitochondrial oxidative metabolism via heme oxygenase-1. Nutr Metab. 2015;12(1):33-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukuda S, Orisaka M, Tajima K, Hattori K, Kotsuji F. Luteinizing hormone-induced Akt phosphorylation and androgen production are modulated by MAP Kinase in bovine theca cells. J Ovarian Res. 2009;2(1):17-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang F, Zhang ZH, Xiao KZ, Wang ZC. Roles of Hypothalamic-Pituitary-Adrenal Axis and Hypothalamus-Pituitary-Ovary Axis in the Abnormal Endocrine Functions in Patients with Polycystic Ovary Syndrome. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2017;39(5):699-704. [DOI] [PubMed] [Google Scholar]
- 35.Venkatesan AM, Dunaif A, Corbould A. Insulin resistance in polycystic ovary syndrome: progress and paradoxes. Recent Prog Horm Res. 2001;56:295-308. [DOI] [PubMed] [Google Scholar]
- 36.Boots AW, Drent M, de Boer VC, Bast A, Haenen GR. Quercetin reduces markers of oxidative stress and inflammation in sarcoidosis. Clin Nutr. 2011;30(4):506-512. [DOI] [PubMed] [Google Scholar]
- 37.Pylypenko D, Gorbach T, Krasnopolsky Y. Study of antioxidant activity of liposomal forms of quercetin and curcumin in ischemic heart disease. Biotechnologia. 2020;101(4):273-282. [Google Scholar]
- 38.Maalik A, Khan FA, Mumtaz A, et al. Pharmacological applications of quercetin and its derivatives: a short review. Trop J Pharmaceut Res. 2014;13(9):1561-1566. [Google Scholar]
- 39.Yang J, Guo J, Yuan J. In vitro antioxidant properties of rutin. LWT - Food Sci Technol. 2008;41(6):1060-1066. [Google Scholar]
- 40.Sirotkin AV, Pelleova B, Fabova Z, Makovicky P, Alwasel S, Harrath AH. Rutin directly affects stimulatory action of FSH on the ovarian cell. PharmaNutrition. 2021;15:100247. [Google Scholar]
- 41.Mihanfar A, Nouri M, Roshangar L, Khadem-Ansari MHJRB. Polyphenols: Natural compounds with promising potential in treating polycystic ovary syndrome. Reprod Biol. 2021;21(2):100500. [DOI] [PubMed] [Google Scholar]
- 42.Singh R, Singh B, Singh S, Kumar N, Kumar S, Arora S. Anti-free radical activities of kaempferol isolated from Acacia nilotica (L.) Willd. Ex. Del. Toxicology In Vitro. 2008;22(8):1965-1970. [DOI] [PubMed] [Google Scholar]