Keywords: cystic kidney disease, autosomal dominant polycystic kidney disease, cardiac valve defect, cardiovascular disease, echocardiography, extrarenal manifestations, heart, left ventricular hypertrophy
Abstract
Key Points
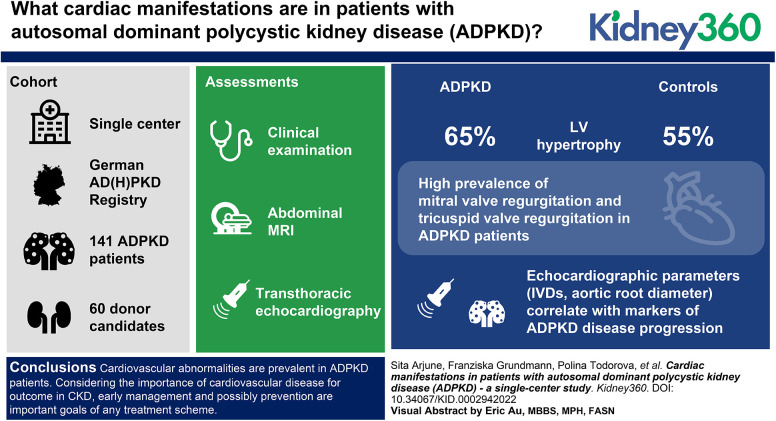
Cardiovascular disease—a key driver of morbidity in CKD—is common in patients with autosomal dominant polycystic kidney disease (ADPKD).
Pathologic echocardiography findings, including valvular defects, aortic root dilation, and hypertrophy, are found in most patients with ADPKD.
These findings correlate with parameters indicating disease progression in ADPKD. Echocardiography should be offered to all patients with ADPKD.
Background
ADPKD is the most common monogenetic kidney disease and results in kidney failure in >75% of affected individuals. As a systemic disorder, ADPKD is associated with a variety of extrarenal manifestations, including cardiac manifestations, that affect the majority of patients. We characterized the cardiac involvement in patients with ADPKD from the German AD(H)PKD registry and compared them with kidney donor candidates as controls.
Methods
In this single-center cohort study, we evaluated 141 patients with ADPKD (44.17±11.23 years) from the German AD(H)PKD registry and 60 kidney donor candidates (55.08±10.21 years). All patients underwent clinical examination, abdominal MRI, and transthoracic echocardiography.
Results
Of the patients with ADPKD, 65% showed hypertrophy of the left ventricle (as defined by an end-diastolic interventricular septal wall thickness [IVSd] >10 mm) compared with 55% in control patients. Mitral regurgitation was the most common finding among 54% of patients with ADPKD who exhibited valvular dysfunction, albeit mild in most patients. Interestingly, left ventricular ejection fraction (LV-EF) differed significantly between both groups, with higher values in patients with ADPKD (64%±6% versus 60%±6%), whereas other parameters, including IVSd, left ventricular end-diastolic diameter (LVEDD), tricuspid annular plane systolic excursion (TAPSE), and pressure gradients across the aortic and tricuspid valve were similar between groups. Correlations of echocardiographic parameters with markers of disease progression revealed statistically significant associations for aortic root diameter (P=0.01), the pressure gradient across the aortic valve (AV dPmax; P=0.0003), and IVSd (P=0.0001), indicating rapid kidney disease progression may also be associated with cardiac findings.
Conclusion
Cardiovascular abnormalities are prevalent in patients with ADPKD. Considering the importance of cardiovascular disease for outcomes in CKD, early management and possibly prevention are important goals of any treatment scheme. Consequently, echocardiography should be offered to all patients with ADPKD in routine management.
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is a chronic, progressive, multisystem disorder with a significant disease burden.1,2 More than 50% of patients with ADPKD reach kidney failure by the age of 60. ADPKD has a genetic prevalence of 1:1000, making it the most common monogenetic kidney disease and being the reason for 5%–10% of all cases of KRT.
ADPKD is characterized by the age-dependent growth of renal cysts and is genetically heterogeneous. Two genes are responsible for most mutations resulting in ADPKD: PKD1, which encodes polycystin-1 (approximately 85% of cases); and PKD2, which encodes polycystin-2 (approximately 15% of cases).3 Further rare cases are explained by variants in other genes.4 Truncating PKD1 variants are associated with more rapid disease progression in contrast to PKD2 and nontruncating PKD1 variants.5 Although enlarged cystic kidneys are the most obvious and loss of kidney function the most severe phenotype of ADPKD, in the vast majority of patients, cardiovascular complications are the primary cause of morbidity and mortality.6 Approximately 60% of patients develop arterial hypertension before a significant decline in eGFR can be detected.7 Patients with ADPKD develop hypertension approximately a decade earlier than the general population.8 Although the mechanism underlying arterial hypertension in ADPKD is not fully understood, it is believed that upregulation of the renin-angiotensin-aldosterone system (RAAS) due to cystic compression of the renal vasculature is a critical factor. This activation of the RAAS is also expected to contribute to vascular resistance and cardiac hypertrophy.9,10 The early onset of hypertension in ADPKD triggers left ventricular (LV) hypertrophy,11 a significant cardiovascular risk factor, and further LV diastolic dysfunction.12 Thus, the RAAS pathway has been the target of several clinical trials (e.g., HALT PKD study) and the benefits of stringent BP targets have been demonstrated.8,13
As a systemic disease, ADPKD is associated with several extrarenal manifestations, including cysts in the liver and, less frequently, the pancreas, seminal vesicles, and the arachnoid membrane.14 Beyond the classic cardiovascular consequences of CKD, such as coronary artery disease and heart failure, ADPKD itself results in a genetically defined increased risk of cardiovascular manifestations. These include both more severe developmental defects, like Fallot tetralogy, and—much more frequently—more subtle phenotypes presenting in adulthood, like cardiac valve defects and dilation of the aortic root.15,16 Moreover, aneurysms of several localizations, including intracranial vessels, are more common in patients with ADPKD, and cardiomyopathy and pericardial effusion have been described in association with this disease.15,17,18
Knowing the association between ADPKD, LV hypertrophy (LVH), and increased cardiovascular risk resulting in cardiovascular complications being the most common cause of death in patients with ADPKD, early management and possibly prevention are important goals of any therapy scheme. Hence, our study explores the frequency of cardiac manifestations in a subgroup of patients with ADPKD from the German AD(H)PKD registry.
Materials and Methods
Patients and Data Extraction
In this cohort study, clinical data of 141 adult (≥18 years) patients with ADPKD enrolled in the AD(H)PKD registry were analyzed. Additionally, 60 kidney donor candidates that had undergone echocardiography from November 2015 to June 2017 were identified retrospectively and included in the analysis as controls. The AD(H)PKD study enrolled adult patients with ADPKD who presented for evaluation of tolvaptan therapy. Patient data were documented longitudinally and included clinical, genetic, and laboratory parameters and radiologic examinations to determine renal volume. Data collection from this cohort was approved by the local institutional review board of the University of Cologne, and written informed consent was obtained from all study participants. The study is registered on clinicaltrials.gov (NCT02497521). The cohort study is conducted in accordance with the Declaration of Helsinki and the good clinical practice guidelines by the International Conference on Harmonization. Approximately 900 participants (as of January 2022) have been enrolled in Cologne, Germany, with a projected follow-up time of 10 years. In a database on clinicalsurveys.net, all relevant clinical data and imaging parameters are collected annually (>2300 variables).
For the subpopulation described here, inclusion and exclusion criteria included the diagnosis of ADPKD, typical renal phenotype of ADPKD (Mayo class 1),19 and availability of transthoracic echocardiography (TTE) performed at the University Hospital Cologne (Cologne, Germany). Patients with severe primary LV dysfunction, as defined by an LV ejection fraction (LV-EF) of <30% (n=3) were excluded from the study. These patients were excluded because severe EF impairment is a complex disease entity with additional associated heart anomalies, which would significantly bias the results of our study. All participants were White.
Echocardiographic Examination Protocol and Equipment
At the University Clinic Cologne’s Heart Center (UHC), all patients underwent standard TTE in the left lateral decubitus position under continuous electrocardiogram recording using either a Philips IE33 or a GE Vivid e95 ultrasound system. Recorded images and loops included parasternal long- and short-axis views; apical four-, two-, and three-chamber views; and subcostal views. Two-dimensional images, Doppler, and pulsed tissue Doppler recordings were acquired and interpreted in accordance with the European and American Society of Cardiology echocardiography guidelines.20,21 Standard parameters, as detailed in the Results section, were obtained. Echocardiographic images were acquired in the echocardiographic laboratories of a tertiary care university hospital by specifically trained personnel under constant supervision of a board-certified cardiologist (certified by the European Society of Cardiovascular Imaging). The images were analyzed by a board-certified cardiologist or by two independent readers who were under constant supervision of a board-certified cardiologist. The Bland–Altman plot for interpretation of interobserver reproducibility can be found in Supplemental Figure 1.
Statistical Analysis
Baseline patient characteristics are reported as mean±SD for normal distributions and median for skewed distributions. Data were tested for normality using the D’Agostino and Pearson test. The P values for statistical difference were computed using the unpaired t test and ANOVA. Statistical significance was set at P<0.05. Echocardiographic parameters were analyzed for their distribution in different patient groups (e.g., age, Mayo class).
Results
Study Population
When defining the study cohort, the AD(H)PKD registry contained 900 patients with ADPKD, 166 (92 men and 74 women) of which had undergone echocardiography at UHC. A total of 141 adult patients with ADPKD were selected after excluding patients with severe primary LV dysfunction, as defined by an LV-EF of <30% (n=3) and/or poor image quality (n=22) (Figure 1). The mean±SD age of patients with ADPKD was 44.17±11.23 (range, 18–67) years. Relevant comorbidities included arterial hypertension (n=129, 91%), hypercholesterolemia (n=48, 34%), and a history of smoking (n= 71, 50%). Patient history revealed pericardial effusion in 12 patients. In addition, 60 kidney donor candidates (31 men and 29 women) were included as controls in the analysis. All controls had an EF >30%. The mean age of the control group was higher than in the ADPKD cohort (55.31±10.04 years, range 29–78 years). Relevant comorbidities included arterial hypertension (n=20, 33%), hypercholesterolemia (n=32, 53%), and history of smoking (n=24, 39%). The baseline characteristics of both cohorts are summarized in Table 1.
Figure 1.
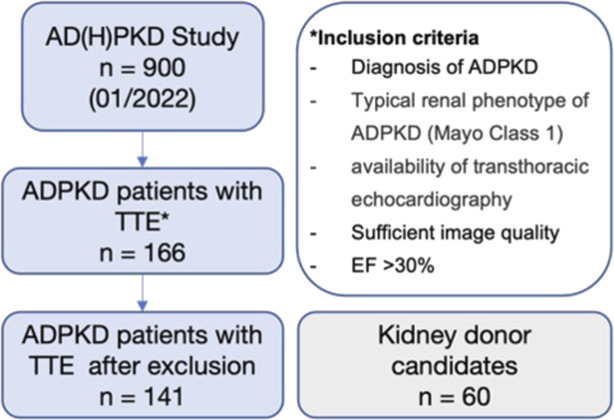
Study flowchart illustrating patient flow and inclusion criteria. ADPKD, autosomal dominant polycystic kidney disease; EF, ejection fraction; TTE, transthoracic echocardiography.
Table 1.
Baseline characteristics of Autosomal Dominant Polycystic Kidney Disease cohort and retrospective kidney donor candidate cohort used as controls
| Characteristics | Autosomal Dominant Polycystic Kidney Disease | Controls | Chi-Squared P Value |
|---|---|---|---|
| Men, n (%) | 141 (45%) | 60 (52%) | |
| Age (yr), mean±SD | 44.17±11.23 | 55.31±10.04 | |
| eGFR (ml/min per 1.73 m2), mean±SD | 70.05±28.68 | 92.42±12.14 | |
| TKV (ml), mean±SD | 1728±1220 | — | |
| Without baseline MRI, n | 1 | 60 | |
| Mayo classification, n | 140 | — | |
| 1A | 1 | — | |
| 1B | 36 | — | |
| 1C | 51 | — | |
| 1D | 41 | — | |
| 1E | 12 | — | |
| 2 | — | — | |
| CKD stage, n | 141 | 60 | |
| 1 (eGFR ≥90 ml/min per 1.73 m2) | 37 | 37 | |
| 2 (eGFR 60–89 ml/min per 1.73 m2) | 45 | 23 | |
| 3 (eGFR 30–59 ml/min per 1.73 m2) | 55 | — | |
| 4 (eGFR 15–29 ml/min per 1.73 m2) | 4 | — | |
| 5 (eGFR <15 ml/min per 1.73 m2) | — | — | |
| Comorbidities, n (%) | |||
| Arterial hypertension | 129 (91) | 20 (33) | <0.001 |
| Hypercholesterolemia | 48 (34) | 32 (53) | 0.01 |
| Ever smoker | 71 (50) | 24 (39) | NS |
| Active smoker | 32 (23) | 9 (15) | NS |
TKV, total kidney volume; MRI, magnetic resonance imaging; NS, not significant.
TTE
Echocardiographic parameters were collected and evaluated systematically from both the ADPKD group and the control cohort. First, the distribution of cardiac valve defects was assessed. Although only 4% (n=6) of patients demonstrated mitral valve prolapse, 63% showed predominantly mild mitral valve regurgitation (n=89, of which 88 were mild), and 62% had tricuspid valve (TV) regurgitation (n=88, of which 87 were mild) (Figure 2). In the control cohort, only one mild TV regurgitation was recorded (2%, data not shown), and no other cardiac valve defects were detected.
Figure 2.
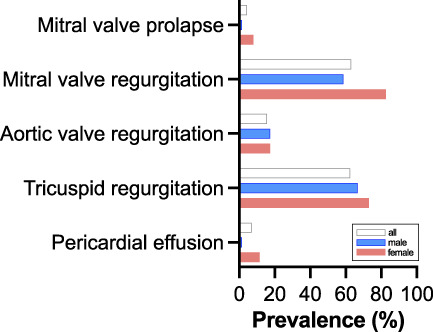
Prevalence of cardiac valve defects and pericardial effusion in patients with ADPKD.
Interestingly, LV-EF was significantly higher in the ADPKD cohort (64%±6% versus 60%±6%; Figure 3). This finding did not appear to be driven by age because age and LV-EF did not correlate (Supplemental Figure 2). Other parameters, such as end-diastolic interventricular septal wall thickness (IVSd), LV end-diastolic diameter (LVEDD), aortic root diameter, tricuspid annular plane systolic excursion (TAPSE), pressure gradients across the aortic valve (AV) and TV, and left arterial volume were within the range of the non-ADPKD controls (Supplemental Figure 3).
Figure 3.
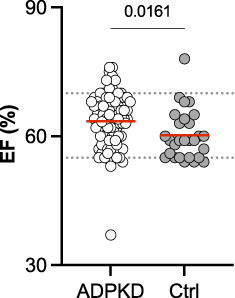
Comparison of left ventricular EF of patients with ADPKD versus controls. Mean values for patients with ADPKD (64%) and controls (Ctrl; 60%) are indicated by red lines.
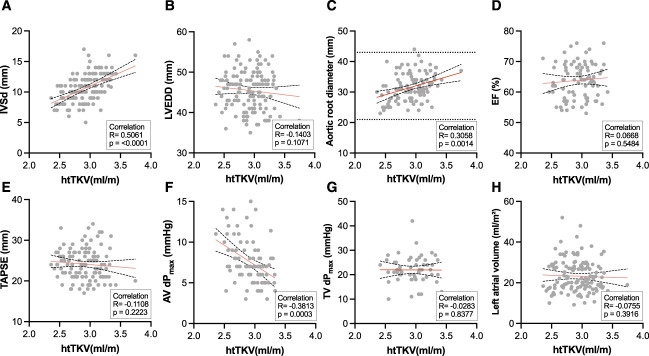
We then went on to characterize the echocardiographic results in relation to total kidney volume (TKV) as a key biomarker in ADPKD. When correlating the same parameters with height-adjusted TKV (htTKV), significant correlations were found for aortic root diameter (P=0.01), pressure gradient across the AV (AV dPmax, P=0.0003), and IVSd (P≤0.001) (Figure 4).
Figure 4.
Correlation of htTKV and echocardiographic parameters. Pearson correlation was conducted after the D’Agostino and Pearson test revealed parametric data distribution. The red line indicates simple linear regressions; black dotted lines indicate the 95% CI. AV, aortic valve; dPmax, pressure gradient; IVSd, end-diastolic interventricular septal wall thickness; LVEDD, left ventricular end-diastolic diameter; htTKV, height-adjusted TKV; TAPSE, tricuspid annular plane systolic excursion; TV, tricuspid valve.
Additionally, all parameters were investigated in relation to further clinical characteristics associated with disease progression in ADPKD: sex, eGFR, and respective CKD stage, Mayo class,19 genetics, and comorbidities/risk factors (smoking history, arterial hypertension, urologic complications). In this cohort, human genetic testing for possible mutations in ADPKD-associated genes had been performed for 131 of the 141 patients with ADPKD.
In particular, considering the clinical importance and the correlation of IVSd with htTKV shown in Figure 4, concentric myocardial hypertrophy was assessed in more detail (Figure 5). A total of 93 patients with ADPKD (66%) had IVSd value >10 mm and were thus classified as having LV myocardial hypertrophy compared with 33 (55%) in the control cohort. IVSd values were significantly higher in the men of both groups (11.55±2.18 mm for men with ADPKD versus 10.82±1.65 mm for men in the control group). A subgroup analysis for differences between the sexes both patients with ADPKD and controls can be found in Supplemental Figure 4. Furthermore, IVSd negatively correlated with eGFR (Figure 5B); therefore, a larger IVSd was found in higher stages of CKD (Figure 5C). Additionally, patients with ADPKD who were classified as Mayo class 1C–1E had a larger IVSd than those classified as Mayo class 1A–1B (Figure 5D). IVSd also had a positive correlation with age in both patients with ADPKD and in controls (Figure 6). Considering the age differences in the two groups, we performed subgroup analyses of patients below and over the age of 50 years (Supplemental Figure 5) and of comorbidities (Supplemental Figure 6). These subgroups showed a tendency, not reaching statistical significance, toward a higher IVSd in patients with ADPKD.
Figure 5.
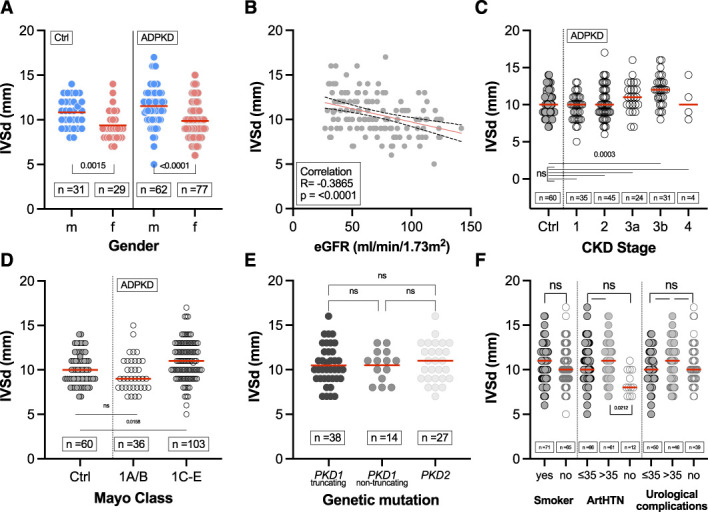
Association of end-diastolic interventricular septal wall thickness (IVSd) with clinical characteristics. (A) Sex distribution of IVSd in patients with ADPKD (mean±SD of 11.55±2.18 mm for men and 9.88±1.97 mm for women) and controls (mean±SD of 10.82±1.65 mm for men and 9.38±1.70 mm for women). (B) Pearson correlation of IVSd and eGFR in patients with ADPKD. (C) IVSd according to CKD stages 1–4 in patients with ADPKD (mean±SD of 9.66±1.78 mm for CKD1, 10.27±2.30 mm for CKD2, 11.04±2.12 mm for CKD3a, 11.93±1.98 mm for CKD3b, 10.50±2.65 mm for CKD4) versus controls (mean±SD of 10.13±1.81 mm). (D) IVSd according to Mayo classification (1A/B [mean±SD of 9.53±1.34 mm] versus 1C/D/E [mean±SD of 11.01±2.19 mm]) in patients with ADPKD versus controls (mean±SD of 10.13±1.81 mm). (E) IVSd in patients with ADPKD among respective gene products (PKD1 and PKD2). Truncating mutations included nonsense, frameshift, splicing mutations, and large rearrangements, whereas nontruncating mutations included missense mutations and in-frame short deletions and insertions. (F) IVSd for smokers and relevant comorbidities (for ArtHTN: mean±SD of 10.63±2.28 mm for ≤35 years, 11.02±2.86 mm for >35, 8.58±1.38 mm for no ArtHTN). Horizontal red lines indicate mean values. ArtHTN, arterial hypertension.
Figure 6.
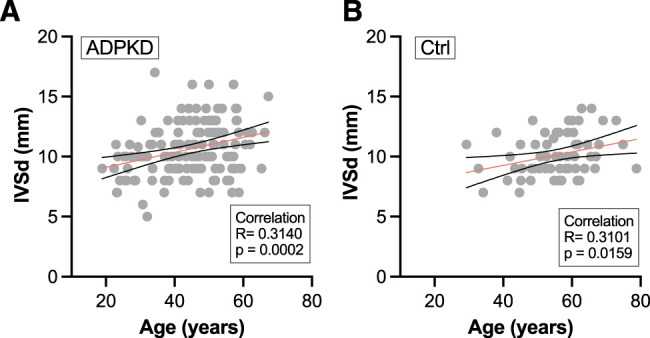
Correlation of IVSd with age in (A) patients with ADPKD and (B) controls. Pearson correlation was conducted after D’Agostino and Pearson test revealed parametric data distribution. The red line indicates simple linear regressions; black dotted lines indicate the 95% CI.
Because some clinical factors are associated with each other (e.g., GFR and age) we performed a multiple linear regression of a model using age, sex, eGFR, and TKV to predict IVSd, resulting in an adjusted R2 of 0.33 (Table 2).
Table 2.
Multiple linear regression models for IVSd using age, sex, eGFR, and total kidney volume (model 1)
| Variable | Estimate | SEM, 95% Confidence Interval (Asymptotic) | |t| | P Value |
|---|---|---|---|---|
| Intercept | 9.004 | 1.454, 6.13 to 11.88 | 6.194 | <0.001 |
| Age | 0.03485 | 0.01974, −0.004 to 0.07 | 1.765 | 0.08 |
| Sex (female) | −1.170 | 0.3313, −1.83 to −0.51 | 3.532 | 0.0006 |
| TKV | 0.0007157 | 0.0001908, 0.0003 to 0.001 | 3.750 | 0.0003 |
| eGFR | −0.007450 | 0.008377, −0.02 to 0.009 | 0.8894 | 0.38 |
Model 1: IVSd=age+sex+TKV+eGFR. Adjusted R2=0.33. For the variable Mayo class, classes were grouped as Mayo class 1A/B and 1C/D/E. |t|, t value, TKV, total kidney volume; IVSd, interventricular septal wall thickness.
Whereas age and eGFR were NS for prediction of IVSd in a model, TKV and sex were highly significant (P=0.0003 and P=0.0006, respectively).
Because aortic root diameter is a predictor of aortic complications, such as aneurysm or dissection, and because of its correlation with htTKV, the diameter of the aortic root was further analyzed (Figure 7).
Figure 7.
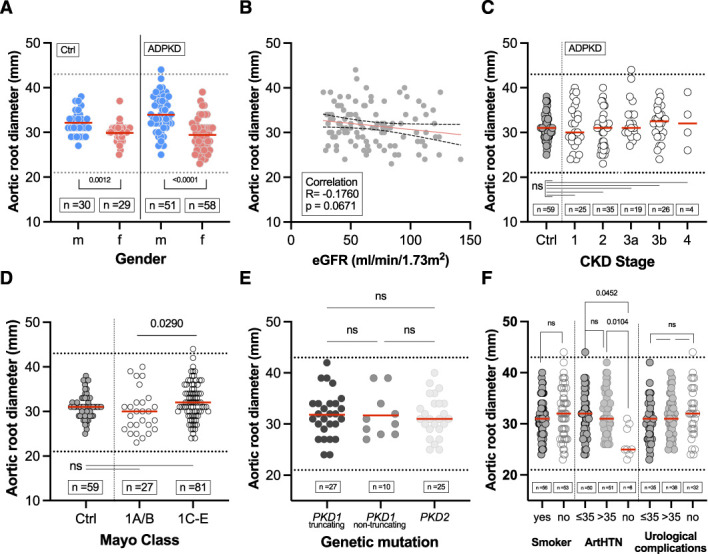
Association of aortic root diameter with clinical characteristics. (A) Sex distribution of aortic root diameter in patients with ADPKD (mean±SD of 33.96±4.06 mm for men and 29.45±3.52 mm for women) and controls (mean±SD of 32.13±2.75 mm for men and 29.90±2.42 mm for women) by sex. (B) Pearson correlation of aortic root diameter and eGFR in patients with ADPKD. (C) Aortic root diameter according to CKD stages 1–4 in patients with ADPKD (mean±SD of 31.40±4.92 mm for CKD1, 30.49±4.17 mm for CKD2, 32.79±4.59 mm for CKD3a, 32.15±3.79 mm for CKD3b, 32.25±5.56 mm for CKD4) versus controls (mean±SD of 31.03±2.74 mm). (D) Aortic root diameter according to Mayo classification (1A/B [mean±SD of 30.04±4.78mm] versus 1C/D/E [mean±SD of 32.15±4.12 mm]) in patients with ADPKD versus controls (mean±SD of 31.03±2.74 mm). (E) Aortic root diameter in patients with ADPKD among respective gene products (PKD1 and PKD2). Truncating mutations included nonsense, frameshift, splicing mutations, and large rearrangements, whereas nontruncating mutations included missense mutations and in-frame short deletions and insertions. (F) Aortic root diameter according to (ever) smokers and relevant comorbidities (for ArtHTN, mean±SD of 31.58±4.34 mm for ≤35 years, 32.33±4.16 mm for >35, 26.50±3.024 mm for no ArtHTN). Horizontal red lines indicate mean values. ArtHTN, arterial hypertension.
The normal range for the diameter of the aortic root is 21–43 mm,22 which was observed in both patients with ADPKD and controls. Only one male patient with ADPKD had a greater value and was thus classified as having aortic root dilation. As expected, male patients had significantly larger values, although there were only minor differences between the two cohorts (Figure 7A). The subgroup analysis for differences between sexes for patients with ADPKD and controls can be found in Supplemental Figure 7. No significant correlation was detected between the aortic root diameter and eGFR (Figure 7B). No significant differences were observed between individual CKD stages or in comparison with the control group (Figure 7C). Significant differences existed between Mayo classes 1A/1B and 1C–1E, but not between controls and either of the classes (Figure 7D). With regard to aortic root diameter, no difference was found between both gene products associated with the ADPKD phenotype (Figure 7E). Patients with ADPKD without arterial hypertension had a significantly smaller aortic root diameter; however, only a small group was available for testing (n=6; Figure 7F). When correlating aortic root diameter with age in both groups, a significant correlation was found (Supplemental Figure 8). As with IVSd, there were larger values in the ADPKD cohort that did not reach statistical significance when subgroup analyses were performed for patients under and over 50 years of age. A further subgroup analysis was performed for comorbidities (Supplemental Figure 9).
To further elucidate the relationship between aortic root diameter and other factors of disease progression in ADPKD, we performed a multiple linear regression using a model containing age, sex, eGFR, and TKV to predict aortic root diameter. This resulted in an adjusted R2 of 0.37 (Table 3).
Table 3.
Multiple linear regression models for aortic root diameter using age, sex, eGFR, and total kidney volume (model 2)
| Variable | Estimate | SEM, 95% Confidence Interval (asymptotic) | |t| | P Value |
|---|---|---|---|---|
| Intercept | 25.16 | 3.100, 19.01 to 31.31 | 8.117 | <0.001 |
| Age | 0.1401 | 0.04484, 0.05 to 0.23 | 3.124 | 0.002 |
| Sex (female) | −4.457 | 0.7489, −5.94 to −2.97 | 5.952 | <0.001 |
| TKV | 0.0005247 | 0.0003174, −0.0001 to 0.001 | 1.653 | 0.10 |
| eGFR | 0.02251 | 0.01805, −0.01 to 0.06 | 1.247 | 0.22 |
Model 2: Aortic root diameter∼age+sex+TKV+eGFR. Adjusted R2=0.37. |t|, t value, TKV, total kidney volume.
For prediction of aortic root diameter, only age (P=0.002) and sex (P<0.001) were significant for the model containing TKV.
Additionally, measurements of LVEDD were routinely taken to detect LV dilation (Supplemental Figure 10). In three patients, the left ventricle was classified as dilated, whereas most patients with ADPKD (n=138, 98%) had an LVEDD within the normal range. As expected, women in both groups had a significantly lower LVEDD (for patients with ADPKD, 47.82±4.55 mm [men] and 43.76±4.66 mm [women] versus 46.60±3.87 mm [men] and 42.59±3.62 mm [women] for controls; Supplemental Figure 10A). No other clinical characteristic examined showed a statistically significant association with LVEDD (Supplemental Figure 10, B–F).
To further assess LV systolic function, the LV-EF was assessed in relation to clinical characteristics (Supplemental Figure 11). Nearly all patients with ADPKD were classified as having a globally good systolic function (n=140, 99%), whereas only one patient had a moderately reduced LV systolic function. Patients with ADPKD who had an EF <30% (n=3) were excluded from the analysis. No wall motion abnormalities were detected. Neither sex, CKD stage, Mayo class, genetics, nor comorbidities showed a significant association with EF. We assessed the LV diastolic function using E/A ratio (the ratio between early filling velocity (E) and and filling velocity during atrial contraction) and E/lat e′ (max. velocity of the lateral mitral valvular annulus during early diastole) (Supplemental Figure 12) and the right ventricular basal diameter as a marker for right ventricular diastolic function (Supplemental Figure 13), and we found no statistically significant differences between patients with ADPKD and control subjects.
Additionally, the association between clinical characteristics and TAPSE as a surrogate of global right ventricular systolic function was examined (Supplemental Figure 14). A value >16 mm was considered normal,23 which was observed in all patients and controls in the cohort. As with LV-EF, no significant differences in sex, CKD stage, Mayo class, genetics, or comorbidities were observed for TAPSE between patients with ADPKD and controls. Only patients with ADPKD who developed arterial hypertension before the age of 35 had a significantly higher TAPSE than those who developed arterial hypertension after the age of 35. The right ventricle was classified by a board-certified cardiologist (using guidelines of the European and American Society of Cardiology echocardiography) as normal sized in 96% (n=136), as borderline in 1% (n=2), and dilated in 1% (n=3) of the 141 patients.23 Similarly, the right atrium was classified as normal sized in 91% (n=121) and dilated in 9% (n=12) of 133 tested patients. Similarly, we investigated the maximum pressure gradient across the AV (Supplemental Figure 15) and the TV (Supplemental Figure 16). On average, women had a greater pressure gradient across the AV in both groups, but this difference was only significant in patients with ADPKD; none of the other clinical characteristics examined were significantly different between groups.
With regards to left atrial volume, none of the clinical characteristics examined were significantly different between groups (Supplemental Figure 17). In 122 patients (87%), the left atrial volume was classified as normal, whereas it was dilated in 19 patients (13%). Patients with ADPKD without arterial hypertension consequently had a lower left atrial volume; however, this was NS.
Discussion
Cardiac hypertrophy and left ventricular dysfunction are caused by RAAS activation resulting in arterial hypertension as well as other mechanisms, including the decline in renal function, increased sympathetic nervous system activity, insulin resistance, disturbances in the fine-tuning of vascular tone.8 Clinical studies have established the importance of LVH in ADPKD.9,11,24 Understanding the relationship between ADPKD, LVH, and increased cardiovascular risk, and the fact that cardiovascular complications are the leading cause of death in patients with ADPKD, early management, and possibly prevention, are critical goals of any therapy scheme. ADPKD has also been reported to be associated with congenital heart defects. However, the precise pathways and interactions involved remain largely unknown.25 We examined cardiac manifestations in a subset of 141 patients with ADPKD participating in the German AD(H)PKD registry who underwent clinical examination, abdominal magnetic resonance imaging, and TTE to determine the frequency, characteristics, and potential correlations of cardiac manifestations of the disease. Echocardiographic parameters, assessing both left and right ventricular cardiac function, were considered and compared with a control cohort of 60 kidney donor candidates. Initially, the prevalence of cardiac valve defects in patients with ADPKD was determined using echocardiography. Whereas other studies reported a lower prevalence of cardiac valve defects,16,26,27 we found predominantly mild mitral valve regurgitation in 63% of patients, and mild TV regurgitation in 62% of patients in this study (see Table 4).
Table 4.
Distribution of cardiac valve defects of this cohort in comparison to the literature
| Mitral Valve | Tricuspid Valve | Aortic Valve | |||||
|---|---|---|---|---|---|---|---|
| Study | Subjects (n) | Year | Regurgitation (%) | Prolapse (%) | Regurgitation (%) | Prolapse (%) | Regurgitation (%) |
| This study | 141 | 2022 | 63 | 4 | 62 | — | 16 |
| Leier et al. (28) | 11 | 1984 | 27 | — | — | — | — |
| Hossack et al. (26) | 163 | 1988 | 31 | 26 | 15 | 6 | 8 |
| Timio et al. (27) | 228 | 1992 | 30 | 25 | — | 5 | 19 |
| Varnero et al. (29) | 21 | 1992 | 23 | 33 | — | 18 | 9 |
| Castiglioni et al. (30) | 25 | 1995 | 5 | 4 | 4 | — | 1 |
| Lumiaho et al. (16) | 109 (PKD1 only) | 2001 | 13 | 26 | 4 | — | — |
Interestingly, we found a significantly higher prevalence of mitral regurgitation than previously reported, although we did not observe mitral prolapse frequently. However, advances in echocardiography in recent years must be considered when directly comparing prevalence in this and earlier studies, especially when detecting minor valvular regurgitations. Although mitral valve prolapse is a characteristic finding in patients with ADPKD, mitral regurgitation can also occur because of hypertension, which was present in 91% of patients. Although most valvular findings were mild, progression of valve disease may occur over years. In any case, the high prevalence again confirms the importance of considering cardiac valve defects in patients with ADPKD.
Regarding LV systolic function, our control cohort showed a significantly lower mean EF (60% versus 64%) than that of patients with ADPKD, despite values being within the normal range. It is important to note that few patients with an EF <30% were excluded and patients with ADPKD were, on average, older than the controls. However, the result was significant despite a clear outlier with a low EF in the ADPKD group. Therefore, this remains an interesting finding that could be linked to the increased RAAS activity associated with ADPKD and, eventually, to activation of the sympathetic nervous system. There was no significant difference regarding EF in relation to stages of disease progression (CKD stage) and rapid or slow disease progression (Mayo classification).
Echocardiography was further used to determine the diameter of the aortic root. The aortic root forms the bulbar part of the ascending aorta and is a typical site for dilation and thus the formation of aortic aneurysms in the context of various diseases (e.g., Marfan syndrome). In addition, AV regurgitation often occurs concomitantly.28 The fact that overall aortic root diameter did not differ between patients with ADPKD and controls may be explained by the age dependency of this parameter. However, a significant correlation was found between aortic root diameter and htTKV, indicating disease progression actively causes, or is associated with, dilation of the aortic root. This is an important finding because it may explain cardiovascular morbidity and mortality in this high-risk cohort of patients.
Chapman et al.11 defined LVH as the maximum LVMI (LV Mass index) plus 2 SDs. This resulted in male patients with ADPKD having an upper limit of 129.22 g/m2 and female patients with ADPKD having an upper limit of 111.18 g/m2. Using these criteria, 2% (n=3, one male and two female patients) of the 128 patients from whom we could obtain LV mass were deemed to have LVH (Supplemental Figure 18A). Using the same methodology as Perrone et al.,13 who defined the normal upper limit as the 95th percentile, the result would be 122.3 g/m2 for male patients and 104.2 g/m2 for female patients with ADPKD. Of the 128 patients from whom we could obtain LV mass, 5% (n=7, three male and four female patients) would be considered to have LVH using these thresholds (Supplemental Figure 18B). Thus, our findings are consistent with both studies. In addition, Oflaz et al.31 reported significant biventricular diastolic dysfunction in both normotensive and hypertensive patients with ADPKD who have preserved renal function. In accordance with the findings of Oflaz et al.,31 we added the interpretation of LV diastolic function using E/A and E/lat e′ (Supplemental Figure 12) and further examined right ventricular basal diameter (Supplemental Figure 13) as a marker for right ventricular diastolic function and found no significant differences between patients with ADPKD and control subjects. Our results for E/A (mean±SD of 1.13±0.36 for controls versus 1.22±0.36 for patients with ADPKD) are in line with Oflaz et al.31 (mean±SD of 1.49±0.21 for controls versus 1.31±0.29 for patients with ADPKD). However, E/lat e′ values are not reported, so our results cannot be directly compared with those of Oflaz et al.31 because different methods were used to determine diastolic dysfunction.
IVSd, aortic root diameter, and the pressure gradient across the AV showed a significant correlation with htTKV, indicating that parameters associated with rapid disease progression in ADPKD may also have an effect on the left ventricle. When analyzing the comorbidities arterial hypertension and hypercholesteremia, a significant difference was only found for arterial hypertension for both IVSd and aortic root diameter in the ADPKD cohort. These findings regarding arterial hypertension are consistent with a previous report by Pietrzak-Nowacka et al.,32 who discovered an increase in IVSd and aortic root diameter in hypertensive patients with ADPKD compared with normotensive patients with ADPKD. Also, Mayo classes 1C–1E showed a larger IVSd than Mayo classes 1A–1B, again indicating an association with disease severity. This was validated when age, sex, eGFR, and TKV were included in a multiple linear regression model to predict IVSd, confirming sex and TKV as independent predictors.
Although polycystin 1 and 2 are essential for the development of the heart,33,34 interestingly, genetics did not show a clear effect on either of the tested echocardiographic parameters in this study. Specifically, polycystin 1 has been shown to regulate L-type calcium channel protein levels and cardiomyocyte contractility in a mouse model, implying this protein plays a critical role in cardiac function,35 whereas reduced polycystin 2 activity in ADPKD resulted in patients being more prone to develop idiopathic dilated cardiomyopathy.33 Furthermore, a recent study investigated PKD2 mutant mice that are characterized by cardiac fibrosis in combination with systolic and diastolic dysfunction.36 Taken together, clinical and experimental data provide compelling evidence that the cardiovascular comorbidity in ADPKD is caused, in part, by the mutant proteins’ primary manifestations. The fact that we did not see a correlation with mutation class may partly be explained by the limited availability of genotype in our cohort. Factors that drive disease progression in ADPKD, independent from mutation type, may also affect cardiac manifestations. This possibility is underlined by the relation between the latter and TKV/Mayo classification. Of course, loss of kidney function also contributes to cardiovascular disease in ADPKD.
In conclusion, our study underlines the cardiac involvement in ADPKD and delineates its association with factors involved in renal disease progression. Most of the detected changes are potentially subclinical; however, the findings are still crucial to understand the cardiac phenotype in ADPKD. Considering the prognostic importance of cardiovascular disease in CKD, echocardiography should be made available to all patients with ADPKD on a regular basis, focusing on the detection of mild forms of valvular regurgitations and signs of cardiac hypertrophy.
Limitations
This study has important limitations. Not all echocardiographic parameters were tested in all patients. As a result, the group size is limited for some parameters. Furthermore, the control cohort, consisting of 60 kidney donor candidates, was smaller (141 versus 60) and significantly older (44.17±11.23 versus 55.31±10.04 years). Additionally, genotype data were only available for a subset of patients (67%). Furthermore, because this was a retrospective analysis, only a post hoc power analysis would be possible. Concerns have been raised over the years that a post hoc power analysis is conceptually flawed and analytically misleading;37–39 therefore, this retrospective analysis was not performed on the basis of sample size calculation.
Supplementary Material
Acknowledgments
We thank Cornelia Böhme and Israa Karimov for excellent technical support. Database generation was supported by “clinicalsurveys.net” (Sebastian Heimann, Jörg Janne Vehreschild).
Footnotes
H.t.F. and R.-U.M. contributed equally to this work.
See related editorial, “Defining Cardiac Dysfunction in ADPKD,” on pages 126–127.
Disclosures
R.-U. Müller reports having consultancy agreements with, and receiving honoraria from, Alnylam and Sanofi; having ownership interest in Bayer, ChemoCentryx, Novartis, Pfizer, Roche, and Santa Barbara Nutrients; serving as chair of the working group "Genes and Kidney" of the European Renal Association (ERA) and member of the working group on AD dysplasias of the European Rare Kidney Disease Reference Network (ERKNet), on the editorial board of Kidney and Dialysis, and on the scientific advisory board of Santa Barbara Nutrients; and receiving research funding from Otsuka Pharmaceuticals and Thermo Fisher Scientific (all research funding was paid to the employer, Department II of Internal Medicine). R. Pfister reports receiving honoraria from Abbot Vascular, AstraZeneca, Bayer, Boehringer Ingelheim, Edwards Lifesciences, Novartis, Occlutech, and Pfizer; having consultancy agreements with Edwards Lifesciences and Occlutech; serving in an advisory or leadership role for Occlutech; and receiving research funding from Pfizer. H. ten Freyhaus reports receiving honoraria from Actelion, Alnylam, Bristol Myers Squibb, CTI, Diaplan, FomF, GlaxoSmithKline, Guerbet, KelCon, Lilly, Medtronic, medupdate, Novartis, Pfizer, Sanofi-Aventis, Servier, TomTec, and Volcano; having consultancy agreements with Alnylam, AstraZeneca, Bayer, GE, and Janssen-Cilag; and receiving research funding from Alnylam and Ionis. P. Todorova reports receiving travel and accommodation costs from Alexion, Astellas, Bristol Myers Squibb, Chemocentryx, Chiesi, Novartis, Omeros, and Otsuka. All remaining authors have nothing to disclose.
Funding
R.-U. Müller was supported by Ministry of Science North Rhine-Westfalia grant Nachwuchsgruppen.NRW 2015-2021, German Research Foundation (DFG) grant DI 1501/9, the Marga und Walter Boll-Stiftung (Marga and Walter Boll Foundation) grant 501100011566, and PKD Foundation grant 100001296. F. Grundmann was supported by the Koeln Fortune Program/Faculty of Medicine, University of Cologne and German Research Foundation grant GR 3932/2-2. The Department II of Internal Medicine received research funding from Otsuka Pharmaceuticals. We acknowledge support for the Article Processing Charge from the DFG (German Research Foundation, 491454339).
Author Contributions
S. Arjune wrote the original draft and was responsible for data curation and visualization; S. Arjune, F. Grundmann, C. Hendrix, R.-U. Müller, and P. Todorova were responsible for investigation; S. Arjune, F. Grundmann, R.-U. Müller, R. Pfister, H. ten Freyhaus, and P. Todorova reviewed and edited the manuscript; S. Arjune, F. Grundmann, R.-U. Müller, and H. ten Freyhaus conceptualized the study; F. Grundmann, C. Hendrix, R.-U. Müller, R. Pfister, H. ten Freyhaus, and P. Todorova were responsible for resources; F. Grundmann and H. ten Freyhaus provided supervision; R.-U. Müller was responsible for funding acquisition, methodology, and project administration; and R. Pfister and H. ten Freyhaus were responsible for validation.
Data Sharing Statement
Partial restrictions to the data and/or materials apply. Individual patient data underly data protection regulations; all aggregated data are reported.
Supplemental Material
This article contains the following supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0002942022/-/DCSupplemental.
Supplemental Figure 1. Bland-Altman plot for interpretation of inter-observer reproducibility.
Supplemental Figure 2. Correlation of ejection fraction and age of ADPKD patients.
Supplemental Figure 3. Comparison of echocardiographic parameters of ADPKD patients versus controls.
Supplemental Figure 4. Association of IVSd with clinical characteristics among male and female ADPKD patients and controls.
Supplemental Figure 5. Association of IVSd with age (A) and eGFR (B) in ADPKD patients (A) and controls (A and B).
Supplemental Figure 6. Association of IVSd with the comorbidities arterial hypertension (A) and hypercholesteremia (B) for ADPKD patients and controls.
Supplemental Figure 7. Association of aortic root diameter with clinical characteristics among male and female ADPKD patients and controls.
Supplemental Figure 8. Association of aortic root diameter and age (A–C) and eGFR (D) in ADPKD patients and controls.
Supplemental Figure 9. Association of aortic root diameter with the comorbidities arterial hypertension (A) and hypercholesteremia (B) for ADPKD patients and controls.
Supplemental Figure 10. Association of left ventricular end-diastolic diameter (LVEDD) with clinical characteristics.
Supplemental Figure 11. Association of ejection fraction (EF) with clinical characteristics.
Supplemental Figure 12. Evaluation of left ventricular function using E/A (A, mean±SD: controls 1.13±0.36 versus ADPKD patients 1.22±0.36) and E/lat e′ (B, mean±SD: controls 7.20±3.23 versus ADPKD patients 6.77±2.86) among control individuals and ADPKD patients.
Supplemental Figure 13. Association of Right ventricular basal diameter (RVD) with clinical characteristics.
Supplemental Figure 14. Association of tricuspid annular plane systolic excursion (TAPSE) with clinical characteristics.
Supplemental Figure 15. Association of maximal pressure gradient across the aortic valve (AV dPmax) with clinical characteristics.
Supplemental Figure 16. Association of peak pressure gradient across the tricuspid valve (TV dPmax) with clinical characteristics.
Supplemental Figure 17. Association of left atrial volume with clinical characteristics.
Supplemental Figure 18. LV mass index among ADPKD patients (mean±SD: male 88.32±20.45 mm and female 74.32±18.43 mm) and kidney donor candidates (mean±SD: male 79.57±17.56 mm and female 64.66±17.02 mm).
References
- 1.Chapman AB Devuyst O Eckardt KU, et al. Conference Participants . Autosomal-dominant polycystic kidney disease (ADPKD): Executive summary from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2015;88:17-27. 10.1038/ki.2015.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Müller RU, Benzing T. Cystic kidney diseases from the adult nephrologist’s point of view. Front Pediatr. 2018;6:65. doi: 10.3389/fped.2018.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007;369:1287-1301. doi: 10.1016/S0140-6736(07)60601-1 [DOI] [PubMed] [Google Scholar]
- 4.Neumann HP Jilg C Bacher J, et al. Else-Kroener-Fresenius-ADPKD-Registry . Epidemiology of autosomal-dominant polycystic kidney disease: An in-depth clinical study for south-western Germany. Nephrol Dial Transplant. 2013;28:1472-1487. doi: 10.1093/ndt/gfs551 [DOI] [PubMed] [Google Scholar]
- 5.Kataoka H Fukuoka H Makabe S, et al. Prediction of renal prognosis in patients with autosomal dominant polycystic kidney disease using PKD1/PKD2 mutations. J Clin Med. 2020;9:146. doi: 10.3390/jcm9010146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helal I Reed B Mettler P, et al. Prevalence of cardiovascular events in patients with autosomal dominant polycystic kidney disease. Am J Nephrol. 2012;36:362-370. 10.1159/000343281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapman AB, Schrier RW. Pathogenesis of hypertension in autosomal dominant polycystic kidney disease. Semin Nephrol. 1991;11:653-660 [PubMed] [Google Scholar]
- 8.Schrier RW Abebe KZ Perrone RD, et al. HALT-PKD Trial Investigators . Blood pressure in early autosomal dominant polycystic kidney disease. 2014;N Engl J Med 371:2255-2266. doi: 10.1056/NEJMoa1402685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schrier RW. Renal volume, renin-angiotensin-aldosterone system, hypertension, and left ventricular hypertrophy in patients with autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2009;20:1888-1893. doi: 10.1681/ASN.2008080882 [DOI] [PubMed] [Google Scholar]
- 10.Ecder T, Schrier RW. Hypertension in autosomal-dominant polycystic kidney disease: Early occurrence and unique aspects. J Am Soc Nephrol. 2001;12:194-200. doi: 10.1681/ASN.V121194 [DOI] [PubMed] [Google Scholar]
- 11.Chapman AB, Johnson AM, Rainguet S, Hossack K, Gabow P, Schrier RW. Left ventricular hypertrophy in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1997;8: 1292-1297. doi: 10.1681/ASN.V881292 [DOI] [PubMed] [Google Scholar]
- 12.Spinelli L Pisani A Giugliano G, et al. ALADIN-Cardiovascular Study Group . Left ventricular dysfunction in ADPKD and effects of octreotide-LAR: A cross-sectional and longitudinal substudy of the ALADIN trial. Int J Cardiol. 2019;275: 145-151. doi: 10.1016/j.ijcard.2018.10.063 [DOI] [PubMed] [Google Scholar]
- 13.Perrone RD Abebe KZ Schrier RW, et al. HALT PKD Study Group . Cardiac magnetic resonance assessment of left ventricular mass in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2011;6: 2508-2515. doi: 10.2215/CJN.04610511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Müller RU, Benzing T. Management of autosomal-dominant polycystic kidney disease-state-of-the-art. Clin Kidney J. 2018;11: i2-i13. doi: 10.1093/ckj/sfy103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chebib FT Hogan MC El-Zoghby ZM, et al. Autosomal dominant polycystic kidney patients may be predisposed to various cardiomyopathies. Kidney Int Rep. 2017;2:913-923. doi: 10.1016/j.ekir.2017.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lumiaho A Ikäheimo R Miettinen R, et al. Mitral valve prolapse and mitral regurgitation are common in patients with polycystic kidney disease type 1. Am J Kidney Dis. 2001;38: 1208-1216. doi: 10.1053/ajkd.2001.29216 [DOI] [PubMed] [Google Scholar]
- 17.Schievink WI, Torres VE, Piepgras DG, Wiebers DO. Saccular intracranial aneurysms in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1992;3: 88-95. doi: 10.1681/ASN.V3188 [DOI] [PubMed] [Google Scholar]
- 18.Qian Q, Hartman RP, King BF, Torres VE. Increased occurrence of pericardial effusion in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2007;2:1223-1227. doi: 10.2215/CJN.01920507 [DOI] [PubMed] [Google Scholar]
- 19.Irazabal MV Rangel LJ Bergstralh EJ, et al. CRISP Investigators . Imaging classification of autosomal dominant polycystic kidney disease: A simple model for selecting patients for clinical trials. J Am Soc Nephrol. 2015;26: 160-172. doi: 10.1681/ASN.2013101138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell C Rahko PS Blauwet LA, et al. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: Recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2019;32:1-64. doi: 10.1016/j.echo.2018.06.004 [DOI] [PubMed] [Google Scholar]
- 21.Lang RM Badano LP Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1-39.e14. doi: 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 22.Devereux RB de Simone G Arnett DK, et al. Normal limits in relation to age, body size and gender of two-dimensional echocardiographic aortic root dimensions in persons ≥15 years of age. Am J Cardiol. 2012;110: 1189-1194. doi: 10.1016/j.amjcard.2012.05.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rudski LG Lai WW Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23: 685-713, quiz 786–788. doi: 10.1016/j.echo.2010.05.010 [DOI] [PubMed] [Google Scholar]
- 24.Martinez-Vea A Valero FA Bardaji A, et al. Left ventricular hypertrophy in hypertensive patients with autosomal dominant polycystic kidney disease: Influence of blood pressure and humoral and neurohormonal factors. Am J Nephrol. 2000;20:193-200. doi: 10.1159/000013583 [DOI] [PubMed] [Google Scholar]
- 25.Kuo IY, Chapman AB. Polycystins, ADPKD, and cardiovascular disease. Kidney Int Rep. 2019;5:396-406. doi: 10.1016/j.ekir.2019.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hossack KF, Leddy CL, Johnson AM, Schrier RW, Gabow PA. Echocardiographic findings in autosomal dominant polycystic kidney disease. N Engl J Med. 1988;319:907-912. doi: 10.1056/NEJM198810063191404 [DOI] [PubMed] [Google Scholar]
- 27.Timio M, Monarca C, Pede S, Gentili S, Verdura C, Lolli S. The spectrum of cardiovascular abnormalities in autosomal dominant polycystic kidney disease: A 10-year follow-up in a five-generation kindred. Clin Nephrol. 1992;37:245-251 [PubMed] [Google Scholar]
- 28.Leier CV, Baker PB, Kilman JW, Wooley CF. Cardiovascular abnormalities associated with adult polycystic kidney disease. Ann Intern Med. 1984;100:683-688. doi: 10.7326/0003-4819-100-5-683 [DOI] [PubMed] [Google Scholar]
- 29.Varnero S, Becchi G, Bormida R, Martinengo E, Carozzi S. [Valvular prolapse in autosomal dominant polycystic kidney [in Italian]. G Ital Cardiol. 1992;22:825-828 [PubMed] [Google Scholar]
- 30.Castiglioni G Gibelli G Milani S Benelli R Riegler P Fasciolo F, et al. Cardiac valvular abnormalities in ADPKD. Preliminary results from the Italian Multicentric Study. Contrib Nephrol. 1995;115:159-162. doi: 10.1159/000424416 [DOI] [PubMed] [Google Scholar]
- 31.Oflaz H Alisir S Buyukaydin B Kocaman O Turgut F Namli S, et al. Biventricular diastolic dysfunction in patients with autosomal-dominant polycystic kidney disease. Kidney Int. 2005;68:2244-2249. doi: 10.1111/j.1523-1755.2005.00682.x [DOI] [PubMed] [Google Scholar]
- 32.Pietrzak-Nowacka M, Safranow K, Czechowska M, Dutkiewicz G, Kornacewicz-Jach Z, Ciechanowski K. Autosomal dominant polycystic kidney disease and hypertension are associated with left ventricular mass in a gender-dependent manner. Kidney Blood Press Res. 2012;36:301-309. doi: 10.1159/000343419 [DOI] [PubMed] [Google Scholar]
- 33.Paavola J Schliffke S Rossetti S, et al. Polycystin-2 mutations lead to impaired calcium cycling in the heart and predispose to dilated cardiomyopathy. J Mol Cell Cardiol 2013;58:199-208. doi: 10.1016/j.yjmcc.2013.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bisgrove BW, Snarr BS, Emrazian A, Yost HJ. Polaris and polycystin-2 in dorsal forerunner cells and Kupffer’s vesicle are required for specification of the zebrafish left-right axis. Dev Biol. 2013;58:199-208. doi: 10.1016/j.ydbio.2005.08.047 [DOI] [PubMed] [Google Scholar]
- 35.Pedrozo Z Criollo A Battiprolu PK, et al. Polycystin-1 is a cardiomyocyte mechanosensor that governs L-type Ca2+ channel protein stability. Circulation. 2015;131:2131-2142. doi: 10.1161/CIRCULATIONAHA.114.013537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amirrad F, Pala R, Shamloo K, Muntean BS, Nauli SM. Arrhythmogenic hearts in PKD2 mutant mice are characterized by cardiac fibrosis, systolic, and diastolic dysfunctions. Front Cardiovasc Med. 2021;8:772961. doi: 10.3389/fcvm.2021.772961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kraemer HC, Mintz J, Noda A, Tinklenberg J, Yesavage JA. Caution regarding the use of pilot studies to guide power calculations for study proposals. Arch Gen Psychiatry. 2006;63:484-489. doi: 10.1001/archpsyc.63.5.484 [DOI] [PubMed] [Google Scholar]
- 38.Levine M, Ensom MH. Post hoc power analysis: An idea whose time has passed? Pharmacotherapy. 2001;21: 405-409. doi: 10.1592/phco.21.5.405.34503 [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Hedo R, Rivera A, Rull R, Richardson S, Tu XM. Post hoc power analysis: Is it an informative and meaningful analysis? Gen Psychiatr. 2019;32:e100069. doi: 10.1136/gpsych-2019-100069 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Partial restrictions to the data and/or materials apply. Individual patient data underly data protection regulations; all aggregated data are reported.