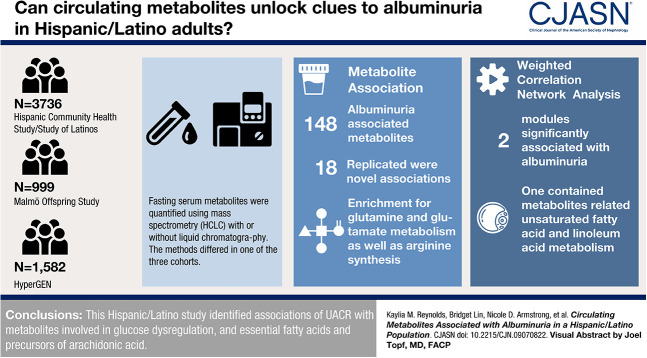
Visual Abstract
Keywords: albuminuria, metabolites, diabetes mellitus, lipids
Abstract
Background
Albuminuria is associated with metabolic abnormalities, but these relationships are not well understood. We studied the association of metabolites with albuminuria in Hispanic/Latino people, a population with high risk for metabolic disease.
Methods
We used data from 3736 participants from the Hispanic Community Health Study/Study of Latinos, of which 16% had diabetes and 9% had an increased urine albumin-to-creatinine ratio (UACR). Metabolites were quantified in fasting serum through nontargeted mass spectrometry (MS) analysis using ultra-performance liquid chromatography-MS/MS. Spot UACR was inverse normally transformed and tested for the association with each metabolite or combined, correlated metabolites, in covariate-adjusted models that accounted for the study design. In total, 132 metabolites were available for replication in the Hypertension Genetic Epidemiology Network study (n=300), and 29 metabolites were available for replication in the Malmö Offspring Study (n=999).
Results
Among 640 named metabolites, we identified 148 metabolites significantly associated with UACR, including 18 novel associations that replicated in independent samples. These metabolites showed enrichment for D-glutamine and D-glutamate metabolism and arginine biosynthesis, pathways previously reported for diabetes and insulin resistance. In correlated metabolite analyses, we identified two modules significantly associated with UACR, including a module composed of lipid metabolites related to the biosynthesis of unsaturated fatty acids and alpha linolenic acid and linoleic acid metabolism.
Conclusions
Our study identified associations of albuminuria with metabolites involved in glucose dysregulation, and essential fatty acids and precursors of arachidonic acid in Hispanic/Latino population.
Podcast
This article contains a podcast at https://dts.podtrac.com/redirect.mp3/www.asn-online.org/media/podcast/CJASN/2023_02_08_CJN09070822.mp3
Introduction
Increased urine albumin excretion (albuminuria) is associated with all-cause and cardiovascular mortality among both diabetic and nondiabetic individuals and is a marker of kidney injury in CKD.1–3 Albuminuria, defined by a urine albumin-to-creatinine ratio (UACR) >30 mg/g, has a prevalence of around 10% in the US adult population.4 Studies have shown that insulin resistance and metabolic syndrome are associated with development of albuminuria.5,6
Albuminuria is also associated with metabolic abnormalities, although the relationships are still poorly understood. Metabolomics are endophenotypes that can capture dysregulated metabolic pathways before disease diagnosis. For example, abnormal lipid metabolism is often observed in proteinuric kidney diseases including diabetic nephropathy and primary glomerular diseases manifesting as nephrotic syndrome.7,8 Prior studies have used high throughput profiling of serum metabolites to study individuals with type 1 diabetes who had prevalent or incident albuminuria9–13 or studied serum metabolites associated with albuminuria to predict incident type 2 diabetes.14 Additional studies have examined one or more candidate metabolites for their association with albuminuria in individuals with prevalent type 2 diabetes.15 Overall, most of these prior studies examined albuminuria-associated metabolomics in the limited context of diabetes or progression of CKD. Metabolomic profiles have not yet been characterized in individuals with mild albuminuria, which is more prevalent in the general population.
The aim of this study is to identify metabolites or metabolite signatures associated with UACR variation in Hispanic/Latino population, a population with a high burden of metabolic disease. We used data from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). This young cohort had a low prevalence of diabetes and CKD at enrollment, with an albuminuria prevalence at 12%.16
In our prior studies of Hispanic/Latino population (HCHS/SOL), we identified 480 metabolites associated with eGFR.17 Given metabolites reflect underlying biochemical activity of cells and tissues, metabolic profiling can also provide insights into abnormal processes related to albuminuria.
Methods
Population
HCHS/SOL is a longitudinal study of 16,415 Hispanic/Latino people (aged 18–74 years) recruited at US field centers (Chicago, Miami, the Bronx, and San Diego) between 2008 and 2011.18 The study includes a complex sampling design of households and cohort selection with oversampling to provide a diverse sample of Hispanic/Latino groups.19 Participants self-reported their Hispanic/Latino background (on the basis of a question of heritage) and then were classified as being of Caribbean (Puerto Rico, Dominican Republic, Cuba) or Mainland (Central America, South America, Mexico) descent. The first HCHS/SOL visit gathered clinical and behavioral measurements and collected fasting blood and spot urine samples. The study was approved by the institutional review boards at each field center and the coordinating center and adheres to the Declaration of Helsinki. All participants provided written informed consent. A random sample of 3972 participants was selected for serum metabolomic profiling using visit-1 fasting serum samples. Of these individuals, 3736 had complete data on metabolites, UACR, and other demographic covariates.
Phenotype and Covariates
UACR was calculated using spot urine albumin (mg/dl)-to-creatinine (g/dl) ratio. Urine albumin was measured using an immunoturbidimetric, and urine creatinine was quantified using a creatinase enzymatic method. Serum creatinine was measured using a creatinase enzymatic method traceable to an isotope dilution mass spectrometry (MS) assay.20 eGFR was estimated from the 2021 Chronic Kidney Disease Epidemiology Collaboration equation, without adjusting for race. Diabetes was defined by a fasting glucose ≥126 mg/dl, a nonfasting glucose ≥200 mg/dl, an A1c ≥6.3, or use of medications. Hypertension was defined as a blood pressure >140/90 mm Hg or use of medications, and current smoking status was assessed using a questionnaire.
Metabolomics
Metabolites (n=1,136, n=782 named metabolites and n=354 unnamed metabolites) were quantified in fasting serum through nontargeted MS analysis using ultra-performance liquid chromatography (UPLC-MS/MS; DiscoveryHD4 platform, Metabolon Inc, NC).21 Samples were randomly allocated across the platform, and duplicates were used to determine endogenous variability, with representative relative SD of 10% across all biochemicals. Raw area counts for each metabolite in each sample were normalized to correct for variation resulting from instrument interday tuning differences. Only named metabolites showing <25% missing values were tested (n=640). Missing values were imputed with the observed minimum value of the metabolite in the sample.
Statistical Analyses
UACR was inverse normally transformed and used as a predictor in all metabolite models. We tested the association of UACR with each metabolite using two models. Model 1 adjusted for age, sex, field center, and Hispanic/Latino background. Model 2 further adjusted for body mass index (BMI), diabetes status, current smoking status, eGFR, and hypertension. Fourteen individuals were missing information on BMI, and three individuals were missing information on current smoking status in model 2. We tested interactions by age (≥50 years versus <50 years), sex (male versus female), Hispanic/Latino background (Caribbean versus Mainland), diabetes (yes versus no), current smoking (yes versus no), eGFR (<30 ml/min/1.73 m2 versus ≥30 ml/min/1.73 m2), and hypertension (yes versus no) in model 1 for significant metabolites, followed by stratified category analyses. All tests were performed in R 3.3.1. Given the complex HCHS/SOL study design and oversampling of Hispanic/Latino subpopulations,19 our linear regression model included strata and sampling weights. Reported P values from models 1 and 2 were adjusted for multiple testing to ensure a false discovery rate (FDR) of 0.05. Interactions were considered significant at a threshold of P<3.0 × 10−4.
Significant model 1 metabolites underwent enrichment analysis using MetaboAnalyst 5.0.22 Metabolite sets were queried against both the Kyoto Encyclopedia of Genes and Genomes (KEGG) and the Small Molecule Pathway Database (SMPDB) libraries. Metabolic pathways were considered significantly associated if their FDR was <0.05. A χ2 test was used to determine whether there is a relationship between super pathways (amino acids, lipids, or other) and UACR associations for significant model 1 metabolites. The significance level for this test was 0.05.
Weighted Correlation Network Analysis
Our previous study of eGFR and metabolite associations revealed that several metabolites are significantly correlated.17 Therefore, we implemented a weighted correlation network analysis (WGCNA) to identify groups (modules) of highly related metabolites.23 Once correlated modules were defined, metabolite area quantities within each module were summarized by an eigenvector and used as the response variable in model 1 and 2 regression analyses. Modules significantly associated with UACR in model 1 analysis were examined for common member metabolite characteristics in super pathways and enrichment analyses. Module P values were FDR-adjusted for multiple testing at 0.05 by accounting for the number of WGCNA modules and ungrouped metabolites.
Replication Datasets and Statistical Models
We replicated our findings in two studies: the Malmö Offspring Study (MOS) and the Hypertension Genetic Epidemiology Network (HyperGEN). We standardized the phenotype definition and covariates and used the same statistical models for discovery and replication studies. Replication used a more stringent Bonferroni correction for the number of metabolites available.
Briefly, MOS is an ongoing population-based cohort study that recruited adult (>18 years) children and grandchildren from the Malmö Diet and Cancer Study.24,25 Participants attended a research clinic where fasting plasma and urine samples were collected and anthropometric measurements were obtained. Spot urine albumin and creatinine were measured using standard procedures. UACR was available for 999 participants with metabolomics data. Metabolite profiling was performed in fasting EDTA plasma using targeted LC-MS with an UHPLC-QTOF-MS System (Agilent Technologies, Santa Clara, CA). Two different LC-MS methods were used in positive and negative ion modes and have both been described previously in more detail.26,27 Metabolite peak integration was performed using Agilent Profinder B.06.00 (Agilent Technologies, Santa Clara, CA). Metabolite areas were normalized to a correction curve calculated from the metabolite area measurements in the quality control samples.26 Metabolites were annotated using synthetic standards or by matching MS/MS fragmentation with the Human Metabolome Database (HMDB)28 and METLIN29 or by matching fragment ions to putative molecule fragments. Metabolites were classified as level 1 or level 2 annotations according to the Metabolomics Standards Initiative.30 Statistical analyses used linear regression models to implement models for the associations between plasma metabolite levels and UACR adjusted for both study-specific variables and covariates included in our model 1 and 2 analyses. Statistical analyses were implemented in R 4.1.1. Twenty-eight metabolites were available for comparison (threshold for replication P<1.8 × 10−3).
HyperGEN is a family-based study of hypertensive sibling pairs and is one of the networks in the National Heart, Lung, and Blood Institute Family Blood Pressure Program.31 From 1995 to 2000, African American sibships with hypertension were recruited from population-based cohorts in Forsyth County, NC, and from the community at large in Birmingham, AL. Sibling pairs with onset of hypertension before age 60 years were recruited in the first phase. The study was later extended to other siblings and the offspring of the hypertensive probands who were unmedicated adults. The study was approved by the institutional review boards of the participating organizations. All HyperGEN participants provided informed consent for use of samples and data for subsequent analyses. The EDTA plasma from 300 African American HyperGEN study participants age ≥40 years were assayed for an untargeted metabolomic analysis using an ultra-high performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) platform from Metabolon (Durham, NC). The median relative SD for the instrument variability in the HyperGEN population was 4%, while the total process variability was 9%; both values met Metabolon's acceptance criteria. Each metabolite was tested for normality, and a rank-based inverse normal transformation was performed. Mixed models using the lmekin function in R were used to test for association between the metabolite and the inverse normalized UACR adjusting for age and sex, as well as family design as a random effect. Model 2 further adjusted for BMI, type 2 diabetes status, smoking status, GFR, and hypertension status, in that order. A Bonferroni threshold for replication was used (P<0.05/148=3.4×10−4).
We also performed a look-up of association in the published Metabolic Syndrome in Men (METSIM) study that reported associations of UACR with metabolites in nondiabetic Finnish participants.14
Glutamine and Glucose Homeostasis
Given results from this study related to glutamine/glutamate metabolism pathways, we tested if the metabolite glutamine was associated with fasting insulin, insulin after a 2-hour oral glucose tolerance test (OGTT), and an indirect measure of insulin resistance using the homeostatic model assessment of insulin resistance (HOMA-IR). HOMA-IR is calculated as the product of fasting insulin (µU/ml) and fasting glucose (mmol/L) divided by 22.32 These analyses were performed on HCHS/SOL participants without diabetes (n=3170) using models adjusted for age, sex, and Hispanic/Latino background. P values were adjusted for the three tests performed.
Results
The demographic and covariate characteristics of 3736 HCHS/SOL participants (weighted), 999 MOS participants, and 1582 HyperGEN participants with complete metabolite and phenotypic data are shown in Table 1.
Table 1.
Descriptive statistics of HCHS/SOL participants at visit 1 and replication studies
| Characteristic | HCHS/SOL n=3842a |
HyperGEN n=300 |
MOS n=999 |
|---|---|---|---|
| Age, yr, mean±SD | 42±15 | 55±10 | 39±15 |
| BMI, kg/m2, mean±SD | 30±6 | 32±7 | 26±5 |
| eGFR, ml/min per 1.73 m 2 , mean±SD | 99±20 | 90±29 | 97±16 |
| Stage 1 (≥90), n (%) | 2695 (70) | 146 (49) | 657 (66) |
| Stage 2 (60–89), n (%) | 1036 (27) | 112 (37) | 334 (33) |
| Stage 3 (30–59), n (%) | 98 (3) | 42 (14) | 8 (1) |
| Stage 4 (15–29), n (%) | 7 (0.2) | 0 (0) | 0 (0) |
| Stage 5 (<15), n (%) | 5 (0.1) | 0 (0) | 0 (0) |
| UACR, mg/g, median (IQR) b | 6 (4.6–12.1) | 6.6 (2.8–25.6) | 3.9 (2.5–7.2) |
| Stage 1 (<30), n (%) | 3512 (91) | 232 (78) | 953 (95) |
| Stage 2 (30–299), n (%) | 273 (7) | 49 (16) | 39 (4) |
| Stage 3 (≥300), n (%) | 57 (2) | 18 (6) | 6 (1) |
| Sex, n (%) | |||
| Male | 1882 (49) | 124 (41) | 510 (51) |
| Female | 1960 (51) | 176 (59) | 489 (49) |
| Smoking status, n (%) c | |||
| Smoker | 945 (25) | 92 (31) | 164 (16) |
| Nonsmoker | 2895 (75) | 205 (69) | 835 (84) |
| Diabetes status, n (%) | |||
| Diabetic | 600 (16) | 92 (31) | 39 (4) |
| Nondiabetic | 3242 (84) | 205 (69) | 960 (96) |
| Hypertension status, n (%) | |||
| Hypertensive | 897 (23) | 281 (94) | 241 (24) |
| Nonhypertensive | 2945 (77) | 19 (6) | 758 (76) |
| Hispanic background, n (%) | |||
| Caribbean | 2057 (53) | — | — |
| Mainland | 1785 (47) | — | — |
HCHS/SOL, Hispanic Community Health Study/Study of Latinos; HyperGEN, Hypertension Genetic Epidemiology Network; MOS, Malmö Offspring Study; BMI, body mass index; UACR, urine albumin-to-creatinine ratio; IQR, interquartile range.
HCHS/SOL descriptive data are weighted to account for the complex study sample design.
The HyperGEN study is missing UACR information for one participant.
The HyperGEN study is missing smoking status information for three participants.
Metabolites Associated with UACR and Interactions
We identified 148 metabolites significantly associated with UACR (FDR <0.05) in the baseline model (model 1), including 29 amino acids and 87 lipids. A χ2 test revealed the significance of UACR with metabolites is significantly dependent on metabolite super pathways (χ2, P<0.001). Amino acids were associated less frequently than expected with UACR, whereas lipids were associated more frequently than expected with UACR (Figure 1). In the fully adjusted models that included BMI, diabetes, current smoking, eGFR, and hypertension (model 2), 55 metabolites remained significant (Supplemental Table 1).
Figure 1.
Volcano plot of the association of known metabolites with urine albumin-to-creatinine ratio (UACR) for model 1 colored by metabolite super pathway. Super pathways represent the role of a metabolite in the body and can provide insight into potential biological mechanisms behind associations with UACR.
Of these metabolites, 1,5-anhydroglucitol (1,5-AG), lactate, and malate had the lowest P values (P=1.97 × 10−9, P=1.58 × 10−6, and P=2.86 × 10−6, respectively). An increase in UACR was associated with a decrease in 1,5-AG levels, whereas lactate and malate levels increase with higher UACR. Among all significant model 1 metabolites tested, there were 33 significant covariate interactions with UACR. One metabolite (nicotinamide) showed a significant UACR interaction with age (P<0.001), one metabolite (4-hydroxychlorothalonil) had a significant UACR interaction with hypertension (P<0.001), and seven metabolites had significant UACR and diabetes interactions (P<0.001; Figure 2, Supplemental Table 2). Twenty-four metabolites had significant UACR and eGFR interactions, although the number of participants with a low eGFR was low (n=13; Supplemental Table 2). With the exception of the metabolite 5alpha-and rostan-3beta,17beta-diol monosulfate, the risk factors of age >50 years, hypertension, and diabetes all resulted in greater absolute effect sizes compared with their opposite class in stratified analyses. No interactions were significant for current smoking status, sex, BMI, or Hispanic/Latino background.
Figure 2.
Forest plot showing the stratified association of UACR for metabolites with significant interaction by type 2 diabetes, hypertension, and age. 95% CI, 95% confidence interval.
Enrichment Analyses
Significant metabolites identified in model 1 were examined for commonly associated metabolic pathways. Two pathways from the KEGG library were significant at an FDR <0.05: D-glutamine and D-glutamate metabolism (P<0.001) and arginine biosynthesis (P<0.001). Pathways from the SMPDB library were not significant.
WGCNA Results
Of the 640 metabolites included in this study, 278 metabolites were not sufficiently correlated to warrant module formation. The remaining 362 metabolites were grouped into four modules (Supplemental Tables 3–6). In model 1 regression analysis, two modules (2 and 3) were significantly associated with UACR (P=0.04 and P=0.02, respectively). In both significant WGCNA modules, all but one metabolite were lipids (Table 2). In the fully adjusted regression analysis, module 2 remained significant (P=0.05). Module 3 was associated with the biosynthesis of unsaturated fatty acids from the KEGG library (P<0.001) and alpha linolenic acid and linoleic acid metabolism in the SMPDB library (P<0.001; Supplemental Figures 1 and 2). No significant pathways were identified for module 2 metabolites.
Table 2.
Model results and metabolite super pathways for WGCNA-defined modules
| Module # | Model 1 | Model 2 | Super Pathway, n (%) | Total | ||||
|---|---|---|---|---|---|---|---|---|
| β | P Value | β | P Value | Amino Acids | Lipids | Other | ||
| No group | — | — | — | — | 80 (29) | 95 (34) | 103 (37) | 278 |
| 1 | −0.007 | 0.86 | 0.02 | 0.65 | 85 (49) | 12 (7) | 77 (44) | 174 |
| 2 | 0.06 | 0.04 | 0.07 | 0.05 | — | 74 (99) | 1 (1) | 75 |
| 3 | 0.07 | 0.02 | 0.05 | 0.17 | — | 64 (98) | 1 (2) | 65 |
| 4 | −0.02 | 0.67 | 0.01 | 0.85 | — | 47 (98) | 1 (2) | 48 |
Metabolites in the module “No Group” were not correlated with a sufficient number of metabolites to either join or form a module. Bolded P values are significant at a false discovery rate of 0.05 (see Methods). Super pathways represent the overarching role of a metabolite in the body and can provide insight into potential biological mechanisms behind correlation groupings. WGCNA, weighted correlation network analysis.
Replication
Among the 148 serum metabolites associated with UACR, four metabolites have been previously reported in the published METSIM study of individuals without diabetics or CKD (1-methylhistidine, argininate, malate and N-acetyltryptophan; Supplemental Table 7).13 Two of the 29 metabolites available in the Malmö Offspring study replicated (glucose and mannose, each P<1.8 × 10−3; Supplemental Table 8), and 18 of 148 metabolites available in HyperGEN were significantly associated with UACR (all with P<3.4 × 10−4; Supplemental Table 9). Overall, 20 metabolites replicated in at least one study, and 18 of these replications were novel associations.
Glutamine Associations among Nondiabetic Individuals
Given findings related to glutamine/glutamate metabolism pathways, we tested the association of glutamine with fasting insulin (P=1.4 × 10−4), insulin after OGTT (P=1.1 × 10−5), and HOMA-IR (8.2 × 10−5), which were all significant (Supplemental Table 10).
Discussion
This study identified 148 metabolites significantly associated with UACR. Four metabolites had previously reported associations with UACR from a Finnish cohort among individuals without diabetes,14 and 18 metabolites were novel associations that replicated in independent studies (HyperGEN, MOS). Two metabolites, mannose and glucose, replicated in both independent studies, although the studies used different metabolomics platforms. In fully adjusted models, 55 metabolites remained significant. Of the 18 novel metabolite associations replicated in the two independent studies, six metabolites remained significant in fully adjusted models in at least one replicating study. Among all identified metabolites, we found enrichment for D-glutamine and D-glutamate metabolism and arginine biosynthesis, pathways that have been previously reported for diabetes and insulin resistance.33,34
1,5-AG, mannose, and glucose were some of the replicated metabolites most significantly associated with UACR. All three of these metabolites are related to type 2 diabetes.35 Glucose and mannose associations were no longer significant in our study when adjusting for diabetes, and there was a significant interaction by diabetes for mannose association with UACR. By contrast, 1,5-AG associations were robust to model 2 adjustments in our study. 1,5-AG is considered a marker of short-term glycemic control and postprandial glucose in type 2 diabetes.36,37 Low 1,5-AG is associated with increased serum glucose.38 In our data, increasing UACR was associated with lower 1,5-AG quantities. This metabolite showed no evidence of a significant interaction between UACR and diabetes, and association findings were robust to adjustments by diabetes and significant among nondiabetic individuals (P=0.007). 1,5-AG has been previously associated with the progression of macroalbuminuria among diabetic patients using untargeted metabolomics.39 However, we are not aware of previous reports of significant associations of this metabolite with UACR among nondiabetic individuals.39 Considering that our study had only a 16% prevalence of diabetes at the visit when metabolomics was measured (weighted estimate), our findings suggest that metabolic pathways related to glucose dysregulation are associated with increased UACR even among participants not meeting criteria for diabetes.
Our study also identified a significant interaction between UACR and diabetes for the metabolite glutamine. Increasing UACR was associated with lower glutamine levels among only individuals with diabetes. Recent studies have shown glutamine is a potent insulin secretagogue in experimental models using cells and animals, through pathways involving the tricarboxylic acid cycle.34 Our study identified significant associations of a higher glutamine with increased fasting insulin and insulin response after OGTT, and insulin resistance as measured by HOMA-IR among Hispanic/Latino people without diabetes. These findings support the role of glutamine in regulation of insulin secretion in populations of high risk for diabetes such as Hispanic/Latino population and its role on albuminuria.40
As expected, a low eGFR (<30 ml/min per 1.73 m2) was the most common factor accounting for significant interactions in the associations of metabolites with UACR. In strata analyses for the 24 metabolites with significant eGFR interactions, there were stronger UACR effect estimates among individuals with a low eGFR compared with those with an eGFR ≥30 ml/min per 1.73 m2, although samples with low eGFR were limited. For 1,5-AG, a low eGFR was associated with a stronger inverse association of UACR compared with higher eGFR. Only one metabolite (nicotinamide) showed interaction by age strata, which may reflect age-related dietary differences. The metabolite 4-hydroxychlorothalonil had significant interactions between UACR and both hypertension status and diabetes status.
In analyses using modules of highly correlated metabolites, two modules were significantly associated with UACR, including one composed of fatty acids. In pathway analyses, the metabolites from this module were associated with the biosynthesis of unsaturated fatty acids, and alpha linolenic acid and linoleic acid metabolism. This includes essential lipids and precursors of arachidonic acid, which have roles in kidney physiology and inflammation.41 The association with this module was no longer significant when adjusted for risk factors including diabetes and BMI, suggesting some mediation of the metabolite effects by these risk factors.
Given the intraindividual and interindividual variability in UACR that has been previously described, the use of a single spot urine sample to measure UACR may be susceptible to bias.42 Another limitation of our study is that we used untargeted metabolomics and thus cannot assess thresholds or interpret the effect estimates beyond direction of effect for associated metabolites. Because of the sparsity of metabolomics studies in Hispanic/Latino population, we were unable to replicate our findings in additional studies of Hispanic/Latino population. However, the relationship of metabolites with UACR is expected to be consistent across populations. The number of participants with eGFR <30 ml/min per 1.73 m2 was low for interaction analyses, but the relationships of blood metabolites with low eGFR are known.
This is the largest study of Hispanic/Latino population to study associations of metabolites with UACR. Hispanic/Latino people are a high-risk population for metabolic diseases, which may have many causes including cultural, lifestyle and behavior, genetics, and metabolic dysregulation, that can be assessed using metabolomics. Our study supports metabolic signatures of glucose dysregulation related to UACR in Hispanic/Latino population with low diabetes prevalence.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Disclosures
E. Boerwinkle reports ownership interest in Codified Genomics and honoraria from The University of Washington, honoraria for participating in the PRIDE program. N. Franceschini reports advisory or leadership roles for the Women's Health Initiative Publication and Presentation Committee and the Women's Health Initiative Ancillary Study Committee. R. Kaplan reports employment with Albert Einstein College of Medicine. J.P. Lash reports serving an advisory or leadership role for Kidney360. B.M. Lin reports ownership interest in ARWR, CNK, and EDIT. D. Muoio reports research funding from Eli Lilly and Co. and honoraria from Eli Lilly and Co. B. Thygarajan reports consultancy agreements with Diversigen Inc. and research funding from Peak Diagnostics. The remaining authors have nothing to disclose.
Funding
The Hispanic Community Health Study/Study of Latinos is a collaborative study supported by contracts from the National Heart, Lung, and Blood Institute (NHLBI) to the University of North Carolina (Grant/Award Number: HHSN268201300001I/N01-HC-65233), University of Miami (Grant/Award Number: HHSN268201300004I/N01-HC-65234), Albert Einstein College of Medicine (Grant/Award Number: HHSN268201300002I/N01-HC-65235), University of Illinois at Chicago (Grant/Award Number: HHSN268201300003I/N01-HC-65236 Northwestern Univ), and San Diego State University (Grant/Award Number: HHSN268201300005I/N01-HC-65237). The following Institutes/Centers/Offices have contributed to the HCHS/SOL through a transfer of funds to the NHLBI: National Institute on Minority Health and Health Disparities, National Institute on Deafness and Other Communication Disorders, National Institute of Dental and Craniofacial Research, National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Neurological Disorders and Stroke, and NIH Institution-Office of Dietary Supplements. Support for metabolomics data was graciously provided by the JLH Foundation as a gift (Houston, TX). This manuscript analyses was supported by NIH R01 DK117445 and R01 MD012765 (to N.F.).
Author Contributions
N. Franceschini and C.B. Newgard conceptualized the study; N. Franceschini and J.P. Lash were responsible for data curation; N.D. Armstrong, B.M. Lin, F. Ottosson, and K.M. Reynolds were responsible for formal analysis; N.D. Armstrong, M.R. Irvin, O. Melander, and F. Ottosson were responsible for validation; E. Boerwinkle, J. Cai, M.L. Daviglus, N. Franceschini, R. Kaplan, J.P. Lash, D. Muoio, C.B. Newgard, B. Thygarajan, A.S. Williams, and B. Yu were responsible for resources; J. Cai, B.M. Lin, and T. Sofer were responsible for methodology; M.L. Daviglus and Q. Qi were responsible for investigation; N. Franceschini was responsible for funding acquisition and project administration and provided supervision; K.M. Reynolds was responsible for visualization; T. Sofer and Y. Zhang were responsible for software; N. Franceschini and K.M. Reynolds wrote the original draft; and N.D. Armstrong, E. Boerwinkle, J. Cai, M.L. Daviglus, N. Franceschini, M.R. Irvin, R. Kaplan, J.P. Lash, B.M. Lin, O. Melander, D. Muoio, C.B. Newgard, F. Ottosson, Q. Qi, T. Sofer, B. Thygarajan, A.S. Williams, and B. Yu reviewed and edited the manuscript.
Data Sharing Statement
Observational data have been deposited to the Database of Genotypes and Phenotypes (dbGaP), phs000810.v1.p1.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/CJN/B562.
Supplemental Table 1. Main metabolite associations with UACR in Models 1 and 2.
Supplemental Table 2. Results from Model 1 UACR interactions and stratification analyses.
Supplemental Table 3. Metabolites and super pathways in WGCNA Module 1.
Supplemental Table 4. Metabolites and super pathways in WGCNA Module 2.
Supplemental Table 5. Metabolites and super pathways in WGCNA Module 3.
Supplemental Table 6. Metabolites and super pathways in WGCNA Module 4.
Supplemental Table 7. Replicated significant metabolites from published METSIM study.
Supplemental Table 8. Replicated significant metabolites from the MOS study.
Supplemental Table 9. Replicated significant metabolites from HyperGEN study.
Supplemental Table 10. Associations of the metabolite glutamine with characteristics of glucose homeostasis among nondiabetic participants.
Supplemental Figure 1. Linoleic acid metabolism pathway.
Supplemental Figure 2. Biosynthesis of unsaturated fatty acids pathway.
References
- 1.Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia. 1989;32(4):219-226. doi: 10.1007/BF00285287 [DOI] [PubMed] [Google Scholar]
- 2.Astor BC, Matsushita K, Gansevoort RT, et al. , the Chronic Kidney Disease Prognosis Consortium. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011;79(12):1331-1340. doi: 10.1038/ki.2010.550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286(4):421-426. doi: 10.1001/jama.286.4.421 [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Chronic Kidney Disease Surveillance System. Available at: www.cdc.gov/ckd. Accessed October 22, 2022 [Google Scholar]
- 5.Pilz S, Rutters F, Nijpels G, et al. ; the RISC Investigators. Insulin sensitivity and albuminuria: the RISC study. Diabetes Care. 2014;37(6):1597-1603. doi: 10.2337/dc13-2573 [DOI] [PubMed] [Google Scholar]
- 6.Thomas G, Sehgal AR, Kashyap SR, Srinivas TR, Kirwan JP, Navaneethan SD. Metabolic syndrome and kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2011;6(10):2364-2373. doi: 10.2215/CJN.02180311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agrawal S, Zaritsky JJ, Fornoni A, Smoyer WE. Dyslipidaemia in nephrotic syndrome: mechanisms and treatment. Nat Rev Nephrol. 2018;14(1):57-70. doi: 10.1038/nrneph.2017.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Appel GB, Valeri A, Appel AS, Blum C. The hyperlipidemia of the nephrotic syndrome. Am J Med. 1989;87(5N):45N-50N [PubMed] [Google Scholar]
- 9.Makinen VP, Tynkkynen T, Soininen P, et al. Metabolic diversity of progressive kidney disease in 325 patients with type 1 diabetes (the FinnDiane Study). J Proteome Res. 2012;11(3):1782-1790. doi: 10.1021/pr201036j [DOI] [PubMed] [Google Scholar]
- 10.Moon S, Tsay JJ, Lampert H, et al. Circulating short and medium chain fatty acids are associated with normoalbuminuria in type 1 diabetes of long duration. Sci Rep. 2021;11:8592. doi: 10.1038/s41598-021-87585-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tofte N, Suvitaival T, Trost K, et al. Metabolomic assessment reveals alteration in polyols and branched chain amino acids associated with present and future renal impairment in a discovery cohort of 637 persons with type 1 diabetes. Front Endocrinol (Lausanne). 2019;10:818. doi: 10.3389/fendo.2019.00818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haukka JK, Sandholm N, Forsblom C, Cobb JE, Groop PH, Ferrannini E. Metabolomic profile predicts development of microalbuminuria in individuals with type 1 diabetes. Sci Rep. 2018;8:13853. doi: 10.1038/s41598-018-32085-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colombo M, Valo E, McGurnaghan SJ, et al. ; on behalf of the FinnDiane Study Group and the Scottish Diabetes Research Network SDRN Type 1 Bioresource Collaboration. Biomarker panels associated with progression of renal disease in type 1 diabetes. Diabetologia. 2019;62(9):1616-1627. doi: 10.1007/s00125-019-4915-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandes Silva L, Vangipurapu J, Smith U, Laakso M. Metabolite signature of albuminuria involves amino acid pathways in 8661 Finnish men without diabetes. J Clin Endocrinol Metab. 2021;106(1):143-152. doi: 10.1210/clinem/dgaa661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kikuchi K, Saigusa D, Kanemitsu Y, et al. Gut microbiome-derived phenyl sulfate contributes to albuminuria in diabetic kidney disease. Nat Commun. 2019;10(1):1835. doi: 10.1038/s41467-019-09735-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ricardo AC, Flessner MF, Eckfeldt JH, et al. Prevalence and correlates of CKD in Hispanics/Latinos in the United States. Clin J Am Soc Nephrol. 2015;10:1757-1766. doi: 10.2215/CJN.02020215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin BM, Zhang Y, Yu B, et al. Metabolome-wide association study of estimated glomerular filtration rates in Hispanics. Kidney Int. 2022 10;101(1):144-151. doi: 10.1016/j.kint.2021.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorlie PD, Aviles-Santa LM, Wassertheil-Smoller S, et al. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 201010;20(8):629-641. doi: 10.1016/j.annepidem.2010.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lavange LM, Kalsbeek WD, Sorlie PD, et al. Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 201010;20(8):642-649. doi: 10.1016/j.annepidem.2010.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thyagarajan B, Howard AG, Durazo-Arvizu R, et al. Analytical and biological variability in biomarker measurement in the Hispanic Community Health Study/Study of Latinos. Clinica Chim Acta. 201610;463:129-137. doi: 10.1016/j.cca.2016.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans AM, Bridgewater BR, Liu Q, et al. High resolution mass spectrometry improves data quantity and quality as compared to unit mass resolution mass spectrometry in high-throughput profiling metabolomics. Metabolomics. 2014; 4(2). doi: 10.4172/2153-0769.1000132 [DOI] [Google Scholar]
- 22.Pang Z, Chong J, Zhou G, et al. MetaboAnalyst 5.0: narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021;49(W1):W388-W396. doi:10.1093/nar/gkab382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9(1):559. doi: 10.1186/1471-2105-9-559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berglund G, Elmstahl S, Janzon L, Larsson SA. Design and feasibility. J Intern Med. 1993;233(1):45-51. doi: 10.1111/j.1365-2796.1993.tb00647.x [DOI] [PubMed] [Google Scholar]
- 25.Brunkwall L, Jonsson D, Ericson U, et al. The Malmo Offspring Study (MOS): design, methods and first results. Eur J Epidemiol. 2021;36(1):103-116. doi: 10.1007/s10654-020-00695-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ottosson F, Brunkwall L, Ericson U, et al. Connection between BMI-related plasma metabolite profile and gut microbiota. J Clin Endocrinol Metab. 2018;103(4):1491-1501. doi: 10.1210/jc.2017-02114 [DOI] [PubMed] [Google Scholar]
- 27.Ottosson F, Ericson U, Almgren P, et al. Postprandial levels of branch chained and aromatic amino acids associate with fasting glycaemia. J Amino Acids. 2016;2016:1-9. doi: 10.1155/2016/8576730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wishart DS, Feunang YD, Marcu A, et al. HMDB 4.0: the human metabolome database for 2018. Nucleic Acids Res. 2018;46(D1):D608-D617. doi: 10.1093/nar/gkx1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guijas C, Montenegro-Burke JR, Domingo-Almenara X, et al. METLIN: a technology platform for identifying knowns and unknowns. Anal Chem. 201810;90(5):3156-3164. doi: 10.1021/acs.analchem.7b04424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sumner LW, Amberg A, Barrett D, et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics. 2007;3:211-221. doi: 10.1007/s11306-007-0082-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams RR, Rao DC, Ellison RC, et al. NHLBI family blood pressure program: methodology and recruitment in the HyperGEN network. Hypertension genetic epidemiology network. Ann Epidemiol. 2000;10(6):389-400. doi: 10.1016/s1047-2797(00)00063-6 [DOI] [PubMed] [Google Scholar]
- 32.Corsino L, Sotres-Alvarez D, Butera NM, et al. Association of the DASH dietary pattern with insulin resistance and diabetes in US Hispanic/Latino adults: results from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). BMJ Open Diabetes Res Care. 2017;5(1):e000402. doi: 10.1136/bmjdrc-2017-000402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 201210;15(5):606-614. doi: 10.1016/j.cmet.2012.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang GF, Jensen MV, Gray SM, et al. Reductive TCA cycle metabolism fuels glutamine- and glucose-stimulated insulin secretion. Cell Metab. 2021;33(4):804-817.e5. doi: 10.1016/j.cmet.2020.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suhre K, Meisinger C, Doring A, et al. Metabolic footprint of diabetes: a multiplatform metabolomics study in an epidemiological setting. PLoS One. 2010;5(11):e13953. doi: 10.1371/journal.pone.0013953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamanouchi T, Minoda S, Yabuuchi M, et al. Plasma 1, 5-anhydro-D-glucitol as new clinical marker of glycemic control in NIDDM patients. Diabetes. 1989;38(6):723-729. doi: 10.2337/diab.38.6.723 [DOI] [PubMed] [Google Scholar]
- 37.Stettler C, Stahl M, Allemann S, et al. Association of 1, 5-anhydroglucitol and 2-h postprandial blood glucose in type 2 diabetic patients. Diabetes Care. 2008;31(8):1534-1535. doi: 10.2337/dc08-0385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saito H, Ohtomo T, Inui K. Na(+)-dependent uptake of 1, 5-anhydro-D-glucitol via the transport systems for D-glucose and D-mannose in the kidney epithelial cell line, LLC-PK1. Nihon Jinzo Gakkai Shi. 1996;38(10):435-440. [PubMed] [Google Scholar]
- 39.Tavares G, Venturini G, Padilha K, et al. 1, 5-anhydroglucitol predicts CKD progression in macroalbuminuric diabetic kidney disease: results from non-targeted metabolomics. Metabolomics. 2018;1439(4):39. doi: 10.1007/s11306-018-1337-9 [DOI] [PubMed] [Google Scholar]
- 40.Haffner SM, Ralph D, Saad MF, et al. Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with non-Hispanic whites. The Insulin Resistance Atherosclerosis Study. Diabetes. 1996;45(6):742-748. doi: 10.2337/diab.45.6.742 [DOI] [PubMed] [Google Scholar]
- 41.Wang T, Fu X, Chen Q, et al. Arachidonic acid metabolism and kidney inflammation. Int J Mol Sci. 2019;20(15):3683. doi: 10.3390/ijms20153683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naresh CN, Hayen A, Weening A, Craig JC, Chadban SJ. Day-to-day variability in spot urine albumin-creatinine ratio. Am J Kidney Dis. 2013;62(6):1095-1101. doi: 10.1053/j.ajkd.2013.06.016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Observational data have been deposited to the Database of Genotypes and Phenotypes (dbGaP), phs000810.v1.p1.