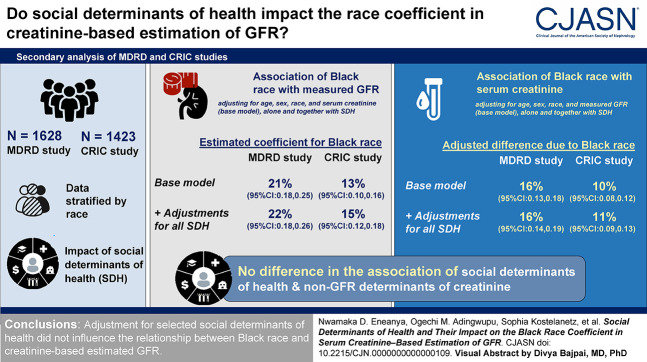
Visual Abstract
Keywords: creatinine, social determinants of health, equity
Abstract
Background
The cause for differences in serum creatinine between Black and non-Black individuals incorporated into prior GFR-estimating equations is not understood. We explored whether social determinants of health can account for this difference.
Methods
We conducted a secondary analysis of baseline data of the Modification of Diet in Renal Disease and Chronic Renal Insufficiency Cohort studies (N=1628 and 1423, respectively). Data in both study cohorts were stratified by race (Black versus non-Black). We first evaluated the extent to which the coefficient of Black race in estimating GFR from creatinine is explained by correlations of race with social determinants of health and non-GFR determinants of creatinine. Second, we evaluated whether the difference between race groups in adjusted mean creatinine can be explained by social determinants of health and non-GFR determinants of creatinine.
Results
In models regressing measured GFR on creatinine, age, sex, and race, the coefficient for Black race was 21% (95% confidence interval, 0.176 to 0.245) in Modification of Diet in Renal Disease and 13% (95% confidence interval, 0.097 to 0.155) in the Chronic Renal Insufficiency Cohort and was not attenuated by the addition of social determinants of health, alone or in combination. In both studies, the coefficient for Black race was larger at lower versus higher income levels. In models, regressing creatinine on measured GFR, age, and sex, mean creatinine was higher in Black versus non-Black participants in both studies, with no effect of social determinants of health.
Conclusions
Adjustment for selected social determinants of health did not influence the relationship between Black race and creatinine-based estimated GFR.
Introduction
Use of race in medical algorithms is facing increasing scrutiny.1,2 Differences observed in race groups that are subsequently assigned to individuals by these algorithms may be secondary to social determinants of health, driven by the effects of structural racism and racial discrimination on biology.3 Social determinants of health are “conditions in which people are born, grow, work, live, and age and the wider set of forces and systems shaping the conditions of daily life”; their effects on health can be profound.4–7 On average, Black individuals in the United States are more likely to live in segregated neighborhoods or below the federal poverty level, have lower rates of educational attainment, and have higher rates of unemployment compared with White individuals.3,8 Differences in socioeconomic status and subsequent social determinants of health can influence individual biology through allostatic load, nutritional status, lifestyle habits, and control of comorbidities.9
In the United States, one example of a medical algorithm that adjusts for race is the serum creatinine–based eGFR equation.10,11 In the Modification of Diet in Renal Disease (MDRD) study and some other studies, Black individuals were observed to have higher average creatinine for the same level of measured GFR compared with non-Black individuals, reflecting differences in determinants of creatinine other than GFR. The inclusion of a Black race coefficient in the previously recommended MDRD study and in the more accurate Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations minimized statistical errors in the development populations for those studies.10–13 The cause of these differences was not investigated at the time the equations were developed and remains incompletely understood. Social determinants of health can lead to variation in muscle mass, physical activity, or dietary protein intake (e.g., animal versus vegetable protein). We hypothesize that if, on average, social determinants of health vary between race groups, these differences could contribute to the racial differences observed in the association of creatinine with measured GFR.14,15 Recently, a new CKD-EPI equation was published that does not include race.16 Despite the availability of this new equation, it is useful to determine whether we can identify factors that contribute to the observed group-level racial differences in creatinine-based eGFR, as any factors that contribute to between race differences could help better interpret potential errors in eGFR for individual patients, regardless of race.
The goals of this study were to determine the extent to which social determinants of health and other factors that influence creatinine account for differences in the relationship between creatinine and GFR previously attributed to Black race. Furthermore, we also sought to characterize variation in the Black race coefficient across two studies (MDRD study and the Chronic Renal Insufficiency Cohort [CRIC]) and social determinants of health.
Methods
Study Populations
The MDRD study was a multicenter, randomized control trial conducted between 1989 and 1991 of people aged 18–70 years with nondiabetic CKD that evaluated the effect of dietary protein restriction and strict BP control on CKD progression.13 We included 1628 participants incorporated in the development of the MDRD study equation (Supplemental Table 1).10 CRIC study is a prospective multicenter, observational cohort study with enrollment from 2003 to 2007 of people aged 21–74 years with CKD.17 We included 1423 participants who had measured GFR using urinary clearance of iothalamate measured at baseline (Supplemental Table 1).12
Variable Descriptions
As previously described, participants were grouped into the originally defined racial categories: Black and non-Black.10,12 The social determinants of health ascertained at baseline in both studies included gross yearly household income, number of persons supported in the household, education, employment, and marital status (Supplemental Item 1). To estimate household income per persons in the household, we divided the mid-point of the household income range in each income category by the number of persons in the household. For categorical analyses, we divided this ratio into two groups: ≤$6800 and >$6800 per person on the basis of the population distribution and federal poverty levels at the time of the MDRD study. The number of persons supported in a household was not reported in the CRIC study, thus we used the mid-point of the household income range to define household income categories, i.e., <$50,000 and ≥$50,000 per household.
We evaluated non-GFR determinants of creatinine, including creatinine excretion, creatinine secretion, and protein intake. Creatinine excretion was defined as the amount of creatinine in a 24-hour urine collection. Creatinine secretion was calculated as measured creatinine clearance minus measured GFR and was adjusted for body surface area. We estimated protein intake from urine nitrogen excretion (UUN) (g/kg per day) = 6.25 (UUN [g/d] + 0.031 [g/kg per day] weight [kg]), where 0.031 g/kg per day is a constant reflecting the rate of urinary excretion of nitrogen in compounds other than urea.18 These variables were treated as continuous variables for covariate adjustment, and median splits were used to define categories for subgroup and interaction analyses.
Statistical Analyses
We conducted two separate analyses to investigate the role of social determinants of health in accounting for differences in the relationship between measured GFR and creatinine by race groups. In the first analysis, we evaluated the extent to which the coefficient of race in estimating GFR from creatinine is explained by correlations of race with social determinants of health and non-GFR determinants of creatinine. In the second analysis, we evaluated the extent to which the association of race with creatinine at a fixed level of GFR is explained by social determinants of health and non-GFR determinants of creatinine.
For the first analysis, we regressed measured GFR as the dependent variable on age, sex, race, and creatinine along with social determinants of health and non-GFR determinants of creatinine as independent predictor variables. We used a base model with age, sex, race, and creatinine. We used log transformation of creatinine and measured GFR to reflect the multiplicative associations between creatinine and GFR. To this base model, we added, as additional predictor variables, each social determinant of health factor alone, then all together, then protein intake, and finally creatinine excretion. Creatinine secretion was not included as an adjustment variable because it cannot be computed independently of the outcome, measured GFR.19
We also investigated the stability of the race coefficient across different study settings and social determinants of health. We used the Wald test to test for statistical difference in coefficients between studies. We repeated the regression models in the first analysis using interaction terms—this was done to examine whether the Black race coefficient differed significantly between subgroups defined by income level, education status, employment status, marital status, protein intake, and creatinine excretion as specified by the authors before the data analysis.
For the second analysis, we regressed creatinine as the dependent variable to measured GFR, age, sex, and race along with social determinants of health as independent variables. Measurement error in measured GFR can be substantial and as such differs from the true GFR. To account for the measurement error, as we have previously performed, we used an errors-in-variables model with a variance of 0.015 on the log scale.20–22 To simplify interpretation, we transformed parameter estimates as the geometric mean percent difference of creatinine from the 25th to 75th percentile of a continuous variable or for the presence versus absence of categorical predictors.
For the evaluation of non-GFR determinants of creatinine other than social determinants of health, we used the errors-in-variables model to obtain estimates of mean levels of creatinine, creatinine excretion, creatinine secretion, and protein intake adjusted for mean age and measured GFR at the median value for each population by race and sex subgroups. We assessed the difference between race groups using nonoverlapping confidence intervals between the two groups.
Results
Baseline Characteristics
Of the MDRD study participants, 12% were Black. Among the Black participants, 43% were female, and the mean (±SD) age and weight were 49±12 years and 84±17 kg, respectively. The non-Black participants had a similar distribution of women and were of similar age, but had lower mean weight (79±17 kg; P<0.001). Of the CRIC participants, 37% were Black. Among the Black participants, 47% were female, and the mean age and weight were 56±13 years and 95±21 kg, respectively. The non-Black participants had the same average age, but were less often female (42%) and had lower mean weight (86±20 kg) (Table 1).
Table 1.
Baseline characteristics of participants in the Modification of Diet in Renal Disease and Chronic Renal Insufficiency Cohort studies by race group
| Baseline Characteristics | MDRD | CRIC | ||
|---|---|---|---|---|
| Black N=197 | Non-Black N=1431 | Black N=528 | Non-Black N=895 | |
| Age, yr | 49±12 | 51±13 | 56±12 | 56±12 |
| Female sex, n (%) | 84 (43) | 561 (39) | 247 (47) | 373 (42) |
| Height, cm | 171±10 | 171±10 | 170±9 | 168±10 |
| Weight, kg | 84±17 | 79±17 | 95±21 | 86±20 |
| BMI, kg/m2 | 29±5 | 27±5 | 33±7 | 30±7 |
| Diabetes, n (%) | 22 (11) | 77 (5) | 263 (50) | 423 (47) |
| Measured GFR, ml/min per 1.73 m2 | 44±21 | 39±21 | 47±19 | 489±20 |
| Estimated protein intake, g/kg per day | 0.9±0.2 | 1.0±0.2 | 0.7±0.3 | 0.9±0.3 |
| Income | ||||
| Low | 45 (23) | 144 (10) | 328 (62) | 425 (47) |
| High | 134 (68) | 1198 (84) | 118 (22) | 347 (38) |
| Education, n (%) | ||||
| College graduate or more | 59 (30) | 543 (38) | 124 (23) | 133 (15) |
| Some college | 51 (26) | 379 (26) | 122 (23) | 140 (16) |
| High school graduate | 51 (26) | 379 (26) | 178 (34) | 223 (25) |
| Less than high school | 35 (18) | 129 (9) | 104 (20) | 398 (44) |
| Employment, n (%) | ||||
| Employed | 121 (61) | 979 (68) | 368 (70) | 763 (85) |
| Unemployed | 25 (13) | 118 (8) | 60 (11) | 54 (6) |
| Disability | 42 (21) | 246 (17) | 100 (19) | 78 (9) |
| Married, n (%) | 103 (52) | 1059 (74) | 232 (44) | 607 (68) |
Data are presented as mean (±SD) or as N and percentage. Household income per persons in household was used to define income groups in MDRD: ≤$6800 for low and >$6800 for high. Mid-point of the household income range was used to define income groups in CRIC: <$50,000 for low and ≥$50,000 for high. For more detailed income categories in each study, see Supplemental Material and Supplemental Table 3. MDRD, Modification of Diet in Renal Disease; CRIC, Chronic Renal Insufficiency Cohort; BMI, body mass index.
In MDRD, a lower proportion of Black compared with non-Black participants had attained a college education or more (30% versus 38%), were employed (61% versus 68%), were married (52% versus 74%), and had a household income per persons in household more than $6800 (68% versus 84%) (Table 1). Similarly, in the CRIC study, a lower percentage of Black participants were employed (70% versus 85%), were married (44% versus 68%), and had a household income more than $50,000 (22% versus 38%) compared with non-Black participants. The proportion of Black participants in the CRIC study with a college education or more was higher compared with non-Black participants (23% versus 15%). Mean protein intake was similar between Black and non-Black participants in both study cohorts (Table 1 and Supplemental Table 2).
In both studies, after adjustments for age, sex, and measured GFR, mean creatinine was higher in Black compared with non-Black participants and protein intake was lower in Black compared with non-Black participants. However, creatinine excretion was higher and creatinine secretion was lower in Black compared with non-Black participants, with inconsistent results by study (Table 2).
Table 2.
Age, sex, measured GFR-adjusted serum creatinine, creatinine excretion, and creatinine secretion by race groups
| Predicted Factors | MDRD | CRIC | ||
|---|---|---|---|---|
| Black | Non-Black | Black | Non-Black | |
| Males | ||||
| Predicted mean serum creatinine, mg/dl | 2.17 (2.12 to 2.22)a | 2.06 (2.04 to 2.08)a | 1.92 (1.90 to 1.95)a | 1.71 (1.69 to 1.73)a |
| Predicted mean creatinine excretion, mg/d | 1518 (1473 to 1564)a | 1383 (1367 to 1399)a | 1331 (1288 to 1375)a | 1253 (1218 to 1287)a |
| Predicted mean creatinine secretion, ml/min per 1.73 m2 | 10 (8 to 12)a | 12 (12 to 13)a | 3 (1 to 5) | 5 (3 to 6) |
| Predicted mean protein intake, g/kg per day | 0.94 (0.91 to 0.98)a | 1.01 (0.99 to 1.02)a | 0.77 (0.74 to 0.79)a | 0.90 (0.88 to 0.93)a |
| Females | ||||
| Predicted mean serum creatinine, mg/dl | 1.84 (1.79 to 1.88)a | 1.66 (1.64 to 1.68)a | 1.53 (1.50 to 1.56)a | 1.36 (1.34 to 1.38)a |
| Predicted mean creatinine excretion, mg/d | 1179 (1138 to 1220)a | 1057 (1041 to 1072)a | 1050 (1004 to 1096) | 979 (945 to 1012) |
| Predicted mean creatinine secretion, ml/min per 1.73 m2 | 8 (6 to 10)a | 11 (10 to 12)a | 3 (1 to 6) | 5 (3 to 6) |
| Predicted mean protein intake, g/kg per day | 0.90 (0.87 to 0.94)a | 0.98 (0.96 to 1.00)a | 0.70 (0.67 to 0.72)a | 0.84 (0.81 to 0.86)a |
The coefficients and 95% confidence intervals in the table are predicted using the mean age (years) and mean measured GFR (ml/min per 1.73 m2) for each group. Mean age and measured GFR in MDRD were 49.5 and 39.7 for Black men, 48.1 and 35.8 for Black women, 51.7 and 34.4 for non-Black men, and 49.5 and 33.0 for non-Black women, respectively, while mean age and measured GFR in CRIC were 55.6 and 43.6 for Black men, 56.4 and 42.5 for Black women, 56.1 and 44.6 for non-Black men, and 55.9 and 44.0 for non-Black women, respectively. MDRD, Modification of Diet in Renal Disease; CRIC, Chronic Renal Insufficiency Cohort.
Coefficients with nonoverlapping confidence intervals are indicated.
In the MDRD and CRIC cohorts, the average measured GFR was 21% (95% confidence interval [CI], 0.176 to 0.245) and 13% (95% CI, 0.097 to 0.155) higher for a given serum creatinine, respectively, in Black compared with non-Black participants when adjusted for age, sex, and creatinine. Additional adjustments for each of the social determinants of health alone or jointly did not substantially change the magnitude of the Black race coefficient in regression models for both study cohorts (Figure 1 and Supplemental Table 3). In the MDRD cohort, adjusting for protein intake and creatinine excretion increased (22.6% [95% CI, 0.192 to 0.261]) and decreased (16.9% [95% CI, 0.137 to 0.201]) the magnitude of the race coefficient, respectively, but this was not observed in the CRIC study. For both study cohorts, adjusting for social determinant of health factors did not affect the magnitude of the age and sex coefficients (Supplemental Table 4).
Figure 1.
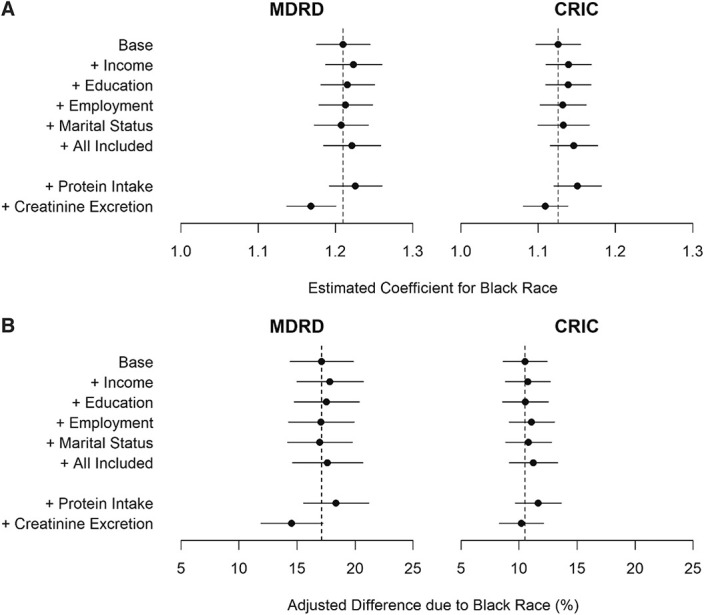
Effect of social determinants of health variables, protein intake, and creatinine excretion on the magnitude of the Black race coefficient in GFR-estimating equations and in association with serum creatinine. (A) Associations of Black race with measured GFR. Estimated coefficient for Black race from regression of measured GFR on age, sex, race, and serum creatinine (base model), alone and together with social determinants of health, protein intake, and creatinine excretion. (B) Associations of Black race with serum creatinine. Coefficient for association of Black race with serum creatinine adjusted for measured GFR, GFR measurement error, age, and sex (base model), alone and together with social determinants of health, protein intake, and creatinine excretion. Left panel shows the results for the MDRD study. Right panel shows the results for CRIC. CRIC, Chronic Renal Insufficiency Cohort; MDRD, Modification of Diet in Renal Disease.
The Black race coefficient in the MDRD study was statistically different from the coefficient in the CRIC study, both in the base model (0.21 versus 0.13; P value from Wald test −0.0003) and in an expanded model that included social determinant of health factors (0.18 versus 0.12; P value from Wald test −0.037). In both studies, the coefficient for Black race was of a greater magnitude at lower versus higher income levels (Table 3) (MDRD: 1.32 [95% CI, −0.71 to 3.34] versus 1.19 [95% CI, −0.80 to 3.19] [P value for the interaction <0.01] and CRIC: 1.16 [95% CI, −0.83 to 3.15] versus 1.10 [95% CI, −0.90 to 3.10] [P value for the interaction of <0.05]).
Table 3.
Variation in coefficient for Black race by subgroups of social determinants of health
| Subgroups of Social Determinants of Health | MDRD | CRIC | ||
|---|---|---|---|---|
| Black Race Coefficient (95% CI) | P Value for Interaction | Black Race Coefficient (95% CI) | P Value for Interaction | |
| Base model | 0.21 (0.18 to 0.25) | — | 0.13 (0.10 to 0.16) | — |
| Income | ||||
| Low | 1.32 (−0.71 to 3.34) | 0.005 | 1.16 (−0.83 to 3.15) | 0.04 |
| High | 1.19 (−0.80 to 3.19) | 1.10 (−0.90 to 3.10) | ||
| Education | ||||
| Some college or more | 1.20 (−0.80 to 3.20) | 0.48 | 1.11 (−0.88 to 3.11) | 0.12 |
| High school or less | 1.23 (−0.78 to 3.23) | 1.16 (−0.84 to 3.16) | ||
| Employment | ||||
| Employed | 1.20 (−0.79 to 3.20) | Ref | 1.14 (−0.85 to 3.13) | Ref |
| Unemployed | 1.29 (−0.76 to 3.33) | 0.13 | 1.09 (−0.98 to 3.15) | 0.34 |
| Disability | 1.16 (−0.86 to 3.19) | 0.59 | 1.17 (−0.87 to 3.20) | 0.50 |
| Marital status | ||||
| Married | 1.21 (−0.79 to 3.22) | 0.86 | 1.10 (−0.90 to 3.10) | 0.09 |
| Not married | 1.20 (−0.80 to 3.20) | 1.16 (−0.84 to 3.15) | ||
| Protein intake | ||||
| Low | 1.23 (−0.76 to 3.23) | 0.37 | 1.13 (−0.86 to 3.13) | 0.42 |
| High | 1.20 (−0.81 to 3.21) | 1.17 (−0.83 to 3.16) | ||
| Creatinine excretion | ||||
| Low | 1.20 (−0.81 to 3.21) | 0.63 | 1.09 (−0.90 to 3.09) | 0.20 |
| High | 1.19 (−0.81 to 3.18) | 1.13 (−0.86 to 3.13) | ||
Low income in CRIC: household income less than the median income range (i.e., <$50,000). High income in CRIC: household income more than the median household income range (≥$50,000). 0.27–0.78 and 0.78–2.70 for low and high estimated protein intake values from urine urea nitrogen, respectively. 25–1290 and 1291–5531 for low and high creatinine excretion values, respectively. Low income in MDRD: household income per persons in household ≤$6800. High income in MDRD: household income per persons in household >$6800. 0.19–0.99 and 1.0–3.47 for low and high dietary protein values, respectively. 0.34–0.96 and 0.97–2.75 for low and high estimated protein intake values from urine urea nitrogen, respectively. 341–1363 and 1365–3325 for low and high creatinine excretion values, respectively. MDRD, Modification of Diet in Renal Disease; CRIC, Chronic Renal Insufficiency Cohort; CI, confidence interval; Ref, reference.
In both studies, younger age, male sex, Black race, higher levels of dietary protein, and creatinine excretion were all associated with higher mean creatinine, after accounting for measured GFR (Supplemental Table 5). The strongest factor that was associated with higher creatinine was creatinine excretion (19% and 11% in MDRD and CRIC, respectively). In MDRD, a greater number of people supported in a household, higher education, and being employed were also associated with higher mean creatinine at the same level of measured GFR.
For the same levels of measured GFR, age, and sex, Black race was associated with 16% and 10% higher mean of creatinine in the MDRD and CRIC studies, respectively (Figure 1 and Supplemental Table 6). Further adjustments for social determinants of health, dietary protein, and creatinine excretion individually and together did not substantially change the magnitude of the association of Black race with creatinine in either study cohort.
Discussion
We sought to explore the effect of social determinants of health and non-GFR determinants of creatinine on the Black race coefficient in GFR equations in two studies of patients with CKD. We found no evidence that correlation of race with social determinants of health explains racial differences in the relationship between measured GFR and creatinine in either the MDRD or CRIC study. However, we did observe that the magnitude of the coefficient of Black race differed between the two studies and that lower income was associated with a greater coefficient for Black race in both studies. Although the cause of the observed racial differences in creatinine remains unknown, this instability in the value of the race coefficient across income groups, in conjunction with other considerations, supports current concerns of using race in GFR estimation and recommendations for use of race-free equations by US national kidney organizations.23
Serum concentrations of all endogenous filtration markers are affected by non-GFR determinants, including factors that influence their generation, tubular handling, and extrarenal elimination.21,22,24–27 For creatinine, the key determinants are generation related to diet and muscle mass, which are both likely to vary according to social determinants of health. For example, the magnitude of lean muscle mass in an individual is dependent on lifestyle factors such as nutrition and physical activity.28,29 Creatinine intake is highly dependent on the quantity and quality of dietary protein intake (e.g., diets containing canned and thoroughly cooked meat) and can vary on the basis of concomitant carbohydrate intake.30–32 Differences in such factors between race groups could have contributed to the observed racial variation in the relationship between measured GFR and serum creatinine concentration. In both studies, after adjusting for measured GFR, we found that higher income, more education, and higher dietary protein intake were associated with higher mean creatinine, which could reflect higher socioeconomic status associated with better physical fitness and better nutrition.33,34 Despite the fact that these social determinants of health differed by race groups, we did not see attenuation of the race coefficient with adjustment for social determinant of health variables in either study. Thus, this study does not support the selected social determinants of health variables fully explaining the coefficient for Black race in creatinine-based GFR-estimating equations; indeed, we observed a slight strengthening of the race coefficient when adjusting for all the social determinant of health variables together. Nevertheless, the MDRD and CRIC studies were performed before the current era where greater attention is paid to how social and environmental factors can affect biological observations; it is likely that the restriction of social determinants of health to demographic factors in these studies did not fully capture all the potential effects of social determinants of health on creatinine. For example, in the United States, racial differences have been observed in source of dietary protein (e.g., animal versus vegetable protein) and physical activity because of costs and other socioeconomic factors.14,15 It is possible that the type of dietary protein or physical activity would not have been captured by the social determinant of health factors ascertained in these studies.
Our findings of variation in the magnitude of the race coefficient between the two studies and by income group may provide some support for variation in the magnitude of non-GFR determinants between groups. For instance, in both studies, we observed higher creatinine excretion in Black versus non-Black participants, but Black participants in the CRIC study had lower levels of creatinine excretion compared with Black participants in MDRD. In MDRD, but not in the CRIC study, we also observed that creatinine secretion was lower in Black versus non-Black participants.35 These findings may be seen as consistent with prior investigations that report that the Black race coefficient is not required for unbiased GFR estimation in Black individuals living in Africa (e.g., a different geographical group than in North America), which could be because of variation in the type of dietary protein intake, physical activity, and other environmental factors.36
In our view, the results from this investigation do not detract from removing race as a component of GFR estimation. There are multiple considerations as to why GFR-estimating equations should not include race, most importantly that race is a social construct with greater biological variability between individuals within racial groups than between racial groups.23,37–40 Future work should investigate endogenous filtration markers that do not vary by race, and recruitment of diverse populations across individuals of different race, sex, socioeconomic strata, and geography to account for the multiple non-GFR factors that determine serum concentrations of filtration markers.41,42
This study has several strengths. We used two cohorts of patients with CKD included in the current recommended CKD-EPI equation for GFR reporting. Measured GFR was determined using a consistent method of urinary iothalamate clearance for all participants, and we used these data to perform multiple analyses to account for statistical and random errors. A major limitation was the inability to account for the wider array of factors, which may reflect the effects of systemic racism and discrimination on health. Tools, such as the Area Deprivation Index, which includes multiple indicators of socioeconomic disadvantage using census tract data (e.g., crowded living situations, proportion of white collar occupations), are increasingly being used to demonstrate the powerful link between neighborhood segregation (as opposed to race alone) and racial disparities in health outcomes.43–45 We were limited in our ability to assess confounding by creatinine secretion given dependence of its measurement on measured GFR and were therefore unable to comment on potential racial differences caused by external factors (e.g., medications that affect creatinine secretion). Other studies of CRIC data have shown substantial error in urinary collection, which would affect estimates of both creatinine excretion and secretion and estimation of protein intake from UUN. The lack of consistent findings between studies may be because of true differences between participants in both studies or variation in error in urine collections and preclude complete exploration of the difference in the magnitude of the coefficient between studies.46 Finally, we acknowledge that populations in the MDRD and CRIC studies were limited by regional variation as well as in diversity and may not accurately represent more contemporary populations of patients with kidney disease.
In conclusion, we were not able to determine that the social determinant of health variables captured in these studies provide an explanation for racial differences in creatinine-based GFR estimation. However, instability in the value of the race coefficient across settings together with health equity considerations is a strong justification for the continued removal of race from the GFR equation.3,23 These analyses serve as an example that can help guide future analyses of GFR estimation or other kidney disease biomarkers as to the potential effect of social determinants of health. Future work should focus on how to best assess and incorporate patients' social and environmental conditions to improve clinical and research standards.41,42
Supplementary Material
Acknowledgments
The CRIC study was conducted by the CRIC investigators and supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Data from the study reported here were supplied by the NIDDK Central Repository. This manuscript was not prepared in collaboration with investigators of the CRIC study and does not necessarily reflect the opinions or views of the study, the NIDDK Central Repository, or the NIDDK.
The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Social Determinants of Health and Estimation of Kidney Function,” on pages 424–426.
Disclosures
S. Beddhu reports research funding from Bayer, Boehringer Ingelheim, and Novartis; royalties from UpToDate; advisory or leadership roles for CJASN and Kidney Reports; and grants to the University of Utah from the National Institute of Health. R. Boucher reports grants to the University of Utah from the National Institute of Health. N.D. Eneanya reports employment with Fresenius Medical Care; consultancy agreements with Somatus and DaVita; ownership interest in Somatus; advisory or leadership roles for Healthcare: The Journal of Delivery Science and Innovation and Kidney Medicine; and other interests or relationships with the National Kidney Foundation of Eastern PA and NJ Advisory Board. T. Greene reports consultancy agreements with AstraZeneca, Invokana, Janssen Pharmaceuticals, Novartis, and Pfizer Inc. and grants to the University of Utah from AstraZeneca, Boehringer-Ingelheim, CSL, and Vertex. L.A. Inker reports consultancy agreements with Diamtrix and Tricida Inc.; funding to the Tufts Medical Center for research and contracts with Chinnocks, the National Institutes of Health, National Kidney Foundation, Omeros, and Reata Pharmaceuticals; advisory or leadership roles for the Alport Foundation Medical Advisory Council and the NKF Scientific Advisory Board; and other interests or relationships as an American Society of Nephrology member and National Kidney Foundation member. S. Kostelanetz reports receiving a grant from the Agency for Healthcare Research and Quality and personal fees from the Cambridge Health Alliance Center for Health Equity Education and Advocacy. A.S. Levey reports grants and contracts paid to the Tufts Medical Center from NIH and NKF, contracts paid to A.S. Levey from AstraZeneca (DSMB for dapagliflozin trials), and honoraria from academic medical centers for visiting professorships. J.B. Lewis reports consultancy agreements with Biovie, CSL Behring, and Veno Stent; ownership interest in Circle Medical Management, a dialysis company; honoraria from Biovie and CSL Bering; and advisory or leadership roles as treasurer and board member of the CSG, Collaborative Study Group, a not-for-profit academic research organization (not paid) and as Chair of the Cardio Renal Advisory Committee for the FDA (paid for time). K.C. Norris reports receiving grants from the American Heart Association and the National Institute of Health, consulting fees from Atlantis Health, and honoraria for grand rounds lectures. All remaining authors have nothing to disclose.
Funding
Research reported in this manuscript was supported by grants (R01DK097020 and 1R01DK116790) to the Tufts Medical Center from the National Institute of Diabetes and Digestive and Kidney Diseases.
Author Contributions
N.D. Eneanya, L.A. Inker, and S. Kostelanetz conceptualized the study; R. Boucher, T. Greene, and S. Miao were responsible for formal analysis; S. Beddhu, R. Boucher, J. Chaudhari, N.D. Eneanya, T. Greene, L.A. Inker, S. Kostelanetz, A.S. Levey, J.B. Lewis, S. Miao, and K.C. Norris were responsible for investigation; O.M. Adingwupu, S. Beddhu, R. Boucher, J. Chaudhari, N.D. Eneanya, T. Greene, L.A. Inker, S. Kostelanetz, A.S. Levey, J.B. Lewis, S. Miao, and K.C. Norris were responsible for methodology; O.M. Adingwupu was responsible for project administration; O.M. Adingwupu, S. Beddhu, N.D. Eneanya, T. Greene, L.A. Inker, S. Kostelanetz, A.S. Levey, J.B. Lewis, and K.C. Norris wrote the original draft; and O.M. Adingwupu, S. Beddhu, N.D. Eneanya, T. Greene, L.A. Inker, S. Kostelanetz, A.S. Levey, J.B. Lewis, and K.C. Norris reviewed and edited the manuscript.
Data Sharing Statement
Observational data have been deposited to the NIDDK Repository, doi: 10.7326/0003-4819-130-6-199903160-00002.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/CJN/B642.
Supplemental Table 1. Measured GFR assessment in the MDRD study and CRIC.
Supplemental Table 2. Social determinants of health categories by race groups.
Supplemental Table 3. Effect of social determinants of health, estimated protein intake, and creatinine excretion on the coefficient for Black race in GFR-estimating equations.
Supplemental Table 4. Effect of social determinants of health, estimated protein intake, and creatinine excretion on age and sex coefficients in GFR-estimating equations.
Supplemental Table 5. Associations of demographic factors, social determinants of health, estimated protein intake, and creatinine excretion on serum creatinine after adjustment for measured GFR and GFR measurement error.
Supplemental Table 6. Effect of social determinants of health, estimated protein intake, and creatinine excretion on the association of Black race with serum creatinine after adjustment for measured GFR and GFR measurement error.
References
- 1.Eneanya ND, Yang W, Reese PP. Reconsidering the consequences of using race to estimate kidney function. JAMA. 2019;322(2):113–114. doi: 10.1001/jama.2019.5774 [DOI] [PubMed] [Google Scholar]
- 2.Vyas DA, Eisenstein LG, Jones DS. Hidden in plain sight—reconsidering the use of race correction in clinical algorithms. N Engl J Med. 2020;383(9):874–882. doi: 10.1056/nejmms2004740 [DOI] [PubMed] [Google Scholar]
- 3.Eneanya ND, Boulware LE, Tsai J, et al. Health inequities and the inappropriate use of race in nephrology. Nat Rev Nephrol. 2021;18(2):84–94. doi: 10.1038/s41581-021-00501-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braveman P, Egerter S, Williams DR. The social determinants of health: coming of age. Annu Rev Public Health. 2011;32(1):381–398. doi: 10.1146/annurev-publhealth-031210-101218 [DOI] [PubMed] [Google Scholar]
- 5.Silverstein M, Hsu HE, Bell A. Addressing social determinants to improve population health: the balance between clinical care and public health. JAMA. 2019;322(24):2379–2380. doi: 10.1001/jama.2019.18055 [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Social Determinants of Health; 2022. Accessed May 12, 2022. https://www.who.int/health-topics/social-determinants-of-health#tab=tab_1 [Google Scholar]
- 7.Hood CM, Gennuso KP, Swain GR, Catlin BB. County health rankings: relationships between determinant factors and health outcomes. Am J Prev Med. 2016;50(2):129–135. doi: 10.1016/j.amepre.2015.08.024 [DOI] [PubMed] [Google Scholar]
- 8.Eneanya ND, Crews DC. “Place, not race”: a focus on neighborhood as a risk factor for hospitalizations in patients receiving maintenance hemodialysis. Am J Kidney Dis. 2020;76(6):749–751. doi: 10.1053/j.ajkd.2020.08.002 [DOI] [PubMed] [Google Scholar]
- 9.Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. In: Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Smedley BD, Stith AY, Nelson AR, editors. National Academies Press (US); 2003. [PubMed] [Google Scholar]
- 10.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002 [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson AH, Yang W, Hsu CY, et al. Estimating GFR among participants in the chronic renal insufficiency cohort (CRIC) study. Am J Kidney Dis. 2012;60(2):250–261. doi: 10.1053/j.ajkd.2012.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. N Engl J Med. 1994;330(13):877–884. doi: 10.1056/nejm199403313301301 [DOI] [PubMed] [Google Scholar]
- 14.Smit E, Nieto FJ, Crespo CJ, Mitchell P. Estimates of animal and plant protein intake in US adults: results from the third national health and nutrition examination survey, 1988-1991. J Am Diet Assoc. 1999;99(7):813–820. doi: 10.1016/s0002-8223(99)00193-5 [DOI] [PubMed] [Google Scholar]
- 15.Saffer H, Dave D, Grossman M, Ann Leung L. Racial, ethnic, and gender differences in physical activity. J Hum Cap. 2013;7(4):378–410. doi: 10.1086/671200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inker LA, Eneanya ND, Coresh J, et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737–1749. doi: 10.1056/nejmoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lash JP, Go AS, Appel LJ, et al. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4(8):1302–1311. doi: 10.2215/CJN.00070109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maroni BJ, Steinman TI, Mitch WE. A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int. 1985;27(1):58–65. doi: 10.1038/ki.1985.10 [DOI] [PubMed] [Google Scholar]
- 19.Garimella PS, Tighiouart H, Sarnak MJ, Levey AS, Ix JH. Tubular secretion of creatinine and risk of kidney failure: the modification of diet in renal disease (MDRD) study. Am J Kidney Dis. 2021;77(6):992–994. doi: 10.1053/j.ajkd.2020.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.R Documentation. Errors-in-variables (EIV) linear regression. Accessed April 7, 2022. https://search.r-project.org/CRAN/refmans/eivtools/html/eivreg.html
- 21.Levey AS, Coresh J, Tighiouart H, Greene T, Inker LA. Measured and estimated glomerular filtration rate: current status and future directions. Nat Rev Nephrol. 2020;16(1):51–64. doi: 10.1038/s41581-019-0191-y [DOI] [PubMed] [Google Scholar]
- 22.Stevens LA, Schmid CH, Greene T, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75(6):652–660. doi: 10.1038/ki.2008.638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delgado C, Baweja M, Crews DC, et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN task force on reassessing the inclusion of race in diagnosing kidney disease. J Am Soc Nephrol. 2021;32(12):2994–3015. doi: 10.1681/ASN.2021070988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fricker M, Wiesli P, Brandle M, Schwegler B, Schmid C. Impact of thyroid dysfunction on serum cystatin C. Kidney Int. 2003;63(5):1944–1947. doi: 10.1046/j.1523-1755.2003.00925.x [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Foster MC, Tighiouart H, et al. Non-GFR determinants of low-molecular-weight serum protein filtration markers in CKD. Am J Kidney Dis. 2016;68(6):892–900. doi: 10.1053/j.ajkd.2016.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foster MC, Levey AS, Inker LA, et al. Non-GFR determinants of low-molecular-weight serum protein filtration markers in the elderly: AGES-kidney and MESA-kidney. Am J Kidney Dis. 2017;70(3):406–414. doi: 10.1053/j.ajkd.2017.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bökenkamp A, van Wijk JA, Lentze MJ, Stoffel-Wagner B. Effect of corticosteroid therapy on serum cystatin C and β2-microglobulin concentrations. Clin Chem. 2002;48(7):1123–1126. doi: 10.1093/clinchem/48.7.1123 [DOI] [PubMed] [Google Scholar]
- 28.Baxmann AC, Ahmed MS, Marques NC, et al. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin J Am Soc Nephrol. 2008;3(2):348–354. doi: 10.2215/CJN.02870707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klahr S, Alleyne GA. Effects of chronic protein-calorie malnutrition on the kidney. Kidney Int. 1973;3(3):129–141. doi: 10.1038/ki.1973.21 [DOI] [PubMed] [Google Scholar]
- 30.Nair S, O'Brien SV, Hayden K, et al. Effect of a cooked meat meal on serum creatinine and estimated glomerular filtration rate in diabetes-related kidney disease. Diabetes Care. 2014;37(2):483–487. doi: 10.2337/dc13-1770 [DOI] [PubMed] [Google Scholar]
- 31.Steenge GR, Simpson EJ, Greenhaff PL. Protein- and carbohydrate-induced augmentation of whole body creatine retention in humans. J Appl Physiol (1985). 2000;89(3):1165–1171. doi: 10.1152/jappl.2000.89.3.1165 [DOI] [PubMed] [Google Scholar]
- 32.Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80(3):1107–1213. doi: 10.1152/physrev.2000.80.3.1107 [DOI] [PubMed] [Google Scholar]
- 33.Ford ES, Merritt RK, Heath GW, et al. Physical activity behaviors in lower and higher socioeconomic status populations. Am J Epidemiol. 1991;133(12):1246–1256. doi: 10.1093/oxfordjournals.aje.a115836 [DOI] [PubMed] [Google Scholar]
- 34.Ingraham C. More money, more fitness: why people in the wealthiest states get more exercise. Washington Post. July 3, 2018. Accessed March 4, 2022. https://www.washingtonpost.com/news/wonk/wp/2018/07/03/more-money-more-fitness-why-people-in-the-wealthiest-states-get-more-exercise/ [Google Scholar]
- 35.Lewis J, Agodoa L, Cheek D, et al. Comparison of cross-sectional renal function measurements in African Americans with hypertensive nephrosclerosis and of primary formulas to estimate glomerular filtration rate. Am J Kidney Dis. 2001;38(4):744–753. doi: 10.1053/ajkd.2001.27691 [DOI] [PubMed] [Google Scholar]
- 36.Fabian J, George JA, Etheredge HR, et al. Methods and reporting of kidney function: a systematic review of studies from sub-Saharan Africa. Clin Kidney J. 2019;12(6):778–787. doi: 10.1093/ckj/sfz089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tishkoff SA, Kidd KK. Implications of biogeography of human populations for “race” and medicine. Nat Genet. 2004;36(11 suppl l):S21–S27. doi: 10.1038/ng1438 [DOI] [PubMed] [Google Scholar]
- 38.Delgado C, Baweja M, Burrows NR, et al. Reassessing the inclusion of race in diagnosing kidney diseases: an interim report from the NKF-ASN task force. J Am Soc Nephrol. 2021;32(6):1305–1317. doi: 10.1681/ASN.2021010039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grubbs V. Precision in GFR reporting: let's stop playing the race card. Clin J Am Soc Nephrol. 2020;15(8):1201–1202. doi: 10.2215/CJN.00690120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmed S, Nutt CT, Eneanya ND, et al. Examining the potential impact of race multiplier utilization in estimated glomerular filtration rate calculation on African-American care outcomes. J Gen Intern Med. 2021;36(2):464–471. doi: 10.1007/s11606-020-06280-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inker LA, Couture SJ, Tighiouart H, et al. A new panel-estimated GFR, including β(2)-microglobulin and β-trace protein and not including race, developed in a diverse population. Am J Kidney Dis. 2021;77(5):673–683.e1. doi: 10.1053/j.ajkd.2020.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eneanya ND, Kostelanetz S, Mendu ML. Race-free biomarkers to quantify kidney function: health equity lessons learned from population-based research. Am J Kidney Dis. 2021;77(5):667–669. doi: 10.1053/j.ajkd.2020.12.001 [DOI] [PubMed] [Google Scholar]
- 43.Hermes Z, Joynt Maddox KE, Yeh RW, Zhao Y, Shen C, Wadhera RK. Neighborhood socioeconomic disadvantage and mortality among medicare beneficiaries hospitalized for acute myocardial infarction, heart failure, and pneumonia. J Gen Intern Med. 2021;37(8):1894–1901. doi: 10.1007/s11606-021-07090-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weissman GE, Teeple S, Eneanya ND, Hubbard RA, Kangovi S. Effects of neighborhood-level data on performance and algorithmic equity of a model that predicts 30-day heart failure readmissions at an urban academic medical center. J Card Fail. 2021;27(9):965–973. doi: 10.1016/j.cardfail.2021.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams DR, Yu Y, Jackson JS, Anderson NB. Racial differences in physical and mental health: socio-economic status, stress and discrimination. J Health Psychol. 1997;2(3):335–351. doi: 10.1177/135910539700200305 [DOI] [PubMed] [Google Scholar]
- 46.Zhang X, Rule AD, McCulloch CE, Lieske JC, Ku E, Hsu CY. Tubular secretion of creatinine and kidney function: an observational study. BMC Nephrol. 2020;21(1):108. doi: 10.1186/s12882-020-01736-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Observational data have been deposited to the NIDDK Repository, doi: 10.7326/0003-4819-130-6-199903160-00002.