Keywords: anti-GBM disease, ESKD, risk factors, goodpasture syndrome, anti–glomerular basement membrane disease, vasculitis, ANCA Renal Risk Score, ARRS
Abstract
Significance Statement
Most patients with anti–glomerular basement membrane (GBM) disease present with rapidly progressive glomerulonephritis, and more than half develop ESKD. Currently, no tools are available to aid in the prognostication or management of this rare disease. In one of the largest assembled cohorts of patients with anti-GBM disease (with 174 patients included in the final analysis), the authors demonstrated that the renal risk score for ANCA-associated vasculitis is transferable to anti-GBM disease and the renal histology is strongly predictive of renal survival and recovery. Stratifying patients according to the percentage of normal glomeruli in the kidney biopsy and the need for RRT at the time of diagnosis improves outcome prediction. Such stratification may assist in the management of anti-GBM disease.
Background
Prospective randomized trials investigating treatments and outcomes in anti–glomerular basement membrane (anti-GBM) disease are sparse, and validated tools to aid prognostication or management are lacking.
Methods
In a retrospective, multicenter, international cohort study, we investigated clinical and histologic parameters predicting kidney outcome and sought to identify patients who benefit from rescue immunosuppressive therapy. We also explored applying the concept of the renal risk score (RRS), currently used to predict renal outcomes in ANCA-associated vasculitis, to anti-GBM disease.
Results
The final analysis included 174 patients (out of a total of 191). Using Cox and Kaplan–Meier methods, we found that the RRS was a strong predictor for ESKD. The 36-month renal survival was 100%, 62.4%, and 20.7% in the low-risk, moderate-risk, and high-risk groups, respectively. The need for renal replacement therapy (RRT) at diagnosis and the percentage of normal glomeruli in the biopsy were independent predictors of ESKD. The best predictor for renal recovery was the percentage of normal glomeruli, with a cut point of 10% normal glomeruli providing good stratification. A model with the predictors RRT and normal glomeruli (N) achieved superior discrimination for significant differences in renal survival. Dividing patients into four risk groups led to a 36-month renal survival of 96.4% (no RRT, N≥10%), 74.0% (no RRT, N<10%), 42.3% (RRT, N≥10%), and 14.1% (RRT, N<10%), respectively.
Conclusions
These findings demonstrate that the RRS concept is transferrable to anti-GBM disease. Stratifying patients according to the need for RRT at diagnosis and renal histology improves prediction, highlighting the importance of normal glomeruli. Such stratification may assist in the management of anti-GBM disease.
Podcast
This article contains a podcast at https://dts.podtrac.com/redirect.mp3/www.asn-online.org/media/podcast/JASN/2023_02_27_JASN0000000000000060.mp3
Introduction
Anti–glomerular basement membrane (GBM) disease is a rare and aggressive vasculitis involving the kidneys and lungs.1–3 It occurs as a result of pathogenic antibodies binding to the α3 chain of type IV collagen in the GBM.1,4,5 Anti-GBM disease is characterized by rapidly progressive glomerulonephritis and alveolar capillaritis, resulting in severe kidney dysfunction and pulmonary hemorrhage.2,6 Renal replacement therapy (RRT) is frequently required at presentation. When left untreated, vasculitis is fatal. Although immunosuppressive treatments with corticosteroids, plasma exchange, and cyclophosphamide have improved outcomes, many patients still progress to ESKD.3,7,8
The incidence is 0.5–1 case per million per year.1,3 Because of the rarity of the disease, prospective randomized controlled trials investigating treatments and clinical outcomes are sparse.1 Early diagnosis and identification of anti-GBM disease remains challenging because of the aggressive nature of the condition. Unlike other glomerulonephritides, severe renal involvement is evident at presentation in most patients. Risk factors, such as oligo-anuria and the degree of renal impairment, have been associated with poor renal survival.1,9,10 The Kidney Disease Improving Global Outcomes (KDIGO) group recommends avoiding immunosuppressive treatment in patients with dialysis dependence and 100% crescents or more than 50% glomerulosclerosis on renal biopsy in the absence of pulmonary hemorrhage11 because of the risks associated with aggressive immunosuppression. Histological changes, including the linear deposition of IgG along the GBM seen on direct immunofluorescence, assist in the diagnosis, and the percentage of normal glomeruli and glomerular crescents have been proposed as predictive for outcome.1,7,12 A significant proportions of patients with anti-GBM present with double positivity for ANCA,9,13,14 particularly against myeloperoxidase (MPO).14,15 Patients with double positive antibodies are reported in 20%–40% of anti-GBM cases and are proposed to exhibit better renal survival.1,14,16–18
Predicting renal outcomes in anti-GBM disease is challenging, and prediction tools are needed to individualize therapy and improve outcomes. Identifying patients with the potential to recover renal function is crucial to be able to tailor therapy to the need of the individual patient. The renal risk score (RRS) was developed to predict renal outcomes in ANCA-associated vasculitis (AAV).19 It is easy to apply and uses routinely collected laboratory and histological data, but it has not been well tested outside of AAV.20,21
In this study, we investigate an international, multicenter, retrospective cohort for clinical and histologic parameters predicting the initial need for RRT, chance of renal recovery, and development of ESKD. We aim to (1) identify patients who may benefit from rescue immunosuppressive therapy to recover kidney function; (2) validate the RRS for its use in anti-GBM disease; and (3) further develop prediction tools aiding the prognostication in anti-GBM disease.
Methods
Patient Cohort
This study was a retrospective longitudinal cohort study. Data were collected from seven renal referral centers and registries (Manchester, Salford, Preston, London, Prague, Baltimore, and the Irish Registry). The data comprise patients presenting with new anti-GBM disease from March 1998 to February 2020, with follow-up data recorded through January 2022. Inclusion criteria were a biopsy-proven anti-GBM diagnosis, evident by linear IgG fluorescence along the GBM, circulating anti-GBM antibodies, and data for renal function at presentation (as measured by eGFR). For the secondary analysis of renal recovery, a subset of patients with an initial need for RRT were included. Patients with missing data and triple antibody positivity were excluded.
In patients with double positivity, ANCA were detected in sera by antigen-specific assays using internationally recognized, commercial ELISA for MPO or proteinase 3 (PR3). The sample size was pragmatic on the basis of all eligible patients from each center. This study was performed in accordance with the Declaration of Helsinki, and data were collected according to the guidelines of the respective local ethics committees (Manchester Biobank, REC 16/NW/0119 and St James'/Tallaght REC 019-09 List 33 [040]).
Baseline characteristics included demographic details at diagnosis, antibody (Ab) status with anti-GBM antibody (mono anti-GBM) or additional MPO and PR3 positivity (double positive Ab), biochemical markers including eGFR and creatinine, initial need for RRT, and induction therapy details. The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation.22 The definition for ESKD was applied as previously published with ESKD defined as RRT for at least 12 weeks and continued until the last follow-up.19 For patients who required RRT at presentation, renal recovery was defined as RRT independence during follow-up for at least 12 weeks. The RRS19 was calculated for each patient using the eGFR at presentation (G0: eGFR>15 ml/min per 1.73 m2, G1: eGFR≤15 ml/min per 1.73 m2), the percentage of normal glomeruli in the kidney biopsy (N0: normal>25%, N1: normal 10%–25%, N2: <10%), and the simplified cutoff for tubular atrophy and interstitial fibrosis (T0: TA/IF none, mild to moderate, T1: TA/IF moderate to severe). Normal glomeruli were defined as glomeruli without any scarring, crescents, or fibrinoid necrosis within the tuft. As previously proposed, these glomeruli were called normal to avoid introducing a new term, such as unaffected or uninvolved glomeruli. Each parameter was assigned points (G1=3, N1=4, N2= 6, T1=2) and summed, creating a total score and risk groups: low (0), moderate (2–7), and high (8–11 points).
Statistics
The primary end point was ESKD (measured by time to ESKD), and the secondary end point was renal recovery (measured by time to recovery), both assessed by the patient's clinical teams. “Time-zero” was the date of the presentation. Censoring was at the last follow-up for the primary end point and at the earlier of the last follow-up or ESKD for the secondary end point. Multivariable models were built using the Cox proportional hazards approach. Model performance was assessed for discrimination using Harrell's C statistic (presented with 95% bootstrap confidence intervals [CIs]). Survival probability was calculated using Kaplan–Meier (KM) estimates.
For the validation of the RRS, the RRS for each patient was calculated from their data and the discrimination of the score was calculated without re-estimating parameters. KM estimates at 36 months were used to calibrate and compare performance against the original publication.
For the development of new models, univariable models and a multivariable model with all predictors (sex, antibody type, age at diagnosis, eGFR, percentage of normal glomeruli, TA/IF, and initial need for RRT) were run, compared with the discrimination of the RRS-only model. Multivariable models were calculated on a complete-case basis, all patients requiring eGFR, percent normal glomeruli, and TA/IF grading as criteria of inclusion. Discrimination estimates were based on continuous parameters. Cutoffs for the risk groups of the new model were chosen to be in line with the cutoffs for the RRS to balance predictive ability and clinical usability. The study team was not blinded to predictor selection. Final proposed models were chosen on the basis of their discrimination with a preference for fewer terms. No formal framework was used in model selection.
The analysis was performed using R v4.0.5 (R Core Team, Vienna, Austria). P<0.05 was considered significant. Data were also presented using KM plots for illustrating survival. This study was reported according to the TRIPOD guidelines.23
Results
Baseline Characteristics and Clinical Outcome
A total of 191 patients were included in the dataset (London=45, Preston=30, Manchester=29, Salford=25, Irish registry=22, Baltimore=20, and Prague=20). Sixteen patients were excluded for missing data and 1 patient for triple antibody positivity, with 174 in the main analysis set and 129 in the recovery analysis set (study flowchart, Supplemental Figure 1). The cohort characteristics are summarized in Table 1. The median follow-up was 39.8 months (interquartile range [IQR] 16.2–89.4 months).
Table 1.
Baseline characteristics
| Total (N=174) | |
|---|---|
| Female sex | 99 (56.9%) |
| Age (mean years (SD)) | 59.0 (45.0–70.0) |
| Antibody | |
| Mono anti-GBM | 107 (61.5%) |
| Double positive Ab | 67 (38.5%) |
| Pulmonary involvement | 47 (27.0%) |
| eGFR (median ml/min per 1.73 m2 (IQR)) | 6.5 (5–13) |
| Glomeruli on biopsy (median number (IQR)) | 16 (10–24) |
| Normal glomeruli (median percentage (IQR)) | 0 (0–20) |
| Tubular atrophy and interstitial fibrosis | |
| ≤25% | 124 (71.3%) |
| >25% | 50 (28.7%) |
| RRS group | |
| High | 115 (66.1%) |
| Moderate | 43 (24.7%) |
| Low | 16 (9.2%) |
mono anti-GBM, anti-GBM antibodies without additional ANCA antibodies; GBM, glomerular basement membrane; Ab, antibody; IQR, interquartile range; RRS, renal risk score comprises eGFR (G0>15 ml/min per 1.73 m2, G1≤15 ml/min per 1.73 m2), percentage of normal glomeruli in the kidney biopsy (N0>25%, N1=10%–25%, N2<10%), and tubular atrophy and interstitial fibrosis (T0≤mild to moderate, T1≥moderate), with points assigned (G1=3, N1=4, N2=6, T1=2), and risk groups created (low 0, moderate 2–7, and high-risk group 8–11 points); double positive Ab, Ab for both anti-GBM and ANCA antibodies.
Of 174 patients, 163 (96.0%) patients received immunosuppressive therapy, 156 (89.7%) received glucocorticoids, 143 (82.2%) received plasma exchange, and 146 (83.9%) received cyclophosphamide (Supplemental Table 1). The median time to biopsy was 6 days (IQR 2–11 days), and 81.0% were biopsied within 14 days of presentation.
At presentation, 129 patients (74.1%) required RRT. Of these, 33 patients (25.6%) recovered renal function (Table 2). A total of 106 patients (60.9%) progressed to ESKD, and approximately one-third of the cohort died during follow-up (n=53, 30.5%).
Table 2.
Clinical Outcomes
| All Patients (N=174) | Low Risk (n=16) | Moderate Risk (n=43) | High Risk (n=115) | |
|---|---|---|---|---|
| Follow-up (median months (IQR)) | 39.8 (16.2–89.4) | 33.5 (25.8–48.2) | 41.0 (17.0–110.0) | 37.5 (11.2–78.8) |
| Initial need for RRT | 129 (74.1%) | 0 | 28 (65.1%) | 101 (87.8%) |
| Renal recovery | 33/129 (25.6%) | 14/28 (50.0%) | 19/101 (18.8%) | |
| ESKD | 106 (60.9%) | 0 | 17 (39.5%) | 89 (77.4%) |
| Mortality | 53 (30.5%) | 2 (12.5%) | 10 (23.3%) | 41 (35.7%) |
IQR, interquartile range; renal risk score (RRS) comprises eGFR (G0>15 ml/min per 1.73 m2, G1≤15 ml/min per 1.73 m2), percentage of normal glomeruli in the kidney biopsy (N0>25%, N1=10%–25%, N2<10%), and tubular atrophy and interstitial fibrosis (T0≤mild to moderate, T1≥moderate), with points assigned (G1=3, N1=4, N2=6, T1=2), and risk groups created (low 0, moderate 2–7, and high-risk group 8–11 points).
RRS in Anti-GBM Disease
The RRS for AAV was used to stratify patients as previously published by Brix et al.19 During follow-up, none of the 16 patients in the low-risk group developed ESKD (Table 2). Seventeen patients (39.5%) in the moderate-risk group and 89 patients (77.4%) in the high-risk group developed ESKD. Analyzing ESKD at 36 months analogue to the initial publication in AAV, kidney survival was 100% in the low-risk group, 62.4% in the moderate group, and 20.7% in the high-risk group. The RRS showed good prediction of kidney survival in patients with anti-GBM disease (Harrell's C=0.760, 95% CI 0.69–0.83, P<0.0001, Figure 1). The score's discriminative power was not influenced by treatment effects, and additional analysis of the plasma exchange treated subcohort did not find a significant reduction in discrimination (n=143, C=0.751). There was no significant difference in mortality across the three groups (P=0.084).
Figure 1.
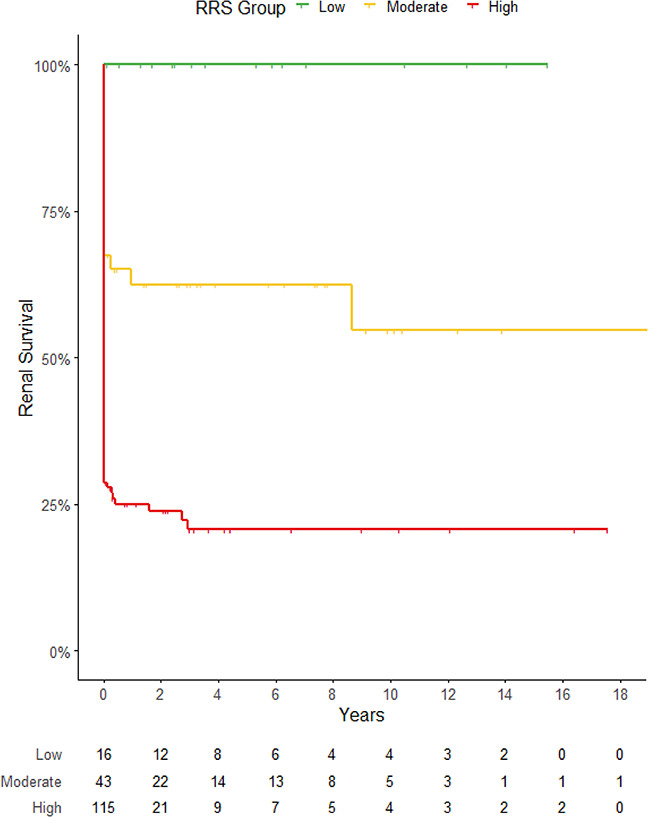
Kidney survival according to the stratification of the RRS. Kaplan–Meier curve depicting the development of ESKD of patients with anti-GBM disease. Patients are assigned points as per the RRS according to the eGFR at presentation (eGFR, G0 >15 ml/min per 1.73 m2, G1 ≤15 ml/min per 1.73 m2), the percentage of normal glomeruli in the kidney biopsy (N0 >25%, N1 0%–25%, N2 <10%), and the simplified cutoff for tubular atrophy and interstitial fibrosis (T0 ≤mild to moderate, T1 ≥moderate). Points are calculated (G1=3, N1=4, N2=6, T1=2) and risk groups created—low-risk (0), moderate-risk (2–7), and high-risk group (8–11 points). Patients’ outcome differs per risk group, C=0.760, P<0.001.
Risk Factors Predicting ESKD
On univariable analyses, risk factors predicting renal outcome showed that eGFR at diagnosis, initial need for RRT, the percentage of normal glomeruli on renal biopsy, and the RRS were all associated with ESKD (Supplemental Table 2a). On further multivariable analysis, the percentage of normal glomeruli on biopsy and the initial need for RRT remained the only significant independent risk factors determining ESKD (P=0.002 and P<0.001, respectively, Table 3). Additional ANCA antibodies and eGFR did not influence the outcome. In the subcohort of patients with serum creatinine at presentation (n=119), the multivariable analysis demonstrated that RRT at the time of diagnosis and the percentage of normal glomeruli remained superior to serum creatinine levels in predicting ESKD (Supplemental Table 2b). Supplemental Figure 2 demonstrates the difference in the development of ESKD between the two groups of patients, patients initially needing RRT versus patients remaining dialysis independent.
Table 3.
Multivariable models for time to ESKD
| β Coefficient | HR (95% CI) | P Value | |
|---|---|---|---|
| Ab (mono anti-GBM, double positive Ab) | −0.164 | 0.848 (0.624 to 1.153) | 0.293 |
| Female sex | −0.193 | 0.824 (0.616 to 1.102) | 0.192 |
| Age at diagnosis | 0.00059 | 1.001 (0.988 to 1.013) | 0.925 |
| eGFR | −0.0231 | 0.977 (0.934 to 1.022) | 0.311 |
| Normal glomeruli | −0.0339 | 0.967 (0.946 to 0.988) | 0.002 |
| TA/IF | 0.353 | 1.424 (0.779 to 2.604) | 0.250 |
| Initial need for RRT | 1.993 | 7.340 (2.699 to 20.0) | <0.001 |
HR, hazard ratio; 95% CI, 95% confidence interval; mono anti-GBM, anti-GBM antibodies without additional ANCA antibodies; GBM, glomerular basement membrane; double Ab, antibodies for both anti-GBM and ANCA antibodies; Ab, antibody; TA/IF, tubular atrophy and interstitial fibrosis.
Renal Outcome of Patients Requiring RRT at Diagnosis
Table 4 illustrates the basic characteristics and outcome data of the 129 patients who required RRT at the time of presentation. Of that cohort, 33 patients (25.6%) recovered renal function and 96 patients (74.4%) developed ESKD. Patients recovering renal function presented with a median eGFR of 6.0 ml/min per 1.73 m2 compared with patients who progressed to ESKD with 5.0 ml/min per 1.73 m2 (P=0.086). Biopsies of patients with renal recovery detected a higher percentage of normal glomeruli (median percentage of normal glomeruli in the recovery cohort: 11% [IQR 0–20] compared with the cohort progressing to ESKD: 0% [IQR 0–7], P=0.008). The degree of TA/IF did not vary between the two groups (27.3% versus 30.2%, respectively, P=0.413). The recovering patients belonged more often to the moderate-risk than high-risk group (42.4% versus 14.6%, respectively, P<0.001). On univariable analyses, the number of normal glomeruli on renal biopsy and the RRS were associated with renal recovery (P<0.001 versus P<0.001, respectively, Supplemental Table 2c). Supplemental Figure 3a demonstrates how the RRS separated renal recovery in that cohort. On further multivariable analysis, the percentage of normal glomeruli on biopsy remained the only significant independent risk factor determining renal recovery (P=0.005, Table 5).
Table 4.
Characteristics of patients initially requiring RRT
| Renal Recovery (n=33) | ESKD (n=96) | |
|---|---|---|
| Female sex | 24 (72.7%) | 52 (54.2%) |
| Age (median years (IQR)) | 61.0 (50.0–71.0) | 61.5 (50.5–71.0) |
| Antibody | ||
| Mono anti-GBM | 16 (48.5%) | 63 (65.6%) |
| Double positive Ab | 17 (51.5%) | 33 (34.4%) |
| Pulmonary involvement | 6 (18.2%) | 26 (27.1%) |
| eGFR (median ml/min per 1.73 m2 (IQR)) | 6.0 (5.0–8.0) | 5.0 (4.0–7.2) |
| Glomeruli on biopsy (median number (IQR)) | 14 (9–21) | 18.5 (11.8–26) |
| Normal glomeruli (median percentage (IQR)) | 11.0 (0.0–20.0) | 0.0 (0.0–7.0) |
| TA/IF | ||
| ≤25% | 24 (72.7%) | 67 (69.8%) |
| >25% | 9 (27.3%) | 29 (30.2%) |
| RRS group | ||
| High | 19 (57.6%) | 82 (85.4%) |
| Moderate | 14 (42.4%) | 14 (14.6%) |
IQR, interquartile range; mono anti-GBM, anti-GBM antibodies without additional ANCA antibodies; GBM, glomerular basement membrane; double Ab, antibodies for both anti-GBM and ANCA antibodies; Ab, antibody; RRS, renal risk score comprises eGFR (G0 >15 ml/min per 1.73 m2, G1 ≤15 ml/min per 1.73 m2), percentage of normal glomeruli in the kidney biopsy (N0 >25%, N1=10%–25%, N2 <10%), and tubular atrophy and interstitial fibrosis (T0≤mild to moderate, T1≥moderate), with points assigned (G1=3, N1=4, N2=6, T1=2), and risk groups created (low 0, moderate 2–7, and high-risk group 8–11 points); TA/IF, tubular atrophy and interstitial fibrosis.
Table 5.
Multivariable models for time to recovery
| β Coefficient | HR (95% CI) | P Value | |
|---|---|---|---|
| Ab (mono anti-GBM, double positive Ab) | 0.256 | 1.292 (0.759 to 2.202) | 0.345 |
| Female sex | 0.154 | 1.167 (0.634 to 2.148) | 0.619 |
| Age at diagnosis | 0.0040 | 1.004 (0.982 to 1.027) | 0.722 |
| eGFR | 0.0297 | 1.030 (0.952 to 1.115) | 0.461 |
| Normal glomeruli | 0.0383 | 1.039 (1.012 to 1.067) | 0.005 |
| TA/IF | −0.564 | 0.569 (0.181 to 1.793) | 0.336 |
HR, hazard ratio; 95% CI, 95% confidence interval; Ab, antibody; mono anti-GBM, anti-GBM antibodies without additional ANCA antibodies; GBM, glomerular basement membrane; double Ab, antibodies for both anti-GBM and ANCA antibodies; TA/IF, tubular atrophy and interstitial fibrosis.
Normal Glomeruli as a Prognostic Factor for Renal Outcome
Investigating the percentage of normal glomeruli as a continuous variable demonstrated that patients with a higher proportion of normal glomeruli showed lower rates of ESKD (P<0.001) and higher rates of renal recovery (P<0.001). Patients were grouped according to the published cutoffs (N0: normal >25%, N1: normal 10%–25%, N2: <10%, Supplemental Table 3a).19 Patients with more than a quarter of normal glomeruli differed in their presentation and outcome from the other patients. Patients with 10%–25% of normal glomeruli presented with similar kidney function and need for RRT compared with patients with <10% of normal glomeruli but recovered more often kidney function and developed less ESKD. To determine whether a small percentage of normal glomeruli would make a difference in outcome, the N2 group was split into patients with zero unaffected glomeruli (100% crescents) and patients with detectable but <10% of normal glomeruli (>0 to <10% normal, Supplemental Table 3b). These patients did not differ in presentation or outcome. The biopsies of the group with detectable normal glomeruli were however bigger and encompassed a higher number of glomeruli (median number of glomeruli, 25.5 versus 14, respectively). The percentage of 10% of normal glomeruli separated outcome and Supplemental Figure 3b depicts how that cutoff separated renal recovery.
Prediction Tool for Anti-GBM Disease
The multivariable analysis showed that the initial need for RRT and the percentage of normal glomeruli were the best predictors for ESKD. Discrimination was superior to the RRS with C=0.840 (95% CI: 0.786–0.894, P<0.0001, Table 6). Categorizing patients according to the need for RRT at presentation and the percentage of normal glomeruli in the kidney biopsy (N≥10% versus N<10%) created four distinct groups with significant difference in renal survival. Figure 2 depicts kidney survival according to the four groups defined as group 1: no RRT, N≥10%; group 2: no RRT, N<10%; group 3: RRT, N≥10%; and group 4: RRT, N<10%. Regarding tissue adequacy, we performed a subanalysis investigating biopsies with at least ten glomeruli (n=134). The sensitivity analysis did not detect a substantial decrease in the tool’s performance (C=0.820). Renal survival was highest in patients who did not require RRT at presentation and presented with a higher number of normal glomeruli. RRT independent patients with little or no normal glomeruli (N<10%), group 2, developed ESKD more often. Patients who initially required RRT but demonstrated more than 10% of normal glomeruli in the biopsy expressed a higher rate of ESKD than group 1/2, but fewer patients developed ESKD than in group 4 (RRT and N<10%). The 36-month KM estimates of kidney survival were 96.4%, 74.0%, 42.3%, and 14.1% for groups 1–4, respectively.
Table 6.
Comparison of models for time to ESKD
| RRS Model | Full Model | Proposed Model | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | C | HR (95% CI) | P Value | C | HR (95% CI) | P Value | C | |
| RRS | 1.326 (1.207 to 1.456) | <0.001 | 0.760 | ||||||
| Female sex | 0.848 (0.624 to 1.153) | 0.293 | 0.868 | ||||||
| Age at diagnosis | 0.824 (0.616 to 1.102) | 0.192 | |||||||
| Ab | 1.001 (0.988 to 1.013) | 0.925 | |||||||
| eGFR | 0.977 (0.934 to 1.022) | 0.311 | |||||||
| Initial RRT | 7.340 (2.699 to 20.0) | <0.001 | 9.931 (3.750 to 23.15) | <0.001 | 0.840 | ||||
| Normal glomeruli | 0.967 (0.946 to 0.988) | 0.002 | 0.964 (0.944 to 0.984) | <0.001 | |||||
| TA/IF | 1.424 (0.779 to 2.604) | 0.250 | |||||||
RRS, renal risk score comprises eGFR (G0>15 ml/min per 1.73 m2, G1≤15 ml/min per 1.73 m2), percentage of normal glomeruli in the kidney biopsy (N0>25%, N1=10%–25%, N2<10%), and tubular atrophy and interstitial fibrosis (T0 ≤mild to moderate, T1 ≥moderate), with points assigned (G1=3, N1=4, N2=6, T1=2), and risk groups created (low 0, moderate 2–7, and high-risk group 8–11 points); HR, hazard ratio; 95% CI, 95% confidence interval; C, Harrell's concordance statistic; Ab, antibody profile with either anti-GBM antibodies alone or with additional ANCA antibodies; GBM, glomerular basement membrane; TA/IF, tubular atrophy and interstitial fibrosis.
Figure 2.
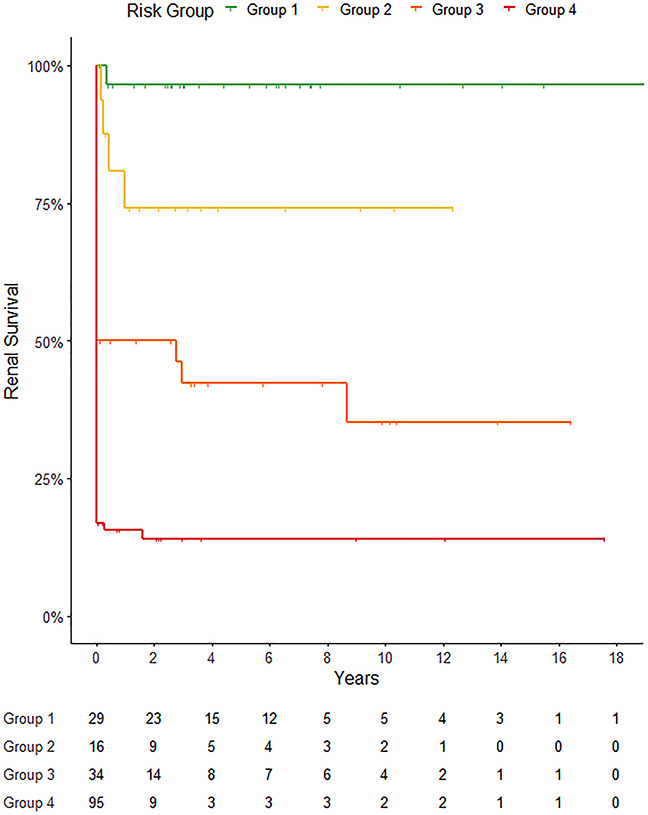
Kidney survival according to the initial need for RRT and the percentage of normal glomeruli in the biopsy. Kaplan–Meier curve demonstrating the development of ESKD of patients with anti-GBM disease. Patients are grouped by their need for RRT at presentation and the percentage of normal glomeruli in the kidney biopsy (N≥10%, N<10%). Group 1: no RRT, N≥10%; group 2: no RRT, N<10%; group 3: RRT, N≥10%; and group 4: RRT, N<10%. Patients’ outcome differs per group, C=0.840, P<0.001.
Double Positive Disease
Of the total cohort, 107 patients (61.5%) expressed antibodies for anti-GBM disease only (mono anti-GBM), whereas an additional ANCA was detected in 67 patients (38.5%). Of patients with double positive antibodies, 48 patients (27.6%) presented with additional MPO antibodies and 19 patients (10.9%) presented with additional PR3 antibodies. Patients with double positive antibodies were older than mono anti-GBM patients (63.7 (IQR 54.2–71.6) versus 55.0 (IQR 39.5–69.0) years, respectively, P=0.002). There was a female predominance (71.6%) in patients with double positive antibodies, as shown in Table 7.
Table 7.
Baseline characteristics and clinical outcomes based on antibody
| Mono Anti-GBM (n=107) | Double Positive Ab (n=67) | P Value | |
|---|---|---|---|
| Female sex | 51 (47.7%) | 48 (71.6%) | 0.003 |
| Age (median years (IQR)) | 55.0 (39.5–69.0) | 63.7 (54.2–71.6) | 0.002 |
| Pulmonary involvement | 33 (30.8%) | 14 (20.9%) | 0.207 |
| eGFR (median ml/min per 1.73 m2 (IQR)) | 6.0 (4.0–13.0) | 7.0 (5.0–13.0) | 0.246 |
| Normal glomeruli (median percentage (IQR)) | 0.0 (0.0–20.0) | 5.0 (0.0–18.5) | 0.036 |
| Tubular atrophy and interstitial fibrosis | 0.353 | ||
| ≤25% | 82 (76.6%) | 42 (62.7%) | |
| >25% | 25 (23.4%) | 25 (37.3%) | |
| RRS group | 0.409 | ||
| High | 69 (64.5%) | 46 (68.7%) | |
| Moderate | 26 (24.3%) | 17 (25.4%) | |
| Low | 12 (11.2%) | 4 (6.0%) | |
| Initial need for RRT | 79 (73.8%) | 50 (74.6%) | 1 |
| Renal recovery | 16/79 (20.3%) | 17/50 (34.0%) | 0.090 |
| ESKD | 68 (63.6%) | 38 (56.7%) | 0.333 |
| Mortality | 32 (29.9%) | 21 (31.3%) | 0.593 |
Mono anti-GBM, anti-GBM antibodies without additional ANCA antibodies; GBM, glomerular basement membrane; double positive Ab, antibodies for anti-GBM and ANCA antibodies; Ab, antibody; IQR, interquartile range; TA/IF, tubular atrophy and interstitial fibrosis; RRS, renal risk score comprises estimated eGFR (G0>15 ml/min per 1.73 m2, G1 ≤15 ml/min per 1.73 m2), percentage of normal glomeruli in the kidney biopsy (N0>25%, N1=10%–25%, N2<10%), and tubular atrophy and interstitial fibrosis (T0≤mild to moderate, T1 ≥moderate), with points assigned (G1=3, N1=4, N2=6, T1=2) and risk groups created (low 0, moderate 2–7, and high-risk group 8–11 points).
Patients with double positive antibodies showed numerically better renal outcomes than mono anti-GBM patients, with more patients recovering renal function (34.0% versus 20.3%, P=0.109) and fewer progressing to ESKD (56.7% versus 63.6%, P=0.333). The median percentage of normal glomeruli on the renal biopsy was higher compared with the mono anti-GBM group: 5 versus 0, respectively (P=0.036). Controlling for the percentage of normal glomeruli, patients with double positive antibodies did not differ in their outcome compared with patients with mono anti-GBM antibodies (Figure 3A/3b, PRecovery=0.230, PESKD=0.146). Separating the cohorts of mono anti-GBM and patients with double positive antibodies according to the percentage of normal glomeruli in the biopsy demonstrated the differing outcome dependent on the percentage of preserved glomeruli (N≥10% versus N<10%). Renal recovery at 12 months was 68% and 10.5% in mono anti-GBM patients compared with 46.5% and 34.4% in patients with double positive antibodies (Figure 3A). ESKD at 36 months was 72.2% and 15.1% in mono anti-GBM patients compared with 52.4% and 35.3% in patients with double positive antibodies (Figure 3B). Both groups presented similarly, with 74.6% of patients requiring dialysis in the double positive group and 73.8% in the mono anti-GBM group (P=1.00). There was no significant difference in mortality between the two groups (29.9% and 31.3%, respectively, P=0.593).
Figure 3.
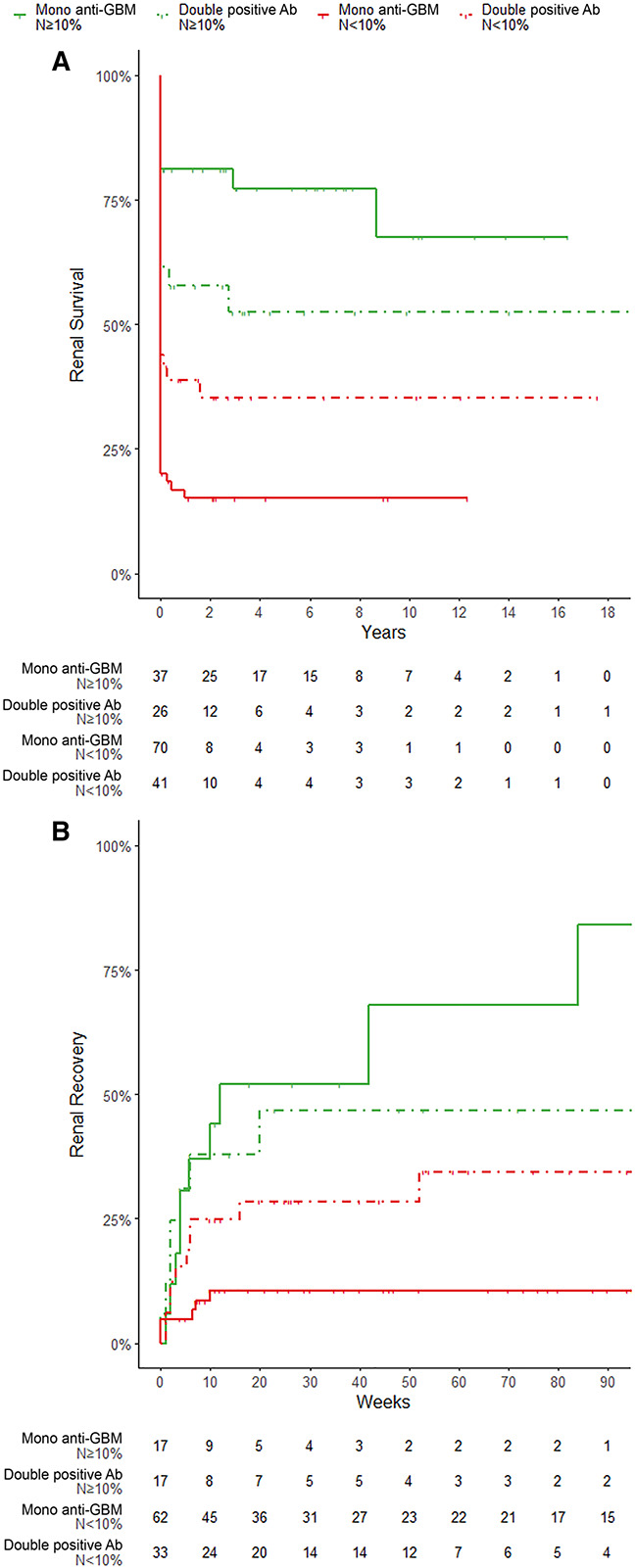
Renal survival and recovery according to autoantibodies and the percentage of normal glomeruli in the biopsy. (A) Renal recovery according to autoantibodies and the percentage of normal glomeruli in the biopsy. Kaplan–Meier (KM) curve demonstrating renal recovery of patients with mono anti-GBM and double positive antibodies (anti-GBM and additional ANCA Ab). Patients are grouped by the percentage of normal glomeruli in the kidney biopsy (N≥10% versus N<10%). Patients with double positive Ab demonstrated a higher median percentage of normal glomeruli on biopsy 5 versus 0, respectively (P=0.036), and they numerically recovered renal function more often than those with mono anti-GBM (34.0% versus 20.3%, P=0.109). Renal recovery at 12 months was 68% and 10.5% in mono anti-GBM patients compared with 46.5% and 34.4% in patients with double positive patients, respectively. (B) Kidney survival according to autoantibodies and the percentage of normal glomeruli in the biopsy. KM curve demonstrating the development of ESKD of patients with mono anti-GBM and double positive antibodies (anti-GBM and additional ANCA Ab). Patients are grouped by the percentage of normal glomeruli in the kidney biopsy (N≥10% versus N<10%). Numerically, fewer patients with double positive Ab progressed to ESKD than those with mono anti-GBM (56.7% versus 63.6%, P=0.333) despite similar numbers of patients requiring dialysis at presentation. ESKD at 36 months was 72.2% and 15.1% in mono anti-GBM patients compared with 52.4% and 35.3% in patients with double positive antibodies, respectively.
Discussion
Anti-GBM disease is a rare disease with very poor outcomes. Most patients present dialysis-dependent and the majority develop ESKD. The KDIGO guideline still recommends withholding active induction therapy in patients with diffuse crescentic lesions and dialysis dependence at presentation.11 The novel approach using endopeptidase cleavage promises an improved outcome, but it will unfortunately take time until widely available.24,25 There is great need to improve outcomes in anti-GBM disease, and a better prognostication promises individualized management preserving kidney function and protecting life.
In this multicenter, international, retrospective study, we showed that the RRS is transferrable to anti-GBM disease. More importantly, however, we identified the combination of the risk factors: (1) the need for RRT at presentation and (2) the percentage of normal glomeruli in the kidney biopsy as superior predictors of long-term kidney survival. A substantial difference in kidney survival was found on either side of 10% of normal glomeruli. Most patients with 10% or more normal glomeruli in the biopsy recovered renal function. Patients with a smaller percentage of normal glomeruli than 10% did not differ in renal outcome compared with patients with all glomeruli affected. Patients with few normal glomeruli (N<10%) developed ESKD in 76.6%, compared with patients with a higher percentage of normal glomeruli (N≥10%) who developed ESKD in 33.3%. Of the former group, only 16.8% recovered kidney function compared with a recovery rate of 50.0% in patients with 10% or more normal glomeruli. Therefore, we propose using 10% of normal glomeruli as a useful cut point. We investigated tissue adequacy by performing a sensitivity analysis using biopsies with at least ten glomeruli and did not detect a significant difference in the performance of the prediction tool. We therefore did not set a minimum total number of glomeruli when using the score but recommend caution in interpreting smaller biopsies with less than ten glomeruli. No other clinical factors, such as age, sex, serum creatinine, eGFR, or additional ANCA antibody, significantly influenced clinical outcomes. Stratification along these two parameters, the need for dialysis and normal glomeruli, assisted in grouping patients improving outcome prediction.
The stepwise stratification effectively stratified patients first by the need for RRT and then by the percentage of normal glomeruli. There was no crossover of risk groups, for example, all patients in group 3 (RRT, N≥10%) demonstrated a higher risk of ESKD compared with group 2 (no RRT, N<10%). Theoretically, a patient from group 3 (RRT, N≥10%) would have needed 61% normal glomeruli to reduce the predicted risk for ESKD to the level of a patient from group 2 (no RRT, N<10%). In anti-GBM disease, the bivariable stratification model demonstrated a high discriminative power and superiority compared with the univariable RRS model. Incorporating eGFR and IFTA did not significantly improve outcome prediction further (Table 6). The GBM prediction model is easy to use, and the pragmatic groupings will allow clinicians to use two readily available parameters to facilitate rational, evidenced-based conversations with patients.
The RRS demonstrated a high discriminative power in anti-GBM disease despite significant differences in the disease presentations. It performed less well than in ANCA-associated glomerulonephritis. The cause for the reduced discriminative ability may lie in the more aggressive nature of anti-GBM disease. The large proportion of patients with rapid loss of kidney function developing ESKD at the time of presentation contorted the time to ESKD. Therefore, the need for RRT at the time of diagnosis became a stronger predictor of outcome than laboratory measures of kidney function.
A significant percentage of patients with anti-GBM present with additional ANCA antibodies. In our study, these patients were older but demonstrated a higher percentage of normal glomeruli. In the multivariable model, ANCA did not influence patient outcomes. In the past, additional ANCA were proposed as a predictor for better outcome in anti-GBM disease.18 We did not detect a significant variance in outcome in the two patient groups after controlling for normal glomeruli. The mechanism behind the action of patients with double positive serology trending toward a better outcome remains unclear. The additional ANCA-associated disease might result in earlier diagnosis. Extrarenal disease, grumbling, and less aggressive disease flares and widely used ANCA assays might enable a prompter intervention in these patients. Our data suggest that the better outcome in double positive serology relies on the percentage of preserved glomeruli in these patients and not on the additional antibody. In double positive patients, the percentage of normal glomeruli with a cutoff at 10% remained a significant predictor of outcome, but the differences in renal outcome seemed less profound than in patients without additional ANCA antibodies. The relapsing–remitting nature of ANCA-associated vasculitides may explain why some patients progressed to ESKD despite a higher percentage of normal glomeruli.
This reiterates the importance of obtaining a kidney biopsy in these patients and not solely managing anti-GBM disease on clinical parameters. Our findings support the use of a prediction tool in anti-GBM disease facilitating informed discussions with patients about their potential for renal survival. Despite the model's good discrimination, 14.1% of patients in the highest risk category remained ESKD-free after 3 years. Furthermore, 17.1% of patients requiring RRT at presentation with <10% normal glomeruli in their biopsy achieved recovery. It is important to stress that the proposed cutoffs separated patients into groups with differing outcome and that the risk groups will assist clinical management, patient education, and trial stratification. These risk groups do, however, not represent a futility threshold. Patients with little or zero normal glomeruli in their biopsies suffered a significantly worse outcome, but it is critical to not abandon all treatment attempts and to discuss treatment options with risks and benefits in diffuse crescentic cases to achieve personalized medicine. We believe that the prediction tool will assist in individualizing the intensity and length of treatment attempts in the clinical context of patients' comorbidities and frailty.
We hope our findings will raise a discussion about treatment in anti-GBM disease and consideration to change the treatment recommendation, highlighting the importance of normal glomeruli even in advanced, diffuse crescentic cases.
Our study was limited by its retrospective character and the rarity of the disease. A significant proportion of patients presented dialysis-dependent and immunosuppressive treatment was felt to be futile in some cases, thus preventing a possible renal recovery. We were unable to investigate the influence of oligo-anuria and the level of anti-GBM antibody on the outcome. Although serum creatinine was not available for all patients, in the subcohort multivariable analysis, RRT and the percentage of normal glomeruli remained superior predictors to serum creatinine at presentation. Previous studies of cohorts with similar number of patients with ANCA-associated glomerulonephritis had detected the percentage of normal glomeruli as the most significant predictor of ESKD and the varied glomerular lesions had failed to improve outcome prediction further. Therefore, we did not investigate glomerular lesions in more detail. Because of the rarity of anti-GBM disease, separating our cohort into a training and validation cohort was not possible and external validation of our findings is needed.
In summary, we demonstrated that renal histology assists in predicting the outcome of anti-GBM disease. We showed that the RRS is transferrable and able to predict recovery in anti-GBM disease. Stratifying patients according to the need for RRT at presentation and the percentage of normal glomeruli in the biopsy improved discrimination and prognostication further. There is great need to improve outcomes in anti-GBM disease and a better prognostication will allow individualized management to preserve kidney function and protect life. This risk stratification hopefully will assist clinicians in the management of patients with anti-GBM disease, individualizing care, improving renal recovery, and reducing the development of ESKD.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the participating physicians and patients enabling the collection of this data. We also acknowledge that part of one participating center's cohort was previously published by van Daalen et al.7 The Irish Rare Kidney Disease databank was supported by grant 17072.211897 and SFI 13/RC/2106_P from the Meath Foundation.
Footnotes
L.F. and S.B. contributed equally to this work.
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Estimating Prognosis in Anti–Glomerular Basement Membrane Disease,” on pages 360–362.
Disclosures
S. Bate reports Employer: Manchester University NHS Foundation Trust, and IQVIA. N. Brown reports Honoraria: Vifor. A. Dhaygude reports Honoraria: Astra Zeneca; and Advisory or Leadership Role: Vifor Pharma. V. Tesar reports Consultancy: Alexion, AstraZeneca, Baxter, Bayer, Boehringer- Ingelheim, Calliditas, Fresenius Medical Care, Eli Lilly, Novartis, Omeros, Otsuka, Pfizer, Sanofi, Swixx BioPharma, and Travere; Honoraria: For consultancy as follows: Alexion, AstraZeneca, Bayer, Boehringer-Ingelheim, Calliditas, Fresenius Medical Care, Eli Lilly, Novartis, Omeros, Pfizer, Swixx Biopharma, and Travere; and Advisory or Leadership Role: member of the B. Braun, Calliditas, Fresenius Medical Care, Novartis, Omeros, and Travere. S. McAdoo reports Consultancy: GSK, and Vifor; Research Funding: Therini Bio; Honoraria: Celltrion, Rigel Pharmaceuticals, and ThermoFisher Scientific; and Advisory or Leadership Role: Cochair United Kingdom & Ireland Vasculitis Society (unpaid). M.A. Little reports Consultancy: AnaptysBio, and LightStone; Research Funding: Vifor pharma; and Patents or Royalties: Euroimmun. D. Geetha reports Consultancy: ChemoCentryx as consultant and as an adjudicator for BVAS/VDI, Consultant Aurinia, and Consultant GSK. S.R. Brix reports consultancy: Vifor. C. Pusey reports Consultancy: Vifor, Alentis; Honoraria: Med Update Europe, Amgen; and Advisory or Leadership Role: CJASN. All remaining authors have nothing to disclose.
Funding
This work was supported by research grant KfL21/SRB03 from Kidneys for Life to SRB.
Author Contributions
S.R. Brix and C. Pusey conceptualized the study; F. Aqeel, S.R. Brix, N. Brown, A. Dhaygude, L. Floyd, D. Frausova, D. Geetha, A. Hadi Kafagi, P.L. Kieu, B. Khurshid, M.A. Little, S. McAdoo, M. Mysilvecek, J. Scott, M. Srikantharajah, and V. Tesar were responsible for data curation; S. Bate, S.R. Brix, M. Kollar, and G. Reid were responsible for formal analysis; S. Bate, S.R. Brix, N. Brown, and M. Kollar were responsible for investigation; S. Bate, S.R. Brix, and G. Reid were responsible for validation; S. Bate and S.R. Brix were responsible for methodology; S. Bate was responsible for resources and software; S.R. Brix was responsible for funding acquisition, project administration, and visualization and provided supervision; S. Bate, N. Brown, S.R. Brix, L. Floyd, and A. Hadi Kafagi wrote the original draft; and F. Aqeel, S. Bate, N. Brown, S.R. Brix, A. Dhaygude, L. Floyd, D. Frausova, D. Geetha, M.A. Little, S. McAdoo, M. Mysilvecek, P.L. Kieu, M. Kollar, B. Khurshid, V. Tesar, G. Reid, J. Scott, and M. Srikantharajah reviewed and edited the manuscript.
Data Sharing Statement
All data used in this study are available in this article.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/JSN/D167.
Supplemental Figure 1. Flowchart showing patient flow through the study.
Supplemental Figure 2. Kidney survival according to the initial need for renal replacement therapy.
Supplemental Figure 3a. Recovery of kidney function according to the renal risk score.
Supplemental Figure 3b. Recovery of kidney function according to the percentage of normal glomeruli in the kidney biopsy.
Supplemental Table 1. Therapeutic intervention.
Supplemental Table 2a. Univariable models for time of ESKD.
Supplemental Table 2b. Multivariable models for time of ESKD in the subcohort of patients with creatinine at presentation.
Supplemental Table 2c. Univariable models for time to recovery.
Supplemental Table 3a. Clinical outcome on the basis of the percentage of normal glomeruli.
Supplemental Table 3b. Clinical outcome on the basis of the percentage of normal glomeruli in patients with less than 10% normal glomeruli.
References
- 1.Kluth DC, Rees AJ. Anti-glomerular basement membrane disease. J Am Soc Nephrol. 1999;10(11):2446-2453. doi: 10.1681/ASN.V10112446 [DOI] [PubMed] [Google Scholar]
- 2.McAdoo SP, Pusey CD. Anti-glomerular basement membrane disease. Clin J Am Soc Nephrol. 2017;12(7):1162-1172. doi: 10.2215/CJN.01380217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang W, McDonald SP, Hawley CM, et al. Anti-glomerular basement membrane antibody disease is an uncommon cause of end-stage renal disease. Kidney Int. 2013;83(3):503-510. doi: 10.1038/ki.2012.375 [DOI] [PubMed] [Google Scholar]
- 4.Peto P, Salama AD. Update on antiglomerular basement membrane disease. Curr Opin Rheumatol. 2011;23(1):32-37. doi: 10.1097/bor.0b013e328341009f [DOI] [PubMed] [Google Scholar]
- 5.Zhao J, Cui Z, Yang R, Jia XY, Zhang Y, Zhao MH. Anti-glomerular basement membrane autoantibodies against different target antigens are associated with disease severity. Kidney Int. 2009;76(10):1108-1115. doi: 10.1038/ki.2009.348 [DOI] [PubMed] [Google Scholar]
- 6.Coche S, Sprangers B, Van Laecke S, et al. Recurrence and outcome of anti−glomerular basement membrane glomerulonephritis after kidney transplantation. Kidney Int Rep. 2021;6(7):1888-1894. doi: 10.1016/j.ekir.2021.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Daalen EE, Jennette JC, McAdoo SP, et al. Predicting outcome in patients with anti-GBM glomerulonephritis. Clin J Am Soc Nephrol. 2018;13(1):63-72. doi: 10.2215/CJN.04290417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levy JB, Hammad T, Coulthart A, Dougan T, Pusey CD. Clinical features and outcome of patients with both ANCA and anti-GBM antibodies. Kidney Int. 2004;66(4):1535-1540. doi: 10.1111/j.1523-1755.2004.00917.x [DOI] [PubMed] [Google Scholar]
- 9.McAdoo SP, Tanna A, Hrušková Z, et al. Patients double-seropositive for ANCA and anti-GBM antibodies have varied renal survival, frequency of relapse, and outcomes compared to single-seropositive patients. Kidney Int. 2017;92(3):693-702. doi: 10.1016/j.kint.2017.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alchi B, Griffiths M, Sivalingam M, Jayne D, Farrington K. Predictors of renal and patient outcomes in anti-GBM disease: clinicopathologic analysis of a two-centre cohort. Nephrol Dial Transplant. 2015;30(5):814-821. doi: 10.1093/ndt/gfu399 [DOI] [PubMed] [Google Scholar]
- 11.Rovin BH, Adler SG, Barratt J, et al. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100(4):S1-S276. doi: 10.1016/j.kint.2021.05.021 [DOI] [PubMed] [Google Scholar]
- 12.Nasr SH, Collins AB, Alexander MP, et al. The clinicopathologic characteristics and outcome of atypical anti-glomerular basement membrane nephritis. Kidney Int. 2016;89(4):897-908. doi: 10.1016/j.kint.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 13.Marques C, Carvelli J, Biard L, et al. Prognostic factors in anti-glomerular basement membrane disease: a multicenter study of 119 patients. Front Immunol. 2019;10:1665. doi: 10.3389/fimmu.2019.01665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jayne DR, Marshall PD, Jones SJ, Lockwood CM. Autoantibodies to GBM and neutrophil cytoplasm in rapidly progressive glomerulonephritis. Kidney Int. 1990;37(3):965-970. doi: 10.1038/ki.1990.72 [DOI] [PubMed] [Google Scholar]
- 15.Wahls TL, Bonsib SM, Schuster VL. Coexistent Wegener’s granulomatosis and anti-glomerular basement membrane disease. Hum Pathol. 1987;18(2):202-205. doi: 10.1016/S0046-8177(87)80340-4 [DOI] [PubMed] [Google Scholar]
- 16.Bosch X, Mirapeix E, Font J, et al. Prognostic implication of anti-neutrophil cytoplasmic autoantibodies with myeloperoxidase specificity in anti-glomerular basement membrane disease. Clin Nephrol. 1991;36(3):107-113. [PubMed] [Google Scholar]
- 17.Mörtzell Henriksson M, Weiner M, Sperker W, et al. Analyses of registry data of patients with anti-GBM and antineutrophil cytoplasmatic antibody-associated (ANCA) vasculitis treated with or without therapeutic apheresis. Transfus Apher Sci. 2021;60(6):103227. doi: 10.1016/j.transci.2021.103227 [DOI] [PubMed] [Google Scholar]
- 18.Philip R, Dumont A, Martin Silva N, de Boysson H, Aouba A, Deshayes S. ANCA and anti-glomerular basement membrane double-positive patients: a systematic review of the literature. Autoimmun Rev. 2021;20(9):102885. doi: 10.1016/j.autrev.2021.102885 [DOI] [PubMed] [Google Scholar]
- 19.Brix SR, Noriega M, Tennstedt P, et al. Development and validation of a renal risk score in ANCA-associated glomerulonephritis. Kidney Int. 2018;94(6):1177-1188. doi: 10.1016/j.kint.2018.07.020 [DOI] [PubMed] [Google Scholar]
- 20.Brix SR, Geetha D. Keeping up with the times: prognostic tools in ANCA-associated glomerulonephritis. Clin J Am Soc Nephrol. 2020;15(8):1078-1080. doi: 10.2215/CJN.09600620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xia M, Yu R, Zheng Z, et al. Meta-analytical accuracy of ANCA renal risk score for prediction of renal outcome in patients with ANCA-associated glomerulonephritis. Front Med. 2021;8:736754. doi: 10.3389/fmed.2021.736754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collins GS, Reitsma JB, Altman DG, Moons KGM. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ 2015;350:g7594. doi: 10.1136/bmj.g7594 [DOI] [PubMed] [Google Scholar]
- 24.Soveri I, Mölne J, Uhlin F, et al. The IgG-degrading enzyme of Streptococcus pyogenes causes rapid clearance of anti-glomerular basement membrane antibodies in patients with refractory anti-glomerular basement membrane disease. Kidney Int. 2019;96(5):1234-1238. doi: 10.1016/j.kint.2019.06.019 [DOI] [PubMed] [Google Scholar]
- 25.Uhlin F, Szpirt W, Kronbichler A, et al. Endopeptidase cleavage of anti-glomerular basement membrane antibodies in vivo in severe kidney disease: an open-label phase 2a study. J Am Soc Nephrol. 2022;33(4):829-838. doi: 10.1681/ASN.2021111460 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used in this study are available in this article.