Abstract
Rituximab is an established therapy in children with idiopathic nephrotic syndrome to sustain short- to medium-term disease remission and avoid steroid toxicities. Recent trials focus on its use as a first-line agent among those with milder disease severity. Rituximab is used in multidrug refractory nephrotic syndrome and post-transplant disease recurrence, although the evidence is much less substantial. Available data suggest that the treatment response to rituximab depends on various patient factors, dosing regimen, and the concomitant use of maintenance immunosuppression. After repeated treatments, patients are found to have an improving response overall with a longer relapse-free period. The drug effect, however, is not permanent, and 80% of patients eventually relapse and many will require an additional course of rituximab. This underpins the importance of understanding the long-term safety profile on repeated treatments. Although rituximab appears to be generally safe, there are concerns about long-term hypogammaglobulinemia, especially in young children. Reliable immunophenotyping and biomarkers are yet to be discovered to predict treatment success, risk of both rare and severe side effects, e.g., persistent hypogammaglobulinemia, and guiding of redosing strategy. In this review, we highlight recent advances in the use of rituximab for childhood nephrotic syndrome and how the therapeutic landscape is evolving.
Keywords: rituximab, steroid dependent nephrotic syndrome, idiopathic nephrotic syndrome, steroid sensitive nephrotic syndrome, children, biologics
Introduction
Childhood idiopathic nephrotic syndrome is an uncommon condition characterized by edema, heavy proteinuria, hypoalbuminemia, and hyperlipidemia. There is substantial variation in the incidence of nephrotic syndrome between different ethnicities, ranging from 1.15 to 16.9 per 100,000 persons.1 Most cases are steroid sensitive, but relapses are common and up to 30%–50% of children are frequently relapsing and/or steroid dependent.2,3 Various steroid-sparing agents are used to sustain disease remission or to reduce the number of relapses. A subset of patients, defined as complicated frequently relapsing nephrotic syndrome, steroid-dependent nephrotic syndrome,4 continue to relapse and develop significant medication toxicities, such as Cushing syndrome, osteopenia, infection, and nephrotoxicity.
Rituximab, a chimeric anti-CD20 monoclonal antibody, is an important treatment for complicated frequently relapsing, steroid-dependent nephrotic syndrome.5–7 After rituximab treatment, most children maintain prolonged remission and can taper or even discontinue other immunosuppressive drugs, including steroids. The drug is also used in the treatment of multidrug refractory nephrotic syndrome and post-transplant recurrence.8,9 In this article, we will review the evolving use of rituximab in childhood nephrotic syndrome and discuss what has been learned over the last decade. We also provide our informed views on unresolved questions pertaining to the treatment regimen, long-term outcomes, and redosing approach.
Immunomodulatory Effects of Rituximab Treatment in Childhood Idiopathic Nephrotic Syndrome
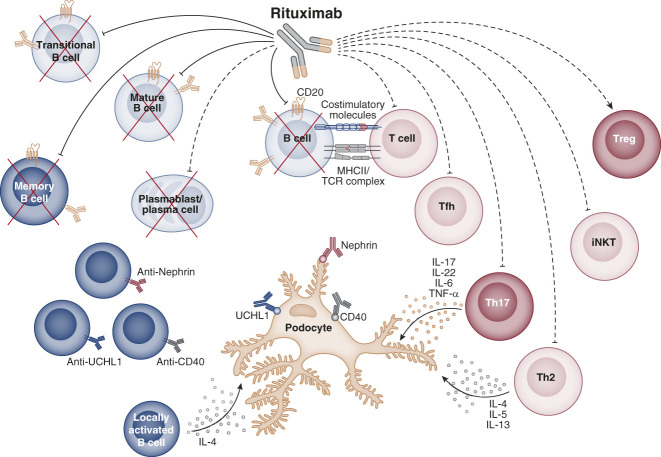
Several alterations in the immune homeostasis of children with nephrotic syndrome have been reported, mainly involving T regulatory, Th2, Th17, and memory B cells (Figure 1, Table 1), and were extensively reviewed elsewhere.21–24 Rituximab depletes all circulating B-cell subsets, and its therapeutic efficacy highlights a key role of B cells in the pathogenesis of nephrotic syndrome. It is understood that an early recovery of B cells, specifically the memory B cells, is associated with disease relapse after rituximab treatment.14,18,20 In addition, rituximab therapy effectively restores the altered balance between regulatory and effector T cells in nephrotic syndrome.14,15 Besides the production of antigen-specific antibodies,10–12 memory B cells can produce specific cytokines and are excellent antigen-presenting cells,13,25 which strongly modify the homeostasis of T lymphocytes.26 Accordingly, the positive effects on T-cell homeostasis exerted by rituximab seem to be indirectly mediated by B-cell depletion occurring in a few hours, rather than through a direct modification of T-cell function, which requires several weeks or months.14–16 Of note, a lower response to ex vivo T-cell activation was found at baseline in children with focal segmental glomerulosclerosis who responded to rituximab compared with nonresponders.19 Whether this T-cell hyporesponsiveness was due to a persistent T-cell activation in vivo needs further investigation.19,27 Low or no modification of the innate immune cell compartment after rituximab administration is reported in children with frequently relapsing, steroid-dependent nephrotic syndrome.15,16,20 The initial hypothesis of a podocyte-specific effect of directly binding to sphingomyelin-phosphodiesterase-acid-like-3b was subsequently disproved by demonstrating the nonspecificity of such binding, and the identical therapeutic efficacy of other anti-CD20 antibodies directed against different CD20 epitopes in childhood nephrotic syndrome.13,16,28
Figure 1.
Potential immune mechanism of action of rituximab in childhood idiopathic nephrotic syndrome. Rituximab treatment can restore the immune homeostasis of pediatric patients with idiopathic nephrotic syndrome by directly (solid lines) or indirectly (dashed lines) affecting different B- and T-cell subsets. Rituximab directly depletes CD20-expressing transitional, mature, and memory B-cell subsets. In some days, also short-lived plasmablasts/plasma cells, which do not express CD20 antigen, are indirectly depleted due to their limited lifespan. B-cell depletion could affect the production of the described nephrotic syndrome–associated podocyte-damaging autoantibodies such as anti-CD40, anti-UCHL1, and antinephrin IgG10–12 or cytokines such as IL-4, produced by B cells activated locally in the glomerulus.13 In addition, B-cell depletion can indirectly modulate the cross talk between B cells and specific T-cell subsets by affecting antigen presentation (MHC class II—TCR complex) and costimulation, resulting in a reduction of effector T cells such as Th2, Th17, T follicular helper (Tfh) cells, and invariant natural killer T (iNKT) cells and in a rise of regulatory T (Treg) cells.14–17 Created with BioRender.com.
Table 1.
Summary of immunological modifications induced by rituximab therapy and relationship with disease relapse in childhood idiopathic nephrotic syndrome
| Study | Study Design | Study Population | No. of Patients Analyzed | No. of Rituximab Courses | Rituximab Regimen per Course, mg/m2 | Maintenance Immunosuppression | Immune Cells | Findings |
|---|---|---|---|---|---|---|---|---|
| Colucci et al.,18 2016 | Retrospective cohort study | FRNS/SDNS | 28 | 1 | 375 × 1 (n=24) 375 × 2 (n=4) |
Steroid MMF CNI For more than 3 months |
B cells T cells |
Complete depletion of total CD19+, transitional, mature-naive, and memory B-cell subsets, which started to reappear at 6 months; normalization of total CD19+, transitional, mature-naive B cells at 12 months; significant reduction of memory B-cell subsets at 12 months. A delayed recovery of total memory B cells and in particular of switched memory B cells was associated with a reduced risk of relapse. No association with relapse was found for the other B-cell subsets. No modification in total CD3+ T cells; normalization of the initially low CD4+/CD8+ T-cell ratio at 12 months. No association with relapse. |
| Chan et al.,19 2016 | Cohort study | SDNS (n=4) SRNS (n=18) |
22 | 1 | 375 × 2 (n=10) 375 × 4 (n=12) |
Steroid and CNI for <3 months MMF for more than 3 months |
B cells T cells |
Total CD19+ B cells started to reappear at 7 months. No association with relapse. At baseline, ex vivo activated T cells (CD154+CD4+CD3+, IFNγ+CD3+, IL-2+CD3+) were lower in responders compared with nonresponders; at 6 months, recovery of all three activated T-cell subsets was observed in responders and no modification was induced in nonresponders. |
| Bhatia et al.,14 2018 | Cohort study | SDNS | 18 | 1 | 375 × 2 (n=18) | Steroid Tapered in 3–5 months |
B cells T cells |
Complete depletion of total CD19+ B cells in 16/18 patients, which started to reappear at 6 months. An earlier recovery of total CD19+ B cells and total memory B cells was associated with relapse. Reduction of Th17 and Th2 cells; increase of regulatory T cells. At relapse, patients had higher Th17 and Th2 cells and lower regulatory T cells. No modification of Th1 cells. |
| Boumediene et al.,15 2018 | Double-blind RCT | CNI- or MMF-dependent FRNS/SDNS |
23 | 1 | 375 × 2 (n=10) Placebo (n=13) |
Steroid MMF CNI For <3 months |
B cells T cells |
Not evaluated. No modification of total CD3+ T cells and of CD4+ and CD8+ T cells; reduction of T follicular helper cells and invariant natural killer T cells, associated with remission; increase of regulatory T cells. At relapse, patients had lower regulatory T cells. |
| Fribourg et al.,20 2021 | Cohort study | CNI-dependent SDNS | 30 | 1 | 375 × 1 (n=15) Ofatumumab 1.50 g/1.73 m2 × 1 (n=15) |
Steroid CNI For < 3 months |
B cells T cells Innate immune cells |
Total CD19+, transitional, naive, regulatory, antibody-secreting and memory B-cell subsets were significantly reduced after 3–7 months. An earlier recovery of switched B-cell subsets was associated with relapse. Reduction of Th17 and T follicular helper cells. No modification of Th1 or regulatory T cells. No association with relapse. No significant modification. No association with relapse. |
| Ravani et al.,16 2021 | Open-label, RCT | CNI-dependent SDNS | 69a | 1 | 375 × 1 (n=33) Ofatumumab 1.50 g/1.73 m2 × 1 (n=36) |
Steroid MMF CNI For <3 months |
B cells |
Complete depletion of total CD19+, transitional, mature-naive, total memory, IgM memory, switched memory B cells and plasmablasts at 1 month, which started to reappear at 3 months in the rituximab arm and at 6 months in the ofatumumab arm; normalization of total CD19+, transitional, mature-naive B cells and plasmablasts at 12 months; significant reduction of memory B-cell subsets at 12 months. The transient more prolonged B-cell depletion with ofatumumab compared with rituximab did not modify the incidence of relapse at 12 and at 24 months. |
| T cells | Transient increase of total CD3+ T cells at 1–3 months, with normalization at 12 months; regulatory T cells started to increase at 1–3 months and were still significantly higher at 12 months. Association with relapse was not evaluated. |
|||||||
| Innate immune cells | Transient increase of total CD56+ natural killer cells at 1–3 months, with normalization at 12 months. Association with relapse was not evaluated. |
FRNS, frequently relapsing nephrotic syndrome; SDNS, steroid-dependent nephrotic syndrome; MMF, mycophenolate mofetil; CNI, calcineurin inhibitor; SRNS, steroid-resistant nephrotic syndrome; RCT, randomized controlled trial.
Data on immunological modifications were available for 69/140 enrolled patients.
Current Evidence for Rituximab Use in Nephrotic Syndrome
Frequently Relapsing, Steroid-Dependent Nephrotic Syndrome
The efficacy of rituximab was first evaluated among children with complicated frequently relapsing, steroid-dependent nephrotic syndrome (Table 2).5–7 In the landmark randomized controlled trial (RCT) of 48 children by Iijima et al.,5 the median relapse-free period was significantly longer in the rituximab group (267 versus 101 days). Subsequent trials confirmed that the use of rituximab permitted steroid withdrawal, prevented recurrences, and improved growth (Table 2).6,7 Although meta-analysis confirmed the role of rituximab in prolonging remission and reducing relapse, trials included were of short observation duration; thus, the long-term efficacy was not adequately addressed.35,36 Recent trials have focused on the role of rituximab as a first-line steroid-sparing agent in children with milder disease, compared with the previous studies.32,33,37 In this patient population, rituximab was more efficacious than tacrolimus, low-dose mycophenolate mofetil (MMF), and oral cyclophosphamide (12-month relapse-free survivals: 90% versus 38%, 80% versus 13%, and 84% versus 59%, respectively).32,33,37 The adverse events were notably not increased in the rituximab groups.32,33 A further trial is underway to evaluate the efficacy of rituximab versus high-dose MMF (1200 mg/m2 per day) in calcineurin inhibitor (CNI)-dependent steroid-dependent nephrotic syndrome.38
Table 2.
Summary of clinical trials on the use of rituximab in childhood idiopathic nephrotic syndrome
| Study | Study Design | Study Population | Age (yr) | No. of Patients Analyzed | Follow-Up Period (mo) | Rituximab Arm | Control/Comparative Arm | Primary End Points | Results |
|---|---|---|---|---|---|---|---|---|---|
| Ravani et al.,29 2011 | Open-label, RCT | CNI-dependent SDNS | 1–16 | 54 | 3 | Add-on RTX 375 mg/m2 × 1–2 (n=27) | Steroid and CNI (n=27) | Proteinuria at 3 months | 3-month proteinuria was 70% lower in the RTX arm (95% CI, 35 to 86); relapse rates were 18.5% (RTX) and 48.1% (control) (P = 0.029) |
| Magnasco et al.,30 2012 | Open-label, RCT | SRNS unresponsive to CNI | 1–16 | 31 | 3 | Add-on RTX 375 mg/m2 × 2 (n=16) | Steroid and CNI (n=15) | Proteinuria reduction at 3 months | 3-month proteinuria was not reduced in the RTX arm (change, −12% [95% CI, −73 to 110]; P = 0.77) |
| Iijima et al.,5 2014 | Double-blind RCT | Complicated FRNS/SDNS | ≥2 | 48 | 12 | 375 mg/m2 × 4 (n=24) | Placebo (n=24) | Relapse-free remission | Remission period, RTX versus placebo: 267 days (95% CI, 223 to 374) versus 101 days (95% CI, 70 to 155); HR, 0.27, 95% CI, 0.14 to 0.53; P<0.0001 |
| Ruggenenti et al.,7 2014 | Off-on trial | FRNS or SDNS, with underlying MCD or FSGS | Not specified | 10 children and 20 adults | 12 | 375 mg/m2 × 1 (n=28) or × 2 (n=2) | Status 1 year prior to RTX | Number of relapses and steroid maintenance dose | Relapse per year decreased from 2.5 (IQR, 2–4) to 0.5 (IQR, 0–1); P<0.001; steroid dose decreased from 0.27 mg/kg (IQR, 0.19–0.60) to 0 mg/kg (IQR, 0–0.23); P<0.001 |
| Ravani et al.,6 2015 | Open-label, noninferiority, RCT | SDNS | 1–16 | 30 | 12 | Add-on RTX 375 mg/m2 × 1 (n=15) | Prednisolone or steroid-sparing agents (n=15) | Proteinuria at 3 months and risk of relapse | 3-month proteinuria was 42% lower in the RTX arm. The risk of relapse was reduced by 98% in RTX arm (HR, 0.02; 95% CI, 0.01 to 0.15) |
| Ahn et al.,31 2018 | Open-label, RCT | CNI-dependent SDNS | <24 | 51 | 12 | Add-on RTX 375 mg/m2 × 1–2 (n=35) | Steroid and CNI tapering (n=16) | Relapse-free remission at 6 months | Remission rates, RTX versus control: 74.3% versus 31.3%; P = 0.003 |
| Basu et al.,32 2018 | Open-label, RCT | SDNS not previously treated with steroid-sparing agent | 3–16 | 120 | 12 | 375 mg/m2 × 2 (n=60) | Tacrolimus with tapering of alternate day steroid (n=60) | Relapse-free remission at 12 months | Remission, RTX versus tacrolimus: 54 (90.0%) versus 38 (63.3%); P<0.001; OR, 5.21; 95% CI, 1.93 to 14.07 |
| Ravani et al.,33 2021 | Open-label, RCT | SDNS | 3–24 | 30 | 12 | 375 mg/m2 × 1 (n=15) | MMF (750 mg/m2 per day) (n=15) | Relapse at 12 months | Relapse, RTX versus MMF: 2 (13%) versus 12 (80%); P = 0.008. A significantly higher odds of relapse among children in the MMF arm (OR, 26; 95% CI, 2.9 to 311.0). The study was terminated early due to unexpectedly high rates of relapse in the MMF arm |
| Ravani et al.,16 2021 | Open-label, RCT | CNI-dependent SDNS | 2–24 | 140 | 24 | 375 mg/m2 × 1 (n=70) | Ofatumumab 1.50 g/1.73 m2 × 1 (n=70) | Relapse at 12 months | Relapse, RTX versus ofatumumab 36 (51%) versus 37 (53%); OR, 1.06; 95% CI, 0.55 to 2.06 |
| Mathew et al.,34 2022 | Open-label, noninferiority, RCT | Complicated FRNS/SDNS | 3–18 | 40 | 12 | 375 mg/m2 × 2 (n=20) | Tacrolimus (n=20) | Relapse-free remission at 12 months | Remission, RTX versus tacrolimus: 11 (55%) versus 11 (55%). Noninferiority of RTX to tacrolimus was not demonstrated |
RCT, randomized controlled trial; CNI, calcineurin inhibitor; SDNS, steroid-dependent nephrotic syndrome; RTX, rituximab; CI, confidence interval; SRNS, steroid-resistant nephrotic syndrome; FRNS, frequently relapsing nephrotic syndrome; HR, hazard ratio; MCD, minimal change disease; FSGS, focal segmental glomerulosclerosis; IQR, interquartile range; MMF, mycophenolate mofetil; OR, odds ratio.
The use of humanized anti-CD20 antibodies, such as ofatumumab, has been considered for the treatment of nephrotic syndrome in children with rituximab resistance, allergy, or development of anti-rituximab antibodies.39,40 However, ofatumumab was not found to be superior to rituximab in maintaining remission in steroid-dependent nephrotic syndrome in a recent RCT.16 Thus, there remains no clear indication for using humanized anti-CD20 antibodies in nephrotic syndrome. Obinutuzumab, a second-generation anti-CD20 monoclonal antibody, has a more profound B-cell depleting effect compared with rituximab due to a different mechanism of B-cell cytotoxicity.41 There is currently only one retrospective study evaluating the efficacy and safety of obinutuzumab, where it was used in combination with daratumumab, an anti-CD38 antibody, among children with multidrug-dependent nephrotic syndrome refractory to rituximab or ofatumumab.42 Nine of 14 patients did not relapse after this global anti–B-cell strategy. Of note, the profound B-cell depletion was associated with a prolonged reduction in serum IgM and IgA, with a lesser extent in IgG. Further studies are required to investigate the drug safety profile and whether such an improved clinical response is due to an effect of obinutuzumab, daratumumab, or a combination of both drugs.
Steroid-Resistant Nephrotic Syndrome and Post-transplant Recurrence
Children with nephrotic syndrome resistant to steroids, CNIs, and supportive therapies are at high risk of kidney failure and may benefit from rituximab therapy.9,43–45 Current International Pediatric Nephrology Association guidelines suggest considering rituximab as a rescue therapy in this patient population.9 A number of retrospective reports showed that 30%–80% of children achieved either complete or partial remission after the use of rituximab.46–48 Such an effect in reduction of proteinuria was not substantiated in the only available clinical trial.30 Nonetheless, the observation period in this work was only 3 months, which was inadequate for a definite conclusion. Details of studies pertaining to the use of rituximab in steroid-resistant nephrotic syndrome are summarized in Table 3. In addition, there are case reports suggesting that ofatumumab may be helpful in a subset of patients who are rituximab-resistant.55–57 Rituximab is used in the treatment of post-transplant recurrences,8 and 75% of patients showed a positive response, yet the evidence is limited to case series.8 Since rituximab is often used alongside therapeutic plasmapheresis, it is not possible to assess the individual efficacy of each treatment and identify the most effective therapy.
Table 3.
Summary of clinical trials and selected observational studies on the use of rituximab in steroid-resistant nephrotic syndrome
| Study | Study Design | Study Population | No. of SRNS Patients Analyzed | Rituximab Dosing, mg/m2 | Control/Comparative Arm | All | Remission | Remarks | |
|---|---|---|---|---|---|---|---|---|---|
| Complete | Partial | ||||||||
| Prytula et al.,49 2010 | Survey | SRNS ± CNI-resistance | 27 | 375–1875 | NA | 18 (66.7%) | 6 (22.2%) | 12 (44.4%) | |
| Magnasco et al.,30 2012 | Open-label, RCT | SRNS unresponsive to steroid and 6 months CNI | 16 | Rituximab 375 × 2 (n=16) | Steroid and CNI (n=15) | 3 (18.8%) | NR | NR | Proteinuria was not reduced at 3 months by rituximab (change, −12% [95% CI, −73 to 110]; P = 0.77) |
| Ito et al.,50 2013 | Survey | SRNS unresponsive to steroid and additional IS | 19 | NR | NA | 12 (63.2%) | 6 (31.6%) | 6 (31.6%) | |
| Kamei et al.,48 2014 | Retrospective cohort | SRNS unresponsive to steroid and CNI | 10 | 375–1500 | NA | 8 (80%) | 7 (70%) | 1 (10%) | |
| Basu et al.,47 2015 | Retrospective cohort | SRNS unresponsive to steroid and 6 months CNI | 24 | 750–1500 | NA | 16 (66.7%) | 5 (20.8%) | 11 (45.8%) | |
| Sinha et al.,51 2015 | Retrospective cohort | SRNS unresponsive to steroid and 6 months CNI | 58 | 750–1500 | NA | 17 (29.3%) | 7 (12.1%) | 10 (17.2%) | |
| Hoseini et al.,52 2018 | Retrospective cohort | SRNS unresponsive to steroid and 6 months CNI | 23 | 1500 | NA | 11 (47.8%) | 8 (34.8%) | 3 (13.3%) | |
| Ahn et al.,31 2018 | Open-label, single arm trial | SRNS unresponsive to steroid and 3 months CNI | 23 | 375–750 | NA | 9 (39.1%) | 7 (30.4%) | 2 (8.7%) | |
| Sinha et al.,53 2020 | Retrospective cohort | SRNS unresponsive to steroid and 6 months CNI | 31 | 750 | NA | 18 (58.1%) | 8 (25.8%) | 10 (32.3%) | |
| Bazargani et al.,54 2022 | Retrospective cohort | SRNS unresponsive to steroid and additional IS | 22 | 1500 | NA | 14 (63.6%) | 5 (22.7%) | 9 (40.9%) | |
SRNS, steroid-resistant nephrotic syndrome; CNI, calcineurin inhibitor; NA, not available; RCT, randomized controlled trial; NR, not reported; CI, confidence interval; IS, immunosuppression.
Patient and Immunological Factors Determining Treatment Efficacy
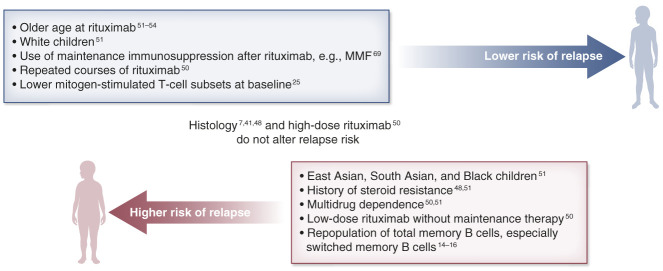
Although there has been no direct comparison in clinical trials, fewer children with complicated frequently relapsing, steroid-dependent nephrotic syndrome previously treated with steroid-sparing immunosuppressive drugs remain relapse-free at 1 year (37% versus 90%), compared with cases receiving rituximab as first-line therapy (Figure 2).5,32 Children are more prone to relapse if they have a history of steroid resistance, multidrug dependence, and higher number of previous immunosuppression before rituximab.46,58–61 These suggest that patients with a complicated disease course tend to respond less favorably to rituximab. In contrast with adolescents who generally experience longer relapse-free survival,61 younger children are associated with a shorter period of B-cell depletion.62–64 Differences by ethnicity in a large multiethnic pediatric cohort demonstrated that White patients had a lower relapse risk than East Asian, South Asian, or Black children.60 Underlying kidney histology is surprisingly an unreliable prognostic factor for relapse.7,46,58 Finally, variations in the immune response remarkably affect treatment outcomes. For instance, earlier relapse was noted in children who had lower circulating regulatory T-cell and higher mitogen-stimulated T-cell subsets before rituximab.17,19 Repopulation of total memory B cells, and particularly of the switched memory B-cell subset, rather than total B cells, was also found to predict nephrotic syndrome relapse.14,18,20 Immunophenotyping, especially at disease onset before immunosuppression, should be investigated for the discovery of biomarkers that aid individualized treatment strategies.
Figure 2.
Clinical and immunological factors that determine the treatment outcomes after rituximab therapy in children with frequently relapsing, steroid-dependent nephrotic syndrome. MMF, mycophenolate mofetil.
Among children with steroid-resistant, CNIs-refractory nephrotic syndrome, higher response rates were observed among patients with early use of rituximab,53 minimal change disease,45,51,65 late steroid resistance,45,65 and concomitant immunosuppression.65
What Is the Optimal Regimen of Rituximab in Nephrotic Syndrome?
Rituximab was originally used to treat B-cell non-Hodgkin lymphoma at 375 mg/m2 with four infusions. An alternative rituximab regimen is 750 mg/m2 for two infusions, which is widely used in different autoimmune diseases such as systemic lupus erythematosus and ANCA-associated vasculitis.66,67 This original dosing regimen (1500 mg/m2 per course) was adopted in the earliest clinical trial for nephrotic syndrome.5 A lower dosing range (375–750 mg/m2 per course) also demonstrated favorable efficacy in subsequent studies.6,31 Until recently, there are very limited data that directly compare the outcomes of different regimens.63,64,68 This has led to a huge variation in prescribed regimens worldwide.49,69 The practice of maintaining immunosuppression to prolong remission is also discrepant.60
Most reports comparing the dose effect of rituximab are limited by retrospective study design, small sample size, and confounding from concurrent immunosuppression.63,64,68,70 While early data suggested that a high-dose regimen (1125–1500 mg/m2) was associated with longer remission,64,68 a recent UK multicenter study reported that low-dose rituximab (375 mg/m2) was equally effective.70 On the other hand, a very low-dose regimen of 100 mg/m2 was reported to be ineffective, with higher risk of relapse.63
To address this long-lasting controversy, we conducted an international, multicenter study with a large pediatric cohort of 511 multiethnic patients with complicated frequently relapsing, steroid-dependent nephrotic syndrome.60 Six different regimens used during the first rituximab treatment were evaluated: low (375 mg/m2), medium (750 mg/m2), and high (1125–1500 mg/m2) dose per course, with or without maintenance immunosuppression. Patients receiving low-dose rituximab without maintenance immunosuppression had the shortest relapse-free survival of only 8.5 months and the highest risk of relapse. Relapse-free survivals (10.9–14.0 months) and relapse risk were otherwise similar among the remaining five treatment regimens. Our unpublished data also demonstrated that whether rituximab was prescribed at 375 mg/m2 for four infusions or 750 mg/m2 for two infusions did not appear to affect treatment outcomes. On the basis of these findings, recent recommendations on rituximab dose have shifted from the original high-dose regimen toward a lower dosing range of 375–750 mg/m2.71–73
Results from a multicenter, randomized, double-blinded, placebo-controlled trial on the role of MMF after rituximab as maintenance therapy have recently become available.74 Patients received either MMF or placebo after rituximab for 505 days. The use of MMF with rituximab reduced the risk of treatment failure by 80%, defined as relapse and/or steroid use, compared with rituximab alone. Although MMF did not increase the frequency of adverse events, its relapse-preventing effect disappeared after drug discontinuation. Although MMF appears to be promising, CNIs may also be efficacious,75 taking into account that this drug is potentially nephrotoxic. An RCT is underway to examine whether monthly intravenous immunoglobulin supplementation together with low-dose rituximab can further improve remission rates (RITUXIVIG, ClinicalTrials.gov identifier: NCT03560011).
In summary, both low-dose rituximab (375 mg/m2/course) with concomitant immunosuppression and medium-dose rituximab (750 mg/m2/course) with tapering of immunosuppression are effective regimens for children with frequently relapsing, steroid-dependent nephrotic syndrome. The use of concurrent immunosuppression extends remission but should be balanced against potential complications such as infection.76 By contrast, the optimal dosing regimen for achieving disease remission among steroid-resistant nephrotic syndrome remains unclear and requires further investigation.
Adverse Events and Long-Term Safety Concerns
Overall, rituximab appears to be safe for most children.5,7,32,49,64,68,77–79 Infusion reactions are prevalent but amenable to the use of premedications such as steroids, antihistamines, and slowing of the infusion rate.60,79 Hypogammaglobulinaemia, neutropenia, and infection are the most common adverse events (Tables 4 and 5).61 Other rare but serious complications are reported, including lung injury, fatal viral hepatitis reactivation, and multifocal leukoencephalopathy.84–88 Although there are isolated reports of neoplasm after rituximab, a definite causal relationship has not been firmly established.61,89–91 One important caveat in interpreting the safety profile of rituximab is that most available data come from older children. Young children often have difficult-to-treat nephrotic syndrome and are also at a potentially higher risk of developing neutropenia and hypogammaglobulinemia.61,92
Table 4.
Summary of adverse events on rituximab use in nephrotic syndrome from prospective studies
| Study/Author | Study Design | No. of Patients Analyzed | No. of Rituximab Courses | Rituximab Regimen per Course, mg/m2 | Any Side Effects | Serious Side Effects | Infusion Reaction | Neutropenia | Infection | Death | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rituximab | Control | Rituximab | Control | Rituximab | Control | Rituximab | Control | Rituximab | Control | Rituximab | Control | |||||
| Magnasco et al.,30 2012 | RCT | 31 | 16 | 750 | 11 | NR | 2 | NR | 11 | — | NR | NR | NR | NR | 0 | 0 |
| Iijima et al.,5 2014 | DBRCT | 48 | 24 | 1500 | 357 | 251 | 16 | 7 | 41 | 26 | 4 | 0 | 105 | 42 | 0 | 0 |
| Ruggenenti et al.,7 2014 | Off-on trial | 30 | 30 | 375–750 | 8 | — | NR | NR | 0 | — | NR | — | 5 | — | 0 | — |
| Ravani et al.,6 2015 | RCT | 30 | 15 | 375 | NR | NR | 0 | 0 | 15 | — | 0 | 0 | 0 | 0 | 0 | 0 |
| Ahn et al.,31 2018 | RCT | 54 | 36 | 375–750 | 92 | 28 | 3 | 1 | 36 | — | NR | NR | 21 | 4 | 0 | 0 |
| Basu et al.,32 2018 | RCT | 120 | 60 | 750 | 123 | 145 | 7 | 4 | 23 | — | NR | NR | 13 | 26 | 0 | 0 |
| Kari et al.,37 2020 | Prospective cohort | 46 | 19 | 750 | 1 | 7 | NR | NR | 1 | — | 0 | 6 | NR | NR | 0 | 0 |
| Ravani et al.,33 2021 | RCT | 30 | 15 | 375 | NR | NR | 0 | 0 | 0 | — | 0 | 0 | 0 | 0 | 0 | 0 |
| Ravani et al.,16 2021 | RCT | 140 | 70 | 750 | 11 | 5 | 0 | 0 | 7 | 4 | 2 | 1 | NR | NR | 0 | 0 |
| Mathew et al.,34 2022 | RCT | 40 | 20 | 750 | 133 | 181 | 1 | 4 | 12 | — | 1 | 0 | 86 | 67 | 0 | 0 |
| Total | 569 | 305 | — | 736/275 | 617/219 | 29/256 | 16/222 | 146/305 | 30/94 | 7/163 | 7/171 | 230/200 | 139/152 | 0/305 | 0/264 | |
| Episode per rituximab course | 2.67 | 2.81 | 0.11 | 0.07 | 0.47 | 0.32 | 0.04 | 0.04 | 1.15 | 0.91 | 0 | 0 | ||||
RCT, randomized controlled trial; NR, not reported; DBRCT, double-blind randomized placebo-controlled trial.
Table 5.
Summary on incidence of hypogammaglobulinaemia after rituximab therapy in nephrotic syndrome from prospective studies
| Study/Author | Study Design | No. of Patients Analyzed | No. of Rituximab Courses | Rituximab Regimen per Course, mg/m2 | Outcome Measures | Rates of Hypogammaglobulinaemia | Remarks | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Baseline IgG Levels | |||||||||
| Normal | Reduced | |||||||||
| Iijima et al.,5,80 2014 | DBRCT | 48 | 24 | 1500 | Patient-based | Low IgG during rituximab | 0 (0%) | 0 (0%) | 0 (0%) | Findings in control group were not reported |
| Ravani et al.,6 2015 | RCT | 30 | 15 | 375 | Patient-based | Low IgG at 3 monthsa | 3/13 (23.1%) | NR | NR | Findings of IgG levels were the same in control group, although numbers were not specified |
| Parmentier et al.,81 2019 | Retrospective cohort study | 107 | NRb | 375–750 | Patient-based | ≥1 low IgG during all rituximab | 37/107 (34.6%) | 25/86 (29.0%) | 12/21 (57.0%) | |
| Colucci et al.,82 2019 | Cohort study | 27 | 41 | 375 | Patient-based | Low IgG at last follow-upc | 11/27 (40.7%) | 5/13 (38.5%) | 6/13 (46.2%) | |
| Chan et al.,60 2020 | Retrospective cohort study | 511 | 511 | 375–1500 | Patient-based | Low IgG at 12 months | 56/400 (14.0%) | NR | NR | |
| Ravani et al.,33 2021 | RCT | 30 | 15 | 375 | Patient-based | Low IgG at last follow-upd | 8/15 (53.3%) | 2/6 (33.3%) | 6/9 (67%) | Findings in control group were not reported |
| Inoki et al.,83 2021 | Retrospective cohort | 103 | 238 | 750 | Course-based | Low IgG during rituximab | 139/238 (58.4%) | 17/74 (23.0%) | 83/98 (84.7%) | |
| Chan et al.,61 2022 | Retrospective cohort | 346 | 1149 | 375–1500 | Course-based | Low IgG during rituximab | 353/693 (50.9%) | NR | NR | |
IgG, immunoglobulin G; DBRCT, double-blind randomized placebo-controlled trial; RCT, randomized controlled trial; NR, not reported.
Since the reference range of IgG was not defined in the study, a cutoff of >500 mg/dl was applied to the reported findings.
Ninety-six patients required additional rituximab therapy.
At least 2 years from last rituximab.
Unpublished data provided upon request by investigators; at last follow-up (median follow-up 286 days).
Hypogammaglobulinemia
Hypogammaglobulinemia is common and ranges from 14% to 58% after rituximab in children treated for nephrotic syndrome.6,33,60,76,81–83 Up to 40% of the treated children experience persistent hypogammaglobulinemia even 2 years after the last rituximab infusion.82 The true incidence and significance of rituximab-induced hypogammaglobulinemia, however, was poorly investigated due to the lack of prospective study that provides consistent immunoglobulin monitoring and a comparative control group of children with nephrotic syndrome who only receive oral immunosuppression. Reduced immunoglobulin G levels at baseline, which is prevalent in nephrotic syndrome due to both the nephrotic state and the use of immunosuppressants,93,94 seem to increase the risk of prolonged hypogammaglobulinemia.83 The history of steroid resistance, young age (<6 years) at rituximab, and previous cyclophosphamide exposure are other reported risk factors.61,81,82,95
Despite the prevalence of hypogammaglobulinemia, only 1% of all hypogammaglobulinemia episodes are associated with infection,61,83 suggesting that most patients have asymptomatic numerical hypogammaglobulinemia. Even very low levels of immunoglobulins below 200 mg/dl do not correlate with clinical infection. Other factors contributing to infectious complications include underlying disease (e.g., lupus), concurrent immunosuppression, and coexisting complications such as neutropenia.76,82,83,95–98 The indications when intravenous immunoglobulin substitution is required are not well defined, and current guidelines do not recommend replacement at a definitive cutoff.61,99
Neutropenia
Neutropenia can occur after rituximab and is more prevalent among young children.92 Kamei et al.92 reported that younger age at first rituximab dose was associated with agranulocytosis (2.5 versus 4.8 years). The incidence of neutropenia and agranulocytosis ranges from 4% to 8% and 2% to 5%, respectively.5,61,92 Although overall outcomes appear favorable, some of these children may develop febrile illness, particularly upper respiratory tract infections and gingivostomatitis, and require treatments including antimicrobials and granulocyte colony–stimulating factor.61,92
Infection
Most infections reported in clinical trials appeared to be mild to moderate in severity according to standard terminology.5,31,32,34 However, serious infections, such as fulminant viral myocarditis and chronic hepatitis infection, have been reported.61,88 Opportunistic infections, such as Pneumocystis Jirovecii pneumonia, can also occur in this patient population.61,100 Tuberculosis remains endemic in many low- and middle-income countries. Thus, screening tests, such as chest radiographs, Mantoux test, and/or IFN-γ release assays, are recommended before rituximab to exclude latent tuberculosis infection.
Fortunately, the risks of acquiring coronavirus disease 2019 (COVID-19) infection, the development of symptomatic illness, and mortality are not increased in children receiving rituximab for nephrotic syndrome.101,102 Nonetheless, COVID-19 vaccination is recommended, and additional doses are required to provide more robust immunological protection.103 Existing guidelines recommend that vaccination should not be delayed despite immunosuppressive treatments and, whenever possible, be provided 2–4 weeks before initiation or resumption of rituximab therapy.104–106 Nonetheless, withholding rituximab to complete vaccination series may not be feasible among patients with difficult-to-treat nephrotic syndrome who are high risk of relapse.106 The timing of vaccination and rituximab administration should thus be individualized, balancing the risk of infection and disease relapse.
Is It Efficacious and Safe to Prescribe Repeated Courses of Rituximab?
Eighty percent of patients treated with rituximab would eventually relapse60; thus, many of these children require multiple courses of rituximab to sustain remission. Redosing rituximab is a decision that requires a thorough understanding of its long-term efficacy and safety profile after repeated treatments. One concern is the development of anti-rituximab antibodies, which occurs in up to 30% of patients after drug exposure.107 Possible hypotheses state that these antibodies could increase the chances of severe infusion reactions or that they potentially could reduce the efficacy of further treatments by binding the active rituximab in vivo.107,108 None have been proven. The impact, if any, of repeated B-cell depletion on the developing immune system is also not well understood.
We recently evaluated 346 children from 16 pediatric nephrology centers in 10 countries, who received two or more courses of rituximab for frequently relapsing, steroid-dependent nephrotic syndrome.61 It was interesting to note that the relapse-free survival time improved with an increasing number of rituximab courses rather than shortened due to drug resistance, even after adjusting for age. This effect was substantial, with the relapse-free period increased from 10.0 months after the first course to 16.0 months after the fourth course. Importantly, about 20%–30% of patients never had a further relapse for 2 years or more after each treatment course. These findings support a disease-modifying effect of rituximab on nephrotic syndrome, possibly due to a cumulative, prolonged impairment of specific pathogenic B-cell subsets more than of total B cells, as evidenced by comparable total B-cell recovery after subsequent rituximab treatments.82 Interestingly, the dosing regimen did not appear to affect treatment efficacy during subsequent rituximab treatments.61 One possible explanation is that B cells repeatedly exposed to depleting therapies are more vulnerable on further treatments, even if rituximab is given at a low dose. Finally, in concordance with previous data,60,78,81 the rates of complications such as infusion reactions, hypogammaglobulinemia, neutropenia, and infections were not increased despite an increasing number of courses and cumulative dose of rituximab. Repeated rituximab therapy, therefore, appears to be effective and safe in frequently relapsing, steroid-dependent nephrotic syndrome. Of note, this study was limited by its retrospective nature and variable policies on rituximab prescription, maintenance immunosuppression, and monitoring strategies between participating centers. Until prospective longitudinal studies with an extended follow-up period are available, applying these data into clinical practice requires judicious interpretation.
Redosing Approaches
There are several strategies for repeated rituximab infusions. One popular strategy is to redose rituximab only with disease relapse, with a consideration of maintenance immunosuppression such as MMF to prolong remission. Preferably, rituximab should be administrated after attaining remission because the serum half-life of rituximab is extremely short due to excessive urinary loss in patients with significant proteinuria.109 An alternative approach would be to repeat rituximab once the B cells reconstitute.32,62,63,110 The temporal correlation between total B-cell population and disease relapse is, however, discrepant, and 30% of patients do not relapse after B-cell recovery.58,61 Relapses can also occur during B-cell depletion.111 A third strategy is prophylactic administration depending on previous treatment response; clinical status, such as steroid toxicity; and social reasons. In a multicenter observation study, among 22 Japanese children with complicated frequently relapsing, steroid-dependent nephrotic syndrome who repeated rituximab every 6 months consecutively for 2 years, half of them had reduced relapse rates.112 However, significant side effects, such as infection (25 episodes) and neutropenia (4 episodes), were observed with this approach,112 indicating overtreatment.
Few studies have directly compared different redosing strategies, and the optimal approach remains controversial. In a retrospective study of 61 children with frequently relapsing, steroid-dependent nephrotic syndrome, patients receiving prophylactic rituximab at B-cell recovery (CD19 >1%) had a significantly longer relapse-free period after last rituximab (667 versus 335 days) compared with the nonprophylaxis group.113 Results from the ongoing RITURNS II study on steroid-dependent nephrotic syndrome evaluating the effect of adding maintenance MMF from 4 months post-rituximab versus prophylactic rituximab therapies at 8 and 18 months (ClinicalTrials.gov identifier: NCT03899103)114 will provide evidence to guide subsequent treatments.
Adult Survivors of Childhood Nephrotic Syndrome
About 78% of children with nephrotic syndrome become disease-free by age 18 years.3 Since the number of relapses during childhood predicts disease activity in adulthood,115 children receiving rituximab for complicated frequently relapsing, steroid-dependent nephrotic syndrome have a higher chance of persistent illness. While the use of rituximab may improve the overall disease stability in glomerular diseases (Table 2),7,31,33,116–119 the tapering/discontinuation of immunosuppression during adulthood should be exercised with caution given the high propensity for relapse in these patients. Having received a considerable burden of immunosuppression (e.g., steroids, cytotoxics, or biologics) during childhood, it is important to properly screen for metabolic, cardiovascular, bone, and neoplastic complications when they enter adulthood.118 New generations of anti-CD20 treatment (e.g., obinutuzumab) demonstrate profound B-cell depletion and apparently promising results in adult glomerular diseases.120 The use of these novel agents in adult survivors of childhood nephrotic syndrome is worthy of prospective study.
Treatment Recommendations
Current evidence clearly supports the use of rituximab in children with complicated frequently relapsing, steroid-dependent nephrotic syndrome. Given the possibility of significant complications, we recommend rituximab after all other second-line treatment options have failed or resulted in side effects, especially among the youngest children. Rituximab as a first-line agent is, as expected, associated with excellent efficacy, but there are valid concerns on safety that justifies a more cautious approach. Ideally, the decision to redose rituximab should be based on nephrotic syndrome relapses. Since a significant proportion of patients do not relapse despite B-cell recovery, redosing solely based on total B-cell population or a regular schedule may subject patients to unnecessary treatments and future risk. Prophylactic rituximab may be considered in selected patients with significant steroid toxicity, history of severe relapses, or poor medication compliance. In such cases, further rituximab should be carefully reassessed every 2–3 cycles as some patients may go into long-term remission.
During initial treatment, low-dose rituximab (375 mg/m2) with maintenance therapy or medium dose (750 mg/m2) with tapering of immunosuppression are both reasonable approaches. A single infusion of rituximab at 375 mg/m2 is reasonable for subsequent treatments, with or without the use of concomitant immunosuppression, notably MMF, to extend relapse-free remission. Part of these recommendations are based on the results of retrospective uncontrolled studies and should be evaluated by prospective controlled trials in the future. Immunoglobulin levels and neutrophil count should be evaluated at baseline and regularly after rituximab. Co-trimoxazole prophylaxis against Pneumocystis Jirovecii is also recommended for 3–6 months.9 The benefit of immunoglobulin substitution to prevent infection is unclear and should be individualized according to the aforementioned risk factors for infection.76
Recent advances have provided important new knowledge on the use of rituximab in nephrotic syndrome, yet many outstanding clinical and research questions remain (Table 6). Although all children receiving rituximab are classified as having frequently relapsing, steroid-dependent nephrotic syndrome, their clinical courses are heterogeneous. The optimal rituximab approach should be individualized, balancing patient factors such as ethnicity and disease severity, treatment efficacy, drug safety, tolerance to comedication, medication compliance, cost, and family as well as physician preference. We are now designing a multiethnic prospective observational study on anti-CD20 therapies in childhood idiopathic nephrotic syndrome, which hopefully can better define their long-term safety and treatment outcomes. New biomarkers and reliable immunophenotyping are also needed to guide the treatment approach.19
Table 6.
Outstanding clinical and research questions pertaining to the use of rituximab in childhood nephrotic syndrome
| • Immunophenotyping to characterize patients' response to rituximab and identify suitable candidates for treatment |
| • Novel biomarkers to predict relapse and guide subsequent treatments |
| • Safety of rituximab as a first-line steroid-sparing agent in children with uncomplicated frequently relapsing, steroid-dependent nephrotic syndrome |
| • Identify the best approach for subsequent treatments, in particular among patients with suboptimal response to the first rituximab therapy |
| • Clinical efficacy and safety among very young children <5 years |
| • Examine and confirm the disease-modifying effect of rituximab in nephrotic syndrome, specifically to evaluate the long-term outcomes of adult survivors |
| • Determine incidence and prevalence of hypogammaglobulinaemia and the infection risk as well as immunological impairment years after last rituximab therapy |
| • Clinical effectiveness of immunoglobulin replacement in asymptomatic numerical hypogammaglobulinaemia to reduce infections |
| • Efficacy and side effect profiles of rituximab biosimilars |
| • Effect and optimal treatment regimen of rituximab in inducing remission among refractory, treatment-resistant nephrotic syndrome |
| • Clinical efficacy of rituximab among post-transplant recurrence of focal segmental glomerulosclerosis or steroid-resistant nephrotic syndrome |
| • The application of newer generation of anti-CD20 for childhood nephrotic syndrome in various clinical scenarios |
Acknowledgments
The authors would like to express their gratitude to Dr. Kjell Tullus for his tremendous contributions to the development and advancement of pediatric nephrology around the world, especially in the field of glomerular disease, urinary tract infection, and renovascular hypertension.
Because Dr. Rulan S. Parekh is an Associate Editor of CJASN, she was not involved in the peer review process for this manuscript. Another editor oversaw the peer review and decision-making process for this manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Disclosures
M. Colucci reports other interests or relationships with Italian Ministry of Health (5 x mille), Fondazione Bambino Gesú and Associazione per la Cura del Bambino Nefropatico ONLUS. R.S. Parekh reports ownership interest in Coramed-stock, SpineFx, and Synaptive-stock; research funding from Canadian Institute of Health Research (CIHR), NIH, and Ontario Ministry; patents or royalties from IZI and SpineFx; serving as an Associate Editor of CJASN, a Board Member of Bishop Strachan School, and a Board Member of Conference of Independent Schools of Ontario; and an advisory or leadership role for ISN Council. R.S. Parekh’s spouse reports patents or royalties from Coramed and serves as an officer of Coramed and SpineFx. K. Tullus reports consultancy agreements with Travere. All remaining authors have nothing to disclose.
Funding
None.
Author Contributions
E.Y.-h. Chan and K. Tullus conceptualized the study; E.Y.-h. Chan was responsible for project administration; M. Colucci was responsible for visualization; A. Lap-tak Ma, R.S. Parekh, and K. Tullus provided supervision; E.Y.-h. Chan, M. Colucci, and D.Y.-h. Yap wrote the original draft; and E.Y.-h. Chan, M. Colucci, A. Lap-tak Ma, R.S. Parekh, K. Tullus, and D.Y.-h. Yap reviewed and edited the manuscript.
References
- 1.Chanchlani R, Parekh RS. Ethnic differences in childhood nephrotic syndrome. Front Pediatr. 2016;4:39. doi: 10.3389/fped.2016.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Webb NJA, Woolley RL, Lambe T, et al. Long term tapering versus standard prednisolone treatment for first episode of childhood nephrotic syndrome: phase III randomised controlled trial and economic evaluation. BMJ. 2019;365:l1800. doi: 10.1136/bmj.l1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carter SA, Mistry S, Fitzpatrick J, et al. Prediction of short- and long-term outcomes in childhood nephrotic syndrome. Kidney Int Rep. 2020;5(4):426–434. doi: 10.1016/j.ekir.2019.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iijima K, Sako M, Nozu K. Rituximab for nephrotic syndrome in children. Clin Exp Nephrol. 2017;21(2):193–202. doi: 10.1007/s10157-016-1313-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iijima K, Sako M, Nozu K, et al. Rituximab for childhood-onset, complicated, frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2014;384(9950):1273–1281. doi: 10.1016/s0140-6736(14)60541-9 [DOI] [PubMed] [Google Scholar]
- 6.Ravani P, Rossi R, Bonanni A, et al. Rituximab in children with steroid-dependent nephrotic syndrome: a multicenter, open-label, noninferiority, randomized controlled trial. J Am Soc Nephrol. 2015;26(9):2259–2266. doi: 10.1681/ASN.2014080799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruggenenti P, Ruggiero B, Cravedi P, et al. Rituximab in steroid-dependent or frequently relapsing idiopathic nephrotic syndrome. J Am Soc Nephrol. 2014;25(4):850–863. doi: 10.1681/ASN.2013030251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weber LT, Tönshoff B, Grenda R, et al. Clinical practice recommendations for recurrence of focal and segmental glomerulosclerosis/steroid-resistant nephrotic syndrome. Pediatr Transplant. 2021;25(3):e13955. doi: 10.1111/petr.13955 [DOI] [PubMed] [Google Scholar]
- 9.Trautmann A, Vivarelli M, Samuel S, et al. IPNA clinical practice recommendations for the diagnosis and management of children with steroid-resistant nephrotic syndrome. Pediatr Nephrol. 2020;35(8):1529–1561. doi: 10.1007/s00467-020-04519-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delville M, Sigdel TK, Wei C, et al. A circulating antibody panel for pretransplant prediction of FSGS recurrence after kidney transplantation. Sci Transl Med. 2014;6(256):256ra136. doi: 10.1126/scitranslmed.3008538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jamin A, Berthelot L, Couderc A, et al. Autoantibodies against podocytic UCHL1 are associated with idiopathic nephrotic syndrome relapses and induce proteinuria in mice. J Autoimmun. 2018;89:149–161. doi: 10.1016/j.jaut.2017.12.014 [DOI] [PubMed] [Google Scholar]
- 12.Watts AJ, Keller KH, Lerner G, et al. Discovery of autoantibodies targeting nephrin in minimal change disease supports a novel autoimmune etiology. J Am Soc Nephrol. 2022;33(1):238–252. doi: 10.1681/ASN.2021060794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim AH, Chung J-J, Akilesh S, et al. B cell–derived IL-4 acts on podocytes to induce proteinuria and foot process effacement. JCI Insight. 2017;2(21):e81836. doi: 10.1172/jci.insight.81836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhatia D, Sinha A, Hari P, et al. Rituximab modulates T- and B-lymphocyte subsets and urinary CD80 excretion in patients with steroid-dependent nephrotic syndrome. Pediatr Res. 2018;84(4):520–526. doi: 10.1038/s41390-018-0088-7 [DOI] [PubMed] [Google Scholar]
- 15.Boumediene A, Vachin P, Sendeyo K, et al. NEPHRUTIX: a randomized, double-blind, placebo vs rituximab-controlled trial assessing T-cell subset changes in minimal change nephrotic syndrome. J Autoimmun. 2018;88:91–102. doi: 10.1016/j.jaut.2017.10.006 [DOI] [PubMed] [Google Scholar]
- 16.Ravani P, Colucci M, Bruschi M, et al. Human or chimeric monoclonal anti-CD20 antibodies for children with nephrotic syndrome: a superiority randomized trial. J Am Soc Nephrol. 2021;32(10):2652–2663. doi: 10.1681/ASN.2021040561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan C-Y, Teo S, Lu L, et al. Low regulatory T-cells: a distinct immunological subgroup in minimal change nephrotic syndrome with early relapse following rituximab therapy. Transl Res. 2021;235:48–61. doi: 10.2215/CJN.11941115 [DOI] [PubMed] [Google Scholar]
- 18.Colucci M, Carsetti R, Cascioli S, et al. B cell reconstitution after rituximab treatment in idiopathic nephrotic syndrome. J Am Soc Nephrol. 2016;27(6):1811–1822. doi: 10.1681/ASN.2015050523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan C-Y, Liu ID, Resontoc LP, et al. T lymphocyte activation markers as predictors of responsiveness to rituximab among patients with FSGS. Clin J Am Soc Nephrol. 2016;11(8):1360–1368. doi: 10.2215/CJN.11941115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fribourg M, Cioni M, Ghiggeri G, et al. CyTOF-enabled analysis identifies class-switched B cells as the main lymphocyte subset associated with disease relapse in children with idiopathic nephrotic syndrome. Front Immunol. 2021;12:726428. doi: 10.3389/fimmu.2021.726428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colucci M, Corpetti G, Emma F, Vivarelli M. Immunology of idiopathic nephrotic syndrome. Pediatr Nephrol. 2018;33(4):573–584. doi: 10.1007/s00467-017-3677-5 [DOI] [PubMed] [Google Scholar]
- 22.Kopp JB, Anders H-J, Susztak K, et al. Podocytopathies. Nat Rev Dis Primers. 2020;6(1):68. doi: 10.1038/s41572-020-0196-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hackl A, Zed SEDA, Diefenhardt P, Binz-Lotter J, Ehren R, Weber LT. The role of the immune system in idiopathic nephrotic syndrome. Mol Cell Pediatr. 2021;8(1):18. doi: 10.1186/s40348-021-00128-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colucci M, Oniszczuk J, Vivarelli M, Audard V. B-cell dysregulation in idiopathic nephrotic syndrome: what we know and what we need to discover. Front Immunol. 2022;13:823204. doi: 10.3389/fimmu.2022.823204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Good KL, Avery DT, Tangye SG. Resting human memory B cells are intrinsically programmed for enhanced survival and responsiveness to diverse stimuli compared to naive B cells. J Immunol. 2009;182(2):890–901. doi: 10.4049/jimmunol.182.2.890 [DOI] [PubMed] [Google Scholar]
- 26.Hoffman W, Lakkis FG, Chalasani G. B cells, antibodies, and more. Clin J Am Soc Nephrol. 2016;11(1):137–154. doi: 10.2215/CJN.09430915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crawford A, Angelosanto JM, Kao C, et al. Molecular and transcriptional basis of CD4+ T cell dysfunction during chronic infection. Immunity. 2014;40(2):289–302. doi: 10.1016/j.immuni.2014.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fornoni A, Sageshima J, Wei C, et al. Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci Transl Med. 2011;3(85):85ra46. doi: 10.1126/scitranslmed.3002231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ravani P, Magnasco A, Edefonti A, et al. Short-term effects of rituximab in children with steroid- and calcineurin-dependent nephrotic syndrome: a randomized controlled trial. Clin J Am Soc Nephrol. 2011;6:1308–1315. doi: 10.2215/CJN.09421010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magnasco A, Ravani P, Edefonti A, et al. Rituximab in children with resistant idiopathic nephrotic syndrome. J Am Soc Nephrol. 2012;23(6):1117–1124. doi: 10.1681/ASN.2011080775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahn YH, Kim SH, Han KH, et al. Efficacy and safety of rituximab in childhood-onset, difficult-to-treat nephrotic syndrome: a multicenter open-label trial in Korea. Medicine (Baltimore). 2018;97(46):e13157. doi: 10.1097/md.0000000000013157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Basu B, Sander A, Roy B, et al. Efficacy of rituximab vs tacrolimus in pediatric corticosteroid-dependent nephrotic syndrome: a randomized clinical trial. JAMA Pediatr. 2018;172(8):757–764. doi: 10.1001/jamapediatrics.2018.1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ravani P, Lugani F, Drovandi S, Caridi G, Angeletti A, Ghiggeri GM. Rituximab vs low-dose mycophenolate mofetil in recurrence of steroid-dependent nephrotic syndrome in children and young adults: a randomized clinical trial. JAMA Pediatr. 2021;175(6):631–632. doi: 10.1001/jamapediatrics.2020.6150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mathew G, Sinha A, Ahmed A, et al. Efficacy of rituximab versus tacrolimus in difficult-to-treat steroid-sensitive nephrotic syndrome: an open-label pilot randomized controlled trial. Pediatr Nephrol. 2022;37(12):3117–3126. doi: 10.1007/s00467-022-05475-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao X, Wang Y, Xu Z, Deng H, Yang H, Zhong F. Systematic review and meta-analysis of rituximab for steroid-dependent or frequently relapsing nephrotic syndrome in children. Front Pediatr. 2021;9:626323. doi: 10.3389/fped.2021.626323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu S, Gui C, Lu Z, Li H, Fu Z, Deng Y. The efficacy and safety of rituximab for childhood steroid-dependent nephrotic syndrome: a systematic review and meta-analysis. Front Pediatr. 2021;9:728010. doi: 10.3389/fped.2021.728010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kari JA, Alhasan KA, Albanna AS, et al. Rituximab versus cyclophosphamide as first steroid-sparing agent in childhood frequently relapsing and steroid-dependent nephrotic syndrome. Pediatr Nephrol. 2020;35(8):1445–1453. doi: 10.1007/s00467-020-04570-y [DOI] [PubMed] [Google Scholar]
- 38.Lugani F, Angeletti A, Ravani P, et al. Randomised controlled trial comparing rituximab to mycophenolate mofetil in children and young adults with steroid-dependent idiopathic nephrotic syndrome: study protocol. BMJ Open. 2021;11:e052450. doi: 10.1136/bmjopen-2021-052450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vivarelli M, Colucci M, Bonanni A, et al. Ofatumumab in two pediatric nephrotic syndrome patients allergic to rituximab. Pediatr Nephrol. 2017;32(1):181–184. doi: 10.1007/s00467-016-3498-y [DOI] [PubMed] [Google Scholar]
- 40.Fujinaga S, Nishino T, Endo S, Umeda C, Watanabe Y, Nakagawa M. Unfavorable impact of anti-rituximab antibodies on clinical outcomes in children with complicated steroid-dependent nephrotic syndrome. Pediatr Nephrol. 2020;35(10):2003–2008. doi: 10.1007/s00467-020-04629-w [DOI] [PubMed] [Google Scholar]
- 41.Mössner E, Brünker P, Moser S, et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell–mediated B-cell cytotoxicity. Blood. 2010;115(22):4393–4402. doi: 10.1182/blood-2009-06-225979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dossier C, Prim B, Moreau C, et al. A global antiB cell strategy combining obinutuzumab and daratumumab in severe pediatric nephrotic syndrome. Pediatr Nephrol. 2021;36(5):1175–1182. doi: 10.1007/s00467-020-04811-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan EYH, Ma ALT, Tullus K. When should we start and stop ACEi/ARB in paediatric chronic kidney disease? Pediatr Nephrol. 2021;36(7):1751–1764 [DOI] [PubMed] [Google Scholar]
- 44.Chan EYH, Yap DYH, Wong WHS, et al. Demographics and long-term outcomes of children with end-stage kidney disease: a 20-year territory-wide study. Nephrology (Carlton). 2022;27(2):171–180. doi: 10.1111/nep.14007 [DOI] [PubMed] [Google Scholar]
- 45.Bagga A, Sinha A. Individualizing treatment of steroid-resistant nephrotic syndrome: registries to the fore. Clin J Am Soc Nephrol. 2020;15(7):920–922. doi: 10.2215/CJN.08080520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sinha A, Bhatia D, Gulati A, et al. Efficacy and safety of rituximab in children with difficult-to-treat nephrotic syndrome. Nephrol Dial Transplant. 2014;30(1):96–106. doi: 10.1093/ndt/gfu267 [DOI] [PubMed] [Google Scholar]
- 47.Basu B, Mahapatra T, Mondal N. Mycophenolate mofetil following rituximab in children with steroid-resistant nephrotic syndrome. Pediatrics. 2015;136(1):e132–e139. doi: 10.1542/peds.2015-0486 [DOI] [PubMed] [Google Scholar]
- 48.Kamei K, Okada M, Sato M, et al. Rituximab treatment combined with methylprednisolone pulse therapy and immunosuppressants for childhood steroid-resistant nephrotic syndrome. Pediatr Nephrol. 2014;29(7):1181–1187. doi: 10.1007/s00467-014-2765-z [DOI] [PubMed] [Google Scholar]
- 49.Prytuła A, Iijima K, Kamei K, et al. Rituximab in refractory nephrotic syndrome. Pediatr Nephrol. 2010;25(3):461–468. doi: 10.1007/s00467-009-1376-6 [DOI] [PubMed] [Google Scholar]
- 50.Ito S, Kamei K, Ogura M, et al. Survey of rituximab treatment for childhood-onset refractory nephrotic syndrome. Pediatr Nephrol. 2013;28(2):257–264. doi: 10.1007/s00467-012-2319-1 [DOI] [PubMed] [Google Scholar]
- 51.Sinha A, Bhatia D, Gulati A, et al. Efficacy and safety of rituximab in children with difficult-to-treat nephrotic syndrome. Nephrol Dial Transplant. 2015;30(1):96–106. doi: 10.1093/ndt/gfu267 [DOI] [PubMed] [Google Scholar]
- 52.Hoseini R, Sabzian K, Otukesh H, et al. Efficacy and safety of rituximab in children with steroid- and cyclosporine-resistant and steroid- and cyclosporine-dependent nephrotic syndrome. Iran J Kidney Dis. 2018;12(1):27–32 [PubMed] [Google Scholar]
- 53.Sinha R, Banerjee S, Mukherjee A, Pradhan S, Akhtar S. Early use of rituximab in calcineurin inhibitor–refractory and steroid-resistant nephrotic syndrome. Kidney Int Rep. 2020;5(12):2354–2357. doi: 10.1016/j.ekir.2020.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bazargani B, Noparast Z, Khedmat L, et al. Efficacy of rituximab therapy in children with nephrotic syndrome: a 10-year experience from an Iranian pediatric hospital. BMC Pediatr. 2022;22(1):36. doi: 10.1186/s12887-022-03109-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang C-S, Liverman RS, Garro R, et al. Ofatumumab for the treatment of childhood nephrotic syndrome. Pediatr Nephrol. 2017;32(5):835–841. doi: 10.1007/s00467-017-3621-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Basu B. Ofatumumab for rituximab-resistant nephrotic syndrome. N Engl J Med. 2014;370(13):1268–1270. doi: 10.1056/nejmc1308488 [DOI] [PubMed] [Google Scholar]
- 57.Bonanni A, Rossi R, Murtas C, Ghiggeri GM. Low-dose ofatumumab for rituximab-resistant nephrotic syndrome. BMJ Case Rep. 2015;2015:bcr2015210208. doi: 10.1136/bcr-2015-210208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kamei K, Ogura M, Sato M, Sako M, Iijima K, Ito S. Risk factors for relapse and long-term outcome in steroid-dependent nephrotic syndrome treated with rituximab. Pediatr Nephrol. 2016;31(1):89–95. doi: 10.1007/s00467-015-3197-0 [DOI] [PubMed] [Google Scholar]
- 59.Chan EYH, Tullus K. Rituximab in children with steroid sensitive nephrotic syndrome: in quest of the optimal regimen. Pediatr Nephrol. 2021;36(6):1397–1405. doi: 10.1681/ASN.2021111472 [DOI] [PubMed] [Google Scholar]
- 60.Chan EYH, Webb H, Yu E, et al. Both the rituximab dose and maintenance immunosuppression in steroid-dependent/frequently-relapsing nephrotic syndrome have important effects on outcomes. Kidney Int. 2020;97(2):393–401. doi: 10.1016/j.kint.2019.09.033 [DOI] [PubMed] [Google Scholar]
- 61.Chan EYH, Yu EL, Angeletti A, et al. Long-term efficacy and safety of repeated rituximab to maintain remission in idiopathic childhood nephrotic syndrome: an international study. J Am Soc Nephrol. 2022;33(6):1193–1207. doi: 10.1681/ASN.2021111472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fujinaga S, Hirano D, Mizutani A, et al. Predictors of relapse and long-term outcome in children with steroid-dependent nephrotic syndrome after rituximab treatment. Clin Exp Nephrol. 2017;21(4):671–676. doi: 10.1007/s10157-016-1328-y [DOI] [PubMed] [Google Scholar]
- 63.Hogan J, Dossier C, Kwon T, et al. Effect of different rituximab regimens on B cell depletion and time to relapse in children with steroid-dependent nephrotic syndrome. Pediatr Nephrol. 2019;34(2):253–259. doi: 10.1007/s00467-018-4052-x [DOI] [PubMed] [Google Scholar]
- 64.Kemper MJ, Gellermann J, Habbig S, et al. Long-term follow-up after rituximab for steroid-dependent idiopathic nephrotic syndrome. Nephrol Dial Transplant. 2011;27(5):1910–1915. doi: 10.1093/ndt/gfr548 [DOI] [PubMed] [Google Scholar]
- 65.Kamei K, Ishikura K, Sako M, Ito S, Nozu K, Iijima K. Rituximab therapy for refractory steroid-resistant nephrotic syndrome in children. Pediatr Nephrol. 2020;35(1):17–24. doi: 10.1007/s00467-018-4166-1 [DOI] [PubMed] [Google Scholar]
- 66.Marlais M, Wlodkowski T, Printza N, et al. Clinical factors and adverse kidney outcomes in children with ANCA-associated glomerulonephritis. Am J Kidney Dis. 2023;81(1):119–122. doi: 10.1053/j.ajkd.2022.05.013 [DOI] [PubMed] [Google Scholar]
- 67.Chan EYH, Yap DYH, Wong WT, et al. Long-term outcomes of children and adolescents with biopsy-proven childhood-onset lupus nephritis. Kidney Int Rep. 2022;8(1):141–150. doi: 10.1016/j.ekir.2022.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Webb H, Jaureguiberry G, Dufek S, Tullus K, Bockenhauer D. Cyclophosphamide and rituximab in frequently relapsing/steroid-dependent nephrotic syndrome. Pediatr Nephrol. 2016;31(4):589–594. doi: 10.1007/s00467-015-3245-9 [DOI] [PubMed] [Google Scholar]
- 69.Deschênes G, Vivarelli M, Peruzzi L. Variability of diagnostic criteria and treatment of idiopathic nephrotic syndrome across European countries. Eur J Pediatr. 2017;176(5):647–654. doi: 10.1007/s00431-017-2891-2 [DOI] [PubMed] [Google Scholar]
- 70.Maxted AP, Dalrymple RA, Chisholm D, et al. Low-dose rituximab is no less effective for nephrotic syndrome measured by 12-month outcome. Pediatr Nephrol. 2019;34(5):855–863. doi: 10.1007/s00467-018-4172-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sinha A, Bagga A, Banerjee S, et al. Steroid sensitive nephrotic syndrome: revised guidelines. Indian Pediatr. 2021;58(5):461–481. doi: 10.1007/s13312-021-2217-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ehren R, Benz MR, Brinkkötter PT, et al. Pediatric idiopathic steroid-sensitive nephrotic syndrome: diagnosis and therapy—short version of the updated German best practice guideline (S2e)—AWMF register no. 166-001, 6/2020. Pediatr Nephrol. 2021;36(10):2971–2985. doi: 10.1007/s00467-021-05135-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zotta F, Vivarelli M, Emma F. Update on the treatment of steroid-sensitive nephrotic syndrome. Pediatr Nephrol. 2022;37(2):303–314. doi: 10.1007/s00467-021-04983-3 [DOI] [PubMed] [Google Scholar]
- 74.Iijima K, Sako M, Oba M, et al. Mycophenolate mofetil after rituximab for childhood-onset complicated frequently-relapsing or steroid-dependent nephrotic syndrome. J Am Soc Nephrol. 2022;33(2):401–419. doi: 10.1681/ASN.2021050643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fujinaga S, Someya T, Watanabe T, et al. Cyclosporine versus mycophenolate mofetil for maintenance of remission of steroid-dependent nephrotic syndrome after a single infusion of rituximab. Eur J Pediatr. 2013;172(4):513–518. doi: 10.1007/s00431-012-1913-3 [DOI] [PubMed] [Google Scholar]
- 76.Chan EYH, Ma ALT, Tullus K. Hypogammaglobulinaemia following rituximab therapy in childhood nephrotic syndrome. Pediatr Nephrol. 2022;37(5):927–931. doi: 10.1007/s00467-021-05345-9 [DOI] [PubMed] [Google Scholar]
- 77.Fujinaga S, Sakuraya K, Yamada A, Urushihara Y, Ohtomo Y, Shimizu T. Positive role of rituximab in switching from cyclosporine to mycophenolate mofetil for children with high-dose steroid-dependent nephrotic syndrome. Pediatr Nephrol. 2015;30(4):687–691. doi: 10.1007/s00467-014-3034-x [DOI] [PubMed] [Google Scholar]
- 78.Bonanni A, Calatroni M, D'Alessandro M, et al. Adverse events linked with the use of chimeric and humanized anti-CD20 antibodies in children with idiopathic nephrotic syndrome. Br J Clin Pharmacol. 2018;84(6):1238–1249. doi: 10.1111/bcp.13548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kamei K, Ogura M, Sato M, Ito S, Ishikura K. Infusion reactions associated with rituximab treatment for childhood-onset complicated nephrotic syndrome. Pediatr Nephrol. 2018;33(6):1013–1018 [DOI] [PubMed] [Google Scholar]
- 80.Iijima K, Sako M, Nozu K, Nakamura H, Ito S. Rituximab for patients with nephrotic syndrome—authors' reply. Lancet. 2015;385(9964):226–227. doi: 10.1016/s0140-6736(15)60052-6 [DOI] [PubMed] [Google Scholar]
- 81.Parmentier C, Delbet J-D, Decramer S, Boyer O, Hogan J, Ulinski T. Immunoglobulin serum levels in rituximab-treated patients with steroid-dependent nephrotic syndrome. Pediatr Nephrol. 2019;35(3):455–462. doi: 10.1007/s00467-019-04398-1 [DOI] [PubMed] [Google Scholar]
- 82.Colucci M, Carsetti R, Serafinelli J, et al. Prolonged impairment of immunological memory after anti-CD20 treatment in pediatric idiopathic nephrotic syndrome. Front Immunol. 2019;10:1653. doi: 10.3389/fimmu.2019.01653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Inoki Y, Kamei K, Nishi K, Sato M, Ogura M, Ishiguro A. Incidence and risk factors of rituximab-associated hypogammaglobulinemia in patients with complicated nephrotic syndrome. Pediatr Nephrol. 2022;37(5):1057–1066. doi: 10.1007/s00467-021-05304-4 [DOI] [PubMed] [Google Scholar]
- 84.Tsutsumi Y, Kanamori H, Mori A, et al. Reactivation of hepatitis B virus with rituximab. Expert Opin Drug Saf. 2005;4(3):599–608. doi: 10.1517/14740338.4.3.599 [DOI] [PubMed] [Google Scholar]
- 85.Chaumais M-C, Garnier A, Chalard F, et al. Fatal pulmonary fibrosis after rituximab administration. Pediatr Nephrol. 2009;24(9):1753–1755. doi: 10.1007/s00467-009-1195-9 [DOI] [PubMed] [Google Scholar]
- 86.Boren EJ, Cheema GS, Naguwa SM, Ansari AA, Gershwin ME. The emergence of progressive multifocal leukoencephalopathy (PML) in rheumatic diseases. J Autoimmun. 2008;30(1-2):90–98. doi: 10.1016/j.jaut.2007.11.013 [DOI] [PubMed] [Google Scholar]
- 87.Gea-Banacloche JC. Rituximab-associated infections. Semin Hematol. 2010;47(2):187–198. doi: 10.1053/j.seminhematol.2010.01.002 [DOI] [PubMed] [Google Scholar]
- 88.Sellier-Leclerc A-L, Belli E, Guérin V, Dorfmüller P, Deschênes G. Fulminant viral myocarditis after rituximab therapy in pediatric nephrotic syndrome. Pediatr Nephrol. 2013;28(9):1875–1879. doi: 10.1007/s00467-013-2485-9 [DOI] [PubMed] [Google Scholar]
- 89.Peuvrel L, Chiffoleau A, Quéreux G, et al. Melanoma and rituximab: an incidental association? Dermatology. 2013;226(3):274–278. doi: 10.1159/000350681 [DOI] [PubMed] [Google Scholar]
- 90.Velter C, Pagès C, Schneider P, Osio A, Brice P, Lebbé C. Four cases of rituximab-associated melanoma. Melanoma Res. 2014;24(4):401–403. doi: 10.1097/cmr.0000000000000074 [DOI] [PubMed] [Google Scholar]
- 91.Tarella C, Passera R, Magni M, et al. Risk factors for the development of secondary malignancy after high-dose chemotherapy and autograft, with or without rituximab: a 20-year retrospective follow-up study in patients with lymphoma. J Clin Oncol. 2011;29(7):814–824. doi: 10.1200/jco.2010.28.9777 [DOI] [PubMed] [Google Scholar]
- 92.Kamei K, Takahashi M, Fuyama M, et al. Rituximab-associated agranulocytosis in children with refractory idiopathic nephrotic syndrome: case series and review of literature. Nephrol Dial Transplant. 2014;30(1):91–96. doi: 10.1093/ndt/gfu258 [DOI] [PubMed] [Google Scholar]
- 93.Kemper MJ, Altrogge H, Ganschow R, Müller-Wiefel DE. Serum levels of immunoglobulins and IgG subclasses in steroid sensitive nephrotic syndrome. Pediatr Nephrol. 2002;17(6):413–417. doi: 10.1007/s00467-001-0817-7 [DOI] [PubMed] [Google Scholar]
- 94.Settipane GA, Pudupakkam R, McGowan JH. Corticosteroid effect on immunoglobulins. J Allergy Clin Immunol. 1978;62(3):162–166. doi: 10.1016/0091-6749(78)90101-x [DOI] [PubMed] [Google Scholar]
- 95.Marco H, Smith RM, Jones RB, et al. The effect of rituximab therapy on immunoglobulin levels in patients with multisystem autoimmune disease. BMC Musculoskelet Disord. 2014;15:178–179. doi: 10.1186/1471-2474-15-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McAtee CL, Lubega J, Underbrink K, et al. Association of rituximab use with adverse events in children, adolescents, and young adults. JAMA Netw Open. 2021;4(2):e2036321. doi: 10.1001/jamanetworkopen.2020.36321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Roberts DM, Jones RB, Smith RM, et al. Immunoglobulin G replacement for the treatment of infective complications of rituximab-associated hypogammaglobulinemia in autoimmune disease: a case series. J Autoimmun. 2015;57:24–29. doi: 10.1016/j.jaut.2014.11.004 [DOI] [PubMed] [Google Scholar]
- 98.Barmettler S, Ong M-S, Farmer JR, Choi H, Walter J. Association of immunoglobulin levels, infectious risk, and mortality with rituximab and hypogammaglobulinemia. JAMA Netw Open. 2018;1(7):e184169. doi: 10.1001/jamanetworkopen.2018.4169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wijetilleka S, Jayne DR, Mukhtyar C, et al. Recommendations for the management of secondary hypogammaglobulinaemia due to B cell targeted therapies in autoimmune rheumatic diseases. Rheumatology (Oxford). 2019;58(5):889–896. doi: 10.1093/rheumatology/key394 [DOI] [PubMed] [Google Scholar]
- 100.Sato M, Ito S, Ogura M, et al. Atypical Pneumocystis jiroveci pneumonia with multiple nodular granulomas after rituximab for refractory nephrotic syndrome. Pediatr Nephrol. 2013;28(1):145–149. doi: 10.1007/s00467-012-2286-6 [DOI] [PubMed] [Google Scholar]
- 101.Marlais M, Wlodkowski T, Al-Akash S, et al. COVID-19 in children treated with immunosuppressive medication for kidney diseases. Arch Dis Child. 2021;106(8):798–801. doi: 10.1136/archdischild-2020-320616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sinha R, Marlais M, Sarkar S, et al. Impact of COVID-19 pandemic on use of rituximab among children with difficult nephrotic syndrome. Pediatr Res. 2022;92(1):3–5. doi: 10.1038/s41390-021-01744-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ma AL-T, Leung D, Chan EY-H, et al. Antibody responses to 2 doses of mRNA COVID-19 vaccine in pediatric patients with kidney diseases. Kidney Int. 2022;101(5):1069–1072. doi: 10.1016/j.kint.2022.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Curtis JR, Johnson SR, Anthony DD, et al. American College of Rheumatology guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases: version 4. Arthritis Rheumatol. 2022;74(5):e21–e36. doi: 10.1002/art.42109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Centers for Disease Control and Prevention. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. Accessed September 18, 2022. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html#considerations-timing-COVID-19-vaccination-immunosuppressive-therapies
- 106.UK Kidney Association. UK Kidney Association guidance on COVID-19 vaccination in highly vulnerable people with kidney disease. Accessed September 18, 2022. https://ukkidney.org/sites/renal.org/files/UKKA%20COVID19%20Vaccination%20Guidance%20for%20HCPs%2006.07.21.pdf
- 107.Bertrand Q, Mignot S, Kwon T, et al. Anti-rituximab antibodies in pediatric steroid-dependent nephrotic syndrome. Pediatr Nephrol. 2022;37(2):357–365. doi: 10.1007/s00467-021-05069-w [DOI] [PubMed] [Google Scholar]
- 108.Ahn YH, Kang HG, Lee JM, Choi HJ, Ha I-S, Cheong HI. Development of antirituximab antibodies in children with nephrotic syndrome. Pediatr Nephrol. 2014;29(8):1461–1464. doi: 10.1007/s00467-014-2794-7 [DOI] [PubMed] [Google Scholar]
- 109.Counsilman CE, Jol-van der Zijde CM, Stevens J, Cransberg K, Bredius RGM, Sukhai RN. Pharmacokinetics of rituximab in a pediatric patient with therapy-resistant nephrotic syndrome. Pediatr Nephrol. 2015;30(8):1367–1370. doi: 10.1007/s00467-015-3120-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim JH, Park E, Hyun HS, et al. Long-term repeated rituximab treatment for childhood steroid-dependent nephrotic syndrome. Kidney Res Clin Pract. 2017;36(3):257–263. doi: 10.23876/j.krcp.2017.36.3.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sato M, Kamei K, Ogura M, Ishikura K, Ito S. Relapse of nephrotic syndrome during post-rituximab peripheral blood B-lymphocyte depletion. Clin Exp Nephrol. 2018;22(1):110–116. doi: 10.1007/s10157-017-1415-8 [DOI] [PubMed] [Google Scholar]
- 112.Takahashi T, Okamoto T, Sato Y, et al. Periodically repeated rituximab administrations in children with refractory nephrotic syndrome: 2-year multicenter observational study. Pediatr Nephrol. 2019;34(1):87–96. doi: 10.1007/s00467-018-4063-7 [DOI] [PubMed] [Google Scholar]
- 113.Okutsu M, Kamei K, Sato M, et al. Prophylactic rituximab administration in children with complicated nephrotic syndrome. Pediatr Nephrol. 2021;36(3):611–619. doi: 10.1007/s00467-020-04771-5 [DOI] [PubMed] [Google Scholar]
- 114.Basu B, Preussler S, Sander A, Mahapatra TKS, Schaefer F. Randomized clinical trial to compare efficacy and safety of repeated courses of rituximab to single-course rituximab followed by maintenance mycophenolate-mofetil in children with steroid dependent nephrotic syndrome. BMC Nephrol. 2020;21:520. doi: 10.1186/s12882-020-02153-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fakhouri F, Bocquet N, Taupin P, et al. Steroid-sensitive nephrotic syndrome: from childhood to adulthood. Am J Kidney Dis. 2003;41(3):550–557. doi: 10.1053/ajkd.2003.50116 [DOI] [PubMed] [Google Scholar]
- 116.Ramachandran R, Bharati J, Rao I, et al. Persistent CD-19 depletion by rituximab is cost-effective in maintaining remission in calcineurin-inhibitor dependent podocytopathy. Nephrology (Carlton). 2019;24(12):1241–1247. doi: 10.1111/nep.13554 [DOI] [PubMed] [Google Scholar]
- 117.Guitard J, Hebral A-L, Fakhouri F, et al. Rituximab for minimal-change nephrotic syndrome in adulthood: predictive factors for response, long-term outcomes and tolerance. Nephrol Dial Transplant. 2014;29(11):2084–2091. doi: 10.1093/ndt/gfu209 [DOI] [PubMed] [Google Scholar]
- 118.Gauckler P, Shin JI, Alberici F, et al. Rituximab in adult minimal change disease and focal segmental glomerulosclerosis—what is known and what is still unknown? Autoimmun Rev. 2020;19(11):102671. doi: 10.1016/j.autrev.2020.102671 [DOI] [PubMed] [Google Scholar]
- 119.DaSilva I, Huerta A, Quintana L, et al. Rituximab for steroid-dependent or frequently relapsing idiopathic nephrotic syndrome in adults: a retrospective, multicenter study in Spain. BioDrugs. 2017;31(3):239–249. doi: 10.1007/s40259-017-0221-x [DOI] [PubMed] [Google Scholar]
- 120.Furie RA, Aroca G, Cascino MD, et al. B-cell depletion with obinutuzumab for the treatment of proliferative lupus nephritis: a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2022;81(1):100–107. doi: 10.1136/annrheumdis-2021-220920 [DOI] [PMC free article] [PubMed] [Google Scholar]