Abstract
Globally, over 103 million individuals are afflicted by CKD, a silent killer claiming the lives of 1.2 million people annually. CKD is characterized by five progressive stages, in which dialysis and kidney transplant are life-saving routes for patients with end stage kidney failure. While kidney damage impairs kidney function and derails BP regulation, uncontrolled hypertension accelerates the development and progression of CKD. Zinc (Zn) deficiency has emerged as a potential hidden driver within this detrimental cycle of CKD and hypertension. This review article will (1) highlight mechanisms of Zn procurement and trafficking, (2) provide evidence that urinary Zn wasting can fuel Zn deficiency in CKD, (3) discuss how Zn deficiency can accelerate the progression of hypertension and kidney damage in CKD, and (4) consider Zn supplementation as an exit strategy with the potential to rectify the course of hypertension and CKD progression.
Keywords: CKD, hypertension, kidney damage, zinc, zinc deficiency, zinc supplementation, zinc wasting
Introduction
Globally, an estimated 103 million individuals (1 in 10) are burdened with CKD,1 a life-threatening condition that has catapulted to the 11th leading cause of global deaths.2 CKD is commonly diagnosed by an estimated GFR of <60 ml/minute per 1.73 m2 or albuminuria lasting for ≥3 months. While CKD progressively worsens in five stages, the National Institute of Diabetes and Digestive and Kidney Diseases reports that approximately 90% of people in stages 1–3 are unaware of their condition,3 earning CKD the grave moniker of being a silent killer.4 In addition to a host of comorbidities, the looming risk of CKD-related deaths and adverse cardiovascular events increases greatly with each stage.4 By stage 5, patients with kidney failure require renal replacement therapy (dialysis or kidney transplant) for life support. Of note, 85% of US patients on the transplant waiting list require a kidney,5 further highlighting an urgent need for effective therapeutic strategies to halt the progression of CKD to end stage kidney failure.
A major risk factor of the development of CKD is uncontrolled hypertension.6,7 Chronically elevated BP damages the kidneys; meanwhile, kidney damage impairs kidney function and derails BP control. This self-perpetuating cycle of kidney damage and hypertension accelerates CKD progression. Despite the clinical use of potent antihypertensive drugs, uncontrolled BP persists in up to 90% of patients with CKD6-9. As an alternative strategy to address this critical demand, the National Institutes of Health Joint National Committee recommended a concurrent dietary approach to lower BP10 in hopes of improving both cardiovascular and kidney health.
CKD is commonly accompanied by a deficiency in the essential dietary micronutrient, zinc (Zn). Several factors contribute to reduced serum Zn levels in patients with CKD11-18 including (1) dietary protein restriction, (2) decreased caloric intake, (3) intestinal malabsorption, (4) hyperuricemia, (5) impaired kidney reabsorption and subsequent urinary wasting, (6) elevated fecal excretion, and (7) hemodialysis. It is worth noting that patients with CKD are often in the elderly population19 and are on multiple medication regimens that alter taste sensation,20 thus contributing to Zn deficiency through decreased caloric intake. In addition to the mechanisms noted above, Zn redistribution can also contribute to Zn deficiency in CKD. Specifically, a study noted recruitment of Zn from both bone and plasma into the bone marrow to stimulate the production of new blood cells in animal models of CKD.21
As the impact of Zn in CKD onset and progression is now being more investigated, Zn supplementation may be recognized as an effective therapeutic strategy. However, progress in integrating Zn supplementation into clinical practice will remain difficult until the interplay between Zn homeostasis, kidney function, and BP regulation is better defined. This review article will (1) highlight mechanisms of Zn procurement and trafficking, (2) provide evidence that urinary Zn wasting can fuel Zn deficiency in CKD, (3) discuss how Zn deficiency can accelerate the progression of hypertension and kidney damage in CKD, and (4) consider Zn supplementation as an exit strategy with the potential to rectify the course of hypertension and CKD progression.
Mechanisms of Zn Procurement and Trafficking
The human body contains 2–3 g of Zn, with the largest fractions found in the skeletal muscle (approximately 50%) and bone (approximately 30%)13,16,22 (Figure 1). Lower Zn fractions are also present in the following tissues: kidney, prostate, liver, gastrointestinal (GI) tract, skin, lung, brain, heart, and pancreas16,23,24 (Figure 1). Three major routes enable Zn entry into the human body12,25,26: (1) inhalation through the lungs, (2) penetrance through the skin, and (3) ingestion through the GI tract. Notably, these organ systems are also responsible for Zn loss. Approximately 0.8–2.7 mg Zn/day are excreted in the feces23 while 500–600 µg Zn/day are excreted in sweat.27 The kidneys also regulate Zn excretion as urinary losses amount to 500–800 µg/day.28
Figure 1.
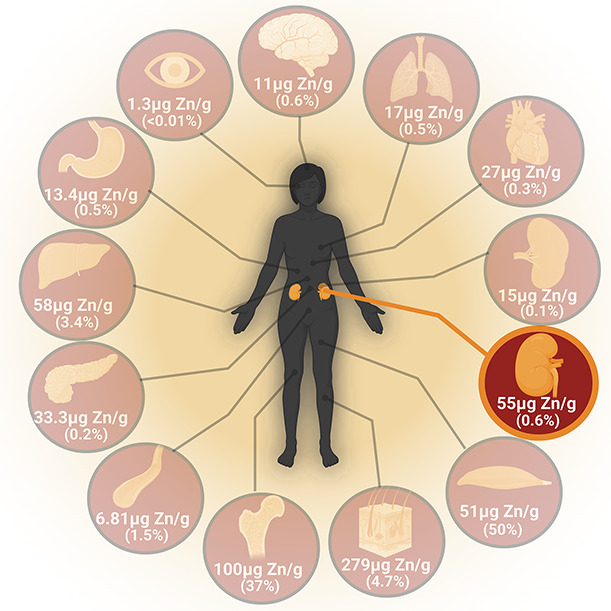
Zinc distribution in organs (clockwise). Brain (11 μgZn/gram, 0.6%), lungs (17 μg Zn/gram, 0.5%), heart (27 μg Zn/gram, 0.3%), spleen (15 μg Zn/gram, 0.1%), kidneys (55 μg Zn/gram, 0.6%), skeletal muscles (51 μg Zn/gram, 50%), hair/skin/nails (279 μg Zn/gram, 4.7%), bone (100 μg Zn/gram, 37%), blood vessels (6.81 μg Zn/gram, 1.5%), pancreas (33.3 μg Zn/gram, 0.2%), liver (58 μg Zn/gram, 3.4%), stomach (13.4 μg Zn/gram, 0.5%), and eyes (1.3 μg Zn/gram, <0.01%).
While the amount required to replenish lost Zn is primarily obtained by adequate dietary intake and proper intestinal absorption, kidney reabsorption is also critical for Zn procurement14,29,30 (Figure 2). Zn trafficking to target organs subsequently occurs through the serum, where it primarily circulates bound to plasma proteins such as albumin, macroglobulins, and transferrin31 (Figure 2). Serum Zn levels in healthy individuals vary from 12 to 16 µM, which corresponds to < 0.1% of total body Zn.32 However, caution is advised when interpreting serum Zn levels because many factors affect plasma Zn concentration32-38—including sex, age, time of the day, meal consumption, medications (thiazides, oral contraceptive use), pregnancy, and inflammation.
Figure 2.
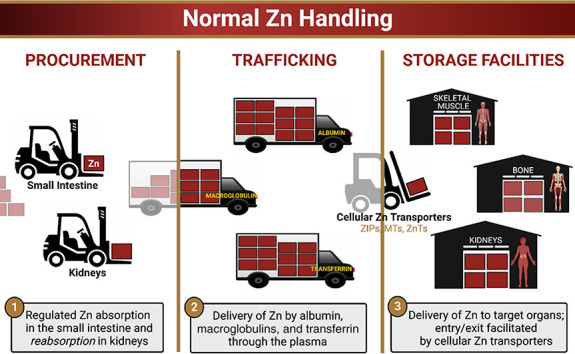
Normal zinc handling. MTs, metallothioneins; ZIPs, Zrt-, Irt-like proteins; ZnTs, Zn transporters.
Cellular uptake of Zn constitutes an efficient homeostatic control mechanism that prevents excess serum Zn levels.12,16,23 Cellular Zn homeostasis is mediated by three main families of Zn transport proteins (Figure 2). The Zrt-, Irt-like protein family facilitates entry of Zn into the cytosol while Zn-binding metallothioneins bind intracellular Zn. Intracellular Zn serves as a cofactor for the catalytic activity and structural integrity of over 300 proteins, including those involved in macromolecule synthesis and cell division39-41. Finally, Zn transporters facilitate exit of unbound, cytosolic Zn into organelles.
Evidence That Urinary Zinc Wasting Can Fuel Zinc Deficiency in CKD
The World Health Organization has deemed Zn deficiency a global health crisis, affecting 31% of the population.42,43 While Zn deficiency is well-documented in patients with CKD,14,17,44-48 it is worth acknowledging that the following factors are associated with Zn deficiency: vegetarian diets, older age, diabetes, diuretics, inflammatory diseases, and digestive disorders. Thus, parsing out whether Zn deficiency is a cause or consequence of CKD is quite complicated given the aforementioned confounding factors in patients with CKD. Multiple mechanisms can contribute to Zn deficiency in patients with CKD, including low dietary intake and increased Zn excretion.18,49,50 A cross-sectional study examining 145 patients at different stages of CKD (stages 1–4) found that serum Zn levels decreased with CKD progression, whereas serum levels of copper, iron, and selenium did not.48 In a case-control study of patients on maintenance hemodialysis, average serum Zn levels were significantly lower (69.2 µg/dl±17.29) than those in healthy controls (82.9 µg/dl±14.75).44 Although dialysis may contribute to Zn deficiency,51 it should be noted that abnormalities in Zn metabolism develop before end stage kidney failure and the initiation of dialysis.47
The progressive decline in serum Zn levels observed in patients with CKD is partially fueled by a newly uncovered phenomenon—urinary Zn wasting (Figure 3). In a cohort study, patients with CKD (regardless of stage) exhibited lower plasma Zn levels (606 µg/L±106.3 versus 664.1 µg/L±101.2), accompanied by higher urinary Zn excretion (612.4 µg/day±425.9 versus 479.2 µg/day±293) than patients without CKD.14 A decline in GFR correlated with enhanced urinary Zn wasting. A sharp increase in urinary Zn was observed at stage 3, when most patients receive a CKD diagnosis. Although hypertension contributes to CKD, hypertensive patients without CKD also had higher urinary Zn excretion than normotensive controls. This indicates that hypertension, in the absence of CKD, can independently promote urinary Zn excretion. Interestingly, these patients were at greater risk of experiencing CKD development within 3 years.14 Taken together, these critical findings indicate that Zn deficiency, accelerated by urinary Zn wasting, is both an early warning sign for the decline in kidney function and a hidden driver of CKD progression.
Figure 3.
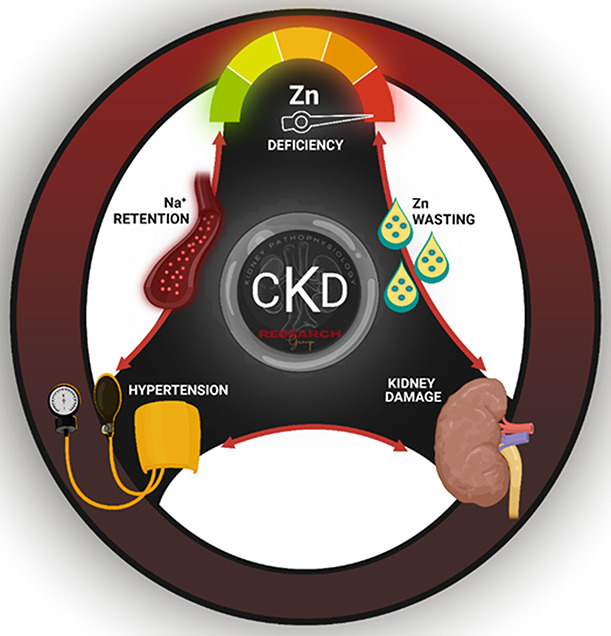
Zinc deficiency can accelerate the detrimental cycle of hypertension and kidney damage in CKD. Zn deficiency is partially fueled by a newly uncovered phenomenon—urinary Zn wasting. Zn deficiency alone causes kidney damage and is also sufficient to induce hypertension. This overlooked culprit drives renal Na+ retention and can consequently promote the self-perpetuating cycle of hypertension and kidney damage that accelerates CKD progression to end stage kidney failure.
Does Zinc Deficiency Accelerate the Detrimental Cycle of Hypertension and Kidney Damage in CKD?
The kidneys are essential in the maintenance of salt-water balance and, subsequently, BP control. Because this critical homeostatic function is impaired in the setting of CKD, up to 90% of patients experience comorbid hypertension.6-9 Notably, resistant hypertension is a common clinical problem, and CKD poses one of the greatest risks of developing treatment-resistant hypertension.52 The prevalence of hypertension increases with advanced CKD stages, with nearly 100% of patients experiencing hypertension in stage 5.53 This stark reality creates a detrimental cycle in which kidney damage causes hypertension, thus further worsening damage to the kidneys. Hypertension in patients with CKD has many etiologies including a hyperactive renin-angiotensin-aldosterone system, reduced GFR, altered vascular reactivity, overactivity of the sympathetic nervous system, and increased Na+ retention.6 As CKD progresses, BP becomes increasingly Na+-sensitive due to the fluid retention caused by salt intake. We present that the disruption of renal Na+ excretory function that causes Na+ retention is fueled by Zn deficiency54 (Figure 3). This overlooked culprit may consequently promote the self-perpetuating cycle of hypertension and kidney damage (Figure 3) that accelerates CKD progression to end stage kidney failure.
Zn is an essential micronutrient present in the diet and readily available as a supplement. The recommended dietary allowance of Zn is approximately 11 mg/day for men and approximately 8 mg/day for women,55 with an increased Zn demand (approximately 10–15 mg) during physiological states of growth such as pregnancy or puberty.31,55 Zn supports numerous aspects of cellular metabolism, and the coronavirus disease 2019 pandemic has highlighted the utility of Zn in immune function.56,57 While Zn's role in both vascular58,59 and cardiac functions31,60 is well established, its impact on kidney function is less known.31 Our laboratory recently established a regulatory role for Zn in renal Na+ reabsorption by the distal nephron.54 Although most of Na+ is reabsorbed in the proximal tubule, renal Na+ handling is fine-tuned in the distal nephron. Specifically, this nephron segment precisely integrates local changes in urinary Na+ with hormonal signals to modulate Na+ excretion, thus maintaining salt-water balance. This sequence of physiological events culminates in BP control.
In CKD, however, distal nephron function is impaired,61 resulting in three of five patients with salt-water retention, thereby fueling the initiation and persistence of hypertension.62 Our preclinical findings provide evidence that Zn deficiency alone causes kidney damage63 and is sufficient to induce hypertension as a direct consequence of impaired renal Na+ excretory function.54 Specifically, mice fed a Zn-deficient diet exhibited hypertension with a concurrent reduction in urinary Na+ excretion.54 Consistent with higher Na+ reabsorption activity in the distal nephron, thiazide treatment promoted natriuresis and importantly restored BP control.54 Mice fed a Zn-deficient diet also exhibited multiple markers of kidney damage including oxidative stress, renal fibrosis, and albuminuria.63,64 These same pathological events likely occur in the context of CKD. However, future studies are necessary to (1) identify renal Zn-sensitive pathways that drive hypertension and accelerate CKD progression and (2) explore strategies using Zn supplementation to provide an offramp from this detrimental cycle of hypertension and kidney damage.
Can Zinc Supplementation Provide an Exit Strategy to Rectify the Course of Chronic Kidney Disease and Hypertension?
Current treatment strategies for hypertension in the CKD population attempt to restore salt balance to lower BP. Particularly, (1) low-salt diets are often recommended to enhance the effects of ACE inhibitors and angiotensin receptor blockers9,65; (2) thiazide diuretics inhibit the distal nephron Na+ reabsorption pathway to reduce Na+ retention mediated by the kidneys9; and (3) mineralocorticoid receptor antagonists block aldosterone-stimulated reabsorption of Na+ by the distal nephron.9,66 Although these treatment strategies exist as monotherapies to exploit renal Na+ excretory function, even their use as combination therapies (three or more antihypertensive drugs) often fail to control BP in patients with CKD.52,62 Notably, these antihypertensives also alter Zn homeostasis.67,68 A systematic review of eight clinical studies, which included patients on antihypertensive therapy, reported reduced serum Zn levels with daily doses of captopril (50–150 mg), hydrochlorothiazide (12.5 mg), and losartan (50 mg). Moreover, urinary Zn wasting was reported with the use of captopril (50–75 mg), enalapril (20 mg), hydrochlorothiazide (12.5–25 mg), furosemide (40 mg), and losartan (50 mg). Further investigation is necessary to determine the extent of this influence because study limitations include small patient sample sizes and a lack of dietary Zn intake reporting.
Zn supplementation is a potential therapeutic strategy for many conditions because of its anti-inflammatory, antifibrotic, and antioxidative properties in the body69-71. Our findings reveal that Zn also exhibits anti-hypertensive and reno-protective properties because of its critical role in renal Na+ excretory function and BP regulation.54,63 There is also evidence that Zn plays a protective role in other organ systems relevant to BP, such as the vasculature and heart. Specifically, the protective effects of Zn include promoting cardiomyocyte redox balance and vascular integrity.31 Interestingly, Zn supplementation in vitro led to the complete restoration of the endothelial cell barrier,58,59,72 an effect not achieved with either calcium or magnesium supplementation.
Collectively, our findings and others support future studies exploring Zn supplementation to disrupt the detrimental cycle of hypertension and kidney damage burdening patients with CKD. Although clinical trial evidence in support of Zn supplementation in patients with CKD is limited, multiple preclinical studies have shown Zn to possess beneficial renal and cardiovascular effects. In rodent models of CKD, Zn supplementation slowed the progression of diabetic nephropathy,73,74 with the antifibrotic effects of Zn reducing renal morphologic changes. These positive outcomes attenuated diabetes-induced proteinuria, a well-known feature of CKD. Furthermore, the antioxidative effects of Zn also protected against diabetes-induced aortic damage and endothelial dysfunction.75 In the same manner that Zn is cardio- and renoprotective in preclinical studies, Zn supplementation likely exerts similar effects in patients with CKD. However, insights from clinical investigation are greatly needed.
In patients with CKD and low serum Zn levels, Zn supplementation may be an effective therapeutic strategy to prevent disease progression. While urinary Zn wasting occurs in CKD, oral Zn supplementation is sufficient to increase serum Zn levels, even in patients on hemodialysis.76 Notably, in patients with CKD and low serum Zn, drugs containing Zn were more renoprotective, leading to a reduction in the risk of disease progression or death by 62%.11 The authors credit these outcomes to the anti-inflammatory and antioxidative benefits of Zn. Although these findings seem encouraging, there is a paucity of evidence directly demonstrating the beneficial effects of Zn supplementation in the CKD population. However, the therapeutic index of Zn supplementation should also be investigated because acute symptoms after uptake of high doses of Zn include abdominal pain, nausea, and vomiting. Additional effects include lethargy, anemia, and neurologic disturbances such as dizziness.12 However, acute Zn intoxication is considered a rare event.12 Long-term, high-dose Zn supplementation interferes with the uptake of copper. Hence, many of the toxic effects of Zn uptake are in fact attributed to copper deficiency.12 Until large-scale clinical trials are conducted, the fate of Zn supplementation remains unknown in the vulnerable CKD population. As such, future studies would provide data directly examining Zn supplementation as a therapeutic intervention—serving as an offramp from the road to end stage kidney failure (Figure 4).
Figure 4.

Zinc supplementation: A possible offramp from the road to end stage kidney failure.
Acknowledgments
The Kidney PathoPhysiology Research Group would like to thank S. Daniel of In Scripto, LLC, for editorial assistance and critical review of the article. We also acknowledge Kidney Krew Members—Cindellynn Rudy and Kelia McMichael—for critically reviewing the article. Lastly, we thank Markel Haralson for contributing to the manuscript preparation by visually depicting the topic of the review article in the painting Zinc Supplementation: An Offramp from the Road to End-Stage Kidney Failure.
Disclosures
The authors have nothing to disclose.
Funding
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Grants: R21 DK119879 (to C.R.W.), R21 DK119879-S (to A.C.U.), and R01 DK-133698 (to C.R.W.); by the William Townsend Porter Predoctoral Fellowship from the American Physiological Society (to A.C.U.); by the American Heart Association Grant 16SDG27080009 (to C. R.W.); and by the American Society of Nephrology KidneyCure Transition to Independence Grant (to C.R.W.).
Author Contributions
Conceptualization: Adaku C. Ume, Clintoria R. Williams.
Funding acquisition: Adaku C. Ume, Clintoria R. Williams.
Writing – original draft: Clintoria R. Williams.
Writing – review & editing: Danielle N. Adams, Sherry E. Adesina, Adaku C. Ume, Tara-Yesomi Wenegieme, Clintoria R. Williams.
References
- 1.GlobalData. EpiCast Report: Chronic Kidney Disease – Epidemiology Forecast to 2026. Available at: https://www.globaldata.com/store/report/chronic-kidney-disease-epidemiology-analysis/#tab-faq-contents. Accessed October 28, 2022. [Google Scholar]
- 2.Naghavi M, Abajobir AA, Abbafati C, et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1151–1210. doi: 10.1016/S0140-6736(17)32152-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plantinga LC, Boulware LE, Coresh J, et al. Patient awareness in chronic kidney disease: trends and predictors. Arch Intern Med. 2008;168(20):2268–2275. doi: 10.1001/ARCHINTE.168.20.2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kopyt NP. Chronic kidney disease: the new silent killer. J Am Osteopath Assoc. 2006;106(3):133–136. doi: 10.7556/jaoa.2006.106.3.133 [DOI] [PubMed] [Google Scholar]
- 5.Health Resources & Services Administration. Organ Donation Statistics. Published online 2022. Available at: https://www.organdonor.gov/learn/organ-donation-statistics. [Google Scholar]
- 6.Ku E, Lee BJ, Wei J, Weir MR. Hypertension in CKD: core curriculum 2019. Am J Kidney Dis. 2019;74(1):120–131. doi: 10.1053/j.ajkd.2018.12.044 [DOI] [PubMed] [Google Scholar]
- 7.Plantinga LC, Miller ER, Stevens LA, et al. Blood pressure control among persons without and with chronic kidney disease: US trends and risk factors 1999-2006. Hypertension. 2009;54(1):47–56. doi: 10.1161/HYPERTENSIONAHA.109.129841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarafidis PA, Li S, Chen SC, et al. Hypertension awareness, treatment, and control in chronic kidney disease. Am J Med. 2008;121(4):332–340. doi: 10.1016/j.amjmed.2007.11.025 [DOI] [PubMed] [Google Scholar]
- 9.Valika A, Peixoto AJ. Hypertension management in transition: from CKD to ESRD. Adv Chronic Kidney Dis. 2016;23(4):255-261. doi: 10.1053/j.ackd.2016.02.002 [DOI] [PubMed] [Google Scholar]
- 10.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2 [DOI] [PubMed] [Google Scholar]
- 11.Tokuyama A, Kanda E, Itano S, et al. Effect of zinc deficiency on chronic kidney disease progression and effect modification by hypoalbuminemia. PLoS One. 2021;16(5):e0251554. doi: 10.1371/journal.pone.0251554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paun S, Tudosie M, Petris R, Macovei R. The effects of zinc on human body, including on renal failure and renal transplantation. J Med Life. 2012;5(Spec Issue):137–140. [PMC free article] [PubMed] [Google Scholar]
- 13.Nakatani S, Mori K, Shoji T, Emoto M. Association of zinc deficiency with development of CVD events in patients with CKD. Nutrients. 2021;13(5):1680. doi: 10.3390/nu13051680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Damianaki K, Lourenco JM, Braconnier P, et al. Renal handling of zinc in chronic kidney disease patients and the role of circulating zinc levels in renal function decline. Nephrol Dial Transplant. 2020;35(7):1163–1170. doi: 10.1093/ndt/gfz065 [DOI] [PubMed] [Google Scholar]
- 15.de Carvalho GB, Brandão-Lima PN, Maia CSC, Barbosa KBF, Pires LV. Zinc’s role in the glycemic control of patients with type 2 diabetes: a systematic review. Biometals. 2017;30(2):151–162. doi: 10.1007/s10534-017-9996-y [DOI] [PubMed] [Google Scholar]
- 16.Kambe T, Tsuji T, Hashimoto A, Itsumura N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol Rev. 2015;95(3):749–784. doi: 10.1152/physrev.00035.2014 [DOI] [PubMed] [Google Scholar]
- 17.Mahajan SK. Zinc in kidney disease. J Am Coll Nutr. 1989;8(4):296–304. doi: 10.1080/07315724.1989.10720305 [DOI] [PubMed] [Google Scholar]
- 18.Joo YS, Kim HW, Lee S, et al. Dietary zinc intake and incident chronic kidney disease. Clin Nutr. 2021;40(3):1039–1045. doi: 10.1016/j.clnu.2020.07.005 [DOI] [PubMed] [Google Scholar]
- 19.O’Hare AM, Choi AI, Bertenthal D, et al. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol. 2007;18(10):2758–2765. doi: 10.1681/ASN.2007040422 [DOI] [PubMed] [Google Scholar]
- 20.Pisano M, Hilas O. Zinc and taste disturbances in older adults: a review of the literature. Consult Pharm. 2016;31(5):267–270. doi: 10.4140/TCP.n.2016.267 [DOI] [PubMed] [Google Scholar]
- 21.Chen YH, Jeng SS, Hsu YC, et al. In anemia zinc is recruited from bone and plasma to produce new red blood cells. J Inorg Biochem. 2020;210:111172. doi: 10.1016/j.jinorgbio.2020.111172 [DOI] [PubMed] [Google Scholar]
- 22.Wastney ME, Aamodt RL, Rumble WF, Henkin RI. Kinetic analysis of zinc metabolism and its regulation in normal humans. Am J Physiol. 1986;251(2):R398–R408. doi: 10.1152/ajpregu.1986.251.2.R398 [DOI] [PubMed] [Google Scholar]
- 23.Maares M, Haase H. A guide to human zinc absorption: general overview and recent advances of in vitro intestinal models. Nutrients. 2020;12(3):762. doi: 10.3390/nu12030762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plum LM, Rink L, Haase H. The essential toxin: impact of zinc on human health. Int J Environ Res Public Health. 2010;7(4):1342–1365. doi: 10.3390/ijerph7041342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwartz JR, Marsh RG, Draelos ZD. Zinc and skin health: overview of physiology and pharmacology. Dermatol Surg. 2006;31(7 Pt 2):837–847. doi: 10.1111/J.1524-4725.2005.31729 [DOI] [PubMed] [Google Scholar]
- 26.Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol Rev. 1993;73(1):79–118. doi: 10.1152/physrev.1993.73.1.79 [DOI] [PubMed] [Google Scholar]
- 27.Brody T. 10 - inorganic nutrients. In: Nutritional Biochemistry (Second Edition). Academic Press; 1999, pp. 693–878. doi: 10.1016/B978-012134836-6/50013-5 [DOI] [Google Scholar]
- 28.Solomons NW. ZINC|physiology. In: Encyclopedia of Food Sciences and Nutrition (Second Edition). Academic Press; 2003, pp. 6272–6277. doi: 10.1016/B0-12-227055-X/01309-2 [DOI] [Google Scholar]
- 29.Abu-Hamdan DK, Migdal SD, Whitehouse R, Rabbani P, Prasad AS, McDonald FD. Renal handling of zinc: effect of cysteine infusion. Am J Physiology-Renal Physiol. 1981;241(5):F487–F494. doi: 10.1152/ajprenal.1981.241.5.F487. [DOI] [PubMed] [Google Scholar]
- 30.Victery W, Smith JM, Vander AJ. Renal tubular handling of zinc in the dog. Am J Physiol. 1981;241(5):F532–F539. doi: 10.1152/AJPRENAL.1981.241.5.F532 [DOI] [PubMed] [Google Scholar]
- 31.Grüngreiff K, Gottstein T, Reinhold D. Zinc deficiency-an independent risk factor in the pathogenesis of haemorrhagic stroke? Nutrients. 2020;12(11):3548. doi: 10.3390/nu12113548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hambidge KM, Goodall MJ, Stall C, Pritts J. Post-prandial and daily changes in plasma zinc. J Trace Elem Electrolytes Health Dis. 1989;3(1):55–57. [PubMed] [Google Scholar]
- 33.Hambidge KM, King JC, Kern DL, English-Westcott JL, Stall C. Pre-breakfast plasma zinc concentrations: the effect of previous meals. J Trace Elem Electrolytes Health Dis. 1990;4(4):229–231. [PubMed] [Google Scholar]
- 34.King JC, Hambidge KM, Westcott JL, Kern DL, Marshall G. Daily variation in plasma zinc concentrations in women fed meals at six-hour intervals. J Nutr. 1994;124(4):508–516. doi: 10.1093/jn/124.4.508 [DOI] [PubMed] [Google Scholar]
- 35.Lifschitz MD, Henkin RI. Circadian variation in copper and zinc in man. J Appl Physiol. 1971;31(1):88–92. doi: 10.1152/jappl.1971.31.1.88 [DOI] [PubMed] [Google Scholar]
- 36.Markowitz ME, Rosen JF, Mizruchi M. Circadian variations in serum zinc (Zn) concentrations: correlation with blood ionized calcium, serum total calcium and phosphate in humans. Am J Clin Nutr. 1985;41(4):689–696. doi: 10.1093/ajcn/41.4.689 [DOI] [PubMed] [Google Scholar]
- 37.Wilson RL, Grieger JA, Bianco-Miotto T, Roberts CT. Association between maternal zinc status, dietary zinc intake and pregnancy complications: a systematic review. Nutrients. 2016;8(10):641. doi: 10.3390/nu8100641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milanino R, Marrella M, Gasperini R, Pasqualicchio M, Velo G. Copper and zinc body levels in inflammation: an overview of the data obtained from animal and human studies. Agents Actions. 1993;39(3-4):195–209. doi: 10.1007/BF01998974 [DOI] [PubMed] [Google Scholar]
- 39.Institute of Medicine (US) Panel on Micronutrients. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, DC: National Academies Press; 2001. doi: 10.17226/10026 [DOI] [PubMed] [Google Scholar]
- 40.Prasad AS. Zinc: an overview. Nutrition. 1995;11(1 suppl l):93–99. [PubMed] [Google Scholar]
- 41.Solomons NW. Mild human zinc deficiency produces an imbalance between cell-mediated and humoral immunity. Nutr Rev. 2009;56(1):27–28. doi: 10.1111/j.1753-4887.1998.tb01656.x [DOI] [PubMed] [Google Scholar]
- 42.Kumera G, Awoke T, Melese T, et al. Prevalence of zinc deficiency and its association with dietary, serum albumin and intestinal parasitic infection among pregnant women attending antenatal care at the University of Gondar Hospital, Gondar, Northwest Ethiopia. BMC Nutr. 2015;1(1):31. doi: 10.1186/s40795-015-0026-6 [DOI] [Google Scholar]
- 43.Caulfield LE. Comparative Quantification of Health Risks: Sexual and Reproductive Health. Available at: https://www.google.com/books/edition/Comparative_Quantification_of_Health_Ris/ACV1jEGx4AgC?hl=en&gbpv=1&pg=PA257&printsec=frontcover. Accessed November 7, 2022. [Google Scholar]
- 44.Dashti-Khavidaki S, Khalili H, Vahedi SM, Lessan-Pezeshki M. Serum zinc concentrations in patients on maintenance hemodialysis and its relationship with anemia, parathyroid hormone concentrations and pruritus severity. Saudi J Kidney Dis Transpl. 2010;21(4):641–645. http://www.ncbi.nlm.nih.gov/pubmed/20587866 [PubMed] [Google Scholar]
- 45.Batista MN, Cuppari L, de Fátima Campos Pedrosa L, et al. Effect of end-stage renal disease and diabetes on zinc and copper status. Biol Trace Elem Res. 2006;112(1):1–12. doi: 10.1385/BTER:112:1:1 [DOI] [PubMed] [Google Scholar]
- 46.Esfahani ST, Hamidian MR, Madani A, et al. Serum zinc and copper levels in children with chronic renal failure. Pediatr Nephrol. 2006;21(8):1153–1156. doi: 10.1007/s00467-006-0119-1 [DOI] [PubMed] [Google Scholar]
- 47.Mahajan SK, Prasad AS, Rabbani P, Briggs WA, McDonald FD. Zinc metabolism in uremia. J Lab Clin Med. 1979;94(5):693–698. [PubMed] [Google Scholar]
- 48.Shih CT, Shiu YL, Chen CA, Lin HY, Huang YL, Lin CC. Changes in levels of copper, iron, zinc, and selenium in patients at different stages of chronic kidney disease. Genomic Med Biomark Health Sci. 2012;4(4):128–130. doi: 10.1016/j.gmbhs.2013.03.001 [DOI] [Google Scholar]
- 49.Mahajan SK, Bowersox EM, Rye DL, et al. Factors underlying abnormal zinc metabolism in uremia. Kidney Int Suppl. 1989;27:S269–S273. http://www.ncbi.nlm.nih.gov/pubmed/2636669 [PubMed] [Google Scholar]
- 50.Kim J, Lee J, Kim KN, et al. Association between dietary mineral intake and chronic kidney disease: the health examinees (HEXA) study. Int J Environ Res Public Health. 2018;15(6):1070. doi: 10.3390/ijerph15061070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tonelli M, Wiebe N, Hemmelgarn B, et al. Trace elements in hemodialysis patients: a systematic review and meta-analysis. BMC Med. 2009;12:1–12. doi: 10.1186/1741-7015-7-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American heart association professional education committee of the council for high blood pressure Research. Circulation. 2008;117(25):e510–e526. doi: 10.1016/s0145-4145(08)79414-0 [DOI] [PubMed] [Google Scholar]
- 53.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function–measured and estimated glomerular filtration rate. New Engl J Med. 2006;354(23):2473–2483. doi: 10.1056/NEJMra054415 [DOI] [PubMed] [Google Scholar]
- 54.Williams CR, Mistry M, Cheriyan AM, et al. Zinc deficiency induces hypertension by promoting renal Na+ reabsorption. Am J Physiol Renal Physiol. 2019;316(4):F646–F653. doi: 10.1152/ajprenal.00487.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trumbo P, Yates AA, Schlicker S, Poos M. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc. 2001;101(3):294–301. doi: 10.1016/S0002-8223(01)00078-5 [DOI] [PubMed] [Google Scholar]
- 56.Skalny AV, Timashev PS, Aschner M, et al. Serum zinc, copper, and other biometals are associated with COVID-19 severity markers. Metabolites. 2021;11(4):244. doi: 10.3390/metabo11040244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pal A, Squitti R, Picozza M, et al. Zinc and COVID-19: basis of current clinical trials. Biol Trace Elem Res. 2021;199(8):2882–2892. doi: 10.1007/s12011-020-02437-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meerarani P, Ramadass P, Toborek M, Bauer HC, Bauer H, Hennig B. Zinc protects against apoptosis of endothelial cells induced by linoleic acid and tumor necrosis factor α. Am J Clin Nutr. 2000;71(1):81–87. doi: 10.1093/ajcn/71.1.81 [DOI] [PubMed] [Google Scholar]
- 59.Hennig B, Meerarani P, Ramadass P, et al. Zinc nutrition and apoptosis of vascular endothelial cells: implications in atherosclerosis. Nutrition. 1999;15(10):744–748. doi: 10.1016/s0899-9007(99)00148-3 [DOI] [PubMed] [Google Scholar]
- 60.Cao JW, Duan SY, Zhang HX, Chen Y, Guo M. Zinc deficiency promoted fibrosis via ROS and TIMP/MMPs in the myocardium of mice. Biol Trace Elem Res. 2020;196(1):145–152. doi: 10.1007/s12011-019-01902-4 [DOI] [PubMed] [Google Scholar]
- 61.Bovée DM, Cuevas CA, Zietse R, Danser AHJ, Mirabito Colafella KM, Hoorn EJ. Salt-sensitive hypertension in chronic kidney disease: distal tubular mechanisms. Am J Physiol Renal Physiol. 2020;319(5):F729–F745. doi: 10.1152/ajprenal.00407.2020. [DOI] [PubMed] [Google Scholar]
- 62.Vidal-Petiot E, Metzger M, Faucon AL, et al. Extracellular fluid volume is an independent determinant of uncontrolled and resistant hypertension in chronic kidney disease: a nephrotest cohort study. J Am Heart Assoc. 2018;7(19):e010278. doi: 10.1161/JAHA.118.010278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li MS, Adesina SE, Ellis CL, Gooch JL, Hoover RS, Williams CR. NADPH oxidase-2 mediates zinc deficiency-induced oxidative stress and kidney damage. Am J Physiol Cell Physiol. 2017;312(1):C47–C55. doi: 10.1152/ajpcell.00208.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang X, Liang D, Lian X, et al. Effect of zinc deficiency on mouse renal interstitial fibrosis in diabetic nephropathy. Mol Med Rep. 2016;14(6):5245–5252. doi: 10.3892/MMR.2016.5870 [DOI] [PubMed] [Google Scholar]
- 65.Vegter S, Perna A, Postma MJ, Navis G, Remuzzi G, Ruggenenti P. Sodium intake, ACE inhibition, and progression to ESRD. J Am Soc Nephrol. 2012;23(1):165–173. doi: 10.1681/ASN.2011040430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shavit L, Lifschitz MD, Epstein M. Aldosterone blockade and the mineralocorticoid receptor in the management of chronic kidney disease: current concepts and emerging treatment paradigms. Kidney Int. 2012;81(10):955–968. doi: 10.1038/ki.2011.505 [DOI] [PubMed] [Google Scholar]
- 67.du Preez MJ, Lockett CJ. Effect of clopamide, a thiazide diuretic, on copper and zinc levels in hypertensive patients. J Am Coll Nutr. 1991;10(1):34–37. doi: 10.1080/07315724.1991.10718123 [DOI] [PubMed] [Google Scholar]
- 68.Braun LA, Rosenfeldt F. Pharmaco-nutrient interactions - a systematic review of zinc and antihypertensive therapy. Int J Clin Pract. 2013;67(8):717–725. doi: 10.1111/ijcp.12040 [DOI] [PubMed] [Google Scholar]
- 69.Prasad AS, Bao B, Beck FWJ, Kucuk O, Sarkar FH. Antioxidant effect of zinc in humans. Free Radic Biol Med. 2004;37(8):1182–1190. doi: 10.1016/j.freeradbiomed.2004.07.007 [DOI] [PubMed] [Google Scholar]
- 70.Jarosz M, Olbert M, Wyszogrodzka G, Młyniec K, Librowski T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology. 2017;25(1):11–24. doi: 10.1007/s10787-017-0309-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moriya K, Nishimura N, Namisaki T, et al. Zinc administration and improved serum markers of hepatic fibrosis in patients with autoimmune hepatitis. J Clin Med. 2021;10(11):2465. doi: 10.3390/jcm10112465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hennig B, Wang Y, Ramasamy S, McClain CJ. Zinc deficiency alters barrier function of cultured porcine endothelial cells. J Nutr. 1992;122(6):1242–1247. doi: 10.1093/jn/122.6.1242 [DOI] [PubMed] [Google Scholar]
- 73.Tang Y, Yang Q, Lu J, et al. Zinc supplementation partially prevents renal pathological changes in diabetic rats. J Nutr Biochem. 2010;21(3):237–246. doi: 10.1016/j.jnutbio.2008.12.010 [DOI] [PubMed] [Google Scholar]
- 74.Li B, Tan Y, Sun W, Fu Y, Miao L, Cai L. The role of zinc in the prevention of diabetic cardiomyopathy and nephropathy. Toxicol Mech Methods. 2013;23(1):27–33. doi: 10.3109/15376516.2012.735277 [DOI] [PubMed] [Google Scholar]
- 75.Liu P, Liu J, Wu Y, et al. Zinc supplementation protects against diabetic endothelial dysfunction via GTP cyclohydrolase 1 restoration. Biochem Biophys Res Commun. 2020;521(4):1049–1054. doi: 10.1016/j.bbrc.2019.11.046 [DOI] [PubMed] [Google Scholar]
- 76.Chevalier CA, Liepa G, Murphy MD, et al. The effects of zinc supplementation on serum zinc and cholesterol concentrations in hemodialysis patients. J Ren Nutr. 2002;12(3):183–189. doi: 10.1053/jren.2002.33515 [DOI] [PubMed] [Google Scholar]


