Keywords: acute kidney injury and ICU nephrology, acute kidney injury, critically ill children, hospital-acquired sepsis
Abstract
Key Points
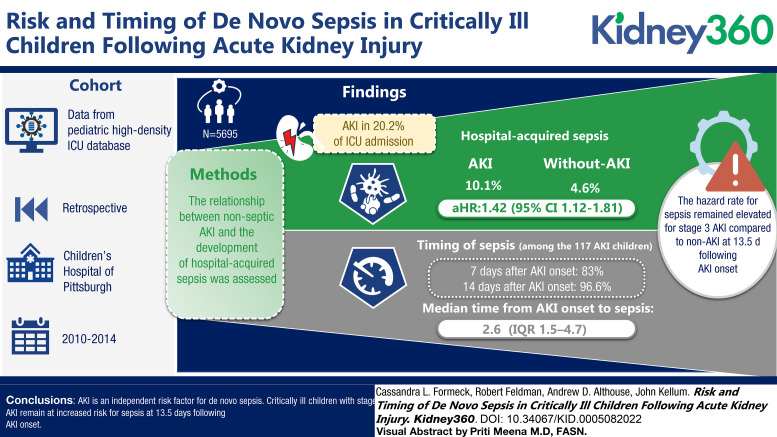
Critically ill children who developed AKI have a 42% increase in the probability of developing subsequent hospital-acquired sepsis when compared with children without AKI.
When evaluating risk of sepsis over time, children with stage 3 AKI remain at increased risk for sepsis for at least 2 weeks after AKI onset.
Medical providers should monitor for signs of sepsis after AKI and limit exposures that may increase the risk for infection.
Background
AKI is common among critically ill children and is associated with an increased risk for de novo infection; however, little is known about the epidemiology and temporal relationship between AKI and AKI-associated infection in this cohort.
Methods
We conducted a single-center retrospective cohort study of children admitted to the pediatric and cardiac intensive care units (ICUs) at a tertiary pediatric care center. The relationship between nonseptic AKI and the development of hospital-acquired sepsis was assessed using Cox proportional hazards models using AKI as a time-varying covariate.
Results
Among the 5695 children included in this study, AKI occurred in 20.2% from ICU admission through 30 days. Hospital-acquired sepsis occurred twice as often among children with AKI compared with those without AKI (10.1% versus 4.6%) with an adjusted hazard ratio of 1.42 (95% confidence interval, 1.12 to 1.81). Among the 117 children who developed sepsis after AKI, 80.3% developed sepsis within 7 days and 96.6% within 14 days of AKI onset, with a median time from AKI onset to sepsis of 2.6 days (interquartile range, 1.5–4.7). When assessing change in risk over time, the hazard rate for sepsis remained elevated for children with stage 3 AKI compared with children without AKI at 13.5 days after AKI onset, after which the estimation of hazard rates was limited by the number of children remaining in the hospital.
Conclusions
AKI is an independent risk factor for de novo sepsis. Critically ill children with stage 3 AKI remain at increased risk for sepsis at 13.5 days after AKI onset.
Introduction
AKI occurs in over a quarter of children admitted to pediatric intensive care units (ICUs) globally and is associated with a 4.5-fold higher 28-day mortality rate when compared with critically ill children without AKI.1 The systemic effects of AKI are far reaching, affecting multiple organ systems.2-8 Several animal studies have shown that AKI has a direct influence on innate immunity.9-13 In experimental murine models of AKI, rhabdomyolysis and nephrotoxic-induced AKI lead to impairment in neutrophil migration and bacterial killing.10,13,14 Clinically, several observational studies in select cohorts of hospitalized children and adults have shown an association between nonseptic AKI and risk for de novo infection after AKI onset.15-19 While these findings support AKI as a clinically relevant immunocompromised state, little is understood about the temporal relationship between AKI and risk for subsequent infection.
Understanding the timing of risk for sepsis after an AKI event is important for patient management. Hospitalized children are exposed to multiple interventions and potential pathogens, and understanding the temporal association between AKI and risk of sepsis can directly influence clinical decision making. Furthermore, little is known about the underlying mechanisms of AKI-associated immune dysfunction, and better characterizing the temporal features of this association may help elucidate the cause(s). The objective of this study was to assess the association between AKI and the development of de novo hospital-acquired sepsis in critically ill children and to describe the timing of sepsis after AKI onset.
Methods
Study Design and Patient Population
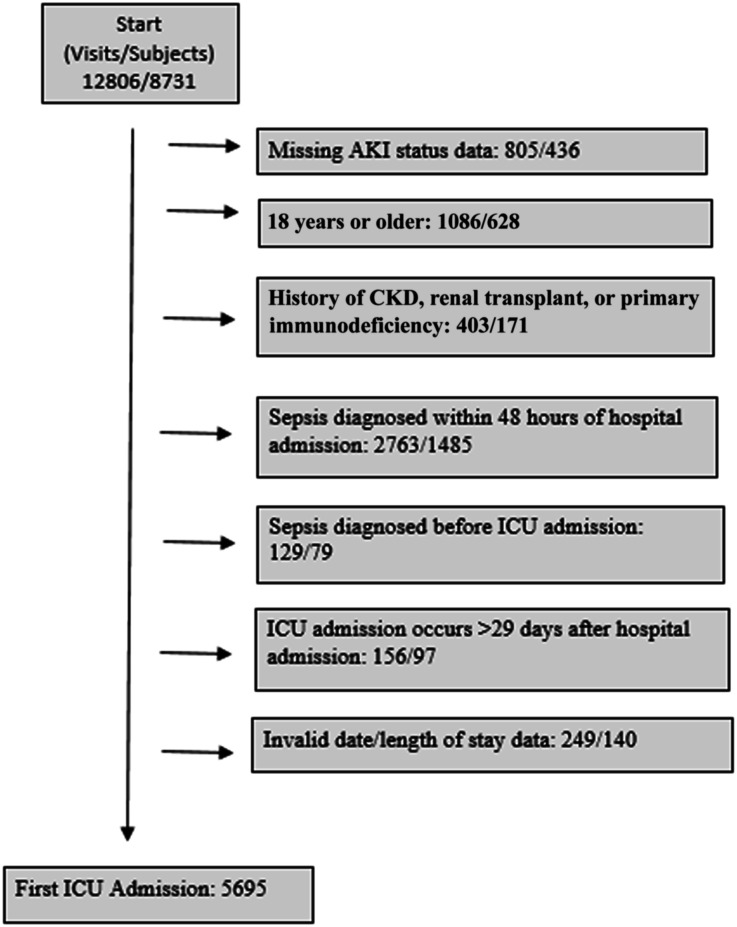
We conducted a retrospective, single-center, observational cohort study assessing the probability of developing hospital-acquired sepsis in critically ill children with and without AKI. Data were obtained from the pediatric high-density ICU (Peds HiDenIC) database, which contains data on 8731 patients admitted to the cardiac or pediatric ICUs at UPMC Children's Hospital of Pittsburgh between 2010 and 2014.20-22 Patients were excluded if they had insufficient data to categorize AKI status; were 18 years or older; had a history of CKD, kidney transplant, or primary immunodeficiency; community-acquired sepsis (sepsis within the first 48 hours of hospital admission); sepsis before ICU admission; or invalid date/time information. To have sufficient information to categorize AKI status, children needed a minimum of one serum creatinine measurement, not including the admission serum creatinine measurement, and/or a minimum of 6 hours of recorded urine output during the exposure window. We identified children with a history of CKD or kidney transplant as those with baseline serum creatinine correlating with an eGFR <60 ml/min per 1.73 m2 or an International Classification of Diseases, Ninth Revision (ICD-9), code for CKD or kidney transplant before or during the incident hospitalization (Supplemental Table 1). Children with primary immunodeficiency were identified by ICD-9 code (Supplemental Table 1). We defined sepsis using the pediatric Sepsis-3 definition.23 The final study cohort comprised 5695 patients (Figure 1). The Institutional Review Board at the University of Pittsburgh approved this study. A waiver of informed consent was granted for this study.
Figure 1.
Flow diagram of study cohort with exclusions. CKD, chronic kidney disease; ICU, intensive care unit.
Definitions
AKI was defined by the Kidney Disease: Improving Global Outcomes (KDIGO) criteria24 as (1) an increase in serum creatinine by ≥0.3 mg/dl, (2) a rise in serum creatinine of >1.5 times baseline, or (3) urine output <0.5 ml/kg per hour for a minimum of 6 hours, occurring from ICU admission through 30 days or hospital discharge if before 30 days. AKI stage was based on the highest serum creatinine value and the lowest urine output on a 6-hour rolling window starting at the time of ICU admission, whichever led to the higher stage. Interpolation and validation of urine output were performed as previously described by Joyce et al.21 Baseline serum creatinine was defined by the median of all serum creatinine values available in the 6 months before ICU admission, excluding the admission creatinine value for the hospitalization. If a measured serum creatinine value was not available in the 6 months before hospital admission, we assumed an estimated eGFR of 100 ml/min per 1.73 m2 and back-calculated a reference serum creatinine value on the basis of the patient's height using the Schwartz bedside estimating equation.25-27 For this calculation, an eGFR of 100 ml/min per 1.73 m2 was chosen, rather than 120 ml/min per 1.73 m2, so as to not overestimate the incidence of AKI.21 If a measured baseline serum creatinine and patient height were not available, then the admission serum creatinine was used as the reference creatinine.
The primary outcome was hospital-acquired sepsis. Sepsis was identified by the pediatric Sepsis-3 criteria,23 defined by the presence of suspected infection and an increase in the pediatric Sequential Organ Failure Assessment score by two points or more within 48 hours before or 24 hours after time zero for infection. For this analysis, we used a modified version of the pediatric Sequential Organ Failure Assessment score excluding the renal subscore (elevation in the serum creatinine level) so as to not have our exposure included in our outcome measure. Occurrence of hospital-acquired sepsis was evaluated from ICU admission through 30 days or hospital discharge if before 30 days. As noted above, patients who developed sepsis in the first 48 hours of their hospital admission were excluded from analysis according to the definition of health care–associated infection.28 Secondary outcomes included need for KRT, days of vasopressor and inotrope use, ICU and hospital length of stay, AKI or acute kidney disease (AKD) at hospital discharge, and hospital mortality. AKD was defined by AKI stage 1 or greater for ≥7 days after AKI onset.
Covariates
Baseline patient characteristics included in the analysis were patient age, sex, race, body mass index (BMI), and admission type. Additional variables that have been associated with the development of AKI and sepsis were included: history of heart failure, liver failure, malignancy, nonkidney solid organ transplant, bone marrow transplant, seizures/epilepsy, exposure to immunosuppressant medications in the 14 days before ICU admission (Supplemental Table 2), surgery before ICU admission, severity of illness at ICU admission, leukopenia, moderate or severe neutropenia, presence of a Foley catheter, and presence of an indwelling central line. Leukopenia, neutropenia, presence of a Foley catheter, and presence of an indwelling central line were included as longitudinal covariates and considered present if occurring before the first episode of sepsis. Severity of illness at ICU admission was defined by the electronic Pediatric Index of Mortality 2 (ePIM2) score.29
Statistical Analyses
Continuous variables are presented as mean±standard deviation; categorical variables are presented as frequencies and percentages. Statistical comparisons of categorical variables were performed using chi-squared tests. The relationship between AKI and development of hospital-acquired sepsis was assessed using Cox proportional hazards models using AKI as a time-varying covariate; we report models that use “any AKI” versus none as well as models computing the risk separately for each AKI stage (1, 2, and 3) versus no AKI. In these models, AKI was treated as a time-varying covariate with the value “0” assigned if the patient had not yet reached AKI onset; if the patient had met criteria for AKI, the value assigned was the highest KDIGO stage reached as of that time since ICU admission (e.g. if a patient had no AKI on ICU admission, KDIGO stage 1 on day 3, and KDIGO stage 3 on day 6, the exposure status would be modeled as 0 for days 0–2, KDIGO stage 1 from days 3–5, and KDIGO stage 3 from day 6 onward). We plotted the hazard function over time from day 0 to day 15 (3-day intervals) to assess the period during which risk for sepsis was highest after AKI onset. Hazard rates were calculated at the midpoint of each time interval using the life-table method. This analysis was limited to 15 days after ICU admission because of the small number of children remaining in the hospital after this time point. Statistical analyses were performed using R version 3.6.0 (https://cran.r-project.org/).
Results
Study Population
Of the 5695 children included in this study, AKI occurred in 1153 (20.2%) children from ICU admission through 30 days. By stage, 738 (64%) children developed a maximum stage 1 AKI, 332 (29%) stage 2 AKI, and 83 (7%) stage 3 AKI. Demographics and clinical characteristics of the cohort are summarized in Table 1.
Table 1.
Cohort characteristics
| Measure | No AKI, n=4542 | AKIa, n=1153 | Total, n=5695 |
|---|---|---|---|
| Age, mean (SD), yr | 6.5±5.6 | 7.7±6.1 | 6.7±5.7 |
| Male patients, n (%) | 2624 (58) | 650 (56) | 3274 (58) |
| Race, n (%) | |||
| White | 3523 (78) | 922 (80) | 4445 (78) |
| Black | 784 (17) | 178 (15) | 962 (17) |
| Other | 235 (5) | 53 (5) | 288 (5) |
| History of heart failure, n (%) | 77 (2) | 50 (4) | 127 (2) |
| History of liver failure, n (%) | 31 (0.7) | 19 (2) | 50 (0.9) |
| History of nonkidney solid organ transplant, n (%) | 274 (6) | 60 (5) | 334 (6) |
| History of bone marrow transplant, n (%) | 6 (0.1) | 8 (0.7) | 14 (0.2) |
| History of malignancy, n (%) | 154 (3) | 54 (5) | 208 (4) |
| Surgery before ICU admission, n (%) | 1612 (36) | 531 (46) | 2143 (38) |
| ePIM2 likelihood of mortality, mean (SD), %b | 1.3±4.3 | 2.6±8.4 | 1.6±5.4 |
ICU, intensive care unit; ePIM2, electronic pediatric index of mortality 2.
For determination of reference serum creatinine: a baseline serum creatinine measurement was available in 254 children (22%), reference serum creatinine was back-calculated in 182 children (15.8%) with an available height, and the admission serum creatinine was used in 717 children (62.2%).
Measured within the first 24 h of ICU admission.
AKI Is an Independent Risk Factor for Hospital-Acquired Sepsis
Hospital-acquired sepsis developed in 10.1% (n=117) of children who previously developed AKI and 4.6% (n=210) of children who did not develop AKI. The risk of sepsis after AKI increased with AKI severity, occurring in 8.7% (n=64) of children who developed stage 1, 10.8% (n=36) of children who developed stage 2, and 20.5% (n=17) of children who developed stage 3 AKI. Clinical characteristics of children with and without sepsis are summarized in Supplemental Table 3. Of the 327 children with hospital-acquired sepsis, 16.2% (n=53) had a positive body fluid culture within 24 hours before or after time zero for suspected infection. Culture positive sepsis occurred in 1.8% (n=21) of children with AKI and 0.7% (n=32) children with no AKI (P<0.001). There was no difference in the frequency of infection with gram positive versus gram negative, encapsulated versus nonencapsulated, or bacterial versus fungal pathogens between children with and without AKI. A list of identified pathogens is summarized in Supplemental Table 4.
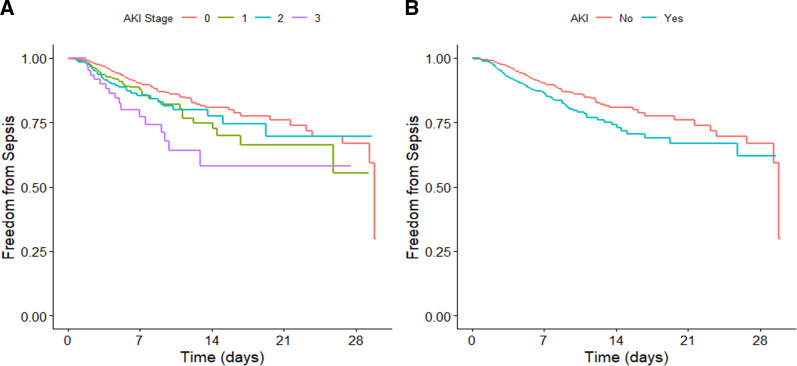
During a median follow-up of 3 days, the unadjusted hazard ratio (HR) for the development of hospital-acquired sepsis in patients with any stage of AKI was 1.61 (95% confidence interval, 1.28 to 2.03). After adjustment for baseline and longitudinal covariates, development of any AKI remained a significant predictor of hospital-acquired sepsis with an hazard ratio (HR) of 1.42 (95% confidence interval, 1.12 to 1.81: Table 2, Figure 2). Children with stage 3 AKI had the highest probability of developing sepsis (Figure 2), with adjusted hazard ratios for stages 1, 2, and 3 AKI of 1.48, 1.20, and 1.91 versus no AKI, respectively (P=0.018).
Table 2.
Multivariable Cox proportional hazards model assessing the association between AKI and the development of hospital-acquired sepsis using AKI as a time-varying covariate
| Variable | Hazard Ratio | 95% Confidence Interval | P Value | Omnibus P Value |
|---|---|---|---|---|
| AKIa | 1.42 | 1.115 to 1.807 | 0.004 | |
| Admission type b , c | 0.001 | |||
| Elective | 0.736 | 0.259 to 2.091 | 0.57 | |
| Emergency | 0.602 | 0.217 to 1.672 | 0.33 | |
| SDSO Elective | 0.271 | 0.094 to 0.785 | 0.02 | |
| Urgent | 0.673 | 0.239 to 1.897 | 0.45 | |
| Missing | 0.566 | 0.198 to 1.618 | 0.29 | |
| Age (yr) | 0.986 | 0.964 to 1.009 | 0.22 | |
| Sex Male | 0.733 | 0.585 to 0.919 | 0.007 | |
| Race d | 0.446 | |||
| White | 0.868 | 0.632 to 1.191 | 0.38 | |
| Other | 1.106 | 0.653 to 1.873 | 0.71 | |
| BMI | 0.983 | 0.958 to 1.009 | 0.20 | |
| ePIM2 (severity of illness) score in first 24 h of ICU admission | 1.257 | 1.149 to 1.376 | <0.001 | |
| History of liver failure | 0.902 | 0.529 to 1.54 | 0.71 | |
| History of heart failure | 1.386 | 0.944 to 2.036 | 0.10 | |
| History of nonkidney solid organ transplant | 1.303 | 0.766 to 2.216 | 0.33 | |
| History of bone marrow transplant | 1.618 | 0.357 to 7.338 | 0.53 | |
| History of seizures/epilepsy | 1.041 | 0.746 to 1.452 | 0.82 | |
| History of malignancy | 1.435 | 0.979 to 2.102 | 0.06 | |
| Surgery before ICU admission | 2.669 | 1.997 to 3.569 | <0.001 | |
| Exposure to immunosuppressant agents in the 14 days before ICU admission | 0.939 | 0.721 to 1.223 | 0.64 | |
| Leukopeniaa | 1.654 | 1.234 to 2.218 | 0.001 | |
| Moderate or severe neutropeniaa | 1.038 | 0.572 to 1.882 | 0.90 | |
| Indwelling Foley cathetera | 1.20 | 0.92 to 1.565 | 0.18 | |
| Indwelling central linea | 2.165 | 1.64 to 2.858 | <0.001 |
N=4975 patients; 720 patients excluded due to missingness.
SDSO, same say surgery outpatient; BMI, body mass index; ePIM2, electronic pediatric index of mortality 2; ICU, intensive care unit.
Longitudinal time-varying covariate.
Reference category: direct admission.
Admission type categories “outside hospital emergency room to floor transfer” (n=2), “same day surgery transfer to inpatient” (n=4), “same day surgery outpatient transfer urgent” (n=10), and “trauma center” (n=3) are not shown.
Reference category: African American.
Figure 2.
Freedom from hospital-acquired sepsis. Cox proportional hazards models showing the probability of remaining sepsis free for children with AKI versus no AKI after ICU admission. In these models, AKI was treated as a time-varying covariate with the value “0” assigned if the patient had not yet reached AKI onset; if the patient had met criteria for AKI, the value assigned was the highest KDIGO stage reached as of that time since ICU admission. (A) Probability according to AKI stage. (B) Probability according to any AKI stage. ICU, intensive care unit; KDIGO, Kidney Disease: Improving Global Outcomes.
Time from AKI Onset to Sepsis
The median time from AKI onset to sepsis was 2.6 days (interquartile range [IQR], 1.5–4.7). Of the 117 children who developed hospital-acquired sepsis after AKI (through 30 days or hospital discharge), 80.3% developed sepsis within 7 days and 96.6% within 14 days of AKI onset.
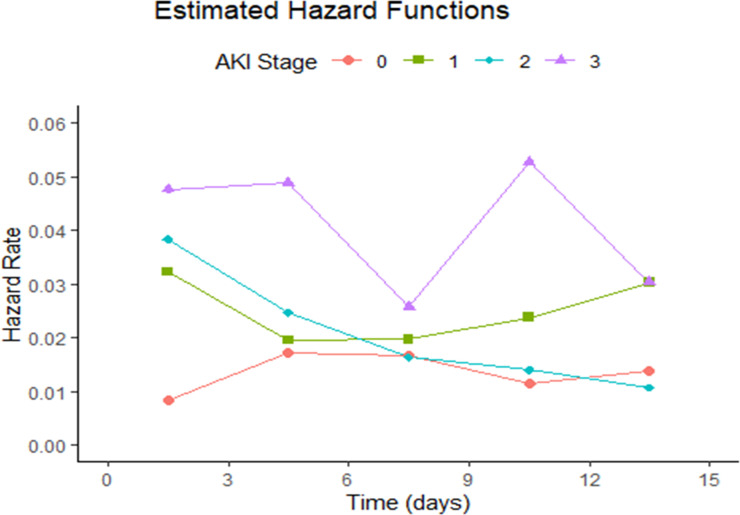
To assess the point in time at which children were at highest risk for developing hospital-acquired sepsis after AKI onset, we plotted the estimated hazard function for the development of sepsis by AKI stage over time. Estimated hazard rates for the occurrences of hospital-acquired sepsis for children with and without AKI are shown in Figure 3. The results of the hazard plot show that the hazard rate for developing hospital-acquired sepsis at 36 hours after ICU admission increased with AKI stage at 0.032, 0.038, and 0.048 for children with stages 1, 2, and 3 AKI, respectively, compared with 0.008 for children without AKI. A similar trend by AKI severity was again seen at 4.5 days. Hazard rates for the development of sepsis remain elevated for children with stage 3 AKI compared with children without AKI at 13.5 days after ICU admission, after which only six children with stage 3 AKI were alive or remained in the hospital. Overall, rates of sepsis over time varied by AKI stage, with no single peak time observed among children with any stage of AKI. Hazard rates for the development of hospital-acquired sepsis among the non-AKI group were stable throughout the first 15 days of ICU admission, ranging from 0.009 to 0.017.
Figure 3.
Hazard rate of hospital-acquired sepsis in critically ill children by AKI stage. This hazard plot shows the time-varying hazard rate for developing hospital-acquired sepsis among critically ill children with and without AKI. Time zero is time at ICU admission. ICU, intensive care unit.
Association of AKI and Outcomes
For children with AKI occurring from ICU admission through 30 days, an increase in the maximum AKI stage was associated with an increase in ICU length of stay, hospital length of stay, days of vasopressor use during the hospitalization, the presence of AKI or AKD at hospital discharge, and patient mortality. When compared with children without AKI, children with AKI also had more days of inotrope use during the hospitalization and more frequent need for KRT (Supplemental Table 5).
Association of Hospital-Acquired Sepsis and Outcomes among Children with AKI
Among children who developed AKI, mortality was higher among those who developed sepsis (5.1% versus 0.6%, P<0.001). Comparatively, hospital mortality among children without AKI was 0.4%. However, there was no statistical association between hospital-acquired sepsis and prevalence of AKI or AKD at hospital discharge (18% versus 14%).
Discussion
AKI is prevalent among children with critical illness and is a major contributor to patient morbidity and mortality. While evidence continues to grow to support AKI as a significant risk factor for infection,10,14-18 little is understood regarding the epidemiology and timing of AKI-associated infection in critically ill children nor the underlying mechanisms responsible for this relationship. In our cohort, children who developed AKI were more than twice as likely to develop hospital-acquired sepsis compared with children who did not develop AKI (10.1% versus 4.6%), with an HR of 1.42 after covariate adjustment. Notably, AKI severity was an important factor in the occurrence of hospital-acquired sepsis, with stage 3 AKI associated with the highest adjusted probability of developing subsequent sepsis when compared with stage 1 and stage 2 AKI. Children with stage 3 AKI had higher rates of hospital-acquired sepsis at 13.5 days after AKI onset compared with children without AKI, after which the estimation of hazard rates was limited by the number of children remaining in the hospital. Overall, the median time from AKI onset to sepsis was 2.6 days for all stages of AKI, with 97% of children with AKI and subsequent sepsis (through 30 days or hospital discharge) developing sepsis within 14 days of AKI onset.
To the best of our knowledge, this is the first study to report timing of sepsis after AKI in a large pediatric cohort. In a study looking at the incidence of postoperative infection in neonates with and without AKI after cardiac surgery, 55% (23 of 42) of children developed postoperative infection after AKI with a median time from AKI to infection of 6 days (IQR, 3–13 days).15 This patient cohort was considerably younger than ours, with a mean age of 5 days at the time of cardiac surgery. Neonates in this group also had a high frequency of exposure to postoperative steroids, prolonged mechanical ventilation, and delayed sternal closure, which may explain the higher incidence of AKI and postoperative infection in this cohort when compared with ours.15 In a study reporting timing of sepsis after AKI in hospitalized adults, among 611 adults with contrast-induced AKI, 40% developed sepsis after AKI diagnosis.16 In this group, sepsis occurred at a median of 5 days after AKI (IQR, 2–9 days), and timing was similar in patients who did and did not require dialysis.16
Importantly, the pathogenesis of AKI-associated infection remains to be elucidated. A variety of animal and human studies have shown inhibition of neutrophil migration and bactericidal activity after nephrotoxic, rhabdomyolysis, and sepsis-induced AKI.9,10,13,14 Whether neutrophil inhibition occurs because of direct organ system crosstalk or is secondary to the accumulation of toxins and cell-signaling proteins in the setting of decreased renal clearance has yet to be determined. In a study evaluating the migratory function of murine neutrophils isolated from AKI-induced mouse models, neutrophil migration measured by in vitro transwell migration assay was reduced as early as 4 hours after AKI induction secondary to rhabdomyolysis and within 24 hours after AKI induction from nephrotoxins.14 Notably, murine models of AKI have shown that preexisting AKI significantly increases the severity of bacterial pneumonia in animals exposed to pathogenic Pseudomonas aeruginosa.10 Mice with AKI demonstrated significantly reduced oxygen levels and higher pulmonary bacterial load compared with mice without AKI, independent of AKI etiology.10 In addition, mice with AKI had markedly reduced global lung tissue neutrophil recruitment compared with mice without AKI.10 In a clinical study including adult patients with AKI and septic shock, plasma from patients with septic shock plus AKI exhibited an inhibitory effect on neutrophil function and migration when compared with patients with septic shock alone.14 Interestingly, the effects on neutrophil activity were not corrected by KRT and persisted for at least four days after KRT initiation.14 In our study, risk of sepsis remained elevated for children who developed stage 3 AKI for up to two weeks after AKI onset, at which time approximately two-thirds of patients had return of kidney function to baseline. This suggests that the mechanisms that lead to AKI-associated immune dysfunction persist beyond normalization of renal clearance.
Looking at other clinical outcomes among children with AKI in our cohort, hospital mortality was 8.5-fold higher among children who developed subsequent sepsis (5.1% versus 0.6%). Comparatively, hospital mortality among children without AKI was 0.4%. In addition, rates of mortality increased with worsening stages of AKI. These findings highlight the contribution of AKI-associated infection to the mortality of critically ill children and the importance of identifying targetable mechanisms to reduce risk of infection in this population.
There are several limitations to our study. Primarily, AKI etiology and daily fluid balance were not available in the database. As a result, we were unable to assess the role of AKI etiology on the occurrence of AKI and risk for subsequent sepsis, nor could we perform creatinine correction for fluid overload. Second, owing to low numbers, we had limited power to assess risk for hospital-acquired sepsis in children with AKI beyond 15 days. Regarding our determination of baseline serum creatinine for defining AKI, if a true measured baseline serum creatinine or patient height were not available, then the admission serum creatinine for the hospitalization was used as the baseline creatinine. This may have resulted in some children with AKI being placed in the non-AKI group, thereby potentially underestimating the association between AKI and subsequent infection. In addition, a modified version of the Sepsis-3 definition was used for this analysis; as such, we may have underestimated the frequency of sepsis in our cohort. Finally, our assessment of timing for sepsis and AKI are limited by the clinical variables used for determination of each. Creatinine changes are known to lag behind injury. However, given an average of 2.6 days, it is unlikely that sepsis caused the AKI event instead of the other way around. Our study also has several strengths. Given the granularity of the Peds HiDenIC database, we were able to identify AKI using both serum creatinine and urine output criteria. Although this is a single-center study, the Peds HiDenIC cohort includes a diverse population of general and cardiac ICU patients with overall rates of AKI comparable with those reported in other pediatric intensive care studies, such as the Assessment of Worldwide Acute Kidney Injury, Renal Angina, and Epidemiology (AWARE) study,30 supporting the generalizability of the results.
In conclusion, these results further support AKI is an independent risk factor for hospital-acquired sepsis among critically ill children, with children with stage 3 AKI remaining at increased risk for hospital-acquired sepsis at 2 weeks after AKI onset. Medical providers should monitor for signs of sepsis after AKI and limit exposures that may increase the risk for infection. Further studies are needed to understand the mechanisms by which AKI modulates immune function in critically ill children and to further understand the risk for AKI-associated infection after hospital discharge. Our results may help inform these investigations by elucidating the temporal relationship between AKI and sepsis.
Supplementary Material
Acknowledgments
We would like to thank the Biostatistical and Data Management Core within the CRISMA (Clinical Research Investigation and Systems Modeling of Acute Illness) Center for their help in variable development as well as data acquisition, management, and storage.
Footnotes
See related editorial, “Sepsis and AKI: A Two-Way Street,” on pages 289-290.
Disclosures
J.A. Kellum reports the following: Employer: Spectral Medical; University of Pittsburgh School of Medicine; University of Pittsburgh Medical Center (on leave); Consultancy: AM Pharma, Astellas, Astute Medical, Baxter, bioMerieux, Cytosorbents, Grifols, Klotho, Mallinckrodt, NxStage, PhotoPhage, Potrero, RenalSense; Ownership Interest: J3RM, Klotho, Photophage, Spectral Medical; Research Funding: Astute Medical, Astellas, Baxter, bioMerieux, Grifols, Cytosorbents, Atox Bio, Astellas, Bioporto, RenalSense; Patents or Royalties: Astute Medical, Cytosorbents, J3RM, Klotho, Photophage; and Advisory or Leadership Role: Editor: Critical Care Clinics of North America; Editorial Boards: Nephrology Dialysis Transplantation; Critical Care; Critical Care Medicine; Blood Purification. All remaining authors have nothing to disclose.
Funding
Financial support provided by an institutional training grant from the University of Pittsburgh School of Medicine, Department of Pediatrics.
Author Contributions
Conceptualization: Cassandra L. Formeck, John A. Kellum.
Data curation: Cassandra L. Formeck.
Formal analysis: Robert Feldman.
Investigation: Cassandra L. Formeck.
Methodology: Andrew D. Althouse, Cassandra L. Formeck.
Validation: Andrew D. Althouse, Robert Feldman, John A. Kellum.
Visualization: Cassandra L. Formeck.
Writing – original draft: Robert Feldman, Cassandra L. Formeck.
Writing – review & editing: Andrew D. Althouse, Robert Feldman, Cassandra L. Formeck, John A. Kellum.
Data Sharing Statement
Partial restrictions to the data and/or materials apply: Deidentified individual participant data will not be made available.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/KN9/A257.
Supplemental Table 1. ICD9 codes for chronic kidney disease, kidney transplantation, and primary immunodeficiency
Supplemental Table 2. Immunosuppressant agents
Supplemental Table 3. Cohort characteristics by hospital-acquired sepsis
Supplemental Table 4. Pathogens associated with culture-positive hospital-acquired sepsis among critically ill children with and without preexisting acute kidney injury
Supplemental Table 5. Hospital/ICU Outcome Measures by AKI Stage
References
- 1.Kaddourah A, Basu RK, Bagshaw SM, et al. Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med. 2017;376(1):11–20. doi: 10.1056/NEJMoa1611391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singbartl K, Joannidis M. Short-term effects of acute kidney injury. Crit Care Clin. 2015;31(4):751–762. doi: 10.1016/j.ccc.2015.06.010 [DOI] [PubMed] [Google Scholar]
- 3.Faubel S, Edelstein CL. Mechanisms and mediators of lung injury after acute kidney injury. Nat Rev Nephrol. 2016;12(1):48–60. doi: 10.1038/nrneph.2015.158. [DOI] [PubMed] [Google Scholar]
- 4.Liu M, Liang Y, Chigurupati S, et al. Acute kidney injury leads to inflammation and functional changes in the brain. J Am Soc Nephrol. 2008;19(7):1360–1370. doi: 10.1681/ASN.2007080901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park SW, Chen SW, Kim M, et al. Cytokines induce small intestine and liver injury after renal ischemia or nephrectomy. Lab Invest. 2011;91(1):63–84. doi: 10.1038/labinvest.2010.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golab F, Kadkhodaee M, Zahmatkesh M, et al. Ischemic and non-ischemic acute kidney injury cause hepatic damage. Kidney Int. 2009;75(8):783–792. doi: 10.1038/ki.2008.683 [DOI] [PubMed] [Google Scholar]
- 7.Kelly KJ. Distant effects of experimental renal ischemia/reperfusion injury. J Am Soc Nephrol. 2003;14(6):1549–1558. doi: 10.1097/01.ASN.0000064946.94590.46 [DOI] [PubMed] [Google Scholar]
- 8.Vieira JM, Jr, Castro I, Curvello-Neto A, et al. Effect of acute kidney injury on weaning from mechanical ventilation in critically ill patients. Crit Care Med. 2007;35(1):184–191. doi: 10.1097/01.CCM.0000249828.81705.65 [DOI] [PubMed] [Google Scholar]
- 9.Singbartl K, Formeck CL, Kellum JA. Kidney-immune system crosstalk in AKI. Semin Nephrol. 2019;39(1):96–106. doi: 10.1016/j.semnephrol.2018.10.007 [DOI] [PubMed] [Google Scholar]
- 10.Singbartl K, Bishop JV, Wen X, et al. Differential effects of kidney-lung cross-talk during acute kidney injury and bacterial pneumonia. Kidney Int. 2011;80(6):633–644. doi: 10.1038/ki.2011.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zarbock A, Schmolke M, Spieker T, et al. Acute uremia but not renal inflammation attenuates aseptic acute lung injury: a critical role for uremic neutrophils. J Am Soc Nephrol. 2006;17(11):3124–3131. doi: 10.1681/ASN.2006040358 [DOI] [PubMed] [Google Scholar]
- 12.Dodd OJ, Hristopoulos M, Scharfstein D, et al. Interactive effects of mechanical ventilation and kidney health on lung function in an in vivo mouse model. Am J Physiol Lung Cell Mol Physiol 2009;296(1):L3–L11. doi: 10.1152/ajplung.00030.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossaint J, Spelten O, Kassens N, et al. Acute loss of renal function attenuates slow leukocyte rolling and transmigration by interfering with intracellular signaling. Kidney Int. 2011;80(5):493–503. doi: 10.1038/ki.2011.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singbartl K, Miller L, Ruiz-Velasco V, Kellum JA. Reversal of acute kidney injury-induced neutrophil dysfunction: a critical role for resistin. Crit Care Med. 2016;44(7):e492–e501. doi: 10.1097/CCM.0000000000001472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.SooHoo M, Griffin B, Jovanovich A, et al. Acute kidney injury is associated with subsequent infection in neonates after the Norwood procedure: a retrospective chart review. Pediatr Nephrol. 2018;33(7):1235–1242. doi: 10.1007/s00467-018-3907-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta RL, Bouchard J, Soroko SB, et al. Sepsis as a cause and consequence of acute kidney injury: program to improve care in acute renal disease. Intensive Care Med. 2011;37(2):241–248. doi: 10.1007/s00134-010-2089-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffin BR, Teixeira JP, Ambruso S, et al. Stage 1 acute kidney injury is independently associated with infection following cardiac surgery. J Thorac Cardiovasc Surg. 2021;161(4):1346–1355. doi: 10.1016/j.jtcvs.2019.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffin BR, You Z, Holmen J, et al. Incident infection following acute kidney injury with recovery to baseline creatinine: a propensity score matched analysis. PLoS One. 2019;14(6):e0217935. doi: 10.1371/journal.pone.0217935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality. A cohort analysis. JAMA 1996;275(19):1489–1494. [PubMed] [Google Scholar]
- 20.Joyce EL, Kane-Gill SL, Priyanka P, et al. Piperacillin/tazobactam and antibiotic-associated acute kidney injury in critically ill children. J Am Soc Nephrol. 2019;30(11):2243–2251. doi: 10.1681/ASN.2018121223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joyce EL, DeAlmeida DR, Fuhrman DY, et al. eResearch in acute kidney injury: a primer for electronic health record research. Nephrol Dial Transplant. 2019;34(3):401–407. doi: 10.1093/ndt/gfy052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Formeck CL, Siripong N, Joyce EL, et al. Association of early hyponatremia and the development of acute kidney injury in critically ill children. Pediatr Nephrol. 2022;37(11):2755–2763. doi: doi: 10.1007/s00467-022-05478-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matics TJ, Sanchez-Pinto LN. Adaptation and validation of a pediatric sequential organ failure assessment score and evaluation of the sepsis-3 definitions in critically ill children. JAMA Pediatr. 2017;171(10):e172352. doi: 10.1001/jamapediatrics.2017.2352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Inter. 2012;2:1–138. [Google Scholar]
- 25.Schwartz GJ, Munoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629–637. doi: 10.1681/ASN.2008030287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz GJ, Work DF. Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol. 2009;4(11):1832–1843. doi: 10.2215/CJN.01640309 [DOI] [PubMed] [Google Scholar]
- 27.Staples A, LeBlond R, Watkins S, et al. Validation of the revised Schwartz estimating equation in a predominantly non-CKD population. Pediatr Nephrol. 2010;25(11):2321–2326. doi: 10.1007/s00467-010-1598-7 [DOI] [PubMed] [Google Scholar]
- 28.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–332. doi: 10.1016/j.ajic.2008.03.002 [DOI] [PubMed] [Google Scholar]
- 29.Joyce EL, Crana CM, Yabes J, Kellum JA. Validation of an electronic pediatric index of mortality 2 score in a mixed quaternary PICU. Pediatr Crit Care Med. 2020;21(8):e572–e575. doi: 10.1097/PCC.0000000000002347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Basu RK, Kaddourah A, Terrell T, et al. Assessment of Worldwide Acute Kidney Injury, Renal Angina and Epidemiology in critically ill children (AWARE): a prospective study to improve diagnostic precision. J Clin Trials. 2015;5(3):222. doi: 10.4172/2167-0870.1000222 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Partial restrictions to the data and/or materials apply: Deidentified individual participant data will not be made available.