Keywords: chronic kidney disease, pregnancy, preeclampsia, gestational hypertension, preterm delivery, low birthweight, severe maternal morbidity indicators
Abstract
Significance Statement
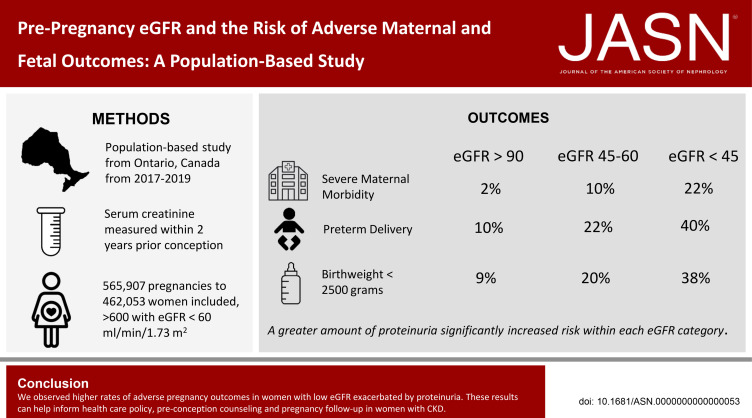
Pregnancies in women with CKD carry greater risk than pregnancies in the general population. The small number of women in prior studies has limited estimates of this risk, especially among those with advanced CKD. We report the results of a population-based cohort study in Ontario, Canada, that assessed more than 500,000 pregnancies, including 600 with a baseline eGFR < 60 ml/min per 1.73 m2. The investigation demonstrates increases in risk of different adverse maternal and fetal outcomes with lower eGFR and further risk elevation with baseline proteinuria.
Background
CKD is a risk factor for pregnancy complications, but estimates for adverse outcomes come largely from single-center studies with few women with moderate or advanced stage CKD.
Methods
To investigate the association between maternal baseline eGFR and risk of adverse pregnancy outcomes, we conducted a retrospective, population-based cohort study of women (not on dialysis or having had a kidney transplant) in Ontario, Canada, who delivered between 2007 and 2019. The study included 565,907 pregnancies among 462,053 women. Administrative health databases captured hospital births, outpatient laboratory testing, and pregnancy complications. We analyzed pregnancies with serum creatinine measured within 2 years of conception up to 30 days after conception and assessed the impact of urine protein where available.
Results
The risk of major maternal morbidity, preterm delivery, and low birthweight increased monotonically across declining eGFR categories, with risk increase most notable as eGFR dropped below 60 ml/min per 1.73 m2. A total of 56 (40%) of the 133 pregnancies with an eGFR <45 ml/min per 1.73 m2 resulted in delivery under 37 weeks, compared with 10% of pregnancies when eGFR exceeded 90 ml/min per 1.73 m2. Greater proteinuria significantly increased risk within each eGFR category. Maternal and neonatal deaths were rare regardless of baseline eGFR (<0.3% of all pregnancies). Only 7% of women with an eGFR <45 ml/min per 1.73 m2 received dialysis during or immediately after pregnancy.
Conclusions
We observed higher rates of adverse pregnancy outcomes in women with low eGFR with concurrent proteinuria. These results can help inform health care policy, preconception counseling, and pregnancy follow-up in women with CKD.
Introduction
Young women with CKD are subject to significantly higher risks during pregnancy compared with healthy young woman at the same life stage. Serious potential adverse outcomes include accelerated loss of kidney function hastening progression to ESKD, a 10-fold higher risk of preeclampsia and a six-fold higher risk of delivering a baby preterm.1,2 Although it is known that more advanced CKD with the coexistence of significant proteinuria and hypertension exacerbates the risks associated with pregnancy, prognostication of an individual woman's pregnancy-associated risk in the setting of CKD remains profoundly challenging, as most studies of pregnancy in women with CKD were published several decades ago, were single-center, or included small numbers of women with more advanced CKD.1,3–6 Much of this published older literature predated the considerable recent advances in neonatal care, which have improved the survival of preterm, very low birthweight, and critically ill term infants.7 Furthermore, more recent shifts in practice patterns, including lowering pre-pregnancy proteinuria with renin-angiotensin blockade until conception, controlling blood pressure, ensuring disease quiescence with pregnancy safe immunosuppression, and the more widespread prescribing of aspirin to prevent preeclampsia, have likely positively affected pregnancy outcomes.8 Notwithstanding, a lack of robust evidence contributes to uncertainty when counseling this high-risk patient population, as a poor pregnancy outcome can have a profound impact on the long-term health of the mother and her child.9
The overall goal of this study was to descriptively characterize the maternal and fetal outcomes of a large population-based cohort in relation to eGFR. We used linked provincial laboratory and administrative data to assess pregnancy outcomes in women by baseline eGFR. We evaluated major maternal morbidity, including acute renal failure and receipt of dialysis, as well as preterm delivery and delivery of a low birthweight baby. Secondary outcomes included rates of maternal and neonatal mortality as well as the receipt of maternal critical care and neonatal intensive care. We also assessed the impact of baseline proteinuria on pregnancy outcomes within each eGFR category.
Methods
Study Design
We conducted a retrospective cohort study of women in the province of Ontario, Canada, where all residents have universal access to medical care. This project was authorized under Section 45 of Ontario's Personal Health Information Protection Act (PHIPA). The conduct and reporting of the study followed the Reporting of Studies Conducted Using Observational Routinely Collected Health Data (RECORD) guidelines for observational studies (Supplemental Table 1).10 To comply with privacy regulations for minimizing the chance of identification of a study participant, numbers of participants are suppressed in the case of five or fewer participants (reported as <6).
Data Sources
Patient characteristics and laboratory data were obtained from multiple health care databases housed at ICES. The datasets were linked using unique encoded identifiers and analyzed at ICES. The MOMBABY cohort, which links mothers and newborns, was used to identify all hospital-based live and stillbirths in the province. Baseline serum creatinine values were determined using the Ontario Laboratory Information System (OLIS). Demographic characteristics and vital statistics were retrieved from the Registered Persons Database (RPDB). The Canadian Institute for Health Information Discharge Abstract Database (CIHI-DAD) was used to determine diagnostic and procedural information from all hospitalizations. Ambulatory visits were obtained from the National Ambulatory Care System (NACRS). The Ontario Health Insurance Plan (OHIP) database, which contains billing claims and diagnostic descriptions for inpatient and outpatient physician services, supplemented CIHI-DAD. The Canadian Organ Replacement Register (CORR) was used to capture information on kidney replacement therapies. These databases have been used extensively for epidemiologic and health services research, including studies of maternal and fetal outcomes.11,12
Population
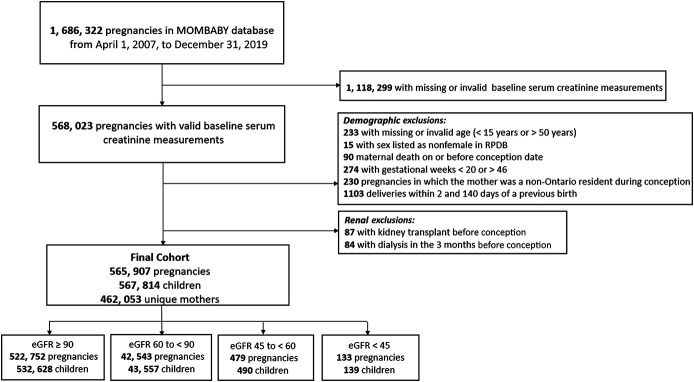
We included all pregnant women in Ontario who had an obstetric delivery between April 1, 2007, and December 31, 2019. Each woman's estimated conception date served as her primary cohort-entry date (first index date) and was estimated by subtracting the gestational weeks from the delivery date. The secondary index date was the date of delivery. Pregnancies were excluded if they did not have a serum creatinine value available in the 2 years before conception, or at the very least, 30 days after conception, as creatinine values during this latter time period remain similar to preconception values.13 Serum creatinine values recorded during a previous pregnancy were excluded. Each pregnancy was considered unique, but a single woman could have contributed more than one pregnancy to the analysis. We accounted for the correlation of multiple pregnancies within women when making inferences. Pregnancies were further excluded if the woman was a non-Ontario resident at the time of conception, had invalid or missing age or sex data, or had a record of any pregnancy with a birth before 20 weeks of gestation or after 46 weeks of gestation. Pregnancies were also excluded if the woman had a history of kidney transplantation or dialysis dependency within 3 months before conception.
Baseline comorbidities, documented within 5 years before the cohort entry date, were ascertained using International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10), as well as the Ontario Health Insurance Plan (OHIP) physician diagnostic and fee codes (Supplemental Table 2). Measures of health care utilization were examined in the 1–2 years before conception up to delivery. Women were followed until end of the observation period (March 31, 2020). Emigration from the province is the only reason for lost follow-up, and the rate is low (<0.5% per year).
Study Exposures
Baseline kidney function was categorized based on serum creatinine data obtained within 2 years before conception or up to 30 days after conception date if a preconception creatinine was not available. Estimated GFR (ml/min per 1.73 m2) was calculated using the CKD-EPI equation without a race coefficient.14 During the study period, serum creatinine values obtained by Ontario laboratories were standardized to the isotope-dilution mass spectrometry (IDMS) method. Kidney disease was classified as preserved kidney function for preconception eGFR ≥90 ml/min per 1.73 m2, mild CKD for preconception eGFR 60 to <90 ml/min per 1.73 m2, moderate CKD for preconception eGFR 45 to <60 ml/min per 1.73 m2, and advanced CKD for preconception eGFR <45 ml/min per 1.73 m2.
Preconception proteinuria within 2 years before the conception date was measured using a hierarchical combination of albumin-to-creatinine ratio (ACR), protein-to-creatinine ratio (PCR), or urine dipstick on the basis of availability. Proteinuria was categorized as severe (ACR value>30 mg/mmol or PCR value>50 mg/mmol or urine dipstick 2+ or more), moderate (ACR value 3–30 mg/mmol or PCR value 15–50 mg/mmol or urine dipstick trace to 1+), normal/mild (ACR value<3 mg/mmol or PCR value<15 mg/mmol or urine dipstick normal), or missing.
Maternal Outcomes
The primary outcome was at least one Severe Maternal Morbidity (SMM) indicator between 20 weeks gestational age and 42 days after delivery. The SMM comprises 44 indicators of maternal morbidity (40 indicators) and mortality (four indicators). See Supplemental Table 3 for the complete list of indicators and corresponding codes. These indicators, on the basis of ICD-10-CA and Canadian Classification of Health Interventions (CCI) procedural codes, have been described in detail elsewhere.11
Secondary outcomes included maternal death, admission to the intensive care unit, development of gestational hypertension, preeclampsia, or postpartum hemorrhage. These outcomes were defined using ICD-10, CCI, special care unit (SCU), and OHIP diagnostic and fee codes (Supplemental Table 4). All outcomes were assessed from the time of conception to 90 days after delivery or death. As the number of eclampsia events was anticipated to be small (incidence <0.1% of pregnancies in the general population), to comply with privacy regulations, such events were categorized as preeclampsia. We also reported the kidney-specific SMM indicators (Acute Renal Failure, Dialysis) given the particular relevance of these outcomes to the study population.
Perinatal Outcomes
The primary neonatal outcomes were preterm delivery (<37 weeks gestational age) and low birthweight (<2500 grams). Additional neonatal outcomes included small for gestational age and severe small for gestational age, defined as birthweight <10th percentile and fifth percentile, respectively, for sex and gestational age in relation to all live births in Ontario (added post priori),15 admission to the neonatal intensive care unit, stillbirth, and neonatal death. A stillbirth was defined as death of the fetus at ≥20 weeks gestational age, and neonatal death was defined as death between 1 and 28 days after delivery. Diagnostic codes for ascertainment of these conditions are detailed in Supplemental Table 4.
Statistical Analysis
Baseline characteristics were reported as frequencies and proportions for categorical measures and mean (SD) or median (25th percentile, 75th percentile) for continuous variables. We calculated the proportion of women and neonates meeting each outcome of interest. The results were stratified by baseline eGFR category. As less sensitive to sample size than traditional hypothesis tests, standardized differences were used to compare pregnancies with preserved kidney function to mild, moderate, and advanced kidney function groups. They provide a measure of the difference between groups in relation to a pooled standard deviation. A standardized difference ≥10% was considered a meaningful intergroup difference. Standardized differences were calculated using preserved kidney function as the referent group (eGFR ≥90 ml/min per 1.73 m2). To further explore outcomes by kidney function, we also looked at events occurring within eGFR categories by proteinuria severity.
Restricted cubic splines with five percentile knots in a logistic regression model were used to visually assess a potential nonlinear relationship between baseline eGFR and our primary outcomes of interest, truncated at an eGFR of 100 ml/min per 1.73 m2. Possible interaction between eGFR and proteinuria on the relative (or multiplicative) scale was assessed for our primary outcomes of interest, by adding an interaction term (eGFR*proteinuria) to our logistic model, restricting to those pregnancies with a proteinuria measurement and adjusting for age, baseline diabetes, baseline hypertension, nulliparous status, and multiple gestations. We used a generalized estimating equation (GEE) assuming an independent working correlation structure and sandwich variance estimates to obtain estimates for interaction and corresponding P values that control for the correlation for pregnancies/babies that occurred within the same woman. We explored other working correlation matrices and identified the most appropriate model using the Quasi likelihood (QIC) goodness-of-fit statistic for GEE models. The additive interaction was assessed using methods described by Knol and Vanderweele.16 Specifically, the odds ratio obtained from the multiplicative interaction model was calculated focusing on two levels of the exposure variables (eGFR and proteinuria) keeping normal eGFR and normal/mild proteinuria categories as our referent groups. The corresponding odds ratio and covariance matrices were then used to compute the Relative Excess Risk due to Interaction (RERI) and corresponding standard error to obtain the P value. We did not adjust our P values and 95% confidence intervals (CI) for multiple testing. The proportion attributable to interaction and the synergy index were also calculated (but not presented) for each of our pairwise comparisons. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Baseline Characteristics
Many of the 1,686,322 pregnancies in Ontario during the study period were excluded because a baseline serum creatinine measurement was not available; 565,907 pregnancies were eligible for our primary analysis (Figure 1). Of these, 522,752 (92.4%) pregnancies were preceded by preserved kidney function, 42,543 (7.5%) with mild CKD, 479 (0.08%) with moderate CKD, and 133 (0.02%) with advanced CKD. Preconception proteinuria assessments were available for 303,001 (58.0%) pregnancies with preserved kidney function, 23,282 (54.7%) pregnancies with mild CKD, 339 (70.8%) pregnancies with moderate CKD, and 115 (86.5%) pregnancies with advanced CKD.
Figure 1.
Study population. Flow diagram demonstrating the creation of the final study cohort.
Baseline characteristics by pregnancies, categorized by kidney disease severity, are summarized in Table 1. Women with kidney disease were more likely to be older at conception (33±5.0 years for mild CKD, 33±5.0 years for moderate CKD, and 32±5.1 years for advanced CKD) compared with women with preserved kidney function (30±5.2 years). Diabetes was similar in pregnancies with preserved kidney function (6.8%) and mild CKD (6.4%); however, it was more common in women with moderate (11.1%) and advanced CKD (22.6%). Women with kidney disease were also more likely to have recorded hypertension (9.8% with mild kidney disease, 22.3% with moderate CKD, and 36.8% with advanced CKD) compared with women with preserved kidney function (7.0%). Health care utilization in the year before conception was similar between groups, with a median of four visits to a family physician in all groups. At least one visit with a nephrologist was recorded in 28.4% of pregnancies with moderate CKD and 71.4% of pregnancies with advanced CKD.
Table 1.
Baseline characteristics by preconception eGFR (ml/min per 1.73 m2)
| Baseline Characteristics | eGFR ≥90 (N=522,752)a | eGFR 60 to <90 (N=42,543)a | eGFR 45 to <60 (N=479)a | eGFR <45 (N=133)a |
|---|---|---|---|---|
| Unique mothers, N (%) | 430,048 (94) | 39,298 (9) | 460 (0.1) | 122 (0.03) |
| Sociodemographic characteristics, N (%) | ||||
| Age at conception, years, mean±SD | 30±5.2 | 33±5.0b | 33±5.0b | 32±5.1b |
| Period of cohort entry | ||||
| 2007–2011 | 116,511 (22.3) | 8799 (20.7) | 97 (20.2) | 23 (17.3) |
| 2012–2015 | 210,792 (40.3) | 14,514 (34.2) | 168 (35.1) | 55 (41.4) |
| 2016–2019 | 195,449 (37.4) | 19,230 (45.2) | 214 (44.7) | 55 (41.4) |
| Income quintile | ||||
| First (lowest) | 114,792 (22.0) | 7760 (18.2) | 123 (25.7) | 31 (23.3) |
| Second | 106,084 (20.3) | 7899 (18.6) | 93 (19.4) | 20 (15.0)b |
| Third | 109,983 (21.0) | 9019 (21.2) | 94 (19.6) | 34 (25.6)b |
| Fourth | 109,745 (21.0) | 9552 (22.5) | 92 (19.2) | 20 (15.0)b |
| Fifth (highest) | 82,148 (15.7) | 8313 (19.5)b | 77 (16.1) | 28 (21.1)b |
| Rural residencec | 37,096 (7.1) | 3025 (7.1) | 37 (7.7) | 11 (8.3) |
| Obstetric and medical history, N (%) | ||||
| Nulliparous | 215, 525 (41.3) | 15,223 (35.8)b | 140 (29.2)b | 52 (39.1) |
| Multiple gestation | 10,773 (2.1) | 1103 (2.6) | 12 (2.5) | 9 (6.8)b |
| Diabetesd | 35,435 (6.8) | 2718 (6.4) | 53 (11.1)b | 30 (22.6)b |
| Hypertensiond | 36,687 (7.0) | 4171 (9.8) | 107 (22.3)b | 49 (36.8)b |
| Health care utilization | ||||
| Family physician visits in year before conception, median (25th, 75th percentile) | 4 (2, 8) | 4 (2, 7) | 4 (2, 8) | 4 (2, 8) |
| One or more nephrologist visits from 2 years before conception up to delivery, N (%) | 10,197 (2.0) | 1302 (3.1) | 136 (28.4)b | 95 (71.4)b |
In accordance with ICES privacy policies, cell sizes <6 cannot be reported.
The N for each eGFR category provides the number of pregnancies. The number of unique mothers is calculated within each eGFR category. It is possible for a mother to have different eGFR categories for each of her pregnancies; therefore, the sum of percentages for unique mothers across eGFR categories will >100%.
Standardized difference ≥10% compared with preserved renal function (eGFR ≥90 ml/min per 1.73 m2) category. Note that the standardized difference has not been adjusted for correlation of pregnancies in women and is intended to be used as a descriptive statistic rather than making inferences about the characteristics.
Rural location was defined as a population <10,000 individuals. Patients with missing location (<0.5%) were coded as urban.
Comorbidities in the 5 years preceding the index date were considered.
Maternal Outcomes
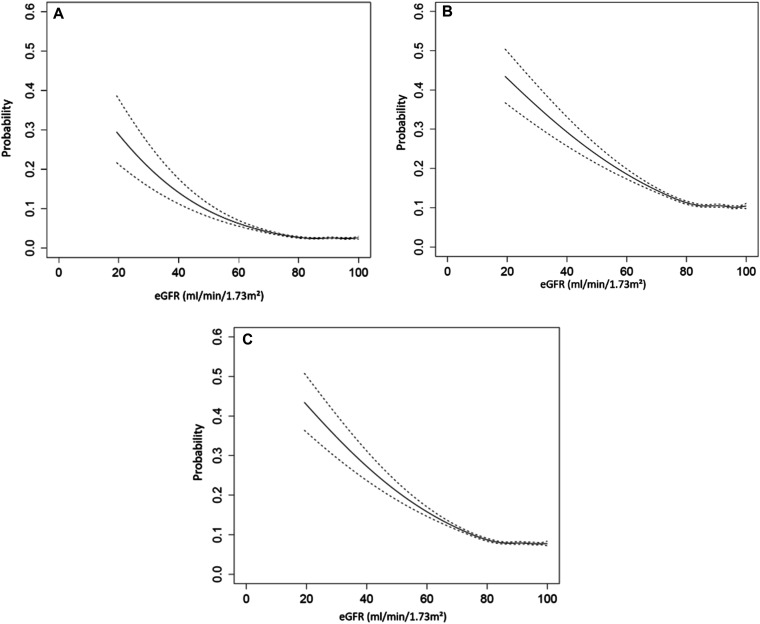
The frequency of at least one SMM indicator was 2.2% in pregnancies with preserved kidney function and not different compared with pregnancies in women with mild CKD at 2.8%; however, we observed an increase to 9.6% in pregnancies with moderate CKD and 21.8% in pregnancies with advanced CKD (results summarized in Table 2). As noted in Figure 2A, the risk of having at least one SMM indicator increased as eGFR declined. There was a nonlinear relationship between eGFR and SMM indicator with a small increase in SMM indicator at more preserved values of eGFR (i.e., eGFR > 80 ml/min per 1.73 m2). An exponential increase in the probability of SMM indicator occurrence was observed with decreasing eGFR values (i.e., eGFR lower than 60 ml/min per 1.73 m2).
Table 2.
Major maternal and fetal adverse outcomes by preconception eGFR (ml/min per 1.73 m2)
| Adverse Outcomes | eGFR ≥90 | eGFR 60 to <90 | eGFR 45 to <60 | eGFR <45 |
|---|---|---|---|---|
| Pregnancies, N (%) | 522, 752 (92.4) | 42, 543 (7.5) | 479 (0.08) | 133 (0.02) |
| Children, N (%) | 532, 628 (92.3) | 43, 557 (7.6) | 490 (0.08) | 139 (0.02) |
| Primary outcomes,a N (%) | ||||
| Presence of at least one SMM indicator | 11,655 (2.2) | 1174 (2.8) | 46 (9.6)b | 29 (21.8)b |
| Preterm delivery (<37 weeks) | 50, 695 (9.5) | 4, 954 (11.4) | 109 (22.2)b | 56 (40.3)b |
| Very preterm delivery (<34 weeks) | 15,511 (2.9) | 1624 (3.7) | 52 (10.6)b | 29 (20.9)b |
| Low birthweightc (<2500 g) | 39,368 (7.4) | 3774 (8.7) | 98 (20.0)b | 53 (38.1)b |
| SGA 10th percentile | 54, 505 (10.2) | 4192 (9.6) | 69 (14.1) | 19 (13.7) |
| SGA fifth percentile | 26, 904 (5.1) | 2034 (4.7) | 39 (8.0) | 7 (5.0) |
| Secondary outcomes,a N (%) | ||||
| Maternal death | ≥39 (0.01) | <6 (0.01) | ||
| Admission to ICU | 3262 (0.6) | 346 (0.8) | 15 (3.1)b | 9 (6.8)b |
| Gestational hypertension | 24,706 (4.7) | 2494 (5.9) | 50 (10.4)b | 24 (18.0)b |
| Preeclampsia | 31,215 (6.0) | 3232 (7.6) | 72 (15.0)b | 33 (24.8)b |
| Acute renal failure (SMM indicator)d | 256 (0.0) | 75 (0.2) | 15 (3.1)b | 12 (9.0)b |
| Dialysis (SMM indicator) | ≥26 (0.0) | <6 (<1.0) | 9 (6.8)b | |
| Postpartum hemorrhage | 31,112 (6.0) | 2647 (6.2) | 32 (6.7) | 10 (7.5) |
| NICU admission | 22,443 (4.2) | 2056 (4.7) | 54 (11.0)b | 15 (10.8)b |
| Stillbirth | 3180 (0.6) | 319 (0.7) | ≥6 (≥1.2)b | <6 (<4.3) |
| Neonatal death | 1348 (0.3) | ≥141 (≥0.3) | <6 (<1.2) | 0 (0.0) |
In accordance with ICES privacy policies, cell sizes < 6 cannot be reported. SMM, Severe Maternal Morbidity; SGA, small for gestational age; NICU, neonatal intensive care unit.
We assessed maternal outcomes from the conception date to 90 days after delivery date and neonatal outcomes up to 28 days after delivery date. The denominators differ for both maternal (eGFR ≥90 N=522,752 pregnancies; eGFR 60 to <90 N=42, 543 pregnancies; eGFR 45 to <60 N=479 pregnancies; and eGFR <45 N=133 pregnancies) and fetal outcomes (eGFR ≥90 N=532,628 babies; eGFR 60 to <90 N=43, 557 babies; eGFR 45 to <60 N=490 babies; and eGFR <45 N=139).
Standardized difference ≥10% compared with preserved renal function (eGFR ≥90 ml/min per 1.73 m2) category. Note that the standardized difference has not been adjusted for correlation of pregnancies in women and is intended to be used as a descriptive statistics rather than making inferences about the characteristics.
<0.5% of deliveries had missing weight. These were classified as normal without loss of generality. The missing weights were fairly evenly distributed across the eGFR categories.
SMM indicators measured between 20 weeks of gestation and 42 days after the index delivery.
Figure 2.
Association between eGFR and at (A) least one SMM indicator, (B) preterm birth (gestational age <37 weeks), and (C) low birthweight (weight <2500 g). SMM indicators were measured between 20 weeks of gestation and 42 days after the index delivery. Restricted cubic splines for eGFR were calculated with five percentile knots in a logistic regression model with the binary outcome for SMM, preterm birth, or low birthweight. The figures provide a visual representation of the relationship between the outcomes and eGFR truncated at 100 ml/min per 1.73 m2
There were 45 maternal deaths in the entire cohort, with ≥39 deaths occurring in pregnancies with preserved kidney function and <6 deaths in pregnancies with mild-to-advanced CKD. The maternal admission rate to the intensive care unit (ICU) from the time of conception to 90 days after delivery was 0.6% in pregnancies with preserved kidney function and 0.8% in those with mild CKD, 3.1% in pregnancies with moderate CKD, and 6.8% in pregnancies with advanced CKD. The proportion of pregnancies complicated by gestational hypertension and preeclampsia increased monotonically across kidney disease severity category (Table 2), with no difference between those with preserved kidney function and mild CKD. Postpartum hemorrhage developed in 6.0% of pregnancies and was similar across all kidney function categories.
Acute renal failure during or immediately after pregnancy increased across categories of kidney disease severity (Table 2). Acute renal failure developed in <0.01% of pregnancies with preserved renal function, 0.2% of pregnancies with mild CKD, 3.1% of pregnancies with moderate CKD, and 9.0% of pregnancies with advanced CKD. Dialysis was initiated in 6.8% of pregnancies with advanced CKD versus <0.01% of pregnancies with preserved kidney function or mild CKD.
Perinatal Outcomes
Both the frequency of preterm delivery before 37 weeks and birthweight <2500 g increased monotonically across kidney disease severity (Table 2). This trend was also observed for early preterm deliveries (<34 weeks gestational age). However, no difference was noted across kidney function groups in SGA babies, suggesting low birthweight to be a correlate of prematurity. In pregnancies with mild CKD, preterm delivery and very preterm delivery occurred in 11.4% and 3.7% of pregnancies, respectively, compared with 9.5% and 2.9%, respectively, in women with preserved renal function. These proportions were remarkably higher in women with moderate or advanced CKD occurring before 37 weeks in 22.2% and 40.3%, respectively, with delivery before 34 weeks occurring in 10.6% and 20.9%, respectively. Similarly, there was a progressive increase in the proportion of babies born with low birthweight at 20.5% in pregnancies complicated by moderate CKD and increasing to 39.8% in those wherein GFR was<45 ml/min per 1.73 m2. We saw a similar nonlinear relationship between eGFR and both perinatal outcomes with an exponential increase in the probability of both prematurity and a low birthweight as eGFR declined below 60 ml/min per 1.73 m2 (Figure 2, B and C).
Fetal demise and neonatal deaths were rare events. In fact, the live birth rate exceeded 98% even for women with eGFR <60 ml/min per 1.73 m2. Stillbirth occurred in 12 pregnancies (2.0%) with moderate or advanced disease compared with 3180 pregnancies (0.6%) with preserved kidney function. Neonatal death occurred in 1348 (0.3%) of pregnancies with preserved kidney function and in only 147 (0.3%) in women with CKD. Use of neonatal ICU was higher only in women with an eGFR <60 ml/min per 1.73 m2 with 11.3% of babies born to women with either moderate or advanced CKD admitted to the neonatal ICU compared with 4.8% in women with mild CKD and 4.3% in those with preserved kidney function.
Pregnancy Outcomes by eGFR and Proteinuria Severity
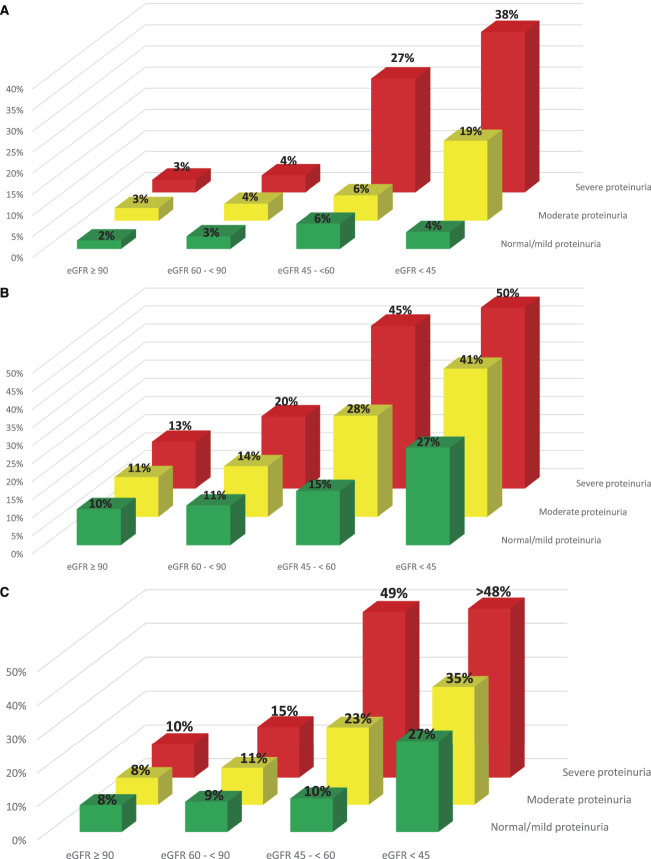
For each of the primary outcomes, proteinuria increased pregnancy related risks within each eGFR category (Figure 3, A–C). Among pregnancies with mild CKD, 4% experienced at least one SMM indicator, but only in the context of either moderate or severe proteinuria. Among pregnancies with moderate CKD, the proportion increased from 6% in pregnancies with mild or moderate proteinuria to 27% in pregnancies with severe proteinuria. Even among pregnancies with advanced CKD, those without significant proteinuria had only a 4% risk of at least one SMM indicator. However, risk increased to 19% of pregnancies with moderate proteinuria and to 38% in those with severe proteinuria (Figure 3A).
Figure 3.
Risk of (A) at least one SMM indicator, (B) preterm birth (gestational age<37 weeks), and (C) low birthweight (weight<2500 grams) by eGFR (ml/min per 1.73 m2) and proteinuria categories. Severe Maternal Morbidity indicators were measured between 20 weeks of gestation and 42 days after the index delivery. Preconception proteinuria in the 1–2 years before conception date was measured using a hierarchical combination of ACR or PCR on the basis of availability. Proteinuria values were categorized as severe (ACR value>30 mg/mmol or PCR value >50mg/mmol or urine dipstick 2+ or more), moderate (ACR value 3–30 mg/mmol or PCR value 15–50 mg/mmol or urine dipstick 1+), normal/mild (ACR value<3 mg/mmol or PCR value<5 mg/mmol or urine dipstick normal), and missing, respectively.
With respect to preterm delivery and low birthweight, similar interactions emerged. The likelihood of preterm delivery and low birthweight babies increased monotonically across eGFR and proteinuria severity categories with 50% of women with advanced CKD and severe proteinuria delivering before 37 weeks (Figure 3B) and almost 50% having a low birthweight baby (Figure 3C). Severe proteinuria did also increase the risk of delivering prematurely in women with only mild CKD with 20% delivering before 37 weeks of gestation and 15% delivering a low birthweight infant.
We explored multiplicative and additive interactions for our three main outcomes (SMM indicator, preterm birth, and low birthweight) adjusting for age, baseline diabetes, baseline hypertension, nulliparous status, and multiple gestations. We observed potential multiplicative and additive interactions between the moderate CKD with severe proteinuria and advanced CKD with severe proteinuria for the SMM indicator, meaning the relative and absolute risk of having at least one SMM indicator increased substantially when both substantial CKD and severe proteinuria were present. While we did not adjust for multiple testing, the RERI estimate indicates a large amount of additional additive risk associated within these interaction groups (RERI of 10.10 and 17.39, respectively) with a corresponding multiplicative risk of 2.92 and 8.54, respectively. More details are present in Supplemental Table 5.
We also observed some multiplicative and additive interactions between moderate CKD with severe proteinuria for preterm birth and low birthweight. Again, we did not adjust for multiple testing, but the RERI estimate indicates an increased additive risk associated within the interaction group (RERI of 5.13 and 10.59 for preterm birth and low birthweight, respectively) with a corresponding multiplicative risk of 2.81 and 5.68 for preterm birth and low birthweight, respectively.
Additional Analyses
We performed several additional post hoc analyses for our primary outcomes (SMM indicator, preterm delivery, and low birthweight offspring). In the first analysis, we restricted the cohort on the basis of serum creatinine availability before the index date of cohort entry (estimated date of conception). We observed a similar trend for each primary outcome when restricting the cohort (1) pregnancies with a serum creatinine within 365 days of conception and (2) pregnancies wherein two serum creatinine values were separated by at least 90 days (3 months) in the 2 years before conception. These data are summarized in Supplemental Figure 1. In a second post hoc analysis, we stratified the cohort by diagnosis of pregestational diabetes or pregestational hypertension. For all eGFR categories, preexisting diabetes or hypertension increased risk of major maternal morbidity, preterm delivery, and low birthweight, but again this relationship was most pronounced in women with an eGFR<60 ml/min per 1.73 m2.
Discussion
In this population-based study of over 560,000 pregnancies, adverse maternal and perinatal outcomes increased in a graded manner across baseline kidney disease severity with the greatest risks for SMM indicators, preterm delivery, and low birthweight occurring in women with moderate to advanced kidney disease (eGFR <45 ml/min per 1.73 m2). Maternal mortality and neonatal mortality were rare events across all categories of kidney function in this study. Preeclampsia occurred in approximately 15% and approximately 25% of pregnancies with moderate or advanced CKD, respectively. However, the outcome of acute renal failure events and receipt of acute dialysis was a rare event even in women with advanced CKD. Among women with advanced CKD, almost one in four had at least one SMM indicator while approximately one in three delivered before 37 weeks of gestation with one in five delivering before 34 weeks. Overall, our results support a maternal baseline eGFR <60 ml/min per 1.73 m2 as a reasonable threshold to intensify surveillance of both mother and fetus (see Figure 2, A–C).
It was notable that many women in this cohort had no quantification of urine protein despite the presence of CKD. Furthermore, more than 70% of women with an eGFR<60 ml/min per 1.73 m2 had not seen a nephrologist within 2 years of pregnancy despite universal health care access. This may stem from a lack of awareness of what constitutes kidney dysfunction in young women. This finding also suggests that physician awareness of the important association between CKD, proteinuria severity, and adverse pregnancy outcomes may be low and education should be prioritized so that expectant mothers with the highest risks receive appropriate care.
To date, this is the largest contemporary population-based study to describe the association between important maternal and perinatal outcomes and preconception eGFR. In North America, there have been three large cohort studies of pregnancy outcomes in women with CKD. Fischer et al. reported on 900 pregnancies from 1989 to 2001 in Colorado using offspring birth certificate coding of maternal renal disease.17 Kendrick et al. reported on 700 pregnancies from 2000 to 2013 from a large health care system in the Midwestern United States using diagnostic codes for CKD and other major comorbidities.18 Harel et al. evaluated over 55,000 pregnancies wherein women with elevated pre-pregnancy creatinine were compared with women with normal pre-pregnancy creatinine on their risk of delivering prematurely.19 Similar to our study, all three cohort studies noted higher rates of maternal and/or fetal complications among women with CKD; however, eGFR category-specific information was not available in these studies, thereby limiting their utility in counseling women across the spectrum of CKD.
Much of our knowledge about maternal and perinatal morbidity in pregnancies in women with CKD is derived from retrospective cohort studies that included few patients with advanced CKD. Specifically, in a large Italian cohort of 504 patients with CKD from 2000 to 2013, only 47 patients had CKD stages 3–5.3 In these women, much higher rates of preterm delivery and early preterm delivery were observed, with >75% of women with CKD 3–5 delivering before 37 weeks gestational age with the vast majority having a birthweight <2500 g. Our findings are in keeping with a recent publication of 178 pregnancies that assessed pregnancy outcomes in women with moderate to severe CKD (eGFR<60 ml/min per 1.73 m2) delivering at 6 centers in the United Kingdom wherein 56% delivered before 37 weeks of gestation.6 The higher rate relative to our study may be due to the inclusion of 43 renal transplant recipients who are known to have high rates of preeclampsia.20 Rates of preterm delivery, which are often iatrogenic, may be influenced by center-specific or region-specific delivery practices. Unfortunately, we had no way to distinguish between induced or spontaneous preterm labor in our data sources. Encouragingly, the live birth rate in women in our cohort with an eGFR <60 ml/min per 1.73 m2 was >98%, similar to that reported in the UK cohort.6
It has been well-documented that lack of physician confidence in caring for women with CKD can result in patient disempowerment because of medical catastrophizing and over approximation of risk.9,21 This notion of heightened risk is best illustrated in an Italian cohort where the association of stage 1 CKD and adverse pregnancy outcomes was attenuated, but persisted, even after controlling for hypertension, proteinuria, and systemic disease in their multivariable models.3 In this study, eGFR was calculated on preconception data; however, when available within 3 months before conception, this was available for just under half of the enrolled patients (224/504) with the rest of the baseline eGFR calculated on the basis of a pregnancy creatinine or a collection for creatinine clearance. The physiological changes associated with pregnancy occur early in gestation and the serum creatinine remains stable for approximately 4 weeks and then decreases significantly, so traditional GFR estimating equations perform poorly.13,22 As such, stage misclassification may have accounted for worse pregnancy outcomes noted in early-stage CKD in the Italian cohort compared with our study wherein 95% of eGFR staging occurred based on a nonpregnant serum creatinine and the remaining 5% occurred within the first 30 days after conception. We demonstrated that pregnancies in women with mild CKD were not substantially different than those with preserved kidney function. This is important new knowledge suggesting that specialized care for some women with mild CKD in the absence of other comorbidities (i.e., hypertension and diabetes) may not be needed, thus reducing resource expenditures for the health care system and undue stress for expectant mothers.
There is robust evidence that both hypertension and proteinuria contribute to adverse pregnancy outcomes. In a systematic review and meta-analysis, hypertension in the absence of CKD increased the relative risk for preeclampsia almost eight-fold.23 Similarly, an Italian cohort study comprising 49 pregnancies in women with stage 3–5 CKD, and proteinuria demonstrated that the combination of proteinuria and reduced GFR was the most potent combination for adverse maternal-fetal outcomes.1 Furthermore, the most contemporary study in advanced CKD, confirmed both as important factors with an odds of delivery before 34 weeks being 16.5 times higher for women with hypertension than without, and the odds of a baby being born below the 10th percentile being 2.6 times higher in women with proteinuria.6 In line with these studies, our study supports a robust association between proteinuria and adverse maternal and fetal outcomes, within each category of CKD, even after adjustment for age, baseline diabetes, baseline hypertension, nulliparous status, and multiple gestations. Specifically, a higher proportion of adverse outcomes particularly occurred in women with moderate to severe CKD and proteinuria and in women with more preserved eGFR in the context of severe proteinuria. Collaborative studies yielding a larger sample size will be required to better understand the relative contribution of all variables of interest to adverse maternal and fetal outcomes.
An important strength of our study is the large number of pregnancies captured in women with moderate to advanced CKD. Our study included 612 pregnancies in patients with a preconception eGFR <60 ml/min per 1.73 m2, a nearly five-fold increase in the number of stage 3–5 CKD pregnancies previously reported. Of note, many women in Ontario did not have a measurement of preconception kidney function. We expect that those without preconception laboratory results are the healthiest women and unlikely to have any adverse maternal or fetal outcomes. Thus, if these women were included in the cohort, the relative difference between more advanced CKD categories and those with normal kidney function may have been accentuated.
Similar to other large database analyses, there are many limitations of this study that warrant review. Because of reporting limitations within ICES, we are unable to report on many rare events due to low outcome numbers. As these data are not prospectively collected or registry quality data, missing data are common in these real-word data sources. This was most notable for proteinuria measurements, which were frequently missing even in patients with advanced stage CKD. Lack of an assessment of proteinuria in women with moderate to severe CKD is concerning and warrants practice reform. Although we have recorded hypertension as a comorbidity, we had no blood pressure measurements and we do not know the independent effect of hypertension on pregnancy outcomes. As such, we cannot untangle the independent effects proteinuria and hypertension by CKD stage despite the larger numbers of patients with CKD. Furthermore, only one serum creatinine measurement was required to be eligible for the study, and we were unable to adjust for body surface area with the information available in our databases, which may have led to some misclassification of pregnancies in their respective baseline eGFR categories. Similarly, there may have been some misclassification of acute kidney injury events. However, we intentionally accepted some risk of misclassification so that we did not exclude a substantial number of pregnancies from our cohort. In an additional post hoc analysis when restricting the cohort to women with at least two creatinine measurements separated by at least 90 days and those with a serum creatinine within the year before conception, we did see similar results (Supplemental Figure 1). Another limitation of our study pertains to the sensitivity of ICD-10 codes for diagnosing preeclampsia, which is of particular concern in women with underlying kidney disease given the diagnostic challenges in this population. Most ICD-10 codes used to identify preeclampsia within a hospital admission have high specificity (97.1%–100.0%) and a moderate-to-high positive predictive value (74.4%–100.0%). While sensitivity is more variable, it appears to improve for coding algorithms used to identify more severe disease.
In our study, we confirm and extend the findings of three previous large cohort studies.17–19 Through stratifying our results by eGFR category, we were able to discern nuanced information that adds to the way we provide care for women with kidney disease. In addition, our study suggests that we must do better with educating physicians on the risks associated with reduced kidney function, especially in the context of proteinuria in pregnancy. We should consider redefining our thresholds for specialist referral so that women with the highest risks may receive appropriate and timely care. Conversely, it may be more appropriate for expectant mothers with mild CKD in the absence of proteinuria or other obstetric issues to be reassured and managed with standard obstetrical care. As a next step toward clinical application, development of a risk index calculator, incorporating a personalized set of clinical characteristics (including pre-pregnancy eGFR and proteinuria), would provide patients personalized information and ultimately improve care for this vulnerable population. This work is currently in progress.
Supplementary Material
ACKNOWLEDGMENTS
J. Tangren was supported by an American Kidney Fund Clinical Scientist in Nephrology Grant and a NIH Career Development Award (K23-DK120874); A.X. Garg was supported by the Dr. Adam Linton Chair in Kidney Health Analytics and a Clinician Investigator Award from the Canadian Institutes of Health Research; MAH holds the Sibbald Chair of Medicine at Sunnybrook Health Sciences Centre.
Footnotes
J.T., L.B., N.J., A.X.G., and M.A.H. contributed equally to this work as primary and supervising authors.
Disclosures
R. Wald reports Research Funding: Baxter; Advisory or Leadership Role: Editorial Board, Clinical Journal of the American Society of Nephrology, Kidney Medicine, Kidney360; and Other Interests or Relationships: Contributor, UpToDate. A. Akbari reports Consultancy: AstraZeneca and Otsuka; Research Funding: AstraZeneca and Otsuka; Honoraria: AstraZeneca; Advisory or Leadership Role: AstraZeneca and Otsuka; and Speakers Bureau: AstraZeneca. S. Huang reports Advisory or Leadership Role: Sanofi; and Speakers Bureau: Norvartis. A.X. Garg reports Research Funding: Astellas, Baxter; Advisory or Leadership Role: Currently on the Editorial Boards of American Journal of Kidney Diseases and Kidney International; and Other Interests or Relationships: Serve on the Data Safety and Monitoring Board for an Anemia Trial Program Funded by Glaxo Smith Kline (activity now complete), Medical Lead Role to Improve Access to Kidney Transplantation and Living Kidney Donation for the Ontario Renal Network (government funded agency located within Ontario Health). M. Hladunewich reports Consultancy: Alnylam Pharmaceuticals; Research Funding: Calliditas Therapeutics, Chemocentryx, Ionis, Pfizer, and Roche; Advisory or Leadership Role: Kidney International, UpToDate; and Other Interests or Relationships: Medical Lead for Glomerular Disease Ontario Renal Network.
Funding
This work was supported by the Ontario Renal Network through funding provided by the Government of Ontario. The opinions, results, view, and conclusions reported in this publication are those of the authors and do not necessarily reflect those of Ontario Renal Network. No endorsement by the Ontario Renal Network is intended or should be inferred. This study was also supported by the ICES Western site. ICES is funded by an annual grant from the Ontario Ministry of Health (MOH) and the Ministry of Long-Term Care (MLTC). Core funding for ICES Western is provided by the Academic Medical Organization of Southwestern Ontario (AMOSO), the Schulich School of Medicine and Dentistry (SSMD), Western University, and the Lawson Health Research Institute (LHRI). Parts of this material are based on data and information compiled and provided by Ontario MOHLTC. The analyses, conclusions, opinions, and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred. Parts of this material are also based on data and information compiled and provided by the Canadian Institutes of Health Information (CIHI). No endorsement by ICES or the Ontario MOH, MLTC is intended or should be inferred. The research was conducted by members of the ICES Kidney, Dialysis and Transplantation team, at the ICES Western facility, who are supported by a grant from the Canadian Institutes of Health Research (CIHR).
Author Contributions
A.X. Garg, M.A. Hladunewich, and J. Tangren conceptualized the study; A.X. Garg, M.A. Hladunewich, and J. Ray were responsible for data curation; M.A. Hladunewich and J. Tangren were responsible for funding acquisition; all authors were responsible for methodology; L. Bathini, S.N. Dixon, A.X. Garg, and N. Jeyakumar were responsible for formal analysis; N. Jeyakumar and A.X. Garg were responsible for resources; N. Jeyakumar was responsible for software; S.N. Dixon was responsible for investigation; A.X. Garg, M.A. Hladunewich, J. Ray, and R. Wald provided supervision; L. Bathini, M.A. Hladunewich, and J. Tangren wrote the original draft; and A. Akbari, S.N. Dixon, A.X. Garg, Z. Harel, M.A. Hladunewich, S. Huang, N. Jeyakumar, A. Mathew, J. Ray, and R. Wald reviewed and edited the manuscript.
Data Sharing Statement
The analysis was conducted by members of the ICES Kidney Dialysis & Transplantation (KDT) team at the ICES Western facility (London, ON). Stephanie N. Dixon was responsible for the data analysis. The protocol can be obtained by emailing Dr. Hladunewich at michelle.hladunewich@sunnybrook.ca.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/JSN/D523.
Supplemental Figure 1. Primary outcomes by serum creatinine availability in the cohort.
Supplemental Figure 2. Primary outcomes by pregestational diabetes and hypertension.
Supplemental Table 1. Checklist of recommendations for reporting of observational studies using the REporting of studies Conducted using Observational Routinely collected health Data (RECORD) statement.
Supplemental Table 2. Codes used in the study to identify baseline characteristics.
Supplemental Table 3. Codes used to identify Severe Maternal Morbidity (SMM) indicators.
Supplemental Table 4. Codes used in the study to identify outcome conditions.
Supplemental Table 5. Adjusted modification of the effect of eGFR categories on the odds of at least one Severe Maternal Morbidity indicator by proteinuria categories.
References
- 1.Imbasciati E, Gregorini G, Cabiddu G, et al. Pregnancy in CKD stages 3 to 5: fetal and maternal outcomes. Am J Kidney Dis. 2007;49(6):753-762. doi: 10.1053/j.ajkd.2007.03.022 [DOI] [PubMed] [Google Scholar]
- 2.Zhang JJ, Ma XX, Hao L, Liu LJ, Lv JC, Zhang H. A systematic review and meta-analysis of outcomes of pregnancy in CKD and CKD outcomes in pregnancy. Clin J Am Soc Nephrol. 2015;10(11):1964-1978. doi: 10.2215/CJN.09250914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piccoli GB, Cabiddu G, Attini R, et al. Risk of adverse pregnancy outcomes in women with CKD. J Am Soc Nephrol. 2015;26(8):2011-2022. doi: 10.1681/ASN.2014050459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jungers P, Chauveau D, Choukroun G, et al. Pregnancy in women with impaired renal function. Clin Nephrol. 1997;47(5):281-288. [PubMed] [Google Scholar]
- 5.Jones DC, Hayslett JP. Outcome of pregnancy in women with moderate or severe renal insufficiency. N Engl J Med. 1996;335(4):226-232. doi: 10.1056/nejm199607253350402 [DOI] [PubMed] [Google Scholar]
- 6.Wiles K, Webster P, Seed PT, et al. The impact of chronic kidney disease Stages 3-5 on pregnancy outcomes. Nephrol Dial Transplant. 2020;36(11):2008-2017. doi: 10.1093/ndt/gfaa247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horbar JD, Carpenter JH, Badger GJ, et al. Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics. 2012;129(6):1019-1026. doi: 10.1542/peds.2011-3028 [DOI] [PubMed] [Google Scholar]
- 8.Oliverio AL, Bramham K, Hladunewich MA. Pregnancy and CKD: advances in care and the legacy of Dr Susan Hou. Am J Kidney Dis. 2021;78(6):865-875. doi: 10.1053/j.ajkd.2021.07.016 [DOI] [PubMed] [Google Scholar]
- 9.Hendren EM, Reynolds ML, Mariani LH, et al. Confidence in women's health: a cross border survey of adult nephrologists. J Clin Med. 2019;8(2):176. doi: 10.3390/jcm8020176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benchimol EI, Smeeth L, Guttmann A, et al. RECORD Working Committee. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015;12(10):e1001885. doi: 10.1371/journal.pmed.1001885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ray JG, Park AL, Dzakpasu S, et al. Prevalence of severe maternal morbidity and factors associated with maternal mortality in Ontario, Canada. JAMA Netw Open. 2018;1(7):e184571. doi: 10.1001/jamanetworkopen.2018.4571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hildebrand AM, Liu K, Shariff SZ, et al. Characteristics and outcomes of AKI treated with dialysis during pregnancy and the postpartum period. J Am Soc Nephrol. 2015;26(12):3085-3091. doi: 10.1681/ASN.2014100954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harel Z, McArthur E, Hladunewich M, et al. Serum creatinine levels before, during, and after pregnancy. JAMA. 2019;321(2):205-207. doi: 10.1001/jama.2018.17948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, et al. for the CKD-EPI Chronic Kidney Disease Epidemiology Collaboration. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jairam JA, Vigod SN, O’ Campo, et al. Neighbourhood income and risk of having an infant with concomitant preterm birth and severe small for gestational age birth weight. JOGC. 2020; 42(2): 156-162. doi: 10.1016/j.jogc.2019.06.014 [DOI] [PubMed] [Google Scholar]
- 16.Knol MJ, VanderWeele TJ. Recommendations for presenting analyses of effect modification and interaction. Int J Epidemiol. 2012;41(2):514-520. doi: 10.1093/ije/dyr218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer MJ, Lehnerz SD, Hebert JR, Parikh CR. Kidney disease is an independent risk factor for adverse fetal and maternal outcomes in pregnancy. Am J Kidney Dis. 2004;43(3):415-423. doi: 10.1053/j.ajkd.2003.10.041 [DOI] [PubMed] [Google Scholar]
- 18.Kendrick J, Sharma S, Holmen J, Palit S, Nuccio E, Chonchol M. Kidney disease and maternal and fetal outcomes in pregnancy. Am J Kidney Dis. 2015;66(1):55-59. doi: 10.1053/j.ajkd.2014.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harel Z, Park AL, McArthur E, et al. Prepregnancy renal function and risk of preterm birth and related outcomes. Can Med Assoc J. 1922020;192(30):E851–E857. doi: 10.1503/cmaj.200089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deshpande NA, James NT, Kucirka LM, et al. Pregnancy outcomes in kidney transplant recipients: a systematic review and meta-analysis. Am J Transplant. 2011;11:2388-2404. doi: 10.1111/j.1600-6143.2011.03656.x [DOI] [PubMed] [Google Scholar]
- 21.Tong A, Jesudason S, Craig JC, Winkelmayer WC. Perspectives on pregnancy in women with chronic kidney disease: systematic review of qualitative studies. Nephrol Dial Transplant. 2015;30(4):652-661. doi: 10.1093/ndt/gfu378 [DOI] [PubMed] [Google Scholar]
- 22.Alper AB, Yi Y, Rahman M, et al. Performance of estimated glomerular filtration rate prediction equations in preeclamptic patients. Am J Perinatol. 2011;28(06):425-430. doi: 10.1055/s-0030-1268712 [DOI] [PubMed] [Google Scholar]
- 23.Bramham K, Parnell B, Nelson-Piercy C, Seed PT, Poston L, Chappell LC. Chronic hypertension and pregnancy outcomes: systematic review and meta-analysis. BMJ. 2014;348:g2301. doi: 10.1136/bmj.g2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analysis was conducted by members of the ICES Kidney Dialysis & Transplantation (KDT) team at the ICES Western facility (London, ON). Stephanie N. Dixon was responsible for the data analysis. The protocol can be obtained by emailing Dr. Hladunewich at michelle.hladunewich@sunnybrook.ca.