Abstract
Renal transplantation is the preferred treatment of ESKD, but the shortage of suitable donor kidneys from the cadaver pool means that many patients with ESKD will not receive a kidney transplant. Xenotransplantation has long represented a solution to the kidney shortage, but the occurrence of antibody-mediated rejection has precluded its clinical development. Developments in somatic cell nuclear transfer in pigs and gene editing tools have led to the creation of new donor pigs with greatly improved crossmatches to patients. In addition, improvements in preclinical kidney xenotransplant survival using new anti-CD40/CD154–based immunosuppression have pushed xenotransplantation to the point where it is reasonable to consider initiating a clinical trial to evaluate this potential therapy in patients.
Keywords: transplantation, basic science, xenotransplantation
Introduction
Kidney transplantation improves both quality and duration of life and is cost effective for patients with ESKD, making it preferred for the treatment of kidney failure. The shortage of donor kidneys for use in clinical transplantation severely restricts the use of this therapy in patients with ESKD. Xenotransplantation could end this shortage by providing an unlimited source of donor kidneys, and the development of somatic cell nuclear transfer (cloning), gene scissors CRISPR-Cas9, and CD40/CD40L costimulatory blockade drugs has positioned the use of pig kidneys to a point where initiation of a clinical trial is reasonable to consider.1-3 Movement to a clinical trial will be warranted if consistent graft survival can be achieved in preclinical models using clinically available immunosuppression without incurring unacceptable infectious morbidity and mortality in recipients.
Pathobiology of Renal Xenografts: The Humoral Barrier
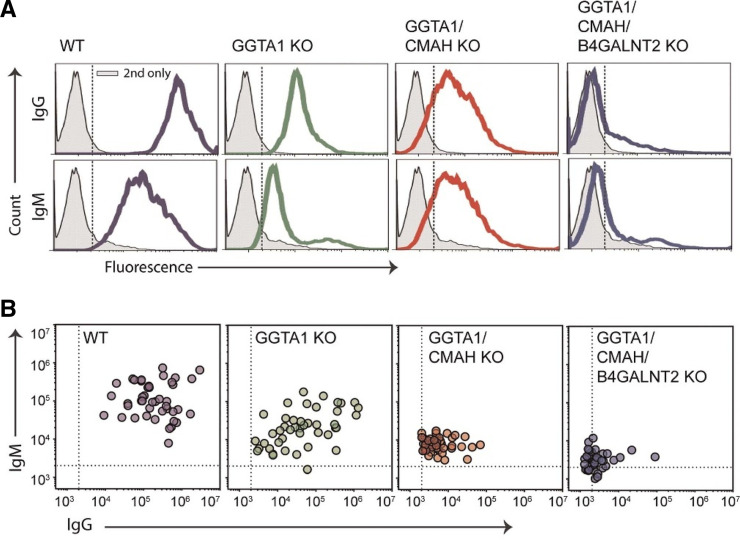
Analogous to allotransplantation, there are two sets of antigens (glycan and MHC) to which humans may have preformed antibodies that can bind to the endothelium and trigger complement activation and coagulation resulting in hyperacute rejection. The initial barrier was the presence of three significant glycan xenoantigens on the surface of pig cells which have been identified1: gal-a1, 3-gal,2 N-glycolylneuraminic acid, and3 Sda antigen.4,5 Serial deletion of the enzymes that produce these antigens, a-1,3-galactosyltransferase (GGTA1), cytidine monophospho-Nacetylneuraminic acid hydroxylase (CMAH), and b1,4-N-acetyl galactosaminyltransferase (b4GalNt2), using CRISPR/Cas9 produces donor pigs that have a negative crossmatch (IgM and IgG) to 30% of waitlisted patients and another 40% have a negative IgG crossmatch. This means that at least 30% of the patients on the transplant waitlist would not be expected to have early graft loss secondary to antibody-mediated rejection (AMR) if they received a GGTA1/CMAH/b4GalNt2 KO pig kidney transplant (Figure 1).6,7
Figure 1.
Analysis of human immunoglobulin binding to pig PBMC from genetically modified swine. PBMC were collected, incubated with human serum, and analyzed for IgM (y axes) and IgG (x axes) binding by flow cytometry. Dotted lines represent an MFI of 2000. (A) A single representative patient is screened on wild type, GGTA1 KO, GGTA1/CMAH KO, and GGTA1/CMAH/b4GalNt2 KO swine PBMC. (B) Sera from 44 randomly selected patients with unknown sensitization were incubated with PBMC from all four glycan backgrounds.
MHC Barrier to Xenotransplantation
The development of anti-HLA antibodies is a significant barrier for patients who need a kidney transplant for finding a human donor with a suitable crossmatch. The Swine Leukocyte Antigen (SLA) has class I and class II alleles that share significant sequence homology with HLA so that some anti-HLA class I and class II antibodies cross react with SLA on the surface of donor pig cells (Figure 2).6-12 This means that some highly HLA-sensitized patients will not be suitable candidates for transplantation with typical triple knockout (TKO) pig kidneys on the basis of a positive crossmatch. This question was answered definitively with the development of SLA I KO pigs and the use of more sophisticated tools to directly evaluate individual SLA antigen binding.9,13-16 If an anti-HLA class I antibody binds to SLA class I, it tends to bind to every class I SLA molecule in the pig (SLA 1, 2, and 3) because the class I alleles in the pig are very homologous. In SLA class II, some patients with anti-HLA antibodies to HLA DR and DQ do have antibodies that are cross reactive. The binding of anti-HLA antibodies to SLA occurs when the antibody binds to a cross-reactive group that is shared between given HLA and SLA alleles. More recently, it is clear that there are some patients who are not HLA sensitized that have anti-SLA antibodies, and both class I and class II reactivity has been demonstrated.9 Clearly, careful and detailed histocompatibility testing will be critical to ensure optimal patient survival and graft function, much like that seen in clinical allotransplantation. The potential importance of histocompatibility in xenotransplantation is further elevated when one considers that unlike allotransplantation, genome editing could be used to direct the creation of a new donor pig with an improved pretransplant crossmatch for any given patient.14
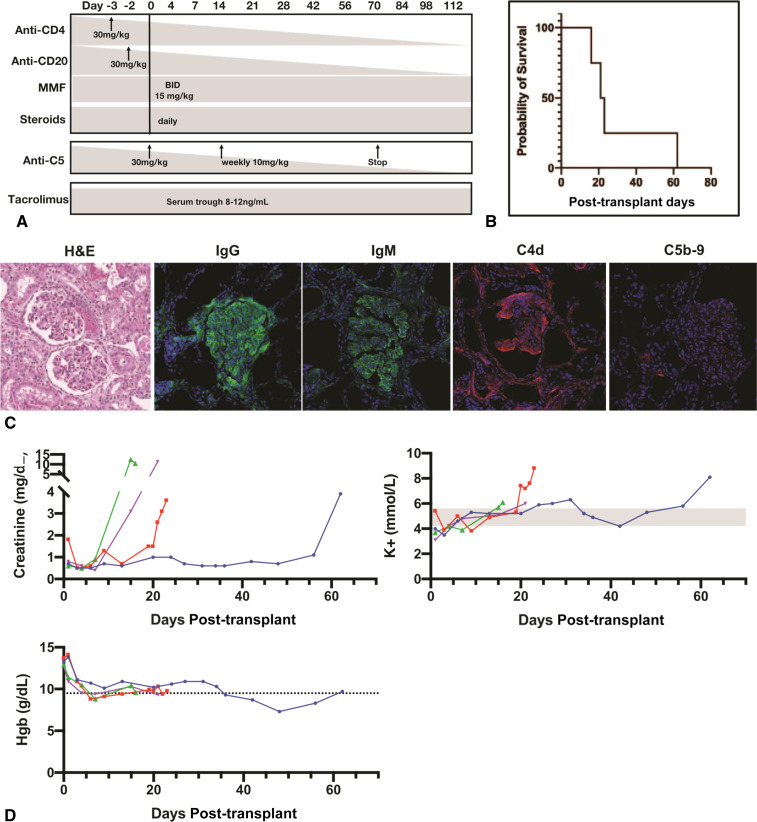
Figure 2.
Tacrolimus-based immunosuppression fails to prolong renal xenograft survival. (A) Immunosuppression regimen including tacrolimus and tesidolumab without CD154 monoclonal antibody therapy. (B) Kaplan-Meier curve analysis reveals that a tacrolimus-based immunosuppression regimen fails to extend xenograft survival beyond 62 days despite the presence of C5 complement inhibition by tesidolumab. (C) Biopsy taken from explanted kidney at day 62 shows parenchymal hemorrhage and thrombotic microangiopathy, prominent IgG, IgM, C4d, and minimal C5b-9. (D) Creatinine and potassium levels rose above normal values days to weeks before rejection. Hemoglobin values remained relatively normal.
Species Incompatibilities in Complement and Coagulation Regulation Systems
Before the development of the GGTA1 KO pig, attention was directed toward potential complement regulatory protein incompatibilities that existed with pig C‐reactive proteins (CRPs) on the surface of the xenograft endothelium. Attention was directed toward CD46, CD55, and CD59, and transgenic pigs were created expressing these CRPs. While kidneys transplanted from these pigs into non‐human primates (NHPs) had improved survival compared with wildtype, they still succumbed to AMR with resultant thrombotic microangiopathy.17 Later work showed that the function of CD46, CD55, and CD59 was not species restricted, but rather the expression of human CRPs could decrease complement-mediated injury because of increased cell surface numbers. The work also showed that in the absence of antibody binding, the impact of complement transgenes would be minimized.18-23
Analysis of rejected renal xenografts showed a heightened susceptibility to intravascular thrombosis that was mainly the result of AMR, but there are some subtle decreases in the ability of the pig thromboregulatory system to keep the human or NHP system from being activated and causing graft thrombosis. There have been a number of transgenes studied to minimize this problem in donor pigs.24 Pigs have been produced that have tissue factor pathway inhibitor (TFPI), CD39, thrombomodulin, and endothelial cell protein receptor C (EPCR), each human transgenes to reduce thrombotic microangiopathy.25,26
Current State of Preclinical Renal Xenotransplantation
Unlike the situation for clinical xenotransplantation where there are significant numbers of patients with a negative crossmatch to genome edited pigs, there is no pig that has a negative crossmatch for rhesus macaques, cynomolgus monkeys, or baboons. This means that all preclinical transplants have been performed in the face of a positive CDC crossmatch.27,28 The result of this imperfect crossmatch is that tacrolimus-based immunosuppression has been ineffective in prolonging survival in preclinical renal xenotransplantation. Transplantation of GGTA1 KO pig kidneys into baboons resulted in 6- to 16-day survival using an immunosuppression regimen with thymoglobulin, tacrolimus, mycophenolic acid, and steroids. Rejection was the result of additional preformed and elicited xenoreactive antibodies, and the pathobiology showed complement activation and thrombotic microangiopathy.29 Transplantation of pig kidneys that were GGTA1 KO/CD55 transgenic using tacrolimus-based immunosuppression did not improve survival in rhesus monkeys beyond the initial attempts nor did the use of GGTA1/b4GalNt2 KO pig kidneys in recipients who were treated with anti-C5 antibody in addition to T cell depletion, tacrolimus, and steroids (Figure 3).30 In order for tacrolimus to be effective in preclinical models, there would need to be a new donor pig with an improved crossmatch for the nonhuman primate. The development of such a donor pig is currently underway in our laboratory.
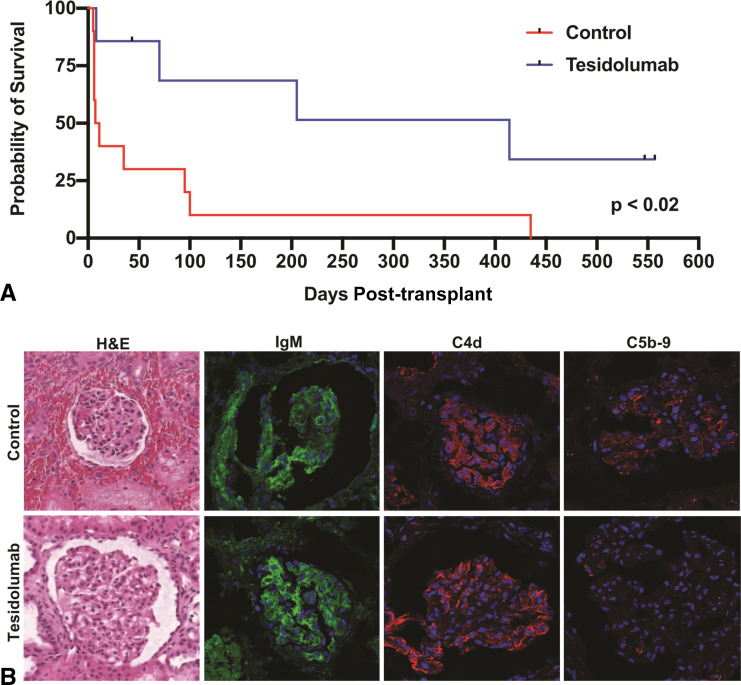
Figure 3.
Analysis of grafts surviving to 557 days. (A) Kaplan-Meier curve analysis of recipients who received genetically engineered porcine kidneys with or without tesidolumab included in the immunosuppression regimen. (B) H&E and confocal microscope analysis of rejected xenografts. Confocal microscopy showing deposition of IgM and C4d in the control and tesidolumab-treated recipients. Tesidolumab-treated recipients had minimal histological injury, while control kidneys had parenchymal hemorrhage and thrombotic microangiopathy. Deposition of C5b-9 was reduced on xenografts in tesidolumab-treated recipients.
Efforts to achieve improved survival in the preclinical model have used one of two approaches: (1) the use of donor pigs with xenoantigen deletion and the inclusion of human multiple transgenes to downregulate the immune response and counteract thrombotic complications or (2) the use of more directed genome editing with simple xenoantigen deletion and heavy reliance on pretransplant crossmatch testing to select recipients with the most favorable crossmatch. CD40/CD40L–based costimulation blockade as baseline immunosuppression was used in both approaches. The use of pig kidneys from donors with xenoantigen deletion and as many as six human transgenes (CD55, CD46, CD59, TFPI, thrombomodulin, CD47, PDL1, and HLA-E) has resulted in mixed results where some recipients reject their kidneys early (<150 days) and others experience more prolonged survival (>180 days).25,31,32 The immunosuppressive regimens used for these studies has used either anti-CD40 or anti-CD154 as its baseline, and ATG, anti-CD20, cell cept, and variably tacrolimus or rapamycin (either short term-2 months, or as part of baseline immunosuppression). Most groups have had more success using anti-CD154 rather than anti-CD40.33. The recipients have had significant issues with infection and wound healing that were complications of the immunosuppressive regimens.30,33 The studies that have used multiple transgenes have not relied on pretransplant crossmatching for recipient selection.
The use of kidneys from less engineered pigs relying on either xenoantigen deletion alone (GGTA1 KO, with or without b4GalNt2 KO) or xenoantigen deletion (GGTA1 KO) and complement regulatory protein transgene CD55 transplanted into recipients with the most favorable pretransplant crossmatches has been studied extensively.27,30,34,35 The immunosuppression in these studies was anti-CD4 T cell depletion, anti-CD20, anti-CD154, cell cept, and steroids. Recipients receiving GGTA1 KO/CD55 Tg kidneys had a median survival of 235 days with two of six recipients surviving for >400 days. Graft loss in all was due to AMR in all cases (Figure 4).35 When the simplest genome editing strategy, xenoantigen deletion alone (GGTA1/b4GalNt2 KO), was used, recipients experienced an early IgM-mediated AMR that resulted in early graft loss in five of six recipients (<100 days), and one recipient survived with good renal function for 435 days.27 Instituting temporary complement inhibition with anti-C5 (weekly injections for 70 days) reduced early graft loss secondary to AMR so that five of seven recipients did not succumb to early AMR, with median survival of 308 days, and all grafts were lost secondary to AMR (two early and five late).30 In this series, there was no graft loss or recipient death because of infectious complications.
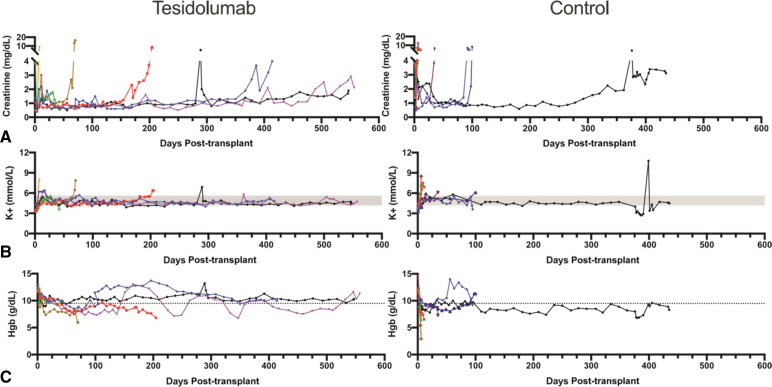
Figure 4.
Anti‐C5 plus anti‐CD40 prolongs renal xenograft survival. (A) Creatinine is shown to be well controlled until acutely rising at time of graft failure. (B) Serum potassium was well controlled throughout, except during late graft failure because of rejection. (C) Early graft failure recipients encounter anemia as shown by decreased hemoglobin levels. Tesidolumab-treated recipients maintained hemoglobin levels at or above normal levels more frequently than control animals for the duration of graft survival.
In each of these series it is clear that the kidney provides life supporting function, normal electrolyte balance, recipients maintain hemoglobin levels above 9 gm/dl without the need for erythropoietin supplementation, and absence of proteinuria.34 The fact that erythropoietin supplementation was not needed suggests that pig erythropoietin supports RBC production in the NHP. Proteinuria was evaluated as a possible species incompatibility,36 but more recent data suggest that proteinuria does not occur unless there is AMR, much like a renal allograft. Recipients have hypercalcemia and hypophosphatemia much like that encountered after clinical renal allotransplantation.34
Critical evaluation of the results of preclinical studies suggests (1) that xenoantigen deletion is an effective strategy to reduce the level of pretransplant donor specific antibody (DSA) to avoid hyperacute rejection; (2) screening for the best pretransplant crossmatches results in more consistent long-term graft survival; (3) complement regulatory protein transgenes can decrease early graft loss, but the use of transgenes has not improved longer-term survival compared with xenoantigen deletion alone; (4) temporary pharmacological complement inhibition minimizes early graft loss due to AMR; and (5) CD40/CD40L costimulation blockade-based immunosuppression is effective in preventing T cell–mediated and B cell–mediated xenograft rejection and required for long-term graft survival.
Use of the Recently Deceased as a Preclinical Model
The use of recently deceased humans with an intact circulation as recipients of renal xenografts has been performed by two groups. The work in decedents followed published guidelines established by the Consensus Panel on Research with the Recently Dead.37 The two published reports show that it is going to be feasible to evaluate pig kidney xenotransplantation in this human preclinical in vivo model but that there are a number of significant variables that will need to be worked out before it is clear what can be evaluated in this model.38,39 The initial report described a decedent whose cause of death was irreversible brain injury secondary to a trauma and had mild-to-moderate AKI and required vasopressor support to maintain normal hemodynamics. Two 10-GE (TKO (GGTA1/CMAH/b4GalNt2 KO) plus GKRKO with 6 human transgenes (CD46, CD55, TBM, EPCR, CD47, and HO1) and pig kidneys were transplanted into the nephrectomized decedent. The patient had a pretransplant crossmatch that evaluated IgG, but not IgM, which is significant because preclinical models suggest that IgM is at least as important in early xenotransplantation.
Postreperfusion, the kidneys initially made urine but failed to clear any creatinine as levels rose from 2 to 6 mg/dl. It is unclear whether this was the result of early AMR or brain death physiology in the decedent who had significant trauma and failure of several organ systems. While hyperacute rejection did not occur, which is reassuring, it is unclear if early AMR occurred because there was early thrombotic microangiopathy. The urine the kidneys did produce appeared to look much like that seen in cases of significant acute tubular necrosis and delayed graft loss in clinical allotransplantation.
The second report from NYU described two cases of a GGTA1 KO thymokidney transplanted into a decedent whose cause of death was secondary to an intracranial bleed.38 The kidneys in these two decedents were not hyperacutely rejected, and while the kidneys were not life supporting, they did increase the eGFR of the decedents significantly. The pathology of the two thymokidneys was also encouraging showing that at very early stages hyperacute rejection is averted. Hence, the initial reports provide confirmation of data from preclinical models that hyperacute rejection does not occur using genetically engineered pig kidneys, but there are still many unanswered questions regarding kidney function.
This report also highlights several other important considerations if the recent decedent kidney xenograft model is going to make important contributions to the understanding of the clinical course and problems encountered in clinical xenotransplantation. Not all decedents with an intact circulation are equivalent, and the choice in this initial case highlights the difficulties in choosing a decedent that succumbs to significant trauma. There were significant difficulties in deciphering whether physiological performance of the kidney and the resultant pathology were reflective of immunological reactions to the xenograft or indicative of physiology of brain death and impending multiple organ dysfunction syndrome. In future attempts, it may be very important to select decedents with a more stable hemodynamic course and with less likelihood of developing multisystem organ dysfunction and hematological failure which will make the interpretation of findings difficult to evaluate from the perspective of what is rejection versus donor physiology. Perhaps the use of decedents with intracranial hemorrhage as a cause of death will make this distinction less likely. The question of whether the use of the decedent model can answer other questions regarding clinical renal xenotransplantation is unclear. Three factors that may limit the model significantly are (1) family and ethical considerations regarding extended duration of invasive monitoring of the decedent; (2) duration that the circulation of a decedent can be kept intact to evaluate events that will impact whether a pig kidney will function in a patient for a longer period; and (3) cost of keeping a decedent's circulation intact for potentially months to evaluate longer-term issues. The decedent model is interesting and could be valuable in the future once some of these issues are worked out. Given that the recent clinical cardiac xenotransplant performed by the University of Maryland survived 2 months, it is likely that the decedent model would need to go out that long to add to any immunosuppression or rejection issues that could be evaluated for moving xenotransplantation to the clinic.
Moving Renal Xenotransplantation to a Clinical Trial
The critical question for moving renal xenotransplantation forward is what are the steps necessary to enter a clinical trial? Recently, the University of Maryland transplanted a 10GE pig heart into a patient with end-stage cardiac failure who was deemed ineligible for cardiac allotransplantation. This patient survived for 2 months post-transplant before dying. The cause of death in this patient will almost certainly be described in forthcoming manuscripts, but likely sources of failure are faulty patient selection choosing someone who was too debilitated receive benefit from a transplant, infection secondary to immunosuppression, or rejection of the cardiac xenograft. This case while exciting and a valiant effort to save a man's life was not successful, and xenotransplantation will not have many more opportunities to demonstrate that it can be used to save patient's lives before it experiences the same long winter that clinical allotransplantation faced before it was finally offered to patients with any degree of regularity. This transplant was performed under an emergency investigational new drug application from the US Food and Drug Administration. This mechanism is operative for a single patient and while helpful for that patient will not lead to a clinical trial and widespread expansion of xenotransplantation. Moving forward, it will be critical to use a formal IND to develop renal xenotransplantation as a therapy that can be used for all patients with ESKD. There are a few components that must be in place to initiate a clinical trial to ensure that this therapy delivers on its promise for the many patients who are desperate to receive a kidney transplant. These components are as follows:
Development of a pig that is healthy and whose kidneys have a favorable crossmatch for patients who are on the transplant waitlist. This pig exists because many groups have adopted the TKO pig which has the negative crossmatch of many waitlisted patients.7
Detailed histocompatibility testing to identify which patients have a chance to benefit from participating in a clinical trial from an immune risk point of view. Renal allotransplantation was severely hampered by early graft loss from AMR until it was understood what antigens were important, and methods were devised to screen potential recipients to determine which donor antigens to avoid. Because some HLA antibodies cross react with SLA and some patients with no HLA sensitization have anti-SLA antibodies, any group proposing to initiate a clinical trial should have very robust histocompatibility testing to minimize the risk of preventable failure for any patients entering into the trial. This is an area that will require intense investigation before clinical implementation to ensure that the initial patients chosen for clinical trials will benefit from renal xenotransplantation.
Consistent extended survival in a preclinical model using an immunosuppressive regimen with drugs that are either US Food and Drug Administration approved or have good safety and toxicity profiles in humans from clinical trials. The regimen should prolong survival with an acceptable infectious profile in recipients. The classes of drugs for use in such a regimen exist and have provided consistent (65% >1-year survival) in the rhesus kidney transplant model.30
The development of renal xenotransplantation has been long and difficult, but there is reason for great optimism with the development of genetic engineering tools and new anti-CD40/CD40L costimulatory blockade reagents. While there are sentiments that moving ahead with laboratory-based preclinical models is simply too hard, it is important to remember that developing xenotransplantation for clinical use was always going to be difficult.33,40 The preclinical models are not perfect, but adherence to principles gleaned from their data has moved transplantation forward for more than 60 years.41 If we continue to adhere to disciplined data-driven evaluation of each barrier encountered, then the quality of life for patients with ESKD will be immeasurably improved sooner rather than later.
Disclosures
None A.J. Tector reports the following: Ownership Interest: founder and equity shareholder of Makana Therapeutics. M. Tector reports the following: Ownership Interest: stock options in Makana Therapeutics. All remaining authors having nothing to disclose.
Funding
A.J. Tector was supported by National Institute of Allergy and Infectious Diseases, National Institutes of Health, grants U01AI126322 and 5U19AI142737.
Author Contributions
A.B. Adams, J.A. Tector, and M. Tector conceptualized the study, wrote the original draft, and reviewed and edited the manuscript; and J.A. Tector was responsible for formal analysis.
References
- 1.Betthauser J, Forsberg E, Augenstein M, et al. Production of cloned pigs from in vitro systems. Nat Biotechnol. 2000;18(10):1055-1059. doi: 10.1038/80242 [DOI] [PubMed] [Google Scholar]
- 2.Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816-821. doi: 10.1126/science.1225829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirk AD, Burkly LC, Batty DS, et al. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat Med. 1999;5(6):686-693. doi: 10.1038/9536 [DOI] [PubMed] [Google Scholar]
- 4.Byrne GW, Stalboerger PG, Davila E, et al. Proteomic identification of non-Gal antibody targets after pig-to-primate cardiac xenotransplantation. Xenotransplantation. 2008;15(4):268-276. doi: 10.1111/j.1399-3089.2008.00480.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper DKC. Modifying the sugar icing on the transplantation cake. Glycobiology. 2016;26(6):571-581. doi: 10.1093/glycob/cww028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Estrada JL, Martens G, Li P, et al. Evaluation of human and non‐human primate antibody binding to pig cells lacking GGTA 1/CMAH/β4Gal NT 2 genes. Xenotransplantation. 2015;22(3):194-202. doi: 10.1111/xen.12161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martens GR, Reyes LM, Sidner RA, et al. Humoral reactivity of renal transplant-waitlisted patients to cells from GGTA1/CMAH/B4GalNT2, and SLA class I knockout pigs. Transplantation. 2017;101(4):e86-e92. doi: 10.1097/tp.0000000000001646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammer SE, Ho CS, Ando A, et al. Importance of the major histocompatibility complex (swine leukocyte antigen) in swine health and biomedical research. Annu Rev Anim Biosci. 2020;8(1):171-198. doi: 10.1146/annurev-animal-020518-115014 [DOI] [PubMed] [Google Scholar]
- 9.Ladowski JM, Martens GR, Reyes LM, et al. Examining the Biosynthesis and Xenoantigenicity of class II swine leukocyte antigen proteins. J Immunol. 2018;200(8):2957-2964. doi: 10.4049/jimmunol.1800022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ladowski JM, Reyes LM, Martens GR, et al. Swine leukocyte antigen class II is a xenoantigen. Transplantation. 2018;102(2):249-254. doi: 10.1097/tp.0000000000001924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martens GR, Ladowski JM, Estrada J, et al. HLA class I-sensitized renal transplant patients have antibody binding to SLA class I epitopes. Transplantation. 2019;103(8):1620-1629. doi: 10.1097/tp.0000000000002739 [DOI] [PubMed] [Google Scholar]
- 12.Mulder A, Kardol MJ, Arn JS, et al. Human monoclonal HLA antibodies reveal interspecies crossreactive swine MHC class I epitopes relevant for xenotransplantation. Mol Immunol. 2010;47(4):809-815. doi: 10.1016/j.molimm.2009.10.004 [DOI] [PubMed] [Google Scholar]
- 13.Gao Q, Davis R, Fitch Z, et al. Anti-thymoglobulin induction improves neonatal porcine xenoislet engraftment and survival. Xenotransplantation. 2021;28(6):e12713. doi: 10.1111/xen.12713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ladowski JM, Martens GR, Reyes LM, Hauptfeld-Dolejsek V, Tector M, Tector J. Examining epitope mutagenesis as a strategy to reduce and eliminate human antibody binding to class II swine leukocyte antigens. Immunogenetics. 2019;71(7):479-487. doi: 10.1007/s00251-019-01123-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reyes LM, Blosser RJ, Smith RF, et al. Characterization of swine leucocyte antigen alleles in a crossbred pig to be used in xenotransplant studies. Tissue Antigens. 2014;84(5):484-488. doi: 10.1111/tan.12430 [DOI] [PubMed] [Google Scholar]
- 16.Reyes LM, Estrada JL, Wang ZY, et al. Creating class I MHC-null pigs using guide RNA and the Cas9 endonuclease. J Immunol. 2014;193(11):5751-5757. doi: 10.4049/jimmunol.1402059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cowan PJ, Aminian A, Barlow H, et al. Renal xenografts from triple-transgenic pigs are not hyperacutely rejected but cause coagulopathy in non-immunosuppressed baboons. Transplantation. 2000;69(12):2504-2515. doi: 10.1097/00007890-200006270-00008 [DOI] [PubMed] [Google Scholar]
- 18.Paul Morgan B, Berg CW, Harris CL. "Homologous restriction" in complement lysis: roles of membrane complement regulators. Xenotransplantation. 2005;12(4):258-265. doi: 10.1111/j.1399-3089.2005.00237.x [DOI] [PubMed] [Google Scholar]
- 19.Hanna SM, Williams GT, Van Den Berg CW, Morgan BP. Characterization in vitro and in vivo of the pig analogue of human CD59 using new monoclonal antibodies. Immunology. 1998;95(3):450-459. doi: 10.1046/j.1365-2567.1998.00623.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinchliffe SJ, Rushmere NK, Hanna SM, Morgan BP. Molecular cloning and functional characterization of the pig analogue of CD59: relevance to xenotransplantation. J Immunol. 1998;160(8):3924-3932. doi: 10.4049/jimmunol.160.8.3924 [DOI] [PubMed] [Google Scholar]
- 21.Morgan BP. Complement regulatory molecules: application to therapy and transplantation. Immunol Today. 1995;16(6):257-259. doi: 10.1016/0167-5699(95)80175-8 [DOI] [PubMed] [Google Scholar]
- 22.Perez de la Lastra JM, Hanna SM, Morgan BP. Distribution of membrane cofactor protein (MCP/CD46) on pig tissues. Relevance to xenotransplantation. Immunology. 1999;98(1):144-151. doi: 10.1046/j.1365-2567.1999.00830.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van den Berg CW, Rix C, Hanna SM, Perez de la Lastra JM, Morgan BP. Role and regulation of pig CD59 and membrane cofactor protein/CD46 expressed on pig aortic endothelial cells. Transplantation. 2000;70(4):667-673. doi: 10.1097/00007890-200008270-00022 [DOI] [PubMed] [Google Scholar]
- 24.Crikis S, Cowan PJ, d'Apice AJF. Intravascular thrombosis in discordant xenotransplantation. Transplantation. 2006;82(9):1119-1123. doi: 10.1097/01.tp.0000238721.88920.ee [DOI] [PubMed] [Google Scholar]
- 25.Iwase H, Liu H, Wijkstrom M, et al. Pig kidney graft survival in a baboon for 136 days: longest life-supporting organ graft survival to date. Xenotransplantation. 2015;22(4):302-309. doi: 10.1111/xen.12174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yue Y, Xu W, Kan Y, et al. Extensive germline genome engineering in pigs. Nat Biomed Eng. 2020;5(2):134-143. doi: 10.1038/s41551-020-00613-9 [DOI] [PubMed] [Google Scholar]
- 27.Adams AB, Kim SC, Martens GR, et al. Xenoantigen deletion and chemical immunosuppression can prolong renal xenograft survival. Ann Surg. 2018;268(4):564-573. doi: 10.1097/sla.0000000000002977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cui Y, Yamamoto T, Raza SS, et al. Evidence for GTKO/β4GalNT2KO pigs as the preferred organ-source for old world nonhuman primates as a preclinical model of xenotransplantation. Transplant Direct. 2020;6(8):e590. doi: 10.1097/txd.0000000000001038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen G, Qian H, Starzl T, et al. Acute rejection is associated with antibodies to non-Gal antigens in baboons using Gal-knockout pig kidneys. Nat Med. 2005;11(12):1295-1298. doi: 10.1038/nm1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adams AB, Lovasik BP, Faber DA, et al. Anti-C5 antibody tesidolumab reduces early antibody-mediated rejection and prolongs survival in renal xenotransplantation. Ann Surg. 2021;274(3):473-480. doi: 10.1097/sla.0000000000004996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma D, Hirose T, Lassiter G, et al. Kidney transplantation from triple-knockout pigs expressing multiple human proteins in cynomolgus macaques. Am J Transplant. 2022;22(1):46-57. doi: 10.1111/ajt.16780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamoto T, Iwase H, Patel D, et al. Old World Monkeys are less than ideal transplantation models for testing pig organs lacking three carbohydrate antigens (Triple-Knockout). Sci Rep. 2020;10(1):9771. doi: 10.1038/s41598-020-66311-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooper DKC. Advancing xenotransplantation to the clinic: How relevant is the pig-to-nonhuman primate kidney transplantation model Today? Transplantation. 2022;106(9):1717-1719. doi: 10.1097/tp.0000000000004097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higginbotham L, Mathews D, Breeden CA, et al. Pre-transplant antibody screening and anti-CD154 costimulation blockade promote long-term xenograft survival in a pig-to-primate kidney transplant model. Xenotransplantation. 2015;22(3):221-230. doi: 10.1111/xen.12166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim SC, Mathews DV, Breeden CP, et al. Long-term survival of pig-to-rhesus macaque renal xenografts is dependent on CD4 T cell depletion. Am J Transplant. 2019;19(8):2174-2185. doi: 10.1111/ajt.15329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tasaki M, Shimizu A, Hanekamp I, Torabi R, Villani V, Yamada K. Rituximab treatment prevents the early development of proteinuria following pig-to-baboon xeno-kidney transplantation. J Am Soc Nephrol. 2014;25(4):737-744. doi: 10.1681/ASN.2013040363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pentz RD, Cohen CB, Wicclair M, DeVita MA, Flamm AL, Youngner SJ, et al. Ethics guidelines for research with the recently dead. Nat Med. 2005;11(11):1145-1149. doi: 10.1038/nm1105-1145 [DOI] [PubMed] [Google Scholar]
- 38.Montgomery RA, Stern JM, Lonze BE, et al. Results of two cases of pig-to-human kidney xenotransplantation. N Engl J Med. 2022;386(20):1889-1898. doi: 10.1056/nejmoa2120238 [DOI] [PubMed] [Google Scholar]
- 39.Porrett PM, Orandi BJ, Kumar V, et al. First clinical-grade porcine kidney xenotransplant using a human decedent model. Am J Transplant. 2022;22(4):1037-1053. doi: 10.1111/ajt.16930 [DOI] [PubMed] [Google Scholar]
- 40.Cooper DK, Hara H. “You cannot stay in the laboratory forever”*: taking pig kidney xenotransplantation from the laboratory to the clinic. EBioMedicine. 2021;71:103562. doi: 10.1016/j.ebiom.2021.103562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knechtle SJ, Shaw JM, Hering BJ, Kraemer K, Madsen JC. Translational impact of NIH-funded nonhuman primate research in transplantation. Sci Transl Med. 2019;11(500):eaau0143. doi: 10.1126/scitranslmed.aau0143 [DOI] [PMC free article] [PubMed] [Google Scholar]