Abstract
Onconephrology is an upcoming and expanding subspecialty that deals with the intersections between hematology/oncology and nephrology. With the paradigm shift in the understanding of cancer immunobiology and mechanisms of oncotherapeutic drug toxicities, it is important for a nephrologist to have a sound understanding of this field. Over the last 5 years, there have been immense developments in our understanding of kidney-related adverse events from various targeted, immuno- and cellular-based therapies. Pathogenic mechanisms of electrolyte imbalance, hypertension (oncohypertension), and AKI from multiple forms of cancer therapies have been explored. Significant research has also been conducted in the field of transplant onconephrology. In this review, we have tried to assimilate the most recent updates in the last 2 years in this ever-growing and fascinating field.
Keywords: Acute kidney injury and ICU nephrology, AKI, amyloidosis, cancer, chemotherapy, immunotherapy, myeloma, oncohypertension, onconephrology, paraproteinemia, stem cell transplant, transplant
Introduction
Advances in hematology-oncology have led to improved long-term outcomes; however, it has become more important to understand the short- and long-term complications of cancer and antineoplastic therapies in the survivor. Cancer survivorship has led to increased CKD and hypertension (HTN). This has paved a pathway for a new subspecialty of onconephrology. In this review, we shall update our readers on the recent studies and trials in various fields of onconephrology: AKI, electrolyte disorders, oncohypertension, glomerular disease, stem cell transplantation, paraprotein and amyloidosis, and transplant onconephrology.
AKI Update
With the advances in anticancer treatment, such as new immunotherapies and other targeted therapies, more patients with cancer are being evaluated in the nephrology services. Nephrologists caring for cancer patients need to be updated on the kidney effects of anticancer treatments and how to manage them, with the ultimate goal of avoiding worsening kidney dysfunction and development of CKD. Classical patterns of AKI seen in these patients are acute tubular injury, tubulointerstitial nephritis, and vascular injury. Several glomerulopathies have also been described. Here, we provide an update on AKI from anticancer therapies.
Immunotherapy-Associated AKI
Immune checkpoint inhibitors (ICI) have dramatically strengthened the treatment of cancer. They work by directly inhibiting immune checkpoints allowing T cells to remain in an active form and eliminate cancer cells. However, many immune-related adverse events have been described with these medications,1 including ICI-associated AKI. Most kidney immune-related adverse events are due to acute interstitial nephritis (AIN).2
A large multicenter retrospective study collected over 400 cases of patients with AKI from immune checkpoint inhibitors.3 These were compared with controls who received ICIs but did not develop AKI. ICI-AKI occurred at 16 weeks (interquartile range [IQR], 8-32). The use of proton pump inhibitor therapy, underlying low eGFR, and presence of extra renal immune-related adverse events were risk factors. AIN was the most common kidney biopsy finding in 83% of patients. Treatment of ICI-AKI with steroids within 14 days after the diagnosis was associated with a higher odd of recovery, with early initiation favoring renal recovery. Of the 121 patients who were rechallenged, 16.5% developed recurrent ICI-AKI. There was no difference in survival among patients rechallenged versus those who were not following ICI-AKI.
A recent meta-analysis examined the effects of AKI on outcomes in patients being treated with ICIs. This meta-analysis included seven studies with a total of 3767 patients demonstrating that ICI-associated AKI is of mild-to-moderate severity in most cases although incidence up to 43% for stage 2 AKI and up to 57% incidence for stage 3 AKI were reported.4 Kidney biopsy was performed in 43% of patients, where the most frequent finding was of tubulointerstitial disease. The presence and severity of AKI have an impact on all-cause mortality, and the absence of kidney recovery was noted to be a predictor of increased mortality. Although these results are not definitive, they emphasize the need for clear management guidelines for AKI during the course of ICI therapy. Moreover, early recognition and proper management may improve the clinical outcome. The duration of steroid therapy has not been well defined. Single-center and multicenter data suggest that a shorter course of steroids with a quick taper is equivalent to a longer course for the treatment of ICI-induced AIN.5,6
The addition of ICIs to standard chemotherapy has improved progression-free and overall survival in patients with metastatic nonsquamous non–small cell lung cancer regardless of programmed death–ligand 1 expression.7 There has been concern for an increased risk of AKI in patients receiving combination therapy such as the triple therapy with pembrolizumab, pemetrexed, and platinum-based chemotherapy. Real-world data published recently showed that both groups (monotherapy and triple therapy) had a similar distribution of AKI severity. However, AKI occurred early in the triple therapy group (105 versus 202 days). The kidney biopsy findings were more suggestive of tubular and interstitial damage than AIN alone.8 Finally, several studies now confirmed the safe use of ICI in patients with ESRD because they had similar incidence of immune-related adverse events compared with the general population. This is likely due to the main mechanism of ICI clearance, through proteolytic catabolism and receptor-mediated endocytosis.9–11
Ideally, a noninvasive test to predict a kidney-related adverse immune event would be an invaluable resource in these patients. A recent novel study by Isik et al. reviewed the role of commonly available laboratory tests, such as C-reactive protein and urine retinol-binding protein, in predicting ICI related-AKI.12 Elevated C-reactive protein with an elevated urine retinol-binding protein/creatinine were helpful in identifying patients with ICI-related AKI. A more recent preprint study showed that soluble interleukin-2 receptor level in peripheral blood was significantly higher in patients with ICI-related nephritis compared with ICI-treated controls and hemodynamic AKI controls.13 A soluble interleukin-2 receptor cutoff point of 1.75-fold upper limit of normal was highly diagnostic of ICI nephritis (area under the curve [AUC] >96%) compared with either ICI-treated or hemodynamic AKI controls. By peripheral blood flow cytometry analysis, lower absolute CD8+ T cells, CD45RA+CD8+ T cells, memory CD27+ B cells, and expansion of plasmablasts were prominent features of ICI nephritis compared with ICI-treated controls. Another emerging biomarker is urine TNF-α as its level was significantly elevated in ICI nephritis compared with other causes of AKI.14 TNF-α in conjunction with specific T cell responses contribute to AKI-ICI injury and could potentially serve as targets for therapeutic intervention as well as potential biomarkers.
Imaging biomarkers using computerized tomography and positron emission tomography have also been investigated recently in a larger cohort at a single center. Bilateral increase in kidney size, new/increasing perinephric stranding, and bilateral wedge-shaped hypoenhancing cortical foci can occur in ICI-related nephritis. On positron emission tomography-computerized tomography, a diffuse increase in radiotracer uptake throughout the renal cortex and a decrease in radiotracer activity in the renal pelvis can be seen.15
Pseudo-AKI from Novel Molecularly Targeted Agents
Three classes of agents, namely mesenchymal-epithelial transition tyrosine kinase inhibitors, inhibitors of polyadenosine diphosphate ribose polymerase, and the cyclin-dependent kinase 4 and 6 inhibitors have been newly associated with pseudo-AKI (an asymptomatic rise in serum creatinine [SCr]).16 The underlying mechanisms of pseudo-AKI are associated with inhibition of renal transporters such as organic cation transporter-2, multidrug and toxin extrusion-1, and multidrug and toxin extrusion2-K17 leading to a reversible increase in SCr.18–21 However, kidney biopsy–proven cases of acute tubular injury have also been reported with cyclin-dependent kinase 4 and 6 inhibitors recently.22
Differentiating “pseudo-AKI” from true AKI can be challenging. Simultaneous measurement of SCr, kidney iothalamate clearance, and/or eGFR based on cystatin C level may help distinguish between the two entities.23 Table 1 summarizes updated kidney toxicities with conventional and new anticancer agents.
Table 1.
Summarized kidney adverse effects from anticancer therapies
| Drug Class | Drug Name | Mechanism of Injury | Kidney Effect |
|---|---|---|---|
| Conventional chemotherapy | |||
| Platinum-based | Cisplatin, carboplatin, oxaliplatin. | Platinum-DNA adducts mediate arrest of cell cycle, initiate apoptosis. ATP depletion. | ATI, renal magnesium wasting, proximal tubulopathy, NDI |
| Antimetabolite | Methotrexate | Intratubular crystal formation. Afferent arteriolar constriction | Crystal nephropathy, ATI |
| Gemcitabine | Endothelial injury | TMA, HTN | |
| Pemetrexed | Unknown | ATI, chronic interstitial fibrosis, proximal tubulopathy, NDI | |
| Alkylating agent | Cyclophosphamide | Toxic metabolite, acrolein | Hemorrhagic cystitis |
| Ifosfamide | Toxic metabolite, chloroacetaldehyde | ATI, proximal tubulopathy, NDI | |
| Melphalan | Increase ADH release | SIADH | |
| Nitrosoureas | Alkylation of tubular cell proteins | Chronic interstitial nephritis | |
| Antitumor antibiotics | Mitomycin C | Endothelial injury | TMA, HTN |
| Targeted therapy | |||
| VEGF inhibitors | Bevacizumab | Endothelial injury | TMA, HTN, ATI |
| Tyrosine kinase inhibitors | Sorafenib, sunitinib, axitinib, pazopanib, lenvatinib. | Endothelial injury, podocyte injury. | ATIN, ATI, TMA, FSGS |
| BCR-ABL tyrosine kinase inhibitor | Imatinib, dasatinib. | Tubular injury, endothelial injury | ATI, TMA |
| BRAF inhibitors | Vemurafenib, dabrafenib | Tubular injury, ERK activation | ATIN, ATI |
| BCL-2 inhibitors | Venetoclax | Tubular injury | AKI, TLS |
| ALK inhibitors | Crizotinib, lorlatinib, alectinib | Inhibition of creatinine secretion, renal arteriolar myocyte vacuolization | Pseudo-AKI, ATIN, ATI, kidney cyst, podocytopathies |
| CDK4/6 inhibitors | Palbociclib, ribociclib | Inhibit MATE1 and MATE2 transporters | Pseudo-AKI, ATI |
| PARP inhibitors | Olaparib, talazoparib | Inhibition of creatinine secretion | Pseudo-AKI |
| MET tyrosine kinase | Capmatinib, tepotinib | Inhibition of creatinine | Pseudo-AKI |
| EFGR monoclonal antibodies | Cetuximab, panitumumab | Inhibition of EGFR signaling at the DCT | Renal magnesium wasting |
| mTOR inhibitors | Everolimus | Decrease cubilin and megalin, VEGF inhibition | ATI, podocytopathies |
| Protease inhibitors | Bortezomib, carfilzomib | Endothelial injury, autoantibody formation | TMA, HTN |
| BTK inhibitors | Ibrutinib | Endothelial injury | ATI, HTN |
| XPO inhibitor | Selinexor | Volume depletion | Hemodynamic AKI, hyponatremia |
| Immunotherapies | |||
| CAR-T therapy | Axicabtagene ciloleucel, idecabtagene vicleucel, brexucabtagene autoleucel, tisagenlecleucel. | Systemic hyperinflammatory state. Ischemic injury | ATI, hemodynamic mediated AKI |
| CTLA-4 inhibitor | Ipilimumab, tremelimumab | Tubular injury, endothelial injury, podocyte injury | ATIN, ATI, MCD, lupus-like GN, necrotizing GN, TMA |
| PD-1 inhibitors | Pembrolizumab, cemiplimab, nivolumab | Tubular injury, endothelial injury, podocyte injury | ATIN, ATI, MCD, IgA nephropathy, FSGS, necrotizing GN, amyloidosis, immune complex–mediated GN |
| PDL-1 inhibitor | Atezolizumab, avelumab, durvalumab | Tubular injury | ATIN, ATI |
ATP, adenosine 5'-triphosphate; ATI, acute tubular injury; NDI, nephrogenic diabetes insipidus; TMA, thrombotic microangiopathy; HTN, hypertension; BTK, Bruton tyrosine kinase; SIADH, syndrome of inappropriate antidiuretic hormone secretion; VEGF, vascular endothelial growth factor; ATIN, acute tubulointerstitial nephritis; FSGS, focal segmental glomerulosclerosis; BCR-ABL, breakpoint cluster region-tyrosine protein kinase ABL-1 gene; BRAF, proto-oncogene B-Raf; BCL-2, B cell lymphoma 2 gene; TLS, tumor lysis syndrome; ALK, anaplastic lymphoma kinase; CDK4/6, cyclin-dependent kinase 4/6; MATE, multidrug and toxin extrusion; PARP, poly-ADP-ribose polymerase; MET, mesenchymal-epithelial transition; EFGR, epidermal growth factor receptor; DCT, distal convoluted tubule; mTOR, mammalian target of rapamycin; XPO, export protein exportin 1; CAR-T, chimeric antigen receptor T cells; CTLA-4, cytotoxic T-lymphocyte–associated protein 4; MCD, minimal change disease; PD-1, programmed cell death protein 1; FSGS, focal segmental glomerulosclerosis; PDL-1, programmed death-ligand 1 ERK, extracellular signal-regulated kinase.
Novel Mechanisms of Tubular Injury for Chemotherapy
Two studies highlight the emerging role of heme and heme metabolism in AKI from two chemotherapy agents. Bai et al. analyzed whether the B-Raf proto-oncogene (BRAF) inhibitor vemurafenib directly causes renal tubular epithelial cell (RTEC) injury in vitro.24 In comparison with the control mice, proximal tubule–specific BRAF knockout mice showed no significant changes in blood urea and creatinine levels or histology before and after vemurafenib administration, indicating the possibility of a BRAF-independent mechanism in vemurafenib-induced nephrotoxicity. Previous chemical proteomic studies suggested that vemurafenib, but not other BRAF inhibitors, inhibits ferrochelatase (FECH).25 FECH is a mitochondrial protein that inserts ferrous ions into a precursor protoporphyrin IX to form protoheme (heme b), which is the final step of heme synthesis.26 The authors found a significant reduction in FECH activity and heme levels and mitochondrial dysfunction in vemurafenib-treated RTECs. In vivo analysis showed that FECH was more abundantly expressed in RTECs than in other renal cells in the normal condition and that the activity of FECH heme levels was downregulated and caspase activity was upregulated in RTECs after vemurafenib treatment. On the basis of these data, they put forth that vemurafenib-induced RTEC injury can be mediated by FECH inhibition and subsequent heme depletion. Furthermore, the in vivo knockdown of FECH did not influence normal renal function but hastened vemurafenib-induced nephrotoxicity. These data suggest that vemurafenib-induced nephrotoxicity is BRAF-independent and can, in part, be explained by reduced FECH activity. Hemopexin, a heme-scavenging protein, accumulates in the kidneys during AKI. In a recent study, the authors found an accumulation of hemoglobin and hemopexin in the kidneys localized to the proximal tubules in mice with cisplatin toxicity.27 There was increased kidney expression of kidney injury molecule-1 and heme oxygenase-1 (an indicator of oxidative stress) in hemopexin wild type compared with knockout mice in both models of AKI. Coincubation of hemopexin with hemoglobin resulted in hemoglobin deposition and exaggerated hemoglobin-induced injury. Deferoxamine, an iron chelator, and ferrostatin-1, a ferroptosis inhibitor, inhibited this deleterious effect of hemoglobin and hemopexin in proximal tubular cells, implicating iron toxicity in the mechanism of hemopexin-mediated injury in cisplatin mediated AKI. Furthermore, the protective effect of deferoxamine in cisplatin-induced AKI was apparent in hemopexin wild type, but not in hemopexin knockout mice.
Electrolyte Disorders Update
Immunotherapy-Associated Electrolyte Disorders
Immune checkpoint inhibitors therapy can lead to hyponatremia by several mechanisms, with the syndrome of inappropriate antidiuresis being the most common. Endocrine causes of hyponatremia are rare.28 Hypokalemia is also common and is associated with both proximal and distal renal tubular acidosis. Hypercalcemia associated with immune checkpoint inhibitors has led to some interesting observations, including immune checkpoint inhibitor–induced parathyroid hormone–related peptide production, sarcoid-like granulomas, and hyperprogression of the disease.29 Hypocalcemia and hyperphosphatemia may be seen with immune checkpoint inhibitors–induced tumor lysis syndrome (TLS). Chimeric antigen receptor T cell therapy–associated electrolyte disorders are also common. This is associated chiefly with hyponatremia, although other electrolyte abnormalities can occur. Table 2 summarizes all novel immunotherapy-associated electrolyte disturbances recently noted in case series and case reports.28,30
Table 2.
Cancer immunotherapy–associated electrolyte disorders
| Electrolyte | Disorder | Cancer Immunotherapy | Mechanism |
|---|---|---|---|
| Sodium | Hyponatremia | Immune checkpoint inhibitors | Hypophysitis |
| Adrenalitis | |||
| Thyroiditis | |||
| SIADH | |||
| CAR-T cell therapy | CRS | ||
| Hypovolemia | |||
| Potassium | Hypokalemia | Immune checkpoint inhibitors | GI losses (Gastritis, Colitis) |
| Distal and proximal RTA | |||
| CAR-T cell therapy | Renal tubular defect | ||
| Calcium | Hypocalcemia | Immune checkpoint inhibitors | Autoimmune hypoparathyroidism |
| TLS | |||
| CAR-T cell therapy | TLS | ||
| Hypercalcemia | Immune checkpoint inhibitors | Hypophysitis | |
| Thyroid disorders | |||
| ICI-related PTHrP | |||
| Hyperprogression of disease | |||
| Sarcoid-like granulomas | |||
| Phosphorous | Hypophosphatemia | Immune checkpoint inhibitors | Proximal tubulopathy |
| GI losses | |||
| CAR-T cell therapy | Unknown (hypotheses GI or kidney losses) | ||
| Hyperphosphatemia | Immune checkpoint inhibitors | TLS | |
| CAR-T cell therapy | TLS | ||
| Magnesium | Hypomagnesemia | Immune checkpoint inhibitors | GI losses, inflammatory diarrhea. |
SIADH, syndrome of inappropriate antidiuretic hormone; CAR-T, chimeric antigen receptor T cells; CRS, cytokine release syndrome; GI, gastrointestinal; RTA, renal tubular acidosis; TLS, tumor lysis syndrome; ICI, immune checkpoint inhibitor; PTHrP, parathyroid-related peptide.
TLS
TLS is an oncological emergency. It occurs secondary to anticancer therapies in patients with high tumor burden or spontaneously. Lysis of tumor cells leads to the release of intracellular ions and metabolites into the systemic circulation, precipitating hyperkalemia, hyperphosphatemia, hypocalcemia, and hyperuricemia. While most of the literature supported a crystalopathy-related AKI as the pathomechanism, a recent study postulated a novel pathophysiologic model through which AKI is associated with endothelial dysfunction from high levels of extracellular histones.31 This animal study proved that extracellular histones are released in vast amounts during TLS, causing a profound endothelial injury in the mouse model. The mechanisms of histone-mediated damage implicate endothelial cell activation mediated by Toll-like receptor 4. In addition, TLS has now been associated with novel agents such as the B cell lymphoma 2 inhibitor venetoclax, the monoclonal antibody obinutuzumab,32,33 and even immune checkpoint inhibitors. Although, data on such associations are related to case reports and case series.28,32,33
Hyponatremia
Selinexor is a selective inhibitor of the nuclear export protein exportin 1; it is approved in combination with dexamethasone for the treatment of multiple relapsed or refractory multiple myeloma and for relapsed/refractory diffuse large B cell lymphoma. Selinexor can cause hyponatremia with incidence ranges from 7% to 26%.34 It is a common grade 3 or 4 adverse event (serum sodium level, <130 mmol/L) in 22% of the patients.35 The mechanism of hyponatremia is not yet elucidated. The observed hyponatremia is likely related to multiple factors, comorbidities, other medications, and selinexor side effects non–kidney-related; nausea and volume depletion, and possible unidentified kidney factor.36
Immune checkpoint inhibitors and chimeric antigen receptor T cell therapy have also been associated with hyponatremia (Table 2).37 While initially believed to be related to endocrinopathies, syndrome of inappropriate antidiuresis is the most common mechanism for hyponatremia.38,39
Magnesium Disorders in Patients with Cancer
Hypomagnesemia is another crucial electrolyte disorder in patients with cancer, and the causes may differ among patients depending on nutritional status and specific cancer therapies. This review will focus on two significant cancer therapies: platinum-based chemotherapy and cetuximab, an epidermal growth factor receptor monoclonal antibody, induced hypomagnesemia.
Platinum-based chemotherapy, in particular cisplatin, is associated with hypomagnesemia. The main mechanism of hypomagnesemia in these patients is renal magnesium wasting due to direct toxicity from an intracellular accumulation of cisplatin, ultimately causing tubular cell injury.40 Cetuximab-induced hypomagnesemia is also secondary to primary renal magnesium wasting, mechanistically from an inhibition of the basolateral epidermal growth factor receptor, which prevents the transcellular magnesium reabsorption through the transient receptor potential cation channel subfamily M member 6 (TRPM6) Mg channels.41 Sodium glucose transporter 2 inhibitors have been used in patients with refractory hypomagnesemia above and beyond the magnesium replenishment.42,43 A meta-analysis showed increased serum Mg levels in patients by 0.15–0.24 mg/dl, but the exact mechanism of this effect is unknown.44 A plausible explanation of improvement in Mg2+ levels is by increased Mg2+ absorption in the intestine or reabsorption in the kidney, possibly by enhancing TRPM6-mediated transport in the intestine and/or the kidney.45
Oncohypertension Update
The topic of HTN in patients with cancer or “oncohypertension” has gained increasing attention over the past years.46 Interestingly, the relationship has been portrayed as bidirectional. HTN has been noticed with several classes of drugs, including conventional agents such as cisplatin.47,48 The topic gained much attention with the introduction of more contemporary targeted therapies such as vascular endothelial growth factor (VEGF) inhibitors.49 This section focuses on the newest anticancer agents inducing HTN.
VEGF Signaling Pathway Inhibitors–Induced HTN
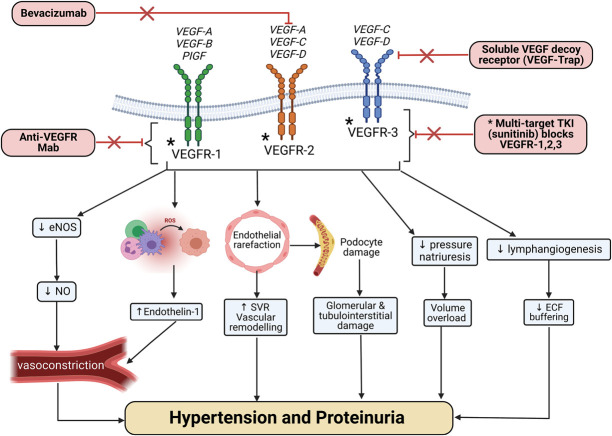
VEGF signaling pathway inhibitors are the prototype of anticancer drug-induced HTN (Figure 1), observed in 20%–90% depending on VEGF signaling pathway inhibitors (VEGFi) type and BP definitions.49,50 Risk factors for the development of HTN include a previous history of HTN, ages 60 years or older, body mass index ≥25 kg/m2, concurrent use of more than one VEGFi medication, as well as tumor type such as renal cell carcinoma.49,51 Not much has been reported on the management of VEGFi-induced HTN in the last few years. A recent review summarizes these findings.52 Table 3 summarizes anti-VEGF signaling pathway agents kidney adverse events.49,53–57 The mechanism of VEGFi-induced HTN is shown in Figure 1.
Figure 1.
Mechanism of action of the different VEGF signaling pathway inhibitors agents and the pathophysiologic changes leading to hypertension and proteinuria. ECF, extracellular fluid; eNOS, endothelial nitric oxide synthase; Mab, monoclonal antibody; NO, nitric oxide; PlGF, placental growth factor; ROS, reactive oxygen species; SVR, systemic vascular resistance; TKI, tyrosine kinase inhibitor; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Table 3.
| Anti-VEGF Agents Subclasses | Drugs | Indications (Approved and Potential) | Proteinuria (Risk Factors: CKD, HTN, RCC) | Hypertension (Risk Factors: Older Than 60 Years, BMI>25 kg/m2, >1 Anti-VEGF Agent) |
|---|---|---|---|---|
| VEGF-Trap (fusion protein) | Alfibercept | Melanoma, prostate cancer, pancreatic cancer, non–small lung cancer | Incidence of 40%–60% | Incidence about 40% |
| VEGF-A monoclonal antibody | Bevacizumab | Glioblastoma, metastatic colorectal cancer, NSCLC, renal cell carcinoma | Incidence of 20%–60% | Incidence about 20% |
| VEGFR2 monoclonal antibody | Ramucirumab | Metastatic colorectal cancer, breast cancer, non–small lung cancer | Incidence about 10% | Incidence about 42% |
| Tyrosine kinase inhibitors | Axitinib | Renal cell carcinoma, thyroid cancer, hepatocellular cancer, gastrointestinal stromal tumors, pancreatic neuroendocrine tumors, soft tissue sarcomas, refractory chronic myelogenous leukemia, and refractory metastatic colorectal cancer | Incidence about 20% | Incidence about 40% |
| Cabozantinib | Incidence about 22% | Incidence about 30%–60% | ||
| Lenvatinib | Incidence about 11% | Incidence about 70% | ||
| Nintedanib | n/a | n/a | ||
| Pazopanib | Incidence about 13% | Incidence about 35% | ||
| Regorafinib | Incidence about 7% | Incidence about 28% | ||
| Sorafenib | Incidence about 11% | Incidence about 19% | ||
| Cabozantinib | Incidence about 8% | Incidence about 20% | ||
| Vandetanib | Incidence about 10% | Incidence about 30% | ||
| Apatinib | Incidence about 15% | Incidence about 9% |
VEGF, vascular endothelial growth factor; HTN, hypertension; RCC, renal cell carcinoma; BMI, body mass index; NSCLC, non–small cell lung cancer; VEGFR, vascular endothelial growth factor receptor; n/a, none available.
Bruton Tyrosine Kinase Inhibitors–Induced HTN
Bruton tyrosine kinase (BTK) inhibitors such as ibrutinib have revolutionized the management of chronic lymphocytic leukemia and mantle cell lymphoma. However, long-term treatment is associated with an increased risk of developing cardiovascular complications, including atrial fibrillation and HTN.58,59 In this systematic review with meta-analysis of randomized controlled trials, the relative risk of HTN with ibrutinib therapy was 2.82 (95% confidence interval [CI], 1.52 to 5.23),58,60 with a range of HTN incidence from 18% to 75%.58,61 The mechanisms of BTK inhibitor–induced HTN are unclear, but a decrease in heat shock protein 70 signaling pathways and inhibition of phosphatidylinositol 3-kinase–dependent and nitric oxide downregulation have been postulated.50 So far, data indicate a lower incidence of HTN with second-generation BTK inhibitors such as acalabrutinib.50,62
Phosphatidylinositol-3 Kinase Inhibitors–Induced HTN
Toxicities from small-molecule phosphatidylinositol-3 kinase (PI3K) inhibitors depend on their PI3K isozyme specificity. Copanlisib is a pan-PI3K with nonselective blockage of PI3K signaling approved for the third-line treatment of follicular non-Hodgkin lymphoma.63 HTN occurs primarily during infusion in almost 30% of the patients, and it is transient, resolving within 24 hours, and usually less prominent with subsequent cycles.63,64 The mechanism is likely related to targeting the PI3K-α isoform.65 PI3K-α is the isoform predominantly mutated in cancer, and studies have shown that selective inactivation of this isoform is enough to block PI3K/AKT signaling in response to different growth factors stimuli.66 Idelalisib and duvelisib target the PI3Kp110δ isoform and are also known to induce HTN.66,67
Immune Checkpoint Inhibitors and HTN
Recently, a systematic review and meta-analysis was performed by Minegishi et al. to look at the incidence of HTN from immune checkpoint inhibitors. Thirty-two randomized controlled trials with around 19,000 patients included in the meta-analysis did not show any increased association with short-term risk of HTN in patients with cancer and the association was similar regardless of concurrent treatment with other anticancer medications.68
Other Miscellaneous Anticancer Agents Associated with HTN
BRAF/mitogen-activated protein kinase kinase (MEK) inhibitors are used in the treatment of BRAF-mutant melanoma and colorectal cancer, with HTN being the most common cardiovascular adverse event reported with these drugs.69 HTN occurs in approximately 14% and 20% of patients treated with BRAF inhibitor monotherapy and BRAF/MEK inhibitors, respectively.69 The mechanism may be related to decrease of nitric oxide bioavailability, causing vasoconstriction and, consequently, HTN.69 Polyadenosine diphosphate ribose polymerase inhibitors have been used to treat ovarian cancer, and HTN has developed in 12%–20% versus 1%–6% of placebo.70,71 However, the mechanism leading to HTN is not entirely understood. The antiandrogens, abiraterone, and enzalutamide, used for prostate cancer, have been associated with the development of all-grade HTN in 26% and 11%, respectively.72 Abiraterone inhibits cytochrome P450 enzymes leading to the accumulation of mineralocorticoid precursors and contributing to its prohypertensive effects.72 However, the mechanisms underlying enzalutamide-induced HTN remain unclear. Aromatase inhibitors such as anastrozole used for estrogen-positive breast cancer have been associated with higher rates of HTN and cardiovascular events.73
Glomerular Diseases and Cancer Update
There is an established relationship between glomerular disease and cancer, associated with both solid and hematological malignancies, and during the course of cancer treatment. Diverse patterns of glomerular injury have been observed and described in the literature. In recent years, we have seen an increased discovery of many new antigens associated with membranous nephropathy (MN). This review aims to provide an update on MN and the new antigens associated with cancer.
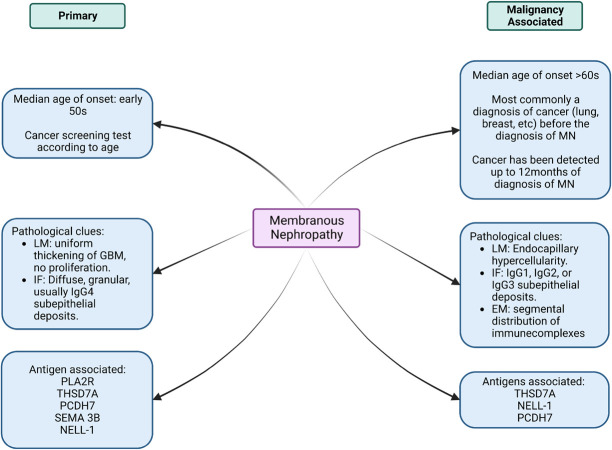
Thrombospondin type-1 domain-containing 7A (THSD7A) accounts for 1%–3% of MN cases in western countries.74 It is also overexpressed in certain malignancies such as gall bladder and endometrial cancers.75 Strong THSD7A staining has also been seen in prostate, breast, kidney, and colorectal cancers.76 Autoantibodies to neural epidermal growth factor–like 1 (NELL-1) protein is another recent addition to the list of antigens implicated in MN. NELL-1–associated MN was identified in approximately 16% of phospholipase A2 receptor (PLA2R)–negative MN cases without any identifiable secondary cause.77 The current literature suggests a connection between NELL-1–associated MN and malignancy.78 In addition, 21.4% of patients with protocadherin 7–associated MN had a malignancy.79 The precise pathology and antigens have not been clearly defined. Figure 2 summarizes the various ways one can differentiate primary versus malignancy-associated MN.
Figure 2.
A concept map of clinical and pathological clues to differentiate primary versus malignancy-associated membranous nephropathy. EM, electron microscopy; GBM, glomerular basement membrane; IF, immunofluorescence; LM, light microscopy; MN, membranous nephropathy; NELL-1, neural epidermal growth factor–like 1 protein; PCDH7, protocadherin 7; PLA2R, phospholipase A2 receptor; SEMA 3B, semaphorin-3B; THSD7A, thrombospondin type I domain–containing 7A.
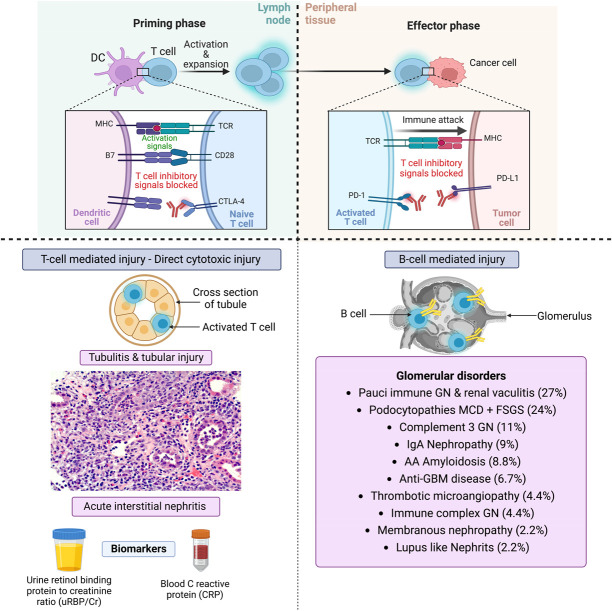
New glomerular diseases have now been reported in association with ICI as well. In an analysis of all published case reports and case series of glomerular pathology findings associated with immune checkpoint inhibitors, several lesion types were observed, with the most frequent being pauci-immune GN and renal vasculitis (27%), podocytopathies (24%), and complement 3 GN (C3GN; 11%).80 Since then, several other glomerular pathologies have been reported and observed (unpublished) in clinical practice such as MN, mesangioproliferative GN, and other podocytopathies (Figure 3).80–83 Treatment of glomerular diseases associated with ICI is challenging.
Figure 3.
The glomerular diseases seen with immune checkpoint inhibitors. CTLA-4, cytotoxic T lymphocyte–associated protein-4; DC, dendritic cell; MCD, minimal change disease; PD-1, programmed cell death protein-1; PD-L1, programmed cell death protein–associated ligand-1; TCR, T cell receptor.
Hematopoietic Stem Cell Transplantation–Related Renal Disease Update
Renal disease after hematopoietic stem cell transplantation (HSCT) includes AKI and CKD, both of which result in high morbidity and mortality.84 Below are two novel concepts noted in the literature on renal diseases associated with HSCT.
“Haplostorm”-Associated AKI
The use of haploidentical donors has expanded the number of patients eligible for this curative therapy. Patients who undergo T cell–replete peripheral blood haploidentical hematopoietic transplantation (Haplo-HSCT) have an additional risk for the development of AKI termed as “haplostorm-associated AKI.” Haplostorm is a cytokine release syndrome (CRS) seen within 14 days of transplantation. It is characterized by fever, rash, diarrhea, vascular leak, hypotension, and multisystem organ dysfunction, including AKI. The high incidence of CRS seen in haploidentical peripheral blood transplants compared with bone marrow transplants is due to the presence of eight-fold more lymphocytes in peripheral blood allografts.85
In a small retrospective study spanning 13 years of a total of 55 cases of confirmed haplostorm, seven were found to have haplostorm associated with AKI (13%). All these patients suffered from severe CRS characterized by hypotension with pressor support requirement and hypoxemia requiring supplemental oxygen.86 In another study by Imus et al.87 of a total of 146 patients who underwent Haplo-HSCT, severe CRS was seen in 25 patients (17%), of which 12 patients suffered severe AKI requiring hemodialysis. Management of these patients is challenging with a focus on supportive therapy, steroid use, and anti–IL-6 therapy, e.g., tocilizumab. Nephrologists need to be aware of this novel cause of AKI post-HSCT in the special situation of a haplo-identical HSCT.
Novel Antigens Associated with HSCT–Associated Nephrotic Syndrome
The development of glomerular diseases post-HSCT is considered by many to represent the kidney manifestation of graft versus host disease.88 The most common pathological lesion seen is MN, followed by minimal change disease. While PLA2R antigen–associated membranous GN has been reported in some HSCT recipients,89,90 most cases are PLA2R-negative, and the target antigen remains unknown. Recently, Nasr et al.91 reported five patients with MN and extensive tubular basement membrane deposits after allogeneic HSCT who presented with acute tubular injury and tubulointerstitial inflammation. Proteomic analysis was negative for routine antigens such as THSD7A, EX 1/2, NELL-1, and SEMA3B. Only one of five patients tested positive for PLA2R, while the identity of the target antigen in the remaining cases remained unknown. NELL-1–associated membranous GN after HSCT has also been described.92 A study published by Sethi et al.93 revealed a novel protein, protocadherin (FAT1), which was detected by microdissection and mass spectrometry in patients with HSCT-associated MN, all of whom were PLA2R antigen–negative. The FAT cadherins comprise four members, FAT1–FAT4, and all are large transmembrane proteins of 500–600 kD.94 The biological function of FAT cadherins is not well understood. FAT1 recessive mutations in humans have been associated with glomerulotubular nephropathy exhibiting extensive podocyte foot process effacement and resulting in steroid-resistant nephrotic syndrome.95 The authors postulate that a somatic/previously hidden FAT1 mutation may cause an immune response as a consequence of graft versus host disease in most of these cases.93,96
Paraproteinemia and Amyloidosis Update
Multiple Myeloma and Other Forms of Monoclonal Gammopathy of Renal Significance
The new International Kidney and Monoclonal Gammopathy Research Group consensus definition of monoclonal gammopathy of renal significance (MGRS) includes all B cell or plasma cell proliferative disorders that produce a nephrotoxic monoclonal immunoglobulin. A recent study demonstrated that 98% of patients with cast nephropathy (CN) diagnosis presented with serum-free light chains (sFLC) levels >500 mg/L.97 As per the International Myeloma Working Group, CN was characterized by the presence of histopathological features or the involved sFLC of more than 1500 mg/L.98 A recently published retrospective French study outlined a distinct subset of patients with MGRS who develop thrombotic microangiopathy. Prognostically, this is an important phenotype, with 70% of patients needing dialysis at the disease onset and median renal survival being 20 months.99 A Chinese single-center large cohort study100 analyzed kidney biopsy specimens of patients with MGRS who underwent a kidney biopsy (N=700). Thirty eight percent of patients had some form of MGRS lesions (63%—Ig-associated amyloidosis, 9% monoclonal immunoglobulin deposition disease, 8%—thrombotic microangiopathy). Membranous nephropathy (40%) was the most common non-MGRS lesion. The above recent studies expand the various known pathologies seen with MGRS.
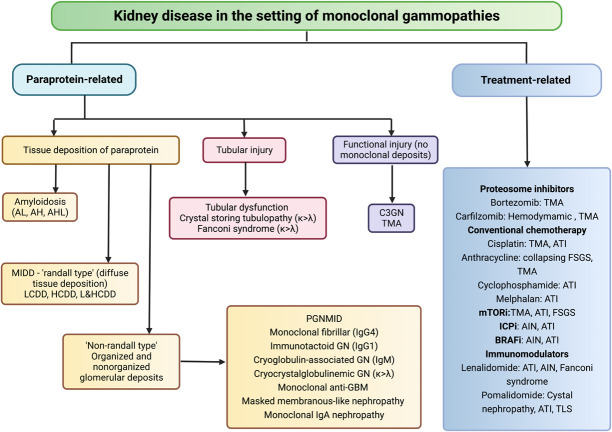
Proteasome inhibitor–based therapies have had a major impact on the survival of patients with both newly diagnosed and relapsed myeloma. A recently published multicenter trial101 compared double (bortezomib+dexamethasone [BD]) versus triple (cyclophosphamide+bortezomib+dexamethasone [C-BD]) regimens in patients with multiple myeloma (MM) and AKI from CN. The study did not show any benefit of C-BD compared with BD on kidney recovery of patients with CN not needing dialysis. The therapeutic drugs for MM and MGRS themselves are not devoid of kidney injury risk (Figure 4). The primary objective in the treatment of MGRS is preserving kidney function, and the treating physician should be mindful of the nephrotoxic effects of the various agents used (Figure 4). In a setting of plasma cell clones, the best results are achieved using proteasome inhibitor–based regimens.102,103 Other therapies are melphalan followed by autologous HSCT in patients with monoclonal Ig deposition disease,104 anti–CD-38 monoclonal antibody, daratumumab for proliferative GN with monoclonal deposits,105 and rituximab-based therapy for patients with B cell clone which express CD-20.102 Daratumumab may become standard therapy for several diseases with the MGRS phenotype based on its recent success in a pilot study for proliferative GN with monoclonal deposits.105
Figure 4.
A flowchart of the spectrum of renal diseases in monoclonal gammopathies, divided into paraprotein-related and treatment-related renal diseases. AH, amyloid heavy chain; AHL, amyloid light and heavy chain; AIN, acute interstitial nephritis; AL, amyloid light chain; ATI, acute tubular injury; BRAFi, v-raf murine sarcoma viral oncogene homolog B1 inhibitor; C3, complement 3; HCDD, heavy chain deposition disease; ICPi, immune checkpoint inhibitor; IgG 1&4, immunoglobulin G type 1&4; IgM, immunoglobulin M; LCDD, light chain deposition disease; LHCDD, light-heavy chain deposition disease; MIDD, monoclonal immunoglobulin deposition disease; mTORi, mammalian target of rapamycin inhibitor; PGMIDD, proliferative N with monoclonal IgG deposits; TMA, thrombotic microangiopathy; TLS, tumor lysis syndrome.
Therapies available for amyloid light chain (AL) amyloidosis are high-dose melphalan followed by autologous stem cell transplant, melphalan-dexamethasone combination, CyBorD (cyclophosphamide-bortezomib-dexamethasone), bortezomib-melphalan-dexamethasone combination, and daratumumab (anti CD-38 antibody).106 In a recent randomized controlled trial with newly diagnosed AL amyloidosis, the addition of daratumumab to CyBorD was associated with higher frequencies of hematological complete response and survival.107
Extracorporeal therapies used for the removal of sFLC are plasma exchange (PLEX) and high cutoff (HCO) dialysis. A Canadian study demonstrated no conclusive evidence of PLEX in reducing composite outcomes of death, dialysis dependence, or eGFR <30 ml/min per 1.73 m2 at 6 months.108 Multiple myeloma and renal failure due to myeloma cast nephropathy109 and the European Trial of Free Light Chain Removal by Extended Hemodialysis in Cast Nephropathy109,110 used HCO dialysis membranes to facilitate sFLC removal and study dialysis primary endpoint of independence at 3 months. Both trials did not demonstrate a significant difference between treatment and control arm. Based on the findings of the trials, the utility of HCO dialysis and PLEX for decreasing sFLC burden to improve kidney outcomes remains elusive and cannot be routinely recommended.
Transplant Onconephrology Update
The field of transplant onconephrology has now been increasingly recognized.111 Recent advancements in cancer treatment have changed the landscape of pretransplant and post-transplant management; however, there is an unmet clinical need to improve patient outcomes.
Immunotherapy in Kidney Transplant Recipients
A multicenter observational study investigated the efficacy and safety of ICIs in kidney transplant recipients.112 The estimated risk of acute rejection was 42%, with median ICI to rejection time being 24 days. The rejection risk was lower if the patients were on a higher number of immunosuppressants (e.g., 3 agent immunosuppression) and mammalian target of rapamycin inhibitors. In patients with advanced cutaneous squamous cell carcinoma (cSCC), the overall survival in those receiving ICIs was significantly longer than that of historical cohorts of advanced cSCC who did not receive ICIs. Another Phase 1 multicenter study (N=17) showed that ongoing immunosuppression along with nivolumab showed less chance of rejection and no change in response to the cancer.113 Both studies conclude that kidney transplant recipients can be given ICIs with ongoing immunosuppression to prevent rejection and no changes in cancer remission.
Several clinical trials are underway to investigate immunosuppression strategies in kidney transplant recipients. One approach is to continue tacrolimus (NCT03816332) for those with skin cancer (cSCC, melanoma, basal cell carcinoma, Merkel cell carcinoma) treated with ipilimumab and/or nivolumab. Another approach includes a combination of mammalian target of rapamycin inhibitor and dynamic steroid dosing for cSCC treated with cemiplimab (NCT04339062). In this study, the patients receive mini prednisone pulse synchronizing with the infusion, modified from a previously reported approach.114 However, acute rejection can still occur in patients on KRT with previously failed kidney transplants. In these cases, patients developed allograft pain and fever 2–4 weeks after the first cycle of ICI therapy, owing to allograft intolerance syndrome.9
Can a Patient with Myeloma and Light/Heavy Chain Amyloidosis Get a Kidney Transplant?
Patients with ESRD from MM have poor outcomes on dialysis. MM has traditionally been a contraindication to kidney transplantation, owing to reported high relapse rates and high risk of allograft loss.9,115 Data on outcomes of patients with MM and amyloidosis-induced ESRD getting a kidney transplant were limited to case reports and case series.116 A recent retrospective study of the United Network for Organ Sharing (UNOS)/Organ Procurement and Transplantation Network (OPTN) dataset (2006–2018) compared patient and graft outcomes of kidney transplant recipients with ESRD due to plasma cell dyscrasias (PCD) versus other causes.117 Among 168,369 first kidney transplants adult recipients, 0.22%–0.43% per year (a very small proportion) had PCD as the cause of ESRD. The PCD group had worse survival than the non-PCD group for both living and deceased donor types (adjusted hazard ratio [aHR], 2.24; 95% CI, 1.67 to 2.99 and 1.40; 95% CI, 1.08 to 1.8). The PCD group had worse survival than the diabetes group but only among living donor types (aHR, 1.87; 95% CI, 1.37 to 2.53 versus 1.16; 95% CI, 0.89 to 1.2). Graft survival in patients with the PCD was worse than non-PCD in both living and deceased donor types (aHR, 1.72; 95% CI, 1.91 to 2.56 versus 1.30; 95% CI, 1.03 to 1.66). Patient and graft survival were worse in amyloidosis but not statistically different in multiple myeloma, compared with the non-PCD group. The comparison of outcomes of patients with ESRD due to PCD in the context of other causes of ESRD will guide future eligibility criteria for patients with PCD to receive kidney transplantation, particularly at the level of transplant centers.
Another study looked at AL amyloidosis and kidney transplantation.118 This multicenter observational study from five countries includes 237 patients with AL amyloidosis who underwent renal transplantation between 1987 and 2020. With a median follow-up of 8.5 years, the median overall survival from renal transplantation was 8.6 years and was significantly longer in patients with complete and very good partial hematologic responses (CR+VGPR) compared with less than VGPR (9 versus 6.8 years; HR, 1.5; 95% CI, 1 to 2.1; P=0.04) at renal transplantation. Median graft survival was 7.8 years and was better in the CR+VGPR group (8.3 versus 5.7 years, HR, 1.4; 95% CI, 1 to 2; P=0.05). The frequency and time to amyloid recurrence in the graft were also lower (16% versus 37%, P=0.01) and longer (median time not achieved versus 10 years, P=0.001) in the CR+VGPR group. Comparing CR versus VGPR, there was no difference in overall or graft survival. Although 69 patients (29%) experienced hematologic relapse, the treatment effectively prevented graft loss in the majority (87%). Kidney transplantation in selected patients with AL amyloidosis is associated with extended overall and renal graft survival. Patients with hematologic CR or VGPR have the most favorable outcomes, and these patients should be considered for kidney transplantation. Similar studies in other forms of paraprotein-mediated diseases receiving more conventional novel treatments and then getting a kidney transplantation are required.
Since the inception of the field of onconephrology over a decade ago, original investigations related to many sections in this field have increased. In the last few years, we saw increasing recognition of novel agents causing pseudo-AKI. We learned about novel agents causing HTN and are moving the glomerular disease field forward with specific cancer markers for paraneoplastic MN. In addition, our understanding of immunotherapy-related AKI and risk factors has been supplemented with the new wealth of data. The field of transplant onconephrology has seen recent advancements in the treatment of patients on immunotherapy and how to navigate the challenges of a patient with paraproteinemia and ESRD now needing a kidney transplant. The field of onconephrology is currently ripe for more ongoing original investigations.
Disclosures
P. Gudsoorkar reports the following: Research Funding: Natera; Advisory or Leadership Role: Editorial Board member for Advances in Chronic Kidney Disease (ACKD) Journal; Medical Advisory Board (MAB) National Kidney Foundation—Northern Kentucky & Southern Ohio; Member of Education and Position statement committee of American Society of Onconephrology (ASON); and Other Interests or Relationships: National Kidney Foundation. K. Jhaveri reports the following: Employer: Northwell Health; Consultancy: Astex Pharmaceuticals; Natera; GSK, ChemoCentryx, and Chinook; George Clinical; Honoraria: Uptodate.com; American Society of Nephrology and International Society of Nephrology; Advisory or Leadership Role: American Journal of Kidney Diseases; Journal of Onconephrology; Clinical Kidney Journal; NDT; CJASN; Kidney International; EIC-ASN Kidney News; and Other Interests or Relationships: President of American Nephrologist of Indian Origin; co-President and Founder of American Society of Onconephrology. R. Wanchoo reports the following: Advisory or Leadership Role: Associate editor Journal of Onconephrology; Editorial board CKJ; Founding member of American Society of Onconephrology. S.M. Herrmann reports the following: Patents or Royalties: Pfizer, unrelated to the current research; Other Interests or Relationships: founding member of the American Society of Onconephrology. The remaining author has nothing to disclose.
Funding
None.
Author Contributions
M. Bonilla conceptualized the study, was responsible for formal analysis, and provided supervision; K.D. Jhaveri was responsible for data curation; M. Bonilla and K.D. Jhaveri were responsible for validation and visualization; M. Bonilla, P. Gudsoorkar, and K. Jhaveri wrote the original draft; and M. Bonilla, P. Gudsoorkar, S.M. Herrmann, K.D. Jhaveri, and R. Wanchoo reviewed and edited the manuscript.
References
- 1.Cortazar FB, Kibbelaar ZA, Glezerman IG, et al. Clinical features and outcomes of immune checkpoint inhibitor-associated AKI: a multicenter study. J Am Soc Nephrol. 2020;31(2):435-446. doi: 10.1681/ASN.2019070676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seethapathy H, Herrmann SM, Sise ME. Immune checkpoint inhibitors and kidney toxicity: advances in diagnosis and management. Kidney Med. 2021;3(6):1074-1081. doi: 10.1016/j.xkme.2021.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta S, Short SAP, Sise ME, et al. Acute kidney injury in patients treated with immune checkpoint inhibitors. J Immunother Cancer. 2021;9(10):e003467. doi: 10.1136/jitc-2021-003467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanbay M, Copur S, Siriopol D, et al. The association between acute kidney injury and outcomes in cancer patients receiving immune checkpoint inhibitor therapy: a systematic review and meta-analysis. Clin Kidney J. 2022:sfac194. doi: 10.1093/ckj/sfac194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee MD, Seethapathy H, Strohbehn IA, et al. Rapid corticosteroid taper versus standard of care for immune checkpoint inhibitor induced nephritis: a single-center retrospective cohort study. J Immunother Cancer. 2021;9(4):e002292. doi: 10.1136/jitc-2020-002292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta S, Garcia-Carro C, Prosek JM, et al. Shorter versus longer corticosteroid duration and recurrent immune checkpoint inhibitor-associated AKI. J Immunother Cancer. 2022;10(9):e005646. doi: 10.1136/jitc-2022-005646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078-2092. doi: 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- 8.Gupta S, Strohbehn IA, Wang Q, et al. Acute kidney injury in patients receiving pembrolizumab combination therapy versus pembrolizumab monotherapy for advanced lung cancer. Kidney Int. 2022;102(4):930-935. doi: 10.1016/j.kint.2022.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitchlu A, Jhaveri KD, Sprangers B, Yanagita M, Wanchoo R. Immune checkpoint inhibitor use in patients with end-stage kidney disease: an analysis of reported cases and literature review. Clin Kidney J. 2021;14(9):2012-2022. doi: 10.1093/ckj/sfab090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirsch JS, Wanchoo R, Ng JH, Khanin Y, Jhaveri KD. Use of immune checkpoint inhibitors in end stage kidney disease patients, single center experience and review of the literature. Kidney360. 2020;1(5):399-402. doi: 10.34067/KID.0000422020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centanni M, Moes DJAR, Trocóniz IF, Ciccolini J, van Hasselt JGC. Clinical pharmacokinetics and pharmacodynamics of immune checkpoint inhibitors. Clin Pharmacokinet. 2019;58(7):835-857. doi: 10.1007/s40262-019-00748-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isik B, Alexander MP, Manohar S, et al. Biomarkers, clinical features, and rechallenge for immune checkpoint inhibitor renal immune-related adverse events. Kidney Int Rep. 2021;6(4):1022-1031. doi: 10.1016/j.ekir.2021.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sise ME, Wang Q, Seethapathy H, et al. Soluble and cell-based markers of immune checkpoint inhibitor associated nephritis. bioRxiv. 2022. doi: 10.1101/2022.10.13.22280966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farooqui N, Zaidi M, Vaughan L, et al. Cytokines and immune cell phenotype in acute kidney injury associated with immune checkpoint inhibitors. Kidney Int Rep. 2022. doi: 10.1016/j.ekir.2022.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Awiwi MO, Abudayyeh A, Abdel-Wahab N, et al. Imaging features of immune checkpoint inhibitor-related nephritis with clinical correlation: a retrospective series of biopsy-proven cases. Eur Radiol. 2022 Oct 18. doi: 10.1007/s00330-022-09158-8. Epub ahead of print. PMID: 36255488. [DOI] [PMC free article] [PubMed]
- 16.Mohan A, Herrmann SM. Capmatinib-induced pseudo-acute kidney injury: a case report. Am J Kidney Dis. 2022;79(1):120-124. doi: 10.1053/j.ajkd.2021.04.009 [DOI] [PubMed] [Google Scholar]
- 17.Chappell JC, Turner PK, Pak YA, et al. Abemaciclib inhibits renal tubular secretion without changing glomerular filtration rate. Clin Pharmacol Ther. 2019;105(5):1187-1195. doi: 10.1002/cpt.1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruin MAC, Korse CM, van Wijnen B, et al. A real or apparent decrease in glomerular filtration rate in patients using olaparib? Eur J Clin Pharmacol. 2020;77(2):179-188. doi: 10.1007/s00228-020-03070-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zibetti DMG, Westin SN, Msaouel P, Gomes LM, Dickens A, Coleman RL. Discrepancy in calculated and measured glomerular filtration rates in patients treated with PARP inhibitors. Int J Gynecol Cancer. 2020;30(1):89-93. doi: 10.1136/ijgc-2019-000714 [DOI] [PubMed] [Google Scholar]
- 20.Sledge GW, Jr, Toi M, Neven P, et al. MONARCH 2: Abemaciclib in combination with Fulvestrant in women with HR+/HER2− advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35(25):2875-2884. doi: 10.1200/JCO.2017.73.7585 [DOI] [PubMed] [Google Scholar]
- 21.Sledge GW, Toi M, Neven P, et al. The effect of Abemaciclib plus Fulvestrant on overall survival in hormone receptor–positive, ERBB2-negative breast cancer that progressed on endocrine therapy—MONARCH 2: a randomized clinical trial. JAMA Oncol. 2020;6(1):116-124. doi: 10.1001/jamaoncol.2019.4782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta S, Caza T, Herrmann SM, Sakhiya VC, Jhaveri KD. Clinicopathologic features of acute kidney injury associated with CDK4/6 inhibitors. Kidney Int Rep. 2021;7(3):618-623. doi: 10.1016/j.ekir.2021.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sy-Go JPT, Yarandi N, Schwartz GL, Herrmann SM. Ribociclib-induced pseudo-acute kidney injury. J Onco-Nephrol. 2022;6(1-2):64-69. doi: 10.1177/10781552211007202 [DOI] [Google Scholar]
- 24.Bai Y, Kim JY, Bisunke B, et al. Kidney toxicity of the BRAF-kinase inhibitor vemurafenib is driven by off-target ferrochelatase inhibition. Kidney Int. 2021;100(6):1214-1226. doi: 10.1016/j.kint.2021.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klaeger S, Gohlke B, Perrin J, et al. Chemical proteomics reveals ferrochelatase as a common off target of kinase inhibitors. ACS Chem Biol. 2016;11(5):1245-1254. doi: 10.1021/acschembio.5b01063 [DOI] [PubMed] [Google Scholar]
- 26.Bloomer J, Bruzzone C, Zhu L, Scarlett Y, Magness S, Brenner D. Molecular defects in ferrochelatase in patients with protoporphyria requiring liver transplantation. J Clin Invest. 1998;102(1):107-114. doi: 10.1172/JCI1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan X, Zhang X, Liu LC, et al. Hemopexin accumulates in kidneys and worsens acute kidney injury by causing hemoglobin deposition and exacerbation of iron toxicity in proximal tubules. Kidney Int. 2022;102(6):1320-1330. doi: 10.1016/j.kint.2022.07.024 [DOI] [PubMed] [Google Scholar]
- 28.Uppal NN, Workeneh BT, Rondon-Berrios H, Jhaveri KD. Electrolyte and acid-base disorders associated with cancer immunotherapy. Clin J Am Soc Nephrol. 2022;17(6):922-933. doi: 10.2215/CJN.14671121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Izzedine H, Chazal T, Wanchoo R, Jhaveri KD. Immune checkpoint inhibitor–associated hypercalcaemia. Nephrol Dial Transpl. 2020;37(9):1598-1608. doi: 10.1093/ndt/gfaa326 [DOI] [PubMed] [Google Scholar]
- 30.Wanchoo R, Sakhiya V, Jhaveri KD. Immune checkpoint inhibitor-associated electrolyte disorders: query of the food and drug administration adverse event reporting system. Kidney Int. 2021;100(4):945-947. doi: 10.1016/j.kint.2021.06.001 [DOI] [PubMed] [Google Scholar]
- 31.Arnaud M, Loiselle M, Vaganay C, et al. Tumor lysis syndrome and AKI: beyond crystal mechanisms. J Am Soc Nephrol. 2022;33(6):1154-1171. doi: 10.1681/ASN.2021070997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wanchoo R, Bernabe RC, Barrientos J, Jhaveri KD. Renal involvement in chronic lymphocytic leukemia. Clin Kidney J. 2018;11(5):670-680. doi: 10.1093/ckj/sfy026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abernathy KM, Perciavalle MA, Gatwood KS, Chen H, Zakhari MM, Byrne M. Real-world analysis of tumor lysis syndrome in patients started on venetoclax combination for acute myeloid leukemia. J Oncol Pharm Pract. 2022; 10781552221118635. doi: 10.1177/10781552221118635. Epub ahead of print. [DOI] [PubMed]
- 34.Jhaveri KD, Wanchoo R. Selinexor for refractory multiple myeloma. N Engl J Med. 2019;381(20):1977. doi: 10.1056/NEJMc1912625 [DOI] [PubMed] [Google Scholar]
- 35.Gavriatopoulou M, Chari A, Chen C, et al. Integrated safety profile of selinexor in multiple myeloma: experience from 437 patients enrolled in clinical trials. Leukemia. 2020;34(9):2430-2440. doi: 10.1038/s41375-020-0756-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kala J, Mamlouk O, Jhaveri KD. Selinexor-associated hyponatremia: single-center, real-world data. Kidney Int. 2020;98(3):789-791. doi: 10.1016/j.kint.2020.06.007 [DOI] [PubMed] [Google Scholar]
- 37.Farooqui N, Sy-Go JPT, Miao J, et al. Incidence and risk factors for acute kidney injury after Chimeric antigen receptor T-cell therapy. Mayo Clin Proc. 2022;97(7):1294-1304. doi: 10.1016/j.mayocp.2022.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seethapathy H, Rusibamayila N, Chute DF, et al. Hyponatremia and other electrolyte abnormalities in patients receiving immune checkpoint inhibitors. Nephrol Dial Transpl. 2021;36(12):2241-2247. doi: 10.1093/ndt/gfaa272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta S, Seethapathy H, Strohbehn IA, et al. Acute kidney injury and electrolyte abnormalities after Chimeric antigen receptor T-cell (CAR-T) therapy for diffuse large B-cell lymphoma. Am J Kidney Dis. 2020;76(1):63-71. doi: 10.1053/j.ajkd.2019.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perazella MA. Onco-nephrology: renal toxicities of chemotherapeutic agents. Clin J Am Soc Nephrol. 2012;7(10):1713-1721. doi: 10.2215/CJN.02780312 [DOI] [PubMed] [Google Scholar]
- 41.Workeneh BT, Uppal NN, Jhaveri KD, Rondon-Berrios H. Hypomagnesemia in the cancer patient. Kidney360. 2021;2(1):154-166. doi: 10.34067/KID.0005622020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ray EC, Boyd-Shiwarski CR, Liu P, Novacic D, Cassiman D. SGLT2 inhibitors for treatment of refractory hypomagnesemia: a case report of 3 patients. Kidney Med. 2020;2(3):359-364. doi: 10.1016/j.xkme.2020.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shah CV, Robbins TS, Sparks MA. SodiumGlucose Cotransporter 2 inhibitors and management of refractory hypomagnesemia without Overt urinary magnesium wasting: a report of 2 cases. Kidney Med. 2022;4(10):100533. doi: 10.1016/j.xkme.2022.100533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang H, Zhang X, Zhang J, et al. Elevated serum magnesium associated with SGLT2 inhibitor use in type 2 diabetes patients: a meta-analysis of randomised controlled trials. Diabetologia. 2016;59(12):2546-2551. doi: 10.1007/s00125-016-4101-6 [DOI] [PubMed] [Google Scholar]
- 45.Srinivasan Sridhar V, Ambinathan JPN, Kretzler M, et al. Renal SGLT mRNA expression in human health and disease: a study in two cohorts. Am J Physiol Ren Physiol. 2019;317(5):F1224-F1230. doi: 10.1152/ajprenal.00370.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gudsoorkar P, Ruf R, Adnani H, Safdar K, Sparks MA. Onco-hypertension: an emerging specialty. Adv Chronic Kidney Dis. 2021;28(5):477-489. doi: 10.1053/j.ackd.2021.09.011 [DOI] [PubMed] [Google Scholar]
- 47.Lees JS, Elyan BMP, Herrmann SM, Lang NN, Jones RJ, Mark PB. The 'other' big complication: how chronic kidney disease impacts on cancer risks and outcomes. Nephrol Dial Transplant. 2022:gfac011. doi: 10.1093/ndt/gfac011. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 48.Boer H, Proost JH, Nuver J, et al. Long-term exposure to circulating platinum is associated with late effects of treatment in testicular cancer survivors. Ann Oncol. 2015;26(11):2305-2310. doi: 10.1093/annonc/mdv369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Touyz RM, Herrmann SMS, Herrmann J. Vascular toxicities with VEGF inhibitor therapies-focus on hypertension and arterial thrombotic events. J Am Soc Hypertens. 2018;12(6):409-425. doi: 10.1016/j.jash.2018.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Dorst DCH, Dobbin SJH, Neves KB, et al. Hypertension and prohypertensive antineoplastic therapies in cancer patients. Circ Res. 2021;128(7):1040-1061. doi: 10.1161/CIRCRESAHA.121.318051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hayman SR, Leung N, Grande JP, Garovic VD. VEGF inhibition, hypertension, and renal toxicity. Curr Oncol Rep. 2012;14(4):285-294. doi: 10.1007/s11912-012-0242-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rashidi A, Wanchoo R, Izzedine H. How I manage hypertension and proteinuria associated with VEGF inhibitor. Clin J Am Soc Nephrol. 2022. doi: 10.2215/CJN.05610522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Estrada CC, Maldonado A, Mallipattu SK. Therapeutic inhibition of VEGF signaling and associated nephrotoxicities. J Am Soc Nephrol. 2019;30(2):187-200. doi: 10.1681/ASN.2018080853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Porta C, Cosmai L, Gallieni M, Pedrazzoli P, Malberti F. Renal effects of targeted anticancer therapies. Nat Rev Nephrol. 2015;11(6):354-370. doi: 10.1038/nrneph.2015.15 [DOI] [PubMed] [Google Scholar]
- 55.Haddad RI, Schlumberger M, Wirth LJ, et al. Incidence and timing of common adverse events in Lenvatinib-treated patients from the SELECT trial and their association with survival outcomes. Endocrine. 2017;56(1):121-128. doi: 10.1007/s12020-017-1233-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Powles T, Motzer RJ, Escudier B, et al. Outcomes based on prior therapy in the phase 3 METEOR trial of cabozantinib versus everolimus in advanced renal cell carcinoma. Br J Cancer. 2018;119(6):663-669. doi: 10.1038/s41416-018-0164-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin Y, Qin S, Li Z, et al. Apatinib vs placebo in patients with locally advanced or metastatic, radioactive iodine–refractory differentiated thyroid cancer. JAMA Oncol. 2022;8(2):242. doi: 10.1001/jamaoncol.2021.6268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Caldeira D, Alves D, Costa J, Ferreira JJ, Pinto FJ. Ibrutinib increases the risk of hypertension and atrial fibrillation: systematic review and meta-analysis. PLoS One. 2019;14(2):e0211228. doi: 10.1371/journal.pone.0211228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coutre SE, Byrd JC, Hillmen P, et al. Long-term safety of single-agent ibrutinib in patients with chronic lymphocytic leukemia in 3 pivotal studies. Blood Adv. 2019;3(12):1799-1807. doi: 10.1182/bloodadvances.2018028761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Woyach JA, Ruppert AS, Heerema NA, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379(26):2517-2528. doi: 10.1056/NEJMoa1812836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dickerson T, Wiczer T, Waller A, et al. Hypertension and incident cardiovascular events following ibrutinib initiation. Blood. 2019;134(22):1919-1928. doi: 10.1182/blood.2019000840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Byrd JC, Harrington B, O’Brien S, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):323-332. doi: 10.1056/NEJMoa1509981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mishra R, Patel H, Alanazi S, Kilroy MK, Garrett JT. PI3K inhibitors in cancer: clinical implications and adverse effects. Int J Mol Sci. 2021;22(7):3464. doi: 10.3390/ijms22073464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dreyling M, Santoro A, Mollica L, et al. Phosphatidylinositol 3-kinase inhibition by Copanlisib in relapsed or refractory Indolent lymphoma. J Clin Oncol. 2017;35(35):3898-3905. doi: 10.1200/JCO.2017.75.4648 [DOI] [PubMed] [Google Scholar]
- 65.Cheson BD, O’Brien S, Ewer MS, et al. Optimal management of adverse events from Copanlisib in the treatment of patients with non-Hodgkin lymphomas. Clin Lymphoma Myeloma Leuk. 2019;19(3):135-141. doi: 10.1016/j.clml.2018.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao JJ, Cheng H, Jia S, et al. The p110alpha isoform of PI3K is essential for proper growth factor signaling and oncogenic transformation. Proc Natl Acad Sci U S A. 2006;103(44):16296-16300. doi: 10.1073/pnas.0607899103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nunnery SE, Mayer IA. Management of toxicity to isoform α-specific PI3K inhibitors. Ann Oncol. 2019;30(suppl_10):x21-x26. doi: 10.1093/annonc/mdz440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Minegishi S, Kinguchi S, Horita N, et al. Immune checkpoint inhibitors do not increase short-term risk of hypertension in cancer patients: a systematic literature review and meta-analysis. Hypertension. 2022;79:2611-2621. doi: 10.1161/HYPERTENSIONAHA.122.19865 [DOI] [PubMed] [Google Scholar]
- 69.Mincu RI, Mahabadi AA, Michel L, et al. Cardiovascular adverse events associated with BRAF and MEK inhibitors: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(8):e198890. doi: 10.1001/jamanetworkopen.2019.8890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu XH, Zhu JQ, Yin RT, et al. Niraparib maintenance therapy in patients with platinum-sensitive recurrent ovarian cancer using an individualized starting dose (NORA): a randomized, double-blind, placebo-controlled phase III trial. Ann Oncol. 2021;32(4):512-521. doi: 10.1016/j.annonc.2020.12.018 [DOI] [PubMed] [Google Scholar]
- 71.Ison G, Howie LJ, Amiri-Kordestani L, et al. FDA approval summary: niraparib for the maintenance treatment of patients with recurrent ovarian cancer in response to platinum-based chemotherapy. Clin Cancer Res. 2018;24(17):4066-4071. doi: 10.1158/1078-0432.CCR-18-0042 [DOI] [PubMed] [Google Scholar]
- 72.Iacovelli R, Ciccarese C, Bria E, et al. The cardiovascular toxicity of abiraterone and enzalutamide in prostate cancer. Clin Genitourin Cancer. 2018;16(3):e645-e653. doi: 10.1016/j.clgc.2017.12.007 [DOI] [PubMed] [Google Scholar]
- 73.Chapman JAW, Shepherd LE, Ingle JN, et al. Competing risks of death in women treated with adjuvant aromatase inhibitors for early breast cancer on NCIC CTG MA.27. Breast Cancer Res Treat. 2016;156(2):343-349. doi: 10.1007/s10549-016-3761-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Herwig J, Skuza S, Sachs W, Sachs M. Thrombospondin type 1 domain–containing 7A localizes to the slit diaphragm and stabilizes membrane dynamics of fully differentiated podocytes. J Am Soc Nephrol. 2019;30(5):824-839. doi: 10.1681/ASN.2018090941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tomas NM, Beck LH, Jr, Meyer-Schwesinger C, et al. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med. 2014;371(24):2277-2287. doi: 10.1056/NEJMoa1409354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Beck LH, Sethi SM, Fervenza FC. M-type phospholipase A2 receptor (PLA2R) and thrombospondin type-1 domain-containing 7A (THSD7A) in membranous nephropathy. In: Molecular Mechanisms in the Pathogenesis of Idiopathic Nephrotic Syndrome. Springer Japan; 2016:181-206. doi: 10.1007/978-4-431-55270-3_11 [DOI] [Google Scholar]
- 77.Sethi S, Debiec H, Madden B, et al. Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney Int. 2020;97(1):163-174. doi: 10.1016/j.kint.2019.09.014 [DOI] [PubMed] [Google Scholar]
- 78.Caza TN, Hassen SI, Dvanajscak Z, et al. NELL1 is a target antigen in malignancy-associated membranous nephropathy. Kidney Int. 2021;99(4):967-976. doi: 10.1016/j.kint.2020.07.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sethi S, Madden B, Debiec H, et al. Protocadherin 7-associated membranous nephropathy. J Am Soc Nephrol. 2021;32(5):1249-1261. doi: 10.1681/ASN.2020081165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kitchlu A, Jhaveri KD, Wadhwani S, et al. A systematic review of immune checkpoint inhibitor-associated glomerular disease. Kidney Int Rep. 2021;6(1):66-77. doi: 10.1016/j.ekir.2020.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Toda MG, Fujii K, Kato A, et al. Minimal change disease associated with Durvalumab. Kidney Int Rep. 2021;6(10):2733-2734. doi: 10.1016/j.ekir.2021.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bonilla M, Maturostrakul B, Khan N, Bijol V, Jhaveri KD, Wanchoo R. Phospholipase A2 receptor antibody mediated membranous nephropathy associated with cemiplimab. J Onco-Nephrol. 2021;5(1):27-30. doi: 10.1177/23993693211004612 [DOI] [Google Scholar]
- 83.Bonilla M, Bijol V, Corona AGDL, Sullivan KM, Jhaveri KD. A case of immune-complex mediated glomerulonephritis associated with pembrolizumab. J Onco-Nephrol. 2022;6(1-2):79-83. 10.1177/23993693211064627 [DOI] [Google Scholar]
- 84.Abudayyeh A, Wanchoo R. Kidney disease following hematopoietic stem cell transplantation. Adv Chronic Kidney Dis. 2022;29(2):103-115. doi: 10.1053/j.ackd.2021.11.003 [DOI] [PubMed] [Google Scholar]
- 85.Raj K, Pagliuca A, Bradstock K, et al. Peripheral blood hematopoietic stem cells for transplantation of hematological diseases from related, haploidentical donors after reduced-intensity conditioning. Biol Blood Marrow Transplant. 2014;20(6):890-895. doi: 10.1016/j.bbmt.2014.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hanna P, Strohbehn I, Wang Q, Frigault M, Sise ME. Acute kidney injury caused by haplostorm after allogenic hematopoietic stem cell transplant. Bone Marrow Transplant. 2022;57(9):1442-1444. doi: 10.1038/s41409-022-01720-8 [DOI] [PubMed] [Google Scholar]
- 87.Imus PH, Blackford AL, Bettinotti M, et al. Severe cytokine release syndrome after haploidentical peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2019;25(12):2431-2437. doi: 10.1016/j.bbmt.2019.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brukamp K, Doyle AM, Bloom RD, Bunin N, Tomaszewski JE, Cizman B. Nephrotic syndrome after hematopoietic cell transplantation: do glomerular lesions represent renal graft-versus-host disease? Clin J Am Soc Nephrol. 2006;1(4):685-694. doi: 10.2215/CJN.00380705 [DOI] [PubMed] [Google Scholar]
- 89.Hiramatsu R. The case | proteinuria in a patient with hematopoietic stem cell transplantation. Kidney Int. 2021;99(5):1249-1250. doi: 10.1016/j.kint.2020.12.005 [DOI] [PubMed] [Google Scholar]
- 90.Huang X, Qin W, Zhang M, Zheng C, Zeng C, Liu Z. Detection of anti-PLA2R autoantibodies and IgG subclasses in post-allogeneic hematopoietic stem cell transplantation membranous nephropathy. Am J Med Sci. 2013;346(1):32-37. doi: 10.1097/MAJ.0b013e318267b5cd [DOI] [PubMed] [Google Scholar]
- 91.Nasr SH, Leung N, Said SM, et al. Membranous nephropathy with extensive tubular basement membrane deposits following allogeneic hematopoietic cell transplant: a report of 5 cases. Am J Kidney Dis. 2022;79(6):904-908. doi: 10.1053/j.ajkd.2021.07.021 [DOI] [PubMed] [Google Scholar]
- 92.Kudose S, Sekulic M, Mehring CJ, et al. NELL1-associated membranous glomerulopathy after hematopoietic stem cell transplantation. Kidney Int Rep. 2021;6(7):1992-1995. doi: 10.1016/j.ekir.2021.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sethi S, Madden B, Casal Moura M, et al. Hematopoietic stem cell transplant-membranous nephropathy is associated with protocadherin FAT1. J Am Soc Nephrol. 2022;33(5):1033-1044. doi: 10.1681/ASN.2021111488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sadeqzadeh E, de Bock CE, Thorne RF. Sleeping giants: emerging roles for the fat cadherins in health and disease. Med Res Rev. 2014;34(1):190-221. doi: 10.1002/med.21286 [DOI] [PubMed] [Google Scholar]
- 95.Gee HY, Sadowski CE, Aggarwal PK, et al. FAT1 mutations cause a glomerulotubular nephropathy. Nat Commun. 2016;7:10822. doi: 10.1038/ncomms10822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lenormand C, Lipsker D. Somatic mutations in “Benign” disease. N Engl J Med. 2021;384(21):2039-2052. doi: 10.1056/NEJMc2110545 [DOI] [PubMed] [Google Scholar]
- 97.Yadav P, Sathick IJ, Leung N, et al. Serum free light chain level at diagnosis in myeloma cast nephropathy—a multicentre study. Blood Cancer J. 2020;10(3):28. doi: 10.1038/s41408-020-0295-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-e548. doi: 10.1016/S1470-2045(14)70442-5 [DOI] [PubMed] [Google Scholar]
- 99.Martins M, Bridoux F, Goujon JM, et al. Complement activation and thrombotic microangiopathy associated with monoclonal Gammopathy: a National French case series. Am J Kidney Dis. 2022;80(3):341-352. doi: 10.1053/j.ajkd.2021.12.014 [DOI] [PubMed] [Google Scholar]
- 100.Yong ZH, Yu XJ, Liu JX, Zhou F, Wang SX, Zhao MH. Kidney histopathologic spectrum and clinical indicators associated with MGRS. Clin J Am Soc Nephrol. 2022;17(4):527-534. doi: 10.2215/CJN.12890921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bridoux F, Arnulf B, Karlin L, et al. Randomized trial comparing double versus triple bortezomib-based regimen in patients with multiple myeloma and acute kidney injury due to cast nephropathy. J Clin Oncol. 2020;38(23):2647-2657. doi: 10.1200/JCO.20.00298 [DOI] [PubMed] [Google Scholar]
- 102.Gumber R, Cohen JB, Palmer MB, et al. A clone-directed approach may improve diagnosis and treatment of proliferative glomerulonephritis with monoclonal immunoglobulin deposits. Kidney Int. 2018;94(1):199-205. doi: 10.1016/j.kint.2018.02.020 [DOI] [PubMed] [Google Scholar]
- 103.Mikhael JR, Schuster SR, Jimenez-Zepeda VH, et al. Cyclophosphamide-bortezomib-dexamethasone (CyBorD) produces rapid and complete hematologic response in patients with AL amyloidosis. Blood. 2012;119(19):4391-4394. doi: 10.1182/blood-2011-11-390930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Angel-Korman A, Stern L, Angel Y, et al. The role of kidney transplantation in monoclonal Ig deposition disease. Kidney Int Rep. 2020;5(4):485-493. doi: 10.1016/j.ekir.2020.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zand L, Rajkumar SV, Leung N, Sethi S, El Ters M, Fervenza FC. Safety and efficacy of daratumumab in patients with proliferative GN with monoclonal immunoglobulin deposits. J Am Soc Nephrol. 2021;32(5):1163-1173. doi: 10.1681/ASN.2020101541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nuvolone M, Merlini G. Emerging therapeutic targets currently under investigation for the treatment of systemic amyloidosis. Expert Opin Ther Targets. 2017;21(12):1095-1110. doi: 10.1080/14728222.2017.1398235 [DOI] [PubMed] [Google Scholar]
- 107.Kastritis E, Palladini G, Minnema MC, et al. Daratumumab-based treatment for immunoglobulin light-chain amyloidosis. N Engl J Med. 2021;385(1):46-58. doi: 10.1056/NEJMoa2028631 [DOI] [PubMed] [Google Scholar]
- 108.Clark WF, Stewart AK, Rock GA, et al. Plasma exchange when myeloma presents as acute renal failure: a randomized, controlled trial. Ann Intern Med. 2005;143(11):777-784. doi: 10.7326/0003-4819-143-11-200512060-00005 [DOI] [PubMed] [Google Scholar]
- 109.Bridoux F, Carron PL, Pegourie B, et al. Effect of high-cutoff hemodialysis vs conventional hemodialysis on hemodialysis independence among patients with myeloma cast nephropathy: a randomized clinical trial. JAMA. 2017;318(21):2099-2110. doi: 10.1001/jama.2017.17924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hutchison CA, Cockwell P, Moroz V, et al. High cutoff versus high-flux haemodialysis for myeloma cast nephropathy in patients receiving bortezomib-based chemotherapy (EuLITE): a phase 2 randomised controlled trial. Lancet Haematol. 2019;6(4):e217-e228. doi: 10.1016/S2352-3026(19)30014-6 [DOI] [PubMed] [Google Scholar]
- 111.Murakami N, Webber AB, Nair V. Transplant onconephrology in patients with kidney transplants. Adv Chronic Kidney Dis. 2022;29(2):188-200. doi: 10.1053/j.ackd.2021.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Murakami N, Mulvaney P, Danesh M, et al. A multi-center study on safety and efficacy of immune checkpoint inhibitors in cancer patients with kidney transplant. Kidney Int. 2021;100(1):196-205. doi: 10.1016/j.kint.2020.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Carroll RP, Boyer M, Gebski V, et al. Immune checkpoint inhibitors in kidney transplant recipients: a multicentre, single-arm, phase 1 study. Lancet Oncol. 2022;23(8):1078-1086. doi: 10.1016/S1470-2045(22)00368-0 [DOI] [PubMed] [Google Scholar]
- 114.Barnett R, Barta VS, Jhaveri KD. Preserved renal-allograft function and the PD-1 pathway inhibitor nivolumab. N Engl J Med. 2017;376(2):191-192. doi: 10.1056/NEJMc1614298 [DOI] [PubMed] [Google Scholar]
- 115.Decourt A, Gondouin B, Delaroziere JC, et al. Trends in survival and renal recovery in patients with multiple myeloma or light-chain amyloidosis on chronic dialysis. Clin J Am Soc Nephrol. 2016;11(3):431-441. doi: 10.2215/CJN.06290615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chitty DW, Hartley-Brown MA, Abate M, et al. Kidney transplantation in patients with multiple myeloma: narrative analysis and review of the last 2 decades. Nephrol Dial Transplant. 2022;37(9):1616-1626. doi: 10.1093/ndt/gfaa361 [DOI] [PubMed] [Google Scholar]
- 117.Ng JH, Izard S, Murakami N, Jhaveri KD, Sharma A, Nair V. Outcomes of kidney transplantation in patients with myeloma and amyloidosis in the US. Nephrol Dial Transplant. 2022;37(12):2569-2580. doi: 10.1093/ndt/gfac196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Havasi A, Heybeli C, Leung N, et al. Outcomes of renal transplantation in patients with AL amyloidosis: an international collaboration through the International Kidney and Monoclonal Gammopathy Research Group. Blood Cancer J. 2022;12(8):119. doi: 10.1038/s41408-022-00714-5 [DOI] [PMC free article] [PubMed] [Google Scholar]