Abstract
Purpose
This study aimed to compare total blood loss (TBL) and hidden blood loss (HBL) in patients undergoing single-level open transforaminal lumbar interbody fusion (O-TLIF) and unilateral biportal endoscopic transforaminal lumbar interbody fusion (ULIF).
Methods
A total of 53 patients who underwent ULIF and 53 patients who underwent O-TLIF from March 2020 to July 2022 were retrospectively reviewed. The Nadler’s formula was employed to estimate the patient’s blood volume (PBV), Gross’s formula to estimate TBL, and Sehat’s formula to estimate HBL. The obtained data were then analyzed with independent t test, chi-squared test, and analysis of covariance.
Results
TBL and measured blood loss (MBL) in ULIF group (326.86 ± 223.45 ml, 99.00 ± 72.81 ml) was significantly lower than O-TLIF group (427.97 ± 280.52 ml, 270.66 ± 102.34 ml). Nevertheless, the HBL in ULIF group was higher than that in O-TLIF group (227.86 ± 221.75 ml vs 157.31 ± 268.08 ml), however this was not statistically significant (p = 0.143). The HBL was 69.71 ± 23.72% of TBL in ULIF group and 36.76 ± 18.79% of TBL in O-TLIF group. Patients in ULIF group had lower TBL and MBL, shorter duration of drainage, lower postoperative anemia, and shorter postoperative hospital stay compared to those in O-TLIF group.
Conclusions
Perioperative HBL should not be neglected in patients undergoing ULIF or O-TILF, as it accounts for a large percentage of TBL in both groups. ULIF is associated with lower TBL and MBL, postoperative anemia, shorter postoperative hospital stays compared with O-TLIF.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12891-023-06393-y.
Keywords: Hidden blood loss, Total blood loss, Unilateral biportal endoscopy, Transforaminal lumbar interbody fusion, Degenerative lumbar disease
Introduction
Posterior lumbar fusion is commonly used to treat degenerative spine disease [1]. Transforaminal lumbar interbody fusion (TLIF) is a surgical technique in which anterior column support is achieved using a posterolateral approach and unilateral cage insertion, along with posterior column stabilization using pedicle screw fixation to preserve posterior ligamentous structures [2, 3]. A literature review and meta-analysis showed that compared with posterior lumbar interbody fusion (PLIF), TLIF has fewer complications and shorter operation time [4]. However, conventional open TLIF (O-TLIF) is associated with significant soft tissue morbidity and a long recovery period, which may cause adverse outcomes [5, 6].
Numerous types of minimally invasive spine surgeries that minimize injury to normal anatomical structures have been proposed for treating lumbar degenerative disease [1, 2, 5, 7–9]. Relative to O-TLIF, minimally invasive TLIF (MI-TLIF) is reported to reduce intraoperative blood loss, postoperative pain, and time to discharge or recovery [10]. Recently, unilateral biportal endoscopic transforaminal lumbar interbody fusion (ULIF) has emerged as an alternative way of managing degenerative lumbar disease [9]. ULIF requires only two small incisions and further decreases muscle injury. Past studies indicate that compared to MI-TLIF, ULIF causes less early postoperative back pain, earlier ambulation, and shorter hospital stay [5].
Previous studies have attributed perioperative bleeding solely to measured blood loss (MBL) which includes intraoperative blood loss (IBL) and postoperative drainage volume. However, the actual total blood loss (TBL) is significantly greater than IBL and postoperative drainage only. Compared with the MBL, the hidden blood loss (HBL) is often ignored. The concept of HBL was first introduced by Sehat et al. [11] and its presence in orthopedic surgery was later confirmed by mounting evidence. Indeed, total blood loss (TBL) is composed of MBL and HBL. Hui Zhang et al. [12] reported that TBL was lower in MI-TLIF than in O-TLIF, while HBL was significantly higher in MI-TLIF. However, to the best of our knowledge, no studies have examined TBL and MBL in patients undergoing ULIF. Here, we compared TBL and MBL in patients undergoing single-level O-TLIF and ULIF.
Patients and methods
Patients
This retrospective study involved consecutive patients with single-level lumbar instability or degenerative disk disease who underwent O-TLIF or ULIF between March 2020 and October 2021. The inclusion criteria were, 1) patients with single operated segment O-TLIF or ULIF, 2) patients with unilateral neurological symptoms, unilateral decompressions and unilateral drainage, and 3) patients with complete clinical data. The exclusion criteria were: 1) previous lumbar surgery, 2) presence of infections and/or cancer, 3) patients on antiplatelet or anticoagulant medication, 4) patients with hematological malignancies, bleeding disorders, or chronic liver disease, 5) patients with missing data, and 6) patients who underwent autologous and allogeneic transfusion. A total of 106 patients met the inclusion criteria. Of these, 53 belonged to the ULIF group and 53 to the O-TLIF group. Two groups of surgery were completed by the same surgeon and surgical team. Ethical approval for this study was granted by the ethics committee of the Second Affiliated Hospital of Soochow University (No: JD-HG-2021–47). All methods were conducted in accordance with the ethical standards of the declaration of Helsinki. The following patient data were collected: gender, age, weight, height, body mass index (BMI), level of fusion, fibrinogen level, American Society of Anesthesiologists (ASA) classification score, operative time, length of postoperative hospital stay, preoperative and postoperative hematocrit (Hct), hemoglobin (Hb), and red blood cells (RBC).
Surgical procedure
ULIF
Taking the left-side approach as an example, patients were placed in prone position under general anesthesia. After intraoperative fluoroscopy, landmarks for skin incision were place 2 cm above and below the target intervertebral disc on the bilateral side central surface projection of the pedicle axis. Subsequently, Four skin incisions, about 1.5 cm long, were made both on the bilateral side according to the landmarks. Contralateral percutaneous pedicle screws were placed under fluoroscopic guidance via contralateral two incisions. The ipsilateral cranial portal was used as a viewing portal and the ipsilateral caudal portal was used as a working portal (Fig. 1A). Intraoperative view of biportal endoscopic spinal surgery was shown in Fig. 1B. Muscle and soft tissue were detached from the left proximal lamina to create workspace. Using an ultrasonic scalpel, ipsilateral laminectomy and facetectomy was performed (Fig. 2A), and the autologous bone harvested during these procedures used as bone grafts. The ligamentum flavum was then resected (Fig. 2B) and herniated disk was exposed (Fig. 2C). Pituitary forceps were then used to remove the intervertebral disc material (Fig. 2D). Endplate preparation was wholly done under endoscopic view (Fig. 2E). Using a bone grafting funnel, autologous bone were inserted and impacted into the intervertebral disc space. Next, using endoscopic guidance, a cage filled with local morselized bone was inserted (Fig. 2F). After cage insertion, the ipsilateral percutaneous pedicle screws were inserted through the viewing and working portals. Ipsilateral percutaneous pedicle screws and rods were then placed through the same incision. A negative pressure drainage tube was placed in the decompression side intraoperatively.
Fig. 1.
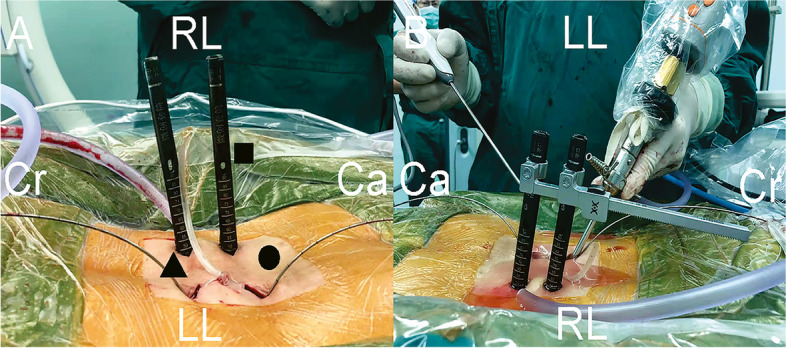
Photographs of the intraoperative scene. A Arrangement of the incisions. ▲A viewing portal.●A working portal. ■Percutaneous pedicle screw. B Intraoperative view of biportal endoscopic spinal surgery
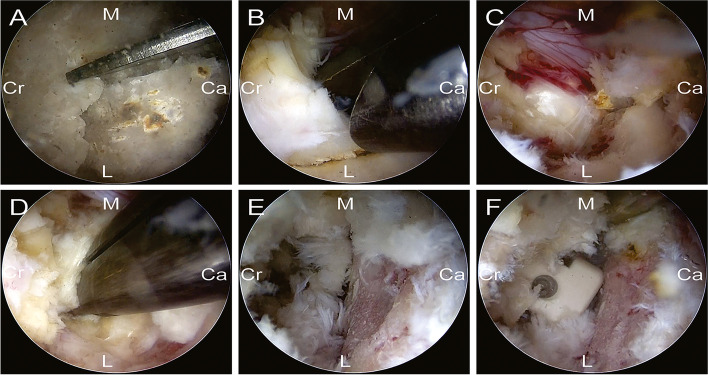
Fig. 2.
Images of the surgical procedure. A An ultrasonic bone scalpel was used for laminectomy and facetectomy. B Flavum ligament was removed by using forceps. C Exposure of disc space. D Pituitary forceps were used for removal of the intervertebral disc material. E Exposure of the disc space. F Endplate preparation. G A cage was filled with bone chips and inserted into the central part of the disc space
O-TLIF
The patient was placed in the prone position after general anesthesia. Then mark the target level under the C-arm guidance. Subperiosteal dissection was carried out to the tips of the spinous processes to expose the entry points for the pedicle screws. Pedicle screws were placed into the upper and subjacent vertebral pedicle of the segmental lesions. Facet joint and lamina were exposed. After unilateral laminectomy and inferior facetectomy, discectomy was done by retracting the traversing nerve root and dura medially. A cage fifilled with autologous bone was inserted in the disc space. The wound was copiously irrigated and closed in layers. A negative pressure drainage tube was placed in the decompression side intraoperatively.
Perioperative fluid management strategy
Both groups adopted the same fluid management strategy. Perioperative fluid management strategy involved two strategies. First, all patients were given intravenous tranexamic acid 1.0 g at the start of the surgical procedure. Secondly, multiple drugs including antibiotics, non-steroidal analgesics, diuretics and proton pump inhibitor are used in the perioperative period. Total fluid infusion volume was 1000 mL approximately.
Management of blood loss
No patient required blood transfusion during or after the operation. Complete blood counts were done on all patients including Hct, RBC, and Hb before surgery and on the second or third postoperative day. By this time, the patients were hemodynamically stable and fluid shifts would have been largely completed [13, 14].
Patients’ height and weight were recorded preoperatively. IBL was recorded by the anesthetist and included blood in suction bottles, as well as blood in weighed sponges used during the procedure. Postoperative drainage volume was recorded every 24 h and the drainage tube removed when the drainage volume in the surgical area was ≤ 50 mL/d.
Patients’ blood volume
TBL was estimated by first determining patient blood volume (PBV) in milliliters using the following formula by Nadler et al. [15].
For men: k1 = 0.3669, k2 = 0.03219, and k3 = 0.6041
For women: k1 = 0.3561, k2 = 0.03308, and k3 = 0.1833.
Total blood loss
TBL was given by the product of PBV and Hct change using the following formula by Gross et al. [16]:
where HctPre is preoperative Hct, HctPost is 2nd or 3rd day postoperative Hct, and Hctave is the mean of HctPre and HctPost.
Measured blood loss
MBL was calculated using the following formula:
IBL of O-TLIF was estimated by the volume of suction and the weight of gauze. Blood loss in gauze pieces was calculated by subtracting weight of dry gauze from the weight of blood soaked gauze pieces [17–19]. However, different from O-TLIF, continuous isotonic saline flow was maintained to provide a clear operative visual field in ULIF. Irrigation and suction were done simultaneously. Intraoperative hemorrhage was mixed with intraoperative irrigation. Because low IBL was detected intraoperatively but could not be calculated, the IBL in ULIF was disregarded. It should be emphasized that total postoperative drainage volume of the second or third postoperative day was not equal to TDV because extubation was not performed on the second or third postoperative day for all patients.
Hidden blood loss
HBL was calculated using the following formula by Sehat et al. [13]:
Anemia measurements
Anemia was indicated by a serum hemoglobin level < 130 g/L for men and < 120 g/L for women based on the World Health Organization’s criteria.
Statistical analysis
All statistical analyses were done on SPSS version 20.0 (IBM). Values are presented as means ± SD. Continuous variables were compared using the independent t-test. Statistical significance of differences between categorical variables was tested using Chi-square test. The statistical significance of differences between groups was tested using univariate general linear model analysis of covariance. P < 0.05 was considered statistically significant.
Results
Demographic data for the 58 patients are shown on Table 1. There was no significant difference between groups with regards to age, sex, weight, height, BMI, preoperative diagnosis, ASA classification, fibrinogen level, and the level of fusion between the 2 groups.
Table 1.
Patients’ demographic information
| Variable | ULIF | O-TLIF | P-value |
|---|---|---|---|
| Number of patients | 53 | 53 | |
| Gender (Male/Female) | 23/30 | 30/23 | 0.174 |
| Age (year) | 54.79 ± 8.54 | 54.92 ± 12.03 | 0.948 |
| Weight (kg) | 64.87 ± 11.52 | 65.24 ± 17.21 | 0.897 |
| Height (m) | 1.63 ± 0.073 | 1.65 ± 0.096 | 0.249 |
| BMI (kg/m2) | 24.19 ± 3.30 | 23.74 ± 4.92 | 0.578 |
| Preoperative diagnosis | |||
| Lumbar spinal stenosis | 47 | 51 | 0.141 |
| Spondylolisthesis | 6 | 2 | |
| Level of fusion | |||
| L2-L3 | 1 | 0 | 0.795 |
| L3-L4 | 3 | 3 | |
| L4-L5 | 29 | 29 | |
| L5-S1 | 20 | 21 | |
| ASA classification | |||
| I | 35 | 29 | 0.233 |
| II | 18 | 24 | |
| Fibrinogen level | 2.86 ± 0.58 | 2.83 ± 0.59 | 0.813 |
| Patient’s blood volume (ml) | 4008.11 ± 626.59 | 4092.18 ± 833.09 | 0.558 |
| Preoperative haematocrit (%) | 39.49 ± 4.44 | 40.85 ± 4.26 | 0.110 |
| Preoperative hemoglobin (g/L) | 133.02 ± 18.14 | 138.04 ± 15.56 | 0.129 |
| Preoperative red blood cells (10^12/L) | 4.48 ± 0.45 | 4.49 ± 0.48 | 0.913 |
| Incidence of pre-operative anemia | 24.53% | 20.75% | 0.643 |
Open transforaminal lumbar interbody fusion O-TLIF, Unilateral biportal endoscopic transforaminal lumbar interbody fusion ULIF, BMI Body mass index, American Society of Anesthesiologists ASA. Data are mean ± standard deviation; *P < 0.05
Relative to the O-TLIF group, ULIF was associated with significantly longer operation time and significantly shorter postoperative hospital stay (p = 0.000 and 0.042, respectively, Table 2). The ULIF group had shorter drainage duration (p = 0.003) and lower TDV (p = 0.000). Relative to the O-TLIF group, drainage volumes in the ULIF group were significantly lower in the first, second, and third postoperative day (p = 0.000, 0.000, and 0.000, respectively). Because the presence of physiological saline during ULIF made IBL difficult to calculate, ULIF-associated IBL was disregarded. The IBL in the O-TLIF group was 85.38 ± 23.20 mL. MBL results are shown in Table 3.
Table 2.
Operative time and postoperative hospital stay information
| Variable | ULIF | O-TLIF | P-value |
|---|---|---|---|
| Operative time (minute) | 176.32 ± 32.89 | 130.87 ± 24.54 | 0.000* |
| Postoperative hospital stay (day) | 5.51 ± 1.51 | 6.55 ± 3.31 | 0.042* |
Open transforaminal lumbar interbody fusion O-TLIF, Unilateral biportal endoscopic transforaminal lumbar interbody fusion ULIF
Data are mean ± standard deviation; *P < 0.05
Table 3.
The results of measured blood loss information
| Variable | ULIF | O-TLIF | P-value |
|---|---|---|---|
| Duration of drainage (day) | 2.21 ± 0.41 | 2.64 ± 0.92 | 0.003* |
| Total amount of postoperative drainage (ml) | 97.09 ± 73.30 | 219.06 ± 140.13 | 0.000* |
| Drainage of first postoperative day (ml) | 57.08 ± 39.61 | 124.91 ± 71.92 | 0.000* |
| Drainage of second postoperative day (ml) | 33.79 ± 31.67 | 61.40 ± 35.30 | 0.000* |
| Drainage of third postoperative day (ml) | 30.00 ± 16.73 | 49.04 ± 25.30 | 0.029* |
| Intraoperative blood loss (ml) | / | 85.38 ± 23.20 | / |
Open transforaminal lumbar interbody fusion O-TLIF, Unilateral biportal endoscopic transforaminal lumbar interbody fusion ULIF
Data are mean ± standard deviation; *P < 0.05
Analysis of perioperative blood revealed that relative to the O-TILF group, the ULIF group had significantly higher levels of postoperative Hct, Hb, and RBC (p = 0.000, 0.000, and 0.000, respectively; Table 4). The 2 groups did not differ significantly with regards to PBV and HBL. However, relative to the O-TLIF group, the HBL of TBL was significantly higher in the ULIF group (36.76 ± 18.79% vs 69.71 ± 23.72%, p = 0.010). MBL was significantly higher in the O-TILF group (270.66 ± 102.34 mL) than in the ULIF group (99.00 ± 72.81 mL). The TBL was 427.97 ± 280.52 ml (10.45 ± 5.87% of PBV) in O-TLIF group and 326.86 ± 223.45 ml (8.15 ± 5.37% of PBV) in ULIF group. TBL were significantly higher in the O-TILF group than in the ULIF group (p = 0.000). The proportions of patients with anemia in the 2 groups are shown in Fig. 3. The composition of total blood loss in the 2 groups is shown in Fig. 4.
Table 4.
The result of perioperative blood changes in patient
| Variable | ULIF | O-TLIF | P-value |
|---|---|---|---|
| Preoperative haematocrit (%) | 39.49 ± 4.43 | 40.85 ± 4.25 | 0.000* |
| Postoperative haematocrit (%) | 36.37 ± 4.24 | 36.64 ± 4.42 | |
| Preoperative hemoglobin (g/L) | 133.02 ± 18.14 | 138.04 ± 15.56 | 0.000* |
| Postoperative hemoglobin (g/L) | 119.07 ± 15.70 | 120.23 ± 15.40 | |
| Preoperative red blood cells (10^12/L) | 4.48 ± 0.45 | 4.49 ± 0.48 | 0.000* |
| Postoperative red blood cells (10^12/L) | 4.05 ± 0.38 | 3.96 ± 0.51 | |
| Patient’s blood volume (ml) | 4008.11 ± 626.59 | 4092.18 ± 833.09 | 0.558 |
| Total blood loss (ml) | 326.86 ± 223.45 | 427.97 ± 280.52 | 0.043* |
| Measured blood loss (ml) | 99.00 ± 72.81 | 270.66 ± 102.34 | 0.000* |
| Hidden blood loss (ml) | 227.86 ± 221.75 | 157.31 ± 268.08 | 0.143 |
| TBL as a % of PBV | 8.15 ± 5.37 | 10.45 ± 5.87 | 0.000* |
| HBL as a % of TBL | 69.71 ± 23.72 | 36.76 ± 18.79 | 0.000* |
Open transforaminal lumbar interbody fusion O-TLIF, Unilateral biportal endoscopic transforaminal lumbar interbody fusion ULIF, Total blood loss TBL, Hidden blood loss HBL, Patient’s blood volume PBV. Data are mean ± standard deviation, *P < 0.05
Fig. 3.
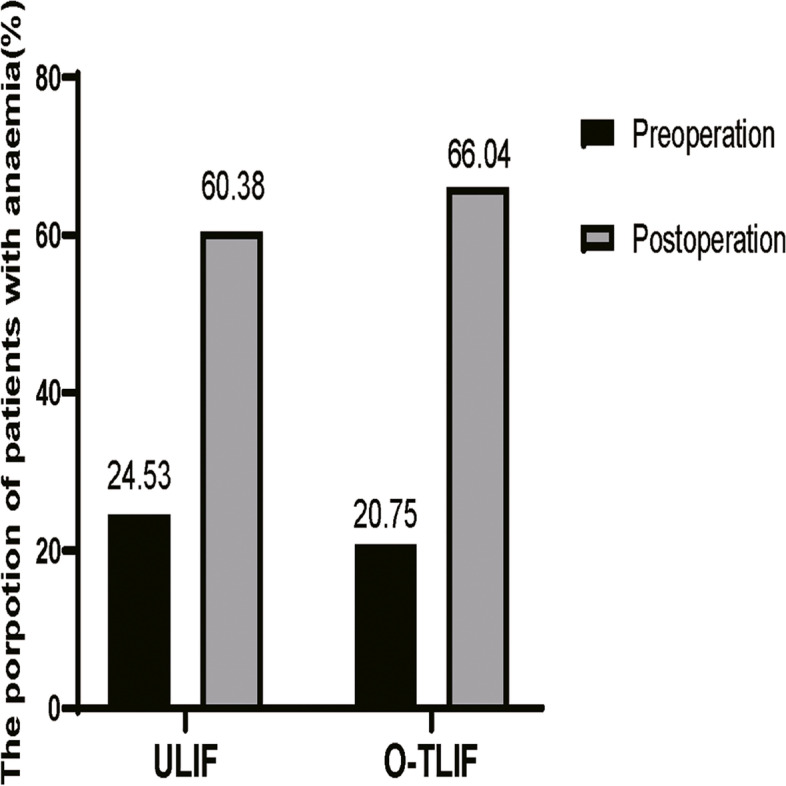
The propotion of patients with anaemia in 2 groups. Open transforaminal lumbar interbody fusion, O-TLIF; Unilateral biportal endoscopic transforaminal lumbar interbody fusion, ULIF
Fig. 4.
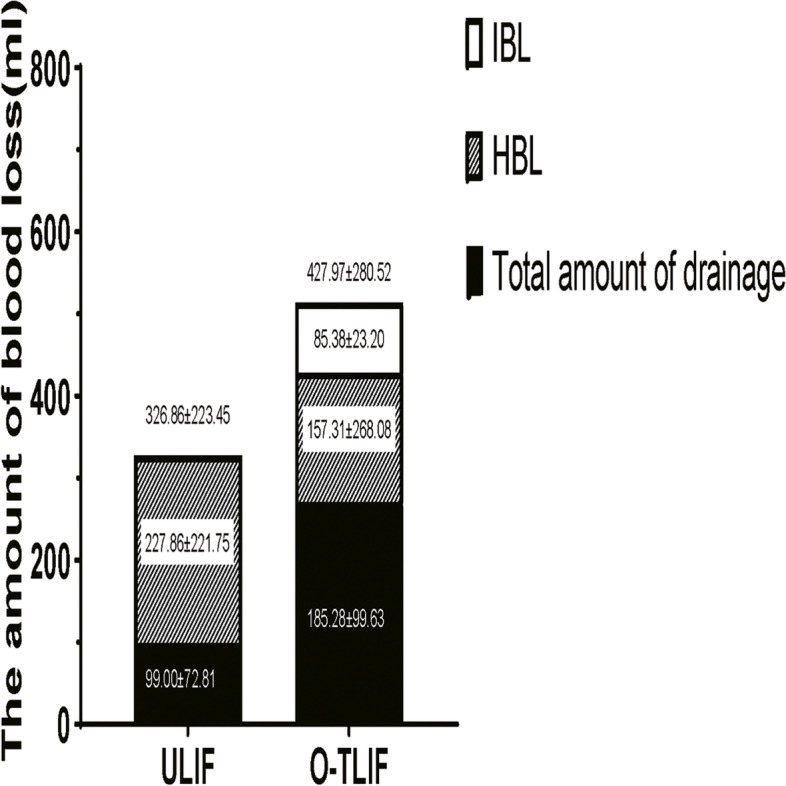
The composition of total blood loss in 2 groups. Open transforaminal lumbar interbody fusion, O-TLIF; Unilateral biportal endoscopic transforaminal lumbar interbody fusion, ULIF; Intraoperative blood loss, IBL; Hidden blood loss, HBL
Discussion
Since its introduction in 2000, several studies have reported HBL in various types of orthopedic surgeries. Sehat et al. [11] found that mean HBL was 735 mL, accounting for 50% of TBL during total knee arthroplasty. Yoji Ogura et al. [20] showed that during 2- to 3-level posterior lumbar fusion, HBL varies from 678–1,267 mL. Importantly, Foss and Kehlet et al. [21] reported that HBL was consistently associated with in-hospital complications and extended length of hospital stay. But as for now, no studies have reported HBL in ULIF. Here, we performed a retrospective analysis of HBL and TBL during ULIF and O-TLIF.
Wang et al. [22] reported that the mean estimated blood loss was 126.03 ± 17.85 ml in ULIF surgery. However, ULIF’s IBL was minimal and difficult to calculate because of the volume of saline solution used for irrigation. Thus, in this study, the IBL of ULIF was neglected and incorporated into the calculations of HBL. HBL may result from blood hemolysis [23, 24], extravasation of blood into tissue compartments [25], and free fatty acids mediated oxidative damage of RBCs and Hb [26].
Previous studies [27–30] indicate that gender, multilevel, operative time, fibrinogen level, ASA classification, autologous and allogeneic transfusion, BMI, and surgical method were independent risk factors for HBL in posterior lumbar interbody fusion. Here, to investigate the effect of surgical method on HBL, we used other variables as control variables. Our data show that mean HBL was 227.86 ± 221.75 mL in ULIF (constituting up to 69.71% of TBL) and 157.31 ± 268.08 ml in O-TLIF (constituting up to 36.76% of TBL). Surprisingly, mean HBL did not differ significantly between the two groups. However, the proportion of HBL in the two groups was significantly different. Our data indicate that HBL level was considerable and that it was the most important contributor to TBL in both ULIF and TLIF, which is consistent with past findings [12, 31]. The difference in HBL composition between the groups may be explained by the following. 1) One non-negligible reason is that IBL in ULIF was neglected and factored into the HBL calculation. 2) The higher radiofrequency used in ULIF may have generated more oxidizing species that damaged RBCs and Hb. 3) HBL might be affected by the “learning curve” that accompanies the introduction of any new surgical method, resulting in higher HBL being observed during ULIF in the initial cases.
In our study, the MBL in O-TLIF (270.66 ± 102.34 mL) was significantly higher than in ULIF (99.00 ± 72.81 mL). ULIF involved two minimally invasive incisions; one for endoscopic viewing and the other for the insertion and manipulation of surgical tools. The advantages of ULIF include being minimally invasive, involving less muscular dissection, better surgical view, and more precise operation. These factors may explain the lower TBL in the ULIF group. Moreover, considering that ULIF was wholly performed in aqueous media, we pulled with all strength to hemostasis because even minor bleeding can obstruct the surgeon field of vision. Moreover, Xu et al. [32] pointed out that the components of drainage changed radically with time. Thus, rather than the drainage volume, the true blood component of the drainage should be taken into account. In contrast with O-TLIF, ULIF leaves a high volume of water in the muscle and spinal space following saline irrigation. From this, it is expected that there was more water in ULIF drainage. Thus, the true blood volume was less than the drainage volume we described and the true difference between the two groups may be larger.
Perioperative anemia is significantly associated with complications and length of hospital stay [33, 34]. TBL only accounted for 8.15 ± 5.37% of PBV in ULIF, which was markedly lower than in the O-TLIF group. As shown on Fig. 3, lower TBL reduced the incidence of postoperative anemia in the ULIF group. As expected, relative to O-TLIF, ULIF was associated with significantly shorter postoperative hospital stay. HBL is the leading cause of perioperative anemia and hidden blood loss and perioperative anemia may be minimized by various interventions. A recent study [35] found that tranexamic acid reduces HBL during posterior lumbar interbody fusion surgery. Hong Qian et al. [36] found out that antioxidants attenuate oxidative stress-induced HBL in rats. However, more research is needed to identify new strategies for reducing HBL.
This study has some limitations. First, being retrospective, this study is inevitably susceptible to bias. Secondly, the study’s sample size was relatively small. Thirdly, disregarding ULIF’s IBL may have influenced our findings. Finally, studies [37, 38] show that fluid shifts may not be completed in all patients in 2 or 3 days after the operation. Thus, the HctPost we used to calculate TBL may not precise.
Conclusion
In summary, perioperative HBL should not be neglected when performing ULIF or O-TILF as it accounts for a large percentage of TBL in both groups. Lower TBL and MBL in the ULIF group lowers the incidence of postoperative anemia in patients, thereby reducing the length of postoperative hospital stay.
Supplementary Information
Acknowledgements
The authors thank all of the participants in the Second Affiliated Hospital of Soochow University, and we thank the support of patients.
Abbreviations
- TBL
Total blood loss
- HBL
Hidden blood loss
- PBV
Patient’s blood volume
- MBL
Measured blood loss
- IBL
Intraoperative blood loss
- BMI
Body mass index
- ASA
American Society of Anesthesiologists
- Hct
Hematocrit
- Hb
Hemoglobin
- RBC
Red blood cells
- O-TLIF
Open transforaminal lumbar interbody fusion
- ULIF
Unilateral biportal endoscopic transforaminal lumbar interbody fusion
- TLIF
Transforaminal lumbar interbody fusion
- PLIF
Posterior lumbar interbody fusion
- MI-TLIF
Minimally invasive Transforaminal lumbar interbody fusion
Authors’ contributions
Yu-Jian Peng: Writing Original draft preparation; Zhi-Yin Fan: Writing- Reviewing and Editing; Qian-liang Wang and Jun Dai: Data analysis and Methodology; Qian-Zhong-Yi Zhang and Jun-Yin Cao: Data curation; Xiao-Feng Liu: Supervision; Jun Yan: Conceptualization. The authors read and approved the final manuscript.
Funding
This study is sponsored by the National Natural Science Foundation of China (81971036,82002345,81902239), the Natural Science Foundation of Jiangsu Province (BK20191169), the Science and Technology Project of Suzhou (KJXW2019011) and the Preliminary Research Project of the Second Affiliated Hospital of Soochow University (SDFEYBS1905).
Availability of data and materials
The original contributions presented in the study are included in the article Supplementary Material, further inquiries can be directed to the corresponding author.
Declarations
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by The Ethics Committee of the Second Affifiliated Hospital of Soochow University(No: JD-HG-2021–47). The ethics committee waived the requirement of written informed consent for participation.All methods were conducted in accordance with the ethical standards of the declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yu-Jian Peng and Zhi-Ying Fan contributed equally to this work.
References
- 1.Heo DH, Son SK, Eum JH, Park CK. Fully endoscopic lumbar interbody fusion using a percutaneous unilateral biportal endoscopic technique: technical note and preliminary clinical results. Neurosurg Focus. 2017;43(2):E8. doi: 10.3171/2017.5.FOCUS17146. [DOI] [PubMed] [Google Scholar]
- 2.Faldini C, Borghi R, Chehrassan M, Perna F, Pilla F, Traina F. Transforaminal lumbar interbody fusion. Eur Spine J. 2017;26(Suppl 3):429–430. doi: 10.1007/s00586-017-5288-z. [DOI] [PubMed] [Google Scholar]
- 3.Mobbs RJ, Phan K, Malham G, Seex K, Rao PJ. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP. LLIF and ALIF J Spine Surg. 2015;1(1):2–18. doi: 10.3978/j.issn.2414-469X.2015.10.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Q, Yuan Z, Zhou M, Liu H, Xu Y, Ren Y. A comparison of posterior lumbar interbody fusion and transforaminal lumbar interbody fusion: a literature review and meta-analysis. BMC Musculoskelet Disord. 2014;15:367. doi: 10.1186/1471-2474-15-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim JE, Yoo HS, Choi DJ, Park EJ, Jee SM. Comparison of Minimal Invasive Versus Biportal Endoscopic Transforaminal Lumbar Interbody Fusion for Single-level Lumbar Disease. Clin Spine Surg. 2021;34(2):E64–E71. doi: 10.1097/BSD.0000000000001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sihvonen T, Herno A, Paljarvi L, Airaksinen O, Partanen J, Tapaninaho A. Local denervation atrophy of paraspinal muscles in postoperative failed back syndrome. Spine (Phila Pa 1976) 1993;18(5):575–81. doi: 10.1097/00007632-199304000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Eun SS, Eum JH, Lee SH, Sabal LA. Biportal Endoscopic Lumbar Decompression for Lumbar Disk Herniation and Spinal Canal Stenosis: A Technical Note. J Neurol Surg A Cent Eur Neurosurg. 2017;78(4):390–396. doi: 10.1055/s-0036-1592157. [DOI] [PubMed] [Google Scholar]
- 8.Hari A, Krishna M, Rajagandhi S, Rajakumar DV. Minimally invasive transforaminal lumbar interbody fusion-indications and clinical experience. Neurol India. 2016;64(3):444–454. doi: 10.4103/0028-3886.181536. [DOI] [PubMed] [Google Scholar]
- 9.Hwa Eum J, Hwa Heo D, Son SK, Park CK. Percutaneous biportal endoscopic decompression for lumbar spinal stenosis: a technical note and preliminary clinical results. J Neurosurg Spine. 2016;24(4):602–607. doi: 10.3171/2015.7.SPINE15304. [DOI] [PubMed] [Google Scholar]
- 10.Ge DH, Stekas ND, Varlotta CG, Fischer CR, Petrizzo A, Protopsaltis TS, et al. Comparative Analysis of Two Transforaminal Lumbar Interbody Fusion Techniques: Open TLIF Versus Wiltse MIS TLIF. Spine (Phila Pa 1976) 2019;44(9):E555–E60. doi: 10.1097/BRS.0000000000002903. [DOI] [PubMed] [Google Scholar]
- 11.Sehat KR, Evans R, Newman JH. How much blood is really lost in total knee arthroplasty? Correct blood loss management should take hidden loss into account. Knee. 2000;7(3):151–5. doi: 10.1016/s0968-0160(00)00047-8. [DOI] [PubMed] [Google Scholar]
- 12.Zhang H, Chen ZX, Sun ZM, Jiang C, Ni WF, Lin Y, et al. Comparison of the Total and Hidden Blood Loss in Patients Undergoing Open and Minimally Invasive Transforaminal Lumbar Interbody Fusion. World Neurosurg. 2017;107:739–743. doi: 10.1016/j.wneu.2017.08.113. [DOI] [PubMed] [Google Scholar]
- 13.Sehat KR, Evans RL, Newman JH. Hidden blood loss following hip and knee arthroplasty. Correct management of blood loss should take hidden loss into account. J Bone Joint Surg Br. 2004;86(4):561–5. doi: 10.1302/0301-620X.86B4.14508. [DOI] [PubMed] [Google Scholar]
- 14.Smorgick Y, Baker KC, Bachison CC, Herkowitz HN, Montgomery DM, Fischgrund JS. Hidden blood loss during posterior spine fusion surgery. Spine J. 2013;13(8):877–881. doi: 10.1016/j.spinee.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224–232. [PubMed] [Google Scholar]
- 16.Gross JB. Estimating allowable blood loss: corrected for dilution. Anesthesiology. 1983;58(3):277–280. doi: 10.1097/00000542-198303000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Braga M, Capretti G, Pecorelli N, Balzano G, Doglioni C, Ariotti R, et al. A prognostic score to predict major complications after pancreaticoduodenectomy. Ann Surg. 2011;254(5):702–7; discussion 7-8. doi: 10.1097/SLA.0b013e31823598fb. [DOI] [PubMed] [Google Scholar]
- 18.Yang LQ, Song JC, Irwin MG, Song JG, Sun YM, Yu WF. A clinical prospective comparison of anesthetics sensitivity and hemodynamic effect among patients with or without obstructive jaundice. Acta Anaesthesiol Scand. 2010;54(7):871–877. doi: 10.1111/j.1399-6576.2010.02222.x. [DOI] [PubMed] [Google Scholar]
- 19.Motoyama S, Sato Y, Wakita A, Kawakita Y, Nagaki Y, Imai K, et al. Extensive Lymph Node Dissection Around the Left Laryngeal Nerve Achieved With Robot-assisted Thoracoscopic Esophagectomy. Anticancer Res. 2019;39(3):1337–42. doi: 10.21873/anticanres.13246. [DOI] [PubMed] [Google Scholar]
- 20.Ogura Y, Dimar Ii JR, Gum JL, Crawford CH, 3rd, Djurasovic M, Glassman SD, et al. Hidden blood loss following 2- to 3-level posterior lumbar fusion. Spine J. 2019;19(12):2003–2006. doi: 10.1016/j.spinee.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Foss NB, Kehlet H. Hidden blood loss after surgery for hip fracture. J Bone Joint Surg Br. 2006;88(8):1053–1059. doi: 10.1302/0301-620X.88B8.17534. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Tian Z, Mansuerjiang M, Younusi A, Xu L, Xiang H, et al. A single-arm retrospective study of the clinical efficacy of unilateral biportal endoscopic transforaminal lumbar interbody fusion for lumbar spinal stenosis. Front Surg. 2022;9:1062451. doi: 10.3389/fsurg.2022.1062451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pattison E, Protheroe K, Pringle RM, Kennedy AC, Dick WC. Reduction in haemoglobin after knee joint surgery. Ann Rheum Dis. 1973;32(6):582–584. doi: 10.1136/ard.32.6.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faris PM, Ritter MA, Keating EM, Valeri CR. Unwashed filtered shed blood collected after knee and hip arthroplasties. A source of autologous red blood cells. J Bone Joint Surg Am. 1991;73(8):1169–78. doi: 10.2106/00004623-199173080-00005. [DOI] [PubMed] [Google Scholar]
- 25.Erskine JG, Fraser C, Simpson R, Protheroe K, Walker ID. Blood loss with knee joint replacement. J R Coll Surg Edinb. 1981;26(5):295–297. [PubMed] [Google Scholar]
- 26.Bao N, Zhou L, Cong Y, Guo T, Fan W, Chang Z, et al. Free fatty acids are responsible for the hidden blood loss in total hip and knee arthroplasty. Med Hypotheses. 2013;81(1):104–107. doi: 10.1016/j.mehy.2013.03.038. [DOI] [PubMed] [Google Scholar]
- 27.Wen L, Jin D, Xie W, Li Y, Chen W, Ding J, et al. Hidden Blood Loss in Posterior Lumbar Fusion Surgery: An Analysis of Risk Factors. Clin Spine Surg. 2018;31(4):180–184. doi: 10.1097/BSD.0000000000000626. [DOI] [PubMed] [Google Scholar]
- 28.Lei F, Li Z, He W, Tian X, Zheng L, Kang J, et al. Hidden blood loss and the risk factors after posterior lumbar fusion surgery: A retrospective study. Medicine (Baltimore) 2020;99(19):e20103. doi: 10.1097/MD.0000000000020103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bai B, Tian Y, Zhang YL, Ma MJ, Yu XR, Huang YG. Prediction of Hidden Blood Loss During Posterior Spinal Surgery. Chin Med Sci J. 2019;34(1):38–44. doi: 10.24920/003463. [DOI] [PubMed] [Google Scholar]
- 30.Wang H, Wang K, Lv B, Li W, Fan T, Zhao J, et al. Analysis of risk factors for perioperative hidden blood loss in unilateral biportal endoscopic spine surgery: a retrospective multicenter study. J Orthop Surg Res. 2021;16(1):559. doi: 10.1186/s13018-021-02698-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou Y, Fu X, Yang M, Ke S, Wang B, Li Z. Hidden blood loss and its possible risk factors in minimally invasive transforaminal lumbar interbody fusion. J Orthop Surg Res. 2020;15(1):445. doi: 10.1186/s13018-020-01971-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu D, Ren Z, Chen X, Zhuang Q, Hui S, Sheng L, et al. The further exploration of hidden blood loss in posterior lumbar fusion surgery. Orthop Traumatol Surg Res. 2017;103(4):527–530. doi: 10.1016/j.otsr.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 33.Clevenger B, Richards T. Pre-operative anaemia. Anaesthesia. 2015;70 Suppl 1:20–8, e6–8. doi: 10.1111/anae.12918. [DOI] [PubMed] [Google Scholar]
- 34.Kotze A, Harris A, Baker C, Iqbal T, Lavies N, Richards T, et al. British Committee for Standards in Haematology Guidelines on the Identification and Management of Pre-Operative Anaemia. Br J Haematol. 2015;171(3):322–331. doi: 10.1111/bjh.13623. [DOI] [PubMed] [Google Scholar]
- 35.Xu D, Chen X, Li Z, Ren Z, Zhuang Q, Li S. Tranexamic acid reduce hidden blood loss in posterior lumbar interbody fusion (PLIF) surgery. Medicine (Baltimore) 2020;99(11):e19552. doi: 10.1097/MD.0000000000019552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qian H, Yuan T, Tong J, Sun WS, Jin J, Chen WX, et al. Antioxidants Attenuate Oxidative Stress-Induced Hidden Blood Loss in Rats. Turk J Haematol. 2017;34(4):334–339. doi: 10.4274/tjh.2016.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miao K, Ni S, Zhou X, Xu N, Sun R, Zhuang C, et al. Hidden blood loss and its influential factors after total hip arthroplasty. J Orthop Surg Res. 2015;10:36. doi: 10.1186/s13018-015-0185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Y, Zhang L, Liu B, Pang M, Xie P, Chen Z, et al. Hidden and overall haemorrhage following minimally invasive and open transforaminal lumbar interbody fusion. J Orthop Traumatol. 2017;18(4):395–400. doi: 10.1007/s10195-017-0464-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article Supplementary Material, further inquiries can be directed to the corresponding author.