Abstract
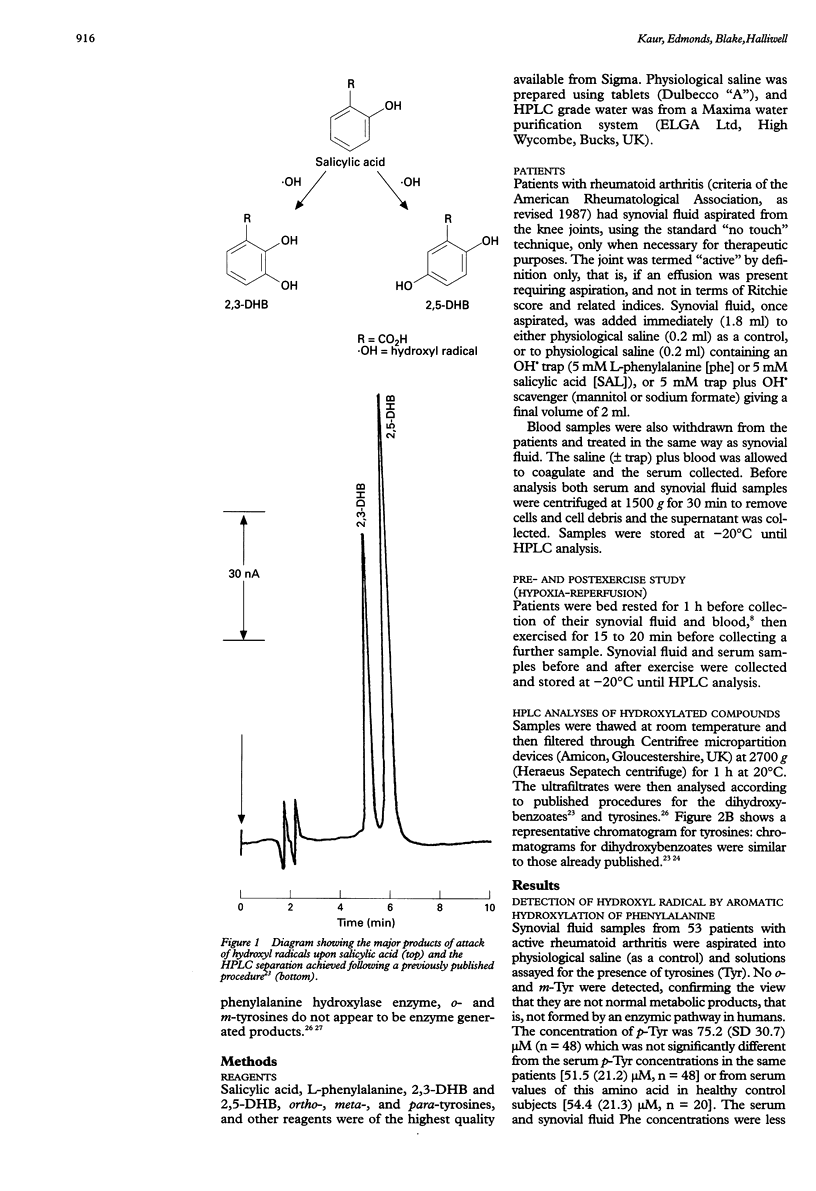
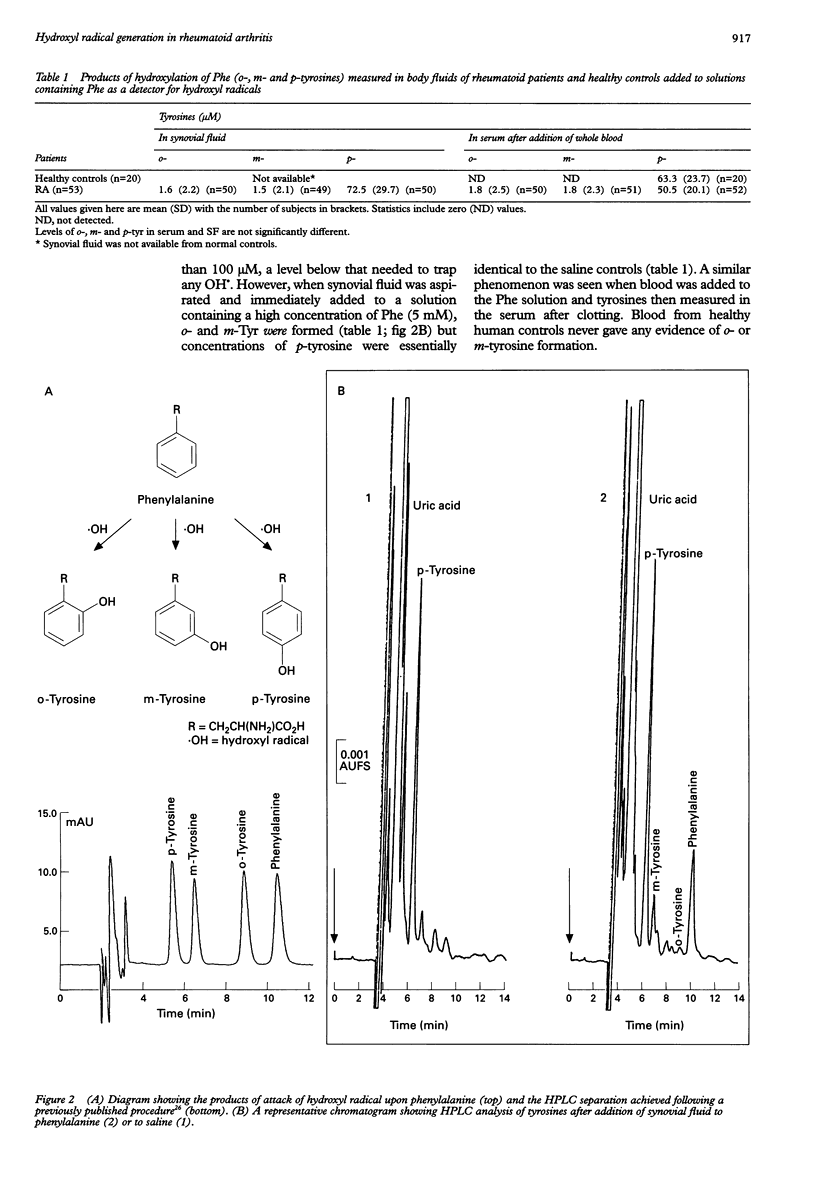
OBJECTIVE: To demonstrate directly that highly reactive hydroxyl radicals (OH.) can be generated in patients with rheumatoid arthritis and contribute to joint damage, and to examine the ability of blood to cause OH. generation. METHODS: The sensitive and specific technique of hydroxylation of aromatic compounds (salicylate and phenylalanine) was used to measure OH.. Synovial fluid and blood from patients with active rheumatoid arthritis were aspirated and immediately added to tubes containing salicylate and phenylalanine as detectors of OH., or to tubes containing saline as a control. Levels of specific products of attack of OH. upon salicylate (2,3- and 2,5-dihydroxybenzoates) and phenylalanine (ortho- and meta-tyrosines) were measured by high performance liquid chromatography. RESULTS: Synovial fluid samples aspirated into saline never contained ortho- or meta-tyrosines or 2,3-dihydroxybenzoate. Of 53 patients examined, synovial fluid and blood from 36 caused formation of ortho- and meta-tyrosines when aspirated into solutions containing phenylalanine. Repeated sampling from three "positive" patients showed consistent evidence of these hydroxylation products. Similarly, of 22 patients examined, synovial fluid and blood from 18 caused formation of 2,3- and 2,5-dihydroxybenzoates when aspirated into salicylate solutions. Further evidence for the role of OH. was provided by inhibition of the hydroxylation by the specific OH. scavengers mannitol and sodium formate. CONCLUSIONS: Aspirated knee joint fluids and blood from rheumatoid arthritis patients can generate OH., consistent with current views on the importance of this radical as a cytotoxic agent in rheumatoid disease. The ability of body fluids to cause OH. formation is not correlated with simple laboratory indices of disease activity, but is reproducible on sequential sampling from the same patients. The mechanism and significance of the phenomenon in rheumatoid arthritis pathology remain to be established.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aruoma O. I., Halliwell B., Butler J., Hoey B. M. Apparent inactivation of alpha 1-antiproteinase by sulphur-containing radicals derived from penicillamine. Biochem Pharmacol. 1989 Dec 15;38(24):4353–4357. doi: 10.1016/0006-2952(89)90642-4. [DOI] [PubMed] [Google Scholar]
- Bashir S., Harris G., Denman M. A., Blake D. R., Winyard P. G. Oxidative DNA damage and cellular sensitivity to oxidative stress in human autoimmune diseases. Ann Rheum Dis. 1993 Sep;52(9):659–666. doi: 10.1136/ard.52.9.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates E. J., Johnson C. C., Lowther D. A. Inhibition of proteoglycan synthesis by hydrogen peroxide in cultured bovine articular cartilage. Biochim Biophys Acta. 1985 Feb 15;838(2):221–228. doi: 10.1016/0304-4165(85)90082-0. [DOI] [PubMed] [Google Scholar]
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biemond P., Swaak A. J., van Eijk H. G., Koster J. F. Intraarticular ferritin-bound iron in rheumatoid arthritis. A factor that increases oxygen free radical-induced tissue destruction. Arthritis Rheum. 1986 Oct;29(10):1187–1193. doi: 10.1002/art.1780291002. [DOI] [PubMed] [Google Scholar]
- Blake D. R., Allen R. E., Lunec J. Free radicals in biological systems--a review orientated to inflammatory processes. Br Med Bull. 1987 Apr;43(2):371–385. doi: 10.1093/oxfordjournals.bmb.a072188. [DOI] [PubMed] [Google Scholar]
- Blake D. R., Merry P., Unsworth J., Kidd B. L., Outhwaite J. M., Ballard R., Morris C. J., Gray L., Lunec J. Hypoxic-reperfusion injury in the inflamed human joint. Lancet. 1989 Feb 11;1(8633):289–293. doi: 10.1016/s0140-6736(89)91305-6. [DOI] [PubMed] [Google Scholar]
- Candeias L. P., Patel K. B., Stratford M. R., Wardman P. Free hydroxyl radicals are formed on reaction between the neutrophil-derived species superoxide anion and hypochlorous acid. FEBS Lett. 1993 Oct 25;333(1-2):151–153. doi: 10.1016/0014-5793(93)80394-a. [DOI] [PubMed] [Google Scholar]
- Cleland L. G., Lowthian P. J., Imhoff D., Bochner F., Betts W. H., O'Callaghan J. Plasma and synovial fluid gentisate in patients receiving salicylate therapy. J Rheumatol. 1985 Feb;12(1):136–139. [PubMed] [Google Scholar]
- Edmonds S. E., Blake D. R., Morris C. J., Winyard P. G. An imaginative approach to synovitis--the role of hypoxic reperfusion damage in arthritis. J Rheumatol Suppl. 1993 Apr;37:26–31. [PubMed] [Google Scholar]
- Eggleton P., Wang L., Penhallow J., Crawford N., Brown K. A. Differences in oxidative response of subpopulations of neutrophils from healthy subjects and patients with rheumatoid arthritis. Ann Rheum Dis. 1995 Nov;54(11):916–923. doi: 10.1136/ard.54.11.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P. J., Cecchini R., Halliwell B. Oxidative damage to lipids and alpha 1-antiproteinase by phenylbutazone in the presence of haem proteins: protection by ascorbic acid. Biochem Pharmacol. 1992 Sep 1;44(5):981–984. doi: 10.1016/0006-2952(92)90131-2. [DOI] [PubMed] [Google Scholar]
- Farrell A. J., Blake D. R., Palmer R. M., Moncada S. Increased concentrations of nitrite in synovial fluid and serum samples suggest increased nitric oxide synthesis in rheumatic diseases. Ann Rheum Dis. 1992 Nov;51(11):1219–1222. doi: 10.1136/ard.51.11.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths H. R., Lunec J. Effects of reactive oxygen species on immunoglobulin G function. Mol Aspects Med. 1991;12(2):107–119. doi: 10.1016/0098-2997(91)90006-8. [DOI] [PubMed] [Google Scholar]
- Grootveld M., Halliwell B. Aromatic hydroxylation as a potential measure of hydroxyl-radical formation in vivo. Identification of hydroxylated derivatives of salicylate in human body fluids. Biochem J. 1986 Jul 15;237(2):499–504. doi: 10.1042/bj2370499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grootveld M., Halliwell B. Measurement of allantoin and uric acid in human body fluids. A potential index of free-radical reactions in vivo? Biochem J. 1987 May 1;243(3):803–808. doi: 10.1042/bj2430803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grootveld M., Henderson E. B., Farrell A., Blake D. R., Parkes H. G., Haycock P. Oxidative damage to hyaluronate and glucose in synovial fluid during exercise of the inflamed rheumatoid joint. Detection of abnormal low-molecular-mass metabolites by proton-n.m.r. spectroscopy. Biochem J. 1991 Jan 15;273(Pt 2):459–467. doi: 10.1042/bj2730459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M. Bleomycin-detectable iron in knee-joint synovial fluid from arthritic patients and its relationship to the extracellular antioxidant activities of caeruloplasmin, transferrin and lactoferrin. Biochem J. 1987 Jul 15;245(2):415–421. doi: 10.1042/bj2450415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M. Iron promoters of the Fenton reaction and lipid peroxidation can be released from haemoglobin by peroxides. FEBS Lett. 1986 Jun 9;201(2):291–295. doi: 10.1016/0014-5793(86)80626-3. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Evans P. J., Kaur H., Chirico S. Drug derived radicals: mediators of the side effects of anti-inflammatory drugs? Ann Rheum Dis. 1992 Nov;51(11):1261–1263. doi: 10.1136/ard.51.11.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. The antioxidants of human extracellular fluids. Arch Biochem Biophys. 1990 Jul;280(1):1–8. doi: 10.1016/0003-9861(90)90510-6. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidative stress, nutrition and health. Experimental strategies for optimization of nutritional antioxidant intake in humans. Free Radic Res. 1996 Jul;25(1):57–74. doi: 10.3109/10715769609145656. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxygen radicals, nitric oxide and human inflammatory joint disease. Ann Rheum Dis. 1995 Jun;54(6):505–510. doi: 10.1136/ard.54.6.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron salts. Its role in degradation of hyaluronic acid by a superoxide-generating system. FEBS Lett. 1978 Dec 15;96(2):238–242. doi: 10.1016/0014-5793(78)80409-8. [DOI] [PubMed] [Google Scholar]
- Hogg N., Darley-Usmar V. M., Wilson M. T., Moncada S. Production of hydroxyl radicals from the simultaneous generation of superoxide and nitric oxide. Biochem J. 1992 Jan 15;281(Pt 2):419–424. doi: 10.1042/bj2810419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humad S., Zarling E., Clapper M., Skosey J. L. Breath pentane excretion as a marker of disease activity in rheumatoid arthritis. Free Radic Res Commun. 1988;5(2):101–106. doi: 10.3109/10715768809066917. [DOI] [PubMed] [Google Scholar]
- Ingelman-Sundberg M., Kaur H., Terelius Y., Persson J. O., Halliwell B. Hydroxylation of salicylate by microsomal fractions and cytochrome P-450. Lack of production of 2,3-dihydroxybenzoate unless hydroxyl radical formation is permitted. Biochem J. 1991 Jun 15;276(Pt 3):753–757. doi: 10.1042/bj2760753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H., Fagerheim I., Grootveld M., Puppo A., Halliwell B. Aromatic hydroxylation of phenylalanine as an assay for hydroxyl radicals: application to activated human neutrophils and to the heme protein leghemoglobin. Anal Biochem. 1988 Aug 1;172(2):360–367. doi: 10.1016/0003-2697(88)90456-3. [DOI] [PubMed] [Google Scholar]
- Kaur H., Halliwell B. Aromatic hydroxylation of phenylalanine as an assay for hydroxyl radicals. Measurement of hydroxyl radical formation from ozone and in blood from premature babies using improved HPLC methodology. Anal Biochem. 1994 Jul;220(1):11–15. doi: 10.1006/abio.1994.1291. [DOI] [PubMed] [Google Scholar]
- Kaur H., Halliwell B. Evidence for nitric oxide-mediated oxidative damage in chronic inflammation. Nitrotyrosine in serum and synovial fluid from rheumatoid patients. FEBS Lett. 1994 Aug 15;350(1):9–12. doi: 10.1016/0014-5793(94)00722-5. [DOI] [PubMed] [Google Scholar]
- Lunec J., Blake D. R., McCleary S. J., Brailsford S., Bacon P. A. Self-perpetuating mechanisms of immunoglobulin G aggregation in rheumatoid inflammation. J Clin Invest. 1985 Dec;76(6):2084–2090. doi: 10.1172/JCI112212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunec J., Herbert K., Blount S., Griffiths H. R., Emery P. 8-Hydroxydeoxyguanosine. A marker of oxidative DNA damage in systemic lupus erythematosus. FEBS Lett. 1994 Jul 11;348(2):131–138. doi: 10.1016/0014-5793(94)00583-4. [DOI] [PubMed] [Google Scholar]
- Pou S., Nguyen S. Y., Gladwell T., Rosen G. M. Does peroxynitrite generate hydroxyl radical? Biochim Biophys Acta. 1995 May 11;1244(1):62–68. doi: 10.1016/0304-4165(94)00197-6. [DOI] [PubMed] [Google Scholar]
- Puppo A., Halliwell B. Formation of hydroxyl radicals from hydrogen peroxide in the presence of iron. Is haemoglobin a biological Fenton reagent? Biochem J. 1988 Jan 1;249(1):185–190. doi: 10.1042/bj2490185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J., Watson F., Bucknall R. C., Edwards S. W. Activation of neutrophil reactive-oxidant production by synovial fluid from patients with inflammatory joint disease. Soluble and insoluble immunoglobulin aggregates activate different pathways in primed and unprimed cells. Biochem J. 1992 Sep 1;286(Pt 2):345–351. doi: 10.1042/bj2860345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley D., Gutteridge J. M., Blake D., Farr M., Halliwell B. Lipid peroxidation in rheumatoid arthritis: thiobarbituric acid-reactive material and catalytic iron salts in synovial fluid from rheumatoid patients. Clin Sci (Lond) 1984 Jun;66(6):691–695. doi: 10.1042/cs0660691. [DOI] [PubMed] [Google Scholar]
- Schalkwijk J., van den Berg W. B., van de Putte L. B., Joosten L. A. An experimental model for hydrogen peroxide-induced tissue damage. Effects of a single inflammatory mediator on (peri)articular tissues. Arthritis Rheum. 1986 Apr;29(4):532–538. doi: 10.1002/art.1780290411. [DOI] [PubMed] [Google Scholar]
- Situnayake R. D., Thurnham D. I., Kootathep S., Chirico S., Lunec J., Davis M., McConkey B. Chain breaking antioxidant status in rheumatoid arthritis: clinical and laboratory correlates. Ann Rheum Dis. 1991 Feb;50(2):81–86. doi: 10.1136/ard.50.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C. R., Benboubetra M., Harrison R., Sahinoglu T., Smith E. C., Blake D. R. Localisation of xanthine oxidase to synovial endothelium. Ann Rheum Dis. 1991 Nov;50(11):760–762. doi: 10.1136/ard.50.11.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J. Z., Kaur H., Halliwell B., Li X. Y., Bolli R. Use of aromatic hydroxylation of phenylalanine to measure production of hydroxyl radicals after myocardial ischemia in vivo. Direct evidence for a pathogenetic role of the hydroxyl radical in myocardial stunning. Circ Res. 1993 Sep;73(3):534–549. doi: 10.1161/01.res.73.3.534. [DOI] [PubMed] [Google Scholar]
- van der Vliet A., O'Neill C. A., Halliwell B., Cross C. E., Kaur H. Aromatic hydroxylation and nitration of phenylalanine and tyrosine by peroxynitrite. Evidence for hydroxyl radical production from peroxynitrite. FEBS Lett. 1994 Feb 14;339(1-2):89–92. doi: 10.1016/0014-5793(94)80391-9. [DOI] [PubMed] [Google Scholar]


