Abstract
Few studies have evaluated the effects of pulmonary arterial hypertension therapies on pericardial effusion. We evaluated hemodynamics, echocardiograms, and outcomes for 119 parenteral prostanoid‐treated patients. We discovered an increased frequency of pericardial effusions posttreatment, and that a moderate‐large pericardial effusion at initiation, but not at 1st follow‐up, was significantly associated with mortality.
Keywords: Echocardiogram, Pericardial Effusion, Prostacylcline Therapy, Pulmonary Arterial Hypertension
INTRODUCTION
In patients with pulmonary arterial hypertension (PAH), prior studies have found that the pericardial effusion at diagnosis is more common in patients with other markers of poor prognosis including particularly elevated right atrial (RA) pressure and that the presence of pericardial effusion associates with increased risk of death. However, few studies have evaluated the effects of PAH therapies on the prevalence of pericardial effusion or evaluated the clinical significance of posttreatment pericardial effusions. We therefore sought to evaluate the frequency, size, and clinical implications of pericardial effusions at baseline and 1st follow‐up in a cohort of PAH patients initiating parenteral prostanoid therapies.
METHODS
This retrospective cohort study included patients with PAH initiated on IV epoprostenol or IV/SC treprostinil between 2007 and 2016 at the University of Texas Southwestern Medical Center. Institutional review board approval was obtained from the University of Texas Southwestern Center Human Research Protection Program (#052015‐041), including a waiver of informed consent. Echocardiographic, right heart catheterization, functional class, 6MWD, and NT‐proBNP results were performed as standard of care tests, with the timing was determined by the treating clinician. First follow‐up was defined as the time of 1st echocardiogram performed after at least 90 days of IV/SC therapy. Survival outcomes by baseline and 1st follow‐up hemodynamics and echocardiographic findings as well as changes in hemodynamics and echocardiogram results between pre and postparenteral prostanoid therapy have been published previously. 1 , 2 Inclusion criteria were treatment with a parenteral prostanoid and results available for a pre‐IV/SC therapy echocardiogram; this results in a slightly different sample size versus prior studies. For the current study, baseline and first follow‐up characteristics for patients with and without pericardial effusion were compared, including demographics, functional class, NTproBNP, 6‐min walk distance (6MWD), echocardiography, and hemodynamic results. For transplant‐free survival, outcomes were assessed both by presence of effusion and by effusion size (none, small, moderate‐severe). Patients undergoing transplant were censored at the time of transplant. Analyses included Cox‐proportional hazards (survival), Students t‐test (pre/post comparison for continuous variables), analysis of variance (ANOVA) with Tukey–Kramer's all pairs comparison (RA pressure by pericardial effusion size), and chi‐square (echocardiography comparisons). Analyses were performed using NCSS 2022.
RESULTS
119 PAH patients initiated on IV/SC therapy were followed for a median of 7 years. Most patients were female (81%) and had idiopathic or CTD‐PAH, 2 and the median age at IV therapy initiation was 47 years. Survival at 1, 2, and 3 years was 86%, 78%, and 67%, respectively. The median time from diagnosis to IV/SC initiation was 625 days (Interquartile range [IQR]: 130–1233 days). Pericardial effusion was present on echocardiogram in 43 of 119 patients before initiation of IV/SC therapy (36%), with similar PAH etiology distribution amongst those with and without an effusion. Patients with a pericardial effusion at the time of IV/SC therapy had greater PAH severity on multiple measures of prognosis, compared to patients without a pericardial effusion, and this was statistically significant for log NTproBNP, RA pressure at catheterization, and TR severity and IVC characteristics on echocardiogram (Table 1).
Table 1.
Comparison between patients before and after receiving IV/SC Therapy.
| N | Severity (echo) | Before IV/SC therapy | p‐value | N | 1st f/u after IV/SC therapy | p‐value | |||
|---|---|---|---|---|---|---|---|---|---|
| Effusion | 119 | Absent | Present | 104 | Absent | Present | |||
| Patient N | 76 | 43 | 57 | 47 | |||||
| Etiology | |||||||||
| Idiopathic PAH | 45 | 26 | 0.99 | 34 | 29 | 0.95 | |||
| CTD PAH | 27 | 16 | 19 | 17 | |||||
| CHD PAH | 4 | 1 | 4 | 1 | |||||
| Log NTproBNP | 99 | 3.16 ± 0.68 | 3.50 ± 0.61 | 0.01* | 104 | 2.89 ± 0.91 | 3.03 ± 0.74 | 0.43 | |
| 6MWD | 97 | 261 ± 132 | 278 ± 140 | 0.54 | 104 | 336.6 ± 107 | 294.8 ± 134 | 0.08 | |
| Catheterization | |||||||||
| RA (mmHg) | 117 | 11.1 ± 6.9 | 14.2 ± 6.8 | 0.018* | 102 | 7.4 ± 5.8 | 8.1 ± 6 | 0.57 | |
| Mean PA (mmHg) | 118 | 55 ± 10.7 | 0.44 | 102 | 43.6 ± 11.5 | 44.7 ± 11.2 | 0.62 | ||
| Wedge (mmHg) | 115 | 9.5 ± 5 | 10.8 ± 4.9 | 0.21 | 102 | 9.4 ± 5.1 | 9.5 ± 4.6 | 0.87 | |
| PVR (Woods) | 115 | 10.8 ± 3.9 | 10.9 ± 5 | 0.86 | 102 | 6.1 ± 3 | 6.7 ± 3.1 | 0.29 | |
| CI (L/min/m2) | 118 | 2.36 ± 0.76 | 2.31 ± 0.79 | 0.68 | 102 | 3.3 ± 0.87 | 3.1 ± 0.79 | 0.23 | |
| Echocardiogram | |||||||||
| RA size | 116 | Normal–mild | 21 | 8 | 0.16 | 102 | 19 | 10 | 0.027* |
| Moderate | 26 | 12 | 20 | 11 | |||||
| Severe | 26 | 23 | 16 | 26 | |||||
| LA size | 116 | Normal–mild | 67 | 40 | 0.54 | 103 | 49 | 43 | 0.79 |
| Moderate | 6 | 3 | 4 | 2 | |||||
| Severe | 2 | 0 | 3 | 2 | |||||
| RV size | 118 | Normal–mild | 12 | 4 | 0.58 | 104 | 16 | 8 | 0.28 |
| Moderate | 25 | 14 | 24 | 19 | |||||
| Severe | 38 | 25 | 17 | 20 | |||||
| RV function | 117 | Normal–mild | 19 | 12 | 0.47 | 103 | 30 | 17 | 0.25 |
| Moderate | 28 | 11 | 14 | 17 | |||||
| Severe | 28 | 19 | 13 | 12 | |||||
| TR severity | 119 | None‐mild | 30 | 14 | 0.048* | 104 | 32 | 17 | 0.051 |
| Moderate | 30 | 11 | 20 | 19 | |||||
| Severe | 16 | 18 | 5 | 11 | |||||
| IVC char. | 79 | Normal | 27 | 7 | 0.032* | 82 | 29 | 18 | 0.092 |
| Dilated | 11 | 12 | 7 | 14 | |||||
| Fails to collapse (±dilated) | 12 | 10 | 8 | 6 |
Note: Statistically p‐values (p < 0.05) are denoted with an *. Size, function, and severity on echocardiogram reported as normal, mildly, moderately, or severely abnormal (qualitative), while IVC characteristics included normal, dilated, and fails to collapse (with or without also being dilated). Abbreviations: 6MWD, 6‐min walk distance; Char., characteristic; CHD congenital heart disease; CI, cardiac index; CTD connective tissue disease; IVC, inferior vena cava; LA, left atrial; PVR, pulmonary vascular resistance; RA, right atrial; PA, pulmonary artery; RV, right ventricular; TR, tricuspid regurgitation.
A moderate or large pericardial effusion before IV/SC therapy initiation, present in only seven patients, was associated with increased mortality risk (hazard ratio [HR]: 2.57, 95% confidence interval [CI] of [1.36–64.87], p = 0.0037), while a small effusion was not associated with increased risk, with an unexpected possible inverse association (HR: 0.37, 95% CI: 0.21–0.64, p = 0.004). Presence of an effusion was particularly common among those dying in the 1st 90 days after IV/SC therapy initiation, with four of seven patients (57%) with a moderate or large effusion dying during this time period compared with early deaths in 2 of 36 (5.5%) with a small effusion and 2 of 76 (2.6%) with no pericardial effusion.
One hundred and four (84.7%) patients underwent a follow‐up echocardiogram after at least 90 days of IV/SC therapy (median 168 [IQR: 120–241 days]); reasons for missing data included death before 90 days (N = 8) and missing data for other reasons (N = 7). Compared with pre‐IV/SC therapy echocardiogram, the number with any pericardial effusion increased from 35 to 47 patients (34% vs. 45%, p < 0.01), including 21 patients who developed a new effusion during this time versus only nine patients whose effusion resolved. The presence of a pericardial effusion at 1st follow‐up was significantly associated with only RA size on echocardiogram (Figure 1), though a trend toward greater TR severity and abnormal IVC characteristics was also seen. There was no significant association between 1st follow‐up pericardial effusion of any size and mortality (small effusion HR 0.74 [95% CI: 0.45–1.22, p = NS]; moderate‐large effusion HR 1.66 [95% CI: 0.76–3.64, p = 0.2]).
Figure 1.
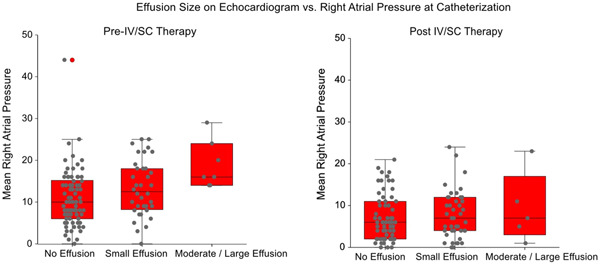
Comparison of mean right atrial pressure to size of pericardial effusion before and after IV/SC therapy.
Finally, we also evaluated pericardial effusion size versus RA pressure in greater detail, as this is the hemodynamic measure reported as most strongly associated with the presence of a pericardial effusion. Before IV/SC therapy, RA pressure increased in a stepwise manner with pericardial effusion size, with mean RA pressure of 11 ± 7, 14 ± 7, and 19 ± 6 mmHg for no effusion, small and moderate‐large effusion (ANOVA p = 0.008; for pair‐wise comparisons RA pressure in the no effusion group was significantly different vs. the RA pressure in the moderate‐large effusion group, p = 0.01). In contrast, much smaller differences were seen in RA pressures at first follow‐up, with RA pressures of 7 ± 6, 8 ± 6, and 9 ± 8 mmHg for those with no effusion, small and moderate‐large effusion (ANOVA p = 0.74).
DISCUSSION
In this study of 119 patients with PAH, pericardial effusion was numerically more common in patients following IV/SC prostanoid therapy (43% at follow‐up vs. 36% before initiation). Although most effusions at follow‐up were small (88%), five patients had moderate or large effusions at first follow‐up.
Prior studies exploring changes in pericardial effusion size after treatment with PAH therapies have had varied results. In the BREATHE‐1 clinical trial of bosentan versus placebo, a possible beneficial effect of bosentan on pericardial effusion score was reported (Echocardiographic substudy; N = 85; treatment effect p‐value = 0.053). In contrast, in the pivotal randomized controlled trial of epoprostenol in idiopathic PAH, there was no change in the pericardial effusion score between baseline and 12 weeks in patients receiving epoprostenol (N = 41), and similar results were seen in patients in the control arm. 3 , 4 , 5
Most pericardial effusions in PAH are thought to occur due to elevations in right‐sided pressures leading to myocardial interstitial edema formation with increased epicardial transudation, and reduced lymphatic outflow related to elevated systemic venous pressures. RA pressure at catheterization is the hemodynamic value most strongly associated with the presence and size of pericardial effusions. 6 , 7 Thus, given the significant hemodynamic improvement seen in our cohort, including RA pressures, we anticipated that fewer pericardial effusions would be present amongst post‐IV/SC treated patients, contrary to what was seen. This then raises the question of whether longstanding pulmonary hypertension may lead to other physiologic changes in the myocardium, pericardium or lymphatic systems that could lead to pericardial effusion. For example, elevated central venous pressure can lead to thoracic duct remodeling due to increased thoracic duct diameter and increased wall thickness. 8 This remodeling could lead to lymphatic valve insufficiency, impairing unidirectional lymph transport and allowing lymph backflow even at lower central venous pressures. The association between pericardial effusion presence and RA size on echocardiogram, a marker of longstanding elevations in RA pressure, indirectly provides support for this hypothesis.
Other possibilities, such as inflammatory pericardial effusions associated with autoimmune conditions, seem unlikely as we did not see a higher rate of pericardial effusion in those with CTD‐PAH in this cohort, though it is still possible that this may have contributed in individual patients. It is possible that alternative mechanisms could be involved. Although we lack a definitive explanation, one other observational study has described a high rate of new pericardial effusion in IV epoprostenol‐treated patients, with 15 of 23 patients (65%) developing a new pericardial effusion during treatment, 9 and additional studies in both prostanoid and non‐prostanoid treated patients would be helpful.
From a survival standpoint, we saw a significantly increased risk of death associated with moderate‐large effusion at IV/SC therapy initiation, versus a more modestly elevated HR at follow‐up that was not statistically significant. We suspect that this latter result may partially relate to the sample size, and the modest number of patients with moderate to large effusions. We also suspect that the inverse association between small effusion size at baseline and outcome may be spurious. Limitations to the study include the small sample size and lack of a validation cohort; as such these results should be considered hypothesis generating. As discussed above, there are numerous reasons why pericardial effusions can form in PAH patients including if elevated central pressures are associated with pericardial effusions and how proteinoids affect this. This study should be used to form larger studies that evaluate this question further.
In summary, in our cohort, we saw an increase in the presence of pericardial effusions among patients treated with IV/SC prostanoids. Most effusions were small and not hemodynamically significant themselves, but new moderate or large effusions did develop in some patients. Further study is warranted to explore both the pathophysiology and reproducibility of this finding.
AUTHOR CONTRIBUTIONS
All authors contributed equally to the development of this manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
This material is the authors' own original work, which has not been previously published elsewhere. The paper is not currently being considered for publication elsewhere. The paper reflects the authors' own research and analysis in a truthful and complete manner. The paper properly credits the meaningful contributions of co‐authors and co‐researchers. The results are appropriately placed in the context of prior and existing research. All sources used are properly disclosed (correct citation). Literally copying of text must be indicated as such by using quotation marks and giving proper references. All authors have been personally and actively involved in substantial work leading to the paper, and will take public responsibility for its content. The violation of the Ethical Statement rules may result in severe consequences. The author agrees with the above statements and declares that this submission follows the policies of Pulmonary Circulation as outlined in the Guide for Authors and in the Ethical Statement.
ACKNOWLEDGMENTS
The author would like to thank all of the contributors for this article and UTSW for help with this research endeavor.
Abu‐Rmaileh M, Mirza O, Patel C, Shah T, Hardin EA, Bartolome SD, Chin KM. Prognosis of pulmonary arterial hypertension patients with pericardial effusion before and after initiation of parenteral prostacyclin therapy. Pulm Circ. 2023;13:e12226. 10.1002/pul2.12226
REFERENCES
- 1. Bartolome SD, Sood N, Shah TG, Styrvoky K, Torres F, Chin KM. Mortality in patients with pulmonary arterial hypertension treated with continuous prostanoids. Chest. 2018;154(3):532–40. [DOI] [PubMed] [Google Scholar]
- 2. Shah T, Manthena P, Patel C, Chuah A, Hardin EA, Torres F, Bartolome SD, Chin KM. Prognostic value of echocardiographic variables prior to and following initiation of parenteral prostacyclin therapy. Chest. 2022;162(3):669–83. [DOI] [PubMed] [Google Scholar]
- 3. Raymond RJ, Hinderliter AL, Willis PW, Ralph D, Caldwell EJ, Williams W, Ettinger NA, Hill NS, Summer WR, de Boisblanc B, Schwartz T, Koch G, Clayton LM, Jöbsis MM, Crow JW, Long W. Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J Am Coll Cardiol. 2002;39(7):1214–9. [DOI] [PubMed] [Google Scholar]
- 4. Hinderliter AL, Willis PW, Barst RJ, Rich S, Rubin LJ, Badesch DB, Groves BM, McGoon MD, Tapson VF, Bourge RC, Brundage BH, Koerner SK, Langleben D, Keller CA, Murali S, Uretsky BF, Koch G, Li S, Clayton LM, Jöbsis MM, Blackburn SD, Crow JW, Long WA. Effects of long‐term infusion of prostacyclin (epoprostenol) on echocardiographic measures of right ventricular structure and function in primary pulmonary hypertension. Circulation. 1997;95(6):1479–86. [DOI] [PubMed] [Google Scholar]
- 5. Hinderliter AL, Willis PW, Long W, Clarke WR, Ralph D, Caldwell EJ, Williams W, Ettinger NA, Hill NS, Summer WR, de Boisblanc B, Koch G, Li S, Clayton LM, Jöbsis MM, Crow JW. Frequency and prognostic significance of pericardial effusion in primary pulmonary hypertension. Am J Cardiol. 1999;84(4):481–4. [DOI] [PubMed] [Google Scholar]
- 6. Stewart RH, Cox CS, Allen SJ, Laine GA. Myocardial edema provides a link between pulmonary arterial hypertension and pericardial effusion. Circulation. 2022;145(11):793–5. [DOI] [PubMed] [Google Scholar]
- 7. Sahay S, Tonelli AR. Pericardial effusion in pulmonary arterial hypertension. Pulm Circ. 2013;3(3):467–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lu X, Wang M, Han L, Krieger J, Ivers J, Chambers S, Itkin M, Burkhoff D, Kassab GS. Changes of thoracic duct flow and morphology in an animal model of elevated central venous pressure. Front Physiol. 2022;13:798284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shimony A, Fox BD, Langleben D, Rudski LG. Incidence and significance of pericardial effusion in patients with pulmonary arterial hypertension. Can J Cardiol. 2013;29(6):678–82. [DOI] [PubMed] [Google Scholar]


