Abstract
Background:
During early development, alcohol exposure causes apoptotic cell death in discrete regions of the embryo which are associated with distinctive patterns of later-life abnormalities. In gastrulation, which occurs during the third week of human pregnancy, alcohol targets the ectoderm, the precursor of the eyes, face, and brain. This midline tissue loss leads to the craniofacial dysmorphologies, such as microphthalmia and a smooth philtrum, which define fetal alcohol syndrome (FAS). An important regulator of alcohol-induced cell death is the pro-apoptotic protein Bax. The current study determines if mice lacking the Bax gene are less susceptible to the pathogenic effects of gastrulation-stage alcohol exposure.
Methods:
Male and female Bax+/− mice mated to produce embryos with full (−/−) or partial (+/−) Bax deletions, or Bax+/+ wild-type controls. On gestational day 7 (GD 7), embryos received two alcohol (2.9 g/kg, four hours apart), or control exposures. A subset of embryos was collected 12 hours later and examined for the presence of apoptotic cell death, while others were examined on GD 17 for the presence of FAS-like facial features.
Results:
Full Bax deletion reduced embryonic apoptotic cell death and the incidence of fetal eye and face malformations, indicating that Bax normally facilitates the development of alcohol-induced defects. An RNA-seq analysis of GD 7 Bax+/+ and Bax−/− embryos revealed 63 differentially expressed genes, some of which may interact with the Bax deletion to further protect against apoptosis.
Conclusions:
Overall, these experiments identify that Bax is a primary teratogenic mechanism of gastrulation-stage alcohol exposure.
Keywords: Apoptosis, Ethanol, Eye Defects, Fetal Alcohol Syndrome, Knockout Mouse-RNA-sequencing
Prenatal alcohol exposure (PAE) is one of the most preventable causes of birth defects. However, fetal alcohol spectrum disorders (FASD) are common; conservative estimates among first graders in the U.S. ranged between 1-5%, and less conservative estimates found as many as 9% of children had FASD (May et al., 2018). The most severe FASD is fetal alcohol syndrome (FAS) which is characterized by distinctive craniofacial appearances that fall within the holoprosencephaly spectrum, including a small or undefined philtrum, a thin upper lip, narrowly spaced eyes, epicanthal folds, short palpebral fissures, micropthalmia, and to a lesser extent, small lower jaws and overall flat midface (Del Campo & Jones, 2017). PAE also alters several brain structures and central nervous system functions that cause people with FAS to have cognitive and socio-emotional difficulties (Kelly, Day, & Streissguth, 2000; Mattson, Crocker, & Nguyen, 2011; Streissguth et al., 1991). These craniofacial and brain malformations are due to alcohol exposures during the gastrulation stage of development, which takes place during the 3rd week of pregnancy, a time when many pregnancies are still unrecognized.
One of the challenges to understanding the etiology of FASD is that PAE affects people differently and not all exposures result in recognizable FASD. In fact, only an estimated 4.3% of children will develop FAS following heavy alcohol exposure (Abel, 1995). Like other teratogenic exposures, the amount, duration, and timing of PAE are factors that are often difficult to discern in clinical studies but that will nonetheless influence developmental outcomes (Petrelli, Weinberg, & Hicks, 2018). Moreover, genetic factors are very important in FASD, as highlighted by a study in alcohol-exposed twins that found that 60% of dizygotic twins had identical diagnoses of FAS and similar IQ scores, while monozygotic twins were 100% concordant for these traits (Streissguth & Dehaene, 1993). These findings of broad genetic influences on PAE outcomes are supported by animal studies demonstrating species and strain differences in response to PAE, as well as those that reveal the role of specific single genes (see, Eberhart & Parnell, 2016; Karacay & Bonthius, 2015). These single gene manipulation studies are also able to uncover the precise mechanisms by which PAE can perturb development and delineate those genes that are protective, and those which confer vulnerability to PAE.
Excessive embryonic cell death is a major pathogenic mechanism for defects cause by PAE. During the gastrulation stage (gestational day 17-20 in human pregnancy), PAE targets the ectodermal tissue that is destined to become the face and the brain, causing apoptosis (Dunty, Chen, Zucker, Dehart, & Sulik, 2001) and disrupting vital cell signaling pathways, including Sonic hedgehog (Shh) (Ahlgren, Thakur, & Bronner-Fraser, 2002; Boschen, Fish, & Parnell, 2021; Hong & Krauss, 2012; Zhang, Ojiaku, & Cole, 2013). Embryonic apoptosis is caused largely by caspase activation following permeabilization of the mitochondrial outer membrane (MOM), as opposed to activation of death receptors through the extrinsic pathway (Voss & Strasser, 2020). Bax (Bcl-2-like protein 4) is a pro-apoptotic effector protein that, like its partner Bak (Bcl-2-antagonist/killer 1), mediates the MOM potential. Bax is a normally inactive monomer prevented from accumulating in the mitochondria by the anti-apoptotic protein Bcl-2. Cytotoxic stimuli activate pro-apoptotic triggers, such as Bad, Puma, and Noxa, which alleviate the repression of Bax by inhibiting Bcl-2, and by directly activating Bax itself. Bax activation involves several conformational changes that allow it to join the mitochondrial membrane and oligomerize to form pores that disrupt the MOM (Pena-Blanco & Garcia-Saez, 2018).
The objective of the current study was to determine if mice lacking the Bax gene are protected from the effects of gastrulation-stage alcohol exposure. Full loss of the Bax gene is not lethal and the mice develop without any major developmental abnormalities, although Bax−/− male mice are infertile (Knudson, Tung, Tourtellotte, Brown, & Korsmeyer, 1995). More subtle effects of Bax deletion have been observed, particularly in the brain and eye, including increased neuronal number (Deckwerth et al., 1996; White, Keller-Peck, Knudson, Korsmeyer, & Snider, 1998), absence of size differences in two sexually dimorphic nuclei (Forger & Peskin, 2005), cerebellar migration defects (Jung et al., 2008), increased retinal cell numbers, glial cell density and altered microglial shape (Kawai et al., 2009; Mac Nair, Schlamp, Montgomery, Shestopalov, & Nickells, 2016; Mosinger Ogilvie, Deckwerth, Knudson, & Korsmeyer, 1998). Importantly, Bax deficiency reduces neuronal and retinal cell death resulting from various injuries (Deckwerth et al., 1996; Robinson, Conley, & Kern, 2003; Semaan, Li, & Nickells, 2010; W. Sun & Oppenheim, 2003), including alcohol exposure. The role of Bax in FASD has been explored in third trimester-equivalent models of cerebellar and neocortical cell death (Britton & Miller, 2018; Heaton, Paiva, Madorsky, Siler-Marsiglio, & Shaw, 2006; Young et al., 2003); in each of these models, Bax deletion protects against neuronal loss. However, it is unclear whether Bax plays a role in alcohol-induced cell death occurring during gastrulation, before the onset of neurogenesis. We hypothesized Bax deletion would protect mice from developing gastrulation alcohol-induced craniofacial and brain dysmorphologies.
Methods
Animals:
Female and male mice on a C57BL/6 background, heterozygous for a Bax gene deletion were bred in the laboratory from founders generously donated by Mohanish Deshmukh (UNC Chapel Hill, originally obtained from Dr. Stanley Korsmeyer [(Knudson et al., 1995)]). Timed pregnancies were established by housing one or two nulliparous female Bax+/− mice with one Bax+/− male for 1-2 h. Gestational day (GD) 0, was defined as the beginning of the breeding period in which a copulation plug was detected. All mice lived in ventilated, polycarbonate cages containing a polycarbonate hut, nesting material, and cob bedding. Food (Purina Isopro RMH 3000, Purina, St. Louis, MO) and water were freely available through a wire food hopper and a drinking spout. The vivarium was 21±1°C and 30-40% humidity and had a 12:12 light:dark cycle (lights on at 7:00 AM). All experiments followed the NIH guidelines using methods approved by the IACUC of University of North Carolina at Chapel Hill.
Genotyping protocol:
Genotyping for Bax DNA was performed on tail or embryonic tissue using the primers: mutant forward CCG CTT CCA TTG CTC AGC GG; wild-type forward GAG CTG ATC AGA ACC ATC ATG; common GTT GAC CAG AGT GGC GTA GG.
Prenatal alcohol exposure (PAE):
Pregnant dams were housed in groups no larger than five. At GD 7, they received two intra-peritoneal (i.p.) injections of 25% ethanol (v/v in lactated Ringer’s solution, LRS; n=33 dams) at a 2.9 g/kg dose, given 4 h apart. This dosing caused peak blood alcohol levels of ~445±29 (SEM) mg/dl, taken 30 min after the 2nd injection from ~30 ul of tail blood analyzed using the Analox AM1 (Analox Instruments, Toronto, ON). Control dams (n=28) were injected similarly with the vehicle alone (1.5 ml/100 g body weight).
Nile Blue experiments:
On GD 7.5 (12 h after alcohol), control and PAE embryos were collected following euthanasia of the dam. Embryos were dissected from extra-embryonic membranes in 37°C LRS and the numbers of live, dead, or resorbed embryos in each uterus were recorded. Nile Blue working solution [1:10,000] was warmed to 37°C in flat-bottom tubes prior to embryo immersion. Embryos were incubated for 30 min in the 37°C oven then rinsed briefly in LRS. Finally, specimens were photographed in LRS and genotyped.
Morphology experiments:
On GD 17, fetuses were collected after CO2 asphyxiation and physical euthanasia of the dam. Fetuses were examined for facial and ocular malformations and tail samples were obtained for genotyping. Based on externally visible malformations, subsets of fetuses were drop-fixed in either 10% buffered formalin or first photographed by a MicroPublisher 5.0 digital camera (QImaging, British Columbia, Canada) on a Nikon SMZ–U Stereoscopic Zoom dissecting microscope (Nikon Corporation, Melville, NY) using QCapture Suite software, and then drop-fixed in Bouin’s fixative. Ocular malformations were evaluated, either at the time of dissection or later in formalin-fixed specimens, by two independent evaluators who were blinded to treatment and genotype, using a 7-point dysmorphology scale modified from Parnell et al.(2006; 2010) and Gilbert et al. (2016). A score for each eye was assigned based on the following criteria: (1) normal globe size, normally shaped pupil; (2) mildly microphthalmic or abnormally-shaped (but non-colobomatous) pupil; (3) mildly microphthalmic with small, abnormally-shaped (but non-colobomatous) pupil; (4) moderately microphthalmic with iridio-retinal coloboma; (5) severely microphthalmic with iridio-retinal coloboma; (6) severely microphthalmic, absent pupil; (7) apparently anophthalmic. For each fetus, the score that reflected the more dysmorphic eye was used for analyses. Image J software (https://imagej.nih.gov/ij/) was used to measure the size of the lens and globe from a subset of photographed fetuses. Heads of Bouin's fixed specimens were cleared with 70% ethanol, then processed using a Leica brand tissue processor and embedded in paraffin. Serial 10 μm coronal sections were cut using a rotary microtome, mounted onto glass slides, and stained with hematoxylin and eosin (H&E). Stained sections were examined using an Olympus BX60 light microscope.
Statistical Analysis:
Data on litter size and number of resorptions were analyzed with t-tests, while Chi-square tests compared the proportion of fetuses with eye defects. Eye and globe sizes were compared with one-way analysis of variance (ANOVA). Fetal dysmorphology data were considered significant at the p<0.05 level. RNA-seq comparisons were considered significant with an adjusted p<0.05.
RNA isolation:
On GD 7, untreated Bax+/− dams were euthanized as above. Under RNAse-free conditions, the embryos were dissected from the placentas and staged for developmental age (see, Boschen, Ptacek, Berginski, Simon, & Parnell, 2021). GD 7-aged embryos were freed from their extra-embryonic tissue and placed in RLT Plus Lysis Buffer (Qiagen, Germantown, MD) plus β-mercaptoethanol and stored at −80°C. Extra-embryonic tissue was placed into 14 ul of DNA lysis buffer (Allele-In-One Mouse Tail Direct Lysis Buffer, Allele Biotechnology, San Diego, CA) and genotyped for Bax. RNA was isolated from Bax+/+ (n=6) and Bax−/− (n=6) embryos using the RNeasy Plus Micro Kit (Qiagen) and RNA concentrations were determined with a Qubit Flex Fluorimeter (Thermo Fisher Scientific, Waltham, MA). No more than two embryos of a given genotype were taken from each litter.
Whole Transcriptome Profiling:
The UNC High Throughput Sequencing Facility performed RNA sequencing (RNA-seq) using libraries prepared with the SMARTr Ultra Low RNA amp (Clontech, Mountain View, CA) and NexteraXT/NON Stranded kits (Illumina, San Diego, CA). Paired-end (75 bp) sequencing was performed on the Illumina HiSeq 4000. Reads were quality-filtered if at least 90% of bases had a quality score greater than 20 using fastq_quality_filter in FASTX 0.0.14 (http://hannonlab.cshl.edu/fastx_toolkit/index.html). Adapter sequences were removed using cutadapt 1.12 (Martin, 2011). Reads were then aligned to the mm9 reference genome using STAR 2.5.2b (Dobin et al., 2013). Salmon 0.11.3 (Patro, Duggal, Love, Irizarry, & Kingsford, 2017) with RefSeq gene annotations quantified post-alignment raw counts and RPKM values. One embryo, originally genotyped as Bax−/−, had detectable Bax mRNA and was not included in the final analysis. Differential expression tests were performed on raw counts using DESeq2 1.22.2 (Anders & Huber, 2010), with significant differences defined by an adjusted p-value < 0.05. The g:profiler toolset (version e104_eg51_p15_3922dba) (Raudvere et al., 2019) was used to assess functional enrichment with the following databases: Gene Ontology: Biological Processes, Cellular Component, and Molecular Function; the Kyoto Encyclopedia of Genes and Genomes; Reactome; Corum; Human Phenotype Ontology; and WikiPathways. Additionally, Gene Set Enrichment Analysis (GSEA) Version 7.4 (Mootha et al., 2003; Subramanian et al., 2005) using the Hallmarks and Gene Ontology databases. Cytoscape V3.8.2 (Shannon et al., 2003), was used to visualize the GSEA data and correlations between differentially expressed genes. For examination of chromosome 7, the location of all genes was identified using the Mouse Genome Informatics database (http://www.informatics.jax.org/), and distance from Bax was designated as the number of base pairs between the start (3’ end), or terminus (5’ end) of Bax, and the nearest end (5’ or 3’) for a particular gene.
Results
Fetal Dysmorphology
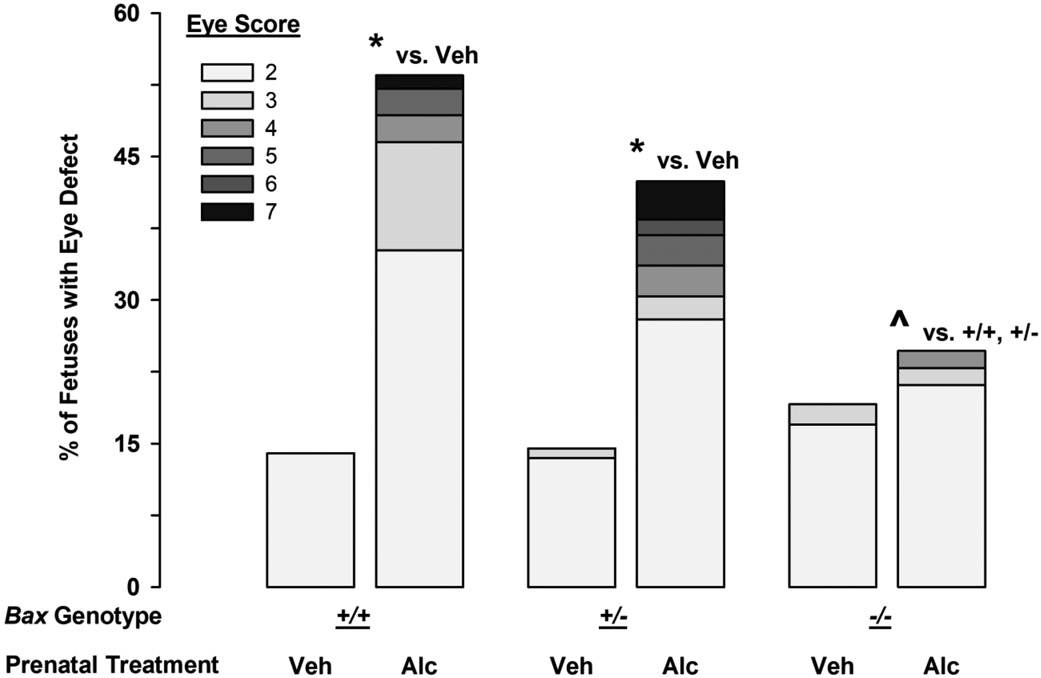
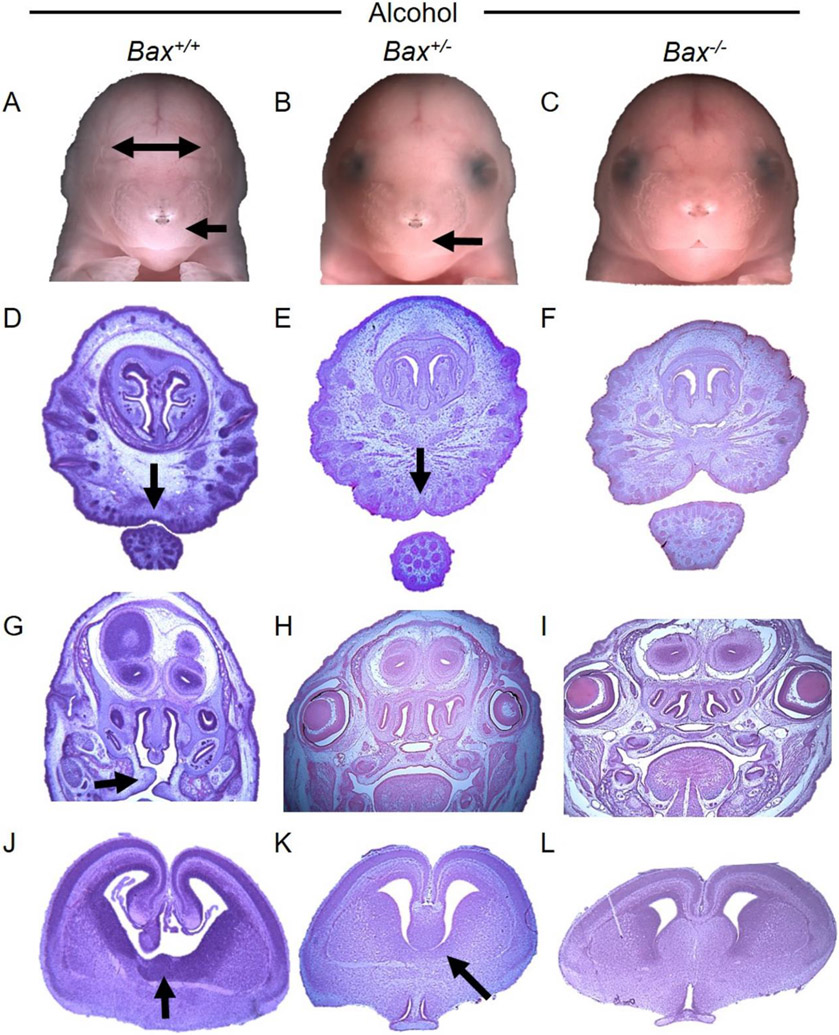
The numbers of Bax+/+, Bax+/−, and Bax−/− mice followed a closely Mendelian ratio after either of the prenatal treatments (Table 1), confirming that full Bax deletion was not lethal. However, alcohol-treated litters were smaller (t59=4.0; p<0.001) and had more resorptions (t59=2.2; p=0.03). The maintenance of Mendelian ratios in the alcohol-treated litters indicates that Bax deletion did not alter the effects of alcohol on fetal survival. About 14% of vehicle-treated Bax+/+ and Bax+/− fetuses had a minor eye defect (Figure 1), primarily on the right side, a rate consistent with our past studies using C57BL/6J mice (Parnell et al., 2006; Parnell et al., 2010). The full Bax deletion had a 5% higher incidence of background eye defects (19.1%, X1=0.19; p=0.66), indicating a modest role of Bax in normal eye development. Analysis of lens and globe size in a subset of fetuses revealed that spontaneous defects in the right eyes were very similar between the Bax+/+ and Bax−/− fetuses, involving a smaller lens, but a normal sized globe, and therefore decreases in the ratio of lens to globe size (Suppl. Fig 1). In contrast, alcohol-induced eye defects tended to be associated with reduced size of both the lens and the globe, and therefore no change in the lens:globe ratio (Suppl. Fig 1). As observed in C57BL/6J mice, about 50% of alcohol-treated Bax+/+ fetuses had an eye defect (53.5%, a 39.5% increase above background; X1=19.7; p<0.001 vs Vehicle; Figure 1) which ranged in severity from minor decreases in globe size to complete anophthalmia (Table 2). Severe FAS-like facial dysmorphologies occurred in 2/71 wild-type fetuses (2.8%) which included tissue loss in the mandible corresponding to the lip notch, cleft palate, and disruptions in the vomeronasal organ, as well as the brain septal area (Figure 2). Holoprosencephaly was also noted in a severely affected fetus (not shown). While partial Bax deletion did not significantly modify alcohol-induced eye and craniofacial defects (42.4% had eye defects, a 28% increase above background; X1=23.3; p<0.001 vs Vehicle; Figure 1, Table 2; 5/125 had FAS-like craniofacial defects [4%]), full Bax deletion provided near complete protection from alcohol-induced eye defects (24.6% had eye defects, 5.5% increase from the background incidence, X1=9.8; p=0.002 vs alcohol-treated Bax+/+; X1=4.6; p=0.03 vs alcohol-treated Bax+/−; Figure 1, Table 2) and only 1/57 fetuses (1.8%) had a minor FAS-like facial dysmorphology (i.e. small reduction in the size of the lip notch, with a minor right eye defect; Suppl. Figure 2). Unfortunately, the small lip notch in the Bax−/− fetus was not identified during the initial dysmorphology exam and the fetus was placed in formalin, which does not provide sufficient drop fixation for the purpose of examining brain sections. Most of the eye defects in Bax+/+ fetuses were in the right eye (a 48% vs a 9% increase from background in the right and left eyes, respectively). A separate analysis of the left and right eyes revealed that the Bax−/− fetuses had significantly fewer right eye defects than did the Bax+/+ fetuses, but there was no difference in the left eye (Left, X1=0.02; p=0,88; Right, X1=8.8; p=0.003; Suppl. Figure 3A). Relatedly, if a Bax+/− or Bax−/− fetus was affected by alcohol, it was more likely to have defects in both the right and left eyes than was a Bax+/+ fetus. Among affected mice, the incidence of bilateral eye defects was 13% for Bax+/+ fetuses, but 47% and 50% for Bax+/− and Bax−/− fetuses, respectively (Suppl. Figure 3B). Thus, while deletion of Bax provided overall protection from alcohol-induced eye defects, its protective effects were most apparent in the more sensitive right eye.
Table 1.
Litter Characteristics for Vehicle and Alcohol Treatments of Bax Heterozygous Dams
| Litter (n) | Live Fetuses | Resorptions | % GT (+/+, +/−, −/−) | n by GT (+/+, +/−, −/−) | |
|---|---|---|---|---|---|
| Vehicle | 28 | 7.4±0.3 | 0.11±0.08 | 27, 50, 23 | 57, 104, 47 |
| Alcohol | 33 | 5.7±0.3* | 0.55±0.17* | 28, 49, 23 | 71, 125, 57 |
Data for live fetuses and resorptions are expressed as the mean litter average (±SEM). Emboldened values with asterisks indicated significant differences between vehicle- and alcohol-treated litters (p<0.05). GT: genotype; +/+: wild-type; +/−: heterozygous; −/−: full knockout.
Figure 1.
Effects of gastrulation-stage alcohol and Bax genotype on the incidence and severity of fetal eye defects. Stacked bars portray the percent of fetuses with at least one affected eye in each of the severity categories ranging from 2 (smaller globe or minor change in pupil shape) to 7 (complete anophthalmia) for Bax+/+, Bax+/−, and Bax−/− fetuses (left, center, and right sets of bars, respectively). The eye score is based on the most severely affected eye for each fetus. * denotes significance vs. vehicle treated fetuses of the same genotype, while the ^ denotes significance vs. the alcohol-treated Bax+/+ and Bax+/− fetuses (p<0.05).
Table 2.
Effects of Bax Genotype and Gastrulation-Stage Alcohol Exposure on the Severity of Eye Defects and Incidence of FAS-like Facial Dysmorphology
| Eye Defect Severity Score | ||||||||
|---|---|---|---|---|---|---|---|---|
| Drug Treatment | 1 | 2 | 3 | 4 | 5 | 6 | 7 | % FAS-like |
| Vehicle | ||||||||
| +/+ | 49 | 8 | 0 | 0 | 0 | 0 | 0 | 0 |
| +/− | 89 | 14 | 1 | 0 | 0 | 0 | 0 | 0 |
| −/− | 38 | 8 | 1 | 0 | 0 | 0 | 0 | 0 |
| Alcohol | ||||||||
| +/+ | 33 | 25 | 8 | 2 | 2 | 0 | 1 | 2.8% |
| +/− | 72 | 35 | 3 | 4 | 4 | 2 | 5 | 4% |
| −/− | 43 | 12 | 1 | 1 | 0 | 0 | 0 | 2% |
The data for the most severely affected eye in each fetus are expressed as the number of fetuses with a score from 1 (unaffected) to 7 (anophthalmia). The incidence of non-ocular craniofacial defects is expressed as the percent of fetus with an FAS-like dysmorphology.
Figure 2.
Effects of Bax genotype on alcohol-induced facial and brain dysmorphology. Photographs of fetal faces (A-C) and of hematoxylin/eosin stained coronal sections through the brain (D-L) are shown for representative Bax+/+, Bax+/−, and Bax−/− fetuses (left, center, and right sets of images, respectively). Arrows indicate significant dysmorphology including narrowly spaced eye sockets (A&B), smooth philtrum, absence of lip notch (A, B, D, & E), cleft palate (G), and tissue loss in the brain septal region (J&K). These severe craniofacial effects of alcohol were not observed in the Bax−/− fetuses (C, F, I, & L). Note, colors have been adjusted to minimize differences in hematoxylin/eosin staining intensity.
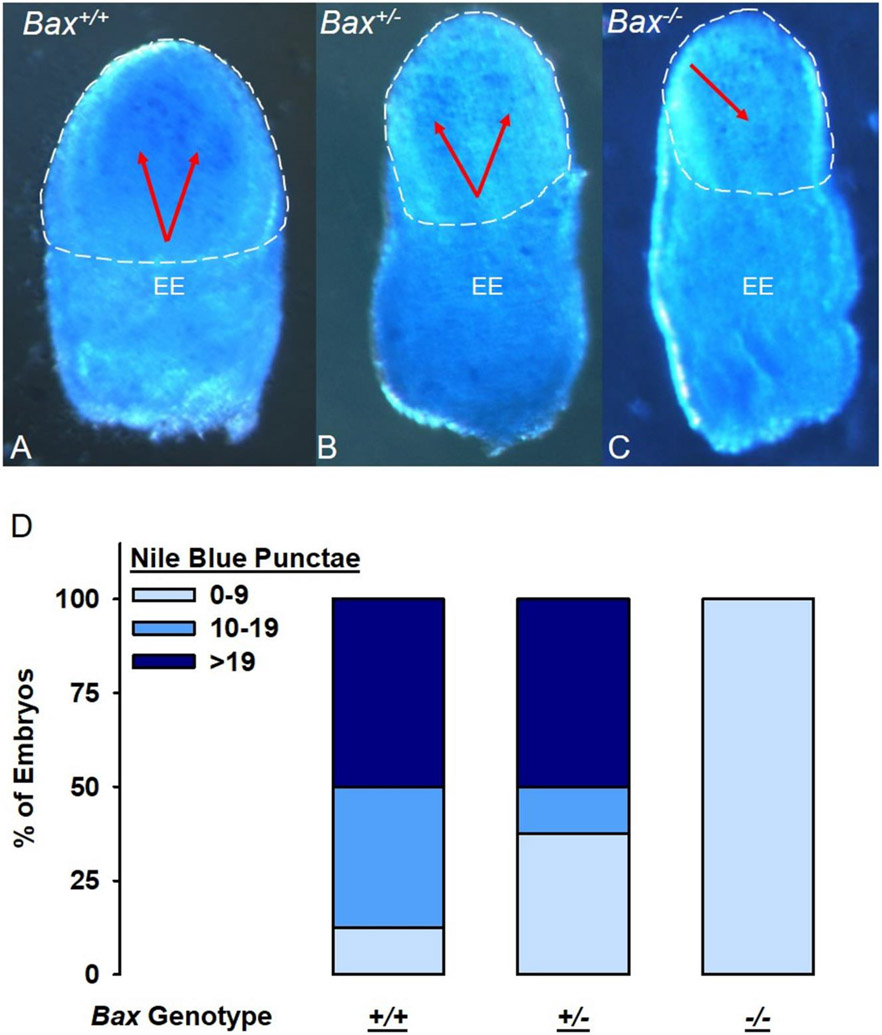
To examine whether Bax deletion affected alcohol-induced apoptosis, embryos were collected and stained with Nile Blue Sulfate (NBS) 12 hours after GD 7 exposure to alcohol or vehicle, a time point corresponding to the highest levels of excessive apoptotic cell death (Kotch & Sulik, 1992). As seen in Figure 3, alcohol-exposed Bax+/+ embryos had the highest observable levels of apoptosis, which was localized to the putative ectoderm, consistent with previous findings (Dunty et al., 2001). The Bax+/− embryos exhibited some apoptosis while little to no apoptosis was observed in the Bax−/− embryos.
Figure 3.
Effects of Bax genotype on alcohol-induced apoptotic cell death. Panels A-C show representative images of the cranial ectoderm side of G7 embryos stained with Nile Blue to detect apoptosis. Panel D portrays pyknotic cell counts for each of the Bax genotypes: Bax+/+ (n=8); Bax+/− (n=8); Bax−/− (n=4), expressed as a % of each genotype with low, moderate, and high levels of cell death.
Differential Gene Expression in GD 7 Bax−/− Embryos
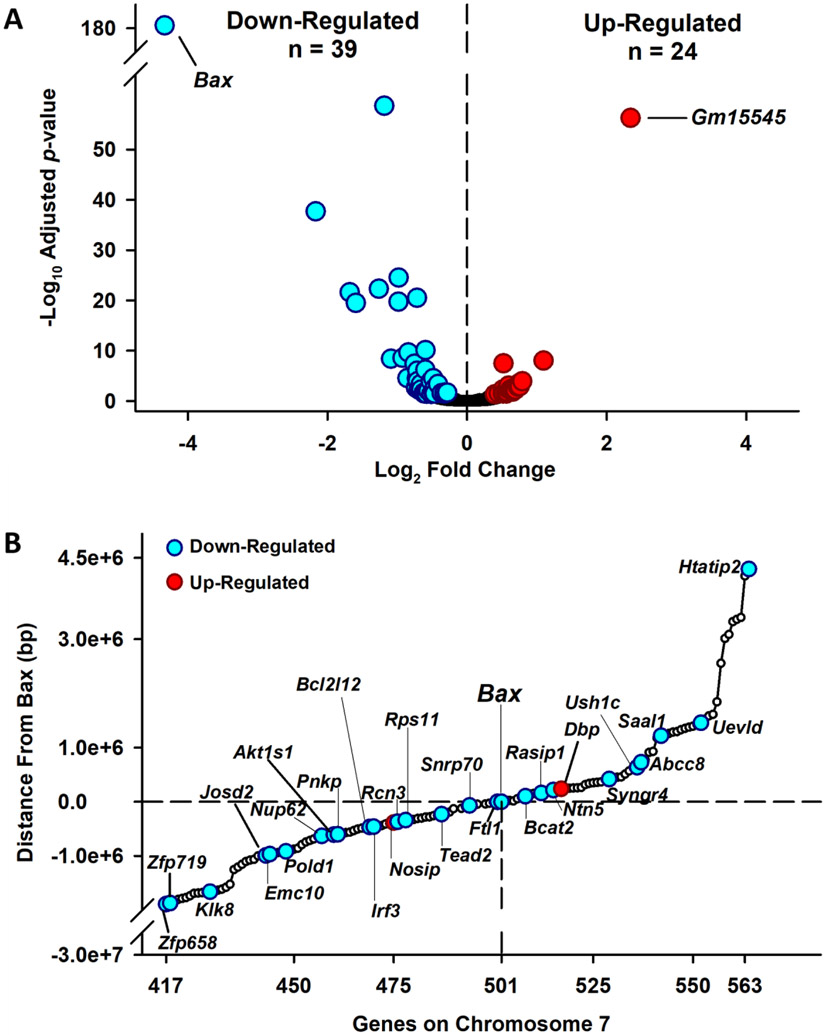
Sixty-three genes were differentially expressed in the Bax−/− embryos compared to the Bax+/+, including 39 down-regulated, and 24 up-regulated genes (Figure 4A, Table S1, S2). Examining the chromosomal location of the differentially expressed genes (DEGs), revealed that 31 of the 63 genes (27 down- and 4 up-regulated) were located on chromosome 7 within relatively close proximity to Bax itself (Suppl. Figure 4; Figure 4B). While most of the DEGs were consistent between individuals of each genotype, one Bax−/− embryo had a pattern of expression for 17 genes that was more similar to the Bax+/+ embryos (Suppl. Figure 5). Examining gene-gene interactions with Cytoscape software, correlations between DEGs were relatively weak, with the strongest correlations between down-regulated genes observed between Bcat2 and Cth; Col1a1 and Rcn3. The strongest correlations between upregulated genes existed between Itga4 and Itga7, Esrrb and Nr0b1, and Gjb3 and Gjb5 (Suppl. Figure 6).
Figure 4.
Differentially expressed genes in Bax−/− and Bax+/+ GD 7 embryos. A. Volcano plot showing the number of genes significantly down-regulated (cyan circles) or up-regulated (red circles) in Bax−/− embryos, as well as genes that were not differentially expressed (black circles). B. Portrayal of differentially expressed genes located on chromosome 7 and their relative proximity (bp) to Bax. Open circles indicate genes that were located near Bax, but were not differentially expressed.
To understand the possible roles of the DEGs, we first examined their function using the published literature (Suppl. Tables S1 and S2). Most of the DEGs (upregulated genes are emboldened, down-regulated genes are underlined) were individually associated with key aspects of embryonic/fetal development including: craniofacial/eye/bone development (Cldn3, Bmp8b, Tmem114, Nosip, Riox1, Tead2, Ptn, Col1a1, Rgs2, Atoh8, and Pnkp); cell adhesion (Itgb4, Itga7, Carmil3, Rasip1, and Ush1c); pluripotency (Plaur, Riox1, Saal1, Josd2, Tead2, Ptn, and Akt1s1); and germ cell expression (Nr0b1, Bmp8b, Esrrb, and Creb3l4). Many of these genes also have roles in the stimulation of cancer cells (Itgb4; Plaur; Sycp3; Cldn3; Inpp1; Bcr; Creb3l4; Josd2; Pold1; Ptn; Col1a1; Pnkp; and Klk8), while others may inhibit cancer cells (Nlrx1, Atoh8, Penk, and Htatip2). Several inflammation signaling-related genes were differentially expressed, including: Itgb4 (anti-inflammatory); Plaur; Nlrx1; Pld4; Carmil3; Irf3; and Penk (each are pro-inflammatory). It is noteworthy that, in addition to Bax, a large number of genes were associated with either the augmentation (Tead2, Irf3, Rgs2, Penk, Htatip2), or the inhibition (Plaur, Ddah1, Bcr, Riox1, Cth, Creb3l4, Rps11, Pold1, Akt1s1, Pnkp, and Bcl2l12) of apoptosis.
To determine if the DEGs were associated with dysregulation of specific pathways, we first used g:profiler. However, owing to the relatively few DEGs, only 10 pathways were significantly altered, most of which shared similar genes (Suppl. Figure 7). We next used GSEA which analyzes all genes based on the correlations in their expression and the ranking of genes within a gene set, rather than simply those that are differentially expressed. Thus GSEA is predicted to uncover more potentially dysregulated pathways than g:profiler. The Hallmark pathway analysis revealed that Bax−/− embryos had 27/50 significantly enriched pathways (21 positively- and six negatively-enriched pathways) as compared to Bax+/+ embryos (Suppl. Figure 8). It was surprising that there was only a trend (FDR-p=0.085) toward negative enrichment of apoptotic pathways in the Bax−/− embryos. The absence of a significant negative enrichment may have been because while Bax and other genes (Bgn, Cav1) were ranked lower in the Bax−/− embryos, their core negative enrichment was offset by other genes in this pathway (e.g. Faslg, Gstm1) that were ranked relatively higher in the Bax−/−, but might play a smaller biological role in the control of apoptosis as compared to Bax itself. Overall, the Hallmark pathway analysis identified several targets that may uncover the potential interplay between Bax and other genes, but functional studies are needed before drawing conclusions on the physiological impact of these enriched gene sets.
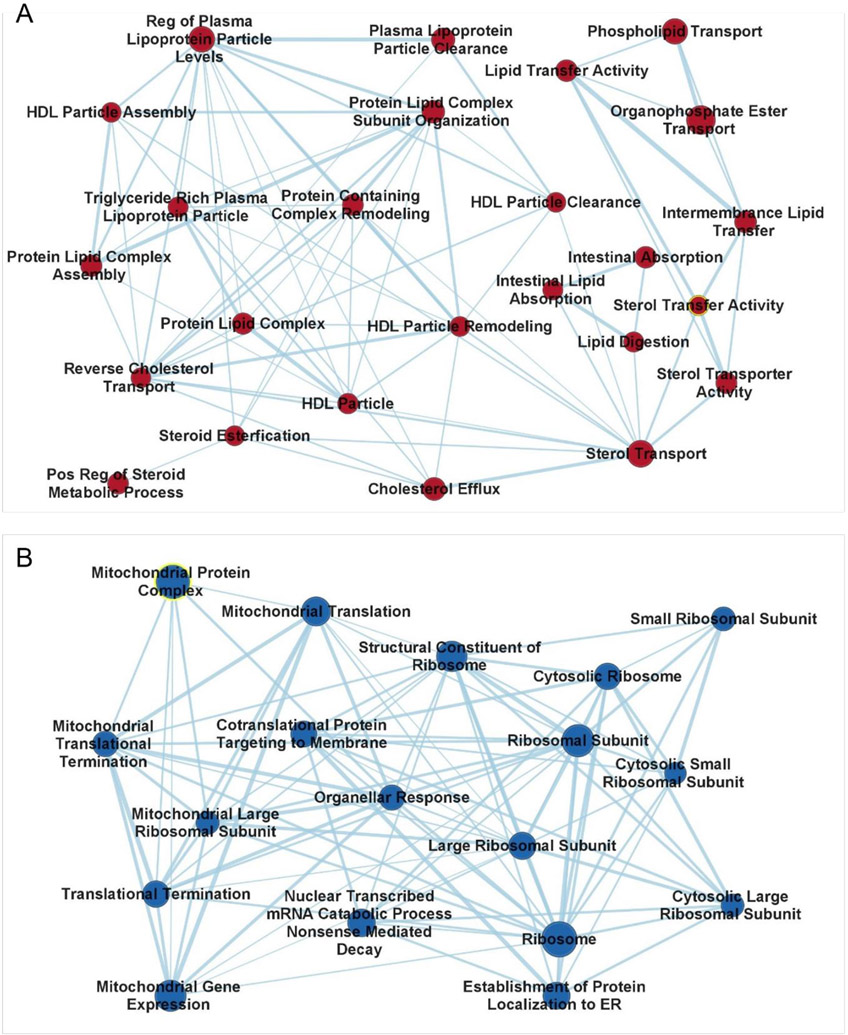
A final GSEA examination of the RNAseq data was conducted using the Gene Ontogeny dataset, which includes many more specialized gene sets than does the Hallmark dataset. Of the 5326 reference gene sets, 98 were found to be significantly enriched in either the Bax−/− or Bax+/+ embryos. Cytoscape identified that many of these sets could be combined into two large clusters (Figure 5). Positively enriched in the Bax−/− is a large group (25) of cholesterol- and lipid-related sets sharing many of the same genes, with the largest enrichment surrounding Apoc2 (apolipoprotein C2), one of the top 50-ranked genes. Several small enrichments are found in other apolipoprotein genes, including Apoa, Apoe, as well as Pltp (phospholipid transfer protein). Negatively enriched in the Bax−/− embryos is a large group (18) of ribosomal- and mitochondrial-related gene sets. These sets are enriched due to the effects a large number of low-ranked genes (e.g. the ribosomal subunit set is enriched in 132/177 genes), rather than a few high-ranked genes, such as Bax itself. Examples of such low-ranking genes include the Mrpls, Mrps, (mammalian mitochondrial ribosomal large and small subunit, respectively), Malsu (mitochondrial assembly of ribosomal large subunit 1), Chchd1 (coiled-coil-helix-coiled-coil-helix domain containing 1), and Rps11 (ribosomal protein S11). The lipid and ribosomal/mitochondrial clusters illustrate the advantage of GSEA over analyses that only include DEGs, in that two key cellular functions are identified that would not have been predicted using DEGs alone.
Figure 5.
Clustering of significantly enriched pathways in Bax−/− embryo identified in GSEA Gene Ontology. A. Relationships between positively enriched gene sets related to lipid membranes- and cholesterol. B. Relationships between negatively enriched gene sets related to mitochondria. Mitochondrial protein complex is highlighted in yellow because it is the only pathway in which Bax contributed significant enrichment. Symbol size reflects the number of genes in each set.
Forty other gene sets were positively enriched in the Bax−/− embryos (Suppl. Table S3), with general functions related to apoptosis, placental development, coagulation, cellular membrane and permeability, gene expression or replication, receptor binding and channel activity, organelles, and other processes. Interestingly, positive enrichment was found in gene sets related to hydrogen peroxide and reactive oxygen species (ROS). Eleven gene sets not related to ribosomes and mitochondria were found to be negatively enriched in the Bax−/− embryos (Suppl. Table 4), related to the general functions of apoptosis, organelles, somite development, and remodeling. Bax itself was associated with all of these pathways, with the exception of somitogenesis and somite development.
Discussion
Alcohol exposure during the developmental stage of gastrulation triggers interacting pathogenic cascades that cause the craniofacial and brain dysmorphologies characteristic of FAS. Excessive ectodermal cell death was the first described pathogenic mechanism (Sulik, Johnston, & Webb, 1981) and more recent research has uncovered the cellular events that orchestrate the transition from developmentally appropriate cell death to the excessive cell death that ultimately compromises the fetus. The present results uphold the role of apoptotic death in the expression of gastrulation stage alcohol-induced craniofacial dysmorphologies, and importantly, highlight the critical, pernicious role of Bax. Full deletion of the Bax gene reduced the incidence of alcohol-induced eye, face, and brain defects, while also minimizing cell death following the alcohol exposure. Other than Bax itself, a relatively small number of genes were differentially expressed between untreated Bax+/+ and Bax−/− embryos and these genes may reflect the direct consequences of Bax deletion or compensatory mechanisms that enable the typical development of Bax−/− embryos. In addition to the DEGs, the GSEA analysis revealed more global changes in gene expression and highlighted several biological functions beyond apoptosis that may be affected by Bax deletion.
The craniofacial and brain dysmorphologies observed in the Bax+/+ and Bax+/− fetuses are consistent in incidence and severity with those previously observed in mice on the C57BL/6J background (Dou et al., 2013; Godin et al., 2010). The most frequent defects observed in the Bax+/+ and Bax+/− fetuses were varying degrees of microphthalmia, a common occurrence in children with FASD (Del Campo & Jones, 2017). Some fetuses were severely affected, demonstrating complete anophthalmia, facial dysmorphologies, and holoprosencephaly, consistent with the idea that FAS falls into the holoprosencephaly spectrum (Hong & Krauss, 2012, 2017; Lipinski et al., 2010; Sulik, 1984). At first glance, these severe dysmorphologies might be surprising given the relatively sparse ectodermal cell death detected by the Nile Blue staining. However, the amount and pattern of staining in the Bax+/+ and Bax+/− embryos was similar to that observed previously in C57BL/6J mice (Dunty et al., 2001). While Nile Blue is rapid and allows post-processing genotyping, it captures only a snap-shot of the total embryonic cell death (Sulik, Cook, & Webster, 1988), which may persist beyond the currently studied time window. At the mid- to late-gastrulation stages measured herein, prior to optic evagination, the ectodermal cell death observed here demonstrates death in the cells that give rise to the forebrain and eyes. Most importantly, apoptotic cell death was attenuated in the Bax−/− embryos, mice whose craniofacies were largely unaffected by a gastrulation-stage alcohol exposure. The very slight protection provided by the Bax+/− genotype on embryonic cell death and the presence of particularly severe facial dysmorphologies in the Bax+/− fetuses do not provide convincing evidence for a gene-dose effect, but instead support the hypothesis that an all-or-none threshold of Bax is required for gastrulation alcohol-induced dysmorphologies, as also demonstrated for retinal damage (Semaan et al., 2010). That Bax−/− was not fully protective against alcohol-induced dysmorphologies (alcohol caused a small increase in the incidence of eye defects and one Bax−/− had a relatively minor FAS-like facial defect) suggests the involvement of other, Bax-independent, mechanisms of teratogenesis. One possibility is a partial substitution by Bak in the induction of mitochondrial cell death, or the involvement of the extrinsic cell death pathway, the activity of which is unaffected by Bax deletion (Mac Nair et al., 2016).
Previous research on the importance of Bax in FASD models has focused on third trimester-equivalent exposure which causes large amounts of neuronal cell death in the cerebellum and regions of the cortex (Ahlers, Karacay, Fuller, Bonthius, & Dailey, 2015; Britton & Miller, 2018; Heaton, Paiva, & Kubovec, 2015; Heaton et al., 2006; Young et al., 2003). The extension of Bax’s apoptotic role to the gastrulation stage embryo raises the question as to whether particular cell phenotypes are spared or remain sensitive to alcohol-induced cell death. Prior studies have shown that Bax affects survival of post-mitotic neurons, and not the neuronal progenitor cells (D'Sa-Eipper et al., 2001; Jung et al., 2008; Lindsten et al., 2000; Shi, Parada, & Kernie, 2005), but it is unclear whether a similar distinction occurs during gastrulation, prior to neurogenesis. Not all cells at gastrulation will have progressed enough to develop the capacity for apoptosis, and it is possible that these may become sensitive only during a second wave of apoptosis (Cartwright, Tessmer, & Smith, 1998; Olney et al., 2002; Young et al., 2003). Nonetheless, there is now converging evidence that Bax is necessary for alcohol-induced apoptosis at different times in development and is therefore critical to many different FASD outcomes. While we report a role for Bax in craniofacial dysmorphology, which is associated with major tissue loss, future studies should examine other, more subtle, sequelae of gastrulation-stage PAE, such as hypothalamic-pituitary-adrenal axis activity and behavioral alterations (Wieczorek, Fish, O'Leary-Moore, Parnell, & Sulik, 2015), to uncover the full contribution of Bax.
The current results suggest that the accumulation of Bax in the mitochondrial membrane is the decisive event determining a cell’s fate after PAE and ultimately whether an individual develops, or is shielded from, an FAS-like dysmorphology. However, a tapestry of cellular signals must interact before this fate is decided and acute alcohol has been shown to affect many of these interactions. First, acute PAE may initiate cell death machinery by inducing metabolic stress, restricting blood flow, and affecting cell adhesion. Previous studies have shown that PAE can activate Tp53 signaling, one of the signals that begin the apoptotic cascade (Anthony, Zhou, Ogawa, Goodlett, & Ruiz, 2008; Flentke, Baulch, Berres, Garic, & Smith, 2019; Ignacio, Mooney, & Middleton, 2014; Kuhn & Miller, 1998), and that genetic deletion of Tp53 is also protective from PAE (Fish, Tucker, Peterson, Eberhart, & Parnell, 2021). Once Tp53, or other signals, initiate the apoptotic cascade, changes in the expression and activity of the BH3 protein family have the dual role to inhibit the pro-survival Bcl-2 signals, which normally keep Bax in check, and directly activate Bax itself. In a transcriptome-wide study, gastrulation-stage PAE up-regulated one of these BH3 signals, Bid (Boschen, Ptacek, et al., 2021), which is also activated by caspase-8 through the extrinsic pathway and is increased following post-natal alcohol exposure (Heaton et al., 2015). Repeated PAE impairs Bcl-2 protein expression (Mooney & Miller, 2001), which may be sufficient to allow Bax to accumulate in the mitochondrial membrane and induce cell death. Taken together, these data strongly support a model where PAE converges on multiple apoptotic mechanisms within the intrinsic cell death pathway, of which Bax is the final arbiter.
We performed whole transcriptomic RNA sequencing to identify genes significantly altered by the Bax deletion, prior to alcohol exposure. While it would have been ideal to focus on a discrete subtype of embryonic cells, such as the ectoderm, using the whole embryo provided more consistent sequencing results. This analysis provided information regarding other genes throughout the embryo that were modified by the Bax deletion and could, like Bax, affect the sensitivity to PAE and provide both alternative interpretations to our results, as well as identify future targets of study. Only 63 genes were differentially expressed between untreated Bax+/+ and Bax−/− GD 7 embryos. Nearly half of the DEGs were found on chromosome 7, in proximity to Bax, suggesting that these are directly due to the insertion of the neo-cassette used to generate the Bax−/− mice (Meier et al., 2010; Pham, MacIvor, Hug, Heusel, & Ley, 1996; Sato et al., 2000; West et al., 2016). None of the DEGs was as dysregulated as Bax itself and several of the upregulated genes, in particular, were expressed at low levels, raising questions of their functional impact. Moreover, there was variation between individuals, suggesting that the alternate gene expression is not a definite consequence of the Bax deletion.
Functional profiling of the up/down-regulated DEGs using g:profiler was limited by the relatively small number of input genes. However, GSEA, which examines the interaction between all expressed genes, identified multiple pathways that were differentially enriched in the Bax+/+ and Bax−/− embryos (see Suppl. Figs 7 & 8, and Tables S3 and S4). Since significant enrichment of a gene does not distinguish functional up- or down-regulation, the gene clusters identified by GSEA serve mainly as rationale for future studies. Two large clusters were identified by the Gene Ontology database: a negatively enriched mitochondria-related set; and a positively enriched lipid-related set. Bax and Bak play a non-apoptotic role in mitochondrial dynamics; Bax deletion shortens mitochondria and reduces rates of fusion (Hoppins et al., 2011; Karbowski, Norris, Cleland, Jeong, & Youle, 2006). An alternative explanation about the critical effect of the Bax deletion could be a background shift in the mitochondrial dynamics of Bax−/− embryos, which might render them less sensitive to the effects of the existing pro-apoptotic proteins Bak and Bid. The viability of this hypothesis remains to be tested. Overall, the negative enrichment of the mitochondrial-related gene set in the Bax−/− embryos spotlights the potential of future studies on the role of mitochondria during gastrulation-stage alcohol exposure.
Apolipoproteins and cholesterol are essential for the development and maintenance of cellular membranes; inhibition of these lipids is associated with embryonic cell death and craniofacial malformations (Homanics et al., 1995; Lanoue et al., 1997). Bax−/− embryos were positively enriched in genes related to cholesterol and lipids and it is possible that these may also promote further resistance to the effects of alcohol. Although gastrulation-stage PAE did not affect gene expression of apolipoproteins in the C57BL/6J or C57BL/6NHsd strains (Boschen, Ptacek, et al., 2021), alcohol has long been shown to disrupt lipid membranes (Goldstein, 1984; Klemm, 1998; Patra et al., 2006) and this disruption could be a signal that triggers the apoptotic cascade. Moreover, cholesterol and apolipoproteins are critical to the synthesis and packaging of Sonic hedgehog (Shh) (Briscoe & Therond, 2013). Since gastrulation-stage alcohol exposure strongly inhibits Shh signaling (Ahlgren et al., 2002; Boschen, Fish, et al., 2021; Hong & Krauss, 2012; Kahn et al., 2017; Zhang et al., 2013), and this inhibition is necessary for craniofacial dysmorphology, examining the interaction between Bax and Shh signaling is a logical extension of the current study. Additionally, positive enrichment in apolipoproteins may further account for the later life enhancement of oligodendrocyte density and axonal density in Bax−/− brains (Kawai et al., 2009; White et al., 1998) and changes in Bax−/− retina (Kawai et al., 2009; Mac Nair et al., 2016; Mosinger Ogilvie et al., 1998).
A critical question is whether the DEGs and/or the enriched pathways, influence the protective effect of Bax deletion on alcohol-induced dysmorphologies. Current experiments are underway employing an Irf3-Bcl2l12 mouse mutant, but Htatip2, Cth, Plaur, Riox1, Creb3l4, Akt1s1, and Pnkp are all sensitive to early PAE in the C57BL/6J mouse strain (Boschen, Ptacek, et al., 2021; Boschen and Parnell, unpublished observations) and their identity as potential mediators of alcohol teratogenesis should also be examined. Additionally, two DEGs, Ddah1 and Nosip, warrant continued investigation since prior studies show a protective role for nitric oxide in FASDs (Bonthius et al., 2002; Karacay & Bonthius, 2015). The potential importance of ROS is supported by the GSEA finding of positive enrichment of genes related to hydrogen peroxide and ROS biosynthetic processes, both of which have roles for Duox2 (dual oxidase maturation factor 2).
In summary, the experiments described herein find a pathogenic role for Bax during gastrulation-stage alcohol exposure. Attenuation of alcohol-induced apoptosis is a mechanism through which Bax gene deletion protects the embryo, but changes in the expression of genes other than Bax reveal that Bax deletion potentially affects other aspects of embryonic physiology. Whether these changes are relevant to the protective effects of Bax deletion on craniofacial dysmorphology can be addressed by future studies using acute pharmacological inhibitors of Bax (Jensen, WuWong, Wong, Matsuyama, & Matsuyama, 2019), which may help pin-point the critical timing of Bax’s deleterious effects on the embryo. Importantly, Bax may be a key gene that modifies susceptibilities to FASD. Several Bax single nucleotide polymorphisms have been linked to cancer risk and treatment response (Peng et al., 2015; Saxena, Moshynska, Sankaran, Viswanathan, & Sheridan, 2002; L. Sun, Wei, Wei, & Li, 2018) and Bax loss of function variants, while elevating risk for cancers, may reduce the risk for at least some FASD symptoms, especially those, like craniofacial malformations, that are caused by apoptotic cell death.
Supplementary Material
Acknowledgements:
The authors appreciate the assistance of Lorinda K. Baker and Laura B. Murdaugh for their initial maintenance of the Bax colony and Rachel L. Peterson for photography of formalin-fixed fetuses. We also thank Melina Steensen and Casey Hunter for performing the specimen sectioning and histological staining. This research was supported by U01-AA021651 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) to SEP. All or part of this work was done in conjunction with the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD), which is funded by grants from the NIAAA. Additional information about CIFASD can be found at www.cifasd.org.
Support:
U01-AA021651 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) to SEP. All or part of this work was done in conjunction with the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD), which is funded by grants from NIAAA. Additional information about CIFASD can be found at: www.cifasd.org. AH and JMS are supported by U54-HD079124 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and P30NS045892 from the National Institute of Neurological Disorders and Stroke (NINDS).
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
References
- Abel EL (1995). An Update on Incidence of Fas - Fas Is Not an Equal-Opportunity Birth-Defect. Neurotoxicology and teratology, 17(4), 437–443. doi:Doi 10.1016/0892-0362(95)00005-C [DOI] [PubMed] [Google Scholar]
- Ahlers KE, Karacay B, Fuller L, Bonthius DJ, & Dailey ME (2015). Transient activation of microglia following acute alcohol exposure in developing mouse neocortex is primarily driven by BAX-dependent neurodegeneration. Glia, 63(10), 1694–1713. doi: 10.1002/glia.22835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlgren SC, Thakur V, & Bronner-Fraser M (2002). Sonic hedgehog rescues cranial neural crest from cell death induced by ethanol exposure. Proceedings of the National Academy of Sciences of the United States of America, 99(16), 10476–10481. doi: 10.1073/pnas.162356199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S, & Huber W (2010). Differential expression analysis for sequence count data. Genome Biol, 11(10), R106. doi: 10.1186/gb-2010-11-10-r106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony B, Zhou FC, Ogawa T, Goodlett CR, & Ruiz J (2008). Alcohol exposure alters cell cycle and apoptotic events during early neurulation. Alcohol and alcoholism, 43(3), 261–273. doi: 10.1093/alcalc/agm166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonthius DJ, Tzouras G, Karacay B, Mahoney J, Hutton A, McKim R, & Pantazis NJ (2002). Deficiency of neuronal nitric oxide synthase (nNOS) worsens alcohol-induced microencephaly and neuronal loss in developing mice. Brain research. Developmental brain research, 138(1), 45–59. doi: 10.1016/s0165-3806(02)00458-3 [DOI] [PubMed] [Google Scholar]
- Boschen KE, Fish EW, & Parnell SE (2021). Prenatal alcohol exposure disrupts Sonic hedgehog pathway and primary cilia genes in the mouse neural tube. Reprod Toxicol, 105, 136–147. doi: 10.1016/j.reprotox.2021.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschen KE, Ptacek TS, Berginski ME, Simon JM, & Parnell SE (2021). Transcriptomic analyses of gastrulation-stage mouse embryos with differential susceptibility to alcohol. Disease models & mechanisms, 14(6). doi: 10.1242/dmm.049012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe J, & Therond PP (2013). The mechanisms of Hedgehog signalling and its roles in development and disease. Nature reviews. Molecular cell biology, 14(7), 416–429. doi: 10.1038/nrm3598 [DOI] [PubMed] [Google Scholar]
- Britton SM, & Miller MW (2018). Neuronal Loss in the Developing Cerebral Cortex of Normal and Bax-Deficient Mice: Effects of Ethanol Exposure. Neuroscience, 369, 278–291. doi: 10.1016/j.neuroscience.2017.11.013 [DOI] [PubMed] [Google Scholar]
- Cartwright MM, Tessmer LL, & Smith SM (1998). Ethanol-induced neural crest apoptosis is coincident with their endogenous death, but is mechanistically distinct. Alcoholism, clinical and experimental research, 22(1), 142–149. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9514299 [PubMed] [Google Scholar]
- D'Sa-Eipper C, Leonard JR, Putcha G, Zheng TS, Flavell RA, Rakic P, … Roth KA (2001). DNA damage-induced neural precursor cell apoptosis requires p53 and caspase 9 but neither Bax nor caspase 3. Development, 128(1), 137–146. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11092819 [DOI] [PubMed] [Google Scholar]
- Deckwerth TL, Elliott JL, Knudson CM, Johnson EM Jr., Snider WD, & Korsmeyer SJ (1996). BAX is required for neuronal death after trophic factor deprivation and during development. Neuron, 17(3), 401–411. doi: 10.1016/s0896-6273(00)80173-7 [DOI] [PubMed] [Google Scholar]
- Del Campo M, & Jones KL (2017). A review of the physical features of the fetal alcohol spectrum disorders. Eur J Med Genet, 60(1), 55–64. doi: 10.1016/j.ejmg.2016.10.004 [DOI] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, … Gingeras TR (2013). STAR: ultrafast universal RNA-seq aligner. Bioinformatics, 29(1), 15–21. doi: 10.1093/bioinformatics/bts635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou X, Wilkemeyer MF, Menkari CE, Parnell SE, Sulik KK, & Charness ME (2013). Mitogen-activated protein kinase modulates ethanol inhibition of cell adhesion mediated by the L1 neural cell adhesion molecule. Proceedings of the National Academy of Sciences of the United States of America, 110(14), 5683–5688. doi: 10.1073/pnas.1221386110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunty WC Jr., Chen SY, Zucker RM, Dehart DB, & Sulik KK (2001). Selective vulnerability of embryonic cell populations to ethanol-induced apoptosis: implications for alcohol-related birth defects and neurodevelopmental disorder. Alcoholism, clinical and experimental research, 25(10), 1523–1535. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11696674 [PubMed] [Google Scholar]
- Eberhart JK, & Parnell SE (2016). The Genetics of Fetal Alcohol Spectrum Disorders. Alcoholism, clinical and experimental research, 40(6), 1154–1165. doi: 10.1111/acer.13066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish EW, Tucker SK, Peterson RL, Eberhart JK, & Parnell SE (2021). Loss of tumor protein 53 protects against alcohol-induced facial malformations in mice and zebrafish. Alcoholism, clinical and experimental research. doi: 10.1111/acer.14688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flentke GR, Baulch JW, Berres ME, Garic A, & Smith SM (2019). Alcohol-mediated calcium signals dysregulate pro-survival Snai2/PUMA/Bcl2 networks to promote p53-mediated apoptosis in avian neural crest progenitors. Birth Defects Res, 111(12), 686–699. doi: 10.1002/bdr2.1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forger DB, & Peskin CS (2005). Stochastic simulation of the mammalian circadian clock. Proceedings of the National Academy of Sciences of the United States of America, 102(2), 321–324. doi: 10.1073/pnas.0408465102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert MT, Sulik KK, Fish EW, Baker LK, Dehart DB, & Parnell SE (2016). Dose-dependent teratogenicity of the synthetic cannabinoid CP-55,940 in mice. Neurotoxicology and teratology, 58, 15–22. doi: 10.1016/j.ntt.2015.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin EA, O'Leary-Moore SK, Khan AA, Parnell SE, Ament JJ, Dehart DB, … Sulik KK (2010). Magnetic resonance microscopy defines ethanol-induced brain abnormalities in prenatal mice: effects of acute insult on gestational day 7. Alcoholism, clinical and experimental research, 34(1), 98–111. doi: 10.1111/j.1530-0277.2009.01071.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DB (1984). The effects of drugs on membrane fluidity. Annu Rev Pharmacol Toxicol, 24, 43–64. doi: 10.1146/annurev.pa.24.040184.000355 [DOI] [PubMed] [Google Scholar]
- Heaton MB, Paiva M, & Kubovec S (2015). Differential effects of ethanol on bid, tBid, and Bax:tBid interactions in postnatal day 4 and postnatal day 7 rat cerebellum. Alcoholism, clinical and experimental research, 39(1), 55–63. doi: 10.1111/acer.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton MB, Paiva M, Madorsky I, Siler-Marsiglio K, & Shaw G (2006). Effect of bax deletion on ethanol sensitivity in the neonatal rat cerebellum. Journal of neurobiology, 66(1), 95–101. doi: 10.1002/neu.20208 [DOI] [PubMed] [Google Scholar]
- Homanics GE, Maeda N, Traber MG, Kayden HJ, Dehart DB, & Sulik KK (1995). Exencephaly and hydrocephaly in mice with targeted modification of the apolipoprotein B (Apob) gene. Teratology, 51(1), 1–10. doi: 10.1002/tera.1420510102 [DOI] [PubMed] [Google Scholar]
- Hong M, & Krauss RS (2012). Cdon mutation and fetal ethanol exposure synergize to produce midline signaling defects and holoprosencephaly spectrum disorders in mice. PLoS genetics, 8(10), e1002999. doi: 10.1371/journal.pgen.1002999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M, & Krauss RS (2017). Ethanol itself is a holoprosencephaly-inducing teratogen. PloS one, 12(4), e0176440. doi: 10.1371/journal.pone.0176440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppins S, Edlich F, Cleland MM, Banerjee S, McCaffery JM, Youle RJ, & Nunnari J (2011). The soluble form of Bax regulates mitochondrial fusion via MFN2 homotypic complexes. Mol Cell, 41(2), 150–160. doi: 10.1016/j.molcel.2010.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignacio C, Mooney SM, & Middleton FA (2014). Effects of Acute Prenatal Exposure to Ethanol on microRNA Expression are Ameliorated by Social Enrichment. Frontiers in pediatrics, 2, 103. doi: 10.3389/fped.2014.00103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K, WuWong DJ, Wong S, Matsuyama M, & Matsuyama S (2019). Pharmacological inhibition of Bax-induced cell death: Bax-inhibiting peptides and small compounds inhibiting Bax. Experimental biology and medicine, 244(8), 621–629. doi: 10.1177/1535370219833624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung AR, Kim TW, Rhyu IJ, Kim H, Lee YD, Vinsant S, … Sun W (2008). Misplacement of Purkinje cells during postnatal development in Bax knock-out mice: a novel role for programmed cell death in the nervous system? The Journal of neuroscience : the official journal of the Society for Neuroscience, 28(11), 2941–2948. doi: 10.1523/JNEUROSCI.3897-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn BM, Corman TS, Lovelace K, Hong M, Krauss RS, & Epstein DJ (2017). Prenatal ethanol exposure in mice phenocopies Cdon mutation by impeding Shh function in the etiology of optic nerve hypoplasia. Disease models & mechanisms, 10(1), 29–37. doi: 10.1242/dmm.026195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karacay B, & Bonthius DJ (2015). The neuronal nitric oxide synthase (nNOS) gene and neuroprotection against alcohol toxicity. Cell Mol Neurobiol, 35(4), 449–461. doi: 10.1007/s10571-015-0155-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski M, Norris KL, Cleland MM, Jeong SY, & Youle RJ (2006). Role of Bax and Bak in mitochondrial morphogenesis. Nature, 443(7112), 658–662. doi: 10.1038/nature05111 [DOI] [PubMed] [Google Scholar]
- Kawai K, Itoh T, Itoh A, Horiuchi M, Wakayama K, Bannerman P, … Lindsten T (2009). Maintenance of the relative proportion of oligodendrocytes to axons even in the absence of BAX and BAK. Eur J Neurosci, 30(11), 2030–2041. doi: 10.1111/j.1460-9568.2009.06988.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SJ, Day N, & Streissguth AP (2000). Effects of prenatal alcohol exposure on social behavior in humans and other species. Neurotoxicology and teratology, 22(2), 143–149. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10758343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm WR (1998). Biological water and its role in the effects of alcohol. Alcohol, 15(3), 249–267. doi: 10.1016/s0741-8329(97)00130-4 [DOI] [PubMed] [Google Scholar]
- Knudson CM, Tung KS, Tourtellotte WG, Brown GA, & Korsmeyer SJ (1995). Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science, 270(5233), 96–99. doi: 10.1126/science.270.5233.96 [DOI] [PubMed] [Google Scholar]
- Kotch LE, & Sulik KK (1992). Patterns of ethanol-induced cell death in the developing nervous system of mice; neural fold states through the time of anterior neural tube closure. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience, 10(4), 273–279. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1414440 [DOI] [PubMed] [Google Scholar]
- Kuhn PE, & Miller MW (1998). Expression of p53 and ALZ-50 immunoreactivity in rat cortex: effect of prenatal exposure to ethanol. Exp Neurol, 154(2), 418–429. doi: 10.1006/exnr.1998.6907 [DOI] [PubMed] [Google Scholar]
- Lanoue L, Dehart DB, Hinsdale ME, Maeda N, Tint GS, & Sulik KK (1997). Limb, genital, CNS, and facial malformations result from gene/environment-induced cholesterol deficiency: further evidence for a link to sonic hedgehog. Am J Med Genet, 73(1), 24–31. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9375918 [PubMed] [Google Scholar]
- Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, … Thompson CB (2000). The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell, 6(6), 1389–1399. doi: 10.1016/s1097-2765(00)00136-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski RJ, Song C, Sulik KK, Everson JL, Gipp JJ, Yan D, … Rowland IJ (2010). Cleft lip and palate results from Hedgehog signaling antagonism in the mouse: Phenotypic characterization and clinical implications. Birth defects research. Part A, Clinical and molecular teratology, 88(4), 232–240. doi: 10.1002/bdra.20656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Nair CE, Schlamp CL, Montgomery AD, Shestopalov VI, & Nickells RW (2016). Retinal glial responses to optic nerve crush are attenuated in Bax-deficient mice and modulated by purinergic signaling pathways. J Neuroinflammation, 13(1), 93. doi: 10.1186/s12974-016-0558-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M (2011). Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. Journal, 17, 10–12. [Google Scholar]
- Mattson SN, Crocker N, & Nguyen TT (2011). Fetal alcohol spectrum disorders: neuropsychological and behavioral features. Neuropsychology review, 21(2), 81–101. doi: 10.1007/s11065-011-9167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Chambers CD, Kalberg WO, Zellner J, Feldman H, Buckley D, … Hoyme HE (2018). Prevalence of Fetal Alcohol Spectrum Disorders in 4 US Communities. JAMA, 319(5), 474–482. doi: 10.1001/jama.2017.21896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier ID, Bernreuther C, Tilling T, Neidhardt J, Wong YW, Schulze C, … Schachner M (2010). Short DNA sequences inserted for gene targeting can accidentally interfere with off-target gene expression. FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 24(6), 1714–1724. doi: 10.1096/fj.09-140749 [DOI] [PubMed] [Google Scholar]
- Mooney SM, & Miller MW (2001). Effects of prenatal exposure to ethanol on the expression of bcl-2, bax and caspase 3 in the developing rat cerebral cortex and thalamus. Brain research, 911(1), 71–81. doi: 10.1016/s0006-8993(01)02718-4 [DOI] [PubMed] [Google Scholar]
- Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, … Groop LC (2003). PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nature genetics, 34(3), 267–273. doi: 10.1038/ng1180 [DOI] [PubMed] [Google Scholar]
- Mosinger Ogilvie J, Deckwerth TL, Knudson CM, & Korsmeyer SJ (1998). Suppression of developmental retinal cell death but not of photoreceptor degeneration in Bax-deficient mice. Invest Ophthalmol Vis Sci, 39(9), 1713–1720. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9699561 [PubMed] [Google Scholar]
- Olney JW, Tenkova T, Dikranian K, Muglia LJ, Jermakowicz WJ, D'Sa C, & Roth KA (2002). Ethanol-induced caspase-3 activation in the in vivo developing mouse brain. Neurobiology of disease, 9(2), 205–219. doi: 10.1006/nbdi.2001.0475 [DOI] [PubMed] [Google Scholar]
- Parnell SE, Dehart DB, Wills TA, Chen SY, Hodge CW, Besheer J, … Sulik KK (2006). Maternal oral intake mouse model for fetal alcohol spectrum disorders: ocular defects as a measure of effect. Alcoholism, clinical and experimental research, 30(10), 1791–1798. doi: 10.1111/j.1530-0277.2006.00212.x [DOI] [PubMed] [Google Scholar]
- Parnell SE, Sulik KK, Dehart DB, & Chen SY (2010). Reduction of ethanol-induced ocular abnormalities in mice through dietary administration of N-acetylcysteine. Alcohol, 44(7–8), 699–705. doi: 10.1016/j.alcohol.2010.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra M, Salonen E, Terama E, Vattulainen I, Faller R, Lee BW, … Karttunen M (2006). Under the influence of alcohol: the effect of ethanol and methanol on lipid bilayers. Biophys J, 90(4), 1121–1135. doi: 10.1529/biophysj.105.062364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patro R, Duggal G, Love MI, Irizarry RA, & Kingsford C (2017). Salmon provides fast and bias-aware quantification of transcript expression. Nat Methods, 14(4), 417–419. doi: 10.1038/nmeth.4197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena-Blanco A, & Garcia-Saez AJ (2018). Bax, Bak and beyond - mitochondrial performance in apoptosis. The FEBS journal, 285(3), 416–431. doi: 10.1111/febs.14186 [DOI] [PubMed] [Google Scholar]
- Peng Y, Wang L, Qing Y, Li C, Ren T, Li Q, … Wang D (2015). Polymorphisms of BCL2 and BAX Genes Associate with Outcomes in Advanced Non-small cell lung cancer Patients treated with platinum-based Chemotherapy. Scientific reports, 5, 17766. doi: 10.1038/srep17766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrelli B, Weinberg J, & Hicks GG (2018). Effects of prenatal alcohol exposure (PAE): insights into FASD using mouse models of PAE. Biochem Cell Biol, 96(2), 131–147. doi: 10.1139/bcb-2017-0280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham CT, MacIvor DM, Hug BA, Heusel JW, & Ley TJ (1996). Long-range disruption of gene expression by a selectable marker cassette. Proceedings of the National Academy of Sciences of the United States of America, 93(23), 13090–13095. doi: 10.1073/pnas.93.23.13090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudvere U, Kolberg L, Kuzmin I, Arak T, Adler P, Peterson H, & Vilo J (2019). g:Profiler: a web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res, 47(W1), W191–W198. doi: 10.1093/nar/gkz369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson AM, Conley DB, & Kern RC (2003). Olfactory neurons in bax knockout mice are protected from bulbectomy-induced apoptosis. Neuroreport, 14(15), 1891–1894. doi: 10.1097/00001756-200310270-00002 [DOI] [PubMed] [Google Scholar]
- Sato M, Suemori H, Hata N, Asagiri M, Ogasawara K, Nakao K, … Taniguchi T (2000). Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity, 13(4), 539–548. doi: 10.1016/s1074-7613(00)00053-4 [DOI] [PubMed] [Google Scholar]
- Saxena A, Moshynska O, Sankaran K, Viswanathan S, & Sheridan DP (2002). Association of a novel single nucleotide polymorphism, G(-248)A, in the 5'-UTR of BAX gene in chronic lymphocytic leukemia with disease progression and treatment resistance. Cancer Lett, 187(1-2), 199–205. doi: 10.1016/s0304-3835(02)00378-6 [DOI] [PubMed] [Google Scholar]
- Semaan SJ, Li Y, & Nickells RW (2010). A single nucleotide polymorphism in the Bax gene promoter affects transcription and influences retinal ganglion cell death. ASN Neuro, 2(2), e00032. doi: 10.1042/AN20100003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, … Ideker T (2003). Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome research, 13(11), 2498–2504. doi: 10.1101/gr.1239303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Parada LF, & Kernie SG (2005). Bax limits adult neural stem cell persistence through caspase and IP3 receptor activation. Cell death and differentiation, 12(12), 1601–1612. doi: 10.1038/sj.cdd.4401676 [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Aase JM, Clarren SK, Randels SP, LaDue RA, & Smith DF (1991). Fetal alcohol syndrome in adolescents and adults. JAMA, 265(15), 1961–1967. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2008025 [PubMed] [Google Scholar]
- Streissguth AP, & Dehaene P (1993). Fetal alcohol syndrome in twins of alcoholic mothers: concordance of diagnosis and IQ. Am J Med Genet, 47(6), 857–861. doi: 10.1002/ajmg.1320470612 [DOI] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, … Mesirov JP (2005). Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proceedings of the National Academy of Sciences of the United States of America, 102(43), 15545–15550. doi: 10.1073/pnas.0506580102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulik KK (1984). Critical periods for alcohol teratogenesis in mice, with special reference to the gastrulation stage of embryogenesis. Ciba Found Symp, 105, 124–141. doi: 10.1002/9780470720868.ch8 [DOI] [PubMed] [Google Scholar]
- Sulik KK, Cook CS, & Webster WS (1988). Teratogens and craniofacial malformations: relationships to cell death. Development, 103 Suppl, 213–231. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/3074910 [DOI] [PubMed] [Google Scholar]
- Sulik KK, Johnston MC, & Webb MA (1981). Fetal alcohol syndrome: embryogenesis in a mouse model. Science, 214(4523), 936–938. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/6795717 [DOI] [PubMed] [Google Scholar]
- Sun L, Wei L, Wei L, & Li D (2018). Correlation between Bax gene polymorphisms and esophagus cancer. Oncol Lett, 16(6), 7097–7101. doi: 10.3892/ol.2018.9511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, & Oppenheim RW (2003). Response of motoneurons to neonatal sciatic nerve axotomy in Bax-knockout mice. Molecular and cellular neurosciences, 24(4), 875–886. doi: 10.1016/s1044-7431(03)00219-7 [DOI] [PubMed] [Google Scholar]
- Voss AK, & Strasser A (2020). The essentials of developmental apoptosis. F1000Res, 9. doi: 10.12688/f1000research.21571.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West DB, Engelhard EK, Adkisson M, Nava AJ, Kirov JV, Cipollone A, … Lloyd KC (2016). Transcriptome Analysis of Targeted Mouse Mutations Reveals the Topography of Local Changes in Gene Expression. PLoS genetics, 12(2), e1005691. doi: 10.1371/journal.pgen.1005691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FA, Keller-Peck CR, Knudson CM, Korsmeyer SJ, & Snider WD (1998). Widespread elimination of naturally occurring neuronal death in Bax-deficient mice. The Journal of neuroscience : the official journal of the Society for Neuroscience, 18(4), 1428–1439. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9454852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieczorek L, Fish EW, O'Leary-Moore SK, Parnell SE, & Sulik KK (2015). Hypothalamic-pituitary-adrenal axis and behavioral dysfunction following early binge-like prenatal alcohol exposure in mice. Alcohol, 49(3), 207–217. doi: 10.1016/j.alcohol.2015.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young C, Klocke BJ, Tenkova T, Choi J, Labruyere J, Qin YQ, … Olney JW (2003). Ethanol-induced neuronal apoptosis in vivo requires BAX in the developing mouse brain. Cell death and differentiation, 10(10), 1148–1155. doi: 10.1038/sj.cdd.4401277 [DOI] [PubMed] [Google Scholar]
- Zhang C, Ojiaku P, & Cole GJ (2013). Forebrain and hindbrain development in zebrafish is sensitive to ethanol exposure involving agrin, Fgf, and sonic hedgehog function. Birth defects research. Part A, Clinical and molecular teratology, 97(1), 8–27. doi: 10.1002/bdra.23099 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.