Abstract
In recent years, significant advances have been made in the field of functionalized membranes. With the functionalization using various materials, such as polymers and enzymes, membranes can exhibit property changes in response to an environmental stimulation, such as heat, light, ionic strength, or pH. The resulting responsive nature allows for an increased breadth of membrane uses, due to the developed functionalization properties, such as smart-gating filtration for size-selective water contaminant removal, self-cleaning antifouling surfaces, increased scalability options, and highly sensitive molecular detection. In this review, new advances in both fabrication and applications of functionalized membranes are reported and summarized, including temperature-responsive, pH-responsive, light-responsive, enzyme-functionalized, and two-dimensional material-functionalized membranes. Specific emphasis was given to the most recent technological improvements, current limitations, advances in characterization techniques, and future directions for the field of functionalized membranes.
Keywords: membrane, functionalization, responsive materials, temperature-responsive, polyelectrolytes, light-responsive, plasmonic particles, thiol-functionalized, graphene oxide
1. Introduction
The field of functionalized membranes, in both extent of applications and fabrication methodology, has seen a continuous growth in recent years. This technological interest is due to membranes being powerful systems that can be functionalized with different materials, such as polymers, chelating groups, enzymes, and reactive nanoparticles, to exhibit a change in certain properties in response to an environmental stimulus1. With a flexible responsive nature, “smart” membrane systems can effectively tackle both new and old environmental, industrial, and community problems, showing certain advantages, such as cost or efficiency, over other existing technologies. While these advantages are not present unanimously in every single situation, functionalized membranes continue to be a leading technology in the field of separations.
Development of functionalization methods has greatly increased the breadth of membrane applications, as the membrane system has additional abilities than traditional filtration/separation2. Depending on the intended application, the surface and/or pores of the membrane are functionalized with the responsive material3 by different means, ranging from physical methods to chemical modifications and incorporation of separate nanomaterials within system matrices4. Furthermore, the environmental stimulus that a responsive membrane can react to varies significantly on the functionalization material, but is typically based on a temperature, pH, or light5 environmental change to produce such a responsive nature1. Additional functionalization can be achieved for the enhancement of a specific functionality (without an expressive property change in the membrane matrix), such as the addition of a thin 2-dimensional layer6 or the addition of certain functional groups (i.e., thiol7).
There are five primary reasons for membrane functionalization, which consist of increasing (1) structural stability of the filtration system, (2) separation selectivity, (3) membrane permeability, (4) reactivity, whether it be degradative or for anti-fouling abilities, and (5) large-scale scalability for industrial applications (Fig. 1). Within membrane science, these enhancements increase the variety of applications that functionalized membranes can apply to. A consistent application of such membranes is the use of smart gating strategies that control permeability and pollutant/ion transport through the system. This allows for high selectivity through porous membranes to improve performance for various applications ranging from separations to drug delivery3,8–10. Other applications include self-cleaning antifouling surface modifications, highly sensitive molecular detection, detoxification of toxic water/air pollutants, and gas separation.
Figure 1.

Summarization of primary functionalized membranes, their responsive nature, and key improvements.
This review provides a broad overview of the most recent technological advances in functionalized membranes for separation applications, ranging from functionalization methods to new membrane uses covering temperature, pH, and light-responsive membranes, as well as enzyme, thiol, and graphene oxide functionalization. This work will focus on the most cutting-edge membrane developments in the field in the past 5 years. Specific attention was given to current limitations, technological improvements in characterization techniques, and future directions of the field of functionalized and responsive membranes.
2. Responsive functionalized membranes
2.1. Temperature-responsive functionalized membranes
2.1.1. Overview of temperature-responsive functionalized membranes
Temperature-responsive membranes are smart materials that can exhibit conformation changes, depending on the temperature of the membrane system, a wide variety of applications, such as environmental remediation, drug delivery, and controllable membrane transport through the membrane. To achieve this reversible responsiveness, the membrane surface and/or pores are typically functionalized with thermo-responsive polymers that undergo volume phase transitions at specific temperature ranges. This transition leads to the swelling/deswelling of the polymer hydrogel, due to changes in hydrophobic/hydrophilic properties, and is governed by fluctuation in hydrogen bonding properties and preferences.
These polymers can be generally categorized into lower critical solution temperature (LCST) materials, such as N-isopropylacrylamide (NIPAm), methyl vinyl ether (MVE), and N,N-diethylacrylamide (DEAM), and upper critical solution temperature (UCST) materials, such as a combination of acrylic acid (AA) and acrylamide (AM)11. These polymers transition from hydrophilic to hydrophobic behavior or vice versa when exceeding their LCST or UCST, respectively (Fig. 2A). This transition is often expressed using the following equation for the polymer’s root-mean-square end-to-end distance1:
| (1) |
Figure 2. Schematic of thermo-responsive membranes functionalized with UCST and LCST polymers.

(A) Change in thermo-responsive membrane permeability and hydrophilicity, based on changes in temperature across the critical solution temperature. (B) Transition between coil-globule phase and (C) example of hydrogen bonding preference of thermo-responsive hydrogel (PNIPAm) on membrane surface and pores across the critical solution temperature.
Where a is the chain expansion factor, is the polymer chain length, is the number of freely jointed links in the -length of hypothetical chain, and is the characteristic ratio (considers valence angles and chain rotation). In the hydrophilic state, the polymer exhibits a coiled structure and swells due to favorable interactions with aqueous solutions (a>1), thus decreasing the effective diameter of the membrane pores. In the hydrophobic state, the polymer retracts upon itself and exhibits a globular structure, as interaction with the aqueous solvent is non-favorable (<1), which increases the effective membrane pore diameter (Fig. 2B). Overall, due to the flexible nature of temperature-responsive polymers, membrane functionalization with such materials is a favorable procedure for large-scale and research purposes.
2.1.2. PNIPAm-functionalized membranes
Poly(N-isopropylacrylamide) (PNIPAm) is a common temperature-responsive polymer, due to its highly studied nature and biocompatibility. PNIPAm contains a hydrophilic amide group and a hydrophobic isopropyl group12, thus creating a sharp behavioral response across its LCST of around 30-34°C. Below the LCST, hydrogen bond interaction between the water and certain groups, such as C=O and N-H moieties13, is preferred. Above the LCST, though, the polymer exhibits preferred intermolecular hydrogen bond interaction between the amide groups of nearby PNIPAm polymer14 (Fig. 2C).
PNIPAm functionalization has been reported with several different membrane materials, such as poly(vinylidene fluoride) (PVDF)15, polypropylene (PP)16, polyethylene (PE)17, and polycarbonate (PC)18. Functionalization is typically achieved using a pore-filling method 19,20, which utilizes the redox free-radical polymerization of the monomer within the membrane pores. Other methods for incorporating PNIPAm into the membrane system include plasma treatment and high energy radiation12. These membranes have shown several different uses, such as enhanced water remediation21,22, the development of antifouling/self-cleaning surfaces23, and effective separation of hydrophobic/hydrophilic liquids24.
The responsive nature of PNIPAm hydrogels is independent of the polymer’s molecular weight and concentration25, but can be limited in certain scenarios of membrane functionalization. Microfiltration PVDF membranes with PNIPAm has shown significant responsive nature below and above the LCST with weight gains post-functionalization ranging from 15-17%21,22. Weight gains greater than such values or with functionalization primarily in membrane pores could, however, inhibit the responsive phase transition of the hydrogel and permeability through the membrane26, as the maximum available volume for the hydrogel to change conformation is limited by the pore volume.
Furthermore, the temperature range that results in PNIPAm’s thermo-responsive behavior can be affected by the ionic strength of the surrounding solution via the Hofmeister series27,28. Essentially, the high presence of salts in the environment can prevent the water from interacting with the amide group of PNIPAm, thus lowering the LCST (salting-out). Vu et al. showed this ionic strength-responsiveness in a PNIPAm-functionalized nanofiltration membrane system, where control of PNIPAm phase (coil or globular) was controlled by change in salt concentration, instead of ambient temperature, via change in the specific value of the polymer’s LCST29.
Several significant advances have been made in the functionalization process of membranes with PNIPAm. Fabricating temperature-responsive membranes using PNIPAm-hydrogel particles and an existing membrane structure has been a common practice, resulting in a mixed-matrix membrane (MMM). This method has several advantages, such as the single step preparation method for the responsive particles and membrane itself, which offers customization and further control of the resulting membrane systems without the need for a complex multi-step process30. Furthermore, this method has shown the potential to yield higher PNIPAm particle presence on certain membranes, which greatly increases the temperature-response of water permeabilities on the resulting membranes31,32.
Creating MMMs with PNIPAm has been achieved with flat-sheet membranes33,34 using the process of non-solvent induced phase separation (NIPS) but has not been applied to hollow fiber membranes (HFM) until recent years. HFM geometry is often favorable for further development after technology validation in flat-sheet applications, as this geometry offers a higher membrane packing density of surface area per volume utilized, thus increasing the scalability and commercial practicality30. Götz et al. recently adapted the NIPS process from flat-sheet membranes to HFM fabrication. This study functionalized a polyether sulfone (PES) HFM (using wet spinning process) with temperature-responsive PNIPAm hydrogels30. PNIPAm presence in the membrane was confirmed using carbon-hydrogen-nitrogen-sulfur (CHNS) elemental analysis, and the membranes showed a 12x increase in water permeability (0.5 to 6 LMH/bar) through the resulting membrane when transitioning above the LCST (at 1 bar of applied pressure). This investigation indicates a significant advance in the fabrication and scalability of thermo-responsive membrane systems for commercial applications.
The technology and understanding of HFM functionalization with PNIPAm have recently been expanded with investigations of microgel mobility and interaction with membranes. Wiese et al. controlled the charge of PNIPAm microgels on a PES-HFM surface and examined how microgel charge affected the functionalization process35, which has not been previously investigated in literature. To achieve this, the authors developed a novel microscopy module, which allows for online movement monitoring of single microgels and layer formation. Furthermore, this study concluded that slightly negatively charged PNIPAm microgels can functionalize a PES membrane without particle loss or rearrangement under specific flow (Reynolds Number Re=100), while highly charged microgels display significant particle loss and rearrangement at Re of 10035. The resulting microscopy tool, along with the discovered effect of microgel charge, has significant current and future implications for the investigation and optimization of PNIPAM hydrogel functionalization of HFMs.
Track-etched membranes with uniform pores have also been a promising medium for PNIPAm functionalization to create responsive membranes with tunable pore sizes. In the past, functionalized ultrafiltration (UF) membranes were shown to achieve an effective pore diameter of 20-100 nm using a controlled surface-initiated atom transfer radical polymerization (SI-ATRP) of PNIPAm36. Ulbright et al. further demonstrated that the effective pore diameter of track-etched poly(ethylene terephthalate) (PET) membrane can be reduced to under 10 nm26. In this study, controlled lysosome rejection (~4 nm in diameter) was validated as 65% and 99% above and below the LCST, respectively, indicating effective tunable filtration of nano-sized material. Furthermore, this study showed that, even in effective nano-diameter pores, effective pore size and functionalization degree was linearly correlated to SI-ATRP reaction time. These recent advances widen the application abilities of thermo-responsive membrane systems for tunable filtration.
The applications of PNIPAm-functionalized membranes have been expanded in recent years with investigations showing uses for environmental remediation and filtration. Temperature swing adsorption/desorption methods have shown promise, as the filtration system can achieve tunable and reusable remediation of water sources from pollutants. Above the LCST of PNIPAm, the functionalized membrane can exhibit enhanced adsorption of contaminants, such as oil24,37, copper38 and proteins39,40, due to the hydrophobic domain that allows greater interaction between the contaminant and membrane system. Below the LCST, PNIPAm exhibits hydrophilic behavior, thus allowing adsorbed contaminants to desorb into a lower volume of solution for further treatment, regenerating the system for further cycles of reuse.
Recent advances have been made to broaden the contaminants that PNIPAm-functionalized membranes can remove. Saad et al. examined the efficacy of temperature-responsive membranes for enhanced removal of harmful perfluorooctanoic acid (PFOA) from water sources22, which had not been previously investigated in literature. With PNIPAm-functionalized PVDF membranes above the LCST, this study achieved an over 4x increase in PFOA adsorbed (ratio of mass of PFOA over mass of membrane) compared to below the LCST. The driving force of this drastic increase in PFOA adsorbed is further explained in Figure 3, where the increased hydrophobic interaction (such as Hansen dispersion parameter) between the isopropyl domain of PNIPAm and the hydrophobic fluorinated tail of PFOA is significantly expressed above the LCST41. This is likely due to the isopropyl groups dehydrating when increasing the temperature above the LCST, where hydrogen bonds of the polymer-bound water (with C=O and N-H) become less favorable and hydrogen bonds (Hansen hydrogen bonding parameter) between the polymer chains themselves become favorable13.
Figure 3. Hydrophobic interaction changes between functional groups of PNIPAm and PFOA below and above the polymer LCST.

Adapted from Saad et al.22
The resulting membranes also displayed stable adsorption/desorption properties over 5 temperature swing cycles (Fig. 4A), indicating high stability and reusability of the system. As previously mentioned, this increased adsorption of PFOA was due to the increased hydrophobic interactions between the polymer’s isopropyl group and the fluorinated chain of the contaminant, while the subsequent desorption of PFOA was caused by the re-hydration of the isopropyl groups below the LCST, thus decreasing the hydrophobic interactions available.
Figure 4. Results of PFOA capture/rejection by recent advances of thermo-responsive functionalized membranes.

(A) PFOA temperature-swing adsorption and desorption using PNIPAm functionalized PVDF400 demonstrates reusability of PFOA-capturing membranes over five adsorption/desorption cycles. Reproduced with permission from Saad et al.22 (B) PFOA adsorption (above LCST) over time in a batch mode using thin film nanofiltration on PNIPAm-functionalized microfiltration composite membrane shows how functionalization with thermo-responsive polymers can add secondary mechanism to further increase removal of contaminants such as PFOA. Reproduced with permission from Léniz-Pizarro et al.43
Furthermore, these adsorption values were comparable to that of granulated activate carbon (GAC)42, an industry standard for PFOA adsorption. Further work on this topic has been conducted by Léniz-Pizarro et al. with the formation of a thin film (polyamide nanofiltration layer) on PNIPAm pore-functionalized microfiltration membranes43. The resulting membrane systems utilized two separation mechanisms, achieving adsorption of 178 mg PFOA adsorbed on the support layer per m2 membrane (Fig. 4B) and exclusion (via thin film layer) of approximately 70% rejection of PFOA at high water fluxes (~100 LMH with applied pressure of 7 bar). Overall, these studies indicate promising new applications for thermo-responsive membranes in the advancement of environmental remediation from perfluoro-organic pollutants.
Further advancements in this field combined PNIPAm and catalytic functionalization for environmental remediation applications. Functionalizing membranes with zero-valent iron (ZVI)-palladium nanoparticles (Fe/Pd NPs) have shown promise for the reductive degradation of certain water pollutants, yet have been shown to be limited in functionality due to mass transfer limitations between the catalyst (Pd) and the contaminants21. Saad et al. incorporated PNIPAm-functionalization into Fe/Pd catalytic PVDF membranes to overcome such limitations for degrading polychlorinated biphenyl (PCB-1). Utilizing PNIPAm functionalization, the resulting Fe/Pd-functionalized membranes yielded first order rate constant (kSA) values of 0.28 and 0.72 LMH at 25°C (below) and 35°C (above the LCST), respectively21. Li et al. have shown similar advantages using PNIPAm-functionalized membranes for enhanced 2-nitroaniline degradation to 1,2-diaminobenzene by embedded Pd NPs. Degradation efficiency increases of 11.6, 4.3, and 0.3% were observed from 30 to 35°C for membranes with Pd loading content of 0.408, 0.556, and 0.685 mg per gram of membrane, respectively37, indicating that PNIPAm-enhancement could allow for a more efficient remediation process. It is important to note that such systems, with magnetic nanoparticles (such as magnetite nanoparticles), are also responsive to magnetic field stimulation. Such responsiveness is often utilized for other applications, such as enhanced separation, drug delivery, and sensing in the field of automation and material separation44.
PNIPAm functionalization is also becoming a promising membrane modification to reduce fouling and add self-cleaning properties. Xu et al. showed that the functionalization of polyamide membranes with PNIPAm exhibited greater resistance to protein adhesion and deposition, due to the hydrophilic properties of the polymer below the LCST, thus reducing long-term fouling23. Above the LCST, PNIPAm functionalization exhibits hydrophobic properties, allowing for the formation of a “buffer layer”. This reduced interaction between the contaminant and membrane, allowing for self-cleaning applications to combat irreversible fouling. Several studies have recently reported similar advances in antifouling and antibacterial properties with PNIPAm-functionalized zwitterionic membranes37,45, random copolymer grafted membranes46, N-isopropylacrylamide grafted silica nanoparticles embedded in membrane systems47, and Bentonite-PNIPAm nanocomposite membranes48. Specifically, Mao et al. developed PVDF membranes with thermo-responsive attapulgite (t-ATP) functionalization by incorporating PNIPAm functionalization with ATP fibers49. Utilizing a temperature-change coupled ultrasonic cleaning process, these membranes systems were able to remove ~89% of bovine serum albumin (BSA) biofouling present on the membrane surface, indicating significant advances for scalable biological waste remediation. Similar reductions in BSA fouling were observed by Zhao et al. with N-isopropylacrylamide grafted silica nanoparticles embedded in ultrafiltration PVDF membranes47.
2.1.2. Other thermo-responsive polymers including poly(N-hydroxymethylacrylamide) and acrylamide/acrylonitrile copolymers
Significant advances in functionalized membranes with other temperature-responsive polymers have been made in recent years. For example, UCST polymers, such as copolymers of acrylic acid, acrylamide and acrylonitrile (AN) at low pH, are less studied than their LCST counterparts. These copolymer hydrogels are fairly temperature-responsive, as increased intermolecular hydrogen bonding is expressed below the UCST (globule-like state), while hydrogen bond interactions between the hydrogel and the surround water are favored above the UCST (coil-like state)50.
Ding et al. successfully created nanofibrous membranes with poly(AAm-co-AN)), utilizing a one-step electrospinning method51. While these membranes displayed a consistent separation efficiency for oil/water separation (99.5% below UCST) over 6 cycles, a permeability of roughly 60,000 LMH/bar was also maintained over these separation cycles (as opposed to only 60% permeability recovery for PVDF membranes). This consistent permeability over 6 cycles was due to reversing the membrane fouling with an acidic high-temperature (55°C) solution above the polymers’ UCST. Another example of an advance in functionalized membranes utilized poly(N-hydroxymethylacrylamide) (PNHMA), an LCST polymer, for enhanced isopropyl alcohol (IPA) dehydration via pervaporation. Baysak et al. recently investigated poly(vinyl alcohol)-graft-PNHMA membranes, showing that membrane flux increased and IPA/water separation factor decreased with increasing operating temperature52. Modeling this observed relationship, this study identified an ideal operating temperature for this membrane system at 30°C, achieving an increased flux (due to LCST polymer response) of 0.0085 kg/m2/h while optimizing the separation factor and selectivity to 362 and 2209, respectively. Furthermore, Işıklan et al. utilized the thermo-responsive polymer poly(N,N-diethylacrylamide) (PDEAAm) and a microwave-induced graft copolymerization to create biocompatible pectin-g-PDEAAm copolymers53 with high grafting, thermal stability, and an LCST of ~31°C, thus advancing previous methods of thermo-responsive membrane functionalization with such responsive polymer material.
2.2. pH-responsive functionalized membranes
2.2.1. Overview of pH-responsive functionalized membranes
pH-responsive membranes allow for certain changes in properties, depending on the pH of the membranes’ surroundings. Such membranes encapsulate a large portion of the functionalized membrane field, due to their wide applicability, such as biomaterials, nanotechnology, drug delivery, controlled transport, and water filtration. These membranes typically achieve their responsive nature via surface and pore functionalization with a pH-responsive material, which is typically a weak polyelectrolyte (PEL) polymer, such as poly(acrylic acid) and poly(methyl methacrylate) (PMMA) and are commonly co-functionalized with another functional material (i.e. temperature-responsive or catalytic functionalization via ion exchange of polyelectrolyte groups). These responsive polymers can swell/deswell and change functional group charge with changes in pH, which, in simple terms, is determined by the pKa value (negative base ten log of acid dissociation constant) of each polymer. pKa is inversely correlated to the strength of the acid, meaning that the lower the pKa value for a pH-responsive polymer, the greater its tendency to deprotonate at a given pH. This responsive polymer nature, similar to temperature-responsive polymeric functionalizations, can be described using root-mean-square end-to-end distance relationships (Equation 1).
Many pH-responsive functionalizations consist of acidic PEL polymers, functionalized via physical blending or chemical grafting54, where co-valent grafting systems can have a higher responsive nature55. More specifically, polyelectrolyte polymers have an electrolyte repeat unit56 and the monomers are typically soluble in water, but become insoluble upon polymerization and crosslinking during the membrane functionalization process57. These now-insoluble anionic polymers on the surface and pores of the membrane can exhibit different charges, due to their weak acid groups (Fig. 5A). In a solution with a pH below the pKa value of the weak PEL polymer’s responsive group, the polymer groups are protonated. This results in the polymer hydrogel collapsing to a globule-like state, due to increased hydrogen bond interactions between the polymer’s carboxylic groups. In a solution with a pH above the respective pKa, the acidic polymer group is deprotonated, thus releasing a positive charge (H+). Due to the negative charge, there is a significant repulsive force that inhibits hydrogen bond interaction between the polymer groups, thus the hydrogel extends into a coil-like state. This interaction change decreases the effective pore diameter. Note that many polymers can have several pKa values, due to multiple electrolyte groups in the repeat unit, thus different protonated and deprotonated group can exist at specific pH ranges. Furthermore, it is important to note that pH levels do not affect the charge distributions of polymers with strong electrolyte groups, only with weak electrolyte groups58. This is because weak PEL polymers contain ionizable groups with a lower degree of dissociation, while strong PEL polymers have groups with such high degree of dissociation that they do not exhibit a significant responsive nature1.
Figure 5. Schematic of pH-responsive membrane section with common polymers.

(A) pH effect on the membrane permeability of membranes functionalized with cationic or anionic polyelectrolytes. Reproduced with permission from Xiao et al62. (B) Different types of charged polymer brushes. Reproduced under Creative Commons License (available at http://creativecommons.org/licenses/by/4.0/) from Durmaz et al63. (C) Differences in structure of common weak anionic PEL polymers with acidic groups.
Additionally, many polyelectrolytes are not responsive to only the concentration of hydrogen ions in the surrounding environment, but to ionic strength overall, such as salt concentration59. The mechanism for responsiveness is also dependent on the type of polyelectrolyte and its responsive mechanism: (1) cationic, such as poly(ethyleneimine and poly(dimethylaminoethyl methacrylate)60, (2) anionic, such as poly(acrylic acid) and poly(methyl methacrylate), and (3) zwitterionic polymers, such as poly[1-vinyl-3(2-carboxyethyl) imidazolium betaine] (PVCIB)61 (Fig. 5B).
2.2.2. PAA/PMAA/PMMA-functionalized membranes
Acrylic acid, methacrylic acid (MAA), and methyl methacrylate (MMA) are common anionic monomers utilized in the functionalization of different membranes (Fig. 5C) via in-situ free radical polymerization and thermal treatment of monomer-modified membranes or via dip-coating membranes with PAA64. These polymers, due to their ion exchange abilities via deprotonated functional groups (carboxylic acid with pKa of 4.565) and fairly hydrophilic properties, are extensively used for various membrane applications, such as metal ion capture21,66–68, nonionic surfactant adsorption69, nanoparticle formation21,70, and enhanced enzyme immobilization on membranes71,72. The weight gain of the resulting membranes after polymerization tends to be lower than that of thermo-responsive membranes, typically less than 15%. This is because lower PAA/PMAA/PMMA polymer density maintains significant membrane permeability and high diffusion capacity for exchangeable ions, thus allowing for higher subsequent functionalization capacity. Control of weight gain, cross-linking density, and desired exchangeable ion concentration allows for specific functionalization capacity to meet the separation criteria for intended application.
Specifically, in the field of environmental catalysis, PAA/PMAA/PMMA-functionalized membranes have been vital for the immobilization of iron and the subsequent formation of ZVI particles, due to the ion-exchange properties of their carboxylic acid component (pKa of 4.565). In an environment with a pH less than ~4.5, the carboxyl group remains protonated (COOH). With a pH above 4.5, though, the group becomes deprotonated (COO−), allowing for the introduction of a positively-charged ion, such as sodium (Na+), into the group. At a high pH, the Na+ can be replaced by ferrous iron, via ion-exchange, and then reduced using a strong reducing agent (i.e., sodium borohydride, NaBH4) or a green reducing agent (i.e., green tea extract73) for the formation of a ZVI nanoparticle. The resulting NP system is more stable long-term in a membrane media than in suspension, and the size of formed NPs in membrane media can be more easily controlled with PAA/PMAA/PMMA cross-linker density74.
PAA, PMAA, and PMMA respond similarly to changes in pH levels, but there are several noticeable differences that are considered with an intended application. At pH levels below the pKa, PAA is not a fully globule structure and is still soluble in water, thus the change in effective pore diameter is less pronounced than other PEL polymers75. Alternatively, PMAA and PMMA display a “hyper-coiled” and significantly globular structure at a low pH, which occurs due to the methyl group that PAA lacks2,75. This allows for a more significant change in mean end-to-end distance with pH change and therefore a stronger response in changing effective pore diameter. Furthermore, PMMA has a considerably lower vapor pressure than other acrylic acid-based options21. This makes PMMA a more viable functionalization material for the industrial scale, as it can increase functionalization efficiency and reduce air/vapor pollution.
There have been significant advances in membrane functionalization with PAA, PMMA, and PMMA. As previously mentioned, in-situ polymerization of PAA is typically performed using thermal initiation of monomer-coated membrane or dip-coating of membranes with polymer solution. These methods, while highly utilized, have certain limitations. Thermal initiation requires an oxygen-free environment and a lengthy functionalization timeline (60-120 minutes)74, which makes it less appealing for scalability. Dip-coating with polymerized solution often results in membranes with agglomeration and clogging of nanoparticles in the membrane system and is limited by polymer compatibility64,76. Microwave (MW) irradiation has been proven to allow for a faster polymerization time with carboxymethyl cellulose membranes77, due to a lower activation energy for initiator formation78, and a more stable membrane matrix. MW irradiation methods have not been previously reported with PVDF membranes, which are a more viable membrane for scalability and highly-selective separation of nanomaterials, respectively.
Silva et al. developed a detailed synthesis method of functionalizing PVDF membranes with PAA utilizing microwave irradiation for water treatment and ZVI applications76. This study extensively investigated controlling functionalization yield (%) by altering the duration of continuous and multi-dose MW treatment. MW exposure times of (2 × 15 s), (3 × 15 s), and (4 × 15 s) resulted in PAA yields of 1.5, 4.5 and 19.4% weight gain, respectively (Fig. 6A). Utilizing multi-dose exposure with longer irradiation times (i.e., 2 x 30 s) yielded significantly lower PAA than that of the (4 × 15 s) dose (Scanning electron microscope-SEM image on Fig. 6B), indicating that doses longer than 15 s can thermally damage the PAA polymer hydrogel76. This damage is most likely due to primary chain scission reactions, which also results in an overall less stable membrane matrix77. Similarly, Ashfaq et al. functionalized graphene-oxide coated RO membranes with PAA using MW radiation for antiscalant purposes79. This method utilized an irradiation power of 600 W for 40 seconds, although a significant subsequent reaction time of 60 minutes still remained. Overall, these studies show significant advances in scalability for PEL polymer functionalization of membranes by overcoming the time limitation associated with traditional methods.
Figure 6. Functionalization of membranes with PAA using microwave radiation.

(A) PAA conversion yield % due to MW continuous and multi-dose irradiation. Average error: 20%. 2 × 15 s; “2” refers to the number of MW doses and “15 s” is the MW dose time (i.e., total MW dose of 2x15 = 30 s), while air-cooling was applied for 30 s between doses. (B) PVDF membrane treated with (4 × 15 s) MW with 30 s of air-cooling: SEM image of bottom surface. Reproduced with permission from Silva et al.76
PAA/PMAA/PMMA-functionalized membranes have a wide range of applications, due to their ability to change charge of certain groups in response to pH level. In recent years, Olimattel et al. reported the use of polyallylamine hydrochloride (PAH)/PAA-functionalized (through fluidic layer-by-layer assembly) UF membranes as a cost-effective alternative to RO membranes for water remediation from perfluorinated toxic pollutants80. Due to size and charge exclusion, the functionalized membranes exhibited roughly 30% higher removal efficiency of PFOA and PFOS with an overall 38% reduction in molecular weight cut-off, showing the promise of lower-cost separation methods than existing commercial options. Liu et al. reported functionalizing carbon nanotube-composite membranes with PAA for oil-water separation81. The highly hydrophilic properties of the membrane (from PAA functionalization) exhibited a high separation efficiency (>95%) of dichloromethane-in-water emulsion over 10 filtration cycles, while maintaining a membrane permeability over 10,000 LMH per bar.
Furthermore, PEL-functionalized membranes have shown advances in removal of hazardous particulate matter filtration from the air phase. Lu et al. fabricated electrospun superhydrophobic polydimethylsiloxane (PDMS) membranes with PMMA as a carrier polymer82. The resulting membranes exhibited almost 100% filtration efficiency of fine inhalable particles (~2.5 μm in size) with miniscule pressure drop (70 Pa). This was achieved in a high humidity environment (96±3% relative humidity), which can often hinder the filtration efficiency of hydrophilic air filters. Additionally, Mishra et al. reported functionalizing poly(amide imide) (PAI) hollow fiber membranes with poly(ethylene oxide) (PEO) and PMAA for carbon dioxide separation83. PMAA was incorporated with the intended goal of overcoming a known limitation of PEO-functionalized membranes, crystallinity formation in layer-by-layer deposition. The resulting membranes achieved one of the highest selectivity factors reported for CO2:N2 (240:1) with a CO2 permeability of 1,018 Barrer, which overcame the previously-tested upper limitation of permeability vs. selectivity balance/trade-off for such applications. These studies exemplify that PEL-functionalized membranes have broadening applications and advances in separation of air-based contaminants as well.
PEL functionalizations are also commonly used to enhance existing membrane systems, specifically for increased scalability and antifouling capabilities. Ansari et al. functionalized RO membranes, which are prone to fouling due to the effective filter pore size (less than 1 nm84), with PAA for enhanced antifoulant properties85. This study found that PAA-functionalized RO membranes, due to the charged surface from the polyelectrolyte, exhibit a slower decrease in water permeability with gypsum (thus less fouling), compared to the unfunctionalized control membrane. The authors also showed that PAA-functionalization does not significantly change the surface roughness of GO-functionalized RO membranes, indicating that this modification process can be used for GO-functionalized membranes without decay in precise structure. Olimattel et al. investigated organic fouling resistance of UF membranes with PAH/PAA functionalization86. Using a fluidic layer-by-layer assembly, the resulting membranes showed significant antifouling properties when tested with humic acid with a lower flux decline of 13.8±12% and a higher flux recovery of 86.2±12%, compared to that of the unfunctionalized commercial UA60 membrane with a flux decline of 22.8±8.8% and flux recovery of 74.4±16.1%. The authors state that this correlation is due to the increased negative charge of the membrane surface from the PAA polymer functionalization, which cause “loose foulant layers” that are more easily reversed. Similar antifouling properties were observed by Ma et al., who fabricated highly hydrophilic and oleophobic membranes with PMAA and hydroxide nanosheets, which were grafted in-situ with alternately arranged sodium 1-dodecanesulfonate and 3-aminopropyltriethoxysilane87. The resulting membranes displayed a drastic resistance to irreversible fouling, while maintaining filtration permeability and separation efficiencies of PEO in water.
As stated, PMMA is a promising option for membrane functionalization with PEL polymer, due to its lower vapor pressure. Membrane functionalization with PMMA has been well-established88,89, but its applications in iron-functionalized membrane systems have not been reported in literature prior to 2020. Zdarta et al. developed a fibrous electrospun membrane with PMMA, 1,1,1,3,3,3-hexafluoro-2-propanol (HFP), and embedded magnetic iron oxide particles for laccase enzyme immobilization90. With 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) and N-hydroxysuccinimide (NHS) chemistry, the methyl group of the PMMA group was utilized for covalent immobilization of laccase, yielding over 60 mg of enzyme loaded per cm2 of membrane area. Due to the PMMA and iron material, the enzymes’ thermostability, reusability, and activity maintenance was improved, compared to non-covalently immobilized forms. This is shown during tetracycline removal, where the immobilized enzyme membrane exhibited higher efficiencies than that of free enzyme. With the incorporation of the pH-responsive polymer, the pH resistance of the enzymes was higher as well. Saad et al. also reported flat-sheet PVDF membranes functionalized with PMMA and iron-palladium nanoparticles for PCB degradation22. Using ion-exchange methods, the PMMA functionalization achieved 0.15 mg of iron immobilized per cm2 of membrane with a subsequent 2.8 mol% of palladium deposition.
Further advances with metal-immobilized systems via PEL functionalization were reported by Heng et al. for titanium functionalization of membranes with PAA91. Specifically, the surfaces of PES membranes were functionalized with PAA, which were then utilized as a bridging agent to add a self-assembled layer of nitrogen-doped carbon quantum dot-modified TiO2. This methodology allowed for precise control of catalyst loading and polymer grafting extent by controlling specific variables, such as concentration of AA monomer or power of plasma (measured in watts) utilized during the polymerization process, while still maintaining a stable membrane system via covalent bonding between the PAA and catalytic components. For Pd-based systems, Wang et al. reported PMAA-functionalized PVDF membranes for the creation of a Pd bilayer composite membrane with a semi-interpenetrating polymer network (IPN)92. The resulting membrane can achieve general separation, catalysis, and in situ product separation in a single step, utilizing the PMAA-IPN layer for controllable filtration of two-component contaminants for subsequent catalytic degradation. A 99.74% degradation of p-nitrophenol solution (10 mg/L) with BSA presence was achieved with a membrane permeability of 27 LMH/bar.
2.2.2. PDM-functionalized membranes
Poly(N,N-dimethylaminoethyl methacrylate) (PDM) is a linear PEL (pKa~7) with tertiary amine groups that exhibits both pH- and temperature-responsive nature. PDM-functionalization of membranes is limited by its difficulty to polymerize93, but has several specific advantages, primarily superior antimicrobial capabilities. Stawski et al. showed that significant antimicrobial capabilities of PDM was maintained at varying degrees of polymerization93, indicating the polymer’s advantage in inhibiting microbial growth over other weak PEL materials used in membrane functionalization.
Several advances in PDM-functionalized membranes have been reported in recent years. Abdellaoui et al. developed a PDM-functionalized cellulose triacetate (CTA) membrane, prepared via free radical polymerization, for ion separation of lead and cadmium94. The structure of the resulting polymer-including membranes was confirmed using multiple characterization methods (SEM, Fourier-transform infrared spectroscopy-FTIR) and achieved permeate concentrations of 51.0 and 24.8 mg/L compared to the feed concentrations of 76.8 and 49.5 mg/L for cadmium and lead ion, respectively, when using two crown-ethers (15-C-5 and DB-24-C-8) for facilitated transport as carriers. Furthermore, Sánchez et al. utilized a cellulose membrane with PDM (polymerized using reversible addition-fragmentation chain transfer-RAFT) for the removal of chromium (Cr-VI)95. Using polymer-enhanced ultrafiltration technique, the increased interaction between the charged metal ion and PEL polymer was investigated at varying pH levels (Fig. 7A). This result, paired with the fractional distribution of different Cr subspecies at varying pH levels (Fig. 7B), indicates that negative or positive charge of PDM (due to pH change) does not simply correlate to increased metal retention, as subspecies can vary between cation and anion.
Figure 7. Removal of chromium and subspecies with pH-responsive membranes.
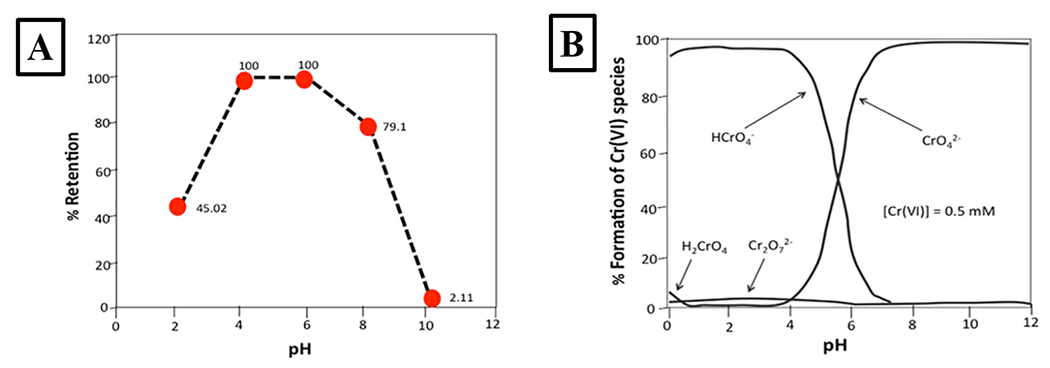
(A) Cr(VI) retention (with an 80:1 polymer-metal ratio) versus pH using 25 mg/L of Cr(VI) at 1 bar of pressure with a filtering factor of 5. (B) Fraction of Cr(VI) species at varying pH (concentration of 0.5 mM). Reproduced with permission from Sánchez et al.95.
With the reason behind this trend elucidated, the authors still achieved significant metal ion retention percentages (~100% in certain pH environments), indicating the promising application of using PDM in membranes for ion separation. Another advance in PDM-functionalized membranes was reported by Zhou et al., who functionalized glass fiber membranes with poly(dimethylsiloxane) and PDM96. The resulting super-hydrophilic membranes achieved high water-oil separations efficiencies (~99%). Overall, there have been several promising recent advances with functionalizing membrane systems to obtain pH-responsiveness, which are summarized in Table 1.
Table 1.
Advances of polymer-functionalized pH-responsive membranes in recent years.
| Base Membrane Material | Functionalizing Polymers | Applications | Reference |
|---|---|---|---|
| Polypiperazine-amide | PAH & PAA | Water remediation from perfluorinated toxic pollutants | Olimattel et al.80 |
| Carboxylated carbon nanotubes | PAA | High efficiency of water-oil separation | Liu et al.81 |
| PDMS (Electrospun) | PMMA | Filtration of fine inhalable particles (low ΔP) | Lu et al.82 |
| PAI (Hollow fiber) | PEO & PMAA | Highly efficient gas separation (CO2:N2) | Mishra et al.83 |
| Composite polyamide (Spiral wound) | PAA | Enhanced antifouling properties of membrane from gypsum | Ansari et al.85 |
| Polypiperazine-amide | PAH & PAA | Enhanced antifouling properties of membrane from humic acid | Olimattel et al.86 |
| PVDF | PMAA | Resistance to irreversible surface and pore fouling | Ma et al.87 |
| HFP (Electrospun) | PMMA | Increased enzyme thermostability, reusability, and activity maintenance (for tetracycline removal) | Zdarta et al.90 |
| PVDF | PMMA | Immobilization of ZVI/Pd for PCB degradation in water sources | Saad et al.22 |
| PES | PAA | Precise catalyst loading during addition of carbon quantum dot-modified TiO2 layer | Heng et al.91 |
| PVDF | PMAA | Addition of Pd bilayer with IPN for filtration control of two-component contaminants | Wang et al.92 |
| CTA | PDM | Ion separation of lead and cadmium in water sources | Abdellaoui et al.94 |
| Cellulose | PDM | Removal of chromium from water | Sánchez et al.95 |
| Glass fiber | PDM and poly(dimethysiloxane) | High efficiency of water-oil separation | Zhou et al.96 |
2.3. Light-responsive functionalization of membranes
Light-responsive materials have been a recent focus of membrane and separation research, given the low energy input of light irradiation for controlling membrane properties. Pantuso et al. recently provided a review with various examples, including numerous applications for photo-switchable wettability, roughness, gating, and self-cleaning properties by varying photo-responsive moieties97. The light-responsive gating properties of photoswitchable components have also been effectively employed in the area of gas separations, as recently reviewed by Rosli and Low, highlighting the advantages of coupling photoresponsive materials with metal-organic frameworks (MOFs) to control transport of gases through dense membranes98. Focused applications and limitations of MOFs with photoresponsive functionality, are summarized by Kanj et al.99. Notably, bio-inspired channels and pores in separations membranes can also be controlled by light, as reviewed recently by Lu et al.100. Overall, there are many advantages of photo-responsive functionalized membranes, making this field’s applications vast (Fig. 8)
Figure 8. Common themes in photoresponsive membrane research.

(A) Photocatalytic materials (purple) are capable of absorbing light to excite electrons and generate electron-hole pairs (e− h+) which allow the excited electron to be capture by nearby molecules to generate radical species (like hydroxyl radicals) that can then participate in oxidative processes to degrade contaminants like oil emulsion droplets (brown circle), as shown in the example membrane which separates and catalytically degrades oil-water emulsions to mitigate fouling and maintain optimal performance in (B). Panel (C) shows two of the most commonly used photoswitchable groups in membrane applications, azobenzene and spiropyrans which are reversibly changed through treatment with Ultraviolet/Vis light. (D) Common applications for azobenzene-functionalized membranes include photo-controlled transport properties (left of dotted line) where the isomerization can alter flux through the membrane, and for spiropyran-functionalized membranes include photo-controlled surface properties (right of dotted line) where the switching controls hydrophobic-hydrophilic surface transitions. (E) Localized surface plasmon resonance (LSPR) occurs in nanoparticles (yellow spheres) when conduction band electrons (e−) are excited by certain wavelengths of light (determined by material properties and nanoparticle dimensions) in an oscillatory fashion, which leads to enhanced local electric fields and ultimately heat generation at the particle surface. (F) Enhanced electric fields can be used for highly sensitive detection as in the case of Surface Enhanced Raman Scattering (above dotted line) for highly sensitive detection of analytes (green pentagon), and the heat generation due to LSPR is also commonly applied for solar energy conversion to heat to vaporize water for desalination applications (below dotted line).
2.3.1. Photocatalytic membranes
A well-studied section of light-responsive membranes is the field of photocatalytic membranes, which are functionalized with materials (i.e., semiconductors or metal organic frameworks-MOFs) that absorb light to generate reactive oxygen species, which have been heavily investigated for water remediation applications (reviewed recently by Mendes-Felipe et al.101). One of the most common materials in photocatalytic membranes functionalization for water treatment is titanium dioxide (TiO2), due to its low cost and well-studied properties as a photocatalyst. Recently, this topic was reviewed in-depth by Riaz and Park with an overview of recent progress in TiO2-containing photocatalytic membranes102. A significant drawback of conventional TiO2 photocatalysts is the limited spectrum (ultraviolet-UV region) and low efficiency (~5%) of solar energy absorption103. However, research in recent years has broadened the absorption spectrum for photocatalytic materials to absorb light in the visible region, leading to expansion of effective membrane applications.
Specifically, biomimetic approaches can allow for broader spectrum light absorption. Fang et al. exhibited a layer-by-layer assembly to create highly porous (~87% porosity) biomimetic porphyrin-based photo-oxidation membranes with high water flux (~387 LMH/bar), achieving >90% removal of three organic dyes under visible light irradiation104. Another example is reported by Zhang et al. with the investigation of a modular system that utilized graphitic carbon nitride (g-C3N4) as the photocatalyst in a polydopamine-functionalized PVDF membrane105. This system, with a separator membrane capable of Fenton catalytic cleaning and a distinct photocatalytic membrane to generate hydrogen peroxide, achieved visible spectrum generation of peroxide that was able to recover 100% of flux after fouling experiments with dextran, comparatively to only ~80% recovery for hydraulic cleaning105. A variety of other photocatalysts with visible light absorption have also been used in membranes in recent years, including various MXenes106,107, metal organic frameworks101,108,109, modified TiO291,110,111, conjugated polyelectrolytes112, Ag-AgBr113, and various mixed metal oxides114–116.
Beyond direct degradation of dissolved pollutants by advanced oxidative processes, one major advantage of photocatalytic membranes is fouling mitigation/prevention, where oxidation of foulants can prevent adsorption, accumulation, and even growth of biofilms that are a major problem with longevity of wastewater treatment systems (reviewed by Nasrollahi et al.117). A recent study showed that functionalizing PVDF membranes with photocatalytic CdS/MIL-101 MOF in a membrane bioreactor setup for microbe-mediated organic pollutant degradation successfully prevented biofouling on the membrane by bacteria and organic foulants under visible light irradiation108. The functionalized membrane exhibited a ~15% flux loss compared to unmodified membrane with ~40% flux loss after 40 min with BSA as a model foulant, thus significantly advancing the field of eco-friendly microbial water remediation. Another recent example found that visible-light photocatalytic g-C3N4/Bi2MoO6 microspheres (blended into polysulfone ultrafiltration membranes) not only aided in removal of protein foulants, but also led to a marked increase in porosity with a subsequent high rejection of protein115. Furthermore, photocatalytic membranes have recently been investigated extensively for the case of oily wastewater treatment106,107,109,118–121, where oxidation of oily contaminants can synergistically aid separation in the production of purified water from oil-in-water emulsions.
2.3.2. Photoswitchable moieties in membranes
Membranes functionalized with photoswitchable materials (that change conformation or chemical structures with light treatment) have also been demonstrated as a powerful tool for oil-in-water separations. One of the most common photoswitchable moiety is azobenzene, as it reversibly changes conformation upon irradiation with UV and visible wavelengths of light, with notable prevalence of other groups, such as spiropyrans that can form or break bonds with visible or UV wavelengths of light (Figure 7). The switchable properties of photoresponsive moieties can control transport through dense membranes in several precise filtration applications, such as gas separation. A recent study showed highly precise control of gas transport properties through a porous steel membrane using a photoswitchable azobenzene based grafted layer combined with a chemically inert liquid gating layer. With this functionalization, these researchers achieved controlled interactions of the membrane substrate with the chemically inert gating liquid (nonpolar functional liquid Krytox® 103), thereby allowing remote spatially-resolved control of gas transport (CO2) through the membrane, while simultaneously preventing corrosion of the metallic porous membrane122.
In addition to controlling transport through dense membranes, photoresponsive moieties have been shown to effectively alter surface properties, resulting in light-controlled separation behavior. Azobenzene-mediated reversal and switching of surface properties between hydrophobic/hydrophilic was recently shown to be effective for purification of oily water, demonstrating a stable reversable switchability for at least 30 days123. Another study investigated a photoswitchable aerophobic Janus membrane for unidirectional transport of air bubbles from water124. Specifically, an asymmetric laser-drilled zinc membrane that can be altered by UV irradiation and heat treatment to modify aerophobicity of one surface, thus resulting in a Janus membrane to control air permeation through the membrane124. Furthermore, asymmetric light irradiation has been used to drive selective cation transport against a concentration gradient125, mimicking active transport of ions across biological membranes. A conducting polymer membrane was functionalized with polystyrene sulfonate anions to allow for generation of a surface charge induced by light. The result was a membrane that could transport ions against a 0.02 mM KCl differential under light irradiation, as measured by the current across the membrane; diffusion in the absence of light demonstrated a 0.1 nA ion current, with a change to −4.5 nA under ~700 mW/cm2 irradiation125.
In comparison with the light-activated cation transport to mimic biological processes, a responsive membrane with enhanced light-responsive functionality was demonstrated recently with the creation of a porous polymer matrix (made of temperature and UV-responsive copolymers using N-isopropylacrylamide and spiropyran as the responsive blocks)126. These developed membranes were utilized to encapsulate an enzyme, resulting in confinement-enhanced reaction rates approximately 3-fold higher than the free enzyme in solution. Similarly, an azobenzene-based functionalization in a porous polymer was found to increase enzymatic reaction rates of asparaginase over 4-fold through confinement effects controlled by UV irradiation127. These studies highlight the potential power of multiple photoresponsive surface functionalities for optimizing efficiency of enzymatic membrane reactors.
2.3.3. Plasmonic material functionalization in membrane applications
Plasmonic materials are generally nanostructured materials with surface electrons that can be excited in an oscillatory fashion on the material surface, resulting in an increased absorption of certain wavelengths of light. This, in turn, enhances local electric fields and ultimately dissipates as heat at the material surface128. One of the most studied applications of plasmonic materials is molecular sensing. Sensing can occur as a direct measurement of plasmonic resonance effects on optical properties like refractive index, as in the case of laboratory SPR equipment129, or by the enhancement of local fields to improve sensitivity of other spectroscopic techniques, as in the case of surface enhanced Raman scattering (SERS)130. Sensing of biological molecular interactions has a long and continuing history of applying plasmonic materials in membranes for highly sensitive and specific sensors131,132. For example, a recent study showed the use of cellulose fiber membranes for monitoring wound healing by functionalization with plasmonic silver to detect the biomarkers matrix metalloproteinase 9 and tumor necrosis factor alpha at nanomolar levels using SERS as a noninvasive measurement technique133. Chiral plasmonic nanoparticles with enantiopure phenylalanine coatings have even been recently shown to detect light polarization at high sensitivity in nanoscale membranes. These membranes generated ~2.4 times the photocurrent for illumination with right-handed circularly polarized light than left-handed, highlighting the diversity of powerful applications for plasmonic membranes in developing futuristic chiral photonics134.
Plasmonic material-functionalized membranes have also seen effective use as sensors for nonbiological molecules like gases, by detection of both direct SPR optical changes (i.e., extinction through a thin membrane) and through spectroscopic detection like SERS. For instance, another recent study showed the use of palladium and Teflon nanocomposite membranes to overcome diffusion limitations of hydrogen into the palladium transducer as part of a remote plasmonic sensor for hydrogen gas resulting in fast response times of 2.5 s at 10% H2, highlighting versatile applications plasmonic materials in membranes for inorganic sensing as well135. The use of a silica microporous membrane with plasmonic silver nanostructures has also been explored for SERS-based sensing of trace gases (i.e. 0.1 ppm hydrazine and 0.5 ppb anisole in the vapor phase) in real time for use in highly sensitive controlled environments like the international space station, demonstrating the vast potential for plasmonic materials in membranes to contribute to next generation of environmental monitoring technologies136. Moreover, a sensor system composed of rare-earth nanosheets assembled into a membrane was shown to separate and detect a mixture of four separate toxic compounds (rhodamine B, crystal violet, bisphenol A, and dichlorophenol) via SERS at concentrations of ~100 nM (with pure component detection in the ~1 pM range), highlighting the flexibility and power of combining plasmonic sensing capabilities with membrane-based separations137.
Photothermal heating of plasmonic materials in membranes has also found various applications, like the innovative example of PNIPAm-coated plasmonic particles exhibiting dual functionality in a porous membrane as both a sensor via SERS and a light-controlled LCST-polymer gating strategy138 (see also Politano et al. for a 2016 review of thermoplasmonic approaches for controlling membrane transport properties139). Importantly, a major segment of recent work regarding plasmonic materials in membrane-based technologies is focused on solar energy harvesting for water purification applications, leveraging the abilities of plasmonic materials to convert light to heat. One recent study showed a high solar-thermal efficiency (66.7%) for purification of simulated seawater (3.5% NaCl) via membrane distillation (1.01 L m−2 h−1 under 1 solar irradiance) using titanium nitride nanoparticles to promote water vaporization precisely at the membrane surface, where the localization of solar energy absorption at the PVDF membrane surface maximizes efficiency140. In another recent study, the size of silver nanoparticles was tuned to generate polyacrylonitrile (PAN) nanofiber membranes with broad plasmonic absorption, resulting in a material with 92.8% absorption efficiency across the solar spectrum that was capable of producing 1.34 kg m−2 h−1 of water vapor flux under 1 solar irradiance141. In another recent example, broadband plasmonic copper telluride nanowires were combined with a cellulose membrane to generate a material for solar-driven desalination, resulting in evaporation efficiencies of 81-90% (4.3 kg m−2 h−1) capable of generating localized heating at the surface to about 56 °C, which is significantly higher than previously established Cu2Te (~50 °C) and graphite (~45 °C) membranes tested in this study142.
In each of these examples, the key feature of the high efficiency for solar energy conversion to water heating/vaporization is the tight coupling between the membrane and plasmonic materials, which maximizes localization of heating effects due to solar radiation at the functional interface. This localized heating has been well explored to this point for application in solar-driven water purification, however, other potential advantages of localized heating in membrane processes are less studied. For example, it is likely that localized heating at the surface of composite nanomaterials with both plasmonic and catalytic properties would provide significant advantages for increasing efficiency of reactions with minimal increase in energy input, especially for plasmonic materials with resonances suited to the solar energy spectrum (as has been shown for solar-thermal water purification research). Indeed, a recent study showed enhanced photocatalytic ROS generation with application as an antimicrobial coating using TiO2 nanoparticle/Au nanorod composites143, lending support for further exploration of the potential for plasmonic-enhanced catalytic materials within membrane science.
2.4. Other stimuli-responsive mechanisms in membrane functionalization
2.4.1. Electro-responsive membranes
Electro-responsive membranes have also been shown to be important in oil-in-water separations. The use of copper-based membranes with rather large pores (2 μm) was shown to efficiently separate ~1 μm oil emulsion droplets from water (water flux of ~2500 LMH/bar with ~98% rejection) via applied negative polarization in the pores. This was shown to reduce oil droplet coalescence and therefore minimize fouling, an effect which was enhanced at low ionic strengths due to screening effects of surfactants in the emulsions144. A particularly interesting case of electro-responsive membranes was recently developed by Dehkordi et al., who employed a pH responsive polymer (PMMA-co-PDEAMA) doped with hydroxyl functionalized spiropyran that had switchable hydrophilicity based on triggers of CO2, electric potential (EP), and/or light. While this switching controlled hydrophilicity (water contact angle changing from 138° to <5° to 121° after CO2 then EP treatment) and water permeability (from ~0 to 1.59 to 0.05 LMH/bar after CO2 then EP) of the membrane with application for oil/water separations, the most interesting feature was the color-changing property imparted by the spiropyran component, which provided a visually noticeable cue for the hydrophilicity change145.
While this discussion is mostly focused on the roles for electroresponsive membranes in oil-in-water separations, there are a variety of other applications for controlling membrane behaviors through applied electric fields (Fig. 9). A recent study showed that conjugating electro-responsive polymers to hexokinase enzyme in a hydrogel matrix could enable reversible control of enzyme reaction rate (Vmax from ~0.6 mmol/min to ~1.7 mmol/min, comparable to free enzyme at ~1.6 mmol/min) with application of external electric fields by tuning accessibility of the enzyme to its substrate with potential applications in bioseparations146. Overall, methods for creating electroresponsive membranes and their advantages have recently been reviewed by Formoso et al.147 and Alayande et al.148. Monovalent ion selective membranes (MIMS), that often employ electric fields and redox reactions149, has recently been reviewed by Wang et al.150 as well. Another thorough recent review by Srimuk et al. was more focused on desalination, ion-selective separations, and element (primarily lithium) recovery, including the use of ion-exchange membranes in concert with charge-transfer materials151. It is important to note that redox-responsive membranes can be considered a subset of electroresponsive membranes, and has been recently reviewed by Gallei & Rüttiger regarding redox-responsive porous membranes and their applications in separations152.
Figure 9. Examples of common methods and applications for electroresponsive membrane functionalization.

Reproduced under Creative Commons License (available at http://creativecommons.org/licenses/by/4.0/) from Alayande et al.153
2.4.2. Mechanical/pressure responsive membranes
An interesting application of mechanically responsive membranes is for enhancing flux of oil/water separations using membranes. A smart material utilizing a hydrophobic thermoplastic polyurethane substrate functionalized with zeolite-imidazole framework (ZIF-L) nanosheets demonstrated strain-responsive flux control (~10,000 LMH to ~35,000 LMH under gravity for hexane) via hydrophobic transition (water contact angle ~47° to ~113°), while maintaining >98% selectivity in oil/water separation for strains from 0% to 100%154. Another study recently showed success in minimizing fouling in oil-water emulsion separations using piezoelectric ceramic membranes with pulsed transmembrane pressure (0-2 bar). This converted mechanical energy from pressure pulses to local high voltage electric fields that demulsify the oil-water mixture and subsequently remove it from the membrane surface by dielectrophoresis155. Conversely, piezoelectric materials in membranes can also be stimulated by applied electric fields to generate a mechanical response, as shown in ultrafiltration membranes made of composite carbon nanotubes/PbZr0.52Ti0.48O3 membranes that were able to achieve a ~80% increase in permeability due to mitigation of fouling during filtration of 2.5 g/L dextran156.
Pressure response can also be more specific, as shown in several examples in the gas separations area. Composite MOF nanosheet/graphene oxide nanosheet membranes were recently shown to enable highly selective CO2 separations (~23 and ~20 for N2 and CH4, respectively) based upon CO2 partial pressure sensitive gating (at ~0.55 bar CO2) in the MOF portion of the composite157. A similar example using hydrogen-bonded organic framework membranes achieved H2/N2 selectivity of ~20 based on differential permeability of the material at higher pressures, a characteristic suggested to be due to stretching of hydrogen bonds and resulting lattice parameter lengthening in the framework at higher pressures158.
3. Enhanced functionality surfaces
Membranes offer a large surface area that provide a great potential for surfaces with enhanced functionality to interact extensively with fluids in a process. Environmental remediation is one field in which the separating ability of membranes coupled with enhanced surfaces has found powerful uses. For example, immobilization of palladium in membranes has shown to be effective for dehalogenation of persistent organic pollutants like polychlorinated biphenyls159. Palladium and other nanoscale zero-valent metal catalytic membrane reactors have been recently reviewed with thorough discussions of functionalization approached160,161.
3.1. Enzyme-functionalized membranes
Porous membranes represent a powerful tool for improving performance of enzymes in catalyzing relevant reactions for industrial scale by providing a highly controllable local environment to enhance stability, control transport, and integrate separations processes for resultant products. A particularly powerful application for enzyme-coupled membranes is in next generation sequencing of nucleic acids, where biological nanopore enzymes are immobilized in an electrically conductive membrane and the current is monitored as nucleic acids are passed through to decode the genetic sequence162. Importantly, the inclusion of stimuli-responsive features, along with enzymes, can further enhance the strengths of enzymes in membranes; Stimuli-responsive membranes functionalized with enzymes were recently reviewed by Qi and Qiao163. More recently, several advances have been made in the area, which are briefly reviewed here.
3.1.1. Advances of enzyme-functionalized membranes
Generally, the methods for attachment of enzymes to membranes have not changed significantly in recent years, with physical approaches (i.e. adsorption, electrostatic, entanglement) and covalent approaches (i.e. aldehyde, carboxyl, epoxide surface chemistries that can crosslink enzymes to the surface) both having strengths and weaknesses for the ultimate function of the membrane reactors (see Figure 10 and also recent review by Cen et al.164). However, several studies have shown advantages for immobilizing enzymes (i.e., lipase, acylase, dehydrogenases) to nanoscale carriers directly with subsequent nanocarrier immobilization on or in membranes for the creation of enzymatic membrane bioreactors. A recent study showed that immobilization of lipase on magnetic nanoparticles (which were then attached to membrane) resulted in ~30-50% improvement of enzyme functionality than direct immobilization on the polyacrylonitrile membrane via similar covalent attachment chemistry using hydrolysis of para-nitrophenyl palmitate to assay enzyme activity165. Moreover, some nanocarriers are themselves functional materials participating in the reactions, such as photocatalytic oxidation of wastewater contaminants, as summarized in the recent review by Kołodziejczak-Radzimska et al.166. In some cases, the functional material may improve separation selectivity with or without participating in reactions, as in the case of a recent study showing the use of carbonic anhydrase enzyme-embedded MOFS in a membrane to improve selectivity by ~20-fold for CO2/N2 gas separation167, or the example of membranes employing MOF-enhanced laccase and micropollutant adsorption that resulted in enhanced selectivity for biocatalysis of pollutant degradation168.
Figure 10. Overview of enzyme functionalization methods and applications in bioremediation of pollutants.

Reproduced under Creative Commons License (available at http://creativecommons.org/licenses/by/4.0/) from Kołodziejczak-Radzimska et al.166.
Importantly, we focus here primarily on enzyme-functionalized membranes and their role in separations processes, but membrane bioreactors (MBRs) incorporating whole microbial organisms are also extremely valuable for advancing various aspects of eco-friendly membrane processes. In these systems, a key aspect of current and future work is reducing biofouling, since microorganisms are quite efficient at colonizing a surface and impeding transport through the membrane as biofilms are formed blocking pores. To combat these issues, a variety of approaches have been investigated, with a notable recent example being the immobilization of quorum-quenching enzymes (like acylase) on the membrane surface (with or without additional composite components like carbon nanotubes) to prevent biofouling169. In contrast, some MBRs target growth of microbial communities on the filter media to optimize efficient pollutant removal, as reviewed by Deng et al.170. Progress in the area of membrane bioreactor process and materials design has been summarized by Qin et al.171, and a focused review comparing traditional versus biofiltration processes for volatile organic and heavy metal separations for polluted water treatment was also recently published by Pachaiappan et al.172.
3.1.2. Applications of enzyme-functionalized membranes
Enzymatic and microbial MBRs have a great potential for producing value-added and high value products in biorefinery processes, but still face significant challenges like scaleup and improving enzyme stability, as reviewed by Akkoyunlu et al.173 and Mazzei et al.174. Hollow fiber membranes can aid in scaleup, as demonstrated recently with a radial MBR for simultaneous enzymatic hydrolysis and product separation in a lignocellulose digestion to glucose. In this study, the radial flow format showed ~3-fold improvement in yield compared to a flat sheet format, which did not simultaneously remove glucose from the reaction mixture175. Another recent instance of simultaneous product removal improving process efficiency used pervaporation membranes to remove methanol from a cascade membrane reactor that converted CO2 to methanol, incorporating MOF composites (including reduced nicotinamide adenine dinucleotide and glutamate dehydrogenase as electron sources) in organized layers (MOF-composites containing formate dehydrogenase, formaldehyde dehydrogenase, and alcohol dehydrogenase) within a microfiltration membrane to enhance methanol production from ~5.8 to ~13.4 μmol176.
With respect to environmental remediation, there has been extensive study on wastewater treatment using biocatalytic membrane reactors (reviewed by Aslam et al.177). Figure 9 shows several examples of pollutants degraded by enzyme-functionalized membranes. For example, enzymatic degradation of formaldehyde to methanol (with alcohol dehydrogenase) was achieved with high efficiency (~90%) through immobilization of alcohol dehydrogenase on PVDF membranes, with significant reusability up to at least seven cycles of water treatment178. Similarly, water purification from oil emulsions also can be aided by enzymatic membrane reactors. Lipase enzymes were recently immobilized on alumina membranes by traditional silane chemistry, as well as via polydopamine coating with and without glutaraldehyde. These membranes showed that the non-glutaraldehyde PDA immobilization provided the highest activity of the enzyme (41% of free enzyme) and also provided twice the flux (~300 LMH/bar vs ~150 LMH/bar) compared to nonactive membranes with a much higher flux recovery (~69% vs ~45%), thus demonstrating the antifouling activity of lipase enzyme for oil-water membrane separations179. Remediation via enzyme-functionalized membranes, though, is not limited to water environments. Mills et al. functionalized PVDF microfiltration membranes with subtilisin Carlsberg and PMAA for the enhanced capture and denaturation of viral particles, specifically spike glycoprotein (S-Protein of SARS-CoV-2) coated aerosols180. These membranes achieved significant denaturation of the particles’ surface proteins, due to enzyme-protein interactions, while exhibiting a protection factor of 540 ± 380 from coronavirus-sized aerosols (higher than established protection factor of 10 for commercial N95 masks).
Enzymatic membrane systems also have powerful applications in sensing, as in the case of a hollow fiber membrane nanosystem designed for in situ blood separation and biosensing that used MOF-enzyme hybrids for detection of important biomarkers, such as cholesterol, glucose, and lactic acid181. In another example, a gold-electrode based amperometric detector for glucose with a detection range of 58 μM to 5 mM was created182. Using supported multilayer liquid membranes decorated with multiwall carbon nanotubes and glucose oxidase enzymes, no interference from uric acid or ascorbic acid was shown. The selectivity of enzyme-functionalized membranes can also be useful for sensing, as in the case of an ultrathin pepsin-functionalized membrane that was shown to retain high molecular weight proteins while selectively allowing peptides from enzymatic digestion through the membrane183, thereby protecting them from further breakdown by the protease. These diverse examples of enzyme-functionalized membranes are just a few instances of the powerful applications for enzyme membrane reactors in a wider variety of important fields.
3.2. Two-dimensional membrane functionalization
Two-dimensional (2-D) functionalized membranes consist of ultrathin membranes with sub-nano/atomic thickness and have been making significant advances in the membrane field, due to their separation abilities, mechanical strength, and flexible yet precise functionalization184. The membranes’ nanolayers allow for the creation of specific interlayer channels (Fig. 11A) to exhibit a high separation efficiency of nano-sized contaminants/ions, while maximizing the membrane permeability that can be achieved, due to minimal thickness. 2-D membranes have additional advantages over traditional membranes, such as lower cost than ceramic membranes185, and they are typically categorized as either porous nanosheet or laminar membranes (Fig. 11B). Typically, these membranes consist of graphene oxide, metal-organic framework nanosheets, covalent-organic frameworks (COF)186, zeolites, and transition metal carbides/carbonitrides, and they have several means of functionalization, such as spin coating, hot dropping filtration, and polymer induction. Overall, 2-D membranes could replace many existing membrane technologies in the future, if known limitations such as scalability and stability185 can be overcome.
Figure 11. Schematic of two types of 2-dimensional membrane systems.

(A) Porous nanosheet membranes and (B) laminar membranes. Reproduced with permission from Cheng et al.185.
In the past years, 2D-functionalized membrane systems have been reviewed extensively, including the works of Zhang et al.187, Loske et al. 188, DuChanois et al.189, Han et al. (with a focus on GO-functionalized membranes and applications)6, as well as Guirguis et al.190 and Cheng et al. (with a focus on biofouling)191. Due to the highly-reviewed nature of 2D membrane materials, this section will focus specifically on recent advances of GO-functionalized membranes. To summarize, graphene oxide has become a popular option for 2-D membrane functionalization because of its thin two-dimensional structure, chemical resistance, mechanical strength, and specific pore size for highly-selective separation6. Furthermore, GO contains several oxygen-containing functional groups, such alcohols, carboxylic acids, and epoxides192, which can serve as locations for further site-directed functionalization with other materials, such as PAA. These functional groups can also introduce charges to the membrane surface, which subsequently increases the rejection of certain contaminants. It is important to note that there are other significant advances in 2-D membrane functionalizations besides those containing GO, such as 2-D double hydroxide for oil-water separations87, metal-organic framework nanosheets for stable thermo-responsive filtration193, and ethanol dehydration using 2-D MXene membranes194.
3.2.1. Advances of graphene oxide functionalization
There are several established methods for GO functionalization, such as carbon nanotube decomposition, mechanical exfoliation, liquid-phase exfoliation, epitaxial growth, and chemical vapor deposition (CVD), the latter being one of the most appealing methods for scalable large-area applications6. There are two main modes of creating separating “pores” for GO-functionalized membranes: formation of porous GO layers via defects or stacking GO layers for molecular sieving using interlayer channels. First, to form porous GO layers, defects are introduced into the system using a variety of methods, such as electron beam nanosculpting195, ion etching196, or block copolymer lithography197. Second, stacked GO layers are functionalized in specific ways to allow for flow through the interlayer channels, thus creating a type of sieve system for filtration. This functionalization is primarily done via vacuum-assisted filtration, layer-by-layer assembly, or continuous centrifugal casting. At this point, it is important to note that weak reduction of oxygen-containing functional groups in GO (to make reduced graphene oxide-rGO) is common to increase membrane stability and control surface hydrophilicity (for increased membrane permeability)6,198.
In recent years, there have been multiple advances in the GO functionalization process and resulting membranes. Fouladivanda et al. functionalized poly(vinylidene fluoride)-co-hexafluoropropylene (PVDF-HFP) electrospun membranes with octadecylamine-reduced graphene oxide (ODA-rGO) for air-gap membrane distillation applications199, which, to the best of our knowledge, is the first ODA-rGO functionalization using hydrothermal methods reported in literature. The resulting functionalization resulted in an X-Ray diffraction (XRD) peak shift from 10.35° to roughly 21°, which was due to the hydrothermal method removing the oxygen-containing functional groups. Additionally, Quanling et al. developed a process for synthesizing thin-film nanofiltration membrane with 2-D GO and 3-D hyperbranched polymer functionalization to achieve higher permeability, selectivity, chemical resistance, and antifouling properties than existing poly(piperazine-amide) (PPA) thin-film options200. This study functionalized polysulfone membranes with a thin-film of PPA (via interfacial polymerization), which also contained hyperbranched polyester-functionalized GO nanosheets (as nanofillerss) with hydrophilic carboxyl functional groups. Due to this novel functionalization, the resulting membranes were able to maintain high salt rejection (above 90% for NaSO4 and MgSO4) with roughly half the flux drop over three BSA filtration cycles than that of traditional options.
Further advances were developed by Qian et al. with a one-step plasma processing method for the addition of polarized nitrogen atoms and amine groups to GO-functionalized membranes201. Membranes were functionalized via plasma treatment (200 W power) in the presence of N2/H2 gas with 5-minute discharge/cool-down operation cycles. The resulting membranes achieved a mono/divalent cation selectivity and binary cation selectivity of 90 and 28.3, respectively, due to electrostatic interactions, while maintaining a high water flux of 120 mol/m2/h. Additionally, Chen et al. recently synthesized GO-functionalized PVDF fibrous membranes via electrospinning202. Using a simple preparation method, this study both dip-coated GO on PVDF membranes and electrospun a GO-PVDF blend solution, resulting in nanofibers with a mean diameter of 991 nm and 551 nm, respectively. Overall, these studies indicate that the methods of membrane functionalization with graphene oxide are significantly advancing the field of separations and taking important steps towards scalable and commercially practical processes.
3.2.2. Applications of graphene oxide functionalization
GO-functionalized membranes have a large variety of applications, such as gas, dye, oil-water, and ion separation. Recently, several advances have been made for self-cleaning and anti-fouling properties of GO-functionalized membranes. Cao et al. developed a PVDF membrane with PNIPAm and GO functionalization for a novel self-cleaning process203. GO can convert near-infrared (NIR) light to heat204, thus utilizing NIR light for reversing pore fouling from BSA resulted in higher flux recovery ratios than traditional back-washing methods. Similarly, Alkhouzaam et al. utilized polydopamine (PDA)-functionalized graphene oxide nanoparticles for nanofiller functionalization of polysulfone membranes205. The resulting functionalized membranes with PDA-GO achieved a 30% increase in flux recovery ratio than the unfunctionalized membranes, as well as a lower contact angle (27.8±3.6° compared to 53.3±1.5° of the GO-functionalized membrane), indicating superior antifouling properties from this nanofiller functionalization. Another interesting advance was made by Karimipour et al. with the investigation of melamine-based dendrimer amine (MDA) and GO functionalization of PES membranes206. The resulting membrane showed an increased flux recovery ratio of 78.5%, compared to the unfunctionalized membrane (50.4%).
GO-functionalized membranes have immense applicability for dye separation, as the minute interlayer channels can effectively filter the extremely low molecular weight dye compounds. This topic was recently reviewed Januário et al. in 2021207, but some new advances are highlighted below. Choi et al. recently synthesized a three-layered diafiltration membrane via a scalable bar-coating approach208. This membrane consisted of a GO nanoribbon selective layer and a functionalized carbon nanotube layer, both on a PES backing material, which achieved ~100% dye rejection rate and a permeability of 138 LMH with stable filtration up to 30 bar of pressure. Previously mentioned for antifouling properties, the work of Karimipour et al. also achieved almost complete rejection of reactive red 198 dye with significant divalent ion retention (77.4% for Na2SO4 and 56.4% for MgSO4) with MDA-GO-functionalized PES membranes206. Another recent advance includes the work of Januário et al. developing a simple method for PES membrane functionalization with polyvinyl alcohol and GO via layer-by-layer assembly209. The resulting membranes achieved over 99% removal of several dyes (sunset yellow, tartrazine yellow, and amaranth), while maintaining a filtration efficiency of 94% or greater over five filtration cycles. In the presence of dissolved salts, 86.17% of sunset yellow dye was removed, indicating effective implementation in real-world conditions.
Furthermore, GO-functionalized membranes are an appealing system for gas separation, due to the size of their interlayer channels and relatively low pressure drop across the membrane. Azizi et al. recently synthesized a copper benzene-1,3,5-tricarboxylic acid/graphene oxide composite membrane (as a filler on a polysulfone substrate)210. The resulting mixed matrix membrane (15% weight gain) achieved reasonably high gas selectivities of 80.03 and 70.46 for H2/CH4 and H2/N2, respectively, compared to the MOF-functionalized membrane without GO (control) with respective selectivities of 44.56 and 40.92. As discussed in the pressure-responsive section, Ying et al. developed 2-D MOF and GO nanosheet membranes for carbon dioxide separation via a “gate-opening” effect157. Similar results were found by Sainath et al. for CO2 separation via functionalized polysulfone hollow fiber membranes with GO nanosheets211. Due to the presence of GO, the developed membranes exhibited a 201% increase in CO2/CH4 separation during mixed gas experimentation, compared to the unfunctionalized membrane systems. Further advances have been achieved by Zhou et al. with the development of 2-D graphitic carbon nitride membranes for hydrogen gas purification212. These 2-D membranes are severely limited in their structural stability and nanosheet formation, thus this research added GO functionalization to overcome existing structural defects. The resulting membranes achieved a high H2/CO2 selectivity of 39.2 with a stable permeability of 2.16 × 10−7 mol m−2 s−1 Pa−1. These studies are great examples of the significant technological advances achieved recently for GO-functionalized membranes, resulting in more scalable options for commercial applications.
3.3. Thiol-functionalized membranes
Thiol-functionalization of membranes is becoming an appealing method for water source remediation from toxic heavy metal ion contamination, such as mercury, copper, nickel, lead and cadmium. This is due to the low energy and material cost of the technology, thus overcoming known limitations with current methods, such as ion exchange, solvent extraction, and precipitation213. Thiol-functionalization of membranes is commonly achieved in several ways. Many studies achieve functionalization via the direct addition of thiol group onto the membrane surface and pores, while other methods incorporate EDC/NHS chemistry/ion exchange for the addition of a thiol-containing amino acid (i.e., cysteine)7. This functionalization yields very high capture efficiencies, as thiol groups (-SH) have a very high affinity for heavy metal ion adsorption. Briefly, this is due to soft Lewis acids (i.e., Hg2+) strongly favoring interactions with soft bases (i.e., SH−) to form a metal-sulfur complex7. This complex is insoluble and held together by a strong covalent bond214, rendering a significant and effective adsorption mechanism. In 2019, Velempini et al. conducted a review on sulfur-functionalized materials for heavy metal ion adsorption213, thus this work will focus on new advances in thiol-functionalized membranes in recent years.
Several advances in approaches for membrane functionalization with thiol groups, as well as increased heavy metal ion capture efficiencies have been achieved in recent years. Specifically for nanotube-containing membrane systems, Qu et al. developed thiol-functionalized and carboxy-functionalized multi-walled carbon nanotubes (MWCNT) for Pb(II) removal215. The resulting membranes were synthesized via grafting of thiol groups onto the carbon nanotubes using 2-mercaptoethanol. This study achieved a 30.5% higher adsorption of Pb(II) with thiol-functionalized MWCNT, compared to COOH-functionalized MWCNT systems (at a pH of 5), showing the higher affinity of thiol than that of the COOH group. This research also found that this adsorption process was both exothermic and spontaneous, and a pseudo-second-order model fit the absorption kinetics, determining that the chemical adsorption is the primary controller of the Pb(II) removal rate. Hezarjaribi et al. achieved a similar functionalization system by functionalizing titanate nanotubes with thiol-containing mercaptosilane (site specific at the free OH groups of the nanotubes) and confirmed the fit of the pseudo-second-order model for Cu(II) and Ni(II) ions216. Using their nanotube systems, Hezarjaribi et al. achieved adsorption capacities of 53.77 and 45.29 mg/g for copper and nickel ions, respectively. The pseudo-second-order model that was applied in these research investigations utilized experimental data to fit the following equation217:
| (2) |
Where is the uptake capacity at time t (mg/g), is the uptake capacity at the equilibrium point (mg/g), and is the second order kinetic constant.
Further advances have been achieved by Hezarjaribi et al. using polyvinyl chloride (PVC) electrospun nanofiber membranes with thiol-functionalized hydrous manganese (1.5% weight gain), which has not been previously reported in literature, to achieve 80% and 89% removal of nickel and copper ions218. Additionally, a more environmentally-friendly approach has been achieved by Thimmiah et al. with the development of a thiol-functionalized Agave americana-based nanofiber membrane for the single-state removal of both heavy metal ions and organic contaminants219. This allowed for a greener fabrication method to achieve a capture efficiency of 97.49% and 99.23% for cadmium and lead ions, respectively. Three-dimensional surface modelling was conducted for the interaction between the resulting membranes and contaminants as well, determining an optimum fiber length of ~1 cm for cadmium ion removal.
Recently, previously-mentioned functionalizations for responsive behavior have been combined with thiol-functionalization to enhance and increase the metal ion capture capabilities of the resulting membranes. In 2020, both Hernández et al. and Islam et al. investigated functionalizing PAA-PVDF membranes with cysteine and cysteamine via both ion exchange and carbodiimide cross-linker chemistry7,220. Upon membrane characterization with X-ray photoelectron spectroscopy (XPS), it was determined from peak intensity differences that EDC/NHS chemistry for thiol functionalization is a stronger method for increasing thiol presence than that of ion exchange. Hernández et al. reported mercury capturing capabilities of 1015 mg/g PAA and 2446 mg/g PAA for membranes functionalized via ion-exchange and EDC/NHS chemistry7, respectively, while Islam et al. investigated long-term capture (over 20 hours) with an achieved mercury adsorption efficiency of 97%220. Overall, there are many new applications of thiol-functionalization in recent years, showing the promising effectiveness of this technology for scalable and commercial heavy metal ion capture from water sources.
3.4. Antimicrobial functionalized membranes
Membrane functionalization with an antimicrobial agent is a common practice for reduction of surface biofouling and for biological/viral air filtration applications. In recent years, such functionalizations have become immensely important, due to the SARS-CoV-2 pandemic, and increased impact of shortcomings of existing personal protective equipment (i.e., N95 masks)221. However, traditional antimicrobial materials, such as silver nanoparticles, are limited by their toxicity and harmful nature upon human exposure/inhalation222. In recent years, several advances have been made in the field of antimicrobial-functionalized membranes, specifically for non-toxic applications.
Briefly, the use of salt functionalization has been investigated as a method of developing anti-microbial membranes and coatings in recent years. In 2021, Rubino et. al. showed that functionalization of polypropylene filters with sodium chloride, potassium chloride, and potassium sulfate to be efficacious in the deactivation of K. pneumoniae223. The salt crystals immobilized on membrane fibers were shown to facilitate cell wall destabilization and cell agglomeration facilitated by the temporary dissolution and subsequent recrystallization of the salt. Functionalization of large pore (~60 μm) polypropylene membranes with various salts (NaCl, K2SO4, KCl) was shown to increase filtration efficiency. Filtration of K. pneumoniae at 15 LPM flowrate increased from 40% for bare membrane to 65%, 43%, and 67% for NaCl, K2SO4, and KCl functionalization’s respectively. The use of quaternary ammonium salts for antibiotic coatings has been investigated more frequently in recent years. In 2018, Jie et. al. integrated chitosan quaternary ammonium salts (CQAS) into polymerized surface coatings of piezoelectric sensors224. In agar plate bacterial growth trials, the CQAS surface coatings showed greater than 99% reduction in cultured bacterial colonies of E. coli, S. aureus, and P. aeruginosa compared to control plates. While allowing for substantial deactivation of bacteria, the addition of the CQAS coating produces little to no reduction in the function of the piezoelectric sensor.
4. Functionalized membrane characterization techniques and advances
Surface and pore characterization is a vital part of the responsive/functionalized membrane field for advancing separation, transport, and structural properties. Functionalized membranes, especially, necessitate advanced characterization techniques to confirm functionalization, as well as investigate details of responsive behavior. There are many established methods for elucidating membrane properties, both dynamic and static in nature, and were recently reviewed by Tanis-Kanbur et al.225 (Fig. 12). Briefly, these methods consist of, but are not limited to, Fourier-transform infrared spectroscopy (FTIR), surface contact angle goniometry, transmission and scanning electron microscopy (TEM and SEM), energy dispersive X-ray spectroscopy (EDX), X-ray photoelectron spectroscopy (XPS), X-Ray diffraction (XRD), and zeta-potential measurements226. Focused ion beams (FIB) are also commonly used to mill membrane samples to image cross-section portions of the system. Advancing these methods is equally important as developing new functionalization techniques to both validate the developed membrane and give vital information for future direction of work. In recent years, several new characterization techniques and adaptations have been achieved to further the field of membrane and separations.
Figure 12. Examples of membrane characterization techniques.

Reproduced with permission from Tanis-Kanbur et al.225.
Cross section imaging is a very important aspect of membrane characterization. Membranes can be simply cut with a sharp blade/microtome or fractured when frozen to reveal the cross-section view, and FIB is frequently utilized for milling membrane samples. However, FIB cross sectioning is limited by the fragile nature of the membrane (compared to solid metal-like samples) and polymeric structure, which requires a low energy beam to reduce sample damage and consequently results in long milling times for complete cross section imaging (6-8 hours for 100-200 μm thickness). These constraints make FIB cross sectioning a costly process with significant time constraints on FIB-SEM instruments. Recently, broad beam ion milling has become a promising alternative to FIB for milling such membrane samples. Using an argon ion gun, the membrane sample is milled with on/off cycles while being cooled with liquid nitrogen to limit heating of sample, resulting in a pristine membrane sample with minimal melting or structural damage227,228. While this method can still take a significant amount of time for milling, broad beam ion milling has several significant benefits as it (1) is a more cost-effective method, (2) does not occupy the SEM instrument for sample prep, (3) allows for a larger milled area, and (4) can reduce the damage done to the sample, which subsequently results in a more accurate characterization in SEM or TEM229.
Tortuosity is one of the more complex variables to characterize in membrane samples. In the past, tortuosity has been roughly calculated from the porosity and effective diffusion coefficient but requires prior knowledge of membrane structure. This method also depends on homogeneous pore structure throughout the membrane, which severely limits the application of this method for asymmetric membranes. Nuclear magnetic resonance (NMR) can also be used to measure the self-diffusion coefficient of a liquid in the pores, but can yield inaccurate results, due to interference with hydrophilic/hydrophobic functionalizations. In recent years, X-ray micro-computed tomography (micro-CT) is becoming an appealing option for overcoming these existing limitations to elucidate and analyze the tortuosity of porous material230. Briefly, the sample is exposed to an X-ray source while on a rotating stage. The X-ray signal is then converted to visible light via the fluorescent crystal plate, and then the subsequent light is analyzed via a cooled charged-couple device (Fig. 13A). Most membrane pore analyses are greatly enhanced by metal nanoparticle coating for micro-CT analysis, such as silver231, and McNeil et al. showed that the complete three-dimensional pore structure of thin membranes can be accurately reconstructed232 (Fig. 13B). This method can capture the functionalization structure inside the membrane pores in relatively short amounts of time (~10 hours) without damaging the sample itself, as long as other metal nanoparticles (such as ZVI functionalization) are not present.
Figure 13. Micro-CT as a characterization technique for membrane tortuosity and pore structure.

(A) Schematic diagram of a micro-computed tomography (micro-CT) scanner. The specimen is mounted on a rotating stage, and the specimen is illuminated at angular increments by an X-ray beam. The transmitted X-ray energy is converted to visible light energy by a fluorescent scintillating crystal plate. The visible light is projected onto a cooled charged-couple device (CCD) by a microscope objective. Reproduced with permission from Ortiz et al.233 (B) Micro-CT 3D analysis of the cross-section of a Ag-plated dual-phase, Al2O3-supported membrane after CO2 and O2 permeation. Reproduced with permission from McNeil et al.232
Other advances in characterization techniques were achieved by Lu et al. who characterized the dehydration process in situ during ion transport through membrane systems with subnano-sized pores234. Using a combination of time-of-flight secondary ion mass spectrometry (ToF-SIMS) and molecular dynamics simulations, this novel research achieved quick and dynamic characterization of ion transport, which is difficult to achieve with other methods (NMR, XRD, vibrational sum frequency spectroscopy). Similarly, Monteleone et al. utilized a mass-spectrometric residual gas analyzer for characterization of mixed gas transport through a polydimethysiloxane membrane with corresponding computational modelling for identifying abnormal transport behavior235. This represents a promising method for analyzing diffusion coefficients of the individual gases present in the mixture, which is not common in literature. Additionally, Barros et al. Recently reviewed ion-exchange membranes and their characterization via chronopotentiometry236, particularly highlighting that many advances in 3D mathematical modeling for these membranes and techniques will be achieved in upcoming years. Overall, these highlighted advances in membrane characterization are significant steps towards better understanding the properties and transport/separation phenomena of membrane systems, as well as developing novel functionalized membranes.
5. Conclusions and future directions
Most traditional membranes lack tunable properties, but the field of advanced membranes continues to see rapid technological developments for membranes functionalized with enzymes, 2-D layers, pH-responsive polymers, temperature-responsive polymers, and light-responsive polymers. The recent advances achieved for these membranes include (1) improvements in the fabrication/functionalization methods for increased membrane stability, switchable hydrophilicity, and overall scalability, (2) expanding applications to environmental remediation and high-efficiency gas separation, and (3) materials characterization methods to elucidate details of surface/pore structure, charge, and functional groups more accurately. These advances further the field of separations, indicating the myriad processes that can be improved using functionalized membrane technology and continuing to push towards developing commercially feasible systems.
Moving forward, the technologies in this field still have several limitations that should be addressed in the future. The two main limitations of functionalized membranes, in a general sense, consist of either 1) operational constraints (scalability, efficiency, or cost) or 2) environmental impact and longevity. In terms of scalability, the transition from flat-sheet and electrospun membranes to modules with higher packing densities, such as spiral wound and hollow fiber membranes, is vital for proving the scalability of functionalized membrane technology, thus advances with such modules in the future are vital to commercial implementation beyond the lab. Existing functionalization tools reviewed here, such as the hydrophobic-hydrophilic transition of thermo-responsive and pH-responsive membranes, as well as the easy switchability and efficient energy capture of light-responsive membranes, can be applied to existing scalable designs to overcome shortcomings of lab-scale formats, such as flat sheet membranes. These continuing innovations will bolster the utility and value of membrane technologies in solving real-world problems now, as well as in the future, as the world and societal needs change with continued population growth.
6. Acknowledgements
The authors acknowledge financial support from the National Science Foundation-RAPID grant 2030217 and the National Institute of Environmental Health Sciences-Superfund Research Program Grant P42ES007380. The authors additionally acknowledge Francisco Léniz-Pizarro, Ronald J. Vogler, and Matthew Whitwer for discussion on technical writing aspects and functionalized membrane research topics.
Footnotes
Conflict of interest
The authors declare no conflict of interest at this time.
9. References
- 1.Wandera D, Wickramasinghe SR & Husson SM Stimuli-responsive membranes. Journal of Membrane Science 357, 6–35, doi: 10.1016/j.memsci.2010.03.046 (2010). [DOI] [Google Scholar]
- 2.Chen J-K & Chang C-J Fabrications and Applications of Stimulus-Responsive Polymer Films and Patterns on Surfaces: A Review. Materials (Basel) 7, 805–875, doi: 10.3390/ma7020805 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brilmayer R, Förster C, Zhao L & Andrieu-Brunsen A Recent trends in nanopore polymer functionalization. Current Opinion in Biotechnology 63, 200–209, doi: 10.1016/j.copbio.2020.03.005 (2020). [DOI] [PubMed] [Google Scholar]
- 4.El-Aswar EI, Ramadan H, Elkik H & Taha AG A comprehensive review on preparation, functionalization and recent applications of nanofiber membranes in wastewater treatment. Journal of Environmental Management 301, 113908, doi: 10.1016/j.jenvman.2021.113908 (2022). [DOI] [PubMed] [Google Scholar]
- 5.Politano A et al. When plasmonics meets membrane technology. Journal of Physics: Condensed Matter 28, 363003 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Han Z. y. et al. A review of performance improvement strategies for graphene oxide-based and graphene-based membranes in water treatment. Journal of Materials Science 56, 9545–9574, doi: 10.1007/s10853-021-05873-7 (2021). [DOI] [Google Scholar]
- 7.Hernández S et al. Thiol-Functionalized Membranes for Mercury Capture from Water. Industrial & Engineering Chemistry Research 59, 5287–5295, doi: 10.1021/acs.iecr.9b03761 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwon T & Chun J ON/OFF Switchable Nanocomposite Membranes for Separations. Polymers 12, 2415 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bandehali S et al. Planning of smart gating membranes for water treatment. Chemosphere 283, 131207, doi: 10.1016/j.chemosphere.2021.131207 (2021). [DOI] [PubMed] [Google Scholar]
- 10.Siekierka A, Smolińska-Kempisty K & Wolska J Enhanced Specific Mechanism of Separation by Polymeric Membrane Modification—A Short Review. Membranes 11, 942 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gandhi A, Paul A, Sen SO & Sen KK Studies on thermoresponsive polymers: Phase behaviour, drug delivery and biomedical applications. Asian Journal of Pharmaceutical Sciences 10, 99–107, doi: 10.1016/j.ajps.2014.08.010 (2015). [DOI] [Google Scholar]
- 12.Meng X, Ji Y, Yu G & Zhai Y Preparation and Properties of Polyvinylidene Fluoride Nanocomposited Membranes based on Poly(N-Isopropylacrylamide) Modified Graphene Oxide Nanosheets. Polymers 11, 473 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Futscher MH, Philipp M, Müller-Buschbaum P & Schulte A The Role of Backbone Hydration of Poly(N-isopropyl acrylamide) Across the Volume Phase Transition Compared to its Monomer. Scientific Reports 7, 17012, doi: 10.1038/s41598-017-17272-7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katsumoto Y, Tanaka T, Sato H & Ozaki Y Conformational Change of Poly(N-isopropylacrylamide) during the Coil–Globule Transition Investigated by Attenuated Total Reflection/Infrared Spectroscopy and Density Functional Theory Calculation. The Journal of Physical Chemistry A 106, 3429–3435, doi: 10.1021/jp0124903 (2002). [DOI] [PubMed] [Google Scholar]
- 15.Fan Q, Sirkar KK & Wu J A thermo-sensitive release system based on polymeric membrane for transdermal delivery of doxycycline HCl. Journal of Membrane Science 337, 175–181, doi: 10.1016/j.memsci.2009.03.032 (2009). [DOI] [Google Scholar]
- 16.Liang L, Shi M, Viswanathan VV, Peurrung LM & Young JS Temperature-sensitive polypropylene membranes prepared by plasma polymerization. Journal of Membrane Science 177, 97–108, doi: 10.1016/S0376-7388(00)00453-1 (2000). [DOI] [Google Scholar]
- 17.Huang J, Wang X-L, Qi W-S & Yu X-H Temperature sensitivity and electrokinetic behavior of a N-isopropylacrylamide grafted microporous polyethylene membrane. Desalination 146, 345–351, doi: 10.1016/S0011-9164(02)00511-8 (2002). [DOI] [Google Scholar]
- 18.Lue SJ et al. Grafting of poly(N-isopropylacrylamide-co-acrylic acid) on micro-porous polycarbonate films: Regulating lower critical solution temperatures for drug controlled release. Journal of Membrane Science 379, 330–340, doi: 10.1016/j.memsci.2011.06.002 (2011). [DOI] [Google Scholar]
- 19.Lue SJ, Hsu J-J, Chen C-H & Chen B-C Thermally on–off switching membranes of poly(N-isopropylacrylamide) immobilized in track-etched polycarbonate films. Journal of Membrane Science 301, 142–150, doi: 10.1016/j.memsci.2007.06.020 (2007). [DOI] [Google Scholar]
- 20.Rzaev ZMO, Dinçer S & Pişkin E Functional copolymers of N-isopropylacrylamide for bioengineering applications. Progress in Polymer Science 32, 534–595, doi: 10.1016/j.progpolymsci.2007.01.006 (2007). [DOI] [Google Scholar]
- 21.Saad A, Mills R, Wan H, Ormsbee L & Bhattacharyya D Thermoresponsive PNIPAm–PMMA-Functionalized PVDF Membranes with Reactive Fe–Pd Nanoparticles for PCB Degradation. Industrial & Engineering Chemistry Research 59, 16614–16625, doi: 10.1021/acs.iecr.0c03260 (2020). [DOI] [Google Scholar]
- 22.Saad A et al. Thermo-responsive adsorption-desorption of perfluoroorganics from water using PNIPAm hydrogels and pore functionalized membranes. Journal of Membrane Science 599, 117821, doi: 10.1016/j.memsci.2020.117821 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu D et al. Mechanistic Insights of a Thermoresponsive Interface for Fouling Control of Thin-Film Composite Nanofiltration Membranes. Environmental Science & Technology 56, 1927–1937, doi: 10.1021/acs.est.1c06156 (2022). [DOI] [PubMed] [Google Scholar]
- 24.Ngang HP, Ahmad AL, Low SC & Ooi BS Adsorption-desorption study of oil emulsion towards thermo-responsive PVDF/SiO2-PNIPAM composite membrane. Journal of Environmental Chemical Engineering 5, 4471–4482, doi: 10.1016/j.jece.2017.08.038 (2017). [DOI] [Google Scholar]
- 25.Bischofberger I & Trappe V New aspects in the phase behaviour of poly-N-isopropyl acrylamide: systematic temperature dependent shrinking of PNiPAM assemblies well beyond the LCST. Scientific Reports 5, 15520, doi: 10.1038/srep15520 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daumann K, Frost S & Ulbricht M Tunable and switchable nanoparticle separation with thermo-responsive track-etched membranes prepared by controlled surface-initiated polymerization of poly(N-isopropylacrylamide). RSC Advances 10, 21028–21038, doi: 10.1039/D0RA03418E (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Du H, Wickramasinghe SR & Qian X Specificity in Cationic Interaction with Poly(N-isopropylacrylamide). The Journal of Physical Chemistry B 117, 5090–5101, doi: 10.1021/jp401817h (2013). [DOI] [PubMed] [Google Scholar]
- 28.Du H, Wickramasinghe R & Qian X Effects of Salt on the Lower Critical Solution Temperature of Poly (N-Isopropylacrylamide). The Journal of Physical Chemistry B 114, 16594–16604, doi: 10.1021/jp105652c (2010). [DOI] [PubMed] [Google Scholar]
- 29.Vu A et al. Oil Deposition on Polymer Brush-Coated NF Membranes. Membranes 9, 168 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Götz T, Landzettel J & Schiestel T Thermo-responsive mixed-matrix hollow fiber membranes. Journal of Applied Polymer Science 138, 50787, doi: 10.1002/app.50787 (2021). [DOI] [Google Scholar]
- 31.Liu Z, Wang W, Xie R, Ju X-J & Chu L-Y Stimuli-responsive smart gating membranes. Chemical Society Reviews 45, 460–475, doi: 10.1039/C5CS00692A (2016). [DOI] [PubMed] [Google Scholar]
- 32.Luo F et al. Effects of fabrication conditions on the microstructures and performances of smart gating membranes with in situ assembled nanogels as gates. Journal of Membrane Science 519, 32–44, doi: 10.1016/j.memsci.2016.07.045 (2016). [DOI] [Google Scholar]
- 33.Wang G, Xie R, Ju X-J & Chu L-Y Thermo-Responsive Polyethersulfone Composite Membranes Blended with Poly(N-isopropylacrylamide) Nanogels. Chemical Engineering & Technology 35, 2015–2022, doi: 10.1002/ceat.201200235 (2012). [DOI] [Google Scholar]
- 34.Lin X, Nguyen Quoc B & Ulbricht M Magnetoresponsive Poly(ether sulfone)-Based Iron Oxide cum Hydrogel Mixed Matrix Composite Membranes for Switchable Molecular Sieving. ACS Applied Materials & Interfaces 8, 29001–29014, doi: 10.1021/acsami.6b09369 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Wiese M, Lohaus T, Haussmann J & Wessling M Charged microgels adsorbed on porous membranes - A study of their mobility and molecular retention. Journal of Membrane Science 588, 117190, doi: 10.1016/j.memsci.2019.117190 (2019). [DOI] [Google Scholar]
- 36.Frost S & Ulbricht M Thermoresponsive ultrafiltration membranes for the switchable permeation and fractionation of nanoparticles. Journal of Membrane Science 448, 1–11, doi: 10.1016/j.memsci.2013.07.036 (2013). [DOI] [Google Scholar]
- 37.Li B et al. Catalytic behavior of a thermo-responsive PVDF/microgel@Pd membrane for 2- nitroaniline degradation. Journal of Environmental Chemical Engineering 9, 104757, doi: 10.1016/j.jece.2020.104757 (2021). [DOI] [Google Scholar]
- 38.Chen JJ, Ahmad AL & Ooi BS Thermo-responsive properties of poly(N-isopropylacrylamide-co-acrylic acid) hydrogel and its effect on copper ion removal and fouling of polymer-enhanced ultrafiltration. Journal of Membrane Science 469, 73–79, doi: 10.1016/j.memsci.2014.05.062 (2014). [DOI] [Google Scholar]
- 39.Meng T et al. A thermo-responsive affinity membrane with nano-structured pores and grafted poly(N-isopropylacrylamide) surface layer for hydrophobic adsorption. Journal of Membrane Science 349, 258–267, doi: 10.1016/j.memsci.2009.11.058 (2010). [DOI] [Google Scholar]
- 40.Morisada S, Namazuda K. i., Kanda H, Hirokawa Y & Nakano Y Temperature-swing adsorption of proteins in water using N-isopropylacrylamide copolymer gel particles. Advanced Powder Technology 21, 28–33, doi: 10.1016/j.apt.2009.10.001 (2010). [DOI] [Google Scholar]
- 41.Pelton R Poly(N-isopropylacrylamide) (PNIPAM) is never hydrophobic. Journal of Colloid and Interface Science 348, 673–674, doi: 10.1016/j.jcis.2010.05.034 (2010). [DOI] [PubMed] [Google Scholar]
- 42.Yao Y, Volchek K, Brown CE, Robinson A & Obal T Comparative study on adsorption of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) by different adsorbents in water. Water Science and Technology 70, 1983–1991, doi: 10.2166/wst.2014.445 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Léniz-Pizarro F et al. Dual-Functional Nanofiltration and Adsorptive Membranes for PFAS and Organics Separation from Water. ACS ES&T Water, doi: 10.1021/acsestwater.2c00043 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Low SC, Ng QH & Tan LS Study of magnetic-responsive nanoparticle on the membrane surface as a membrane antifouling surface coating. Journal of Polymer Research 26, 70, doi: 10.1007/s10965-019-1734-4 (2019). [DOI] [Google Scholar]
- 45.Maity S, Mishra B, Nayak K, Dubey NC & Tripathi BP Zwitterionic microgel based anti(-bio)fouling smart membranes for tunable water filtration and molecular separation. Materials Today Chemistry 24, 100779, doi: 10.1016/j.mtchem.2022.100779 (2022). [DOI] [Google Scholar]
- 46.Li D, Niu X, Yang S, Chen Y & Ran F Thermo-responsive polysulfone membranes with good anti-fouling property modified by grafting random copolymers via surface-initiated eATRP. Sep Purif Technol 206, 166–176, doi: 10.1016/j.seppur.2018.05.046 (2018). [DOI] [Google Scholar]
- 47.Zhao Y et al. Thermo-responsive separation membrane with smart anti-fouling and self-cleaning properties. Chemical Engineering Research and Design 156, 333–342, doi: 10.1016/j.cherd.2020.02.006 (2020). [DOI] [Google Scholar]
- 48.Yaghoubi Z & Basiri-Parsa J Modification of ultrafiltration membrane by thermo-responsive Bentonite-poly(N-isopropylacrylamide) nanocomposite to improve its antifouling properties. Journal of Water Process Engineering 34, 101067, doi: 10.1016/j.jwpe.2019.101067 (2020). [DOI] [Google Scholar]
- 49.Mao H et al. Anti-fouling and easy-cleaning PVDF membranes blended with hydrophilic thermo-responsive nanofibers for efficient biological wastewater treatment. Sep Purif Technol 281, 119881, doi: 10.1016/j.seppur.2021.119881 (2022). [DOI] [Google Scholar]
- 50.Yang M, Liu C, Li Z, Gao G & Liu F Temperature-Responsive Properties of Poly(acrylic acid-co-acrylamide) Hydrophobic Association Hydrogels with High Mechanical Strength. Macromolecules 43, 10645–10651, doi: 10.1021/ma1022555 (2010). [DOI] [Google Scholar]
- 51.Ding Y et al. One-Step Fabrication of a Micro/Nanosphere-Coordinated Dual Stimulus-Responsive Nanofibrous Membrane for Intelligent Antifouling and Ultrahigh Permeability of Viscous Water-in-Oil Emulsions. ACS Applied Materials & Interfaces 13, 27635–27644, doi: 10.1021/acsami.1c05896 (2021). [DOI] [PubMed] [Google Scholar]
- 52.Baysak FK & Işıklan N Pervaporation performance of poly(vinyl alcohol)-graft-poly(N-hydroxymethyl acrylamide) membranes for dehydration of isopropyl alcohol-water mixture. Journal of Applied Polymer Science 139, 51976, doi: 10.1002/app.51976 (2022). [DOI] [Google Scholar]
- 53.Işıklan N & Tokmak Ş Microwave based synthesis and spectral characterization of thermo-sensitive poly(N,N-diethylacrylamide) grafted pectin copolymer. International Journal of Biological Macromolecules 113, 669–680, doi: 10.1016/j.ijbiomac.2018.02.155 (2018). [DOI] [PubMed] [Google Scholar]
- 54.Fan X-X et al. Dual pH-responsive smart gating membranes. Journal of Membrane Science 555, 20–29, doi: 10.1016/j.memsci.2018.03.028 (2018). [DOI] [Google Scholar]
- 55.Ito Y, Park YS & Imanishi Y Visualization of Critical pH-Controlled Gating of a Porous Membrane Grafted with Polyelectrolyte Brushes. Journal of the American Chemical Society 119, 2739–2740, doi: 10.1021/ja9641057 (1997). [DOI] [Google Scholar]
- 56.Reddy KR et al. Self-healing polymers: Synthesis methods and applications. Nano-Structures & Nano-Objects 23, 100500, doi: 10.1016/j.nanoso.2020.100500 (2020). [DOI] [Google Scholar]
- 57.Madhav H, Singh N & Jaiswar G in Materials for Biomedical Engineering (eds Valentina Grumezescu& Alexandru Mihai Grumezescu) 105–143 (Elsevier, 2019). [Google Scholar]
- 58.Padeste C & Neuhaus S in Polymer Micro- and Nanografting (eds Celestino Padeste& Sonja Neuhaus) 1–10 (William Andrew Publishing, 2015). [Google Scholar]
- 59.Nava-Ocampo MF et al. Sacrificial coating development for biofouling control in membrane systems. Desalination 496, 114650, doi: 10.1016/j.desal.2020.114650 (2020). [DOI] [Google Scholar]
- 60.Roy SG & De P pH responsive polymers with amino acids in the side chains and their potential applications. Journal of Applied Polymer Science 131, doi: 10.1002/app.41084 (2014). [DOI] [Google Scholar]
- 61.Davari S, Omidkhah M & Abdollahi M Improved antifouling ability of thin film composite polyamide membrane modified by a pH-sensitive imidazole-based zwitterionic polyelectrolyte. Journal of Membrane Science 564, 788–799, doi: 10.1016/j.memsci.2018.07.050 (2018). [DOI] [Google Scholar]
- 62.Xiao L, Davenport DM, Ormsbee L & Bhattacharyya D Polymerization and Functionalization of Membrane Pores for Water Related Applications. Industrial & Engineering Chemistry Research 54, 4174–4182, doi: 10.1021/ie504149t (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Durmaz EN et al. Polyelectrolytes as Building Blocks for Next-Generation Membranes with Advanced Functionalities. ACS Applied Polymer Materials 3, 4347–4374, doi: 10.1021/acsapm.1c00654 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li X, Sotto A, Li J & Van der Bruggen B Progress and perspectives for synthesis of sustainable antifouling composite membranes containing in situ generated nanoparticles. Journal of Membrane Science 524, 502–528, doi: 10.1016/j.memsci.2016.11.040 (2017). [DOI] [Google Scholar]
- 65.Wiśniewska M, Urban T, Grządka E, Zarko VI & Gun’ko VM Comparison of adsorption affinity of polyacrylic acid for surfaces of mixed silica-alumina. Colloid Polym Sci 292, 699–705, doi: 10.1007/s00396-013-3103-x (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rivas B, Jara M & Pereira E Preparation and adsorption properties of the chelating resins containing carboxylic, sulfonic, and imidazole groups. Journal of Applied Polymer Science 89, 2852–2856, doi: 10.1002/app.12524 (2003). [DOI] [Google Scholar]
- 67.Lewis S, Smuleac V, Montague A, Bachas L & Bhattacharyya D Iron-Functionalized Membranes for Nanoparticle Synthesis and Reactions. Sep Sci Technol 44, 3289–3311, doi: 10.1080/01496390903212805 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Y et al. High-Affinity Detection and Capture of Heavy Metal Contaminants using Block Polymer Composite Membranes. ACS Cent Sci 4, 1697–1707, doi: 10.1021/acscentsci.8b00690 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ladhe AR, Radomyselski A & Bhattacharyya D Ethoxylated nonionic surfactants in hydrophobic solvent: interaction with aqueous and membrane-immobilized poly(acrylic acid). Langmuir 22, 615–621, doi: 10.1021/la052221u (2006). [DOI] [PubMed] [Google Scholar]
- 70.Xu J, Dozier A & Bhattacharyya D Synthesis of Nanoscale Bimetallic Particles in Polyelectrolyte Membrane Matrix for Reductive Transformation of Halogenated Organic Compounds. Journal of Nanoparticle Research 7, 449–467, doi: 10.1007/s11051-005-4273-3 (2005). [DOI] [Google Scholar]
- 71.Datta S, Cecil C & Bhattacharyya D Functionalized Membranes by Layer-By-Layer Assembly of Polyelectrolytes and In Situ Polymerization of Acrylic Acid for Applications in Enzymatic Catalysis. Industrial & Engineering Chemistry Research 47, 4586–4597, doi: 10.1021/ie800142d (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sarma R, Islam MS, Running MP & Bhattacharyya D Multienzyme Immobilized Polymeric Membrane Reactor for the Transformation of a Lignin Model Compound. Polymers 10, 463 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smuleac V, Varma R, Sikdar S & Bhattacharyya D Green synthesis of Fe and Fe/Pd bimetallic nanoparticles in membranes for reductive degradation of chlorinated organics. Journal of Membrane Science 379, 131–137, doi: 10.1016/j.memsci.2011.05.054 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Islam MS, Hernández S, Wan H, Ormsbee L & Bhattacharyya D Role of membrane pore polymerization conditions for pH responsive behavior, catalytic metal nanoparticle synthesis, and PCB degradation. Journal of Membrane Science 555, 348–361, doi: 10.1016/j.memsci.2018.03.060 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Swift T, Swanson L, Geoghegan M & Rimmer S The pH-responsive behaviour of poly(acrylic acid) in aqueous solution is dependent on molar mass. Soft Matter 12, 2542–2549, doi: 10.1039/C5SM02693H (2016). [DOI] [PubMed] [Google Scholar]
- 76.Silva LLS et al. Novel microwave-driven synthesis of hydrophilic polyvinylidene fluoride/polyacrylic acid (PVDF/PAA) membranes and decoration with nano zero-valent-iron (nZVI) for water treatment applications. Journal of Membrane Science 620, 118817, doi: 10.1016/j.memsci.2020.118817 (2021). [DOI] [Google Scholar]
- 77.Mishra S, Usha Rani G & Sen G Microwave initiated synthesis and application of polyacrylic acid grafted carboxymethyl cellulose. Carbohydrate Polymers 87, 2255–2262, doi: 10.1016/j.carbpol.2011.10.057 (2012). [DOI] [Google Scholar]
- 78.Jovanovic J & Adnadjevic B Influence of microwave heating on the kinetic of acrylic acid polymerization and crosslinking. Journal of Applied Polymer Science 116, 55–63, doi: 10.1002/app.31230 (2010). [DOI] [Google Scholar]
- 79.Ashfaq MY, Al-Ghouti MA & Zouari N Functionalization of reverse osmosis membrane with graphene oxide and polyacrylic acid to control biofouling and mineral scaling. Science of The Total Environment 736, 139500, doi: 10.1016/j.scitotenv.2020.139500 (2020). [DOI] [PubMed] [Google Scholar]
- 80.Olimattel K, Zhai L & Sadmani AHMA Enhanced removal of perfluorooctane sulfonic acid and perfluorooctanoic acid via polyelectrolyte functionalized ultrafiltration membrane: Effects of membrane modification and water matrix. Journal of Hazardous Materials Letters 2, 100043, doi: 10.1016/j.hazl.2021.100043 (2021). [DOI] [Google Scholar]
- 81.Liu C et al. Multifunctional CNTs-PAA/MIL101(Fe)@Pt Composite Membrane for High-throughput Oily Wastewater Remediation. Journal of Hazardous Materials 403, 123547, doi: 10.1016/j.jhazmat.2020.123547 (2021). [DOI] [PubMed] [Google Scholar]
- 82.Lu N et al. Superwetting Electrospun PDMS/PMMA Membrane for PM2.5 Capture and Microdroplet Transfer. Langmuir 37, 12972–12980, doi: 10.1021/acs.langmuir.1c02038 (2021). [DOI] [PubMed] [Google Scholar]
- 83.Mishra NK et al. Highly selective hollow fiber membranes for carbon capture via in-situ layer-by-layer surface functionalization. Journal of Membrane Science 633, 119381, doi: 10.1016/j.memsci.2021.119381 (2021). [DOI] [Google Scholar]
- 84.in Synthetic Polymeric Membranes: Characterization by Atomic Force Microscopy (eds Khulbe KC, Feng CY, & Matsuura Takeshi) 101–139 (Springer Berlin Heidelberg, 2008). [Google Scholar]
- 85.Ansari A et al. Polyacrylic acid-brushes tethered to graphene oxide membrane coating for scaling and biofouling mitigation on reverse osmosis membranes. Journal of Membrane Science 630, 119308, doi: 10.1016/j.memsci.2021.119308 (2021). [DOI] [Google Scholar]
- 86.Olimattel K et al. Enhanced Fouling Resistance and Antimicrobial Property of Ultrafiltration Membranes Via Polyelectrolyte-Assisted Silver Phosphate Nanoparticle Immobilization. Membranes 10, 293 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ma Z et al. Superhydrophilic and oleophobic membrane functionalized with heterogeneously tailored two-dimensional layered double hydroxide nanosheets for antifouling. Journal of Membrane Science 577, 165–175, doi: 10.1016/j.memsci.2019.01.054 (2019). [DOI] [Google Scholar]
- 88.Temtem M et al. Development of PMMA membranes functionalized with hydroxypropyl-β-cyclodextrins for controlled drug delivery using a supercritical CO2-assisted technology. International Journal of Pharmaceutics 376, 110–115, doi: 10.1016/j.ijpharm.2009.04.029 (2009). [DOI] [PubMed] [Google Scholar]
- 89.Uyar T et al. Cyclodextrin functionalized poly(methyl methacrylate) (PMMA) electrospun nanofibers for organic vapors waste treatment. Journal of Membrane Science 365, 409–417, doi: 10.1016/j.memsci.2010.09.037 (2010). [DOI] [Google Scholar]
- 90.Zdarta J et al. A promising laccase immobilization using electrospun materials for biocatalytic degradation of tetracycline: Effect of process conditions and catalytic pathways. Catalysis Today 348, 127–136, doi: 10.1016/j.cattod.2019.08.042 (2020). [DOI] [Google Scholar]
- 91.Heng ZW, Chong WC, Pang YL, Sim LC & Koo CH Novel visible-light responsive NCQDs-TiO2/PAA/PES photocatalytic membrane with enhanced antifouling properties and self-cleaning performance. Journal of Environmental Chemical Engineering 9, 105388, doi: 10.1016/j.jece.2021.105388 (2021). [DOI] [Google Scholar]
- 92.Wang S et al. One-step selective separation and catalytic transformation of an organic pollutant from pollutant mixture via a thermo-responsive semi-IPN/PVDF@Pd bilayer composite membrane. Sep Purif Technol 286, 120493, doi: 10.1016/j.seppur.2022.120493 (2022). [DOI] [Google Scholar]
- 93.Stawski D et al. Antibacterial properties of poly (N,N-dimethylaminoethyl methacrylate) obtained at different initiator concentrations in solution polymerization. Royal Society Open Science 9, 211367, doi:doi: 10.1098/rsos.211367 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Abdellaoui N, Laoui FM, Cerbah H & Arous O Preparation of poly (N,N-dimethylaminoethyl methacrylate) (PDAEM) membranes: Application for water purification. Journal of Applied Polymer Science 135, 46592, doi: 10.1002/app.46592 (2018). [DOI] [Google Scholar]
- 95.Sánchez J et al. Poly(N,N-dimethylaminoethyl methacrylate) for removing chromium (VI) through polymer-enhanced ultrafiltration technique. Reactive and Functional Polymers 127, 67–73, doi: 10.1016/j.reactfunctpolym.2018.04.002 (2018). [DOI] [Google Scholar]
- 96.Zhou X & He C Tailoring the surface chemistry and morphology of glass fiber membranes for robust oil/water separation using poly(dimethylsiloxanes) as hydrophobic molecular binders. Journal of Materials Chemistry A 6, 607–615, doi: 10.1039/C7TA09411F (2018). [DOI] [Google Scholar]
- 97.Pantuso E, De Filpo G & Nicoletta FP Light-Responsive Polymer Membranes. Advanced Optical Materials 7, 1900252–1900252, doi: 10.1002/adom.201900252 (2019). [DOI] [Google Scholar]
- 98.Rosli A & Low SC Molecularly engineered switchable photo-responsive membrane in gas separation for environmental protection. Environmental Engineering Research 25, 447–461, doi: 10.4491/eer.2019.090 (2020). [DOI] [Google Scholar]
- 99.Kanj AB, Müller K & Heinke L Stimuli-Responsive Metal-Organic Frameworks with Photoswitchable Azobenzene Side Groups. Macromolecular Rapid Communications 39, 1700239–1700239, doi: 10.1002/marc.201700239 (2018). [DOI] [PubMed] [Google Scholar]
- 100.Lu J, Jiang Y, Yu P, Jiang W & Mao L Light-Controlled Ionic/Molecular Transport through Solid-State Nanopores and Nanochannels. Chemistry – An Asian Journal n/a, doi: 10.1002/asia.202200158 (2022). [DOI] [PubMed] [Google Scholar]
- 101.Mendes-Felipe C, Veloso-Fernández A, Vilas-Vilela JL & Ruiz-Rubio L Vol. 12 (2022). [Google Scholar]
- 102.Riaz S & Park S-J An overview of TiO2-based photocatalytic membrane reactors for water and wastewater treatments. Journal of Industrial and Engineering Chemistry 84, 23–41, doi: 10.1016/j.jiec.2019.12.021 (2020). [DOI] [Google Scholar]
- 103.Dong H et al. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Research 79, 128–146, doi: 10.1016/j.watres.2015.04.038 (2015). [DOI] [PubMed] [Google Scholar]
- 104.Fang H et al. Electrostatic Assembly of Porphyrin-Functionalized Porous Membrane toward Biomimetic Photocatalytic Degradation Dyes. ACS Omega 5, 8707–8720, doi: 10.1021/acsomega.0c00135 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang H, Zhang J, Luo J & Wan Y A novel paradigm of photocatalytic cleaning for membrane fouling removal. Journal of Membrane Science 641, 119859–119859, doi: 10.1016/j.memsci.2021.119859 (2022). [DOI] [Google Scholar]
- 106.Hu J et al. Durable and super-hydrophilic/underwater super-oleophobic two-dimensional MXene composite lamellar membrane with photocatalytic self-cleaning property for efficient oil/water separation in harsh environments. Journal of Membrane Science 637, 119627–119627, doi: 10.1016/j.memsci.2021.119627 (2021). [DOI] [Google Scholar]
- 107.Lin Q et al. Self-cleaning photocatalytic MXene composite membrane for synergistically enhanced water treatment: Oil/water separation and dyes removal. Chemical Engineering Journal 427, 131668–131668, doi: 10.1016/j.cej.2021.131668 (2022). [DOI] [Google Scholar]
- 108.Ni L, Zhu Y, Ma J & Wang Y Novel strategy for membrane biofouling control in MBR with CdS/MIL-101 modified PVDF membrane by in situ visible light irradiation. Water Research 188, 116554–116554, doi: 10.1016/j.watres.2020.116554 (2021). [DOI] [PubMed] [Google Scholar]
- 109.Zhang L et al. Photocatalytic GO/M88A “interceptor plate” assembled nanofibrous membrane with photo-Fenton self-cleaning performance for oil/water emulsion separation. Chemical Engineering Journal 427, 130948–130948, doi: 10.1016/j.cej.2021.130948 (2022). [DOI] [Google Scholar]
- 110.Li J et al. Removal of volatile organic compounds from air using supported ionic liquid membrane containing ultraviolet-visible light-driven Nd-TiO2 nanoparticles. Journal of Molecular Structure 1231, 130023–130023, doi: 10.1016/j.molstruc.2021.130023 (2021). [DOI] [Google Scholar]
- 111.Yang C et al. Photocatalytic PVDF ultrafiltration membrane blended with visible-light responsive Fe(III)-TiO2 catalyst: Degradation kinetics, catalytic performance and reusability. Chemical Engineering Journal 417, 129340–129340, doi: 10.1016/j.cej.2021.129340 (2021). [DOI] [Google Scholar]
- 112.Jeong E, Byun J, Bayarkhuu B & Hong SW Hydrophilic photocatalytic membrane via grafting conjugated polyelectrolyte for visible-light-driven biofouling control. Applied Catalysis B: Environmental 282, 119587–119587, doi: 10.1016/j.apcatb.2020.119587 (2021). [DOI] [Google Scholar]
- 113.Wang Y et al. Novel Ag-AgBr decorated composite membrane for dye rejection and photodegradation under visible light. Frontiers of Chemical Science and Engineering 15, 892–901, doi: 10.1007/s11705-020-2011-0 (2021). [DOI] [Google Scholar]
- 114.Wan J et al. Fabrication of self-assembled 0D-2D Bi2MoO6-g-C3N4 photocatalytic composite membrane based on PDA intermediate coating with visible light self-cleaning performance. Journal of Colloid and Interface Science 601, 229–241, doi: 10.1016/j.jcis.2021.05.038 (2021). [DOI] [PubMed] [Google Scholar]
- 115.Meng M, Li B, Zhu Y, Yan Y & Feng Y A novel mixed matrix polysulfone membrane for enhanced ultrafiltration and photocatalytic self-cleaning performance. Journal of Colloid and Interface Science 599, 178–189, doi: 10.1016/j.jcis.2021.04.082 (2021). [DOI] [PubMed] [Google Scholar]
- 116.Zhao X, You Y, Ma Y, Meng C & Zhang Z Ti3C2/W18O49 hybrid membrane with visible-light-driven photocatalytic ability for selective dye separation. Separation and Purification Technology 282, 120145–120145, doi: 10.1016/j.seppur.2021.120145 (2022). [DOI] [Google Scholar]
- 117.Nasrollahi N, Ghalamchi L, Vatanpour V & Khataee A Photocatalytic-membrane technology: a critical review for membrane fouling mitigation. Journal of Industrial and Engineering Chemistry 93, 101–116, doi: 10.1016/j.jiec.2020.09.031 (2021). [DOI] [Google Scholar]
- 118.Li J-J, Zhou Y-N & Luo Z-H Polymeric materials with switchable superwettability for controllable oil/water separation: A comprehensive review. Progress in Polymer Science 87, 1–33, doi: 10.1016/j.progpolymsci.2018.06.009 (2018). [DOI] [Google Scholar]
- 119.Nascimbén Santos É, László Z, Hodúr C, Arthanareeswaran G & Veréb G Photocatalytic membrane filtration and its advantages over conventional approaches in the treatment of oily wastewater: A review. Asia-Pacific Journal of Chemical Engineering 15, e2533–e2533, doi: 10.1002/apj.2533 (2020). [DOI] [Google Scholar]
- 120.Shrestha B, Ezazi M & Kwon G Vol. 11 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sun F et al. Multi-scaled, hierarchical nanofibrous membrane for oil/water separation and photocatalysis: Preparation, characterization and properties evaluation. Progress in Organic Coatings 152, 106125–106125, doi: 10.1016/j.porgcoat.2020.106125 (2021). [DOI] [Google Scholar]
- 122.Chen B et al. Light-responsive and corrosion-resistant gas valve with non-thermal effective liquid-gating positional flow control. Light: Science & Applications 10, 127–127, doi: 10.1038/s41377-021-00568-9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen Z et al. Smart light responsive polypropylene membrane switching reversibly between hydrophobicity and hydrophilicity for oily water separation. Journal of Membrane Science 638, 119704–119704, doi: 10.1016/j.memsci.2021.119704 (2021). [DOI] [Google Scholar]
- 124.Wu S et al. Dual-Responsive Janus Membrane by One-Step Laser Drilling for Underwater Bubble Selective Capture and Repelling. Advanced Materials Interfaces 6, 1901176–1901176, doi: 10.1002/admi.201901176 (2019). [DOI] [Google Scholar]
- 125.Nie X et al. Light-Powered Ion Pumping in a Cation-Selective Conducting Polymer Membrane. Angewandte Chemie International Edition 61, e202201138–e202201138, doi: 10.1002/anie.202201138 (2022). [DOI] [PubMed] [Google Scholar]
- 126.Shen J, Qiao J, Zhang X & Qi L Dual-stimuli-responsive porous polymer enzyme reactor for tuning enzymolysis efficiency. Microchimica Acta 188, 435–435, doi: 10.1007/s00604-021-05095-3 (2021). [DOI] [PubMed] [Google Scholar]
- 127.Shen J, Qiao J, Kim D-P & Qi L Study on controllable enzymolysis by chiral capillary electrophoresis with an ultraviolet-visible responsive polymer membrane based l-asparaginase reactor. Talanta 234, 122676–122676, doi: 10.1016/j.talanta.2021.122676 (2021). [DOI] [PubMed] [Google Scholar]
- 128.Jauffred L, Samadi A, Klingberg H, Bendix PM & Oddershede LB Plasmonic Heating of Nanostructures. Chemical Reviews 119, 8087–8130, doi: 10.1021/acs.chemrev.8b00738 (2019). [DOI] [PubMed] [Google Scholar]
- 129.Assis M et al. Vol. 11 (2021). [Google Scholar]
- 130.Langer J et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 14, 28–117, doi: 10.1021/acsnano.9b04224 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Boysen RI, Schwarz LJ, Nicolau DV & Hearn MTW Molecularly imprinted polymer membranes and thin films for the separation and sensing of biomacromolecules. Journal of Separation Science 40, 314–335, doi: 10.1002/jssc.201600849 (2017). [DOI] [PubMed] [Google Scholar]
- 132.Xu F, Xuan M, Ben Z, Shang W & Ma G Surface enhanced Raman scattering analysis with filter-based enhancement substrates: A mini review. Reviews in Analytical Chemistry 40, 75–92, doi:doi: 10.1515/revac-2021-0126 (2021). [DOI] [Google Scholar]
- 133.Perumal J et al. novel cellulose fibre-based flexible plasmonic membrane for point-of-care SERS biomarker detection in chronic wound healing. International Journal of Nanomedicine 16, 5869–5869 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cai J et al. Polarization-sensitive optoionic membranes from chiral plasmonic nanoparticles. Nature Nanotechnology, doi: 10.1038/s41565-022-01079-3 (2022). [DOI] [PubMed] [Google Scholar]
- 135.Östergren I et al. Highly Permeable Fluorinated Polymer Nanocomposites for Plasmonic Hydrogen Sensing. ACS Applied Materials & Interfaces 13, 21724–21732, doi: 10.1021/acsami.1c01968 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Snitka V et al. Surface-enhanced Raman scattering sensors for biomedical and molecular detection applications in space. CEAS Space Journal 13, 509–520, doi: 10.1007/s12567-021-00356-6 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li J et al. Plasmonic Rare-Earth Nanosheets as Surface Enhanced Raman Scattering Substrates with High Sensitivity and Stability for Multicomponent Analysis. ACS Nano 16, 1160–1169, doi: 10.1021/acsnano.1c08961 (2022). [DOI] [PubMed] [Google Scholar]
- 138.Sousa-Castillo A et al. Plasmonic Retrofitting of Membrane Materials: Shifting from Self-Regulation to On-Command Control of Fluid Flow. Advanced Materials 30, 1707598–1707598, doi: 10.1002/adma.201707598 (2018). [DOI] [PubMed] [Google Scholar]
- 139.Politano A et al. When plasmonics meets membrane technology. Journal of Physics: Condensed Matter 28, 363003–363003 (2016). [DOI] [PubMed] [Google Scholar]
- 140.Farid MU, Kharraz JA & An AK Plasmonic Titanium Nitride Nano-enabled Membranes with High Structural Stability for Efficient Photothermal Desalination. ACS Applied Materials & Interfaces 13, 3805–3815, doi: 10.1021/acsami.0c17154 (2021). [DOI] [PubMed] [Google Scholar]
- 141.Liu Y et al. Plasmonic silver nanoparticle-decorated electrospun nanofiber membrane for interfacial solar vapor generation. Textile Research Journal 91, 2624–2634, doi: 10.1177/00405175211014966 (2021). [DOI] [Google Scholar]
- 142.Chen C, Liu H, Wang H, Zhao Y & Li M A scalable broadband plasmonic cuprous telluride nanowire-based hybrid photothermal membrane for efficient solar vapor generation. Nano Energy 84, 105868–105868, doi: 10.1016/j.nanoen.2021.105868 (2021). [DOI] [Google Scholar]
- 143.Marín-Caba L, Bodelón G, Negrín-Montecelo Y & Correa-Duarte MA Sunlight-Sensitive Plasmonic Nanostructured Composites as Photocatalytic Coating with Antibacterial Properties. Advanced Functional Materials 31, 2105807–2105807, doi: 10.1002/adfm.202105807 (2021). [DOI] [Google Scholar]
- 144.Shi Y et al. Electro-Enhanced Separation of Microsized Oil-in-Water Emulsions via Metallic Membranes: Performance and Mechanistic Insights. Environmental Science & Technology 56, 4518–4530, doi: 10.1021/acs.est.2c00336 (2022). [DOI] [PubMed] [Google Scholar]
- 145.Dehkordi TF, Shirin-Abadi AR, Karimipour K & Mahdavian AR CO2-, electric potential-, and photo-switchable-hydrophilicity membrane (x-SHM) as an efficient color-changeable tool for oil/water separation. Polymer 212, 123250–123250, doi: 10.1016/j.polymer.2020.123250 (2021). [DOI] [Google Scholar]
- 146.Mallawarachchi S, Gejji V, Sierra LS, Wang H & Fernando S Electrical Field Reversibly Modulates Enzyme Kinetics of Hexokinase Entrapped in an Electro-Responsive Hydrogel. ACS Applied Bio Materials 2, 5676–5686, doi: 10.1021/acsabm.9b00748 (2019). [DOI] [PubMed] [Google Scholar]
- 147.Formoso P, Pantuso E, De Filpo G & Nicoletta FP Vol. 7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Alayande AB et al. Vol. 11 (2021). [Google Scholar]
- 149.Besha AT, Tsehaye MT, Aili D, Zhang W & Tufa RA Design of Monovalent Ion Selective Membranes for Reducing the Impacts of Multivalent Ions in Reverse Electrodialysis. Membranes (Basel) 10, doi: 10.3390/membranes10010007 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang W et al. Recent advances in monovalent ion selective membranes towards environmental remediation and energy harvesting. Separation and Purification Technology 297, 121520, doi: 10.1016/j.seppur.2022.121520 (2022). [DOI] [Google Scholar]
- 151.Srimuk P, Su X, Yoon J, Aurbach D & Presser V Charge-transfer materials for electrochemical water desalination, ion separation and the recovery of elements. Nature Reviews Materials 5, 517–538, doi: 10.1038/s41578-020-0193-1 (2020). [DOI] [Google Scholar]
- 152.Gallei M & Rüttiger C Recent Trends in Metallopolymer Design: Redox-Controlled Surfaces, Porous Membranes, and Switchable Optical Materials Using Ferrocene-Containing Polymers. Chemistry – A European Journal 24, 10006–10021, doi: 10.1002/chem.201800412 (2018). [DOI] [PubMed] [Google Scholar]
- 153.Alayande AB et al. Recent Progress in One- and Two-Dimensional Nanomaterial-Based Electro-Responsive Membranes: Versatile and Smart Applications from Fouling Mitigation to Tuning Mass Transport. Membranes 11, 5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hu L et al. Mechanical response of surface wettability of Janus porous membrane and its application in oil–water separation. Nanotechnology 33, 245704–245704, doi: 10.1088/1361-6528/ac5ca7 (2022). [DOI] [PubMed] [Google Scholar]
- 155.Zhao Y, Gu Y & Gao G Piezoelectricity induced by pulsed hydraulic pressure enables in situ membrane demulsification and oil/water separation. Water Research 215, 118245–118245, doi: 10.1016/j.watres.2022.118245 (2022). [DOI] [PubMed] [Google Scholar]
- 156.Mao H, Qiu M, Chen X, Verweij H & Fan Y Fabrication and in-situ fouling mitigation of a supported carbon nanotube/γ-alumina ultrafiltration membrane. Journal of Membrane Science 550, 26–35, doi: 10.1016/j.memsci.2017.12.050 (2018). [DOI] [Google Scholar]
- 157.Ying Y et al. Pressure-Responsive Two-Dimensional Metal–Organic Framework Composite Membranes for CO2 Separation. Angewandte Chemie International Edition 60, 11318–11325, doi: 10.1002/anie.202017089 (2021). [DOI] [PubMed] [Google Scholar]
- 158.Feng S et al. Fabrication of a Hydrogen-Bonded Organic Framework Membrane through Solution Processing for Pressure-Regulated Gas Separation. Angewandte Chemie International Edition 59, 3840–3845, doi: 10.1002/anie.201914548 (2020). [DOI] [PubMed] [Google Scholar]
- 159.Ji Y et al. Effect of silica-core gold-shell nanoparticles on the kinetics of biohydrogen production and pollutant hydrogenation via organic acid photofermentation over enhanced near-infrared illumination. International Journal of Hydrogen Energy 46, 7821–7835, doi: 10.1016/j.ijhydene.2020.11.257 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Habib MA et al. Palladium-Alloy Membrane Reactors for Fuel Reforming and Hydrogen Production: A Review. Energy & Fuels 35, 5558–5593, doi: 10.1021/acs.energyfuels.0c04352 (2021). [DOI] [Google Scholar]
- 161.Amiri S, Vatanpour V, Mansourpanah Y & Khataee A Recent trends in application of nanoscale zero-valent metals and metal single atoms in membrane processes. Journal of Environmental Chemical Engineering 10, 107457–107457, doi: 10.1016/j.jece.2022.107457 (2022). [DOI] [Google Scholar]
- 162.Leggett RM & Clark MD A world of opportunities with nanopore sequencing. Journal of Experimental Botany 68, 5419–5429, doi: 10.1093/jxb/erx289 (2017). [DOI] [PubMed] [Google Scholar]
- 163.Qi L & Qiao J Design of Switchable Enzyme Carriers Based on Stimuli-Responsive Porous Polymer Membranes for Bioapplications. ACS Applied Bio Materials 4, 4706–4719, doi: 10.1021/acsabm.1c00338 (2021). [DOI] [PubMed] [Google Scholar]
- 164.Cen Y-K, Liu Y-X, Xue Y-P & Zheng Y-G Immobilization of Enzymes in/on Membranes and their Applications. Advanced Synthesis & Catalysis 361, 5500–5515, doi: 10.1002/adsc.201900439 (2019). [DOI] [Google Scholar]
- 165.Aghababaie M, Beheshti M, Bordbar A-K & Razmjou A Novel approaches to immobilize Candida rugosa lipase on nanocomposite membranes prepared by covalent attachment of magnetic nanoparticles on poly acrylonitrile membrane. RSC advances 8, 4561–4570 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kołodziejczak-Radzimska A, Nghiem LD & Jesionowski T Functionalized Materials as a Versatile Platform for Enzyme Immobilization in Wastewater Treatment. Current Pollution Reports 7, 263–276, doi: 10.1007/s40726-021-00193-5 (2021). [DOI] [Google Scholar]
- 167.Zhang Y, Wang H, Liu J, Hou J & Zhang Y Enzyme-embedded metal–organic framework membranes on polymeric substrates for efficient CO2 capture. Journal of Materials Chemistry A 5, 19954–19962, doi: 10.1039/C7TA03719H (2017). [DOI] [Google Scholar]
- 168.Ren Z, Luo J & Wan Y Highly permeable biocatalytic membrane prepared by 3D modification: Metal-organic frameworks ameliorate its stability for micropollutants removal. Chemical Engineering Journal 348, 389–398, doi: 10.1016/j.cej.2018.04.203 (2018). [DOI] [Google Scholar]
- 169.Kim TH, Lee I, Yeon K-M & Kim J Biocatalytic membrane with acylase stabilized on intact carbon nanotubes for effective antifouling via quorum quenching. Journal of Membrane Science 554, 357–365, doi: 10.1016/j.memsci.2018.03.020 (2018). [DOI] [Google Scholar]
- 170.Deng L et al. Recent advances in attached growth membrane bioreactor systems for wastewater treatment. Science of The Total Environment 808, 152123–152123, doi: 10.1016/j.scitotenv.2021.152123 (2022). [DOI] [PubMed] [Google Scholar]
- 171.Qin L, Zhang Y, Xu Z & Zhang G Advanced membrane bioreactors systems: New materials and hybrid process design. Bioresource Technology 269, 476–488, doi: 10.1016/j.biortech.2018.08.062 (2018). [DOI] [PubMed] [Google Scholar]
- 172.Pachaiappan R et al. A review on biofiltration techniques: recent advancements in the removal of volatile organic compounds and heavy metals in the treatment of polluted water. Bioengineered 13, 8432–8477, doi: 10.1080/21655979.2022.2050538 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Akkoyunlu B, Daly S & Casey E Membrane bioreactors for the production of value-added products: Recent developments, challenges and perspectives. Bioresource Technology 341, 125793–125793, doi: 10.1016/j.biortech.2021.125793 (2021). [DOI] [PubMed] [Google Scholar]
- 174.Mazzei R et al. Enzyme catalysis coupled with artificial membranes towards process intensification in biorefinery- a review. Bioresource Technology 335, 125248–125248, doi: 10.1016/j.biortech.2021.125248 (2021). [DOI] [PubMed] [Google Scholar]
- 175.Al-Mardeai S et al. Vol. 27 (2022). [Google Scholar]
- 176.Zhu D et al. Ordered Coimmobilization of a Multienzyme Cascade System with a Metal Organic Framework in a Membrane: Reduction of CO2 to Methanol. ACS Applied Materials & Interfaces 11, 33581–33588, doi: 10.1021/acsami.9b09811 (2019). [DOI] [PubMed] [Google Scholar]
- 177.Aslam M, Ahmad R & Kim J Recent developments in biofouling control in membrane bioreactors for domestic wastewater treatment. Separation and Purification Technology 206, 297–315, doi: 10.1016/j.seppur.2018.06.004 (2018). [DOI] [Google Scholar]
- 178.Ismail FH, Marpani F, Othman NH & Nik Him NR Simultaneous separation and biocatalytic conversion of formaldehyde to methanol in enzymatic membrane reactor. Chemical Engineering Communications 208, 636–645, doi: 10.1080/00986445.2019.1705795 (2021). [DOI] [Google Scholar]
- 179.Mulinari J et al. Lipase immobilization on alumina membranes using a traditional and a nature-inspired method for active degradation of oil fouling. Separation and Purification Technology 287, 120527–120527, doi: 10.1016/j.seppur.2022.120527 (2022). [DOI] [Google Scholar]
- 180.Mills R et al. Aerosol capture and coronavirus spike protein deactivation by enzyme functionalized antiviral membranes. Communications Materials 3, 34, doi: 10.1038/s43246-022-00256-0 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wu H et al. MOF-enzyme hybrid nanosystem decorated 3D hollow fiber membranes for in-situ blood separation and biosensing array. Biosensors and Bioelectronics 190, 113413–113413, doi: 10.1016/j.bios.2021.113413 (2021). [DOI] [PubMed] [Google Scholar]
- 182.Zhao H & Ju H Multilayer membranes for glucose biosensing via layer-by-layer assembly of multiwall carbon nanotubes and glucose oxidase. Analytical Biochemistry 350, 138–144, doi: 10.1016/j.ab.2005.11.034 (2006). [DOI] [PubMed] [Google Scholar]
- 183.Raaijmakers MJT et al. Enzymatically Active Ultrathin Pepsin Membranes. Angewandte Chemie International Edition 54, 5910–5914, doi: 10.1002/anie.201411263 (2015). [DOI] [PubMed] [Google Scholar]
- 184.Wang Y et al. Enhanced water flux through graphitic carbon nitride nanosheets membrane by incorporating polyacrylic acid. AIChE Journal 64, 2181–2188, doi: 10.1002/aic.16076 (2018). [DOI] [Google Scholar]
- 185.Cheng L, Liu G, Zhao J & Jin W Two-Dimensional-Material Membranes: Manipulating the Transport Pathway for Molecular Separation. Accounts of Materials Research 2, 114–128, doi: 10.1021/accountsmr.0c00092 (2021). [DOI] [Google Scholar]
- 186.Zhang Y et al. Molecularly soldered covalent organic frameworks for ultrafast precision sieving. Science Advances 7, eabe8706, doi:doi: 10.1126/sciadv.abe8706 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhang J et al. Two dimensional nanomaterial-based separation membranes. Electrophoresis 40, 2029–2040, doi: 10.1002/elps.201800529 (2019). [DOI] [PubMed] [Google Scholar]
- 188.Loske L, Nakagawa K, Yoshioka T & Matsuyama H 2D Nanocomposite Membranes: Water Purification and Fouling Mitigation. Membranes 10, 295, doi: 10.3390/membranes10100295 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.DuChanois RM, Porter CJ, Violet C, Verduzco R & Elimelech M Membrane Materials for Selective Ion Separations at the Water–Energy Nexus. Advanced Materials 33, 2101312, doi: 10.1002/adma.202101312 (2021). [DOI] [PubMed] [Google Scholar]
- 190.Guirguis A et al. Applications of nano-porous graphene materials – critical review on performance and challenges. Materials Horizons 7, 1218–1245, doi: 10.1039/C9MH01570A (2020). [DOI] [Google Scholar]
- 191.Cheng W et al. Graphene Oxide-Functionalized Membranes: The Importance of Nanosheet Surface Exposure for Biofouling Resistance. Environmental Science & Technology 54, 517–526, doi: 10.1021/acs.est.9b05335 (2020). [DOI] [PubMed] [Google Scholar]
- 192.Tiginyanu I, Ursaki V & Popa V in Nanocoatings and Ultra-Thin Films (eds Abdel Salam Hamdy Makhlouf& Ion Tiginyanu) 330–354 (Woodhead Publishing, 2011). [Google Scholar]
- 193.Jia W, Wu B, Sun S & Wu P Interfacially stable MOF nanosheet membrane with tailored nanochannels for ultrafast and thermo-responsive nanofiltration. Nano Research 13, 2973–2978, doi: 10.1007/s12274-020-2959-6 (2020). [DOI] [Google Scholar]
- 194.Wu Y, Ding L, Lu Z, Deng J & Wei Y Two-dimensional MXene membrane for ethanol dehydration. Journal of Membrane Science 590, 117300, doi: 10.1016/j.memsci.2019.117300 (2019). [DOI] [Google Scholar]
- 195.Merchant CA et al. DNA Translocation through Graphene Nanopores. Nano Letters 10, 2915–2921, doi: 10.1021/nl101046t (2010). [DOI] [PubMed] [Google Scholar]
- 196.Celebi K et al. Ultimate permeation across atomically thin porous graphene. Science 344, 289–292, doi: 10.1126/science.1249097 (2014). [DOI] [PubMed] [Google Scholar]
- 197.Bai J, Zhong X, Jiang S, Huang Y & Duan X Graphene nanomesh. Nature Nanotechnology 5, 190–194, doi: 10.1038/nnano.2010.8 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Su Y et al. Impermeable barrier films and protective coatings based on reduced graphene oxide. Nature Communications 5, 4843, doi: 10.1038/ncomms5843 (2014). [DOI] [PubMed] [Google Scholar]
- 199.Fouladivanda M, Karimi-Sabet J, Abbasi F & Moosavian MA Step-by-step improvement of mixed-matrix nanofiber membrane with functionalized graphene oxide for desalination via air-gap membrane distillation. Sep Purif Technol 256, 117809, doi: 10.1016/j.seppur.2020.117809 (2021). [DOI] [Google Scholar]
- 200.Xie Q et al. A Novel Thin-Film Nanocomposite Nanofiltration Membrane by Incorporating 3D Hyperbranched Polymer Functionalized 2D Graphene Oxide. Polymers 10, 1253 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Qian Y et al. Enhanced Ion Sieving of Graphene Oxide Membranes via Surface Amine Functionalization. Journal of the American Chemical Society 143, 5080–5090, doi: 10.1021/jacs.1c00575 (2021). [DOI] [PubMed] [Google Scholar]
- 202.Chen M et al. Graphene oxide functionalized polyvinylidene fluoride nanofibrous membranes for efficient particulate matter removal. Journal of Membrane Science 635, 119463, doi: 10.1016/j.memsci.2021.119463 (2021). [DOI] [Google Scholar]
- 203.Cao R et al. Photo- and Thermosensitive Polymer Membrane with a Tunable Microstructure Doped with Graphene Oxide Nanosheets and Poly(N-isopropylacrylamide) for the Application of Light-Cleaning. ACS Applied Materials & Interfaces 12, 14352–14364, doi: 10.1021/acsami.0c00410 (2020). [DOI] [PubMed] [Google Scholar]
- 204.Wang P et al. NIR-Light- and pH-Responsive Graphene Oxide Hybrid Cyclodextrin-Based Supramolecular Hydrogels. Langmuir 35, 1021–1031, doi: 10.1021/acs.langmuir.8b03689 (2019). [DOI] [PubMed] [Google Scholar]
- 205.Alkhouzaam A, Qiblawey H & Khraisheh M Polydopamine Functionalized Graphene Oxide as Membrane Nanofiller: Spectral and Structural Studies. Membranes 11, 86 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Karimipour H, Shahbazi A & Vatanpour V Fouling decline and retention increase of polyethersulfone membrane by incorporating melamine-based dendrimer amine functionalized graphene oxide nanosheets (GO/MDA). Journal of Environmental Chemical Engineering 9, 104849, doi: 10.1016/j.jece.2020.104849 (2021). [DOI] [Google Scholar]
- 207.Januário EFD et al. Advanced graphene oxide-based membranes as a potential alternative for dyes removal: A review. Science of The Total Environment 789, 147957, doi: 10.1016/j.scitotenv.2021.147957 (2021). [DOI] [PubMed] [Google Scholar]
- 208.Choi Y et al. Carbon nanotube-supported graphene oxide nanoribbon bilayer membrane for high-performance diafiltration. Chemical Engineering Journal 427, 131805, doi: 10.1016/j.cej.2021.131805 (2022). [DOI] [Google Scholar]
- 209.Januário EFD, Vidovix TB, Calsavara MA, Bergamasco R & Vieira AMS Membrane surface functionalization by the deposition of polyvinyl alcohol and graphene oxide for dyes removal and treatment of a simulated wastewater. Chemical Engineering and Processing - Process Intensification 170, 108725, doi: 10.1016/j.cep.2021.108725 (2022). [DOI] [Google Scholar]
- 210.Azizi A, Feijani EA, Ghorbani Z & Tavasoli A Fabrication and characterization of highly efficient three component CuBTC/graphene oxide/PSF membrane for gas separation application. International Journal of Hydrogen Energy 46, 2244–2254, doi: 10.1016/j.ijhydene.2020.10.025 (2021). [DOI] [Google Scholar]
- 211.Sainath K, Modi A & Bellare J CO2/CH4 mixed gas separation using graphene oxide nanosheets embedded hollow fiber membranes: Evaluating effect of filler concentration on performance. Chemical Engineering Journal Advances 5, 100074, doi: 10.1016/j.ceja.2020.100074 (2021). [DOI] [Google Scholar]
- 212.Zhou Y et al. Graphene oxide-modified g-C3N4 nanosheet membranes for efficient hydrogen purification. Chemical Engineering Journal 420, 129574, doi: 10.1016/j.cej.2021.129574 (2021). [DOI] [Google Scholar]
- 213.Velempini T & Pillay K Sulphur functionalized materials for Hg(II) adsorption: A review. Journal of Environmental Chemical Engineering 7, 103350, doi: 10.1016/j.jece.2019.103350 (2019). [DOI] [Google Scholar]
- 214.Gu B et al. Mercury reduction and complexation by natural organic matter in anoxic environments. Proceedings of the National Academy of Sciences 108, 1479–1483, doi:doi: 10.1073/pnas.1008747108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Qu G et al. Thiol-functionalized multi-walled carbon nanotubes for effective removal of Pb(II) from aqueous solutions. Materials Chemistry and Physics 278, 125688, doi: 10.1016/j.matchemphys.2021.125688 (2022). [DOI] [Google Scholar]
- 216.Hezarjaribi M et al. Assessment of Adsorption Capacity of Thiol-functionalized Titanate Nanotubes for Removal of Cu(II) and Ni(II) from Aqueous Solution via Static Adsorption. Iranian (Iranica) Journal of Energy & Environment 12, 1–9, doi: 10.5829/ijee.2021.12.01.01 (2021). [DOI] [Google Scholar]
- 217.Ho YS & McKay G Pseudo-second order model for sorption processes. Process Biochemistry 34, 451–465, doi: 10.1016/S0032-9592(98)00112-5 (1999). [DOI] [Google Scholar]
- 218.Hezarjaribi M et al. New strategy to enhance heavy metal ions removal from synthetic wastewater by mercapto-functionalized hydrous manganese oxide via adsorption and membrane separation. Environmental Science and Pollution Research 28, 51808–51825, doi: 10.1007/s11356-021-14326-2 (2021). [DOI] [PubMed] [Google Scholar]
- 219.Thimmiah BR & Nallathambi G Development of high-performance filter from Agave americana fibre/polyacrylonitrile nanofibre membrane for Cd, Pb (II) and organic contaminants removal from aqueous solution. Journal of Industrial Textiles, doi: 10.1177/15280837221095203 (2022). [DOI] [Google Scholar]
- 220.Islam MS et al. Mercury Removal from Wastewater Using Cysteamine Functionalized Membranes. ACS Omega 5, 22255–22267, doi: 10.1021/acsomega.0c02526 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lee SA, Grinshpun SA & Reponen T Respiratory performance offered by N95 respirators and surgical masks: human subject evaluation with NaCl aerosol representing bacterial and viral particle size range. Ann Occup Hyg 52, 177–185, doi: 10.1093/annhyg/men005 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Hadrup N, Sharma AK & Loeschner K Toxicity of silver ions, metallic silver, and silver nanoparticle materials after in vivo dermal and mucosal surface exposure: A review. Regul Toxicol Pharmacol 98, 257–267, doi: 10.1016/j.yrtph.2018.08.007 (2018). [DOI] [PubMed] [Google Scholar]
- 223.Rubino I et al. Salt coatings functionalize inert membranes into high-performing filters against infectious respiratory diseases. Scientific Reports 10, 13875, doi: 10.1038/s41598-020-70623-9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Li J et al. Facile synthesis and characterization of cross-linked chitosan quaternary ammonium salt membrane for antibacterial coating of piezoelectric sensors. International Journal of Biological Macromolecules 120, 745–752, doi: 10.1016/j.ijbiomac.2018.08.153 (2018). [DOI] [PubMed] [Google Scholar]
- 225.Tanis-Kanbur MB, Peinador RI, Calvo JI, Hernández A & Chew JW Porosimetric membrane characterization techniques: A review. Journal of Membrane Science 619, 118750, doi: 10.1016/j.memsci.2020.118750 (2021). [DOI] [Google Scholar]
- 226.Khulbe KC & Matsuura T in Nanotechnology in Membrane Processes (eds Kailash Chandra Khulbe & Takeshi Matsuura) 89–133 (Springer International Publishing, 2021). [Google Scholar]
- 227.Fürjes P Controlled Focused Ion Beam Milling of Composite Solid State Nanopore Arrays for Molecule Sensing. Micromachines (Basel) 10, 774, doi: 10.3390/mi10110774 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Zhang P et al. Broad ion beam-scanning electron microscopy pore microstructure and multifractal characterization of shale oil reservoir: A case sample from Dongying Sag, Bohai Bay Basin, China. Energy Exploration & Exploitation 38, 613–628, doi: 10.1177/0144598719893126 (2020). [DOI] [Google Scholar]
- 229.Baldridge KC et al. Demonstration of Hollow Fiber Membrane-Based Enclosed Space Air Remediation for Capture of an Aerosolized Synthetic SARS-CoV-2 Mimic and Pseudovirus Particles. ACS ES&T Engineering 2, 251–262, doi: 10.1021/acsestengg.1c00369 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Fu J, Thomas HR & Li C Tortuosity of porous media: Image analysis and physical simulation. Earth-Science Reviews 212, 103439, doi: 10.1016/j.earscirev.2020.103439 (2021). [DOI] [Google Scholar]
- 231.Ashton JR, West JL & Badea CT In vivo small animal micro-CT using nanoparticle contrast agents. Frontiers in Pharmacology 6, doi: 10.3389/fphar.2015.00256 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.McNeil LA et al. Dendritic silver self-assembly in molten-carbonate membranes for efficient carbon dioxide capture. Energy & Environmental Science 13, 1766–1775, doi: 10.1039/C9EE03497H (2020). [DOI] [Google Scholar]
- 233.Bentley MD, Ortiz MC, Ritman EL & Romero JC The use of microcomputed tomography to study microvasculature in small rodents. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 282, R1267–R1279, doi: 10.1152/ajpregu.00560.2001 (2002). [DOI] [PubMed] [Google Scholar]
- 234.Lu C et al. In Situ Characterization of Dehydration during Ion Transport in Polymeric Nanochannels. Journal of the American Chemical Society 143, 14242–14252, doi: 10.1021/jacs.1c05765 (2021). [DOI] [PubMed] [Google Scholar]
- 235.Monteleone M et al. Advanced methods for analysis of mixed gas diffusion in polymeric membranes. Journal of Membrane Science 648, 120356, doi: 10.1016/j.memsci.2022.120356 (2022). [DOI] [Google Scholar]
- 236.Barros KS et al. Investigation of ion-exchange membranes by means of chronopotentiometry: A comprehensive review on this highly informative and multipurpose technique. Advances in Colloid and Interface Science 293, 102439, doi: 10.1016/j.cis.2021.102439 (2021). [DOI] [PubMed] [Google Scholar]


