Abstract
Preclinical evidence suggests that the actions of ovarian steroid hormones and brain-derived neurotrophic factor (BDNF) are highly convergent on brain function. Studies in humanized mice document an interaction between estrus cycle-related changes in estradiol secretion and BDNF Val66Met genotype on measures of hippocampal function and anxiety-like behavior. We believe our multimodal imaging data provide the first demonstration in women that the effects of the BDNF Val/Met polymorphism on hippocampal function are selectively modulated by estradiol. In a 6-month pharmacological hormone manipulation protocol, healthy, regularly menstruating, asymptomatic women completed positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) scans while performing the n-back working memory task during three hormone conditions: ovarian suppression induced by the gonadotropin-releasing hormone agonist, leuprolide acetate; leuprolide plus estradiol; and leuprolide plus progesterone. For each of the three hormone conditions, a discovery data set was obtained with oxygen-15 water regional cerebral blood flow PET in 39 healthy women genotyped for BDNF Val66Met, and a confirmatory data set was obtained with fMRI in 27 women. Our results, in close agreement across the two imaging platforms, demonstrate an ovarian hormone-by-BDNF interaction on working memory-related hippocampal function (PET: F2,37 = 9.11, P = 0.00026 uncorrected, P = 0.05, familywise error corrected with small volume correction; fMRI: F2,25 = 5.43, P = 0.01, uncorrected) that reflects differential hippocampal recruitment in Met carriers but only in the presence of estradiol. These findings have clinical relevance for understanding the neurobiological basis of individual differences in the cognitive and behavioral effects of ovarian steroids in women, and may provide a neurogenetic framework for understanding neuropsychiatric disorders related to reproductive hormones as well as illnesses with sex differences in disease expression and course.
INTRODUCTION
There is mounting evidence that sex steroids play an important role in a number of serious neuropsychiatric disorders, such as depression, anxiety and schizophrenia, which are characterized by sex–related differences in onset, severity and course of disease. There is, thus, growing interest in defining mechanisms by which these hormones affect the genesis or modulation of such illnesses. Moreover, reproductive- and menstrual cycle-related disorders make clear that ovarian steroids have the capacity to induce changes in affective and cognitive states in some women, but not in others.1 Several contextual factors have been identified that contribute to the individual variability in the impact of ovarian hormones on brain function, including age, environmental influences (for example, exposure to early life stress) and variations in ovarian steroid-regulated genes.1 However, very few studies have tested for potential interaction between genotype and hormonal state in the human brain.2
Variation in the gene coding for brain-derived neurotrophic factor (BDNF) is a particularly promising candidate that could mediate effects of ovarian steroids on central nervous system function.3 Preclinical evidence suggests that the actions of ovarian steroid hormones and BDNF on the brain are highly convergent. Both exert a wide range of neuromodulatory and neuroprotective effects including neural differentiation,4,5 synaptic plasticity6,7 and dendritic arborization.8,9 In addition, both play critical roles in prefrontal (PFC)10,11 and in hippocampal processes including activity-dependent synaptic plasticity involved in learning and memory.12-14 The BDNF gene is most abundantly expressed in the medial temporal lobe, specifically in the hippocampus, as well as in the PFC.15 Moreover, BDNF tyrosine kinase receptors (TrkB) and steroid hormone receptors are co-localized in both the hippocampus and PFC,16 indicating a potential for the physiologically relevant coupling of their individual functions. Finally, in animals, ovarian hormones affect the expression of both BDNF and TrkB,16,17 and the BDNF gene contains a putative estrogen response element.18 Thus, while interactions between ovarian steroid hormones and BDNF are well documented in animal studies, and while effects in the PFC and hippocampus appear particularly relevant in humans, the impact of this interaction on neurophysiologic systems underpinning behavior in women is less well characterized.
A uniquely human, functional single-nucleotide polymorphism (SNP) in the BDNF gene provides an opportunity to examine the effects of variations in BDNF function on the neuroregulatory actions of ovarian steroids. The BDNF Val66Met SNP (rs6265) results in the substitution of methionine (Met) for valine (Val) in the 5′ pro-region of the BDNF protein in 20–30% of Caucasians,19 and this variant affects intracellular trafficking and secretion of BDNF12,20 in addition to long-term changes in hippocampal synapses.12,13,20 Neuroimaging studies in humans document (1) altered hippocampal recruitment in BDNF Met carriers during both working12 and episodic memory,21 (2) an altered relationship between resting regional cerebral blood flow (rCBF) and anxious temperament22 and (3) sex-dependent changes in resting rCBF and resting-state functional connectivity.11 Finally, studies using humanized BDNF Met knock-in mice showed that this BDNF allelic variation interacts with ovarian steroids to affect cognitive and behavioral functions.23,24
To characterize the effects of ovarian steroid hormones and BDNF genotype on brain circuits underlying PFC- and hippocampal-dependent processes, we used oxygen-15 water rCBF positron emission tomography (PET) in our discovery data set and functional magnetic resonance imaging (fMRI) in our confirmatory data set to study healthy women who participated in a 6-month long hormone manipulation protocol (that is, pharmacologically induced hypogonadism and standardized physiologic ovarian steroid replacement) in which three sets of scans were performed in each of three separate hormone conditions in every woman. PET was considered the discovery data set because, to the best of our knowledge, our study was first initiated with this gold-standard method, while fMRI measurements were begun later. The choice of a working memory task was based not only on the fundamental and well documented role of the PFC in this cognitive function,25-27 but also on the importance of the hippocampus in short-term working memory, as now demonstrated in lesion studies.28-30 Importantly, neuroimaging investigations suggest a reciprocal relationship between the PFC and hippocampus, in which activation of dorsolateral prefrontal cortex (DLPFC) is accompanied (at least in healthy participants) by hippocampal deactivation in a working memory load-dependent manner.31 We selected the n-back working memory paradigm because it is widely employed in neuroimaging studies to target our regions of focus, the DLPFC and hippocampus,32-35 and because it is a robust cognitive imaging probe of these regions, even with repeated scan sessions,36-38 as was necessary in this study. We hypothesized that an ovarian hormone-by-BDNF genotype interaction would be observed in the working memory network, specifically in the hippocampus and PFC.
MATERIALS AND METHODS
Subject selection
Healthy, regularly menstruating women aged 18–50 years provided oral and written consent and were paid for participation as per approved NIH IRB procedures (Table 1). All had normal physical exams, structural MRIs and laboratory results including negative pregnancy tests. Absence of current or past psychiatric illness was confirmed by the Structured Clinical Interview for DSM-IV, and daily symptom self-ratings for 2 months prior to the study established the absence of menstrual-related mood and behavioral symptoms. In addition, the Beck Depression Inventory39 confirmed the absence of depressive symptoms in all participants.
Table 1.
Subject demographics
|
PET discovery data set (N = 39) |
fMRI confirmatory data set (N = 27) |
|||||
|---|---|---|---|---|---|---|
|
Val/Val
homozygotes |
Met carriers |
Statistical
significance |
Val/Val
homozygotes |
Met carriers |
Statistical
significance |
|
| n | 29 | 10 | Genotype frequency: χ2 = 0.94; P = 1.00 | 20 | 7 | Genotype frequency: χ2 = 0.66 P = 1.00 |
| Age (years) mean ± s.d. | 33.9 ± 8.2 | 37.6 ± 8.3 | t(37) = 0.25; P = 0.80 | 30.9 ± 7.4 | 34.8 ± 10.9 | t(25) = 0.37; P = 0.87 |
| Race | 20C/8AA/1A | 7C/2AA/1A | Z = 0.06; P = 0.95 | 15C/5AA | 6C/1AA | Z = 0.15; P = 0.88 |
| BMI (kg/m2) mean ± s.d. | 24.61 ± 5.15 | 24.82 ± 4.74 | t(37) = 0.91; P = 0.37 | 23.71 ± 4.65 | 25.59 ± 4.99 | t(25) = 0.38; P = 0.71 |
| Handedness | 24R/5L (82.8%R) | 9R/1L (90%R) | χ2 = 0.30; P = 0.58 | 18R/3L (86.3%R) | 6R/1L (85.7%R) | χ2 = 0.00; P = 1.00 |
Abbreviations: A, Asians; AA, African Americans; BMI, body mass index; C, Caucasians; fMRI, functional magnetic resonance imaging; L, left handed; PET, positron emission tomography; R, right handed. DNA was extracted from peripheral blood, and BDNF Val66Met (rs6265) genotype was determined by TaqMan 5′ custom-designed exonuclease assay (Applied Biosystems, Foster City, CA, USA). To test for occult genetic stratification, participants were also genotyped for a common functional polymorphism in COMT Val158Met (rs4680), and no significant variation in allele frequency was found in the study populations. As is common in studies of genotype–phenotype associations with the BDNF Val66Met SNP, BDNF Val/Met heterozygotes and Met/Met homozygotes were combined into a ‘Met Carrier’ group because of the rarity of Met homozygotes ( < 5% in Caucasian samples19). For both PET and fMRI data sets, there were no significant differences between Val homozygotes and Met carriers in age, racial distribution, BMI and handedness.
BDNF genotyping
DNA was extracted from peripheral blood, and BDNF Val66Met (rs6265) genotype was determined (Table 1 for methods).
Pharmacological hormone manipulation
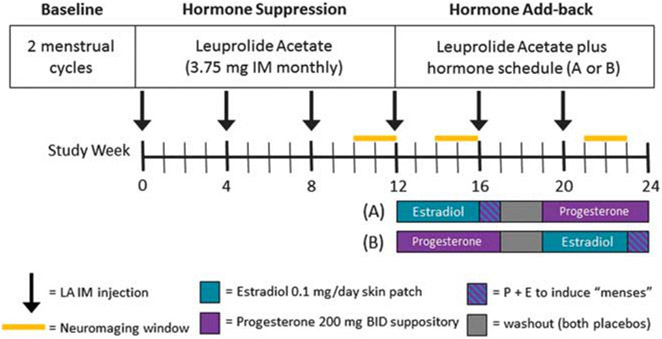
Participants received monthly injections of the GnRH agonist leuprolide acetate (Lupron, TAP Pharmaceuticals, Chicago, IL, USA, 3.75 mg IM), for 6 months to suppress endogenous production of the ovarian steroids, estradiol and progesterone (Figure 1). Following 3 months of Lupron alone, women were randomly assigned to additionally receive transdermal estradiol and progesterone vaginal suppositories separately, each for 5 weeks, after which they were switched to the alternative hormonal replacement in a double-blind, crossover design with a 2-week washout between hormone add-back periods. Plasma estradiol and progesterone levels were measured before each imaging session (Figure 1 and Table 2). Estradiol was assayed by liquid chromatography/mass spectrometry. Plasma estradiol and progesterone levels were confirmed (by clinical assays) to have returned to levels comparable to those during the Lupron alone condition after the 2-week washout.
Figure 1.
Schematic diagram of GnRH agonist-induced hypogonadism and gonadal steroid replacement. Following a 2-month baseline evaluation period, women received 3.75 mg of Lupron (leuprolide acetate, purchased from TAP Pharmaceuticals, Chicago, IL, USA) by intramuscular injection every 4 weeks for 6 months. Lupron alone was administered for the first 12 weeks. After the Lupron-alone period, women received, in addition to Lupron, 17β estradiol (0.1 mg per day) by skin patch or progesterone suppositories (200 mg BID) for 5 weeks each. Women then were crossed-over to the alternative treatment (in a double blind, counterbalanced design). During the fifth week of estradiol add-back, progesterone suppositories (200 mg twice daily) were added to provide progesterone withdrawal-induced shedding of the endometrium and menses in order to prevent prolonged exposure of the endometrium to unopposed estrogen. The two replacement regimens were separated by a 2-week washout period. Three PET and three fMRI sessions were acquired: during Lupron alone, estradiol add-back and progesterone add-back periods. fMRI, functional magnetic resonance imaging; PET, positron emission tomography.
Table 2.
Hormone levels, Beck Depression Inventory scores and n-back working memory performance
| Lupron | Progesterone | Estradiol |
ANOVA-R main effect of hormone condition F (P-value) |
ANOVA-R main effect of genotype F (P-value) |
ANOVA-R interaction of hormone by genotype F (P-value) |
||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||
| Val | Met | Val | Met | Val | Met | ||||
| Plasma estradiol (pg ml−1) | |||||||||
| PET | 8.21 (6.88) | 9.13 (6.18) | 7.15 (4.82) | 7.84 (5.91) | 129.63 (75.4) | 140.30 (73.86) | 662.30 (P = 0.002) | 1.55 (P = 0.34) | 0.12 (P = 0.89) |
| fMRI | 10.62 (11.27) | 6.87 (4.94) | 6.62 (3.76) | 8.69 (7.28) | 138.17 (102.04) | 127.87 (63.30) | 541.79 (P = 0.002) | 1.25 (P = 0.38) | 0.07 (P = 0.94) |
| Plasma progesterone (ng ml−1) | |||||||||
| PET | 0.37 (0.16) | 0.39 (0.13) | 13.66 (6.07) | 13.15 (5.97) | 0.39 (0.17) | 0.37 (0.23) | 2640.4 (P < 0.001) | 0.99 (P = 0.43) | 0.05 (P = 0.95) |
| fMRI | 0.38 (0.17) | 0.66 (0.87) | 13.10 (4.26) | 15.77 (8.31) | 0.37 (0.15) | 0.36 (0.19) | 121.44 (P = 0.008) | 1.34 (P = 0.37) | 1.03 (P = 0.36) |
| Beck Depression Inventory | |||||||||
| PET | 0.67 (1.44) | 1.40 (1.84) | 0.89 (1.45) | 0.70 (1.57) | 0.70 (1.54) | 0.40 (0.97) | 1.69 (P = 0.19) | 0.09 (P = 0.77) | 2.30 (P = 0.11) |
| fMRI | 0.73 (1.54) | 1.56 (2.07) | 1.19 (2.25) | 1.00 (2.00) | 0.50 (1.27) | 0.56 (1.13) | 1.28 (P = 0.29) | 1.14 (P = 0.30) | 1.01 (P = 0.37) |
| 0-Back performance (% accuracy) | |||||||||
| PET | 98.80 (1.70) | 99.11 (1.39) | 98.05 (3.34) | 99.54 (0.77) | 99.07 (1.05) | 99.24 (1.17) | 0.34 (P = 0.71) | 1.84 (P = 0.18) | 0.75 (P = 0.48) |
| fMRI | 98.80 (2.85) | 95.72 (7.60) | 98.97 (2.07) | 98.01 (2.43) | 99.00 (1.85) | 96.36 (3.96) | 1.34 (P = 0.28) | 4.35 (P = 0.06) | 1.28 (0.30) |
| 2-Back performance (% accuracy) | |||||||||
| PET | 84.51 (12.80) | 79.05 (16.06) | 87.11 (12.52) | 81.31 (15.47) | 85.84 (12.57) | 83.75 (10.23) | 1.47 (P = 0.24) | 1.03 (P = 0.32) | 1.28 (P = 0.29) |
| fMRI | 89.60 (10.70) | 85.38 (14.97) | 92.67 (8.82) | 91.61 (9.84) | 91.48 (9.44) | 89.29 (13.45) | 1.21 (P = 0.32) | 0.44 (P = 0.52) | 0.14 (P = 0.87) |
Abbreviations: fMRI, functional magnetic resonance imaging; PET, positron emission tomography. Blood samples were centrifuged, aliquoted and stored at − 70 °C until time of assay. Plasma levels of progesterone were analyzed by radioimmunoassay (Diagnostic Systems Laboratory, Webster, TX, USA). Intra- and inter-assay coefficients of variation for progesterone were 7.0–7.3% and 8.0–9.2%, respectively. Because plasma levels of estradiol during both the Lupron alone and progesterone add-back conditions were anticipated to be at the lower limits of detectability for standard RIA, estradiol was assayed by liquid chromatography/mass spectrometry.73 For both PET and fMRI data sets, expected significant main effects of hormone condition were observed. Post hoc analyses showed pairwise differences between hormone condition in both plasma estradiol levels (Estradiol versus Lupron, P < 0.01; Estradiol versus Progesterone, P < 0.01; Lupron versus Progesterone, P = NS) and plasma progesterone levels (Estradiol versus Progesterone, P < 0.01; Lupron versus Progesterone, P < 0.01; Estradiol versus Lupron, P = NS). There were no significant main effects of genotype and no genotype-by-hormone interaction effects on either estradiol or progesterone plasma levels. No main effects of genotype or hormone condition, and no significant hormone-by-genotype interactions were observed for the Beck Depression Inventory scores, 0-back working memory accuracy or 2-back working memory accuracy (all post hoc pairwise comparisons P = NS). All values are mean ± s.d. Bold values signify statistical significance.
PET rCBF and fMRI BOLD data acquisition and preprocessing
PET and fMRI were performed during each of the three separate hormonal conditions: after at least 6 weeks of Lupron alone (hypogonadism), after at least 2 weeks of Lupron plus estradiol, and after at least 2 weeks of Lupron plus progesterone. Subjects were instructed to refrain from alcohol, nicotine or caffeine for 4 h prior to scanning, as well as over-the-counter medications that could affect rCBF or blood-oxygenation-level-dependent (BOLD) signal for the preceding 24 h.
The same n-back working memory paradigm was used for both PET rCBF and fMRI BOLD signal measurements. Subjects were shown a series of diamond-shaped number arrays, with one of four numbers highlighted in random sequences with a 2 s inter-trial interval. For the 0-back sensorimotor control task, participants pushed a button corresponding to the number shown at the time of the trial. For the 2-back working memory task, participants pushed a button corresponding to the number displayed two trials previously. To avoid practice effects that could confound interpretations of the imaging data, participants were intensively trained on this task prior to every scanning session. The n-back working memory paradigm reliably affects both DLPFC and hippocampus and is commonly used in neuroimaging. Importantly, it is well documented that in healthy subjects performing this task, DLPFC is activated (see review in Owen et al.26), whereas hippocampal regions are ‘deactivated’ (that is, have less neuronal recruitment during working memory than at baseline), possibly reflecting the necessary reliance on short-term, DLPFC memory circuits, rather than hippocampal episodic memory mechanisms for optimal performance of the working memory task.40,41
PET rCBF measurements.
During each hormone condition, fourteen 60-s scans (seven 0-back and seven 2-back scans in alternating order) were independently collected 6-min apart to allow entirely independent analyses of 0-back sensorimotor and 2-back working memory rCBF (a particular advantage of the PET rCBF technique), in addition to activation analyses comparing rCBF during the two tasks. rCBF data were collected with a GE Advance PET scanner (Waukesha, WI, USA) in 3D mode (4.25 mm slice separation, 35 slices, axial field of view 15.3 cm). Each scan was preceded by an intravenous bolus of 10 mCi of oxygen-15 water. Scans were corrected for background counts and attenuation (via a transmission scan) and were reconstructed into 32 axial planes (6.5 mm full-width at half-maximum). With Statistical Parametric Mapping 5 (SPM5; Wellcome Department of Cognitive Neurology), the reconstructed PET data were anatomically normalized to an average template, scaled proportionally to remove variations in global blood flow, and smoothed using a 10 mm Gaussian kernel, and first-level single-subject activation maps (2-back versus 0-back) were calculated for each scan session (one activation/deactivation statistical map per hormone condition for each woman). Because altered activation (2-back versus 0-back) could reflect rCBF changes in either the 0-back control or 2-back working memory conditions or in both, the 2-back and 0-back rCBF maps were also analyzed separately to disambiguate the activation/deactivation genotype-by-hormone findings.
fMRI BOLD signal measurements.
During each hormone condition, two runs of the n-back working memory task were acquired for each subject on a GE 3-Tesla scanner using T2*-weighted gradient-echo planar imaging (36 axial slices, 4 mm thickness, 1 mm gap; repetition time/echo time = 3000/35 ms, field of view = 24 cm, matrix = 64 × 64). Each run consisted of fourteen 30-s blocks, switching between 2-back and 0-back tasks. After preprocessing using SPM5 (slice-timing and motion-correction, coregistration to a standard template, alignment to the first volume for each subject, spatial normalization to the Montreal Neurological Institute T1-weighted template, and, as in the PET data, smoothed with a Gaussian kernel of 10 mm full-width at half-maximum to improve signal-to-noise ratios and to ameliorate differences in inter-subject localization. First-level single-subject activation maps (2-back versus 0-back) were created in similar manner to the PET analysis.
Hormone-by-BDNF genotype analyses of PET and fMRI data
The same analytic design was used in both the PET and fMRI data sets to test for differences in Met carriers compared to Val homozygotes that varied according to hormone condition. A first-level 2-back versus 0-back activation/deactivation map for each hormone condition for each woman was entered as a within-subject repeated measure, and genotype was entered as a between-groups measure in a full-factorial design within SPM5. Because BDNF and ovarian steroids have been shown in animal studies to interactively affect PFC and hippocampal function, these brain areas were chosen a priori as regions of interest, and voxel-wise analyses within these regions were carried out to test the hypothesis that BDNF Val66Met genotype and hormone status interactively effect cognitively related brain function in women. To restrict the findings to these regions of interest (that is, hippocampal region and PFC, specifically DLPFC), a bilateral hippocampal mask (made using the Wake Forest Pick-Atlas tool (Winston-Salem, NC, USA) in SPM) and an independently derived DLPFC mask (as cytoarchitechtonically defined in standard stereotaxic space in postmortem human brain by Rajkowska and Goldman-Rakic42) were applied. For analysis of the PET discovery data set, genotype-by-ovarian hormone interactions in working-memory activation/deactivation (2-back versus 0-back) were evaluated with a voxel-wise statistical threshold of P < 0.001, uncorrected, within our a priori-chosen, region-specific masks and small volume correction for familywise error (FWE) was also applied. For the relatively smaller fMRI cohort, considered to be a confirmatory data set, we accepted a voxel-wise statistical threshold of P < 0.05, uncorrected, and small volume correction for FWE was tested within regions of interest. In both data sets, for post hoc between-genotype, between-hormone decomposition of the gene-by-hormone interaction analyses, we extracted and graphed average activation/deactivation values from a 3 mm diameter sphere surrounding identified foci in the hippocampus. We chose a sphere with a diameter of 3 mm because in some locales the cross-sectional dimensions of the hippocampus are as small as 3 mm.43 In addition to these regionally focused, hypothesis-driven analyses, we performed whole brain voxel-wise gene-by-hormone interaction analyses of both the fMRI and PET data sets to test for unpredicted results outside of the DLPFC and hippocampus using a voxel-wise statistical threshold of P < 0.001, uncorrected. Because in some formulations the hippocampus is considered to be a part of the default-mode network (DMN), we specially examined the data within (1) a ‘task deactive’ (0-back–2-back) mask and (2) a literature-based DMN mask that was derived from 1000 subjects.44
Because altered activation (2-back versus 0-back) could reflect rCBF changes in either the 0-back or 2-back conditions or in both, for PET data, the independently collected 2-back and 0-back rCBF maps were also analyzed separately to disambiguate the activation/deactivation genotype-by-hormone findings. The procedures were identical to the 2-back versus 0-back activation/deactivation analysis except that for each woman, one average first-level 2-back or 0-back rCBF map per hormone condition per woman was entered and separate task-specific (0-back alone and 2-back alone) second-level full-factorial analyses were performed.
Finally, to assess the adequacy of our sample size, we performed a sensitivity power analysis.45 We assumed 80% power and an α of 0.05 using the G*power program.46,47
RESULTS
Participants
For the PET analyses (N = 39), 29 women were Val homozygotes and 10 were Met carriers (mean ages ± s.d. = 33.9 ± 8.2 and 37.6 ± 8.3 years, respectively); for the fMRI analyses (N = 27), there were 20 Val homozygotes and seven Met carriers (ages = 30.9 ± 7.4 and 34.8 ± 10.9 years, respectively). Genotype frequencies were in Hardy–Weinberg equilibrium, and there were no between-genotype differences in age, racial distribution, or handedness (Table 1). In addition, Beck Depression Inventory scores did not differ across genotype groups and remained in the asymptomatic range across all hormone conditions (Table 2). There were no significant differences between the two genotype groups in the variances of all measures (Levene’s test: P’s > 0.1). One woman was a smoker, but neither performance nor imaging results changed when she was excluded from the data.
Plasma estradiol and progesterone levels, and n-back performance
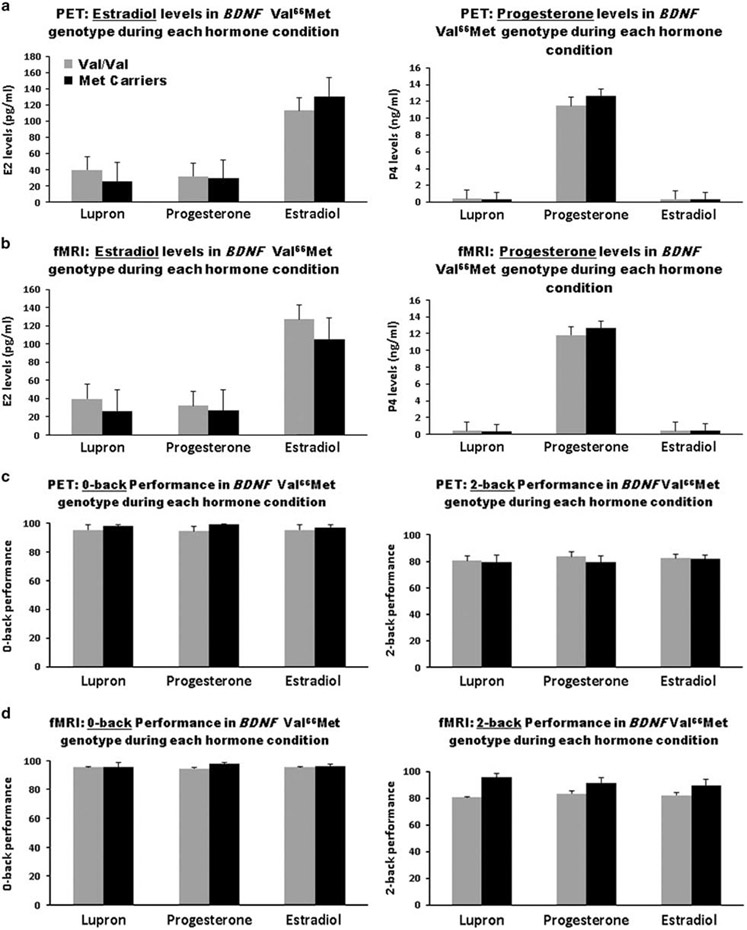
In the PET study, 55% of the Val homozygotes received estradiol add-back first, and 40% of the Met carriers had estradiol add-back first (χ2 = 0.66, P = 0.41) (Figure 2 and Table 2) . In the fMRI study, 55% of the Val homozygotes received estradiol first, and 43% of the Met carriers received estradiol first (χ2 = 0.31, P = 0.58). No effects of add-back order on imaging or performance results were observed. As predicted from the pharmacological manipulation, during hypogonadism (Lupron alone), plasma levels of estradiol and progesterone were suppressed ( < 20 pg ml−1 and < 0.6 ng ml−1, respectively), whereas during estradiol replacement plasma levels of estradiol were in the mid-follicular range and during progesterone replacement plasma levels of progesterone were comparable to those in the mid-luteal phase. Hormone levels did not differ between genotypes during any of the three hormone conditions in PET or fMRI. There were no significant differences in plasma hormone levels between Val homozygotes and Met carriers and no significant genotype-by-plasma hormone level interactions (Figure 2).
Figure 2.
Plasma hormone levels at the time of the PET and fMRI scans and n-back performance scores during scanning during three conditions: hormone suppression (Lupron alone), estradiol add-back, and progesterone add-back (mean ± s.e.m.). Also see Table 2. (a, b) As expected, during hypogonadism (Lupron alone), plasma levels of estradiol and progesterone were suppressed ( < 20 pg ml−1 and < 0.6 ng ml−1, respectively), whereas during estradiol replacement, plasma levels of estradiol were in the mid-follicular phase range, and during progesterone replacement plasma levels of progesterone were comparable to those in the mid-luteal phase. Hormone levels did not differ significantly between genotypes during any of the three hormone conditions in PET or fMRI. (c, d) No significant main or interactive effects of hormone and genotype were observed in either the 0-back or the 2-back performance scores. fMRI, functional magnetic resonance imaging; PET, positron emission tomography.
All participants performed the n-back working memory task well above chance (25%) on all runs in all hormone conditions. As expected in light of the intensive pre-scan training on the task, there were no performance differences between genotype groups or across hormone conditions, and no genotype-by-hormone interactions. Finally, there were no effects of age or education on n-back working memory performance scores.
PET rCBF findings
Activation/deactivation results.
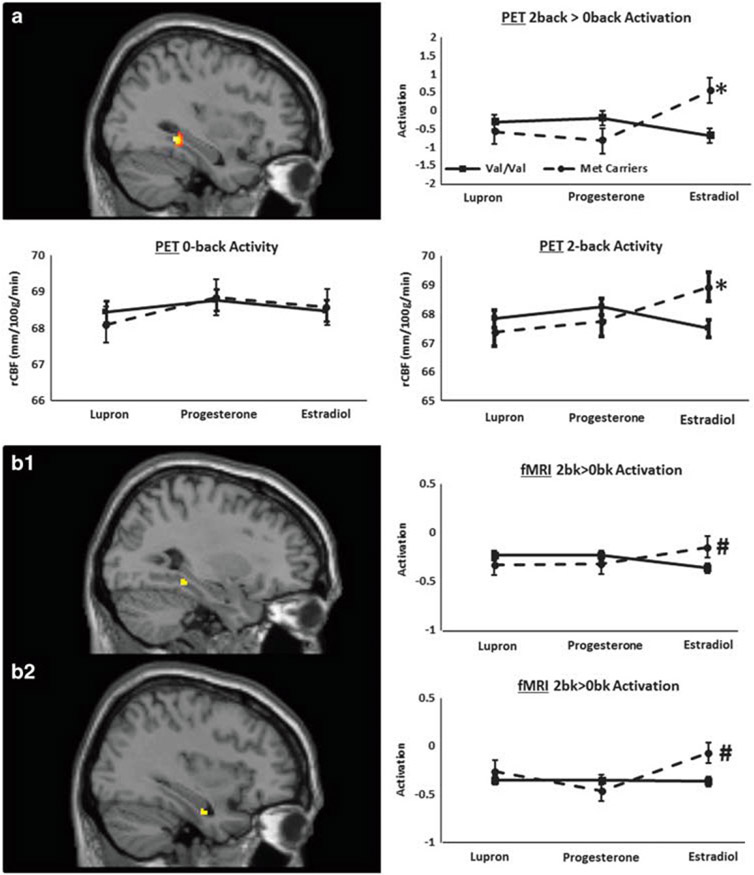
No significant genotype-by-hormone interactions were observed in the PFC (Figure 3a, upper). However, within the hippocampus, a region typically deactivated in the n-back working memory paradigm,26,41 an interaction was observed in the right hemisphere for 2-back versus 0-back activation/deactivation (F2,37 = 9.11, P = 0.00026 uncorrected, P = 0.05 FWE corrected with small volume correction, effect size: partial eta square ; MNI x,y,z coordinates: 24, − 36, − 4). A similar finding was also observed in the left hippocampus (F2,37 = 5.83, P = 0.004 uncorrected; x,y,z = − 34, − 14, − 18) but did not survive correction for multiple comparisons. Sensitivity power analyses of the hippocampal findings indicated that we were sufficiently powered to detect an effect size of 0.20 (actual observed effect size = 0.41). Whole-brain voxel-wise analysis revealed only a single, 10-voxel cluster outside of our a priori regions of interest, in the medial prefrontal cortex (mPFC; F2,37 = 8.44, P = 0.0004, uncorrected; x,y,z = 22,54, − 8). This region was not in the task deactive mask and was formally outside the DMN mask that we investigated44 (although in some formulations mPFC may be included in the DMN).
Figure 3.
Working memory-related right hippocampal rCBF and BOLD activation/deactivation. (a) Top left: statistical parametric map showing differential BDNF-by-hormone interaction on PET rCBF changes in the 2-back versus 0-back activation/deactivation map (F2,37 = 9.11, P = 0.00026 uncorrected, P = 0.05 small volume correction for FWE; MNI x,y,z coordinates: 24, − 36, − 4). Top right: post hoc analyses demonstrated that for Val homozygous women there was no significant change in activation across hormone conditions (F2,27 = 1.58, P = 0.21), whereas Met carriers showed hormone-specific changes (F2,8 = 4.57, P = 0.02): activated (not deactivated) hippocampal function during estradiol add-back compared to Lupron alone (t9 = 3.01, P = 0.01) and to the progesterone replacement phase (t9 = 3.5, P = 0.007). Between genotype comparison revealed that, compared to Val homozygotes, Met carriers showed atypically elevated right hippocampal activation (t38 = 3.16, P = 0.003) during estradiol replacement. (a) Bottom right: the hippocampal rCBF values during the 2-back working memory condition analyzed entirely independently showed a significant genotype-by-hormone interaction with a between-group and across-hormone pattern remarkably similar to that seen in the 2-back versus 0-back activation/deactivation analysis in an almost identical hippocampal locale (F2,37 = 7.86, P = 0.0006 uncorrected; x,y,z = 26, − 40, − 4). Bottom left: in contrast, no BDNF-by-hormone interaction was observed when the 0-back control condition was analyzed alone (P>0.2, NS). (b1, b2) Statistical parametric maps and graphs showing differential BDNF-by-hormone interaction on fMRI BOLD changes in 2-back versus 0-back activation/deactivation maps in two foci in the right hippocampal region (x,y,z = 30, − 38, − 12 and x,y,z = 35, − 10, − 27) within which interactions were observed (F2,25 = 5.43, P = 0.01 uncorrected and F2,25 = 5.11, P = 0.02 uncorrected, respectively). The patterns of interaction in both loci were remarkably similar to that observed in the PET rCBF data (Figure 3a). BOLD, blood-oxygenation-level-dependent; fMRI, functional magnetic resonance imaging; FWE, familywise error; PET, positron emission tomography; rCBF, regional cerebral blood flow.
Post hoc activation/deactivation analyses.
Post hoc analyses of the right hippocampal cluster that was identified in the interaction analyses revealed that for Val homozygous women there was no significant change in activation across hormone conditions (F2,27 = 1.58, P = 0.21), whereas Met carriers showed hormone-specific changes (F2,8 = 4.57, P = 0.02). In Met carriers the hippocampus was activated (not deactivated) during estradiol add-back, and this finding differed from that in the hypogonadal (Lupron alone) state (t9 = 3.01, P = 0.01) and the progesterone replacement phase (t9 = 3.5, P = 0.007), with no difference between Lupron alone and progesterone add-back (t9 = 0.51, P = 0.62). Finally, there was a significant effect of genotype during estradiol replacement, with Met carriers having elevated activation compared to Val homozygotes (t38 = 3.16, P = 0.003), but there were no genotype effects during Lupron alone or progesterone add-back (Figure 3). Post hoc analysis of the mPFC findings, which were neither predicted nor replicated in the fMRI data, and were not in the task deactive mask, showed a between-genotype difference during estradiol add-back that was different in direction than that in the hippocampus. In contrast to the hippocampus, in the mPFC activation was greater in Val homozygotes than in Met carriers (P < 0.01, uncorrected).
Within-task rCBF results.
Analyses exploring the BDNF-by-hormone effects during 0-back and 2-back separately made possible by the task-specific, independent PET measurements, indicated that the genotype-by-hormone interaction in the activation data was due to neural activity during the working memory condition and not during the sensorimotor control task (Figure 3a, lower). Specifically, the PET hippocampal rCBF changes in the 2-back working memory condition analyzed entirely independently showed a significant genotype-by-hormone interaction with a between-group and across-hormone pattern remarkably similar to that seen in the 2-back versus 0-back activation/deactivation analysis in an almost identical hippocampal locale (F2,37 = 7.86, P = 0.0006 uncorrected; x,y,z = 26, − 40, − 4). In contrast, no BDNF-by-hormone interaction was observed when the 0-back control condition was analyzed alone (P>0.2, NS).
fMRI findings
As in the PET discovery data set, there were no significant BDNF-by-ovarian hormone interactions in the PFC. However, in the right hippocampal region there were two foci (x,y,z = 30, − 38, − 12 and x,y,z = 35, − 10, − 27; Figure 3b1 and b2) within which interactions were observed (F2,25 = 5.43, P = 0.01 uncorrected, effect size ; and F2,25 = 5.11, P = 0.02 uncorrected, , respectively; not significant with small volume correction for FWE). Moreover, the patterns of interaction in both loci were remarkably similar to that observed in the PET data. Specifically, post hoc analyses of the two right hippocampal clusters that were identified in the interaction analyses showed that in Met carriers, activation was higher than in the Val homozygotes, but only during estradiol add-back (both loci: t25 = 2.13, P = 0.04). There were no between-group activation differences during Lupron alone or progesterone add-back (P’s>0.4). There was no significant change in activation across hormone conditions in the Val homozygotes (P’s>0.2), whereas in the Met carriers a trend-level interaction was observed in one of the right hippocampal loci (P = 0.1, Figure 3b2). In this smaller cohort, there were no left hippocampal findings. Sensitivity power analyses of the hippocampal findings indicated that we were sufficiently powered to detect an effect size of 0.25 (actual observed effect sizes = 0.24 and 0.31 for the two hippocampal loci). Whole-brain analysis of this smaller data set revealed only one cluster in BA19 (F2,25 = 9.88, P = 0.0002, uncorrected; x,y,z = 18, − 56, − 4). Post hoc analyses showed nominal (P = 0.05) and opposite-in-direction, between-genotype differences during estradiol and progesterone add-back conditions. This finding, like the mPFC result, was neither predicted, nor replicated in the alternative data set (here, PET).
DISCUSSION
By combining a 6-month hormonal manipulation protocol with two different neuroimaging modalities, fMRI and a gold standard PET rCBF method, we demonstrated that hippocampal function in women is differentially modulated by estradiol in a genotypically specific manner, that is, in BDNF Met carriers only. These data extend to humans previous preclinical findings that estradiol and BDNF allelic variations both affect hippocampal physiology, and also delineate interactive effects between these two factors. These results additionally identify biological contributors to individual differences in the cognitive and affective effects of ovarian steroids in women, and thus suggest a new conceptual framework within which to view the pathophysiological mechanism by which variations in genes such as BDNF may be a substrate of risk in women with reproductive-related mood disorders and a modifier in neuropsychiatric illnesses with sexually dimorphic presentation and course.
The n-back working memory paradigm, which has consistently shown PFC activation and hippocampal ‘deactivation’ (that is, less neuronal recruitment during working memory than at baseline),26,40,41 was utilized to examine neurofunctional changes mediated by the interaction between BDNF and ovarian steroids. We observed this expected pattern of hippocampal deactivation during working memory in all three hormonal states in Val homozygotes and in all hormonal states except estradiol add-back in the Met carriers. Importantly, atypical hippocampal activation (not deactivation) during estradiol add-back in Met carriers was identified with PET rCBF; moreover, consistent with these findings, fMRI also demonstrated altered BOLD signal in the hippocampus only in Met carriers and only during estradiol add-back, albeit with the limitation that the findings in this smaller data set did not survive correction for multiple comparisons. The results from these two neuroimaging platforms (direct measurement of rCBF in the PET data, change in BOLD signal in the fMRI data) were highly convergent with regard to selectively identifying atypical activation in the Met carriers during estradiol exposure—convergent in both anatomical distribution and with regard to specificity in participants’ BDNF status and hormone conditions. This close agreement between the rCBF and BOLD signal data (related, but quite distinct neurophysiological parameters of brain function that could be differentially affected by hormone state and/or vascular changes48) lends credence to our results. By showing specific neuromodulatory effects of estradiol in women with the functionally less efficient BDNF Met allele, our data also inform previous observations of differential hippocampal recruitment during working memory for Met carriers compared to Val homozygotes in both men and women.12
Pre-clinical studies offer several mechanistic insights into our data. At the molecular level, estradiol increases BDNF function by binding to a putative estrogen response element on the BDNF gene and/or by inducing the BDNF receptor, TrkB,18 and estradiol and BDNF activate similar signaling cascades and pathways through estrogen receptors and trkB, respectively.49 At the cellular level, both BDNF and estradiol alone facilitate neural activity in the hippocampus5,50 and conjointly influence neural growth, survival, and plasticity in the hippocampus.51,52 In contrast, the BDNF Met protein has been shown to impair intracellular processing and activity-dependent modulation of BDNF in transfected hippocampal neuronal cultures.12,20 Moreover, in a humanized knock-in mouse model (that is, mice made homozygous for the human BDNF Met allele), both wild-type and Met carriers showed a proestrus-related increase in BDNF gene expression (but not TrkB expression).23 However, differential estrous cycle-related changes in the hippocampus also were observed in the quantities of two molecules related to hippocampal neuroplasticity. Met knock-in mice had decreased phosphorylated Akt and decreased PSD-95 expression during proestrus in contrast to proestrus-related increases in both measures in the wild-type mice. Behaviorally, in this mouse model, estrous cycle-related sensitivity was observed in both cognitive and anxiety-like measures.23,24 Compared with wild-type mice, BDNF Met knock-in mice displayed enhanced mnemonic function (that is, object placement) during proestrus (when estradiol levels are high), whereas anxiety-like behaviors (that is, elevated-plus maze, open field) were increased during estrus (when estradiol levels are declining) compared with wild-type mice. Together, these findings in Met knock-in mice suggest that hippocampal BDNF function, and potentially the regulation of hippocampal neuroplasticity, differ from wild-type mice both constitutionally (that is, trait-like) and in an estradiol-dependent manner, and that these differences have behavioral implications. Thus, preclinical studies suggest that trait differences in BDNF/TrkB activity could be regulated by estradiol levels to alter optimal hippocampal function, as we observed here in Met-carrying women.
Our data also suggest a potential explanation for observations of individual differences in the effects of estradiol on hippocampal performance in women. Declines in hippocampal-based memory performance have been reported in some but not all postmenopausal women, as have both beneficial and no effects of estradiol therapy on verbal and visual-spatial memory performances in hypogonadal women.53-63 In addition, estradiol exposure in animal studies alters the preferred neurocircuitry employed to solve hippocampal tasks, with high physiologic levels of estradiol favoring place learning and impairing response learning in a reward-based (that is, food) maze navigation task.64,65 Indeed, altered hippocampal-related task performances in the presence of differing levels of estradiol secretion have been observed in both the humanized Met knock-in mouse23 as well as the BDNF (+/−) heterozygote mouse.66
In women in the present study, we did not observe differences in working memory performance in the Met carriers during estradiol administration. However, we trained our participants to optimal working memory performance prior to each scan session during the three hormonal states in an effort to minimize the potential of performance differences to confound interpretation of the observed impacts of hormone and genotype on neural recruitment. Several previously published behavioral studies demonstrate that both sex and the presence of ovarian steroids (estradiol specifically) influence working memory performance, but that the effect is small67-69 and is likely to be modulated by a number of contextual factors. Our results suggest that genotypic variation is among those contextual factors. Had we not repeatedly trained, or if we had employed a more focused hippocampal task or a more difficult working memory task, altered performance scores could have emerged in the Met carriers during estradiol treatment.
In contrast to results of several preclinical studies that demonstrate the ability of progesterone to regulate BDNF function,70-72 we observed neither effects of progesterone on the pattern of cortical activation during the working memory task, nor genotype-related differences in hippocampal function related to this ovarian steroid. Thus, our data would suggest that the mechanisms (or brain regions) involved in progesterone’s effects on BDNF function are not dysregulated by BDNF allelic variation. It is possible that our relatively small sample size, while sufficient to demonstrate genotype-by-estradiol effects in the hippocampus, may have limited our ability to identify similar progesterone effects, as well as main effects of estradiol or progesterone on working-memory related PFC function as we had demonstrated in a previous study using a PFC task more related to executive function.10 As we were sufficiently powered, our data suggest that the mechanisms (or brain regions) involved in progesterone’s effects are not dysregulated by BDNF allelic variation.
Our work demonstrates that the effects of BDNF genotype on hippocampal function can be modulated by estradiol (either as a part of working memory circuitry or possibly as a component of the DMN). Harboring a BDNF Met allele conveys a robust estradiol-related sensitivity in the hippocampus that may have important clinical implications both for our understanding of individual differences in the effects of estradiol on cognitive performance and for the biological underpinnings of the risk for affective disorders in women. Together these clinical and preclinical findings underscore the physiological relevance of the convergence of estrogen receptor signaling and BDNF system function. Thus, failure to consider gene by hormone interactions may lead to spurious conclusions about main effects of either variable. Finally, our results extend previous findings demonstrating the modulatory effects of both estradiol and BDNF allelic variations on hippocampal physiology, and offer key and clinically relevant translation from an extensive body of animal research.
ACKNOWLEDGMENTS
We thank the National Institutes of Health 2013 Bench-to-Bedside award program (290698), the Positron Emission Tomography Department, and the Functional Magnetic Resonance Imaging Core Facility of the National Institutes of Health for their help and support. This research was supported by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health; NIH protocols: NCT00004571, NCT00001258 and NCT00001322; NIMH project #: ZIAMH002717 and ZIAMH002874.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Schiller CE, Johnson AL, Abate AC, Schmidt PJ, Rubinow DR. Reproductive steroid regulation of mood and behavior. Compr Physiol 2016; 6: 1135–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobs E, D'Esposito M. Estrogen shapes dopamine-dependent cognitive processes: implications for women's health. J Neurosci 2011; 31: 5286–5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Epperson CN, Bale TL. BDNF Val66Met polymorphism and brain-derived neurotrophic factor levels across the female life span: implications for the sex bias in affective disorders. Biol Psychiatry 2012; 72: 434–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pilgrim C, Hutchison JB. Developmental regulation of sex-differences in the brain - can the role of gonadal-steroids be redefined. Neuroscience 1994; 60: 843–855. [DOI] [PubMed] [Google Scholar]
- 5.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 2001; 24: 677–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ninan I, Bath KG, Dagar K, Perez-Castro R, Plummer MR, Lee FS et al. The BDNF Val66Met polymorphism impairs NMDA receptor-dependent synaptic plasticity in the hippocampus. J Neurosci 30: 8866–8870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang F, Je HS, Ji YY, Nagappan G, Hempstead B, Lu B. Pro-BDNF-induced synaptic depression and retraction at developing neuromuscular synapses. J Cell Biol 2009; 185: 727–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji YY, Lu Y, Yang F, Shen WH, Tang TTT, Feng LY et al. Acute and gradual increases in BDNF concentration elicit distinct signaling and functions in neurons. Nat Neurosci 2010; 13: 302–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tyler WJ, Pozzo-Miller L. Miniature synaptic transmission and BDNF modulate dendritic spine growth and form in rat CA1 neurones. J Physiol 2003; 553: 497–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berman KF, Schmidt PJ, Rubinow DR, Danaceau MA, VanHorn JD, Esposito G et al. Modulation of cognition-specific cortical activity by gonadal steroids: a positron-emission tomography study in women. Proc Natl Acad Sci USA 1997; 94: 8836–8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei S-M, Eisenberg DP, Kohn PD, Kippenhan JS, Kolachana BS, Weinberger DR et al. Brain-derived neurotrophic factor Val(66)Met polymorphism affects resting regional cerebral blood flow and functional connectivity differentially in women versus men. J Neurosci 2012; 32: 7074–7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 2003; 112: 257–269. [DOI] [PubMed] [Google Scholar]
- 13.Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem 2003; 10: 86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology 2003; 144: 2836–2844. [DOI] [PubMed] [Google Scholar]
- 15.Murer MG, Yan Q, Raisman-Vozari R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer's disease and Parkinson's disease. Prog Neurobiol 2001; 63: 71–124. [DOI] [PubMed] [Google Scholar]
- 16.Miranda RC, Sohrabji F, Toranallerand CD. Neuronal colocalization of messengerrnas for neurotrophins and their receptors in the developing central-nervous-system suggests a potential for autocrine interactions. Proc Natl Acad Sci USA 1993; 90: 6439–6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibbs RB. Estrogen replacement enhances acquisition of a spatial memory task and reduces deficits associated with hippocampal muscarinic receptor inhibition. Horm Behav 1999; 36: 222–233. [DOI] [PubMed] [Google Scholar]
- 18.Sohrabji F, Miranda RCG, Toranallerand CD. Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proc Natl Acad Sci USA 1995; 92: 11110–11114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimizu E, Hashimoto K, Iyo M. Ethnic difference of the BDNF 196G/A (val66met) polymorphism frequencies: the possibility to explain ethnic mental traits. Am J Med Genet B 2004; 126B: 122–123. [DOI] [PubMed] [Google Scholar]
- 20.Chen ZY, Patel PD, Sant G, Meng CX, Teng KK, Hempstead BL et al. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J Neurosci 2004; 24: 4401–4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF et al. Brain-derived neurotrophic factor val(66)met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci 2003; 23: 6690–6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei S-M, Eisenberg DP, Nabel KG, Kohn PD, Kippenhan JS, Dickinson D et al. Brain-derived neurotrophic factor Val66Met polymorphism affects the relationship between an anxiety-related personality trait and resting regional cerebral blood flow. Cereb Cortex 2016; bhw072; doi: 10.1093/cercor/bhw072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spencer JL, Waters EM, Milner TA, Lee FS, McEwen BS. BDNF variant Val66Met interacts with estrous cycle in the control of hippocampal function. Proc Natl Acad Sci USA 2010; 107: 4395–4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bath KG, Chuang J, Spencer-Segal JL, Amso D, Altemus M, McEwen BS et al. Variant brain-derived neurotrophic factor (Valine66Methionine) polymorphism contributes to developmental and estrous stage-specific expression of anxiety-like behavior in female mice. Biol Psychiatry 2012; 72: 499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage 1997; 5: 49–62. [DOI] [PubMed] [Google Scholar]
- 26.Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging. Hum Brain Mapp 2005; 25: 46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eriksson J, Vogel EK, Lansner A, Bergstrom F, Nyberg L. Neurocognitive architecture of working memory. Neuron 2015; 88: 33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olson IR, Page K, Moore KS, Chatterjee A, Verfaellie M. Working memory for conjunctions relies on the medial temporal lobe. J Neurosci 2006; 26: 4596–4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartley T, Bird CM, Chan D, Cipolotti L, Husain M, Vargha-Khadem F et al. The hippocampus is required for short-term topographical memory in humans. Hippocampus 2007; 17: 34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ezzyat Y, Olson I. The medial temporal lobe and visual working memory: comparisons across tasks, delays, and visual similarity. Cogn Affect Behav Neurosci 2008; 8: 32–40. [DOI] [PubMed] [Google Scholar]
- 31.Axmacher N, Mormann F, Fernandez G, Cohen MX, Elger CE, Fell J. Sustained neural activity patterns during working memory in the human medial temporal lobe. J Neurosci 2007; 27: 7807–7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bahner F, Demanuele C, Schweiger J, Gerchen MF, Zamoscik V, Ueltzhoffer K et al. Hippocampal-dorsolateral prefrontal coupling as a species-conserved cognitive mechanism: a Human Translational Imaging Study. Neuropsychopharmacology 2015; 40: 1674–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cousijn H, Rijpkema M, Qin SZ, van Wingen GA, Fernandez G. Phasic deactivation of the medial temporal lobe enables working memory processing under stress. Neuroimage 2012; 59: 1161–1167. [DOI] [PubMed] [Google Scholar]
- 34.Finn AS, Sheridan MA, Kam CLH, Hinshaw S, D'Esposito M. Longitudinal evidence for functional specialization of the neural circuit supporting working memory in the human brain. J Neurosci 2010; 30: 11062–11067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dixson L, Walter H, Schneider M, Erk S, Schafer A, Haddad L et al. Identification of gene ontologies linked to prefrontal-hippocampal functional coupling in the human brain. Proc Natl Acad Sci USA 2014; 111: 9657–9662. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.McEvoy LK, Smith ME, Gevins A. Dynamic cortical networks of verbal and spatial working memory: effects of memory load and task practice. Cereb Cortex 1998; 8: 563–574. [DOI] [PubMed] [Google Scholar]
- 37.Gradin V, Gountouna VE, Waiter G, Ahearn TS, Brennan D, Condon B et al. Between- and within-scanner variability in the CaliBrain study n-back cognitive task. Psychiatry Res Neuroimaging 2010; 184: 86–95. [DOI] [PubMed] [Google Scholar]
- 38.Baller EB, Wei SM, Kohn PD, Rubinow DR, Alarcon G, Schmidt PJ et al. Abnormalities of dorsolateral prefrontal function in women with premenstrual dysphoric disorder: a Multimodal Neuroimaging Study. Am J Psychiatry 2013; 170: 305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beck AT, Erbaugh J, Ward CH, Mock J, Mendelsohn M. An inventory for measuring depression. Arch Gen Psychiatry 1961; 4: 561–571. [DOI] [PubMed] [Google Scholar]
- 40.Axmacher N, Elger CE, Fell J. Working memory-related hippocampal deactivation interferes with long-term memory formation. J Neurosci 2009; 29: 1052–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyer-Lindenberg A, Poline JB, Kohn PD, Holt JL, Egan MF, Weinberger DR et al. Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. Am J Psychiatry 2001; 158: 1809–1817. [DOI] [PubMed] [Google Scholar]
- 42.Rajkowska G, Goldmanrakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex.2. Variability in locations of areas 9 and 46 and relationship to the Talairach Coordinate System. Cereb Cortex 1995; 5: 323–337. [DOI] [PubMed] [Google Scholar]
- 43.Pluta J, Avants BB, Glynn S, Awate S, Gee JC, Detre JA. Appearance and incomplete label matching for diffeomorphic template based hippocampus segmentation. Hippocampus 2009; 19: 565–571. [DOI] [PubMed] [Google Scholar]
- 44.Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 2011; 106: 1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cohen J. Statistical Power Analysis for the Behavioral Sciences (2nd Edition). Lawrence Erlbaum Associates: Mahwah, NJ, USA, 1988. [Google Scholar]
- 46.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav Res Methods 2009; 41:1149–1160. [DOI] [PubMed] [Google Scholar]
- 47.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007; 39: 175–191. [DOI] [PubMed] [Google Scholar]
- 48.Pike GB. Quantitative functional MRI: concepts, issues and future challenges. Neuroimage 2012; 62: 1234–1240. [DOI] [PubMed] [Google Scholar]
- 49.Scharfman HE, MacLusky NJ. Estrogen and brain-derived neurotrophic factor (BDNF) in hippocampus: complexity of steroid hormone-growth factor interactions in the adult CNS. Front Neuroendocrinol 2006; 27: 415–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol 2008; 29: 219–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Henderson CE. Role of neurotrophic factors in neuronal development. Curr Opin Neurobiol 1996; 6: 64–70. [DOI] [PubMed] [Google Scholar]
- 52.Benraiss A, Chmielnicki E, Lerner K, Roh D, Goldman SA. Adenoviral brain-derived neurotrophic factor induces both neostriatal and olfactory neuronal recruitment from endogenous progenitor cells in the adult forebrain. J Neurosci 2001; 21: 6718–6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Resnick SM, Maki PM, Golski S, Kraut MA, Zonderman AB. Effects of estrogen replacement therapy on PET cerebral blood flow and neuropsychological performance. Horm Behav 1998; 34: 171–182. [DOI] [PubMed] [Google Scholar]
- 54.Maki PM, Resnick SM. Longitudinal effects of estrogen replacement therapy on PET cerebral blood flow and cognition. Neurobiol Aging 2000; 21: 373–383. [DOI] [PubMed] [Google Scholar]
- 55.Mulnard RI, Cotman CW, Kawas C, van Dyck CH, Sano H, Doody R et al. Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease - a randomized controlled trial. JAMA 2000; 283: 1007–1015. [DOI] [PubMed] [Google Scholar]
- 56.Hogervorst E, Yaffe K, Richards M, Huppert F. Hormone replacement therapy for cognitive function in postmenopausal women (Review). Cochrane Database Syst Rev 2002; CD003122. [DOI] [PubMed] [Google Scholar]
- 57.Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE et al. Effect of estrogen plus progestin on global cognitive function in postmenopausal women - The Women's Health Initiative Memory Study: a randomized controlled trial. JAMA 2003; 289: 2663–2672. [DOI] [PubMed] [Google Scholar]
- 58.Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB et al. Conjugated equine estrogens and global cognitive function in postmenopausal women - Women's Health Initiative Memory Study. JAMA 2004; 291: 2959–2968. [DOI] [PubMed] [Google Scholar]
- 59.Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women - Women's Health Initiative Memory Study. JAMA 2004; 291: 2947–2958. [DOI] [PubMed] [Google Scholar]
- 60.Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women - The Women's Health Initiative Memory Study: a randomized controlled trial. JAMA 2003; 289: 2651–2662. [DOI] [PubMed] [Google Scholar]
- 61.Low LF, Anstey KJ. Hormone replacement therapy and cognitive performance in postmenopausal women - a review by cognitive domain. Neurosci Biobehav Rev 2006; 30: 66–84. [DOI] [PubMed] [Google Scholar]
- 62.Yaffe K, Vittinghoff E, Ensrud KE, Johnson KC, Diem S, Hanes V et al. Effects of ultra-low-dose transdermal estradiol on cognition and health-related quality of life. Arch Neurol 2006; 63: 945–950. [DOI] [PubMed] [Google Scholar]
- 63.Lethaby A, Hogervorst E, Richards M, Yesufu A, Yaffe K. Hormone replacement therapy for cognitive function in postmenopausal women. Cochrane Database Syst Rev 2008; CD003122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Korol DL, Kolo LL. Estrogen-induced changes in place and response learning in young adult female rats. Behav Neurosci 2002; 116: 411–420. [DOI] [PubMed] [Google Scholar]
- 65.Zurkovsky L, Brown SL, Boyd SE, Fell JA, Korol DL. Estrogen modulates learning in female rats by acting directly at distinct memory systems. Neuroscience 2007; 144: 26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu YWC, Du X, van den Buuse M, Hill RA. Analyzing the influence of BDNF heterozygosity on spatial memory response to 17 beta-estradiol. Transl Psychiatry 2015; 5: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grigorova M, Sherwin BB. No differences in performance on test of working memory and executive functioning between healthy elderly postmenopausal women using or not using hormone therapy. Climacteric 2006; 9: 181–194. [DOI] [PubMed] [Google Scholar]
- 68.Owens JF, Matthews KA, Everson SA. Cognitive function effects of suppressing ovarian hormones in young women. Menopause 2002; 9: 227–235. [DOI] [PubMed] [Google Scholar]
- 69.Hampson E, Morley EE. Estradiol concentrations and working memory performance in women of reproductive age. Psychoneuroendocrinology 2013; 38: 2897–2904. [DOI] [PubMed] [Google Scholar]
- 70.Singh M, Meyer EM, Simpkins JW. The effect of ovariectomy and estradiol replacement on brain-derived neurotrophic factor messenger-ribonucleic-acid expression in cortical and hippocampal brain-regions of female Sprague-Dawley rats. Endocrinology 1995; 136: 2320–2324. [DOI] [PubMed] [Google Scholar]
- 71.Gonzalez SL, Labombarda F, Deniselle MCG, Guennoun R, Schumacher M, De Nicola AF. Progesterone up-regulates neuronal brain-derived neurotrophic factor expression in the injured spinal cord. Neuroscience 2004; 125: 605–614. [DOI] [PubMed] [Google Scholar]
- 72.Kaur P, Jodhka PK, Underwood WA, Bowles CA, de Fiebre NC, de Fiebre CM et al. Progesterone increases brain-derived neurotrophic factor expression and protects against glutamate toxicity in a mitogen-activated protein kinase- and phosphoinositide-3 kinase-dependent manner in cerebral cortical explants. J Neurosci Res 2007; 85: 2441–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rosner W, Hankinson SE, Sluss PM, Vesper HW, Wierman ME. Challenges to the measurement of estradiol: an endocrine society position statement. J Clin Endocrinol Metab 2013; 98: 1376–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]