Abstract
Background
Studies in women of European descent showed an inverse association of dietary vitamin A (retinol and carotenoids) intake with breast cancer risks, mainly in premenopausal women.
Objectives
We examined whether higher compared with lower levels of dietary vitamin A are associated with reduced breast cancer risks among Black women by estrogen receptor (ER) and menopausal statuses.
Methods
In this pooled analysis, data were from 3564 breast cancer cases and 11,843 controls (mean ages = 56.4 and 56.3 years, respectively) in the African American Breast Cancer Epidemiology and Risk (AMBER) Consortium. Dietary intake was assessed by FFQs. Multivariable logistic regressions were performed to estimate ORs and 95% CIs for study-specific quintiles of total vitamin A equivalents and individual carotenoids, and a pooled OR was estimated by a random-effect model.
Results
We observed an inverse association of total vitamin A equivalents with ER-positive breast cancer (quintiles 5 compared with 1: pooled OR: 0.82; 95% CI: 0.67–1.00; P-trend = 0.045). The association was seen among premenopausal women (pooled OR: 0.60; 95% CI: 0.43–0.83; P-trend = 0.004), but not among postmenopausal women (pooled OR: 0.99; 95% CI: 0.77–1.28; P-trend = 0.78). Additionally, there were inverse associations of dietary β-carotene (quintiles 5 compared with 1: pooled OR: 0.70; 95% CI: 0.51–0.95; P-trend = 0.08) and lutein (pooled OR: 0.63; 95% CI: 0.45–0.87; P-trend = 0.020) with ER-positive breast cancer among premenopausal women. There was no evidence for an association of total vitamin A equivalents or individual carotenoids with ER-negative breast cancer, regardless of menopausal status.
Conclusions
Our findings on dietary vitamin A and breast cancer risks in Black women are consistent with observations in women of European descent and advance the literature showing an inverse association for ER-positive disease.
Abbreviations: AMBER, African American Breast Cancer Epidemiology and Risk; BWHS, Black Women's Health Study; ER, estrogen receptor; HT, hormone therapy; MEC, Multiethnic Cohort Study; MV, multivitamin supplements; Q, quintile; RAR, retinoic acid receptors; RXR, retinoid X receptor; WCHS, Women's Circle of Health Study.
Introduction
Vitamin A, a group of fat-soluble micronutrients including retinol and carotenoids acquired through dietary sources and supplements, has been shown to have anti–breast cancer properties. Preformed vitamin A (retinol) promotes cell differentiation in human mammary ducts by binding with retinoid X receptor (RXR) and retinoic acid receptors (RAR) with its oxidized form, 9-cis-retinoic acid (1). Also, the inhibition of breast cancer growth by retinoic acid can be dependent on the estrogen receptor (ER) status, because RAR is mediated by ER (2). Additionally, provitamin A carotenoids, such as β-carotene and lutein, may protect against carcinogenesis by intervening with oxidative stress to DNA, lipids, and proteins (3, 4). In the United States, vitamin A intake and vitamin A status differ between racial groups. African-American/Black women have both lower vitamin A intake and status, measured as serum retinol concentrations, compared with White women (5). Individual studies and pooled analyses have shown inverse associations of dietary intake of total vitamin A—that is, retinol plus carotenoids—and carotenoids, particularly β-carotene, with the risk of breast cancer overall and in premenopausal women (6., 7., 8., 9.). In most of these studies, however, the associations for breast cancer subtypes defined by ER status and the inclusion of Black women were unclear. By far, the largest study using dietary intake was a pooled analysis of 18 prospective cohorts reporting an inverse association of carotenoid intake with the ER-negative, but not ER-positive, breast cancer risk (8). In the study, Black women represented only 0.05% (826/33,380) of breast cancer cases (8). The only study included that focused on Black women was the Black Women's Health Study (BWHS). For studies that utilized biomarkers—that is, serum or plasma concentrations of vitamin A—significant inverse associations were observed for serum/plasma α-carotene and β-carotene in most studies, with few additional studies having significance for lutein and total carotenoids (10., 11., 12., 13., 14., 15., 16., 17., 18.). Again, these study populations were mainly conducted among White women. Investigating vitamin A intake in relation to breast cancer subtypes among women of African descent is important because they are at a higher risk of ER-negative breast cancer than White women (19), and low vitamin A intake may play a role in the etiology of ER-negative breast cancer (8).
In this paper, we examined the association of dietary vitamin A intake with breast cancer risks according to tumor ER status in a large consortium of Black women. We hypothesized that vitamin A intake is associated with lower ER-positive and ER-negative breast cancer risks. In addition, previous research has observed the association in premenopausal women but not in postmenopausal women (6, 7, 9, 20). Therefore, we also examined the associations by menopausal status.
Methods
Study population
Data were from 3 studies that were part of the African American Breast Cancer Epidemiology and Risk (AMBER) consortium (21) and collected dietary data: BWHS (22), the Multiethnic Cohort Study (MEC) (23), and the Women's Circle of Health Study (WCHS) (24, 25). Study details for the AMBER consortium have been previously described (21). BWHS is a prospective cohort study of 59,000 Black women from around the United States enrolled by mailed questionnaire starting in 1995, with follow-up questionnaires administered every other year. Cases were identified by self-report and confirmed by medical record review or linkage with state cancer registries. MEC is a prospective cohort study based in Hawaii and Los Angeles, California, consisting of women from 5 different racial-ethnic groups with over 16,000 Black women enrolled from 1993–1996. Cases were identified via linkage to the Los Angeles County Cancer Surveillance Program, the State of California Cancer Registry, and the Hawaii State Cancer Registry. WCHS is a case-control study started in 2002 in New York City hospitals and expanded into 10 counties in New Jersey, with cases identified by rapid case ascertainment by the New Jersey State Cancer Registry. Controls were identified by Random Digit Dialing in both sites, complemented in New Jersey with community-based recruitment (24, 25). WCHS cases and controls included in these analyses were recruited between 2002 and 2012. The MEC and BWHS were sampled as nested case-control studies, with cases and controls frequency matched by 5-year age categories, geographic location, and most recent questionnaire completed (23, 26). Research protocols for each study were approved by the Institutional Review Boards at the respective institutions. All subjects provided informed consent for study enrollment. Under the AMBER Consortium, eligible cases were women with a first diagnosis of invasive breast cancer. Tumor subtypes for ER, progesterone receptor, and Human Epidermal Growth Factor Receptor 2 classifications were based on pathology data from hospital records or cancer registry records. A total of 3564 cases with a known ER status and 11,843 controls with complete dietary intake data were included in this analysis.
Exposure assessment
Dietary intake of retinol and carotenoids, including α-carotene, β-carotene, β-cryptoxanthin, and lutein, was assessed by FFQs. The WCHS FFQ was developed at the Fred Hutchinson Cancer Research Center (27, 28). During home interviews, women reported their usual frequency of intake and portion size (small, medium, or large, with reference to a specified medium portion size for each item) for approximately 125 food and beverages consumed during the 12 months prior to diagnosis for cases and to a comparable reference date for controls. Food nutrient content values were obtained from the Nutrient Database, Minnesota Nutrient Data System for Research (University of Minnesota's Nutrition Coordination Center, Minneapolis). The average daily intakes of these nutrients were computed by multiplying the standard serving frequency of each food or beverage item by its nutrient content of the specified standard portion size and then summing the nutrients for all foods and beverages. In the BWHS, diet was assessed in 1995 and again in 2001 using a modified version of the National Cancer Institute–Block short-form FFQ (29). Data were collected based on the usual frequencies and portion sizes (1995: small, medium, or large, relative to the stated medium portion size; 2001: small, medium, large, or supersize) of foods and beverages consumed during the previous 12 months. The average daily intakes of nutrients were calculated by multiplying the serving size–adjusted frequency of intake for each specific food by its vitamin A content, as determined by DIETSYS software (version 4.01) for the 1995 FFQ and DIETCALC (version 1.4.1; National Cancer Institute) for the 2001 FFQ. The energy-adjusted, de-attenuated Pearson correlation between the estimated intake of β-carotene from the 1995 FFQ and three 24-hour recalls plus a 3-consecutive day diary was 0.60 (30). In the MEC, nutrient intakes were calculated on the basis of responses to the 180 questions included in a FFQ mailed to participants at baseline in 1993–1996 (31, 32). For each food item, the frequency of consumption and usual portion size were indicated, assisted by food photographs printed. The energy-adjusted correlations between three 24-hour recalls and the FFQ were 0.51 for vitamin A and 0.45 for β-carotene among the female Black participants (31). In each study, the dietary total vitamin A equivalent was defined as retinol plus carotenoids, indicated by μg retinol-equivalent (RE) values. The intake value of retinol alone was available in the WCHS only, and thus a risk estimate for retinol alone was not provided.
BWHS participants reported their use of multivitamin supplements (MVs) in the prior year on all follow-up questionnaires. The current data were obtained from the questionnaire implemented in 1999. In the MEC, MV use over the prior year was self-reported in the mailed questionnaire; an MV was defined as any product containing ≥2 vitamins, with or without minerals and with or without herbal or botanical components (32). The WCHS did not collect information on MV use.
Covariates
Detailed methods of data collection for smoking, alcohol use, reproductive factors, hormone use, and body size have been reported elsewhere (33., 34., 35., 36.). Reproductive factors included age at menarche, age at first birth, number of births, and menopausal status (37, 38). In the BWHS and MEC, self-reported anthropometric measurements, including height and weight, were collected at baseline and in follow-up questionnaires, while they were measured during in-person home interviews in the WCHS (36). Current weight and height were used to calculate BMI as kg/m2.
Statistical analyses
Descriptive statistics were generated for each study and for the AMBER consortium. For each study, categorical variables were created to indicate the quintiles (Qs) of dietary total vitamin A equivalents and individual carotenoids based on the distributions among the control participants. Logistic regression models were used to calculate the ORs and 95% CIs for the breast cancer risks associated with dietary intake levels. Two-sided tests and a significance level of 0.05 were used for all tests of statistical significance. Covariates in the regression models were selected based on epidemiologic knowledge of breast cancer risks. Covariates were coded as follows: age (continuous), level of education (<12 years, 12 years, some college, college graduate, or any graduate or professional school), BMI (<25.0, 25.0–29.9, ≥30.0 kg/m2), history of breast cancer in first-degree relatives (yes or no for mother, daughter, or sister), age at menarche (continuous), age at first birth (continuous), menopausal status (premenopausal or postmenopausal), postmenopausal hormone therapy (HT) use (never or ever used estrogen and progesterone as combined therapy), duration of oral contraceptive use (never, 1–9 years, 10 or more years), smoking status (never, former, or current smoker), alcohol use (never or ever used), and total energy intake from FFQs (kcal; continuous). Fat intake was not included in the model to avoid multicollinearity with BMI and total energy intake measures. Because MVs often contained retinol and carotenoids that might affect the hypothesized associations, a subgroup analysis was conducted by evaluating the associations of dietary total vitamin A equivalents and individual carotenoids with breast cancer risks among women who did not use MVs in the BWHS and MEC. In addition, as a sensitivity analysis, a model of mutual adjustment of 4 carotenoids was performed to account for the correlation between carotenoids. Tests for trends were conducted by treating the quintiles as ordinal variables in regression models, with the P value of Wald tests serving as the measure of significance. Lastly, a pooled OR was estimated using a random-effects model to summarize study-specific ORs, weighted by the inverse of variances (39). We chose the random-effect approach because we expected heterogeneity due to different designs and data collection methods between the studies (40). I2 values, which measure the percentage of variability in risk estimates due to between-study heterogeneity rather than chance, and P values were estimated. All analyses were planned, and the results were not adjusted for multiplicity. All analyses were performed in RStudio 1.2.1335.
Results
Table 1 lists selected characteristics of study participants. Among the 3 individual studies, the mean age ranged from 51.2–68.4 years among cases and 49.8–67.8 years for controls, with MEC participants, on average, older than those in the other studies. Obesity (BMI ≥30 kg/m2) was 42.6% among cases and 41.4% among controls in the AMBER consortium. A first-degree family member who previously had a breast cancer diagnosis was reported by 15.9% of cases and 9.8% of controls. Both cases and controls were more likely to be postmenopausal than premenopausal, with the exception of participants in the WCHS. Cases and controls did not differ significantly in MV use and duration of MV use. Two-thirds (68.4%) of women with breast cancer had ER-positive tumors, and one-third (32.6%) had ER-negative disease. Table 2 lists median intake levels by dietary total vitamin A equivalents, retinol, and carotenoids and by study. Participants in the BWHS had a lower median intake level of each carotenoid compared to the other studies.
Table 1.
Selected characteristics of study participants by case and control status in individual studies and the AMBER Consortium
| BWHS | WCHS | MEC | AMBER (total) | |||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls |
| Participants, n | 1984 | 7900 | 772 | 830 | 808 | 3113 | 3564 | 11843 |
| Age, years | 53.4 (10.7) | 52.5 (10.9) | 51.2 (10.3) | 49.8 (9.8) | 68.4 (9.4) | 67.8 (9.3) | 56.4 (12.2) | 56.3 (12.5) |
| Education | ||||||||
| 12 years | 43 (2.2) | 194 (2.5) | 109 (14.1) | 94 (11.3) | 91 (11.3) | 414 (13.3) | 243 (6.8) | 702 (5.9) |
| 12 years | 308 (15.5) | 1205 (15.3) | 233 (30.2) | 213 (25.7) | 200 (24.8) | 904 (29.0) | 741 (20.8) | 2322 (19.6) |
| Some college | 583 (29.4) | 2376 (30.1) | 206 (26.7) | 241 (29.0) | 302 (37.4) | 1149 (36.9) | 1091 (30.6) | 3766 (31.8) |
| College graduate | 449 (22.6) | 1814 (23.0) | 137 (17.7) | 174 (21.0) | 100 (12.4) | 338 (10.9) | 686 (19.2) | 2326 (19.6) |
| Any graduate or professions | 601 (30.3) | 2311 (29.3) | 87 (11.3) | 108 (13.0) | 115 (14.2) | 308 (9.9) | 803 (22.5) | 2727 (23.0) |
| BMI, kg/m2 | ||||||||
| 25.0 | 507 (25.6) | 1942 (24.6) | 152 (19.7) | 172 (20.7) | 191 (23.6) | 761 (24.4) | 850 (23.8) | 2875 (24.3) |
| 25.0–29.9 | 685 (34.5) | 2644 (33.5) | 235 (30.4) | 252 (30.4) | 277 (34.3) | 1173 (37.7) | 1197 (33.6) | 4069 (34.4) |
| ≥30.0 | 792 (39.9) | 3314 (41.9) | 385 (49.9) | 406 (48.9) | 340 (42.1) | 1179 (37.9) | 1517 (42.6) | 4899 (41.4) |
| First-degree family history of breast cancer | ||||||||
| No | 1671 (84.2) | 7157 (90.6) | 657 (85.1) | 731 (88.1) | 671 (83.0) | 2793 (89.7) | 2999 (84.1) | 10681 (90.2) |
| Yes | 313 (15.8) | 743 (9.4) | 115 (14.9) | 99 (11.9) | 137 (17.0) | 320 (10.3) | 565 (15.9) | 1162 (9.8) |
| Menopausal status | ||||||||
| Premenopausal | 788 (45.3) | 3263 (46.1) | 396 (54.4) | 429 (54.1) | 95 (12.2) | 298 (10.0) | 1279 (39.4) | 3990 (36.7) |
| Postmenopausal | 951 (54.7) | 3821 (53.9) | 332 (45.6) | 364 (45.9) | 683 (87.8) | 2695 (90.0) | 1966 (60.6) | 6880 (63.3) |
| Alcohol use | ||||||||
| Ever | 1176 (59.3) | 4835 (61.2) | 254 (32.9) | 289 (34.8) | 319 (39.5) | 1204 (38.7) | 1749 (49.1) | 6328 (53.4) |
| Never | 808 (40.7) | 3065 (38.8) | 518 (67.1) | 541 (65.2) | 489 (60.5) | 1909 (61.3) | 1815 (50.9) | 5515 (46.6) |
| Cigarette use | ||||||||
| Current smoker | 354 (17.8) | 1426 (18.1) | 111 (14.4) | 165 (19.9) | 165 (20.4) | 621 (19.9) | 630 (17.7) | 2212 (18.7) |
| Past smoker | 460 (23.2) | 1897 (24.0) | 177 (22.9) | 179 (21.6) | 290 (35.9) | 1075 (34.5) | 927 (26.0) | 3151 (26.6) |
| Never smoked | 1170 (59.0) | 4577 (57.9) | 484 (62.7) | 486 (58.6) | 353 (43.7) | 1417 (45.5) | 2007 (56.3) | 6480 (54.7) |
| Multivitamin use | ||||||||
| Current use | 977 (57.0) | 4270 (60.7) | — | — | 350 (84.5) | 1328 (82.2) | 1327 (62.4) | 5598 (64.7) |
| Past | 190 (11.1) | 925 (13.2) | — | — | 54 (13.0) | 248 (15.3) | 244 (11.5) | 1173 (13.6) |
| Never | 546 (31.9) | 1833 (26.1) | — | — | 10 (2.5) | 40 (2.5) | 556 (26.1) | 1873 (21.7) |
| Duration of multivitamin use, years | ||||||||
| 2 | 104 (6.6) | 400 (6.3) | — | — | 82 (20.5) | 396 (26.3) | 186 (9.5) | 796 (10.1) |
| 2–4 | 548 (34.9) | 2149 (33.5) | — | — | 97 (24.3) | 372 (24.7) | 645 (32.7) | 2521 (31.8) |
| 5–9 | 493 (31.4) | 2202 (34.3) | — | — | 221 (55.2) | 738 (49.0) | 714 (36.2) | 2940 (37.1) |
| ≥10 | 426 (27.1) | 1662 (25.9) | — | — | 0 | 0 | 426 (21.6) | 1662 (21.0) |
| Tumor ER status | ||||||||
| ER+ | 807 (65.2) | — | 415 (68.9) | — | 479 (74.0) | — | 1701 (68.4) | — |
| ER− | 431 (34.8) | — | 187 (31.1) | — | 168 (26.0) | — | 786 (32.6) | — |
Numbers are n (column %) or means (SD). Abbreviations: AMBER, African American Breast Cancer Epidemiology and Risk; BWHS, Black Women's Health Study; ER−, estrogen receptor negative; ER+, estrogen receptor positive; MEC, Multiethnic Cohort Study; WCHS, Women's Circle of Health Study.
Table 2.
Intake levels of dietary total vitamin A equivalents, carotenoids, and retinol in individual studies and the AMBER Consortium
| Quintiles | ||||||
|---|---|---|---|---|---|---|
| Study/vitamin A species | Total | Quintile 1 (low) | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 (high) |
| BWHS | ||||||
| Total vitamin A equivalents | ||||||
| Cases/Controls, n/N | 1983/7896 | 418/1576 | 381/1580 | 385/1580 | 387/1580 | 412/1580 |
| Intake, μg RE/d | 989 (2.59–4743) | 374 (2.59–532) | 676 (>532–823) | 989 (>823–1173) | 1391 (>1173–1720) | 2305 (>1720–4743) |
| α-Carotene | ||||||
| Cases/Controls, n/N | 1958/7808 | 430/1557 | 385/1563 | 387/1562 | 371/1563 | 385/1563 |
| Intake, μg/d | 396 (0.01–3115) | 88.4 (0.01–160) | 235 (>160–310) | 399 (>310–497) | 694 (>497–944) | 1438 (945–3115) |
| β-Carotene | ||||||
| Cases/Controls, n/N | 1976/7860 | 400/1570 | 382/1572 | 418/1573 | 374/1572 | 402/1573 |
| Intake, μg/d | 2883 (0.72–15,244) | 943 (0.72–1435) | 1882 (>1435–2351) | 2885 (2352–3499) | 4318 (>3499–5410) | 7478 (5413–15,244) |
| β-Cryptoxanthin | ||||||
| Cases/Controls, n/N | 1974/7864 | 392/1574 | 387/1574 | 398/1574 | 406/1571 | 391/1571 |
| Intake, μg/d | 112 (0.01–643) | 28 (0.01–45.4) | 65.1 (45.7–87.1) | 112 (>87.1–142) | 174 (>142–220) | 293 (>220–643) |
| Lutein | ||||||
| Cases/Controls, n/N | 1950/7751 | 376/1547 | 391/1551 | 411/1551 | 397/1551 | 375/1551 |
| Intake, μg/d | 2099 (0.02–10,959) | 745 (0.02–1139) | 1440 (>1139–1757) | 2099 (>1757–2497) | 3041 (>2497–3916) | 5480 (3919–10,959) |
| WCHS | ||||||
| Total vitamin A equivalents | ||||||
| Cases/Controls, n/N | 772/830 | 137/165 | 177/164 | 137/166 | 189/168 | 132/167 |
| Intake, μg RE/d | 1083 (28.4–17,419) | 430 (28.4–615) | 770 (>615–925) | 1077 (>925–1269) | 1560 (1273–1964) | 2754 (>1964–17,419) |
| α-Carotene | ||||||
| Cases/Controls, n/N | 772/830 | 143/166 | 135/166 | 160/165 | 165/166 | 169/167 |
| Intake, μg/d | 477 (5.40–8141) | 79.3 (5.40–144) | 228 (145–315) | 449 (317–600) | 852 (602–1165) | 1740 (1166–8141) |
| β-Carotene | ||||||
| Cases/Controls, n/N | 772/830 | 176/166 | 136/165 | 160/166 | 138/165 | 162/168 |
| Intake, μg/d | 3450 (67.2–37,333) | 1212 (67.2–1870) | 2329 (>1870–2864) | 3464 (2865–4224) | 5136 (4228–6282) | 8420 (6300–37,333) |
| β-Cryptoxanthin | ||||||
| Cases/Controls, n/N | 772/830 | 139/166 | 153/166 | 139/166 | 163/166 | 178/166 |
| Intake, μg/d | 116.5 (0.43–1878) | 26.4 (0.43–45.4) | 66.7 (45.7–88.6) | 112 (88.8–146) | 194 (>146–246) | 347 (247–1878) |
| Lutein | ||||||
| Cases/Controls, n/N | 772/830 | 196/166 | 149/166 | 150/166 | 154/166 | 123/166 |
| Intake, μg/d | 2299 (95.2–28,829) | 902 (95.2–1355) | 1680 (>1355–2002) | 2374 (2003–2834) | 3500 (2835–4385) | 6393 (4393–28,829) |
| Retinol | ||||||
| Cases/Controls, n/N | 772/830 | 142/166 | 141/166 | 160/166 | 172/166 | 157/166 |
| Intake, μg/d | 351 (2.54–15,629) | 108 (2.54–171) | 228 (>171–279) | 341 (281–407) | 521 (408–695) | 1136 (698–15,629) |
| MEC | ||||||
| Total vitamin A equivalents | ||||||
| Cases/Controls, n/N | 808/3113 | 160/622 | 163/622 | 174/622 | 159/623 | 152/624 |
| Intake, μg RE/d | 1204 (40.1–12,291) | 494 (40.1–678) | 846 (>678–1014) | 1207 (>1014–1460) | 1787 (1461–2182) | 2996 (>2182–12,291) |
| α-Carotene | ||||||
| Cases/Controls, n/N | 808/3113 | 156/623 | 161/622 | 163/623 | 164/622 | 164/623 |
| Intake, μg/d | 592 (2.03–13,662) | 163 (2.03–264) | 362 (>264–467) | 590 (>467–755) | 1069 (>755–1430) | 2359 (1432–13,662) |
| β-Carotene | ||||||
| Cases/Controls, n/N | 808/3113 | 149/623 | 182/622 | 143/623 | 161/623 | 173/622 |
| Intake, μg/d | 3882 (25.7–46,833) | 1340 (25.7–1957) | 2504 (1960–3162) | 3881 (3163–4691) | 6062 (4697–7687) | 11,197 (7691–46,833) |
| β-Cryptoxanthin | ||||||
| Cases/Controls, n/N | 808/3112 | 182/622 | 160/622 | 169/623 | 148/622 | 159/623 |
| Intake, μg/d | 140 (0.01–6274) | 19.2 (0.01–39.1) | 68.6 (39.2–97.7) | 142 (97.8–187) | 251 (>187–358) | 687 (359–6274) |
| Lutein | ||||||
| Cases/Controls, n/N | 808/3113 | 157/623 | 173/622 | 167/622 | 161/623 | 150/623 |
| Intake, μg/d | 2776 (32.1–35,383) | 1075 (32.1–1520) | 1907 (1521–2293) | 2786 (2297–3357) | 4102 (3359–5178) | 7332 (5202–35,383) |
| AMBER1 | ||||||
| Total vitamin A equivalents | ||||||
| Cases/Controls, n/N | 3564/11,842 | 716/2367 | 721/2366 | 696/2368 | 735/2369 | 696/2372 |
| Intake, μg RE/d | 1048 (0.0–17,418) | 408 (0.0–678) | 725 (532–1014) | 1048 (822–1460) | 1500 (1173–2181) | 2525 (1720–17,418) |
| α-Carotene | ||||||
| Cases/Controls, n/N | 3564/11,843 | 731/2352 | 681/2351 | 710/2350 | 700/2351 | 742/2439 |
| Intake, μg/d | 444.7 (0.0–13,662) | 101 (0.0–264) | 260 (145–467) | 437 (310–755) | 781 (497–1430) | 1691 (945–13,662) |
| β-Carotene | ||||||
| Cases/Controls, n/N | 3556/11,806 | 725/2363 | 700/2358 | 721/2362 | 673/2360 | 737/2363 |
| Intake, μg/d | 3144 (0.0–46,833 | 1054 (0.0–1957) | 2069 (1435–3162) | 3141 (2352–4691) | 4739 (3499–7687) | 8489 (5413–46,833) |
| β-Cryptoxanthin | ||||||
| Cases/Controls, n/N | 3555/11,813 | 714/2369 | 700/2362 | 696/2363 | 717/2359 | 728/2360 |
| Intake, μg/d | 117 (0.0–6274) | 25.3 (0.0–45.8) | 66 (39.2–97.7) | 116 (87.1–187) | 189 (142–358) | 356 (220–6274) |
| Lutein | ||||||
| Cases/Controls, n/N | 3530/11,699 | 729/2341 | 713/2339 | 728/2339 | 712/2340 | 648/2340 |
| Intake, μg/d | 2266 (0.00–35,383) | 851 (0.00–1520) | 1578 (1139–2293) | 2273 (1757–3357) | 3367 (2497–5178) | 6062 (3919–35,383) |
Values are median (minimum-maximum) unless otherwise indicated. Abbreviations: AMBER, African American Breast Cancer Epidemiology and Risk; BWHS, Black Women's Health Study; MEC, Multiethnic Cohort Study; RE, retinol-equivalent; WCHS, Women's Circle of Health Study.
The intake ranges in AMBER may overlap between quintiles because of study-specific values.
The associations of dietary total vitamin A equivalents and individual carotenoids with ER-positive and ER-negative breast cancer risks are presented in Table 3. Intake of total vitamin A equivalents (μg RE/d) was inversely associated with ER-positive breast cancer (Q5 compared with Q1 pooled OR: 0.82; 95% CI: 0.67–1.00; P-trend = 0.045). In addition, there was an inverse association with dietary lutein intake and the ER-positive breast cancer risk (Q5 compared with Q1 pooled OR: 0.80; 95% CI: 0.66–0.96; P-trend = 0.034). The association remained significant in a model additionally adjusting for the other carotenoids (Q5 compared with Q1 pooled OR: 0.70; 95% CI: 0.54–0.91; P-trend = 0.011; Supplemental Table 1). There was no association of dietary total vitamin A equivalents or individual carotenoids with the ER-negative breast cancer risk.
Table 3.
Associations of dietary total vitamin A equivalents and carotenoids with ER-positive and ER-negative breast cancer risk in AMBER
| Quintiles | ||||||
|---|---|---|---|---|---|---|
| Quintile 1 (low) | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 (high) | P-trend | |
| ER-positive breast cancer vs. controls | ||||||
| Total vitamin A equivalents | ||||||
| Cases/Controls, n/N | 311/2140 | 322/2160 | 317/2176 | 322/2200 | 305/2193 | |
| Pooled OR (95% CI)1 | 1.00 | 0.97 (0.81–1.15) | 0.92 (0.77–1.10) | 0.89 (0.75–1.08) | 0.82 (0.67–1.00) | 0.045 |
| α-Carotene | ||||||
| Cases/Controls, n/N | 306/2134 | 287/2144 | 325/2159 | 320/2181 | 339/2252 | |
| Pooled OR (95% CI) | 1.00 | 0.90 (0.75–1.07) | 1.00 (0.85–1.19) | 0.97 (0.82–1.16) | 0.97 (0.82–1.17) | 0.85 |
| β-Carotene | ||||||
| Cases/Controls, n/N | 314/2146 | 297/2161 | 319/2159 | 310/2178 | 336/2192 | |
| Pooled OR (95% CI) | 1.00 | 0.90 (0.76–1.07) | 0.95 (0.80–1.13) | 0.90 (0.75–1.07) | 0.95 (0.79–1.14) | 0.61 |
| β-Cryptoxanthin | ||||||
| Cases/Controls, n/N | 296/2141 | 320/2173 | 315/2160 | 316/2180 | 329/2191 | |
| Pooled OR (95% CI) | 1.00 | 1.04 (0.87–1.23) | 1.01 (0.85–1.20) | 1.00 (0.84–1.19) | 1.02 (0.85–1.22) | 0.99 |
| Lutein | ||||||
| Cases/Controls, n/N | 307/2132 | 320/2135 | 331/2152 | 327/2147 | 283/2170 | |
| Pooled OR (95% CI) | 1.00 | 0.99 (0.83–1.17) | 1.00 (0.84–1.19) | 0.97 (0.82–1.16) | 0.80 (0.66–0.96) | 0.034 |
| ER-negative breast cancer vs. controls | ||||||
| Total vitamin A equivalents | ||||||
| Cases/Controls, n/N | 131/2140 | 149/2160 | 143/2176 | 150/2200 | 136/2193 | |
| Pooled OR (95% CI) | 1.00 | 1.11 (0.87–1.42) | 1.05 (0.82–1.36) | 1.08 (0.83–1.40) | 0.95 (0.71–1.27) | 0.71 |
| α-Carotene | ||||||
| Cases/Controls, n/N | 141/2134 | 138/2144 | 139/2159 | 156/2181 | 135/2252 | |
| Pooled OR (95% CI) | 1.00 | 0.98 (0.77–1.25) | 0.98 (0.76–1.25) | 1.07 (0.84–1.37) | 0.89 (0.69–1.16) | 0.68 |
| β-Carotene | ||||||
| Cases/Controls, n/N | 137/2146 | 154/2161 | 137/2159 | 131/2178 | 150/2192 | |
| Pooled OR (95% CI) | 1.00 | 1.11 (0.87–1.41) | 0.99 (0.77–1.27) | 0.95 (0.74–1.23) | 1.07 (0.82–1.40) | 0.93 |
| β-Cryptoxanthin | ||||||
| Cases/Controls, n/N | 137/2141 | 136/2173 | 135/2160 | 139/2180 | 159/2191 | |
| Pooled OR (95% CI) | 1.00 | 0.97 (0.76–1.25) | 0.97 (0.76–1.25) | 1.02 (0.79–1.31) | 1.13 (0.88–1.46) | 0.32 |
| Lutein | ||||||
| Cases/Controls, n/N | 142/2132 | 139/2135 | 144/2152 | 135/2147 | 142/2170 | |
| Pooled OR (95% CI) | 1.00 | 0.98 (0.77–1.26) | 1.02 (0.79–1.30) | 0.96 (0.74–1.24) | 1.00 (0.76–1.30) | 0.92 |
AMBER, African American Breast Cancer Epidemiology and Risk; BWHS, Black Women's Health Study; ER, estrogen receptor; MEC, Multiethnic Cohort Study; WCHS, Women's Circle of Health Study.
Adjusted for age, education, BMI, family history of breast cancer, age at menarche, parity, age at first birth, menopausal status, HT use, and duration of oral contraceptive use, smoking status, alcohol use, and total energy intake.
After stratification by menopausal status, we observed an inverse association between dietary total vitamin A equivalents and the ER-positive breast cancer risk among premenopausal women (Q5 compared with Q1 pooled OR: 0.60; 95% CI: 0.43–0.83; P-trend = 0.0036; Figure 1A), although these results appeared primarily driven by data from the WCHS (Q5 compared with Q1 OR: 0.44; 95% CI: 0.23–0.85; P-trend = 0.047). We observed no significant association between higher dietary total vitamin A equivalents and the risk of ER-negative breast cancer by menopausal status (Figure 1B). Also, higher compared with lower dietary β-carotene intake was associated with a lower risk of ER-positive breast cancer among premenopausal women (Q5 compared with Q1 pooled OR: 0.70; 95% CI: 0.51–0.95; P-trend = 0.08; Figure 2A). Lastly, there was an inverse association between dietary lutein and the ER-positive breast cancer risk among premenopausal women (Q5 compared with Q1 pooled OR: 0.63; 95% CI 0.45–0.87; P-trend = 0.022; Figure 3A). The I2 values (15.1% for premenopausal women and 24.4% for all women, both P values > 0.05; Figure 3A) suggested a low degree of non-significant heterogeneity in the association of lutein intake and the ER-positive breast cancer risk between the studies. Similar to the results of dietary total vitamin A equivalents, the WCHS was the only study that showed an association between dietary lutein intake and the ER-positive breast cancer risk among premenopausal women (Q5 compared with Q1 OR: 0.31; 95% CI: 0.16–0.58; P-trend = 0.002; Figure 3A). There was no association of dietary α-carotene or β-cryptoxanthin with breast cancer risks in any of the strata.
Figure 1.
The association of dietary total vitamin A equivalents intake with (A) ER+ and (B) ER− breast cancer risk, overall and by menopausal status, adjusted for age, education, BMI, family history of breast cancer, age at menarche, parity, age at first birth, menopausal status, HT use, and duration of oral contraceptive use, smoking status, alcohol use, and total energy intake. All I2= 0 (P > 0.05). Abbreviations: BWHS, Black Women's Health Study; ER−, estrogen receptor negative; ER+, estrogen receptor positive; HT, hormone therapy; MEC, Multiethnic Cohort Study; Q, quintile; WCHS, Women's Circle of Health Study.
Figure 2.
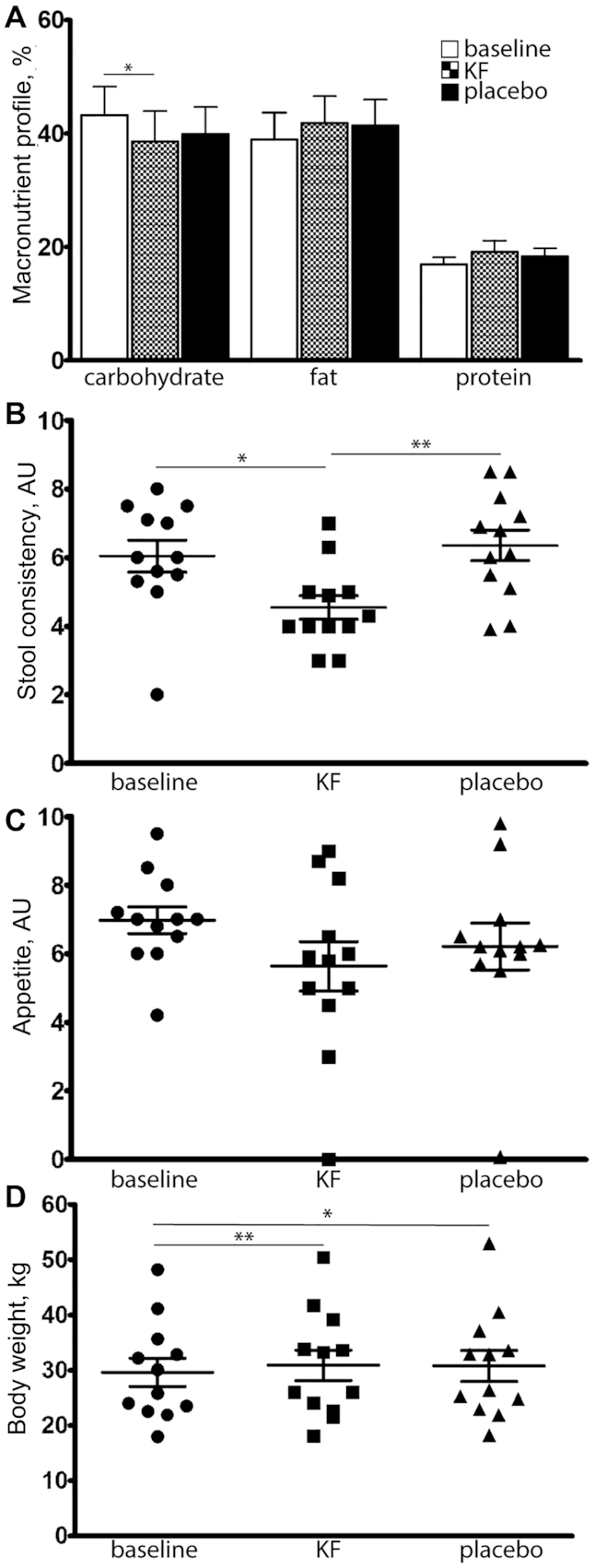
The association of dietary β-carotene intake with (A) ER+ and (B) ER− breast cancer risk, overall and by menopausal status, adjusted for age, education, BMI, family history of breast cancer, age at menarche, parity, age at first birth, menopausal status, HT use, and duration of oral contraceptive use, smoking status, alcohol use, and total energy intake. All I2 = 0 (P > 0.05). Abbreviations: BWHS, Black Women's Health Study; ER−, estrogen receptor negative; ER+, estrogen receptor positive; HT, hormone therapy; MEC, Multiethnic Cohort Study; Q, quintile; WCHS, Women's Circle of Health Study.
Figure 3.
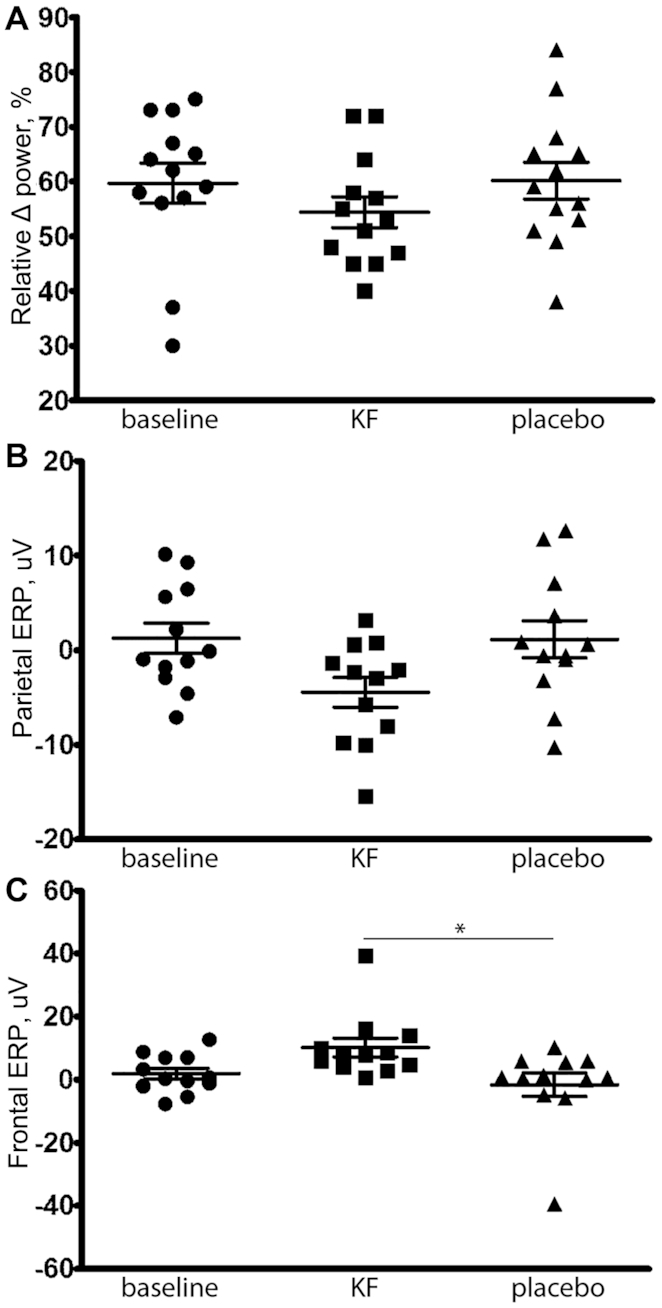
The association of dietary lutein intake with ER+ (A) and ER− (B) breast cancer risk, overall and by menopausal status, adjusted for age, education, BMI, family history of breast cancer, age at menarche, parity, age at first birth, menopausal status, HT use, and duration of oral contraceptive use, smoking status, alcohol use, and total energy intake. I2 = 15.1% for premenopausal ER+ and 24.4% for overall ER+; I2 = 0 for all other estimates (all P > 0.05). Abbreviations: BWHS, Black Women's Health Study; ER−, estrogen receptor negative; ER+, estrogen receptor positive; HT, hormone therapy; MEC, Multiethnic Cohort Study; Q, quintile; WCHS, Women's Circle of Health Study.
We stratified the analysis by those who had ever compared with never used MVs among BWHS and MEC participants who had information on the variable. The patterns of inverse associations between dietary intakes of vitamin A, β-carotene, and lutein and ER-positive breast cancer in premenopausal women remained similar among those who never used an MV. However, there was no significant association in the strata (Supplemental Table 2).
Discussion
Our analysis showed inverse associations of dietary intake of vitamin A in μg RE, β-carotene, and lutein with ER-positive breast cancer risks overall and among premenopausal Black women enrolled in the AMBER consortium. There was no evidence for an association of any carotenoids with the ER-negative breast cancer risk. To our knowledge, this study is the first that focuses on the association of dietary vitamin A intake and breast cancer risks in US Black women. The sample size of our study was large enough to provide risk estimates for ER-positive and ER-negative breast cancer by menopausal status.
Our results are consistent with biological findings regarding the effects of retinoids on breast cancer cells of different ER statuses. 9-Cis retinoic acid modulates retinoid receptor RNAs, which decrease expression of ER RNA and protein (41). Also, retinoids inhibit ER-positive, but not ER-negative, breast cancer cells (42), which may also explain our finding on the ER-positive breast cancer risk. It is notable that ER status is an important but not essential factor for breast cancer cells in response to carotenoid and retinol, because other mechanisms may be involved in tumorigenesis (43). Why the associations are mainly in premenopausal women, but not postmenopausal women, is not completely clear. In vitro evidence shows that dietary carotenoids can attenuate cell proliferation that is promoted by estrogen (44). Because circulating estrogen levels are much higher in premenopausal than postmenopausal women, the potential mechanism may be estrogen-related.
Individual studies and a meta-analysis showed inverse associations between dietary intake of total vitamin A equivalents, with or without accounting for retinol, and breast cancer risks (6, 7, 9, 45). Significant associations were observed in premenopausal women (7, 45) in general and in premenopausal women who had a family history of breast cancer or who were current smokers (6, 9). Our findings on total vitamin A equivalents were largely consistent with these studies and advance the literature by showing an inverse association with ER-positive breast cancer. Only 1 study reported a breast cancer risk by tumor ER status in relation to dietary retinol intake in Hispanic and non-Hispanic White women, and it did not observe an association (46). These studies have also consistently observed that dietary intake of retinol alone was not associated with breast cancer risks (6, 45., 46., 47.). Also, studies examining serum or plasma retinol concentrations in relation to breast cancer risks did not find any association (12, 14, 15, 17, 48, 49). Thus, these findings on retinol suggest that the association of vitamin A with a decreased risk of breast cancer may mainly come from carotenoids, not retinol. Our study was unable to evaluate retinol alone in a pooled analysis, as the variable was unavailable in BWHS or MEC data. In the WCHS, we did not observe a significant association of dietary retinol intake with the ER-positive breast cancer risk in premenopausal women (OR: 0.66; 95% CI: 0.33–1.29; data not shown).
Our results that the highest quintile of dietary β-carotene intake was associated with a 30% lower risk of ER-positive breast cancer among premenopausal women compared with the lowest quintile of intake are consistent with previous findings that utilized dietary intake measurements (6, 7, 13). Among carotenoids, β-carotene is consistently observed to be associated with breast cancer risks, and our study strengthens the evidence by adding data from Black women. A meta-analysis consisting of 25 observational studies examining the associations of 6 carotenoids in the diet and breast cancer risks observed an association only with dietary intake of β-carotene, while the analysis of blood concentrations of the carotenoids found associations with β-carotene, total carotenoids, α-carotene, and lutein (11). A limitation of the meta-analysis was that analyses by tumor ER status or menopausal status were not reported. A large cohort study that stratified data by menopausal status found a modest inverse association of dietary β-carotene intake with breast cancer risks among premenopausal women, and the association was stronger among those with a family history of breast cancer and those who consumed 15 g or more of alcohol per day (6). One pooled analysis of 18 prospective cohort studies showed an inverse association between dietary β-carotene intake and ER-negative breast cancer (8). However, the finding of ER-negative breast cancer was not observed in our study of Black women.
Our results relating to lutein are consistent with previous studies [3 measured dietary lutein intake (6, 7, 17); 2 measured circulating lutein (10, 18); and 2 meta- and pooled analyses of circulating lutein (8, 11)] showing that lutein exposure was inversely associated with breast cancer risks overall or in premenopausal women. Lutein measurements in these studies were combined with zeaxanthin due to the limited ability of nutrient databases or laboratory measurements to discriminate between the 2 carotenoids (8, 50). The evidence for an association between lutein exposure and breast cancer subtypes is limited; a pooled analysis of prospective cohorts reported an inverse association of dietary lutein intake with the ER-negative breast cancer risk (8). It should be noted that in dietary studies, the equations used to convert carotenoid levels to retinol equivalents often do not include lutein (51). Thus, the observed association for dietary lutein in our study should be separately considered from the association for dietary intake of total vitamin A equivalents. In addition, several other studies observed inverse associations of α-carotene and lycopene with breast cancer risks (10, 16, 18), but we did not observe an association for α-carotene in Black women, and lycopene intake data were unavailable.
An examination of vitamin A in breast tissue may be important, but the data are very limited. Zhang et al. (52) observed an inverse association between breast adipose tissue concentrations of β-carotene and lycopene, but not of retinol (retinyl palmitate) and lutein/zeaxanthin, with breast cancer risks. A main limitation of the study was a small sample size (46 cases and 63 controls).
Of importance, the study-specific associations for dietary total vitamin A equivalents and lutein with ER-positive breast cancer risks overall and among premenopausal women were only significant for the WCHS, but not the other 2 studies. The heterogeneity of findings between the studies could be in part due to the differences in study designs. The WCHS was a case-control study in which the dietary data potentially suffered from differential recall between cases and controls, and the BWHS and MEC were prospective cohort studies in which dietary intake was measured before a breast cancer diagnosis. The heterogeneity seemed stronger for the ER-positive overall associations than those among premenopausal women. For ER-positive breast cancer in premenopausal women, all 3 studies showed an inverse association (Figures 1A, 2A, and 3A), suggesting that the significant pooled ORs could also result from the larger sample size in AMBER than individual studies. Other sources of study heterogeneity included temporal and geographic aspects of the studies. The dietary intake data were collected in 1993–1996 in the MEC, 1995 and again in 2001 in the BWHS, and 2002–2012 in the WCHS. Also, the MEC and WCHS recruited participants in relatively geographically restricted areas, while the BWHS recruited participants around the United States. Factors related to vitamin A intake, such as obesity rates, may have changed over time or been different between geographic locations, and thus contributed to the between-study heterogeneity.
Our study had other limitations. First, like other nutritional epidemiologic studies examining associations between diet and chronic diseases, our study has inherent limitations from potential measurement errors related to recall and the inability to evaluate specific nutrients. The present analysis did not use biomarkers for the exposure assessment. Research has suggested that blood concentrations of carotenoids are more accurate measurements of exposure than dietary intake information on carotenoids (11, 13). Second, the lower intake values in the BWHS might reflect less comprehensive estimates of retinol or carotenoid intake from its FFQ. We were unable to calculate total carotenoid values in part due to the different versions of FFQs and nutrient databases and the lack of data on lycopene intake. Third, the generalizability of our study may be limited, indicated by the fact that our study participants had higher educational attainment than US Black women in general (53). Lastly, although we performed planned analyses with a priori hypotheses, the investigation of carotenoids in different strata may have resulted in multiple comparisons, potentially leading to false-positive results.
In conclusion, data from the AMBER Consortium show an inverse association of dietary intake of vitamin A, including carotenoids, with ER-positive breast cancer risks among premenopausal Black women. There is some heterogeneity between the individual studies in AMBER, and the findings may warrant further confirmation.
Acknowledgments
KRB and T-YDC contributed equally to this work.
The authors’ responsibilities were as follows—KRB and T-YDC: designed and conducted the research and wrote the paper; KRB and GZ: analyzed data; EVB, LNK, LR, AFO, JRP, and CBA: provided essential materials; SEM and SY: provided essential comments and edits; T-YDC: had primary responsibility for the final content; and all authors: read and approved the final manuscript.
Footnotes
This work was supported by the National Cancer Institute (grant number P01CA151135 to JRP, CBA, and AFO; R01CA058420 to LR; UM1CA164974 to LR; R01CA098663 to JRP; R01CA100598 to CBA and EVB; P50CA58223 to AFO; K07CA201334 to T-YDC) and the Breast Cancer Research Foundation (to CBA).
Author disclosures: The authors report no conflicts of interest.
Supplementary Tables 1–2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn.
Supplementary Material
References
- 1.van der Leede BJ, Folkers GE, van den Brink CE, van der Saag PT, van der Burg B. Retinoic acid receptor alpha 1 isoform is induced by estradiol and confers retinoic acid sensitivity in human breast cancer cells. Mol Cell Endocrinol. 1995;109:77–86. doi: 10.1016/0303-7207(95)03487-r. [DOI] [PubMed] [Google Scholar]
- 2.Sheikh MS, Shao ZM, Chen JC, Hussain A, Jetten AM, Fontana JA. Estrogen receptor-negative breast cancer cells transfected with the estrogen receptor exhibit increased RAR alpha gene expression and sensitivity to growth inhibition by retinoic acid. J Cell Biochem. 1993;53:394–404. doi: 10.1002/jcb.240530417. [DOI] [PubMed] [Google Scholar]
- 3.Maillard V, Hoinard C, Arab K, Jourdan ML, Bougnoux P, Chajès V. Dietary beta-carotene inhibits mammary carcinogenesis in rats depending on dietary alpha-linolenic acid content. Br J Nutr. 2006;96:18–21. doi: 10.1079/bjn20061781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paiva SA, Russell RM. Beta-carotene and other carotenoids as antioxidants. J Am Coll Nutr. 1999;18:426–433. doi: 10.1080/07315724.1999.10718880. [DOI] [PubMed] [Google Scholar]
- 5.Hanson C, Lyden E, Abresch C, Anderson-Berry A. Serum retinol concentrations, race, and socioeconomic status in of women of childbearing age in the united states. Nutrients. 2016;8:508. doi: 10.3390/nu8080508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang S, Hunter DJ, Forman MR, Rosner BA, Speizer FE, Colditz GA, Manson JE, Hankinson SE, Willett WC. Dietary carotenoids and vitamins A, C, and E and risk of breast cancer. J Natl Cancer Inst. 1999;91:547–556. doi: 10.1093/jnci/91.6.547. [DOI] [PubMed] [Google Scholar]
- 7.Mignone LI, Giovannucci E, Newcomb PA, Titus-Ernstoff L, Trentham-Dietz A, Hampton JM, Willett WC, Egan KM. Dietary carotenoids and the risk of invasive breast cancer. Int J Cancer. 2009;124(12):2929–2937. doi: 10.1002/ijc.24334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X, Spiegelman D, Baglietto L, Bernstein L, Boggs DA, van den Brandt PA, Buring JE, Gapstur SM, Giles GG, Giovannucci E, et al. Carotenoid intakes and risk of breast cancer defined by estrogen receptor and progesterone receptor status: a pooled analysis of 18 prospective cohort studies. Am J Clin Nutr. 2012;95:713–725. doi: 10.3945/ajcn.111.014415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho E, Spiegelman D, Hunter DJ, Chen WY, Zhang SM, Colditz GA, Willett WC. Premenopausal intakes of vitamins A, C, and E, folate, and carotenoids, and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:713–720. [PubMed] [Google Scholar]
- 10.Eliassen AH, Hendrickson SJ, Brinton LA, Buring JE, Campos H, Dai Q, Dorgan JF, Franke AA, Gao YT, Goodman MT, et al. Circulating carotenoids and risk of breast cancer: pooled analysis of eight prospective studies. J Natl Cancer Inst. 2012;104:1905–1916. doi: 10.1093/jnci/djs461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aune D, Chan DS, Vieira AR, Navarro Rosenblatt DA, Vieira R, Greenwood DC, Norat T. Dietary compared with blood concentrations of carotenoids and breast cancer risk: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr. 2012;96:356–373. doi: 10.3945/ajcn.112.034165. [DOI] [PubMed] [Google Scholar]
- 12.Bakker MF, Peeters PH, Klaasen VM, Bueno-de-Mesquita HB, Jansen EH, Ros MM, Travier N, Olsen A, Tjønneland A, Overvad K, et al. Plasma carotenoids, vitamin C, tocopherols, and retinol and the risk of breast cancer in the European Prospective Investigation into Cancer and Nutrition cohort. Am J Clin Nutr. 2016;103:454–464. doi: 10.3945/ajcn.114.101659. [DOI] [PubMed] [Google Scholar]
- 13.Prentice RL, Pettinger M, Neuhouser ML, Tinker LF, Huang Y, Zheng C, Manson JE, Mossavar-Rahmani Y, Anderson GL, Lampe JW. Application of blood concentration biomarkers in nutritional epidemiology: example of carotenoid and tocopherol intake in relation to chronic disease risk. Am J Clin Nutr. 2019;109:1189–1196. doi: 10.1093/ajcn/nqy360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kabat GC, Kim M, Adams-Campbell LL, Caan BJ, Chlebowski RT, Neuhouser ML, Shikany JM, Rohan TE, Investigators W. Longitudinal study of serum carotenoid, retinol, and tocopherol concentrations in relation to breast cancer risk among postmenopausal women. Am J Clin Nutr. 2009;90:162–169. doi: 10.3945/ajcn.2009.27568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sato R, Helzlsouer KJ, Alberg AJ, Hoffman SC, Norkus EP, Comstock GW. Prospective study of carotenoids, tocopherols, and retinoid concentrations and the risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2002;11:451–457. [PubMed] [Google Scholar]
- 16.Eliassen AH, Liao X, Rosner B, Tamimi RM, Tworoger SS, Hankinson SE. Plasma carotenoids and risk of breast cancer over 20 y of follow-up. Am J Clin Nutr. 2015;101:1197–1205. doi: 10.3945/ajcn.114.105080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tamimi RM, Hankinson SE, Campos H, Spiegelman D, Zhang S, Colditz GA, Willett WC, Hunter DJ. Plasma carotenoids, retinol, and tocopherols and risk of breast cancer. Am J Epidemiol. 2005;161:153–160. doi: 10.1093/aje/kwi030. [DOI] [PubMed] [Google Scholar]
- 18.Yan B, Lu MS, Wang L, Mo XF, Luo WP, Du YF, Zhang CX. Specific serum carotenoids are inversely associated with breast cancer risk among Chinese women: a case-control study. Br J Nutr. 2016;115:129–137. doi: 10.1017/S000711451500416X. [DOI] [PubMed] [Google Scholar]
- 19.Kurian AW, Fish K, Shema SJ, Clarke CA. Lifetime risks of specific breast cancer subtypes among women in four racial/ethnic groups. Breast Cancer Res. 2010;12:R99. doi: 10.1186/bcr2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bohlke K, Spiegelman D, Trichopoulou A, Katsouyanni K, Trichopoulos D. Vitamins A, C and E and the risk of breast cancer: results from a case-control study in Greece. Br J Cancer. 1999;79:23–29. doi: 10.1038/sj.bjc.6690006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmer JR, Ambrosone CB, Olshan AF. A collaborative study of the etiology of breast cancer subtypes in African American women: the AMBER consortium. Cancer Causes Control. 2014;25:309–319. doi: 10.1007/s10552-013-0332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenberg L, Rao RS, Palmer JR. A case-control study of acetaminophen use in relation to the risk of first myocardial infarction in men. Pharmacoepidemiol Drug Saf. 2003;12:459–465. doi: 10.1002/pds.867. [DOI] [PubMed] [Google Scholar]
- 23.Kolonel LN, Henderson BE, Hankin JH, Nomura AM, Wilkens LR, Pike MC, Stram DO, Monroe KR, Earle ME, Nagamine FS. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol. 2000;151:346–357. doi: 10.1093/oxfordjournals.aje.a010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ambrosone CB, Ciupak GL, Bandera EV, Jandorf L, Bovbjerg DH, Zirpoli G, Pawlish K, Godbold J, Furberg H, Fatone A, et al. Conducting molecular epidemiological research in the age of HIPAA: a multi-institutional case-control study of breast cancer in African-American and European-American women. J Oncol. 2009;2009:871250. doi: 10.1155/2009/871250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bandera EV, Chandran U, Zirpoli G, McCann SE, Ciupak G, Ambrosone CB. Rethinking sources of representative controls for the conduct of case-control studies in minority populations. BMC Med Res Method. 2013;13:71. doi: 10.1186/1471-2288-13-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenberg L, Adams-Campbell L, Palmer JR. The Black Women's Health Study: A follow-up study for causes and preventions of illness. J Am Med Womens Assoc. 1995;50:56–58. [PubMed] [Google Scholar]
- 27.Gong Z, Ambrosone CB, McCann SE, Zirpoli G, Chandran U, Hong CC, Bovbjerg DH, Jandorf L, Ciupak G, Pawlish K, et al. Associations of dietary folate, vitamins B6 and B12 and methionine intake with risk of breast cancer among African American and European American women. Int J Cancer. 2014;134:1422–1435. doi: 10.1002/ijc.28466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin B, Xu B, Ji N, Yao S, Pawlish K, Llanos AAM, Lin Y, Demissie K, Ambrosone CB, Hong CC, et al. Intake of vitamin D and calcium, sun exposure, and risk of breast cancer subtypes among Black women. Am J Clin Nutr. 2020;111:396–405. doi: 10.1093/ajcn/nqz302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wise LA, Radin RG, Palmer JR, Kumanyika SK, Rosenberg L. A prospective study of dairy intake and risk of uterine leiomyomata. Am J Epidemiol. 2010;171:221–232. doi: 10.1093/aje/kwp355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumanyika SK, Mauger D, Mitchell DC, Phillips B, Smiciklas-Wright H, Palmer JR. Relative validity of food frequency questionnaire nutrient estimates in the Black Women's Health Study. Ann Epidemiol. 2003;13:111–118. doi: 10.1016/s1047-2797(02)00253-3. [DOI] [PubMed] [Google Scholar]
- 31.Stram DO, Hankin JH, Wilkens LR, Pike MC, Monroe KR, Park S, Henderson BE, Nomura AM, Earle ME, Nagamine FS, et al. Calibration of the dietary questionnaire for a multiethnic cohort in Hawaii and Los Angeles. Am J Epidemiol. 2000;151:358–370. doi: 10.1093/oxfordjournals.aje.a010214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy SP, White KK, Park SY, Sharma S. Multivitamin-multimineral supplements' effect on total nutrient intake. Am J Clin Nutr. 2007;85:280S–284S. doi: 10.1093/ajcn/85.1.280S. [DOI] [PubMed] [Google Scholar]
- 33.Park SY, Palmer JR, Rosenberg L, Haiman CA, Bandera EV, Bethea TN, Troester MA, Viscidi E, Kolonel LN, Olshan AF, et al. A case-control analysis of smoking and breast cancer in African American women: findings from the AMBER Consortium. Carcinogenesis. 2016;37:607–615. doi: 10.1093/carcin/bgw040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams LA, Olshan AF, Hong CC, Bandera EV, Rosenberg L, Cheng TD, Lunetta KL, McCann SE, Poole C, Kolonel LN, et al. Alcohol intake and breast cancer risk in African American women from the AMBER Consortium. Cancer Epidemiol Biomarkers Prev. 2017;26:787–794. doi: 10.1158/1055-9965.EPI-16-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenberg L, Bethea TN, Viscidi E, Hong CC, Troester MA, Bandera EV, Haiman CA, Kolonel LN, Olshan AF, Ambrosone CB, et al. Postmenopausal female hormone use and estrogen receptor-positive and -negative breast cancer in African American women. J Natl Cancer Inst. 2016;108:djv361. doi: 10.1093/jnci/djv361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bandera EV, Chandran U, Hong CC, Troester MA, Bethea TN, Adams-Campbell LL, Haiman CA, Park SY, Olshan AF, Ambrosone CB, et al. Obesity, body fat distribution, and risk of breast cancer subtypes in African American women participating in the AMBER Consortium. Breast Cancer Res Treat. 2015;150:655–666. doi: 10.1007/s10549-015-3353-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ambrosone CB, Zirpoli G, Hong CC, Yao S, Troester MA, Bandera EV, Schedin P, Bethea TN, Borges V, Park SY, et al. Important role of menarche in development of estrogen receptor-negative breast cancer in African American women. J Natl Cancer Inst. 2015;107:djv172. doi: 10.1093/jnci/djv172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palmer JR, Viscidi E, Troester MA, Hong CC, Schedin P, Bethea TN, Bandera EV, Borges V, McKinnon C, Haiman CA, et al. Parity, lactation, and breast cancer subtypes in African American women: results from the AMBER Consortium. J Natl Cancer Inst. 2014;106:dju237. doi: 10.1093/jnci/dju237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Basagana X, Pedersen M, Barrera-Gomez J, Gehring U, Giorgis-Allemand L, Hoek G, Stafoggia M, Nieuwenhuijsen MJ, Brunekreef B, Slama R, et al. Analysis of multicentre epidemiological studies: contrasting fixed or random effects modelling and meta-analysis. Int J Epidemiol. 2018;47:1343–1354. doi: 10.1093/ije/dyy117. [DOI] [PubMed] [Google Scholar]
- 40.Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011;342:d549. doi: 10.1136/bmj.d549. [DOI] [PubMed] [Google Scholar]
- 41.Rubin M, Fenig E, Rosenauer A, Menendez-Botet C, Achkar C, Bentel JM, Yahalom J, Mendelsohn J, Miller WH. 9-Cis retinoic acid inhibits growth of breast cancer cells and down-regulates estrogen receptor RNA and protein. Cancer Res. 1994;54:6549–6556. [PubMed] [Google Scholar]
- 42.Prakash P, Krinsky NI, Russell RM. Retinoids, carotenoids, and human breast cancer cell cultures: a review of differential effects. Nutr Rev. 2000;58:170–176. doi: 10.1111/j.1753-4887.2000.tb01856.x. [DOI] [PubMed] [Google Scholar]
- 43.Prakash P, Russell RM, Krinsky NI. In vitro inhibition of proliferation of estrogen-dependent and estrogen-independent human breast cancer cells treated with carotenoids or retinoids. J Nutr. 2001;131:1574–1580. doi: 10.1093/jn/131.5.1574. [DOI] [PubMed] [Google Scholar]
- 44.Hirsch K, Atzmon A, Danilenko M, Levy J, Sharoni Y. Lycopene and other carotenoids inhibit estrogenic activity of 17beta-estradiol and genistein in cancer cells. Breast Cancer Res Treat. 2007;104:221–230. doi: 10.1007/s10549-006-9405-7. [DOI] [PubMed] [Google Scholar]
- 45.Fulan H, Changxing J, Baina WY, Wencui Z, Chunqing L, Fan W, Dandan L, Dianjun S, Tong W, Da P, et al. Retinol, vitamins A, C, and E and breast cancer risk: a meta-analysis and meta-regression. Cancer Causes Control. 2011;22:1383–1396. doi: 10.1007/s10552-011-9811-y. [DOI] [PubMed] [Google Scholar]
- 46.Wang C, Baumgartner RN, Yang D, Slattery ML, Murtaugh MA, Byers T, Hines LM, Giuliano AR, Baumgartner KB. No evidence of association between breast cancer risk and dietary carotenoids, retinols, vitamin C and tocopherols in Southwestern Hispanic and non-Hispanic White women. Breast Cancer Res Treat. 2009;114:137–145. doi: 10.1007/s10549-008-9979-3. [DOI] [PubMed] [Google Scholar]
- 47.Kushi LH, Fee RM, Sellers TA, Zheng W, Folsom AR. Intake of vitamins A, C, and E and postmenopausal breast cancer. The Iowa Women's Health Study. Am J Epidemiol. 1996;144:165–174. doi: 10.1093/oxfordjournals.aje.a008904. [DOI] [PubMed] [Google Scholar]
- 48.Dorjgochoo T, Gao YT, Chow WH, Shu XO, Li H, Yang G, Cai Q, Rothman N, Cai H, Franke AA, et al. Plasma carotenoids, tocopherols, retinol and breast cancer risk: results from the Shanghai Women Health Study (SWHS). Breast Cancer Res. Treat. 2009;117:381–389. doi: 10.1007/s10549-008-0270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Epplein M, Shvetsov YB, Wilkens LR, Franke AA, Cooney RV, Le Marchand L, Henderson BE, Kolonel LN, Goodman MT. Plasma carotenoids, retinol, and tocopherols and postmenopausal breast cancer risk in the Multiethnic Cohort Study: a nested case-control study. Breast Cancer Res. 2009;11:R49. doi: 10.1186/bcr2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mangels AR, Holden JM, Beecher GR, Forman MR, Lanza E. Carotenoid content of fruits and vegetables: an evaluation of analytic data. J Am Diet Assoc. 1993;93:284–296. doi: 10.1016/0002-8223(93)91553-3. [DOI] [PubMed] [Google Scholar]
- 51.Office of Dietary Supplements (ODS). Vitamin A–fact sheet for health professionals. Bethesda (MD): National Institutes of Health; 2021.[Internet].Available from https://ods.od.nih.gov/factsheets/VitaminA-HealthProfessional/
- 52.Zhang S, Tang G, Russell RM, Mayzel KA, Stampfer MJ, Willett WC, Hunter DJ. Measurement of retinoids and carotenoids in breast adipose tissue and a comparison of concentrations in breast cancer cases and control subjects. Am J Clin Nutr. 1997;66:626–632. doi: 10.1093/ajcn/66.3.626. [DOI] [PubMed] [Google Scholar]
- 53.U.S. Census Bureau. Educational attainment in the United States. [Internet]. Suitland (MD): U.S. Census Bureau; 2013. Available from https://www.census.gov/data/tables/2013/demo/educational-attainment/cps-detailed-tables.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.