Abstract
Electrospray ionization is a powerful and prevalent technique used to ionize analytes in mass spectrometry. The distribution of charges that an analyte receives (charge state distribution, CSD) is an important consideration for interpreting mass spectra. However, due to an incomplete understanding of the ionization mechanism, the analyte properties that influence CSDs are not fully understood. Here, we employ a machine learning-based high-throughput approach and analyze CSDs of hundreds of thousands of peptides. Interestingly, half of the peptides exhibit charges that differ from what one would naively expect (number of basic sites). We find that these peptides can be classified into two regimes—undercharging and overcharging—and that these two regimes display markedly different charging characteristics. Strikingly, peptides in the overcharging regime show minimal dependence on basic site count, and more generally, the two regimes exhibit distinct sequence determinants. These findings highlight the rich ionization behavior of peptides and the potential of CSDs for enhancing peptide identification.
Introduction
Over the years, electrospray ionization1 (ESI) has become a leading ionization technique for pairing with liquid chromatography tandem mass spectrometry (LC-MS/MS)2,3. ESI’s ability to ionize a wide range of biomolecules, and to process samples in a high-throughput manner4–8 has greatly broadened the scope of mass spectrometry9,10, enabling applications to proteomics11–14, clinical biology15,16, drug discovery17, and more18.
ESI ionizes aqueous solutions through maintaining a high voltage potential across a capillary, which vaporizes the solution into a mist of highly charged droplets19. As the solvent continues to evaporate, the droplets experience an increased charge density, and fissure into smaller droplets upon reaching their Rayleigh limit20, the point at which the Coulombic forces overcome the surface tension. The charges are ultimately deposited onto the analytes, through mechanisms that are still not fully understood3,21, producing gaseous ionic molecules that arrive at the mass analyzer. Several theories have been proposed to explain the ionization mechanism, such as the ion evaporation model22,23, the charge residue model24,25, and the chain-ejection model26,27, which differ in their assumptions of how the analyte interacts with the droplet. It is believed that these evaporation models are appropriate for different types of analytes, depending on their size and structure27. However, the dynamic nature of ESI and the inability to directly observe ESI at the molecular scale have made it challenging to fully characterize the determinants of analyte ionization.
Here we utilize a high-throughput approach to investigate the ESI ionization of peptides. We reasoned that a systematic analysis of a large-scale dataset would not only complement existing studies on select analytes, but also provide more insights than previous “black box”28 deep learning approaches29. We therefore generated a dataset containing charging information on hundreds of thousands of peptides, using both new and published LC-MS/MS runs. For each peptide, the resulting dataset includes the measured charge state distribution (CSD), defined as the relative intensities of the ions produced by that peptide.
We next employed machine learning on this dataset to gain insights into the relationship between peptide sequence and CSD. Our analysis revealed that half of the peptides exhibited charges that did not correspond to their number of basic sites. Classifying these peptides into two regimes, namely undercharging and overcharging, we identified striking differences in their ionization characteristics. Specifically, we discovered that for overcharged peptides, mass takes precedence over basic site count, and that charging in the two regimes is affected by distinct amino acid features.
Overall, our findings offer new insights into the complex dynamics of peptide ionization, highlight that CSDs contain rich information about peptide sequences, and may open opportunities for applications to identification pipelines in proteomics.
Results
Overview of peptide CSD dataset.
To facilitate a machine learning approach, we developed an extraction scheme to extract CSD readings from MS1 scans (see methods). In each LC-MS/MS run, a single CSD reading was assigned to each MS2-identified peptide by averaging CSD readings across the peptide’s elution.
To cover a wide range of experimental settings, we applied our extraction scheme to 326 positive-ion mode LC-MS/MS runs acquired from three sources: our own, Confetti30, and Meier et al.31 These data sources differ in their choice of experimental parameters, protease, organism, and type of mass spectrometry instrument (Orbitrap32 and timsTOF33,34). The resulting dataset contained CSD readings of 261,667 unique peptides (Table 1).
Table 1.
Overview of peptide CSD dataset.
| Data source | ||||
|---|---|---|---|---|
| Ours | Confetti | Meier et al. | Total | |
|
| ||||
| LC-MS/MS runs | 20 | 18 | 288 (39*) | 326 (77*) |
|
| ||||
| Total peptides | 264,259 | 166,543 | 416,306 | 847,108 |
|
| ||||
| Unique peptides | 41,594 | 80,402 | 183,340 | 261,667 |
|
| ||||
| Varied parameters | gradient length, voltage, flow rate | protease | organism, protease | |
|
| ||||
| MS instrument | Orbitrap | Orbitrap | timsTOF | |
Breakdown of extracted CSDs from each of the three data sources (ours, Confetti, Meier et al.).
Asterisk (*) indicates the number of LC-MS/MS runs after aggregating over fractionations.
In order to confirm the reproducibility of the extracted CSD readings, we compared CSDs obtained from various LC-MS/MS runs. We found that CSD readings of the same peptide were generally consistent across different runs (Supplementary Fig. 1), and especially consistent among experimental replicates (~3.7% error Orbitrap, ~5.2% error timsTOF). After applying a one-parameter batch correction (see methods), errors across replicates dropped slightly (~3.4% error Orbitrap, ~4.7% timsTOF). Batch correction was not used in downstream analysis as LC-MS/MS runs were analyzed separately. Furthermore, the datasets from timsTOF instruments had slightly higher errors (Supplementary Fig. 1), which may be due to the extraction scheme being insufficiently optimized to that technology (as it does not employ ion mobility information). Together, these results demonstrate the robustness of the extraction scheme, and that peptide CSDs are highly consistent across replicates.
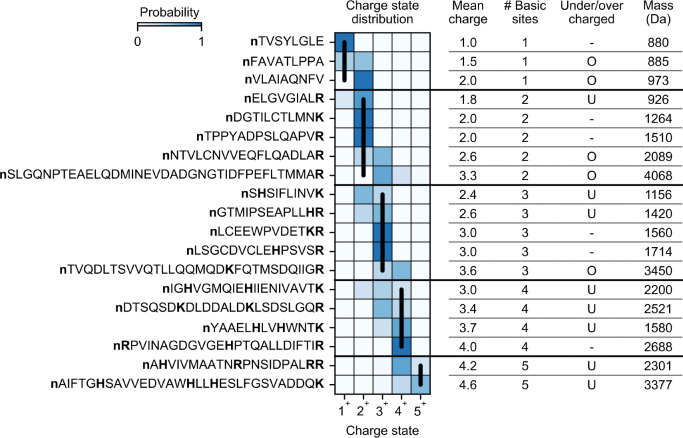
Peptides in our dataset exhibited a variety of CSDs (select peptides shown in Fig. 1). It is known that the basic site count serves as a rough estimate for the charge a peptide receives in positive-ion mode ESI35. Indeed, 51% of the peptides in the dataset have a CSD concentrated solely on the charge state equal to the basic site count. On the other hand, 40% of peptides exhibit undercharging (mean charge less than basic site count), and 9% exhibit overcharging (mean charge greater than basic site count). In downstream analysis, we explore factors that explain why some peptides receive fewer charges or more charges than their basic site count.
Figure 1. CSDs of select peptides.
Extracted CSDs and properties of select peptides from one of our HeLa trypsin runs (2.5 kV ESI voltage, 160 min gradient length, 400 nL/min flow rate). Basic sites are bolded. Vertical black lines denote the charge state equal to basic site count. U = undercharged (mean charge < basic site count). O = overcharged (mean charge > basic site count).
Under- and overcharged peptides exhibit different dependence on mass and number of basic sites.
Grouping peptides by their basic site count, we observed that mean charge increases with mass, and exhibits transitions from under- to overcharging in all LC-MS/MS runs (representative runs shown in Fig. 2, Supplementary Figs. 3, 4). For instance, in one of our HeLa runs, among peptides with three basic sites and mass less than 2600 Da, 98% exhibited charges of 3+ or lower (undercharging, Fig. 2b). In contrast, among peptides with three basic sites and mass greater than 2600 Da, 94% exhibited charges 3+ or higher (overcharging, Fig. 2b). We confirmed that this transition phenomenon cannot be explained by the mass spectrometer’s cutoff, since the presence of a charge state recedes well before reaching the cutoff. Moreover, the phenomenon is present in runs performed with other proteases (Supplementary Fig. 3) and with the timsTOF instrument (Supplementary Fig. 4), suggesting it is independent of the peptide distribution or the choice of mass spectrometer instrument.
Figure 2. Visualizing CSD dataset versus mass.
Plots of mean charge versus mass for peptides from a representative run (our HeLa trypsin run; 2.5 kV ESI voltage, 160 min gradient length, 400 nL/min flow rate), colored by number of basic sites. Data points are shown as (a) a scatter plot with jittering in the y-axis, uniformly chosen from −0.02 to 0.02, and (b) 2D hexagonal binning plots separated by basic site count. Colored curves show spline-interpolation of mean charge versus mass, performed with 20 cubic-splines, a smoothing parameter of 5, and a monotonicity constraint. Curves are dashed when the interpolated mean charge is less than the basic site count (undercharging) and solid otherwise (overcharging). Hatched regions correspond to unobservable mean charge due to cutoff.
Prior studies on select protein complexes36–38 and synthetic peptides39 have also observed correlations between size and charging. Moreover, the relationship is consistent with proposed ionization theories27 as larger analytes would be enclosed in larger, more highly charged droplets20, may have higher solvent-exposed surface area37,38, and may have more functional groups that can stabilize charge through intramolecular solvation40–42. Our findings suggest that this correlation holds universally for peptides, and provide quantitative dependencies between the number of basic sites, mass, and mean charge.
One striking observation is that in the overcharging regime, the number of basic sites has little effect on the mean charge trend as a function of mass (Fig. 2a, solid lines). In contrast, in the undercharging regime, the number of basic sites has a significant effect on the mean charge trend (Fig. 2a, dashed lines). Thus, in the overcharging (but not in the undercharging) regime, mass takes precedence over the number of basic sites. For instance, among peptides of mass 1800 Da, those with 1 or 2 basic sites exhibit, on average, similar mean charges of 2.1 and 2.2, respectively (overcharging), whereas those with 3, 4, or 5 basic sites exhibit, on average, distinct mean charges of 2.9, 3.4, and 4.0, respectively (undercharging). These observations suggest differences in ionization factors at play for the over- and undercharging regimes.
These findings also demonstrate the potential benefits that CSDs can offer to peptide identification. Aside from peptides that exhibit overcharging, peptides sharing the same mass but having a different number of basic sites generally exhibit different CSDs (Supplementary Fig. 5), allowing them to be distinguished based on the MS1 scan. For example, among peptides with mass 1600 ± 25 Da, 99% of those with two basic sites have mean charge < 2.5, while 98% of those with three basic sites have mean charge > 2.5. As such, when one is searching for potential peptide candidates for a given collection of ion peaks, the observed mass and CSD can be used to infer the peptide’s basic site count. This example showcases a preliminary use-case for peptide CSDs, and suggests that incorporating more sequence-dependent insights may provide further identification opportunities.
Distinct sequence determinants underlie peptide ionization in the under- and overcharging regimes.
A peptide’s ionization depends on many factors, including Coulombic forces43,44, its gas-phase conformation41,45, its protonatable locations46,47, and intramolecular forces40–42. To provide further insights into the sequence factors that influence peptide ionization, we analyzed the sequence determinants of CSDs within the under- and overcharging regimes.
For the analysis, we considered the 20 amino acid counts and the identity of the N-terminal amino acid as the sequence features of interest. To account for possible dependence on the number of basic sites or charge states, we performed a separate analysis for each of four regions, labelled #2O, #3O, #3U, and #4U (O : overcharged, U : undercharged, Fig. 3a). Region #2O consists of peptides with two basic sites and considers charging across 2+ and 3+. We similarly define region #3O (three basic sites, charging across 3+ and 4+), region #3U (three basic sites, charging across 2+ to 3+), and region #4U (four basic sites, charging across 3+ and 4+). For each of our 12 LC-MS/MS runs (with sufficiently many data points, see methods) and each of the four regions, we calculated a feature’s contribution using an “intrinsic basicity” score (Fig. 3b), derived from coefficients of a logistic regression (see methods). Interestingly, clustering the region-run pairs by their calculated intrinsic basicities, we identified two distinct clusters (Fig. 3c): there was strong agreement only between regions #2O and #3O, and between regions #3U and #4U. This result suggests that under- and overcharging are influenced by different sequence features, and that these sequence features do not depend strongly on the number of basic sites.
Figure 3. CSD sequence determinants for under- and overcharged peptides.
(a) Schematic representation of the four peptide charging regions of interest, determined by number of basic sites and pair of consecutive charge states (see text). (b) Scatter plots of calculated amino acid intrinsic basicities (see text) versus mass for two representative charging regions (top: region #3U; bottom: region #2O). Panels (a–b) use our HeLa trypsin run with 2.5 kV ESI voltage, 160 min gradient length, 400 nL/min flow rate. (c) Heatmap showing correlations between calculated amino acid intrinsic basicities among all pairs of region-run combinations for our runs with sufficiently many data points (see methods). Along both axes, all 12 runs are aligned four times, once for each of the four regions. (d) Box plot of estimated mass-adjusted intrinsic basicities (given by residual intrinsic basicities after subtracting trend in mass, see methods) across runs with sufficiently many data points, separated by data source and charging region. Runs from Meier et al. were aggregated across fractionations (average taken for duplicate readings). C* denotes carbamidomethyl-cysteine in our and Meier et al.’s runs, and N-ethylmaleimide modified cysteine in Confetti’s runs. (e) Box plot of intrinsic basicities for the N-terminal identity of select amino acids (region #3U). Box plot elements: centerline, median; boxplot limits, upper and lower quartiles; whiskers, 1.5x interquartile range; points, in whisker range with jitters (outliers not shown).
To understand the sequence determinants of overcharging, we examined some of the more prominent calculated intrinsic basicities for region #2O across all runs. We observed that glutamine (Q) and aspartic acid (D) have the highest and lowest mass-adjusted intrinsic basicities, respectively (Fig. 3d, bottom; Supplementary Fig. 6a, bottom). These observations are consistent with previous work: glutamine (Q) has been reported to have high gas-phase basicity due to its amide group46,48 and can be a potential charge carrier during ESI46; aspartic acid (D) has been observed to form salt bridges with basic sites in molecular dynamics simulations49. Interestingly, despite having the same functional group, glutamine (Q) displays notably higher calculated intrinsic basicity than asparagine (N); similarly, glutamic acid (E) displays notably higher calculated intrinsic basicity than aspartic acid (D) (Fig. 3b,d). Lastly, we observed that amino acids with nonaromatic hydrocarbon side chains (alanine (A), valine (V), isoleucine (I), and leucine (L)) have high mass-adjusted intrinsic basicities.
Next, to understand the impact of sequence features on undercharging, we examined some of the more prominent calculated intrinsic basicities for region #3U across all runs. We observed that the presence of an arginine (R), lysine (K), or histidine (H) at the N-terminal greatly reduces charging (Fig. 3e; Supplementary Fig. 6b, top). This suggests that undercharging occurs due to Coulombic repulsion between the N-terminus and an N-terminal basic side chain. In particular, this effect can be observed in sequential isomers TNSTFNQVVLKR and RTNSTFNQVVLK, which have identical sequences apart from an arginine at the C- or N-terminal. In the four runs that contain CSD readings for both peptides, the former peptide has a CSD of (mean ± s.d.; not batch corrected), while the latter, with an N-terminal arginine, has a CSD of . In addition to these Coulombic effects, we observed that proline (P) has a notably high intrinsic basicity (Fig. 3d, top; Supplementary Fig. 6a, top), especially if located at the N-terminal. Moreover, the runs from Meier et al. demonstrated higher intrinsic basicity for strongly hydrophobic amino acids (isoleucine (I), leucine (L), phenylalanine (F)) than did the Orbitrap runs (Fig. 3d, top).
In conclusion, our analysis demonstrates that the under- and overcharging regimes are influenced by distinct sequence determinants. These sequence determinants are consistent across different LC-MS/MS runs, and align with previously documented effects. Moreover, our findings indicate that peptide ionization depends on many factors beyond mass and basic site count, including both composition and position of the constituent amino acids. Collectively, these results underscore the value of CSDs in providing information about peptide sequences, and suggest that CSDs may complement other measured analyte properties for proteomics identification.
CSD variations offer opportunities for identification.
It is well known that certain LC-MS/MS experimental parameters change the overall degree of charging experienced by analytes3,35,50,51. Indeed, in our own experiments, we observed that increasing flow rate and gradient length generally resulted in higher overall charging (Supplementary Fig. 7). While such experimental variations may be perceived as undesirable, they can be potentially harnessed to improve peptide identification. Specifically, peptides with similar CSDs may exhibit more distinct CSDs under different charging conditions, allowing for easier separation.
To illustrate this concept, consider the two peptides ASGQAFELILSPR and ACANPAAGSVILLENLR. In the low flow rate runs (200 nL/min), these peptides exhibited CSDs of and (88%, 12%), respectively, a difference of 8%. In the high flow rate runs (800 nL/min), these peptides exhibited CSDs of and (74%, 26%), respectively, a difference of 16%. In other words, increasing the flow rate resulted in a twofold increase in the difference between these two CSDs. In fact, this example occurs throughout our dataset (Supplementary Fig. 2a,b). For instance, among peptides with mean charge between 2 and 2.15 in the low flow rate run, more than half of the pairwise differences (59%) increased by twofold or more when flow rate increased (Supplementary Fig. 2a). Similarly, among peptides with mean charge between 2.85 and 3 in the high flow rate run, more than half of of the pairwise differences (52%) increased by two fold or more when flow rate decreased. Lastly, CSD variations across other pairs of runs exhibited similar trends (Supplementary Fig. 2b). Together, these findings highlight how varying experimental parameters can magnify differences in CSDs.
Instead of varying experimental parameters across runs, one can also imagine inducing variations in charging within a single run, possibly on a scan-to-scan basis. In fact, we observed that in many runs, mild scan-to-scan charging fluctuations already exist. While the reasons for these fluctuation are not clear, our findings indicate that they are not due to noise and have potential for improving identification (Supplementary Text 1).
In summary, these findings suggest that varying charging conditions across runs and within a single run may enhance the utility of CSDs for peptide identification.
Discussion
In this work, we demonstrate that utilizing a high-throughput approach can reveal novel, general patterns underlying peptide ionization. Previous work has demonstrated that the ionization of native proteins, due to their tightly compacted structure in their folded state, is well-predicted by their surface-exposed area37,38. In contrast, our study indicates that denatured peptides, typically encountered in shotgun proteomics52, exhibit complex ionization behavior: the interplay of mass and basic site count determines under- and overcharging, with further fine-tuning based on additional sequence-dependent factors.
Notably, our findings demonstrate that the under- and overcharging regimes display different dependencies on mass and basic site count. We found that basic site count has little effect on the typical mean charge exhibited by overcharged (but not undercharged) peptides. One potential explanation for this phenomenon is that overcharged peptides, having high mass relative to their basic site count, contain enough backbone carbonyls for solvating excess protons42. As such, additional basic sites may not have a strong effect on overcharging. There may be other potential explanations for this phenomenon, even causes arising outside of ESI such as the efficiency of transporting ions into the mass analyzer. Regardless, the striking contrast in basic site count dependence suggests there are differences in the underlying ionization processes that govern under- and overcharging of peptides.
Additionally, the distinct sequence determinants that we derived for the two regimes align with factors related to gas-phase interactions and dynamics, suggesting possible mechanistic connections. Specifically, for overcharged peptides, our findings align with previously documented effects of glutamine (Q) having high gas-phase basicity46, and aspartic acid (D) forming salt bridges41,49. Interestingly, we observed that both glutamine (Q) and glutamic acid (E) had notably higher intrinsic basicities than asparagine (N) and aspartic acid (D), respectively. Both pairs of amino acids share the same functional group, but differ only in side-chain length, suggesting that the observed effect is associated with differences in conformational entropy53. Additionally, we discovered that amino acids with non-aromatic hydrocarbon side chains (A, V, I, L) promote overcharging. As non-polar moieties increase a peptide’s affinity for the droplet-air interface50, this finding suggests that peptides positioned closer to the droplet surface may exhibit a stronger overcharging response. For undercharged peptides, our results align with the effects of Coulombic repulsion for basic amino acids at the N-terminal. We also found unexpected features that counteract undercharging, such as the presence of proline, especially when located at the N-terminal, and hydrophobic amino acids (I, L, F), specifically in the timsTOF datasets. We speculate that internal prolines may reduce undercharging by introducing a kink in the peptide chain54, thereby promoting charge solvation42 and protecting from Coulombic repulsion. Further studies would be necessary to elucidate the mechanisms underlying the aforementioned observations, and why the sequence determinants differ so much in the two regimes.
Our findings suggest that peptide CSDs—through their rich sequence determinants and their differential response to experimental conditions—contain information that may benefit shotgun proteomics, information that is mostly neglected in state-of-the-art identification pipelines55,56. In addition to data-dependent acquisition (DDA)57, our findings may especially be useful for data-independent acquisition (DIA)58 and MS1-only59 approaches, where there are no fragmentation spectra dominated by one peptide.
Of the many machine learning techniques available to analyze large-scale datasets, deep learning has had tremendous progress in recent years, as demonstrated by accurate predictions for retention time29,60–62, collisional cross section62,63, fragmentation spectra31,62, and charge state distributions29. However, interpreting the predictions of black box neural networks still remains a challenge28,64. While we have experimented with deep learning in the early stages of this study, we show here that classic machine learning approaches still have merit in providing easily communicable and readily interpretable insights. In fact, these two approaches are not mutually exclusive: the insights derived here from analyzing peptide CSDs can potentially inform model design and improve deep learning predictions.
Our study has several inherent limitations. Firstly, the extraction scheme cannot extract intensities that fall below the mass spectrometer’s intensity threshold. This may introduce biases in CSD readings for less abundant peptides and for scans located at the tails of elution profiles. We address this through our extraction scheme’s filtering steps which favor the extraction of high intensity peptides; we verified that the intensity threshold only accounts for < 2% error for most CSD readings (data not shown). Secondly, the distribution of peptide sequences in our dataset is affected by the proteome, the experimental setup, and the extraction scheme’s filtering steps. To address this, we used LC-MS/MS runs across different experimental parameters, proteases, cell lines, and instruments. This issue can be further addressed through incorporating synthetic peptides. Thirdly, our analysis on calculated intrinsic basicity does not capture pairwise, nor higher-order, interactions between amino acids. Although we explored including these higher-order terms, we had difficulty interpreting the results due to overparameterization. A deeper mechanistic understanding may provide opportunities for better feature engineering that can overcome these challenges. Fourthly, CSDs are influenced by all factors that occur in and downstream of ESI, including ionization efficiency, transport efficiency (from ESI to MS detection)50, and possible interactions within the drift tube65. These factors can limit the interpretation of our analysis. We partly addressed this by using runs from both Orbitrap and timsTOF instruments. Lastly, our analysis can only establish correlations, not causal relationships. Further experiments and molecular dynamics simulations are necessary to fully establish the mechanisms underlying the insights we have identified.
In conclusion, our high-throughput study of peptide CSDs has yielded informative results for the field of ESI and LC-MS/MS-based proteomics. The insights we have generated for peptide ionization contribute to our understanding of ESI mechanisms, and offer opportunities for improved peptide identification.
Methods
Sample preparation & mass spectrometry.
To include LC-MS/MS runs with different experimental parameters, we ran our own experiments varying ESI voltage, gradient length, and sample flow rate.
HeLa cells were grown to 90% confluency and washed twice with PBS before direct lysis on the plate. Total proteins were extracted using a urea lysis buffer (8 M Urea, 75 mM NaCl, 50 mM Tris/HCl pH 8.0, 1 mM EDTA). The protein concentration was determined by Pierce BCA assay. 20 μg of total protein was processed further. Disulfide bonds were reduced with 5 mM dithiothreitol and cysteines were subsequently alkylated with 10 mM iodoacetamide. Samples were diluted 1:4 with 50 mM Tris/HCl (pH 8.0) and sequencing grade modified trypsin (Promega) was added in an enzyme-to-substrate ratio of 1:50. After 16 h of digestion, samples were acidified with 1% formic acid (final concentration). Tryptic peptides were desalted on C18 StageTips according to Rappsilber et al.67 and evaporated to dryness in a vacuum concentrator. Desalted peptides were reconstituted in Buffer A (3% acetonitrile, 0.2% formic acid).
For mass spectrometer runs that were run in April 2021, 2 μg of peptides were analyzed on a Thermo Scientific Orbitrap Q Exactive HF mass spectrometer coupled via a 25 cm long, 1.6 μm particle size Aurora C18 column (IonOpticks) to an Acuity M Class UPLC system (Waters). For the long gradient, peptides were separated at a flow rate of 400 nL/min with a linear gradient spanning 2 min from 5% to 8% solvent B (100% acetonitrile, 0.1% formic acid), followed by a 87 min linear gradient from 8% to 22% solvent B, a 20 min linear gradient from 22% to 30% solvent B, a 14 min linear gradient from 30% to 60% solvent B, and a 1 min linear gradient from 60% to 90% solvent B. Each sample was run for 160 min, including sample loading and column equilibration times. For the short gradient, peptides were separated at a flow rate of 400 nL/min in linear steps from 2% to 8% solvent B over 1 min, from 8% to 30% solvent B over 33 min, from from 30% to 60% solvent B over 5 min, and from 60% to 90% solvent B over 5 min. Each sample was run for 90 min, including sample loading and column equilibration times.
For mass spectrometer runs that were run in August 2021, 2 μg of peptides were analyzed on a Thermo Scientific Orbitrap Q Exactive HF mass spectrometer coupled via a 15 cm long, 3 μm particle size EASY-Spray C18 column (ThermoFisher Scientific) to an Acuity M Class UPLC system (Waters). Peptides were separated at varying flow rates ranging from 200 nL/min to 800 nL/min in 200 nL/min increments. The peptides were separated in linear steps from 5% to 8% solvent B over 4 min, from 8% to 14% solvent B over 45 min, from 14% to 22% solvent B over 45 min, from 22% to 30% solvent B over 20 min, from 30% to 60% solvent B over 9 min, and from 60% to 90% solvent B over 1 min. Each sample was run for 190 min, including sample loading and column equilibration times.
Data was acquired in data dependent mode using the Xcalibur 4.1 software. MS1 spectra were measured with a resolution of 120,000, an AGC target of 3e6 and a mass range from 300 to 1800 . Up to 12 MS2 spectra per duty cycle were triggered at a resolution of 15,000, an AGC target of 1e5, an isolation window of 1.6 and a normalized collision energy of 28.
Gathering published LC-MS/MS raw files.
To supplement our LC-MS/MS runs, we gathered published raw files from two sources: Confetti30 and Meier et al.31. These raw files are located at the ProteomeXchange Consortium via PRIDE partner repository68 with data identifier PXD000900 (Confetti) and PXD019086 (Meier et al.).
Preprocessing steps for LC-MS/MS raw files.
To identify peptides that were present in the LC-MS/MS runs, the raw LC-MS/MS files were analyzed with the MaxQuant software55. MaxQuant was run with the following parameters: maximum of two missed cleavages, methionine oxidation and N-terminal acetylation as variable modifications, cysteine carbamidomethylation (ours, Meier et al.) or N-ethylmaleimidation (Confetti) as a fixed modification, minimum peptide length of 7 amino acids, maximum peptide mass of 4600 Da, and a false discovery rate of 1%. MaxQuant was run with version 1.6.10.43 (ours, Confetti) and version 1.6.5.0 (Meier et al., original analysis retained).
Additional preprocessing steps were performed to access MS1 profile peaks from the raw LC-MS/MS files. The Thermo .raw files from Orbitrap runs were converted to .mzML format using MSConvert from ProteoWizard version 3.069, with the vendor peak picking setting enabled to obtain centroided MS1 peaks. The MS1 spectra of Bruker .d folders from timsTOF runs were accessed using alphatims v. 1.0.070. The MS1 profile peaks were centroided using our own procedure (Supplementary Text 2). These centroided MS1 peaks were fed into our downstream CSD extraction scheme.
CSD extraction scheme.
Per-scan CSD readings were extracted from MS1 scans for MS2-identified peptides using the following scheme. For each peptide and each charge state from 1+ to 5+, relevant MS1 scans were searched for peaks that matched the theoretical isotope envelope and passed stringent filtering requirements. From these peaks, the charge states’ intensities were estimated, and then normalized to obtain the peptide’s CSD reading for that scan. Finally, these per-scan CSDs were combined into one CSD estimate per peptide by performing an intensity-weighted average across scans. The full details of the extraction scheme are provided in Supplementary Text 2.
One of the main design goals was to achieve high quality CSD readings. As such, we applied stringent filtering and only retained CSD readings which contained confident intensity estimates for every charge state. To determine whether a peptide’s charge state was present in an MS1 spectrum, we verified that (i) the theoretical isotope peaks had low offset from the observed isotope peaks, (ii) the shape of the theoretical isotope distribution matched the observed isotope distribution (cosine similarity >0.98), and (iii) there was an absence of peaks that might belong to the isotope distributions of other peptides (chimeric peaks). On the other hand, a charge state was denoted absent and thereby assigned an extracted intensity of 0 if no peaks were in a sufficiently large vicinity. Through tuning the thresholds used for filtering, the extraction scheme favors the extraction of highly confident peptide CSD readings.
We analyzed a total of 326 raw LC-MS/MS files which resulted in CSD readings extracted for a total of 261,667 unique peptides (Table 1). The resulting CSD dataset has a similar distribution of peptides to those typically identified using MS/MS, with masses ranging from 700 Da–4600 Da, and varying numbers of basic sites (Fig. 2).
Measuring error between CSD readings.
Error between CSD readings of the same peptide was measured through total variation. The total variation between two distributions and is given by the sum of absolute differences between probabilities divided by two:
Total variation ranges from 0 to 1, signifying equivalent or disjoint distributions, respectively.
Correcting experimental batch effects.
To better assess CSD errors across pairs of runs, we applied a one-parameter batch correction (Supplementary Figs. 1,2). Peptide CSDs were transformed according to the following scheme. For each charge state , we scaled its probability by a factor of , for some global batch parameter . The scaled probabilities were then renormalized to sum to one, forming the transformed CSD. In other words, our batch correction maps peptide CSDs of the form to
This transformation can be interpreted as additive shifts in the energy scale (i.e., log probability scale), where the magnitude of the shift scales linearly with the charge state.
Errors across pairs of runs were measured before and after applying a batch correction (Supplementary Fig. 1). The batch correction parameter was chosen to minimize the total error, calculated as the sum of the total variation in CSD readings across all peptides common to both runs. We found this batch correction to be reasonably effective given that it only depends on one parameter.
Calculating and analyzing intrinsic basicity scores.
In the analysis of sequence effects on charging, we calculated the intrinsic basicities of amino acid features for each of four charging regions (Fig. 3). Each charging region considered a subset of peptides (those with a given number of basic sites and with no variable modifications) and charging across consecutive charge state probabilities and for some from 2 to 4.
The calculated intrinsic basicities were given by the coefficients of a logistic regression. The input variables were chosen as follows: 20 numerical variables for each of the 20 amino acid counts, and 20 binary variables to denote the identity of the N-terminal amino acid. The target variable for the regression was chosen as . The logistic regression was performed using the pyGAM library71, with binary cross entropy as the loss function and mild L2 regularization
To compare across region-run pairs (Fig. 3c), we calculated the Pearson correlation coefficient of the calculated intrinsic basicities for the 17 non-basic amino acids. Only our LC-MS/MS runs with sufficiently many data points in all four charging regions were considered; that is, each charging region needed to contain >100 peptides that exhibited nonzero probabilities on both charge states in question. The resulting LC-MS/MS runs, which were used in Fig. 3c, are the 12 runs from the April 2021 experiment.
To calculate mass-adjusted intrinsic basicities for each region-run pair (Fig. 3d), we subtracted from the overall intrinsic basicities the portion that could be explained by mass. Specifically, the mass-adjusted intrinsic basicities were derived as the residuals of a linear regression between the calculated intrinsic basicities and the masses of the 17 non-basic amino acids plus an intercept term. The linear regression used a Huber loss72 with parameter . We selected the Huber loss, which is equal to the L1 loss for large values and the L2 loss for small values , to ensure that amino acids with intrinsic basicities significantly different from the mass trend were not overly penalized, and to guarantee uniqueness of the coefficients.
Supplementary Material
Acknowledgements
We are grateful to Lars Konermann, Brian Chait, David Fenyő, and Nikolai Slavov for their insightful comments. We thank Andrew Lemoff and the UTSW Proteomics Core for their assistance with technical queries. We also thank Susan Liao for feedback on the manuscript. We acknowledge funding from Simons Investigator Award (O.R., A.M.X.); NSF MCB-2226731 (O.R.); NSF (Award 2224211) (M.J.); NIH (R35GM128802, R01AG071869 and R01HG012216) (M.J.); Columbia startup funding (M.J.); and NSF-GRFP (Award DGE2036197) (L.C.T.).
Footnotes
Competing interests The authors declare no competing interests.
Code availability Extraction scheme and figure generating code are available on GitHub (https://github.com/regev-lab/extract-csd).
Data availability
Raw files and MaxQuant analyses for our LC-MS/MS runs are available at the MassIVE data repository with ID MSV000091473. The CSD dataset generated and analyzed in this study is available at figshare66.
References
- 1.Fenn J. B., Mann M., Meng C. K., Wong S. F. & Whitehouse C. M. Electrospray ionization for mass spectrometry of large biomolecules. Science 246, 64–71 (1989). [DOI] [PubMed] [Google Scholar]
- 2.Snyder A. P. in ACS Symposium Series 1–20 (American Chemical Society, Washington, DC, 1996). [Google Scholar]
- 3.Banerjee S. & Mazumdar S. Electrospray ionization mass spectrometry: a technique to access the information beyond the molecular weight of the analyte. Int. J. Anal. Chem. 2012, 282574 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ventura R. et al. High-throughput and sensitive screening by ultra-performance liquid chromatography tandem mass spectrometry of diuretics and other doping agents. Eur. J. Mass Spectrom. (Chichester, Eng.) 14, 191–200 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Kushnir M. M., Komaromy-Hiller G., Shushan B., Urry F. M. & Roberts W. L. Analysis of dicarboxylic acids by tandem mass spectrometry. High-throughput quantitative measurement of methylmalonic acid in serum, plasma, and urine. Clin. Chem. 47, 1993–2002 (2001). [PubMed] [Google Scholar]
- 6.Rafii M. et al. High-throughput and simultaneous measurement of homocysteine and cysteine in human plasma and urine by liquid chromatography-electrospray tandem mass spectrometry. Anal. Biochem. 371, 71–81 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Sun S. & Kennedy R. T. Droplet electrospray ionization mass spectrometry for high throughput screening for enzyme inhibitors. Anal. Chem. 86, 9309–9314 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wleklinski M. et al. High throughput reaction screening using desorption electrospray ionization mass spectrometry. Chem. Sci. 9, 1647–1653 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho C. S. et al. Electrospray ionisation mass spectrometry: principles and clinical applications. Clin. Biochem. Rev. 24, 3–12 (2003). [PMC free article] [PubMed] [Google Scholar]
- 10.Pitt J. J. Principles and applications of liquid chromatography-mass spectrometry in clinical biochemistry. Clin. Biochem. Rev. 30, 19–34 (2009). [PMC free article] [PubMed] [Google Scholar]
- 11.Roepstorff P. Mass spectrometry in protein studies from genome to function. Curr. Opin. Biotechnol. 8, 6–13 (1997). [DOI] [PubMed] [Google Scholar]
- 12.Oda Y., Huang K., Cross F. R., Cowburn D. & Chait B. T. Accurate quantitation of protein expression and site-specific phosphorylation. Proc. Natl. Acad. Sci. U. S. A. 96, 6591–6596 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loo J. A. Electrospray ionization mass spectrometry: a technology for studying noncovalent macromolecular complexes. Int. J. Mass Spectrom. 200, 175–186 (2000). [Google Scholar]
- 14.Chowdhury S. K., Katta V. & Chait B. T. Probing conformational changes in proteins by mass spectrometry. J. Am. Chem. Soc. 112, 9012–9013 (1990). [Google Scholar]
- 15.Rashed M. S. et al. Screening blood spots for inborn errors of metabolism by electrospray tandem mass spectrometry with a microplate batch process and a computer algorithm for automated flagging of abnormal profiles. Clin. Chem. 43, 1129–1141 (1997). [PubMed] [Google Scholar]
- 16.Mayr B. M. et al. Absolute myoglobin quantitation in serum by combining two-dimensional liquid chromatography-electrospray ionization mass spectrometry and novel data analysis algorithms. J. Proteome Res. 5, 414–421 (2006). [DOI] [PubMed] [Google Scholar]
- 17.Hofstadler S. A. & Sannes-Lowery K. A. Applications of ESI-MS in drug discovery: interrogation of noncovalent complexes. Nat. Rev. Drug Discov. 5, 585–595 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Wang J., Chow W. & Leung D. Applications of LC/ESI-MS/MS and UHPLC QqTOF MS for the determination of 148 pesticides in fruits and vegetables. Anal. Bioanal. Chem. 396, 1513–1538 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Kebarle P. & Tang L. From ions in solution to ions in the gas phase - the mechanism of electrospray mass spectrometry. Anal. Chem. 65, 972A–986A (1993). [Google Scholar]
- 20.Lord R. XX. On the equilibrium of liquid conducting masses charged with electricity. Lond. Edinb. Dublin Philos. Mag. J. Sci. 14, 184–186 (1882). [Google Scholar]
- 21.Konermann L., Ahadi E., Rodriguez A. D. & Vahidi S. Unraveling the mechanism of electrospray ionization. Anal. Chem. 85, 2–9 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Iribarne J. V. On the evaporation of small ions from charged droplets. J. Chem. Phys. 64, 2287 (1976). [Google Scholar]
- 23.Fernandez de la Mora J. Electrospray ionization of large multiply charged species proceeds via Dole’s charged residue mechanism. Anal. Chim. Acta 406, 93–104 (2000). [Google Scholar]
- 24.Dole M. et al. Molecular Beams of Macroions. J. Chem. Phys. 49, 2240–2249 (1968). [Google Scholar]
- 25.Iavarone A. T. & Williams E. R. Mechanism of charging and supercharging molecules in electrospray ionization. J. Am. Chem. Soc. 125, 2319–2327 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahadi E. & Konermann L. Modeling the behavior of coarse-grained polymer chains in charged water droplets: implications for the mechanism of electrospray ionization. J. Phys. Chem. B 116, 104–112 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Konermann L., Rodriguez A. D. & Liu J. On the formation of highly charged gaseous ions from unfolded proteins by electrospray ionization. Anal. Chem. 84, 6798–6804 (2012). [DOI] [PubMed] [Google Scholar]
- 28.Novakovsky G., Dexter N., Libbrecht M. W., Wasserman W. W. & Mostafavi S. Obtaining genetics insights from deep learning via explainable artificial intelligence. Nat. Rev. Genet. 24, 125–137 (2023). [DOI] [PubMed] [Google Scholar]
- 29.Guan S., Moran M. F. & Ma B. Prediction of LC-MS/MS properties of peptides from sequence by deep learning. Mol. Cell. Proteomics 18, 2099–2107 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo X., Trudgian D. C., Lemoff A., Yadavalli S. & Mirzaei H. Confetti: a multiprotease map of the HeLa proteome for comprehensive proteomics. Mol. Cell. Proteomics 13, 1573–1584 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meier F. et al. Deep learning the collisional cross sections of the peptide universe from a million experimental values. Nat. Commun. 12, 1185 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zubarev R. A. & Makarov A. Orbitrap mass spectrometry. Anal. Chem. 85, 5288–5296 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Meier F. et al. Parallel accumulation-serial fragmentation (PASEF): Multiplying sequencing speed and sensitivity by synchronized scans in a trapped ion mobility device. J. Proteome Res. 14, 5378–5387 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Meier F. et al. diaPASEF: parallel accumulation-serial fragmentation combined with data-independent acquisition. Nat. Methods 17, 1229–1236 (2020). [DOI] [PubMed] [Google Scholar]
- 35.Cole R. B. Electrospray and MALDI mass spectrometry 2nd ed. (ed Cole R. B.) (Wiley-Blackwell, Chichester, England, 2010). [Google Scholar]
- 36.Tolić L. P. et al. Electrospray ionization Fourier transform ion cyclotron resonance mass spectrometric characterization of high molecular mass Starburst™ dendrimers. Int. J. Mass Spectrom. Ion Process. 165–166, 405–418 (1997). [Google Scholar]
- 37.Kaltashov I. A. & Mohimen A. Estimates of protein surface areas in solution by electrospray ionization mass spectrometry. Anal. Chem. 77, 5370–5379 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Testa L., Brocca S. & Grandori R. Charge-surface correlation in electrospray ionization of folded and unfolded proteins. Anal. Chem. 83, 6459–6463 (2011). [DOI] [PubMed] [Google Scholar]
- 39.Breibeck J. et al. PAS-cal: a generic recombinant peptide calibration standard for mass spectrometry. J. Am. Soc. Mass Spectrom. 25, 1489–1497 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen J., Im W. & Brooks C. L. 3rd Balancing solvation and intramolecular interactions: toward a consistent generalized Born force field. J. Am. Chem. Soc. 128, 3728–3736 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marchese R., Grandori R., Carloni P. & Raugei S. On the zwitterionic nature of gas-phase peptides and protein ions. PLoS Comput. Biol. 6, e1000775 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Warnke S., von Helden G. & Pagel K. Protein structure in the gas phase: the influence of side-chain microsolvation. J. Am. Chem. Soc. 135, 1177–1180 (2013). [DOI] [PubMed] [Google Scholar]
- 43.Douglass K. A. & Venter A. R. Predicting the highest intensity ion in multiple charging envelopes observed for denatured proteins during electrospray ionization mass spectrometry by inspection of the amino acid sequence. Anal. Chem. 85, 8212–8218 (2013). [DOI] [PubMed] [Google Scholar]
- 44.Krusemark C. J., Frey B. L., Belshaw P. J. & Smith L. M. Modifying the charge state distribution of proteins in electrospray ionization mass spectrometry by chemical derivatization. J. Am. Soc. Mass Spectrom. 20, 1617–1625 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim D., Wagner N., Wooding K., Clemmer D. E. & Russell D. H. Ions from solution to the gas phase: A molecular dynamics simulation of the structural evolution of Substance P during desolvation of charged nanodroplets generated by electrospray ionization. J. Am. Chem. Soc. 139, 2981–2988 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Schnier P. D., Gross D. S. & Williams E. R. On the maximum charge state and proton transfer reactivity of peptide and protein ions formed by electrospray ionization. J. Am. Soc. Mass Spectrom. 6, 1086–1097 (1995). [DOI] [PubMed] [Google Scholar]
- 47.Wysocki V. H., Tsaprailis G., Smith L. L. & Breci L. A. Mobile and localized protons: a framework for understanding peptide dissociation. J. Mass Spectrom. 35, 1399–1406 (2000). [DOI] [PubMed] [Google Scholar]
- 48.Gorman G. S., Speir J. P., Turner C. A. & Amster I. J. Proton affinities of the 20 common .alpha.-amino acids. J. Am. Chem. Soc. 114, 3986–3988 (1992). [Google Scholar]
- 49.Konermann L. Molecular dynamics simulations on gas-phase proteins with mobile protons: Inclusion of all-atom charge solvation. J. Phys. Chem. B 121, 8102–8112 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Cech N. B. & Enke C. G. Practical implications of some recent studies in electrospray ionization fundamentals. Mass Spectrom. Rev. 20, 362–387 (2001). [DOI] [PubMed] [Google Scholar]
- 51.Prabhu G. R. D., Ponnusamy V. K., Witek H. A. & Urban P. L. Sample flow rate scan in electrospray ionization mass spectrometry reveals alterations in protein charge state distribution. Anal. Chem. 92, 13042–13049 (2020). [DOI] [PubMed] [Google Scholar]
- 52.Meyer J. G. Qualitative and quantitative shotgun proteomics data analysis from data-dependent acquisition mass spectrometry. Methods Mol. Biol. 2259, 297–308 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Doig A. J. & Sternberg M. J. Side-chain conformational entropy in protein folding. Protein Sci. 4, 2247–2251 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Von Heijne G. Proline kinks in transmembrane alpha-helices. J. Mol. Biol. 218, 499–503 (1991). [DOI] [PubMed] [Google Scholar]
- 55.Cox J. & Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nature Biotechnology 26, 1367–1372 (2008). [DOI] [PubMed] [Google Scholar]
- 56.Brosch M., Yu L., Hubbard T. & Choudhary J. Accurate and sensitive peptide identification with mascot percolator. J. Proteome Res. 8, 3176–3181 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bantscheff M., Lemeer S., Savitski M. M. & Kuster B. Quantitative mass spectrometry in proteomics: critical review update from 2007 to the present. Anal. Bioanal. Chem. 404, 939–965 (2012). [DOI] [PubMed] [Google Scholar]
- 58.Bruderer R. et al. Optimization of experimental parameters in data-independent mass spectrometry significantly increases depth and reproducibility of results. Mol. Cell. Proteomics 16, 2296–2309 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ivanov M. V. et al. DirectMS1: MS/MS-free identification of 1000 proteins of cellular proteomes in 5 minutes. Anal. Chem. 92, 4326–4333 (2020). [DOI] [PubMed] [Google Scholar]
- 60.Bouwmeester R., Gabriels R., Hulstaert N., Martens L. & Degroeve S. DeepLC can predict retention times for peptides that carry as-yet unseen modifications. Nat. Methods 18, 1363–1369 (2021). [DOI] [PubMed] [Google Scholar]
- 61.Ma C. et al. Improved peptide retention time prediction in liquid chromatography through deep learning. Anal. Chem. 90, 10881–10888 (2018). [DOI] [PubMed] [Google Scholar]
- 62.Zeng W.-F. et al. AlphaPeptDeep: a modular deep learning framework to predict peptide properties for proteomics. Nat. Commun. 13, 7238 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gessulat S. et al. Prosit: proteome-wide prediction of peptide tandem mass spectra by deep learning. Nat. Methods 16, 509–518 (2019). [DOI] [PubMed] [Google Scholar]
- 64.Gilpin L. H. et al. Explaining explanations: An overview of interpretability of machine learning in 2018 IEEE 5th International Conference on Data Science and Advanced Analytics (DSAA) (IEEE, Turin, Italy, 2018). [Google Scholar]
- 65.Daub C. D. & Cann N. M. How are completely desolvated ions produced in electrospray ionization: insights from molecular dynamics simulations. Anal. Chem. 83, 8372–8376 (2011). [DOI] [PubMed] [Google Scholar]
- 66.Xu A. M., Tang L. C., Jovanovic M. & Regev O. A high-throughput approach reveals distinct peptide charging behaviors in electrospray ionization mass spectrometry. doi: 10.6084/m9.figshare.22268587.v1 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rappsilber J., Mann M. & Ishihama Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat. Protoc. 2, 1896–1906 (2007). [DOI] [PubMed] [Google Scholar]
- 68.Perez-Riverol Y. et al. The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res. 47, D442–D450 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chambers M. C. et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 30, 918–920 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Willems S., Voytik E., Skowronek P., Strauss M. T. & Mann M. AlphaTims: Indexing trapped ion mobility spectrometry-TOF data for fast and easy accession and visualization. Mol. Cell. Proteomics 20, 100149 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Servén D. & Brummitt C. pyGAM: Generalized Additive Models in Python 2018.
- 72.Huber P. J. Robust estimation of a location parameter. Ann. Math. Stat. 35, 73–101 (1964). [Google Scholar]
- 73.Wang G. & Cole R. B. Mechanistic interpretation of the dependence of charge state distributions on analyte concentrations in electrospray ionization mass spectrometry. Anal. Chem. 67, 2892–2900 (1995). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw files and MaxQuant analyses for our LC-MS/MS runs are available at the MassIVE data repository with ID MSV000091473. The CSD dataset generated and analyzed in this study is available at figshare66.