Abstract
To determine whether upright bicycle exercise could provide useful information about disabling exertional dyspnea in the absence of severe abnormalities (as shown by traditional testing methods), we evaluated 13 such patients. There were 3 men and 10 women with a mean age of 49 ± 15 (SD) years. We used pulmonary artery catheterization at rest and during upright bicycle exercise to evaluate these patients. All patients had normal left ventricular function except for 1, who had an ejection fraction of 45%. The mean duration to peak exercise was 9 ± 6 minutes.
Normal systolic pulmonary artery pressure was defined as 25 ± 5 mmHg. Four patients had normal systolic pulmonary pressure, and 9 exhibited pulmonary hypertension with exercise. In those 9, the mean mixed pulmonary venous oxygen saturation at rest was 61% ± 9% and fell to 32% ± 9% at peak exercise. Six of the 9 patients also had some degree of resting pulmonary hypertension that worsened with exercise: their mean pulmonary artery systolic pressure at rest was 47 ± 14 mmHg and rose to 75 ± 25 mmHg at peak exertion (P = 0.01). The other 3 patients showed no pulmonary hypertension at rest; their mean pulmonary artery systolic pressure was 27 ± 6 mmHg. However, this level rose to 53 ± 4 mmHg at peak exertion (P = 0.04).
In this pilot study of patients with dyspnea, 9 of 13 (69%) displayed marked pulmonary hypertension with exercise. The resting hemodynamic levels were normal in 3 (33%) of those with exercise pulmonary hypertension. We conclude that hemodynamic data from bicycle exercise tests can provide additional information regarding the mechanisms of exertional dyspnea.
Key words: Catheterization; dyspnea; exercise test; hemodynamics; hypertension, pulmonary/diagnosis
Diagnosis of patients who have exertional dyspnea that dramatically limits their lifestyles, yet whose basic cardiopulmonary evaluation does not disclose abnormalities severe enough to explain the degree of limitation, is problematic. Establishing a diagnosis for such patients is an ongoing challenge in cardiology and pulmonary practices. Frequently, patients with dyspnea gain weight, and the dyspnea is therefore attributed to deconditioning and obesity.
Beyond the basic evaluation (consisting of stress testing or angiography, echocardiography, pulmonary function testing, and ventilation/perfusion scanning), resting pulmonary artery catheterization is often performed at our institution to exclude pulmonary hypertension not detected echocardiographically. In order to help in the diagnosis and treatment of patients with cryptogenic dyspnea, we have recently added bicycle exercise to the pulmonary artery catheterization protocol. Exercise-induced pulmonary hypertension has been an unexpectedly frequent finding in a number of patients with exertional dyspnea.
Patients and Methods
Patients included in this pilot study were adults, at least 18 years of age, with limiting exertional dyspnea that was not fully explained by pulmonary and cardiac examinations. These evaluations included the exclusion of ischemia by stress testing (exercise or chemical radionuclide or stress echocardiography) or exclusion of significant (≥70%) coronary stenosis by angiography. Echocardiographic examinations excluded significant left ventricular dysfunction (ejection fraction <50%) or significant valvular stenosis or insufficiency (>2+ valvular insufficiency). Only 1 patient departed from this standard with a left ventricular ejection fraction of 45%. Mitral inflow evaluations of the peak early filling (E) and the atrial contraction (A) velocities and deceleration times excluded severe diastolic abnormalities. 1 Finally, ventilation/perfusion scans were used to exclude patients whose results showed an intermediate or high probability of pulmonary emboli.
Cardiopulmonary (metabolic) stress testing was not performed to measure maximal oxygen consumption, because the patients had already undergone basic cardiac and pulmonary testing to search for specific causes of their dyspnea. We hypothesized that pulmonary artery catheterization followed by exercise might yield greater insight into the pathogenesis of the exertional dyspnea.
Pulmonary Artery Catheterization. The patients were asked not to eat, drink, or take their medications for 12 hours before testing. Each patient signed an informed consent. Under sterile conditions, a local anesthetic was administered to the right (or left) neck area, followed by percutaneous insertion of a venous sheath using the Seldinger technique. An 8-F Oximetric 3® (Abbott Laboratories; Abbott Park, Ill) thermodilution catheter was then advanced from the right atrium to the pulmonary artery, and intracardiac pressures were recorded as the catheter traversed each chamber of the right heart. Serial cardiac outputs in triplicate were then obtained with the catheter in the pulmonary artery, using a standard thermodilution technique including 10 cc of dextrose solution with each injection. When more than 10% variability was found, an additional value was obtained and the outlying measurement was excluded. Continuous pulmonary arterial mixed venous oxygenation was measured through the catheter during rest and exercise. Continuous peripheral systemic oxygen saturation levels were also monitored with a digital oximeter.
Exercise Hemodynamics. The indwelling thermodilution catheter was taped securely in place with the tip remaining in the pulmonary artery. The patient was carefully assisted onto an upright bicycle exercise ergometer that was adjacent to the fluoroscopy table. With no previous training on the exercise bicycle, the patient exercised to the point of limiting fatigue, beginning at a workload of 20 watts; the workload was increased by 10 watts every 3 minutes. During exercise, heart rhythm and arterial and pulmonary artery pressure were monitored continuously; pulmonary capillary wedge pressure and arterial pressures were measured every minute. Cardiac output was measured in triplicate by the thermodilution technique during the final 2 minutes of the most advanced exercise stage that was completed.
Analysis. Right heart resting pressures and cardiac output were compared with the same parameters that were measured during bicycle exercise. A paired t-test was used to perform the comparisons. A P value of <0.05 was considered significant.
Results
Thirteen patients underwent bicycle hemodynamic evaluation from December 1997 through August 1998. The group consisted of 3 men and 10 women with a mean age of 49 ± 15 (SD) years. Coexisting medical conditions included current tobacco use in 2, diabetes mellitus in 1, hypertension in 6, and obesity in 4. Underlying connective tissue disease was present in 3 patients: systemic lupus erythematosus in 1, scleroderma in 1, and Sjögren's syndrome in the 3rd. The patients were taking various medications: 6 were taking diuretics; 2, vasodilators; 3, beta-blockers; 3, central alpha agonists; and 2, nitrates.
Chest Radiographs. Chest radiographs showed evidence of interstitial lung disease in 2 patients. It was mild and nonspecific in both.
Pulmonary Function Assessment. The following results refer to the 9 patients who had pulmonary hypertension with exercise. Results of pulmonary function tests were abnormal in some patients (see below), although often not enough to explain the patients' marked exercise limitation.The forced vital capacity (FVC) was ≥70% of the predicted value for age, sex, and weight in 6 (67%) of the patients. In the others, the FVC ranged from 52% to 68% of the predicted value. The forced expiratory volume at 1 second (FEV1) was ≥70% of the predicted value in 6 (67%) of the patients. In the remainder, the FEV1 ranged from 49% to 65% of the predicted value.
The forced expiratory flow, midexpiratory phase (FEF25%–75%) was ≥70% of the predicted value in 5 (56%) of the patients; in the others it ranged from 20% to 61% of the predicted value. The diffusing capacity of the lung for carbon monoxide (DLCO) was 80% of the predicted value in only 1 patient; the DLCO ranged from 20% to 66% of the predicted value in the remaining 8 patients.
Echocardiographic Parameters of Diastole and Right Ventricular Pressures. Normal diastolic mitral filling patterns were present in all but 3 patients. In these 3, delayed relaxation was seen with deceleration times exceeding 240 msec; however, these findings were expected in 2 of the 3 patients because of age.
Right ventricular systolic function was normal in 9 patients, as shown by echocardiography. Qualitatively, the function was mildly impaired in 1, moderately impaired in 1, and moderately-to-severely impaired in 2 patients.
With the use of tricuspid insufficiency data recorded by Doppler ultrasound, 2 right ventricular systolic pressures (RVSPs) were estimated, yielding a range of 31 to 65 mmHg, with a mean of 44 ± 12 mmHg. Those values were then used to screen for resting pulmonary hypertension. Estimated RVSPs were unobtainable in 4 patients due to the absence of tricuspid insufficiency. In 3 others, the echocardiographic RVSP was quite disparate with the resting pulmonary artery pressures (PAPs), a finding that was inconsistent with previously published data. 2
Bicycle Hemodynamics. Normal resting systolic PAP was defined as ≤25 ± 5 mmHg. 3 There were 4 patients with normal PAPs and 9 with exercise-induced PH. Six of these 9 also had some degree of resting PH; the other 3 had normal resting systolic PAPs as defined above 3 but exceeded normal peak exertion systolic PAPs (>34 ± 6 mmHg), as defined by Higginbotham 4 for upright bicycle exercise. The mean duration to peak exercise for the group was 9 ± 6 minutes. The average exercise duration was 11.7 minutes for the 4 patients with normal PAPs, 12.7 minutes for the 3 with normal resting PAPs that rose excessively with exercise, and 4.5 minutes for the 6 with resting PH.
Table I and Figures 1 through 5 present the hemodynamic results, excluding the 4 patients who did not develop exertional PH. In those with normal resting PAPs (n = 3), the PA systolic pressure rose from 27 ± 6 to 53 ± 4 mmHg at peak exercise (P = 0.04). In those with some resting PH (n = 6), the PAP rose from 47 ± 14 to 75 ± 25 mmHg at peak exercise (P = 0.01).
Table I. Hemodynamics Data in Patients with Exercise Pulmonary Hypertension (n = 9)
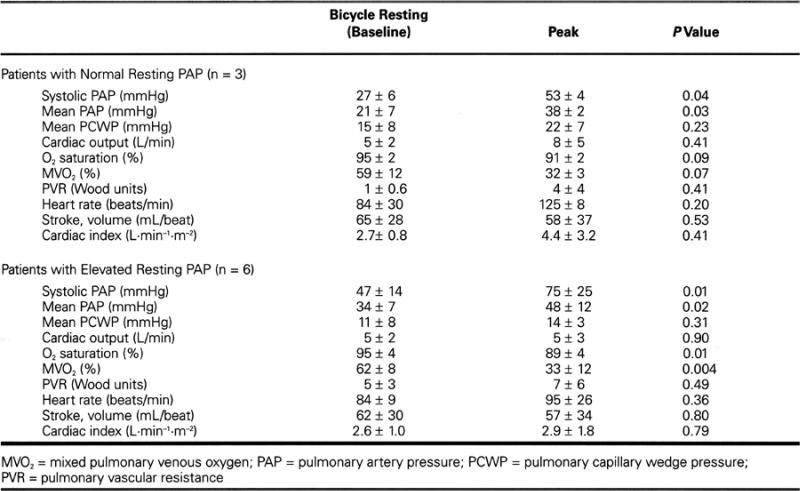
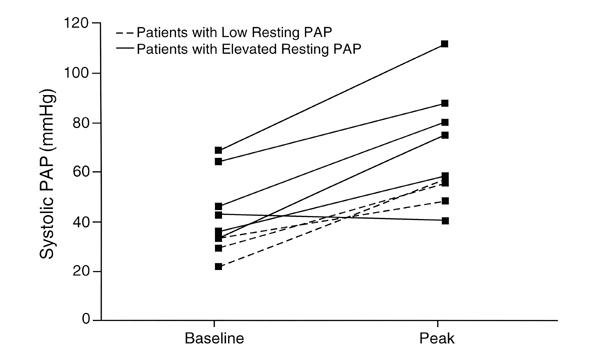
Fig. 1 Bicycle resting and peak pulmonary artery systolic pressures.
PAP = pulmonary artery pressure
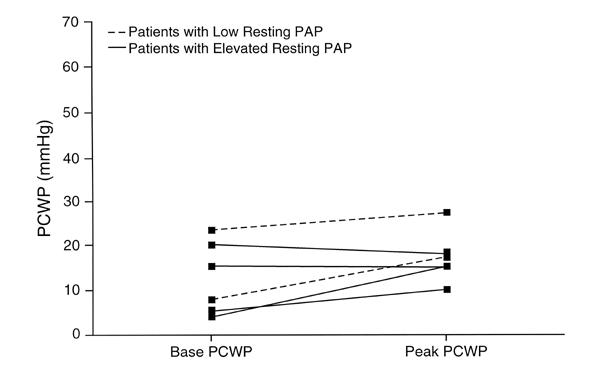
Fig. 2 Bicycle resting and peak pulmonary capillary wedge pressure.
PAP = pulmonary artery pressure; PCWP = pulmonary capillary wedge pressure
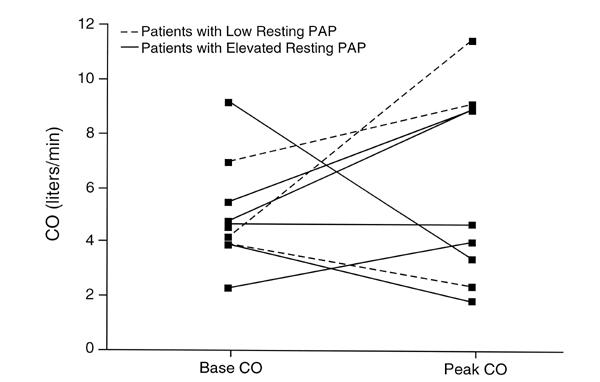
Fig. 3 Bicycle resting and peak cardiac output.
CO = cardiac output; PAP = pulmonary artery pressure
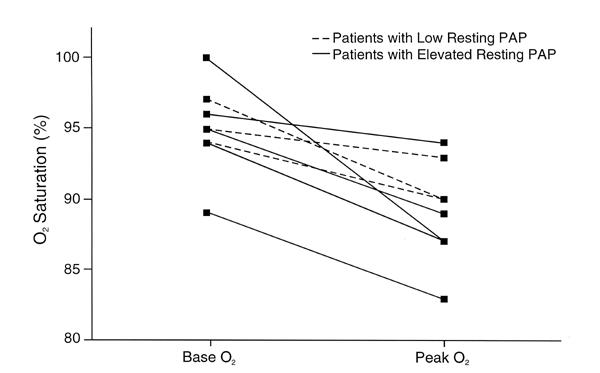
Fig. 4 Bicycle resting and peak systemic oxygen saturation.
O2 = oxygen; PAP = pulmonary artery pressure
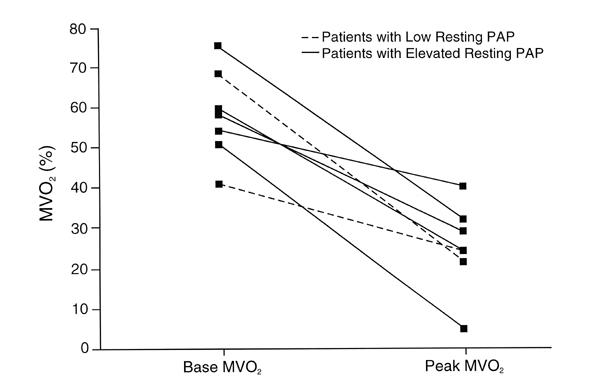
Fig. 5 Bicycle resting and peak mixed venous oxygenation.
MVO2 = mixed venous oxygenation; PAP = pulmonary artery pressure
In the 4 patients who did not develop exercise-induced PH, results of the exercise studies were normal in 3. The 4th patient displayed volume depletion with a low pulmonary capillary wedge pressure and an otherwise negative exercise study.
Desaturation of mixed pulmonary venous oxygen with exercise occurred in those patients with PH: the mean resting level of mixed pulmonary venous oxygen was 61% ± 9% and fell to 32% ± 9% at peak exercise (P = 0.001). The response of peripheral arterial oxygen saturation is shown in Table I and Figure 4.
Discussion
Much of the literature on exercise hemodynamics is written about patients with congestive heart failure. There are few data concerning patients who have exertional dyspnea in the absence of heart failure. As reported by Chandrashekhar and Anand, 5 the resting hemodynamics variables in patients with heart failure do not correlate well with their exercise capacity, as measured by treadmill maximal oxygen consumption. Exercise hemodynamic responses, on the other hand, provide additional physiologic and prognostic information.
Bicycle exercise testing has been performed in healthy patients, describing the normal hemodynamic response to exertion. Thadani and Parker 6 reported that in 10 healthy patients at supine rest, the mean PAP was 13 ± 1 mmHg and the pulmonary capillary wedge pressure was 6 ± 1 mmHg. These pressures rose to a mean PAP of 22 ± 1 mmHg and a pulmonary capillary wedge pressure of 8 ± 1 mmHg with bicycle exercise. The cardiac index rose from 3.5 ± 0.3 to 7.3 ± 0.5 L.min−1.m−2.
Higginbotham and co-authors 4 described the hemodynamic response to upright bicycle exercise in “normal man.” In their series, the mean systolic PAP in patients at rest in an upright position was 15 ± 4 mmHg and rose to 34 ± 6 mmHg at peak exertion. The cardiac index increased 3.2-fold, from 3.0 to 9.7 L.min−1.m−2.
Treadmill exercise has been shown to elicit greater circulatory reserve than bicycle exercise, with higher peak oxygen consumption. 7 However, the increase in oxygen uptake is more regular with bicycle than with treadmill exercise. Page and coworkers 7 reported that the coefficient of correlation between oxygen uptake and time was greater with bicycle than with treadmill exercise (r = 0.97, P <0.001). Moreover, stationary bicycle exercise is technically easier than treadmill exercise and more accurate when done in conjunction with hemodynamic studies due to less motion artifact and tendency for catheter dislodgment and migration. In terms of the best position for bicycle exercise, Kramer's group 8 has shown that exercise capacity is greater when the exercise is performed in the upright position rather than the supine position.
In this pilot study, we applied a testing method not generally used in dyspnea evaluations to demonstrate an actual mechanism for the dyspnea. It is remarkable that exercise-induced PH was diagnosed in 70% of these preselected patients, indicating that its occurrence may not be rare in patients with these clinical symptoms. Of note, during the initial evaluations by echocardiography, the RVSPs did not consistently predict the resting PAPs. In 4 patients with RVSPs ranging from 46 to 65 mmHg, 2 had normal resting PAPs. Furthermore, 4 patients did not have tricuspid insufficiency recorded by Doppler ultrasound; therefore, we were not able to calculate the RVSP to exclude pulmonary hypertension.
The pulmonary vascular resistance rose in our patients (Table I), suggesting reduced pulmonary vasodilatory capacity. Exercise-induced mitral insufficiency was another possible explanation for elevated PAPs, although the hemodynamic data did not strongly support this.
In most of our patients, pulmonary function testing revealed FVC and FEV1 values that were not, in our opinion, abnormal enough to explain the marked exertional dyspnea in terms of obstructive or restrictive lung disease alone. We specified a cutoff of ≥70% predicted (as opposed to higher percentages) to avoid overly rigid diagnoses of mild vital capacity and expiratory volume abnormalities. Certainly, the FEF25%–75% and diffusing capacity did suggest important underlying abnormalities in several of the patients, although the exercise hemodynamic responses provided additional physiologic information.
Exercise-induced PH may represent an early clinical stage of pulmonary hypertension, including primary pulmonary hypertension. Primary pulmonary hypertension is characterized by extensive remodeling of the pulmonary vessels. 9 Of note, there is a sexual bias in this pilot study, since 10 of the 13 patients were women; however, primary pulmonary hypertension predominantly affects young to middle-aged women. The median survival rate for patients with primary pulmonary hypertension is 2.6 years from the time of diagnosis. A genetic predisposition may be involved, triggered by a stimulus such as medication or even HIV infection. 9 Treatments include calcium antagonists, anticoagulants, prostacyclin, and lung transplantation in cases refractory to medical therapy. 10–12
Preexisting connective tissue disease was present in 3 of our patients. Their systolic PAPs at peak exertion ranged from 40 to 88 mmHg. These results may support the hypothesis that exercise-induced PH represents an early stage of PH in the setting of connective tissue disease.
In all the patients with exercise pulmonary hypertension, there was a decline in mixed pulmonary venous oxygenation during bicycle exercise testing. This decline indicates increased oxygen extraction, likely due to the inadequacy of the cardiac output to meet the peripheral circulatory demands. Higginbotham and colleagues 4 have previously reported that cardiac index increases 3.2-fold with exercise in normal subjects; Table I and Figure 3 indicate that the cardiac output response appeared subnormal in a number of our patients.
Stress echocardiography is another method of evaluating dyspnea that deserves mention. Bach 13 has reported that stress echocardiography makes possible the assessment of right ventricular systolic pressures with exercise. This may be a useful screening technique in some patients, although the exercise hemodynamic evaluation yields more exacting PAP data in addition to other direct hemodynamic measurements.
This small pilot study has several limitations. We did not measure peak oxygen consumption with cardiopulmonary stress testing in most patients; instead, we proceeded directly to invasive bicycle hemodynamics after the basic evaluation. Our rationale was that the bicycle study would potentially yield more specific etiologic information in terms of PAPs and left ventricular filling pressures, as well as cardiac output. Certainly, including gas analysis for oxygen consumption and adding exercise pulmonary function tests would have provided further information and may be added to future investigations.
The small sample size is another limitation, even though this was a pilot study. Only 3 of the original 13 patients (23%) had PH only with exercise; we nonetheless concluded that PH was demonstrable in this small subset of patients using the bicycle studies. Another limitation is the heterogeneity of the study group. Ideally, a group of “cryptogenic” patients with dyspnea who have totally normal pulmonary function should be studied, but it is difficult in clinical practice to select patients with completely negative backgrounds. Nevertheless, this dissimilarity complicates the interpretation of the data.
Finally, abnormalities of diastolic function remain in the differential diagnosis of dyspnea. In this study, we excluded severe diastolic impairment noninvasively, by use of mitral inflows. We did not measure pulmonary venous flows or isovolumic relaxation times; nor did we measure diastolic function invasively, other than for evaluation of the right ventricular waveforms to exclude constrictive or restrictive patterns.
Conclusion
In this pilot study, the use of bicycle hemodynamics in patients with exertional dyspnea led to the finding of exertional PH that might not otherwise have been diagnosed. Whether earlier treatment has any impact on the prognosis for patients with pulmonary hypertension remains to be determined. However, the ability to provide a diagnosis and the possibility of symptom improvement constitutes a step forward for some patients with limiting dyspnea. Further investigation is necessary to establish conclusive data and to gain additional insight into unexplained exertional dyspnea.
Footnotes
Address for reprints: Karen B. James, MD, Desk F25, Section of Heart Failure, Department of Cardiology, Cleveland Clinic Foundation, 9500 Euclid Avenue, Cleveland, OH 44195
References
- 1.Appleton CP, Hatle LK, Popp RL. Relation of transmitral flow velocity patterns to left ventricular diastolic function: new insights from a combined hemodynamic and Doppler echocardiographic study. J Am Coll Cardiol 1988;12:426–40. [DOI] [PubMed]
- 2.Yock PG, Popp RL. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation 1984;70:657–62. [DOI] [PubMed]
- 3.Grossman W, Braunwald E. Pulmonary hypertension. In: Braunwald E, editor. Heart disease: a textbook of cardiovascular medicine. Philadelphia: WB Saunders, 1988:793–818.
- 4.Higginbotham MB, Morris KG, Williams RS, McHale PA, Coleman RE, Cobb FR. Regulation of stroke volume during submaximal and maximal upright exercise in normal man. Circ Res 1986;58:281–91. [DOI] [PubMed]
- 5.Chandrashekhar Y, Anand IS. Do hemodynamic indices correlate with maximum exercise capacity and oxygen consumption in chronic congestive heart failure? Indian Heart J 1992;44:19–22. [PubMed]
- 6.Thadani U, Parker JO. Hemodynamics at rest and during supine and sitting bicycle exercise in normal subjects. Am J Cardiol 1978;41:52–9. [DOI] [PubMed]
- 7.Page E, Cohen-Solal A, Jondeau G, Douard H, Roul G, Kantelip JP, Bussiere JL. Comparison of treadmill and bicycle exercise in patients with chronic heart failure. Chest 1994;106:1002–6. [DOI] [PubMed]
- 8.Kramer B, Massie B, Topic N. Hemodynamic differences between supine and upright exercise in patients with congestive heart failure. Circulation 1982;66:820–5. [DOI] [PubMed]
- 9.Rubin LJ. Pathology and pathophysiology of primary pulmonary hypertension. Am J Cardiol 1995;75:51A–54A. [DOI] [PubMed]
- 10.Mesa RA, Edell ES, Dunn WF, Edward WD. Human immunodeficiency virus infection and pulmonary hypertension: two new cases and a review of 86 reported cases. Mayo Clinic Proc 1998;73:37–45. [DOI] [PubMed]
- 11.Rich S. Medical treatment of primary pulmonary hypertension: a bridge to transplantation? Am J Cardiol 1995; 75:63A–66A. [DOI] [PubMed]
- 12.Rubin LJ. Primary pulmonary hypertension. N Engl J Med 1997;336:111–7. [DOI] [PubMed]
- 13.Bach DS. Stress echocardiography for evaluation of hemodynamics: valvular heart disease, prosthetic valve function, and pulmonary hypertension. Prog Cardiovasc Dis 1997; 39:543–54. [DOI] [PubMed]


